Abstract
AIM
To investigate the morphological characteristics of retinal vessels in patients with different severity of diabetic retinopathy (DR) and in patients with or without diabetic macular edema (DME).
METHODS
The 239 eyes of DR patients and 100 eyes of healthy individuals were recruited for the study. The severity of DR patients was graded as mild, moderate and severe non-proliferative diabetic retinopathy (NPDR) according to the international clinical diabetic retinopathy (ICDR) disease severity scale classification, and retinal vascular morphology was quantitatively analyzed in ultra-wide field images using RU-net and transfer learning methods. The presence of DME was determined by optical coherence tomography (OCT), and differences in vascular morphological characteristics were compared between patients with and without DME.
RESULTS
Retinal vessel segmentation using RU-net and transfer learning system had an accuracy of 99% and a Dice metric of 0.76. Compared with the healthy group, the DR group had smaller vessel angles (33.68±3.01 vs 37.78±1.60), smaller fractal dimension (Df) values (1.33±0.05 vs 1.41±0.03), less vessel density (1.12±0.44 vs 2.09±0.36) and fewer vascular branches (206.1±88.8 vs 396.5±91.3), all P<0.001. As the severity of DR increased, Df values decreased, P=0.031. No significant difference between the DME and non-DME groups were observed in vascular morphological characteristics.
CONCLUSION
In this study, an artificial intelligence retinal vessel segmentation system is used with 99% accuracy, thus providing with relatively satisfactory performance in the evaluation of quantitative vascular morphology. DR patients have a tendency of vascular occlusion and dropout. The presence of DME does not compromise the integral retinal vascular pattern.
Keywords: diabetic retinopathy, vascular morphology, deep learning, ultra-wide field imaging, diabetic macular edema
INTRODUCTION
Globally, diabetes is estimated to have affected 415 million people in 2015, and by 2040, that number is expected to rise to 642 million[1]. The most prevalent complication of diabetes mellitus, diabetic retinopathy (DR), causes the majority of visual impairment in working-age adults around the world[2]. DR is traditionally considered a disease of retinal vascular abnormalities since the clinically detectable lesions are primarily vascular alterations and manifests differently in different stages, including microaneurysms, intraretinal hemorrhages and venous dilation[2]. One of the most prevalent complications associated with DR is diabetic macular edema (DME), which can develop at any stage of DR and seriously impair central vision[3]. Anti-vascular endothelial growth factor (anti-VEGF) is the most important drug for the treatment of DME. Until now, vascular changes in ophthalmoscopy and color fundus photography have been the main basis for staging and determining the severity of DR. Therefore, understanding the fundamental characteristics of retinal blood vessels may be crucial to comprehending the clinical development of DR.
Recent advances in imaging techniques in retinal diseases have enabled a deeper understanding of the diagnosis and treatment of DR. Microvascular morphological parameters of DR in optical coherence tomography angiography (OCTA) have been quantified through studies, including capillary plexus vessel density, vessel tortuosity, vascular caliber, foveal avascular zone area, etc[4]–[8]. However, few studies have investigated the quantitative vascular morphological changes in DR at the whole retinal scale. With the advent of ultra-wide field (UWF) imaging in clinical practice, it has become possible to provide up to 200° imaging of the retina, covering more than 80% area of the fundus. Researches have proven that when assessing DR severity, UWF imaging and the early treatment diabetic retinopathy study (ETDRS) area accord satisfactorily, and the identification of peripheral lesions will affect the assessment of further DR progression[9]–[11].
Artificial intelligence (AI) has been extensively used in ophthalmic diseases, e.g., glaucoma[12], age-related macular degeneration[13]. AI systems using deep learning can detect and analyze the features of retinal lesions automatically, enabling an automatic diagnosis. The use of deep learning for fundus color map blood vessel segmentation has made very significant progress. Zhang et al[14] used a cascaded network to perform very fine segmentation of arteries and veins in fundus color images taken by dual-modal fundus cameras. In our previous studies, automatic vascular segmentation for familial exudative vitreoretinopathy[15] and retinopathy of prematurity[16] had been accomplished via the deep learning method. Compared to color fundus photography, which focused on the posterior retina, and OCTA, which focused on the macula, UWF imaging can cover more than 80% of the fundus. Thus, the aim of this study was to investigate the quantitative vascular morphological alterations in DR patients from a corporate perspective.
