Abstract
Objective
The aim of our study was to evaluate the safety and effectiveness of the hybrid method off-pump for closure of isolated ventricular septal defect (VSD) compared with the traditional method of on-pump of children.
Methods
This research was a retrospective cohort study. Data were collected from 500 patients with isolated VSD (or residual VSD after a previous repair) who underwent surgery at the National Scientific Medical Center from May 2016 to December 2020. Patients were operated with 1 of 2 methods of surgery: the traditional method of on-pump or the hybrid method of off-pump. This study assessed the safety and efficacy of the hybrid method by comparing it with the traditional method for the treatment of patients with isolated VSD.
Results
The procedural success rate reached 93.2% in the hybrid method, with a 6.4% conversion rate to the traditional method and 0.4% hospital mortality. The mean operation time was 84 minutes (31; 160 minutes) in the hybrid group (n = 250) and 168 minutes (70; 300 minutes) in the traditional group (n = 250) (P = .000). Hospital mortality was 0.43% in the first group and 1.5% in the second group (P = .000).
Conclusions
The hybrid method of VSD closure is safe and effective in a selected group of patients. The advantages of the hybrid method are improved cosmetics and shorter operation time and overall hospital stay.
Key Words: ventricular septal defect, hybrid method, minimally invasive, surgery, Kazakhstan

Our team.
Central Message.
The hybrid method of closing the ventricular septal results in shorter hospitalization and better cosmetic as compared with the traditional method.
Perspective.
The hybrid method was introduced 7 years ago in Kazakhstan by our team. This article is a summary of the comparative analysis of defect correction by 2 different methods.
Currently, there are 3 surgical methods: the traditional method of on-pump, the interventional method, and the hybrid (minimally invasive perventricular device closure) method off-pump.1, 2, 3 Moreover, minimally invasive cardiac surgery techniques have some types of incisions, such as inferior median sternotomy and right/left anterior thoracotomy or vertical axillary. Minimally invasive cardiac surgery often leads to less pain, smaller scars, lower risk of bleeding or infection, and faster recovery.4 Each method has advantages and disadvantages. The traditional method of on-pump is considered the “gold standard” treatment; however, post-on-pump neurologic outcomes cannot be ignored, which would have an impact on patients’ quality of life.5,6 The interventional method is the alternative way of treatment.7 Nevertheless, it is a difficult implantation procedure with an unsatisfactory high level of postoperative arrhythmia and vascular complications.8,9 The hybrid method is a minimally invasive ventricular septal defect (VSD) closure on a beating heart. The advantages are shortening the duration of the hospitalization, rehabilitation, no X-ray exposure, and good cosmetic effects. However, there are cons, where mentioned, of the development of arrhythmia and, dislocation of the occluder after implantation.10, 11, 12, 13
The aim of our study was to evaluate the safety and effectiveness of the hybrid method off-pump for treating isolated VSD compared with the traditional method of on-pump. We present the following article in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology reporting checklist.
Methods
Patient Population
This research was a retrospective cohort study. Data were collected from 500 patients with isolated VSD (or residual VSD after a previous repair) who underwent surgery at the National Scientific Medical Center in Astana, Kazakhstan from May 2016 to December 2020. Patients were operated via 2 methods of surgery: the traditional method of on-pump and the hybrid method of off-pump. This study assessed the safety and efficacy of the hybrid method by comparing it with the traditional method for the treatment of isolated VSDs. The study protocol was approved by the Ethics Committee of National Scientific Medical Center, Astana, Kazakhstan (protocol number: 081/CR-75; assigned number: 053/СТ-63) and carried out in accordance with the principles set out in the Declaration of Helsinki 1964.
