Abstract
Tumor tissue is densely packed with cancer cells, non‐cancerous cells, and ECM, forming functional structures. Cancer cells transfer extracellular vesicles (EVs) to modify surrounding normal cells into cancer‐promoting cells, establishing a tumor‐favorable environment together with other signaling molecules and structural components. Such tissue environments largely affect cancer cell properties, and so as EV‐mediated cellular communications within tumor tissue. However, current research on EVs focuses on functional analysis of vesicles isolated from the liquid phase, including cell culture supernatants and blood draws, 2D‐cultured cell assays, or systemic analyses on animal models for biodistribution. Therefore, we have a limited understanding of local EV transfer within tumor tissues. In this review, we discuss the need to study EVs in a physiological tissue context by summarizing the current findings on the impacts of tumor tissue environment on cancer EV properties and transfer and the techniques required for the analysis. Tumor tissue environment is likely to alter EV properties, pose physical barriers, interactions, and interstitial flows for the dynamics, and introduce varieties in the cell types taken up. Utilizing physiological experimental settings and spatial analyses, we need to tackle the remaining questions on physiological EV‐mediated cancer–host cell interactions. Understanding cancer EV‐mediated cellular communications in physiological tumor tissues will lead to developing interaction‐targeting therapies and provide insight into EV‐mediated non‐cancerous cells and interspecies interactions.
Keywords: cellular spatial distribution, exosomes, extracellular vesicles, organotypic 3D culture models, tumor microenvironment
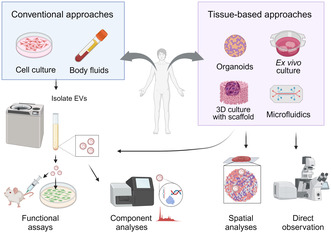
We highlight the importance of studying cancer extracellular vesicles (EVs) within physiological tissue contexts to deepen our understanding of their roles in cancer progression and malignancy. The schematic contrasts current approaches based on culture systems and emerging tissue‐based approaches with physiological tissue models in EV studies. These novel approaches can address how the tissue context can modify EV characteristics, create barriers to EV transfer, and influence the selection of recipient cells.
Abbreviations
- BBB
blood‐brain barrier
- BRET
Bioluminescence Resonance Energy Transfer
- DDS
drug delivery systems
- ECM
extracellular matrix
- EV
extracellular vesicle
- HIFs
Hypoxia‐inducible factors
- HSPG
heparan sulfate proteoglycans
- MMPs
metalloproteinase
- MSC
mesenchymal stem cells
- TME
tumor microenvironment
- TNF‐α
Tumor Necrosis Factor alpha
- YAP
yes‐associated protein
1. INTRODUCTION: WHY STUDYING CANCER EXTRACELLULAR VESICLES IN A TISSUE CONTEXT IS IMPORTANT?
Tumor tissues consist of not only cancer cells but also non‐cancerous host cells, such as fibroblasts, endothelial cells, and immune cells, together with structural support for the ECM. Within the tissues, cellular and non‐cellular components interact with each other to develop a tumor‐favorable TME. 1 They transduce intercellular signals via direct contact and humoral factors such as growth factors, cytokines, metabolites, and EVs. EVs are nanosized lipid bilayer vesicles that are packed with cellular components such as DNA, RNA, proteins, and metabolites (Figure 1). EVs are secreted from one cell and taken up to another cell through endocytosis, micropinocytosis, and direct fusion to the plasma membrane, 2 and release their cargo to induce signals at the recipient cells. 3 EVs also interact with cells through surface structures to induce signals, posing multimodal mechanisms of action to cause changes in cancer cells. EVs are a collection of varieties of vesicle subtypes based on size and their origin. 4 Exosomes are 100–200 nm in diameter and originate from endocytotic multivesicular bodies, whereas microvesicles and apoptotic bodies are generated from the plasma membrane and are larger in diameter (microvesicles: 150–1000 nm, apoptotic bodies: 100 nm to 5 μm). 2 , 4
FIGURE 1.
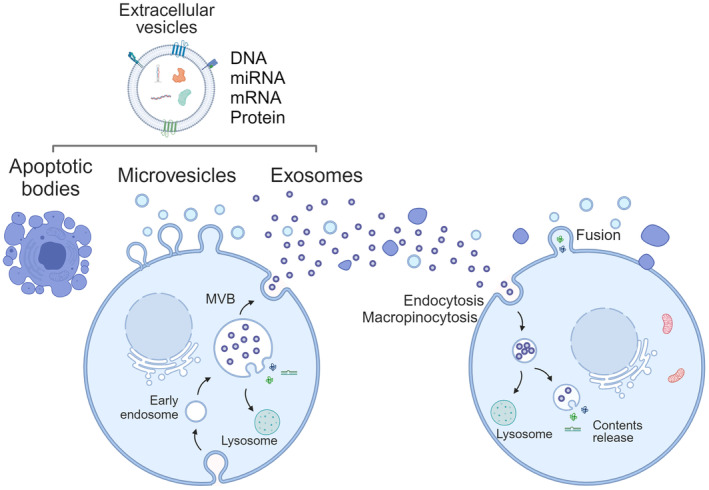
Secretion and internalization of extracellular vesicles (EVs). EVs are a collection of varieties of vesicle subtypes, i.e. exosomes, microvesicles, apoptotic bodies, and others. Exosomes are approximately 100 nm in diameter and originate from endocytotic multivesicular bodies, whereas microvesicles and apoptotic bodies are larger vesicles generated from the plasma membrane. The EVs are internalized through endocytosis, micropinocytosis, or direct fusion to plasma membrane. The internalized EVs release their cargoes to the recipient cytoplasm.
Recent studies have reiterated that the tissue context of TME, including interactions with the stromal cells and ECM, humoral factors, mechanical stresses, hypoxic environment, and lower pH, collectively alters cancer cell properties in proliferation, invasion, drug resistance, and metastasis. 5 Given that TME impacts cancer cell properties, it is reasonable to assume that the properties of cancer‐derived EVs are also affected. Additionally, as EVs induce cellular responses after either releasing their cargo into the recipient or interacting through surface receptors, it is essential to determine the destination of the secreted EVs to understand the function of cancer EVs in tumor progression. However, widely used approaches of supplementing isolated EVs to 2D cell culture hardly address EV‐mediated cell–cell communication in physiological TME (Figure 2). We have many questions about EV transfer within tumor tissue: How different are EVs secreted from cancer cells in tissue context from currently analyzed EVs from culture? How far do the EVs from one cell reach within the tissue architecture, and how long does it take? Which cells preferentially take up cancer EVs? Answering these questions is essential to understanding physiological EV‐mediated cellular communications in TME in the tumor tissue context. In this review, we will discuss the importance of studying EVs in a tumor tissue context, possible tissue factors affecting EV transfer, and experimental strategies for studying EV‐mediated cell–cell communication in tumor tissue.
FIGURE 2.
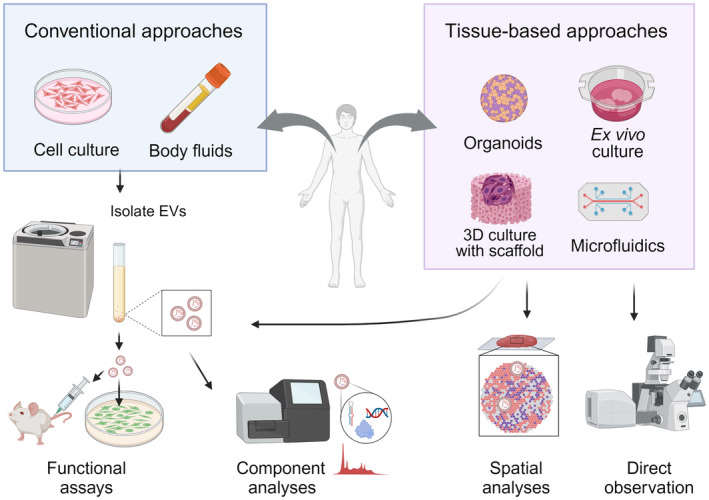
A schematic of approaches for extracellular vesicles (EV) functional studies. Conventional approaches isolate EVs from cell culture supernatants or body fluids for functional assays on 2D‐grown cells and animal models, and component analyses. Physiological tumor tissue models can provide EVs that represent physiological conditions for components and functional analyses. Further, the models allow direct observations and spatial analyses of EV transfer.
