Abstract
Valgus instability can occur after total knee arthroplasty (TKA) due to traumatic medial collateral ligament (MCL) injury, component malpositioning, or progressive ligamentous laxity. Although revision TKA with exchange of the polyethylene to a varus–valgus-constrained liner can reduce laxity due to MCL insufficiency, isolated liner exchange in the setting of collateral ligament insufficiency may lead to greater strain at the cement–bone or implant–cement interface and possibly a greater rate of aseptic loosening. Anatomic MCL reconstruction can be performed in conjunction with liner exchange to restore stability and reduce strain compared with liner exchange alone. The purpose of this Technical Note is to describe a technique for MCL reconstruction and liner exchange for treatment of valgus instability after TKA.
Technique Video
Valgus instability can occur after total knee arthroplasty (TKA) due to a traumatic medial collateral ligament (MCL) injury, component malpositioning, or progressive ligamentous laxity. Instability has been reported to account for 10% to 20% of indications for revision TKA.1 Although revision TKA with exchange of the polyethylene liner to a varus–valgus-constrained (VVC) liner can reduce laxity due to MCL insufficiency, isolated liner exchange in the setting of collateral ligament insufficiency may lead to greater strain at the implant–bone or implant–cement–bone interface and possibly a greater rate of aseptic loosening.2 MCL reconstruction can be performed along with liner exchange to restore stability and reduce strain at the implant–bone interface as compared with isolated liner exchange. In this Technical Note and associated video (Video 1), we describe a technique for MCL reconstruction and liner exchange for treatment of valgus instability after TKA.
Surgical Technique (With Video Illustration)
Patient Evaluation, Imaging, and Indications
MCL reconstruction in the setting of TKA is indicated for chronic valgus laxity or irreparable MCL injuries after TKA and in the same setting as the primary TKA for iatrogenic MCL injuries or MCL incompetence to avoid using a rotating hinge implant. Although a VVC liner can be used either during primary TKA or revision TKA, the addition of MCL reconstruction may minimize strain at the implant–bone or implant–cement–bone interface, restore the soft-tissue balance, and possibly reduce the rate of aseptic loosening. Although metal artifact reduction sequence magnetic resonance imaging can be used to evaluate the MCL preoperatively, this is not routinely obtained, since the diagnosis can be made clinically. Advantages and disadvantages of this technique are shown in Table 1.
Table 1.
Advantages and Disadvantages of the Proposed Technique
| Advantages | Disadvantages |
|---|---|
| Minimize stress at the implant–bone interface with isolated liner exchange to VVC liner | Increased surgical time compared with isolated liner exchange Risk of disturbing implant fixation with graft fixation |
| Can be used in both primary knees with significant valgus deformity and incompetent MCL or postoperative MCL injuries | |
| Entire procedure can be performed in single standard incision used for total knee arthroplasty |
MCL, medial collateral ligament; VVC, varus–valgus constrained.
Patient Positioning
Patients are positioned in the standard fashion for primary or revision TKA, supine with a lateral thigh post and a foot bump to maintain the knee in flexion. An examination under anesthesia is performed on the operative knee, including valgus and varus laxity testing at 0 and 30° and anterior and posterior laxity testing at 90°.
Approach and Liner Exchange
A standard midline incision and medial parapatellar arthrotomy are used. Once the prosthesis is exposed, it is important to intraoperatively evaluate for component wear, breakage, or loosening, as any of these also can lead to patient-reported instability. A large synovectomy is not usually performed, as this can contribute to a greater degree of instability, and extensive exposure is not required for an isolated liner exchange. The polyethylene liner is removed, and the knee is trialed with VVC liners of varying thickness. Once the size is confirmed, the final VVC liner is placed. Repeat examination is performed to confirm acceptable range of motion, laxity, and patellar tracking.
