Significance
While dopaminergic medications effectively treat motor symptoms in Parkinson’s disease (PD), their impact on speech—a critical yet often overlooked nonmotor symptom—has been ambiguous. Our study elucidates a neural mechanism revealing that such dopamine modulates functional connectivity between specific subdivisions of the subthalamic nuclei (STN) and language networks. We found this connectivity to be uniquely correlated with changes in speech functions, especially those requiring vocal responses, while remaining dissociated from cognitive tasks that required manual responses. Our work offers insights into the neurocircuitry governing speech in PD and lays the foundation for targeted, more efficacious treatment strategies, thereby filling a crucial gap in our understanding of dopaminergic modulation of speech functions in PD.
Keywords: speech, language, dopamine, subthalamic nuclei, functional connectivity
Abstract
Speech impediments are a prominent yet understudied symptom of Parkinson’s disease (PD). While the subthalamic nucleus (STN) is an established clinical target for treating motor symptoms, these interventions can lead to further worsening of speech. The interplay between dopaminergic medication, STN circuitry, and their downstream effects on speech in PD is not yet fully understood. Here, we investigate the effect of dopaminergic medication on STN circuitry and probe its association with speech and cognitive functions in PD patients. We found that changes in intrinsic functional connectivity of the STN were associated with alterations in speech functions in PD. Interestingly, this relationship was characterized by altered functional connectivity of the dorsolateral and ventromedial subdivisions of the STN with the language network. Crucially, medication-induced changes in functional connectivity between the STN’s dorsolateral subdivision and key regions in the language network, including the left inferior frontal cortex and the left superior temporal gyrus, correlated with alterations on a standardized neuropsychological test requiring oral responses. This relation was not observed in the written version of the same test. Furthermore, changes in functional connectivity between STN and language regions predicted the medication’s downstream effects on speech-related cognitive performance. These findings reveal a previously unidentified brain mechanism through which dopaminergic medication influences speech function in PD. Our study sheds light into the subcortical-cortical circuit mechanisms underlying impaired speech control in PD. The insights gained here could inform treatment strategies aimed at mitigating speech deficits in PD and enhancing the quality of life for affected individuals.
Parkinson’s disease (PD) is typically characterized by motor abnormalities, though it also manifests nonmotor symptoms including speech and cognitive difficulties that significantly impact the patients’ quality of life (1, 2). The underlying cause of motor symptoms in PD is primarily due to nigrostriatal degeneration of dopamine neurons (3) and is associated with hyperactivity in the subthalamic nucleus (STN) (4). This neurodegenerative process not only impacts motor function but also gives rise to a broad spectrum of nonmotor symptoms (5). While dopaminergic medication is widely used to alleviate motor symptoms, such as bradykinesia and rigidity, its impact on nonmotor symptoms, specifically changes in speech and cognition, remains poorly understood (6–9). Considering the high prevalence of speech impairments in PD (10, 11), and the clinical importance of addressing both nonmotor and motor impairments, there is a critical need for elucidating the neural circuit mechanisms by which dopaminergic medication influences speech and cognitive functions in PD. Here, we address critical gaps in the literature by investigating the relationship between STN functioning, dopaminergic medication, and their collective influence on speech and cognitive impairments.
Speech, an intricate cognitive-motor function unique to humans, relies on precise control of orofacial muscles and well-orchestrated cognitive processes for accurate and efficient articulation. Disturbances in these motor and nonmotor components can contribute to speech production problems, a common phenomenon in PD (10). Indeed, over 90% of PD patients have speech difficulties, such as hypophonia, dysarthria, and stuttering. However, the etiological mechanism underlying speech deficits in PD remains unclear.
The language network, central to speech and to the production and comprehension of oral language, is primarily composed of cortical areas including Broca’s area in the left inferior frontal gyrus (12, 13), ventral sensorimotor cortex (14), and Wernicke’s area in the superior temporal gyrus (15, 16). Besides these well-documented cortical areas, subcortical regions also play a crucial role in speech motor control (17, 18). Disturbances and lesions in the basal ganglia often accompany a variety of speech problems, such as dysarthria and dysphonia (19–21). However, our understanding of the complex cortical–subcortical circuits underlying speech control remains inadequate. A deeper exploration of these circuits, especially in the context of PD and the influence of dopaminergic medication, is crucial to developing a more mechanistic understanding of speech production in general and its deficits in PD.
The STN is a central region in basal ganglia-cortical circuits that underpin movement and cognitive control in humans (22–25). It receives inhibitory GABAergic input from the external segment of the globus pallidus through the indirect pathway and excitatory glutamatergic input from the cortex via the hyperdirect pathway of cortical–basal ganglia loop. It sends glutamatergic output to both the external and internal segments of the globus pallidus (26, 27). Excitation of the STN increases neuronal activity in the internal segment of the globus pallidus, which then sends inhibitory signals to the thalamus, consequently reducing motor output (Fig. 1A). In the context of PD, this suggests that hyperactivity in the STN can lead to excessive inhibitory output from the globus pallidus internus to the thalamus, disrupting normal cortical–basal ganglia signaling (4). However, the precise relationship between nigrostriatal dopamine deficiency and STN hyperactivity is not fully understood. The STN is the primary target of deep brain stimulation (DBS), a treatment method that alleviates specific motor symptoms, such as bradykinesia and resting tremor, in PD patients (28). However, speech impairment is a common side effect of STN stimulation in PD patients (29–34), and increased dysarthria has also been reported (2), suggesting that altered STN functioning may directly affect speech production (35, 36). However, the exact relation between STN pathophysiology and speech problems in PD and how this may be modulated by dopaminergic medication remains unclear.
Fig. 1.
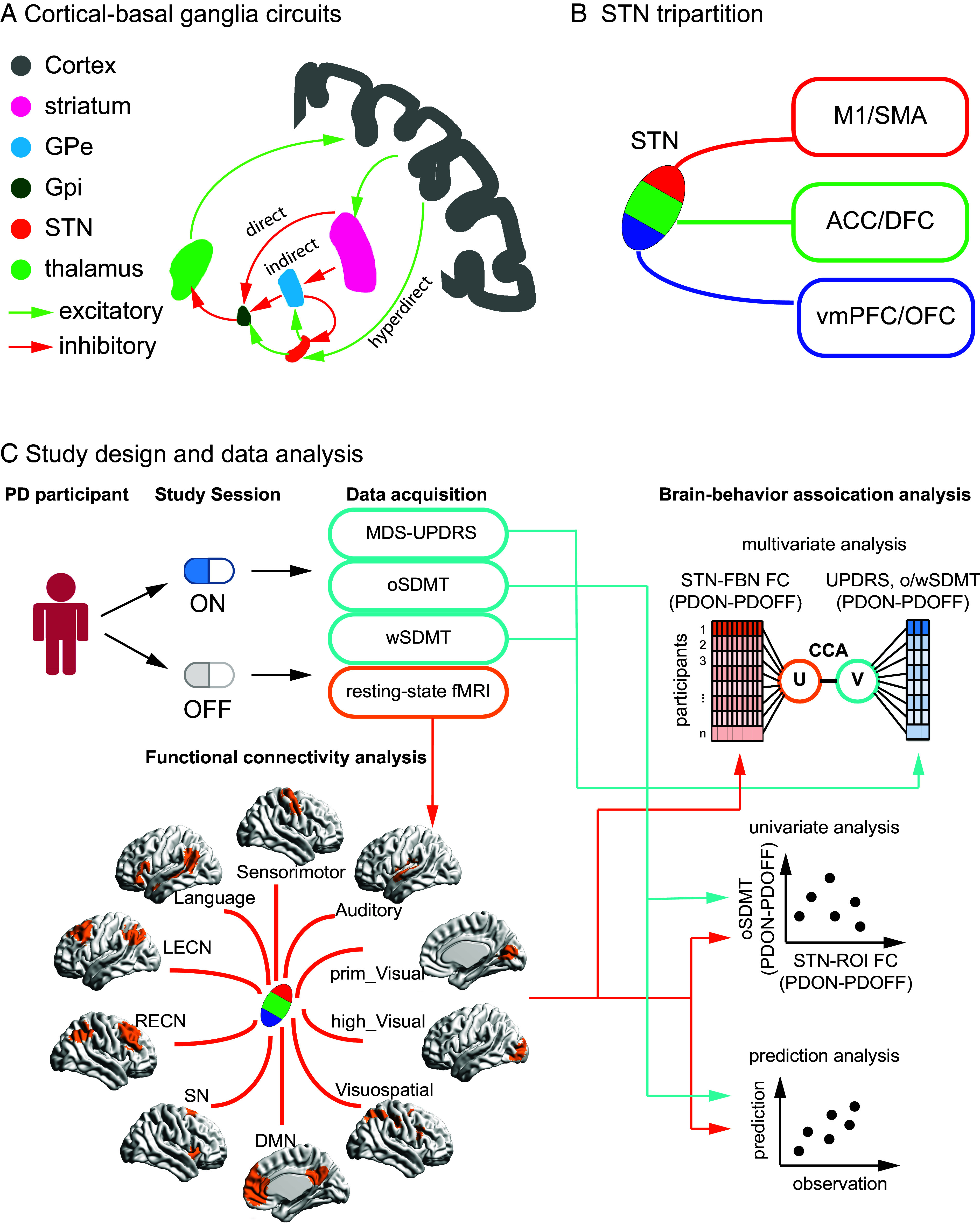
Model, anatomy, study design, and main results. (A) Hyperdirect, indirect, and direct pathways of cortical–basal ganglia circuitry. (B) STN tripartition is composed of dorsolateral (red), central (green), and ventromedial (blue) subdivisions, which connects M1/SMA, ACC/DFC, VMPFC/OFC, respectively. (C) Study design and data analysis. STN: subthalamic nucleus; GPe: globus pallidus externus; GPi: globus pallidus internus; M1: primary motor cortex; SMA: supplementary motor area; STN-FBN FC: subthalamic nuclei-functional brain network functional connectivity; SN: salience network; DMN: default mode network; LECN: left executive central network; RECN: right executive central network; Visuospatial: visuospatial network; Language: language network; Auditory: auditory network; high_Visual: high visual network; prim_Visual: prime visual network; sensorimotor: sensorimotor network; MDS-UPDRS: the Movement Disorders Society-Unified Parkinson’s disease Rating Scale motor assessment; oSDMT: oral the Symbol Digit Modalities Test; wSDMT: written the Symbol Digit Modalities Test.