2. FACTORS AFFECTING CELLULAR COMMUNICATION VIA EVs IN TUMOR TISSUE
TME and tissue structures will affect EV‐mediated cellular transfer in several ways. Based on the current reports, the following factors are mainly assumed to be affected by TME: (1) change in cancer cell status resulting in EV properties; (2) physical barriers, ECM and cellular attachment, and interstitial flow affecting EV transfer distance; (3) targeted delivery and preference in EV uptake among the surrounding cells (Figure 3). I will summarize current knowledge on these effects and discuss possible impacts on EV transfer in tumor tissues.
FIGURE 3.
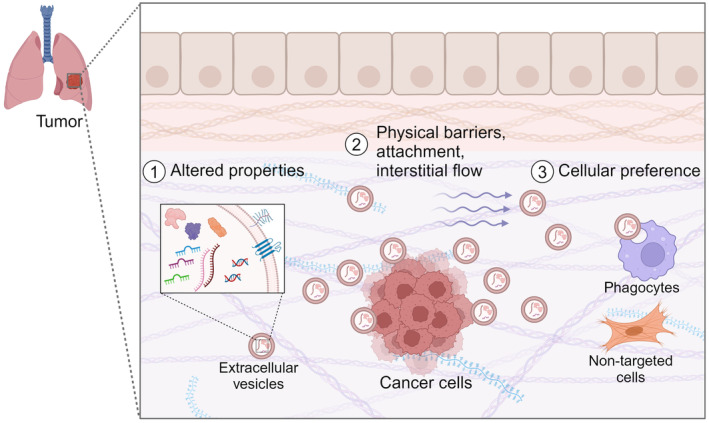
Impact of tissue microenvironment on EV‐mediated cancer‐noncancerous cell communication. Possible effects are: (1) Altered cancer cell properties affect EV components and characteristics. (2) Physical barriers, attachment to cell surface and ECM, and interstitial flow. (3) Preferences in cell types that take up EVs. Selecting optimal physiological models that retain/mimic tissue environment is required to address these questions.
2.1. Changes in EV properties due to changes in cancer cell properties
Several researchers reported that the EVs collected from cancer cells cultured in 3D structures, including patent specimens and spheroids, are smaller in diameter and higher in number, compared with EVs from 2D culture. Furthermore, a tissue‐based environment mostly alters EV cargoes toward tumor‐promoting phenotypes (summarized in Table 1).
TABLE 1.
Altered extracellular vesicle (EV) properties by tissue environment.
| Altered EV properties | Tissue environmental EVs compared with 2D EVs | Stimuli | References |
|---|---|---|---|
| Size | Smaller in diameter | 3D culture |
(6) Ewing's sarcoma (7) Cervical cancer (8) Gastric cancer |
| Hypoxia | Reviewed in (21) | ||
| No change | Spheroids | (9) Breast cancer | |
| Number | Increase | 3D culture | (6, 7, 8, 9, 22) Breast cancer |
| Stiff matrix | (24) Hepatocellular carcinoma, breast, and pancreatic cancer | ||
| Alginate hydrogels | (25) MSCs | ||
| Hypoxia |
Reviewed in (21) (23) MSC |
||
| Mechanical stress | (26) MSCs and muscle cell | ||
| Cargoes | Similar to patient EVs | 3D culture | (7) |
| Tumor‐promoting miRNAs | 3D culture | (8) | |
| Tumor supportive phenotype | Hypoxia | Reviewed in (21) | |
| Regenerative | 3D culture | (13, 14, 15, 16, 17, 18) MSCs | |
| Less regenerative | 3D culture | (19) MSCs | |
| Uptake by the recipient cells | Increased | Low pH | (20) HeLa cells |
Villasante et al. found that the size distribution of EVs from Ewing's sarcoma type 1 cultured in 3D with native tumor ECM components was equivalent to the size of sarcoma patients' plasma EVs (average mean ± SD: 88.7 ± 22 nm), and smaller and increased particle number compared with EVs from 2D monolayer culture (average mean ± SD: 149.2 ± 19 nm) or 3D culture in a generic polypropylene. 6 They further loosen cellular tension with blebbistatin to observe larger vesicles, suggesting that tension affects EV particle size. Thippabhotla et al. reported that EVs from cervical cancer cells (HeLa) cultured in 3D spheroid with hydrogels had smaller diameters with increased particle number per cell than EVs from 2D culture. 7 EVs from 3D‐cultured HeLa gained miRNA profiles more alike (~96% similarity) to cervical cancer patient plasma EVs. 7 Rocha et al. compared the EVs collected from gastric cancer cell lines cultured in 2D and spheroids developed in 3D microwells, and found that the average diameter of EVs became 85–135 nm, which was much smaller than 2D EVs with 100–180 nm, with increased particle number per cells. 8 EVs from 3D cultures contained several specific miRNAs compared with 2D EVs, which are related to p53, MAPK, TGFβ, and RAS signaling pathways. However, not all 3D cultures follow the trends in size and amount. Rima et al. cultured breast cancer spheroids within microgels captured in a microfluidic device, collected EVs from 20 μL of supernatant, and characterized EVs from low‐, middle‐, and high‐density spheroids. 9 The size of the EVs was similar among the spheroids with different sizes. EV number per cell also remained similar but increased compared with 2D‐cultured EVs. These differences could be due to the cell types, various 3D culture models, and measuring methods that introduce size distribution variations (NTA, electron microscopy, etc.).
Besides cancer, EVs from 3D culture have been mostly studied with mesenchymal stem cells (MSC) due to potential clinical applications for immunoregulation and regenerative medicine. 10 MSCs grown in 3D with or without matrix support increase secretion of smaller EV size. 11 , 12 Kim et al. showed that high‐density MSC 2D culture equivalent to 3D spheroid even lowered EV secretion efficiency than 2D low‐density culture, suggesting that the 3D tissue structure is essential for the EVs to stimulate the EV secretion. 12 Generally, EVs from MSCs cultured in 3D or with shear stress enhance regenerative functions such as skin wound closure, 13 bone regeneration, 14 heart repair, 15 and neuronal growth, 16 by enhancing angiogenesis, anti‐inflammatory, anti‐apoptotic, and anti‐fibrotic effects. 17 , 18 These observations suggest that tissue environmental effects on EV properties are generalized to MSC and possibly non‐cancerous cells. Although a report from Kusuma et al. showed 3D‐cultured bone marrow‐derived cells lost their regenerative activity, 19 these results reiterate the impact of cell culture conditions on EV cargo and their biological activities, and therefore underline the importance of studying EVs under the physiological context to understand the true biological functions of EVs.
Studies from cell cultures recapitulating part of the characteristics of TME can identify which stimuli cause changes in tumor EVs. Nakase et al. showed that a lower pH environment (pH 5) stimulated both the secretion of CD63‐expressing EVs by HeLa cells, and the take up of EVs by human epidermoid (A431). 20 Hypoxic environment, which is a hallmark of TME, stimulates EV secretion by various cancer cells with decreased size and altered cargoes, and could induce EV transport, cell recognition, and internalization to act against hypoxia (ex. angiogenesis; reviewed in 21 ). Hypoxia‐inducible factors (HIFs) upregulate RAB22A expression and promote microvesicle shedding in breast cancer cells. 22 Salomon et al. found that a lower oxygen environment increases EV secretion using placental MSC cells cultured under low oxygen atmosphere at 1%, 3%, or 8% O2. 23 The EVs stimulated the endothelial cell migration by 1.6‐fold, tube formation by 7.2‐fold, and increased cargoes involved in cytoskeleton organization and immunomodulation. Cancer and non‐cancerous cells will utilize EVs to act against hypoxic environments.
