Abstract
Objective:
To evaluate the use of a computer-based biodex balance exercise system (BBS) on balance, neuropathic pain, clinical presentation and nerve function in patients with diabetic peripheral neuropathy (DPN).
Methods:
A total of 32 participants with DPN were randomly assigned in a 1:1 ratio to an intervention group (IG) or control group (CG). The IG performed exercises using the BBS twice weekly for 8 weeks, while CG were informed regarding diabetes self-management. At baseline and after study completion, participants underwent balance (postural stability and fall risk) and neuropathic pain assessment (DN4 questionnaire) and were screened using the Michigan Neuropathy Screening Instrument and nerve conduction test.
Results:
Among the baseline participants, 14 in the IG and 13 in the CG completed the study. Balance training improved postural stability (overall, p<0.001), fall risk (p<0.001), neuropathic pain (p=0.01) and symptoms (p<0.001), and clinical presentation (p=0.02), but not nerve function, within the IG. At follow-up, IG displayed significantly improved stability (p<0.001) and fall risk (p=0.02) and decreased neuropathic symptoms (p=0.01) compared to the CG.
Conclusion:
Computer-based balance exercises improve balance, pain, and clinical presentation of DPN, but not nerve function, in patients with DPN. ClinicalTrials.gov ID: NCT05255497.
Keywords: Balance, Diabetes Complications, Diabetic Neuropathy, Exercise, Nerve Conduction Test
Introduction
Diabetes is a rapidly rising global health issue[1]. Diabetic peripheral neuropathy (DPN) is a common complication, resulting from prolonged high blood sugar damaging peripheral nerves. Around 50% of diabetes patients may experience neuropathy in their lifetime[2]. DPN is characterized as length-dependent damage to the peripheral nerves that progresses proximally from the feet to the hands[3]. Sensory symptoms often manifest first, encompassing sensations of numbness, tingling, or burning that can progress to feelings of pain or extreme sensitivity to touch. As the conditions progresses, motor symptoms may emerge, including muscle weakness, difficulty with coordination, and even muscle atrophy[3]. In more advanced stages, the loss of sensation can lead to injuries or wounds going unnoticed, increasing the risk of infections and ulcers. Severe cases might require amputation if these complications become unmanageable[3]. Clinical signs and symptoms of DPN are indicative of the clinical presentation of DPN[3].
A significant consequence of DPN is the development of balance issues due to distruption of sensory feedback and proprioception process necessary for maintaining equilibrium[4]. Proprioception, the body’s awareness of its position in space, heavily relies on signals from peripheral nerves that provide information about joint angles, muscle tension and pressure on the feet[5]. When these nerves are damaged, as is the case in DPN, the brain receives incomplete or inaccurate signals which can lead to difficulties in coordinating movements and adjusting posture thus increasing the risk of falls[5]. The loss of sensation in the feet can make it challenging to detect changes in ground texture or obstacles, further compromising stability[4]. Ultimately, individuals with DPN might experience unsteadiness, stumbling and reduced overall balance control[6].
Exercise can help enhance muscle strength, flexibility, and coordination, which are crucial for maintaining stability[7]. It can also promote blood circulation, delivering essential nutrients and oxygen to peripheral nerves, and assist in managing blood sugar levels, potentially slowing down the progression of neuropathy[7]. Previous studies investigating the impact of exercise on nerve health have focused on aerobic and resistance exercises[8-11]. While positive effects of balance exercises have been demonstrated for enhancing neuromuscular function, their effectiveness in directly improving nerve function, particularly in the context of DPN, requires further investigation[12-16].
The Biodex Balance System (BBS) is a modern computer system for enhancing balance, stability, and body awareness[17]. It uses sensors on a computerized platform to track movements in real-time and offers diverse interactive balance exercises[17]. Previous studies report that balance training using the BBS improve balance and stability in patients with DPN[17,18]. However, it is not clear whether balance training can reverse neuropathy symptoms or restore nerve function. Therefore, we aimed to assess the effects of balance exercises on balance, neuropathic pain, clinical presentation of neuropathy, and nerve functions in patients with DPN.
