Abstract
Metabolic dysfunction-associated fatty liver disease (MAFLD) and chronic kidney disease (CKD) present notable health challenges, however, abdominal obesity has received scant attention despite its potential role in exacerbating these conditions. Thus, we conducted a retrospective cohort study using the National Health and Nutrition Examination Surveys III (NHANES III) of the United States from 1988 to 1994 including 9161 participants, and mortality follow-up survey in 2019. Statistical analyze including univariable and multivariable Logistic and Cox regression models, and Mediation effect analyze were applied in study after adjustment for covariates. Our findings revealed that individuals with both abdominal obesity and MAFLD were more likely to be female, older and exhibit higher prevalence of advanced liver fibrosis (7.421% vs. 2.363%, p < 0.001), type 2 diabetes mellitus (T2DM) (21.484% vs. 8.318%, p < 0.001) and CKD(30.306% vs. 16.068%, p < 0.001) compared to those with MAFLD alone. MAFLD (adjusted OR: 1.392, 95% CI 1.013–1.913, p = 0.041), abdominal obesity (adjusted OR 1.456, 95% CI 1.127–1.880, p = 0.004), abdominal obesity with MAFLD (adjusted OR 1.839, 95% CI 1.377–2.456, p < 0.001), advanced fibrosis(adjusted OR 1.756, 95% CI 1.178–2.619, p = 0.006) and T2DM (adjusted OR 2.365, 95% CI 1.758–3.183, p < 0.001) were independent risk factors of CKD. The abdominal obese MAFLD group had the highest all-cause mortality as well as mortality categorized by disease during the 30-year follow-up period. Indices for measuring abdominal obesity, such as waist circumference (WC), waist-hip ratio (WHR), and lipid accumulation product (LAP), elucidated a greater mediation effect of MAFLD on CKD compared to BMI on CKD (proportion mediation 65.23%,70.68%, 71.98%, respectively vs. 32.63%). In conclusion, the coexistence of abdominal obesity and MAFLD increases the prevalence and mortality of CKD, and abdominal obesity serves as a mediator in the association between MAFLD and CKD.
Keywords: Abdominal obesity, Metabolic dysfunction-associated fatty liver disease (MAFLD), Chronic kidney disease (CKD), Diabetes mellitus, Liver fibrosis, Mortality, NHANES III
Subject terms: Chronic kidney disease, Non-alcoholic fatty liver disease, Obesity, Risk factors
Introduction
Non-alcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases, affecting nearly 30% of the global adult population and having a significant impact on global health and the economy1. In 2020, there was a proposal to rename and redefine NAFLD as metabolic-associated fatty liver disease (MAFLD) in order to better characterize the underlying pathophysiology and associated metabolic abnormalities. The proposed criteria for diagnosing MAFLD include evidence of hepatic steatosis along with one of the following three criteria: overweight or obesity, presence of type 2 diabetes mellitus (T2DM), or evidence of metabolic dysregulation2. This condition has been recognized as a significant global health concern due to its increasing prevalence and potential to progress to more severe liver diseases, including advanced liver fibrosis and liver cancer3.
Chronic kidney disease (CKD) is another prevalent and progressive condition that affects millions of individuals worldwide4. It is characterized by a gradual loss of kidney function, leading to various complications and an increased risk of cardiovascular events and mortality5. The etiology of CKD is multifactorial, involving both non-modifiable factors such as age and genetic predisposition, as well as modifiable factors such as diabetes mellitus, hypertension, and obesity, which are also the metabolic risk factors shared with MAFLD6. Several studies have demonstrated that individuals with MAFLD are more likely to have and develop CKD compared with those without MAFLD7,8.
Abdominal obesity, typified by the accumulation of visceral fat, has been found to have a stronger correlation with metabolic abnormalities and is considered a more reliable predictor of metabolic and cardiovascular diseases compared to overall obesity, which may also encompass MAFLD and CKD9. Previous studies have substantiated a strong association between obesity and both MAFLD and CKD10,11. However, these studies have predominantly focused on general obesity measures such as body mass index (BMI), which do not offer a comprehensive assessment of abdominal fat distribution. Waist circumference(WC) is commonly used to measure abdominal obesity, and meanwhile newer indices such as body shape index (ABSI), body roundness index (BRI), visceral fat index (VAI), and lipid accumulation product (LAP) have emerged in recent years to measure abdominal obesity or visceral fat12. Although previous research has extensively examined the independent links between MAFLD and CKD with obesity, the potential mediating role of abdominal obesity between MAFLD and CKD remains underexplored. Understanding this potential association is crucial in identifying effective interventions and developing targeted strategies to prevent and manage both MAFLD and CKD.
Therefore, the objective of this national retrospective cohort study is to investigate the relationship between MAFLD, CKD and abdominal obesity, as well as to analyze mortality outcomes stratified by cause of death during a 30-year follow-up period. We extracted pertinent associations from extensive population data to enhance our understanding of the intricate mechanisms linking MAFLD, abdominal obesity, and CKD, shed light on the potential mediation effects of abdominal obesity of these conditions, and ultimately provide valuable insights for clinical practice and public health interventions.
Materials and methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is a national survey managed by the National Center for Health Statistics (NCHS) at the U.S. Centers for Disease Control and Prevention (CDC). Samples in NHANES represented the health and nutritional status of the general U.S. population well and employed a carefully conducted multistage and stratification probability design. NHANES is designed to monitor health and nutritional status in the US through the collection of demographic, dietary, physical examination, hepatic ultrasound, laboratory and questionnaire data from adults and children. All participants gave written informed consent. NHANES is publicly available at www.cdc.gov/nchs/nhanes/.
For this study, we utilized the Third National Health and Nutrition Examination Survey (NHANES III) and the Third National Health and Nutrition Examination Survey Mortality Follow-up studies. The survey was conducted from 1988 to 1994, and the mortality follow-up study was a prospective study of the vital status of all participants aged 20 and older to December 2019. The Participants’ length of survival was determined by the amount of time between the date of completion of the NHANESIII survey to time of death or 31 December 2019, whichever came first. All-cause mortality was defined as any reason for death. Data on mortality due to specific causes was collected either, such as cardiovascular and cerebrovascular diseases (CVD), diabetes mellitus and kidney diseases (include nephritis, nephrotic syndrome and nephrosis) related mortality.
General demographic and socioeconomic characteristics such as age, sex, race and ethnicity (Non-Hispanic White, Non-Hispanic Black, Mexican American, Other), marital status (single, married, divorced/separated, other/widowed), military service, ratio of family income to poverty (PIR), and sedentary behavior, smoke and alcohol use were obtained. All these variables were self-reported as per the design of NHANES. Physical and blood measurements including BMI, WC, VAI, LAP, white blood cell (WBC), red blood cell (RBC), hemoglobin(HB), platelet (PLT), Na+, K+, Cl−, Ca2+, blood urea nitrogen (BUN), total bilirubin (TB), serum creatinine (Scr), γ-glutamyl transpeptidase (GGT) , lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), albumin, globulin, total protein (TP), total cholesterol(Tc), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides(TG), estimated glomerular filtration rate (eGFR), serum glucose, homeostatic model assessment of insulin resistance (HOMA-IR), urinary albumin, albumin-to-creatinine ratio (ACR) were obtained from the laboratory tests.
Inclusion/exclusion criteria
33197 participants in total subjects from the NHANES III were included. Among them, we excluded subjects who were aged below 20 years old (N = 1225), pregnant(as pregnancy can significantly impact waist circumference and other body measurements, leading to potential confounding factors in our study) or lacked laboratory and ultrasound data(N = 22811), after which a total of 9161 participants remained. Finally, we categorized participants based on the presence or absence of MAFLD and abdominal obesity (Fig. 1).
Figure 1.
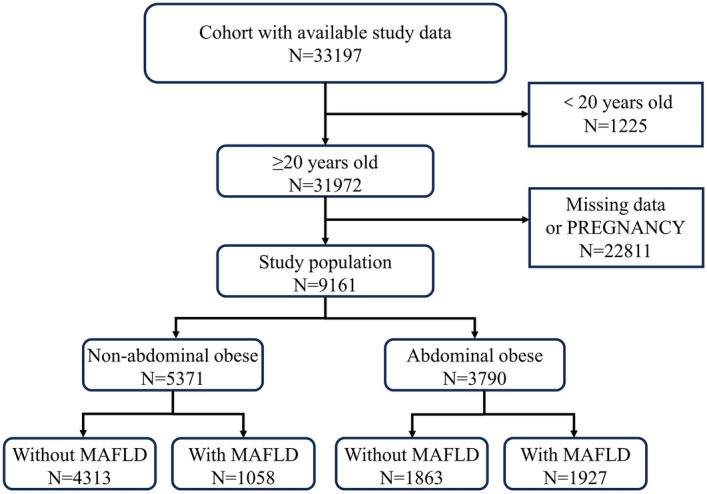
Flowchart.