Moreover, mechanical stimuli arising from TME also stimulate EV secretion amount. Recently, Wu et al. showed that EV secretion was approximately tripled when cultured on a stiffer matrix, which most solid tumors have, compared with a softer matrix prepared by collagen‐coated stiffness‐adjusted polyacrylamide gels in several types of cancer (hepatocellular carcinoma, breast, and pancreatic cancer). 24 Stiffer matrix activates Akt and promotes GTP loading to Rab8 to drive EV secretion. The induced EVs promoted tumor growth in mouse models by enhanced Jagged 1 loading and activating the Notch signaling pathway in the recipient cancer cells. In addition, a stiffer platform does not always stimulate EVs. Lenzini et al. showed that EV secretion from MSCs cultured on alginate‐based hydrogels increased ~10‐fold compared with cells cultured on a rigid plastic platform, and two‐fold compared with cells cultured on a stiffer matrix without altering particle size, cargoes, and biological activities. 25 Lower integrin‐ligand interaction promoted MVB fusion to the plasma membrane in an actin‐dependent manner. Guo et al. explicitly showed that mechanical force stimulates EV secretion in their microfluidic bioreactor, culturing MSCs with flow and muscle cells with stretching. 26 EV induction was mediated by yes‐associated protein (YAP) mechanosensing pathway, and the 3D‐cultured MSC EVs gained axonal sprouting capability. Overall, these observations indicated that the mechanical stimuli from scaffold stiffness regulate EV secretion.
The mechanisms of the increase in the number of smaller vesicles in 3D culture are not known. One simple hypothesis is that there is an increase in small EVs, including exosomes (30–150 nm in diameter), and a decrease in larger EVs, such as microvesicles (~1000 nm) and oncosomes (1–10 μm). 27 Rocha et al. uncovered miRNAs and proteins relating to ARF6 signaling pathways, which relate to microvesicle shedding, were significantly downregulated in EVs from 3D culture. 8 This finding may partially explain the mechanisms of smaller vesicle size by 3D culture. Recently found non‐membrane particles, exomeres (≤50 nm),28 and supermeres (~35 nm) 29 may also account for smaller particle counts, as nanoparticle tracking analysis includes counts from non‐membrane components. Another possibility is that vesicle size was regulated. For exosomes, the vesicle size may be regulated through ESCRT‐dependent and ‐independent budding into a multivesicular body. 30 Lipid composition may contribute to vesicle size regulation by curvature of lipids. 31 , 32 , 33 Together with the control on EV secretion number, the overall mechanisms of regulating EV formation are being actively investigated.
2.2. Impact of tissue structure on EV delivery‐ physical barriers, incorporation, and EV surface interaction
Within the tissue, cancer EVs are secreted to the interstitial space among crowded cells aligned with extracellular matrix and connective tissues. 34 These tissue structures will pose physical barriers to EV dissemination within tissues. Interactions between EV surface structure and cells or ECM will further maintain the secreted EVs in proximity to the cells of origin and alter EV behaviors within tissues. In addition, EVs are known to travel systematically through the bloodstream and lymphatic flow. Understanding the destination of cancer EVs and the range of communication is essential to elucidate cell–cell interactions within tissue context. This field is still emerging, but I will summarize current reports, including indicative results, to discuss what factors will affect EV dynamics in tumor tissues.
2.2.1. Physical barrier by tissue architecture
Several tissue structures will pose physical barriers to EV dissociation. ECM confines EVs and acts as a reservoir because ECM fills extracellular space. Basement membrane that aligns tumor tissues, and tissue structures, such as blood vessels that consist of cell–cell or cell‐ECM adhesion, could insulate EV diffusion. Therefore, most of the EVs secreted are assumed to be retained at proximity, and the range of the EV‐mediated information transfer seems to be mostly limited.
Studies on the secretory protein provide insight into EV transfer within the tissue. Dr. Malanchi's group identified niche cells in mouse metastatic lungs using cancer cells secreting fluorescent proteins fused with cell‐penetrating protein. 35 They found that the cancer‐derived secretory fluorescent protein penetrated about five cell layers. Bagnall et al. showed the range of macrophage activation by tumor necrosis factor‐α (TNF‐α) by integrating experimental reporter assay and in silico approaches. 36 In the in vitro connective tissue model, propagation of TNF‐α signaling of macrophages is limited to a few cells diameters, and computational modeling suggested that competitive uptake limits TNF‐α propagation. These results suggest that humoral cellular communication mainly happens locally within tissue. As EVs are larger than secretory proteins, the range of EV transfer from one cell could be smaller than the secretory proteins. Competitive uptake may also apply to EVs as macrophages and other phagocytes remove cancer EVs, 37 which will also limit the distance of EV dissemination.
Recently, Colombo et al. reported an interesting observation in their preprint that cancer cells with EV labels cocultured in 2D monolayer transferred EVs only to the proximal cells, mostly within. 38 Culture with agitation did not increase EV transfer in distant orientation. Their observation indicates that EV transfer is limited even in 2D culture. The mechanisms of limiting EV transfer in 2D could be ECM or fast EV uptake by the surrounding cells to deplete EVs from the environment. Together with the studies introduced above, these results indicate that the majority of EV‐mediated cellular interactions within tumor tissues may occur among proximal cells.
However, cancer EVs systemically travel by penetrating the endothelial cell layer and aligning the basement membrane to reach the blood and lymphatic flow to the distal organs to establish a premetastatic niche. 39 There should be mechanisms to circumvent physical barriers of ECM, basement membrane, and cell layers. Lenzini et al. examined the mechanisms of EVs bypassing the ECM barrier. They showed that EVs can pass the mesh of the engineered hydrogel scaffold that nanoparticles of the same size could not by deforming their shape by aquaporin‐1. 40 This result indicates that the EVs spontaneously travel across the ECM network by controlling their size. Additionally, EVs possess ECM‐degrading and modulating enzymes such as metalloproteinase (MMPs) and their inhibitor TIMPS, ADAMs ADAMTS, heparanases, hyaluronidases, and LOX inside and at the surface. 41 , 42 Malignant cancer cells secrete more EVs with MMPs to enhance tumor invasion and metastasis, angiogenesis, and stromal cell modification. 41 Hoshino et al. showed that EV secretion was stimulated at invadopodia and MT1‐MMP associated with EVs is likely to degrade ECM at the cell protrusion tip to stimulate cell migration. 43 These results suggest that cancer EVs will degrade and rewire ECM to travel to the distance. At the blood–brain barrier (BBB), breast cancer EVs penetrate the barrier to stimulate brain metastasis. 44 Cancer and non‐cancerous EVs can also mutually cross a layer of endothelial cells by transcytosis of the endothelial cells. 45
Tissue deformation by cytoskeleton followed by interstitial flow pressure will stimulate EV diffusion. Koomulli et al. computationally simulated EV transfer within the TME, by including the impact of EV particle size, concentration, diffusion, interstitial flow pressure, and surrounding cellular uptake, in a mixed cell population, although in a 2D platform. 46 They predicted that concentration around EVs will be ~3.5 times higher at later stages of tumors because of increased cell obstacles, increased cancer EV release, and higher interstitial fluid pressure. Sariano et al. suggested by combining experiments on microfluidics with the interstitial flow and in silico approaches that convection and ECM bindings are the dominant mechanisms of EV interstitial transport. 47 By integrating these strategies and unknown mechanisms, EVs will travel across cell layers and basal membranes for systemic dissemination.
