Abstract
Background
The newly discovered intronic repeat expansions in the genes encoding replication factor C subunit 1 (RFC1) and fibroblast growth factor 14 (FGF14) frequently cause late‐onset cerebellar ataxia.
Objectives
To investigate the presence of RFC1 and FGF14 pathogenic repeat expansions in Serbian patients with adult‐onset cerebellar ataxia.
Methods
The study included 167 unrelated patients with sporadic or familial cerebellar ataxia. The RFC1 repeat expansion analysis was performed by duplex PCR and Sanger sequencing, while the FGF14 repeat expansion was tested for by long‐range PCR, repeat‐primed PCR, and Sanger sequencing.
Results
We identified pathogenic repeat expansions in RFC1 in seven patients (7/167; 4.2%) with late‐onset sporadic ataxia with neuropathy and chronic cough. Two patients also had bilateral vestibulopathy. Repeat expansions in FGF14 were found in nine unrelated patients (9/167; 5.4%) with ataxia, less than half of whom presented with neuropathy and two‐thirds with global brain atrophy. Tremor and episodic features were the most frequent additional characteristics in carriers of uninterrupted FGF14 repeat expansions. Among the 122 sporadic cases, 12 (9.8%) carried an expansion in either RFC1 or FGF14, comparable to 4/45 (8.9%) among the patients with a positive family history.
Conclusions
Pathogenic repeat expansions in RFC1 and FGF14 are relatively frequent causes of adult‐onset cerebellar ataxia, especially among sporadic patients, indicating that family history should not be considered when prioritizing ataxia patients for testing of RFC1 or FGF14 repeat expansions.
Keywords: ataxia, CANVAS, RFC1, FGF14
Until recently, there has been an unsatisfactory gap in the genetic diagnosis of patients with late‐onset (age at onset [AAO] >30 years), sporadic, presumably degenerative ataxia. 1 A new milestone in the field of late‐onset cerebellar ataxias (LOCA) was reached in 2019 when biallelic repeat expansions (mainly involving an AAGGG repeat, but also AAAGG, AGGGC, AAGGC, or AGAGG) in the second intron of the RFC1 gene, encoding the Replication Factor C subunit, were found to cause cerebellar ataxia‐neuropathy‐vestibular areflexia syndrome (CANVAS, RFC1 repeat expansion). 2 , 3 , 4 A second exciting step forward was recently achieved when two independent research teams discovered GAA repeat expansions in the first intron of the gene encoding the Fibroblast Growth Factor 14 (FGF14) as a cause of another LOCA subtype (SCA27B, GAA‐FGF14‐related ataxia, FGF14 repeat expansion). 5 , 6
Here, we report the results of genetic screening for these two novel pathogenic repeat expansions in RFC1 and FGF14 in a large group of Serbian patients with cerebellar ataxia of unknown cause.
Methods
Patients
Patients with progressive ataxia, referred to the Department for Neurodegenerative Diseases and Movement Disorders at the Neurology Clinic at the University Clinical Center of Serbia, were recruited for this study. All patients were examined by movement disorder specialists (M.S, N.D.M, V.K), and acquired causes of ataxia were excluded. Likewise, we excluded patients suggestive of multiple system atrophy and those positive for other well‐established hereditary ataxias (spinocerebellar ataxias 1, 2, 3, 6, 7, Friedreich's ataxia, and fragile X‐associated tremor ataxia syndrome (FXTAS) when appropriate). In total, we included 167 unrelated (index) patients in this study. There were 45 patients (45/167; 26.9%) with a positive family history. Brain magnetic resonance imaging (MRI) was performed to evaluate cerebellar and cerebral atrophy in 154 patients, while the remainder underwent computed tomography. Nerve conduction studies (NCS) were carried out in all but 10 patients. Available patients (n = 4) with newly identified repeat‐expansions in RFC1 or FGF14 were reexamined for the presence of vestibulopathy with the head impulse test (HIT) or the video‐HIT (vHIT), and the presence of cough was documented by the patient or family member reports in all genetically confirmed patients.
To better understand the frequency of pathogenic expansions in RFC1 and FGF14 in certain subgroups of ataxia patients, we divided the entire patient group (n = 167) into an early‐onset group (AAO < 30 years, n = 52) and a late‐onset group (n = 115) and subdivided them further by the presence of neuropathy based on NCS (Group A and C) and absence (Group B or D) (Fig. 1). 1 AAO was defined by the first occurrence of cerebellar symptoms (ie, gait unsteadiness, difficulties with speaking). The 10 patients in whom NCS could not be performed were assigned to the group without defined neuropathy, according to their AAO.
