Abstract
Purpose:
Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors radically changed the treatment paradigm for breast cancer. Similar to estrogen receptor in breast cancer, androgen receptor signaling activates cyclin D–CDK4/6, driving proliferation and resistance to hormonal manipulation in prostate cancer. This study was designed to detect signals of clinical activity for abemaciclib in treatment-refractory metastatic castration-resistant prostate cancer (mCRPC).
Patients and Methods:
Eligible patients had progressive mCRPC, measurable disease, and previously received ≥1 novel hormonal agent(s) and 2 lines of taxane chemotherapy. Abemaciclib 200 mg twice daily was administered on a continuous dosing schedule. Primary endpoint was objective response rate (ORR) without concurrent bone progression. This study was designed to detect a minimum ORR of 12.5%.
Results:
At trial entry, 40 (90.9%) of 44 patients had objective radiographic disease progression, 4 (9.1%) had prostate-specific antigen (PSA)–only progression, and 20 (46.5%) had visceral metastases (of these, 60% had liver metastases). Efficacy analyses are as follows: ORR without concurrent bone progression: 6.8%; disease control rate: 45.5%; median time to PSA progression: 6.5 months [95% confidence interval (CI), 3.2–NA]; median radiographic PFS; 2.7 months (95% CI, 1.9–3.7); and median OS, 8.4 months (95% CI, 5.6–12.7). Most frequent grade ≥3 treatment-emergent adverse events (AE) were neutropenia (25.0%), anemia, and fatigue (11.4% each). No grade 4 or 5 AEs were related to abemaciclib.
Conclusions:
Abemaciclib monotherapy was well tolerated and showed clinical activity in this heavily pretreated population, nearly half with visceral metastases. This study is considered preliminary proof-of-concept and designates CDK4/6 as a valid therapeutic target in prostate cancer.
Translational Relevance.
Persistent oncogenic addiction to androgen receptor (AR) signaling underlies the development and progression of castration‐resistant prostate cancer (CRPC). Preclinical evidence suggests a key role for cyclin D–CDK4/6 in AR-induced mitogenic signaling and in resistance to hormonal agents. As evidence of the relationship between the cyclin D-CDK4/6 and AR pathways, cyclin D1 is a transcriptional regulator of AR, and conversely, AR elicits upregulation of cyclin D1 and downregulates CDK4/6 inhibitors p21 and p27. Abemaciclib is a potent oral inhibitor of CDK4/6 that significantly augments the efficacy of hormonal therapy in high-risk early-stage and metastatic breast cancer. In prostate cancer models, abemaciclib induces cell-cycle arrest and tumor growth inhibition. This study of abemaciclib in heavily pretreated patients with metastatic CRPC provides the first clinical proof-of-concept for targeting CDK4/6 in prostate cancer and supports further clinical investigations to leverage the expected synergy of CDK4/6 and AR dual inhibition.
Introduction
Uncontrolled cellular proliferation is a hallmark of cancer and the cyclin D–CDK4/6 and Rb–E2F pathway plays a key role in controlling G1–S transition of the cell cycle that irreversibly commits cells to division (1–3). Rb acts as a co-repressor that binds E2F transcription factors. When Rb becomes phosphorylated by cyclin D–CDK4/6, the Rb–E2F repressive complex is disrupted, enabling the expression of E2F target genes involved in S-phase entry and cell-cycle progression (4).
CDK4/6 inhibitors (abemaciclib, palbociclib, and ribociclib) have transformed the treatment paradigm for hormone receptor–positive (HR+), HER2− metastatic breast cancer. When combined with endocrine therapy, all three CDK4/6 inhibitors demonstrated significantly increased progression-free survival (PFS) over endocrine therapy alone, and overall survival (OS) was improved with abemaciclib and ribociclib (5–8). Mechanistically, CDK4/6 may also represent a promising therapeutic target in prostate cancer. Preclinical evidence indicates that androgen receptor (AR) signaling activates CDK4/6 to drive prostate cancer cell proliferation (1). In addition, increased expression of cyclin D, and subsequent activation of CDK4/6, has been proposed as a resistance mechanism to novel hormonal agents (NHA; ref. 9). Furthermore, pharmacological inhibition of CDK4/6 led to robust cell-cycle arrest and tumor growth inhibition in prostate cancer models (10).
Abemaciclib is a potent, orally administered inhibitor of CDK4/6 that is 14 times more potent against CDK 4/cyclin D1 than CDK 6/cyclin D3 in enzymatic assays (11, 12). It is approved as a single agent and in combination with endocrine therapy for patients with high-risk early stage or metastatic HR+, HER2− breast cancer. Distinct in its class due to hematologic toxicities not being dose limiting, abemaciclib is administered on a continuous dosing schedule (13).
