Abstract
Background
Weightlifting exposes athletes to significant loads, potentially placing the knee joint in an abnormal mechanical environment and leading to anterior cruciate ligament (ACL) injuries. Once an ACL injury occurs, it can affect athletes’ competitive ability to varying degrees and even prematurely end their career. Understanding the biomechanical mechanisms of ACL injuries in weightlifters helps in comprehensively understanding the stress patterns and degrees on ACL during human movement, and identifying potential injury-causing factors, thereby enabling the implementation of appropriate preventive measures to reduce the occurrence of injuries. This study aimed to explore the biomechanical mechanisms of ACL injuries during the jerk dip phase of clean and jerk in weightlifters, providing a theoretical basis for the prevention of ACL injuries in weightlifting sports.
Methods
This study utilized the German SIMI Motion 10.2 movement analysis system and the AnyBody simulation system to analyze the kinematic and dynamic parameters of a 109 kg + class weightlifter (height: 191 cm, age: 22 years, weight: 148 kg, athletic level: elite) performing a 205 kg clean and jerk (non-injured) and a 210 kg clean and jerk (ACL injury occurred). The differences in kinematic and dynamic indicators of lower limb joints under injured and non-injured jerk dip conditions were investigated.
Results
Knee joint torque during non-injured clean and jerk was consistently positive (i.e., external rotation) but turned from positive to negative (i.e., from external rotation to internal rotation) during injured clean and jerk and reached a maximum internal rotation torque of 21.34 Nm at the moment of injury. At every moment, the muscle activation and simulated muscle force of the quadriceps and gastrocnemius during the injured clean and jerk were higher than those during the non-injured clean and jerk. By contrast, the muscle activation and simulated muscle force of the semitendinosus, semimembranosus, biceps femoris, and soleus during non-injured clean and jerk were higher than those during injured clean and jerk. The knee joint internal rotation angle during injured clean and jerk first increased and then declined, reaching a peak at 46.93° at the moment of injury, whereas it gradually increased during non-injured clean and jerk. The proximal tibia on the left side during the injured clean and jerk moved forward faster by 0.76 m/s compared with that during the non-injured clean and jerk.
Conclusions
The small muscle activation and simulated muscle force of the hamstring and soleus could not resist timely and effectively the large muscle activation and simulated muscle force of the quadriceps (especially the medial quad) and gastrocnemius. As such, the force applied to the ACL could exceed its ultimate load-bearing capacity. Kinematic indicators in the athlete's injured lift demonstrated certain disparities from those in their non-injured lift. Knee internal rotation and tibial anterior translation during the jerk dip phase of weightlifting might be the kinematic characteristics of ACL injuries.
Keywords: Clean & jerk, Jerk dip, Anterior cruciate ligament injury, AnyBody simulation, Inverse dynamics
1. Introduction
The uniqueness of weightlifting lies in the fact that athletes bear enormous loads in each phase of the movement, often exceeding their individual limit load, thereby increasing the risk of injury. Related research [1]indicates that the injury rate for elite weightlifters is as high as 100 %, with a knee injury rate of 0.33/1000 h. Among the types of knee injuries, ACL injuries account for a significant proportion. As one of the most representative ligaments of the knee joint, ACL plays important roles in controlling the anterior translation of the tibia relative to the femur, preventing hyperextension of the knee joint, and enhancing the stability of the knee joint. Previous studies have found that the treatment of ACL injuries is complex, recovery is difficult, and the treatment often causes damage to other tissues within the joint. Postoperatively, a serious risk of osteoarthritis still exists, including damage to the meniscus and cartilage surface, leading to osteoarthritis, which can affect an athlete's competitive ability to varying degrees [2]and even prematurely end their career [3]. The clean and jerk movement is a competitive sport, and many sports use it as a training method to improve athletes' competitive performance [[4], [5], [6]].
Currently, no effective ACL injury prevention measures are available specifically for weightlifting, and generic preventive measures are employed, including wearing knee braces, technical training, neuromuscular training, rapid muscle contraction compound training, strength training, balance training, and comprehensive training. However, which measure is most effective in preventing ACL injuries is still unknown, mainly due to differences in training arrangements and research subjects [[7], [8], [9], [10], [11], [12], [13]]. Therefore, exploring the mechanisms of ACL injuries in weightlifters under real conditions is crucial to provide coaches and athletes with safe training, competition guidelines, and effective preventive measures.
Current research classifies ACL injuries into three types, namely, non-contact, contact, and intermediate, with non-contact injuries accounting for the highest proportion [14]. Noyes [15] et al. surveyed 103 high school and college athletes with ACL injuries and found that 80 (78 %) of the ACL ruptures occurred during non-contact. Boden [14] reported that 72 % of ACL injuries observed in sports game footage occurred while athletes were performing an action on their own. Non-contact ACL injuries are common in sports, such as basketball [14], football [[16], [17]], and rugby [18], occurring during specific displacement movements like landing, cutting, and changing direction. Previous studies on the mechanisms of non-contact ACL injuries have focused on several directions [19]. Some studies [13] suggest that when athletes cannot fully use their muscles to absorb ground reaction forces, they passively use bones, cartilage, and ligaments to absorb these forces, resulting in increased impact forces on the ligaments and joints in a short time, which may lead to ligament rupture. Other studies [[20], [21], [22]] suggest that athletes are more accustomed to using the quadriceps to stabilize the knee joint rather than using the posterior chain muscles to maintain stability and control during actions like landing, cutting, and changing direction. When the quadriceps contract, athletes pull the tibia forward relative to the femur, generating an anterior shear force on the tibia and thus shear stress on the ACL, leading to injury. Some authors [[23], [24], [25]]believe that when athletes cannot accurately control their torso in three-dimensional space, it leads to uncontrolled lateral movement due to a lack of balance, resulting in internal and external rotation torque on the knee joint caused by ground reaction forces. Although many injury mechanisms have been identified, the decisive and risk factors remain debatable. Some scholars believe that sagittal plane loading is the most important loading mechanism for ACL injuries (including knee joint shear forces and smaller knee joint angles), whereas others believe that frontal plane loading (varus and valgus states of the knee joint) is the main contributor to ACL injuries, especially in women. Some scholars believe that loading in a single plane cannot easily cause ACL injuries, and that the combined action of abnormal loads in the sagittal, coronal, and horizontal planes of the knee joint is an important reason for ACL injuries [[26], [27], [28], [29], [30], [31]]. Compared with displacement movements (e.g., landing, cutting, changing direction), weightlifting has the characteristic of completing movements in place (both feet are in contact with the ground and fixed for most of the time), and few studies have been conducted on the mechanisms of ACL injuries related to weightlifting. Laboratory studies on weightlifting suggest that compared with other sports, weightlifting involves lifting heavy weights overhead, and the slightest improper movement can directly bring heavy loads to the joints and ligaments. If the knee joint is subjected to a high load, an imbalance between the strength of the rectus femoris and the biceps femoris may occur, which can reduce the ability to control the anterior movement of the tibia relative to the femur, leading to ACL injuries [32]. However, current research has only extensively studied the mechanisms of ACL injuries in displacement movements, and limited research on the mechanisms of ACL injuries in weightlifting, where both feet are relatively fixed, is available. Whether the injury mechanisms and preventive measures for these two types of movements can be interchangeable remains to be verified.
Quantitative biomechanical analysis of ACL injury cases is an effective method to understand the mechanisms and risk factors of ACL injuries. However, collecting valid biomechanical data during the occurrence of ACL injuries is challenging. Many researchers have attempted to obtain kinematic data from videos of ACL injury events [14,23,[33], [34], [35], [36]]. However, the kinematic data collected from these videos are questionable, because most of the collected data are 2D, and the athletes are not strictly parallel to the camera's shooting plane (completely perpendicular to the ground). Additionally, when converting 2D data into 3D data, the lack of camera calibration can considerably compromise the accuracy of the data.
Krosshaug and Bahr [36] developed a model-based image-matching technique that can reconstruct 3D body movements from uncalibrated cameras. This method has been applied to collect kinematic data from video recordings of ACL injuries in sports such as football, skiing, handball, and basketball [[37], [38], [39]]. However, even with the use of multiple cameras, the minimum root mean square errors for knee flexion angle, knee valgus/varus angle, and knee internal/external rotation angle are 7.5° ± 12.4°, 3.9° ± 9.6°, and 7.5° ± 13.9°, respectively [36]. This finding significantly reduces the validity of the kinematic data. Collecting movement data with minimal error by using effective measurement methods is necessary to truly understand the biomechanical characteristics of ACL injury cases.
In this study, two cameras were used to analyze a video of a non-contact ACL injury occurring in a competition involving an elite male weightlifter by using Direct Linear Transformation (DLT). This method has been proven to be a reliable and effective measurement method with high accuracy [40,41]. Therefore, this case provides a unique opportunity to understand the mechanisms and risk factors of ACL injuries in weightlifters. Human motion simulation based on musculoskeletal models is another practical method for estimating muscle states, and it is useful for athletes’ physical training, sports optimization, and injury risk prediction. This technique has been applied to various competitive sports such as basketball [42], golf [43], cross-country sit skiing [44], and swimming [45]. In the present study, relevant research on the improved simulation method based on inverse dynamics was referenced, and the dynamic data during the ACL injury process of the weightlifter were simulated, including ground reaction forces, joint torques, muscle forces, and activation levels [46].