Figure 1.
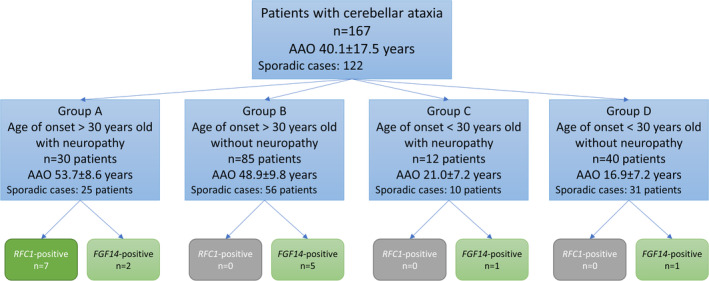
Patients included in the study were divided into four groups based on AAO and the presence of neuropathy. Genetic results are indicated. AAO, age at onset.
All patients signed an informed consent to participate in the study. The Ethics Committee of the Medical Faculty, University of Belgrade approved the study. The work was carried out in accordance with the ethical standards of the Declaration of Helsinki.
Genetic Analysis of Repeat Expansions in the RFC1 and FGF14 Genes
Genetic analysis was performed at the Institute of Neurogenetics, University of Lübeck, Lübeck, Germany. For RFC1, we applied genetic analyses for all patients as described, 6 including duplex PCR and Sanger sequencing. For FGF14, we performed long‐range PCR followed by fragment length analysis as described. 6 In addition, repeat‐primed PCR was used to test for interruptions of the GAA repeat in FGF14 using the following primers (F: CACGACGTTGTAAAACGACCTTCTTCTTCTTCTTCTTCTT, R: AGCAATCGTCAGTCAGTGTAAGC) and confirmed with Sanger sequencing of a repeat‐spanning PCR product. FGF14 repeat expansions were considered disease‐causing with a pure GAA repeat number > 250. 7
Statistical Analysis
Most of the data is presented on a descriptive level. For statistical analysis, we used SPSS version 23. We explored the association between the repeat number and AAO by Spearman correlation. P values <0.05 were considered significant.
Results
Clinical Description and Genetic Analysis of RFC1 and FGF14 Repeat Expansions
In total, 167 patients (women: 90, men: 77) underwent genetic testing for repeat expansions in RFC1 and FGF14. The average AAO (± standard deviation, STD) was 40.1 (±17.5) years, and the mean disease duration (±STD) was 10.0 (±8.2) years. Signs of neuropathy were found in 25.1% (42/167) of the patients, including 12 patients with early disease onset. Family history was positive in 26.9% (45/167), while the other patients, 73.1% (122/167), had sporadic onset of the disease.
We found pathogenic biallelic AAGGG repeat expansions in the RFC1 gene in seven (7/167, 4.2%) patients. All seven patients belonged to subgroup A and had onset of the disease after 45 years of age, a negative family history, and the presence of neuropathy. Thus, among the 25 sporadic patients from Group A (Fig. 1), pathogenic expansions in RFC1 were found in 28% (7/25) of the patients. We did not find carriers of other expanded repeat motifs.
Heterozygous repeat expansions (>250 repeats) in the FGF14 gene were found in 11 patients (11/167, 6.6%). Repeat‐primed PCR indicated pure GAA repeats in nine patients (9/167, 5.4%) and interrupted repeats in the remaining two individuals. Only uninterrupted repeats were considered disease‐causing. Among the carriers of uninterrupted repeats, four patients had a positive family history compatible with an autosomal dominant pattern of inheritance. Thus, pathogenic repeat expansions in FGF14 accounted for 8.8% (4/45) of the patients with a positive family history. Among the sporadic patients, 4.1% (5/122) were found to have uninterrupted repeat expansions in FGF14. With respect to AAO, two patients had an AAO before 30 years of age, which represent 3.8% of the tested patients with early onset ataxia (2/52, Groups C + D in Fig. 1). Among the carriers of pathogenic FGF14 repeat expansions, 22.2% (2/7) showed an early onset. Neuropathy was present in three patients, accounting for 7.1% of all included patients with neuropathy (3/42, Groups A + C in Fig. 1) and for 33.3% (3/9) of FGF14‐positive patients. The two early‐onset patients, with AAO at 18 and 27 years, had ~280 and ~450 GAA repeats, respectively. Three patients had repeat numbers between 250 and 300, while six patients had >300 repeats. We did not find any significant correlation between AAO and the number of GAA repeats among these nine carriers (r = −0.290, P = 0.449).