Materials and Methods
Study Design and Populations
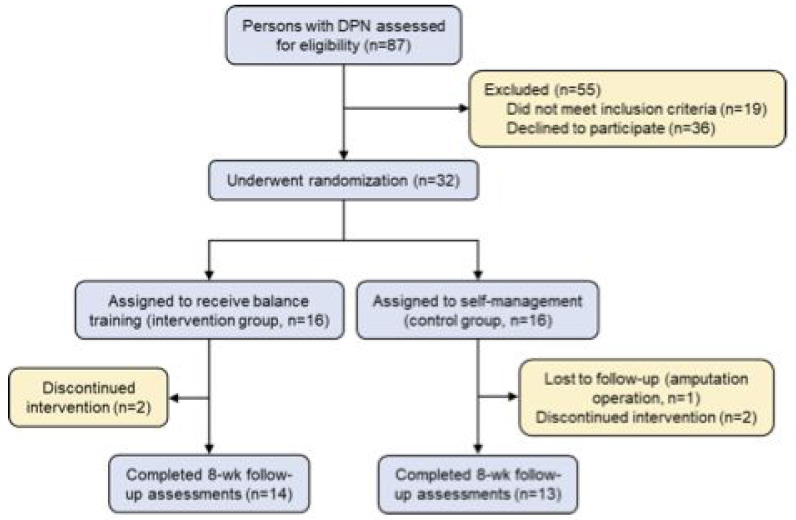
An outline of the study design can be found in Figure 1. From September 2017 to June 2019, participants were enrolled from Dokuz Eylul University. Inclusion criteria were age between 45 and 76 years old and diabetes-related neuropathy based on laboratory tests, clinical findings, and nerve conduction test (NCT)[19]. Exclusion criteria were history of autoimmune, cerebrovascular, or chronic infectious disease, cancer, chemotherapy and/or radiotherapy, radicular neuropathy, chronic kidney and liver failure, alcoholism or other substance abuse, and mental or physical disabilities (including blindness)[19]. Persons who didn’t speak Turkish were also excluded. Exclusion criteria did not include the use of antidepressant or anticonvulsant medications.
Figure 1.
Study Flow Diagram. Study participation and loss to follow-up or discontinued intervention. DPN denotes diabetic peripheral neuropathy.
Participants were examined by an endocrinologist and a neurologist. Detailed medical history and sociodemographic data, including age, weight, height, and education level, were collected from each participant. Type and duration of diabetes, physical examination findings, and results of laboratory tests, such as fasting blood glucose and HbA1c, were recorded.
Outcome measurements for balance, neuropathic pain, clinical presentation and nerve function were performed for all participant at baseline and after the 8 weeks of balance training.
Balance Exercise Program Intervention
Patients were randomized into the intervention group (IG) or control group (CG) using an online list randomizer (https://www.random.org/lists/). Participants listed in odd-numbered positions were included in the intervention group, while those in even-numbered positions were included in the control group.
The intervention group (IG) performed balance exercises using the recommended BBS training program for peripheral neuropathy patients, attending two sessions per week for eight weeks[20]. Each 30 min BBS session included a 4 min warm-up prior to the exercise program and 2 min rest periods between each exercise segment. Participants were instructured to stand on a cursor and stabilize themselves to keep the cursor in the middle of a co-centered circle on the display screen[17]. Participants were asked to maintain balance while doing functional tasks with the help of a visual target. The balance exercise protocol is detailed in Figure 2. The level of platform stability was adjusted over the course of the 8 weeks to progressively increase difficulty of the program.
Figure 2.
Biodex Balance System exercise protocol. A) Intervention group (IG) and control group participants underwent balance, neuropathic pain (Douleur Neuropathique 4, DN4), clinical presentation (Michigan Neuropathy Screening Instrument, MNSI), and nerve function (nerve conduction test, NCT) assessments and examinations prior to start of intervention. Participants in the IG performed exercises using the computerized Biodex Balance System (BBS) twice a week for 8 weeks. Participants in the CG received education regarding diabetes self-management. After 8 weeks of intervention, participants were again assessed and examined for balance, neuropathic pain, clinical presentation, and nerve function. B) Postural stability was the first training and asked participants keep the cursor at the midpoint at that time, in second exercise requested from participants to turn the target randomly illuminated. Participants stayed in while the ring moving on the screen random training as third, and participants followed circularly located targets at the last.
Participants in both groups maintained their prescribed medical therapy and received diabetes self-management education. The importance of weight management through dietary modifications and regular physical activity was explained. A support platform was created using social networks and twice-weekly notifications were used to encourage participants to measure their blood glucose and increase their daily physical activity for weight management.
Balance Outcomes
Balance outcomes were postural stability and fall risk as measured using the BBS. The BBS (SD 12.1 Display 115 VAC) platform can be used as a static or a dynamic surface ranging in level from 1 (most movable) to 12 (most stable)[21]. During the tests, participants were positioned barefoot on the platform without any hand support. Participants were informed that the ground reaction force they created on the platform was converted into a visual cursor and that they had to keep the cursor on the screen at the middle of the target. Participants underwent a familiarization test prior to the testing and training protocol, with the procedure thoroughly explained. Following the initial trial test measurements, actual values were recorded. The most comfortable position was determined, foot angle was measured and mean values were recorded from three repetitions. The literature supports the demonstrated validity and reliability of measurements obtained from the BBS[22].
To measure postural stability, participants were asked to hold the cursor (projected on the computer screen) on the target at the 8th stability level for 20 sec. Deviations from the target were recorded. Measures of postural stability included overall (OA), anteroposterior (AP), and mediolateral (ML) stability scores. A higher OA score indicates weaker balance. To measure fall risk, participants were asked to stay on target while the platform stability was gradually changed by the system over 20 sec (from level 12 to level 8). Excess of deviations from the target indicated poor adaptation to the dynamic platform[17].
