SUMMARY
BACKGROUND:
TB is the leading cause of mortality among people living with HIV (PLHIV), for whom isoniazid preventive therapy (IPT) has a proven mortality benefit. Despite WHO recommendations, countries have been slow in scaling up IPT. This study describes processes, challenges, solutions, outcomes and lessons learned during IPT scale-up in Kenya.
METHODS:
We conducted a desk review and analyzed aggregated Ministry of Health (MOH) IPT enrollment data from 2014 to 2018 to determine trends and impact of program activities. We further analyzed IPT completion reports for patients initiated from 2015 to 2017 in 745 MOH sites in Nairobi, Central, Eastern and Western Kenya.
RESULTS:
IPT was scaled up 75-fold from 2014 to 2018: the number of PLHIV covered increased from 9,981 to 749,890. The highest percentage increases in the cumulative number of PLHIV on IPT were seen in the quarters following IPT pilot projects in 2014 (49%), national launch in 2015 (54%), and HIV treatment acceleration in 2016 (158%). Among 250,069 patients initiating IPT from 2015 to 2017, 97.5% completed treatment, 0.2% died, 0.8% were lost to follow-up, 1.0% were not evaluated, and 0.6% discontinued treatment.
CONCLUSIONS:
IPT can be scaled up rapidly and effectively among PLHIV. Deliberate MOH efforts, strong leadership, service delivery integration, continuous mentorship, stakeholder involvement, and accountability are critical to program success.
Keywords: tuberculosis, prevention, HIV-positive
RÉSUMÉ
CADRE:
La TB est la première cause de mortalité parmi les personnes vivant avec le VIH (PLHIV), alors que le traitement préventif par isoniazide (IPT) a un benefice prouvé en termes de mortalité. En dépit des recommandations de l’OMS, les pays ont été lents à accélérer l’IPT. Cette étude décrit les processus, les défis, les solutions, les résultats et les leçons apprises lors de l’accélération de l’IPT au Kenya.
MÉTHODES:
Nous avons réalisé une revue de dossiers et analysé les données compilées d’enrôlement à l’IPT du ministère de la santé (MOH) de 2014 è 2018 pour déterminer les tendances et l’impact des activités du programme. Nous avons ensuite analysé les rapports d’achèvement de l’IPT pour les patients mis sous traitement entre 2015 et 2017 dans 745 sites du MOH à Nairobi, ainsi que dans les régions du Centre, de l’Est et de l’Ouest du Kenya.
RÉSULTATS:
Entre 2014 et 2018, l’IPT a été multiplié par 75 passant de 9 981 à 749 890 PLHIV. Les augmentations les plus élevées en nombre cumulés ont été vues au cours des trimestres suivant des projets pilotes en 2014 (49%), du lancement national en 2015 (54%) et lors de l’accélération du traitement du VIH en 2016 (158%). Parmi les 250 069 patients initiant l’IPT entre 2015 et 2017, 97,5% ont achevé le traitement, 0,2% sont décédés, 0,8% ont été perdus de vue, 1,0% n’ont pas été évalués et 0,6% ont arrêté je traitement.
CONCLUSION:
L’IPT peut être accéléré rapidement et efficacement parmi les PLHIV. Les efforts délibérés du MOH, un leadership fort, une intégration de la prestation de services, un tutorat continu, l’implication des parties prenantes et la reddition de comptes sont cruciaux pour le succès du programme.
