Abstract
Objectives:
The current study investigated performance fatigability (PF) and time course of changes in force, electromyographic amplitude (EMG AMP) and frequency (EMG MPF), and neuromuscular efficiency (NME) during a sustained, isometric, handgrip hold to failure (HTF) using the rating of perceived exertion (RPE)-Clamp Model.
Methods:
Twelve males performed a handgrip HTF anchored to RPE=5. The time to task failure (Tlim), force (N), EMG AMP and MPF, and NME (normalized force/ normalized EMG AMP) were recorded. Analyses included a paired samples t-test for PF at an alpha of p<0.05, 1-way repeated measures ANOVA across time and post-hoc t-tests (p<0.0025) for force, EMG AMP and MPF, and NME responses.
Results:
The PF (pre- to post- maximal force % decline) was 38.2±11.5%. There were decreases in responses, relative to 0% Tlim, from 40% to 100% Tlim (force), at 30%, 60%, and 100% Tlim (EMG AMP), from 10% to 100% Tlim (EMP MPF), and from 50% to 65%, and 80% to 100% Tlim (NME) (p<0.0025).
Conclusions:
The RPE-Clamp Model in this study demonstrated that pacing strategies may be influenced by the integration of anticipatory, feedforward, and feedback mechanisms, and provided insights into the relationship between neuromuscular and perceptual responses, and actual force generating capacity.
Keywords: Electromyography, Handgrip, Muscle Fatigue, Performance Fatigability, Rating of Perceived Exertion
Introduction
Anchoring exercise to a rating of perceived exertion (RPE-Clamp Model) has been used to examine complex fatigue-induced responses[1-5]. Kluger et al.[6], reported that fatigue can be explained by two independent characteristics including perceived fatigability and performance fatigability (PF). Perceived fatigability encompasses subjective perceptions of fatigue, effort, or discrepancies between expended exertion and achieved performance[6]. In contrast, PF refers to the magnitude of changes in performance-associated measurements (e.g., force/power decrease) after a given fatiguing task. Furthermore, it has been suggested that PF is affected by peripheral factors (e.g., uncoupling of excitation/contraction due to an accumulation of metabolic byproducts in active muscles) as well as central factors (e.g., decrease in central drive, and cortical and subcortical networks)[6,7]. In this regard, the RPE-Clamp Model has been utilized to investigate the impacts of perception of fatigue on adjustments in force throughout a task and on the resultant PF[2-4,7]. Previous studies have reported continuous reductions in force to maintain the required RPE and significant reductions in maximal voluntary isometric contraction (MVIC) force from pre- to post- exercise (PF of 34.7% [women] to 47.5% [men]) for unilateral, isometric leg extensions[2,8]. However, the amount of muscle mass engaged can also play an important role in the magnitude of perceived fatigability and PF[9,10]. Thomas et al.[10], suggested that as the active muscle mass is smaller, the exerciser is able to tolerate greater local muscular stress, leading to greater magnitude of PF (i.e., greater reduction in MVIC) than in exercise involving greater active muscle mass. Therefore, it is possible that a fatiguing task requiring a muscle contraction of small muscle group (e.g., forearm muscles related to unilateral handgrip) would bring about greater magnitude of PF compared to a muscle contraction of a larger muscle group (e.g., quadriceps muscles related to leg extension)[10]. Koral et al.[11], not only demonstrated that there was a significantly lower post- exercise MVIC in a unilateral condition compared to bilateral condition after a 1-min sustained maximal leg extension exercise, but cautioned that other factors such as an exercise intensity (maximal vs. submaximal muscle contraction), a contribution of assistive muscles, and psychobiological processes should be also considered when assessing the global fatigue response. The PF has not been examined for RPE-clamp exercise involving the relatively small muscles of the forearm during submaximal, isometric handgrip exercise.
The application of RPE-Clamp exercise allows for the examination of mechanisms underlying pacing strategies reflected by the selection of an initial force and the continuous modulations in force throughout a task to maintain the required RPE[4,5,7,12,13]. Tucker[5] suggested that the initial exercise intensity for RPE-Clamp exercise is informed by an anticipatory or feedforward component that involves physio-psychological inputs (e.g., previous experiences, motivation, skin temperature, muscle glycogen) which are processed within the pre-motor and/or motor areas of the brain. However, it has been proposed that the continued reductions in force throughout exercise at a fixed RPE are related to both feedforward and feedback mechanisms[4,5,7,12,13]. The Corollary Discharge Model from previous studies[13,14] supports the feedforward mechanism, and proposed that the corollary discharge may arise from premotor and primary motor areas that can produce an internal signal that traverses outward via efferent pathways, thus the perception of effort may be generated from central motor commands and integrated within the supplementary motor area (SMA) to regulate the exercise intensity. During continuous exercise, however, it has been suggested that feedback from the exercising muscle, via group III and IV afferent neurons which are sensitive to mechanical and metabolic changes within the muscles, respectively, may inform modulations in the exercise intensity and reduce central motor drive[12]. These feedforward and feedback mechanisms are integrated in the Sensory Tolerance Limit (STL) Model which identifies a global model of fatigue where the sum of feedback from primary and indirectly involved muscles during an exercise are combined with feedforward corollary discharges to regulate the magnitude of central motor command and ultimately inform the voluntary modulations in force generation that lead to task failure[15]. Therefore, the RPE-Clamp Model allows for the examination of how pacing strategies are influenced by the integration of these anticipatory, feedforward, and feedback mechanisms[4,5,7,12,13].
The RPE-Clamp Model has been also used to examine the fatigue-induced neuromuscular responses as an alternative method of anchoring to force[2-4,7]. Previous studies have reported that during prolonged, submaximal, isometric muscle contractions anchored to force, there were fatigue induced increases in electromyographic (EMG) amplitude (AMP) that reflect an increase in muscle excitation (i.e., motor unit recruitment, firing rate, synchronization), and decreases in EMG mean power frequency (MPF) due to the decreases in action potential conduction velocity along the sarcolemma as a result of metabolic perturbations[2,16,17]. Moreover, neuromuscular efficiency (NME), defined as normalized force divided by normalized EMG AMP, has been shown to decrease during prolonged contractions because more muscle excitation of the fatigued muscle is required to maintain the same voluntary tension during or after fatiguing muscle contraction[18-20]. However, during RPE-clamp exercise, where force is modulated to maintain a fixed RPE, evidence of neuromuscular fatigue diverges from the typical manifestations present during constant force exercise[2-4,7]. Specifically, Keller et al.[2], demonstrated that there was a decrease in EMG AMP, but no change in EMG MPF during a sustained, isometric leg extension muscle contraction anchored to RPE=5 (OMNI-RES) in males. It is plausible that it was necessary to decrease force production which was accompanied by reductions in muscle excitation (EMG AMP) to maintain the assigned RPE=5 during the isometric, fatiguing task, and no clear evidence of metabolic perturbations based on the lack of change in EMG MPF, which may assume that there would be little change in NME (force/EMG AMP)[2]. However, this study[2] set the maximal time of exercise at 5 min. Specifically, the isometric, fatiguing leg extension exercise was terminated at a predetermined 5 min endpoint even though four of the 10 subjects had not reached zero force output and, therefore, could have continued to reduce force to maintain the assigned RPE. It is possible that a fatiguing task sustained for longer periods of time (more than 5 min), until subjects reach zero force output, could alter the accompanied neuromuscular patterns and NME responses.
No previous studies have reported the effects of RPE-clamp exercise on the neuromuscular responses (EMG AMP, EMG MPF, and NME) and PF (i.e., absolute force difference between pre-MVIC to post-MVIC, and percent decline of MVIC) during sustained, isometric, fatiguing handgrip holds. This information may provide insight into how the subjective sensations (perceived fatigability) influence the actual force production (PF) during the fatiguing task. The purpose of this study was to investigate PF as well as the time course of changes in force and neuromuscular responses (EMG AMP, EMG MPF, and NME) during a sustained, isometric, handgrip hold to failure (HTF) using the RPE-Clamp Model at an RPE level equal to 5 on the OMNI-RES 0-10 scale. Based on previous reports[2,10], it was hypothesized that there would be reductions in force and EMG AMP to maintain the predetermined RPE=5, but there would be no changes in EMG MPF. It was also hypothesized that the magnitude of PF of the forearm muscles would be greater than previously reported for the leg extensors[2].