Study Methods
Surgery was indicated if patients (aged 0-3 years) had a VSD of >4 mm, were older than 3 years, VSD of >3 mm with a pulmonary/systemic blood flow of >1.5, and/or signs of heart failure.14,15 Children who had a VSD of >4 mm between the ages of 0 and 3 years and shunts greater than 1.5:1 generally have mild-to-moderate elevations of pulmonary artery pressure and resistance. They can be monitored until they are up to 5 years of age to maximize the chance of spontaneous closure. If failing the latter, surgical treatment may be performed.16
Inclusion criteria for both methods included (1) patients with congenital isolated VSD (perimembranous, muscular, atrioventricular conal type [inlet], and subarterial [outlet]); (2) patients with residual VSD postsurgical correction of a congenital VSD; and (3) clinical signs: symptoms of heart failure, recurrent respiratory infection, developmental delay, or history of bacterial endocarditis.17
Echocardiographic inclusion criteria for hybrid method included (1) distance to the pulmonary valve >2 mm; (2) no prolapse of the aortic valve into the defect18; (3) subaortic rim (distance from the defect to the aortic valve) of >2 mm; (4) distance to the tricuspid valve >2 mm; (5) defect size from 4 to 12 mm; and (6) for perimembranous defects, the ratio of the size of the VSD and the weight of the patient was taken into account: (a) ≤6 mm with a weight of 4 to 8 kg; (b) ≤8 mm with a weight of 9 to 12 kg; and (c) ≤10 mm greater than 13 kg.19
Exclusion criteria for the hybrid method included (1) large, nonrestrictive VSDs with indistinct margins or high pulmonary hypertension and bidirectional shunting; (2) prolapse of the aortic valve leaflet into the defect; (3) infective endocarditis; (4) concomitant anomalies requiring correction using cardiopulmonary bypass; (5) the presence of cardiac arrhythmias (in particular, AV block); (6) aortic dextraposition; (7) aortic valve regurgitation (more than mild); (8) tricuspid valve regurgitation (more than mild); and (9) aneurysm of the interventricular septum.18, 19, 20, 21
The Traditional VSD Closure of On-Pump
All patients in the traditional method group were operated on through median sternotomy incision under general anesthesia. The standard cardiopulmonary bypass (on pump) was with bicaval cannulation and under normothermia. Then cold blood cardioplegia was used for myocardial protection in all operations. The majority of VSDs were performed through a right transatrial access, by using a porous polytetrafluoroethylene patch “Ecoflon” with a continuous running 6/0 polypropylene suture. Transesophageal echocardiography (TEE) was used in all circumstances to evaluate the residual shunts and the competency of the tricuspid and aortic valves.
The Hybrid VSD Closure Off-Pump
After general anesthesia, all patients were placed in a supine position (Video 1). The operation was performed entirely under TEE guidance. Before the operation, the location and size of the VSDs were carefully assessed by TEE. Different types of occluders (symmetric, asymmetric, eccentric, and muscular) may be chosen based on the specific characteristics of the VSD as determined by the TEE. A 2- to 4-cm inferior median sternotomy or vertical axillary incision was done and a pericardiotomy was made. After the pericardium was opened and the free wall of the right ventricle was exposed, the puncture site was identified under continuous TEE control. A purse string suture was placed around the chosen location. Then, that place was punctured by a trocar. The 0.035-inch guidewire was introduced into the right ventricle and then through the defect to the left ventricle by the trocar. After the trocar was removed, the delivery sheath was introduced along the guidewire to the left ventricle. After the inner sheath of the delivery sheath and the guidewire were removed, the occluder was deployed through the loading sheath under TEE. Finally, The TEE was used to detect residual shunt and valve dysfunction (especially for the aortic valve). If, after evaluation with TEE, there were signs such as atrioventricular block, residual shunt greater than 2 mm, and new aortic or tricuspid regurgitation, patients were converted to traditional treatment on-pump.
Devices and Delivery System
The occluder used in this study was the Cera Occluders (LifeTech Scientific Co). The Cera Occluders are a self-expandable, double-disc device made from a nitinol wire mesh. The 2 discs are linked together by a short cylindrical waist matching the size of the VSD. The discs and waist are filled with polytetrafluoroethylene membranes securely sewn to the device by nylon threads to increase the closing ability of the occlude and reduce the residual shunts. The metallic skeleton is plated with a biological ceramic coating to improve biocompatibility and induce better and faster endothelialization of the device. The ceramic coating reduces electrochemical erosion and the nickel ion concentration in blood and endocardium.22
The occluder size was selected in accordance with the anatomical conditions (the position, diameter of the VSD, and thickness of the interventricular septum) by doctors. The occluder must be used in combination with the introducer to advance the VSD occluder to the proper position. When the occluder was released from the sheath, one disc expanded on each side of the defect, and the expanding waist closed the VSD tunnel in the septum between the left and right ventricles.
Four different Cera occluders were used, as follows:
-
•
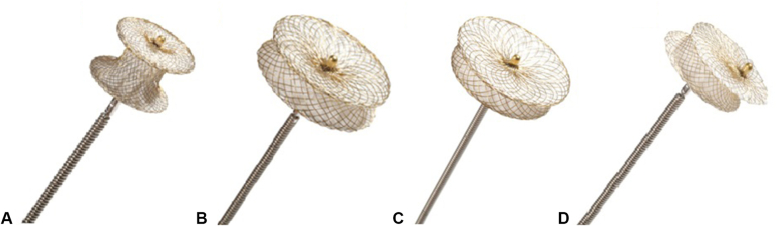
Muscular VSD occluders were used in 50 (20%) cases (Figure 1, A).
