Abstract
Background
Feasibility of exercise in patients with metastatic cancer is still a challenge. This study aimed to determine the feasibility and preliminary efficacy of an exercise intervention based on a patient-preferred delivery mode in patients affected by metastatic cancer.
Materials and Methods
Forty-four patients with a confirmed diagnosis of metastatic cancer were recruited in a 3-month exercise program. Whereas the exercise program consisted of aerobic and resistance activities performed twice a week, the participants may choose the mode of delivery: home based, personal training, or group based. The primary endpoint was the feasibility, defined by recruitment rate, attendance, adherence, dropout rate, tolerability (comparing the session RPE with the target RPE), and safety (using the Common Terminology Criteria for Adverse Events, version 5.0). Secondary endpoints included cardiorespiratory fitness (six minutes walking test), muscle strength (handgrip strength test and isometric leg press test), flexibility (the back scratch and chair sit and reach tests), anthropometric parameters (body mass index and waist-hip ratio), quality of life (EORTC QLQ C-30 questionnaire), and amount of physical exercise (Godin’s Shepard Leisure Time Exercise Questionnaire). Descriptive statistics, Student t test, and Wilcoxon signed rank test were used to analyze data.
Results
The study recruitment rate was 81%. Out of 44 recruited patients, 28 chose the personal training program, 16 chose the home-based program, and none chose the group-based program. Nine dropouts occurred (20%), 6 in the personal training program, and 3 in the home-based intervention. The median attendance rate was 92%, adherence was 88%, tolerability was 100%, and 9 nonsevere adverse events were registered during the exercise sessions. An increase in cardiorespiratory fitness (P < .001) and flexibility (P = .011 for chair sit and reach; P = .040 for back scratch) was observed at the end of the intervention, while no changes in anthropometric values and muscle strength were detected. Different quality-of-life domains were improved following the intervention, including physical (P = .002), emotional (P < .001), and role functioning (P = .018), fatigue (P = .030), and appetite loss (P = .005).
Conclusion
A 3-month exercise program based on a patient-preferred delivery mode is feasible in patients with metastatic cancer and may improve physical function and quality of life.
Trial Registration
Keywords: exercise, metastatic cancer, patient’s preferences, feasibility
This study aimed to determine the feasibility and preliminary efficacy of an exercise intervention based on a patient-preferred delivery mode in patients affected by metastatic cancer.
Implications for Practice.
The authors proposed a program based on the patient-preferred delivery mode, allowing patients with metastatic cancer to choose if to perform exercise at home, with personal training, or in a group-based program. The program has proved to be feasible without serious adverse events and a preliminary efficacy in cardiorespiratory fitness and quality of life has been detected. Although further evaluations of its efficacy have been required, an exercise intervention based on the patient-preferred delivery mode may be a feasible strategy to deliver exercise in the metastatic setting.
Introduction
Although approximately 67% of cancer-related deaths are due to metastatic disease,1 the introduction of innovative anticancer treatment has led to an increasing number of patients living with metastatic cancer, which is projected to grow.2,3 Metastatic cancer is characterized by a high burden of symptoms, with about one-quarter of patients reporting moderate-severe physical and psychological symptoms at diagnosis, such as tiredness, anxiety, fatigue, breathlessness, and pain, which may negatively impact the quality of life and prognosis.4,5
It is now recognized that physical exercise may confer significant enhancements in a broad array of symptom control outcomes for patients with cancer. Nevertheless, most available evidence derives from early-stage settings, whereas minor attention has been dedicated to the metastatic context. Few investigations have been conducted on patients affected by a metastatic disease6-14; most of them tested combined aerobic and resistance training delivered through a supervision-based approach7,8,10,12,14 or with a home-based program,6,9,11 whereas just one explored the impact of aerobic training.13 Preliminary evidence deriving from these researches reported mixed findings regarding benefits in physical fitness and quality of life, which are the most studied outcomes.6-14 In addition, another concern regards the feasibility of the intervention; although the safety profile of exercise in patients with metastasis has been reported,15,16 compliance and adherence to the program are still challenging, and some studies also find a high dropout rate.10,13,17 Different features may obstacle exercise participation, including disease specific (eg, symptoms burden and treatment toxicity) and general barriers (eg, lack of time and interest).18 On the contrary, patients’ preferences may facilitate the adoption of an exercise intervention, especially in the context of metastatic cancer. Whereas the majority of patients prefer a supervised program, the favorite mode of delivery tends to vary: a quarter of patients would like an individual program to follow at home, a quarter an individual program in a gym with a personal trainer, and 40% a group-based program supervised by a kinesiologist.19 Thus, it is possible to speculate that proposing different delivery modalities may be a strategy to increase the patient’s compliance with the exercise program.
The present study aimed to determine the feasibility and safety of a combined exercise program in patients with metastatic cancer based on the patient-preferred delivery mode. The secondary aims were to explore the impact on physical fitness, symptoms, and quality of life. We hypothesized that an exercise intervention that allows patients to choose the delivery modality would be safe, feasible, and produce significant benefits.
Materials and Methods
Study Design
A prospective single-arm feasibility study was conducted between December 2019 and March 2023 at the University of Verona to investigate the effect of a 3-month exercise program in patients with metastatic cancer. The single-arm design was chosen to specifically evaluate each component of the feasibility of the new proposed exercise intervention.20 The project was approved by Verona University Ethics Committee (Prot. No. 33320) and was registered at ClinicalTrials.gov (NCT04226508). The study was conducted following the principles of the Declaration of Helsinki as well as the Declaration of Oviedo. The current report complies with the CONSORT Statement: extension to randomized pilot and feasibility trials.21
Participants and Procedures
Potential eligible patients were recruited by invitation from their attending oncologist or dietitian at the Oncology Units of the University of Verona Hospital Trust. Patients were eligible if they (i) were ≥18 years old, (ii) had a histologically confirmed diagnosis of metastatic cancer, (iii) had an Eastern Cooperative Oncology Group performance status of 0 to 1, 2 (or Karnofsky index ≥ 50), (iv) had medical clearance for study participation, and (v) signed the written informed consent. Exclusion criteria included: (i) pregnancy status, (ii) a time less than 8 weeks from any surgical procedure, (iii) participant’s inability to walk, and (iv) comorbidities that could inhibit patients from exercising as determined by their clinicians.