Methods
Research Design
This study utilized a repeated measure, experimental design that consisted of 2 visits separated by a minimum of 24 hours. Testing procedures involved performing isometric muscle actions using a handgrip dynamometer (TSD121C; BioPac Systems Inc), which had a sensitivity rating of 0.022N. During the familiarization visit (visit 1), the subjects were oriented to the RPE scale, completed MVIC testing of the dominant hand for reliability purposes, and were asked to set anchors to the RPE scale. At visit 2, the subjects performed a continuous isometric handgrip hold anchored by RPE at an RPE=5 on the OMNI-RES 0-10 scale to task failure (i.e., force = 0N). The HTF during visit 2 was performed on the dominant hand and pre- and post-MVICs were performed to examine PF which was expressed as absolute newton (N) values for analyses and as a relative value for descriptive purposes (i.e., % change). During all handgrip exercises, neuromuscular responses (EMG AMP and MPF) were measured from the brachioradialis of the dominant limb. NME (normalized force/normalized EMG AMP) was examined for each MVIC and throughout the RPE hold. After the fatiguing handgrip task, the subjects were asked to identify the specific factors that contributed to the task failure via a post-test failure questionnaire. Specifically, the subjects were asked to indicate 1) muscles (forearm muscles, hand, biceps, triceps) or organs (lungs/out of breath), 2) psychological factors (loss of mental focus, loss of motivation, boredom), 3) levels of pain or discomfort that were all categorized from 1 (no contribution at all) to 5 (extreme contribution).
Subjects
Twelve males (mean±SD: 28.2±3.8yr, 179.2±7.0cm, 82.5±17.5kg) were recruited and completed testing for this study. Inclusion in the study comprised subjects who were free of musculoskeletal injuries and neuromuscular diseases, currently were participating in resistance training (for at least 6 months, 1 time per week), demonstrated readiness for physical activity via an informed consent form, had no medical contraindications as determined via a health history form. The subjects were asked to maintain their current level of physical activity, but to abstain from upper body resistance training exercise for at least 24 hours, and from consuming caffeine within 3 hours prior to their testing session. All of the subjects completed a health history form.
Familiarization Session and MVIC Determination
The first visit included a familiarization session to orient the subjects to the RPE scale. In addition, MVIC testing was performed, which was used for test-retest reliability determination along with the pre-MVIC measurement during visit 2. During the familiarization and experimental visits, the following RPE instructions, which were modified from Keller et al.[2], were read to subjects:
“You will be asked to set an anchor point for both the lowest and highest value on the RPE scale. To determine the lowest anchor, you will be asked to sit quietly and relax your whole body without contracting your forearm muscles to familiarize yourself with a zero. You will be then asked to perform two maximal voluntary isometric contractions to familiarize yourself with a 10. When instructed to perform the handgrip task corresponding to this scale, perceived exertion at a given RPE level should be relative to these defined anchors”.
Following these instructions and prior to the MVIC trials, a warm-up was performed with 3-5, 6-s holds at a perceived effort of 30-80% of maximum. After three minutes of rest from the last warm-up contraction, the subjects performed 3-5 MVICs for the dominant hand, each consisting of a 3-s contraction at 90° of forearm flexion, with the hand in the neutral position. For the MVICs, a 1-s epoch corresponding with the middle 1/3 of the contraction was isolated for signal analysis. Two trials of MVICs within 5% of one another were selected, and the test that resulted in the greatest peak force was used as the test 1 MVIC force.
Experimental Visit: Handgrip exercise anchored to a rating of perceived exertion
During the experimental visit, the subjects performed 3-5 MVIC trials (the greatest force was recorded as test 2 for reliability analyses) before and one MVIC trial immediately after a continuous isometric hold exercise anchored to RPE 5. Prior to the MVIC trials, the subjects performed the standardized warm-up and were read the same RPE instructions previously described. After the MVIC trials, the subjects completed the RPE=5 HTF. The subjects were instructed to adjust force production to maintain a predetermined level of exertion equivalent to RPE=5 (between somewhat easy and somewhat hard). During the hold, the subjects were blinded to the force output to mitigate any potential pacing strategies from visual feedback during the trial. Specifically, the subjects did not receive visual feedback associated to force production, so that the perception of exertion would not be confounded with visual information of force. Task failure was determined as the timepoint at which subjects indicated that they could no longer sustain the assigned RPE (i.e., increase in perceived exertion) despite continued reductions in force (i.e., zero force production). The time to task failure (Tlim) was recorded in seconds. The changes in force were examined in standardized segments every 5% of time to failure to examine the changes across time. Specifically, a 1-sec epoch at the beginning and end (0% and 100% Tlim) of each HTF and 500 ms before and after each 5% of Tlim (1-second epoch from the center of each 5% segment) were used to examine the changes in force over time, providing 21 timepoints.
Electromyography Measurements
During the test 1 MVIC as well as the test 2 pre- and post-MVICs and the RPE=5 HTF trial of the experimental visit, pre-gelled surface EMG electrodes were placed in a bipolar arrangement (20mm center-to-center) on the brachioradialis of the dominant arm. The brachioradialis was selected based on pilot data that demonstrated pronounced changes in EMG signals, despite this muscle not being the primary muscle in handgrip, and to minimize cross-talk between electrodes placed on the intrinsic muscles. Prior to electrode placement, the site on the skin was shaved, carefully abraded, and cleaned with isopropyl alcohol. According to recommendations from Barbero et al.[21], a line from the styloid process of the radius to a midpoint on the line between the lateral and medial epicondyles were indicated. One electrode was then placed at 75% of this line (i.e., proximal part of the muscle belly), and another electrode was placed 2 cm distally from the attached electrode on the same line. A reference electrode was placed on the radial styloid process.
Signal Processing
The raw EMG signals were digitized at 2000 Hz and stored in a personal computer for analysis. The recorded signals were processed with a custom code written in MATLAB (The MathWorks) program. The EMG signals were amplified (EMG 100c, BIOPAC Systems, Inc., gain: x1,000) and bandpass-filtered (zero-phase shift fourth-order Butterworth) at 10-500 Hz. The EMG AMP and MPF were recorded in standardized segments of 5% of Tlim during the fatiguing testing session and normalized to the respective values at the pre-MVIC. A 1-sec epoch at the beginning (0% Tlim) and end (100% Tlim) of the HTF and 500 ms before and after each 5% of Tlim (1-second epoch from the center of each 5% segment) were used to calculate the EMG AMP (microvolts root mean square, µVrms). For the EMG MPF analysis, each data segment was processed with a Hamming window and a Fast Fourier transform (FFT) algorithm. The MPF was selected to represent the power spectrum based on the recommendations of Hermens et al[22]. In addition, NME was calculated for the initial (0% Tlim), final (100% Tlim), and each 5% segment by determining the ratio of the normalized force to normalized EMG AMP[20].
Statistical Analyses
Anthropometric data (age, height, and weight) were used for descriptive purposes. The reliability of the MVIC force from test 1 and test 2 was examined using a one-way repeated measures ANOVA, ICC (2,1), standard error of the measurement (SEM), minimal difference to be considered real (MD)[23], and coefficient of variation (CoV)[24]. A paired samples t-test was used to compare the pre- to post-MVIC force (N) (i.e., PF). Analyses were performed to examine the time course of changes in force, EMG AMP and MPF responses as well as NME. Since each subject had a different Tlim, time was normalized as a percentage of Tlim, and 21 timepoints (5% segments, 0%-100% Tlim) were used for the analyses of force, EMG AMP and MPF as well as NME responses. Specifically, the time course of changes in force, EMG AMP, EMG MPF, and NME were examined using separate, 1-way repeated measures ANOVAs across time and post-hoc t-tests with a Bonferroni corrected alpha level to determine changes across time, relative to the initial value (p<0.0025). An alpha level of p≤0.05 was used to determine statistical significance for all ANOVAs. Measures of effect size included partial eta square (ηp2) and Cohen’s d. All statistical analyses were conducted using the Statistical Package for Social Sciences software (v.28.0 IBM SPSS Inc.).
