Abstract
In musculoskeletal and sports medicine, pain has traditionally been linked to tissue injury, often assuming a linear correlation between tissue damage and pain intensity. However, modern pain science has illuminated the complexity of the human pain experience, incorporating psychosocial elements, nervous system sensitization, immune responses, and structural changes in the brain as factors. This contemporary understanding of pain has proven highly beneficial for both clinicians treating individuals in pain and those experiencing pain.
Pain neuroscience education (PNE) provides individuals in pain with an understanding of the underlying neurobiology and neurophysiology of their pain experience, which has been shown to result in decreased self-reported pain, reduced disability, the alleviation of fear and fear-avoidance behaviors, diminished pain catastrophizing, and improved movement. Currently, research on PNE predominantly focuses on interventions with individuals with persistent or chronic pain conditions. However, those who experience acute, sub-acute, and perioperative pain also have the potential for elevated levels of fear, fear-avoidance, and pain catastrophizing, indicating potential benefits from PNE.
This invited commentary seeks to inform readers about the latest advancements in pain science and propose a conceptual model for delivering PNE in acute pain experiences.
Level of Evidence
5
Keywords: acute pain, athletes, fear avoidance, pain catastrophizing, pain neuroscience education
INTRODUCTION
Pain is an everyday human experience designed to warn an individual in the case of danger and ultimately have them take action to reduce or eliminate the threat.1 For example, suppose a football player hurts their knee in a tackle. In that case, pain is a critical warning sign to reduce the threat by stopping what they’re doing (running on the leg) and take action by getting it further evaluated by the medical team. Throughout the history of pain science, human pain experiences, especially in musculoskeletal medicine have been tied to the health of the person’s tissues, including the stages and duration of healing.2 Traditionally, society has been taught and expects pain to be present in the event of an injury, and as tissues heal, pain eases, and a person returns to their prior level of activity.3 Persistent pain does not follow this trajectory, and often, despite tissue healing, the pain experience continues.1 This not only significantly impacts the person dealing with pain but also increases challenges faced by medical providers when seeking ways to address persistent pain.4
Biomedical models for understanding pain, tying tissue health to pain, have been scrutinized in the last two to three decades based on significant advances in pain neuroscience. Apart from the relationship to tissue health, it is now well understood that a human’s pain experience is complex, unique to each person, consisting of a delicate interplay between tissue-related issues, peripheral neuropathic processes, immune function, brain processing, psychosocial variables, sensitization of the peripheral and central nervous system, neuroplasticity, endogenous mechanisms, and more.1,5 The progress made in pain science, particularly concerning persistent pain, has propelled research forward and this progress in pain science has undergone rigorous testing and validation in clinical settings. Additionally, pain science been integrated into entry-level programs for medical professionals, and has shown positive changes towards patients with persistent pain.
One treatment at the forefront of non-pharmacological treatment of persistent pain is pain neuroscience education (PNE).6 Currently, almost twenty systematic reviews and meta-analyses examining PNE for various persistent pain conditions have consistently demonstrated significant benefits in reducing self-reported pain, disability, fear-avoidance, pain catastrophizing, and positively impacting physical movement and healthcare expenses.7–9 In contrast to the increasing evidence of PNE effectiveness for persistent pain, only a limited number of studies have explored its potential benefits for acute pain experiences.10,11 Given that pain, including acute and sub-acute forms, is a universal human experience, this invited commentary seeks to delve into the application of PNE for athletes experiencing acute pain episodes.
PAIN SCIENCE UPDATE
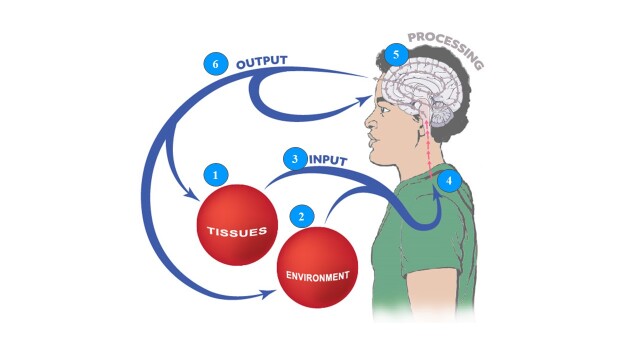
PNE seeks to provide individuals in pain with an understanding of the underlying neurobiology and neurophysiology of their pain experience, as well as the psychosocial dimensions of their pain experience, collectively known as the neuroscience of pain.12 A critical element of this approach is the provider’s knowledge and understanding of modern pain science.13 Given the recent advances in pain science, a starting point for all clinicians is an updated review of pain science, which will then allow for an enhanced ability to educate people they encounter who experience pain. The model chosen for this education is the Mature Organism Model, described by Gifford,14 which represents the critical biological and psychosocial processes underpinning a human pain experience (Figure 1).
Figure 1. Mature Organism Model of pain – adapted from Gifford.14 Used with permission.