Healthcare providers sent the information of the interested patients to the research staff. Interested patients were then contacted by the research staff to provide a detailed description of the study and to fix a first appointment at the Department of Sport Science at the University of Verona to confirm eligibility, obtain written informed consent, and perform baseline evaluations.
Exercise Training Intervention
The 3-month exercise program was conducted at the Department of Sport Science facilities at the University of Verona. Patients could choose one of the following exercise delivery modalities based on their preferences and needs:
Home-based program consisted of a personalized written exercise program to perform on patients’ own. In such programs, the activities’ type, frequency, intensity, and duration were detailed, and an exercise log diary was proposed to monitor the intervention. Periodical meetings every 2, 4, and 6 weeks were scheduled in order to hand the new exercise program and teach patients the exercise and how to self-monitor the intensity. A weekly phone call by the research staff was additionally made to check and support patients.
Personal training program, in which each session was supervised and delivered by a dedicated kinesiologist with a kinesiologist-patient ratio of 1:1, at the facilities of the Department of Sport Science.
Group-based program, where participants could exercise with other cancer patients. Each session was supervised and delivered by a dedicated kinesiologist with a kinesiologist-patient ratio of 1:4-6 at the Department of Sport Science facilities.
Patients could not switch the exercise delivery modalities throughout the 3 months.
The exercise prescription was similar for all delivery modalities and was individually tailored to each patient according to functional baseline evaluation and symptom burden.
The program included aerobic and resistance exercises preceded by 5 minutes of warm-up (stretching and light aerobic activity) and 5 minutes of cooldown (stretching). The aerobic component comprised cardiovascular exercises, such as treadmill and cycle-ergometer, for those patients who chose to perform the personal training or group-based program, while patients who followed the home-based program were allowed to perform their preferred activity (swimming, jogging, walking, and cycling). Aerobic exercise duration started at 10-20 minutes and progressively increased every 2 weeks until it reached 30 minutes. The intensity was set at a moderate level and was checked using the 10-point Borg Rating of the Perceived Exertion Scale (ie, 3-5 RPE). Resistance training included 6 exercises using bodyweight and elastic resistance bands (Thera-Bands, Hygienic Corp. Akron, OH), for all 3 exercise delivery modalities. Resistance activity involved the major upper and lower body muscle groups, utilizing a variety of exercises, including, for example, squats, lunges, chest press, should press, biceps curls, pulley, triceps extension, row, calf raise, and crunch, which were adapted on patient’s ability. Participants were instructed to perform 2-3 sets of 8-12 repetitions for each exercise which progressively increased over the weeks, at moderate intensity (ie, 3-5 RPE). Aerobic and strength exercises were performed twice a week each, and patients were allowed to choose whether to perform them in the same session or on 2 separate days based on their preferences, symptoms, and needs.
Outcomes
The primary study endpoint was feasibility assessed by the following: (i) recruitment and completion rate (the ratio of patients who were enrolled and completed the intervention, ie, performed the post-intervention evaluations); (ii) attendance to the aerobic and resistance program (ie, number of the attended sessions compared to the total programmed); attendance were also defined as missed session (ie, missing 1-2 consecutive sessions), treatment interruption (ie, missing ≥3 consecutive sessions), or permanent discontinuation (ie, loss to follow-up); (iii) adherence to the program (ie, the number of completed planned exercise dosage compared to the total programmed); adherence was also classified as dose modification (ie, number of patients who requiring ≥10% of sessions dose escalation/reduction), early session interruption (ie, interruption of session before the planned intensity/duration); (iv) program tolerance (comparing the session RPE with the target RPE)22,23; (iv) patient safety, checking the exercise related adverse events, (using the Common Terminology Criteria for Adverse Events, version 5.0).24 According to the prior literature, the study was considered feasible in the absence of severe life-treating adverse events and if achieved 3 or more of the following: recruitment rate > 50%, lost to follow-up rate < 20%, median attendance > 80%, median adherence > 75%, and tolerance to the planned RPE > 70%.25,26
Secondary endpoints, performed at baseline and postintervention, included the evaluation of the patient’s health-related skills through a series of standardized tests. Cardiorespiratory fitness was estimated using the “six minutes walking test,” according to the guidelines of the American Thoracic Society.27 Muscle strength was evaluated using the handgrip strength test for upper limb,28 and the isometric leg press test for lower limb,29 following standard procedures. The sit and reach test,30 aiming to assess the lower body flexibility, and back scratch test30 for upper body flexibility, were proposed. Anthropometric measures involved height, weight, body mass index, and waist and hip circumferences, evaluated according to the procedures of the World Health Organization.31 Patients reported outcomes included the European Organization for Research and Treatment of Cancer Quality of Life and Core Questionnaire (EORTC QLQ C-30) to determine the quality of life (QoL)32 and exercise level using the Godin’s Shepard Leisure Time Exercise Questionnaire.33 The EORTC QLQ C-30 incorporates 5 functional scales (physical, role, cognitive, emotional, and social), 3 symptom scales (fatigue, pain, nausea and vomiting), and a global health and quality-of-life scale. The remaining single items assess additional symptoms commonly reported by cancer patients (dyspnea, appetite loss, sleep disturbance, constipation, and diarrhea). The scores range from 0 to 100, with higher scores indicating a better function, and a worsening for what concerning symptoms. The Godin’s Shepard Leisure Time Exercise Questionnaire assesses the weekly duration and frequencies of physical activity across strenuous, moderate, and mild intensities. Clinical information was extracted at baselines by reviewing medical charts, and socio-demographic data were collected using a dedicated questionnaire.
Statistical Analysis
Since this was an feasibility study, a formal sample size analysis was not performed. A sample of 44 patients was considered appropriate to estimate the feasibility and explore the efficacy of the exercise intervention.34 Baseline data and feasibility outcomes were analyzed using descriptive statistics (mean, standard deviation, median, interquartile range, frequencies, and percentage). The Shapiro-Wilk test was used for secondary endpoints to test the normality assumption. Changes from baseline to postintervention were explored using the Student’s t test or Wilcoxon signed rank test, as appropriate. Data were analyzed using SigmaStat v. 4.0 (Systat Software Inc). All tests were 2 tailed, and P-values of <.05 were considered statistically significant.