Results
Reliability
There were no mean differences between the test 1 (Mean±SD: 452.95±93.82 N) and test 2 (453.63± 94.01 N) pre-MVICs (F=0.011, p=0.920, ηp2=0.001). The ICC values of pre-MVICs (R=0.970) from test 1 and 2 indicated ‘excellent’ test-retest reliability[25]. In addition, the SEM and MD were 15.89 N, and 44.15 N, respectively. Furthermore, the CoV (3.51%) was below the 10% threshold to be considered sufficiently reliable[24].
Time to Task Failure and Time Course of Changes in Force
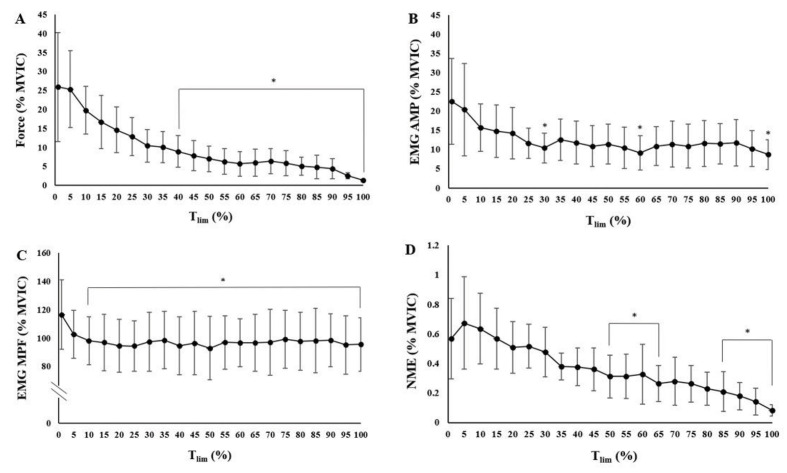
The Tlim for the handgrip HTF at RPE 5 was 512.4±245.9 sec (range: 156 – 1033 sec). All of the subjects reached 0 N in the last millisecond during the handgrip holds at RPE 5. The contributing factors that led to the task failure were indicated in Table 1 via the post-test failure questionnaire. A 1(RPE 5) x 21(time: 0-100% MVIC) repeated measures ANOVA indicated that there were significant differences across time (F=24.989, p<0.001, ηp2=0.694) for the relative force response (% MVIC). The post-hoc t-tests with a Bonferroni corrected alpha level (p<0.0025) indicated that there were decreases in force, relative to the initial value (0% Tlim), from 40% to 100% Tlim (Figure 1A and Table 2).
Table 1.
The results of the post-test failure questionnaire after the handgrip task anchored to a rating of perceived exertion at=5. The number of reports was indicated among 12 subjects.
| No Contribution | Slight Contribution | Moderate Contribution | Significant Contribution | Extreme Contribution | |
|---|---|---|---|---|---|
| Forearm Muscle Groups | 2 | 3 | 3 | 4 | |
| Handgrip-involved Muscles | 1 | 1 | 5 | 5 | |
| Biceps | 6 | 5 | 1 | ||
| Triceps | 10 | 1 | 1 | ||
| Loss of Mental Focus | 8 | 3 | 1 | ||
| Loss of Motivation | 10 | 2 | |||
| Boredom | 10 | 1 | 1 | ||
| Pain | 8 | 2 | 1 | 1 | |
| Discomfort | 7 | 2 | 1 | 2 |
Figure 1.
Time course of changes for the mean ± SD normalized force and neuromuscular responses (% pre-maximal voluntary isometric contraction [MVIC]) during the sustained, isometric handgrip holds anchored to RPE 5. A) Force, B) Electromyographic amplitude (EMG AMP), C) Electromyographic mean power frequency (EMG MPF), D) Neuromuscular efficiency (NME). Data are presented using means (dots) and standard deviation (error bars). * Indicates a significant (p<0.0025) decrease, relative to the initial time point (0% Tlim).
Table 2.
The post-hoc t-tests with a Bonferroni corrected alpha level for comparisons across time (p<0.0025) for the force indicating mean difference (% MVIC), 95% confidence interval of the difference (uncorrected), effect size (Cohen’s d), and p-value between each time point.
| Time | Mean difference | 95% CI | Effect size | p- value | |
|---|---|---|---|---|---|
| 0% - 5% | 0.551 | -12.277 | 13.378 | 0.027 | 0.926 |
| 0% - 10% | 6.115 | -5.259 | 17.489 | 0.342 | 0.262 |
| 0% - 15% | 9.253 | -2.264 | 20.771 | 0.510 | 0.105 |
| 0% - 20% | 11.264 | 0.304 | 22.225 | 0.653 | 0.045 |
| 0% - 25% | 13.056 | 2.551 | 23.561 | 0.790 | 0.019 |
| 0% - 30% | 15.488 | 5.837 | 25.138 | 1.020 | 0.005 |
| 0% - 35% | 15.824 | 6.023 | 25.625 | 1.026 | 0.005 |
| 0% - 40% | 16.954 | 7.387 | 26.521 | 1.126 | * 0.002 |
| 0% - 45% | 18.066 | 9.299 | 26.832 | 1.309 | * <0.001 |
| 0% - 50% | 18.930 | 9.486 | 28.374 | 1.274 | * 0.001 |
| 0% - 55% | 19.608 | 10.042 | 29.173 | 1.302 | * <0.001 |
| 0% - 60% | 20.247 | 10.754 | 29.739 | 1.355 | * <0.001 |
| 0% - 65% | 19.876 | 11.203 | 28.549 | 1.456 | * <0.001 |
| 0% - 70% | 19.518 | 10.466 | 28.569 | 1.370 | * <0.001 |
| 0% - 75% | 20.058 | 10.841 | 29.274 | 1.383 | * <0.001 |
| 0% - 80% | 20.802 | 11.684 | 29.919 | 1.450 | * <0.001 |
| 0% - 85% | 21.058 | 12.229 | 29.888 | 1.515 | * <0.001 |
| 0% - 90% | 21.537 | 12.243 | 30.830 | 1.472 | * <0.001 |
| 0% - 95% | 23.335 | 14.210 | 32.460 | 1.625 | * <0.001 |
| 0% - 100% | 24.588 | 15.427 | 33.748 | 1.705 | * <0.001 |
Indicates a significant (p<0.0025) decrease in force, relative to the initial time point (0% Tlim).
Performance Fatigability
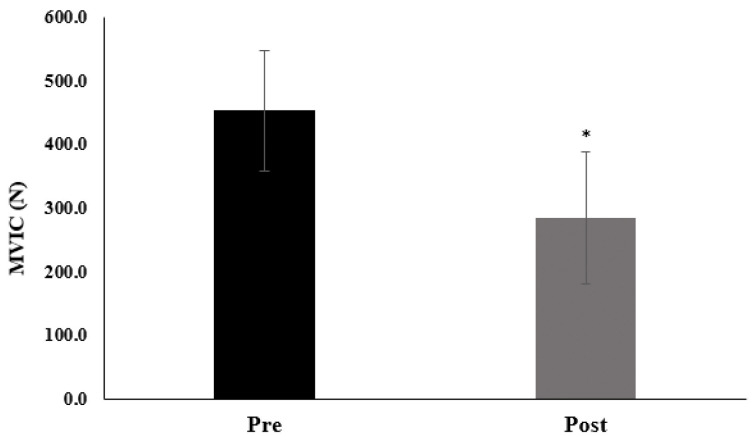
The absolute values of pre- and post- MVIC values (N) and % decrease for each subject for the handgrip HTF at RPE 5 are indicated in Table 3 and Figure 2. The PF for the handgrip HTF at RPE 5 was 38.2±11.5% (range: 19.5% – 50.9%). There was a significant decrease (p<0.001, 95% CI=13.997–20.408, d=3.412) in MVIC force from pre- to post- HTF.