- •
Figure 1.
Types of occluders for closure VSD (Cera Occluders, LifeTech Scientific Co).22 A, Muscular occluder; (B) symmetric occluder; (C) asymmetric occluder; (D) eccentric occluder.
Statistical Analysis
Continuous variables are presented means and standard deviation and were analyzed using the Student t test. Median and interquartile ranges are described by the Mann–Whitney U test. Categorical variables are expressed as numbers (%) and frequencies and compared using the χ2 test. Statistical analysis was performed by SPSS 26.0 (IBM Corp) and Excel 2016 software (Microsoft Corp).
Results
Overall, the 500 consecutive patients with VSDs were divided into 2 groups of 250 patients. In the first group, patients were operated on by the hybrid method (aged 2 months to 18 years and weighing 4.7-100 kg). In the second group, patients were operated on by the traditional method (aged 1 month to 16 years and weighing 3-60 kg) (Table 1).
Table 1.
Patients’ baseline data and the characteristics of VSD, for the period from 2016 to 2020
| Data on patients | Hybrid method (n = 250) | Traditional method (n = 250) |
|---|---|---|
| Sex (men/women), n | 116/134 | 133/117 |
| Age, y, n (%) | ||
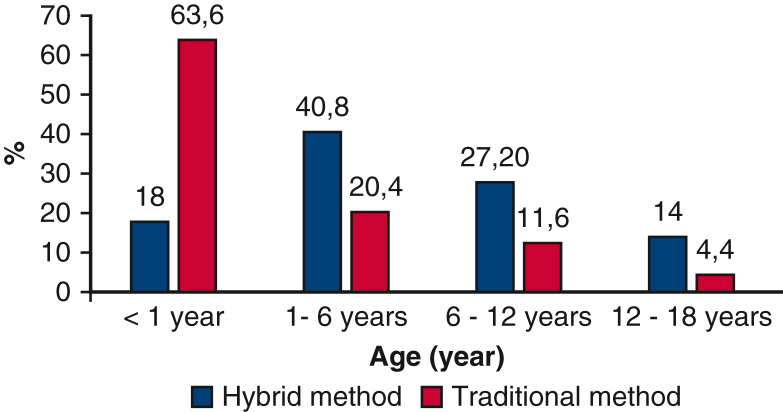
| <1 | 45 (18) | 159 (63.6) |
| >1-< 6 | 102 (40.8) | 51 (20.4) |
| >6- < 12 | 68 (27.2) | 29 (11.6) |
| >12-< 18 | 35 (14) | 11 (4.4) |
| Height, cm (min; max) | 107 (55; 176) | 79 (50; 178) |
| Weight, kg (min; max) | 22 (4.7; 100) | 11.4 (3; 60) |
| Up to 10 kg, n (%) | 64 (25.6) | 177 (70.8) |
| From 10 to 20 kg, n (%) | 81 (32.4) | 37 (14.8) |
| From 20 to 30 kg, n (%) | 49 (19.6) | 16 (6.4) |
| Over 30 kg, n (%) | 56 (22.4) | 20 (8) |
| VSD location, n (%) | ||
| Perimembranous | 200 (80) | 220 (88) |
| Muscular | 19 (7.6) | 4 (1.6) |
| Inlet | 4 (1.6) | 4 (1.6) |
| Subarterial (outlet) | 15 (6) | 12 (4.8) |
| Residual | 10 (4) | 6 (1.6) |
| Multiple | 2 (0.8) | 4 (1.6) |
| Defect diameter, mm | ||
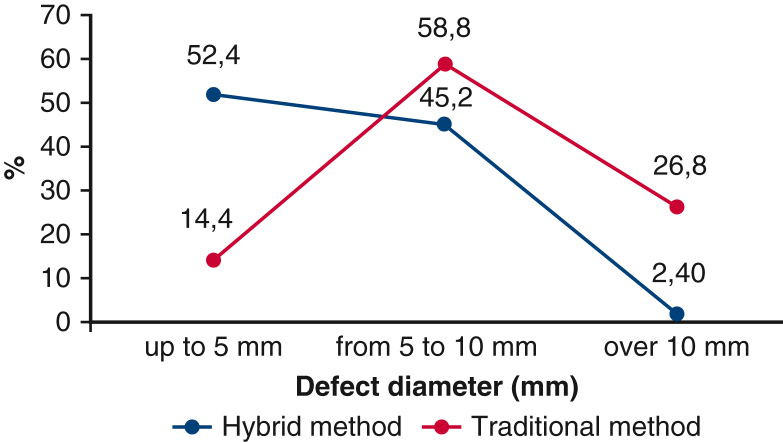
| Up to 5 mm, n (%) | 131 (52.4) | 36 (14.4) |
| From 5 to 10 mm, n (%) | 113 (45.2) | 147 (58.8) |
| Over 10 mm, n (%) | 6 (2.4) | 67 (26.8) |
VSD, Ventricular septal defect.