Results
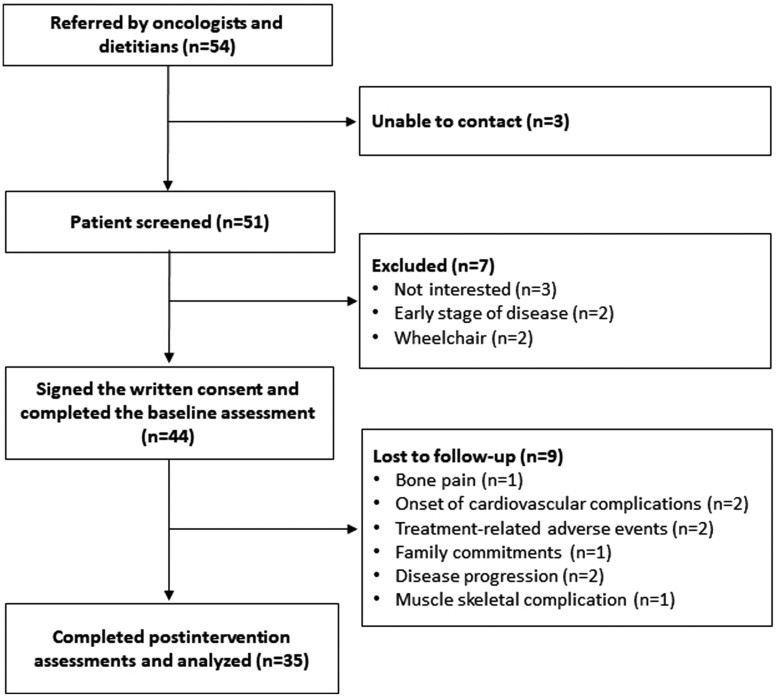
Figure 1 shows the study flow. Of the 54 referred patients, 44 consented to participate and undertook the baseline assessments. Lack of interest (n = 3) and inability to contact patients (n = 3) were the main reasons for exclusion. Baseline participants’ characteristics are displayed in Table 1. Patients had a mean age of 60.5 ± 10.7 years old, 56.8% were male, 43.2% had at least an undergraduate degree, and 81.8% were married. The most represented tumor was pancreatic (27.3%) and breast (18.2%) cancer, and the most common metastatic site was the liver (47.7%). The median time since diagnosis was 17.0 (IQR = 6.0-37.5) months, all patients were undergoing systemic treatments, and 68.2% presented at least one comorbidity. Out of 44 patients, 28 chose to participate in the personal training program and 16 in the home-based program. None chose the group-based program.
Figure 1.
Study flowchart.
Table 1.
Baseline demographics and clinical characteristics.
| Characteristics | Participants (n = 44) |
|---|---|
| Age, mean (SD) | 60.5 (10.7) |
| Male, n (%) | 19 (43.2) |
| Female, n (%) | 25 (56.8) |
| White race, n (%) | 44 (100.0) |
| Education, n (%) | |
| Secondary | 10 (22.7) |
| High school degree | 15 (34.1) |
| Undergraduate degree | 16 (36.4) |
| Postgraduate degree | 3 (6.8) |
| Marital status, n (%) | |
| Single | 4 (9.1) |
| Married | 36 (81.8) |
| Divorced | 3 (6.8) |
| Widow | 1 (2.3) |
| Employment, n (%) | |
| Part-time employed | 5 (11.4) |
| Full-time employed | 13 (29.5) |
| Retired | 26 (59.1) |
| Family income, n (%) | |
| Inadequate | 1 (2.3) |
| Barely adequate | 5 (11.4) |
| Adequate | 24 (54.5) |
| More than adequate | 14 (31.8) |
| Tumor site, n (%) | |
| Pancreas | 12 (27.3) |
| Breast | 8 (18.2) |
| Lung | 7 (15.9) |
| Colon | 6 (13.6) |
| Esophagus | 3 (6.8) |
| Prostate | 3 (6.8) |
| Ovary | 2 (4.5) |
| Thymus | 1 (2.3) |
| Melanoma | 1 (2.3) |
| Liver | 1 (2.3) |
| Metastases sites, n (%) | |
| Liver | 21 (47.7) |
| Bone | 14 (31.8) |
| Lymph nodes | 8 (20.9) |
| Lung | 8 (18.2) |
| Brain | 3 (6.8) |
| Peritoneum | 2 (4.5) |
| Rectum | 1 (2.3) |
| Bladder | 1 (2.3) |
| Metastatic involvement | |
| Single organ | 29 (65.9) |
| Multiorgan | 15 (34.1) |
| Months since diagnosis, median (IQR) | 17.0 (6.0; 37.5) |
| Type of treatment, n (%) | |
| Chemotherapy | 32 (72.7) |
| Radiotherapy | 12 (27.3) |
| Surgery | 17 (38.6) |
| Immunotherapy | 4 (9.1) |
| Target therapy | 7 (15.9) |
| Hormone therapy | 10 (22.7) |
| Current treatments status, n (%) | |
| Ongoing | 44 (100.0) |
| Completed | 0 (0.0) |
| Concomitant comorbidities, n (%) | |
| Yesa | 30 (68.2) |
| No | 14 (31.8) |
aType of comorbidities: hypertension (22.7%), diabetes (13.6%), osteoporosis (11.4%), hypercholesterolemia (2.3%), polyradiculopathy (2.3%), kidney stones (2.3%), anxious-depressive syndrome (2.3%), migraine (4.5%), demyelinating neuropathy (2.3%), gastroesophageal reflux (2.3%), hip vasculitis (2.3%), cardiopathy (11.4%), myasthenia gravis (2.3%), scoliosis (2.3%), dyslipidemia (6.8%), hyperthyroidism (2.3%), metabolic syndrome (2.3%), neuropathy (2.3%), Hashimoto’s thyroiditis (2.3%), Graves-Basedow’s disease (2.3%), and chronic gastritis (2.3%).