Table 3.
The pre- and post- maximal voluntary isometric contraction (MVIC) expressed in absolute (N) and relative (% decrease) terms for each subject.
| Subject | pre (N) | post (N) | % decrease |
|---|---|---|---|
| 1 | 543.3 | 433.6 | 20.2 |
| 2 | 425.9 | 235.4 | 44.7 |
| 3 | 525.0 | 338.3 | 35.6 |
| 4 | 357.4 | 204.2 | 42.9 |
| 5 | 669.0 | 538.7 | 19.5 |
| 6 | 390.1 | 233.9 | 40.0 |
| 7 | 439.7 | 237.0 | 46.1 |
| 8 | 374.9 | 188.9 | 49.6 |
| 9 | 417.6 | 275.1 | 34.1 |
| 10 | 432.8 | 214.8 | 50.4 |
| 11 | 348.2 | 263.6 | 24.3 |
| 12 | 519.7 | 255.2 | 50.9 |
| Mean | 453.6 | 284.9 | 38.2 |
| SD | 94.0 | 103.9 | 11.5 |
Figure 2.
The pre- and post-maximal voluntary isometric contraction (MVIC) values for handgrips holds anchored at RPE 5. The mean ± SD values of pre- and post-MVIC were 453.6±94.0 N, and 284.9±103.9 N, respectively. * Indicates a significant (p<0.001) decrease in force from pre- to post-MVIC.
Electromyographic Responses
A 1(RPE: 5) x 21(time: 0-100% MVIC) repeated measures ANOVA indicated that there were significant changes across time (F=8.416, p<0.001, ηp2=0.433) for EMG AMP. The post-hoc t-tests with Bonferroni corrected alpha level (p<0.0025) indicated that there were decreases in EMG AMP, relative to the initial value (0% Tlim), at 30%,60%, and 100% Tlim (Figure 1B) (Table 4).
Table 4.
The post-hoc t-tests with a Bonferroni corrected alpha level for comparisons across time (p<0.0025) for the normalized electromyographic amplitude (EMG AMP) responses including the mean difference between time points, 95% confidence interval of the difference, effect size (Cohen’s d), and p-value between each time point.
| Time | Mean difference | 95% CI | Effect size | p- value | |
|---|---|---|---|---|---|
| 0% - 5% | 2.120 | 16.127 | 4.655 | 0.131 | 0.658 |
| 0% - 10% | 6.798 | 12.097 | 3.492 | 0.122 | 0.078 |
| 0% - 15% | 7.732 | 12.713 | 3.670 | 0.562 | 0.059 |
| 0% - 20% | 8.254 | 12.246 | 3.535 | 0.523 | 0.040 |
| 0% - 25% | 10.866 | 11.238 | 3.244 | 0.608 | 0.006 |
| 0% - 30% | 12.077 | 10.651 | 3.075 | 0.566 | * 0.002 |
| 0% - 35% | 9.933 | 11.549 | 3.334 | 0.674 | 0.013 |
| 0% - 40% | 10.692 | 11.784 | 3.402 | 0.627 | 0.009 |
| 0% - 45% | 11.595 | 10.896 | 3.145 | 0.967 | 0.004 |
| 0% - 50% | 11.065 | 10.436 | 3.013 | 0.899 | 0.004 |
| 0% - 55% | 12.091 | 10.887 | 3.143 | 1.134 | 0.003 |
| 0% - 60% | 13.384 | 10.698 | 3.088 | 1.054 | * 0.001 |
| 0% - 65% | 11.591 | 10.717 | 3.094 | 0.86 | 0.003 |
| 0% - 70% | 11.079 | 11.562 | 3.338 | 0.8 | 0.007 |
| 0% - 75% | 11.590 | 11.245 | 3.246 | 0.907 | 0.004 |
| 0% - 80% | 10.903 | 11.296 | 3.261 | 0.844 | 0.007 |
| 0% - 85% | 11.027 | 10.745 | 3.102 | 1.064 | 0.005 |
| 0% - 90% | 10.720 | 11.507 | 3.322 | 0.99 | 0.008 |
| 0% - 95% | 12.247 | 12.071 | 3.485 | 1.06 | 0.005 |
| 0% - 100% | 13.812 | 11.261 | 3.251 | 0.986 | * 0.001 |
Indicates a significant (p<0.0025) decrease in EMG AMP, relative to the initial time point (0% Tlim).
A 1(RPE: 5) x 21(time: 0-100% MVIC) repeated measures ANOVA indicated that there were significant changes across time (F=5.415, p<0.001, η p2=0.330) for EMG MPF. The post-hoc t-tests with a Bonferroni corrected alpha level (p<0.0025) indicated that there were decreases in EMG MPF, relative to the initial value (0% Tlim), from 10% to 100% Tlim (Figure 1C) (Table 5).
Table 5.
The post-hoc t-tests with a Bonferroni corrected alpha level for comparisons across time (p<0.0025) for the normalized electromyographic mean power frequency (EMG MPF) responses including the mean difference between time points, 95% confidence interval of the difference, effect size (Cohen’s d), and p-value between each time point.
| Time | Mean difference | 95% CI | Effect size | p- value | |
|---|---|---|---|---|---|
| 0% - 5% | 13.861 | 13.637 | 3.937 | 1.016 | 0.005 |
| 0% - 10% | 18.322 | 13.918 | 4.018 | 0.945 | * <0.001 |
| 0% - 15% | 19.634 | 10.565 | 3.050 | 1.316 | * <0.001 |
| 0% - 20% | 21.832 | 9.956 | 2.874 | 1.224 | * <0.001 |
| 0% - 25% | 22.090 | 12.150 | 3.507 | 1.858 | * <0.001 |
| 0% - 30% | 19.175 | 8.890 | 2.566 | 1.728 | * <0.001 |
| 0% - 35% | 17.919 | 10.533 | 3.041 | 2.193 | * <0.001 |
| 0% - 40% | 22.048 | 8.860 | 2.558 | 2.039 | * <0.001 |
| 0% - 45% | 20.016 | 9.690 | 2.797 | 1.818 | * <0.001 |
| 0% - 50% | 23.576 | 12.585 | 3.633 | 1.691 | * <0.001 |
| 0% - 55% | 19.512 | 14.380 | 4.151 | 2.157 | * <0.001 |
| 0% - 60% | 19.833 | 12.925 | 3.731 | 2.006 | * <0.001 |
| 0% - 65% | 19.671 | 10.394 | 3.000 | 1.701 | * <0.001 |
| 0% - 70% | 19.496 | 6.270 | 1.810 | 1.582 | * <0.001 |
| 0% - 75% | 17.281 | 13.223 | 3.817 | 2.488 | * <0.001 |
| 0% - 80% | 18.679 | 14.882 | 4.296 | 2.314 | * 0.001 |
| 0% - 85% | 18.314 | 14.385 | 4.153 | 2.066 | * 0.001 |
| 0% - 90% | 18.031 | 15.046 | 4.343 | 1.921 | * 0.002 |
| 0% - 95% | 21.326 | 11.873 | 3.427 | 1.873 | * <0.001 |
| 0% - 100% | 20.990 | 14.709 | 4.246 | 1.742 | * <0.001 |
Indicates a significant (p<0.0025) decrease in EMG MPF, relative to the initial time point (0% Tlim).
Neuromuscular Efficiency Response
A 1(RPE 5) x 21(time: 0-100% MVIC) repeated measures ANOVAs indicated that there were significant changes across time (F=22.368, p<0.001, ηp2=0.670) for NME (normalized force/normalized EMG AMP). The post-hoc t-tests with a Bonferroni corrected alpha level (p<0.0025) indicated that there were decreases in NME, relative to the initial value (0% Tlim), from 50% to 65%, and from 80% to 100% Tlim (Figure 1D and Table 6).