The STN is a heterogeneous structure with a tripartite organization consisting of the dorsolateral, central, and ventromedial subdivisions (37, 38). Each of these subdivisions receives distinct white matter projections from various cortical regions. Specifically, the dorsolateral STN primarily interfaces with the motor cortex, the central STN with the dorsal prefrontal and anterior cingulate cortices, and the ventromedial STN with the ventral prefrontal and orbital frontal cortices (37, 38) (Fig. 1B). By virtue of their distinct anatomical wiring, these STN subdivisions are thought to serve distinct motor and cognitive functions (39). In support of this, studies have demonstrated that stimulation of different STN subdivisions elicits distinct patterns of motor and cognitive responses in PD patients (40–42). Furthermore, the structural and functional connectivity of STN stimulation targets has been shown to predict therapeutic improvements in motor symptoms (43). However, key knowledge gaps persist. Particularly, it is not known which STN subdivision is primarily involved in speech control, and the degree to which dopaminergic medication differentially modulates its cortical circuits in PD remains poorly understood. Understanding the dynamical circuit mechanisms associated with individual STN subdivisions could yield significant insights into speech impairments in PD and inform more targeted and effective treatment strategies.
We had three objectives in this study. Our first objective was to investigate the impact of dopaminergic medication on the functional connectivity between the STN and canonical large-scale functional brain networks (FBN) involved in motor, speech, and cognitive functions, and their links to medication-induced changes in behavior. We employed a within-subject design, where each PD participant was assessed ON and OFF dopaminergic medication, along with a between-subject comparison to age-, sex-, education-matched healthy controls (HC) (Fig. 1C). Each PD participant completed Movement Disorders Society-Unified Parkinson’s disease Rating Scale motor assessment (MDS-UPDRS part III), the Symbol Digit Modalities Test (SDMT), and resting-state fMRI scanning in both the ON and OFF medication sessions in a within-subject design, serving as their own controls. Each HC participant completed one session. This design strategy allowed us to explore the effects of dopaminergic medication on STN functional connectivity and its relation to standardized motor, speech, and cognitive function measures.
The MDS-UPDRS provided quantitative assessments of motor function (44), while the SDMT evaluated working memory, attention switching, and processing speed. Importantly, the SDMT offers both an oral (oSDMT) and a written (wSDMT) version, involving different response modalities but equivalent cognitive demands (45). The oSDMT requires participants to verbally state the responses whereas the wSDMT requires participants to write down the responses. This allowed us to test the differential effects of dopaminergic medication on motor, speech, and cognitive functions. We hypothesized that medication effects on speech and cognitive functions are modulated by functional connectivity between STN and brain systems integral to speech and language and cognitive control (46).
Our second objective was to probe the functional heterogeneity of STN subdivisions, particularly in terms of how dopaminergic medication modulates speech function. First, we examined whether the human STN has functionally heterogeneous subdivisions characterized by distinct spontaneous fMRI signals. To this end, we leveraged a large Human Connectome Project (HCP) dataset (N = 801) to identify functionally distinct clusters within the STN, based on the temporal fluctuation patterns. Next, we examined how dopaminergic medication alters the relationship between each STN subdivision’s connectivity with FBNs and motor, speech, and cognition. We hypothesized that dopamine-induced changes in functional connectivity between the dorsolateral subdivision of the STN and language network would be associated with altered speech-related behaviors. We further predicted that functional connectivity between the STN and individual language regions would predict the effect of dopaminergic medication on speech.
Our final objective was to construct a reliable statistical model capable of predicting the impact of dopaminergic medication on speech in PD participants, based on the functional connectivity between the STN and language networks. We hypothesized that dopaminergic modulation on functional connectivity and language networks can accurately predict its modulation on PD participants’ speech function.
Results
Dopaminergic Medication Alleviates Motor Symptoms.
Motor performance in PD was assessed using the MDS-UPDRS (44). As expected, dopaminergic medication significantly reduced motor symptoms in PD participants (t26 = 9.42, P = 7e−10, paired t test, SI Appendix, Table S1).
PD-OFF MDS-UPDRS scores were significantly correlated with PD-ON scores (r = 0.86, P = 6e−09, Pearson’s correlation, SI Appendix, Fig. S1A) as well as medication-induced change in MDS-UPDRS score (i.e., PD-ONUPDRS – PD-OFFUPDRS) in a marginally significant manner (r = −0.34, P = 0.08, Pearson’s correlation); this relationship was significant after removal of one outlier, defined by 3 SDs of group mean, (r = −0.45, P = 0.01, Pearson’s correlation, SI Appendix, Fig. S1B) and remained significant after controlling for age, sex, and levodopa equivalent daily dosage (LEDD; P = 0.03, SI Appendix, Table S2). These results suggest that dopaminergic medication alleviates motor symptoms in PD and participants with more severe motor problems had greater benefits from dopaminergic medication treatment.
Effect of Dopaminergic Medication on Cognitive Function.
Cognitive functions in PD were assessed using oral and written versions of the SDMT (45). Dopaminergic medication did not significantly affect performance on the oSDMT or wSDMT (all ps > 0.5). The PD-OFF and PD-ON showed significant correlation in both oSDMT (r = 0.82, P = 4e−07, Pearson’s correlation, SI Appendix, Fig. S1C) and wSDMT (r = 0.89, P = 8e−10, Pearson’s correlation, SI Appendix, Fig. S1E), indicating high stability and consistency of SDMT assessment on PD participants in ON and OFF sessions. Interestingly, PD-OFF oSDMT scores were significantly correlated with medication-induced change in oSDMT (i.e., PD-ONoSDMT – PD-OFFoSDMT; r = −0.46, P = 0.01, Pearson’s correlation, SI Appendix, Fig. S1D), but this relationship was not significant in wSDMT scores (r = −0.11, P = 0.6, Pearson’s correlation, SI Appendix, Fig. S1F). This association between PD-OFF oSDMT and medication-induced changes in oSDMT remained significant after controlling for age, sex, and LEDD (P = 0.007, SI Appendix, Table S3). These results demonstrate that PD participants with worse performance on the spoken version of the SDMT had greater benefits from the dopaminergic medication. No such effect was observed for the written version of the SDMT.
Abnormal Functional Connectivity between STN and Functional Brain Networks in PD.
We examined functional connectivity between the STN and 10 canonical FBNs (Fig. 2A) in PD-OFF and HC and found that, among the 10 FBNs, the left STN had greater functional connectivity with the salience and sensorimotor networks in PD-OFF than HC (all ps < 0.05, Bonferroni corrected). No significant group differences were found in the functional connectivity seeded in the right STN.
Fig. 2.