There were 6 patients with larger defects than 10 mm who received an occluder (Table 1). The results included 3 conversions and 3 successful closures. The reason for conversions in 3 patients were 1 patient with complete atrioventricular block and 2 patients with residual shunt (>2 mm) (Table 2). The symmetric occluder with size of 10 to 14 mm was used in children at the age of 10 to 14 years.
Table 3.
Operative and postoperative data
| Operative and postoperative data | Hybrid method (n = 234) | Traditional method (n = 266) | P value |
|---|---|---|---|
| Size of device used, range, mm | 4-14 | – | – |
| Success rate, n (%) | 233 (93.2) | 250 (100) | .001 |
| Hospital mortality, n (%) | 1 (0.4) | 4 (1.5) | <.000 |
| Conversion cases, n (%) | 16 (6,4) | – | – |
| Intraoperative blood loss, mL | 27 | 31 | .463 |
| Operation time, min (min; max) | 84 (31; 160) | 168 (70; 300) | <.000 |
| CPB times, min (min; max) | – | 72 (28; 150) | – |
| Crossclamping time, min (min; max) | – | 36 (10; 70) | – |
| Left ventricular ejection fraction (%) (before surgery) (min; max) | 65.5 (59; 78) | 65 (40; 78) | .465 |
| Left ventricular ejection fraction (%) (after surgery) (min; max) | 60.7 (55; 68) | 59.6 (45; 67) | <.000 |
| Residual shunts, n (%) | 16 (6.84) | 19 (7.14) | .612 |
| Before operation stay, d | 3.12 | 6.24 | <.000 |
| After the operation stay, d | 6.76 | 11.13 | <.000 |
| Intensive care unit stay, d | 1.03 | 2.5 | <.000 |
| Total hospital stay with first step rehabilitation, d | 9.88 | 17.35 | <.000 |
CPB, Cardiopulmonary bypass.
Patients between 1 and 6 years dominated (40.8%) in the first group, whereas the second group was patients under 1 year (63.6%) (Figure 2). Patients were divided into 4 subgroups according to their weight (1, up to 10 kg; 2, from 10 to 20 kg; 3, from 20 to 30 kg; 4, >30 kg). The average weight was 22 kg in the off-pump group, which included 2 subgroups (32.4%), and 11 kg in the on-pump group, which included 1 subgroup (70.8%) (Table 1). The perimembranous VSDs were the most common VSD location in both groups (80%; 88%).
Figure 2.
Patients’ data were divided by age in both groups, for the period from 2016 to 2020.
VSD were classified according to the diameter of the defect’s 3 subgroups (A, up to 5 mm; B, from 5 to 10 mm; C, >10 mm) (Table 1 and Figure 3). Most patients were in subgroups A and B. Patients with a large VSD (>0 mm) was 10 times more likely to undergo the traditional method (26.8%) than the hybrid method (2.40%).
Figure 3.
Patients’ data depending on the diameter of the defect of VSD in both groups, for the period from 2016 to 2020. VSD, Ventricular septal defect.
The success rate of the operation was 93.2% (233/250) in the off-pump group, and a total of 50 muscular occluders (20%) and 200 perimembranous occluders (80%) were implanted. Three types of perimembranous occluders were used: symmetric (in 100 cases: 40%), eccentric (in 88 cases: 35.2%), and asymmetric (in 12 cases: 4.8%) (Table 1 and Figure 1). The average intraoperative blood loss was 27 mL in the first group and 31 mL in the second group (P = .463). Echocardiography upon discharge from the hospital showed that 16 (6.84%) patients in the off-pump group and 19 (7.14%) in the on-pump group had residual shunts (<2 mm). There was no difference in the occurrence of the residual shunts between 2 groups (P = .612) (Table 3).
Of the 250 patients, 16 patients (6.4%) were converted to traditional surgery. The main reason for the conversion was a residual shunt >2 mm wide in 9 patients (3.6%). Then, in 6 patients was new aortic regurgitation 2 (0.8%), complete atrioventricular block 2 (0.8%), dislocation 2 (0.8%) and new tricuspid regurgitation (Table 2). As mentioned previously, perimembranous VSDs comprised 80% of patients (200∖250) of which 5% of patients (10∖200) were converted to the traditional procedure. A total of 0.8% of patients (2/250) had multiple VSDs, and there was noted 100% (2/2) conversion (Table 2). There were 15 cases with subarterial (outlet) VSDs (Table 1), of which 11 cases were successful closures and 4 cases were converted to the traditional method. The cause of conversions of 4 cases were 2 cases of new aortic regurgitation and 2 cases of residual shunt (>2 mm) (Table 2). No cardiac arrhythmias were noted in this case.