Clinical Findings in Patients Harboring Pathogenic Repeat Expansions in the RFC1 and FGF14 Genes
The range and mean (±STD) AAO of the disease were 46 to 69 and 54.1 (±7.6) years in RFC1 and 18 to 66 and 49.7 (±13.9) years in FGF14 repeat expansion‐positive patients. The mean disease duration (±STD) was 10.7 ± 6.0 and 7.8 ± 4.5 years in RFC1 and FGF14 repeat expansion‐positive patients, respectively. Clinical features and genetic findings are presented in detail in Tables 1 and 2, as well as in Video 1.
TABLE 1.
Clinical and diagnostic features of the RFC1 repeat expansion carriers
| Patient ID | SCN 329 | SCN 345 | SCN 366 | SCN 34 | SCN 204 | HNPP 398 | SCN 179 |
|---|---|---|---|---|---|---|---|
| Family history | No | No | No | No | No | No | No |
| Sex | F | F | M | F | F | F | M |
| AAO | 50 | 50 | 58 | 51 | 46 | 69 | 55 |
| AAE | 60 | 70 | 63 | 68 | 56 | 72 | 65 |
| DD | 10 | 20 | 5 | 17 | 10 | 3 | 10 |
| First symptoms | Instability | Instability | Instability (increased with closed eyes) | Instability | Instability (increased with closed eyes) | Tingling in hands and feet, instability | Instability (increased with closed eyes) |
| Cough | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Gait ataxia | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Walk assistance | No | Yes (walker) | No | No | No | No | Yes |
| Limb ataxia | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Dysarthria | Yes | Yes | No | No | Yes | No | Yes |
| Oculomotor findings | ISP, downbeat nystagmus | ISP, GEN | ISP | GEN | Normal | Normal | Normal |
| Vestibulopathy | Yes | Yes | N/A | N/A | N/A | N/A | N/A |
| Spasticity | No | No | No | No | No | No | No |
| DTR | Absent on the LL | Absent on the LL | Normal | Absent on the LL | Absent on the LL | Absent on the LL | Decreased on the LL |
| Extensor plantar response | No | No | No | No | No | No | No |
| Other | Distal hypo‐trophy, episodic diplopia | Head tremor, paraparesis, impaired vibration sense | N/A | Headache, impaired vibration sense | Dysphagia, impaired vibration sense | Head tremor, distal hypo‐trophia, impaired vibration sense, pes cavus, global muscle weakness | Dysphagia, incontinence, dystal hypo‐trophy and muscle weakness |
| MRI findings | Cerebellar atrophy | Cerebellar atrophy | Cerebellar atrophy | Cerebellar atrophy | Normal | Normal | Cerebellar atrophy |
| Neuropathy (NCS) | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Abbreviation: AAO, age at onset (in years); AAE, age at examination (in years); DD, disease duration (in years); ISP, impaired smooth pursuit; DTR, deep tendon reflexes; GEN, gaze‐evoked nystagmus; LL, lower limbs; NA, not assessed; NCS, nerve conductions studies; MRI, magnetic resonance imaging; UL, upper limbs.
TABLE 2.
Clinical and diagnostic features of the FGF14 repeat expansion carriers
| Patient ID | SCN 412 | SCN 352 | SCN 88 | HSP 185 | SCN 486 | SCN 46 | SCN 400 | FAN 46 | SCN 305 |
|---|---|---|---|---|---|---|---|---|---|
| Family history | No | No | Yes | Yes | Yes | No | No | Yes | No |
| Sex | F | M | F | F | F | M | M | F | M |
| AAO | 53 | 56 | 54 | 35 | 36 | 54 | 66 | 27 | 18 |
| AAE | 69 | 57 | 59 | 43 | 46 | 66 | 74 | 33 | 35 |
| DD | 16 | 1 | 5 | 8 | 10 | 12 | 8 | 6 | 17 |
| First symptoms | Episodic vertigo and nausea | Instability | Instability | Incoordination with hands, episodic vertigo | Instability | Instability | Instability | Instability | Episodic vertigo and nausea |
| Cough | No | No | No | No | No | No | No | No | No |
| Gait ataxia | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Walk assistance | Yes | No | No | No | No | Yes (walker) | Yes (walker) | No | No |
| Limb ataxia | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Dysarthria | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes |
| Oculomotor findings | ISP, DBN | GEN | DBN | Normal | GEN, skew deviation, ISP | DBN | GEN, slow saccades | GEN, ISP | Normal |
| Vestibulo‐pathy | N/a | N/a | N/a | No | No | N/a | N/a | N/a | N/a |
| Spasticity | No | No | No | No | No | No | No | No | No |
| DTR | Absent on the LL | Normal | Normal | Increased on UL and LL | Increased | Decreased on the LL | Normal | Increased patellar reflex, absent achillis reflex | Normal |
| Extensor plantar response | No | No | No | No | No | No | No | Yes | No |
| Other | Head tremor, distal hypo‐trophy, tremor of hands and legs, inconti‐nence | Tremor of the right hand, distal hypo‐trophy | Inter‐mittent tremor of the head and hands, urinary urgency | Muscle weakness of LL decreased vibrational sense, dysphagia, inconti‐nence, headache | Tremor of the hands | Diplopia, hypo‐trophy of the LL and weakness | Positional vertigo | N/a | N/a |
| MRI findings | Marked global atrophy | Marked global atrophy (more severe on the left side) | Marked global atrophy | Mild global atrophy | Normal | Frontal and cerebellar atrophy | Marked global atrophy | Moderate global atrophy | Normal |
| Neuropathy (NCS) | No | Yes | No | No | No | Yes | No | Yes | No |
| Number of repeats | 480 | 330 | 280 | 300 | 400 | 350 | 270 | 450 | 280 |
Abbreviations: AAO, age at onset (in years); AAE, age at examination (in years); DD, disease duration (in years); ISP, impaired smooth pursuit; DBN, downbeat nystagmus; DTR, deep tendon reflexes; GEN, gaze‐evoked nystagmus; LL, lower limbs; NA, not assessed; NCS, nerve conductions studies; MRI, magnetic resonance imaging; UL, upper limbs.
Video 1.
The clinical findings (downbeat nystagmus, dysmetria, and gait ataxia, tremor of the extremities with static and action component, titubation) in one patient carrying FGF14 repeat expansion (Patient ID SCN412).
All patients had gait ataxia. Further, 85.7% and 100% had limb ataxia among the RFC1‐ and FGF14‐patients, respectively. Dysarthria was present in 57.1% of the RFC1 and in 77.8% of the FGF14 pathogenic expansion carriers.
Among the seven RFC1 repeat expansion‐positive patients, three patients had impaired smooth pursuit, two gaze‐evoked nystagmus, and one downbeat nystagmus, while three patients had normal findings on oculomotor examination. As for the nine FGF14 expansion carriers, seven patients had oculomotor disturbances, including four with gaze‐evoked nystagmus, three with downbeat nystagmus, three with impaired smooth pursuit, and two patients also had skew deviation and slow saccades in all directions. In the RFC1 repeat expansion‐positive group, two patients had vestibular system dysfunction on vHIT. We found head tremor in two patients from both groups and prominent hand tremor in four FGF14 repeat expansion carriers. MRI of the brain in RFC1 repeat expansion‐positive patients showed cerebellar atrophy in five and normal findings in two patients. In the FGF14 group, 66.6% of patients had global brain atrophy (Fig. 2), from mild to marked; one patient had combined frontal and cerebellar atrophy, and two had normal findings. The atrophy was more pronounced than it would be expected for the respective age group. Signs of sensory neuropathy were present in all RFC1‐positive patients and in 33.3% of the FGF14‐positive patients. All but one RFC1 repeat expansion‐positive patient reported chronic cough without known etiology, while no one reported this feature among the FGF14 repeat expansion‐positive patients.
Figure 2.
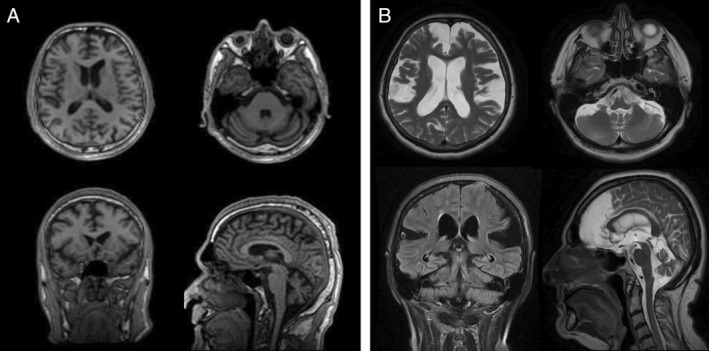
MRI of the brain of two patients with pathogenic repeat expansions in FGF14. (A) Patient SCN46, who is 10 years after disease onset and shows frontal and cerebellar atrophy. (B) Patient SCN412, who is 15 years after disease onset and shows marked global brain and cerebellar atrophy.