MCL Reconstruction
An all-soft tissue Achilles allograft is prepared by removing the Achilles bone block and preparing that end to a diameter of 8 mm and placing an adjustable loop cortical button (ProCinch ST; Stryker, Kalamazoo, MI). A mark is placed on the 8-mm end of the graft at 20 mm from the end. The other broader end of the graft is whipstitched with high-strength braided nonabsorbable suture (#2 FiberWire; Arthrex, Naples, FL). Once the total femoral tunnel length from the medial to lateral cortices is determined, that distance is marked on the adjustable loop sutures from the button to assist with flipping the button during femoral graft fixation.
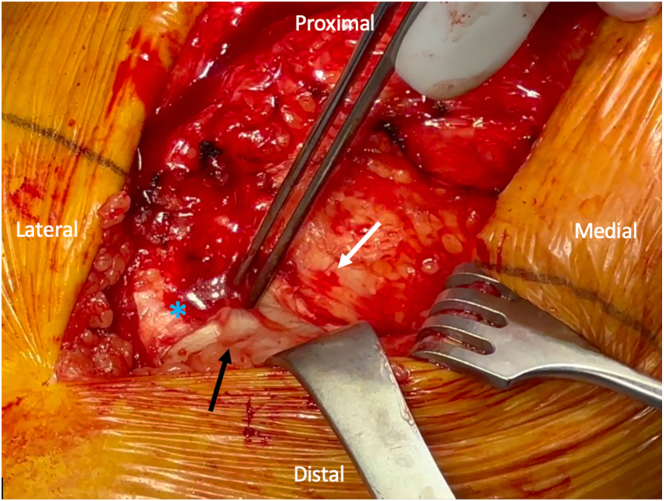
The same anterior incision is used for the MCL reconstruction. It is recommended to avoid a separate medial incision to minimize the risk of wound complications. The medial skin flap is elevated superficial to the capsule to expose the medial femur and tibia. The sartorius fascia is identified and obliquely incised, and the distal insertion of the superficial medial collateral ligament (sMCL) is identified just posterior to the tendinous insertions of the gracilis and semitendinosus and approximately 6 cm distal to the joint line (Fig 1). Often, the remnant MCL fibers can be visualized. A Beath pin is drilled into the tibia at the tibial sMCL insertion site, taking care to aim slightly distally to avoid the tibial keel.
Fig 1.
Identifying the medial collateral ligament (MCL) insertion. An intraoperative image of a right knee is shown, with the orientation as labeled in the figure. The fibers of the MCL insertion (black arrow) in layer two of the medial knee are identified underneath the sartorius fascia. The MCL insertion is identified just posterior to the tendons of the pes anserinus (blue asterisk) underneath the sartorius fascia, approximately 6 cm distal to the joint line. A tonsil is used to develop the plane underneath the sartorius fascia (white solid arrow) and layer one of the knee for later graft passage.
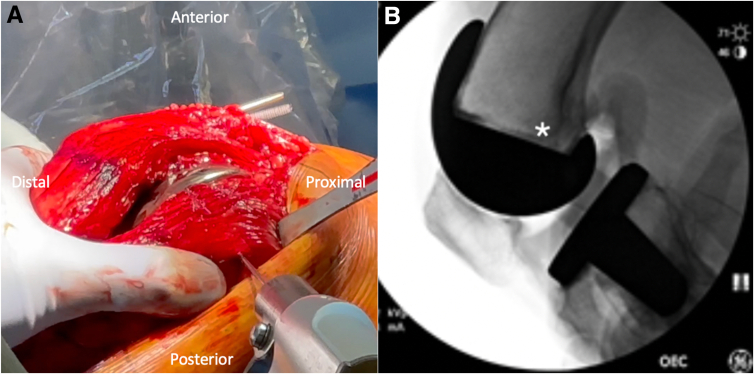
Attention is then turned to identifying the anatomic sMCL femoral origin. The medial epicondyle is palpated. The sMCL origin, which is proximal and posterior to the medial epicondyle, is identified on a lateral fluoroscopic image of the knee,3 and a Beath pin is drilled into the femur at this point, with care taken to aim slightly proximally to avoid the femoral component (Fig 2).
