Abstract
Ionizing radiation can have a wide range of impacts on tumor–immune interactions, which are being studied with the greatest interest and at an accelerating pace by the medical community. Despite its undeniable immunostimulatory potential, it clearly appears that radiotherapy as it is prescribed and delivered nowadays often alters the host's immunity toward a suboptimal state. This may impair the full recovery of a sustained and efficient antitumor immunosurveillance posttreatment. An emerging concept is arising from this awareness and consists of reconsidering the way of designing radiation treatment planning, notably by taking into account the individualized risks of deleterious radio-induced immune alteration that can be deciphered from the planned beam trajectory through lymphocyte-rich organs. In this review, we critically appraise key aspects to consider while planning immunologically fitted radiotherapy, including the challenges linked to the identification of new dose constraints to immune-rich structures. We also discuss how pharmacologic immunomodulation could be advantageously used in combination with radiotherapy to compensate for the radio-induced loss, for example, with (i) agonists of interleukin (IL)2, IL4, IL7, IL9, IL15, or IL21, similarly to G-CSF being used for the prophylaxis of severe chemo-induced neutropenia, or with (ii) myeloid-derived suppressive cell blockers.
Introduction
Radiotherapy (RT) is now a well-recognized promotor of immunomodulatory effects. Briefly, ionizing radiation can induce an “immunogenic cell death,” a phenomenon that promotes T cell–mediated immune response against antigens present in dying cells (1). Irradiated tissues elicit “damage‐associated molecular patterns” (DAMP)—that is, calreticulin translocation to the cell surface, activation of the cGAS/STING pathway, release of the nuclear protein HMGB1, and release of adenosine triphosphate—that directly contribute to the immunostimulant tumor–directed effects of RT (2–4). This process enhances the uptake of tumor‐derived antigens by antigen‐presenting cells (such as dendritic cells (DC) and macrophages), including the uptake of neoantigens from radiation-driven immunogenic mutations that are released after RT.
These observations have fueled innovative approaches to immuno-radiotherapy combinations, mostly with immune-checkpoint inhibitors. Those were strongly supported by the fact that 50% to 80% of patients do not respond at all to immune-checkpoint blockers administered alone (5), and all hopes and expectations have been pinned on finding interventions that will turn their disease into an immunoresponsive phenotype.
For years, RT has been a top candidate for pairing with immunotherapy. This has effectively culminated in the approval of the PD-L1 inhibitor durvalumab as a consolidation therapy after definitive platinum-based chemoradiation in unresectable stage III non–small cell lung cancer (NSCLC), with drastic improvements in both overall survival (OS) and progression-free survival (PFS) in the randomized PACIFIC trial (6, 7). In light of this, the recent setbacks of clinical trials exploring chemoradiation combined with anti-PD(L)1 therapy in locally advanced head and neck cancers (HNSCC) are intriguing and counterintuitive: three well-powered randomized trials (GORTEC-REACH, PembroRad, KEYNOTE-412) have unexpectedly failed to reach their primary endpoint when evaluating combined anti-PD(L)1 therapy and chemoradiation versus chemoradiation alone in locally advanced HNSCC (Table 1; refs. 8–11).
Table 1.
Characteristics of selected randomized trials evaluating chemoradiation with and without immune-checkpoint inhibition.
| Trial | Patient population | Standard of care (SOC) | Total RT dose | Immunotherapy intervention | Comparative arm | Outcome |
|---|---|---|---|---|---|---|
| PACIFIC (6, 7) | Locally advanced unresectable NSCLC (N = 709) | Platinum-based chemoradiation | 54–66 Gy | SOC + durvalumab (anti–PD-L1) 10 mg/kg every 2 weeks for up to 12 months, start 1–42 days after RT completion | SOC + placebo | mPFS 16.8 months (SOC-durva) vs. 5.6 months (SOC-placebo); HR 0.52; 95% CI, 0.42–0.65; P < 0.001—Response rate 28.4% (SOC-durva) vs. 16.0% (SOC-placebo); P < 0.001 |
| GORTEC-REACH (9) | Locally advanced unresectable HNSCC (N = 707) | Cisplatin- or cetuximab-based chemoradiation | 70 Gy | Cetuximab-based chemoradiation + avelumab (anti–PD-L1) 10 mg/kg at day −7 and every 2 weeks during RT followed by avelumab for up to 12 months | SOC | Cisplatin-unfit patients: 2-year PFS rate 44% (Cetux-RT-Ave) vs. 31% (Cetux-SOC); HR 0.85; P = 0.15); 2-year OS rate 58% (Cetux-RT-Ave) vs. 54% (Cetux-SOC); HR 1.08, P = 0.69)/cisplatin-fin patients: 1-year PFS rate 64% (Cetux-RT-Ave) vs. 73% (SOC-cisplatin); HR 1.27, 0.83–1.93 |
| PembroRad (8, 10) | Locally advanced unresectable HNSCC (N = 707) | Cetuximab-based chemoradiation | 70 Gy | Radiotherapy + pembrolizumab (anti–PD-1) every 3 weeks during RT | SOC | 2-year PFS rate 40% (Cetux-RT) vs. 42% (Pembro-RT); HR = 0.83, 95% CI, 0.53–1.29, P = 0.41/2-year OS rate 55% (Cetux-RT) vs. 62% (Pembro-RT); HR = 0.83; 95% CI, 0.49–1.40; P = 0.49 |
| KEYNOTE-412 (11) | Locally advanced unresectable HNSCC (N = 804) | Cisplatin-based chemoradiation | 70 Gy | SOC + pembrolizumab (anti–PD-1) every 3 weeks during RT followed by pembrolizumab every 3 weeks for up to 14 cycles | SOC + placebo | 2-Year EFS rate 63.2% (SOC-pembro) vs. 56.2% (SOC-placebo); HR 0.83; 95% CI, 0.68–1.03; P = 0.0429 (superiority threshold = 0.0242)/2-year OS rate 77.9% (SOC-pembro) vs. 76.8 (SOC-placebo); HR 0.90; 95% CI, 0.71–1.15; P = NS |
Note: Whether immunotherapy was administered sequentially or concomitantly to chemoradiation is underlined.
Abbreviations: EFS, event-free survival; HR, hazard ratio; HNSCC, head and neck squamous cell carcinoma; (m)PFS, (median) progression-free survival; NS, nonsignificant; NSCLC, non–small cell lung cancer; SOC, standard of care.
Digging into the differences between locally advanced NSCLC and HNSCC as a starting point, we emphasize key elements that may have influenced the lack of significant benefit observed in HNSCC settings. The immuno-suppressive potential of radiotherapy appears to have a strong random component. Notably, lymphocytes are exquisitely sensitive to radiation as compared with other blood cells, a phenomenon that has been overlooked so far and that is presumed to influence concurrent immunotherapy outcomes. Studies reported that a high fraction of lymphocytes eventually undergoes cell death as an immediate consequence of radiation-induced DNA damages (assessed by γH2AX marker) and can die in interphase within a few hours after exposure to doses as low as 0.125 Gy, irrespective of intervening mitosis (12–14). By contrast, circulating monocytes, immature dendritic cells and monocyte-derived macrophages show low levels of radiation-induced cell death, with 0% to 10% of radiation-induced cell deaths observed 24 hours after 2 Gy delivered ex vivo (12). In patients receiving total body irradiation (TBI) prior to bone marrow transplantation (6 fractions of 2 Gy in 3 consecutive days), the count of circulating lymphocytes drastically drops by about 95% at one day out from 2 fractions of 2 Gy, whereas the count of monocytes declines by about 50% and the counts of neutrophils and total leukocytes transiently increase, reflecting their radioresistance in vivo (12).
Accordingly, RT-induced lymphopenia is a frequent adverse event related to radiation, observed across all tumor types, that often lasts several months after completion of radiotherapy, and that directly affects survival outcomes (15–19). The severity, kinetics, and duration of RT-induced lymphopenia have been linked to significantly poorer PFS and OS among patients treated with radiotherapy. In a meta-analysis pooling, 21 cohorts with a total of 5,383 patients treated with RT and in which the incidence of grade ≥3 radiotherapy-induced lymphopenia ranged from 14% to 87%, grade ≥3 lymphopenia had a detrimental effect on pan-cancer OS [pooled adjusted hazard ratio (aHR), 1.65; 95% confidence interval (CI), 1.43–1.90; ref. 20), with a risk even more pronounced when RT-treated sites were located in the brain, thorax, and upper abdomen.
Here, we extract a comprehensive picture of what will be necessary to adjust in next-generation radiotherapy planning to take an immunologically fitted shape, maximizing both local and systemic radiation-triggered antitumor effects. We consider different aspects of radiation's influence on immune cells, balanced with what is presumed to be needed for immuno-refractory disease states to be moved toward immune-responsiveness. Finally, we introduce the concept of lymphocyte-sparing artificial intelligence (AI)–guided radio-immunotherapy and we present the design of a multifeatured ongoing project that aims at leveraging immunologically fitted radiotherapy to a clinically operational level.
The Physiologic Journey of Lymphocytes
To fully appreciate the potential of affecting the immune response in a patient undergoing radiotherapy, one must have in mind the body map of lymphocytes’ distribution. Beyond the vascular compartment, several solid tissues are considered "lymphocyte-rich." Depending on the organ, the level of maturation and the relative proportion of each subtype of lymphocytes may substantially vary (Table 2), reflecting the natural histories of T, natural killer (NK), and B cells from genesis to death (21–30). These factors are important to weigh, because for the same dose and same volume, irradiating an organ enriched with T progenitors will not have the same impact as an organ with mature B cells, for example.
Table 2.
Relative abundance of lymphocytes within lymphocyte-rich tissues in humans, according to their maturation level.
| Repartition of median count within lymphocytes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T-cell subsets (% among T cells) | B-cell subsets (% among B cells) | |||||||||||
| % of Lymphocytes among total leukocytes | T cells | Progenitor or immature | CD4+ | CD8+ | B cells | Progenitor or immature | Memory | Plasma | NK cells | Ref. | ||
| Tonsil | NS | + | ∼ | ++++ | + | ++ | ∼ | + | +++++ | + | (21) | |
| Thymus | +++++ | +++++ | +++++ | + | + | ∼ | ∼ | ∼ | ∼ | + | (22, 23) | |
| Lymph node | +++++ | ++++ | ∼ | +++ | ++ | + | ∼ | ∼ | ∼ | + | (22, 23) | |
| Lung | ++ | +++ | ∼ | +++ | ++ | + | ∼ | ∼ | ++ | +++ | (24) | |
| Liver | + | ++ | ∼ | ++ | +++ | + | ∼ | ∼ | ∼ | +++ | (25, 26) | |
| Spleen | ++++ | ++ | ∼ | + | + | +++ | ∼ | ∼ | ∼ | + | (22, 27) | |
| Kidney | NS | +++ | ∼ | +++ | +++ | + | ∼ | ∼ | ∼ | ++ | (23, 28) | |
| Gut (Peyer) | NS | +++ | ∼ | ++++ | + | +++ | + | + | ∼ | + | (23, 29) | |
| Blood | ++ | ++++ | ∼ | +++ | + | ++ | ∼ | +++ | +++ | + | ||
| Bone marrow | + | +++ | +++ | ++ | ++ | + | +++++ | ∼ | ∼ | + | (30) | |
Note: Green columns indicate the relative abundance of the main subpopulations of T or B lymphocytes among the complete cohorts of T or B cells (black columns).
Abbreviations: Negligible fraction, ∼; <20%, +; (20%–40%), ++; (40%–60%), +++; (60%–80%), ++++; >80%, +++++; NS, not specified.