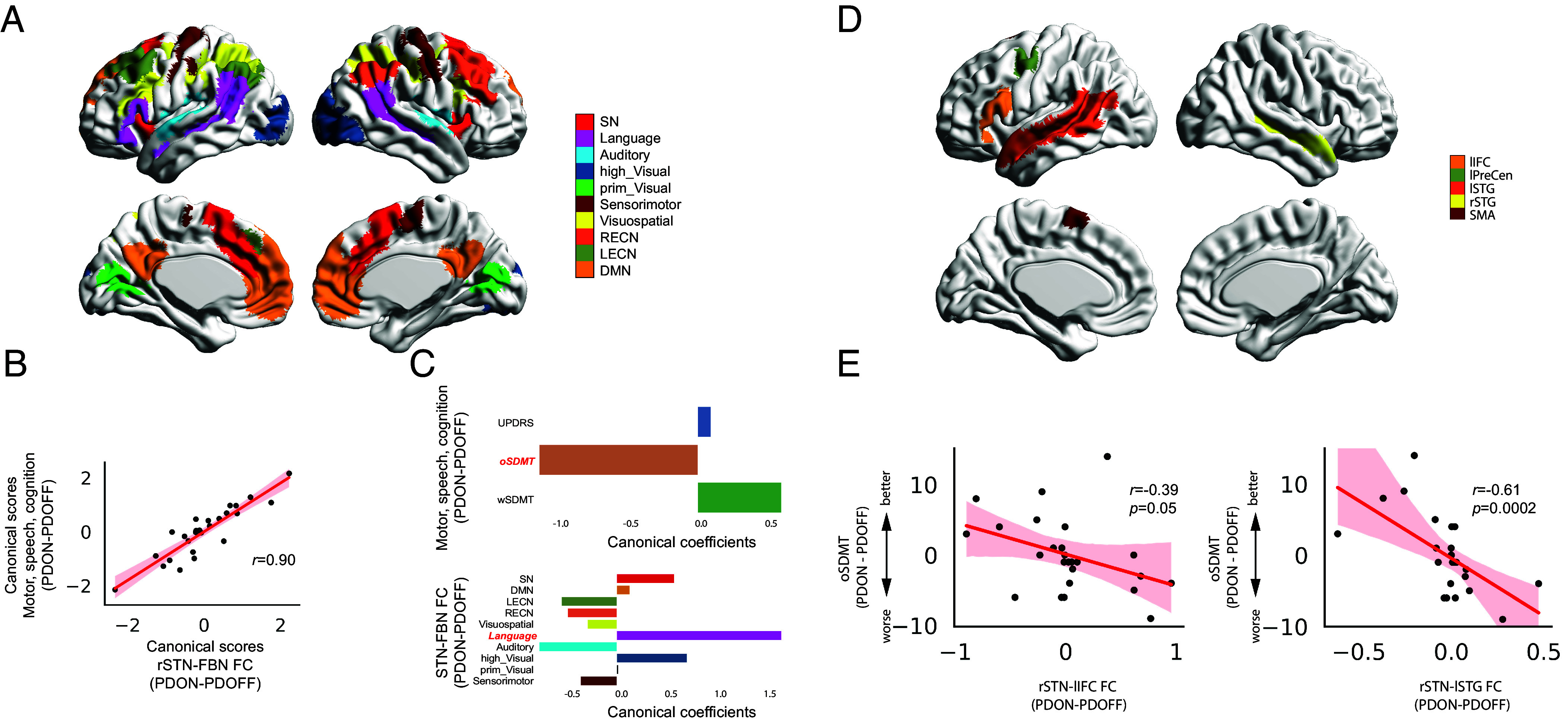
Functional connectivity in the rSTN-cortical circuits in relation to medication effect on motor, speech, and cognition functions in PD. (A) Canonical functional brain networks obtained from Shirer et al. (47) including SN, DMN, LECN, RECN, Visuospatial, Language, Auditory, high_Visual, prim_Visual, and Sensorimotor networks. (B) CCA revealed a marginally significant multivariate relationship between dopaminergic modulation of rSTN-FBN FC and dopaminergic modulation of motor-cognitive function (r = 0.90, P = 0.05 FWER correction). (C) Multivariate relationship was characterized by high canonical coefficients of rSTN-Language and oSDMT. Significant canonical loading factors were highlighted in red and bold-italic font. (D) Key regions in the language network obtained from Lipkin et al. (48), including lIFC, lPreCen, lSTG, rSTG, and SMA. (E) Pearson’s correlation analysis revealed that Medication-induced changes in oSDMT scores (PDON – PDOFF) are associated with medication-induced changes in rSTN-lIFC FC (r = −0.39, P = 0.05) and rSTN-lSTG FC (r = −0.61, P = 0.0002). STN-FBN FC: subthalamic nuclei-functional brain network functional connectivity; SN: salience network; DMN: default mode network; LECN: left executive central network; RECN: right executive central network; Visuospatial: visuospatial network; Language: language network; Auditory: auditory network; high_Visual: high visual network; prim_Visual: prime visual network; sensorimotor: sensorimotor network; UPDRS: the Movement Disorders Society-Unified Parkinson’s disease Rating Scale motor assessment; oSDMT: oral the Symbol Digit Modalities Test; wSDMT: written the Symbol Digit Modalities Test; lIFC: left inferior frontal cortex; lPreCen: left precentral gyrus; lSTG: left superior temporal gyrus; rSTG: right superior temporal gyrus; SMA: supplementary motor area.
There was no significant medication effect on functional connectivity between STN and FBNs in PD participants (ps > 0.05).
Functional Connectivity in the Cortical-STN Circuits in Relation to Medication Effect on Motor, Speech, and Cognitive Functions in PD.
We used canonical correlation analysis (CCA) to examine the multivariate relationship between the effect of dopaminergic medication on functional connectivity of STN and FBNs and medication effect on motor, speech, and cognitive functions (see Materials and Methods for details). CCA revealed marginally significant canonical correlations with clinical variables for the right (r = 0.90, P = 0.05, FWER corrected, Fig. 2B), but not the left STN (P = 0.33, FWER corrected). Multivariate contributions to the CCA model showed a high negative canonical coefficient of oSDMT and a high positive canonical coefficient of functional connectivity between the rSTN and Language network (Fig. 2C). Canonical loading analysis revealed that canonical loadings of oSDMT (r = −0.83, P < 0.001, Pearson’s correlation) and STN-Language (r = 0.44, P = 0.02, Pearson’s correlation) are significant. Taken together, these results suggest that medication-induced functional connectivity changes in rSTN and Language network are negatively associated with medication-induced changes in speech function in PD participants.
Functional Connectivity between STN and Language Regions in Relation to Dopaminergic Modulation of oSDMT Performance.
As the multivariate brain–behavior association was dominated by the relationship between STN-Language network connectivity and oSDMT performance, we further examined whether functional connectivity between STN and key regions in the Language system can predict oSDMT performance. To demonstrate the robustness of our findings, instead of using the Language network derived from whole brain parcellation using ICA (47), we used an independent Language-specific brain atlas (48) (see details in Materials and Methods) to identify five key regions in the Language system, including the left inferior frontal cortex (lIFC), left precentral gyrus (lPreCen), left superior temporal gyrus (lSTG), right superior temporal gyrus (rSTG), and supplementary motor area (SMA) (Fig. 2D).
We examined functional connectivity between left or right STN and five key regions in the Language system in association with the effect of medication on oSDMT performance. Dopaminergic medication on oSDMT was significantly correlated with medication-induced changes on the functional connectivity between rSTN and lSTG (r = −0.69, P = 0.0002, Pearson’s correlation, Fig. 2E), between lSTN and lSTG (r = −0.49, P = 0.02, Pearson’s correlation, SI Appendix, Fig. S2), and marginally significant between rSTN and lIFC (r = −0.39, P = 0.05, Pearson’s correlation, Fig. 2E). Multiple linear regression analyses revealed that, after controlling for age, sex, education, LEDD, and head motion, the effect of medication on oSDMT performance is significantly associated with functional connectivity between rSTN and lSTG (P = 0.007) and between lSTN and lSTG (P = 0.01). No significant correlation was found in functional connectivity between STN and other regions in the Language system.
Functional Parcellation of the STN.
We then examined functional heterogeneous subdivisions in the STN using the HCP resting-state fMRI dataset. Resting-state time series was extracted from each voxel in the left and right STN separately for each participant and voxel-wise temporal patterns were clustered individually and consensus was reached in the group level. The number of clusters varied from 2 to 5 for left and right STN and the optimal number of clusters was determined using the probability rand index (PRI, SI Appendix, Fig. S3A). Resultantly, we clustered the left and right STN into three subdivisions each [i.e., left dorsolateral STN (lSTN_dl), left central STN (lSTN_cen), left ventromedial STN (lSTN_vm), right dorsolateral STN (rSTN_dl), right central STN (rSTN_cen), right ventromedial STN (rSTN_vm)] (SI Appendix, Fig. S3B).
STN Subdivisions’ Connectivity to Functional Brain Networks in Relation to Medication Effect on Motor, Speech, and Cognitive Functions in PD.
Next, we used CCA to examine the multivariate relationship between effect of dopaminergic medication on STN subdivisions’ connectivity with FBNs and medication effect on motor, speech, and cognitive functions (see Materials and Methods for details). This analysis revealed significant canonical correlation models for the rSTN_dl (r = 0.89, P = 0.03, FWER corrected, Fig. 3B) and the rSTN_vm (r = 0.92, P = 0.04, FWER corrected, Fig. 3C) but not for the other STN subdivisions (all ps > 0.05, FWER corrected).
Fig. 3.