Feasibility
The study recruitment rate was 81%. During the trial, 35 patients (80%) completed the study, whereas 9 (20%) patients were lost to follow-up, 6 (21%) in the personal-training program group, and 3 (19%) in the home-based program group (Table 2). No significant difference in clinical, sociodemographic, physical, and quality of life data was observed in patients who were lost to follow-up and those who completed the program (Supplementary Material). The median individual session attendance rate among patients who completed the study was 92% (IQR = 75%-100%; 1431 of 1680 planned sessions), with a minimal difference between aerobic (723 of 840 planned sessions) and resistance (709 of the 840 planned sessions) components. The median attendance rates for the personal training program and home-based program were 90% (IQR = 78%-97%; 900 of 1056 planned sessions) and 96% (IQR = 71%-100%; 541 of 624 planned sessions), respectively. A total of 249 sessions were missed; the mean number of missed sessions per patients was 6.8 ± 6.9 due to principally non–health-related reasons (38.6%) and fatigue (10.8%; Supplementary Material). The treatment interruption rate was 31% (11 of 35 patients), and reasons for interruption were health related (treatment adverse events n = 1, undergoing a nephrostomy n = 1, bone pain n = 1, hospital appointments n = 2, hospitalization n = 1) and non–health related (lack of motivation n = 1 and personal reasons n = 2).
Table 2.
Feasibility outcomes of the entire cohort, and according to the delivery modality.
| Variable | Total cohort | Personal training program | Home-based program | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Aerobic training | Resistance training | Overall | Aerobic training | Resistance training | Overall | Aerobic training | Resistance training | |
| Lost to follow-up, n (%) | 9 (20) | – | – | 6 (21) | – | – | 3 (19) | – | – |
| Attendance, median (IQR) | 92% (75%-100%) | 92% (79%-100%) | 92% (75%-100%) | 90% (78%-97%) | 90% (79%-97%) | 90% (78%-97%) | 96% (71%-100%) | 96% (77%-100%) | 96% (65%-100%) |
| Treatment interruption, n (%) | 11 (31) | 10 (29) | 11 (31) | 6 (26) | 6 (26) | 6 (26) | 5 (38) | 4 (31) | 5 (38) |
| Missed session, mean (SD) | 6.8 (6.9) | 3.2 (3.2) | 3.7 (4.1) | 7.1 (6.7) | 3.3 (3.1) | 3.8 (3.9) | 6.4 (7.5) | 2.9 (3.3) | 3.5 (4.5) |
| Adherence, median (IQR) | 88% (75%-100%) | 88% (79%-100%) | 88% (75%-100%) | 85% (77%-97%) | 85% (79%-97%) | 85% (75%-97%) | 90% (71%-100%) | 96% (77%-100%) | 92% (65%-100%) |
| Dose modification, n (%) | 1 (3) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 1 (8) | 0 (0) | 1 (8) |
| Early session termination, n (%) | 1 (0) | – | – | 1 (0) | – | – | 0 (0) | – | – |
| Tolerability, % | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Definition: Lost to follow-up, number of patients who did not complete the study; attendance, number of attended sessions compared to the total; treatment interruption, number of patients who missed ≥3 continuous sessions; missed session, number of sessions not attended by the patients; adherence, number of completed planned exercise dosage compared to the total programmed; dose modification, number of patients that required ≥10% of sessions dose escalation/reduction; early session termination, number of sessions interrupted before the planned intensity/duration; tolerability, number sessions performed at the planned intensity.
Median adherence to the prescribed exercise program was overall 88% (IQR = 75%-100%; 1427 of 1680 planned exercise dosage), 88% (IQR = 79%-100%; 727 of 840 planned exercise dosage) for the aerobic training component and 88% (IQR = 75%-100%; 700 of 840 planned exercise dosage) for resistance exercise. Personal training program showed a median adherence of 85% (IQR = 77%-97%; 892 of 1056 planned exercise dosage), while the home-based program of 90% (IQR = 71%-100%; 535 of 624 planned exercise dosage). One patient in the home-based program required a dose modification for the resistance component, whereas another in the personal training program had to terminate the session early due to tachycardia onset. Overall, patients reported to have performed the sessions at the prescribed intensity (ie, 3-5 RPE). Nine nonserious adverse events were registered during the exercise sessions (Table 3), 5 in the personal training group and 4 in the home-based group; just in one case, the onset of an adverse event required interrupting the session as reported.
Table 3.
Adverse events registered during the exercise sessions.
| Variable | Total cohort | Personal training program | Home-based program |
|---|---|---|---|
| Adverse events, n (%) | 9 (100) | 5 (100) | 4 (100) |
| Dizziness | 2 (22.2) | 2 (40.0) | 0 (0.0) |
| Back pain | 1 (11.1) | 0 (0.0) | 1 (25.0) |
| Leg pain | 3 (33.3) | 0 (0.0) | 3 (75.0) |
| Fatigue | 1 (811.1) | 1 (20.0) | 0 (0.0) |
| Palpitation | 2 (22.2) | 2 (40.0) | 0 (0.0) |
Exploratory Endpoints
The effect of the intervention on physical function is displayed in Table 4. There were no significant changes in the anthropometric measures and strength. Significant increases in the six minutes walking test (+34.5 m, 95% CI: 19.8-49.2; P < .001), the chair sit and reach test—right leg (+3.1 cm, 95% CI: 0.8-5.5; P = .011), and back scratch test—left arm (+1.0 cm, 95% CI: 0.1-2.0; P = .040) were observed. Patients reported outcomes are reported in Table 5. At the end of the intervention, several domains of QoL improved, including physical functioning (80.0 points [pts] 95% CI: 73.3-93.3 vs. 86.7 pts 95% CI: 80.0-93.3; P = .002), role functioning (83.3 pts 95% CI: 66.7-100.0 vs. 100.0 pts 95% CI: 83.3-100.0; P = .018), emotional functioning (75.0 pts 95% CI: 58.3-83.3 vs. 83.3 pts 95% CI: 75.0-31.7; P < .001), fatigue (33.3 pts 95% CI: 22.2-55.6 vs. 22.2 pts 95% CI: 11.1-33.3; P = .030), and appetite loss (0.0 pts 95% CI: 0.0-33.3 vs. 0.0 pts 95% CI:0.0-0.0; P = .005). The total physical activity (225.0 minutes/week 95% CI: 0.0-420.0 vs. 330.0 pts 95% CI: 135.0-720.0; P = .019) and activity performed at moderate intensity (0.0 minutes/week 95% CI: 0.0-120.0 vs. 120.0 pts 95% CI: 60.0-240.0; P = .004) increased significantly.