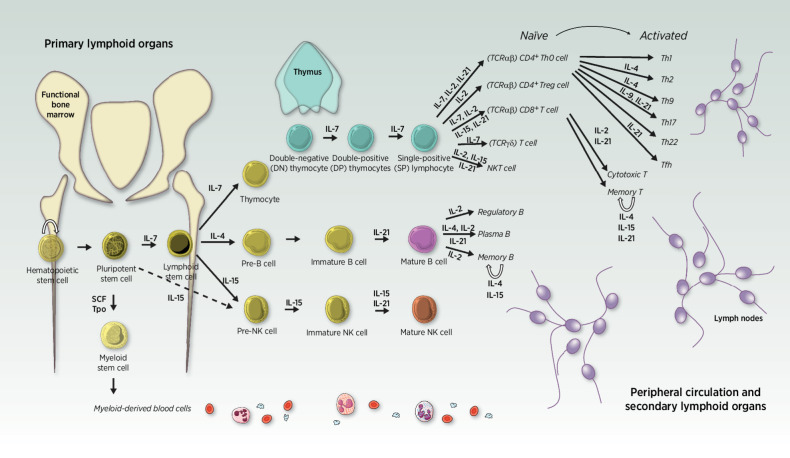
Hematopoietic stem cells will preferably differentiate to become the earliest multilymphoid progenitors upon stimulation by interleukin (IL)-7 (31). From that point, common lymphoid progenitor cells can continue dividing and eventually differentiate themselves to become thymocytes (“pre-T” cells), pre-B cells, or pre-NK cells, dedicated progenitors of the three main types of lymphocytes. Importantly, the differentiation pathway into which progenitors are guided depends on the chemokine landscape composition that surrounds them at key moments of their differentiation journey (Fig. 1). Importantly, within each subtype of mature lymphocytes, multiple states of activation (from naïve to terminally exhausted) and multiple landscapes of functions can be discriminated, some of which are linked to effective antitumor finality and others are rather immune-suppressive and responsible for “runaway reactions.”
Figure 1.
Lymphopoiesis differentiation pathways. Hematopoietic stem cells and progenitors are mostly found in the bone marrow of adults. Upon stimulation by surrounding cytokines, cells entering the lymphoid lineage will eventually migrate from primary (light yellow) to secondary lymphoid organs and peripheral circulation (light purple) while differentiating themselves into more mature phenotypes. Their differentiation fate depends on the cytokine composition of their microenvironment at every step of their journey. Thymocytes rapidly migrate from the bone marrow to the thymus gland, where they undergo maturation in an antigen-free environment, followed by a drastic positive and negative selection during which T cells that are reactive to self-peptides are removed from the pool. Ultimately, the 2% competent survivors leave the thymus as differentiated T cells. They predominantly reach the peripheral circulation as one of the two main T subtypes, characterized by the expression on their cell surface of either CD4 or CD8, respectively, categorizing them as “helper” or “cytotoxic” lymphocytes, historically. Of note, several other types of T cells exist and are rather considered “innate-like” (as opposed to the adaptive immune capacities of T CD4+, T CD8+, and B cells), such as γδ T cells, mucosal-associated invariant T cells or NK T cells (that are different from NK cells), although they each account for less than 2% of circulating lymphocytes. Back to the bone marrow, common lymphoid progenitors can also differentiate into pre-B cells or pre-NK cells, respectively, upon IL4 or IL15 predominant exposure. Pre-B cells mature in the marrow until they develop a functional B-cell receptor (BCR), then migrate to a secondary lymphoid organ (such as the spleen) to upgrade their maturation against self-antigens before reaching the periphery (55). Similarly, pre-NK cells develop within the bone marrow, although it remains unclear whether they need to enter secondary lymphoid organs to achieve full maturation (characterized by the expression of the CD56 on their cell surface). For now, the privileged hypothesis is that CD56+ NK cells can mature in secondary lymphoid organs but those are not absolutely required to complete their education, which is continuously upgraded throughout their lifetime. IL, interleukin; SCF, stem cell factor; Tpo, Thrombopoietin
The Physiopathology of Lymphocytes in Response to Chronic Inflammatory Stimuli
As the body gets older, mature lymphocytes endure a particular type of aging that is referred to as “immunosenescence” and is linked to their cumulative solicitation over their lifetime. Quantitatively, physiologic immunosenescence implies a change in the relative proportions of lymphocytes in peripheral blood, which is at least partly linked to the physiologic decrease of IL7 secretion with age and subsequent involution of the thymus (32). A study performed on 962 healthy blood donors ages 20 to 95 years showed that the counts of peripheral T cells and B cells were both significantly decreased in individuals ages over 60 years compared with younger donors (both P < 0.001), whereas the count of NK cells was significantly increased in the population over 60 (P < 0.001; ref. 33). The decrease in T cells was rather linked to the CD4+ subset and interestingly, the CD8+ count was found unchanged upon aging in this study. As another direct consequence of IL7 level decline, the fraction of myeloid-derived cells and the neutrophil-to-lymphocyte ratio (NLR) increase with age (34). In patients with cancer, a large meta-analysis of over 17,000 cases found no correlation between (chronological) age and efficacy of immune-checkpoint inhibitors (35).
Qualitatively, aging is linked to a sharp decline in lymphocytes’ function. Immunosenescence hallmarks gather the features of highly educated lymphocytes and also those of lymphocytes that would have also completely lost their capacity to proliferate (36, 37). Phenotypically, this implies (i) both the loss of expression of CD28 and CD27 cell-surface markers in T cells (33, 38, 39) as seen in advanced differentiation and (ii) the concomitant increase of KLRG1 expression and decrease of TCF1, which together reflect the cells’ loss of proliferative capacity (40–44).
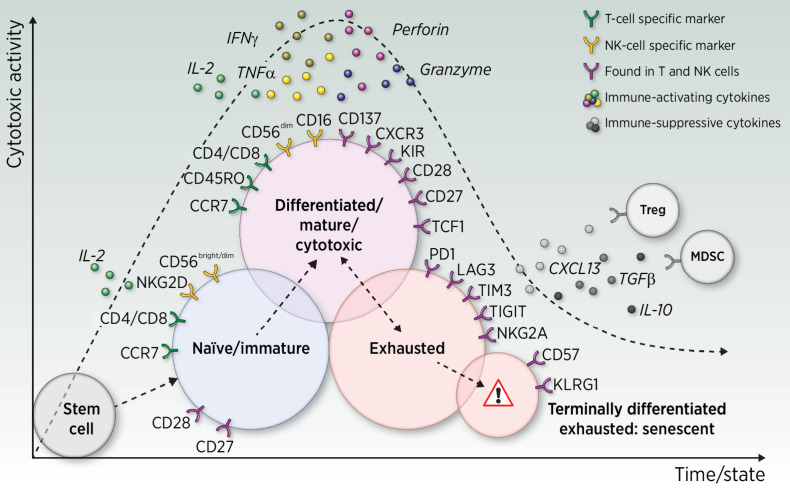
In NK cells—which are characterized by either a CD56dimCD16bright phenotype (mostly circulating NKs), or a CD56brightCD16dim (mostly tissue-resident NKs)—the CD56bright phenotype is almost never observed in senescent cells while CD56dim can be observed at all states, including senescence (45–47). Both T and NK cells can express high levels of CD57 and KLRG1, markers which are linked, respectively, to a late-differentiated state and an impaired proliferative ability in response to stimuli (Fig. 2; refs. 46–49).
Figure 2.
Panels of representative surface markers found on T and/or NK cells according to their actionable state. Single arrows indicate that the cell's progress toward the next state is believed to be irreversible, whereas the double-headed arrow indicates possible reversion, for example, upon exposure to immune-checkpoint inhibitors. Treg, regulatory T cell; MDSC, myeloid-derived suppressive cell.
As a direct consequence of aging, lymphocytes lose their capacity to release cytokines in response to danger (infection, cancer) as they reach a senescent state. Aged NK cells show a significantly reduced ability to secrete lytic molecules required for their target cell suppression, including perforin and granzyme enzymes (50, 51). In addition, secretion of IL2—a key pleiotropic cytokine mainly produced by T cells—shows quantitative decreases of 20% to 90% with age (52), which directly impairs the capacity of NK and T cells to induce cytotoxicity in the cells they monitor (47).
In the case of cancer, an intermediate state broadly called “lymphocytes exhaustion” has been described and is believed to occur after several weeks of chronic exposure to tumor-associated antigens (TAA; ref. 53). With respect to what is known about the lymphocyte's journey upon carcinogenesis, exhaustion is presumed to exist between the “mature and activated” state and the senescent state (Fig. 2). Therefore, it appears to be an exaggerated and abnormally sustained negative feedback loop that T and NK cells may develop when chronically overexposed to TAA stimuli, leading to unavoidable chronic overinflammation.
Exhaustion seems mostly driven by the TOX pathway that, when overactivated, leads to a weakening of effector functions of lymphocytes and to the parallel upregulation of multiple inhibitory receptors on their surface, such as PD1, TIM3, LAG3, CTLA4, and TIGIT (42, 54–56). Schematically speaking, exhausted populations may evolve toward two possible outcomes: they either cross the senescence barrier by progressively and irreversibly losing their capacity for cytotoxic cytokine release and their proliferative ability (42) or may be driven back to an activated state, for example, when exposed to an immune-checkpoint inhibitor.
Lymphopoiesis upon Radiation Exposure
The effects of radiation on lymphocytes depend on the dose received and the timing of exposure, with differences observed among lymphocytes’ subpopulation groups and according to their anatomic location at the time of exposure. In 2023, H. Paganetti summarized lymphocyte radiosensitivity studies performed in vitro and in vivo in humans and in mice, which all consistently indicated that B lymphocytes are the most radiosensitive subtype, followed by T and NK cells, with helper T cells (CD4+) being more radiosensitive than cytotoxic T cells (CD8+; ref. 14). Evidence also suggests that naïve lymphocytes are more radiosensitive than educated ones, whereas CD34+ progenitor circulating counts remain relatively stable after 2 fractions of 2 Gy in patients undergoing TBI prior to bone marrow transplantation (57, 58).
For doses received that are greater than 1 Gy, one can expect severe DNA and protein damage that often leads to cell death (59–61). For lower doses, it has been observed that exposed T cells that survive exposure to radiations might reroute their phenotype toward a more differentiated state and possibly until reaching a compromised immunosenescent state—resembling an “acute aging” phenomenon—with a more pronounced effect observed following low radiation doses in the range of 0.01 –0.1 Gy, as first observed in atomic bomb survivors who experienced accidental TBI (62–65). This can be explained by the known tendency of mature lymphocytes to become less active following chronic exposure to stress, given that the radio-induced generation of reactive oxygen species, DNA damages, chromosomal aberrations, and telomere shortening all represent important levels of cell stress (53, 66, 67). The timing of exposure relative to the cell cycle is also important, as lymphocytes that are exposed to radiation during their proliferation phase are more sensitive to irreparable DNA damage than those that are not actively dividing (61).
In the case of patients who are candidates for immuno-radiotherapy treatment, it is not yet fully understood whether the net effects are positive or negative. For example, a gene set enrichment analysis performed on RNA sequencing data of circulating lymphocytes collected before and after RT in patients treated for prostate cancer revealed an upregulation of CD28 expression and PD1 signaling (68), suggesting that RT can direct lymphocytes toward a state amenable to efficient anti–PD-1 treatment as the CD28 costimulatory signaling is known to be essential for effective anti-PD(L)1 therapy (69, 70). However, this may be countered by the concomitant upregulation of the KLRG1 and KLRC1 senescence markers (68, 71, 72). Surviving lymphocytes exposed to radiation displayed significantly activated DNA damage response pathways (68), the high burden of endogenous DNA damage being a direct contributor to senescence induction (73).
From the immuno-stimulatory perspective, radiation-driven tumor lysis also affects lymphocytic fate. In a prospective study on 89 patients with treatment-naïve NSCLC, the characterization of circulating T-cell populations before and after stereotactic ablative RT (SABR) revealed that despite the significant decrease of lymphocyte counts, surviving CD4+ T cells were rerouted toward a more activated “helper” state and the proportion of circulating FOXP3+ (“regulatory”) immunosuppressive CD4+ (Treg) cells was significantly decreased posttreatment (74). However, the impact of RT on Tregs is controversial, and it remains unclear whether observations of circulating cells truly reflect what happens within the tumor microenvironment (75, 76). The proportion of CD8+ T cells as compared with the whole lymphocyte pool seems increased after RT both in the tumor and in the periphery even though their total count is diminished (74, 76, 77), suggesting that RT generates a condition favoring expansion and effector functions of surviving T cells.