Tissue-Related Issues
Tissue injury is well described and understood by medical providers and often follows the predicted stages and timelines of healing.3 Nociceptors are stimulated (mechanically, chemically, or thermally), initiating an electrochemical impulse into the central nervous system (CNS), passed onto the brain for processing, and typically a pain experience ensues, which garners the attention of the individual.1 Most pain experiences in life, including those that occur during sport participation, fit within this category and are referred to as a nociceptive driven pain experience. In fact, epidemiological research data from outpatient physical therapy (PT) indicates that approximately 55% of patients attending PT fit into this category.15,16 In a nociceptive driven pain experience, the pain experienced is typically proportionate to the input stimulus, presents with very definite aggravating and easing factors, is often described as a dull ache or a throb at rest, and does not include neurological symptoms associated with sensory changes (numbness, tingling, loss of sensation, etc.).17 Although the aforementioned process is the most common, some key exceptions should be mentioned. First, injury and pain are not synonymous – people can experience an injury and no pain, and conversely, many people experience pain with no tissue injury.1,2 A poignant example is the growing body of evidence from imaging studies on pain-free individuals demonstrating various tissue anomalies, including bulging discs, arthritic age-related changes, rotator cuff tears, hip labral issues, and more.18,19 Second, and perhaps more critical, is to acknowledge that when nociceptors are stimulated from the tissues, they will only send nociceptive information (or danger messages) into the CNS for the brain to process. They do not send pain messages to the brain.20 It is the brain that processes this information, adds context to it from various other sources, and ultimately produces pain (or not). Examples include noticing a bruise on one’s body or blood (tissue injury), but not knowing where it came from.
Environmental Issues
All pain experiences occur in the context of varied environments. Environmental factors powerfully influence pain – by either increasing or decreasing the pain experience. For example, an athlete who sprains their ankle while their team is winning will represent a different experience (including pain experience), compared to when they are losing. Support for this notion arises from research demonstrating that children engaged in contact sports exhibit decreased sensitivity later in life to painful stimuli, i.e., injections.21 Additionally, despite experiencing repetitive and forceful whiplash injuries, demolition derby drivers often report minimal to no lasting pain.22 Furthermore, recent findings linking pain to psychosocial factors such as stress and anxiety reveal that children involved in individual sports face a significantly higher risk of mental health issues compared to those participating in team sports or none at all.23 This is important to consider in athletes because stress and anxiety are known to increase pain experiences. These examples underscore how psychosocial factors, beyond mere tissue health, significantly influence the pain experience.
Peripheral Nervous System
The human body is thought to contain over 400 individual nerves, with sensory nerves designed to electrochemically transmit information to the CNS and, ultimately, the brain for processing.24 Complex biological processes during a pain experience such as demyelination, ion channel upregulation, blood flow changes, and glial cell activation can result in an upregulation of the peripheral nervous system.24 This is normal and part of the architecture of the “pain system” to warn the individual of danger. This “waking” or senstizing of the nervous system is designed to protect. As pain eases and tissues heal, typically, the sensitivity of the nervous system decreases allowing a person to return to their prior activities “pain-free.” For example, when an athlete sprains their ankle, the nervous system in and around the ankle increases its sensitivity (hyperalgesia) to protect the ankle. As the ankle recovers, it remains “sensitive” as the nervous system also adapts its response to the injury and healing process. In approximately 25% of patients attending outpatient PT, the peripheral nervous system does not calm down and becomes the main driver of the pain experience, even when tissues have healed.15,16,25 This is referred to as peripheral neuropathic pain and includes conditions such as radiculopathy, and nerve compression syndromes such as carpal tunnel and cubital syndrome. Clinically, these patients display symptoms in a dermatomal or cutaneous nerve distribution, positive neurodynamic tests, sensitivity to nerve palpation, and neurological symptoms (numbness, pins and needles, etc.).26
Central Nervous System
The next process, which highlights the interaction between the peripheral nervous system and the central nervous system, has garnered considerable attention in the field of pain science. In the dorsal horn of the spinal cord, information from the periphery is received with the intent to pass that information on to the brain for processing.1 Inhibitory and excitatory neurotransmitters interact with receptors, allowing the information to be blocked (gating) or continue on to second-order neurons to pass the information on to the brain. In acute, sub-acute, and postoperative pain experiences, the dorsal horn becomes bombarded with nociceptive information from the target tissue; the dorsal horn plays an essential role in the acute pain experience and then reverts back to a normal state as tissues heal. In persistent pain, however, continued bombardment from the periphery results in permanent changes at the dorsal horn, including loss of inhibitory interneurons, expansion of receptor fields, upregulation of second-order neurons, decreased endogenous analgesia, and more, resulting in a heightened sensitization of the CNS to peripheral input – hyperalgesia and allodynia.1,2,5,20 This process is labeled as a nociplastic pain, with a clinical presentation of disproportionate pain, diffuse tenderness to palpation, disproportionate aggravating and easing factors, and psychosocial issues – typically high levels of fear, fear-avoidance, depression, and pain catastrophizing.27