Table 4.
Functional assessments and change over 3 months.
| Variables (n = 35) | Baseline, mean (SD) | Postintervention, mean (SD) | Mean difference (95% CI) | P-value |
|---|---|---|---|---|
| Anthropometric measures | ||||
| Body weight (kg)* | 70.9 (13.3) | 71.7 (12.6) | 0.83 (−0.29 to 1.94) | .140 |
| Body mass index (kg/m2)* | 26.2 (4.4) | 26.3 (4.0) | 0.08 (−0.49 to 0.65) | .781 |
| Waist (cm)* | 90.8 (13.9) | 89.5 (12.8) | −1.25 (−3.01 to 0.51) | .159 |
| Hip (cm)* | 102.5 (8.0) | 101.8 (6.3) | −0.74 (−2.21 to 0.74) | .317 |
| Waist-hip ratio (cm)* | 0.88 (0.09) | 0.87 (0.04) | 0.00 (−0.02 to 0.01) | .586 |
| Chair sit and reach (cm) | ||||
| Right leg* | −3.3 (10.7) | −0.1 (12.2) | 3.14 (0.78 to 5.51) | .011 |
| Left leg* | −3.3 (11.5) | −0.8 (12.3) | 2.44 (−0.03 to 4.93) | .052 |
| Back scratch (cm) | ||||
| Right arm* | −3.7 (12.1) | −2.6 (11.9) | 0.46 (−1.54 to 2.45) | .645 |
| Left arm* | −9.7 (12.3) | −8.4 (12.8) | 1.03 (0.05 to 2.01) | .040 |
| Handgrip (kg) | ||||
| Right arm* | 28.7 (8.1) | 29.1 (7.3) | 1.21 (−0.20 to 2.62) | .090 |
| Left arm* | 26.8 (8.5) | 27.1 (7.3) | 0.77 (−0.35 to 1.88) | .171 |
| Leg press (kg)* | 89.8 (44.9) | 114.3 (85.4) | 24.40 (−0.28 to 49.08) | .052 |
| Six minutes walking test (m)* | 489.0 (79.5) | 519.1 (71.4) | 34.51 (19.82 to 49.21) | <.001 |
*Data analyzed using the Student’s t test.
Table 5.
Patient-reported outcomes and change over 3 months.
| Variables (n = 35) | Baseline, median (IQR) | Postintervention, Median (IQR) | P-value |
|---|---|---|---|
| EORTC QLQ-C30 | |||
| Physical functioning* | 80.0 (73.3-93.3) | 86.7 (80.0-93.3) | .002 |
| Role functioning* | 83.3 (66.7-100.0) | 100.0 (83.3-100.0) | .018 |
| Emotional functioning* | 75.0 (58.3-83.3) | 83.3 (75.0-91.7) | <.001 |
| Cognitive functioning* | 83.3 (66.7-100.0) | 100.0 (83.3-100.0) | 1.000 |
| Social functioning* | 66.7 (50.0-83.3) | 83.3 (66.7-100.0) | .057 |
| Global health status* | 66.7 (50.0-83.3) | 66.7 (50.0-83.3) | .322 |
| Fatigue* | 33.3 (22.2-55.6) | 22.2 (11.1-33.3) | .030 |
| Nausea/vomiting* | 0.0 (0.0-16.7) | 0.0 (0.0-16.7) | .893 |
| Pain* | 16.7 (0.0-33.3) | 16.7 (0.0-33.3) | .265 |
| Dyspnea* | 33.3 (0.0-33.3) | 0.0 (0.0-33.3) | .353 |
| Insomnia* | 0.0 (0.0-33.3) | 0.0 (0.0-33.3) | .252 |
| Appetite loss* | 0.0 (0.0-33.3) | 0.0 (0.0-0.0) | .005 |
| Constipation* | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | .164 |
| Diarrhea* | 0.0 (0.0-33.3) | 0.0 (0.0-0.0) | .250 |
| Financial problems* | 0.0 (0.0-33.3) | 0.0 (0.0-0.0) | .250 |
| Physical activity level (minutes/week) | |||
| Vigorous* | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | .383 |
| Moderate* | 0.0 (0.0-120.0) | 120.0 (60.0-240.0) | .004 |
| Light* | 75.0 (0.0-280.0) | 120.0 (60.0-240.0) | .440 |
| Total* | 225.0 (0.0-420.0) | 330.0 (135.0-720.0) | .019 |
*Data analyzed using the Wilcoxon signed rank test.
An exploratory analysis of the 2 exercise program modalities was performed (Supplementary Material). At baseline, the 2 groups were balanced for sociodemographic and clinical data, except for family income (P = .022) and type of anticancer treatment (P = .15). Moreover, at baseline a significant better profile for waist-hip ratio (P = .044), handgrip strength (P = .008, right arm; P = .007, left arm), six minutes walking test (P < .001), and some domains of quality of life (ie, physical functioning pain, dyspnea, insomnia, and constipation) was observed in patients who chose the home-based program. Both groups exhibited a significant increase in the six minutes walking test (+31.9 m 95% CI: 11.8-52.2; P = .005 home-based program; +35.9 m 95% CI: 14.9-56.9; P = .001 personal training program), and additionally, the home-based program reported improvement in upper (+2.0 cm 95% CI: 0.6-3.5; P = .010 right arm; + 2.0 cm 95% CI: 0.4-3.6; P = .021 left arm) and lower (+3.8 cm 95% CI: 0.5-4.8; P = .025 right leg) limb flexibility. Patients in the personal training program reported an increase in the handgrip strength test—right arm (+1.6 kg 95% CI: 0.1-3.2; P = .021) and significant enhancements in physical functioning (P = .013), role functioning (P = .010), emotional functioning (P = .014), appetite loss (P = .021), and moderate intensity physical activity (P = .010).