Table 6.
The post-hoc t-tests with a Bonferroni corrected alpha level for comparisons across time (p<0.0025) for the neuromuscular efficiency (normalized force/ normalized electromyographic amplitude) responses including the mean difference between time points, 95% confidence interval of the difference, effect size (Cohen’s d), and p-value between each time point.
| Time | Mean difference | 95% CI | Effect size | p- value | |
|---|---|---|---|---|---|
| 0% - 5% | -0.105 | 0.221 | 0.064 | -0.476 | 0.127 |
| 0% - 10% | -0.066 | 0.201 | 0.058 | -0.443 | 0.280 |
| 0% - 15% | 0.002 | 0.161 | 0.047 | -0.328 | 0.962 |
| 0% - 20% | 0.060 | 0.216 | 0.062 | -0.305 | 0.359 |
| 0% - 25% | 0.053 | 0.245 | 0.071 | 0.014 | 0.471 |
| 0% - 30% | 0.094 | 0.210 | 0.061 | 0.013 | 0.152 |
| 0% - 35% | 0.190 | 0.258 | 0.075 | 0.276 | 0.027 |
| 0% - 40% | 0.193 | 0.230 | 0.066 | 0.257 | 0.014 |
| 0% - 45% | 0.208 | 0.245 | 0.071 | 0.215 | 0.013 |
| 0% - 50% | 0.258 | 0.180 | 0.052 | 0.2 | * <0.001 |
| 0% - 55% | 0.257 | 0.207 | 0.060 | 0.445 | * 0.001 |
| 0% - 60% | 0.242 | 0.170 | 0.049 | 0.414 | * <0.001 |
| 0% - 65% | 0.305 | 0.260 | 0.075 | 0.735 | * 0.002 |
| 0% - 70% | 0.290 | 0.304 | 0.088 | 0.683 | 0.007 |
| 0% - 75% | 0.307 | 0.276 | 0.080 | 0.839 | 0.003 |
| 0% - 80% | 0.340 | 0.261 | 0.075 | 0.78 | * <0.001 |
| 0% - 85% | 0.360 | 0.287 | 0.083 | 0.85 | * 0.001 |
| 0% - 90% | 0.390 | 0.277 | 0.080 | 0.791 | * <0.001 |
| 0% - 95% | 0.427 | 0.222 | 0.064 | 1.43 | * <0.001 |
| 0% - 100% | 0.488 | 0.252 | 0.073 | 1.33 | * <0.001 |
Indicates a significant (p<0.0025) decrease in neuromuscular efficiency (normalized force/ normalized EMG AMP), relative to the initial time point (0% Tlim).
Discussion
Performance fatigability
The present study examined the effects of sustained, isometric, fatiguing handgrip HTF anchored to RPE=5 on PF, Tlim, force alterations, neuromuscular patterns of response (i.e., EMG AMP and MPF), and NME. The PF was examined based on the decrease in absolute force (N) and expressed as a percent change in the pre- to post-MVIC force output for descriptive purposes[26]. This was in line with the previous recommendation of Enoka and Duchateau[27] who suggested that rather than dichotomizing fatigue into peripheral and central factors, measures that reflect “real-world performance” (p. 2229) such as time to task failure, rating of perceived exertion, and changes in pre- and post-maximal voluntary contraction should be used to describe global fatigue. The magnitude of PF in the current study (38.2±11.5%) was consistent with the magnitude of PF reported for sustained, isometric leg extension anchored to an RPE=5 in females[8] and males[2], where the percent reductions in MVIC were 34.7±17.1% and 47.5±19.6%, respectively. In these studies[2,8], however, the RPE holds were ended at a maximal time-limit duration of 5 min, instead of allowing subjects to reach the zero-force output. In the current study, the Tlim was approximately 9 min (512.4±245.9 sec) and subjects were instructed to continue to alter force as necessary to maintain the RPE=5 and the task was not ended until the force output (N) was zero. The current findings did not support the hypothesis that the smaller muscle mass (i.e., handgrip-involved muscles) engaged likely resulted in greater magnitude of PF compared to relatively bigger muscle mass (i.e., quadriceps)[2]. In addition, despite the different endpoints between the current study and previous findings[2,8], the similar magnitude of PF may be explained by the intensities above >20% MVIC of the isometric muscle contractions during the initial phases (~5% Tlim) in this study and throughout the RPE anchored isometric leg extensions previously reported[2,8]. Previous studies have reported that during a sustained, isometric muscle contraction, blood flow begins to decrease at forces higher than 20% MVIC[28] due to an increased occlusion of vascular beds caused by an increase in intramuscular pressure[29]. These alterations in blood flow result in buildup of metabolic byproducts (e.g., H+ and inorganic phosphates) within the muscle, which impacts excitation-contraction coupling and decrease force generating capacity[4,30]. In addition, the metabolic perturbations elicit feedback of group III/IV afferents that may alter the level of central motor drive and reduce the force generating capacity[2,8,12]. In the current study, subjects sustained the handgrip holds above 20% MVIC from the onset of exercise through 5% Tlim, which corresponded to ~30 sec of the task and may have been sufficient to cause a partial accumulation of metabolic byproducts during the initial phase. Thus, it is possible the metabolic perturbations at the beginning of the handgrip holds in this study were sufficient to alter the intracellular environment to a level that contributed to a decrease in force generating capacity at task failure that reflected a similar magnitude of PF to previous tasks anchored to RPE=5.
Force and Neuromuscular Responses
It has been suggested that the perceptually determined force levels when anchored to RPE underestimate the values related to an expected % of MVIC[3,5,7]. In the current study, the initial (0% Tlim) force (26.3±7.6% MVIC) was underestimated relative to the expected force (% MVIC) corresponding to RPE=5 (50% MVIC). This response was consistent with other RPE anchored studies[3,7]. The dissociation between the expected force and actual force may be explained by an anticipatory or feedforward component[5]. During RPE-clamp exercise, Tucker[5] indicated that an anticipatory or feedforward component may integrate previous training and experience, motivation, expected time to task failure, and physiological inputs (e.g., stored muscle glycogen, skin temperature), and these combined physio-psychological factors are integrated to inform the self-selected initial exercise intensity. At the onset of exercise, subjects in this study could perceive the sensations of tension from the handgrip-involved muscles[31], and after the initial force was selected, they consciously adjusted the force to maintain the anticipated duration of exercise at the prescribed RPE. These reductions in force were associated with conscious decreases in central motor command, which are descending neural signals (i.e., efferent copy) from higher brain centers including the primary motor- and pre-motor areas[4].
After the initial force selection, despite the no significant changes of forces, the qualitative pattern of force response demonstrated ~26% decrease from 0% to 10% Tlim at which point the relative force output was <20% MVIC and continued to decrease at a consistent rate until ~40% Tlim (Figure 1A). In addition to the anticipatory or feedforward components, the magnitude of force reductions during the initial phase may also be affected by inhibitory feedback from mechano-sensitive (i.e., group III afferent) and metabosensitive neurons (i.e., group IV afferent)[12]. It is possible that as the exercise started, the perceived sensations of mechanical tension generated from the handgrip-involved muscles were detected by group III afferent neurons[12,31]. Moreover, the reduction in force during the first 5% Tlim accounted for ~30 sec and it is likely that the continuous muscle contraction above 20% MVIC led to the generation of metabolic perturbations which mainly came from reliance on the adenosine triphosphate (ATP), creatine phosphate (CP) system (i.e., APT-PCr system), and anaerobic glycolysis[32]. The inhibitory feedback from group IV muscle afferents may have decreased the neural drive to avoid the additional accumulation of intramuscular metabolites[12].