Pain Outcomes
The Douleur Neuropathique 4 (DN4) questionnaire, utilized to evaluate neuropathic components of pain, was administered, employing the Turkish validated version of DN4[23]. The DN4 questionnaire consists of 10 items answered with a “Yes” (score=1) or “No” (score=0). The first 7 items ask participants to self-assess subjective complaints related to pain and sensational variations. The last 3 items relate to sensory examination. Scores range from 0-10 with higher scores suggestive of neuropathic pain.
DPN Outcomes
DPN outcomes included the Michigan Neuropathy Screening Instrument (MNSI) questionnaire, examination, and combined index and nerve conduction studies. The MNSI consists of two components: a self-administered questionnaire (MNSI-Q) and a clinical examination (MNSI-E). The MNSI-Q consists of 15 items answered with a “Yes” or “No”. Responses for each item are assigned a point value and the total score is calculates. A score of 4 or higher indicates presence of neuropathy symptoms. The MSNI-E involves clinical assessment of vibration perception and ankle reflexes and examination of the foot for ulcers and deformities. Specific criteria are used to assign scores for each clinical assessment and the total score is the sum of those values. A score of 2.5 or higher (out of 8 total points) is considered abnormal. Turkish version was used[24].
NCTs were performed by a clinical neurophysiology fellow. In the upper extremity, unilateral median and ulnar nerve sensory and motor potentials were recorded. In the lower extremity, unilateral peroneal and tibial nerves were used for motor assessment and the sural nerve was used for sensory assessment. Single sensory and motor nerve potentials from opposite extremities were also assessed to rule out asymmetric polyneuropathy and plexopathy. Diagnosis of peripheral neuropathy was based on alterations in conduction velocity, amplitude, and/or F-wave latency in a least 2 different nerves.
Statistical Analysis
Sample size of the study was determined using G*Power software (version 3.1) and based on a study conducted with computerized balance systems in the geriatric population[25]. Based on the results of fall risk test after exercise training, which were 2.4 ± 1.9 in the exercise group and 4.6 ± 2.5 in the control group (effect size 0.990), a minimum sample size of 28 participants was calculated for the study to have 80% power at a significance level of p=0.05. Anticipating a 15% drop-out rate, a total of 32 patients were recruited for the study (Figure 1). The randomization process, NCTs, and statistical analyses were carried out by a blinded researcher.
Data was analyzed using SPSS software (version 22) for Windows. Normality was tested using the Shapiro-Wilk test. Descriptive statistics was used to present median, minimum, maximum, and percentage values of data not normally distributed. Independent samples were tested for significant difference between IG and CG by Mann-Whitney U test. Wilcoxon signed-rank tests were used for dependent samples. Discrete data was analyzed by chi-square tests. Statistical significance was determined using a p value threshold of 0.05.
Results
Study participants and demographics
A total of 87 individuals were assessed for eligibility, of whom 19 did not meet inclusion criteria and 36 declined to participate. The remaining 32 participants were assigned 1:1 into the intervention group (IG) or the control, self-management group (CG). Fourteen participants (42.9% women, 57.1% men) in the IG and 13 participants (53.8% women, 46.2% men) in the CG completed the study. Study participation and loss to follow-up are summarized in Figure 1. There were no statistically significant differences between study groups in baseline participant characteristics (Table 1).
Table 1.
Demographic and Clinical Baseline Characteristics of Study Participants.
| Intervention Group (n = 14) | Control Group (n = 13) | p Value | |
|---|---|---|---|
| Variable (median, min-max) | |||
| Age (years) | 64 (50-70) | 61 (50-69) | 0.40 a |
| BMI (kg/m2) | 31.48 (20.34-38.06) | 28.87 (25.40-39.56) | 0.35 a |
| Diabetes duration (years) | 18 (8-22) | 10 (1-20) | 0. 75 a |
| HbA1c (%) | 7.6 (6.5-10.5) | 7.4 (6.5-13.3) | 0.61 a |
| Fasting blood glucose (mg/dL) | 151 (105-279) | 164 (110-308) | 0.51 a |
| Category (n, %) | |||
| Gender | |||
| Female | 6 (42.9) | 7 (53.8) | 0.56 c |
| Male | 8 (57.1) | 6 (46.2) | |
| Marital status | |||
| Married | 10 (71.4) | 13 (100) | 0.09c |
| Single/Widow | 4 (28.6) | 0 (0) | |
| Education | |||
| None | 1 ( 7.1) | 3 (23.1) | 0.45c |
| Primary/Secondary | 8 (57.1) | 4 (30.8) | |
| High school | 1 (7.1) | 2 (15.4) | |
| University | 4 (28.6) | 4 (30.8) | |
| Professional status | |||
| Working | 2 (14.3) | 1 (7.7) | 0.55c |
| Not working | 2 (14.3) | 4 (30.8) | |
| Retired | 10 (71.4) | 8 (61.5) | |
| Insulin usage | |||
| Yes | 10 (71.4) | 5 (38.5) | 0.08c |
| No | 4 (28.6) | 8 (61.5) | |
Significant P values are presented in boldface.