Patients with metastatic castration-resistant prostate cancer (mCRPC) experiencing disease progression after failure of life-prolonging NHAs and taxanes have limited therapeutic options and very poor prognoses (14). The pressing need for treatments with novel modes of action, and the biological rationale for CDK4/6 inhibition against prostate cancer prompted the design of CYCLONE 1, a single-arm, signal-finding, phase 2 study of abemaciclib monotherapy in heavily pretreated patients with refractory mCRPC.
Patients and Methods
Patient characteristics
Patient disposition and eligibility criteria are presented in Supplementary Fig. S1. Eligible patients had measurable disease and progressive mCRPC by at least two rising prostate-specific antigen (PSA) values and/or imaging per RECIST v1.1 for soft tissue and Prostate Cancer Clinical Trials Working Group 3 (PCWG3) criteria for bone. Prior therapy with one or more NHA (abiraterone, apalutamide, darolutamide, or enzalutamide) and two taxane-based chemotherapy regimens (docetaxel and cabazitaxel) were required. Exceptions for not receiving the second taxane due to hypersensitivity/intolerance or contraindications to taxanes, or discontinuation of the first taxane due to adverse events (AE), required sponsor approval. Up to three prior systemic therapy regimens in the mCRPC setting were permitted [of note: luteinizing hormone-releasing hormone (LHRH) analogues, first-generation antiandrogens, diethylstilbesterol, or other estrogens, corticosteroids, ketoconazole, and bone loss prevention were not considered prior systemic therapies]. Metastatic tumor tissue collection was required before enrollment (fresh biopsy or ≤12-week-old archival formalin-fixed, paraffin-embedded block/slides).
The study was conducted in accordance with Good Clinical Practice Guidelines and in compliance with the Declaration of Helsinki. Written informed consent was provided from all patients before study enrollment. The study protocol was approved by all sites’ ethical review boards and all patients gave written, informed consent.
Study design
CYCLONE 1 (NCT04408924) was a multicenter, single-arm, open-label, phase 2 study that evaluated the safety and efficacy of abemaciclib monotherapy in heavily pretreated patients with mCRPC. Patients received study treatment until disease progression (symptomatic and/or radiographic), unacceptable treatment-related toxicity, or until another discontinuation criterion was met.
Procedures
Eligibility was assessed during a 28-day screening period. Abemaciclib 200 mg twice daily was administrated orally in a continuous dosing schedule on 28-day cycles. Standard androgen deprivation therapy with an LHRH analogue was continued for patients who had not undergone bilateral orchiectomy. Safety was evaluated using the National Cancer Institute Common Toxicity Criteria for AEs version 5 (NCI CTCAE v5.0). Contrast-enhanced CT of the chest, CT or MRI of the abdomen and pelvis, and whole-body bone scans were obtained at baseline and repeated every 8 weeks from cycle 1 day 1 until documented radiographic progression, start of new anticancer therapy, withdrawal of consent, death, or study completion. Tumor response was assessed according to RECIST v1.1 for soft tissue and PCWG3 criteria for bone.
Study endpoints
Definitions of primary and secondary endpoints are provided in Supplementary Table S1.
The primary endpoint was investigator-assessed objective response rate (ORR) defined as the proportion of all treated patients with a confirmed complete response (CR) or confirmed partial response (PR) in soft tissue per RECIST v1.1 without concurrent bone progression per PCWG3. Key secondary endpoints included radiographic PFS (rPFS), OS, time to PSA progression, time to symptomatic progression, pharmacokinetics, and safety. The nature and severity of AEs were assessed according to CTCAE v5.0.
Statistical analysis
All patients who received at least one dose of abemaciclib were included in the safety and efficacy analyses.
For the primary efficacy analysis, an ORR of ≥12.5% was considered promising in this highly treatment-refractory setting with no accepted standard of care. Patients with a non-evaluable tumor or unknown response were considered non-responders. Time-to-event efficacy endpoints such as rPFS, time to PSA progression, OS, and time to symptomatic progression were estimated using the Kaplan–Meier method and respective confidence intervals (CI) were reported with a 2-sided 95% level.
The primary analysis was planned to occur approximately 6 months after the last patient entered treatment. Because of sparse pharmacokinetic sampling, a population pharmacokinetic modeling approach matching CYCLONE 1 patient characteristics was used to simulate the pharmacokinetic parameters of interest based on a final population pharmacokinetic model previously developed (15).
Data availability
Eli Lilly and Company provides access, after anonymization, to all individual participant data collected during the trial, except for pharmacokinetic and genetic data. Data can be requested 6 months after the indication studied has been approved in the United States and European Union or after primary publication acceptance, whichever is later. No expiration date for data requests is set once the data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data-sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data-sharing environment. For details on submitting a request, see instructions at https://vivli.org.
Results
Patients
Between January and October 2021, 44 patients from France, Spain, and the United States were enrolled and received abemaciclib 200 mg twice daily on a continuous schedule (Supplementary Fig. S1). Representativeness of study participants is reflected in Supplementary Table S2.