Table 2.
Characteristics of conversion cases
| Hybrid method (n = 250) | Conversion cases |
|---|---|
| Patients, n (%) | 16 (6.4) |
| Male, n | 10 |
| Female, n | 6 |
| Age, mo (min; max) | 61 (7; 192) |
| Weight, kg (min; max) | 22.5 (5.9; 65) |
| Defect diameter, mm (min; max) | 7.7 (4; 13) |
| VSD location, n (%) | |
| Perimembranous | 10 (5) |
| Muscular | 0 |
| Atrioventricular conal type (inlet) | 0 |
| Subarterial (outlet) | 4 (27) |
| Re-VSD | 0 |
| Multiple | 2 (100) |
| Conversion cause | |
| Residual shunt, n (%) | 9 (3.6) |
| New aortic regurgitation, n (%) | 2 (0.8) |
| CAVB, n (%) | 2 (0.8) |
| Dislocation, n (%) | 2 (0.8) |
| New tricuspid regurgitation, n (%) | 1 (0.4) |
VSD, Ventricular septal defect; CAVB, complete atrioventricular block.
Hospital mortality was noted in both groups: 0.43% of patients (1/234) in the first group and 1.5% of patients (4/266) in the second (P = .000) (Tables 3 and 4). According to the conclusion of pathologic autopsies, cause of death was a pulmonary hypertensive crisis in the off-pump group. The second group had different reasons for death, 2 patients with sepsis, 1 patient with arterial hypertension crisis (with the development of ischemic stroke), and 1 patient with a complete atrioventricular block (Table 4).
Table 4.
Characteristics of hospital mortality cases
| Data on patients | Hybrid method (n = 234) | Traditional method (n = 266) |
|---|---|---|
| Patients, n (%) | 1 (0.4) | 4 (1.5) |
| Male, n | 1 | 3 |
| Female, n | – | 1 |
| Age, mo (min; max) | 6 | 56 (2; 192) |
| Weight, kg (min; max) | 5.6 | 29.7 (3.67; 100) |
| Defect diameter, mm | 5 | 10.25 (5; 17) |
| VSD location, n | ||
| Perimembranous | 1 | 3 |
| Muscular | – | – |
| Atrioventricular conal type (inlet) | – | – |
| Subarterial (outlet) | – | 1 |
| Re-VSD | – | – |
| Multiple | – | – |
| Cause of hospital mortality, n | ||
| Sepsis | – | 2 |
| Pulmonary hypertension crisis | 1 | – |
| Ischemic stroke | – | 1 |
| Atrioventricular block (complete) (CAVB) | – | 1 |
VSD, Ventricular septal defect; CAVB, complete atrioventricular block.
Furthermore, the off-pump group showed significantly decreased operation time, ventilation time, pre-/postoperative hospitalization days, intensive care unit days, and total hospital days than that in the traditional group (P < .05, Table 3).
Discussion
This is the first study, to our knowledge, in Central Asia that demonstrates the hybrid method is equally effective as the traditional method for infants through 18-year-old patients. Although the hybrid method has been widely used in China and some European countries since 2000,23, 24 our medical center was implemented in 2016 by Professor Xiangbin Pan from China. Compared with the traditional method, the advantages of the hybrid method are evident. First, this method eliminates the potential complications of on-pump, with less surgical trauma and lower risks of mediastinitis. In this study, the hybrid group underwent partial sternotomy, whereas the traditional group had midline sternotomy. Second, there is less psychological anxiety due to favorable cosmetic results. In our study, the wound size was 2 to 4 cm in the hybrid group and approximately 10 cm in the traditional group. Third, the procedure presents an economic benefit as the result of shorter hospitalization days.25, 26, 27 In our case, the total hospital stay with the first-step rehabilitation was 9.88 days in the hybrid group and 17.35 days in the traditional group.
Our series included a heterogeneous group of patients with VSD of various localizations. Most of the patients in both groups had perimembranous defects (hybrid: 200/250, traditional: 220/250) and only 23 muscle defects (hybrid: 19/250, traditional: 4/250). The success rate of the operation was 93.2% in the hybrid group and 100% in the traditional group (Table 3).