In addition, RT-driven immunogenic cell death is accompanied by the increased release of proinflammatory cytokines, such as CXCL10 and CXCL16, which together with the RT‐promoted process of vascular normalization, can enhance the subsequent tumor infiltration with activated CD8+ cells (78, 79). Thus, newly recruited T cells can contribute to RT efficacy within the irradiated field via a de novo RT-driven guidance of cytotoxic lymphocytes (80). RT also enhances the expression of major histocompatibility complex‐I molecules favoring antigen presentation (81) and therefore possible tumor recognition by immune cells at distant sites. Such abscopal effects are rare but documented in metastatic patients treated with focal RT alone who experience distant response in nonirradiated lesions (4, 82).
Altogether, there is growing evidence that RT can lead lymphocytes toward a phenotype that can be effectively targeted by PD(L)1 blockade (among other immune-checkpoint inhibition strategies), whereas it can also have systemic immune activation properties by its own and also bring about lymphosenescence that implies failure of proliferating enough to raise a well-dimensioned antitumor response. One of the main challenges of next-generation radiotherapy will therefore be to limit the irretrievable crossing of mature lymphocytes to a terminally senescent state, which most likely could be achieved by constraining the dose delivered to lymphocytes during RT.
The Optimal Radiotherapy Settings
Beyond the fact that RT is often accompanied by concurrent cytotoxic chemotherapy that also affects negatively blood cell counts, several aspects of RT settings have been proven to significantly influence the risk of developing radio-induced lymphopenia (83), including the radiation delivery technique, the radiation schedule and the clinical target volume (CTV) choice that is deemed to encompass subclinical microscopic malignant disease.
Briefly and implicitly, the less irradiated are the target-surrounding healthy tissues, the less significant the RT-related side effects, including decreases in lymphocyte counts. Accordingly, proton therapy has been shown to reduce the risk of RT-induced toxicity, including lymphopenia, as compared with modulated photon treatment (IMRT, VMAT) in several indications, including esophageal, brain, and NSCLC (84–88). Using protons, one can expect a 30% to 71% reduction of the risk of developing grade 4 lymphopenia, which could be explained by smaller irradiated volumes and by their radiobiological properties (89). Importantly, however, proton therapy has been shown to cause lymphocyte cell death by necrosis more intensely than do X-ray photons (89). Even though chromosomal aberrations in lymphocytes of patients treated with oral brachytherapy have been described in the 1980s (90), few studies have reported the lymphotoxicity potential of brachytherapy, which could be considered lymphocyte sparing due to its localized action using radiation sources with short path lengths.
In addition, novel unconventional approaches such as spatially fractionated radiotherapy including GRID, LATTICE, PATHY, and minibeam/microbeam technics could also theoretically maintain tumor control effectiveness while increasing normal tissue-dose tolerance (91). Those technics use grids or tiny collimated beams to create a heterogenous pattern of hot spots and cold spots within the tumor target, with the aim of being able to safely deliver higher radiation doses per fraction. The impact on the incidence of lymphopenia remains to be explored but spatially fractionated radiotherapy is presumed to combine favorably with immune-checkpoint inhibitors (92), suggesting enhanced immune fitness compared with homogenous beam distribution. One hypothesis—mainly built on nonclinical experiments—is that the heterogeneous peak and valley dose deposition may induce higher levels of tumor immunologic cell death that can propagate to underirradiated tumor cells in the vicinity through by-stander effects while relatively sparing the regionally circulating immune effector cells (93, 94). The massive release of TAA in peak valleys is presumed to foster tumor infiltration by lymphocytes, assuming the maintenance of their actionability at this stage of the disease, which may be the source of considerable interpatient disparities in terms of immune response.
A greater number of RT fractions has also been linked to a significantly increased risk of lymphopenia, implying that stereotactic body radiotherapy (SBRT), and more broadly hypofractionated regimens, appear less lymphotoxic than conventional fractionated RT in several tumor types, with and without concomitant chemotherapy (95–100). The biological explanation underlying this observation remains unclear; valid hypotheses include that fewer fractions may lead to diminished exposure of blood and lymph circulating lymphocytes and also that hypofractionation involves radiation target volumes that are typically much smaller than with conventional fractionation. In line with this, stereotactic ablation of one or several tumors in the metastatic setting combined with immunotherapy has shown promising efficacy results, for example, in NSCLC and melanoma (101), suggesting that a reduced lymphotoxic effect may contribute to the post-RT induction of an effective antitumor immunosurveillance. The optimal timing and the tumor lesion(s) target selection remain to be defined (102). Pushing hypofractionation to its limit, the upcoming clinical evaluation of ultra-high dose rate FLASH radiotherapy that should allow delivering several Gy in less than a tenth of a second may limit the volume of circulating blood entering the radiation field (103).
Currently, lymphocyte-rich tissues are not all considered organs at risk (OAR) for routine RT planning, which suggests that volume delineation, radiation beam trajectory, and dose-distribution improvements can be achieved toward a more lymphocyte-sparing delivery of radiation. This would involve a drastically complex change in the way RT is routinely prescribed, mostly because by nature, lymphocytes can be found almost everywhere within the body, making complete avoidance of lymphocytes impossible for external radiation beams. However, there are areas enriched in lymphocytes that would constitute relevant “immune organs at risk” (iOAR), mostly in primary and secondary lymphoid organs, that is, tissues in which lymphocytes are developed, matured (primary), and then activated and possibly solicited (secondary; ref. 104). For instance, as compared with the blood where the lymphocyte normal concentration range is 1 to 4 G/L, lymph nodes contain approximately 190 G/L of lymphocytes, and organs such as the bone marrow, thymus, kidney, and gut are estimated to contain 50 to 70 G/L of lymphocytes, each (105).
In locally advanced HNSCC, patients usually receive 70 Gy over 6.5 weeks, which leads to severe (grade ≥3 according to CTCAE V5) lymphopenia in 73% to 88% of cases according to studies (106). Elective lymph node irradiation (ENI) is a mainstay in locally advanced HNSCC, which in practice translates into larger radiation volumes that encompass lymph nodes where lymphocytes are 100 times more concentrated than in the peripheral blood circulation (105). Studies showed that in mice and in humans, ENI ablates the systemic immune response to combined radiation and immunotherapy by reducing tumor-antigen–specific T-cell priming, expansion, and capacity to infiltrate the tumor (107, 108). Importantly, in Darragh and colleagues, they failed to reduce the risk of regional recurrence when irradiating only the primary tumor, whereas none of the mice treated with ENI developed regional metastases, similar to what is observed in HNSCC patients (109). Altogether, their data support delayed surgical resection of sentinel lymph node 3 to 6 weeks after completing neoadjuvant immunotherapy and RT to allow priming and expansion of tumor-antigen–specific T cells in lymph nodes prior to their removal. According to their data, delayed neck resection was a better option than (i) “sterilizing” ENI, (ii) upfront surgery followed by immunotherapy, or (iii) immediate surgery after immunotherapy. Of course, this observation would warrant clinical validation with randomized trials in the settings for which there is a rationale to combine RT and immunotherapy. This would need to be balanced with the potential risk of delaying an effective and needed treatment.
In contrast with HNSCC, in locally advanced NSCLC, deescalating efforts have been made to optimize subclinical dose reduction, such as with 18F-FDG PET/CT-driven omission of ENI. The PET-Plan study demonstrated that in patients with inoperable stage II–III NSCLC receiving chemoradiation, the risk of locoregional progression in patients who had an 18F-FDG PET–based RT planning was noninferior to—and almost statistically lower than, with a hazard ratio of 0.57 (95% CI, 0.30–1.06)—patients who had systematic ENI (110, 111). In this situation, involved-field irradiation has become the standard of care in NSCLC with a low risk of isolated nodal relapse and presumably a minimized impact on immunity.
Altogether, several variables that may influence the risk of radio-induced lymphotoxicity to a certain extent are presumably adjustable. One can add to those the considerable room that exists for reducing uncertainties in gross tumor volume (GTV) detection, for example, by exploiting AI to drastically reduce uncertainties in areas in which the human eye is incapable of highly precise tumor-healthy tissue perception (112). Emerging fields such as digital pathology (113) can be effectively used to drastically reduce margins, extracting valuable hidden information from CT-scans, MRI, or PET as if the tumor–healthy tissue boundary was observed under the microscope, which should significantly improve tumor control while reducing radiation-induced side effects (114).
The Timing in Favor of Sequential Delivery and Early-Stage Disease
The placebo-controlled PACIFIC trial demonstrated that durvalumab (anti—PD-L1), when received as consolidation therapy after platinum-based chemoradiation, significantly improved PFS from 5.6 months to 16.9 months (HR 0.52; 95% CI, 0.42–0.65; P < 0.001) and OS from 29.1 months to 47.5 months (HR, 0.72; 95% CI, 0.59–0.89) in patients with locally advanced unresectable NSCLC (6, 7), irrespectively of the baseline PD-L1 tumor expression. Importantly in this trial, randomization (and durvalumab start) was possible 1 to 42 days after chemoradiation therapy completion, meaning that durvalumab has been initiated sequentially at least a couple of days after the last irradiation session. Inclusion criteria for the PACIFIC trial required patients to have displayed normal absolute counts of neutrophil, platelet, and red blood cells (inclusion criteria N°10), suggesting that patients experiencing radio(chemo)-induced hematologic toxicities at the time of screening were excluded, although lymphopenia was not specifically screened. In contrast, in HNSCC studies, the immune-checkpoint blocker was administered concomitantly and adjuvantly to radio/chemoradiotherapy.
In lung cancer, radio-immunotherapy combinations also triggered significantly better clinical outcomes in the early-stage setting, when compared with immunotherapy alone (115) and when compared with radiotherapy alone (116). Interestingly, Monjazeb and colleagues showed that in the early-stage lung disease treated with SBRT plus atezolizumab, the functional effector capacity of T cells declines following exposure to immunotherapy in patients who rapidly progress whereas it is boosted in patients who do not progress, independently from PD-L1 tumor expression (117). This is in line with the notion that terminally lymphosenescent immune systems fail to be reinvigorated after immune-checkpoint blockade with or without combined RT, which alter clinical outcomes in this population. Altogether, these data suggest that the less the disease is immunologically advanced (low fraction of terminally senescent effectors), the more are the chances of an effective response to radio-immunotherapy combinations, even in early tumor stages. Results from trials evaluating neoadjuvant, definitive, or adjuvant immunotherapy plus radio(chemo)therapy in early-stage HNSCC are awaited.
The Blood Values at the Time of Immunotherapy Initiation
According to the RTOG-9410 study results, the expected rates of acute grade 3 or worse granulocytopenia, leukopenia, and thrombocytopenia related to chemoradiation in locally advanced NSCLC are, respectively, around 82%, 84%, and 9% of patients (118). Therefore, it appears plausible that by choosing to use the highly common selection criterion of “adequate organ and marrow function …” in NSCLC patients who just completed chemoradiation, the PACIFIC trial investigators might have selected a population of patients more capable of blood cell regeneration. In other words, one could presume that bone marrows capable of rapidly compensating chemoradiation-induced blood cell loss—that is, patients with normal hematologic values immediately after 6 to 7 weeks of chemoradiation—might also be more likely to positively reengage a rapid and effective immune antitumor targeting upon anti-PD(L)1 blockade. In addition, patients who had progressed while undergoing chemoradiation therapy were excluded from the PACIFIC study (exclusion criterion N°7), which may have influenced immunotherapy outcomes compared with HNSCC settings in which this selection could not have been made.
In the PACIFIC study results published by Antonia and colleagues, the screening failure rate is not indicated, only is the fact that the protocol has been amended to enlarge the period of recruitment to 1 to 42 days after chemoradiation completion, instead of 1 to 14 days as planned (7). In the observational PACIFIC-R study, which follows NSCLC patients who received durvalumab consolidation therapy after chemoradiation, the median time to start of durvalumab from the end of RT was 56.0 days, with 30.1% of patients having started within 42 days (119). Median PFS was 21.7 months (95% CI, 19.1–24.5) and 2-year OS rate reached 71.2% (95% CI, 68.8–73.6) of patients, which align with another real-world study (120) and are even higher than outcomes observed in the intervention group in PACIFIC. Notably in the PACIFIC-R trial, 31.6% of patients were >70 years old at the time of inclusion and median PFS was similar among patients aged less than 70 years and those aged between 70 and 75 (22.8 and 22.4 months, respectively); however, it was shorter in patients over 75 (19.2 months).