The median age was 68 (range, 48–94) years and 75% of patients had an Eastern Cooperative Oncology Group performance status of 1. The Gleason score was ≥8 for 64.3% of the patients and 50.0% had metastatic disease at the initial diagnosis of prostate cancer. Median time from mCRPC diagnosis to study entry was 2.1 years (range, 0.7–6.6) and median PSA at study entry was 96.0 ng/mL. Most patients (40/44; 93.0%) had three or more metastatic lesions. All patients had measurable disease. Notably, nearly half of the patients (20/44; 46.5%) had visceral metastases, of whom 60.0% (12/20) had liver lesions. Patients predominantly experienced radiographic progression (RECIST/PCWG3) at study entry (40/44; 90.9%), whereas only four (9.1%) had PSA-only progression (Table 1). Baseline expression of the Ki-67 proliferation marker assessed by IHC was high (cutoff score ≥20%) for 74.2% (23/31) of the 31 patients with evaluable baseline metastatic tumor samples.
Table 1.
Patient characteristics.
| Characteristics | n (%) |
|---|---|
| Median age (range) | 68 (48–94) |
| Gleason score (n = 42) | |
| ≤7 | 15 (35.7) |
| 8–10 | 27 (64.3) |
| Initial diagnosis (n = 42) | |
| Localized | 21 (50.0) |
| Metastatic | 21 (50.0) |
| ECOG PS (n = 44) | |
| 0 (fully active) | 11 (25.0) |
| 1 (restricted) | 33 (75.0) |
| Metastatic sites (n = 43) | |
| 2 | 3 (7.0) |
| ≥3 | 40 (93.0) |
| Site of disease (n = 43) | |
| Viscerala | 20 (46.5) |
| Liver | 12 (60.0) |
| Lung | 9 (45.0) |
| Other | 3 (15.0) |
| Non-visceral | 23 (53.5) |
| Type of progression at trial entry (n = 44) | |
| PSA-only | 4 (9.1) |
| Radiographic (by RECIST/PCWG3) ± PSA | 40 (90.9) |
| Baseline Ki-67 expression by IHC (n = 31) | |
| Low (<20%) | 8 (25.8) |
| High (≥20%) | 23 (74.2) |
| Prior therapy | n (%) |
| Type of prior therapyb (n = 44) | |
| Chemotherapy | 44 (100) |
| Docetaxel | 44 (100) |
| Cabazitaxel | 40 (90.9) |
| Carboplatin | 2 (4.5) |
| Paclitaxel | 1 (2.3) |
| Novel hormonal agent | 44 (100) |
| 1 | 37 (84.1) |
| 2c | 7 (15.9) |
| Abiraterone | 24 (54.5) |
| Enzalutamide | 24 (54.5) |
| Apalutamide | 2 (4.5) |
| Darolutamide | 1 (2.3) |
| Radiopharmaceuticals | 2 (4.5) |
| Radium-223 | 1 (2.3) |
| 177Lu-PSMA | 1 (2.3) |
| Immunotherapy | 6 (13.6) |
| Pembrolizumab | 3 (6.8) |
| Atezolizumab | 1 (2.3) |
| Durvalumab | 1 (2.3) |
| Ipilimumab | 1 (2.3) |
| Nivolumab | 1 (2.3) |
| Number of prior therapies for mCRPC (n = 44) | |
| ≤2 | 19 (43.2) |
| ≥3d | 25 (56.8) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; mHSPC, metastatic hormone-sensitive prostate cancer; nmCRPC, nonmetastatic castration–resistant prostate cancer; PCWG3, Prostate Cancer Clinical Trials Working Group 3; RECIST, Response Evaluation Criteria in Solid Tumors.
aFour patients had both liver and lung lesions.
bPatients may have received multiple prior therapies in any setting, including mHSPC, nmCRPC, or mCRPC.
cOne patient received the two NHAs in combination.
dThree patients had four prior therapies for mCRPC.
All patients were previously treated with at least one NHA, most commonly abiraterone and enzalutamide (54.5% each). Seven patients (15.9%) received two prior NHAs. In addition, all patients previously received docetaxel, and all but 4 (40/44; 90.9%) also received cabazitaxel. Other prior systemic therapies included radiopharmaceuticals (radium-223 and 177Lutetium-PSMA-617, 2.3% each) and immune checkpoint inhibitors (pembrolizumab, 6.8%; atezolizumab, durvalumab, ipilimumab, and nivolumab, 2.3% each). The median number of prior lines of systemic treatments for mCRPC was 3 (range, 1–4; Table 1).