2.2.2. Extracellular matrix‐binding EVs
EVs interact with and bind to ECM, which will affect EV dynamics within tumor tissue. Matrix vesicles, a type of EVs that initiate the mineralization of bones and cartridges by interacting with ECM, have been studied for more than 40 years. 48 Recently, other EVs have been gradually recognized to be integrated as a component of ECM in other tissues and cell cultures in general. 49 , 50 , 51 As discussed above, EVs display ECM‐binding proteins such as integrins. Sariano et al. suggested using a series of human breast cell lines (MCF10) that laminin‐binding integrins α3β1 and α6β1 at the surface of EVs enhanced the local concentration and gradient of EVs. 47 The amount of integrins on EVs correlated with malignancy of the cancer cells. Dr. Badylak's group investigated the properties of ECM‐bound EVs. 51 They collected matrix‐bound vesicles by partial enzymatic digestion from porcine dermal, urinary bladder, and small intestinal matrix. The vesicles are distributed in sizes of 50–400 nm in diameter and contain miRNA to activate macrophages and induce neuroblastoma differentiation. The group further investigated ECM‐bound EVs with mouse fibroblast (3 T3), and identified differential miRNAs and lipid components among liquid‐phase EVs and ECM‐bound EVs. 52 Their results indicate that ECM‐unbound EVs may be intended to be delivered to distal organs with selective cargoes.
The biological functions of the ECM‐bound EVs remain mostly unknown. Dr. Weaver's group showed that ECM‐bound EVs have roles in cell migration. They observed under intravital imaging of chick embryos that cancer cells locate their EVs on ECM to the direction of the invasion to stimulate cell motility. 53 EVs utilize fibronectin at the surface to help cells form matrix attachment through integrins. Downregulating EV secretion by knocking down RAB27A decreased cellular protrusion, and supplementing EVs rescued cell migration. Although we only have limited reports, a population of EVs seems to be ECM‐bound and remain proximal to the donor cells, and exert unique functions. Studying matrix‐bound vesicles will uncover a novel aspect of the functions and transfer mechanisms of EVs.
2.2.3. EV surface properties and their interacting partners
As EV surface is at the forefront of interacting with the recipient cells and tissue structures, EV surface properties affect EV uptake, removal, and motility, resulting in an altered EV destination. The surface of EVs has many structures: protein receptors, membrane proteins, lipid bilayer with lipid rafts, and glycosyl modification. 54 Additionally, recently, Dr. Buzás’ group suggested that the EV surface is coated with proteins in a physiological environment and called “protein corona.” 55 The importance of EV surface property is reiterated by drug delivery systems (DDS) studies utilizing surface‐modulated EVs as a carrier. Strategies to utilize EVs as DDS carriers, including natural and modified EVs, are summarized in a review by Dr. Vader. 56
As discussed in the previous section, EVs bind to ECM through integrins and CD44, which bind to collagens and hyaluronan, respectively. 54 Interactions with ECM through these proteins will affect the spatial distribution and cellular destination of local and systemic delivery of EVs. Hoshino et al. showed that integrins at the surface of cancer EVs determine organ tropisms through binding to organ‐specific ECM. 39 Integrins are also involved in cellular uptake. EVs secreted from MDA‐MB‐231 human breast cancer cells carry αvβ3 integrin, and inhibiting αvβ3 integrins inhibits the uptake to non‐malignant MCF10A cells. 57 Tetraspanins at the surface of EVs, such as CD9, CD63, and CD81, are recognized as EV marker proteins, although their expression is heterogeneous among vesicles. 58 Tetraspanins accumulate in microdomains and are involved in exosome biogenesis, cargo sorting, and antigen presentation, as well as uptake by target cells. 59 The tetraspanin‐enriched domain recruits adhesion molecules such as ICAM and integrins and regulates cellular adhesion. Anchoring proteins, tetherin, in HeLa cells retain EVs to form EV clusters on the cell surface. 60
The surface of EVs is enriched in glycosylation on their protein and lipids, 61 , 62 and cancer EVs display altered glycosylation. Disrupting the surface N‐ or O‐glycosylation stimulated EV uptake in vitro or in vivo, 63 , 64 , 65 suggesting that this prevents EVs from promiscuous uptake by the proximal cells for targeted delivery. The negative charge of the EV surface derived from glycosylation, together with lipids such as phosphatidylserine and proteins, 66 causes electric repulsion against the negatively charged plasma membrane and ECM. In addition, specific glycosyl modifications stimulate the uptake of EVs. Sialylation on EVs is recognized by Siglecs and stimulates uptake on macrophages and other phagocytotic cells, 67 , 68 and initiates dendritic cell activation. 69 Zheng et al. engineered a glycosylation domain in CD63, introducing sialyl‐Lewis X and Lewis X to achieve EV targeting to endothelial cells and dendritic cells. 70 Both cancer EVs and the cancer cell surface are covered with heparan sulfate proteoglycans (HSPG), but only the cancer cell surface HSPG serves as receptors for EV internalization and functional activity. 71
Phagocytotic removal of macrophages and monocytes will also largely affect EV behaviors. In exogenous EV administration to animals, EVs are mostly removed by phagocytotic cells such as macrophages and monocytes. 37 Phosphatidylserine, which is an apoptotic marker of the cells, is displayed at the surface of cancer and non‐apoptotic cell‐derived EVs, 72 and serves as an “eat me” signal for phagocytotic removal. 73 In addition, cancer EVs display “Don't eat me” signals (CD47) to regulate phagocytotic removal. Dr. Kalluri's group showed that EVs from pancreatic cancer cells display CD47 to protect themselves from phagocytosis and that they utilized the EVs for DDS to deliver miRNAs, suppressing oncogenes in preclinical models. 74 Harnessing “eat me” and “don't eat me” signals will be added to a DDS toolbox to develop EVs as a carrier of anti‐cancer therapies. 75 Altogether, multiple EV surface components play critical roles in EV targeting and uptake by the cells.
2.3. Cellular preferences on EV uptake
The tissue environment introduces heterogeneity in surrounding cell types that incorporate cancer EVs. EVs are taken up through multiple pathways: direct fusion to plasma membrane, endocytosis (Caveolin, clathrin‐dependent, lipid raft‐mediated), micropinocytosis, and phagocytosis. 2 These incorporating speeds will vary among the cell types and cause differences in the EV amount taken up. Additionally, cell surface receptors that interact with the EV surface discussed in the previous sections will define the preference for uptake of cancer EVs.
There have been a few reports that address the selective incorporation of EVs by different cell types. Sancho‐Albero et al. demonstrated that EVs were more likely to be taken up to the cells of the same origin, at the endpoint and by time‐lapse microscopy with particle track analyses. 76 They observed selective incorporation of MSC EVs labeled with hollow gold nanoparticles to MSCs rather than monocytes or melanoma cells in coculture conditions; monocyte EV‐encapsulated gold nanoparticles were not efficiently incorporated into MSCs. Jurgielewicz et al. compared the uptake of HEK293T EVs labeled with CD63‐GFP by HEK293T, liver (C3A), endothelial (HUVEC), and glioblastoma (SH‐SY5Y) cells, using imaging flow cytometry. 77 HEK293 took up most HEK 293 EVs. Although their experimental design cannot exclude the possibility that the difference is simply due to the internalization speed of the cell types, their findings align with other reports to support EV uptake by the same origin. The synergy of EVs to their cellular origin may explain systemic EV homing to the organs from which they are derived, although the concept is still controversial. 78 Jurgielewicz et al. also found that neural stem cells with higher metabolic activities were more potent to take up HEK293 EVs than mature neurons, 77 suggesting that fast‐growing cells, including cancers, will take up more EVs than stable cells.
Additionally, target delivery to other types of cells exists. Rossaint et al. showed directed and mutual EV transport between platelets and neutrophils for metabolite shuttling and full immune activation during bacterial infections. 79 Sadovska et al. studied cellular uptake under a relatively physiological condition, a mixed spheroid of prostate cancer cells (PC3) and PBMC. 80 They analyzed GFP‐labeled PC3 EV uptake of the PBMCs using flow cytometry, and found that B cells bind to more EVs; approximately half of the B cells took up PC‐3 EVs, whereas only about 20% of CD3+T cells and 6% of CD8+ T cells took up the EVs. Their results explicitly showed that cells had preferences for cancer EV uptake under exactly the same culture conditions. Zoller's group showed that tetraspanins and integrin associations at the surface of EVs strongly affected target cell selection both in vitro and in vivo. 81 They modulated Tspan 8 and integrin β4 in rat pancreatic cancer cells to cause different combinations of tetraspanin–integrin complexes, causing differential uptake by lymphocytes, fibroblast, endothelial cells, and peritoneal cells. Furthermore, Zoller's group showed selective uptake of EVs from rat pancreatic adenocarcinoma cells to leukocyte populations and showed that other than phagocytotic populations of CD11b+ cells (monocytes and macrophages) and CD11c+ cells (dendritic cells), T or B cells take up some amount, while granulocytes took up the lowest amount. 82 Adhesion molecules at the surface of EVs, such as CD11b, CD11c, CD44, CD49d, CD54, and CD62L contribute to EV binding together with tetraspanins. These findings further identify surface proteins that contribute to selectivity in EV uptake.