Interestingly, the ongoing PACIFIC-2 trial (NCT03519971) is a randomized, double-blinded, and placebo-controlled study that is currently evaluating the concurrent administration of durvalumab and platinum-based chemoradiation in patients with locally advanced unresectable NSCLC. This trial will provide precious insight into the link between the onset of hematologic toxicity at the time of immunotherapy and effectiveness of immunotherapy in this context.
This notwithstanding, to our knowledge there is no available robust demonstration that would show that patients who are either rapidly recovering from severe chemoradiation-related hematologic toxicities or not experiencing them at all would represent a population of patients more prone to respond to immune-checkpoint blockers than the others; although, multiple studies reported that patients with low lymphocyte counts at baseline of an immune-checkpoint inhibition therapy had significantly shortened PFS and OS, including in NSCLC (121–123). Other baseline blood cell counts such as high neutrophils and high monocyte CD14low seem to be negatively correlated with OS in patients treated with immune-checkpoint blockers (124).
Chen and colleagues also observed that among patients undergoing combined immuno-radiotherapy, patients with a lymphocyte count above the median post-RT had a significantly higher risk of experiencing out-of-the-field tumor regression compared with patients with post-RT lymphocyte count below the median (abscopal response rates 34.2% vs. 3.9%, respectively; P < 0.0001; ref. 125). Post-RT lymphocyte count was also correlated with PFS and OS, suggesting that in this cohort of 162 patients affected with various tumor types at the metastatic stage including NSCLC, small cell lung cancer, HNSCC, and renal cell carcinoma, the way the immune system handled radiation had a significant impact on prognosis, similarly to what has been observed in response to immune-checkpoint blockers (117). In this situation, blood monitoring of the dynamic changes between before and after the onset of treatment seems highly relevant to anticipate any risk of treatment failure.
Optimizing Radiation Target Volumes: The iOAR Component
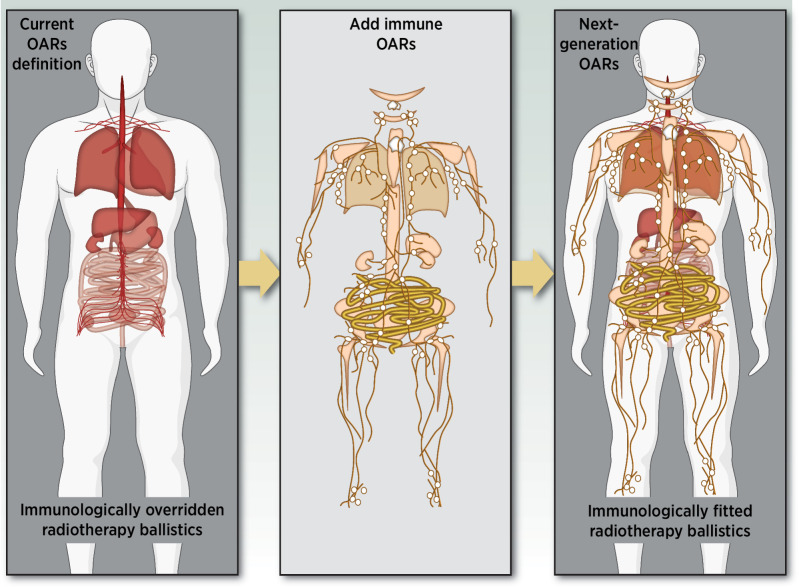
As stated above, lymphocytes can be found everywhere within the body, which technically makes complete lymphocyte-sparing an impossible challenge for RT planning with contemporary machines and external beams. However, every effort should be made to spare lymphocyte-rich tissues (including lymph nodes, large blood vessels, heart, spleen, bones containing red bone marrow, and thymus, especially in children) as much as possible as a new standard approach. Those structures, also called lymphocyte-related OAR (LOAR; ref. 126) or immune organs at risk (iOAR), mostly include conventional OAR that are already being considered at the treatment planning stage such as the heart, lung, aorta, and spinal cord (Fig. 3) but would add an additional level of constraints to weight their lymphocyte enrichment beyond the risk of alteration of tissue-specific cells.
Figure 3.
Schematic representation of organs at risk (red) currently considered for establishing the treatment plan for conventional radiotherapy planning in mirror with the schematic distribution of lymphocytes within the body (yellow). The intensity of the red color correlates with the value of the volume-dose constraints established by the stereotactic ablative body radiotherapy (SABR UK Consortium), version 6.1 (2019): the deeper the color, the more restricted is the volume-dose limit in Gy. Similarly, the intensity of the yellow color correlates with the estimated concentration of lymphocytes within a particular tissue. The stacked representation (right) combines conventional and lymphocyte-rich organs at risk for an immunologically fitted radiotherapy treatment planning.
Models have been developed to estimate the dose received by the pool of circulating blood cells while considering the blood flow [such as ED(R)IC models, effective dose (of radiation) to immune cells] with a high prognostic value for decreased OS and PFS (127–129). However, those models do not take into account the relative abundance of T and NK cells in each of the main lymphoid structures. They also underestimate the out-of-the-field dose despite the known effect of low doses on lymphocyte count and function. Indeed, the current treatment planning systems (TPS) used in clinical routine underestimate radiation doses delivered “far” from the target volumes and do not allow dosimetric evaluations of the doses outside the field of view of the planning CT. To fill this gap, analytical and Monte–Carlo models are currently being developed to accurately estimate out-of-the-field radiation doses delivered during treatment (130–133).
To date, the impact of EDIC computation on survival has only been assessed retrospectively in noninterventional studies and no randomized clinical trial has ever robustly evaluated the effects of adapting RT treatment plans to avoid lymphocyte-rich structures. To make it easily translatable to clinical routine, next-generation TPSs should be updated to include the possibility of considering iOAR for delineation, with predefined dose constraints (15).
The Myeloid–Lymphoid Balance in Response to Radiation
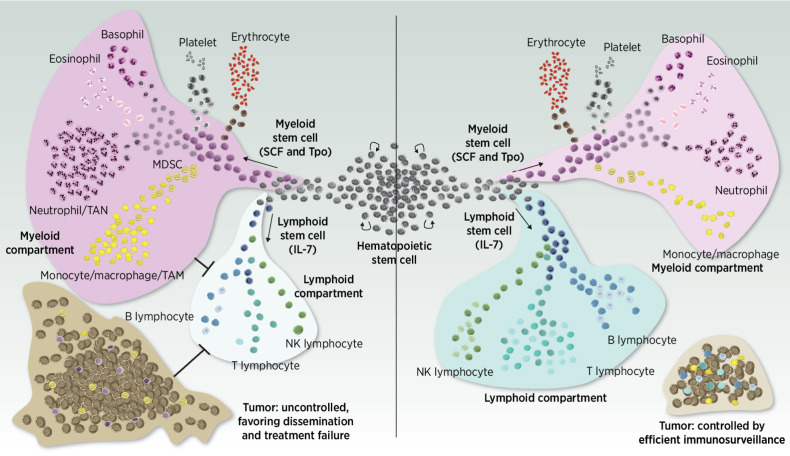
Immune systems being exposed to significant amounts of radiation can evolve toward a senescent condition that is characterized by a diminished IL7 production while stem cell factor (SCF). Thrombopoietin (TPO) levels are, however, maintained, and thus, the myeloid compartment became overrepresented at the expense of lymphoid cells (Fig. 4). This is compounded by the fact that myeloid cells are more radioresistant than lymphocytes, contributing to the increase of the NLR post-RT. Importantly, the NLR has been proposed as a biomarker of systemic inflammation, with a high NLR indicating an inflammatory reactionary phenotype with a negative impact on survival prognosis in patients with cancer (134–137).
Figure 4.
A balanced “ménage à trois” between the tumor (brown), lymphocytes (blue), and myeloid cells (purple) is crucial to achieve tumor control posttreatment and ensure the establishment of an efficient antitumor immunosurveillance in the long term. Reaching a disproportionate overrepresentation of the myeloid compartment at some point of the treatment journey is detrimental and feeds a vicious cycle in which innate and adaptive immune abilities are inhibited, a phenomenon that is complex to turn over.
More specifically, exposure to irradiation has been linked to the expansion of myeloid-derived suppressor cells (MDSC; refs. 138–140), which are a heterogeneous population of immature myeloid cells that have the ability to suppress tumor-directed immunity, including by inhibiting cytotoxic lymphoid activities. Their abundance has been linked to severe lymphopenia and to poor clinical outcomes in multiple tumor types (139, 141).
MDSCs are recruited to the tumor site by immunosuppressive chemoattractants produced by tumor cells themselves, but also by stromal cells and some subpopulations of lymphocytes that have reached an advanced activation state. In return, MDSCs have been shown to inhibit the function of cytotoxic lymphocytes and even inducing their apoptosis through the secretion of various factors such as IDO, arginase, nitric oxide, reactive oxygen species, and TGFβ, which induce T- and NK-cell anergy and drive the differentiation of CD4+ T cells to immunosuppressive Tregs (142–146).
MDSCs have also been shown to promote T-cell exhaustion (145), feeding a detrimental loop toward global immune inactivation within the tumor microenvironment. However, whether MDSC recruitment within the tumor is a cause or a consequence of tumor-infiltrating lymphocyte exhaustion upon exposure to radiation remains unclear. A relationship has been found between chemoradiation-induced lymphopenia and increased tumor infiltration of both G-MDSCs and M-MDSCs in patients treated for glioblastoma (139). In preclinical experiments performed in glioblastoma-bearing mice treated with RT, Ghosh and colleagues showed that tumor irradiation led to an aberrant myelopoiesis in the bone marrow and in the spleen and that pharmacologic inhibition of MDSCs using an arginase-1 inhibitor (CB1158) or a phosphodiesterase-5 inhibitor (tadalafil) concomitantly to RT abrogated radiation-induced lymphopenia and improved survival (139). Altogether, these data suggest that inhibiting MDSC could directly mitigate radio-induced lymphopenia and restore a myeloid-lymphoid balance in favor of an effective recovery of immunosurveillance.
A “ménage à trois” Requiring Pharmacologic Support to Reach Immunologic Fitness
Roughly speaking, what the medical community would ultimately target is a radiotherapy approach that is capable of (i) accurately identifying the GTV and associated CTV with an emphasis placed on the characterization of lymph node invasion and tumor cell transit through lymphatic channels to cover the whole tumor volume without undue effects on healthy tissues, while (ii) establishing an effective posttreatment tumor-directed immunosurveillance, and (iii) without favoring the myeloid compartment over lymphocytes, which are guarantors of the immunosurveillance. Therefore, the next generation of treatment planning must deal with a complex interplay between tumor cells, lymphocytes, and myeloid populations, a “ménage à trois” with multidimensional specific constraints requiring opposite actions (full dose or zero dose) and may, in some cases, be impossible to reach with the sole improvement of personalized dosimetry.
One part of such a treatment approach will be to attain a favorable myeloid-lymphoid balance that may be hit pharmacologically, via two main possible routes: either by promoting the proliferation, activation, and maintenance of cytotoxic lymphocytes, or by suppressing the deleterious influence of myeloid cells.
The first option would require taking advantage of clinically relevant derivatives of natural interleukins implicated in the progress of lymphopoiesis. As of mid-2023, IL2, IL4, IL7, IL9, IL15, and IL21—each with its specificities (Fig. 1)—have been shown to positively regulate the lymphocytic compartment toward a more tumor-directed cytotoxic state. Depending on the interleukin used, a multiple range of outcomes can be expected including numerical expansion of the CD4+ helper and/or CD8+ T cells and/or NK subpopulations, enhancement of their cytotoxic effector and/or their memory functions, and extension of their life expectancy (147–149).