Definition of MAFLD, abdominal obesity and CKD
The established 2020 criteria for MAFLD include evidence of hepatic steatosis plus one of the following: overweight (BMI ≥ 25 and < 30 kg/m2)/obesity (BMI ≥ 30 kg/m2), T2DM, or t at least two metabolic risk abnormalities. Metabolic risk abnormalities consisted of: (1) waist circumference ≥ 102 cm in male and ≥ 88 cm female, (2) blood pressure ≥ 130/85 mmHg or specific drug treatment, (3) plasma triglycerides ≥ 150 mg/dl (≥ 1.70 mmol/L) or specific drug treatment, (4) plasma HDL-cholesterol < 40 mg/dl (< 1.0 mmol/L) for male and < 50 mg/dl (< 1.3 mmol/L) for female or specific drug treatment, (5) prediabetes (fasting glucose levels 100–125 mg/dl [5.6 to 6.9 mmol/L] or hemoglobin A1c [HbA1c[ 5.7–6.4% [39 to 47 mmol/mol]), (6) homeostasis model assessment of insulin resistance (HOMA-IR) score ≥ 2.5, (7) and/or plasma high-sensitivity C-reactive protein level > 2 mg/L. T2DM in this study was defined as a history of diabetes mellitus or HbA1c ≥ 6.5% or serum glucose level ≥ 7.0mmol/L13. Hypertension was defined as a history of hypertension or blood pressure ≥ 140/90 mmHg2.
Hepatic steatosis was determined in NHANES III participants using the Hepatic Steatosis Ultrasound Examination (HSUE). The ultrasonographic assessments were reported as normal, mild, moderate, or severe hepatic steatosis. All ultrasound personnel received training in the standardized procedures, and they were supervised periodically. Abiding by quality control procedures, reliability results (intra-rater and inter-rater) were calculated. The intra-rater reliability was found to be 91.3% (kappa 0.77) and the inter-rater reliability was found to be 88.7% (kappa 0.70)14 .
The advanced fibrosis was defined as Fibrosis-4 index (FIB-4) ≥ 2.67, NAFLD fibrosis score (NFS) ≥ 0.676 and AST to platelet ratio index (APRI) ≥ 0.91815,16. FIB-4 = Age[years] × AST[U/L])/(platelet [109] × ALT[U/L]); NFS = − 1.675 + (0.037 × Age[years]) + (0.094 × BMI) + (1.13 × IFG/diabetes [yes = 1, no = 0]) + (0.99 × AST/ALT) − (0.013 × platelet [109/l]) − (0.66 × albumin[g/dL]); APRI = ([AST/upper limit of normal]/platelet count [109/l]) × 10017.
Abdominal obesity is determined according to the WC thresholds. The cut-off points for abdominal obesity for men and women were ≥ 102 cm and ≥ 88 cm, respectively18. In addition to BMI and WC, there are many other obesity measurement indexes, including traditional indexes such as waist-hip ratio (WHR) and waist-to-height ratio (WHtR), as well as new indexes such as a ABSI, BRI, VAI and LAP. WHR = WC (cm)/ Hip Circumference (cm), WHtR = WC (cm)/Height (cm). ABSI = WC (m)/[BMI (kg/m2)2/3 × Height (m)1/2]; BRI = 364.2 − 365.5 × [1 − (WC(m)/2π)2/(0.5 × Height (m)2]1/219. VAI is the integration of BMI, WC, TG and HDL: for male, VAI = [WC (cm)/[39.68 + 1.88 × BMI (kg/m2)]] × TG (mmol/L)/1.03 × [1.31/HDL − C (mmol/L)], for female, VAI = [WC (cm)/[36.58 + 1.89 × BMI (kg/m2)]] × TG (mmol/L)/ 0.81 × [1.52/HDL − C (mmol/L)]20. LAP is the indicator used to evaluate lipid accumulation, and it combined WC and triglycerides (TGs): for males, LAP = (WC[cm] − 65) × TG[mmol/L]; for females, LAP = (WC [cm] − 58) × TG[mmol/L]21.
CKD was defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 (CKD-EPI) and/or ACR ≥ 30 mg/g22,23.
Statistical analyses
We followed CDC guidelines rigorously during all statistical analyses and used a suitable sample weight for each participant to account for the NHANES complex multistage cluster survey design24.
The included subjects were divided into four groups: the non- abdominal obese and non-MAFLD group, non-abdominal obese and MAFLD group, the abdominal obese and non-MAFLD group, the abdominal obese and MAFLD group. Characteristics of participants by the presence of abdominal obese and MAFLD group were reported as weighted percentages(95%CI) or mean with standard error (SE), aimed to make the sample better reflect the characteristics of the population. The continuous variables were compared using Student’s t-test, and categorical variables using χ2 test. Multivariable logistics regression analysis was used to assess independent risk factors. All tests were two-tailed and results with a p < 0.05 were considered statistically significant. All calculations were conducted by SPSS version 25.0 (IBM Corp., Armonk, NY, USA) and STATA version 18.0 (Stata Corp., College Station, TX, USA).
We calculated the prevalence of our divided four groups among the overall cohort of patients in relevant subgroups such as by T2DM, CKD, and advanced fibrosis by NFS, we also calculated the prevalence of CKD groups in subgroups as well. We further performed univariable and adjusted multivariable logistic regression models to determine risks factors associated with CKD. We reported the univariate and multivariate odd ratios (OR) as 95% confidence intervals (CI).
Next, we estimated overall mortality rates in study population and in subgroups using the Kaplan–Meier methods. Cox regression analysis was used to compare mortality of different groups and Cox regression models were used to test hazard ratio (HR) of known risk factors for kidney disease-related mortality after adjustment for potential confounders (sex, race, age, marital status, military service, sedentary behavior, weight category by BMI, advanced fibrosis by NFS, proteinuria, LDL, TRI, Tc, albumin).
Mediation analysis is a statistical method used to assess the underlying mechanisms through which an independent variable affects a dependent variable. We used mediation analysis to explore whether the associations of MAFLD with CKD were mediated by abdominal obesity (mediator: BMI, WC, WHR, WHtR, ABSI, BRI, LAP, and VAP). The direct effect (DE) represented the effects of MAFLD on CKD without a mediator. The indirect effect (IE) represented the effects of MAFLD on CKD through the mediator. A significant IE is indicative of a mediation effect. The proportion of mediation was calculated by using IE divided by TE (total effect).
Results
Baseline characteristics of subjects
Baseline characteristics are shown in Table 1. Among the 9161 participants, 48.030% were men, the mean age was 43.227 ± 0.164 years old. Participants with MAFLD were found to be older (p < 0.001) and had a higher incidence of CKD (p < 0.001) compared to those without MAFLD. Several significant differences were observed in other demographic factors among the four groups, such as sex, age, race, PIR, marital status, and military service (p < 0.001). When categorizing MAFLD patients based on abdominal obesity presence, the abdominal obese MAFLD group tended to be female, older, exhibiting sedentary behavior, and having higher values of BMI, LAP, VAP, Tc, TRI, LDL, et al. compared to the non-abdominal obese MAFLD group (p < 0.001). As expected, the abdominal obese MAFLD group exhibited a higher prevalence of T2DM, advanced liver fibrosis (by NFS and APRI), proteinuria and CKD than other three groups (p < 0.001).
Table 1.