Among all operated patients in the hybrid group, 6.84% intraoperatively (Table 3), according to the TEE, revealed the presence of a residual shunt. Typically, a device averaging 1 mm larger than the VSD, as measured by TEE, was used. The use of an occluder that exceeds the size of the device by more than 2 mm has a high risk of developing cardiac arrhythmias and damage to adjacent intracardiac structures.28 The decision to complete the procedure was required if a single shunt did not exceed 3 mm, and each of the multiple shunts did not exceed 1 to 2 mm, and also if the faults were in close proximity to the occluder.28 During the first hours after the operation, blood cells settle on the devices, and during the first months after implantation, they gradually endothelize-blood cells form a single cell layer, which organizes the growth and development of connective tissue cells, which leads to the closure of most of the residual defects.29
Aortic regurgitation is one of the most serious complications in the postoperative period of VSD closure.30 A total of 15 patients of 250 operated by the hybrid method had subarterial (outlet) VSDs, of which 4 were transformed to the traditional method (reason for converting in 2 patients developed new aortic regurgitation and in 2 patients residual shunts) (Table 2).
Intraoperative development of cardiac arrhythmias during occluder implantation may be associated with mechanical trauma to the edge of the defect by the device itself or the delivery system. Immediately after the operation, both discs of the occluder may press on the posterior–inferior edge of the defect, causing local edema that affects the proximal conduction system.31 Various studies dealing with the development of arrhythmias after correction of isolated perimembranous VSDs32, 33, 34 did not reveal a significant dependence of the development of complete AV block on age, weight, size of the defect, the ratio of occluder sizes, and VSD.35,36 Therefore, the question of predicting the development of cardiac arrhythmias in the hybrid method VSD closure remains open. Cases reported in the literature of complete AV block months and even years after implantation are practically resistant to drug therapy and require the implantation of a permanent pacemaker.37,38 This fact necessitates a long-term, perhaps even lifelong, follow-up of patients with electrocardiography.
Study Limitations
We acknowledge that this study is limited by its retrospective, single-center design. We wanted to share an important report on the experience of our medical center—the National Scientific Medical Center, which is located in Astana, Republic of Kazakhstan. The National Scientific Medical Center is one of the leading medical centers not only in Kazakhstan but in Central Asia for the treatment of congenital heart defects. We are currently conducting a retrospective study with long-term results to evaluate the efficacy and safety of the hybrid method. The results of the study will be published in an original research article that will be written by Dr Adilbekova.
Conclusions
The hybrid method of ventricular septal defect closure is safe and effective in a selected group of patients. The advantages of the hybrid method are improved cosmetic and shorter operation time and overall hospital stay.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
The National Scientific Medical Center of the Republic of Kazakhstan provided data for this study.
Footnotes
This study was funded via the National Scientific Medical Center, Astana City, the Republic of Kazakhstan for International Publication. The funding agencies did not have any role in the registry design, analysis, and interpretation of data.
Data-Sharing Statement: The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Supplementary Data
The hybrid ventricular septal defect closure off-pump. Video available at: https://www.jtcvs.org/article/S2666-2507(24)00042-7/fulltext.
References
- 1.Yang J., Yang L., Yu S., et al. Transcatheter versus surgical closure of perimembranous ventricular septal defects in children: a randomized controlled trial. J Am Coll Cardiol. 2014;63(12):1159–1168. doi: 10.1016/j.jacc.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Gan C., An Q., Lin K., et al. Perventricular device closure of ventricular septal defects: six months results in 30 young children. Ann Thorac Surg. 2008;86(1):142–146. doi: 10.1016/j.athoracsur.2008.03.058. [DOI] [PubMed] [Google Scholar]