Efficacy
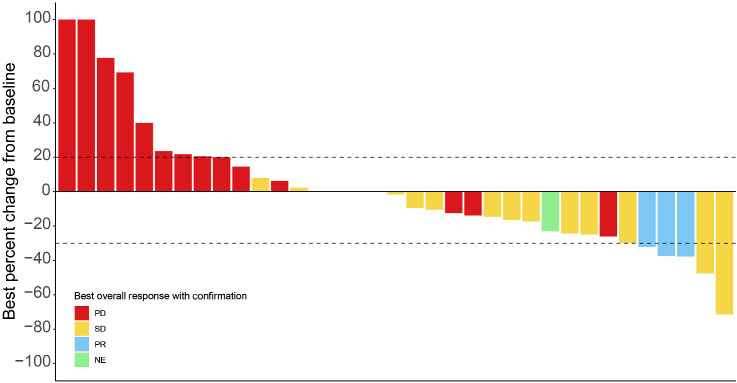
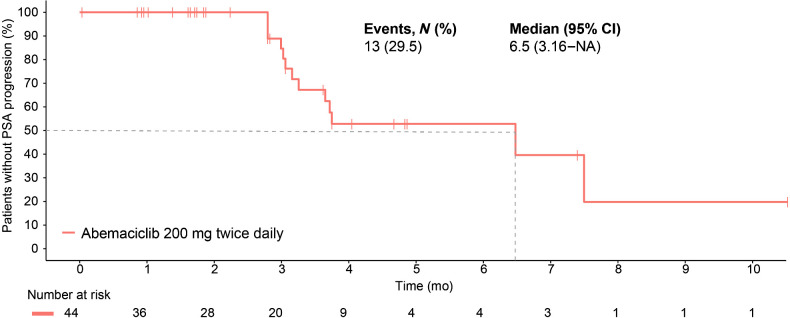
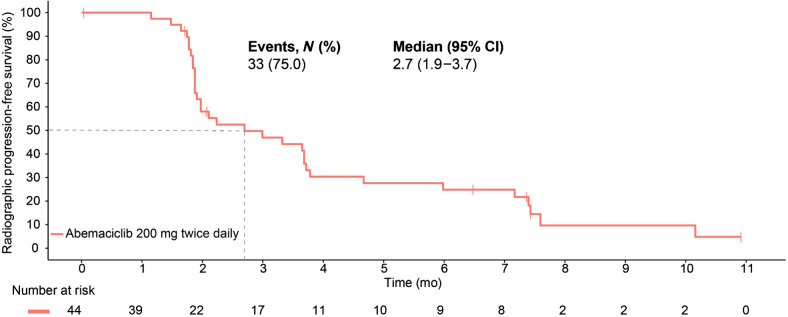
At the time of data cutoff on April 27, 2022, 3 of the 44 patients had a confirmed PR without concurrent bone progression for an ORR of 6.8% (95% CI, 0.0–14.3). Figure 1 and Supplementary Table S3 summarize the best percentage of change in tumor size (waterfall plot) and best overall response by RECIST v1.1 criteria without concurrent bone progression. Notably, 17 (38.6%) patients experienced radiographic stabilization of their disease that was durable for ≥6 months for over a third (6/17; 35.3%) of these patients. The soft tissue disease control rate (CR+PR+stable disease) without concurrent bone progression was 45.5% (95% CI, 30.7–60.2). The clinical benefit rate (CR+PR+stable disease ≥6 months) without concurrent bone progression was 20.5% (95% CI, 8.5–32.4). The median time to PSA progression was 6.5 months (95% CI, 3.2–NA; Fig. 2). Two (4.6%) patients experienced ≥50% decline in PSA from baseline, confirmed with a second assessment conducted at least 3 weeks later. The median rPFS (soft tissue or bone progression, or death from any cause) was 2.7 months (95% CI, 1.9–3.7) and the 6-month rPFS rate was 24.9% (Fig. 3). The median time to symptomatic progression was 4.1 months (95% CI, 3.7–NA; Supplementary Fig. S2). Other secondary outcomes of duration of response, change in PSA, and PSA response are reported in Supplementary Table S4 and Supplementary Fig. S2. Because of the limited sample size, correlation between efficacy outcomes and baseline Ki-67 expression in the metastatic tumor samples could not be established.
Figure 1.
Best percentage of change in tumor size. Two patients had a greater than 30% reduction in their target lesions but did not have their responses confirmed without concurrent bone progression.
Figure 2.
Time to prostate-specific antigen progression.
Figure 3.
Radiographic progression-free survival.
Forty-one of the 44 patients (93.2%) discontinued abemaciclib study treatment: 30 (73.2%) due to progressive disease and 6 (14.6%) due to AEs (Supplementary Fig. S1). A total of 36 patients entered post-discontinuation follow-up, of whom 15 (41.7%) received subsequent treatment. Most common post-discontinuation treatments were palliative radiotherapy (4/15, 26.7%), radium-223 (3/15, 20%), and carboplatin (3/15, 20%; Supplementary Table S5).