Fig 2.
Identifying the medial collateral ligament (MCL) origin: An intraoperative image of a right knee is shown in (A), with the orientation as labeled in the figure. The medial epicondyle is palpated, and the MCL origin is identified approximately 3.2 mm proximal and 4.8 mm posterior to the medial epicondyle. An intraoperative lateral fluoroscopic image of a right knee is shown in (B), and fluoroscopy is used to confirm the MCL origin (white asterisk), and a Beath pin is drilled into the MCL femoral origin (A), with care taken to aim proximally to avoid the prosthesis.
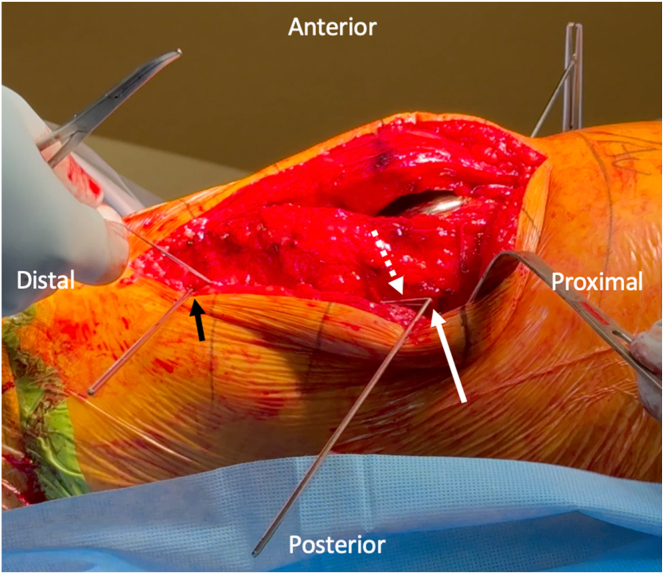
A tonsil is used to develop the plane underneath layer one of the medial aspect of the knee, including the sartorius fascia, from the tibial to the femoral MCL insertion sites, and a suture is passed in this plane between the 2 pins. The suture is wrapped around the pins (Fig 3), and the knee is taken through a range of motion to check isometry, with maximal tension at 30° and reduced suture tension at greater degrees of flexion.
Fig 3.
Evaluating isometry for the planned medial collateral ligament (MCL) graft fixation sites: An intraoperative image of a right knee is shown in (A), with the orientation as labeled in the figure. Beath pins are placed at the planned tibial (black solid arrow) and femoral (white solid arrow) insertion sites for the MCL allograft. A high strength suture (white dotted arrow) is wrapped around the femoral pin and the tibial pin. The knee is taken through a range of motion while attention is paid to whether the suture shortens or lengthens to confirm appropriate isometry.
Once isometry and tension are confirmed, an 8-mm cannulated reamer (Stryker, Kalamazoo, MI) is used to drill a femoral socket to a depth of 30 mm. A second cannulated 4.5-mm reamer (Stryker) is reamed bicortically through the lateral femoral cortex for button passage. The 8-mm end of the graft is then passed into the femoral socket using a passing stitch, the button is flipped on the cortex, and the adjustable loop is used to load 20 mm of the graft into the femoral tunnel (Fig 4).
Fig 4.
Medial collateral ligament (MCL) graft passage into the femoral tunnel. An intraoperative image of a right knee is shown, with the orientation as labeled in the figure. An 8-mm tunnel with 30 mm depth has been reamed. The Achilles tendon allograft (white dotted arrow), which has been previously prepared with an adjustable loop cortical button (ProCinch ST; Stryker, Kalamazoo, MI) after removing the bone block and trimming that end to 8 mm, is passed into the femoral tunnel (white solid arrow). The cortical button is flipped, and 20 mm of the graft is loaded into the tunnel. By only loading 20 mm of graft into a 30-mm tunnel, this allows for additional tensioning after the tibial end of the graft is fixed.