This study aimed to understand the mechanisms of ACL injuries in weightlifting by comparing the kinematics and dynamics of a male weightlifter's injured and non-injured trials in the same competition. The results could provide important information for understanding the specific mechanisms and risk factors of ACL injuries in weightlifting, as well as the general mechanisms and risk factors of ACL injuries in other similar movements. The findings of this study could provide insights into the feasibility of improving weightlifting techniques to prevent ACL injuries without affecting weightlifting performance. However, this research is a case study, and its findings are limited by individual differences such as the anatomical structure, technique, and training history of the participating athlete. Despite these limitations, the study offers insights into the mechanisms of ACL injuries during weightlifting activities. Through the analysis of biomechanical data from a specific athlete, it has revealed the injury mechanisms, enhancing our understanding of the conditions under which ACL injuries occur. It is hoped that the findings of this study will lay the groundwork for subsequent large-scale research and be validated and expanded upon in a broader population.
In this study, athletes’ ACL injury was hypothesized to be attributed to larger knee joint torque in all three axes during injury lift than during normal lift and a greater simulated force ratio between the quadriceps and the hamstring muscles. During injury lift, the knee joint angle in the sagittal plane is larger than during normal lift, whereas the angles in the coronal and horizontal planes are smaller.
2. Methods
2.1. Injury case in clean and jerk
This study captured an ACL injury event at the 2023 Chinese National Men's Weightlifting Championships in the 109 kg + category. The athlete, having a height of 191 cm, age of 22 years, and weight of 148 kg, sustained an ACL injury during the initial stage of a 210 kg clean and jerk. This study focused on the athlete's kinematic and kinetic parameters during a successful 205 kg clean and jerk (non-injury lift) and 210 kg clean and jerk (ACL injury lift) because of the scarcity of research on ACL injuries in the clean and jerk, the unique nature of this movement, and significant technical variations among athletes. Variables studied were eight indicators related to the risk of ACL injury (frontal, sagittal, and horizontal plane kinetics and kinematics, impact loading, and simulated muscle forces).
Our study received ethical approval from Zhejiang Normal University (No. ZSRT2024019).
2.2. Kinematic data collection
Two SONY Z90 cameras were used to capture fixed-point, fixed-focus footage of the athletes during the competition at a frame rate of 50 frames per second. The camera setup and PEAK three-dimensional calibration framework layout are consistent with related studies [47]. The Direct Linear Transform (DLT) algorithm was used for image synthesis. The videos were imported into SIMI Motion10.2 (SIMI, Germany) motion analysis system, where 42 bony landmarks of the athlete (Fig. 1) were manually marked to complete the collection and processing of kinematic data.
Fig. 1.
Marking of 42 bony landmarks on the human body.
2.3. Creation of the Barbell–Human model
Anybody 7.4 motion modeling simulation software (AnyBody Technology A/S, Denmark) was used for human simulation modeling and inverse dynamics calculations. Modifications were made to the FullBody_GRFPrediction model in the AMMR—v2.4.3 model library. A barbell model was created using Solidworks software according to the national standard GB 23177-2008 (total length of the barbell bar 2200 mm, diameter of the barbell bar 28 mm, diameter of the barbell plates = 450 mm) and imported into the Standing Model in STL format. Two nodes were established in the barbell, linked to the first joint of the index finger of the model, and spaced at the same distance as the athlete's grip. The first joint of the index finger was connected to the barbell nodes by using a hinge joint (1DOF*). The Scaling_Length_Mass_Fat mode was used for the model's body type, and the model's segment lengths were adjusted based on the subject's height, weight, and kinematic data from the Markers to ensure maximum alignment with the subject for accurate results [48].
2.4. Kinetic data collection
Kinematic data output from the SIMI Motion10.2 motion analysis system was converted into C3D files recognizable by Anybody simulation software 7.4 (AnyBody Technology, Aalborg, Denmark). Bony landmarks were imported into the Anybody standing model in C3D format, and the height and weight were adjusted to match the experimental subject. Finally, inverse dynamics analysis was run (Fig. 2).
Fig. 2.
Schematic diagram of muscle simulation in weightlifting clean and jerk movement.
The following function is used to calculate maximal muscle force and involves minimizing muscle activity, muscle recruitment, and objective function G. The calculation formula is as follows.
Objective Function:
| (1) |
Constraints:
| (2) |
Minimization of the maximal muscle activity:
| (3) |
P between 1 and 5:
| (4) |
Muscle activity :
| (5) |
here C is the system matrix; d is the external force of the human system; is the force of muscle during a period of movement; is the maximal muscle force; is the tensile strength of a single muscle and represents the maximum force provided by a muscle at its optimal length; is the muscle activity and represents the ratio of muscle force to muscle strength; R is the ground reaction force; and is the number of muscles [49].
2.5. Model validity verification
A male athlete (height 169 cm, weight 67 kg, age 22 years) was selected to perform the jerk dip phase of the clean and jerk to verify the reliability and accuracy of the FullBody_GRFPrediction model. The barbell weight was 40 kg. The athlete warmed up for 20 min before the test. Two high-speed cameras (SONY Z90) were used to collect the kinematic data of the athlete at a sampling frequency of 50 Hz, and a 3D force plate (Kistler, Switzerland) was employed at a sampling frequency of 1000 Hz. Surface electromyography (BTS, Italy) also had a sampling frequency of 1000 Hz. All equipment was synchronized using a HAM synchronizer. The human kinematic parameters obtained from SIMI Motion analysis were imported into Anybody simulation software for inverse dynamics analysis. The ground reaction force prediction and lower limb muscle activation curves obtained from inverse dynamics analysis were correlated with data simultaneously collected from the 3D force plate and surface electromyography during the experimental process. The similarity between the predicted vertical ground reaction force (VGRF) and the actual VGRF curves and the correlation between model muscle activation and actual human muscle activation were assessed [50,51]. Coefficient of multiple correlation (CMC) was used to evaluate the fit of simulated muscle forces to actual electromyography signals and simulate VGRF to actual VGRF [52].
| (6) |
is the number of curves, is the number of data points in each curve, is the th data point of the th curve, is the average of the th data points across curves, and is the overall average of the th data point across curves [53].
2.6. Division of movement phases
The clean and jerk consists of two parts, namely, the clean and the jerk. The jerk can be divided into five phases: the jerk dip, the jerk drive, the unsupported split under the bar, the supported split under the bar, and the recovery from the jerk. The moment of injury was determined to be 0.04 s before the end of the jerk dip braking (at the 0.30 s mark) through kinetic analysis. To further analyze the injury mechanism, this study divided the entire jerk dip phase into two stages, namely, the active jerk dip (from the start of the jerk dip to the moment of maximum barbell descent speed, M0–M1) and the jerk dip braking (from the moment of maximum barbell descent speed to the moment when the barbell descent speed is zero, M1–M3). Four moments were identified, namely, the start of active jerk dip (M0), the start of jerk dip braking (M1), the moment of injury (M2), and the end of jerk dip braking (M3). The total duration of the jerk dip phase (M0–M3) for non-injury and injury lifts was 0.34 s, and the active jerk dip stage (M0–M1) and the jerk dip braking stage (M1–M3) lasted for 0.22 and 0.12 s, respectively.
3. Results
3.1. Simulation validation results
3.1.1. VGRF validation
The effectiveness of AnyBody Modeling System was verified by the acquisition system and 3D force table of SIMI Motion. The model was found to accurately simulate VGRF, with a similarity of 0.958 to the F(Z) from the force plate (Fig. 3). The validation results showed a peak difference of 17.9 N between the simulation and force plate, with a minor difference in the timing of peak occurrence. This finding indicates that collecting kinematic parameters for dynamic simulation is feasible, and the accuracy can be assured. Collecting human kinematic parameters, analyzing joint degrees of freedom and characteristics to establish corresponding mechanical models, and solving them using mathematical equations are scientifically feasible [54].
Fig. 3.
VGRF curve from the jerk dip experiment by comparing 3D force plate and simulation.
3.1.2. Muscle activation simulation validation
As shown in Table 1, the CMC of the simulated muscle force curve and the surface electromyography signal curve was above 0.75, indicating a high similarity between simulated lower limb muscle force and electrophysiological data. This finding demonstrates the validity of the Anybody musculoskeletal model and its ability to accurately perform human–machine ergonomics analysis and research [51].
Table 1.
Correlation analysis between surface electromyography signals and simulated muscle force.
| Muscle | CMC |
|---|---|
| Rectus femoris | 0.905 |
| Gluteus Maximus | 0.792 |
| Semimembranosus | 0.751 |
| Gastrocnemius medialis | 0.855 |
3.2. Kinetic indicators
In Anybody human motion simulation software, the athlete's non-injury and injury lifts during the entire jerk dip process were modeled, and inverse dynamics calculations were performed over 0.34 s. Muscle activation (MA) [55] and simulated muscle force (MF) [56] during activity were calculated based on inverse dynamics optimization methods. The muscle activation data in each frame of motion were optimized to determine muscle strength and other indicators [57].
3.2.1. GRF
The GRF trends in the x-axis for non-injury and injury lifts were similar, showing an inverted bell-shaped curve. However, the timing of peak GRF values differed; the peak (285.46 N) and lowest value (−221.46 N) in the non-injury lift were higher and lower than those in the injury lift (251.42 N, −90.22 N), respectively. The GRF trends on the y-axis for both lifts were similar, displaying a double-peaked curve; however, the magnitudes and timings of peaks and valleys differed. The GRF changes in the z-axis were similar, with the main difference being that the injury lift reached its peak at moment M2 (5170.41 N) and then decreased; meanwhile, the non-injury lift continued to increase and reached its peak at M3 (5587.36 N, Table 2, Fig. 4).
Table 2.