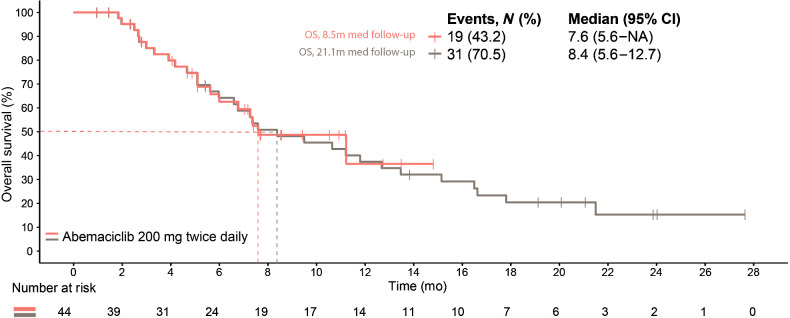
At the primary outcome data cutoff, the median follow-up time was 8.5 months and OS was immature (19 events, median OS, 7.6 months; 95% CI, 5.6–not reached; Fig. 4). At the final OS analysis, the median follow-up was 21.1 months, there were 31 death events, and the median OS was 8.4 months (95% CI, 5.6–12.7).
Figure 4.
Overall survival.
Safety
All patients experienced ≥1 treatment-emergent AE (TEAE) that were mostly low grade (Table 2). The most frequently reported any-grade TEAEs were diarrhea (81.8%), fatigue (63.6%), decreased appetite (54.5%), nausea (47.7%), neutropenia (38.6%), anemia (36.4%), vomiting (36.4%), and constipation (20.5%). The most frequent grade ≥3 TEAEs were neutropenia (25.0%), anemia (11.4%), and fatigue (11.4%).
Table 2.
Safety results.
| TEAEs >10% of patients | N = 44 | |
|---|---|---|
| Any Grade | Grade ≥3 | |
| Diarrhea | 36 (81.8) | 4 (9.1) |
| Fatiguea | 28 (63.6) | 5 (11.4) |
| Decreased appetite | 24 (54.5) | 1 (2.3) |
| Nausea | 21 (47.7) | 0 (0.0) |
| Neutropeniaa | 17 (38.6) | 11 (25.0) |
| Anemia | 16 (36.4) | 5 (11.4) |
| Vomiting | 16 (36.4) | 0 (0.0) |
| Constipation | 9 (20.5) | 0 (0.0) |
| Blood creatinine increased | 8 (18.2) | 0 (0.0) |
| Thrombocytopeniaa | 8 (18.2) | 2 (4.5) |
| Back pain | 7 (15.9) | 0 (0.0) |
| Pyrexia | 7 (15.9) | 1 (2.3) |
| Edema peripheral | 6 (13.6) | 0 (0.0) |
| Arthralgia | 5 (11.4) | 0 (0.0) |
| TRAEs >10% of patients | N = 44 | |
| Any grade | Grade 3 b | |
| Diarrhea | 35 (79.5) | 3 (6.8) |
| Decreased appetite | 23 (52.3) | 1 (2.3) |
| Fatiguea | 22 (50.0) | 3 (6.8) |
| Nausea | 19 (43.2) | 0 (0.0) |
| Neutropeniaa | 15 (34.1) | 10 (22.7) |
| Vomiting | 15 (34.1) | 0 (0.0) |
| Anemia | 13 (29.5) | 3 (6.8) |
| Thrombocytopeniaa | 7 (15.9) | 1 (2.3) |
| Blood creatinine increased | 5 (11.4) | 0 (0.0) |
aConsolidated terms: fatigue, fatigue and asthenia; neutropenia, neutropenia and neutrophil count decreased; thrombocytopenia, thrombocytopenia, and platelet count decreased.
bNo Grade 4 or 5 TRAEs (as assessed by investigators) were reported.
The most frequently reported any-grade treatment-related AEs (TRAE) occurring in >10% of patients were diarrhea (79.5%), decreased appetite (52.3%), fatigue (50.0%), nausea (43.2%), neutropenia (34.1%), vomiting (34.1%), anemia (29.5%), thrombocytopenia (15.9%), and blood creatinine increased (11.4%; Table 2). There were no grade 4 or 5 TRAEs.
Serious AEs (SAE) were reported for 16 patients (36.4%), with pyrexia (19%), renal failure (19%), anemia (13%), and serum creatinine increase (13%) being the most common. Two patients experienced SAEs deemed possibly related to abemaciclib: one patient had two SAEs (serum creatinine increase and renal failure) and discontinued study treatment; the second patient had a SAE of anemia.
The median relative dose intensity was 69.5% (Q1–Q3: 48.6%–92.6%). Thirty-four patients (34/44; 77.3%) had a dose suspension of abemaciclib due to an AE, most commonly diarrhea (11/34, 25.0%), neutropenia (7/34, 15.9%), and blood creatinine increased (4/34, 9.1%). Nineteen patients (19/44; 43.2%) required ≥1 dose reduction of abemaciclib due to an AE. The most frequent AEs leading to dose reductions were diarrhea (7/19, 36.8%), blood creatinine increased (3/19, 15.8%), and neutropenia (3/19, 15.8%). Of the 44 patients, 6 (13.6%) discontinued treatment due to an AE/SAE (anemia, neutropenia, vomiting, or renal failure). AEs leading to treatment discontinuation were deemed possibly related to abemaciclib for 4 patients (4/44; 9.1%).