Mann Whitney U test.
Chi Square test.
Change in balance
Intragroup analysis of baseline and post-intervention assessments of balance indicated that balance training significantly improved postural stability (overall, p<0.001; antero-posterior, p<0.001; medio-lateral, p<0.001) and fall risk (p<0.001) in the IG (Table 2). In contrast, all balance results showed no significant differences in CG in the intragroup analysis at baseline and follow-up (Table 2). The IG displayed improved postural stability (overall, p<0.001; antero-posterior, p<0.001; medio-lateral, p<0.001) and fall risk (p=0.02) compared to the CG in the intergroup analysis at follow-up (Table 2).
Table 2.
Baseline and Post-Study Balance Assessment.
| Intervention Group | Control Group | Intergroup p-valuea | ||
|---|---|---|---|---|
| Postural stability (median, min-max) | ||||
| Overall (OASI) | Baseline | 1.65 (0.50-2.20) | 1.40 (1.30-2.70) | 0.37 |
| Post | 0.80 (0.30-1.5) | 1.40 (1.20-2.50) | p<0.001 | |
| Intragroup p-valueb | p<0.001 | 0.63 | ||
| Antero-posterior(APSI) | Baseline | 1.05 (0.40-1.50) | 0.90 (0.80-1.70) | 0.55 |
| Post | 0.45 (0.20-1.20) | 1 (0.80-1.70) | p<0.001 | |
| Intragroup p-valueb | p<0.001 | 0.40 | ||
| Medio-lateral(MLSI) | Baseline | 0.75 (0.20-1.60) | 0.80 (0.70-1.80) | 0.58 |
| Post | 0.40 (0.10-1) | 0.80(0.50-1.90) | p<0.001 | |
| Intragroup p-valueb | p<0.001 | 0.58 | ||
| Fall risk (median, min-max) | Baseline | 1.35 (0.90-1.90) | 1.10 (0.90-2.10) | 0.20 |
| Post | 0.95 (0.40-1.40) | 1.20 (0.80-1.50) | p<0.001 | |
| Intragroup p-valueb | p<0.001 | 0.68 | ||
Significant p values presented in bold.
Mann Whitney U test, bWilcoxon test.
Change in neuropathic pain and clinical presentation of DPN
A significant decrease in neuropathic pain (p<0.01), as assessed by the DN4 questionnaire, was observed in the IG following balance training (Table 3). Additionally, MNSI-Q (p<0.001) and MNSI-E (p=0.02) scores, assessments of neuropathic symptoms and evaluations of clinical presentation, also improved post-balance training in the IG in the intragroup analysis (Table 3). In the intragroup comparison of CG, neuropathic pain (DN4, p=0.60) and neuropathic evaluation part of clinical presentation (MNSI-E, p=0.08) remained stable at follow-up, while the number of symptoms worsened (MNSI-Q, p<0.04; Table 3). In the intergroup comparison between IG and CG, improvements were detected only in the neuropathic symptoms assessment component of the clinical presentation, represented by MNSI-Q (p<0.01), during follow-up however, there were no significant improvements observed in the DN4 (p=0.08) or the neuropathic evaluation component of the clinical presentation, as indicated by MNSI-E (p=0.08) (Table 3).
Table 3.
Baseline and Post-Study Neuropathic Pain Assessment and Clinical Presentation.
| Intervention Group | Control Group | Intergroup p-valuea | ||
|---|---|---|---|---|
| DN4 (median, min-max) | Baseline | 5 (1-9) | 5 (2-9) | 0.90 |
| Post | 2 (0-9) | 4 (2-9) | 0.08 | |
| Intragroup p-valueb | 0.01 | 0.60 | ||
| MNSI-Q (median, min-max) | Baseline | 5.50 (1-8) | 5 (1-10) | 0.75 |
| Post | 1 (0-6) | 6 (1-10) | 0.01 | |
| Intragroup p-valueb | p<0.001 | 0.04 | ||
| MNSI-E (median, min-max) | Baseline | 4.50 (1-7) | 3.5(1-6.5) | 0.18 |
| Post | 4 (7-13) | 4 (2-6.5) | 0.08 | |
| Intragroup p-valueb | 0.02 | 0.28 | ||
Significant p values presented in bold.
Mann Whitney U test.
Wilcoxon test.
Change in nerve function
The primary outcomes of nerve function, NCTs, the IG and CG didn’t show any significant improvement in the intragroup analysis (Table 4). Similarly, no differences in peroneal or tibial nerve amplitude, latency, or velocity were observed between IG and CG at follow-up in intergroup measurements (Table 4).
Table 4.