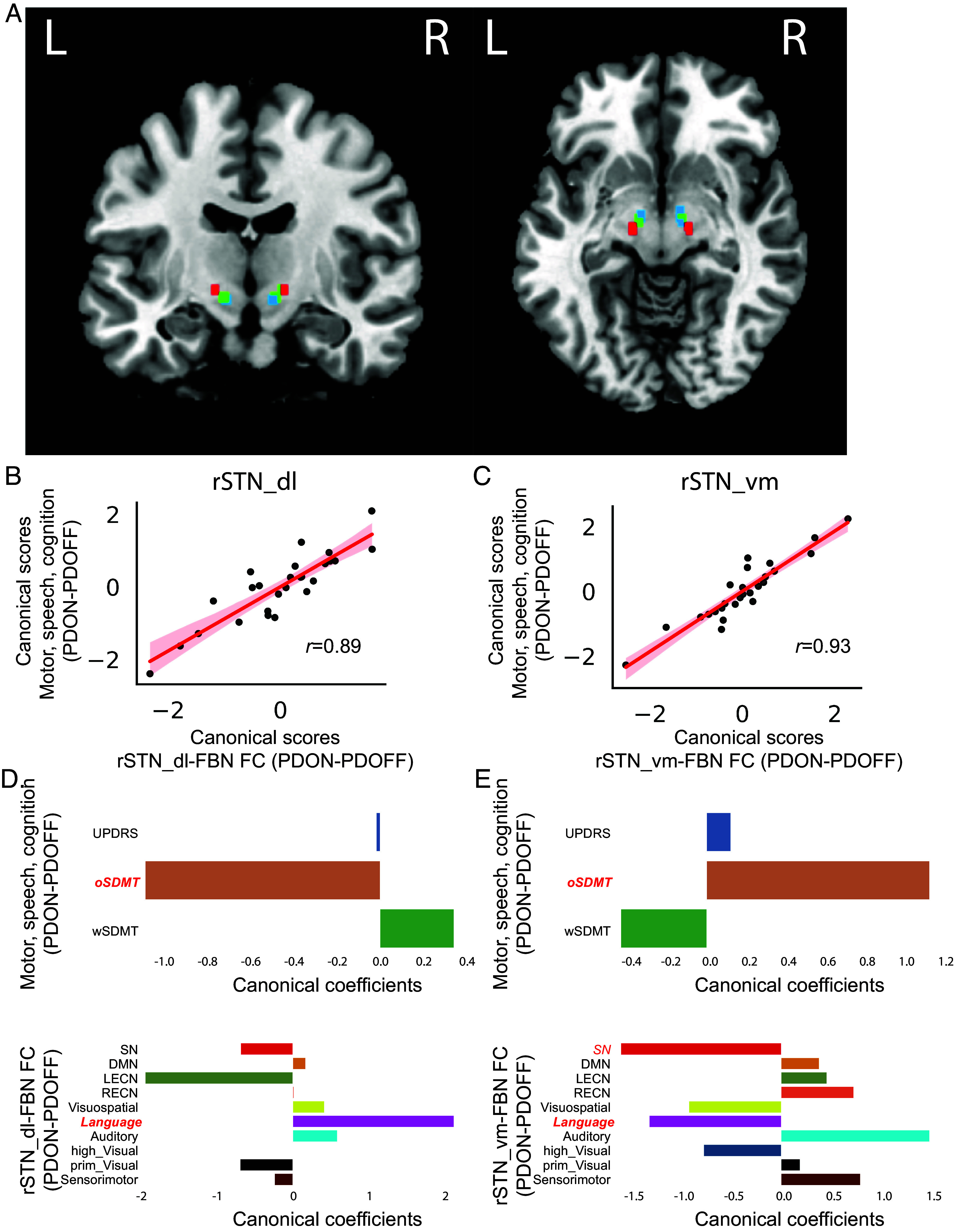
STN subdivisions’ connectivity to functional brain networks in relation to medication effect on motor and cognitive functions in PD. (A) Parcellation analysis revealed that the left and right STN ROIs were composed of dorsolateral (red), central (green), and ventromedial (blue) subdivisions. (B and C) CCA revealed significant multivariate relationship between dopaminergic modulation of STN subdivisions-FBN FC and dopaminergic modulation of motor, speech, and cognition for both (B) rSTN_dl and (C) rSTN_vm. (D and E) Multivariate relationship was characterized by high canonical coefficients from (D) rSTN_dl and (E) rSTN_vm STN to Language and oSDMT. Significant canonical loading factors were highlighted in red and bold-italic font. STN-FBN FC: subthalamic nuclei-functional brain network functional connectivity; SN: salience network; DMN: default mode network; LECN: left executive central network; RECN: right executive central network; Visuospatial: visuospatial network; Language: language network; Auditory: auditory network; high_Visual: high visual network; prim_Visual: prime visual network; sensorimotor: sensorimotor network; UPDRS: the Movement Disorders Society-Unified Parkinson’s disease Rating Scale motor assessment; oSDMT: oral the Symbol Digit Modalities Test; wSDMT: written the Symbol Digit Modalities Test; rSTN_dl: right subthalamic nucleus dorsolateral subregion; rSTN_vm: right subthalamic nucleus ventromedial subregion.
For the rSTN_dl, multivariate contributions to the CCA model were featured by a high negative canonical coefficient in oSDMT, high positive canonical coefficient in functional connectivity with the Language network, and high negative canonical coefficient in functional connectivity with the left executive central network (LECN) (Fig. 3D). Canonical loading analysis revealed that canonical loading of the oSDMT is significant (r = −0.93, P < 0.001, Pearson’s correlation) and the canonical loading of the STN-Language is marginally significant (r = 0.36, P = 0.06, Pearson’s correlation). The canonical loading of the STN-LECN was not significant.
For the rSTN_vm, multivariate contributions to the CCA model were featured by a high positive canonical coefficient in oSDMT, high positive canonical coefficient in functional connectivity with the auditory network, and high negative canonical coefficients in functional connectivity with the Salience and Language networks (Fig. 3E). Canonical loading analysis revealed that canonical loading of the oSDMT is significant (r = 0.91, P < 0.001, Pearson’s correlation), canonical loading of the STN-Language is significant (r = −0.42, P = 0.03, Pearson’s correlation) and the canonical loading of the STN-Salience is marginally significant (r = −0.36, P = 0.07, Pearson’s correlation). The canonical loading of the STN-Auditory was not significant.
Taken together, these results demonstrate regional specificity of medication-induced changes on functional connectivity of STN with FBNs in relation to the changes in motor, speech, and cognitive functions. In particular, medication-induced connectivity changes in rSTN_dl and rSTN_vm with Language network were associated with medication-induced changes in speech function in PD participants. Canonical loadings of all variables were summarized in SI Appendix, Table S4.
Functional Connectivity between STN Subdivisions and Language Regions Predicts Dopaminergic Modulation of oSDMT Performance.
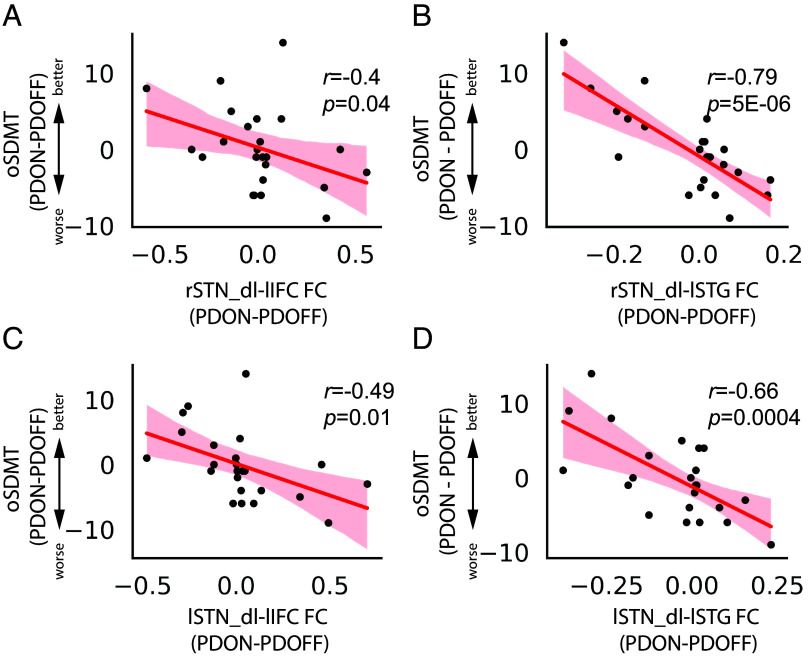
We examined the functional connectivity between STN subdivisions and five key regions in the Language system in association with the effect of medication on oSDMT performance. We found that the effect of dopaminergic medication on oSDMT is significantly correlated with medication-induced changes on the functional connectivity between rSTN_dl and lSTG (r = −0.79, P = 5E−06, Pearson’s correlation, Fig. 4B), between rSTN_dl and lIFC (r = −0.4, P < 0.05, Pearson’s correlation, Fig. 4A), between rSTN_cen and lSTG (r = −0.42, P < 0.05, Pearson’s correlation), between rSTN_cen and lIFC (r = −0.4, P < 0.05, Pearson’s correlation), between rSTN_vm and lSTG (r = −0.69, P = 0.0002, Pearson’s correlation), between lSTN_dl and lSTG (r = −0.66, P = 0.0004, Pearson’s correlation, Fig. 4D) and between lSTN_dl and lIFC (r = −0.49, P = 0.01, Pearson’s correlation, Fig. 4C). Multiple linear regression analyses revealed that, after controlling for age, sex, education, LEDD, and head motion, the effect of medication on oSDMT performance is significantly associated with functional connectivity between rSTN_dl and lSTG (P = 3e−05) and between rSTN_cen and lSTG (P = 0.0003), between lSTN_dl and lSTG (P = 0.0003) and between lSTN_dl and lIFC (P < 0.05) (SI Appendix, Table S5). No significant correlation was found in functional connectivity between STN subdivisions and other regions in the Language system.
Fig. 4.
Functional connectivity between STN subdivisions and key regions in language network in association with medication-induced changes in verbal fluency. Medication-induced changes in oSDMT scores (PDON – PDOFF) are correlated with medication-induced changes in (A) rSTN_dl-lIFC FC (r = −0.4, P = 0.04), (B) rSTN_dl-lSTG FC (r = −0.79, P = 5E−06), (C) lSTN_dl-lIFC FC (r = −0.49, P = 0.01) and (D) lSTN_dl-lSTG FC (r = −0.66, P = 0.0004). FC: functional connectivity; STN_dl: subthalamic nuclei dorsolateral subregion; lIFC: left inferior frontal cortex; lSTG: left superior temporal gyrus; oSDMT: oral the Symbol Digit Modalities Test.