Pharmacokinetics
Plasma concentration-time data for abemaciclib were available for 43/44 patients (97.7%). In CYCLONE 1, the starting dose of abemaciclib was 200 mg twice daily, and sequential reductions by 50-mg dose levels were permitted. Summary exposures of 178 mg were calculated on the basis of the average dose received throughout the study and are consistent with previous studies in breast cancer (data on file; Supplementary Table S6).
Discussion
CYCLONE 1 enrolled a heavily pretreated population with no real treatment alternatives. Although the primary endpoint of a 12.5% ORR in soft tissue without concurrent bone progression was not met, results from this signal-finding study provided the first evidence of abemaciclib's clinical activity in a late and refractory mCRPC disease setting, after patients had progressed on NHA and taxanes. No new safety signals were identified in this study. Abemaciclib was well tolerated in this advanced patient population and demonstrated predictable toxicity, consistent with that previously observed in metastatic cancer (16, 17). Toxicities were generally low grade, manageable, and reversible with supportive care and dose adjustments. Altogether, the CYCLONE 1 findings are considered preliminary proof-of-concept and represent the first clinical validation of CDK4/6 as a therapeutic target in prostate cancer.
CDK4/6 inhibitors have transformed outcomes for patients with early-stage and metastatic HR+, HER2− breast cancer by significantly augmenting the clinical benefit of hormone therapy. Similar to the estrogen receptor in breast cancer, AR signaling, a key driver of mCRPC progression, elicits CDK4/6 activation to sustain prostate cancer cell uncontrolled proliferation and resistance to AR signaling directed therapies (1). Early attempts to evaluate the role for CDK4/6 inhibition in mCRPC were conducted with palbociclib monotherapy (NCT02905318) and ribociclib in combination with docetaxel (NCT02494921). These investigations demonstrated either no evidence of clinical activity or dose-limiting toxicities in the mCRPC setting. The phase 2 trial evaluating palbociclib monotherapy in previously treated mCRPC demonstrated a 0% ORR and no patients experienced any PSA decrease from baseline (18). The association of ribociclib and docetaxel in a similar mCRPC population demonstrated dose-limiting neutropenia and febrile neutropenia during the escalation portion, prompting a reduced and intermittent dosing schedule of the combination (17).
Abemaciclib is the only CDK4/6 inhibitor dosed continuously and approved as monotherapy based on its clinical activity in patients with heavily pretreated refractory HR+, HER2− metastatic breast cancer. Uniquely, continuous inhibition of CDK4/6 may prevent cell-cycle rebound and development of resistance that may lead to durable responses, particularly in patients with aggressive disease features and rapidly growing tumors.
The clinical activity in this late mCRPC setting may have been hampered by the heavily pretreated and advanced nature of the disease population that often presents with significant morbidity. In addition, there was a relatively high proportion of patients with visceral metastases, including to the liver, and almost all patients had objective radiographic disease progression at study entry, a clinical scenario associated with a very poor prognosis and short survival. Consistently, about a fifth of the patients discontinued study treatment early, before the first scheduled post-baseline imaging assessment, mostly due to death before progressive disease and subject withdrawals. Because of differences in the measurement intervals and event definitions, as well as the high censoring rate for time to PSA progression driven by confirmation requirements, the median time to PSA progression was slightly greater than the median rPFS time. The small sample size of this non-randomized study in a heavily pretreated mCRPC population also limits broad interpretation of the clinical activity observed for abemaciclib.
Nonetheless, the tumor responses observed in this study provide rationale for further exploration of abemaciclib in earlier disease settings. The opportunity to leverage the potential compounding effect of dual inhibition of the AR axis and CDK4/6 to maximize the therapeutic benefit and delay hormonal resistance and progression served as the basis for CYCLONE 2 (NCT03706365), a randomized, placebo-controlled, adaptive, phase 2/3 study of abiraterone with abemaciclib in first-line mCRPC. CYCLONE 2 expanded from phase 2 to 3 based on an adaptive interim efficacy analysis and triggered further interest in evaluating this drug combination in patients with high-risk metastatic hormone-sensitive prostate cancer in CYCLONE 3, a randomized, placebo-controlled, phase 3 trial (NCT05288166). Primary results from CYCLONE 2 and CYCLONE 3 are awaited to confirm whether, similar to augmenting ER signaling inhibition in hormonally driven breast cancer, abemaciclib will meaningfully augment the clinical benefit of AR signaling pathway inhibition.
Supplementary Material
Summary of patient disposition and list of study inclusion and exclusion criteria.
Kaplan-Meier curve of time to symptomatic progression.
List of study key endpoints and definitions.
List for representativeness of study participants.
Best overall response by RECIST v1.1 criteria without concurrent bone progression.
List of secondary endpoints and corresponding outcomes.
Post-discontinuation therapies.
Pharmacokinetic Summary.