TB remains the leading cause of death from a single infectious agent globally, including among people living with HIV (PLHIV).1 TB control efforts to date have focused on finding and treating active TB; however, these have had only modest impact on reducing the global TB burden. At the current 2% annual decline in TB incidence, we are not on track to meet 2035 global End TB targets. Achieving the necessary rate of TB incidence decline requires additional strengthening of TB prevention through treatment of latent TB infection.2
HIV infection is associated with a 30–50% lifetime risk of active TB disease.3 While antiretroviral therapy (ART) reduces the risk of TB among PLHIV, their annual risk of TB remains higher than for those without HIV, regardless of immunosuppression.4,5 Isoniazid preventive therapy (IPT), a form of TB preventive therapy (TPT), reduces 5-year mortality by 37% independent of ART.6 The WHO has thus recommended a 6–9-month course of IPT for PLHIV since 2003.7 Despite demonstrated benefits and long-standing policy guidance, countries worldwide have been slow in implementing IPT. In 2018, less than two million PLHIV newly enrolled in care had received TPT, compared to an estimated 21.7 million on ART.1,8
In 2018, Kenya had an estimated 1.5 million PLHIV, with 1.2 million on ART.8 Annually, an estimated 40,000 PLHIV develop TB and 13,000 of these die.1 To curb the TB-HIV epidemic, Kenya has been scaling up IPT among PLHIV on ART over the past decade. As countries prepare for massive TPT scale-up in line with global End TB goals, lessons from Kenya’s experience may aide in fast-tracking and optimizing their efforts. We describe the processes, challenges, outcomes and lessons learned in the rollout and scale-up of IPT in Kenya.
METHODS
We conducted a desk review of the Kenya Ministry of Health (MOH) guidelines, standard operating procedures (SOPs), registers and technical working group (TWG) meeting notes and examined MOH, Global Fund and U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) work plans to understand the scale-up of IPT in Kenya. We describe the process of IPT implementation, including evolution of policy, data collection and flow, MOH leadership, program coordination, stakeholder engagement, supply chain management, service delivery, pharmacovigilance, and monitoring and evaluation (M&E), and identified barriers to and facilitators of program implementation.
Using MS Excel 2016 (Microsoft, Redmond, WA, USA), we analyzed aggregated IPT enrollment data for all ages in the MOH District Health Information System (DHIS2) from January 2014 to December 2018 to determine the quarterly increase in enrollment of PLHIV on IPT over time. Quarterly percentage increase was calculated using cumulative IPT uptake in previous periods as the denominator to determine trends and their relationship with program events. Quarterly implementing-partner reports from 735 sites submitted to the U.S. Centers for Disease Control and Prevention (CDC), Nairobi, Kenya, were analyzed to determine IPT completion for patients enrolled from January 2015 to December 2017 in Nairobi, Central, Eastern and Western Kenya regions. Treatment outcomes for patients who initiated 12 months earlier were defined as treatment completed (6 months’ IPT), died, lost to follow-up, discontinued treatment, or not evaluated.
Ethical clearance
This project was cleared by Kenyatta National Hospital Institutional Review Board (Nairobi, Kenya), reviewed in accordance with CDC human research protection procedures and was determined to be research, but CDC investigators did not interact with human subjects or have access to identifiable data.
RESULTS
Processes
Policy evolution
Between 2009 and 2013, with engagement of civil societies, the 2009, 2012, and 2013 guidelines were revised to remove restrictions on IPT use and were progressively aligned with global TPT guidelines to facilitate access for eligible populations, including PLHIV.
Policy implementation
To translate policy into program, the National AIDS and STI Control Program (NASCOP) and National TB Program (NTP) needed assurance that the time was right for IPT scale-up. This required strengthening TB screening as part of routine HIV care, which was initiated in 2009 with development and operationalization of a TB symptom screening tool. This was later aligned to the 2011 WHO-recommended 4-symptom TB screening questionnaire.7 In addition, an integrated intensified case finding (ICF)/IPT tool and IPT register were developed.
Availability of supportive policies, recording tools and subsequent standardization of TB screening among PLHIV paved the way for IPT pilot projects from 2012 to 2014. Documentation of high (92%) IPT completion, and low (1%) loss to follow-up and adverse drug reactions (ADRs) in these projects demonstrated feasibility for national scale-up.9 IPT drugs were integrated into the existing national TB drug register and consumption reporting tools, SOPs developed to guide implementation, and program and facility level staff trained for IPT national scale-up.
Concerns about emerging isoniazid (INH) resistance among healthcare workers were initially a threat to IPT scale-up. Introduction of the Xpert® MTB-Rif assay (Cepheid, Sunnyvale, CA, USA) in 2011 improved healthcare workers’ confidence in diagnosing TB among PLHIV, allaying anxiety over potential INH monotherapy, although there remains ongoing need for evidence sharing, TPT championship, and engagement with healthcare workers to ensure future misperceptions are addressed. Selection of sites that were likely to succeed during initial IPT scale-up was also critical for demonstrating positive outcomes to later adopters.