Ground reaction forces at different moments of the two lifts (N)
Note: The positive and negative values on the x-axis and y-axis indicate the direction of the force. A positive value on the x-axis means that the force is directed backward, and a positive value on the y-axis means that the force is directed to the right. “NI” represents non-injured, and “I” represents injured.
| X-axis |
Y-axis |
Z-axis |
||||
|---|---|---|---|---|---|---|
| NI | I | NI | I | NI | I | |
| M0 | −221.46 | −90.22 | −40.19 | −100.30 | 3525.44 | 3576.26 |
| M1 | 132.63 | 251.42 | 174.41 | 30.59 | 3496.57 | 3194.76 |
| M2 | 124.38 | 55.92 | 22.43 | 64.33 | 5401.21 | 5170.41 |
| M3 | −117.34 | −60.95 | 49.05 | 22.84 | 5587.36 | 4690.42 |
Fig. 4.
Ground reaction force changes in the x, y, and z axes from the active jerk dip to the moment of injury in the two lifts.
3.2.2. Joint torques
Throughout the Jerk Dip phase, the flexion–extension, torsional, and eversion–inversion torques of the knee, hip, and ankle joints in the non-injury lift were higher than those in the injury lift. The knee joint flexion–extension torque in the two lifts differed; the knee extension torque in the injury lift increased from 50 % of the period to moment M2 and peaked at 376.73 Nm, while the peak extension torque in the non-injury lift occurred later and reached 481.46 Nm at M3. The hip and knee joint torsional torques in the two lifts differed in magnitude and direction; the injury lift's hip joint torsional torque first increased and then decreased (all negative values, i.e., internal rotation), peaking at M2 at −91.31 Nm. The hip joint torsional torque of the non-injury lift showed an inverted U-shape, changing from negative to positive values and then reverting back to negative, with a peak of 114.06 Nm. The knee joint torsional torque of the non-injury lift was multi-peaked and had positive values (i.e., external rotation), peaking at 60.11 Nm; meanwhile, that of the injury lift showed a single peak, changing from positive to negative values (i.e., from external to internal rotation), with the maximum internal rotation torque of 21.34 Nm at M2 (Table 3, Fig. 5). The knee joint eversion torque of the injury lift peaked at M2 (130.16Nm), while that of the non-injury lift continued to increase and peaked at M3 (195.42 Nm).
Table 3.
Lower limb joint torques at different moments of the two lifts (Nm).
| Variable | M0 |
M1 |
M2 |
M3 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| NI | I | NI | I | NI | I | NI | I | ||
| Hip joint (Nm) | external rotation(+) | −9.96 | −8.51 | 71.71 | −59.38 | 14.98 | −91.31 | −73.12 | −82.6 |
| Flexion (+) | 95.75 | 77.68 | 47.61 | 10.17 | 164.95 | 81.03 | 269.2 | 80.07 | |
| Eversion(+) | −3.22 | −1.32 | −559.64 | −111.56 | −276.21 | −45.45 | −77.03 | −33.18 | |
| Knee joint (Nm) | external rotation(+) | 15.42 | 10.44 | 60.11 | 2.32 | 8.68 | −17.95 | 24.23 | −21.34 |
| extension (+) | 28.02 | 22.32 | −3.25 | 73.43 | 206.45 | 354.1 | 435.2 | 355.46 | |
| Eversion(+) | 0.62 | 1.02 | 34.07 | 63.88 | 141.32 | 130.16 | 179.4 | 121.15 | |
| Ankle Joint (Nm) | external rotation(+) | 14.16 | 15.67 | 152.81 | 85.85 | 139.88 | 80.11 | 94.41 | 65.51 |
| Dorsiflexion (+) | −40.71 | −47.32 | −380.42 | −305.22 | −389.55 | −278.94 | −262.91 | −229.05 | |
| Eversion(+) | 28.57 | 19.22 | 281.35 | 152.03 | 264.53 | 151.07 | 183.57 | 124.13 | |
Fig. 5.
Changes in lower limb joint flexion–extension and torsional torques from the active jerk dip to the moment of injury in the two lifts. (a): knee rotation moment; (b): Knee Joint Flexion-Extension and Inversion-Eversion; (c): Hip Joint Flexion-Extension, Abduction-Adduction, and Rotation Moments; (d) Ankle Joint Flexion-Extension, Inversion-Eversion, and Rotation Moments.
3.2.3. Muscle activation and simulated forces
AnyBody simulation software introduced the concept of muscle activation (MA) to express the relative use of a muscle according to its length. MA directly reflects the level of muscle activation; that is, higher MA indicates greater relative use of the muscle [58]. In the injury lift, the muscle activation and simulated forces of the quadriceps and gastrocnemius at each moment were higher than those in the non-injury lift. Compared with those in the non-injury lift, the MA and MF of the quadriceps in the injury lift at M2 increased by 100.93 % and 25.37 %, respectively; those of the gastrocnemius increased by 26.76 % and 58.69 %, respectively. The MA and MF of the semitendinosus, semimembranosus, biceps femoris, and soleus in the non-injury lift were higher than those in the injury lift. Compared with those in the non-injury lift, the MA and MF of the biceps femoris in the injury lift at M2 decreased by 70.00 % and 73.80 %, respectively; and those of the soleus decreased by 35.85 % and 38.95 %, respectively (Table 4, Fig. 6).
Table 4.
Comparison of lower limb muscle activity and simulated forces in the two lifts.
| Variable | M0 |
M1 |
M2 |
M3 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| NI | I | NI | I | NI | I | NI | I | ||
| Muscle activity | Quads | 0.01 | 0.23 | 0.58 | 1.51 | 2.15 | 4.32 | 3.1 | 4.19 |
| ST | 0.02 | 0.01 | 0.18 | 0.11 | 0.23 | 0.16 | 0.22 | 0.14 | |
| SM | 0.03 | 0.02 | 0.21 | 0.10 | 0.23 | 0.13 | 0.21 | 0.12 | |
| BF | 0.06 | 0.03 | 0.42 | 0.13 | 0.60 | 0.18 | 0.48 | 0.15 | |
| GA | 0.40 | 0.44 | 3.56 | 3.62 | 2.69 | 3.41 | 1.72 | 2.31 | |
| S | 0.21 | 0.08 | 1.23 | 0.45 | 1.59 | 1.02 | 1.36 | 0.89 | |
| Muscle simulated forces(N) | Quads | 120.60 | 654.3 | 3006.57 | 4718.69 | 11132.74 | 13956.62 | 16035.01 | 13229.12 |
| ST | 18.52 | 4.98 | 283.45 | 175.70 | 261.56 | 134.55 | 252.08 | 128.20 | |
| SM | 68.44 | 30.32 | 332.92 | 202.36 | 376.91 | 203.36 | 332.90 | 245.10 | |
| BF | 173.90 | 138.00 | 2268.83 | 586.80 | 3373.30 | 883.97 | 1607.45 | 488.40 | |
| GA | 367.82 | 1132.32 | 5298.04 | 5627.03 | 5636.35 | 8944.12 | 5112.44 | 4543.31 | |
| S | 653.23 | 457.23 | 4778.22 | 2917.48 | 4778.22 | 2917.48 | 3736.26 | 2715.84 | |
Note: Quads: Quadriceps; ST: Semitendinosus; SM: Semimembranosus; BF: Biceps Femoris; GA: Gastrocnemius; S: Soleus.
Fig. 6.
Comparison of lower limb muscle activation simulation in the two lifts. (a): The comparison of muscle activation levels for Quadriceps femoris muscle, Biceps femoris muscle, Gastrocnemius muscle, and Soleus muscle; (b): Activation of the Vastus Lateralis Muscle.
Each muscle's MA and MF were analyzed to further explore the effect of the quadriceps group on the ACL. In the injury lift, the MA and MF of the vastus medialis, rectus femoris, and vastus intermedius were higher than those in the non-injury lift. Compared with those in the non-injury lift, the MA and MF of the vastus medialis at M2 increased by 100 % and 84.99 %, respectively (Table 5, Fig. 6).
Table 5.
Comparison of quadriceps muscle activity and simulated forces in the two lifts.
| Variable | M0 |
M1 |
M2 |
M3 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| NI | I | NI | I | NI | I | NI | I | ||
| Muscle activity | L Quads | 0.01 | 0.01 | 0.15 | 0.03 | 0.27 | 0.14 | 0.26 | 0.20 |
| M Quads | 0.01 | 0.04 | 0.09 | 0.25 | 0.36 | 0.72 | 0.52 | 0.70 | |
| VM | 0.01 | 0.03 | 0.07 | 1.06 | 1.25 | 2.35 | 2.58 | 2.22 | |
| RF | 0.58 | 0.66 | 0.13 | 0.32 | 0.42 | 0.79 | 0.59 | 0.79 | |
| Muscle simulated forces(N) | L Quads | 178.93 | 27.44 | 2555.54 | 976.18 | 4852.44 | 4006.04 | 5782.00 | 4715.37 |
| M Quads | 10.23 | 55.34 | 235.33 | 351.75 | 655.43 | 1212.46 | 712.46 | 1053.25 | |
| VM | 2.76 | 8.33 | 52.16 | 75.04 | 167.92 | 187.58 | 234.09 | 187.92 | |
| RF | 493.87 | 544.99 | 62.60 | 543.65 | 1061.96 | 1202.35 | 2197.74 | 1132.24 | |
Note: L Quads: Lateral Quadriceps; M Quads: Medial Quadriceps; VM: Vastus Medialis; RF: Rectus Femoris.