SUBJECTS AND METHODS
Ethical Approval
This study adhered to the Declaration of Helsinki and received approval by the Ethics Review Board of Zhejiang Provincial People's Hospital (No.KY2022063). DR cases (239 subjects, 239 eyes) and healthy participants (controls; 100 subjects, 100 eyes) were recruited from February 2022 to September 2022 in Zhejiang Provincial People's Hospital. All the participants underwent comprehensive ocular examination, including slit lamp examination, UWF imaging (Optos 200Tx Imaging System,), OCT (Spectralis, Heidelberg Engineering).
Inclusion criteria were age ≥18 years old, having either type 1 or type 2 diabetes, and clinically diagnosed non-proliferative diabetic retinopathy (NPDR). Ocular trauma, any other vitreoretinal diseases, or systemic disease like hypertension that could affect the eyes, as well as considerable refractive media opacity which impair image quality, were all excluded.
Ultra-Wide Field Retinal Imaging Examination
UWF retinal imaging was performed on all the DR patients and healthy participants with dilated pupils. A single imaging consists of a green laser image at a wavelength of 532 nm, a red laser image at a wavelength of 633 nm, and a pseudo-color image synthesized from both of the above. In this study, green laser images were collected for blood vessel segmentation in this study due to the favorable contrast between the retinal blood vessels and the background. Following that, the vascular characteristics including vessel angle, vascular density, fractal dimension (Df), and vascular branches, were examined.
DR Severity Grading and Division of DME Subgroups
The DR severity was graded according to the international clinical diabetic retinopathy (ICDR) disease severity scale into 5 grades: no retinopathy, mild NPDR, moderate NPDR, severe NPDR, and proliferative DR (PDR)[17]. The present study included mild, moderate, and severe NPDR. The PDR was excluded because of vascular disorganization, vitreous hemorrhage, and retinal detachment, which made vascular segmentation difficult for AI. Two of the authors (Deng XY and Mao JB) performed the grading job. When there was a disagreement, a senior specialist in ophthalmology (Shen LJ) made the final decision. According to the presence of DME in OCT scans, the DR group was divided into the DME group and the non-DME group, and the vascular morphology of these two groups was compared.
Analysis of Vascular Characteristic by Artificial Intelligence
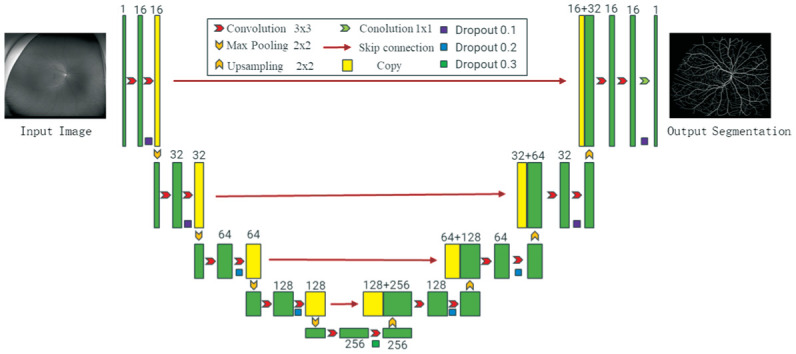
In this study, we optimized the RU-net (Figure 1) by transferring learning, and we performed transfer training on the network using a small number of labeled UWF images of DR patients. A total of 380 well-annotated fundus photography (with a 45° field of view) that were publicly available were applied to pre-train the RU-network. After training, the network was able to detect over 90% of the vessels in UWF images. Then, we fine-tuned the network using 50 well-annotated UWF images to further improve the segmentation of vessels[18]–[19]. Before segmentation, we uniformly cropped the UWF image of DR patients to a size of 3900×3072 pixels, and then sliced it to a size of 576×576 pixels. Finally, the output vascular segmentation map is reorganized, and the vascular segmentation map with a size of 3900×3072 pixels is output.