As for the two patients with interrupted repeat expansions in FGF14, they both have progressive cerebellar ataxia with a disease duration of more than 15 years, but without any episodic features, a negative family history, downbeat nystagmus and absent neuropathy. Both had cerebellar atrophy on MRI of the brain.
Discussion
Here, we investigated the frequency of newly reported repeat expansion disorders, ie, RFC1 and FGF14, in a large group of Serbian patients with cerebellar ataxia. In this group of patients with previously unknown disease etiology, we were able to provide 9.6% (16/167) of the patients with a genetic diagnosis of either an FGF14 repeat expansion (5.4%, n = 9) or an RFC1 repeat expansion (4.2%, n = 7). Among the sporadic patients, the diagnostic yield was 9.8% (12/122).
Notably, all our RFC1 repeat expansion‐positive patients had an AAO after the age of 45 years and signs of neuropathy. Thus, the diagnostic yield with respect to RFC1 repeat expansions was 23.3% (7/30) within the group of patients with LOCA and the presence of neuropathy. The proportion was even higher among sporadic patients with these two features, ie, 28.0% (7/25). This confirms previous clinical descriptions suggesting that RFC1 repeat expansion‐positive patients frequently have LOCA and neuropathy (and often also additional vestibulopathy [CANVAS]), 2 , 8 but AAOs as early as 19 years have also been reported. 9
Although the previous studies mainly reported that FGF14 repeat expansion carriers had an onset later than 30 years of age, 5 , 6 , 10 , 11 , 12 we found expansions in one patient in whom symptoms started when she was 27 years old and another one who had episodic features from the age of 18 years. These patients harbored 450 and 280 GAA repeats in the FGF14 gene, respectively. On the contrary, another patient, carrying 480 repeats in the FGF14 gene, had a late onset of the disease (after the age of 50 years). However, this individual presented with a complex clinical manifestation in the form of a severe gait ataxia and a high‐amplitude hand and leg tremor combined with head tremor, leading to pronounced disability in daily activities, while previous studies mainly reported slow progression in FGF14‐repeat expansion carriers, ie, slow increase of the score on the Scale for the Assessment and Rating of Ataxia (SARA) and rare dependence on a wheelchair. 11 , 12 One explanation for the faster and atypical disease progression and increased severity of FGF14 repeat expansion disorder in the above‐mentioned patient could be the presence of another neurological disorder with a different etiology. 11 We did not find any correlation between the repeat number and AAO in the FGF14 repeat expansion carriers, as previously suggested, 5 , 6 maybe due to the small sample size. 11
While neuropathy was present in 100% of the RFC1 repeat expansion‐positive patients, more than half of the FGF14 repeat expansion‐positive patients had normal findings on NCS. Thus, we suggest that all patients with LOCA associated with neuropathy should be first tested for RFC1 repeat expansions (Fig. 1). Based on this and other studies, the highest predictive value would be achieved with additional vestibulopathy and cough. 13 , 14 , 15 In previous studies, the frequency of biallelic RFC1 repeat expansion‐positive patients was reported to range from 1.8% in Japanese patients 16 to approximately 30% of patients with adult‐onset ataxia at European neurological centers. 9 The frequency of RFC1 repeat expansion‐positive patients in our entire cohort lies in between these numbers, reflecting heterogeneity in terms of AAO, presence of neuropathy, and bilateral vestibulopathy. 9 The phenotype of RFC1 repeat expansion carriers has been expanded and is referred to as incomplete CANVAS when one feature of the previously mentioned triad is not present. 8 Since the first papers, it was noted that the most prominent sign is the presence of neuropathy, and subsequent studies described RFC1 repeat expansion carriers with only isolated neuropathy, 9 , 17 , 18 even though neuropathological studies revealed loss of Purkinje and other cells in the cerebellum. 2 , 19 , 20 Additional features may include chronic cough, autonomic dysfunction, and parkinsonism. 21 , 22 , 23 , 24 A few studies reported a high percentage of RFC1 repeat expansion carriers (~65%) in groups of patients with at least two of the three CANVAS features or in combination with cough. 18 , 21 In our cohort, two patients had full‐blown CANVAS, and five had incomplete CANVAS. Nevertheless, according to new findings, in patients with full‐blown or incomplete CANVAS, FGF14 repeat expansion should also be considered in the differential diagnosis. 25
Certain point and frameshift variants in the coding region of the FGF14 gene lead to SCA27A, an autosomal dominant form of spinocerebellar ataxia, while the more recently found intronic repeat expansion in this gene can cause LOCA, often referred to as SCA27B. 5 , 26 This novel pathogenic repeat expansion was found in 5.4% (9/167) of all tested Serbian patients, involving 4.8% (5/122) of the tested patients with sporadic ataxia. Three of our patients had an expansion between 250 and 300 repeats in the FGF14 gene. These repeat sizes are considered to be pathogenic with reduced penetrance in the initial studies, given that they were also found in a small percentage among controls. 5 , 7 On the other hand, the purity of GAA repeats is an important factor, as only uninterrupted repeats seem to be pathogenic. 7 , 27 , 28 Of our 11 initially identified patients with expansions, only nine carried uninterrupted, ie, uninterrupted, disease‐causing expansions. Among the two carriers of interrupted FGF14 repeat expansions, no specific clinical features were found.