3.3. Kinematic indicators
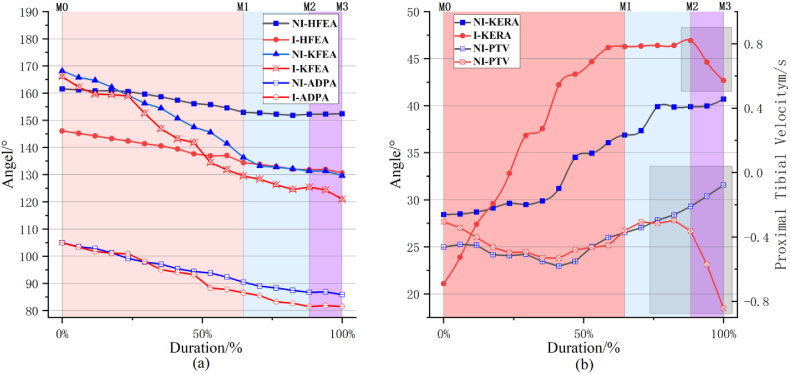
The kinematic characteristics of the lower limbs were compared between the athlete's injury and non-injury lifts. During the entire jerk dip phase, the flexion angles of the hip, knee, and ankle joints in both lifts showed a gradual decreasing trend, with the three joint angles in the non-injury lift being larger at all four moments (Fig. 4). The ankle joint angle difference became significantly larger at M3 (4.30°); at this moment, the left ankle joint angular velocity of the injury lift was 63.90°/s, while that of the non-injury lift was only −0.13°/s. The eversion angle values of the knee joint in both lifts changed from negative to positive values (from inversion to eversion), and that of the injury lift was higher, peaking at 5.90°. The knee joint torsional angle of the injury lift first increased and then decreased (from external to internal rotation), peaking at M2 (46.93°), while that of the non-injury lift showed a gradually increasing (external rotation) trend. The forward speed of the proximal left tibia at M3 differed between the two lifts. The speed of the injury lift was 0.76 m/s higher than that of the non-injury lift (Table 6, Fig. 7).
Table 6.
Kinematic indicators of the jerk dip phase in the two lifts at different moments.
| M0 |
M1 |
M2 |
M3 |
|||||
|---|---|---|---|---|---|---|---|---|
| NI | I | NI | I | NI | I | NI | I | |
| Hip Flexion and Extension Angle (°) | 161.56 | 146.06 | 152.94 | 137.01 | 152.25 | 131.85 | 152.43 | 130.61 |
| Knee Flexion and Extension Angle (°) | 168.23 | 166.12 | 136.33 | 131.78 | 131.35 | 125.45 | 129.66 | 121.00 |
| Ankle Dorsiflexion and Plantarflexion Angle (°) | 105.00 | 104.92 | 90.45 | 87.75 | 86.71 | 81.53 | 85.89 | 81.59 |
| Knee Varus/Valgus Angle (°) | −5.44 | −3.32 | 3.18 | 4.78 | 3.61 | 5.01 | 3.25 | 3.68 |
| Knee External/Internal Rotation Angle (°) | 30.94 | 21.09 | 36.88 | 46.18 | 39.89 | 46.93 | 40.70 | 42.68 |
| Proximal Tibial Velocity (m/s) | −0.46 | −0.31 | −0.37 | −0.45 | −0.21 | −0.36 | −0.08 | −0.84 |
Note: A positive value for knee valgus angle indicates valgus (outward angulation), while a negative value indicates varus (inward angulation). The gradual increase in the knee rotation angle represents external knee rotation, whereas a decrease indicates internal rotation.
Fig. 7.
Kinematic indicator changes during the jerk dip process in the two lifts. (a): Hip, Knee, Ankle Joint Flexion Angle; (b): knee joint internal rotation angle and proximal tibia velocity
Note: NI-Hip Flexion and Extension Angle: NI-HFEA; I-Hip Flexion and Extension Angle: I-HFEA; NI-Knee Flexion and Extension Angle: NI-KFEA; I-Knee Flexion and Extension Angle: I-KFEA; NI-Ankle Dorsiflexion and Plantarflexion Angle: NI-ADPA; I-Ankle Dorsiflexion and Plantarflexion Angle: I-ADPA; NI-Knee Ex/Internal Rotation Angle: NI-KERA; I-Knee Ex/Internal Rotation Angle: I-KERA; NI-Proximal Tibial Velocity: NI-PTV; I-Proximal Tibial Velocity: I-PTV.
4. Discussion
The video footage of the athlete's injury process captured on-site was converted into 3D by using the DLT algorithm, and inverse dynamics simulation was performed through musculoskeletal modeling to obtain the kinematic and dynamic data of the athlete during normal and injury lifts for analysis. The results showed differences in the kinematic and dynamic data between the two lifts. At the moment of injury, the knee joint flexion-extension torque is consistent with the hypothesis, whereas the torque magnitudes in the other two axes and the angles in the three planes do not match the hypothesis. The ratio between the quadriceps and the hamstring muscles is consistent with the previous hypothesis.
4.1. GRF analysis of ACL injury
During the active jerk dip phase, an athlete actively flexes the knees and hips, accelerating the body and the barbell downward. In this phase, the body and barbell are in a state of weightlessness, and the VGRF is less than the combined force of the body and barbell. In the jerk dip braking phase, the athlete decelerates the descent through the extensor muscles of the lower limbs, resulting in an overweight state, where VGRF exceeds the combined force. After M1, the vertical GRF continuously increases and peaks at M3. The VGRF of the injury lift differs mainly after M1, where it starts to decline at M2, suggesting the possibility of ACL injury. The load on the ACL is proportional to VGRF [59,60]. However, the small difference in VGRF between the two lifts and the lower VGRF at M3 in the injury lift suggest that VGRF is not the direct cause of ACL injury.
Previous works did not discuss GRF in the x and y axes. In the present study, the changes in the GRF in the x and y axes were relatively minor. The GRF of the non-injury and injury lifts showed an inverted bell-shaped curve in the X-axis, with the peak occurring later in the non-injury lift. This finding indicates that the center of gravity of the injury lift was more forward during the jerk dip braking phase. The GRF of both lifts in the y-axis showed minor differences in amplitude, suggesting similar stability [47].
4.2. Joint torque analysis of ACL injury
From a mechanical perspective, non-contact ACL injuries occur when the knee joint experiences significant forces or torques generated by the individual, causing the ACL to exceed its ultimate load-bearing capacity. The anterior shear force at the proximal tibia is a primary contributor to ACL injuries [61]. Zhang Meizhen et al. [59,62]pointed out a significant correlation between knee joint extension torque and anterior shear force at the proximal tibia. The results of this study showed differences in the knee joint extension torque during the two attempts; at M2, when the extension torque during the injured attempt reached its maximum, the difference between the two was only 33.56 Nm. Furthermore, at M3, the non-injured attempt reached a peak extension torque of 481.46 Nm, which is higher than that of the injured attempt. The increase in shear force due to knee joint extension torque during non-injured attempts is insufficient to cause ACL injuries. Additionally, the relationship between knee joint flexion–extension torque and shear force is not clear. Therefore, the combined effect of internal–external varus–valgus torque and internal–external rotation torque on the knee joint leads to ACL injuries. However, some studies [61,63] support the notion that isolated internal–external varus–valgus torque and internal–external rotation torque cannot lead to ACL injuries. They stated that the combined effect of internal–external varus–valgus torque, internal–external rotation torque, and knee joint shear force are the key factors in causing ACL injuries. At present, the combined effects of internal–external varus–valgus torque, internal–external rotation torque, and shear force on the ACL remain debatable. Markolf et al. [63] suggested that external rotation torque can, to some extent, reduce ACL load, while internal rotation and internal–external varus–valgus torque can increase ACL load. However, other research [64] indicated that only internal rotation torque increased ACL load, with other torques having a less significant effect on the ACL. In the present study, from M0 to M3, both attempts at the knee joint were in the form of eversion torque; the peak external rotation torque of the non-injured attempt (195.42 Nm) was greater than that of the injured attempt (130.16 Nm). Studies [64,65] confirmed that the MCL is the primary structure responsible for bearing external rotation torque. In cases where the MCL is intact, the increase in external rotation torque may not significantly affect ACL load. A significant difference in twisting torque was found at the knee joint during the two attempts. Starting from M1, the twisting torque at the knee joint during the injured attempt changed from external rotation torque to internal rotation torque (torque values changed from positive to negative, Fig. 8), and the internal rotation torque gradually increased. By contrast, the non-injured attempt consistently had external rotation torque. Therefore, the combined effect of knee joint internal rotation torque and shear force may be the key factor that leads to ACL injuries. The results also showed that athletes during the injured attempt had hip joint internal rotation, while athletes during the non-injured attempt had hip joint external rotation. Knee joint injuries are related to kinematic and dynamic abnormalities of the hip joint, and excessive adduction and internal rotation of the hip joint under load can lead to knee joint internal rotation [[66], [67], [68], [69], [70]].
Fig. 8.
Muscle mechanisms leading to ACL injuries.
4.3. MA and MF analysis of ACL injury
Non-contact ACL injury risks are related to neuromuscular factors that influence the mechanical load on the knee joint. These factors affect the size and activation sequence of the muscles around the knee joint, thereby affecting its stability and ultimately leading to ACL injuries [[71], [72], [73]]. The quadriceps [[71], [72], [73]], hamstring [75], gastrocnemius [76], and soleus muscles [77] are considered to be related to ACL injuries.
The contraction force of the quadriceps muscles significantly contributes to the generation of anterior shear force at the knee joint, especially during closed-chain movements, where the foot is fixed on the ground. This phenomenon results in oblique anterior upward tension on the tibia [78,79]. In this study, the MA and MF of the quadriceps during injured and non-injured clean and jerk movements were compared; the results revealed that from M0 to M2, MA and MF were higher in the injured clean and jerk than in the non-injured clean and jerk. At M2, MA during the injured clean and jerk was 4.32, which was 2.01 times that of the non-injured clean and jerk, with a 25.37 % increase in MF. These findings suggest that the increase in MA and MF of the quadriceps may be one of the contributing factors to ACL injuries [74,79,80]. Furthermore, differences in attachment points and moment arms of individual quadriceps muscles affected ACL load [81]. Wünschel et al. [82] reported a significant correlation between high medial quadriceps muscle strength and knee joint internal rotation. This study confirmed that injured clean-and-jerk athletes had higher MA and MF in the medial quadriceps compared with non-injured clean-and-jerk athletes. Therefore, knee joint internal rotation torque during the jerk dip phase of weightlifting may be associated with the contraction of the medial quadriceps.