Figure 1. The schematic diagram of the RU-net used in this study.
Dou et al[20] first performed arteriovenous segmentation of color fundus images before analyzing the vascular morphological parameters of blood vessel segmentation. Based on the work, the vascular segmentation results of UWF images were quantitatively evaluated for vascular morphological parameters. In the present study, the vessel angle, Df, vascular density, and number of vascular branches were all evaluated. In order to determine the angle of blood vessels, we used the reference axis that was created by joining the centers of the macula and the optic disc. For each vessel, we fitted a line with 5 pixels around it and then measured the angle of each blood vessel with respect to the axis. Stosic et al[21] standard box calculation method was utilized to calculate the Df in the skeletonized blood vessels. After removing the non-vascular area, the proportion of the number of vascular pixels to the total number of pixels in the image is calculated as a measure of vascular density. By counting the connecting regions of the interrupted vascular segmentation map, we determined the number of vascular branches. In our earlier work, we provided a full explanation of the calculation process[22].
Statistical Analysis
Data were analyzed using SPSS (version 26.0, USA). All the continuous variables were presented in the form of means±standard deviations. The Chi-square test was used for categorical analyses (sex). The age and vascular morphological characteristics were compared between the groups using one-way analysis of variance. A P-value of less than 0.05 was considered statistically different.
RESULTS
Segmentation of Retinal Vessels in UWF Images
Totally 50 UWF images were used as the test set. The accuracy of the vascular segmentation network reached 0.99 on the test set, and the Dice metrics reached 0.76, which can well segment the blood vessels in the UWF fundus image. Figure 2 depicts representative examples of skeletonized blood vessels from healthy subjects and DR of different severity.
Figure 2. Representative examples of healthy subject and DR in different severity.
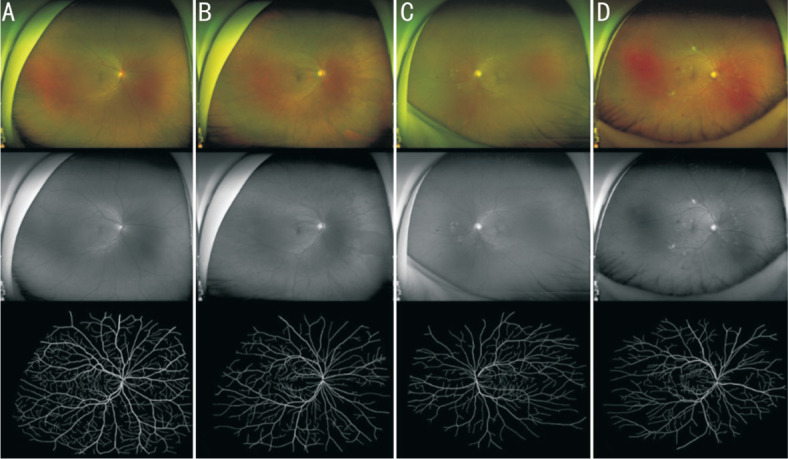
A: Healthy participants; B: Mild NPDR; C: Moderate NPDR; D: Severe NPDR. Upper panel (in color): pseudo-color images; middle panel (in grey): green laser images; lower panel (in black and white): segmented images of binarized skeletonized retinal vessels. NPDR: Non-proliferative diabetic retinopathy.