Notably, 75% of patients with GAA expansions in the FGF14 gene had early episodic ataxia and downbeat nystagmus in previous reports. 5 Among our patients, one‐third manifested paroxysmal symptoms early on in the disease. Oculomotor abnormalities were present in a higher percentage of FGF14 repeat expansion‐positive patients than in those with biallelic RFC1 repeat expansions, with gaze‐evoked nystagmus being the most frequent presentation. 17 , 18 , 29
Nearly half of our patients with a GAA expansion in FGF14 had either limb or head tremor, which is consistent with the phenotype of SCA27A, 30 although it was less frequent in previously reported cohorts with FGF14 repeat expansions. 5 Some authors found isolated cerebellar atrophy, 5 , 11 others reported mostly global brain atrophy in FGF14 repeat expansion carriers, 6 which is similar to our findings of mild to marked global atrophy. 11
Today's genetic and bioinformatic analyses have made it possible to discover new disease‐causing repeat expansions, especially in non‐coding regions. 31 Since its identification in 2019, repeat expansions in the RFC1 gene have been reported in populations worldwide. 16 , 19 , 24 , 32 Similarly, FGF14 repeat expansions were found in ataxia patients in French‐Canadian, Australian, Indian, German, French, Spanish, and Brazilian populations. 5 , 6 , 10 , 12 , 33 Notably, our study expands the findings of both repeats to Serbian ataxia patients, revealing that they represent a relevant disease cause among individuals with LOCA also in the Southeastern European population, especially in sporadic patients. This discovery is of great prognostic and treatment value for newly diagnosed patients, particularly considering potential therapeutic trials and the suggested beneficial effect of amantadine, acetazolamide in SCA27A, 30 , 34 and 4‐aminopyridine in FGF14 repeat expansion carriers. 5 , 11
In summary, our data confirm the known clinical features of RFC1 repeat expansion carriers regarding AAO, neuropathy combined with cerebellar signs, and chronic cough in most patients. As for the novel GAA repeat expansion in FGF14, we broaden the spectrum of the clinical manifestation in the context of a more frequent presence of tremor and significant global brain atrophy on MRI scans, which can be a distinguishing factor in the group of LOCA, as well as earlier manifestation of the disease.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the first draft, B. Review and Critique.
AM: 1B, 1C, 1C, 2A, 2B, 3A.
ND‐M: 1B, 1C, 1C, 2A, 2B, 3A.
MT: 1C, 3B.
MB: 1A, 3B.
FH: 1C, 3B.
AW: 1B, 3B.
CK: 1B, 3B.
NB: 1A, 3B.
MS: 1C, 3B.
MB: 1C, 3A,B.
AM: 1C, 3B.
VSK: 1A, 1C, 3B.
KL: 1A, 1B, 2C, 3A, 3B.
Disclosures
Ethical Compliance Statement: The study was approved by the Ethics Committee of the Medical Faculty, University of Belgrade. All patients signed a written informed consent prior to inclusion in the study. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: This study was funded by the University of Lübeck and the German Research Foundation (FOR 2488 to A.W., N.B., C.K., and K.L.). The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the previous 12 months: A.W. and C.K. serve as advisors to CENTOGENE GmbH. C.K. received grants from the Michael J. Fox Foundation (MJFF) and Aligning Science Across Parkinson's, speaking honoraria from Desitin and Bial, and royalties from Oxford University Press. K.L. received grants from MJFF, Damp foundation, and Parkinson's Foundation. A.M., N.D‐M., M.T., M.B., F.H., N.B., M.B., A.M., M.S., V.S.K. declare that there are no additional disclosures to report.
Acknowledgment
We thank the patients and their relatives for their participation in this study. This study was funded by the University of Luebeck and the German Research Foundation (FOR 2488).