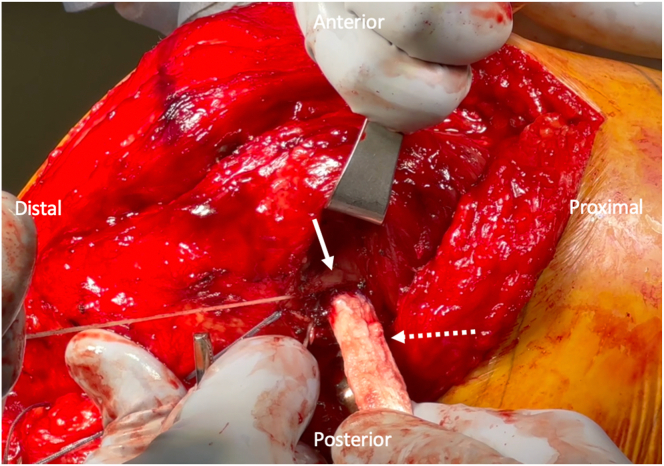
The broader end of the allograft, which corresponds to the proximal part of the Achilles tendon, is then passed underneath layer one of the knee using a passing stitch. The Beath pin is removed, and a 3.2-mm drill (Synthes, Westchester, PA) is used to drill a bicortical hole, with care taken to avoid the tibial keel. The tendon is then placed over the hole, and a 1-cm longitudinal incision in line with the graft is made with a #15 blade over the previously drilled hole. A 4.5-mm solid cortical screw (Synthes, Westchester, PA) with a 13.5-mm spiked PEEK (polyether ether ketone) washer (Synthes) is then placed through the graft and into the previously drilled hole to secure the tibial end of the graft while the knee is at 30°of flexion with a varus force applied (Fig 5). The knee is taken through a range of motion and graft tension and isometry are evaluated. The femoral end of the graft can be additionally tensioned as needed, given that only 20 mm has been loaded into a 30-mm tunnel. We prefer this method of graft fixation on the