Brain Processing
Since the emergence of brain scan technology in the early 1990s, scientists have significantly enhanced their understanding of the role of the brain in human pain experiences.28,29 It is now well established that a human pain experience consists of widespread brain activation of various functional parts of the brain, including key areas such as the amygdala (fear-conditioning), anterior cingulate cortex (focus and concentration), hippocampus (memory), motor and pre-motor corteces (planning and execution of movement), and more.30,31 The distributed brain activity observed during a painful experience signifies a functional change within the brain, known as the pain neuromatrix.30,31 Each involved brain area is part of the pain experience, potentially resulting in the sub-optimal function of the tasks normally associated with each area, which can lead to altered motor control (motor and pre-motor cortex), decreased memory (hippocampus), reduced focus and concentration (anterior cingulate cortex) as examples.30 What renders the human pain experience unique is the activation of various regions of the brain, which not only process the perceived threat but also interact with other existing cognitive maps linked to memory, beliefs, and past experiences. This extensive activation across multiple brain regions renders the pain experience distinctive for each individual, influencing the optimal functioning of the brain.30,31
Output Mechanisms
The concluding stage of the mature organism model is output, encompassing diverse biological and physiological reactions to the input and processing of information, including motor, immune, linguistic, sympathetic, and other responses.1 For example, for a soccer player who sustains an ankle sprain, information is sent to the CNS and brain for processing, and if the brain perceives a threat, pain will be produced by the brain to protect the ankle, and ultimately the athlete. Pain is an output of the brain to get a person to stop, pay attention to the situation, and seek help. Likewise, an individual might experience anger (sympathetic response), utter a choice word or two (linguistic expression), or clutch their leg (motor action). The athlete, in this case a soccer player who sprains their ankle, experiences pain, vocalizes expletives, and instinctively grasps their leg as they collapse to the ground. Finally, since this is a feedback model, these physiological processes also impact tissue, the peripheral nervous system, and senses, which feed into the CNS.14
The mature organism model (Figure 1) provides a quick, updated view of pain science and is a great place for medical providers to develop an understanding of modern pain science, with two key takeaways. First, research indicates that healthcare providers who deepen their understanding of pain tend to exhibit increased empathy and compassion toward individuals experiencing pain. They also tend to employ less provocative biomedical language and approaches when addressing pain, adjust their clinical practices to better treat individuals in pain, and ultimately achieve better patient outcomes.13,32,33 Second, and perhaps of greater significance, studies have demonstrated that enhancements in pain knowledge among patients have a beneficial impact on various aspects including self-reported pain ratings, disability, fear and fear-avoidance, pain catastrophizing, willingness and ability to move, as well as healthcare expenditures.7–9 For athletes this is very important, since pain, fear and catastrophizing all have the potential to impact movement, performance, motor control, concentration, which are essential for optimial performance.
PAIN NEUROSCIENCE EDUCATION
Pain Neuroscience Education
The process of teaching a patient about the underlying biology and physiology of pain is referred to as PNE.12,34 PNE emerged due to the inadequacies of the traditional biomedical educational model in addressing pain in light of current scientific knowledge, the complexities of treating persistent pain, and rising rates of pain prevalence.35 PNE has gained considerable scrutiny from various systematic reviews and meta-analyses, with increasing evidence for its efficacy, especially for those with persistent pain. However, more recently, it has been shown that the combination of PNE and a physical treatment, i.e., exercise, manual therapy, etc., is superior to PNE alone.36,37 This concept of PNE plus (PNE+) aligns with behavioral medicine research showing that education-only approaches are not successful in a achieving change in patient behaviors.38 In musculoskeletal medicine, this would imply that providers who use movement-based therapy, with the addition of PNE, deliver PNE+, which aligns with current best-evidence care for people with pain, especially those with persistent pain.38
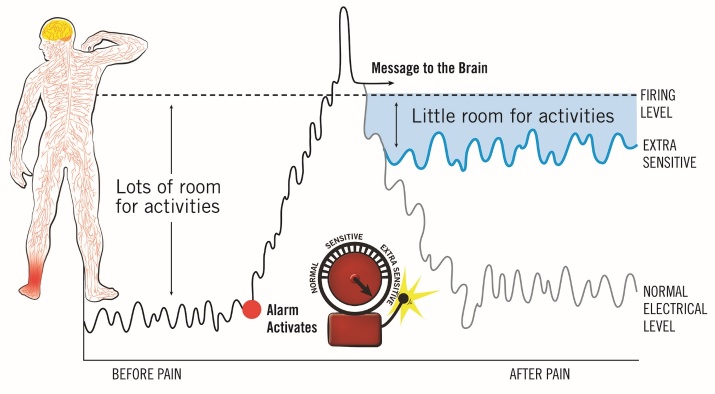
PNE is best delivered using metaphors, examples, and images (Figure 2).6,9 This can be done verbally in one-on-one, group sessions, or via digital therapeutics, i.e., telehealth or virtual reality. As with exercise, education should also be paced, and it is recommended that PNE be delivered in short, digestible sessions, complimented with physical treatments (i.e., stretches, exercises, neurodynamics, manual therapy, etc.), resulting in the PNE+ approach.6 During subsequent clinical interactions, different or additional metaphors are used to teach the patient about various aspects of their pain experience, carefully matching their clinical presentation with a metaphor designed to systematically increase the patient’s understanding of their pain and steadily reduce fear and catastrophizing, which allows them to move more and more over time, thereby allowing for pacing and graded exposure to movement and return to function.
Figure 2. Pain neuroscience education example using a metaphorical sensitive alarm system analogy – used with permission.6.