Demographic Characteristics
A total of 239 eyes from DR patients and 100 eyes from healthy individuals were enrolled. The mean ages in DR group were 60.6±11.8y (ranged from 26 to 88y). The mean ages of controls were 52.0±13.0y (ranged from 28 to 83y). In the DR and healthy groups, the male-to-female ratios were 128:111 (1.15) and 47:53 (0.89), respectively. Age and gender between the two groups were comparable (P=0.137 and 0.271, respectively).
Retinal Vascular Morphology in DR and Control Group
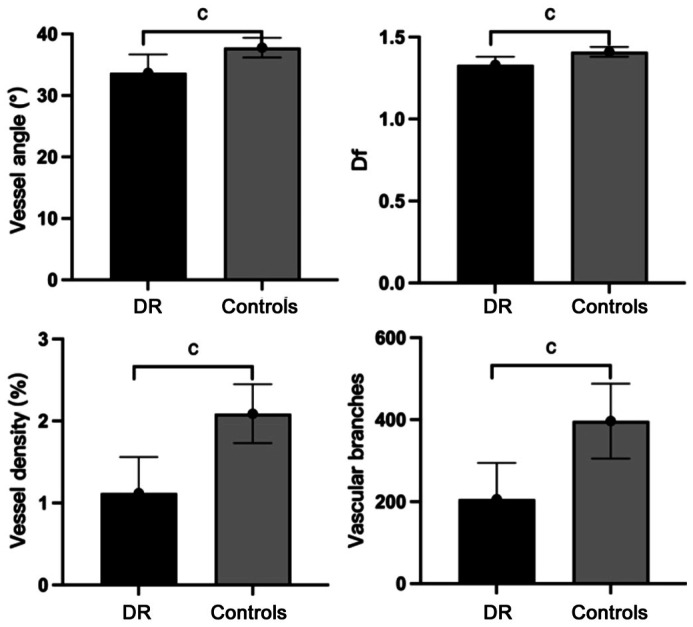
The DR group had smaller vessel angles (33.68±3.01 vs 37.78±1.60), smaller Df values (1.33±0.05 vs 1.41±0.03), less vessel density (1.12±0.44 vs 2.09±0.36) and fewer vascular branches (206.1±88.8 vs 396.5±91.3) as compared to the controls (all P<0.001; Figure 3).
Figure 3. Morphological characteristics between DR group and controls.
DR: Diabetic retinopathy; Df: Fractal dimension. cP<0.001.
Retinal Vascular Morphology in Various DR Severities
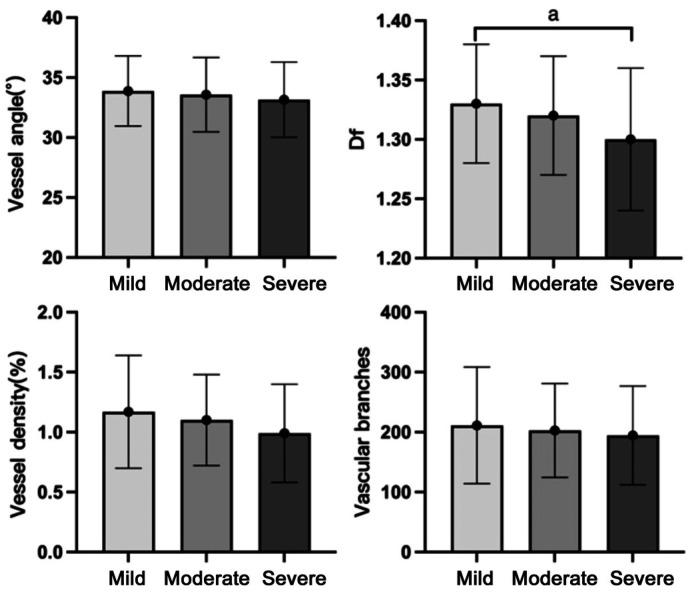
There was significant intergroup difference of Df values in different severity of DR (P=0.031). As the DR severity increased, vessel angle, vessel density, and vascular branches all tended to decrease, although these differences did not reach significance (P=0.463, 0.117, 0.594 respectively; Table 1). In pairwise comparisons (Figure 4), there were significant differences between mild and severe DR in Df values (P=0.028).