Demographic, clinical, and laboratory characteristics of participants divided by abdominal obesity and MAFLD.
| Characteristics | Total | Non-abdominal obesity | Abdominal obesity | P-Value | ||
|---|---|---|---|---|---|---|
| without MAFLD | with MAFLD | without MAFLD | with MAFLD | |||
| Group1 | Group2 | Group3 | Group4 | |||
| N = 9161 | N = 4313 | N = 1058 | N = 1863 | N = 1927 | ||
| Sex | < 0.001 | |||||
| Male | 48.030(47.007–49.054) | 57.060(55.577–58.531) | 69.282(66.434–71.990) | 24.047(22.160–26.041) | 39.336(37.177–41.537) | |
| Female | 51.970(50.946–52.993) | 42.940(41.469–44.423) | 30.718(28.010–33.566) | 75.953(73.959–77.840) | 60.664(58.463–62.823) | |
| Age | < 0.001 | |||||
| < 65 | 86.355(85.637–87.043) | 90.633(89.726–91.467) | 87.240(85.091–89.119) | 82.179(80.375–83.851) | 80.332(78.497–82.047) | |
| ≥ 65 | 13.645(12.957–14.363) | 9.367(8.533–10.274) | 12.760(10.881–14.909) | 17.821(16.149–19.625) | 19.668(17.953–21.503) | |
| Race | < 0.001 | |||||
| Non-Hispanic White | 37.965(36.977–38.964) | 40.111(38.658–41.583) | 31.096(28.378–33.952) | 37.037(34.872–39.256) | 37.831(35.691–40.019) | |
| Non-Hispanic Black | 28.469(27.553–29.402) | 28.634(27.304–30.002) | 24.669(22.164–27.358) | 36.286(34.131–38.496) | 22.626(20.812–24.549) | |
| Mexican American | 29.451(28.526–30.393) | 26.617(25.319–27.957) | 39.698(36.79–42.68) | 23.564(21.691–25.546) | 35.859(33.747–38.027) | |
| Other race | 4.115(3.727–4.542) | 4.637(4.048–5.307) | 4.537(3.435–5.970) | 3.113(2.414–4.006) | 3.684(2.930–4.624) | |
| PIR | < 0.001 | |||||
| Blank but applicable | 8.733(8.172–9.328) | 8.161(7.381–9.017) | 9.735(8.089–11.674) | 9.232(7.999–10.634) | 8.978(7.780–10.339) | |
| < 1.0 | 20.238(19.428–21.073) | 17.389(16.287–18.550) | 23.062(20.622–25.698) | 21.846(20.028–23.781) | 23.508(21.668–25.454) | |
| ≥ 1.0 | 71.029(70.092–71.95) | 74.449(73.126–75.729) | 67.202(64.313–69.967) | 68.921(66.781–70.983) | 67.514(65.389–69.570) | |
| Marital status | < 0.001 | |||||
| Legally married | 60.059(59.052–61.058) | 56.504(55.019–57.977) | 63.989(61.048–66.828) | 61.138(58.903–63.327) | 64.816(62.655–66.918) | |
| Divorced/separated | 12.149(11.496–12.834) | 10.758(9.868–11.719) | 11.909(10.091–14.004) | 14.654(13.119–16.334) | 12.974(11.545–14.549) | |
| Never married | 17.935(17.162–18.734) | 24.67(23.406–25.979) | 16.635(14.51–19.002) | 12.185(10.775–13.75) | 9.133(7.926–10.504) | |
| Other(a) | 9.857(9.263–10.485) | 8.069(7.292–8.920) | 7.467(6.029–9.214) | 12.024(10.623–13.581) | 13.077(11.644–14.658) | |
| Military service | < 0.001 | |||||
| Blank but applicable | 0.546(0.414–0.719) | 0.533(0.355–0.801) | 0.567(0.255–1.257) | 0.537(0.289–0.995) | 0.571(0.316–1.028) | |
| No | 15.642(14.913–16.401) | 15.419(14.371–16.527) | 21.55(19.175–24.131) | 11.648(10.268–13.186) | 16.762(15.159–18.497) | |
| Yes | 83.812(83.043–84.552) | 84.048(82.925–85.111) | 77.883(75.281–80.283) | 87.815(86.25–89.225) | 82.667(80.911–84.293) | |
| Sedentary behavior | < 0.001 | |||||
| No | 71.608(70.675–72.522) | 77.556(76.286–78.777) | 70.794(67.980–73.457) | 66.130(63.948–68.245) | 64.037(61.868–66.151) | |
| Yes | 28.392(27.478–29.325) | 22.444(21.223–23.714) | 29.206(26.543–32.020) | 33.870(31.755–36.052) | 35.963(33.849–38.132) | |
| Weight category by BMI | < 0.001 | |||||
| Non-obese | 74.097(73.189–74.984) | 97.728(97.238–98.133) | 95.274(93.818–96.400) | 49.705(47.436–51.975) | 33.160(31.093–35.295) | |
| Obese | 25.903(25.016–26.811) | 2.272(1.867–2.762) | 4.726(3.600–6.182) | 50.295(48.025–52.564) | 66.840(64.705–68.907) | |
| Advanced fibrosis by FIB-4 | 0.129 | |||||
| No | 99.465(99.293–99.596) | 99.629(99.395–99.773) | 99.338(98.619–99.684) | 99.463(99.005–99.711) | 99.170(98.649–99.491) | |
| Yes | 0.535(0.404–0.707) | 0.371(0.227–0.605) | 0.662(0.316–1.381) | 0.537(0.289–0.995) | 0.830(0.509–1.351) | |
| Advanced fibrosis by NFS | < 0.001 | |||||
| No | 96.420(96.019–96.781) | 98.609(98.212–98.918) | 97.637(96.526–98.399) | 94.632(93.512–95.569) | 92.579(91.320–93.668) | |
| Yes | 3.580(3.219–3.981) | 1.391(1.082–1.788) | 2.363(1.601–3.474) | 5.368(4.431–6.488) | 7.421(6.332–8.680) | |
| Advanced fibrosis by APRI | < 0.001 | |||||
| No | 98.843(98.602–99.043) | 99.258(98.953–99.475) | 97.732(96.638–98.475) | 99.249(98.735–99.554) | 98.132(97.421–98.650) | |
| Yes | 1.157(0.957–1.398) | 0.742(0.525–1.047) | 2.268(1.525–3.362) | 0.751(0.446–1.265) | 1.868(1.350–2.579) | |
| T2DM | < 0.001 | |||||
| No | 91.169(90.570–91.733) | 97.079(96.532–97.541) | 91.682(89.860–93.202) | 90.284(88.854–91.549) | 78.516(76.625–80.293) | |
| Yes | 8.831(8.267–9.430) | 2.921(2.459–3.468) | 8.318(6.798–10.140) | 9.716(8.451–11.146) | 21.484(19.707–23.375) | |
| IFG | < 0.001 | |||||
| No | 94.400(93.910–94.853) | 97.055(96.507–97.520) | 92.344(90.580–93.800) | 94.096(92.930–95.079) | 89.881(88.452–91.150) | |
| Yes | 5.600(5.147–6.090) | 2.945(2.480–3.493) | 7.656(6.200–9.420) | 5.904(4.921–7.070) | 10.119(8.850–11.548) | |
| Proteinuria | < 0.001 | |||||
| Normal range | 90.372(89.751–90.960) | 94.134(93.392–94.797) | 91.304(89.449–92.859) | 87.332(85.743–88.767) | 84.38(82.689–85.933) | |
| Microalbuminuria | 8.012(7.474–8.586) | 5.078(4.461–5.774) | 7.561(6.114–9.317) | 10.199(8.904–11.657) | 12.714(11.299–14.277) | |
| Macroalbuminuria | 1.616(1.377–1.895) | 0.788(0.564–1.101) | 1.134(0.645–1.987) | 2.469(1.854–3.281) | 2.906(2.243–3.758) | |