The EMG AMP and MPF responses in the present study supported the possible explanations that metabolic byproducts may have been generated, despite the decreases in force during the initial phases. The amplitude of the EMG signal, which is associated with muscle excitation (i.e., motor unit recruitment, firing rate, synchronization)[16,17,33], has previously been suggested to track the decreases in force required to maintain an assigned RPE during isometric muscle actions anchored to an RPE[2,4,7]. In the present study, qualitatively, there were gradual decreases in the EMG AMP responses for the initial 25% of Tlim (~53% change from 0% to 25% Tlim) that became significantly lower than the initial value at 30% Tlim (Figure 1B). These changes in muscle excitation (EMG AMP) tracked the decreases in force output. In addition, the EMG MPF, qualitatively, showed a ~12% decrease at 5% Tlim, and significantly decreased from 10% Tlim to the end time point of task failure (100% Tlim), relative to the 0% Tlim (Figure 1C). During a fatiguing task, decreases in EMG MPF have been suggested to be an indirect indicator of decreases in muscle fiber action potential conduction velocity (APCV) associated with metabolic perturbations (increases in extracellular potassium and decreases in intracellular pH) that cause the loss of membrane excitability, resulting in slower conduction velocities of active muscle fibers[3,34,35]. In addition, these changes in EMG MPF may have reflected de-recruitment of higher threshold motor units with faster APCV and increased reliance on lower threshold motor units with slower APCV, which resulted in a decrease in the global APCV[36-40]. Thus, after the initial force was selected based on the anticipatory feedforward mechanism, it is possible the continued reductions in force during the first ~30% of Tlim were the result of feedback from mechanical and metabolic perturbations detected by group III/IV afferents.
In the current study, the gradual decreases in force became significantly lower at 40% Tlim, relative to 0% Tlim. Qualitatively, the rate of change in the force from 40% to 90% Tlim appeared to slow, relative to the early phase (0-35% Tlim) before a more abrupt decrease during the last 10% of the RPE=5 holds. A previous study from Smith et al.[4], indicated that during isometric forearm flexion exercise anchored to RPE 7 (OMNI-RES), the force outputs across the majority of the task were above 20% MVIC where blood flow alterations could occur, leading to an accumulation of intramuscular metabolites (e.g., H+ and inorganic phosphates). In the present study, however, the forces from mid- to end phases were less than 10% MVIC (Figure 1A), which may be due to the different RPE anchor level (RPE 5) or muscle group tested. After first 30 seconds, the force generations below 20% MVIC in this study may have allowed for greater perfusion of the active muscles and resulted in clearance metabolic byproducts. Thus, it is unlikely that during mid- to end phases, the reduced force responses were affected by the additional production of intramuscular metabolites caused by alterations of blood flow. Nevertheless, the responses of EMG AMP, NME, and EMG MPF suggested that fatigue-induced metabolic responses still existed throughout the handgrip holds. In this study, qualitatively, there were ~52% decreases in EMG AMP during mid- to end phases, (significantly decreased at 60%, and 100% Tlim), relative to 0% Tlim, and these continuous reductions in muscle excitation tracked the decreases in force necessary to sustain the moderate exercise intensity (RPE 5) (Figure 1B). It should be noted that the NME response was also significantly reduced, from 50% to 65% Tlim and from 85% to 100% Tlim, relative to 0% Tlim (Figure 1D), which suggested that greater levels of muscle excitation (EMG AMP) were required for a given force to compensate for the reductions of force generating capacity of the fatigued muscle fibers during the mid- to end phases[18]. Moreover, considering that there were significantly decreased EMG MPF responses, from initial- to end phases, relative to the 0% Tlim (Figure 1C), it is also possible that metabolic perturbations at the cellular levels that occurred in the initial phases still remained at the mid- and end-phases[3,34,35]. Thus, we hypothesized that the generation (decrease in NME and EMG MPF) and clearance (forces < 20% MVIC) of metabolic byproducts could occur at the same time during the mid- to end-phase (40% to 100% Tlim), and the residual effects of metabolic perturbations could be detected by group III and group IV afferent neurons, leading to a decrease in central motor drive[2,8,12].
The force reductions in the mid- and end-phases may also be partially explained by the corollary discharge model. Previous studies[13,31] reported that the perception of effort is regulated by a neuronal process of an efferent copy derived from the centrifugal motor commands (i.e., activity of premotor and motor area regulating voluntary muscle actions). According to the corollary discharge model, when muscle fatigue response is produced independently from exercise-induced metabolites, the perception of effort results from the efferent copy of central motor command without afferent feedback. Considering the relatively low force levels during mid- and end-phases (< 10% MVIC), in the current study, the fatigue responses from metabolic perturbation would be minimal, thus the corollary discharge model may, in part, explain the continued force decreases. However, Drouin et al.[41], demonstrated that when the neural drive was held constant, effort increased, suggesting that perception was not entirely modulated by neural drive. Rather, while efferent drive may inform some of perceptual responses, local muscle oxygenation may also play an important role in regulating perception of effort during exercise[41]. In concert, it seems plausible that force regulation to maintain an RPE=5 during the mid- and end-phases resulted from the combination of afferent feedback and corollary discharge model. Thus, the eventual task failure in the current study may be explained by “…a sensory or tolerance limit…” as proposed by Gandevia (p. 1766)[42]. Hureau et al.[15], conceptualized the STL as the sum of feedforward signals (i.e., corollary discharges) and afferent feedback (e.g., active muscle, respiratory muscles, and indirectly involved muscles and organs) that are integrated within the brain and ultimately regulate work rate or the magnitude of central motor command, which eventually leads to the termination of the task. Keller et al.[43], hypothesized that during isometric leg extension exercise anchored to RPE 5 and 8, the metabolic perturbations and afferent feedback from both the involved and indirectly engaged muscles (e.g., postural stabilizer muscles) would overcome the reduction in feedforward motor command, eventually resulting in task failure by the STL. Smith et al.[4], also demonstrated that during a fatiguing, isometric forearm flexion anchored to RPE 7, both the sensory feedback primarily from forearm flexors (i.e., biceps brachii) and synergistic muscles (i.e., handgrip-involved muscles) and psychological factors (e.g., pain, motivation) caused subjects to reach their STL, and ultimately led to zero force output for 10 of 12 subjects. In the current study, all 12 subjects reached 0 N force in the last millisecond of the handgrip task, and on the post-test failure questionnaire, 8 of 12 subjects reported that indirectly involved muscles (e.g., biceps, triceps) were engaged (slight to significant contribution), 9 of 12 subjects reported the discomfort or pain in their wrist joints, hand/fingers, or forearm muscles (slight to significant contribution), and 8 of 12 subjects reported loss of motivation and mental focus, or boredom during the handgrip task (slight to moderate contribution) (Table 1). Therefore, in the current study, it is assumed that afferent feedback from the primary muscles (i.e., handgrip-involved muscle) and indirectly engaged muscles (i.e., biceps, triceps) along with corollary discharge related to central command could have contributed to decreases in the voluntary reductions in force. Moreover, factors associated with perceived fatigability such as loss of motivation, pain, or discomfort may have also contributed to the reductions in force to zero[6,27]. Interestingly, the task was terminated by the investigator when the subjects reached 0N force, but the subjects were still holding the handgrip dynamometer and perceived that they were still producing force, which implied that there were dissociations in actual force production and perceptual responses. Kluger et al.[6], described the distinctions between perceptions of fatigue and PF. Perceptions of fatigue are defined as “subjective sensations of weariness, increasing sense of effort, mismatch between effort expended and actual performance, or exhaustion” (p 411)[6]. In contrast, PF is an objective measure of declined performance (e.g., pre- to post- MVIC)[6,26]. Therefore, in the current study, the perception of fatigue such as pain, discomfort, and motivation may have influenced the magnitude of PF during the handgrip holds, but the subjects demonstrated a mismatch in perceived fatigability and PF responses such that the perceived force production did not reflect the actual force production.