Baseline and Post-Study Nerve Conduction Tests.
| Intervention Group | Control Group | Intergroup p-valuea | ||
|---|---|---|---|---|
| Peroneal nerve | ||||
| Latancy (ms) | Baseline | 4.20 (3.40-5.10) | 4.70 (3.45-5.60) | 0.17 |
| Post | 3.87 (3.25-5.25) | 4.60 (3.40-8.00) | 0.76 | |
| Intragroup p-valueb | 0.40 | 0.17 | ||
| Amplitude (µV) | Baseline | 2.15 (1.10-5.40) | 2.60 (0.90-6.90) | 0.58 |
| Post | 2.20 (1.10-4.40) | 3.40 (0.60-6.80) | 0.28 | |
| Intragroup p-valueb | 0.75 | 0.62 | ||
| Velocity (m/s) | Baseline | 41.05 (30.20-50) | 40.60 (36.3-55.20) | 1.00 |
| Post | 40.60 (33.90-50) | 43.93 (33.50-54.20) | 0.14 | |
| Intragroup p-valueb | 0.53 | 0.33 | ||
| Tibial nerve | ||||
| Latancy (ms) | Baseline | 4.80 (3.50-6.25) | 4.85 (3.55-5.65) | 0.55 |
| Post | 4.47 (3.05-6.20) | 3.75 (3.40-6.20) | 0.62 | |
| Intragroup p-valueb | 0.15 | 0.42 | ||
| Amplitude (µV) | Baseline | 4.70 (0.40-9.80) | 5.20 (2.60-9.70) | 0.62 |
| Post | 5.00 (0.40-8.80) | 5.80 (3.20-11.30) | 0.40 | |
| Intragroup p-valueb | 0.88 | 0.46 | ||
| Velocity (m/s) | Baseline | 40.20 (34.00-46.90) | 39.60 (33.00-43.50) | 0.52 |
| Post | 39.90 (30.90-48.40) | 40.59 (35.40-42.70) | 0.94 | |
| Intragroup p-valueb | 0.33 | 0.08 | ||
Mann Whitney U test.
Wilcoxon test.
Discussion
Here, we evaluated the effect of balance exercise training performed with a BBS on balance and nerve function in patients with DPN. To our knowledge, this is the first randomized controlled trial investigating the impact of balance exercises on neuropathic pain, clinical presentation, and nerve function in DPN. This study also represents the first use of the BBS rehabilitation program specifically recommended for peripheral neuropathy[21]. We demonstrated that balance training successfully improved balance, neuropathy pain and clinical presentation in patients with DPN. However, nerve function was unaffected by balance training.
After 8 weeks of twice weekly balance training sessions on the BBS, we found that all balance outcomes were improved in the IG. This finding is consistent with the results of other studies examining the effectiveness of the BBS in participants with DPN[13,17,26]. Instability in patients with DPN is believed to be primarily due to impaired somatosensory systems and ankle movement strategies[27]. Balance training exercises with the BBS offer a multifaceted approach to address the challenges posed by compromised balance. By engaging the somatosensory system, the BBS stimulates neural pathways responsible for proprioception and tactile perception, crucial components of balance control. Through controlled movements and perturbations, the training exercises aid in the gradual adaptation and recalibration of these sensory pathways, fostering heightened sensitivity to body positioning and weight distributions[28]. Additionally, the BBS places particular emphasis on ankle movements. Finally, small type 2 afferents that carry information from the sole of the feet play a key role in postural control[29]. Use of the BBS barefoot with varying levels of platform movability and visual biofeedback improves ankle strategy and further enhances somatosensory stimulation. Overall, the BBS training approach synergistically refines the intricate interplay between sensory input and motor response, gradually ameliorating balance deficient and potentially reducing the risk of falls among individuals with DPN.
For metabolically induced neuropathies, such as DPN, exercise can increase blood flow and oxygenation to peripheral tissue, reduce glucose levels, and decrease oxidative stress, which subsequently prevents additional neuronal damage and improves regeneration[7]. Indeed, several studies reported the benefits of aerobic, endurance, and resistance exercise for management of DPN[8,9,11,30]. Here, we demonstrated that, despite the short duration of our study, 8 weeks of balance training also improved neuropathic pain, symptoms and clinical presentation in patients with DPN. Unfortunately, the precise mechanisms underlying the favorable impacts of balance training on neuropathic symptoms and presentation in patients with DPN remain incompletely understood. These improvements may be due to regenerative effects on nerve fibers through modified growth factor expression, initiation of remyelination, or accelerated axonal regeneration[30-32]. It is also thought that balance training may increase receptor density, activate deafferented neurons through increased metabolism, reduce excitability thresholds, and promote supraspinal learning effects[30,33]. In fact, a recent systematic analysis suggested that balance exercises may be the most effective type of exercise for improving motor and sensory symptoms in patients with peripheral neuropathies[30]. However additional research is needed to fully understand the mechanisms underlying balance training-induced neuropathic improvements.
Surprisingly, while neuropathic pain and clinical presentation improved after balance training within the IG, differences in neuropathic pain and clinical examination were not observed between IG and CG at follow-up. Our study is one of the few studies examining the impact of exercise on DPN that includes a control group for intergroup comparison. The lack of difference in neuropathic pain and clinical examination at follow-up could be due to our small sample size. Also, while patient demographics and clinical presentation between the two groups were similar at baseline, we did not control for diabetes duration.