PNE that has gained considerable attention for the clinical application of identifying patients that would respond favorably to PNE. Various studies have shown that patients presenting with some or all the clinical presentations in Table 1 are ideally suited for a PNE or PNE+ approach.6,39,40
Table 1. Clinical presentations that respond favorably to a PNE/PNE+ approach with clinical characteristics and proposed screening tools.
| Central sensitization27 | |
| Widespread pain; diffuse palpation tenderness; disproportionate aggravating and easing factors and psychosocial issues (i.e., fear avoidance, depression, etc.) | Central sensitization inventory (CSI) ≥ 40 |
| Persistent pain20 | |
| Ongoing, persistent pain beyond normal expected healing phases | Persistent pain > 3-6 months |
| High fear-avoidance41 | |
| Expressing fear related to pain, including movement, activities, work, etc. Displaying fear and fear-avoidance in the presence of pain. |
Fear-avoidance beliefs questionnaire (FABQ)42
Tampa Scale of Kinesiophobia (TSK)
|
| High pain catastrophizing43 | |
| Inability to foresee anything other than a negative outcome. Heavily tied to poor beliefs and expression regarding pain, including hyper focus on pain, biomedical views of pain, etc. |
Pain catastrophizing scale (PCS)43
|
| Readiness for change44 | |
| Patients beyond pre-contemplation phase – contemplative, planning and action phase. Interest in changing their clinical situation and being an active participant. | Stages of Change Readiness and Treatment Eagerness Scale45 Subjective clues regarding their interest in changing situation, including active participation in treatment. |
An examination of Table 1 unmistakably suggests that individuals exhibiting persistent, widespread pain characterized by elevated levels of fear and fear-avoidance, as well as pain catastrophizing, would likely benefit from PNE. An intriguing inquiry arises: Do any of the items listed in Table 1 serve as stronger indicators of the success of PNE? Remarkably, and crucially for this commentary, it has been demonstrated that both high initial levels of fear-avoidance and pain catastrophizing can potentially predict a favorable outcome to PNE.6,39,40 This holds significance as elevated levels of fear and pain catastrophizing have been linked to unfavorable outcomes in orthopedic surgery, acute and sub-acute pain episodes, and return to sport.46–49 This prompts the question: Can PNE offer benefits for those experiencing acute and sub-acute pain experiences, such as an athlete with an acute ankle sprain?11
To date, only a small number of studies have investigated the use of PNE for acute, sub-acute, and perioperative pain. Research on preoperative PNE interventions for lumbar surgery, total knee arthroplasty, and shoulder surgery has demonstrated positive effects on surgical outcomes, patient experiences, nervous system sensitization, and healthcare costs post-surgery.50–53 Zimney and Louw have suggested that individuals with higher fear-avoidance levels during the acute phase of low back pain can derive benefits from PNE interventions.10,11 Although the evidence for PNE in treatment of those with acute and sub-acute pain is not as extensive as for those with persistent pain, emerging research supports its clinical application in cases where individuals exhibit high levels of fear-avoidance and/or pain catastrophizing. A notable distinction, however, is that while PNE typically de-emphasizes anatomical education in persistent pain cases, some degree of anatomical education may be necessary in acute pain scenarios due to patient expectations.54
CLINICAL APPLICATION
Considering that the majority of research on PNE focuses on persistent pain, there is a clear need for invited commentary on this topic. This commentary seeks to demonstrate how PNE can and should be utilized for individuals experiencing acute pain, such as athletes with ankle sprains, who exhibit high levels of fear-avoidance and pain catastrophizing. Such individuals are at risk of experiencing prolonged recovery periods, which can delay their return to regular activities and sport participation.
Case study: Soccer player with an acute ankle sprain
Clinical presentation
Hailey is an 18-year-old high school senior soccer player who was referred to physical therapy (PT). She plays as a striker and sustained a right ankle sprain two days ago during a tackle in a soccer game. Immediately after the incident, she experienced acute pain and swelling. The athletic trainer attended to her on the field, and she had to leave the game due to the severity of the ankle sprain. The Ottawa Ankle Rules screen was inconclusive regarding potential foot and ankle fractures, so she underwent x-ray imaging. She was provided with crutches to maintain non-weight bearing status until she saw the team’s physician the following day. The x-rays showed no fractures, and she was diagnosed with a grade II inversion ankle sprain. She was advised to continue using crutches, gradually transitioning to weight-bearing as tolerated, given an ace wrap for edema control, and referred for outpatient PT.
During her examination, Hailey reported a pain rating of 5 out of 10 on the Numeric Pain Rating Scale. She exhibited limited range-of-motion in her right ankle, ankle swelling, and significant fear of moving her ankle. The athletic trainer informed the attending PT that Hailey was deeply upset about the injury because she is a senior and is concerned about playing during her final year of high school. Additionally, she is worried that the injury could impact her chances of receiving a collegiate scholarship. As part of the examination, the attending PT conducted a Pain Catastrophizing Scale (PCS) and Fear-Avoidance Beliefs Questionnaire (FABQ) screening survey, resulting in a PCS score of 32, FABQ-Physical Activity (PA) score of 17, and FABQ-Work Subscale (WS) score of 12.