The contraction and activation of the hamstring muscles in relation to the protection of the anterior cruciate ligament (ACL) has been a subject of debate. Clinically, the hamstring muscles (including the biceps femoris, semimembranosus, and semitendinosus) are considered to be antagonistic to the quadriceps muscles. Many studies, including in vivo and muscle simulation experiments, suggest that the co-contraction of the hamstring and quadriceps muscles can protect the ACL by reducing its strain [[83], [84], [85]], anterior tibial traction force [75,86,87], anterior tibial translation [74,75,88], and internal tibial rotation [74,75,88].However, when the knee joint flexion angle was small (<30°), the hamstring muscles may not provide protective effects [89]. The results of this research indicated that the MA and MF of hamstring muscles, including the biceps femoris, semimembranosus, and semitendinosus, were smaller during injured clean and jerk than during non-injured clean and jerk. At M2, the peak MA (0.60) and MF (3373.30 N) of the non-injured clean and jerk hamstring muscles were 3.33 and 3.82 times higher than those of the injured clean and jerk, suggesting that hamstring muscles, particularly the biceps femoris, contraction may play a role in reducing ACL load. These findings are consistent with prior research, and the knee joint flexion angle during M2 in this study was approximately 50° (>30°). Some experimental studies have used the hamstring/quadriceps (H/Q) ratio to compare the risk of injury in athletes performing different actions; a lower H/Q ratio indicates a higher risk of injury [85,90]. In the present study, the maximum H/Q ratio during injured clean and jerk was only 6.33 %, and that for non-injured clean and jerk was 30.30 %. These results are in line with previous research, where injured clean-and-jerk athletes exhibited a significantly lower H/Q ratio than non-injured clean-and-jerk athletes. Further research [81,86,88,91] has shown that isolated quadriceps contraction can cause tibial internal rotation, whereas concurrent contraction of the hamstring muscles, particularly the biceps femoris, can counteract knee internal rotation, thereby effectively reducing ACL load. The biceps femoris was found to be more effective in generating muscle strength than the semitendinosus and semimembranosus muscles.
The gastrocnemius muscle, spanning the knee joint, plays a crucial role in ACL mechanics, actively contracting when the knee joint is flexed [64,76,92]. This contraction leads to an increase in posterior shear force on the tibia, causing an increase in ACL stress. The results indicated that from M0 to M2, MA and MF of the gastrocnemius muscle of the injured clean and jerk were higher than those of the non-injured clean and jerk. At M2, the MA and MF of the gastrocnemius muscle of the injured clean and jerk increased by 26.76 % and 58.69 %, respectively, suggesting an association between gastrocnemius muscle contraction and ACL injuries. Further experimental studies have found that during the completion of single-leg landing [93] and lateral jumping [94] actions, the forward shear force generated by the gastrocnemius is comparable to the strength of the quadriceps muscle group (up to 334 N and 342 N, respectively). However, when completing side-cutting [52] and double-leg landing [95] actions (117 N vs 233 N and 144 N vs 577 N, respectively), the forward shear force generated by the gastrocnemius is relatively less. The emergence of these controversies may be related to the specific knee joint actions selected in previous studies when examining the effect of the gastrocnemius on ACL stress. Although the soleus muscle does not cross the knee joint, an increasing number of studies [77,92,96,97] indicate that the soleus muscle can effectively reduce the load on the ACL. Sritharan et al. [77] suggest that the soleus muscle, in conjunction with the ankle dorsiflexors, plays an important role in stabilizing the knee joint and resisting anterior tibial translation. When the soleus muscle is severed, the ability of the ankle dorsiflexors to resist anterior tibial translation disappears. In this study, the MA and MF of the soleus muscle during injury trials at moments M0 to M2 were less than those during non-injury trials, consistent with related research [92,96,97], indicating that the contraction of the soleus muscle during the squatting process generates a backward shear force on the tibia, reducing the load on the ACL.
4.4. Kinematic analysis of ACL injury
In the jerk dip phase of weightlifting, athletes use this movement to transfer the ground reaction force through their body to the barbell. During the force application phase, rapid knee extension, hip extension, and barbell thrusting are employed to give the barbell an appropriate initial velocity at the end of this phase. Li et al. [98]suggested that the risk of ACL (anterior cruciate ligament) injury increases as the knee flexion angle decreases. Our study results indicate that, compared with non-injury trials, injury trials exhibit a greater knee flexion angle, which may be related to the specificity of the weightlifting jerk dip movement. Additionally, our results reveal a marked increase in the ankle joint angle at moment M3 in injury trials, differing from non-injury trials. This findings suggests a plantarflexion motion in athletes during injury trials. The dynamical analysis at this moment shows that the ankle's flexion-extension torque is less than in non-injury trials, indicating that the plantarflexion motion in kinematics is not due to active force generation by the athlete, but possibly a stress response due to ACL rupture.
Previous research [14,63,78,99] pointed out that most non-contact ACL injuries occur in positions with slight knee flexion and torsion, leading to increased ACL loading. From moment M0 to M3, the rotational angle of the knee joint in non-injury trials continuously increases, indicating persistent external rotation of the knee joint. By contrast, the rotational angle in injury trials first increases and then decreases, indicating a shift from external to internal rotation of the knee joint. The peak rotational velocity of the knee joint at moment M3 reaches 193.46°/s, suggesting that internal rotation may be one of the kinematic characteristics of ACL injury during the jerk dip phase. During knee flexion, one of the primary functions of the anterior cruciate ligament is to control the anterior translation of the tibia [100,101]. The anterior shear force experienced by the proximal tibia is a crucial factor in determining the tension in the anterior–middle bundle of the ACL [61]. Our study confirms that at the moment M3, the forward speed of the proximal tibia in injury trials is significantly higher than in non-injury trials, indicating that the ACL may be subjected to a greater forward shear force load at this time, thus suggesting anterior tibial translation as another kinematic characteristic of ACL injury in athletes. Furthermore, our results show that the knee joint inversion-eversion angle in both types of trials exhibits a trend from inversion to eversion, with a larger range of change in the injury trials. Similar studies [102,103] reported findings consistent with our results, where knee eversion and internal rotation, along with anterior tibial translation, are the primary kinematic characteristics of ACL injury.
In summary, during the jerk dip phase in weightlifting, the kinematic manifestations leading to ACL injury in athletes include large internal and external rotation angles of the knee joint in a flexed state, as well as rapid anterior movement of the tibia. Dynamic simulation results suggest that overactivation of the quadriceps and gastrocnemius muscles, coupled with insufficient activation of the hamstrings and soleus muscles, are the primary causes of ACL injury in athletes. The quadriceps and gastrocnemius muscles jointly provide anterior shear force on the knee joint, promoting anterior tibial translation relative to the femur. The adductor muscles of the thigh primarily contribute to internal rotation torque, thereby increasing the load on the ACL. The smaller activation and contraction of the hamstrings (particularly the biceps femoris) and the soleus muscle are insufficient to counteract the forward shear force and internal rotation torque on the knee joint, thus failing to effectively reduce the load on the ACL (Fig. 8).
4.5. Limitations
Due to the unpredictability of when and where an athlete's injury will occur, especially in competitive settings, capturing data on ACL injuries in weightlifting is challenging, and simulations under laboratory conditions are not feasible. Consequently, One of the limitations of this study is the single subject sample size, which means the results are subject to individual variations such as the athlete's anatomical structure, technique, and training history. We plan to address this issue in future research by collecting a larger sample size. Moreover, the videos in this study were taken under competitive conditions by using two cameras, and 3D kinematic data were synthesized using the DLT algorithm. During the digitalization process of the video, body joints were inevitably occluded by partial limbs, making them invisible in the video. The joints were locally magnified to make the body joints clearer, which helped improve the accuracy of the data.
5. Conclusions
-
1)
Kinematic parameters collected through high-speed videography can be effectively applied in the AnyBody motion simulation system to explore the mechanisms of injuries occurring during athletic movements. This approach is feasible and reasonable.
-
2)
Compared with the non-injury lift, the injury lift of the weightlifting athlete shows differences in kinematic indicators. Notably, the injury lift exhibits a larger knee internal rotation angle and anterior tibial translation, which may be kinematic characteristics of ACL injuries.
-
3)
Compared with the non-injury lift, the injury lift of the weightlifting athlete demonstrates differences in joint torques, muscle activation, and simulated muscle forces. Specifically, the smaller MF and MA of the hamstrings and soleus are unable to effectively counteract the larger MF and MA of the quadriceps (especially the vastus medialis) and gastrocnemius. This phenomenon results in increased anterior shear force and internal rotation torque on the knee joint, leading to the ACL being subjected to forces beyond its ultimate load-bearing capacity.
Funding
The present study was funded by the Department of Science and Technology of Zhejiang Province (No. 2021C03128 and No. 2023C03197).