femoral end, as this allows for additional graft tensioning in the event that the tibial end of the graft was not appropriately tensioned with the screw and washer insertion.
Fig 5.
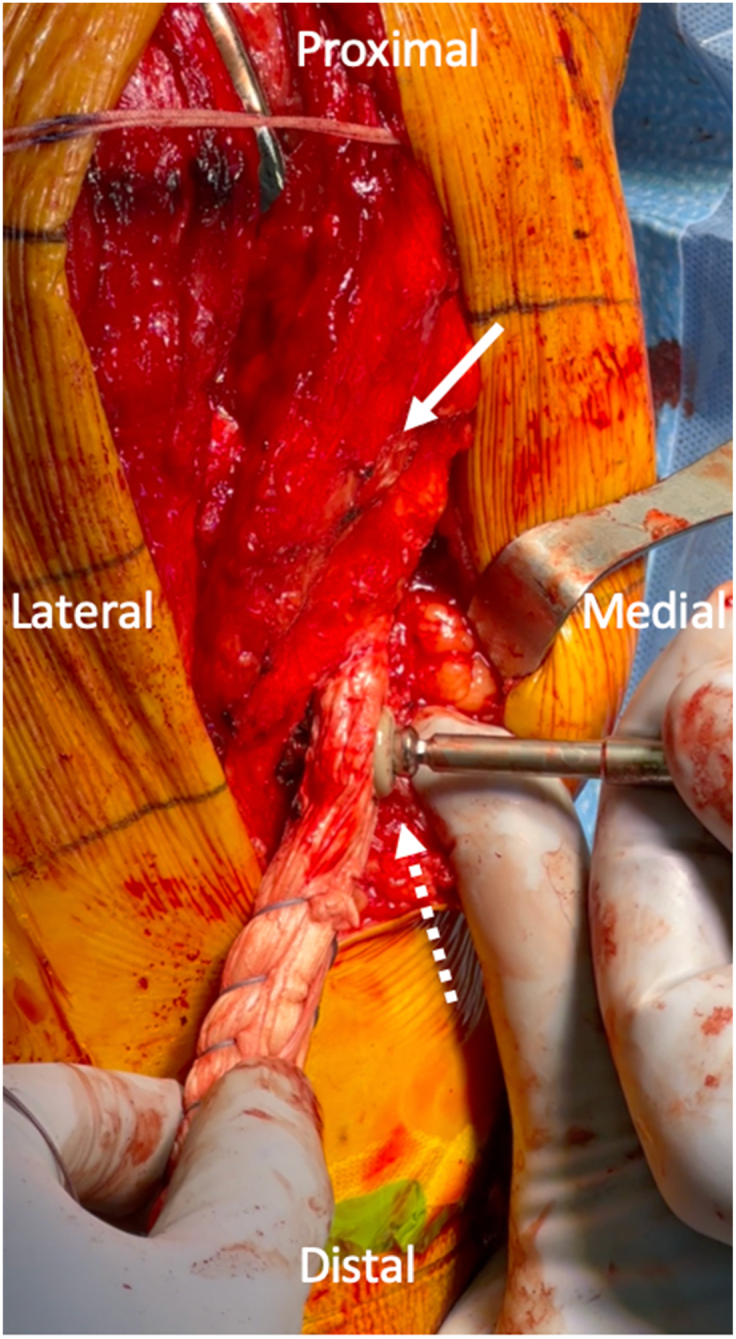
Medial collateral ligament (MCL) graft fixation on the tibia: An intraoperative image of a right knee is shown, with the orientation as labeled in the figure. After the MCL graft is fixed on the femur, the broader end of the graft is passed underneath layer one of the knee (white solid arrow) with a passing suture. A 4.5-mm solid cortical screw with a spiked PEEK washer (Synthes, Westchester, PA) is used to secure the tibial end of the graft (white dotted arrow) while the knee is at 30° of flexion with a varus force applied.
Fluoroscopy is used to confirm hardware position and that the cortical button is located on the cortex with no interposed soft tissue (Fig 6). The wounds are thoroughly irrigated and closed in the standard layered fashion. Pearls and pitfalls of this technique are shown in Table 2.
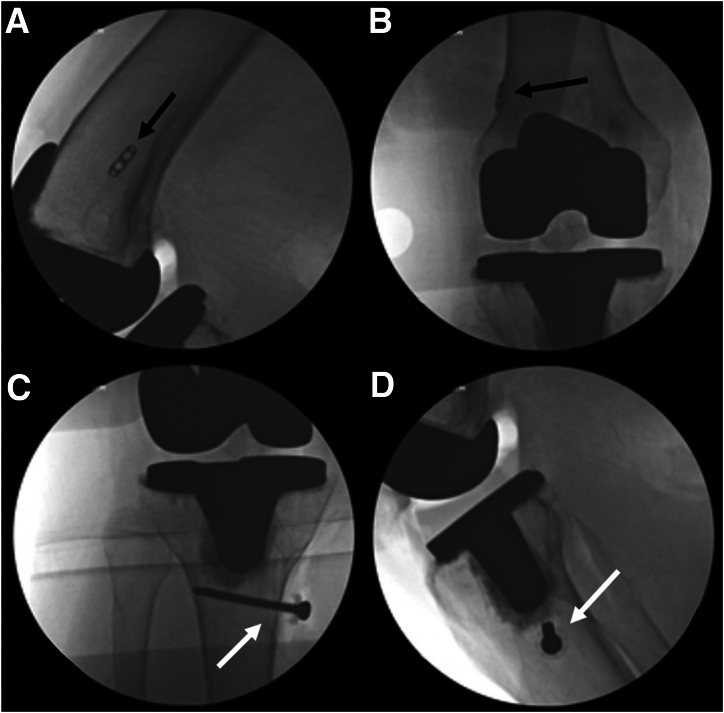
Fig 6.