Interpretation
Traditional biomedical education for a case like this typically involves a detailed explanation of ankle anatomy, ligaments, biomechanics, and injury mechanisms. This explanation often utilizes anatomical models or posters mounted in the clinic.55 Commonly used terms such as “tear” or “ripped” may be employed, along with in-depth anatomical terminology like “anterior talofibular ligament”.56 However, research suggests that this approach may not be beneficial in reducing fear or promoting recovery; in fact, it could increase fear and catastrophizing, both of which are linked to heightened pain experiences.41,56 The results of the screening tools indicate that the athlete is experiencing high levels of acute pain, fear of movement, and catastrophizing, likely exacerbated by concerns about missing the remainder of the season.42,43
Focusing solely on a biomedical approach to education could potentially prolong recovery in this scenario.2,57 Therefore, it is suggested that clinicians adopt a dual-model approach. This approach would involve addressing the biomedical aspects to aid in tissue healing and recovery, while also integrating PNE and a PNE+ approach to facilitate holistic healing and recovery individualized to the patient.
Biomedical education
Although biomedical education has faced criticism, clinicians must still recognize the value of providing a thorough explanation of the patient’s injury and diagnosis. Several qualitative studies have highlighted that patients desire more information about their diagnosis. When this need is not met, patients often resort to seeking additional information, including from the internet and websites.54,58 Biomedical education needs to evolve by providing patients with essential information while avoiding language that triggers catastrophizing and fear (“words that harm”). It should use explanations that fulfill patient needs without inducing fear and catastrophizing by using “words that heal”, and should incorporate reassurance (refer to Table 2).56 In the case of this soccer player, it’s recommended to use the term “sprain,” which is less alarming than “tear” and accurately describes the current situation.56 Additionally, it is crucial to acknowledge tissue injury and validate the patient’s current condition. Education should encompass information on diagnosis (what is wrong with me?), prognosis (how long with it take?), self-help strategies to empower the patient and boost self-efficacy (what can I do for it?), and the overall treatment plan from the medical team (what will you do for it?). (Refer to Table 2 for details).54
Table 2. Example biomedical patient education.
| What is wrong with me? | How long will it take? |
| Ankles are designed to move. When we suddenly move in a direction, we may sprain a ligament, resulting in a sprained ankle, just like what you experienced. With an ankle sprain there is some swelling and bleeding, which is normal and expected, but over time this will get better, along with the pain. | Over time the pain will ease, swelling come down and the ankle will get better. With an ankle sprain like yours, we expect this to be much better in 1-2 weeks. You’re young, healthy, and eager to get back to soccer, all of which will help recovery. We will see you three times a week for two weeks and expect you to be much better in two weeks. |
| What can I do for it? | What will you do for it? |
| In therapy we will work on getting you better but there’s a lot you can do to help at home. Ice as needed to help with swelling and pain; do the exercises we provide; keep moving; do not stress about the ankle and focus on getting yourself in the best position to get back to sport. | We will see you three times a week; a session will last 40-45 minutes and will focus on getting swelling down; restoring movement; putting weight on the ankle; getting rid of the crutches and walking normally. Later we will focus on sport-specific exercises to get you ready for return to play. You will see the doctor in three weeks for a follow-up. |
The updated biomedical education may vary in terminology, explanations, and timelines among clinicians and clinical settings due to their individual experiences. However, the primary goal of this education should be to alleviate fear and anxiety, offer reassurance, and provide clear guidance for the patient’s path forward. However, it is crucially important to note that this is just one aspect of the educational model. In light of the latest advancements in pain neuroscience, the authors propose implementing a secondary, complementary PNE approach. This approach aims to further educate the patient about her pain, as well as to diminish fear and anxiety, thereby facilitating an optimal recovery process.
Pain Neuroscience Education
From a clinical standpoint, heightened sensitization of the nervous system around the ankle is likely to result in increased pain, particularly during movement.1,24 During her assessment, her elevated score on the Fear-Avoidance Beliefs Questionnaire for Physical Activity (FABQ-PA) suggests a high probability of it hindering her recovery and return to sports.42 Furthermore, her elevated score on the Pain Catastrophizing Scale (PCS) score indicates a diminished sense of hope, particularly concerning her status as a senior and her concerns about participating in her final high school season.43 These factors alone justify considering the incorporation of PNE, given its capacity to mitigate fear-avoidance and pain catastrophizing, while also facilitating movement, which will be integral to her upcoming rehabilitation process. Table 3 and Figure 2 provide an illustration of a PNE session tailored for this athlete, utilizing a metaphorical depiction of a sensitized alarm system.6
Table 3. Pain neuroscience education example using a metaphorical sensitive alarm system analogy.
| The extra sensitive alarm system6 |
|---|
|
Conclusions and Take-Aways for the Clinician
By integrating PNE into the revised biomedical educational model, a foundation is established for clinicians to refer to during rehabilitation. This is important even for athletes with apparently minor injuries. While pain during movement is anticipated during the recovery process, understanding this pain diminishes fear. As the clinician reminds the patient during rehabilitation that the overly sensitive alarm system is gradually settling down, fear and pain catastrophizing decrease further. This is crucial for achieving optimal recovery from pain, encompassing acute, sub-acute, and perioperative pain experiences.