| CKD | < 0.001 | |||||
| No | 80.559(79.736–81.357) | 87.572(86.554–88.524) | 83.932(81.594–86.024) | 73.645(71.596–75.596) | 69.694(67.603–71.706) | |
| Yes | 19.441(18.643–20.264) | 12.428(11.476–13.446) | 16.068(13.976–18.406) | 26.355(24.404–28.404) | 30.306(28.294–32.397) | |
| Age | 43.227 ± 0.164 | 38.953 ± 0.230 | 42.121 ± 0.471 | 46.797 ± 0.359 | 49.948 ± 0.329 | < 0.001 |
| BMI(kg/m2) | 27.208 ± 0.060 | 23.658 ± 0.046 | 24.953 ± 0.102 | 30.82 ± 0.111 | 32.898 ± 0.132 | < 0.001 |
| WC(cm) | 92.891 ± 0.151 | 83.324 ± 0.137 | 87.933 ± 0.289 | 102.067 ± 0.230 | 108.153 ± 0.271 | < 0.001 |
| VAI | 2.315 ± 0.026 | 1.555 ± 0.024 | 2.511 ± 0.075 | 2.532 ± 0.051 | 3.697 ± 0.078 | < 0.001 |
| LAP | 54.754 ± 0.605 | 28.356 ± 0.386 | 48.218 ± 1.304 | 69.117 ± 1.164 | 103.541 ± 1.891 | < 0.001 |
| WHR | 0.915 ± 0.001 | 0.877 ± 0.001 | 0.918 ± 0.002 | 0.938 ± 0.002 | 0.976 ± 0.002 | < 0.001 |
| WHtR | 0.557 ± 0.001 | 0.495 ± 0.001 | 0.522 ± 0.002 | 0.619 ± 0.001 | 0.654 ± 0.002 | < 0.001 |
| ABSI | 0.0798 ± 0.0001 | 0.0781 ± 0.0001 | 0.0796 ± 0.0001 | 0.0813 ± 0.0001 | 0.0823 ± 0.0001 | < 0.001 |
| BRI | 4.644 ± 0.021 | 3.32 ± 0.014 | 3.841 ± 0.030 | 5.943 ± 0.034 | 6.794 ± 0.041 | < 0.001 |
| WBC | 7.143 ± 0.024 | 6.9 ± 0.032 | 6.975 ± 0.062 | 7.37 ± 0.064 | 7.557 ± 0.048 | < 0.001 |
| RBC | 4.669 ± 0.005 | 4.674 ± 0.007 | 4.752 ± 0.015 | 4.576 ± 0.01 | 4.702 ± 0.011 | < 0.001 |
| HB(g/dL) | 13.99 ± 0.016 | 14.107 ± 0.022 | 14.294 ± 0.048 | 13.522 ± 0.033 | 14.011 ± 0.034 | < 0.001 |
| PLT | 278.771 ± 0.733 | 272.104 ± 0.992 | 271.613 ± 2.139 | 291.172 ± 1.75 | 285.635 ± 1.675 | < 0.001 |
| Tc(mmol/L) | 5.268 ± 0.012 | 5.037 ± 0.016 | 5.22 ± 0.036 | 5.521 ± 0.026 | 5.567 ± 0.026 | < 0.001 |
| TG(mmol/L) | 1.578 ± 0.013 | 1.239 ± 0.012 | 1.807 ± 0.042 | 1.606 ± 0.024 | 2.186 ± 0.039 | < 0.001 |
| LDL(mmol/L) | 1.446 ± 0.018 | 1.405 ± 0.026 | 1.313 ± 0.052 | 1.588 ± 0.043 | 1.471 ± 0.041 | < 0.001 |
| HDL(mmol/L) | 1.314 ± 0.004 | 1.392 ± 0.006 | 1.246 ± 0.013 | 1.318 ± 0.009 | 1.174 ± 0.008 | < 0.001 |
| Na(mmol/L) | 141.363 ± 0.025 | 141.414 ± 0.035 | 141.456 ± 0.071 | 141.248 ± 0.06 | 141.312 ± 0.056 | 0.023 |
| K(mmol/L) | 4.023 ± 0.003 | 4.029 ± 0.005 | 4.038 ± 0.01 | 4.016 ± 0.007 | 4.007 ± 0.008 | 0.140 |
| Cl(mmol/L) | 104.67 ± 0.034 | 104.71 ± 0.047 | 104.53 ± 0.101 | 104.871 ± 0.078 | 104.463 ± 0.079 | < 0.001 |
| Ca(mmol/L) | 2.32 ± 0.001 | 2.326 ± 0.002 | 2.324 ± 0.003 | 2.311 ± 0.003 | 2.316 ± 0.002 | < 0.001 |
| BUN(mmol/L) | 4.934 ± 0.019 | 4.873 ± 0.026 | 4.897 ± 0.050 | 4.945 ± 0.045 | 5.08 ± 0.044 | 0.001 |
| TB(μmol/L) | 10.158 ± 0.060 | 10.74 ± 0.090 | 10.942 ± 0.208 | 8.753 ± 0.103 | 9.786 ± 0.132 | < 0.001 |
| Scr(μmol/L) | 94.026 ± 0.246 | 94.873 ± 0.337 | 97.199 ± 1.004 | 91.531 ± 0.5 | 92.8 ± 0.501 | < 0.001 |
| AST(U/L) | 22.497 ± 0.169 | 21.577 ± 0.225 | 26.104 ± 0.683 | 20.006 ± 0.284 | 24.982 ± 0.403 | < 0.001 |
| ALT(U/L) | 18.568 ± 0.179 | 16.451 ± 0.231 | 23.153 ± 0.659 | 15.695 ± 0.303 | 23.564 ± 0.463 | < 0.001 |
| GGT(U/L) | 25.504 ± 0.482 | 20.946 ± 0.490 | 34.906 ± 2.314 | 23.645 ± 1.113 | 32.338 ± 1.101 | < 0.001 |
| LDH(U/L) | 158.053 ± 0.405 | 151.669 ± 0.573 | 159.748 ± 1.281 | 161.443 ± 0.84 | 168.131 ± 0.893 | < 0.001 |
| ALP(U/L) | 85.019 ± 0.328 | 79.677 ± 0.412 | 86.232 ± 0.947 | 87.958 ± 0.889 | 93.468 ± 0.702 | < 0.001 |
| TP(g/dl) | 7.413 ± 0.005 | 7.409 ± 0.007 | 7.473 ± 0.015 | 7.381 ± 0.011 | 7.421 ± 0.010 | < 0.001 |
| Albumin(g/dL) | 4.184 ± 0.004 | 4.263 ± 0.005 | 4.22 ± 0.011 | 4.062 ± 0.008 | 4.106 ± 0.008 | < 0.001 |
| Globulin(g/dL) | 2.367 ± 0.016 | 2.287 ± 0.023 | 2.51 ± 0.045 | 2.382 ± 0.037 | 2.452 ± 0.035 | < 0.001 |
| Serum glucose(mmol/L) | 5.458 ± 0.020 | 5.059 ± 0.018 | 5.545 ± 0.061 | 5.494 ± 0.045 | 6.272 ± 0.062 | < 0.001 |
| HOMAIR | 3.441 ± 0.098 | 1.977 ± 0.045 | 3.387 ± 0.351 | 4.287 ± 0.377 | 5.929 ± 0.175 | < 0.001 |
| eGFR | 80.598 ± 0.188 | 83.752 ± 0.261 | 82.4 ± 0.543 | 77.123 ± 0.423 | 75.908 ± 0.410 | < 0.001 |
| FIB-4 | 0.618 ± 0.005 | 0.515 ± 0.006 | 0.718 ± 0.021 | 0.585 ± 0.011 | 0.826 ± 0.012 | < 0.001 |
| NFS | − 2.312 ± 0.017 | − 2.763 ± 0.022 | − 2.563 ± 0.045 | − 1.877 ± 0.038 | − 1.584 ± 0.036 | < 0.001 |
| APRI | 0.224 ± 0.003 | 0.215 ± 0.003 | 0.284 ± 0.016 | 0.19 ± 0.004 | 0.245 ± 0.007 | < 0.001 |
| Urinary albumin(ug/mL) | 38.261 ± 3.237 | 24.834 ± 3.812 | 32.935 ± 5.595 | 46.346 ± 6.593 | 63.423 ± 10.654 | < 0.001 |
| ACR(mg/g) | 36.09 ± 3.580 | 22.824 ± 3.715 | 34.809 ± 11.299 | 41.73 ± 5.793 | 61.031 ± 12.260 | < 0.001 |
(a) Other marital status is widowed, living separately.
Values for categorical variables, as percentage (95% CI); values for continuous variables, as mean ± standard error.
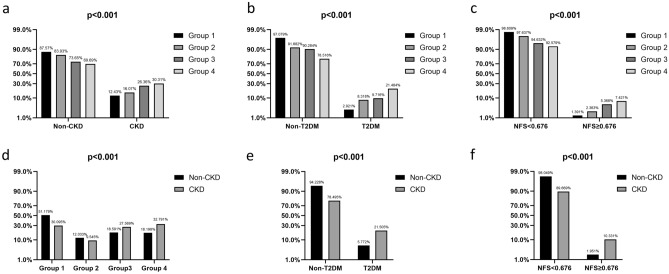
In the subgroup analysis, participants with T2DM, advanced fibrosis and CKD were found to have a higher likelihood of being abdominal obese, having MAFLD, or having both abdominal obese and MAFLD (p < 0.001). The proportion of participants with T2DM, advanced fibrosis and CKD increased from Group 1 to Group 4 (Fig. 2a,b,c). Correspondingly, participants who had abdominal obesity and MAFLD, T2DM, and advanced liver fibrosis were more likely to have CKD (p < 0.001), either(Fig. 2d,e,f).
Figure 2.
Distribution of participant groups by (a) CKD, (b) T2DM, (c) NFS category and distribution of CKD by (d) groups divided by abdominal obesity and MAFLD, (e) T2DM, (f) NFS category.