Then, we trained a linear support vector regression model in which dopaminergic modulation of functional connectivity between STN and regions in the Language system was used as features to predict the effect of dopaminergic medication on oSDMT. The model was tested using the leave-one-out cross-validation procedure, and its performance was assessed by the correlation between predicted oSDMT scores and observed oSDMT scores. We found that model trained on the functional connectivity between rSTN_dl and regions in the Language system can accurately predict medication-induced changes in the oSDMT performance (r = 0.47, P = 0.02, Pearson’s correlation, SI Appendix, Fig. S4) and models trained on the functional connectivity between other STN subdivisions and Language regions were not predictive of medication-induced changes in the oSDMT performance.
Functional Connectivity between STN and Language Regions Was Not Associated with Dopaminergic Modulation of wSDMT Performance.
Finally, to demonstrate that the functional connectivity between STN and Language regions was specifically associated with speech function in the oSDMT performance rather than working memory and executive function, we conducted the same correlation analyses to examine the relationship between the functional connectivity pattern and wSDMT performance. As expected, we found that medication-induced changes in functional connectivity between STN and any Language region were not associated with medication-induced changes in wSDMT (all ps > 0.1).
Discussion
Speech difficulties are a common feature among PD patients, often worsening after surgical interventions involving the STN (10, 11, 19). However, the pathophysiology underlying PD-related speech deficits is not fully understood. In this study, we examined the impact of dopaminergic medication on the relationship between functional connectivity of the STN, a critical node of the basal ganglia-cortical motor and cognitive control system (22–25), and medication-induced changes in speech and cognitive functions. We found that medication-induced alterations in the functional connectivity between the STN and language network corresponded with changes in speech-related behaviors. Analysis of the functional specificity of STN subdivisions further revealed a tripartite organization of the STN and an anatomical locus of connectivity deficits with the language network in PD. Specifically, medication-induced changes in functional connectivity between the dorsolateral and ventromedial subdivisions of the right STN and the language network were strongly associated with behavioral performance requiring a vocal response. Crucially, functional connectivity between the dorsolateral subdivision of the right STN and key regions in the left-lateralized language network, including the inferior frontal cortex and superior temporal gyrus, were uniquely associated with the effects of medication on vocal responses during cognitive testing. Our study provides insights into the STN-cortical circuits involved in speech control and elucidate the brain mechanisms through which dopaminergic medication modulates speech function in PD.
Functional Hyperconnectivity of STN to Motor and Salience Networks in PD.
Because PD is primarily known for its impact on the motor systems, most neuroimaging research on the STN has focused on its links with the motor cortex (49–52). PD-related abnormalities in functional connectivity between the STN and nonmotor cortical regions have been grossly understudied, and extant findings have been inconsistent (50, 53, 54). Some studies have reported hyperconnectivity between the STN and ventromedial prefrontal cortex in PD patients compared to controls along with hypoconnectivity with parietal and visual regions (53), while other studies report the opposite—reduced connectivity between STN and prefrontal cortex but increased connectivity with visual and parietal cortex (50, 54). In the present study, we took a broader view and investigated functional connectivity of the STN with 10 large-scale FBNs consistently implicated in motor, speech, and cognitive functions.
Our analysis revealed that PD participants showed increased connectivity between the left STN and sensorimotor network, aligning with prior findings of hyperconnectivity between the STN and motor regions (49–52). Additionally, compared to the HC group, PD participants demonstrated heightened functional connectivity between the left STN and the salience network. Prior research in neurotypical individuals has linked functional connectivity between the STN and the anterior insula and anterior cingulate cortex—two key nodes in the salience network (55) and revealed a hyperdirect pathway between the STN and salience network crucial for efficient inhibitory control (23). Our findings suggest that aberrant functional connectivity of the STN with distinct networks may underpin motor and inhibitory control deficits in PD patients.
Dopaminergic Modulation of STN Network Connectivity Is Related to Medication-Induced Behavioral Changes in PD.
Dopaminergic medication altered functional connectivity between the STN and the 10 large-scale FBNs in PD participants in a clinically relevant manner. Functional connectivity of cortical-STN circuits has been associated with motor and inhibitory control functions (23, 51), but little is known about how dopaminergic modulation on cortical-STN connectivity impacts motor, speech, and cognitive functions. Here, we took an exploratory approach to examine multivariate brain–behavior association in relation to the effect of dopaminergic medication in PD. Specifically, we employed CCA to determine the association between dopaminergic medication effects on functional connectivity of the right and left STN with large-scale FBNs and medication-induced changes in motor, speech, and cognitive functions.
CCA revealed that clinical responsiveness to dopaminergic medication depends on the extent of medication-induced modulation of network functional connectivity with the STN (Fig. 2B). Notably, functional connectivity changes between the STN and the language network was the major contributor to multivariate associations with behavioral changes (Fig. 2C). On the behavioral side, network connectivity changes in PD patients were most prominently associated with medication-induced changes in the oSDMT (Fig. 2C), which requires participants to make a vocal response. In contrast, the relation with medication-induced changes in the wSDMT, which requires written responses, was much weaker. The oSDMT and wSDMT are essentially the same test and the major difference is that oSDMT requires vocal responses whereas wSDMT requires written responses. These results suggest a specific link between dopaminergic modulation of STN-Language network connectivity and medication-induced behavioral changes in speech function in PD.
Next, we used an independent language network atlas (48) to examine the direct relationship between medication-induced changes in STN-Language Regions of interest (ROI) connectivity and changes in the oSDMT performance. The ROIs were determined by an independent probabilistic atlas for Language network (48), encompassing lIFC, lPreCen, lSTG, rSTG, and SMA (Fig. 2D). We found that medication-induced changes in the oSDMT were significantly correlated with changes in functional connectivity between STN and both left IFC and left STG (Fig. 2E and SI Appendix, Fig. S2). Importantly, such brain–behavior association was not observed in the wSDMT performance, suggesting the specific contribution of the STN-Language ROI connectivity on speech but not on manual motor control.
Our findings are consistent with deterioration of speech after STN DBS procedures, which suggests the critical role of the STN in speech control (56). The clinical side effect of deteriorated speech function following STN DBS has inspired more research to investigate the association between STN and speech, particularly with the PD model and DBS approaches. It has been shown that speech production is accompanied by modulation in neuronal firing rate (36), beta power (57), and high gamma power in STN (35). PD patients with speech deficits had weaker neuronal activity in the STN during speech production than those without speech deficits (58). Beyond these previous findings, our study provided evidence on the brain mechanism by which dopaminergic medication affects speech function in PD patients. Together, it indicates that PD patients’ speech deficits could be alleviated by improving functional connectivity between STN and language network, which also speaks to the underlying mechanism why patients’ speech function deteriorates after STN DBS operation.
Dopaminergic Medication Modulates Functional Connectivity between STN Subdivisions and Language Network in Relation to Speech.
STN is a functionally heterogeneous region. Location of the DBS electrodes within the STN has a significant impact on its clinical outcome in PD patients (59, 60). However, whether dopaminergic medication has different modulatory effects on STN subregions in relation to clinical outcomes remains unknown. Previous studies suggest that the subregions within the STN may contribute differently to motor, cognitive, and emotion functions (39). Accordingly, we sought to characterize the functional heterogeneity of STN subdivisions in order to more precisely evaluate the behavioral effects of dopaminergic medication on STN circuits in PD. Studies in nonhuman primates have suggested a tripartite organization of the STN, composed of dorsolateral, central, and ventromedial subdivisions, associated with motor, cognitive, and affective functions, respectively (37, 38). This structure is mirrored in human diffusion weighted imaging (DWI) studies, which have highlighted similar subdivisions within the STN, each displaying distinct patterns of structural connectivity with other cortical areas (61). However, the precise boundaries of the DWI-derived subdivisions of the human STN have been questioned because of the difficulty of tracking fibers from the STN (62).
We addressed this question here by conducting a parcellation analysis to identify subdivisions within the STN based on temporal patterns of voxel-wise spontaneous neural activity. Importantly, the parcellation was conducted in an independent large-scale resting-state fMRI data obtained from the HCP (63). We employed a consensus clustering algorithm (64, 65), which identified three functional clusters within the STN in each hemisphere (SI Appendix, Fig. S3). Notably, these partitions aligned with the dorsolateral, central, and ventromedial subdivisions observed in nonhuman primates (37, 38).
We then employed CCA to determine the association between dopaminergic medication effects on functional connectivity of STN subdivisions with large-scale functional networks and changes in medication-induced changes in motor, speech, and cognitive functions. By utilizing a rigorous and confound-controlled CCA algorithm (66), we identified significant canonical correlation models for the right dorsolateral and right ventromedial subdivisions of the STN. The multivariate relationships were primarily influenced by medication-induced changes between these STN subdivisions and the language network and alterations in the oSDMT (Fig. 3).
These results demonstrate that dopaminergic medication effects on functional connectivity of the STN are associated with both the sensorimotor and limbic subdivisions of the STN. The dorsolateral STN is considered the sensorimotor subdivision and is most directly involved in motor function (37, 38). It receives input from the motor areas of the cortex and is the target for DBS treatments for movement disorder in PD (59, 60). The ventromedial STN receives inputs from limbic areas of the brain and is differentially involved in affective processing (37, 38). Our findings suggest that dopaminergic medication impacts speech processing via changes in both the motor and limbic subdivisions of the STN.
Next, we further examined which language region’s functional connectivity with the STN subdivisions is associated with dopaminergic modulation on speech function in PD. Analysis with the independent language network atlas (48) confirmed that medication-induced changes in the oSDMT were significantly correlated with changes in functional connectivity between the dorsolateral subdivision of the right STN and both left IFC and left STG, two key nodes in the cortical language system (Fig. 4).