Ethics statement
Our study received ethical approval from Zhejiang Normal University (No. ZSRT2024019). The participants provided their written informed consent to participate in this study.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Binyong Ye: Writing – original draft, Methodology, Data curation. Gongju Liu: Writing – review & editing, Funding acquisition. Zhanyang He: Writing – original draft, Software, Methodology. Jun Xu: Software. Huiju Pan: Writing – review & editing. Houwei Zhu: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Raske A., Norlin R. Injury incidence and prevalence among elite weight and power lifters. Am. J. Sports Med. 2002;30:248–256. doi: 10.1177/03635465020300021701. [DOI] [PubMed] [Google Scholar]
- 2.Sherbondy P.S., Queale W.S., McFarland E.G., Mizuno Y., Cosgarea A.J. Soleus and gastrocnemius muscle loading decreases anterior tibial translation in anterior cruciate ligament intact and deficient knees. J. Knee Surg. 2003;16:152–158. [PubMed] [Google Scholar]
- 3.Mazza D., Viglietta E., Monaco E., Iorio R., Marzilli F., Princi G., Massafra C., Ferretti A. Impact of anterior cruciate ligament injury on European professional soccer players. Orthopaedic Journal of Sports Medicine. 2022;10 doi: 10.1177/23259671221076865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huyghe T., Goriss B., Delosangeles E., Bird S.P. vol. 1. 2021. pp. 1–10. (Exploring the Power Clean, International Universities Strength and Conditioning Association). [Google Scholar]
- 5.Zecchin A., Puggina E.F., Hortobágyi T., Granacher U. Association between foundation strength and weightlifting exercises in highly trained weightlifters: support for a general strength component. J. Strength Condit Res. 2022;37(7):1375–1381. doi: 10.1519/JSC.0000000000004433. [DOI] [PubMed] [Google Scholar]
- 6.Verhoeff W., Millar S., Oldham A., Cronin J. Coaching the power clean: a constraints-led approach. Strength Condit. J. 2019;42:16–25. [Google Scholar]
- 7.Yu B., Herman D., Preston J., Lu W., Kirkendall D., Garrett W. Immediate effects of a knee brace with a constraint to knee extension on knee kinematics and ground reaction forces in a stop-jump task. Am. J. Sports Med. 2004;32:1136–1143. doi: 10.1177/0363546503262204. [DOI] [PubMed] [Google Scholar]
- 8.Lin C., Liu H., Garrett W., Yu B. Effects of a knee extension constraint brace on selected lower extremity motion patterns during a stop-jump task. J. Appl. Biomech. 2008;24:158–165. doi: 10.1123/jab.24.2.158. [DOI] [PubMed] [Google Scholar]
- 9.Dempsey A.R., Lloyd D., Elliott B., Steele J., Munro B. Changing sidestep cutting technique reduces knee valgus loading. Am. J. Sports Med. 2009;37:2194–2200. doi: 10.1177/0363546509334373. [DOI] [PubMed] [Google Scholar]
- 10.Lephart S., Abt J., Ferris C.M., Sell T., Nagai T., Myers J., Irrgang J. Neuromuscular and biomechanical characteristic changes in high school athletes: a plyometric versus basic resistance program. Brit. J. Sport Med. 2005;39:932–938. doi: 10.1136/bjsm.2005.019083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myer G., Ford K., Brent J.L., Hewett T. The effects of plyometric vs.dynamic stabilization and balance training on power, balance, and landing force in female athletes. J. Strength Condit Res. 2006;20:345–353. doi: 10.1519/R-17955.1. [DOI] [PubMed] [Google Scholar]
- 12.Paterno M., Myer G., Ford K., Hewett T. Neuromuscular training improves single-limb stability in young female athletes. J. Orthop. Sport Phys. 2004;34:305–316. doi: 10.2519/jospt.2004.34.6.305. [DOI] [PubMed] [Google Scholar]
- 13.Hewett T., Stroupe A.L., Nance T., Noyes F. Plyometric training in female athletes. Am. J. Sports Med. 1996;24:765–773. doi: 10.1177/036354659602400611. [DOI] [PubMed] [Google Scholar]
- 14.Boden B.P., Dean G.S., Feagin J.A., Garrett W.E. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23:573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 15.Noyes F.R., Mooar P.A., Matthews D.S., Butler D.L. The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. JBJS. 1983;65:154–162. doi: 10.2106/00004623-198365020-00003. [DOI] [PubMed] [Google Scholar]
- 16.Agel J., Arendt E., Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am. J. Sports Med. 2005;33:524–531. doi: 10.1177/0363546504269937. [DOI] [PubMed] [Google Scholar]
- 17.Renström P., Ljungqvist A., Arendt E., Beynnon B., Fukubayashi T., Garrett W., Georgoulis T., Hewett T., Johnson R., Krosshaug T., Mandelbaum B., Micheli L., Myklebust G., Roos E., Roos H., Schamasch P., Shultz S., Werner S., Wojtys E., Engebretsen L. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Brit. J. Sport Med. 2008;42:394–412. doi: 10.1136/bjsm.2008.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fairley J., Wollen E., Gomez A., Vega N., Oaties W. Impact of fifa 11+ program on acl injury rates in collegian female rugby players. Med. Sci. Sports Exerc. 2020;52:793. 793. [Google Scholar]
- 19.Rourke K.O., Quinn F., Mun S., Browne M., Sheehan J., Cusack S., Molloy M. A comparison of paediatric soccer, gaelic football and rugby injuries presenting to an emergency department in Ireland. Injury. 2006;38:104–111. doi: 10.1016/j.injury.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Huston L.J., Wojtys E.M. Neuromuscular performance characteristics in elite female athletes. Am. J. Sports Med. 1996;24:427–436. doi: 10.1177/036354659602400405. [DOI] [PubMed] [Google Scholar]
- 21.Draganich L.F., Vahey J.W. An in vitro study of anterior cruciate ligament strain induced by quadriceps and hamstrings forces. J. Biomech. 1990;23:717. doi: 10.1002/jor.1100080107. 717. [DOI] [PubMed] [Google Scholar]
- 22.Dürselen L., Claes L., Kiefer H. The influence of muscle forces and external loads on cruciate ligament strain. Am. J. Sports Med. 1995;23:129–136. doi: 10.1177/036354659502300122. [DOI] [PubMed] [Google Scholar]
- 23.Hewett T., Torg J., Boden B. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Brit. J. Sport Med. 2009;43:417–422. doi: 10.1136/bjsm.2009.059162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zazulak B.T., Hewett T., Reeves N.P., Goldberg B., Cholewicki J. The effects of core proprioception on knee injury. Am. J. Sports Med. 2007;35:368–373. doi: 10.1177/0363546506297909. [DOI] [PubMed] [Google Scholar]
- 25.Zazulak B.T., Hewett T., Reeves N.P., Goldberg B., Cholewicki J. Deficits in neuromuscular control of the trunk predict knee injury risk. Am. J. Sports Med. 2007;35:1123–1130. doi: 10.1177/0363546507301585. [DOI] [PubMed] [Google Scholar]
- 26.Amp B.D., Yu B. Prevention of ACL injury, Part I: injury characteristics, risk factors, and loading mechanism. Res. Sports Med. 2012;20:180–197. doi: 10.1080/15438627.2012.680990. [DOI] [PubMed] [Google Scholar]
- 27.Yu B., Garrett W. Mechanisms of non-contact ACL injuries. Brit. J. Sport Med. 2007;41:47–51. doi: 10.1136/bjsm.2007.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai B., Herman D., Liu H., Garrett W., Yu B. Prevention of ACL injury, Part II: effects of ACL injury prevention programs on neuromuscular risk factors and injury rate. Res. Sports Med. 2012;20:198–222. doi: 10.1080/15438627.2012.680987. [DOI] [PubMed] [Google Scholar]
- 29.Dai B., Mao D., Garrett W., Yu B. Anterior cruciate ligament injuries in soccer: loading mechanisms, risk factors, and prevention programs. J. Sport Health Sci. 2014;3:299–306. [Google Scholar]
- 30.Quatman C.E., Hewett T. The anterior cruciate ligament injury controversy: is “valgus collapse” a sex-specific mechanism? Brit. J. Sport Med. 2009;43:328–335. doi: 10.1136/bjsm.2009.059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLean S., Huang X., Su A., van den Bogert A.J. Sagittal plane biomechanics cannot injure the ACL during sidestep cutting. Clin. BioMech. 2004;19:828–838. doi: 10.1016/j.clinbiomech.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Mundt D.J., Kelsey J.L., Golden A.L., Panjabi M.M., Pastides H., Berg A.T., Sklar J., Hosea T. An epidemiologic study of sports and weight lifting as possible risk factors for herniated lumbar and cervical discs. Am. J. Sports Med. 1993;21:854. doi: 10.1177/036354659302100617. [DOI] [PubMed] [Google Scholar]
- 33.Cochrane J.L., Lloyd D.G., Buttfield A., Seward H., Mcgivern J. Characteristics of anterior cruciate ligament injuries in Australian football. J. Sci. Med. Sport. 2007;10:96–104. doi: 10.1016/j.jsams.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Olsen O., Myklebust G., Engebretsen L., Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball. Am. J. Sports Med. 2004;32:1002–1012. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- 35.Bere T., Flørenes T.W., Krosshaug T., Koga H., Nordsletten L., Irving C., Muller E., Reid R.C., Senner V., Bahr R. Mechanisms of anterior cruciate ligament injury in world cup alpine skiing: a systematic video analysis of 20 cases. Am. J. Sports Med. 2011;45:326–327. doi: 10.1177/0363546511405147. [DOI] [PubMed] [Google Scholar]