Table 1. Vascular morphological characteristics in different severity of DR groups.
| Characteristics | DR severity |
P | ||
| Mild (n=123) | Moderate (n=85) | Severe (n=31) | ||
| Vessel angle | 33.88±2.92 | 33.58±3.10 | 33.16±3.14 | 0.463 |
| Df | 1.33±0.05 | 1.32±0.05 | 1.30±0.06 | 0.031 |
| Vessel density | 1.17±0.47 | 1.10±0.38 | 0.99±0.41 | 0.117 |
| Vascular branches | 211.2±97.2 | 202.9±78.3 | 194.5±82.2 | 0.594 |
DR: Diabetic retinopathy; Df: Fractal dimension.
Figure 4. Differences in morphological characteristics in different severity of DR group.
DR: Diabetic retinopathy; Df: Fractal dimension. aP<0.05.
Comparison between Groups with and without DME
We further divided the DR group into DME (n=120) and non-DME (n=119) groups based on OCT scans. The Df was significantly different between DME and non-DME groups (P=0.044; Table 2). In different severity of DR, no significant difference between the DME and non-DME groups were observed (Table 3).
Table 2. Vascular morphological characteristics between DME and non-DME groups.
| Characteristics | DME (n=120) | Non-DME (n=119) | P |
| Vessel angle | 33.47±3.02 | 33.89±3.00 | 0.289 |
| Df | 1.32±0.05 | 1.33±0.06 | 0.044 |
| Vessel density | 1.08±0.40 | 1.17±0.47 | 0.107 |
| Vascular branches | 200.0±88.4 | 212.2±89.2 | 0.291 |
DME: Diabetic macular edema; Df: Fractal dimension.
Table 3. Vascular morphological characteristics between DME and non-DME groups in different severity of DR groups.
| Characteristics | DME (n=120) | Non-DME (n=119) | P |
| Vessel angle | |||
| Mild | 34.15±3.31 | 33.77±2.76 | 0.521 |
| Moderate | 33.34±2.98 | 34.13±3.35 | 0.281 |
| Severe | 32.87±2.63 | 34.67±5.21 | 0.248 |
| Df | |||
| Mild | 1.33±0.05 | 1.34±0.05 | 0.345 |
| Moderate | 1.32±0.05 | 1.33±0.06 | 0.704 |
| Severe | 1.30±0.05 | 1.32±0.09 | 0.736 |
| Vessel density | |||
| Mild | 1.15±0.48 | 1.17±0.47 | 0.867 |
| Moderate | 1.08±0.36 | 1.16±0.43 | 0.359 |
| Severe | 0.96±0.35 | 1.13±0.68 | 0.625 |
| Vascular branches | |||
| Mild | 218.2±116.9 | 208.4±88.7 | 0.616 |
| Moderate | 194.7±75.1 | 221.4±83.7 | 0.149 |
| Severe | 187.6±69.8 | 230.6±135.3 | 0.523 |
DME: Diabetic macular edema; Df: Fractal dimension.
DISCUSSION
In this study, we investigated the quantified vascular morphology characteristics of DR in UWF retinal imaging using deep learning along with transfer learning techniques. We found a reduction in vessel angle, Df value, vessel density, and vascular branches in eyes with DR. In addition, Df values and vessel density differed between mild and severe DR. However, the presence of DME did not impact the vascular morphology.
Compared with healthy subjects, DR patients had reduced Df values, vessel density and vascular branches, showing a trend toward vascular atrophy and occlusion, which was in line with previous studies. Previous researchers used OCTA to quantify the microvascular density in the macular region, confirming the decrease in vascular density and the increase in avascular area in DR patients[4]–[6],[23]–[24]. Reduced vessel angle indicated increased retinal vascular rigidity and decreased compliance[25].