Discussion
The present study indicates that a physical exercise program, based on patients-preferred delivery mode, on the basis of predefined criteria is feasible in patients with metastatic cancer; indeed our intervention has reported an 81% recruitment rate, a median attendance of 92%, and adherence of 88%, an optimal tolerance, a good safety profile, and a 20% of dropout rate. A recent systematic review on patients with advanced cancer reported a median recruitment rate of 68% and attendance rate of 86%35; focusing on patients with metastatic disease, recruitment, dropout, and attendance rates varied, ranging from 25% to 75%, 17.8% to 50%, and 50% to 89%, respectively,35 suggesting that feasibility is highly heterogeneous across the trials. Most of the studies included in such revision were conducted under a fully supervised context, and among the reasons for nonparticipation, the travel distance to the study site was found relevant. Home-based programs could break down this barrier, offering patients a more flexible intervention; on the other hand, patients have a greater degree of autonomy to manage training with potential implications in its attendance and completion. In this sense, a 12-week home-based program, including walking and resistance training with elastic bands, in men with metastatic prostate cancer showed a 47% of recruitment rate, 27% of patients who withdrew, and 80% of adherence to the walking program, and only 63% of resistance component.9 Similarly, another trial testing the feasibility of a walking program in patients with different metastatic cancer reported a 38% of recruitment rate and a 45% of attrition rate.36 Our study reported high rates of recruitment, adherence and attendance, and a low dropout; additionally, no important difference for such feasibility outcomes was observed between the personal training program and the home-based program and neither the aerobic and resistance components. This finding is particularly interesting and may suggest that the intervention design may have favored these aspects. Indeed, allowing patients to choose their preferred exercise modality may allow patients to have a proactive role in their care pathways without being conditioned by the impositions of others, leading to an increase in their empowerment. Another concern related to the proposal of a single exercise modality is that it may not suit all patients, especially in the metastatic cancer setting. The most deconditioned patients may feel particularly uncomfortable in a home-based program, mainly because of safety issues; vice versa, patients with a preserved psycho-physical condition may not need or prefer a close supervision. Our exploratory analysis confirmed this hypothesis: at baseline, patients who have chosen the home-based program had a better profile in the anthropometric values, in strength, cardiorespiratory fitness, and some domains of quality of life, while patients in the personal training program were more deconditioned. In this light, from the healthcare and kinesiologists’ perspectives, the proposal of multiple modalities of exercise delivery may have several advantages, including supervising the frailest patients and having a time-saving intervention (ie, a home-based program) that allows following several patients simultaneously. Another in point in favor of the present study is the lack of serious adverse events which highlights a good safety profile. Only 9 nonserious events were observed and just in one case it require a dose modification and an early session termination. Scott et al13 in their trial investigating aerobic exercise in patients with metastatic breast cancer reported an higher rate of adverse events during the training sessions, however, even in this case, the side effects were nonserious, confirming the acceptable safe profile of exercise.
Regarding the exploratory efficacy outcomes, no significant effect of exercise was found for muscle strength and anthropometric parameters. Compared to our study, a prior investigation found a positive impact of exercise on strength.14 Specifically, a 12-week intervention combining aerobic and strength training was able to significantly improve upper and lower limb strength, both, in patients with advanced cancer. A possible explanation could be related to the strength protocol; whereas such a study used strength training based on isotonic machines, we have preferred to deliver a program based on exercises performed using body weight or elastic bands. In this sense, it is possible to speculate that strength training on isotonic machines may produce higher stimulus to increase strength compared to exercises performed using body weight.
We found that exercise improved flexibility, cardiorespiratory fitness, and some QoL domains. The favorable effect of exercise on cardiorespiratory fitness was also consistent in the sub-analysis of the 2 exercise modalities, suggesting that the programs may be equally effective in increasing this parameter. Whereas prior meta-analyses and systematic reviews investigating the impact of exercise on cardiorespiratory fitness found inconsistent results,35,37 other studies found positive findings.14,38 For instance, a single-arm trial involving patients with locally advanced or metastatic disease reported that a 12-week combined aerobic and resistance training program significantly improved cardiorespiratory, strength, and QoL.14 This results may be particularly encouraging and assume an important clinical implication since cardiorespiratory fitness is a prognostic factor in cancer39 and the vast majority of patients present impairment in this parameter.40
In our study, several domains of QoL associated with functioning, such as physical and emotional functioning, and symptoms, including fatigue and appetite loss, improved after exercise intervention. Moreover, the exploratory analysis has shown that significant improvements occurred in patients attending the personal training program; nevertheless, it may be related to the low baseline levels reported in the personal training program, making it more sensitive to the improvements. Although the impact of exercise on QoL, and symptoms such as nausea,41 and fatigue42 are well established, our findings may be particularly relevant, since most of the available studies analyzing the effect of exercise on QoL and symptoms are conducted in early-stage context. Indeed, QoL assumes considerable importance in daily oncology practice, especially in the treatment pathway of patients with incurable disease, in which more consideration is oriented to the trade-off between patients’ quality and quantity of life, and the decision-making process is often oriented toward extending the patient’s life without reducing QoL.43
Limitations of the present work may be related to the heterogeneity of the study population, the lack of follow-up, and measurements dedicated to the body composition analysis, given its relevance in the oncological setting. Additionally, the COVID-19 outbreak period in which this study was conducted has discouraged the implementation of the group-based program, which no one has chosen, consequently losing valuable information. The study’s strengths are primarily in the exercise intervention, being the first study proposing a program based on a patient-preferred delivery mode, and in the large amount of collected feasibility outcomes, which provided a large amount of data enables the replication of this study in a large scale and a randomized design.
Conclusion
In conclusion, a 12-week exercise program based on the patient-preferred delivery mode is safe and feasible in patients with metastatic cancer. Further, the intervention appears to improve cardiorespiratory fitness, flexibility, and some domains of QoL. A future large randomized controlled trial should be addressed to evaluate the real efficacy of this intervention.
Supplementary Material
Contributor Information
Alice Avancini, Section of Innovation Biomedicine-Oncology Area, Department of Engineering for Innovation Medicine (DIMI), University of Verona and University and Hospital Trust (AOUI) of Verona, Verona, Italy.
Anita Borsati, Biomedical, Clinical and Experimental Sciences, Department of Medicine, University of Verona, Verona, Italy.