- 36.Krosshaug T., Bahr R. A model-based image-matching technique for three-dimensional reconstruction of human motion from uncalibrated video sequences. J. Biomech. 2005;38:919–929. doi: 10.1016/j.jbiomech.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 37.Koga H., Bahr R., Myklebust G., Engebretsen L., Grund T., Krosshaug T. Estimating anterior tibial translation from model-based image-matching of a noncontact anterior cruciate ligament injury in professional football: a case report. Clin. J. Sport Med. 2011;21:271–274. doi: 10.1097/JSM.0b013e31821899ec. [DOI] [PubMed] [Google Scholar]
- 38.Bere T., Mok K., Koga H., Krosshaug T., Nordsletten L., Bahr R. Kinematics of anterior cruciate ligament ruptures in world cup alpine skiing. Am. J. Sports Med. 2013;41:1067–1073. doi: 10.1177/0363546513479341. [DOI] [PubMed] [Google Scholar]
- 39.Koga H., Nakamae A., Shima Y., Iwasa J., Myklebust G., Engebretsen L., Bahr R., Krosshaug T. Mechanisms for noncontact anterior cruciate ligament injuries. Am. J. Sports Med. 2010;38:2218–2225. doi: 10.1177/0363546510373570. [DOI] [PubMed] [Google Scholar]
- 40.Chen L., Armstrong C.W., Raftopoulos D.D. An investigation on the accuracy of three-dimensional space reconstruction using the direct linear transformation technique. J. Biomech. 1994;27:493–500. doi: 10.1016/0021-9290(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 41.Dai B., Leigh S., Li H., Mercer V.S., Yu B. The relationships between technique variability and performance in discus throwing. J. Sport Sci. 2012;31:219–228. doi: 10.1080/02640414.2012.729078. [DOI] [PubMed] [Google Scholar]
- 42.R. Akhundov, D.J. Saxby, L.E. Diamond, S. Snodgrass, P. Clausen, M. Drew, K. Dooley, T. Pizzari, E. Rio, A. Schultz, L. Donnan, T. McGann, S. Edwards, Game-play affects hamstring but not adductor muscle fibre mechanics in elite U20 basketball athletes, Sport Biomech, 1-17. [DOI] [PubMed]
- 43.Mahadas S., Mahadas K., Hung G.K. Biomechanics of the golf swing using OpenSim. Comput. Biol. Med. 2019;105:39–45. doi: 10.1016/j.compbiomed.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Chen X., Huang Y., Jiang L., Sun Q., Tian Y., Zhou Z., Yin J., Gao Y., Liu C., Huo B. Bilateral upper extremity trunk model for cross-country sit-skiing double poling propulsion: model development and validation. Med. Biol. Eng. Comput. 2023;61:445–455. doi: 10.1007/s11517-022-02724-8. [DOI] [PubMed] [Google Scholar]
- 45.Langholz J.B., Westman G., Karlsteen M. Musculoskeletal modelling in sports - evaluation of different software tools with focus on swimming. Procedia Eng. 2016;147:281–287. [Google Scholar]
- 46.Shi H., Ding L., Ren S., Jiang Y., Zhang H., Hu X., Huang H., Ao Y. Prediction of knee kinematics at the time of noncontact anterior cruciate ligament injuries based on the bone bruises. Ann. Biomed. Eng. 2020;49:162–170. doi: 10.1007/s10439-020-02523-y. [DOI] [PubMed] [Google Scholar]
- 47.Liu G., Zhu H., Ma J., Pan H., Pan X., Zhang Y., Hu T., Fekete G., Guo H., Liang M. A biomechanical study on failed snatch based on the human and bar combination barycenter. Appl. Bionics Biomech. 2022;2022:927–938. doi: 10.1155/2022/9279638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frankenfield D., Rowe W.A., Cooney R.N., Smith J.S., Becker D. Limits of body mass index to detect obesity and predict body composition. Nutrition. 2001;17:26–30. doi: 10.1016/s0899-9007(00)00471-8. [DOI] [PubMed] [Google Scholar]
- 49.Damsgaard M., Rasmussen J., Christensen S.T., Surma E., de Zee M. Analysis of musculoskeletal systems in the AnyBody modeling system. Simul Model Pract. Th. 2006;14:1100–1111. [Google Scholar]
- 50.Kar J., Quesada P. A musculoskeletal modeling approach for estimating anterior cruciate ligament strains and knee anterior–posterior shear forces in stop-jumps performed by young recreational female athletes. Ann. Biomed. Eng. 2012;41:338–348. doi: 10.1007/s10439-012-0644-y. [DOI] [PubMed] [Google Scholar]
- 51.Sasaki K., Neptune R. Individual muscle contributions to the axial knee joint contact force during normal walking. J. Biomech. 2010;43:2780–2784. doi: 10.1016/j.jbiomech.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maniar N., Schache A., Sritharan P., Opar D. Non-knee-spanning muscles contribute to tibiofemoral shear as well as valgus and rotational joint reaction moments during unanticipated sidestep cutting. Sci. Rep-Uk. 2018;8:2501. doi: 10.1038/s41598-017-19098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kadaba M.P., Ramakrishnan H.K., Wootten M.E., Gainey J., Gorton G., Cochran G.V.B. Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. J. Orthop. Res. 1989;7:849–860. doi: 10.1002/jor.1100070611. [DOI] [PubMed] [Google Scholar]
- 54.Pandy M. Computer modeling and simulation of human movement. Annu. Rev. Biomed. Eng. 2001;3:245–273. doi: 10.1146/annurev.bioeng.3.1.245. [DOI] [PubMed] [Google Scholar]
- 55.Trinler U., Schwameder H., Baker R., Alexander N. Muscle force estimation in clinical gait analysis using AnyBody and OpenSim. J. Biomech. 2019:86. doi: 10.1016/j.jbiomech.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 56.Kim Y., Jung Y., Choi W., Lee K., Koo S. Similarities and differences between musculoskeletal simulations of OpenSim and AnyBody modeling system. J. Mech. Sci. Technol. 2018;32:6037–6044. [Google Scholar]
- 57.Chander D.S., Cavatorta M. Multi-directional one-handed strength assessments using AnyBody Modeling Systems. Appl. Ergon. 2018;67:225–236. doi: 10.1016/j.apergo.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 58.Millard M., Uchida T.K., Seth A., Delp S. Flexing computational muscle: modeling and simulation of musculotendon dynamics. J. Biomech. Eng. 2013;135 doi: 10.1115/1.4023390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu B., Lin C.F., Garrett W.E. Lower extremity biomechanics during the landing of a stop-jump task. Clin. BioMech. 2006;21:297–305. doi: 10.1016/j.clinbiomech.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Terada M., Pietrosimone B., Gribble P. Individuals with chronic ankle instability exhibit altered landing knee kinematics: potential link with the mechanism of loading for the anterior cruciate ligament. Clin. BioMech. 2014;29:1125–1130. doi: 10.1016/j.clinbiomech.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 61.Gregory S., Berns M.L., Hull HughA. Patterson, Strain in the anteromedial bundle of the anterior cruciate ligament under combination loading. J. Orthop. Res. 1992;10:167–176. doi: 10.1002/jor.1100100203. [DOI] [PubMed] [Google Scholar]
- 62.Lin C., Gross M., Ji C., Padua D., Weinhold P., Garrett W.E., Yu B. A stochastic biomechanical model for risk and risk factors of non-contact anterior cruciate ligament injuries. J. Biomech. 2009;42(4):418–423. doi: 10.1016/j.jbiomech.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Markolf K.L., Burchfield D.M., Shapiro M.M., Shepard M.F., Finerman G.A.M., Slauterbeck J.L. Combined knee loading states that generate high anterior cruciate ligament forces. J. Orthop. Res. 1995;13:930–935. doi: 10.1002/jor.1100130618. [DOI] [PubMed] [Google Scholar]
- 64.Fleming B.C., Renstrom P.A., Ohlen G., Johnson R.J., Peura G.D., Beynnon B.D., Badger G.J. The gastrocnemius muscle is an antagonist of the anterior cruciate ligament. J. Orthop. Res. 2001;19:1178–1184. doi: 10.1016/S0736-0266(01)00057-2. [DOI] [PubMed] [Google Scholar]
- 65.Zukor S.A. Finite element analysis of human knee joint in varus-valgus. Clin. BioMech. 1997;12(3):139–148. doi: 10.1016/s0268-0033(97)00072-7. [DOI] [PubMed] [Google Scholar]
- 66.Howard J.S., Fazio M.A., Mattacola C.G., Uhl T.L., Jacobs C.A. 2011. Structure, Sex, and Strength and Knee and Hip Kinematics during Landing, J ATHL TRAINING. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Powers M. Christopher. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J. Orthop. Sports Phys. Ther. 2003;33:639–646. doi: 10.2519/jospt.2003.33.11.639. [DOI] [PubMed] [Google Scholar]
- 68.Cannon J., Cambridge E.D.J., Mcgill S.M. Anterior cruciate ligament injury mechanisms and the kinetic chain linkage: the effect of proximal joint stiffness on distal knee control during bilateral landings. J. Orthop. Sports Phys. Ther. 2019;49:601–610. doi: 10.2519/jospt.2019.8248. [DOI] [PubMed] [Google Scholar]
- 69.Jacobs C.A., Uhl T.L., Mattacola C.G., Shapiro R., Rayens W.S. 2007. Hip Abductor Function and Lower Extremity Landing Kinematics: Sex Differences, J ATHL TRAINING. [PMC free article] [PubMed] [Google Scholar]
- 70.Sigward S., Ota S., Powers C. Predictors of frontal plane knee excursion during a drop land in young female soccer players. J. Orthop. Sports Phys. Ther. 2008;38:661–667. doi: 10.2519/jospt.2008.2695. [DOI] [PubMed] [Google Scholar]