References
- The biology of chronic pain and its implications for Pain Neuroscience Education: State of the art. Zimney K., Van Bogaert W., Louw A. 2023J Clin Med. 12(13):4199. doi: 10.3390/jcm12134199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinking beyond muscles and joints: therapists' and patients' attitudes and beliefs regarding chronic musculoskeletal pain are key to applying effective treatment. Nijs J., Roussel N., Paul van Wilgen C., Koke A., Smeets R. 2013Man Ther. 18(2):96–102. doi: 10.1016/j.math.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Presidential address, North American Spine Society: Failure of the pathology model to predict back pain. Haldeman S. 1990Spine. 15(7):718–724. doi: 10.1097/00007632-199007000-00019. [DOI] [PubMed] [Google Scholar]
- Diagnosing and treating chronic pain: Are we doing this right? Carnago L., O'Regan A., Hughes J. M. 2021J Prim Care Community Health. 12:21501327211008055. doi: 10.1177/21501327211008055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Central sensitisation in chronic pain conditions: latest discoveries and their potential for precision medicine. Nijs J., George S. Z., Clauw D. J., et al. 2021Lancet Rheumatol. 3(5):e383–e392. doi: 10.1016/S2665-9913(21)00032-1. [DOI] [PubMed] [Google Scholar]
- The clinical application of teaching people about pain. Louw A., Zimney K., O'Hotto C., Hilton S. 2016Physiother Theory Pract. 32(5):385–395. doi: 10.1080/09593985.2016.1194652. [DOI] [PubMed] [Google Scholar]
- Short-term impact of combining pain neuroscience education with exercise for chronic musculoskeletal pain: a systematic review and meta-analysis. Siddall B., Ram A., Jones M. D., Booth J., Perriman D., Summers S. J. 2021Pain. doi: 10.1097/j.pain.0000000000002308. [DOI] [PubMed]
- Pain neuroscience education for adults with chronic musculoskeletal pain: a mixed-methods systematic review and meta-analysis. Watson J. A., Ryan C. G., Cooper L., et al. 2019J Pain. doi: 10.1016/j.jpain.2019.02.011. [DOI] [PubMed]
- The efficacy of therapeutic neuroscience education on musculoskeletal pain – a systematic review of the literature. Louw A., Zimney K., Puentedura E.J., Diener I. 2016Physiother Theory Pract. 32(5):332–355. doi: 10.1080/09593985.2016.1194646. [DOI] [PubMed] [Google Scholar]
- Use of Therapeutic Neuroscience education to address psychosocial factors associated with acute low back pain: a case report. Zimney K., Louw A., Puentedura E. J. 2014Physiother Theory Pract. 30(3):202–209. doi: 10.3109/09593985.2013.856508. [DOI] [PubMed] [Google Scholar]
- Immediate effect of pain neuroscience education for recent onset low back pain: an exploratory single arm trial. Louw A., Farrell K., Choffin B., et al. 2019J Man Manip Ther. :1–10. doi: 10.1080/10669817.2019.1624006. [DOI] [PMC free article] [PubMed]
- A randomized controlled trial of intensive neurophysiology education in chronic low back pain. Moseley G. L., Hodges P. W., Nicholas M. K. 2004Clin J Pain. 20:324–330. doi: 10.1097/00002508-200409000-00007. [DOI] [PubMed] [Google Scholar]
- Interdisciplinary pain neuroscience continuing education in the veterans affairs. Louw A., Vogsland R., Marth L., Marshall P., Cox T., Landers M. 2019Clin J Pain. 35(11) doi: 10.1097/AJP.0000000000000756. [DOI] [PubMed] [Google Scholar]
- Pain, the tissues and the nervous system: a conceptual model. Gifford L. 1998Physiother. 84(1):27–36. doi: 10.1016/S0031-9406(05)65900-7. [DOI] [Google Scholar]
- Diagnostic triage for low back pain: a practical approach for primary care. Bardin L. D., King P., Maher C. G. 2017Med J Aust. 206(6):268–273. doi: 10.5694/mja16.00828. [DOI] [PubMed] [Google Scholar]
- Central sensitization in patients attending physical therapy for musculoskeletal disorders. Louw A. H. D., Weber B., Shuda D., Obert C., Louw H., Farrell K. 2021Phys Med Rehabil Int. 8(5) [Google Scholar]
- Mechanisms-based classifications of musculoskeletal pain: Part 3 of 3: Symptoms and signs of nociceptive pain in patients with low back (+/-leg) pain. Smart K. M., Blake C., Staines A., Thacker M., Doody C. 2012Man Ther. 17(4):352–357. doi: 10.1016/j.math.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. Brinjikji W., Luetmer P. H., Comstock B., et al. 2015AJNR Am J Neuroradiol. 36(4):811–816. doi: 10.3174/ajnr.A4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study) Guermazi A., Niu J., Hayashi D.., et al. 2012BMJ Med. 345:e5339. doi: 10.1136/bmj.e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unravelling the barriers to reconceptualisation of the problem in chronic pain: the actual and perceived ability of patients and health professionals to understand the neurophysiology. Moseley G. L. 2003J Pain. 4(4):184–189. doi: 10.1016/S1526-5900(03)00488-7. [DOI] [PubMed] [Google Scholar]