Intraoperative fluoroscopy. Fluoroscopic images of a right knee are shown. Final anteroposterior (A) and lateral (B) fluoroscopic images of the femur demonstrate the position of the cortical button (black arrow), which is noted to be directly on the lateral cortex of the femur. Final anteroposterior (C) and lateral (D) fluoroscopic images of the tibia demonstrate the position of the tibial screw and washer (white arrow). Note that the head of the screw and washer are not directly on the tibial cortex due to the interposed allograft tissue.
Table 2.
Pearls and Pitfalls of the Proposed Technique
| Pearls | Pitfalls |
|---|---|
| By using the soft-tissue portion of this graft end with suspensory fixation, this allows for additional graft tensioning if the preliminary graft tension is not appropriate after tibial fixation. When drilling for the MCL graft insertion, aim distal in the tibia and proximal in the femur to avoid disturbing the implant–bone interface. The final graft fixation and tensioning should be performed after the final implants, including the varus–valgus-constrained liner, are placed |
Failure to check isometry with a suture running between the planned femoral and tibial insertion sites. Using a separate incision, which can increase the risk for wound complications. |
MCL, medial collateral ligament.
Postoperative Rehabilitation Protocol
Postoperatively, the patient is kept weight-bearing as tolerated in a hinged knee brace. Physical therapy and full range of motion in the unlocked brace, including active and passive range-of-motion exercises, are begun immediately. At 6 weeks postoperatively, the brace is discontinued.
Discussion
In this Technical Note, the management of MCL injury in the setting of TKA is demonstrated. MCL injuries can either occur intraoperatively due to iatrogenic causes or postoperatively as a result of trauma, or the MCL may be incompetent in the setting of significant valgus deformities.4 Although VVC liners can be used to restore valgus stability in these cases, the lack of a competent MCL may lead to greater strain at the cement–bone or implant–cement interface, early post wear, and possibly a greater rate of aseptic loosening when compared with unconstrained liners.2 Although there are case reports of patients with early TKA failure due to valgus laxity who were treated with an MCL reconstruction with allograft,5 there is limited reporting of the long-term results of this technique.
There are some key considerations when performing MCL reconstruction in the setting of TKA. First, we recommend performing the reconstruction within the same anterior incision rather than a separate medial incision to minimize the risk of wound complications, given that the blood supply to the anterior aspect of the soft tissues originates from the medial aspect of the knee.6 Second, it is important when placing the femoral tunnel and distal tibial screw to aim proximally and distally, respectively, to avoid the prosthesis. Lastly, the reconstruction should be performed after the final implants are placed to ensure appropriate graft tensioning and isometry.