We also found that 8 weeks of balance training with BBS had no effect on peroneal and tibial nerve conduction velocity, latencies, or amplitudes. While the benefits of aerobic exercise on nerve regeneration is frequently reported, studies demonstrating the therapeutic impact of different exercise types on nerve damage are lacking[8-10,34,35]. Additionally, when changes in nerve damage, as measured by NCTs, are reported, results are variable. For example, one study demonstrated a significant increase in sural and peroneal nerve conduction velocity, but no difference in distal latency or amplitude, in individuals with DPN after 8 weeks of treadmill training[33]. Similarly, participation in a 12 week Tai Chi program significantly improved bilateral median and tibial nerve motor nerve conduction velocities and bilateral ulnar nerve distal sensory latencies in diabetic patients[36]. However, another study found that participation in aerobic and resistance exercises had no impact on tibial and peroneal nerve conduction velocity, amplitude, or latency in participants with DPN[10]. Variability in NCTs in response to exercise may be due to differences in exercise type, duration and frequency of exercise, or duration of diabetes. Therefore, additional work is needed to determine the optimal exercise regimen to improve nerve function in patients with DPN.
Several mechanisms have been proposed for the positive impact of exercise of nerve conduction. DPN-induced reliance on the anaerobic polyl-sorbital pathway may lead to chronic nerve hypoxia through increased intracellular sorbital concentrations and decreased endoneurial blood flow[37]. Hyperglycemia also impairs production of the vasodilator nitric oxide through activation of protein kinase C[37]. Exercise stimulates nitric oxide synthesis, thus improving blood flow, facilitating better oxygen diffusion into nerve cells, and enabling repair of endothelial damage[39]. Exercise-induced Na-K-ATPase activation may also improve nerve conduction as use of K (ATP) channel openers restores blood flow, motor conduction velocity, and sciatic nerve Na-K-ATPase activity in experimental models of DPN[40]. The lack of improvement in nerve function following balance training may be because balance exercises do not target these physiological pathways.
A strength of the current study is that participants were supervised and trained individually, and a control group was included. This ensured the quality of data collection procedures, clinical findings, and neurophysiological assessments and decreased a possible Hawthorne effect of attention. Second, to the best of our knowledge, this is the first study to use a computer-based balance training program specifically designed for patients with DPN. In our investigation into neuropathic pain, clinical presentation and nerve functions of patients with DPN using Biodex Balance training, we noted the Biodex Balance System’s (BBS) enjoyable and user-friendly nature with positive effects. However, despite its effectiveness, the system’s high cost and the necessity for a clinical environment, along with the challenge of maintaining patients’ high motivation for regular outings, pose disadvantages leading to a high dropout rate. Study limitations included a relatively small sample size and we also did not measure HbA1c levels after intervention to determine if balance training improved glycemic control. In the present study, balance training did not improve nerve function. The duration and frequency of the balance training sessions may need to be increased to observe a positive response in motor nerves. Likewise, pain and the clinical examination part of MNSI, where no change was observed between the groups, may undergo positive changes for the same reasons. We believe there is a need for clinical studies focusing on cost-effective programs that utilize virtual reality technology, are home-based, and do not require expensive devices, enabling patients to engage in longer and more comfortable training sessions without being tethered to a specific location. Additionally, changes in sensory NCTs could not be analyzed as 97% of the participants did not respond to the sural nerve. Our findings underscore the necessity for future research to identify an easily accessible biomarker facilitating the objective evaluation of the sural nerve in cases where it cannot be measured with NCS. Finally, use of thin fiber nerve biopsy in future studies may allow a more sensitive assessment of changes in sensory and motor nerves in response to balance training.
Conclusions
Here, we present a comprehensive study evaluating the effect of BBS training exercises on balance and neuropathic responses in patients with DPN. We found that participation in a BBS balance training program significantly improved balance, neuropathic pain and symptoms, and clinical presentation, but not nerve function, in patients with DPN. Our results suggest that computer-based balance training could be an effective and reasonable treatment tool to enhance the management of neuropathic pain and the clinical signs-symptoms are represented as clinical presentation associated with diabetic peripheral neuropathy.
Ethics approval
The study was approved by the Dokuz Eylul University Ethics Committee (no:2019 GOA/2015-13-05). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Consent to participate
All participants provided oral and written informed consent to participate in the study.
Acknowledgments
We would like to thank Dilber Karacasu Coskunsu and Gul Dıkec whose guidance and feedback improved our research. We also thank Eva L. Feldman and Emily J. Koubek at the University of Michigan NeuroNetwork for Emerging Therapies for expert editorial assistance.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
Authors’ contributions
Concept - DAR, BK; Design - DAR, BK; Supervision - ISS; Resources - ISS; Materials -DAR, GY; Data Collection and/or Processing - DAR, GY; Analysis and/or Interpretation - GY; Writing - DAR, GY; Critical Review – BK. All authors read and approved the final version of the manuscript.