Metabolic states may regulate EV targeting. Crewe et al. showed EV‐mediated caveolin‐1 transfer from endothelial cells to adipose tissue in adipocyte‐specific knockout mice. 83 Furthermore, they observed EV‐mediated transfer of a HaloTag‐labeled adipocyte membrane component to endothelial cells in mice, and found that 35% of endothelial and macrophage populations were positive for HaloTag, whereas 10% or less were positive for CD45+ hematopoietic cells and Pdgfrb+ preadipocytes or mural cells. The authors also showed that EV transfer from endothelial cells to adipose cells was increased by fasting and that feeding reversed the effect. These results suggested that the metabolic activities of the cells regulate EV exchange among specific cell types.
3. EXPERIMENTAL SYSTEMS TO STUDY EVs IN A TUMOR TISSUE CONTEXT
To address EV function and transfer within a tissue context, analyzing EV in a physiological tissue model is essential (Figure 2). However, both the complexity of the tissue and the size of the EVs pose technical hurdles to examining EV transfer in detail. Here, we discuss the experimental systems that enable EV studies in tissue contexts.
3.1. Physiological tumor models and strategies for studying tissue‐derived EVs
Clinical specimens and animal models are the most relevant to the physiological tumors. Many studies have collected EVs from organs and tumor tissues, both from animals and patient surgical tissues. The simplest way of collecting tissue EV is soaking tissues in a medium, with or without mild ECM degradation. 34 Another strategy is from interstitial fluids. A group led by Drs. Polsky and Glaros extracted dermal interstitial fluids through microneedles from rats and humans and characterized EVs with transcriptome and proteome. 84 , 85 They found similar profiles of dermal interstitial fluid‐derived EVs with serum and plasma EVs, but their concentration was 12–13 times higher. Intravital microscopic observation has been performed to investigate EV transfer. 86 , 87 However, clinical specimens are scarce, and intravital imaging of EVs demands advanced microscopic and animal handling techniques, which limits availability for researchers. Furthermore, some molecular analyses of the EV transfer are incapable of in vivo study.
To study EVs within physiological tissue architecture in the experimental systems, we can utilize many tissue models that have been developed to mimic a physiological tumor microenvironment. 5 , 88 , 89 This includes 2D cocultures, 3D cultures such as multicellular spheroids, natural and artificial scaffolds, bioprinting, microfluidics devices to mimic tissue structure, explants and organoids derived from tumors, and animal models. Each tissue model has a range of physiological relevance with pros and cons, so we should use the optimal model for our research aims. A prior review by Dr. Congtag's group provided an overview of cutting‐edge 3D models that can be used for visualizing EVs in a tumor tissue context. 90
Ex vivo culture can also be a powerful tool to address EV transfer in a physiological context. As introduced in the previous sections, EVs collected from organoids, spheroids, and other 3D cultures have been analyzed to identify the effect of tissue architecture on EV properties. Organoids prepared from patient tumors will retain patient‐derived immune cells and fibroblasts. 88 Spheroids formed from cancer cell lines alone or mixed with other cells are more manipulable to include cells of interest to produce three‐dimensional structures. To study the ECM effect, tissue engineering such as scaffolds of ECM or synthetic hydrogels are powerful tools. 91 Organotypic tissue culture is another explant that can be used. Organotypic tissue slice cultures cannot be stored or expanded but maintain close‐to‐original tissue profiling and have a larger surface area suitable for microscopic observation. More recently, 3D bioprinting with ECM support and microfluidic devices to reconstruct biological flow has become a cutting‐edge tool to study EVs. 5 Nguyen et al. developed multiple organs‐on‐a‐chip that mimics organ–organ connections for injured kidney disease connected with the liver, and found that injured kidney tissues took up and retained more EVs than the control. 92 The authors further showed that interorgan communication targeted deliveries to a specific type of cell. These models will provide versatile, manipulative, yet physiological platforms to study EV‐based cellular communication in a physiological context.
3.2. Imaging and detection techniques for EVs
As EVs are nanoscale, detecting EVs within a tissue context is technically challenging. Direct observation of EV transfer is the most straightforward way to understand EV behavior in a tissue context. A comprehensive review of in vivo imaging of EVs by Verweij et al. covers in vivo imaging techniques of EVs, using animal models such as rodents and zebrafish, with multiple EV labeling methods. 93
Integrating fluorescent protein to EV marker proteins (CD63, CD9, CD81, Alix, Hsp70) or to membrane anchors such as palmitoyl modification are the most common strategies to label EVs. To aim for higher sensitivity and in vivo detection, later systems use bioluminescence resonance energy transfer (BRET) instead of a single fluorescent protein. Dr. Ishii observed real‐time EV transfer from osteoblasts to the surrounding cells in mouse bone tissues using intravital multiphoton imaging. 87 They labeled bone‐forming mature osteoblasts with an enhanced cyan fluorescent protein (ECFP) and tracked small and large osteoblast vesicles to find that smaller vesicles traveled faster and were taken up by mature osteoblasts within 20 min in an autocrine and paracrine manner.
Reporter systems are effective tools to detect EV uptake by their recipient cells. Zomer et al. pioneered the detection of EV transfer in vivo using Cre‐loaded EVs and reporter cells expressing fluorescent proteins under the loxP sequence. 86 They showed mutual EV transport among transplanted cancer cells and mouse host cells, and from malignant to less malignant cancer cells, although the efficiency seemed very low. Other reporter systems such as nano luciferase, 94 , 95 TEV‐protease‐based reporters, 96 and CRISPR‐based RNA reporters 97 have been utilized to detect EV transfer. As the reporter expresses after cargo release and function, reporter expression will be lower than the amount taken up, as some of the EVs are transported to the lysosome degradation pathway or release pathway. The reports on cargo release rate vary from <1% 98 to ~30% of the uptake. 99 Therefore, the reporter systems are suitable for the interest in the activity of EV cargoes.
Dr. Boppart's group developed label‐free intravital imaging EVs based on intrinsic metabolic and structural contrast of multiphoton images. They demonstrated the distribution of a NAD(P)H‐rich EV, which was likely to have been derived from tumors, enriched within the tumor, tumor boundary, and around vessel structures in mouse and human breast cancer tissue. 100 Through intracellular imaging of 2D culture cells, Liebel et al. tracked 3D EV movement inside the cells using holographic fluorescent imaging, 101 which could potentially be deployed to intercellular EV transfer. By combining physiological models and imaging techniques, together with recent spatial analyzing tools (spatial RNA‐seq, imaging‐MS), we can explore cancer EVs‐mediated cancer–non‐cancerous cell communications within bona fide tumor tissue.
4. CONCLUSION
Tissue environments affect EV‐mediated cell–cell communications in tumor tissues on their cargoes, recipient cells, their localization, and dynamics. Therefore, to understand the EV function and transfer in a physiological environment, physiological tumor tissue platforms with microscopic and spatial molecular analyzing techniques are required. As EV transfer is a fundamental biological activity, the concept and the strategies will be applicable to non‐cancerous physiological interactions in organs and even interspecies interactions such as microbes or edible plants and the human gut.
AUTHOR CONTRIBUTIONS
Nao Nishida‐Aoki: Conceptualization; funding acquisition; writing – original draft. Takahiro Ochiya: Supervision; writing – review and editing.
FUNDING INFORMATION
This work was supported by the following funding: JSPS KAKENHI Grant‐in‐Aid for Young Scientists (Start‐up) (22K20805), Grant‐in‐Aid for Scientific Research (C) (23K06680), the Noguchi Institute research fund, Waseda University personal research funding, Waseda University Grants for Special Research Project, and collaborative research fund from Asfreya. Inc.