- 3.Lu W., Zhang F., Fan T., et al. Minimally-invasive-perventricular-device-occlusion versus surgical-closure for treating perimembranous-ventricular-septal-defect: 3-year outcomes of a multicenter randomized clinical trial. J Thorac Dis. 2021;13(4):2106–2115. doi: 10.21037/jtd-20-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An K., Li S., Yan J., et al. Minimal right vertical infra-axillary incision for repair of congenital heart defects. Ann Thorac Surg. 2022;113(3):896–902. doi: 10.1016/j.athoracsur.2021.01.052. [DOI] [PubMed] [Google Scholar]
- 5.Tao K., Lin K., Shi Y., et al. Perventricular device closure of perimembranous ventricular septal defects in 61 young children: early and midterm follow-up results. J Thorac Cardiovasc Surg. 2010;140(4):864–870. doi: 10.1016/j.jtcvs.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Gessler P., Schmitt B., Pretre R., et al. Inflammatory response and neurodevelopmental outcome after open-heart surgery in children. Pediatr Cardiol. 2009;30:301–305. doi: 10.1007/s00246-008-9354-5. [DOI] [PubMed] [Google Scholar]
- 7.Lock J., Block P., De Wolf D., et al. Transcatheter closure of ventricular septal defects. Circulation. 1988;78(2):361–368. doi: 10.1161/01.cir.78.2.361. [DOI] [PubMed] [Google Scholar]
- 8.Pedra C.A.C., Pedra S.R.F., Esteves C.A., et al. Percutaneous closure of perimembranous ventricular septal defects with the amplatzer device: technical and morphological considerations. Catheter Cardiovasc Interv. 2004;61(3):403–410. doi: 10.1002/ccd.10797. [DOI] [PubMed] [Google Scholar]
- 9.Knauth A.L., Lock J.E., Perry S.B., et al. Transcatheter device closure of congenital and postoperative residual ventricular septal defects. Circulation. 2004;110(5):501–507. doi: 10.1161/01.CIR.0000137116.12176.A6. [DOI] [PubMed] [Google Scholar]
- 10.Voitov A., Omelchenko A., Gorbatykh Y., et al. Outcomes of perventricular off-pump versus conventional closure of ventricular septal defects: a prospective randomized study. Eur J Cardiothoracic Surg. 2017;51(5):980–986. doi: 10.1093/ejcts/ezx002. [DOI] [PubMed] [Google Scholar]
- 11.Adilbekova A., Marasulov S., Nurkeyev B., et al. Evolution of surgery of ventricular septal defect closure. J Clin Med Kaz. 2022;19(5):4–8. [Google Scholar]
- 12.Thakkar B., Patel N., Shah S., et al. Perventricular device closure of isolated muscular ventricular septal defect in infants: a single centre experience. Indian Heart J. 2012;64(6):559–567. doi: 10.1016/j.ihj.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li D., Zhou X., Li M., et al. Comparisons of perventricular device closure, conventional surgical repair, and transcatheter device closure in patients with perimembranous ventricular septal defects: a network meta-analysis. BMC Surg. 2020;20(1):115. doi: 10.1186/s12893-020-00777-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schipper M., Slieker M.G., Schoof P.H., et al. Surgical repair of ventricular septal defect; contemporary results and risk factors for a complicated course. Pediatr Cardiol. 2017;38(2):264–270. doi: 10.1007/s00246-016-1508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.David L.S., Brandi B.S., Charles D.F., et al. In: Congenital Cardiac Surgery. 3rd ed. David D.Y., Luca A.V., Stephen C.Y., editors. McGraw Hill Medical; 2014. Ventricular septal defect; p. 65. [Google Scholar]
- 16.Muralidaran A., Shen I. In: Critical Heart Disease in Infants and Children. 3rd ed. Ross M.U., Kristen N.M., editors. Elsevier Health Sciences; 2019. Ventricular septal defects; pp. 597–605.e2. [Google Scholar]
- 17.Ou-Yang W. Bin, Wang S.Z., Zhang D.W., et al. Echocardiographic guided closure of perimembranous ventricular septal defects. Ann Thorac Surg. 2015;100(4):1398–1402. doi: 10.1016/j.athoracsur.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 18.Michel-Behnke I., Ewert P., Koch A., et al. Device closure of ventricular septal defects by hybrid procedures: a multicenter retrospective study. Catheter Cardiovasc Interv. 2011;77(2):242–251. doi: 10.1002/ccd.22666. [DOI] [PubMed] [Google Scholar]
- 19.Plotnikov M.V., Tkachev I.V., Tungusova M.A., et al. Perventricular closure of ventricular septal defects without cardiopulmonary bypass. Clin Exp Surg. 2017;5(4):138–141. [Google Scholar]
- 20.Xing Q., Wu Q., Shi L., et al. Minimally invasive transthoracic device closure of isolated ventricular septal defects without cardiopulmonary bypass: long-term follow-up results. J Thorac Cardiovasc Surg. 2015;149(1):257–264. doi: 10.1016/j.jtcvs.2014.07.078. [DOI] [PubMed] [Google Scholar]
- 21.Pedra C.A.C., Pedra S.R.F., Chaccur P., et al. Perventricular device closure of congenital muscular ventricular septal defects. Expert Rev Cardiovasc Ther. 2010;8(5):663–674. doi: 10.1586/erc.10.31. [DOI] [PubMed] [Google Scholar]
- 22.Lifetechmed.com [internet]. China: CeraTM Occluders. LifeTech Scientific Co. http://www.lifetechmed.com/en/product/p1/cera/282.aspx