Factors associated with CKD
In the univariable and multivariable regression analysis, we found that MAFLD (adjusted OR: 1.392, 95% CI 1.013–1.913, p = 0.041), abdominal obesity (adjusted OR 1.456, 95% CI 1.127–1.880, p = 0.004), and abdominal obesity with MAFLD (adjusted OR 1.839, 95% CI 1.377–2.456, p < 0.001) were all independent risk factors for CKD in fully adjusted model(Table 2). Other factors independently associated with a higher risk of CKD were being female (adjusted OR 1.974, 95% CI 1.575–2.473, p < 0.001), being aged 65 or older (adjusted OR 8.735, 95% CI 7.027–10.858, p < 0.001), advanced fibrosis(adjusted OR 1.756, 95% CI 1.178–2.619, p = 0.006), T2DM (adjusted OR 2.365, 95% CI 1.758–3.183, p < 0.001), triglycerides (adjusted OR 1.089, 95% CI 1.010–1.175, p = 0.026) and total cholesterol (adjusted OR 1.308, 95% CI 1.205–1.421, p < 0.001) (Tab. S1).
Table 2.
Association of abdominal obesity-MAFLD with risk of CKD.
| CKD | Univariable Model | Multivariable Model 1 | Multivariable Model 2 | Multivariable Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| OR(95%CI) | p | OR(95%CI) | p | OR(95%CI) | p | OR(95%CI) | p | |
| Group 1 | 1(reference) | 1(reference) | 1(reference) | 1(reference) | ||||
| Group 2 | 1.563(1.178–2.074) | 0.002 | 1.61(1.193–2.174) | 0.002 | 1.548(1.138–2.104) | 0.005 | 1.392(1.013–1.913) | 0.041 |
| Group 3 | 2.758(2.269–3.353) | < 0.001 | 2.164(1.734–2.701) | < 0.001 | 2.042(1.636–2.549) | < 0.001 | 1.456(1.127–1.880) | 0.004 |
| Group 4 | 3.952(3.256–4.795) | < 0.001 | 3.381(2.718–4.205) | < 0.001 | 3.140(2.526–3.905) | < 0.001 | 1.839(1.377–2.456) | < 0.001 |
Model 1 was adjusted for: age, sex, race.
Model 2 was adjusted for model 1 plus marital status, military service, sedentary behavior.
Model 3 was further adjusted for model 2 plus weight category by BMI, advanced fibrosis by NFS, T2DM, LDL, TG, Tc, albumin.
OR, odds ratio; CI, confidence interval.
Long‑term mortality in MAFLD and abdominal obese participant
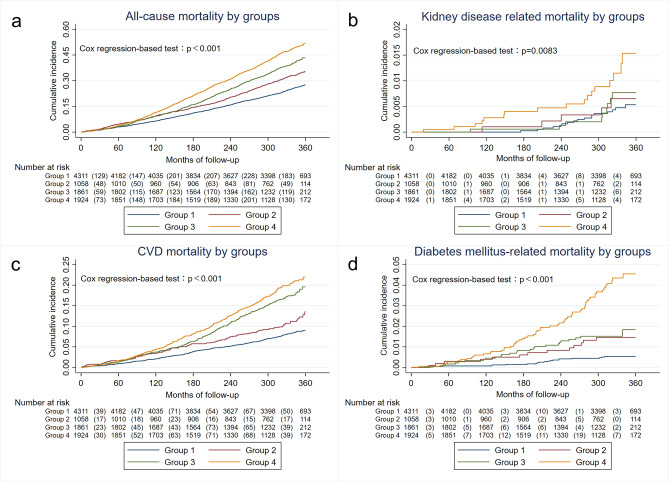
We investigated higher 30-years cumulative all-cause mortality among participants among the four groups. We used cox regression analyze and found all-cause mortality of abdominal obese MAFLD group is consistently higher than other groups (p < 0.001) (Fig. 3a). Additionally, when categorized by the cause of death, we observed that the cumulative incidence of the abdominal obese MAFLD group was significantly higher than the other groups in kidney disease-related mortality (p = 0.0083) (Fig. 3b), cardiovascular and cerebrovascular-related diseases (p < 0.001) (Fig. 3c), and diabetes mellitus-related mortality (p < 0.001) (Fig. 3d).
Figure 3.
Cumulative mortality among participants in MAFLD-abdominal obesity groups for 30 years. (a) all-cause mortality; (b) nephritis, nephrotic syndrome and nephrosis related mortality; (c) cardiovascular and cerebrovascular related diseases; (d) diabetes mellitus related mortality.
Risk factors of kidney disease-related mortality
As shown in Table 3, abdominal obesity in conjunction with MAFLD was a independent risk factor for kidney-related mortality (adjusted HR: 3.448, CI 1.058–11.239, p = 0.040) after adjustment for confounders, while only MAFLD or only abdominal obesity were not. Other risk factors of kidney disease-related mortality were microalbuminuria (adjusted HR: 5.384, 95% CI 1.952–14.845, p = 0.001) and total cholesterol (adjusted HR: 1.829, 95% CI 1.555–2.894, p = 0.010). In contrast, triglycerides (adjusted HR: 0.545, 95% CI 0.361–0.822, p = 0.004), LDL(adjusted HR: 0.796, 95% CI 0.662–0.957, p = 0.015), and serum albumin(adjusted HR: 0.141, 95% CI 0.034–0.591, p = 0.007) were protective factors of kidney-related mortality (Tab. S2).
Table 3.
Association of abdominal obesity-MAFLD with risk of kidney disease-related mortality.
| kidney disease-related mortality | Univariable model | Multivariable Model 1 | Multivariable Model 2 | Multivariable model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| HR(95%CI) | p | HR(95%CI) | p | HR(95%CI) | p | HR(95%CI) | p | |
| Group 1 | 1(reference) | 1(reference) | 1(reference) | 1(reference) | ||||
| Group 2 | 1.716(0.400–7.365) | 0.468 | 1.730(0.378–7.922) | 0.480 | 1.599(0.356–7.186) | 0.541 | 1.788(0.391–8.185) | 0.454 |
| Group 3 | 2.358(0.803–6.923) | 0.119 | 2.485(0.837–7.377)) | 0.101 | 2.126(0.715–6.321) | 0.175 | 1.348(0.414–4.394) | 0.620 |
| Group 4 | 5.316(2.031–13.913) | 0.001 | 5.617(2.090–15.098) | 0.001 | 4.646(1.698–12.712) | 0.003 | 3.448(1.058–11.239) | 0.040 |
HR, hazard ratio; CI, confidence interval.
Model 1 was adjusted for: age, sex, race.
Model 2 was adjusted for model 1 plus marital status, military service, sedentary behavior.
Model 3 was further adjusted for model 2 plus weight category by BMI, advanced fibrosis by NFS, proteinuria, LDL, TG, Tc, albumin.
The mediation effect of abdominal obesity in the association between MAFLD and CKD
To further explore the association between MAFLD and CKD, we conducted covariate-adjusted causal mediation analyses. As shown in Table 4, we observed a significant indirect mediation effect of MAFLD on CKD through several obesity measurement indexes, Notably, indicators that represent abdominal obesity count higher proportion of mediation effect than traditional BMI(32.63%), such as WC(65.23%), WHR(70.68%), WHtR(68.21%) and BRI(62.68%). Moreover, LAP, which represents lipid accumulation, accounts for the highest proportion mediated, amounting to 71.98% of the total effect.
Table 4.
Mediation analysis for the associations between MAFLD and CKD.
| Independent variable | Mediator | Direct effect | Indirect effect | Proportion of mediation (%) |
|---|---|---|---|---|
| Coefficient (95% CI) | Coefficient (95% CI) | |||
| MAFLD | BMI | 0.02001 (0.00623, 0.03340) | 0.00969 (0.00524, 0.01426) | 32.63 |
| WC | 0.01033 (− 0.00547, 0.02504) | 0.01937 (0.01438, 0.02467) | 65.23 | |
| WHR | 0.00871 (− 0.00620, 002,271) | 0.02099 (0.01623, 0.02554) | 70.68 | |
| WHtR | 0.00944 (− 0.00438, 0.02388) | 0.02026 (0.01520, 0.02519) | 68.21 | |
| ABSI | 0.02019 (0.00735, 0.03417) | 0.00951 (0.00691, 0.01219) | 32.03 | |
| BRI | 0.01108 (− 0.00438,0.02608) | 0.01862(0.01328, 0.02423) | 62.68 | |
| VAI | 0.01775 (− 0.00493,0.03245) | 0.01196(0.00785, 0.02885) | 40.25 | |
| LAP | 0.00832 (− 0.00681, 0.02137) | 0.02138 (0.01494, 0.02085) | 71.98 |
Number of bootstrap samples for percentile bootstrap confidence intervals: 1000.