CONFLICT OF INTEREST STATEMENT
N.N.‐A. and T.O. declare no conflict of interest related to the research. T.O. is an associate editor of Cancer Science.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: N/A.
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.
ACKNOWLEDGMENTS
I would like to thank Ochiya Laboratory members for their support. The figures were prepared using BioRender.
Nishida‐Aoki N, Ochiya T. Impacts of tissue context on extracellular vesicles‐mediated cancer–host cell communications. Cancer Sci. 2024;115:1726‐1737. doi: 10.1111/cas.16161
REFERENCES
- 1. de Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41(3):374‐403. doi: 10.1016/J.CCELL.2023.02.016 [DOI] [PubMed] [Google Scholar]
- 2. Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3(1):24641. doi: 10.3402/JEV.V3.24641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022;23(5):369‐382. doi: 10.1038/s41580-022-00460-3 [DOI] [PubMed] [Google Scholar]
- 4. Buzas EI. The roles of extracellular vesicles in the immune system. Nat Rev Immunol. 2023;23:236‐250. doi: 10.1038/s41577-022-00763-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodrigues J, Heinrich MA, Teixeira LM, Prakash J. 3D in vitro model (R)evolution: unveiling tumor–stroma interactions. Trends Cancer. 2021;7(3):249‐264. doi: 10.1016/j.trecan.2020.10.009 [DOI] [PubMed] [Google Scholar]
- 6. Villasante A, Marturano‐Kruik A, Ambati SR, et al. Recapitulating the size and cargo of tumor exosomes in a tissue‐engineered model. Theranostics. 2016;6(8):1119‐1130. doi: 10.7150/thno.13944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thippabhotla S, Zhong C, He M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci Rep. 2019;9(1):13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rocha S, Carvalho J, Oliveira P, et al. 3D cellular architecture affects microRNA and protein cargo of extracellular vesicles. Adv Sci. 2019;6(4):1800948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rima XY, Zhang J, Nguyen LTH, et al. Microfluidic harvesting of breast cancer tumor spheroid‐derived extracellular vesicles from immobilized microgels for single‐vesicle analysis. Lab Chip. 2022;22(13):2502‐2518. doi: 10.1039/D1LC01053K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holkar K, Kale V, Ingavle G. Well‐orchestrated physico‐chemical and biological factors for enhanced secretion of osteogenic and angiogenic extracellular vesicles by mesenchymal stem cells in a 3D culture format. Biomater Sci. 2022;10(16):4458‐4473. doi: 10.1039/D2BM00750A [DOI] [PubMed] [Google Scholar]
- 11. Haraszti RA, Miller R, Stoppato M, et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol Ther. 2018;26(12):2838‐2847. doi: 10.1016/j.ymthe.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim M, Yun HW, Park DY, Choi BH, Min BH. Three‐dimensional spheroid culture increases exosome secretion from mesenchymal stem cells. Tissue Eng Regen Med. 2018;15(4):427‐436. doi: 10.1007/S13770-018-0139-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Min LK, Kim S, Yeom J, et al. Advanced 3D dynamic culture system with transforming growth factor‐β3 enhances production of potent extracellular vesicles with modified protein cargoes via upregulation of TGF‐β signaling. J Adv Res. 2022;47:57‐74. doi: 10.1016/J.JARE.2022.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu W, Li S, Guan X, et al. Higher yield and enhanced therapeutic effects of exosomes derived from MSCs in hydrogel‐assisted 3D culture system for bone regeneration. Biomater Adv. 2022;133:112646. doi: 10.1016/J.MSEC.2022.112646 [DOI] [PubMed] [Google Scholar]
- 15. Sun L, Ji Y, Chi B, et al. A 3D culture system improves the yield of MSCs‐derived extracellular vesicles and enhances their therapeutic efficacy for heart repair. Biomed Pharmacother. 2023;161:114557. doi: 10.1016/J.BIOPHA.2023.114557 [DOI] [PubMed] [Google Scholar]
- 16. Jalilian E, Massoumi H, Bigit B, et al. Bone marrow mesenchymal stromal cells in a 3D system produce higher concentration of extracellular vesicles (EVs) with increased complexity and enhanced neuronal growth properties. Stem Cell Res Ther. 2022;13(1):425. doi: 10.1186/S13287-022-03128-Z/FIGURES/5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim JY, Rhim W‐K, Cha S‐G, et al. Bolstering the secretion and bioactivities of umbilical cord MSC‐derived extracellular vesicles with 3D culture and priming in chemically defined media. Nano Converg. 2022;9(1):57. doi: 10.1186/S40580-022-00349-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuan X, Sun L, Jeske R, et al. Engineering extracellular vesicles by three‐dimensional dynamic culture of human mesenchymal stem cells. J Extracell Vesicles. 2022;11(6):e12235. doi: 10.1002/JEV2.12235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kusuma GD, Li A, Zhu D, et al. Effect of 2D and 3D culture microenvironments on mesenchymal stem cell‐derived extracellular vesicles potencies. Front Cell Dev Biol. 2022;10:819726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakase I, Ueno N, Matsuzawa M, et al. Environmental pH stress influences cellular secretion and uptake of extracellular vesicles. FEBS Open Bio. 2021;11(3):753‐767. doi: 10.1002/2211-5463.13107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He G, Peng X, Wei S, et al. Exosomes in the hypoxic TME: from release, uptake and biofunctions to clinical applications. Mol Cancer. 2022;21(1):19. doi: 10.1186/S12943-021-01440-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang T, Gilkes DM, Takano N, et al. Hypoxia‐inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci USA. 2014;111(31):E3234‐E3242. doi: 10.1073/PNAS.1410041111/SUPPL_FILE/PNAS.201410041SI.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salomon C, Ryan J, Sobrevia L, et al. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PloS One. 2013;8(7):e68451. doi: 10.1371/JOURNAL.PONE.0068451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu B, Liu DA, Guan L, et al. Stiff matrix induces exosome secretion to promote tumour growth. Nat Cell Biol. 2023;25(3):415‐424. doi: 10.1038/s41556-023-01092-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lenzini S, Debnath K, Joshi JC, et al. Cell–matrix interactions regulate functional extracellular vesicle secretion from mesenchymal stromal cells. ACS Nano. 2021;15(11):17439‐17452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo S, Debbi L, Zohar B, et al. Stimulating extracellular vesicles production from engineered tissues by mechanical forces. Nano Lett. 2021;21(6):2497‐2504. doi: 10.1021/ACS.NANOLETT.0C04834/ASSET/IMAGES/LARGE/NL0C04834_0004.JPEG [DOI] [PubMed] [Google Scholar]
- 27. Morello M, Minciacchi VR, De Candia P, et al. Large oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle. 2013;12(22):3526‐3536. doi: 10.4161/CC.26539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang H, Freitas D, Kim HS, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field‐flow fractionation. Nat Cell Biol. 2018;20(3):332‐343. doi: 10.1038/s41556-018-0040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Q, Jeppesen DK, Higginbotham JN, et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nat Cell Biol. 2021;23(12):1240‐1254. doi: 10.1038/s41556-021-00805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edgar JR, Eden ER, Futter CE. Hrs‐ and CD63‐dependent competing mechanisms make different sized endosomal intraluminal vesicles. Traffic. 2014;15(2):197‐211. doi: 10.1111/TRA.12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skotland T, Hessvik NP, Sandvig K, Llorente A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J Lipid Res. 2019;60(1):9‐18. doi: 10.1194/jlr.R084343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Skotland T, Sagini K, Sandvig K, Llorente A. An emerging focus on lipids in extracellular vesicles. Adv Drug Deliv Rev. 2020;159:308‐321. doi: 10.1016/j.addr.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 33. Sapoń K, Mańka R, Janas T, Janas T. The role of lipid rafts in vesicle formation. J Cell Sci. 2023;136(9):jcs260887. doi: 10.1242/JCS.260887 [DOI] [PubMed] [Google Scholar]