Beyond aldesleukin—a recombinant IL2 approved in the early 1990s for the treatment of melanoma and kidney cancer with a narrow therapeutic window that restricts its use in practice nowadays—several agonists and superagonists of antitumor interleukins are currently under clinical development. The most advanced, N-803 (nogapendekin alfa inbakicept, aka NAI), is an IL15 superagonist consisting of an IL15 mutant linked to an IL15 receptor α/IgG1 Fc fusion protein. In 2022, N-803 received “Breakthrough Therapy” and “Fast Track” designations by the FDA for the treatment of BCG-unresponsive nonmuscle-invasive bladder cancer (150). Other promising compounds include SOT101 (nanrilkefusp alfa), another IL15 agonist that showed efficacy signals in combination with pembrolizumab in advanced solid tumors (151), and also THOR-707, ALKS 4230 (nemvaleukin alfa) and NKTR-214 (bempegaldesleukin, aka bempeg), all being IL2-derived drugs that have passed early-phase development, while over a dozen of other IL2-based proteins are currently being evaluated in phase I studies (152). Products targeting IL7 receptors also exist but have shown disappointing results so far in the treatment of cancer and are rather studied for the management of other inflammatory conditions, such as septic shock (153, 154).
As of mid-2023, none of these antitumor interleukins have been evaluated in combination with RT, despite a strong rationale and preclinical data in favor of positive impacts on outcomes when overcoming the radio-induced lymphopenia with IL15 agonism (155). The rapid development of this new pharmacologic class will help in better understanding the compatibility of toxicity profiles in the near future.
Another valuable approach for restoring immunologic fitness after RT would be to rather rely on the suppression of MDSC's deleterious effects. Multiple ways of targeting MDSCs have been described to date, including in the context of patients treated with radiotherapy (139, 156, 157). Briefly, either (i) MDSC can be functionally inactivated by inhibitors targeting phosphodiesterase-5 (PDE-5, such as tadalafil), arginine-1 (ARG-1), class 1 histone deacetylase (HDAC, mostly entinostat), IDO or CSF1R (139, 158–160), or (ii) their recruitment to tumor sites can be obstructed using chemotaxis blockers (e.g., targeting CCR2, CCR5, CXCL2, or CXCR2; refs. 156, 161), or (iii) they can be forced to differentiate and mature into an unharmful state using low doses of taxanes (docetaxel, paclitaxel; refs. 162, 163) or all transretinoic acid (ATRA; ref. 164), which is approved for the treatment of acute promyelocytic leukemia.
Several clinical studies have shown that targeting MDSCs in patients with cancer effectively leads to a decrease of the intratumor population of myeloid-suppressive cells, proportionally reflected by a decrease of MDSC and CD4+ regulatory T cells in peripheral blood (PBMC) and the concomitant increase of the CD8+ T cells population (139, 158, 165–168). More well-powered randomized studies evaluating standard of care with and without MDSC inhibition are warranted to establish the potential of this strategy both on preventing and reversing treatment-induced lymphopenia and on improving survival.
The advantage of this second option (combining RT with MDSC suppression) is that several compounds with MDSC inhibition potential are already available in the clinic, most of which are well tolerated, making them reliable candidates for combination trials. Among the compounds that have shown MDSC suppression activity and that are currently approved for their anticancer action are sunitinib (159), docetaxel (162), paclitaxel (163), and ATRA (164, 169). In addition, several drugs currently approved for nononcology indications have a significant suppressive effect on MDSC, including the PDE5 inhibitors tadalafil (139, 158, 165) and sildenafil (170) and the antiviral maraviroc (171).
Combining Next-Generation Volume Delineation and Dosimetry with Controlled Lymphocytemia
The last two decades have witnessed phenomenal progress in multiple fields related to the radiation oncology practice, including the introduction of new imaging sequences providing an enhanced definition of target volumes combined with improved spatial resolution and contrast, beam delivery precision, radio-immuno-biology knowledge, and above all, with the acknowledgment that AI-guided tools can be valuable assets to support physicians’ decisions for tasks that require a lot of time, resource, and energy.
As of now, none of these capabilities has been fully exploited. To our knowledge, the only ongoing project that aims at evaluating lymphocyte-sparing RT is conducted by the lymphocyte-sparing artificial intelligence-guided radio-immunotherapy (LySAIRI) research program (172), which is a 5-year effort that gathers internationally recognized cancer centers, research organizations, and a company that develops a TPS and AI-powered web-based solutions for the automatic contouring of anatomic regions from planning images of patients with cancer. The project is primarily focused on head and neck tumors (pharynx and larynx)—that is, a population of patients who are severely affected by high-grade treatment-induced lymphopenia and for whom improvement of post(chemo)radiation immunosurveillance may be key for achieving long-term response—with the ambition to pave the way for broader indications.
The LySAIRI project includes four main modules of optimization. The first is a virtual biopsy module that uses digital pathology to automatically detect the GTV from routine imaging with an accuracy that surpasses the human eye's competence. State-of-the-art linear accelerators that are now accessible almost at every RT center worldwide offer the ability to target tissue in a highly precise fashion and are endowed with embedded imaging capabilities that can allow treatment adaptation on the fly. The virtual biopsy module will thereby enable an accurate identification of the tumor contours and eventually lymphatic spread to fully individualize CTV definition.
The second module consists of developing operational dosimetry models for the computation of risks related to the radiation dose received by the immune compartment and more precisely, by lymphocytes. This module will enable both an AI-driven assessment of the out-of-field dose, using as input data the in-field dose estimated by the clinical TPS, and a calculation tool based on Markov chains to characterize the doses received by peripheral blood lymphocytes. The combination of the two systems will provide extensive new insights into the doses received by lymphocytes throughout the patient's body and will help to define new dose constraints for iOARs.
Treatment planning is time consuming, tedious, expertise-dependent, and imprecise due to the inability of existing solutions to encompass/account for (i) tumor characteristics, (ii) precise delineation and modeling of target volume, and (iii) fine-grained full-scale simulation and dose optimization. Consequently, existing treatment planning for more than 60% patients with cancer still relies on a two-decade-old principle that considers treatment as a “one tumor one dose distribution” objective, hampering efficiency while at the same time causing lifelong side effects and sequelae. This is due to two main limiting factors: the lack of straightforward clinical evidence for microscopic-level tumor irradiation and the lack of computationally efficient models that can handle treatment planning at high resolution. This aspect is addressed by the third module of optimization of the LySAIRI project, which focuses on developing turnkey solutions for a next-generation TPS that is accessible to all, which could homogenize practices across cancer centers and could offer a fully personalized treatment planning for each patient.
The fourth and last module of therapeutic optimization comprises a well-powered clinical evaluation with the ultimate goal to show that lymphocyte-sparing radiotherapy improves clinical outcomes and can have positive medico-economic impacts from perspectives of the social security system, the hospital and society more generally, both in terms of cost-effectiveness (survival) and of cost-utility (quality of life). This module will be used to assess the clinical efficacy of enhanced volume delineation and dosimetry with or without combined pharmacologic support aimed at reinforcing the cytotoxic lymphocyte population. The clinical proof-of-concept is expected by 2027 and will be performed in patients with stage II–IV squamous cell carcinoma of the oropharynx, larynx or hypopharynx, up to, but not crossing the midline.
Conclusion
Multiple reports have exposed radiotherapy as an undeniable and critical regulator of immune homeostasis, playing a paradoxical role fostering antitumor immunogenicity on the one hand and constraining the cytotoxic capacities of innate and adaptive immune systems on the other. Those aspects are all the more complex to evaluate when concomitant chemotherapy is given together with RT—cytotoxic chemotherapy also being highly hematotoxic—which is often the case in clinical practice. Although the blood toxicity of chemotherapy seems inevitable with most of the standard therapeutic arsenal, we estimate that there remains considerable scope for improvement on the immune effects of radiation. Here, we summarized the current knowledge supporting the evolution of radiotherapy practice toward a more immunologically fitted approach, which may improve the outcomes of radio-immunotherapy combinations. Several other research avenues including antigen-directed therapies such as antitumor vaccines (173) or CAR-T cells (174) and consideration of cancer-associated fibroblasts (175) and the tumor–nerve axis (176) may also influence the way RT will be envisaged in the years to come and will undoubtedly benefit from immunologically fitted radiotherapy.
Controlling the effects of radiation treatments on the immune system is an urgent medical need that should lead to significantly improved outcomes in patients for whom effective immunosurveillance can be maintained posttreatment. Fortunately, the technology has evolved in conjunction with this knowledge and now allows the community to actively develop and evaluate next-generation strategies that take into account the immune fitness of every medical intervention involving radiotherapy. Large-scale initiatives such as the LySAIRI project are paving the way for smooth integration into the clinical routine of lymphocyte-sparing radiotherapy, while taking full advantage of radio-immunotherapy combinations.
Acknowledgments
The authors gratefully thank Dr Baris Ungun for his careful proofreading and editing. This work was supported by the French National Research Agency (French ANR) within the FRANCE2030 investment plan (grant number ANR-21-RHU5-0005).
Authors' Disclosures
D. Morel reports grants from French ANR during the conduct of the study. C. Robert reports grants from Agence Nationale de la Recherche (ANR-21-RHU5-0005) during the conduct of the study; in addition, C. Robert has a patent for dosimetric tool for assessing lymphocytic irradiation pending. N. Paragios reports grants from Agence National de la Recherche during the conduct of the study as well as personal fees and other support from TheraPanacea outside the submitted work. No disclosures were reported by the other authors.