In the present study, as the DR severity increased, the Df values decreased, which was an indicator of vascular complexity. Previous study[26] using OCTA had confirmed that lower Df values was associated with more severe DR, which was consistent with our findings. Other studies of OCTA had confirmed decrease in macular microvascular density with increasing severity of DR. In our study, although there was an overall decreasing trend in vascular density, the difference was not significant. This may be partly due to differences in grouping methods. For instance, Grag et al[6] and Scheive et al[24] classified DR patients into three groups: mild, moderate-severe NPDR, and PDR, whereas the present study did not include patients with PDR to ensure the accuracy of the overall vascular segmentation. Laser Doppler retinal flowmetry also confirmed decreased blood flow in PDR patients, in comparison with NPDR and pre-PDR[27]. In addition, Song et al[28] investigated fundus photographs and suggested that increased retinal arterial tortuosity was significantly associated with both DR genesis and progress.
Our research suggested that the presence of DME did not influence the retinal vascular morphological characteristics. Guan et al[29] quantified different DME risk groups using Canon laser blood flowmeter and indicated no significant differences between the groups with respect to vessel caliber or flow, which suggested a reduction in the compliance of the arteriolar circulation with increasing risk of DME. Existing studies suggested that plasma proteins accumulation due to the breakdown of the blood-retinal barrier (BRB) may be a factor in the development of capillary nonperfusion and the development of DME[30]. However, the present study aimed at the morphological analysis of medium- to large-sized vessels in the retina in general, which may account for the possible fact that no significant effect of DME on vascular morphology was found in any severity of NPDR.
The current study has several drawbacks. To start with, this was a single-center study with a limited sample size, especially for severe NPDR patients. Thus, the results should be considered cautiously. Moreover, the accuracy of AI for vessel segmentation required to be improved further before it can be used in PDR patients. Further studies may enlarge the sample size through multi-center testing and the continuing improvements in AI and deep learning techniques to eliminate these problems.
Footnotes
Foundation: Supported by Zhejiang Medical Health Science and Technology Project (No.2023KY490).
Conflicts of Interest: Deng XY, None; Liu H, None; Zhang ZX, None; Li HX, None; Wang J, None; Chen YQ, None; Mao JB, None; Sun MZ, None; Shen LJ, None.
REFERENCES
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, Ying GS. Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology. 2020;127(1):P66–P145. doi: 10.1016/j.ophtha.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Bandello F, Battaglia Parodi M, Lanzetta P, Loewenstein A, Massin P, Menchini F, et al. Diabetic Macular Edema. In: Coscas G, Loewenstein A, Cunha-Vaz J, Soubrane G, editors. Developments in Ophthalmology. S. Karger AG; 2017. [cited 2022 Dec 1]. p. 102-138. https://www.karger.com/Article/FullText/455277. Accessed on: 2023 Nov. [DOI] [PubMed] [Google Scholar]