- 34. Crescitelli R, Lässer C, Lötvall J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat Protoc. 2021;16(3):1548‐1580. doi: 10.1038/s41596-020-00466-1 [DOI] [PubMed] [Google Scholar]
- 35. Ombrato L, Nolan E, Kurelac I, et al. Metastatic‐niche labelling reveals parenchymal cells with stem features. Nature. 2019;572(7771):603‐608. doi: 10.1038/s41586-019-1487-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bagnall J, Boddington C, England H, et al. Quantitative analysis of competitive cytokine signaling predicts tissue thresholds for the propagation of macrophage activation. Sci Signal. 2018;11(540):eaaf3998. doi: 10.1126/SCISIGNAL.AAF3998/SUPPL_FILE/AAF3998_SM.PDF [DOI] [PubMed] [Google Scholar]
- 37. Imai T, Takahashi Y, Nishikawa M, et al. Macrophage‐dependent clearance of systemically administered B16BL6‐derived exosomes from the blood circulation in mice. J Extracell Vesicles. 2015;4:26238. doi: 10.3402/JEV.V4.26238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Colombo F, Norton EG, Cocucci E. Extracellular vesicle exchange is favored by cell proximity. BioRxiv 2022:2022.05.10.491399. doi: 10.1101/2022.05.10.491399 [DOI]
- 39. Hoshino A, Costa‐Silva B, Shen T‐L, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329‐335. doi: 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lenzini S, Bargi R, Chung G, Shin JW. Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat Nanotechnol. 2020;15(3):217‐223. doi: 10.1038/s41565-020-0636-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shimoda M, Khokha R. Metalloproteinases in extracellular vesicles. Biochim Biophys Acta Mol Cell Res. 2017;1864(11 Pt A):1989‐2000. doi: 10.1016/J.BBAMCR.2017.05.027 [DOI] [PubMed] [Google Scholar]
- 42. Nawaz M, Shah N, Zanetti BR, et al. Extracellular vesicles and matrix remodeling enzymes: the emerging roles in extracellular matrix remodeling, progression of diseases and tissue repair. Cells. 2018;7(10):167. doi: 10.3390/CELLS7100167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hoshino D, Kirkbride KC, Costello K, et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5(5):1159‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tominaga N, Kosaka N, Ono M, et al. Brain metastatic cancer cells release microRNA‐181c‐containing extracellular vesicles capable of destructing blood‐brain barrier. Nat Commun. 2015;6(1):6716. doi: 10.1038/ncomms7716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saint‐Pol J, Gosselet F, Duban‐Deweer S, Pottiez G, Karamanos Y. Targeting and crossing the blood‐brain barrier with extracellular vesicles. Cells. 2020;9(4):851. doi: 10.3390/CELLS9040851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koomullil R, Tehrani B, Goliwas K, et al. Computational simulation of exosome transport in tumor microenvironment. Front Med (Lausanne). 2021;8:452. doi: 10.3389/FMED.2021.643793/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sariano PA, Mizenko RR, Shirure S, et al. Convection and extracellular matrix binding control interstitial transport of extracellular vesicles. J Extracell Vesicles. 2023;12(4):e12323. doi: 10.1002/JEV2.12323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Golub EE. Role of matrix vesicles in biomineralization. Biochim Biophys Acta. 2009;1790(12):1592‐1598. doi: 10.1016/J.BBAGEN.2009.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rilla K, Mustonen AM, Arasu UT, Härkönen K, Matilainen J, Nieminen P. Extracellular vesicles are integral and functional components of the extracellular matrix. Matrix Biol. 2019;75–76:201‐219. doi: 10.1016/J.MATBIO.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 50. Debnath K, Las HK, Rivera A, Lenzini S, Shin J‐W. Extracellular vesicle–matrix interactions. Nat Rev Mater. 2023;8(6):390‐402. doi: 10.1038/s41578-023-00551-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huleihel L, Hussey GS, Naranjo JD, et al. Matrix‐bound nanovesicles within ECM bioscaffolds. Sci Adv. 2016;2(6):e1600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hussey GS, Molina CP, Cramer MC, et al. Lipidomics and RNA sequencing reveal a novel subpopulation of nanovesicle within extracellular matrix biomaterials. Sci Adv. 2020;6(12):eaay4361. doi: 10.1126/SCIADV.AAY4361/SUPPL_FILE/AAY4361_SM.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun. 2015;6:7164. doi: 10.1038/ncomms8164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Buzás EI, Tóth E, Sódar BW, Szabó‐Taylor K. Molecular interactions at the surface of extracellular vesicles. Semin Immunopathol. 2018;40(5):453‐464. doi: 10.1007/S00281-018-0682-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tóth E, Turiák L, Visnovitz T, et al. Formation of a protein corona on the surface of extracellular vesicles in blood plasma. J Extracell Vesicles. 2021;10(11):e12140. doi: 10.1002/JEV2.12140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Murphy DE, de Jong OG, Brouwer M, et al. Extracellular vesicle‐based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51(3):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Altei WF, Pachane BC, Dos Santos PK, et al. Inhibition of αvβ3 integrin impairs adhesion and uptake of tumor‐derived small extracellular vesicles. Cell Commun Signal. 2020;18(1):158. doi: 10.1186/S12964-020-00630-W/FIGURES/6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Karimi N, Dalirfardouei R, Dias T, Lötvall J, Lässer C. Tetraspanins distinguish separate extracellular vesicle subpopulations in human serum and plasma – Contributions of platelet extracellular vesicles in plasma samples. J Extracell Vesicles. 2022;11(5):e12213. doi: 10.1002/JEV2.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Andreu Z, Yáñez‐Mó M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. doi: 10.3389/FIMMU.2014.00442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Edgar JR, Manna PT, Nishimura S, Banting G, Robinson MS. Tetherin is an exosomal tether. elife. 2016;5:e17180. doi: 10.7554/ELIFE.17180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Williams C, Royo F, Aizpurua‐Olaizola O, et al. Glycosylation of extracellular vesicles: current knowledge, tools and clinical perspectives. J Extracell Vesicles. 2018;7(1):1442985. doi: 10.1080/20013078.2018.1442985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Martins ÁM, Ramos CC, Freitas D, Reis CA. Glycosylation of cancer extracellular vesicles: capture strategies, functional roles and potential clinical applications. Cells. 2021;10(1):109. doi: 10.3390/CELLS10010109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Williams C, Pazos R, Royo F, et al. Assessing the role of surface glycans of extracellular vesicles on cellular uptake. Sci Rep. 2019;9(1):11920. doi: 10.1038/s41598-019-48499-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Royo F, Cossío U, Ruiz De Angulo A, Llop J, Falcon‐Perez JM. Modification of the glycosylation of extracellular vesicles alters their biodistribution in mice. Nanoscale. 2019;11(4):1531‐1537. doi: 10.1039/C8NR03900C [DOI] [PubMed] [Google Scholar]
- 65. Nishida‐Aoki N, Tominaga N, Kosaka N, Ochiya T. Altered biodistribution of deglycosylated extracellular vesicles through enhanced cellular uptake. J Extracell Vesicles. 2020;9(1):1713527. doi: 10.1080/20013078.2020.1713527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Midekessa G, Godakumara K, Ord J, et al. Zeta potential of extracellular vesicles: toward understanding the attributes that determine colloidal stability. ACS Omega. 2020;5(27):16701‐16710. doi: 10.1021/ACSOMEGA.0C01582/SUPPL_FILE/AO0C01582_SI_003.AVI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014;123(2):208‐216. doi: 10.1182/BLOOD-2013-03-489732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shimoda A, Tahara Y, Sawada SI, Sasaki Y, Akiyoshi K. Glycan profiling analysis using evanescent‐field fluorescence‐assisted lectin array: importance of sugar recognition for cellular uptake of exosomes from mesenchymal stem cells. Biochem Biophys Res Commun. 2017;491(3):701‐707. doi: 10.1016/J.BBRC.2017.07.126 [DOI] [PubMed] [Google Scholar]