- Pain threshold and tolerance differences among intercollegiate athletes: implication of past sports injuries and willingness to compete among sports teams. Raudenbush B., Canter R. J., Corley N., et al. 2012N Am J Psychol. 14(1) [Google Scholar]
- Neck pain in demolition derby drivers. Simotas A.C., Shen T. 2005Arch Phys Med Rehabil. 86(4):693–696. doi: 10.1016/j.apmr.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Associations between organized sport participation and mental health difficulties: Data from over 11,000 US children and adolescents. Hoffmann M. D., Barnes J. D., Tremblay M. S., Guerrero M. D. 2022PLoS One. 17(6):e0268583. doi: 10.1371/journal.pone.0268583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reappraising entrapment neuropathies--mechanisms, diagnosis and management. Schmid A. B., Nee R. J., Coppieters M. W. 2013Man Ther. 18(6):449–457. doi: 10.1016/j.math.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Prevalence and location of neuropathic pain in lumbar spinal disorders: Analysis of 1804 consecutive patients with primary lower back pain. Orita S., Yamashita T., Ohtori S., et al 2016Spine. 41(15):1224–1231. doi: 10.1097/BRS.0000000000001553. [DOI] [PubMed] [Google Scholar]
- Mechanisms-based classifications of musculoskeletal pain: Part 2 of 3: Symptoms and signs of peripheral neuropathic pain in patients with low back (+/-leg) pain. Smart K. M., Blake C., Staines A., Thacker M., Doody C. 2012Man Ther. 17(4):345–351. doi: 10.1016/j.math.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Mechanisms-based classifications of musculoskeletal pain: Part 1 of 3: Symptoms and signs of central sensitisation in patients with low back (+/-leg) pain. Smart K. M., Blake C., Staines A., Thacker M., Doody C. 2012Man Ther. 17(4):336–344. doi: 10.1016/j.math.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Preoperative therapeutic neuroscience education for lumbar radiculopathy: a single-case fMRI report. Louw A., Puentedura E. J., Diener I., Peoples R. R. 2015Physiother Theory Pract. 31(7):496–508. doi: 10.3109/09593985.2015.1038374. [DOI] [PubMed] [Google Scholar]
- Widespread brain activity during an abdominal task markedly reduced after pain physiology education: fMRI evaluation of a single patient with chronic low back pain. Moseley G. L. 2005Aust J Physiother. 51(1):49–52. doi: 10.1016/S0004-9514(05)70053-2. [DOI] [PubMed] [Google Scholar]
- A neuroscience approach to managing athletes with low back pain. Puentedura E. J., Louw A. 2012Phys Ther Sport. 13(3):123–133. doi: 10.1016/j.ptsp.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Pain and the neuromatrix in the brain. Melzack R. 2001J Dent Educ. 65:1378–1382. [PubMed] [Google Scholar]
- Pain neuroscience education for physiotherapy receptionists. Louw A., Neilson B., Freund B., Cox T. 2019Pain and Rehab. 46(Winter 2019):24–31. [Google Scholar]
- The clinical impact of pain neuroscience continuing education on physical therapy outcomes for patients with low back and neck pain. Louw A., Puentedura E. J., Denninger T. R., et al. 2022PLoS One. 17(4):e0267157. doi: 10.1371/journal.pone.0267157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teaching patients about pain: It works, but what should we call it? Louw A, Puentedura E L, Zimney K. 2016Physiother Theory Pract. 32(5):328–331. doi: 10.1080/09593985.2016.1194669. [DOI] [PubMed] [Google Scholar]
- Combined physiotherapy and education is efficacious for chronic low back pain. Moseley L. 2002Aust J Physiother. 48(4):297–302. doi: 10.1016/S0004-9514(14)60169-0. [DOI] [PubMed] [Google Scholar]
- A systematic review and meta-analysis of pain neuroscience education for chronic low back pain: Short-and long-term outcomes of pain and disability. Wood L., Hendrick P.A. 2019Eur J Pain. 23(2):234–249. doi: 10.1002/ejp.1314. [DOI] [PubMed] [Google Scholar]
- The impact of combining pain education strategies with physical therapy interventions for patients with chronic pain: A systematic review and meta-analysis of randomized controlled trials. Marris D., Theophanous K., Cabezon P., Dunlap Z., Donaldson M. 2021Physiother Theory Pract. 37(4):461–472. doi: 10.1080/09593985.2019.1633714. [DOI] [PubMed] [Google Scholar]
- Revisiting the provision of pain neuroscience education: An adjunct intervention for patients but a primary focus of clinician education. Louw A., Sluka K. A., Nijs J., Courtney C. A., Zimney K. 2021J Orthop Sports Phys Ther. 51(2):57–59. doi: 10.2519/jospt.2021.9804. [DOI] [PubMed] [Google Scholar]
- Fifteen years of explaining pain: the past, present, and future. Moseley G. L., Butler D. S. 2015J Pain. doi: 10.1016/j.jpain.2015.05.005. [DOI] [PubMed]