Elisabetta Baldo, Department of Neurosciences, Biomedicine and Movement, University of Verona, Verona, Italy.
Christian Ciurnelli, Department of Neurosciences, Biomedicine and Movement, University of Verona, Verona, Italy.
Ilaria Trestini, Dietetic Service, Medical Direction, Azienda Ospedaliera Universitaria Integrata di Verona, Verona, Italy.
Daniela Tregnago, Section of Innovation Biomedicine-Oncology Area, Department of Engineering for Innovation Medicine (DIMI), University of Verona and University and Hospital Trust (AOUI) of Verona, Verona, Italy.
Lorenzo Belluomini, Section of Innovation Biomedicine-Oncology Area, Department of Engineering for Innovation Medicine (DIMI), University of Verona and University and Hospital Trust (AOUI) of Verona, Verona, Italy.
Marco Sposito, Section of Innovation Biomedicine-Oncology Area, Department of Engineering for Innovation Medicine (DIMI), University of Verona and University and Hospital Trust (AOUI) of Verona, Verona, Italy.
Jessica Insolda, Section of Innovation Biomedicine-Oncology Area, Department of Engineering for Innovation Medicine (DIMI), University of Verona and University and Hospital Trust (AOUI) of Verona, Verona, Italy.
Alessandra Auriemma, Section of Oncology, University of Verona Hospital Trust (AOUI) Verona, Verona, Italy.
Elena Fiorio, Section of Oncology, University of Verona Hospital Trust (AOUI) Verona, Verona, Italy.
Michela Piacentini, Section of Oncology, University of Verona Hospital Trust (AOUI) Verona, Verona, Italy.
Federico Schena, Department of Neurosciences, Biomedicine and Movement, University of Verona, Verona, Italy.
Michele Milella, Section of Innovation Biomedicine-Oncology Area, Department of Engineering for Innovation Medicine (DIMI), University of Verona and University and Hospital Trust (AOUI) of Verona, Verona, Italy.
Sara Pilotto, Section of Innovation Biomedicine-Oncology Area, Department of Engineering for Innovation Medicine (DIMI), University of Verona and University and Hospital Trust (AOUI) of Verona, Verona, Italy.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
Michele Milelia reports personal fees from Pfizer, EUSA Pharma, and AstraZeneca. Sara Pilotto received honoraria or speakers’ fee from AstraZeneca, Eli Lilly, BMS, Boehringer Ingelheim, MSD, Roche, and Istituto Gentili. The other authors indicated no financial relationships.
Author Contributions
Conception/design: All authors. Provision of study material or patients: All authors. Collection and/or assembly of data: A.A., A.B., E.B., C.C., S.P. Data analysis and interpretation: A.A., A.B., M.M., S.P. Manuscript writing: A.A., A.B., E.B., S.P. Final approval of manuscript: All authors.
Data Availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Dillekas H, Rogers MS, Straume O.. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019;8(12):5574-5576. 10.1002/cam4.2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gallicchio L, Devasia TP, Tonorezos E, Mollica MA, Mariotto A.. Estimation of the number of individuals living with metastatic cancer in the United States. J Natl Cancer Inst. 2022;114(11):1476-1483. 10.1093/jnci/djac158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hudock NL, Mani K, Khunsriraksakul C, et al. Future trends in incidence and long-term survival of metastatic cancer in the United States. Commun Med (Lond). 2023;3(1):76. 10.1038/s43856-023-00304-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cuthbert CA, Boyne DJ, Yuan X, Hemmelgarn BR, Cheung WY.. Patient-reported symptom burden and supportive care needs at cancer diagnosis: a retrospective cohort study. Support Care Cancer. 2020;28(12):5889-5899. 10.1007/s00520-020-05415-y [DOI] [PubMed] [Google Scholar]
- 5. Batra A, Yang L, Boyne DJ, et al. Associations between baseline symptom burden as assessed by patient-reported outcomes and overall survival of patients with metastatic cancer. Support Care Cancer. 2021;29(3):1423-1431. 10.1007/s00520-020-05623-6 [DOI] [PubMed] [Google Scholar]
- 6. Cheville AL, Kollasch J, Vandenberg J, et al. A home-based exercise program to improve function, fatigue, and sleep quality in patients with Stage IV lung and colorectal cancer: a randomized controlled trial. J Pain Symptom Manage. 2013;45(5):811-821. 10.1016/j.jpainsymman.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cormie P, Newton RU, Spry N, et al. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(4):328-335. 10.1038/pcan.2013.22 [DOI] [PubMed] [Google Scholar]
- 8. Galvao DA, Taaffe DR, Spry N, et al. Exercise preserves physical function in prostate cancer patients with bone metastases. Med Sci Sports Exerc. 2018;50(3):393-399. 10.1249/MSS.0000000000001454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanson ED, Alzer M, Carver J, et al. Feasibility of home-based exercise training in men with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2023;26(2):302-308. 10.1038/s41391-022-00523-8 [DOI] [PubMed] [Google Scholar]
- 10. Oldervoll LM, Loge JH, Lydersen S, et al. Physical exercise for cancer patients with advanced disease: a randomized controlled trial. Oncologist. 2011;16(11):1649-1657. 10.1634/theoncologist.2011-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rief H, Omlor G, Akbar M, et al. Feasibility of isometric spinal muscle training in patients with bone metastases under radiation therapy - first results of a randomized pilot trial. BMC Cancer. 2014;14:67. 10.1186/1471-2407-14-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zimmer P, Trebing S, Timmers-Trebing U, et al. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: a randomized controlled trial. Support Care Cancer. 2018;26(2):615-624. 10.1007/s00520-017-3875-5 [DOI] [PubMed] [Google Scholar]
- 13. Scott JM, Iyengar NM, Nilsen TS, et al. Feasibility, safety, and efficacy of aerobic training in pretreated patients with metastatic breast cancer: a randomized controlled trial. Cancer. 2018;124(12):2552-2560. 10.1002/cncr.31368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gil Herrero L, McNeely ML, Courneya KS, et al. Safety, feasibility, and effectiveness of implementing supervised exercise into the clinical care of individuals with advanced cancer. Clin Rehabil. 2022;36(12):1666-1678. 10.1177/02692155221114556 [DOI] [PubMed] [Google Scholar]