Adjusted for sex, age, race, PIR, marital status, military service and sedentary behavior.
Proportion mediated = indirect effect/ (direct effect + indirect effect).
Discussion
As ongoing research continues to explore the utilization of MAFLD as a more comprehensive and refined term and definition for characterizing what appears to be a metabolically based fatty liver disease, our study aimed to examine the demographic and clinical characteristics, mortality, and the complex association among MAFLD, abdominal obesity and CKD. In our study, we found that 32.584% of individuals had MAFLD, and 41.371% were abdominal obese. Among the abdominal obese participants, 50.844% had MAFLD, which was significantly higher than the 19.698% observed in non-abdominal obese participants. Among those with MAFLD, abdominal obese MAFLD individuals were more likely to be female, older than 65 years, and exhibit higher prevalence of T2DM, advanced liver fibrosis and CKD. Additionally, we observed a higher distribution of advanced liver fibrosis, T2DM, abdominal obesity, and MAFLD in the CKD population. These findings were highlighted in our multivariate logistic analysis where we identified several independent risk factors for CKD, including MAFLD, abdominal obesity, T2DM, being female, age over 65, and sedentary behavior, advanced fibrosis, triglycerides and total cholesterol.
In our study, we found participants with abdominal obesity and MAFLD were more likely to be female. Similar findings have been reported in other literatures. In the research conducted by Dao et al., there was a higher prevalence of obesity MAFLD in females than males (62.6% vs. 47.6%; p < 0.001)25. Previous research has demonstrated that there were significantly more females than males with MAFLD in age subgroups older than 40 years and there was a sharp rise in the prevalence of MAFLD in perimenopausal and postmenopausal women26,27, a period during which the decrease in oestrogen levels can lead to fat redistribution and lead to metabolic disorders, including MAFLD28. Furthermore, abdominal obesity in women is a well-documented risk factor for polycystic ovary syndrome(PCOS) while a number of studies have suggested the close correlation between PCOS and NAFLD29. A meta-analyze involving 7148 participants has reported that premenopausal PCOS patients are associated with 2.5-fold increase in the risk of NAFLD30. In conclusion, it is reasonable to suggest that the high proportion of women with abdominal obesity and MAFLD may be associated with decreased oestrogen levels and co-morbid of PCOS.
We also examined the mortality and found that over a follow-up time of 30 years, the all cause mortality among participants with abdominal obesity and MAFLD was always higher compared to the other groups. Combined with the results of previous baseline data and correlation analysis, we further followed up mortality related to T2DM, cardiovascular and cerebrovascular disease, and kidney disease, and found that participants with both abdominal obesity and MAFLD had the highest mortality among these three cause of mortality. Therefore, we took one additional step to perform a cox regression analysis and confirmed that abdominal obesity plus MAFLD was indeed a risk factor for kidney-related mortalityafter fully adjusted for known prognostic factors. However, only abdominal obesity, as well as MAFLD alone, were not found to be independent risk factors for kidney disease-related death in our study. This finding suggests that the increased mortality associated with kidney disease may be attributed to possible combined effect of abdominal obesity and MAFLD. This relationship has not been demonstrated in previous studies, thus motivated us to conduct causal mediation effect analysis to investigate the potential role of abdominal obesity as a mediator in the association between MAFLD and CKD.
In causal mediation analysis, we found that obesity mediate the relationship between MAFLD and CKD. Notably, when compared to BMI, obesity measurement indexes that better represent abdominal obesity or accumulation of lipid, such as WC, WHR, WHtR, BRI and LAP12,21,31,32, exhibit higher proportion of mediation. This intriguing observation provides a novel perspective on the potential role of visceral fat or accumulated lipid in influencing the association between MAFLD and CKD. It is important to recognize that fat deposition is not limited to adipose tissue but can also occur in non-adipose tissues such as the liver and kidneys and consequently impacts organ function, which is closely associated with both MAFLD and CKD. In both conditions, excessive lipid accumulation exacerbates inflammation, oxidative stress, and organ structural damage by lipotoxicity33–35. Hence, it can be argued that the use of WC as a diagnostic criterion for metabolic disorders in MAFLD proves to be a superior measure of obesity compared to BMI, which is the diagnostic basis for obesity in MAFLD, in exploring the association of MAFLD with patients with CKD.
Abdominal obesity may also contribute to the cross-linking of MAFLD and CKD by increasing the risk of insulin resistance and diabetes mellitus36,37. In our multivariate analysis, we found that T2DM, abdominal obesity, and MAFLD are all independent risk factors of CKD. There are several studies that supported our results to certain extent and found the influential role of abdominal obesity/adiposity in diabetic kidney disease or CKD caused by diabetes mellitus38–41. It is noteworthy that both abdominal obesity and diabetes mellitus are included in the diagnostic criteria for MAFLD, and their association with MAFLD has been demonstrated in many studies42. A cross-sectional study using of 12,571 individuals data from NHANES III has found that patients with MAFLD with coexisting T2DM had a higher prevalence of CKD than their counterparts without diabetes(46.99% vs. 24.22%)43. Another longitudinal study has shown that the clustering of obesity, visceral obesity, and fatty liver disease markedly increased the risk of T2DM in men (adjusted HR 10.5, 95% CI 8.0–13.8) and women (adjusted HR 30.0, 95% CI 18.0–50.0)44. What’s more, the patatin-like phospholipase domain-containing 3 (PNPLA3) rs738409 gene is considered the strongest genetic determinant of fatty liver disease, is also highly expressed on renal podocytes and contributes to renal dysfunction35,45,46. Several studies have found that this genetic variant is primarily associated with insulin resistance or T2DM patients with obesity47. Consequently, extrapolating based on our results and previous studies, abdominal obesity may further enhance the connection between MAFLD and CKD partly through the presence of T2DM.
Our study revealed that liver fibrosis is more prevalent and severe in individuals with abdominal obese MAFLD compared to non-abdominal individuals with MAFLD, as well as in those with CKD compared to those without CKD. Our results are consistent with a community-based prospective study with an average follow-up duration of 4.4 years indicated that the development of NAFLD and progression of fibrosis are linked to an elevated risk of incident CKD48. In addition, NAFLD may exacerbate systemic and hepatic insulin resistance, cause atherogenic dyslipidemia and adipose accumulation, and release a variety of pro-inflammatory, pro-coagulant, pro-oxidant, and pro-fibrogenic mediators that may contributes directly to endothelial dysfunction and tubulointerstitial fibrosis and result in the development and progression of CKD49,50. It has been observed that visceral obesity, rather than elevated BMI, has a stronger correlation with the degree of fibrosis in patients with chronic hepatitis C51, suggesting a potential connection between abdominal obesity and hepatic fibrosis. Several studies have shown that the PNPLA3 I148M variant was also associated with an increased risk of steatosis and fibrosis in liver and kidney, and fat accumulation in this process is causally linked with liver fibrosis and kidney disease progression35,52,53. Thus, our findings highlight the potential role of liver fibrosis relationship between abdominal obesity, MAFLD and CKD. However, understanding the mechanisms and genetic factors that contribute to the onset and progression of these diseases necessitates further investigation through basic medical and prospective studies.
There still existed some limitations in this study. Firstly, NHANES III did not include an over-sampling of Asian Americans and participants with race and ethnicities other than white, black, and Hispanic, so our data may not be generalizable to the Asian American and other race and ethnicity groups. Second, until now, liver biopsy has been the gold standard for dentification of steatosis and advanced hepatic fibrosis, but due to limitations in the NHANES study, we were only able to assess this using noninvasive tests with as much specificity and sensitivity as possible. Third, our study did not include alcohol consumption, smoking, and other possible confounders in the analysis, given that the high level of missing data in this section would have significantly reduced the sample size we included, but these factors may have some influence on the progression of the development of abdominal obesity, MAFLD, and CKD. Moreover, according to the KDIGO Clinical Practice Guidelines, a diagnosis of CKD requires a decline in eGFR and/or presence of kidney damage (such as proteinuria) persisting a minimum of three months. However, due to the limitations of the NHANES III database, the diagnosis of CKD in this article is limited to the criteria of eGFR < 60 mL/min/1.73 m2 and/or ACR ≥ 30 mg/g, without the criterion of duration of not less than 3 months.