Crucially, both multivariate and univariate analyses converged on the finding that medication-induced changes in functional connectivity between the STN and the language network were associated with medication-induced changes in the oSDMT, but not in the wSDMT. The oSDMT and wSDMT are identical tests, with the sole distinction being that oSDMT demands vocal responses while wSDMT requires written responses. This unique association with oSDMT, but not wSDMT, implies that medication-induced changes in functional connectivity between the STN and language network are not primarily influencing overall cognition. Instead, they specifically contribute to speech function. These findings provide compelling evidence for a more precise understanding of the dorsolateral STN’s role and the influence of dopaminergic medication on speech function in PD patients.
Our final objective was to develop a machine learning model that evaluates whether changes in functional connectivity between the STN and key areas of the language network can predict medication-induced changes in the oSDMT performance with medication. A support vector regression model was trained and tested using the leave-one-out cross-validation procedure. We found that multivariate pattern of medication-induced functional connectivities between the dorsolateral STN and key regions in the language network can accurately predict dopaminergic modulation on the oSDMT performance (SI Appendix, Fig. S4). This prediction analysis demonstrates the robustness of our findings as the multivariate features learned from STN-Language network functional connectivity have significant prediction power on the unseen speech performance data.
A striking finding of our study was that dopaminergic medication-induced changes in functional connectivity between the STN and both the left IFC and left STG were significant predictors of enhancements in oSDMT scores. Both these regions are integral components of the left-lateralized classical speech network model, as the left IFC specializes in speech production and the left STG plays a critical role in auditory processing and speech comprehension (16, 67, 68). Prior studies of the language network have primarily focused on the neocortex, leaving the role of subcortical regions in speech production unclear. Interestingly, our findings echo the results of two recent intracranial EEG studies in PD patients with DBS of the STN (46, 69). These studies reported that STN stimulation evoked neuronal responses in the left inferior frontal cortex and superior temporal gyrus (46) and identified the propagation of high-gamma activity from the superior temporal gyrus to the STN prior to speech onset (69). This hints at a key involvement of the STN in the language network. Our investigation provides further evidence for functional connectivity between the STN and the language network and demonstrates that this STN-language network connection plays a vital role in modulating speech functions in PD patients via dopaminergic medication.
Conclusion
Our study provides insights into the neural mechanisms underlying the effects of dopaminergic medication on speech impediments in PD, an underexplored aspect of the disorder. We investigated the effect of dopaminergic medication on STN circuits, specifically its association with speech and cognitive functions in PD patients. We found that medication-induced changes in the intrinsic functional connectivity of the STN predicted changes in speech and cognitive function, which were characterized by altered connectivity of the STN’s dorsolateral and ventromedial subdivisions with the language network. Notably, medication-induced changes in functional connectivity between the dorsolateral subdivision of the STN and key regions within the language network, particularly the left inferior frontal cortex and the left superior temporal gyrus, showed a strong correlation with performance changes on standardized neuropsychological tests requiring verbal responses. Interestingly, such a relation was absent in the written form of the same test, underscoring a potentially unique association between dopaminergic medication and speech functions in PD. Additionally, our findings highlight the predictive value of dopaminergic medication-induced changes in functional connectivity between the STN and language regions, indicating their role as a potential biomarker for determining the medication’s downstream effects on speech-related cognitive performance. These results reveal a previously unidentified brain mechanism, shedding light on the neural underpinnings of medication-induced alterations of speech in PD patients. Our findings that medication has different impact on STN subdivisions and its circuity with the language network in PD may improve treatment strategies for speech and cognitive deficits in PD.
Materials and Methods
Participants.
All participants were enrolled in the Stanford Alzheimer’s Disease Research Center. Inclusion criteria for HC included age ≥60 y; no neurological, psychiatric, or medical conditions causing cognitive impairment determined through history and neurological examination; and cognitively normal as determined by clinical consensus after formal testing that included the National Alzheimer’s Coordinating Center Uniform Data Set (version 3) neuropsychological battery. PD was determined by UK Brain Bank criteria (70) after a comprehensive neurological exam and the MDS-UPDRS part III (44) both OFF and ON dopaminergic medications. PD participants completed formal neuropsychological testing with the Uniform Data Set version 3 battery which occurred within 6 mo of the fMRI session. A total of 44 HCs completed a resting-state fMRI session and 38 PD participants completed two separate ON and OFF resting-state fMRI sessions. The session order was arranged to accommodate the participants’ convenience, which does not influence the significance of the findings. After screening head motion (max displacement < 3 mm and mean frame-wise displacement < 0.25 mm), a total of 42 HCs (71 ± 6 y old; 23F/19 M) and 27 PD participants (69 ± 7 y old; 14F/13 M; 22 PD with no cognitive impairment, 5 PD with mild cognitive impairment) were included in the final analyses. The final samples of HCs and PD participants were well matched in age, sex, education, and head motion, and head motion was not significantly different between ON and OFF sessions for PD participants (SI Appendix, Table S1).
All participants provided written consent and the Stanford University Institutional Review Board approved all study protocols.
Motor, Cognitive, and Speech Functioning.
Motor functioning in PD participants both ON and OFF dopaminergic medication was quantified by MDS-UPDRS part III scores. Cognitive functioning was measured with the SDMT. The SDMT is a neuropsychological test of executive functioning that is sensitive to cognitive changes in PD (71–73). There is both an oral (oSDMT) and a written (wSDMT) version of the SDMT and the oral version is often given to minimize motor demands, which is especially important for PD because tremor can significantly interfere with writing ability. For this study, each PD participant completed both oSDMT and wSDMT versions of the SDMT during the ON and OFF sessions. Raw scores were converted to demographically corrected standardized scores using previously published norms (74).
Data Acquisition.
The fMRI images were collected using a 3 T scanner. A total of 790 functional images were acquired using multiband echo-planar imaging with the following parameters: 47 slices, repetition time (TR) = 490 ms, flip angle = 45°, echo time = 30 ms, field of view = 220 × 220 mm, matrix = 74 × 74, 3 mm slice thickness, and voxel size = 2.97 × 2.97 × 3 mm. The first 12 time points were removed to allow for signal equilibration, leaving 778 time points for each participant. Each participant’s T1-weighted anatomical scan had been acquired using a magnetization-prepared rapid-acquisition gradient echo (MPRAGE) sequence (256 slices with a 176*256 matrix; voxel size 1.00 × 0.977 × 0.977 mm3).
fMRI Preprocessing.
A standard preprocessing pipeline was implemented using SPM12 software package (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/), as well as in-house programs in MATLAB (MathWorks). Functional MRI data were first slice time corrected, aligned to the averaged time frame to correct for head motion, and co-registered with each participant’s T1-weighted images. Structural MRI images were segmented into gray matter, white matter, and cerebrospinal fluid. Based on the transformation matrix from the structural image, the functional images were then transformed to the standard Montreal Neurological Institute (MNI) template in 2 × 2 × 2 mm3 by using the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) toolbox (75). A 2-mm Gaussian kernel was used to spatially smooth the functional images.
HCP Data.
Minimally preprocessed resting-state fMRI data were obtained from the HCP for STN parcellation. We selected 801 individuals based on the following criteria: 1) range of head motion in any translational direction is less than 1 mm; 2) average scan-to-scan head motion is less than 0.2 mm, and 3) maximum scan-to-scan head motion is less than 1 mm. For each individual, 1,200 frames were acquired using multiband, gradient-echo planar imaging with the following parameters: TR = 720 ms, TE = 33.1 ms; flip angle = 52°, field of view = 280 × 180 mm, matrix = 140 × 90, voxel size = 2 × 2 × 2. During scanning, individuals were eye-fixated on a projected crosshair on the screen.
Minimal preprocessing was implemented using fMRIVolume pipeline (76), including correction of gradient-nonlinearity-induced distortion, realignment for motion correction, registration, and normalization in 2 mm MNI space. Details of the preprocessing steps are described in previous studies (76). We applied spatial smoothing with a Gaussian kernel of 2 mm FWHM in minimally preprocessed HCP data to improve signal-to-noise ratio.
ROI.
STN.
The left and right STN (lSTN and rSTN) ROIs were constructed using a high-resolution probabilistic atlas of subcortical regions (77). With a probabilistic threshold of 0.1, lSTN was composed of 38 voxels and rSTN was composed of 39 voxels in the 2 mm spatial-resolution MNI space (SI Appendix, Fig. S2B).
Functional brain networks.
ROIs for functional brain networks were determined from an independent study (47), including sensorimotor (Sensorimotor), primary visual (prim_Visual), high visual (high_Visual), auditory (Auditory), language (Language), visuospatial (Visuospatial), left executive central (LECN), right executive central (RECN), salience (Salience), and dorsal default model network (DMN) (Figs. 1C and 2A).
Language network.
To further test functional connectivity between STN and Language network, we constructed ROIs using a probabilistic atlas for Language network (48). With a probabilistic threshold of 0.25, the language network was composed of the left inferior frontal cortex (lIFC), left precentral gyrus (lPreCen), left superior temporal gyrus (lSTG), right superior temporal gyrus (rSTG), and SMA (Fig. 2D).
Functional Parcellation of the STN.