Acknowledgments
This work was funded by Eli Lilly and Company. Medical writing was provided by Trish Huynh, employee of Eli Lilly and Company. Eli Lilly and Company contracted with Syneos Health for editorial support from Antonia Baldo. We thank the participants and their families or caregivers for participating in this trial. CYCLONE 1 would not have been possible without the investigators and their support staff who participated in this work.
This article is featured in Highlights of This Issue, p. 2295
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
N. Agarwal reports other support from Lilly during the conduct of the study; N. Agarwal also reports consultancy to Astellas, AstraZeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Gilead, Janssen, Merck, MEI Pharma, Nektar, Novartis, Pfizer, Pharmacyclics, and Seattle Genetics, as well as research funding to institution from Arnivas, Astellas, AstraZeneca, Bavarian Nordic, Bayer, Bristol Myers Squibb, Calithera, Celldex, Clovis, Crispr, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Gilead, GlaxoSmithKline, Immunomedics, Janssen, Lava, Medivation, Merck, Nektar, Neoleukin, New Link Genetics, Novartis, Oric, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda, and Tracon. D. Castellano reports other support from Pfizer, Roche, AstraZeneca, MSD, Merck, Novartis, Astellas, Johnson & Johnson, Ipsen, BMS, Bayer, and GSK outside the submitted work. T. Alonso-Gordoa reports grants and personal fees from Johnson & Johnson and Ipsen, as well as personal fees from Lilly, MSD, Eisai, Astellas, Bayer, Adacap, and Novartis during the conduct of the study. J.A. Arranz Arija reports personal fees from BMS, MSD, Astellas, Johnson & Johnson, Bayer, Ipsen, and Merck during the conduct of the study. E. Colomba reports grants from Ipsen, personal fees from Eisai, and other support from MSD outside the submitted work; E. Colomba also reports payment or honoraria for lectures, presentations, speakers bureaus or educational events (to institution) from BMS, GSK, AstraZeneca, Bayer, and Ipsen, as well as support for attending meetings and/or travel from MSD, Eisai, Ipsen, and BMS. L. Mourey reports personal fees and non-financial support from Sanofi, Astellas, Janssen, MSD, BMS, Ipsen, AstraZeneca, and Pfizer, as well as personal fees from Merck outside the submitted work. A. Fléchon reports personal fees and other support from Janssen, Astellas, Bayer, Novartis, Adacap, AstraZeneca, and Pfizer during the conduct of the study, as well as personal fees and other support from Gilead, Merck, MSD, Ipsen, and BMS outside the submitted work. M.T. Schweizer reports grants from Eli Lilly during the conduct of the study. M.T. Schweizer also reports personal fees from Sanofi and Fibrogen; grants and personal fees from AstraZeneca, Janssen, and Pfizer; and grants from Novartis, Zenith Epigenetics, BMS, Merck, Immunomedics, Hoffmann-La Roche, Tmunity, SignalOne Bio, Epigenetix, Xencor, Incyte, and Ambrx outside the submitted work. E. Gallardo reports personal fees from Lilly during the conduct of the study. E. Gallardo also reports personal fees from Sanofi, Pfizer, Eisai, Recordati, BMS, Rovi, and GSK; grants from Janssen; grants and personal fees from Astellas and Advanced Accelerator Applications; grants, personal fees, and non-financial support from Bayer and Ipsen; and personal fees and non-financial support from AstraZeneca and Leo Pharma outside the submitted work. E. Johnston reports other support from Eli Lilly & Company outside the submitted work. A. Balar reports other support from Eli Lilly during the conduct of the study. N. Haddad reports grants and other support from Eli Lilly and Company outside the submitted work. K. Nacerddine reports other support from Eli Lilly during the conduct of the study; other support from Eli Lilly outside the submitted work; and a patent for WO/2023/114264 pending. J.M. Piulats reports personal fees from Lilly during the conduct of the study. J.M. Piulats also reports grants and personal fees from BMS, MSD, Pfizer, and Immunocore; grants and non-financial support from BeiGene; grants from Mirati and Regeneron; and personal fees from Janssen, Astellas, and AstraZeneca outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
N. Agarwal: Conceptualization, data curation, formal analysis, investigation, visualization, methodology, writing–review and editing. D. Castellano: Conceptualization, data curation, formal analysis, investigation, methodology, writing–original draft, writing–review and editing. T. Alonso-Gordoa: Investigation, writing–review and editing. J.A. Arranz Arija: Investigation, writing–review and editing. E. Colomba: Data curation, investigation, writing–review and editing. G. Gravis: Data curation, investigation, writing–review and editing. L. Mourey: Data curation, writing–review and editing. S. Oudard: Data curation, formal analysis, investigation, writing–review and editing. A. Fléchon: Data curation, formal analysis, investigation, writing–review and editing. M. González: Data curation, writing–review and editing. P.M. Rey: Data curation, writing–review and editing. M.T. Schweizer: Data curation, investigation, writing–review and editing. E. Gallardo: Data curation, writing–review and editing. E. Johnston: Conceptualization, formal analysis, investigation, methodology, writing–review and editing. A. Balar: Formal analysis, investigation, writing–original draft, writing–review and editing. N. Haddad: Writing–original draft, writing–review and editing, interpretation of data for the work. A.K. Appiah: Formal analysis, writing–original draft, interpretation of data for the work. K. Nacerddine: Formal analysis, investigation, methodology, writing–original draft, writing–review and editing. J.M. Piulats: Investigation, writing–review and editing.