References
- 1. van Gaalen J, van de Warrenburg BPC. A practical approach to late‐onset cerebellar ataxia: putting the disorder with lack of order into order. Pract Neurol 2012;12(1):14–24. 10.1136/practneurol-2011-000108. [DOI] [PubMed] [Google Scholar]
- 2. Cortese A, Simone R, Sullivan R, et al. Biallelic expansion of an intronic repeat in RFC1 is a common cause of late‐onset ataxia. Nat Genet 2019;51(4):649–658. 10.1038/s41588-019-0372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rafehi H, Szmulewicz DJ, Bennett MF, et al. Bioinformatics‐based identification of expanded repeats: a non‐reference Intronic Pentamer expansion in RFC1 causes CANVAS. Am J Hum Genet 2019;105(1):151–165. 10.1016/j.ajhg.2019.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dominik N, Magri S, Currò R, et al. Normal and pathogenic variation of RFC1 repeat expansions: implications for clinical diagnosis. Brain 2023;146(12):5060–5069. 10.1093/brain/awad240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pellerin D, Danzi MC, Wilke C, et al. Deep Intronic FGF14 GAA repeat expansion in late‐onset cerebellar ataxia. N Engl J Med 2023;388(2):128–141. 10.1056/NEJMoa2207406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rafehi H, Read J, Szmulewicz DJ, et al. An intronic GAA repeat expansion in FGF14 causes the autosomal‐dominant adult‐onset ataxia SCA50/ATX‐FGF14. Am J Hum Genet 2023;110(1):105–119. 10.1016/j.ajhg.2022.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonnet C, Pellerin D, Roth V, et al. Optimized testing strategy for the diagnosis of GAA‐FGF14 ataxia/spinocerebellar ataxia 27B. Sci Rep 2023;13(1):9737. 10.1038/s41598-023-36654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gisatulin M, Dobricic V, Zühlke C, et al. Clinical spectrum of the pentanucleotide repeat expansion in the RFC1 gene in ataxia syndromes. Neurology 2020;95(21):e2912–e2923. 10.1212/WNL.0000000000010744. [DOI] [PubMed] [Google Scholar]
- 9. Cortese A, Tozza S, Yau WY, et al. Cerebellar ataxia, neuropathy, vestibular areflexia syndrome due to RFC1 repeat expansion. Brain 2020;143(2):480–490. 10.1093/brain/awz418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iruzubieta P, Pellerin D, Bergareche A, et al. Frequency and phenotypic spectrum of spinocerebellar ataxia 27B and other genetic ataxias in a Spanish cohort of late‐onset cerebellar ataxia. Eur J Neurol 2023;30(12):3828–3833. 10.1111/ene.16039. [DOI] [PubMed] [Google Scholar]
- 11. Wilke C, Pellerin D, Mengel D, et al. GAA‐FGF14 ataxia (SCA27B): phenotypic profile, natural history progression and 4‐aminopyridine treatment response. Brain 2023;146(10):4144–4157. 10.1093/brain/awad157. [DOI] [PubMed] [Google Scholar]
- 12. Wirth T, Clément G, Delvallée C, et al. Natural history and phenotypic Spectrum of GAA‐FGF14 sporadic late‐onset cerebellar ataxia (SCA27B). Mov Disord 2023;38(10):1950–1956. 10.1002/mds.29560. [DOI] [PubMed] [Google Scholar]
- 13. Cortese A, Curro’ R, Vegezzi E, Yau WY, Houlden H, Reilly MM. Cerebellar ataxia, neuropathy and vestibular areflexia syndrome (CANVAS): genetic and clinical aspects. Pract Neurol 2022;22(1):14–18. 10.1136/practneurol-2020-002822. [DOI] [PubMed] [Google Scholar]
- 14. Beijer D, Dohrn MF, De Winter J, et al. RFC1 repeat expansions: a recurrent cause of sensory and autonomic neuropathy with cough and ataxia. Eur J Neurol 2022;29(7):2156–2161. 10.1111/ene.15310. [DOI] [PubMed] [Google Scholar]
- 15. Hadjivassiliou M, Currò R, Beauchamp N, et al. Can CANVAS due to RFC1 biallelic expansions present with pure ataxia? J Neurol Neurosurg Psychiatry 2024;95(2):171–174. 10.1136/jnnp-2023-331381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ando M, Higuchi Y, Yuan JH, et al. Genetic and clinical features of cerebellar ataxia with RFC1 biallelic repeat expansions in Japan. Front Neurol 2022;13:952493. 10.3389/fneur.2022.952493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Currò R, Salvalaggio A, Tozza S, et al. RFC1 expansions are a common cause of idiopathic sensory neuropathy. Brain 2021;144(5):1542–1550. 10.1093/brain/awab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sánchez‐Tejerina D, Alvarez PF, Laínez E, et al. RFC1 repeat expansions and cerebellar ataxia, neuropathy and vestibular areflexia syndrome: experience and perspectives from a neuromuscular disorders unit. J Neurol Sci 2023;446:120565. 10.1016/j.jns.2023.120565. [DOI] [PubMed] [Google Scholar]