References
- 1.Sun H, Saeedi P, Karuranga S. IDF Diabetes Atlas:Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hicks CW, Selvin E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr Diab Rep. 2019;19:86. doi: 10.1007/s11892-019-1212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elafros MA, Andersen H, Bennett DL, et al. Towards prevention of diabetic peripheral neuropathy:clinical presentation, pathogenesis, and new treatments. Lancet Neurol. 2022;21:922–936. doi: 10.1016/S1474-4422(22)00188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reeves ND, Orlando G, Brown SJ. Sensory-Motor Mechanisms Increasing Falls Risk in Diabetic Peripheral Neuropathy. Medicina (Kaunas) 2021;57:457. doi: 10.3390/medicina57050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ettinger LR, Boucher A, Simonovich E. Patients with type 2 diabetes demonstrate proprioceptive deficit in the knee. World J Diabetes. 2018;9:59–65. doi: 10.4239/wjd.v9.i3.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menz HB, Lord SR, St George R, Fitzpatrick RC. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch Phys Med Rehabil. 2004;85(2):245–52. doi: 10.1016/j.apmr.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Singleton JR, Foster-Palmer S, Marcus RL. Exercise as Treatment for Neuropathy in the Setting of Diabetes and Prediabetic Metabolic Syndrome:A Review of Animal Models and Human Trials. Curr Diabetes Rev. 2022;18:e230921196752. doi: 10.2174/1573399817666210923125832. [DOI] [PubMed] [Google Scholar]
- 8.Billinger SA, Sisante JV, Alqahtani AS, Pasnoor M, Kluding PM. Aerobic exercise improves measures of vascular health in diabetic peripheral neuropathy. Int J Neurosci. 2017;127:80–85. doi: 10.3109/00207454.2016.1144056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu Y, Dennis SM, Kiernan MC, Harmer AR. Aerobic exercise training may improve nerve function in type 2 diabetes and pre-diabetes:A systematic review. Diabetes Metab Res Rev. 2019;35:e3099. doi: 10.1002/dmrr.3099. [DOI] [PubMed] [Google Scholar]
- 10.Kluding PM, Pasnoor M, Singh R, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012;26:424–429. doi: 10.1016/j.jdiacomp.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaribeygi H, Butler AE, Sahebkar A. Aerobic exercise can modulate the underlying mechanisms involved in the development of diabetic complications. J Cell Physiol. 2019;234(8):12508–12515. doi: 10.1002/jcp.28110. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad I, Noohu MM, Verma S, Singla D, Hussain ME. Effect of sensorimotor training on balance measures and proprioception among middle and older age adults with diabetic peripheral neuropathy. Gait Posture. 2019;74:114–120. doi: 10.1016/j.gaitpost.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Akbari M, Jafari H, Moshashaee A, Forugh B. Do diabetic neuropathy patients benefit from balance training?J Rehabil Res Dev. 2012;49:333–338. doi: 10.1682/jrrd.2010.10.0197. [DOI] [PubMed] [Google Scholar]
- 14.Akhtar S, Nasir JA, Abbas T, Sarwar A. Diabetes in Pakistan:A systematic review and meta-analysis. Pak J Med Sci. 2019;35:1173–1178. doi: 10.12669/pjms.35.4.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown SJ, Handsaker JC, Bowling FL, Boulton AJ, Reeves ND. Diabetic peripheral neuropathy compromises balance during daily activities. Diabetes Care. 2015;38:1116–1122. doi: 10.2337/dc14-1982. [DOI] [PubMed] [Google Scholar]
- 16.Thukral N, Kaur J, Malik M. A Systematic Review and Meta-analysis on Efficacy of Exercise on Posture and Balance in Patients Suffering from Diabetic Neuropathy. Curr Diabetes Rev. 2021;17:332–344. doi: 10.2174/1573399816666200703190437. [DOI] [PubMed] [Google Scholar]
- 17.Eftekhar-Sadat B, Azizi R, Aliasgharzadeh A, Toopchizadeh V, Ghojazadeh M. Effect of balance training with Biodex Stability System on balance in diabetic neuropathy. Ther Adv Endocrinol Metab. 2015;6:233–240. doi: 10.1177/2042018815595566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daud SAH, Rahman MU, Arsh A, Junaid M. Effect of balance training with Biodex Balance System to improve balance in patients with diabetic neuropathy:A quasi experimental study. Pak J Med Sci. 2021;37:389–392. doi: 10.12669/pjms.37.2.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boulton A, Vinik AI, Arezzo JC, et al. Diabetic neuropathies:a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 20.Lindemann U, Rupp K, Muche R, Nikolaus T, Becker C. Improving balance by improving motor skills. Z Gerontol Geriatr. 2004;37:20–26. doi: 10.1007/s00391-004-0206-5. [DOI] [PubMed] [Google Scholar]
- 21.Thompson N, Wilcox D, Jackson K. Peripheral neuropathy. Diagnosis Specific Testing and Treatment Guide. 2014. [Accessed:20 July 2017]. https: //www.iprsmediquipe.com/assets/Uploads/clinical-guideline-peripheral-neuro-14298.pdf .