- 23.Hong Z.N., Chen Q., Huang L.Q., et al. A meta-analysis of perventricular device closure of perimembranous ventricular septal defect. J Cardiothorac Surg. 2019;14(1):119. doi: 10.1186/s13019-019-0936-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu L., Tanidir I.C., Ye D., et al. Hybrid transthoracic periventricular device closure of ventricular septal defects: single-center experience. Brazilian J Cardiovasc Surg. 2021;36(1):48–56. doi: 10.21470/1678-9741-2020-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adilbekova A., Marassulov S., Nurkeev B., et al. Mortality rates of ventricular septal defect for children in Kazakhstan: spatio temporal epidemiological appraisal. Congenital Heart Dis. 2023;18(4):447–459. [Google Scholar]
- 26.Li D., Zhang Z., Li M. Comparisons of periventricular device closure, conventional surgical repair, and transcatheter device closure in patients with congenital ventricular septal defects: a protocol for systematic review. Medicine (Baltimore) 2020;99(4) doi: 10.1097/MD.0000000000018901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y., Zhu P., Zhou P., et al. Intra-operative device closure of perimembranous ventricular septal defect without cardiopulmonary bypass under guidance of trans-epicardial echocardiography: a single center experience. J Cardiothorac Surg. 2016;11(1):87. doi: 10.1186/s13019-016-0483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren C., Wu C., Pan Z., et al. Minimally invasive closure of transthoracic ventricular septal defect: postoperative complications and risk factors. J Cardiothorac Surg. 2021;16(1):30. doi: 10.1186/s13019-021-01415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang-Shan H., Kai-Peng S., Ning X., et al. Transthoracic closure of ventricular septal defects guided by transesophageal echocardiography. Turkish J Thorac Cardiovasc Surg. 2020;28(2):250–256. doi: 10.5606/tgkdc.dergisi.2020.18745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang G.H., Chen Q., Hong Z.N., et al. The comparison of perventricular device closure with transcatheter device closure and the surgical repair via median sternotomy for perimembranous ventricular septal defect. Ann Thorac Cardiovasc Surg. 2018;24(6):308–314. doi: 10.5761/atcs.oa.18-00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ou-Yang W. Bin, Wang S.Z., Hu S.S., et al. Perventricular device closure of perimembranous ventricular septal defect: effectiveness of symmetric and asymmetric occluders. Eur J Cardiothoracic Surg. 2017;51(3):478–482. doi: 10.1093/ejcts/ezw352. [DOI] [PubMed] [Google Scholar]
- 32.Liu H., Lu F.X., Zhou J., et al. Minimally invasive perventricular versus open surgical ventricular septal defect closure in infants and children: a randomised clinical trial. Heart. 2018;104(24):2035–2043. doi: 10.1136/heartjnl-2017-312793. [DOI] [PubMed] [Google Scholar]
- 33.Huang J.S., Sun K.P., Huang S.T., et al. A meta-analysis of perventricular device closure of doubly committed subarterial ventricular septal defects. J Cardiothorac Surg. 2020;15(1):28. doi: 10.1186/s13019-020-1062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J.S., Huang S.T., Sun K.P., et al. Health-related quality of life in children and adolescents undergoing intraoperative device closure of isolated perimembranous ventricular septal defects in southeastern China. J Cardiothorac Surg. 2019;14(1):218. doi: 10.1186/s13019-019-1040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Q., Hong Z.N., Zhang G.C., et al. Intraoperative device closure of isolated ventricular septal defects: experience on 1,090 cases. Ann Thorac Surg. 2018;105(6):1797–1802. doi: 10.1016/j.athoracsur.2018.02.059. [DOI] [PubMed] [Google Scholar]
- 36.Zhang G.C., Chen Q., Cao H., et al. Minimally invasive perventricular device closure of ventricular septal defect in infants under transthoracic echocardiograhic guidance: feasibility and comparison with transesophageal echocardiography. Cardiovasc Ultrasound. 2013;11(1):8. doi: 10.1186/1476-7120-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang S.L., Tometzki A., Caputo M., Morgan G., Parry A., Martin R. Longer-term outcome of perventricular device closure of muscular ventricular septal defects in children. Catheter Cardiovasc Interv. 2015;85(6):998–1005. doi: 10.1002/ccd.25821. [DOI] [PubMed] [Google Scholar]
- 38.Huang X.S., Luo Z.R., Chen Q., et al. A comparative study of perventricular and percutaneous device closure treatments for isolated ventricular septal defect: a Chinese single-institution experience. Brazilian J Cardiovasc Surg. 2019;34(3):344–351. doi: 10.21470/1678-9741-2018-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The hybrid ventricular septal defect closure off-pump. Video available at: https://www.jtcvs.org/article/S2666-2507(24)00042-7/fulltext.