- 19. Montaut S, Diedhiou N, Fahrer P, et al. Biallelic RFC1‐expansion in a French multicentric sporadic ataxia cohort. J Neurol 2021;268(9):3337–3343. 10.1007/s00415-021-10499-5. [DOI] [PubMed] [Google Scholar]
- 20. Huin V, Coarelli G, Guemy C, et al. Motor neuron pathology in CANVAS due to RFC1 expansions. Brain 2022;145(6):2121–2132. 10.1093/brain/awab449. [DOI] [PubMed] [Google Scholar]
- 21. Traschütz A, Cortese A, Reich S, et al. Natural history, phenotypic Spectrum, and discriminative features of multisystemic RFC1 disease. Neurology 2021;96(9):e1369–e1382. 10.1212/WNL.0000000000011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sullivan R, Yau WY, Chelban V, et al. RFC1‐related ataxia is a mimic of early multiple system atrophy. J Neurol Neurosurg Psychiatry 2021;92(4):444–446. 10.1136/jnnp-2020-325092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davies K, Szmulewicz DJ, Corben LA, Delatycki M, Lockhart PJ. RFC1‐related disease molecular and clinical insights. Neurol Genet. 2022;8(5):e200016. 10.1212/NXG.0000000000200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kontogeorgiou Z, Kartanou C, Tsirligkani C, et al. Biallelic RFC1 pentanucleotide repeat expansions in Greek patients with late‐onset ataxia. Clin Genet 2021;100(1):90–94. 10.1111/cge.13960. [DOI] [PubMed] [Google Scholar]
- 25. Pellerin D, Wilke C, Traschütz A, et al. Intronic FGF14 GAA repeat expansions are a common cause of ataxia syndromes with neuropathy and bilateral vestibulopathy. J Neurol Neurosurg Psychiatry 2024;95(2):175–179. 10.1136/jnnp-2023-331490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Swieten JC, Brusse E, de Graaf BM, et al. A mutation in the fibroblast growth factor 14 gene is associated with autosomal dominant cerebellar ataxia [corrected]. Am J Hum Genet 2003;72(1):191–199. 10.1086/345488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saffie Awad P, Lohmann K, Hirmas Y, et al. Shaking up ataxia: FGF14 and RFC1 repeat expansions in affected and unaffected members of a Chilean family. Mov Disord 2023;38(6):1107–1109. 10.1002/mds.29390. [DOI] [PubMed] [Google Scholar]
- 28. Pellerin D, Iruzubieta P, Tekgül Ş, et al. Non‐GAA repeat expansions in FGF14 are likely not pathogenic—reply to: “shaking up ataxia: FGF14 and RFC1 repeat expansions in affected and unaffected members of a Chilean family”. Mov Disord 2023;38(8):1575–1577. 10.1002/mds.29552. [DOI] [PubMed] [Google Scholar]
- 29. Kheradmand A, Zee D. Cerebellum and ocular motor control. Frontiers in Neurology 2011;2:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Groth CL, Berman BD. Spinocerebellar ataxia 27: a review and characterization of an evolving phenotype. Tremor Other Hyperkinet Mov (N Y) 2018;8:534. 10.7916/D80S0ZJQ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Depienne C, Mandel JL. 30 years of repeat expansion disorders: what have we learned and what are the remaining challenges? Am J Hum Genet 2021;108(5):764–785. 10.1016/j.ajhg.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ghorbani F, de Boer‐Bergsma J, Verschuuren‐Bemelmans CC, et al. Prevalence of intronic repeat expansions in RFC1 in Dutch patients with CANVAS and adult‐onset ataxia. J Neurol 2022;269(11):6086–6093. 10.1007/s00415-022-11275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Novis LE, Frezatti RS, Pellerin D, et al. Frequency of GAA‐FGF14 ataxia in a large cohort of Brazilian patients with unsolved adult‐onset cerebellar ataxia. Neurol Genet 2023;9(5):e200094. 10.1212/NXG.0000000000200094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schesny M, Joncourt F, Tarnutzer AA. Acetazolamide‐responsive episodic ataxia linked to novel splice site variant in the FGF14 gene. Cerebellum 2019;18(3):649–653. 10.1007/s12311-018-0997-3. [DOI] [PubMed] [Google Scholar]