We used a consensus clustering evidence accumulation method to identify stable and robust clusters in the STN (64, 65). Voxel-wise time series in the STN was extracted from each participant and used as the feature in the parcellation. Let be the features, where consists of T observations at each voxel i for a subject s; M is the total number of voxels and S is the total number of subjects. In step 1, we generated 100 different partitions of data from each subject for each k ranging from 2 to 5 using different initializations of K-means algorithm (78). In step 2, we computed a coassociation matrix C, for each k and S that finds similarities between these 100 different partitions. In step 3, we applied hierarchical clustering with average linkage using C as the similarity matrix for each k and S. In step 4, we computed the Probability Rand Index (PRI) (79) to quantify the similarity of the clusters across all the subjects S for a given k. The optimal number of clusters ( was determined by the max value of the PRI. In step 5, we computed the group level average coassociation matrix by averaging the coassociation matrices for the optimal clustering solution k* across all the S subjects. We then obtained the stable clusters at the group level by applying hierarchical clustering method with average linkage using this coassociation as on the similarity matrix. More method details can be found in our previous studies (64, 65).
Functional Connectivity.
To investigate the functional circuits associated with individual STN clusters we conducted seed-based functional connectivity analysis. First, time series across all the voxels within the thresholded cluster was extracted and averaged. The resulting averaged time series was then used as a covariate of interest in a linear regression of the whole-brain analysis. A global time series, computed across all brain voxels, along with six motion parameters were used as additional covariates to remove confounding effects of physiological noise and participant movement. Linear regression was conducted at the individual subject level.
Canonical Correlation Analysis.
We use CCA to investigate the effect of dopaminergic medication on cortical-STN connectivity in relation to medication effect on motor and cognitive function (80). The medication-induced difference in functional connectivity between STN and functional brain networks (PD-ON-PD-OFF) were used as the set of X variables and the medication-induced difference in motor and cognitive measures, including UDPRS, oSDMT, and wSDMT, were used as the set of Y variables. All neuropsychological measures were transformed such that higher scores were indicative of better performance and positive values in the contrast of PD-ON versus PD-OFF were indicative of improved function induced by dopaminergic medication. Age, sex, and education were included in the model as nuisance variables. CCA and its statistical testing were conducted using “permcca” algorithm implemented in Matlab (66). The “permcca” algorithm was developed to address the concerns about shared variation on nuisance variables and repeatedly explained variations between canonical variables. Permutation tests (1,000 times) were used to build a null distribution of canonical correlations from which statistical significance was defined by P < 0.05, corrected with the familywise error rate (FWER). The permutation test for CCA involves randomly shuffling the rows of either X or Y variables. With each permutation of the data denoted by π, a fresh set of canonical correlation rπ would be calculated. Subsequently, a P-value could be computed as P = (∑I(rπ >r0))/np, where np is the number of permutations, r0 is the canonical correlation from nonpermuted (original) data, and I = 1 if rπ >r0, otherwise I = 0. Last, P values were FWER-corrected for all the canonical correlation tests. Canonical coefficients were reported to characterize the multivariate relationship between two sets of variables. The contribution of each original variable on the canonical scores was assessed using Pearson’s correlation.
Univariate Association Analysis.
We used Pearson’s correlation to test whether medication-induced changes in functional connectivity between STN and regions in the Language network are significantly associated with medication-induced changes in the oSDMT performance. Next, multiple linear regression analyses were used to further examine the relation between medication-induced changes in functional connectivity between STN and regions in the Language network is significantly associated with medication-induced changes in the oSDMT performance while controlling for age, sex, education, LEDD, and head motion.
Prediction Analysis.
To examine whether medication-induced changes in functional connectivity between STN and regions in Language network can predict medication-induced changes in oSDMT performance in PD patients, we conducted multivariate regression analysis using linear support vector regression (SVR). Differences between PD-ON and PD-OFF in functional connectivity between STN and all regions in the language network were used as features to predict the difference between PD-ON and PD-OFF in oSDMT. The prediction model was tested using leave-one-out cross-validation. Each time, one participant’s data (PD-ON and PD-OFF) was selected as a test set, and the rest of the data were used as a training set. The training set was then used to train an SVR model, which was then applied on the test set for prediction. This procedure was repeated N times with each data point used exactly once as a test set. The model’s performance was evaluated using Pearson’s correlation between predicted scores and observed scores. Python package scikit-learn was used for SVR and cross-validation (https://github.com/scikit-learn/scikit-learn).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This research was supported by grants from the NIH (P50 AG047366, P30 AG066515, RF1 NS086085, R21 DC017950-S1, R01 NS115114, R01 MH121069, and K99 AG071837) and the Alzheimer’s Association (AARFD-21-849349, AARGD-NTF-21-850781, and AARFD-21-848178).
Author contributions
W.C. and V.M. designed research; W.C., S.R., J.K., L.Y., T.F.L., V.W.H., and K.L.P. performed research; W.C., R.Y., and B.L. analyzed data; and W.C., C.B.Y., K.L.P., and V.M. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Weidong Cai, Email: wdcai@stanford.edu.
Vinod Menon, Email: menon@stanford.edu.
Data, Materials, and Software Availability
Data used for parcellation of the Subthalamic Nucleus are available from the HCP (https://www.humanconnectomeproject.org/) (81). Anonymized PD and HC data will be made available on request to qualified researchers, but this will be contingent upon the authors’ ability to secure Stanford University’s institutional review board approval, and upon both parties signing a Data Usage Agreement. Code used in the analysis can be found at (https://github.com/scsnl/Cai_PD_Dopamine_STN_Language_RSFC_2023) (82).
Supporting Information
References
- 1.Galperin I., et al. , Associations between daily-living physical activity and laboratory-based assessments of motor severity in patients with falls and Parkinson’s disease. Parkinsonism Relat. Disord. 62, 85–90 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Pfeiffer R. F., Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat. Disord. 22, S119–S122 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Fearnley J. M., Lees A. J., Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain 114, 2283–2301 (1991). [DOI] [PubMed] [Google Scholar]
- 4.Jahanshahi M., Obeso I., Baunez C., Alegre M., Krack P., Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Movement Disord. 30, 128–140 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Chaudhuri K. R., Healy D. G., Schapira A. H., National Institute for Clinical E, Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurol. 5, 235–245 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Cools R., Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci. Biobehav. Rev. 30, 1–23 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Cools R., Barker R. A., Sahakian B. J., Robbins T. W., Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex 11, 1136–1143 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Norel R., et al. , Speech-based characterization of dopamine replacement therapy in people with Parkinson’s disease. Npj Parkinsons Disease 6, 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tykalova T., Novotny M., Ruzicka E., Dusek P., Rusz J., Short-term effect of dopaminergic medication on speech in early-stage Parkinson’s disease. Npj Parkinsons Disease 8, 22 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohl A., et al. , Speech dysfunction, cognition, and Parkinson’s disease. Prog. Brain Res. 269, 153–173 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart C., et al. , Speech dysfunction in early Parkinson’s disease. Mov. Disord. 10, 562–565 (1995). [DOI] [PubMed] [Google Scholar]
- 12.Flinker A., et al. , Redefining the role of Broca’s area in speech. Proc. Natl. Acad. Sci. U.S.A. 112, 2871–2875 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Indefrey P., Levelt W. J. M., The spatial and temporal signatures of word production components. Cognition 92, 101–144 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Bouchard K. E., Mesgarani N., Johnson K., Chang E. F., Functional organization of human sensorimotor cortex for speech articulation. Nature 495, 327–332 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mesgarani N., Cheung C., Johnson K., Chang E. F., Phonetic feature encoding in human superior temporal gyrus. Science 343, 1006–1010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oganian Y., Bhaya-Grossman I., Johnson K., Chang E. F., Vowel and formant representation in the human auditory speech cortex. Neuron 111, 2105–2118.e4 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manes J. L., et al. , Connectivity of the subthalamic nucleus and globus pallidus pars interna to regions within the speech network: A meta-analytic connectivity study. Hum. Brain Mapping 35, 3499–3516 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watkins K. E., Jenkinson N., "The anatomy of basal ganglia" in Neurobiology of Language, Hickok G., Small S. L., Eds. (Elsevier, 2016), pp. 85–94. [Google Scholar]