Disclosures
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: A.C.K. reports Editorial Board member for Arthroscopy. A.F.V. reports a relationship with American Academy of Orthopaedic Surgeons that includes: board membership; a relationship with American Orthopaedic Society for Sports Medicine that includes: board membership; a relationship with Arthrex that includes: funding grants; and a relationship with Stryker that includes: consulting or advisory. J.C.R. reports a relationship with American Academy of Orthopaedic Surgeons that includes: board membership; a relationship with Aerobiotix that includes: consulting or advisory; a relationship with DePuy Orthopaedics that includes: consulting or advisory; and a relationship with Zimmer that includes: consulting or advisory. M.T.P. reports a relationship with American Academy of Orthopaedic Surgeons that includes: board membership; a relationship with American Orthopaedic Society for Sports Medicine that includes: board membership; a relationship with American Shoulder and Elbow Surgeons that includes: board membership; a relationship with Arthrex that includes: consulting or advisory and funding grants; a relationship with Arthroscopy Association of North America that includes: board membership; a relationship with Elsevier that includes: consulting or advisory and funding grants; a relationship with International Society of Arthroscopy Knee Surgery and Orthopaedic Sports Medicine that includes: board membership; a relationship with JRF Ortho that includes: consulting or advisory; a relationship with Knee that includes: board membership; a relationship with Orthopedics that includes: board membership; a relationship with San Diego Shoulder Institute that includes: board membership; a relationship with Slack Incorporated that includes: board membership, consulting or advisory, and funding grants; and a relationship with The Society of Military Orthopaedic Surgeons that includes: board membership. R.H.K. reports a relationship with DJO Global that includes: consulting or advisory; a relationship with Innomed that includes: consulting or advisory; and a relationship with International Orthopedic Education Network that includes: board membership. T.R.H. reports a relationship with Arthrex that includes: consulting or advisory and funding grants; and a relationship with NICE Recovery Solutions that includes: equity or stocks. All other authors (C.C.L., R.J.W.) declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Data
This video demonstrates the technique for medial collateral ligament (MCL) reconstruction with allograft and liner exchange after total knee arthroplasty (TKA). The patient is a 69-year-old woman who is status post-right TKA approximately 6 years earlier and subsequently developed valgus knee laxity atraumatically. She was indicated for liner exchange to a varus–valgus-constrained (VVC) liner and MCL reconstruction. On examination under anesthesia, the knee is found to be unstable to valgus stress with significant gapping of the medial side at 30° of flexion. The previous midline incision is used and a standard approach, including a medial parapatellar arthrotomy, is performed. The polyethylene liner is removed, and the knee is trialed with VVC liners of varying thickness, and the final VVC liner is placed. The graft is prepared by removing the bone block from the Achilles tendon allograft and trimming the bone block soft-tissue end to a diameter of 8 mm, which is attached to an adjustable loop cortical button for femoral fixation. Through the same midline anterior incision, a medial subcutaneous flap is elevated. Layer two of the knee is identified and developed underneath the sartorius fascia. After incising the fascia and identifying the remnant MCL on the tibia, a tension assessment suture is shuttled under the fascia from proximal to distal with the loop end proximal. Under fluoroscopy, a Beath pin is drilled at the MCL origin through the femur aiming slightly proximally to avoid the femoral component. A second pin is placed at the MCL insertion on the tibia, approximately 6 cm distal to the joint line just posterior to the pes anserine insertion. The previously placed suture is looped over the pins to help assess isometry as the knee was taken through range of motion. The femoral pin is overdrilled with an 8-mm reamer to a depth of 30 mm for graft passage. This is followed by a 4.5-mm reamer through the far cortex to allow for button passage. The femoral Beath pin is then passed through the femur, leaving a passing suture. The femoral tunnel length is measured to help to guide button passage, and the aperture is cleared of soft tissue with electrocautery. The space under the sartorius fascia is gently expanded, and the loop of a shuttling suture is passed proximally. The proximal end of the graft previously prepared with a cortical button is then passed through the femur, and the button is flipped over the lateral cortex. The sutures through the button are then sequentially tightened to load 20 mm of the graft into the femoral socket. Using the shuttling sutures under the sartorius fascia, the free end of the graft is passed under the fascia. A 3.2-mm hole was drilled bicortically at the tibial footprint. A small slit is made in the graft for the passage of a 4.5-mm solid screw with a spiked washer. After assessing hardware positioning with fluoroscopy, the knee is cycled. The graft is retensioned on the femoral end. Final fluoroscopic images are taken, and excess sutures and graft are trimmed.