- 15. Wilk M, Kepski J, Kepska J, Casselli S, Szmit S.. Exercise interventions in metastatic cancer disease: a literature review and a brief discussion on current and future perspectives. BMJ Support Palliat Care. 2020;10(4):404-410. 10.1136/bmjspcare-2020-002487 [DOI] [PubMed] [Google Scholar]
- 16. Avancini A, Benato G, Borsati A, et al. Exercise and bone health in cancer: enemy or ally? Cancers (Basel). 2022;14(24):6078. 10.3390/cancers14246078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Avancini A, Sartori G, Gkountakos A, et al. Physical activity and exercise in lung cancer care: will promises be fulfilled? Oncologist. 2020;25(3):e555-e569. 10.1634/theoncologist.2019-0463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Avancini A, Tregnago D, Rigatti L, et al. Factors influencing physical activity in cancer patients during oncological treatments: a qualitative study. Integr Cancer Ther. 2020;19:1–10. 10.1177/1534735420971365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Avancini A, Pala V, Trestini I, et al. Exercise levels and preferences in cancer patients: a cross-sectional study. Int J Environ Res Public Health. 2020;17(15):5351. 10.3390/ijerph17155351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones LW. Precision oncology framework for investigation of exercise as treatment for cancer. J Clin Oncol. 2015;33(35):4134-4137. 10.1200/JCO.2015.62.7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eldridge SM, Chan CL, Campbell MJ, et al. ; PAFS consensus group. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. 10.1136/bmj.i5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taaffe DR, McCombie SP, Galvão DA, et al. Efficacy and feasibility of presurgical exercise in bladder cancer patients scheduled for open radical cystectomy. Med Sci Sports Exerc. 2023;55(7):1123-1132. 10.1249/MSS.0000000000003137 [DOI] [PubMed] [Google Scholar]
- 23. Crosby BJ, Newton RU, Galvão DA, et al. Feasibility of supervised telehealth exercise for patients with advanced melanoma receiving checkpoint inhibitor therapy. Cancer Med. 2023;12(13):14694-14706. 10.1002/cam4.6091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Institute, NC. Common Terminology Criteria for Adverse Events (CTCAE). 2022. Available from: CTEP (cancer.gov). [Google Scholar]
- 25. Dittus KL, Gramling RE, Ades PA.. Exercise interventions for individuals with advanced cancer: a systematic review. Prev Med. 2017;104:124-132. 10.1016/j.ypmed.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 26. Heywood R, McCarthy AL, Skinner TL.. Safety and feasibility of exercise interventions in patients with advanced cancer: a systematic review. Support Care Cancer. 2017;25(10):3031-3050. 10.1007/s00520-017-3827-0 [DOI] [PubMed] [Google Scholar]
- 27. Laboratories, ACoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117. [DOI] [PubMed] [Google Scholar]
- 28. Innes E. Handgrip strength testing: a review of the literature. Aust Occup Ther J. 1999;46(3):120-140. 10.1046/j.1440-1630.1999.00182.x [DOI] [Google Scholar]
- 29. Impellizzeri FM, Rampinini E, Maffiuletti N, Marcora SM.. A vertical jump force test for assessing bilateral strength asymmetry in athletes. Med Sci Sports Exerc. 2007;39(11):2044-2050. 10.1249/mss.0b013e31814fb55c [DOI] [PubMed] [Google Scholar]
- 30. Rikli RE, Jones CJ.. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist. 2013;53(2):255-267. 10.1093/geront/gns071 [DOI] [PubMed] [Google Scholar]
- 31. Organization, WH. The use and interpretation of anthropometry. Report of a World Health Organization Expert Comitee. World Health Organization technical report series. Vol. 854. WHO; 1995. [PubMed] [Google Scholar]
- 32. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-376. 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 33. Amireault S, Godin G, Lacombe J, Sabiston CM.. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: a systematic review. BMC Med Res Methodol. 2015;15:60. 10.1186/s12874-015-0045-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaye DR, Schafer C, Thelen-Perry S, et al. The feasibility and impact of a presurgical exercise intervention program (prehabilitation) for patients undergoing cystectomy for bladder cancer. Urology. 2020;145:106-112. 10.1016/j.urology.2020.05.104 [DOI] [PubMed] [Google Scholar]
- 35. De Lazzari N, Niels T, Tewes M, Götte M.. A systematic review of the safety, feasibility and benefits of exercise for patients with advanced cancer. Cancers (Basel). 2021;13(17):4478. 10.3390/cancers13174478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsianakas V, Harris J, Ream E, et al. CanWalk: a feasibility study with embedded randomised controlled trial pilot of a walking intervention for people with recurrent or metastatic cancer. BMJ Open. 2017;7(2):e013719. 10.1136/bmjopen-2016-013719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Avancini A, Sperduti I, Borsati A, et al. Effect of exercise on functional capacity in patients with advanced cancer: a meta-analysis of randomized controlled trials. Crit Rev Oncol Hematol. 2022;175:103726. 10.1016/j.critrevonc.2022.103726 [DOI] [PubMed] [Google Scholar]
- 38. Mikkelsen MK, Lund CM, Vinther A, et al. Effects of a 12-week multimodal exercise intervention among older patients with advanced cancer: results from a randomized controlled trial. Oncologist. 2022;27(1):67-78. 10.1002/onco.13970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Groarke JD, Payne DL, Claggett B, et al. Association of post-diagnosis cardiorespiratory fitness with cause-specific mortality in cancer. Eur Heart J Qual Care Clin Outcomes. 2020;6(4):315-322. 10.1093/ehjqcco/qcaa015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kirkham AA, Haykowsky MJ, Beaudry RI, et al. Cardiac and skeletal muscle predictors of impaired cardiorespiratory fitness post-anthracycline chemotherapy for breast cancer. Sci Rep. 2021;11(1):14005. 10.1038/s41598-021-93241-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375-2390. 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fabi A, Bhargava R, Fatigoni S, et al. ; ESMO Guidelines Committee. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol. 2020;31(6):713-723. 10.1016/j.annonc.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 43. Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;7:102. 10.1186/1477-7525-7-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.