Conclusion
The findings of the current study demonstrated that during a sustained, isometric handgrip HTF anchored to RPE=5, there were decreases in force and EMG AMP that likely reflected a decrease in central motor command due to an accumulation of intramuscular metabolic byproducts (i.e., decrease in EMG MPF and NME). In addition, the initial force production was likely regulated by anticipatory feedforward mechanisms, and then modulated by combination of corollary discharge and feedback from group III/IV muscle afferents. Moreover, the accumulations of metabolic byproducts at the beginning of handgrip holds (~30 sec) due to the alteration of blood flow at high intensities of isometric muscle contraction (> 20% MVIC) may cause force reductions at task failure, which led to similar PF to previous tasks[2,8] anchored to RPE=5. The current study also demonstrated that despite the recorded force output indicating 0 N, the subjects continuously felt they were exerting force, which implies there was a dissociation in perceived fatigability and PF responses.
This study was not without limitations. This study examined response only in male subjects due to the potential for differences in fatigue response (e.g., muscle size, strength, endurance time, or metabolism on muscle performance) between males and females[44-46]. Moreover, the EMG AMP and MPF responses were measured from the brachioradialis which is not the primary muscle for handgrip exercise. In addition, the handgrip dynamometer was not adjusted based on each subject’s hand size, and therefore, the subsequent muscle performance and neuromuscular responses may reflect slight variations in the grip width among individuals.
The physical work capacity during submaximal, sustained, isometric handgrip tasks is important in workplaces (e.g., assembly line, agricultural field, or machine operation)[47] and various sports (e.g., race car driving, or rock-climbing)[48], and handgrip-related musculoskeletal disorders or injuries derived from repetitive motion or strain have been prevalent in these settings[49,50]. Therefore, it is crucial to identify the underlying fatigue mechanisms of sustained, isometric handgrip tasks. In this regard, the application of RPE-Clamp model to handgrip exercise may be beneficial because it considers the role of perception of effort thereby suggesting unique psycho-physiological mechanisms in regulating force production, which may better provide an appropriate intensity of physical workloads to decrease the risk of hand/wrist musculoskeletal disorders and injuries. To investigate the unrevealed findings from the current study, future studies should examine how the factors of perceived fatigability (e.g., discomfort/pain, loss of motivation/boredom) contribute to the decision to terminate an exercise anchored to a constant RPE. Future studies should also investigate the relationship between perceptions of fatigue and PF across various modes (resistance exercise vs whole-body continuous dynamic exercise) and levels of engaged muscle mass to further elucidate the integration of perceived and performance fatigability in both sexes.
Ethics approval
The study was approved by the Institutional Review Board of The University of Kentucky (78542).
Consent to participate
All subjects signed a written informed consent.
Funding
MAS is funded by National Institutes of Health (K01-AG073698).
Authors’ contributions
Devising research (MK, HCB, MGA, MAS), data collection (MK, PJS, BB), data analysis (MK, PJS, BB), manuscript writing (MK, HCB), editing the manuscript (all authors). All authors provided suggestions, revisions and edits to the manuscript and approved the final version.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Cochrane K, Housh T, Bergstrom H, Jenkins N, Johnson G, Schmidt R, Cramer JT. Physiological responses during cycle ergometry at a constant perception of effort. International journal of sports medicine. 2015:466–473. doi: 10.1055/s-0034-1396826. [DOI] [PubMed] [Google Scholar]
- 2.Keller JL, Housh TJ, Hill EC, Smith CM, Schmidt RJ, Johnson GO. Self-regulated force and neuromuscular responses during fatiguing isometric leg extensions anchored to a rating of perceived exertion. Applied Psychophysiology and Biofeedback. 2019;44:343–350. doi: 10.1007/s10484-019-09450-2. [DOI] [PubMed] [Google Scholar]
- 3.Keller JL, Housh TJ, Hill EC, Smith CM, Schmidt RJ, Johnson GO. Are there sex-specific neuromuscular or force responses to fatiguing isometric muscle actions anchored to a high perceptual intensity? Journal of strength and conditioning research. 2022;36(1):156–161. doi: 10.1519/JSC.0000000000003394. [DOI] [PubMed] [Google Scholar]
- 4.Smith RW, Housh TJ, Anders JPV, Neltner TJ, Arnett JE, Schmidt RJ, Johnson GO. Application of the ratings of perceived exertion-clamp model to examine the effects of joint angle on the time course of torque and neuromuscular responses during a sustained, isometric forearm flexion to task failure. The Journal of Strength &Conditioning Research. 2022:10.1519. doi: 10.1519/JSC.0000000000004357. [DOI] [PubMed] [Google Scholar]
- 5.Tucker R. The anticipatory regulation of performance:the physiological basis for pacing strategies and the development of a perception-based model for exercise performance. British journal of sports medicine. 2009;43(6):392–400. doi: 10.1136/bjsm.2008.050799. [DOI] [PubMed] [Google Scholar]
- 6.Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses:proposal for a unified taxonomy. Neurology. 2013;80(4):409–416. doi: 10.1212/WNL.0b013e31827f07be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith RW, Anders JPV, Neltner TJ, Arnett JE, Keller JL, Housh TJ, Schmidt RJ, Johnson GO. Perceptual fatigability and neuromuscular responses during a sustained, isometric forearm flexion muscle action anchored to a constant level of perceived exertion. NeuroSports. 2021;1(2):2. [Google Scholar]
- 8.Keller JL, Housh TJ, Hill EC, Smith CM, Schmidt RJ, Johnson GO. Neuromuscular responses of recreationally active women during a sustained, submaximal isometric leg extension muscle action at a constant perception of effort. European journal of applied physiology. 2018;118:2499–2508. doi: 10.1007/s00421-018-3976-y. [DOI] [PubMed] [Google Scholar]
- 9.Hunter SK. Performance fatigability:mechanisms and task specificity. Cold spring harbor perspectives in medicine. 2018;8(7) doi: 10.1101/cshperspect.a029728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas K, Goodall S, Howatson G. Performance fatigability is not regulated to a peripheral critical threshold. Exercise and sport sciences reviews. 2018;46(4):240–246. doi: 10.1249/JES.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 11.Koral J, Oranchuk DJ, Wrightson JG, Twomey R, Millet GY. Mechanisms of neuromuscular fatigue and recovery in unilateral versus bilateral maximal voluntary contractions. Journal of Applied Physiology. 2020;128(4):785–794. doi: 10.1152/japplphysiol.00651.2019. [DOI] [PubMed] [Google Scholar]
- 12.Amann M, Wan H-Y, Thurston TS, Georgescu VP, Weavil JC. On the influence of group III/IV muscle afferent feedback on endurance exercise performance. Exercise and sport sciences reviews. 2020;48(4):209. doi: 10.1249/JES.0000000000000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcora S. Perception of effort during exercise is independent of afferent feedback from skeletal muscles, heart, and lungs. Journal of applied physiology. 2009;106(6):2060–2062. doi: 10.1152/japplphysiol.90378.2008. [DOI] [PubMed] [Google Scholar]
- 14.De Morree HM, Klein C, Marcora SM. Perception of effort reflects central motor command during movement execution. Psychophysiology. 2012;49(9):1242–1253. doi: 10.1111/j.1469-8986.2012.01399.x. [DOI] [PubMed] [Google Scholar]
- 15.Hureau TJ, Romer LM, Amann M. The 'sensory tolerance limit':A hypothetical construct determining exercise performance? European journal of sport science. 2018;18(1):13–24. doi: 10.1080/17461391.2016.1252428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck TW, Housh TJ, Johnson GO, Weir JP, Cramer JT, Coburn JW, Malek MH. Mechanomyographic amplitude and mean power frequency versus torque relationships during isokinetic and isometric muscle actions of the biceps brachii. Journal of Electromyography and Kinesiology. 2004;14(5):555–564. doi: 10.1016/j.jelekin.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Smith CM, Housh TJ, Herda TJ, Zuniga JM, Camic CL, Bergstrom HC, Smith DB, Weir JP, Hill EC, Cochrane KC. Time course of changes in neuromuscular parameters during sustained isometric muscle actions. Journal of strength and conditioning research. 2016;30(10):2697–2702. doi: 10.1519/JSC.0000000000001547. [DOI] [PubMed] [Google Scholar]