- 4.Jung JJ, Yu DJG, Zeng A, Chen MH, Shi Y, Nassisi M, Marion KM, Sadda SR, Hoang QV. Correlation of quantitative measurements with diabetic disease severity using multiple en face OCT angiography image averaging. Ophthalmol Retina. 2020;4(11):1069–1082. doi: 10.1016/j.oret.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan BY, Chua J, Lin E, Cheng J, Gan A, Yao XW, Wong DWK, Sabanayagam C, Wong D, Chan CM, Wong TY, Schmetterer L, Tan GS. Quantitative microvascular analysis with wide-field optical coherence tomography angiography in eyes with diabetic retinopathy. JAMA Netw Open. 2020;3(1):e1919469. doi: 10.1001/jamanetworkopen.2019.19469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg I, Uwakwe C, Le R, Lu ES, Cui Y, Wai KM, Katz R, Zhu Y, Moon JY, Li CY, Laíns I, Eliott D, Elze T, Kim LA, Wu DM, Miller JW, Husain D, Vavvas DG, Miller JB. Nonperfusion area and other vascular metrics by wider field swept-source OCT angiography as biomarkers of diabetic retinopathy severity. Ophthalmol Sci. 2022;2(2):100144. doi: 10.1016/j.xops.2022.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng QZ, Li SY, Yao YO, Jin EZ, Qu JF, Zhao MW. Comparison of 24×20 mm2 swept-source OCTA and fluorescein angiography for the evaluation of lesions in diabetic retinopathy. Int J Ophthalmol. 2022;15(11):1798–1805. doi: 10.18240/ijo.2022.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qu S, Niu YL, Liu X, Zhang YS, Liu CY, Bi YL. Reproducibility of macular perfusion parameters in non-proliferative diabetic retinopathy patients by two different OCTA sweep modes. Int J Ophthalmol. 2022;15(9):1483–1487. doi: 10.18240/ijo.2022.09.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kernt M, Hadi I, Pinter F, Seidensticker F, Hirneiss C, Haritoglou C, Kampik A, Ulbig MW, Neubauer AS. Assessment of diabetic retinopathy using nonmydriatic ultra-widefield scanning laser ophthalmoscopy (Optomap) compared with ETDRS 7-field stereo photography. Diabetes Care. 2012;35(12):2459–2463. doi: 10.2337/dc12-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aiello LP, Odia I, Glassman AR, Melia M, Jampol LM, Bressler NM, Kiss S, Silva PS, Wykoff CC, Sun JK, Diabetic Retinopathy Clinical Research Network Comparison of Early Treatment Diabetic Retinopathy Study Standard 7-Field imaging with ultrawide-field imaging for determining severity of diabetic retinopathy. JAMA Ophthalmol. 2019;137(1):65–73. doi: 10.1001/jamaophthalmol.2018.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma A, Alagorie AR, Ramasamy K, van Hemert J, Yadav NK, Pappuru RR, Tufail A, Nittala MG, Sadda SR, Raman R, Indian Retina Research Associates (IRRA) Distribution of peripheral lesions identified by mydriatic ultra-wide field fundus imaging in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2020;258(4):725–733. doi: 10.1007/s00417-020-04607-w. [DOI] [PubMed] [Google Scholar]
- 12.Asaoka R, Murata H, Hirasawa K, Fujino Y, Matsuura M, Miki A, Kanamoto T, Ikeda Y, Mori K, Iwase A, Shoji N, Inoue K, Yamagami J, Araie M. Using deep learning and transfer learning to accurately diagnose early-onset glaucoma from macular optical coherence tomography images. Am J Ophthalmol. 2019;198:136–145. doi: 10.1016/j.ajo.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Burlina PM, Joshi N, Pacheco KD, Alvin Liu TYA, Bressler NM. Assessment of deep generative models for high-resolution synthetic retinal image generation of age-related macular degeneration. JAMA Ophthalmol. 2019;137(3):258–264. doi: 10.1001/jamaophthalmol.2018.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang SL, Zheng R, Luo YH, Wang XW, Mao JB, Roberts CJ, Sun MZ. Simultaneous arteriole and venule segmentation of dual-modal fundus images using a multi-task cascade network. IEEE Access. 2019;7:57561–57573. [Google Scholar]
- 15.Ye Y, Mao JB, Liu L, Zhang SL, Shen LJ, Sun MZ. Automatic diagnosis of familial exudative vitreoretinopathy using a fusion neural network for wide-angle retinal images. IEEE Access. 2020;8:162–173. [Google Scholar]