References
- 1. Wennerberg E, Vanpouille-Box C, Bornstein S, Yamazaki T, Demaria S, Galluzzi L. Immune recognition of irradiated cancer cells. Immunol Rev 2017;280:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 2014;41:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ 2018;25:486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deutsch E, Chargari C, Galluzzi L, Kroemer G. Optimising efficacy and reducing toxicity of anticancer radioimmunotherapy. Lancet Oncol 2019;20:e452–63. [DOI] [PubMed] [Google Scholar]
- 5. Sharma P, Hu-Lieskovan S, Wargo JA, RA. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017;168:707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. J Clin Oncol 2022;40:1301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–50. [DOI] [PubMed] [Google Scholar]
- 8. Bourhis J, Sire C, Tao Y, Martin L, Alfonsi M, Prevost JB, et al. LBA38 pembrolizumab versus cetuximab, concomitant with radiotherapy (RT) in locally advanced head and neck squamous cell carcinoma (LA-HNSCC): results of the GORTEC 2015-01 “PembroRad” randomized trial. Ann Oncol 2020;31:S1168. [DOI] [PubMed] [Google Scholar]
- 9. Bourhis J, Tao Y, Sun X, Sire C, Martin L, Liem X, et al. LBA35 avelumab-cetuximab-radiotherapy versus standards of care in patients with locally advanced squamous cell carcinoma of head and neck (LA-SCCHN): randomized phase III GORTEC-REACH trial. Ann Oncol 2021;32:S1310. [Google Scholar]
- 10. Tao Y, Biau J, Sun XS, Sire C, Martin L, Alfonsi M, et al. Pembrolizumab versus cetuximab concurrent with radiotherapy in patients with locally advanced squamous cell carcinoma of head and neck unfit for cisplatin (GORTEC 2015-01 PembroRad): a multicenter, randomized, phase II trial. Ann Oncol 2023;34:101–10. [DOI] [PubMed] [Google Scholar]
- 11. Machiels J-P, Tao Y, Burtness B, Tahara M, Rischin D, Alves GV, et al. LBA5 primary results of the phase III KEYNOTE-412 study: pembrolizumab (pembro) with chemoradiation therapy (CRT) vs placebo plus CRT for locally advanced (LA) head and neck squamous cell carcinoma (HNSCC). Ann Oncol 2022;33:S1399. [Google Scholar]
- 12. Heylmann D, Ponath V, Kindler T, Kaina B. Comparison of DNA repair and radiosensitivity of different blood cell populations. Sci Rep 2021;11:2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Falcke S, Rühle P, Deloch L, Fietkau R, Frey B, Gaipl U. Clinically relevant radiation exposure differentially impacts forms of cell death in human cells of the innate and adaptive immune system. Int J Mol Sci 2018;19:3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paganetti H. A review on lymphocyte radiosensitivity and its impact on radiotherapy. Front Oncol 2023;13:1201500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol 2018;123:42–51. [DOI] [PubMed] [Google Scholar]
- 16. Abravan A, Faivre-Finn C, Kennedy J, McWilliam A, van Herk M. Radiotherapy-related lymphopenia affects overall survival in patients with lung cancer. J Thorac Oncol 2020;15:1624–35. [DOI] [PubMed] [Google Scholar]
- 17. Taguchi A, Furusawa A, Ito K, Nakajima Y, Shimizuguchi T, Hara K, et al. Postradiotherapy persistent lymphopenia as a poor prognostic factor in patients with cervical cancer receiving radiotherapy: a single-center, retrospective study. Int J Clin Oncol 2020;25:955–62. [DOI] [PubMed] [Google Scholar]
- 18. Lin AJ, Gang M, Rao YJ, Campian J, Daly M, Gay H, et al. Association of posttreatment lymphopenia and elevated neutrophil-to-lymphocyte ratio with poor clinical outcomes in patients with human papillomavirus-negative oropharyngeal cancers. JAMA Otolaryngol Head Neck Surg 2019;145:413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jensen GL, Blanchard P, Gunn GB, Garden AS, David Fuller C, Sturgis EM, et al. Prognostic impact of leukocyte counts before and during radiotherapy for oropharyngeal cancer. Clin Transl Radiat Oncol 2017;7:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Damen PJJ, Kroese TE, van Hillegersberg R, Schuit E, Peters M, Verhoeff JJC, et al. The influence of severe radiation-induced lymphopenia on overall survival in solid tumors: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys 2021;111:936–48. [DOI] [PubMed] [Google Scholar]
- 21. Sada-Ovalle I, Talayero A, Chavéz-Galán L, Barrera L, Castorena-Maldonado A, Soda-Merhy A, et al. Functionality of CD4+ and CD8+ T cells from tonsillar tissue. Clin Exp Immunol 2012;168:200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kerbrat S, Vingert B, Junier M-P, Castellano F, Renault-Mihara F, Dos Reis Tavares S, et al. Absence of the adaptor protein PEA-15 is associated with altered pattern of Th cytokines production by activated CD4+ T lymphocytes in vitro, and defective red blood cell alloimmune response in vivo. Unutmaz D (ed): PLoS One 2015;10:e0136885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carrega P, Bonaccorsi I, Di Carlo E, Morandi B, Paul P, Rizzello V, et al. CD56brightPerforinlow noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J Immunol 2014;192:3805–15. [DOI] [PubMed] [Google Scholar]
- 24. Travaglini KJ, Nabhan AN, Penland L, Sinha R, Gillich A, Sit RV, et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 2020;587:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen Y, Tian Z. Innate lymphocytes: pathogenesis and therapeutic targets of liver diseases and cancer. Cell Mol Immunol 2021;18:57–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shi F-D, Ljunggren H-G, La Cava A, Van Kaer L. Organ-specific features of natural killer cells. Nat Rev Immunol 2011;11:658–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gualdrón-López M, Díaz-Varela M, Toda H, Aparici-Herraiz I, Pedró-Cos L, Lauzurica R, et al. Multiparameter flow cytometry analysis of the human spleen applied to studies of plasma-derived EVs from plasmodium vivax patients. Front Cell Infect Microbiol 2021;11:596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park J-G, Na M, Kim M-G, Park SH, Lee HJ, Kim DK, et al. Immune cell composition in normal human kidneys. Sci Rep 2020;10:15678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fenton TM, Jørgensen PB, Niss K, Rubin SJS, Mörbe UM, Riis LB, et al. Immune profiling of human gut-associated lymphoid tissue identifies a role for isolated lymphoid follicles in priming of region-specific immunity. Immunity 2020;52:557–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clark P, Normansell D, Innes D, Hess C. Lymphocyte subsets in normal bone marrow. Blood 1986;67:1600–6. [PubMed] [Google Scholar]
- 31. Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med 1994;180:1955–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol 2004;5:133–9. [DOI] [PubMed] [Google Scholar]
- 33. Sun H, Kang X, Chen X, Cai L, Li Y, Yu J, et al. Immunosenescence evaluation of peripheral blood lymphocyte subsets in 957 healthy adults from 20 to 95 years old. Exp Gerontol 2022;157:111615. [DOI] [PubMed] [Google Scholar]
- 34. Li J, Chen Q, Luo X, Hong J, Pan K, Lin X, et al. Neutrophil-to-lymphocyte ratio positively correlates to age in healthy population. J Clin Lab Anal 2015;29:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim CM, Lee JB, Shin SJ, Ahn JB, Lee M, Kim HS. The efficacy of immune checkpoint inhibitors in elderly patients: a meta-analysis and meta-regression. ESMO Open 2022;7:100577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quinn KM, Fox A, Harland KL, Russ BE, Li J, Nguyen THO, et al. Age-related decline in primary CD8+ T cell responses is associated with the development of senescence in virtual memory CD8+ T cells. Cell Rep 2018;23:3512–24. [DOI] [PubMed] [Google Scholar]
- 37. Goronzy JJ, Weyand CM. Mechanisms underlying T cell ageing. Nat Rev Immunol 2019;19:573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease: CD8+ CD28− (CD8+ CD57+) T cells in health and disease. Immunology 2011;134:17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vicente R, Mausset-Bonnefont A-L, Jorgensen C, Louis-Plence P, Brondello J-M. Cellular senescence impact on immune cell fate and function. Aging Cell 2016;15:400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1). Blood 2002;100:3698–702. [DOI] [PubMed] [Google Scholar]
- 41. Borys SM, Bag AK, Brossay L, Adeegbe DO. The yin and yang of targeting KLRG1+ tregs and effector cells. Front Immunol 2022;13:894508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, et al. Defining ‘T cell exhaustion. Nat Rev Immunol 2019;19:665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao X, Shan Q, Xue H-H. TCF1 in T cell immunity: a broadened frontier. Nat Rev Immunol 2022;22:147–57. [DOI] [PubMed] [Google Scholar]
- 44. Held W, Siddiqui I, Schaeuble K, Speiser DE. Intratumoral CD8 + T cells with stem cell–like properties: Implications for cancer immunotherapy. Sci Transl Med 2019;11:eaay6863. [DOI] [PubMed] [Google Scholar]
- 45. Le Garff-Tavernier M, Béziat V, Decocq J, Siguret V, Gandjbakhch F, Pautas E, et al. Human NK cells display major phenotypic and functional changes over the life span: human NK cell features over the life span. Aging Cell 2010;9:527–35. [DOI] [PubMed] [Google Scholar]
- 46. Gayoso I, Sanchez-Correa B, Campos C, Alonso C, Pera A, Casado JG, et al. Immunosenescence of human natural killer cells. J Innate Immun 2011;3:337–43. [DOI] [PubMed] [Google Scholar]
- 47. Brauning A, Rae M, Zhu G, Fulton E, Admasu TD, Stolzing A, et al. Aging of the immune system: focus on natural killer cells phenotype and functions. Cells 2022;11:1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu RC, Hwu P, Radvanyi LG. New insights on the role of CD8 + CD57 + T-cells in cancer. OncoImmunology 2012;1:954–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Müller-Durovic B, Lanna A, Covre LP, Mills RS, Henson SM, Akbar AN, et al. Killer cell lectin-like receptor G1 inhibits NK cell function through activation of adenosine 5’-monophosphate-activated protein kinase. J Immunol 2016;197:2891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hazeldine J, Hampson P, Lord JM. Reduced release and binding of perforin at the immunological synapse underlies the age-related decline in natural killer cell cytotoxicity. Aging Cell 2012;11:751–9. [DOI] [PubMed] [Google Scholar]
- 51. Wendt K, Wilk E, Buyny S, Buer J, Schmidt RE, Jacobs R. Gene and protein characteristics reflect functional diversity of CD56 dim and CD56 bright NK cells. J Leukocyte Biol 2006;80:1529–41. [DOI] [PubMed] [Google Scholar]
- 52. Pahlavani MA, Richardson A. The effect of age on the expression of Interleukin-2. Mech Ageing Dev 1996;89:125–54. [DOI] [PubMed] [Google Scholar]
- 53. McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol 2019;37:457–95. [DOI] [PubMed] [Google Scholar]
- 54. Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature 2019;571:211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim K, Park S, Park SY, Kim G, Park SM, Cho J-W, et al. Single-cell transcriptome analysis reveals TOX as a promoting factor for T cell exhaustion and a predictor for anti-PD-1 responses in human cancer. Genome Med 2020;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun 2020;11:3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Clave E, Socié G, Cosset JM, Chaillet MP, Tartour E, Girinsky T, et al. Multicolor flow cytometry analysis of blood cell subsets in patients given total body irradiation before bone marrow transplantation. Int J Radiat Oncol Biol Phys 1995;33:881–6. [DOI] [PubMed] [Google Scholar]
- 58. Arina A, Beckett M, Fernandez C, Zheng W, Pitroda S, Chmura SJ, et al. Tumor-reprogrammed resident T cells resist radiation to control tumors. Nat Commun 2019;10:3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yovino S, Grossman SA. Severity, etiology and possible consequences of treatment-related lymphopenia in patients with newly diagnosed high-grade gliomas. CNS Oncol 2012;1:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest 2013;31:140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Georgakilas AG, O'Neill P, Stewart RD. Induction and repair of clustered DNA lesions: what do we know so far? Radiat Res 2013;180:100–9. [DOI] [PubMed] [Google Scholar]
- 62. Yamaoka M, Kusunoki Y, Kasagi F, Hayashi T, Nakachi K, Kyoizumi S, et al. Decreases in percentages of naïve CD4 and CD8 T cells and increases in percentages of memory CD8 T-cell subsets in the peripheral blood lymphocyte populations of A-bomb survivors. Radiat Res 2004;161:290–8. [DOI] [PubMed] [Google Scholar]
- 63. Kusunoki Y, Yamaoka M, Kubo Y, Hayashi T, Kasagi F, Douple EB, et al. T-cell immunosenescence and inflammatory response in atomic bomb survivors. Radiat Res 2010;174:870–6. [DOI] [PubMed] [Google Scholar]
- 64. Candéias SM, Mika J, Finnon P, Verbiest T, Finnon R, Brown N, et al. Low-dose radiation accelerates aging of the T-cell receptor repertoire in CBA/Ca mice. Cell Mol Life Sci 2017;74:4339–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lumniczky K, Impens N, Armengol G, Candéias S, Georgakilas AG, Hornhardt S, et al. Low dose ionizing radiation effects on the immune system. Environ Int 2021;149:106212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Metcalfe JA, Parkhill J, Campbell L, Stacey M, Biggs P, Byrd PJ, et al. Accelerated telomere shortening in ataxia telangiectasia. Nat Genet 1996;13:350–3. [DOI] [PubMed] [Google Scholar]