Conclusions
Using the data from NHANES III from 1988 to 1994 and the mortality follow-up survey in 2019, we found participants with abdominal obesity and MAFLD were more likely to be female, older and have higher prevalence of T2DM, advanced liver fibrosis and CKD. The abdominal obesity in conjunction with MAFLD had about 3.5-fold increase in the risk of kidney disease-related mortality and the highest cumulative mortality in the 30 years follow-up period. Abdominal obesity may mediate the association between MAFLD and CKD. WC, WHR, LAP are superior indicators of the mediation effect of MAFLD on CKD compared to BMI. Consequently, it is crucial to identify and select appropriate indicators to assess abdominal obesity. Individuals with either MAFLD or abdominal obesity should undergo screening CKD at an early stage, allowing for the implementation of effective interventions to reduce the associated risk of mortality.
Supplementary Information
Acknowledgements
We thank all participants in NHANES and NCHS for providing the data from the National Health and Nutrition Examination Survey III (NHANES III) used in this study.
Author contributions
C.C. and Y.L. conceived and designed research, Z.F. and X.D. performed experiments, C.C., Z.F. and X.D. analyzed data, Z.F. and X.T. prepared tables and figures, Z.F. and C.C. wrote original draft, X.D., X.T and Y.L. revised manuscript, all authors approved final version of manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Zhejiang Provincial Natural Science Foundation of China (LZ24H030001) and National Natural Science Foundation of China (81970543).
Data availability
The datasets used in the current study are publicly available at https://wwwn.cdc.gov/nchs/nhanes.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chao Cen and Zhongwen Fan.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-63386-0.
References
- 1.Xiao J, Wang F, Wong N-K, He J, Zhang R, Sun R, Xu Y, Liu Y, Li W, Koike K, He W, You H, Miao Y, Liu X, Meng M, Gao B, Wang H, Li C. Global liver disease burdens and research trends: Analysis from a chinese perspective. J. Hepatol. 2019;71(1):212–221. doi: 10.1016/j.jhep.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour J-F, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan J-G, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng M-H, Fouad Y, Chan W-K, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Bae SDW, George J, Qiao L. From MAFLD to hepatocellular carcinoma and everything in between. Chin. Med. J. (Engl.) 2022;135(5):547–556. doi: 10.1097/CM9.0000000000002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sundström J, Bodegard J, Bollmann A, Vervloet MG, Mark PB, Karasik A, Taveira-Gomes T, Botana M, Birkeland KI, Thuresson M, Jäger L, Sood MM, VanPottelbergh G, Tangri N. Prevalence, outcomes, and cost of chronic kidney disease in a contemporary population of 2·4 million patients from 11 countries: The CaReMe CKD study. Lancet Reg. Health - Eur. 2022;20:100438. doi: 10.1016/j.lanepe.2022.100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. The Lancet. 2021;398(10302):786–802. doi: 10.1016/S0140-6736(21)00519-5. [DOI] [PubMed] [Google Scholar]
- 6.Pan Z, Alqahtani SA, Eslam M. MAFLD and chronic kidney disease: Two sides of the same coin? Hepatol. Int. 2023;17(3):519–521. doi: 10.1007/s12072-023-10526-9. [DOI] [PubMed] [Google Scholar]
- 7.Association of MAFLD with end-stage kidney disease: a prospective study of 337,783 UK Biobank participants | SpringerLink. https://link.springer.com/article/10.1007/s12072-023-10486-0 (accessed 2023–09–07). [DOI] [PubMed]
- 8.Agustanti N, Soetedjo NNM, Damara FA, Iryaningrum MR, Permana H, Bestari MB, Supriyadi R. The association between metabolic dysfunction-associated fatty liver disease and chronic kidney disease: a systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2023;17(5):102780. doi: 10.1016/j.dsx.2023.102780. [DOI] [PubMed] [Google Scholar]
- 9.Després J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 10.Machado MV, Cortez-Pinto H. NAFLD, MAFLD and obesity: Brothers in arms? Nat. Rev. Gastroenterol. Hepatol. 2023;20(2):67–68. doi: 10.1038/s41575-022-00717-4. [DOI] [PubMed] [Google Scholar]
- 11.Yun H-R, Kim H, Park JT, Chang TI, Yoo T-H, Kang S-W, Choi KH, Sung S, Kim SW, Lee J, Oh K-H, Ahn C, Han SH, Park S, Jhee JH, Kee YK, Chae DW, Chin HJ, Park HC, Lee K, Kim Y-S, Chung W, Hwang Y-H, Kim YH, Kang SW. Obesity, metabolic abnormality, and progression of CKD. Am. J. Kidney Dis. 2018;72(3):400–410. doi: 10.1053/j.ajkd.2018.02.362. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Zhang Y, Liu Y, Li H, Xu R, Fu H, Yan C, Qu B. Comparison between traditional and new obesity measurement index for screening metabolic associated fatty liver disease. Front. Endocrinol. 2023;14:1163682. doi: 10.3389/fendo.2023.1163682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association Professional Practice Committee 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2022. Diabetes Care. 2021;45(Supplement_1):S17–S38. doi: 10.2337/dc22-S002. [DOI] [PubMed] [Google Scholar]
- 14.National Health and Nutrition Examination Survey (NHANES) III, Hepatic Steatosis Ultrasound Images Assessment Procedures Manual 2010. https://www.cdc.gov/nchs/data/nhanes/nhanes3/%20hepatic_steatosis_ultrasound_procedures_manual.pdf. (accessed 2023-10-23).
- 15.Lomonaco R, Godinez Leiva E, Bril F, Shrestha S, Mansour L, Budd J, Portillo Romero J, Schmidt S, Chang K-L, Samraj G, Malaty J, Huber K, Bedossa P, Kalavalapalli S, Marte J, Barb D, Poulton D, Fanous N, Cusi K. Advanced liver fibrosis is common in patients with type 2 diabetes followed in the outpatient setting: the need for systematic screening. Diabetes Care. 2021;44(2):399–406. doi: 10.2337/dc20-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EASL–EASD–EASO Clinical practice guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol.64(6), 1388–1402. (2016) 10.1016/j.jhep.2015.11.004. [DOI] [PubMed]
- 17.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009;7(10):1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311(6998):158–161. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N, Chang Y, Guo X, Chen Y, Ye N, Sun Y. A body shape index and body roundness index: Two new body indices for detecting association between obesity and hyperuricemia in rural area of China. Eur. J. Intern. Med. 2016;29:32–36. doi: 10.1016/j.ejim.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, Galluzzo A. Visceral adiposity index. Diabetes Care. 2010;33(4):920–922. doi: 10.2337/dc09-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahn HS. The lipid accumulation product is better than BMI for identifying diabetes. Diabetes Care. 2006 doi: 10.2337/diacare.29.01.06.dc05-1805. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh JA. New equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens PE, Ahmed SB, Carrero JJ, Foster B, Francis A, Hall RK, Herrington WG, Hill G, Inker LA, Kazancıoğlu R, Lamb E, Lin P, Madero M, McIntyre N, Morrow K, Roberts G, Sabanayagam D, Schaeffner E, Shlipak M, Shroff R, Tangri N, Thanachayanont T, Ulasi I, Wong G, Yang C-W, Zhang L, Levin A. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024;105(4):S117–S314. doi: 10.1016/j.kint.2023.10.018. [DOI] [PubMed] [Google Scholar]
- 24.National Health and Nutrition Examination Survey: Analytic Guidelines, 1999–2010; National Center for Health Statistics (U.S.), Ed.; Vital and health statistics. Series 2; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, Maryland, 2013.