- 71.Hewett T.E., Myer G.D., Ford K.R., Heidt R.S., Colosimo A.J., McLean S.G., van den Bogert A.J., Paterno M.V., Succop P. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am. J. Sport Med. 2005;33:492. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 72.Hewett T.E., Zazulak B.T., Myer G.D., Ford K.R. A review of electromyographic activation levels, timing differences, and increased anterior cruciate ligament injury incidence in female athletes. Br. J. Sports Med. 2005;39:347–350. doi: 10.1136/bjsm.2005.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zebis M.K., Andersen L.L., Bencke J., Kjaer M., Aagaard P. Identification of athletes at future risk of anterior cruciate ligament ruptures by neuromuscular screening. Am. J. Sports Med. 2009;37:1967–1973. doi: 10.1177/0363546509335000. [DOI] [PubMed] [Google Scholar]
- 74.More R.C., Karras B.T., Neiman R., Fritschy D., Woo S.L., Daniel D.M. Hamstrings—an anterior cruciate ligament protagonist: an in vitro study. Am. J. Sports Med. 1993;21:231–237. doi: 10.1177/036354659302100212. [DOI] [PubMed] [Google Scholar]
- 75.MacWilliams B., Wilson D., DesJardins J., Romero J., Chao E. Hamstrings cocontraction reduces internal rotation, anterior translation, and anterior cruciate ligament load in weight‐bearing flexion. J. Orthop. Res. 1999;17:817–822. doi: 10.1002/jor.1100170605. [DOI] [PubMed] [Google Scholar]
- 76.Adouni M., Shirazi-Adl A., Marouane H. Role of gastrocnemius activation in knee joint biomechanics: gastrocnemius acts as an ACL antagonist. Comput. Methods Biomech. Biomed. Eng. 2016;19:376–385. doi: 10.1080/10255842.2015.1032943. [DOI] [PubMed] [Google Scholar]
- 77.Sritharan P., Lin Y.C., Pandy M.G. Muscles that do not cross the knee contribute to the knee adduction moment and tibiofemoral compartment loading during gait. J. Orthop. Res. 2012;30:1586–1595. doi: 10.1002/jor.22082. [DOI] [PubMed] [Google Scholar]
- 78.Bodor M. Quadriceps protects the anterior cruciate ligament. J. Orthop. Res. 2001;19:629–633. doi: 10.1016/S0736-0266(01)00050-X. [DOI] [PubMed] [Google Scholar]
- 79.Alkjr T., Wieland M.R., Andersen M.S., Simonsen E.B., Rasmussen J. Computational modeling of a forward lunge: towards a better understanding of the function of the cruciate ligaments. J. Anat. 2012;221 doi: 10.1111/j.1469-7580.2012.01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Solomon R., Micheli L. Strain within the anterior cruciate ligament during hamstring and quadriceps activity. Am. J. Sports Med. 1986;14:83–90. doi: 10.1177/036354658601400114. [DOI] [PubMed] [Google Scholar]
- 81.Arnold E.M., Ward S.R., Lieber R.L., Delp S.L. A model of the lower limb for analysis of human movement. Ann. Biomed. Eng. 2009;38:269–279. doi: 10.1007/s10439-009-9852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wünschel M., Leichtle U., Obloh C., Wülker N., Müller O. The effect of different quadriceps loading patterns on tibiofemoral joint kinematics and patellofemoral contact pressure during simulated partial weight-bearing knee flexion. Knee Surg. Sports Traumatol. Arthrosc. 2011;19:1099–1106. doi: 10.1007/s00167-010-1359-y. [DOI] [PubMed] [Google Scholar]
- 83.Li G., Zayontz S., Most E., Defrate L.E., Suggs J.F., Rubash H.E. In situ forces of the anterior and posterior cruciate ligaments in high knee flexion: an in vitro investigation. J. Orthop. Res. 2010;22:293–297. doi: 10.1016/S0736-0266(03)00179-7. [DOI] [PubMed] [Google Scholar]
- 84.Withrow T., Huston L., Wojtys E., Ashton-Miller J. Effect of varying hamstring tension on anterior cruciate ligament strain during in vitro impulsive knee flexion and compression loading. J. Bone Jt. Surg. Am. Vol. 2008;90:815–823. doi: 10.2106/JBJS.F.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hanson A., Padua D., Blackburn J.T., Prentice W., Hirth C.J. Muscle activation during side-step cutting maneuvers in male and female soccer athletes. J. Athl. Training. 2008;43:133–143. doi: 10.4085/1062-6050-43.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Biscarini A., Botti F.M. Selective contribution of each hamstring muscle to anterior cruciate ligament protection and tibiofemoral joint stability in leg-extension exercise: a simulation study. Eur. J. Appl. Physiol. 2013;113:2263–2273. doi: 10.1007/s00421-013-2656-1. [DOI] [PubMed] [Google Scholar]
- 87.Biscarini A., Benvenuti P., Botti F.M., Brunetti A., Brunetti O., Pettorossi V.E. Voluntary enhanced cocontraction of hamstring muscles during open kinetic chain leg extension exercise: its potential unloading effect on the anterior cruciate ligament. Am. J. Sports Med. 2014;42:2103–2112. doi: 10.1177/0363546514536137. [DOI] [PubMed] [Google Scholar]
- 88.Victor J., Labey L., Wong P., Innocenti B., Bellemans J. The influence of muscle load on tibiofemoral knee kinematics. J. Orthop. Res. 2009;28:419–428. doi: 10.1002/jor.21019. [DOI] [PubMed] [Google Scholar]
- 89.Li G., Rudy T., Sakane M., Kanamori A., Ma C., Woo S. The importance of quadriceps and hamstring muscle loading on knee kinematics and in-situ forces in the ACL. J. Biomech. 1999;32:395–400. doi: 10.1016/s0021-9290(98)00181-x. [DOI] [PubMed] [Google Scholar]
- 90.Ebben W., Fauth M.L., Petushek E., Garceau L.R., Hsu B.E., Lutsch B.N., Feldmann C.R. Gender-based analysis of hamstring and quadriceps muscle activation during jump landings and cutting. J. Strength Cond. Res. 2010;24:408–415. doi: 10.1519/JSC.0b013e3181c509f4. [DOI] [PubMed] [Google Scholar]
- 91.Guelich D.R., Xu D., Koh J.L., Nuber G.W., Zhang L.Q. Different roles of the medial and lateral hamstrings in unloading the anterior cruciate ligament. Knee. 2016;23:97–101. doi: 10.1016/j.knee.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 92.Elias J.J., Faust A.F., Chu Y., Chao E.Y., Cosgarea A.J. The soleus muscle acts as an agonist for the anterior cruciate ligament: an in vitro experimental study. Am. J. Sports Med. 2003;31:241–246. doi: 10.1177/03635465030310021401. [DOI] [PubMed] [Google Scholar]
- 93.Maniar N., Schache A.G., Pizzolato C., Opar D.A. Muscle contributions to tibiofemoral shear forces and valgus and rotational joint moments during single leg drop landing. Scand. J. Med. Sci. Sports. 2020;30:1664–1674. doi: 10.1111/sms.13711. [DOI] [PubMed] [Google Scholar]
- 94.Nasseri A., Lloyd D.G., Bryant A.L., Headrick J., Saxby D.J. Mechanism of anterior cruciate ligament loading during dynamic motor tasks. Med. Sci. Sports Exerc. 2021;53(6):1235–1244. doi: 10.1249/MSS.0000000000002589. [DOI] [PubMed] [Google Scholar]
- 95.Pflum M.A., Shelburne K.B., Torry M.R., Decker M.J., Pandy M.G. Model prediction of anterior cruciate ligament force during drop-landings. Med. Sci. Sports Exerc. 2004;36:1949. doi: 10.1249/01.mss.0000145467.79916.46. [DOI] [PubMed] [Google Scholar]
- 96.Hossein Mokhtarzadeh, And Chen, Hua Yeow, And James, Cho Hong. Contributions of the Soleus and Gastrocnemius muscles to the anterior cruciate ligament loading during single-leg landing. J. Biomech. 2013 doi: 10.1016/j.jbiomech.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 97.Maniar N., Schache A.G., Cole M.H., Opar D.A. Lower-limb muscle function during sidestep cutting. J. Biomech. 2019;82:186–192. doi: 10.1016/j.jbiomech.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 98.Li G., DeFrate L., Rubash H., Gill T. In vivo kinematics of the ACL during weight‐bearing knee flexion. J. Orthop. Res. 2005;23:340–344. doi: 10.1016/j.orthres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 99.Ingersoll C.D.P.A., Grindstaff T.L.D.A., Pietrosimone B.G.M.A., Hart J.M.P.A. Neuromuscular consequences of anterior cruciate ligament injury. Clin. Sport Med. 2008;27:383–404. doi: 10.1016/j.csm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 100.Beynnon B.D., Fleming B.C., Labovitch R., Parsons B. Chronic anterior cruciate ligament deficiency is associated with increased anterior translation of the tibia during the transition from non-weightbearing to weightbearing. J. Orthop. Res. 2002;20:332–337. doi: 10.1016/S0736-0266(01)00115-2. [DOI] [PubMed] [Google Scholar]
- 101.Rachmat H.H., Janssen D., Verkerke G.J., Diercks R.L., Verdonschot N. In-situ mechanical behavior and slackness of the anterior cruciate ligament at multiple knee flexion angles. Med. Eng. Phys. 2016;38:209–215. doi: 10.1016/j.medengphy.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 102.Lu Z., Li X., Rong M., Baker J.S., Gu Y. Effect of rearfoot valgus on biomechanics during barbell squatting: a study based on OpenSim musculoskeletal modeling. Front. Neurorobotics. 2022;16:832005. doi: 10.3389/fnbot.2022.832005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dai B., Mao M., Garrett W.E., Yu B. Biomechanical characteristics of an anterior cruciate ligament injury in javelin throwing. J. Sport Health Sci. 2015;4:333–340. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.