- 18.Arabadzhiev TI, Dimitrov VG, Dimitrova NA, Dimitrov GV. Interpretation of EMG integral or RMS and estimates of “neuromuscular efficiency”can be misleading in fatiguing contraction. Journal of electromyography and kinesiology. 2010;20(2):223–232. doi: 10.1016/j.jelekin.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Edwards R, Lippold O. The relation between force and integrated electrical activity in fatigued muscle. The Journal of physiology. 1956;132(3):677. doi: 10.1113/jphysiol.1956.sp005558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones AA, Power GA, Herzog W. History dependence of the electromyogram:Implications for isometric steady-state EMG parameters following a lengthening or shortening contraction. Journal of Electromyography and Kinesiology. 2016;27:30–38. doi: 10.1016/j.jelekin.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Barbero M, Merletti R, Rainoldi A. Atlas of muscle innervation zones:understanding surface electromyography and its applications. Springer Science &Business Media. 2012 [Google Scholar]
- 22.Hermens HJ, Bruggen T, Baten CT, Rutten W, Boom H. The median frequency of the surface EMG power spectrum in relation to motor unit firing and action potential properties. Journal of Electromyography and Kinesiology. 1992;2(1):15–25. doi: 10.1016/1050-6411(92)90004-3. [DOI] [PubMed] [Google Scholar]
- 23.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. The Journal of Strength &Conditioning Research. 2005;19(1):231–240. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- 24.Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports medicine. 1998;26:217–238. doi: 10.2165/00007256-199826040-00002. [DOI] [PubMed] [Google Scholar]
- 25.Buckthorpe MW, Hannah R, Pain T, Folland JP. Reliability of neuromuscular measurements during explosive isometric contractions, with special reference to electromyography normalization techniques. Muscle &nerve. 2012;46(4):566–576. doi: 10.1002/mus.23322. [DOI] [PubMed] [Google Scholar]
- 26.Keller JL, Housh TJ, Anders JPV, Neltner TJ, Schmidt RJ, Johnson GO. Similar performance fatigability and neuromuscular responses following sustained bilateral tasks above and below critical force. European Journal of Applied Physiology. 2021;121:1111–1124. doi: 10.1007/s00421-020-04588-y. [DOI] [PubMed] [Google Scholar]
- 27.Enoka RM, Duchateau J. Translating fatigue to human performance. Medicine and science in sports and exercise. 2016;48(11):2228. doi: 10.1249/MSS.0000000000000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes WS. The relationship between maximum isometric strength and intramuscular circulatory occlusion. Ergonomics. 1980;23(4):351–357. doi: 10.1080/00140138008924748. [DOI] [PubMed] [Google Scholar]
- 29.Thompson BC, Fadia T, Pincivero DM, Scheuermann BW. Forearm blood flow responses to fatiguing isometric contractions in women and men. American Journal of Physiology-Heart and Circulatory Physiology. 2007;293(1):H805–H812. doi: 10.1152/ajpheart.01136.2006. [DOI] [PubMed] [Google Scholar]
- 30.Broman H, Bilotto G, De Luca C. Myoelectric signal conduction velocity and spectral parameters:influence of force and time. Journal of applied physiology. 1985;58(5):1428–1437. doi: 10.1152/jappl.1985.58.5.1428. [DOI] [PubMed] [Google Scholar]
- 31.Pageaux B. Perception of effort in exercise science:definition, measurement and perspectives. European journal of sport science. 2016;16(8):885–894. doi: 10.1080/17461391.2016.1188992. [DOI] [PubMed] [Google Scholar]
- 32.Knuttgen HG, Komi PV. Basic considerations for exercise. Strength and power in sport. 2003:3–7. [Google Scholar]
- 33.Coburn JW, Housh TJ, Cramer JT, Weir JP, Miller JM, Beck TW, Malek MH, Johnson GO. Mechanomyographic and electromyographic responses of the vastus medialis muscle during isometric and concentric muscle actions. The Journal of Strength &Conditioning Research. 2005;19(2):412–420. doi: 10.1519/15744.1. [DOI] [PubMed] [Google Scholar]
- 34.Enoka RM, Duchateau J. Muscle fatigue:what, why and how it influences muscle function. The Journal of physiology. 2008;586(1):11–23. doi: 10.1113/jphysiol.2007.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fortune E, Lowery M. The effect of extracellular potassium concentration on muscle fiber conduction velocity examined using model simulation. 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society:IEEE. 2007:2726–2729. doi: 10.1109/IEMBS.2007.4352892. [DOI] [PubMed] [Google Scholar]
- 36.Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126(3287):1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- 37.Hu X, Rymer WZ, Suresh NL. Motor unit pool organization examined via spike-triggered averaging of the surface electromyogram. Journal of neurophysiology. 2013;110(5):1205–1220. doi: 10.1152/jn.00301.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Contessa P, De Luca CJ, Kline JC. The compensatory interaction between motor unit firing behavior and muscle force during fatigue. Journal of neurophysiology. 2016;116(4):1579–1585. doi: 10.1152/jn.00347.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kossler F, Lange F, Caffier G, Kuchler G. External potassium and action potential propagation in rat fast and slow twitch muscles. Gen Physiol Biophys. 1991;10(5):485–98. [PubMed] [Google Scholar]
- 40.Potvin JR, Fuglevand AJ. A motor unit-based model of muscle fatigue. PLoS computational biology. 2017;13(6):e1005581. doi: 10.1371/journal.pcbi.1005581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drouin PJ, Kohoko ZIN, Mew OK, Lynn MJT, Fenuta AM, Tschakovsky ME. Fatigue-independent alterations in muscle activation and effort perception during forearm exercise:role of local oxygen delivery. Journal of Applied Physiology. 2019;127(1):111–121. doi: 10.1152/japplphysiol.00122.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiological reviews. 2001 doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 43.Keller JL, Housh TJ, Anders JPV, Neltner TJ, Schmidt RJ, Johnson GO. Anchor Scheme, Intensity, and Time to Task Failure do not Influence Performance Fatigability or Changes in Neuromuscular Responses following Bilateral Leg Extensions. Journal of Exercise Physiology Online. 2020;23(4) [Google Scholar]
- 44.Keller JL, Anders JPV, Neltner TJ, Housh TJ, Schmidt RJ, Johnson GO. Sex differences in muscle excitation and oxygenation, but not in force fluctuations or active hyperemia resulting from a fatiguing, bilateral isometric task. Physiological Measurement. 2021;42(11):115004. doi: 10.1088/1361-6579/ac3e86. [DOI] [PubMed] [Google Scholar]
- 45.Hunter SK. Sex differences and mechanisms of task-specific muscle fatigue. Exercise and sport sciences reviews. 2009;37(3):113. doi: 10.1097/JES.0b013e3181aa63e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. Journal of Applied Physiology. 2002;93(5):1813–1823. doi: 10.1152/japplphysiol.00091.2002. [DOI] [PubMed] [Google Scholar]
- 47.Liu B, Ma L, Zhang W, Zhang Z. Subject-specific hand grip fatigability indicator determined using parameter identification technique. Human Factors and Ergonomics in Manufacturing &Service Industries. 2019;29(1):86–94. [Google Scholar]
- 48.Kwak M, Succi PJ, Benitez B, Bergstrom HC. Sustainability and perceptual responses during handgrip holds to failure at two fatigue thresholds. European Journal of Applied Physiology. 2023:1–11. doi: 10.1007/s00421-023-05248-7. [DOI] [PubMed] [Google Scholar]
- 49.Silverstein BA, Fine LJ, Armstrong TJ. Hand wrist cumulative trauma disorders in industry. Occupational and Environmental Medicine. 1986;43(11):779–784. doi: 10.1136/oem.43.11.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barr AE, Barbe MF, Clark BD. Work-related musculoskeletal disorders of the hand and wrist:epidemiology, pathophysiology, and sensorimotor changes. Journal of orthopaedic &sports physical therapy. 2004;34(10):610–627. doi: 10.2519/jospt.2004.34.10.610. [DOI] [PMC free article] [PubMed] [Google Scholar]