- 69. Dusoswa SA, Horrevorts SK, Ambrosini M, et al. Glycan modification of glioblastoma‐derived extracellular vesicles enhances receptor‐mediated targeting of dendritic cells. J Extracell Vesicles. 2019;8(1):1648995. doi: 10.1080/20013078.2019.1648995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zheng W, He R, Liang X, et al. Cell‐specific targeting of extracellular vesicles though engineering the glycocalyx. J Extracell Vesicles. 2022;11(12):12290. doi: 10.1002/JEV2.12290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell‐surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci USA. 2013;110(43):17380‐17385. doi: 10.1073/PNAS.1304266110/SUPPL_FILE/PNAS.201304266SI.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Perez GI, Bernard MP, Vocelle D, et al. Phosphatidylserine‐exposing annexin A1‐positive extracellular vesicles: potential cancer biomarkers. Vaccines (Basel). 2023;11(3):639. doi: 10.3390/VACCINES11030639/S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fadok VA, Bratton DL, Frasch SC, Warner ML, Henson PM. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998;5(7):551‐562. doi: 10.1038/sj.cdd.4400404 [DOI] [PubMed] [Google Scholar]
- 74. Kamerkar S, Lebleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498‐503. doi: 10.1038/NATURE22341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Belhadj Z, He B, Deng H, et al. A combined “eat me/don't eat me” strategy based on extracellular vesicles for anticancer nanomedicine. J Extracell Vesicles. 2020;9(1):1806444. doi: 10.1080/20013078.2020.1806444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sancho‐Albero M, Navascués N, Mendoza G, et al. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J Nanobiotechnol. 2019;17(1):16. doi: 10.1186/S12951-018-0437-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jurgielewicz BJ, Yao Y, Stice SL. Kinetics and specificity of HEK293T extracellular vesicle uptake using imaging flow cytometry. Nanoscale Res Lett. 2020;15(1):170. doi: 10.1186/S11671-020-03399-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Edelmann MJ, Kima PE. Current understanding of extracellular vesicle homing/tropism. Zoonoses. 2022;2(1):14. doi: 10.15212/ZOONOSES-2022-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rossaint J, Kühne K, Skupski J, et al. Directed transport of neutrophil‐derived extracellular vesicles enables platelet‐mediated innate immune response. Nat Commun. 2016;7(1):13464. doi: 10.1038/ncomms13464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sadovska L, Zandberga E, Sagini K, et al. A novel 3D heterotypic spheroid model for studying extracellular vesicle‐mediated tumour and immune cell communication. Biochem Biophys Res Commun. 2018;495(2):1930‐1935. doi: 10.1016/j.bbrc.2017.12.072 [DOI] [PubMed] [Google Scholar]
- 81. Rana S, Yue S, Stadel D, Zöller M. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. 2012;44(9):1574‐1584. doi: 10.1016/J.BIOCEL.2012.06.018 [DOI] [PubMed] [Google Scholar]
- 82. Zech D, Rana S, Büchler MW, Zöller M. Tumor‐exosomes and leukocyte activation: An ambivalent crosstalk. Cell Commun Signal. 2012;10(1):37. doi: 10.1186/1478-811X-10-37/FIGURES/11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Crewe C, Joffin N, Rutkowski JM, et al. An endothelial‐to‐adipocyte extracellular vesicle axis governed by metabolic state. Cell. 2018;175(3):695‐708.e13. doi: 10.1016/j.cell.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Miller PR, Taylor RM, Tran BQ, et al. Extraction and biomolecular analysis of dermal interstitial fluid collected with hollow microneedles. Commun Biol. 2018;1(1):173. doi: 10.1038/s42003-018-0170-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tran BQ, Miller PR, Taylor RM, et al. Proteomic characterization of dermal interstitial fluid extracted using a novel microneedle‐assisted technique. J Proteome Res. 2018;17(1):479‐485. doi: 10.1021/acs.jproteome.7b00642 [DOI] [PubMed] [Google Scholar]
- 86. Zomer A, Maynard C, Verweij FJ, et al. In vivo imaging reveals extracellular vesicle‐mediated phenocopying of metastatic behavior. Cell. 2015;161(5):1046‐1057. doi: 10.1016/j.cell.2015.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Uenaka M, Yamashita E, Kikuta J, et al. Osteoblast‐derived vesicles induce a switch from bone‐formation to bone‐resorption in vivo. Nat Commun. 2022;13(1):1066. doi: 10.1038/s41467-022-28673-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tuveson D, Clevers H. Cancer modeling meets human organoid technology. Science. 2019;364(6444):952‐955. [DOI] [PubMed] [Google Scholar]
- 89. Gengenbacher N, Singhal M, Augustin HG. Preclinical mouse solid tumour models: status quo, challenges and perspectives. Nat Rev Cancer. 2017;17(12):751‐765. doi: 10.1038/nrc.2017.92 [DOI] [PubMed] [Google Scholar]
- 90. Ural EE, Toomajian V, Hoque AE, et al. Visualizing extracellular vesicles and their function in 3D tumor microenvironment models. Int J Mol Sci. 2021;22(9):4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sood D, Tang‐Schomer M, Pouli D, et al. 3D extracellular matrix microenvironment in bioengineered tissue models of primary pediatric and adult brain tumors. Nat Commun. 2019;10(1):4529. doi: 10.1038/s41467-019-12420-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Nguyen VVT, Ye S, Gkouzioti V, et al. A human kidney and liver organoid‐based multi‐organ‐on‐a‐chip model to study the therapeutic effects and biodistribution of mesenchymal stromal cell‐derived extracellular vesicles. J Extracell Vesicles. 2022;11(11):12280. doi: 10.1002/JEV2.12280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Verweij FJ, Balaj L, Boulanger CM, et al. The power of imaging to understand extracellular vesicle biology in vivo. Nat Methods. 2021;18(9):1013‐1026. doi: 10.1038/s41592-021-01206-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Somiya M, Kuroda S. Real‐time luminescence assay for cytoplasmic cargo delivery of extracellular vesicles. Anal Chem. 2021;93(13):5612‐5620. doi: 10.1021/ACS.ANALCHEM.1C00339/SUPPL_FILE/AC1C00339_SI_003.AVI [DOI] [PubMed] [Google Scholar]
- 95. van Solinge TS, Mahjoum S, Ughetto S, et al. Illuminating cellular and extracellular vesicle‐mediated communication via a split‐Nanoluc reporter in vitro and in vivo. Cell reports. Methods. 2023;3(2):100412. doi: 10.1016/J.CRMETH.2023.100412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Somiya M, Kuroda S. Reporter gene assay for membrane fusion of extracellular vesicles. J Extracell Vesicles. 2021;10(13):e12171. doi: 10.1002/JEV2.12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. de Jong OG, Murphy DE, Mäger I, et al. A CRISPR‐Cas9‐based reporter system for single‐cell detection of extracellular vesicle‐mediated functional transfer of RNA. Nat Commun. 2020;11(1):1113. doi: 10.1038/s41467-020-14977-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. O'Brien K, Ughetto S, Mahjoum S, Nair AV, Breakefield XO. Uptake, functionality, and re‐release of extracellular vesicle‐encapsulated cargo. Cell Rep. 2022;39(2):110651. doi: 10.1016/J.CELREP.2022.110651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bonsergent E, Grisard E, Buchrieser J, Schwartz O, Théry C, Lavieu G. Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nat Commun. 2021;12(1):1864. doi: 10.1038/s41467-021-22126-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. You S, Barkalifa R, Chaney EJ, et al. Label‐free visualization and characterization of extracellular vesicles in breast cancer. Proc Natl Acad Sci USA. 2019;116(48):24012‐24018. doi: 10.1073/PNAS.1909243116/SUPPL_FILE/PNAS.1909243116.SM09.AVI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Liebel M, Arroyo JO, Beltrán VS, et al. 3D tracking of extracellular vesicles by holographic fluorescence imaging. Sci Adv. 2020;6(45):eabc2508. doi: 10.1126/SCIADV.ABC2508/SUPPL_FILE/ABC2508_SM.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]