- 22.Cachupe WJ, Shifflett B, Kahanov L, Wughalter EH. Reliability of biodex balance system measures. Meas Phys Educ Exerc Sci. 2001;5:97–108. [Google Scholar]
- 23.Cevik U, Isin S, Sarioglu Ay S, Evcik DA. comparison of the DN4 and LANSS questionnaires in the assessment of neuropathic pain:validity and reliability of the Turkish version of DN4. J Pain. 2010;11:1129–1135. doi: 10.1016/j.jpain.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Aktar Reyhanioglu D, Adiyaman SC, Bektas M, et al. Validity and reliability of the Turkish version of the Michigan Neuropathy Screening Instrument. Turk J Med Sci. 2020;50(4):789–797. doi: 10.3906/sag-1906-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gusi N, Carmelo Adsuar J, Corzo H, Del Pozo-Cruz B, Olivares PR, Parraca JA. Balance training reduces fear of falling and improves dynamic balance and isometric strength in institutionalised older people:a randomised trial. J Physiother. 2012;58:97–104. doi: 10.1016/S1836-9553(12)70089-9. [DOI] [PubMed] [Google Scholar]
- 26.Salsabili H, Bahrpeyma F, Forogh B, Rajabali S. Dynamic stability training improves standing balance control in neuropathic patients with type 2 diabetes. J Rehabil Res Dev. 2011;48:775–786. doi: 10.1682/jrrd.2010.08.0160. [DOI] [PubMed] [Google Scholar]
- 27.Bonnet C, Carello C, Turvey MT. Diabetes and postural stability:review and hypotheses. J Mot Behav. 2009;41:172–190. doi: 10.3200/JMBR.41.2.172-192. [DOI] [PubMed] [Google Scholar]
- 28.Lin LF, Liou TH, Hu CJ, et al. Balance function and sensory integration after mild traumatic brain injury. Brain Inj. 2015;29:41–46. doi: 10.3109/02699052.2014.955881. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Zhang S, Dobson J. The contribution of small and large sensory afferents to postural control in patients with peripheral neuropathy. J Sport Health Sci. 2019;8(3):218–227. doi: 10.1016/j.jshs.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Streckmann F, Zopf EM, Lehmann HC, et al. Exercise intervention studies in patients with peripheral neuropathy:a systematic review. Sports Med. 2014;44:1289–1304. doi: 10.1007/s40279-014-0207-5. [DOI] [PubMed] [Google Scholar]
- 31.Sabatier MJ, Redmon N, Schwartz G, English AW. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol. 2008;211:489–493. doi: 10.1016/j.expneurol.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molteni R, Zheng JQ, Ying Z, Gómez-Pinilla F, Twiss JL. Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci U S A. 2004;101:8473–8478. doi: 10.1073/pnas.0401443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taube W, Gruber M, Gollhofer A. Spinal and supraspinal adaptations associated with balance training and their functional relevance. Acta Physiol (Oxf) 2008;193:101–116. doi: 10.1111/j.1748-1716.2008.01850.x. [DOI] [PubMed] [Google Scholar]
- 34.Balducci S, Iacobellis G, Parisi L, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20:216–223. doi: 10.1016/j.jdiacomp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Dixit S, Maiya A, Shastry B. Effect of aerobic exercise on quality of life in population with diabetic peripheral neuropathy in type 2 diabetes:a single blind, randomized controlled trial. Qual Life Res. 2014;23:1629–1640. doi: 10.1007/s11136-013-0602-7. [DOI] [PubMed] [Google Scholar]
- 36.Hung JW, Liou CW, Wang PW, et al. Effect of 12-week tai chi chuan exercise on peripheral nerve modulation in patients with type 2 diabetes mellitus. J Rehabil Med. 2009;41:924–929. doi: 10.2340/16501977-0445. [DOI] [PubMed] [Google Scholar]
- 37.Kikkawa Y, Kuwabara S, Misawa S, et al. The acute effects of glycemic control on nerve conduction in human diabetics. Clin Neurophysiol. 2005;116:270–274. doi: 10.1016/j.clinph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Creager MA. Inhibition of protein kinase Cbeta prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ Res. 2002;90:107–111. doi: 10.1161/hh0102.102359. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka LY, Bechara LR, dos Santos AM, et al. Exercise improves endothelial function:a local analysis of production of nitric oxide and reactive oxygen species. Nitric Oxide. 2015;45:7–14. doi: 10.1016/j.niox.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Hohman TC, Cotter MA, Cameron NE. ATP-sensitive K(+) channel effects on nerve function, Na(+), K(+) ATPase, and glutathione in diabetic rats. Eur J Pharmacol. 2000;397:335–341. doi: 10.1016/s0014-2999(00)00227-2. [DOI] [PubMed] [Google Scholar]