- 16.Mao JB, Luo YH, Liu L, Lao JM, Shao YR, Zhang M, Zhang CY, Sun MZ, Shen LJ. Automated diagnosis and quantitative analysis of plus disease in retinopathy of prematurity based on deep convolutional neural networks. Acta Ophthalmol. 2020;98(3):e339–e345. doi: 10.1111/aos.14264. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT, Global Diabetic Retinopathy Project Group Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 18.Yu F, Xian W, Chen Y, Liu F, Liao M, Madhavan V, et al. BDD100K: a diverse driving video database with scalable annotation tooling. CoRR. 2018;abs/1805.04687 http://arxiv.org/abs/1805.04687. Accessed on: 2023 Nov. [Google Scholar]
- 19.Gargeya R, Leng T. Automated identification of diabetic retinopathy using deep learning. Ophthalmology. 2017;124(7):962–969. doi: 10.1016/j.ophtha.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Dou P, Zhang Y, Zheng R, Ye Y, Mao J, Liu L, Wu M, Sun M. Retinal imaging and analysis using machine learning with information fusion of the functional and structural features based on a dual-modal fundus camera. J Mech Med Biol. 2021;21(6):2150030. [Google Scholar]
- 21.Stosić T, Stosić BD. Multifractal analysis of human retinal vessels. IEEE Trans Med Imaging. 2006;25(8):1101–1107. doi: 10.1109/tmi.2006.879316. [DOI] [PubMed] [Google Scholar]
- 22.Mao JB, Deng XY, Ye Y, Liu H, Fang YY, Zhang ZX, Chen N, Sun MZ, Shen LJ. Morphological characteristics of retinal vessels in eyes with high myopia: Ultra-wide field images analyzed by artificial intelligence using a transfer learning system. Front Med. 2022;9:956179. doi: 10.3389/fmed.2022.956179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mané V, Dupas B, Gaudric A, Bonnin S, Pedinielli A, Bousquet E, Erginay A, Tadayoni R, Couturier A. Correlation between cystoid spaces in chronic diabetic macular edema and capillary nonperfusion detected by optical coherence tomography angiography. Retina. 2016;36 Suppl 1:S102–S110. doi: 10.1097/IAE.0000000000001289. [DOI] [PubMed] [Google Scholar]
- 24.Scheive M, Reinhart KL, Hajrasouliha AR. Using optical coherence tomography angiography as a biomarker of retinopathy severity and treatment for diabetic retinopathy. Mol Vis. 2022;28:220–229. [PMC free article] [PubMed] [Google Scholar]
- 25.Chaqour B, Karrasch C. Eyeing the extracellular matrix in vascular development and microvascular diseases and bridging the divide between vascular mechanics and function. Int J Mol Sci. 2020;21(10):3487. doi: 10.3390/ijms21103487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang FY, Chan EO, Sun ZH, Wong R, Lok J, Szeto S, Chan JC, Lam A, Tham CC, Ng DS, Cheung CY. Clinically relevant factors associated with quantitative optical coherence tomography angiography metrics in deep capillary plexus in patients with diabetes. Eye Vis (Lond) 2020;7:7. doi: 10.1186/s40662-019-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuypers MH, Kasanardjo JS, Polak BC. Retinal blood flow changes in diabetic retinopathy measured with the Heidelberg scanning laser Doppler flowmeter. Graefes Arch Clin Exp Ophthalmol. 2000;238(12):935–941. doi: 10.1007/s004170000207. [DOI] [PubMed] [Google Scholar]
- 28.Song Y, Zhou Z, Liu H, Du R, Zhou Y, Zhu S, Chen S. Tortuosity of branch retinal artery is more associated with the genesis and progress of diabetic retinopathy. Front Endocrinol. 2022;13:972339. doi: 10.3389/fendo.2022.972339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan K, Hudson C, Wong T, Kisilevsky M, Nrusimhadevara RK, Lam WC, Mandelcorn M, Devenyi RG, Flanagan JG. Retinal hemodynamics in early diabetic macular edema. Diabetes. 2006;55(3):813–818. doi: 10.2337/diabetes.55.03.06.db05-0937. [DOI] [PubMed] [Google Scholar]
- 30.Zhang XY, Zeng H, Bao SA, Wang NL, Gillies MC. Diabetic macular edema: new concepts in patho-physiology and treatment. Cell Biosci. 2014;4:27. doi: 10.1186/2045-3701-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]