- 67. Li P, Hou M, Lou F, Björkholm M, Xu D. Telomere dysfunction induced by chemotherapeutic agents and radiation in normal human cells. Int J Biochem Cell Biol 2012;44:1531–40. [DOI] [PubMed] [Google Scholar]
- 68. El-Saghire H, Vandevoorde C, Ost P, Monsieurs P, Michaux A, De Meerleer G, et al. Intensity modulated radiotherapy induces pro-inflammatory and pro-survival responses in prostate cancer patients. Int J Oncol 2014;44:1073–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, et al. Rescue of exhausted CD8 T cells by PD-1–targeted therapies is CD28-dependent. Science 2017;355:1423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, et al. T cell costimulatory receptor CD28 is a primary target for PD-1–mediated inhibition. Science 2017;355:1428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Greenberg SA, Thompson E, Gulla SV. Abstract 3227: differential expression of T cell co-inhibitory receptors: KLRG1 alignment with cytotoxicity and adaptive resistance mechanisms. Cancer Res 2019;79(13_Supplement):3227–. [Google Scholar]
- 72. Guo Y, Feng Y, Fan P, Yao X, Peng Y, Wang R, et al. Expression and clinical significance of KLRG1 and 2B4 on T cells in the peripheral blood and tumour of patients with cervical cancer. Immunol Invest 2022;51:670–87. [DOI] [PubMed] [Google Scholar]
- 73. Yousefzadeh MJ, Flores RR, Zhu Y, Schmiechen ZC, Brooks RW, Trussoni CE, et al. An aged immune system drives senescence and ageing of solid organs. Nature 2021;594:100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rutkowski J, Ślebioda T, Kmieć Z, Zaucha R. Changes of systemic immune response after stereotactic ablative radiotherapy (SABR) - first results of a prospective study in early lung cancer patients. Pol Arch Intern Med 2017;127:245–53. [DOI] [PubMed] [Google Scholar]
- 75. Beauford SS, Kumari A, Garnett-Benson C. Ionizing radiation modulates the phenotype and function of human CD4+ induced regulatory T cells. BMC Immunol 2020;21:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Muroyama Y, Nirschl TR, Kochel CM, Lopez-Bujanda Z, Theodros D, Mao W, et al. Stereotactic radiotherapy increases functionally suppressive regulatory T cells in the tumor microenvironment. Cancer Immunol Res 2017;5:992–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lai J-Z, Zhu Y-Y, Ruan M, Chen L, Zhang Q-Y. Local irradiation sensitized tumors to adoptive T cell therapy via enhancing the cross-priming, homing, and cytotoxicity of antigen-specific CD8 T cells. Front Immunol 2019;10:2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013;24:589–602. [DOI] [PubMed] [Google Scholar]
- 79. Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 2008;181:3099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hoover AR, Kaabinejadian S, Krawic JR, Sun X-H, Naqash AR, Yin Q, et al. Localized ablative immunotherapy drives de novo CD8 + T-cell responses to poorly immunogenic tumors. J Immunother Cancer 2022;10:e004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203:1259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer 2016;40:25–37. [DOI] [PubMed] [Google Scholar]
- 83. De Kermenguy F, Meziani L, Mondini M, Clémenson C, Morel D, Deutsch E, et al. Radio-induced lymphopenia in the era of anti-cancer immunotherapy. Int Rev Cell Mol Biol 2023;378:1–30. [DOI] [PubMed] [Google Scholar]
- 84. Chang JY, Zhang X, Wang X, Kang Y, Riley B, Bilton S, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;65:1087–96. [DOI] [PubMed] [Google Scholar]
- 85. Zhang X, Li Y, Pan X, Xiaoqiang L, Mohan R, Komaki R, et al. Intensity-modulated proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIB non-small-cell lung cancer: a virtual clinical study. Int J Radiat Oncol Biol Phys 2010;77:357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shiraishi Y, Fang P, Xu C, Song J, Krishnan S, Koay EJ, et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: a propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol 2018;128:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kim N, Myoung Noh J, Lee W, Park B, Park H, Young Park J, et al. Proton beam therapy reduces the risk of severe radiation-induced lymphopenia during chemoradiotherapy for locally advanced non-small cell lung cancer: a comparative analysis of proton versus photon therapy. Radiother Oncol 2021;156:166–73. [DOI] [PubMed] [Google Scholar]
- 88. Liu KX, Ioakeim-Ioannidou M, Susko MS, Rao AD, Yeap BY, Snijders AM, et al. A multi-institutional comparative analysis of proton and photon therapy-induced hematologic toxicity in patients with medulloblastoma. Int J Radiat Oncol Biol Phys 2021;109:726–35. [DOI] [PubMed] [Google Scholar]
- 89. Miszczyk J, Rawojć K, Panek A, Borkowska A, Prasanna PGS, Ahmed MM, et al. Do protons and X-rays induce cell-killing in human peripheral blood lymphocytes by different mechanisms? Clin Transl Radiat Oncol 2018;9:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Matsubara S, Horiuchi J, Okuyama T, Takeda M, Shibuya H, Suzuki S, et al. Chromosome aberrations in the peripheral lymphocytes induced by brachytherapy and external cobalt teletherapy. Int J Radiat Oncol Biol Phys 1985;11:1085–94. [DOI] [PubMed] [Google Scholar]
- 91. Tubin S, Vozenin MC, Prezado Y, Durante M, Prise KM, Lara PC, et al. Novel unconventional radiotherapy techniques: current status and future perspectives: report from the 2nd international radiation oncology online seminar. Clin Transl Radiat Oncol 2023;40:100605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lu Q, Yan W, Zhu A, Tubin S, Mourad WF, Yang J, et al. Combining spatially fractionated radiation therapy (SFRT) and immunotherapy opens new rays of hope for enhancing therapeutic ratio. Clin Transl Radiat Oncol 2024;44:100691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Moghaddasi L, Reid P, Bezak E, Marcu LG. Radiobiological and treatment-related aspects of spatially fractionated radiotherapy. Int J Mol Sci 2022;23:3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chi M-S, Tien D-C, Chi K-H. Inhomogeneously distributed ferroptosis with a high peak-to-valley ratio may improve the antitumor immune response. Front Oncol 2023;13:1178681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Crocenzi T, Cottam B, Newell P, Wolf RF, Hansen PD, Hammill C, et al. A hypofractionated radiation regimen avoids the lymphopenia associated with neoadjuvant chemoradiation therapy of borderline resectable and locally advanced pancreatic adenocarcinoma. J Immunother Cancer 2016;4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wild AT, Herman JM, Dholakia AS, Moningi S, Lu Y, Rosati LM, et al. Lymphocyte-sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2016;94:571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wu G, Baine MJ, Zhao N, Li S, Li X, Lin C, et al. Lymphocyte-sparing effect of stereotactic body radiation therapy compared to conventional fractionated radiation therapy in patients with locally advanced pancreatic cancer. BMC Cancer 2019;19:977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wang S-J, Jhawar SR, Rivera-Nunez Z, Silk AW, Byun J, Miller E, et al. The association of radiation dose-fractionation and immunotherapy use with overall survival in metastatic melanoma patients. Cureus 2020;12:e8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sun G-Y, Wang S-L, Song Y-W, Jin J, Wang W-H, Liu Y-P, et al. Radiation-induced lymphopenia predicts poorer prognosis in patients with breast cancer: a post hoc analysis of a randomized controlled trial of postmastectomy hypofractionated radiation therapy. Int J Radiat Oncol Biol Phys 2020;108:277–85. [DOI] [PubMed] [Google Scholar]
- 100. McLaughlin MF, Alam M, Smith L, Ryckman J, Lin C, Baine MJ, et al. Stereotactic body radiation therapy mitigates radiation induced lymphopenia in early stage non-small cell lung cancer. PLoS One 2020;15:e0241505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Damen PJJ, Verhoeff JJC. Efficacy of stereotactic ablative radiotherapy (SABR) during anti-PD-1 in oligoprogressive non-small cell lung cancer and melanoma-a prospective multicenter observational study pointing out new unmet needs. Transl Cancer Res 2023;12:688–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Spaas M, Sundahl N, Kruse V, Rottey S, De Maeseneer D, Duprez F, et al. Checkpoint inhibitors in combination with stereotactic body radiotherapy in patients with advanced solid tumors: the CHEERS phase 2 randomized clinical trial. JAMA Oncol 2023;9:1205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jin J-Y, Gu A, Wang W, Oleinick NL, Machtay M, (Spring) Kong F-M, et al. Ultra-high dose rate effect on circulating immune cells: a potential mechanism for FLASH effect? Radiother Oncol 2020;149:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol 2006;7:344–53. [DOI] [PubMed] [Google Scholar]
- 105. Fischer S, Proschmann U, Akgün K, Ziemssen T. Lymphocyte counts and multiple sclerosis therapeutics: between mechanisms of action and treatment-limiting side effects. Cells 2021;10:3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dai D, Tian Q, Shui Y, Li J, Wei Q. The impact of radiation induced lymphopenia in the prognosis of head and neck cancer: a systematic review and meta-analysis. Radiother Oncol March 2022;168:28–36. [DOI] [PubMed] [Google Scholar]
- 107. Marciscano AE, Ghasemzadeh A, Nirschl TR, Theodros D, Kochel CM, Francica BJ, et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin Cancer Res 2018;24:5058–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Darragh LB, Gadwa J, Pham TT, Van Court B, Neupert B, Olimpo NA, et al. Elective nodal irradiation mitigates local and systemic immunity generated by combination radiation and immunotherapy in head and neck tumors. Nat Commun 2022;13:7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ma TM, Wong DJ, Chai-Ho W, Mendelsohn A, St John M, Abemayor E, et al. High recurrence for HPV-positive oropharyngeal cancer with neoadjuvant radiation therapy to gross disease plus immunotherapy: analysis from a prospective phase Ib/II clinical trial. Int J Radiat Oncol Biol Phys 2023;117:348–54. [DOI] [PubMed] [Google Scholar]
- 110. Faivre-Finn C. Less is more in radiotherapy target volume planning: lessons from the PET-plan trial. Lancet Oncol 2020;21:481–3. [DOI] [PubMed] [Google Scholar]
- 111. Nestle U, Schimek-Jasch T, Kremp S, Schaefer-Schuler A, Mix M, Küsters A, et al. Imaging-based target volume reduction in chemoradiotherapy for locally advanced non-small-cell lung cancer (PET-Plan): a multicentre, open-label, randomised, controlled trial. Lancet Oncol 2020;21:581–92. [DOI] [PubMed] [Google Scholar]
- 112. Leroy A, Lerousseau M, Henry T, Cafaro A, Paragios N, Grégoire V, et al. End-to-end multi-slice-to-volume concurrent registration and multimodal generation. In:Wang L, Dou Q, Fletcher PT, Speidel S, Li S. editors. Medical image computing and computer assisted intervention – MICCAI 2022 Cham: Springer Nature Switzerland; 2022. [cited July 13, 2023];152–62. (Lecture Notes in Computer Science; vol. 13436). Available at: https://link.springer.com/10.1007/978-3-031-16446-0_15 [Google Scholar]
- 113. Shmatko A, Ghaffari Laleh N, Gerstung M, Kather JN. Artificial intelligence in histopathology: enhancing cancer research and clinical oncology. Nat Cancer 2022;3:1026–38. [DOI] [PubMed] [Google Scholar]
- 114. Apolle R, Rehm M, Bortfeld T, Baumann M, Troost EGC. The clinical target volume in lung, head-and-neck, and esophageal cancer: lessons from pathological measurement and recurrence analysis. Clin Transl Radiat Oncol 2017;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Altorki NK, McGraw TE, Borczuk AC, Saxena A, Port JL, Stiles BM, et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol 2021;22:824–35. [DOI] [PubMed] [Google Scholar]
- 116. Chang JY, Lin SH, Dong W, Liao Z, Gandhi SJ, Gay CM, et al. Stereotactic ablative radiotherapy with or without immunotherapy for early-stage or isolated lung parenchymal recurrent node-negative non-small-cell lung cancer: an open-label, randomised, phase 2 trial. Lancet 2023;402:871–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Monjazeb AM, Daly ME, Luxardi G, Maverakis E, Merleev AA, Marusina AI, et al. Atezolizumab plus stereotactic ablative radiotherapy for medically inoperable patients with early-stage non-small cell lung cancer: a multi-institutional phase I trial. Nat Commun 2023;14:5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Curran WJ, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Girard N, Bar J, Garrido P, Garassino MC, McDonald F, Mornex F, et al. Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: findings from the PACIFIC-R study. J Thorac Oncol 2023;18:181–93. [DOI] [PubMed] [Google Scholar]