- 19.Dashtipour K., Tafreshi A., Lee J., Crawley B., Speech disorders in Parkinson’s disease: Pathophysiology, medical management and surgical approaches. Neurodegener Dis. Man 8, 337–348 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Simonyan K., Berman B. D., Herscovitch P., Hallett M., Abnormal striatal dopaminergic neurotransmission during rest and task production in spasmodic dysphonia. J. Neurosci. 33, 14705–14714 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walter U., Blitzer A., Benecke R., Grossmann A., Dressler D., Sonographic detection of basal ganglia abnormalities in spasmodic dysphonia. Eur. J. Neurol. 21, 349–352 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Aron A. R., Poldrack R. A., Cortical and subcortical contributions to Stop signal response inhibition: Role of the subthalamic nucleus. J. Neurosci. 26, 2424–2433 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai W., et al. , Hyperdirect insula-basal-ganglia pathway and adult-like maturity of global brain responses predict inhibitory control in children. Nat. Commun. 10, 4798 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W., et al. , Prefrontal-subthalamic hyperdirect pathway modulates movement inhibition in humans. Neuron 106, 579–588.e573 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forstmann B. U., et al. , Cortico-subthalamic white matter tract strength predicts interindividual efficacy in stopping a motor response. Neuroimage 60, 370–375 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Nambu A., Tokuno H., Takada M., Functional significance of the cortico-subthalamo-pallidal "hyperdirect" pathway. Neurosci. Res. 43, 111–117 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Polyakova Z., Chiken S., Hatanaka N., Nambu A., Cortical control of subthalamic neuronal activity through the hyperdirect and indirect pathways in monkeys. J. Neurosci. 40, 7451–7463 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong J. K., et al. , STN vs. GPi deep brain stimulation for tremor suppression in Parkinson disease: A systematic review and meta -analysis. Parkinsonism Relat. Disord. 58, 56–62 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alegret M., et al. , Effects of bilateral subthalamic stimulation on cognitive function in Parkinson disease. Arch. Neurol.-Chicago 58, 1223–1227 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Costentin G., et al. , White matter tracts lesions and decline of verbal fluency after deep brain stimulation in Parkinson’s disease. Hum. Brain Mapp. 40, 2561–2570 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okun M. S., et al. , Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: The COMPARE trial. Ann. Neurol. 65, 586–595 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsons T. D., Rogers S. A., Braaten A. J., Woods S. P., Troster A. I., Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson’s disease: A meta-analysis. Lancet Neurol. 5, 578–588 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Schroeder U., et al. , Subthalamic nucleus stimulation affects a frontotemporal network: A PET study. Ann. Neurol. 54, 445–450 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Witt K., et al. , Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in Parkinson’s disease: Results from a randomized trial. Brain 136, 2109–2119 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Chrabaszcz A., et al. , Subthalamic nucleus and sensorimotor cortex activity during speech production. J. Neurosci. 39, 2698–2708 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipski W. J., et al. , Subthalamic nucleus neurons differentially encode early and late aspects of speech production. J. Neurosci. 38, 5620–5631 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haynes W. I. A., Haber S. N., The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: Implications for basal ganglia models and deep brain stimulation. J. Neurosci. 33, 4804–4814 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monakow K. H., Akert K., Kunzle H., Projections of the precentral motor cortex and other cortical areas of the frontal lobe to the subthalamic nucleus in the monkey. Exp. Brain Res. 33, 395–403 (1978). [DOI] [PubMed] [Google Scholar]
- 39.Temel Y., Blokland A., Steinbusch H. W., Visser-Vandewalle V., The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog. Neurobiol. 76, 393–413 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Hershey T., et al. , Mapping Go-No-Go performance within the subthalamic nucleus region. Brain 133, 3625–3634 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mallet L., et al. , Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proc. Natl. Acad. Sci. U.S.A. 104, 10661–10666 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasquereau B., Turner R. S., A selective role for ventromedial subthalamic nucleus in inhibitory control. Elife 6, e31627 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horn A., et al. , Connectivity predicts deep brain stimulation outcome in Parkinson disease. Ann. Neurol. 82, 67–78 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goetz C. G., et al. , Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Smith A., Symbol Digit Modalities Test (Western Psychological Services, Los Angeles, CA, 1982). [Google Scholar]
- 46.Jorge A., et al. , Hyperdirect connectivity of opercular speech network to the subthalamic nucleus. Cell Rep. 38, 110477 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shirer W. R., Ryali S., Rykhlevskaia E., Menon V., Greicius M. D., Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex 22, 158–165 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lipkin B., et al. , Probabilistic atlas for the language network based on precision fMRI data from >800 individuals. Sci. Data 9, 529 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baudrexel S., et al. , Resting state fMRI reveals increased subthalamic nucleus-motor cortex connectivity in Parkinson’s disease. Neuroimage 55, 1728–1738 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Fernandez-Seara M. A., et al. , Resting state functional connectivity of the subthalamic nucleus in Parkinson’s disease assessed using arterial spin-labeled perfusion fMRI. Hum. Brain Mapp. 36, 1937–1950 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurani A. S., et al. , Subthalamic nucleus–sensorimotor cortex functional connectivity in de novo and moderate Parkinson’s disease. Neurobiol. Aging 36, 462–469 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen B., et al. , Resting state fMRI reveals increased subthalamic nucleus and sensorimotor cortex connectivity in patients with Parkinson’s disease under medication. Front Aging Neurosci. 9, 74 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathys C., et al. , Functional connectivity differences of the subthalamic nucleus related to Parkinson’s disease. Hum. Brain Mapp. 37, 1235–1253 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z., et al. , Resting-state functional connectivity of subthalamic nucleus in different Parkinson’s disease phenotypes. J. Neurol. Sci. 371, 137–147 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Anteraper S. A., et al. , Resting-state functional connectivity of the subthalamic nucleus to limbic, associative, and motor networks. Brain Connect. 8, 22–32 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Wertheimer J., et al. , The impact of STN deep brain stimulation on speech in individuals with Parkinson’s disease: The patient’s perspective. Parkinsonism Relat. Disord. 20, 1065–1070 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Hebb A. O., Darvas F., Miller K. J., Transient and state modulation of beta power in human subthalamic nucleus during speech production and finger movement. Neuroscience 202, 218–233 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tankus A., Fried I., Degradation of neuronal encoding of speech in the subthalamic nucleus in Parkinson’s disease. Neurosurgery 84, 378–387 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Hacker M. L., et al. , Connectivity profile for subthalamic nucleus deep brain stimulation in early stage Parkinson disease. Ann. Neurol. 94, 271–284 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang F., et al. , Relationship between electrode position of deep brain stimulation and motor symptoms of Parkinson’s disease. Bmc Neurol. 21, 122 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lambert C., et al. , Confirmation of functional zones within the human subthalamic nucleus: Patterns of connectivity and sub-parcellation using diffusion weighted imaging. Neuroimage 60, 83–94 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alkemade A., Forstmann B. U., Do we need to revise the tripartite subdivision hypothesis of the human subthalamic nucleus (STN)? Neuroimage 95, 326–329 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Van Essen D. C., et al. , The Human Connectome Project: A data acquisition perspective. Neuroimage 62, 2222–2231 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai W., Ryali S., Chen T., Li C. S., Menon V., Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: Evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. J. Neurosci. 34, 14652–14667 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ryali S., Chen T., Padmanabhan A., Cai W., Menon V., Development and validation of consensus clustering-based framework for brain segmentation using resting fMRI. J. Neurosci. Methods 240, 128–140 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winkler A. M., Renaud O., Smith S. M., Nichols T. E., Permutation inference for canonical correlation analysis. Neuroimage 220, 117065 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Graves R. E., The legacy of the Wernicke-Lichtheim model. J. Hist. Neurosci. 6, 3–20 (1997). [DOI] [PubMed] [Google Scholar]
- 68.Poeppel D., Hickok G., Towards a new functional anatomy of language. Cognition 92, 1–12 (2004). [DOI] [PubMed] [Google Scholar]
- 69.Weiss A. R., et al. , Lexicality-modulated influence of auditory cortex on subthalamic nucleus during motor planning for speech. Neurobiol. Lang. (Camb) 4, 53–80 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Litvan I., et al. , Movement disorders society scientific issues committee report: SIC task force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov. Disord. 18, 467–486 (2003). [DOI] [PubMed] [Google Scholar]
- 71.Gabrieli J. D. E., Singh J., Stebbins G. T., Goetz C. G., Reduced working memory span in Parkinson’s disease: Evidence for the role of a frontostriatal system in working and strategic memory. Neuropsychology 10, 322–332 (1996). [Google Scholar]
- 72.Pascoe M., Alamri Y., Dalrymple-Alford J., Anderson T., MacAskill M., The symbol-digit modalities test in mild cognitive impairment: Evidence from Parkinson’s disease patients. Eur. Neurol. 79, 206–210 (2018). [DOI] [PubMed] [Google Scholar]
- 73.Starkstein S. E., et al. , Depression and cognitive impairment in Parkinsons-disease. Brain 112, 1141–1153 (1989). [DOI] [PubMed] [Google Scholar]
- 74.Fellows R. P., Schmitter-Edgecombe M., Symbol digit modalities test: Regression-based normative data and clinical utility. Arch. Clin. Neuropsych. 35, 105–115 (2020). [DOI] [PubMed] [Google Scholar]
- 75.Ashburner J., A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Glasser M. F., et al. , The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pauli W. M., Nili A. N., Tyszka J. M., Data Descriptor: A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci. Data 5, 180063 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.MacQueen J. B., "Some methods for classification and analysis of multivariate observations" in Proceedings of the Symposium on Mathematics and Probability (University of California Press, Berkely, 1967), pp. 281–297. [Google Scholar]
- 79.Carpineto C., Romano G., Consensus clustering based on a new Probabilistic Rand Index with application to subtopic retrieval. IEEE Trans. Pattern Anal. Mach. Intell. 34, 2315–2326 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Hotelling H., Relations between two sets of variates. Biometrika 28, 321–377 (1936). [Google Scholar]
- 81.Van Essen D. C., et al. , Data from “The Human Connectome Project: a data acquisition perspective.” Neuroimage 62, 2222–2231 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cai W., Cai_PD_Dopamine_STN_Language_RSFC_2023. GitHub. https://github.com/scsnl/Cai_PD_Dopamine_STN_Language_RSFC_2023. Deposited 30 March 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Data used for parcellation of the Subthalamic Nucleus are available from the HCP (https://www.humanconnectomeproject.org/) (81). Anonymized PD and HC data will be made available on request to qualified researchers, but this will be contingent upon the authors’ ability to secure Stanford University’s institutional review board approval, and upon both parties signing a Data Usage Agreement. Code used in the analysis can be found at (https://github.com/scsnl/Cai_PD_Dopamine_STN_Language_RSFC_2023) (82).