References
- 1.Petrie J., Haidukewych G. Instability in total knee arthroplasty: Assessment and solutions. Bone Joint J. 2016;98:116–119. doi: 10.1302/0301-620X.98B1.36371. [DOI] [PubMed] [Google Scholar]
- 2.Easley M.E., Insall J.N., Scuderi G.R., Bullek D.D. Primary constrained condylar knee arthroplasty for the arthritic valgus knee. Clin Orthop Relat Res. 2000;380:58–64. doi: 10.1097/00003086-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Wijdicks C.A., Griffith C.J., LaPrade R.F., et al. Radiographic identification of the primary medial knee structures. J Bone Joint Surg. 2009;91:521–529. doi: 10.2106/JBJS.H.00909. [DOI] [PubMed] [Google Scholar]
- 4.Cheung A., Yan C.H., Chan P.K., Chiu K.Y. The medial collateral ligament in primary total knee arthroplasty: Anatomy, biomechanics, and injury. J Am Acad Orthop Surg. 2020;28:e510–e516. doi: 10.5435/JAAOS-D-19-00355. [DOI] [PubMed] [Google Scholar]
- 5.Wierer G., Runer A., Hoser C., Gföller P., Fink C. Anatomical MCL reconstruction following TKA. Knee. 2016;23:911–914. doi: 10.1016/j.knee.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Vince K., Chivas D., Droll K.P. Wound complications after total knee arthroplasty. J Arthroplasty. 2007;22:39–44. doi: 10.1016/j.arth.2007.03.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video demonstrates the technique for medial collateral ligament (MCL) reconstruction with allograft and liner exchange after total knee arthroplasty (TKA). The patient is a 69-year-old woman who is status post-right TKA approximately 6 years earlier and subsequently developed valgus knee laxity atraumatically. She was indicated for liner exchange to a varus–valgus-constrained (VVC) liner and MCL reconstruction. On examination under anesthesia, the knee is found to be unstable to valgus stress with significant gapping of the medial side at 30° of flexion. The previous midline incision is used and a standard approach, including a medial parapatellar arthrotomy, is performed. The polyethylene liner is removed, and the knee is trialed with VVC liners of varying thickness, and the final VVC liner is placed. The graft is prepared by removing the bone block from the Achilles tendon allograft and trimming the bone block soft-tissue end to a diameter of 8 mm, which is attached to an adjustable loop cortical button for femoral fixation. Through the same midline anterior incision, a medial subcutaneous flap is elevated. Layer two of the knee is identified and developed underneath the sartorius fascia. After incising the fascia and identifying the remnant MCL on the tibia, a tension assessment suture is shuttled under the fascia from proximal to distal with the loop end proximal. Under fluoroscopy, a Beath pin is drilled at the MCL origin through the femur aiming slightly proximally to avoid the femoral component. A second pin is placed at the MCL insertion on the tibia, approximately 6 cm distal to the joint line just posterior to the pes anserine insertion. The previously placed suture is looped over the pins to help assess isometry as the knee was taken through range of motion. The femoral pin is overdrilled with an 8-mm reamer to a depth of 30 mm for graft passage. This is followed by a 4.5-mm reamer through the far cortex to allow for button passage. The femoral Beath pin is then passed through the femur, leaving a passing suture. The femoral tunnel length is measured to help to guide button passage, and the aperture is cleared of soft tissue with electrocautery. The space under the sartorius fascia is gently expanded, and the loop of a shuttling suture is passed proximally. The proximal end of the graft previously prepared with a cortical button is then passed through the femur, and the button is flipped over the lateral cortex. The sutures through the button are then sequentially tightened to load 20 mm of the graft into the femoral socket. Using the shuttling sutures under the sartorius fascia, the free end of the graft is passed under the fascia. A 3.2-mm hole was drilled bicortically at the tibial footprint. A small slit is made in the graft for the passage of a 4.5-mm solid screw with a spiked washer. After assessing hardware positioning with fluoroscopy, the knee is cycled. The graft is retensioned on the femoral end. Final fluoroscopic images are taken, and excess sutures and graft are trimmed.