- The clinical implementation of pain neuroscience education: A survey study. Louw A., Puentedura E. J., Zimney K., Cox T., Rico D. 2017Physiother Theory Pract. 33(11):869–879. doi: 10.1080/09593985.2017.1359870. [DOI] [PubMed] [Google Scholar]
- Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Vlaeyen J. W. S., Linton S. J. 2000Pain. 85:317–322. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- Identifying psychosocial variables in patients with acute work-related low back pain: the importance of fear-avoidance beliefs. Fritz J. M., George S. Z. 2002Phys Ther. 82(10):973–983. doi: 10.1093/ptj/82.10.973. [DOI] [PubMed] [Google Scholar]
- The pain catastrophizing scale: Development and validation. Sullivan M. J. L., Bishop S. R., Pivak J. 1995Psychol Assess. 7:524–532. doi: 10.1037/1040-3590.7.4.524. [DOI] [Google Scholar]
- Stages of change. Prochaska J.O., Norcross J.C. 2001Psychother. 38(4):443–448. doi: 10.1037/0033-3204.38.4.443. [DOI] [Google Scholar]
- The psychometric properties of the Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES) in a clinical sample of active duty military service members. Mitchell D., Francis J. P., Tafrate R. C. 2005Mil Med. 170(11):960–963. doi: 10.7205/MILMED.170.11.960. [DOI] [PubMed] [Google Scholar]
- The effects of fear-avoidance beliefs on anterior knee pain and physical therapy visit count for young individuals: A retrospective study. Mansfield C.B., Selhorst M. 2018Phys Ther Sport. 34:187–191. doi: 10.1016/j.ptsp.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Early postoperative fear of movement predicts pain, disability, and physical health six months after spinal surgery for degenerative conditions. Archer K. R., Seebach C. L., Mathis S. L., Riley L. H., 3rd, Wegener S. T. 2014Spine J. 14(5):759–767. doi: 10.1016/j.spinee.2013.06.087. [DOI] [PubMed] [Google Scholar]
- Association of preoperative pain catastrophizing with postoperative pain after lower limb trauma surgery. Subedi A., Pokharel K., Sah B.P., Chaudhary P. 2021J Psychosom Res. 149:110575. doi: 10.1016/j.jpsychores.2021.110575. [DOI] [PubMed] [Google Scholar]
- The fear avoidance model predicts short-term pain and disability following lumbar disc surgery. Alodaibi F. A., Fritz J. M., Thackeray A., Koppenhaver S. L., Hebert J. J. 2018PLoS One. 13(3):e0193566. doi: 10.1371/journal.pone.0193566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preoperative pain neuroscience education for lumbar radiculopathy: a multicenter randomized controlled trial with 1-year follow-up. Louw A., Diener I., Landers M. R., Puentedura E. J. 2014Spine. 39(18):1449–1457. doi: 10.1097/BRS.0000000000000444. [DOI] [PubMed] [Google Scholar]
- Three-year follow-up of a randomized controlled trial comparing preoperative neuroscience education for patients undergoing surgery for lumbar radiculopathy. Louw A., Diener I., Landers M. R., Zimney K., Puentedura E. J. 2016J Spine Surg. 2(4):289–298. doi: 10.21037/jss.2016.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A controlled clinical trial of preoperative pain neuroscience education for patients about to undergo total knee arthroplasty. Louw A., Puentedura E. J., Reed J., Zimney K., Grimm D., Landers M. R. 2019Clin Rehabil. :269215519857782. doi: 10.1177/0269215519857782. [DOI] [PubMed]
- Preoperative pain neuroscience education for shoulder surgery: A case series. Louw A., Rico D., Langerwerf L., Maiers N., Diener I., Cox T. 2020S Afr J Physiother. 76(1):1417. doi: 10.4102/sajp.v76i1.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- What do patients referred to physical therapy for a musculoskeletal condition expect? A qualitative assessment. Subialka J. A., Smith K., Signorino J. A., Young J. L., Rhon D. I., Rentmeester C. 2022Musculoskelet Sci Pract. 59:102543. doi: 10.1016/j.msksp.2022.102543. [DOI] [PubMed] [Google Scholar]
- The fall of the postural-structural-biomechanical model in manual and physical therapies: Exemplified in lower back pain. Lederman E. 2010CPDO Online Journal. :1–14. doi: 10.1016/j.jbmt.2011.01.011. [DOI] [PubMed]
- Sticks and stones: the impact of language in musculoskeletal rehabilitation. Stewart M., Loftus S. 2018J Orthop Sports Phys Ther. 48(7):519–522. doi: 10.2519/jospt.2018.0610. [DOI] [PubMed] [Google Scholar]
- Biopsychosocial model of disease: 40 years on. Which way is the pendulum swinging? Jull G. 2017Br J Sports Med. 51(16):1187–1188. doi: 10.1136/bjsports-2016-097362. [DOI] [PubMed] [Google Scholar]
- Patient expectations of treatment for back pain: a systematic review of qualitative and quantitative studies. Verbeek J., Sengers M. J., Riemens L., Haafkens J. 2004Spine. 29(20):2309–2318. doi: 10.1097/01.brs.0000142007.38256.7f. [DOI] [PubMed] [Google Scholar]