- 120. Zhang Y, Tian Y, Zheng L, Sun X, Zhao Z, Zheng Y, et al. Efficacy and safety of consolidation durvalumab after chemoradiation therapy for stage III non-small-cell lung cancer: a systematic review, meta-analysis, and meta-regression of real-world studies. Front Pharmacol 2023;14:1103927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ménétrier-Caux C, Ray-Coquard I, Blay J-Y, Caux C. Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with cytokines? J Immunotherapy Cancer 2019;7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lee YJ, Park YS, Lee HW, Park TY, Lee JK, Heo EY. Peripheral lymphocyte count as a surrogate marker of immune checkpoint inhibitor therapy outcomes in patients with non-small-cell lung cancer. Sci Rep 2022;12:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tanizaki J, Haratani K, Hayashi H, Chiba Y, Nakamura Y, Yonesaka K, et al. Peripheral blood biomarkers associated with clinical outcome in non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol 2018;13:97–105. [DOI] [PubMed] [Google Scholar]
- 124. Zhou J-G, Donaubauer A-J, Frey B, Becker I, Rutzner S, Eckstein M, et al. Prospective development and validation of a liquid immune profile-based signature (LIPS) to predict response of patients with recurrent/metastatic cancer to immune checkpoint inhibitors. J Immunother Cancer 2021;9:e001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chen D, Verma V, Patel RR, Barsoumian HB, Cortez MA, Welsh JW, et al. Absolute lymphocyte count predicts abscopal responses and outcomes in patients receiving combined immunotherapy and radiation therapy: analysis of 3 phase 1/2 trials. Int J Radiat Oncol Biol Phys 2020;108:196–203. [DOI] [PubMed] [Google Scholar]
- 126. Lambin P, Lieverse RIY, Eckert F, Marcus D, Oberije C, van der Wiel AMA, et al. Lymphocyte-sparing radiotherapy: the rationale for protecting lymphocyte-rich organs when combining radiotherapy with immunotherapy. Semin Radiat Oncol 2020;30:187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kong FM, Zhang H, Liu Y, Yao H, Cerra-Franco A, Shiue K, et al. Radiation to the immune system may be an important risk factor for long-term survival after SBRT in early stage non-small cell lung cancer: a role of RT plan optimization. Int J Radiat Oncol Biol Phys 2018;102:e689–90. [Google Scholar]
- 128. Jin JY, Hu C, Xiao Y, Zhang H, Ellsworth S, Schild SE, et al. Higher radiation dose to immune system is correlated with poorer survival in patients with stage iii non–small cell lung cancer: a secondary study of a phase 3 cooperative group trial (NRG Oncology RTOG 0617). Int J Radiat Oncol Biol Phys 2017;99:S151–2. [Google Scholar]
- 129. Ladbury CJ, Rusthoven CG, Camidge DR, Kavanagh BD, Nath SK. Impact of radiation dose to the host immune system on tumor control and survival for stage III non-small cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys 2019;105:346–55. [DOI] [PubMed] [Google Scholar]
- 130. Benzazon N, Colnot J, De Kermenguy F, Achkar S, De Vathaire F, Deutsch E, et al. Analytical models for external photon beam radiotherapy out-of-field dose calculation: a scoping review. Front Oncol 2023;13:1197079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. De Saint-Hubert M, Suesselbeck F, Vasi F, Stuckmann F, Rodriguez M, Dabin J, et al. Experimental validation of an analytical program and a Monte Carlo simulation for the computation of the far out-of-field dose in external beam photon therapy applied to pediatric patients. Front Oncol 2022;12:882506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cravo Sá A, Barateiro A, Bednarz B, Borges C, Pereira J, Baptista M, et al. Assessment of out-of-field doses in radiotherapy treatments of paediatric patients using Monte Carlo methods and measurements. Phys Med 2020;71:53–61. [DOI] [PubMed] [Google Scholar]
- 133. Colnot J, Barraux V, Loiseau C, Berejny P, Batalla A, Gschwind R, et al. A new Monte Carlo model of a Cyberknife® system for the precise determination of out-of-field doses. Phys Med Biol 2019;64:195008. [DOI] [PubMed] [Google Scholar]
- 134. Bojaxhiu B, Templeton AJ, Elicin O, Shelan M, Zaugg K, Walser M, et al. Relation of baseline neutrophil-to-lymphocyte ratio to survival and toxicity in head and neck cancer patients treated with (chemo-) radiation. Radiat Oncol 2018;13:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Bartlett EK, Flynn JR, Panageas KS, Ferraro RA, Sta Cruz JM, Postow MA, et al. High neutrophil-to-lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with PD-1 inhibitor monotherapy. Cancer 2020;126:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wolfe AR, Siedow M, Nalin A, DiCostanzo D, Miller ED, Diaz DA, et al. Increasing neutrophil-to-lymphocyte ratio following radiation is a poor prognostic factor and directly correlates with splenic radiation dose in pancreatic cancer. Radiother Oncol 2021;158:207–14. [DOI] [PubMed] [Google Scholar]
- 137. Ng SP, Bahig H, Jethanandani A, Sturgis EM, Johnson FM, Elgohari B, et al. Prognostic significance of pre-treatment neutrophil-to-lymphocyte ratio (NLR) in patients with oropharyngeal cancer treated with radiotherapy. Br J Cancer 2021;124:628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kho VM, Mekers VE, Span PN, Bussink J, Adema GJ. Radiotherapy and cGAS/STING signaling: impact on MDSCs in the tumor microenvironment. Cell Immunol 2021;362:104298. [DOI] [PubMed] [Google Scholar]
- 139. Ghosh S, Huang J, Inkman M, Zhang J, Thotala S, Tikhonova E, et al. Radiation-induced circulating myeloid-derived suppressor cells induce systemic lymphopenia after chemoradiotherapy in patients with glioblastoma. Sci Transl Med 2023;15:eabn6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Rao E, Hou Y, Huang X, Wang L, Wang J, Zheng W, et al. All-trans retinoic acid overcomes solid tumor radioresistance by inducing inflammatory macrophages. Sci Immunol 2021;6:eaba8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Choi N, Kim JH, Chie EK, Gim J, Kang H-C. A meta-analysis of the impact of neutrophil-to-lymphocyte ratio on treatment outcomes after radiotherapy for solid tumors. Medicine (Baltimore) 2019;98:e15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009;16:183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest 2015;125:3356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol 2016;37:208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Law AMK, Valdes-Mora F, Gallego-Ortega D. Myeloid-derived suppressor cells as a therapeutic target for cancer. Cells 2020;9:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity 2019;50:924–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Briukhovetska D, Dörr J, Endres S, Libby P, Dinarello CA, Kobold S. Interleukins in cancer: from biology to therapy. Nat Rev Cancer 2021;21:481–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Coppola C, Hopkins B, Huhn S, Du Z, Huang Z, Kelly WJ. Investigation of the impact from IL-2, IL-7, and IL-15 on the growth and signaling of activated CD4+ T cells. Int J Mol Sci 2020;21:7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kim J-H, Lee K-J, Lee S-W. Cancer immunotherapy with T-cell targeting cytokines: IL-2 and IL-7. BMB Rep 2021;54:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Chamie K, Chang SS, Kramolowsky E, Gonzalgo ML, Agarwal PK, Bassett JC, et al. IL-15 superagonist NAI in BCG-unresponsive non–muscle-invasive bladder cancer. NEJM Evidence 2023;2:EVIDoa2200167. [DOI] [PubMed] [Google Scholar]
- 151. Garralda E, Naing A, Galvao V, LoRusso P, Grell P, Cassier PA, et al. Interim safety and efficacy results from AURELIO-03: a phase 1 dose escalation study of the IL-2/IL-15 receptor βγ superagonist SOT101 as a single agent and in combination with pembrolizumab in patients with advanced solid tumors. JCO 2022;40(16_suppl):2502. [Google Scholar]
- 152. Dolgin E. IL-2 upgrades show promise at ASCO. Nat Biotechnol 2022;40:986–8. [DOI] [PubMed] [Google Scholar]
- 153. Yu EY, Fling S, Salim B, Sweis RF, Chatta GS, Jain RK, et al. A randomized phase II study of atezolizumab plus recombinant human IL-7 (CYT107) or atezolizumab alone in patients with locally advanced or metastatic urothelial carcinoma (mUC): a cancer immunotherapy trials network trial (CITN-14). JCO 2019;37(15_suppl):TPS4586. [Google Scholar]
- 154. Daix T, Mathonnet A, Brakenridge S, Dequin P-F, Mira J-P, Berbille F, et al. Intravenously administered interleukin-7 to reverse lymphopenia in patients with septic shock: a double-blind, randomized, placebo-controlled trial. Ann Intensive Care 2023;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Sharma A, Neri S, Venkatesulu BP, Lin SH. Abstract 473: preclinical model of radiation induced lymphopenia to identify abrogation strategies to enhance cancer therapy. Cancer Res 2020;80(16_Supplement):473. [Google Scholar]
- 156. Kang C, Jeong S-Y, Song SY, Choi EK. The emerging role of myeloid-derived suppressor cells in radiotherapy. Radiat Oncol J 2020;38:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Jiménez-Cortegana C, Galassi C, Klapp V, Gabrilovich DI, Galluzzi L. Myeloid-derived suppressor cells and radiotherapy. Cancer Immunol Res 2022;10:545–57. [DOI] [PubMed] [Google Scholar]
- 158. Weed DT, Vella JL, Reis IM, De La Fuente AC, Gomez C, Sargi Z, et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res 2015;21:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Orillion A, Hashimoto A, Damayanti N, Shen L, Adelaiye-Ogala R, Arisa S, et al. Entinostat neutralizes myeloid-derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal cell carcinoma. Clin Cancer Res 2017;23:5187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, et al. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res 2010;70:3526–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Sun L, Clavijo PE, Robbins Y, Patel P, Friedman J, Greene S, et al. Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight 2019;4:e126853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY, et al. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res 2010;16:4583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Michels T, Shurin GV, Naiditch H, Sevko A, Umansky V, Shurin MR, et al. Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in vitro in a TLR4-independent manner. J Immunotoxicol 2012;9:292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res 2006;66:9299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res 2015;21:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Gebhardt C, Simon SCS, Weber R, Gries M, Mun DH, Reinhard R, et al. Potential therapeutic effect of low-dose paclitaxel in melanoma patients resistant to immune checkpoint blockade: a pilot study. Cell Immunol 2021;360:104274. [DOI] [PubMed] [Google Scholar]
- 167. Luginbuhl AJ, Johnson JM, Harshyne LA, Linnenbach AJ, Shukla SK, Alnemri A, et al. Tadalafil enhances immune signatures in response to neoadjuvant nivolumab in resectable head and neck squamous cell carcinoma. Clin Cancer Res 2022;28:915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Tobin RP, Cogswell DT, Cates VM, Davis DM, Borgers JSW, Van Gulick RJ, et al. Targeting MDSC differentiation using ATRA: a phase I/II clinical trial combining pembrolizumab and all-trans retinoic acid for metastatic melanoma. Clin Cancer Res 2023;29:1209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI, et al. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res 2007;67:11021–8. [DOI] [PubMed] [Google Scholar]
- 170. Andersson K-E. PDE5 inhibitors: pharmacology and clinical applications 20 years after sildenafil discovery. Br J Pharmacol 2018;175:2554–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ban Y, Mai J, Li X, Mitchell-Flack M, Zhang T, Zhang L, et al. Targeting autocrine CCL5–CCR5 axis reprograms immunosuppressive myeloid cells and reinvigorates antitumor immunity. Cancer Res 2017;77:2857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Gustave Roussy. LySAIRI: towards an innovative radiotherapy that facilitates immune sparing to improve overall clinical efficacy. https://www.gustaveroussy.fr/en/lysairi-towards-innovative-radiotherapy-facilitates-immune-sparing-improve-overall-clinical-efficacy.
- 173. Mondini M, Nizard M, Tran T, Mauge L, Loi M, Clémenson C, et al. Synergy of radiotherapy and a cancer vaccine for the treatment of HPV-associated head and neck cancer. Mol Cancer Ther 2015;14:1336–45. [DOI] [PubMed] [Google Scholar]
- 174. Laurent P-A, Morel D, Meziani L, Depil S, Deutsch E. Radiotherapy as a means to increase the efficacy of T-cell therapy in solid tumors. Oncoimmunology 2023;12:2158013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Nicolas AM, Pesic M, Engel E, Ziegler PK, Diefenhardt M, Kennel KB, et al. Inflammatory fibroblasts mediate resistance to neoadjuvant therapy in rectal cancer. Cancer Cell 2022;40:168–84. [DOI] [PubMed] [Google Scholar]
- 176. Shi DD, Guo JA, Hoffman HI, Su J, Mino-Kenudson M, Barth JL, et al. Therapeutic avenues for cancer neuroscience: translational frontiers and clinical opportunities. Lancet Oncol 2022;23:e62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]