- 25.Dao AD, Nguyen VH, Ito T, Cheung R, Nguyen MH. Prevalence, characteristics, and mortality outcomes of obese and nonobese MAFLD in the United States. Hepatol. Int. 2023;17(1):225–236. doi: 10.1007/s12072-022-10436-2. [DOI] [PubMed] [Google Scholar]
- 26.Prabhakar T, Prasad M, Kumar G, Kaushal K, Shenoy PS, Dubey S, Sarin SK. High prevalence of MAFLD in general population: A large cross-sectional study calls for concerted public health action. Aliment. Pharmacol. Ther. 2024;59(7):843–851. doi: 10.1111/apt.17892. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Li H, Li S, Xu Z, Tian S, Wu J, Liang X, Li X, Liu Z, Xiao J, Wei J, Ma C, Wu K, Ran L, Kong L. Prevalence of and risk factors for metabolic associated fatty liver disease in an urban population in China: A cross-sectional comparative study. BMC Gastroenterol. 2021;21(1):212. doi: 10.1186/s12876-021-01782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutierrez-Grobe Y, Ponciano-Rodríguez G, Ramos MH, Uribe M, Méndez-Sánchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. Role Estrogens. Ann. Hepatol. 2010;9(4):402–409. doi: 10.1016/S1665-2681(19)31616-3. [DOI] [PubMed] [Google Scholar]
- 29.Arefhosseini S, Ebrahimi-Mameghani M, Najafipour F, Tutunchi H. Non-alcoholic fatty liver disease across endocrinopathies: Interaction with sex hormones. Front. Endocrinol. 2022;13:1032361. doi: 10.3389/fendo.2022.1032361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shengir M, Chen T, Guadagno E, Ramanakumar AV, Ghali P, Deschenes M, Wong P, Krishnamurthy S, Sebastiani G. Non-alcoholic fatty liver disease in premenopausal women with polycystic ovary syndrome: A systematic review and meta-analysis. JGH Open. 2021;5(4):434–445. doi: 10.1002/jgh3.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motamed N, Sohrabi M, Poustchi H, Maadi M, Malek M, Keyvani H, Amoli MS, Zamani F. The six obesity indices, which one is more compatible with metabolic syndrome? A population based study. Diabetes Metab. Syndr. Clin. Res. Rev. 2017;11(3):173–177. doi: 10.1016/j.dsx.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 32.Duan Y, Zhang W, Li Z, Niu Y, Chen Y, Liu X, Dong Z, Zheng Y, Chen X, Feng Z, Wang Y, Zhao D, Liu Q, Li H, Peng H, Sun X, Cai G, Jiang H, Chen X. Predictive ability of obesity- and lipid-related indicators for metabolic syndrome in relatively healthy Chinese adults. Front. Endocrinol. 2022;13:1016581. doi: 10.3389/fendo.2022.1016581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clare K, Dillon JF, Brennan PN. Reactive oxygen species and oxidative stress in the pathogenesis of MAFLD. J. Clin. Transl. Hepatol. 2022;10(5):939–946. doi: 10.14218/JCTH.2022.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meléndez-Salcido CG, Ramírez-Emiliano J, Pérez-Vázquez V. Hypercaloric diet promotes metabolic disorders and impaired kidney function. Curr. Pharm. Des. 2022;28(38):3127–3139. doi: 10.2174/1381612829666221020162955. [DOI] [PubMed] [Google Scholar]
- 35.Wang T-Y, Wang R-F, Bu Z-Y, Targher G, Byrne CD, Sun D-Q, Zheng M-H. Association of metabolic dysfunction-associated fatty liver disease with kidney disease. Nat. Rev. Nephrol. 2022;18(4):259–268. doi: 10.1038/s41581-021-00519-y. [DOI] [PubMed] [Google Scholar]
- 36.Ascaso J. Abdominal obesity, insulin resistance, and metabolic syndrome in a southern European population. Eur. J. Intern. Med. 2003;14(2):101–106. doi: 10.1016/S0953-6205(03)00022-0. [DOI] [PubMed] [Google Scholar]
- 37.Muche Ewunie T, Sisay D, Kabthymer RH. Diabetes mellitus and its association with central obesity, and overweight/obesity among adults in Ethiopia. A systematic review and meta-analysis. PLoS One. 2022;17(6):e0269877. doi: 10.1371/journal.pone.0269877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu J, Yang S, Zhang A, Yang P, Cao X, Li X, Goswami R, Wang Y, Luo T, Liao K, Cheng Q, Xiao X, Li Q. Abdominal obesity is more closely associated with diabetic kidney disease than general obesity. Diabetes Care. 2016;39(10):e179–e180. doi: 10.2337/dc16-1025. [DOI] [PubMed] [Google Scholar]
- 39.Shen Y, Xie L, Chen X, Mao L, Qin Y, Lan R, Yang S, Hu J, Li X, Ye H, Luo W, Gong L, Li Q, Mao Y, Wang Z. Renal fat fraction is significantly associated with the risk of chronic kidney disease in patients with type 2 diabetes. Front. Endocrinol. 2022;13:995028. doi: 10.3389/fendo.2022.995028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace AS, Chang AR, Shin J-I, Reider J, Echouffo-Tcheugui JB, Grams ME, Selvin E. Obesity and chronic kidney disease in US adults with type 1 and type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2022;107(5):1247–1256. doi: 10.1210/clinem/dgab927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma C, Yuan M. 14-LB: abdominal obesity indices predict incident renal events in a 10-year cohort with type 2 diabetes mellitus. Diabetes. 2023 doi: 10.2337/db23-14-LB. [DOI] [Google Scholar]
- 42.Jeeyavudeen MS, Khan SKA, Fouda S, Pappachan JM. Management of metabolic-associated fatty liver disease: The diabetology perspective. World J. Gastroenterol. 2023;29(1):126–143. doi: 10.3748/wjg.v29.i1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun D-Q, Jin Y, Wang T-Y, Zheng KI, Rios RS, Zhang H-Y, Targher G, Byrne CD, Yuan W-J, Zheng M-H. MAFLD and risk of CKD. Metabolism. 2021;115:154433. doi: 10.1016/j.metabol.2020.154433. [DOI] [PubMed] [Google Scholar]
- 44.Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: A population-based longitudinal study. Int. J. Obes. 2019;43(1):139–148. doi: 10.1038/s41366-018-0076-3. [DOI] [PubMed] [Google Scholar]
- 45.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mantovani A, Taliento A, Zusi C, Baselli G, Prati D, Granata S, Zaza G, Colecchia A, Maffeis C, Byrne CD, Valenti L, Targher G. PNPLA3 I148M Gene variant and chronic kidney disease in type 2 diabetic patients with NAFLD: Clinical and experimental findings. Liver Int. 2020;40(5):1130–1141. doi: 10.1111/liv.14419. [DOI] [PubMed] [Google Scholar]
- 47.Palmer CNA, Maglio C, Pirazzi C, Burza MA, Adiels M, Burch L, Donnelly LA, Colhoun H, Doney AS, Dillon JF, Pearson ER, McCarthy M, Hattersley AT, Frayling T, Morris AD, Peltonen M, Svensson P-A, Jacobson P, Borén J, Sjöström L, Carlsson LMS, Romeo S. Paradoxical lower serum triglyceride levels and higher type 2 diabetes mellitus susceptibility in obese individuals with the PNPLA3 148m variant. PLoS One. 2012;7(6):e39362. doi: 10.1371/journal.pone.0039362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuo G, Xuan L, Xin Z, Xu Y, Lu J, Chen Y, Dai M, Zhang D, Wang W, Li M, Bi Y, Ning G, Xu M. New nonalcoholic fatty liver disease and fibrosis progression associate with the risk of incident chronic kidney disease. J. Clin. Endocrinol. Metab. 2021;106(10):e3957–e3968. doi: 10.1210/clinem/dgab425. [DOI] [PubMed] [Google Scholar]
- 49.Sinn DH, Kang D, Jang HR, Gu S, Cho SJ, Paik SW, Ryu S, Chang Y, Lazo M, Guallar E, Cho J, Gwak G-Y. Development of chronic kidney disease in patients with non-alcoholic fatty liver disease: A cohort study. J. Hepatol. 2017;67(6):1274–1280. doi: 10.1016/j.jhep.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 50.Whaley-Connell A, Sowers JR. Obesity and kidney disease: From population to basic science and the search for new therapeutic targets. Kidney Int. 2017;92(2):313–323. doi: 10.1016/j.kint.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 51.The impact of insulin resistance, serum adipocytokines and visceral obesity on steatosis and fibrosis in patients with chronic hepatitis C. https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1365-2036.2007.03309.x (accessed 2023–10–29). [DOI] [PubMed]
- 52.Romeo S, Sanyal A, Valenti L. Leveraging human genetics to identify potential new treatments for fatty liver disease. Cell Metab. 2020;31(1):35–45. doi: 10.1016/j.cmet.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Mantovani A, Zusi C, Sani E, Colecchia A, Lippi G, Zaza GL, Valenti L, Byrne CD, Maffeis C, Bonora E, Targher G. Association between PNPLA3rs738409 polymorphism decreased kidney function in postmenopausal type 2 diabetic women with or without non-alcoholic fatty liver disease. Diabetes Metab. 2019;45(5):480–487. doi: 10.1016/j.diabet.2019.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in the current study are publicly available at https://wwwn.cdc.gov/nchs/nhanes.