References
- 1. Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res 2006;66:7783–92. [DOI] [PubMed] [Google Scholar]
- 2. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov 2022;12:31–46. [DOI] [PubMed] [Google Scholar]
- 3. Balk SP, Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl Recept Signal 2008;6:e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knudsen ES, Wang JY. Dual mechanisms for the inhibition of E2F binding to RB by cyclin-dependent kinase-mediated RB phosphorylation. Mol Cell Biol 1997;17:5771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hortobagyi GN, Stemmer SM, Burris HA, Yap Y-S, Sonke GS, Hart L, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med 2022;386:942–50. [DOI] [PubMed] [Google Scholar]
- 6. Sledge GW Jr, Masakazu T, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: a randomized clinical trial. JAMA Oncol 2020;6:116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med 2020;382:514–24. [DOI] [PubMed] [Google Scholar]
- 8. Im S-A, Lu Y-S, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. New Engl J Med 2019;381:307–16. [DOI] [PubMed] [Google Scholar]
- 9. Pal SK, Patel J, He M, Foulk B, Kraft K, Smirnov DA, et al. Identification of mechanisms of resistance to treatment with abiraterone acetate or enzalutamide in patients with castration-resistant prostate cancer (CRPC). Cancer 2018;124:1216–24. [DOI] [PubMed] [Google Scholar]
- 10. Torres-Guzmán R, Baquero C, Ganado MP, Marugán C, Bian H, Zeng Y, et al. Abstract 4850: targeting prostate cancer with the CDK4 and CDK6 inhibitor abemaciclib. Cancer Res 2020;80:4850. [Google Scholar]
- 11. Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in vivo cell-cycle–dependent/independent antitumor activities alone/in combination with gemcitabine. Invest New Drugs 2014;32:825–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Torres-Guzman R, Calsina B, Hermoso A, Baquero C, Alvarez B, Amat J, et al. Preclinical characterization of abemaciclib in hormone receptor positive breast cancer. Oncotarget 2017;8:69493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rugo HS, O'Shaughnessy J, Boyle F, Toi M, Broom R, Blancas I, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: safety and patient-reported outcomes from the monarchE study. Ann Oncol 2022;33:616–27. [DOI] [PubMed] [Google Scholar]
- 14. de Wit R, de Bono J, Sternberg CN, Fizazi K, Tombal B, Wulfing C, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med 2019;381:2506–18. [DOI] [PubMed] [Google Scholar]
- 15. Chigutsa E, Kambhampati SRP, Karen Sykes A, Posada MM, van der Walt JS, Turner PK. Development and application of a mechanistic population modeling approach to describe abemaciclib pharmacokinetics. CPT Pharmacometrics Syst Pharmacol 2020;9:523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rugo HS, Huober J, Garcia-Saenz JA, Masuda N, Sohn JH, Andre VAM, et al. Management of abemaciclib-associated adverse events in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: safety analysis of MONARCH 2 and MONARCH 3. Oncologist 2021;26:e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Kouchkovsky I, Rao A, Carneiro BA, Zhang L, Lewis C, Phone A, et al. A phase Ib/II study of the CDK4/6 inhibitor ribociclib in combination with docetaxel plus prednisone in metastatic castration–resistant prostate cancer. Clin Cancer Res 2022;28:1531–9. [DOI] [PubMed] [Google Scholar]
- 18. Chi KN, Mukherjee S, Saad F, Winquist E, Ong M, Kolinsky MP, et al. Prostate cancer biomarker enrichment and treatment selection (PC-BETS) study: a Canadian cancer trials group phase II umbrella trial for metastatic castration–resistant prostate cancer (mCRPC). J Clin Oncol 2020;38:5551. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of patient disposition and list of study inclusion and exclusion criteria.
Kaplan-Meier curve of time to symptomatic progression.
List of study key endpoints and definitions.
List for representativeness of study participants.
Best overall response by RECIST v1.1 criteria without concurrent bone progression.
List of secondary endpoints and corresponding outcomes.
Post-discontinuation therapies.
Pharmacokinetic Summary.
Data Availability Statement
Eli Lilly and Company provides access, after anonymization, to all individual participant data collected during the trial, except for pharmacokinetic and genetic data. Data can be requested 6 months after the indication studied has been approved in the United States and European Union or after primary publication acceptance, whichever is later. No expiration date for data requests is set once the data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data-sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data-sharing environment. For details on submitting a request, see instructions at https://vivli.org.