Leadership and coordination
Strong leadership within MOH was critical in ensuring that national HIV and TB programs deliberately aligned priorities, harmonized guidelines and jointly planned, implemented and monitored IPT scale-up. Commitment by the highest level of MOH leadership was pivotal in providing a much-needed catalyst. IPT scale-up was built into the 2015–2018 National TB Strategic Plan, and stakeholders were engaged to identify and address program and resource gaps. A TB-HIV TWG with membership from MOH, and civil societies, and implementing and development partners was established and tasked with national coordination, and development of SOPs and training materials. IPT tools, including registers, ICF/IPT cards and SOPs were printed centrally and distributed.
National IPT scale-up was officially launched on World TB Day 2015. Ambitious targets were jointly set by NTP and NASCOP and included in performance contracts for senior staff. Targets were cascaded to counties and health facilities, with site-level performance reporting. Implementing-partners supported training and mentorship of facility-level staff and provided required tools. In March 2016, NASCOP launched an HIV treatment acceleration initiative that included county targets to place 50% of PLHIV in care on IPT within 100 days. Implementing-partners whose sites had already achieved this target were encouraged to work towards achieving 90% coverage. Progress was tracked by the Director of Medical Services, MOH.
Stakeholder engagement
Multiple stakeholders were engaged from design through implementation of IPT scale-up. The WHO, PEPFAR and other partners provided technical assistance and support for policy development, program planning, commodity procurement, and program monitoring. PEPFAR supported healthcare workers training, identification of TPT champions, continuous mentorship, and commodity distribution and consumption reporting. The 2015 PEPFAR requirement for IPT reporting and intensified site monitoring prompted implementing partners to prioritize IPT scale-up.
Civil societies provided patient perspective in program design and implementation. Beginning 2009, civil societies engaged NTP and NASCOP to demand policy change to facilitate access to IPT. The participation of civil societies in guidelines development promoted buy-in and demand by PLHIV communities, and was instrumental in allaying community-level anxiety about IPT-related ADRs.
Supply chain management
The need for INH and pyridoxine was met by the MOH, the Global Fund and PEPFAR through annual joint TB and HIV commodity forecasting and quantification, and procurement initiated centrally by the NTP, regardless of funding source. Commodities were initially distributed by NTP, but existing NASCOP mechanisms for ART distribution were adopted in 2015 to facilitate mass distribution to HIV clinics. An initial supply was provided to health facilities from the national store based on ambitious scale-up targets. Subsequent monthly orders were placed by sites based on consumption, with county governments engaged to anticipate demand and ensure adequate buffer stocks. Monthly TWG meetings were held to review stock status, address challenges in commodity distribution, and plan for subsequent procurement.
Despite adequate funding, a shortage of INH occurred in 2016 due to higher-than-anticipated demand, delay in procurement, and a global shortage of raw materials. This was mitigated by redistribution from sites with excess supply, procurement of buffer stocks by implementing partners and the MOH, and PEPFAR intervention at the global level requesting fast-tracking of Kenya’s drug orders. Although site-level enrollment slowed, adequate supplies had been secured for patients already initiated on IPT to complete their full courses.
Service delivery
Rather than establishing a stand-alone program, IPT was integrated into the standard package of clinical HIV care and reporting systems, and providing patient-centered HIV care. At sites, patient files were systematically reviewed and those without documentation of prior IPT or other key HIV services were color-tagged for ease of identification and action. Patients without prior IPT were contacted and evaluated for IPT initiation. Among these, ≈90% had good (≥95%) ART adherence, were without visible jaundice, rash, neuropathy or excessive alcohol use, and were initiated on a 6-month course of IPT, with a follow-up visit scheduled within a month. Subsequent monthly appointments were harmonized with ART clinic/drug pickup as a norm during IPT (Figure 1). Missed appointments were addressed using ART guidelines.
Figure 1.
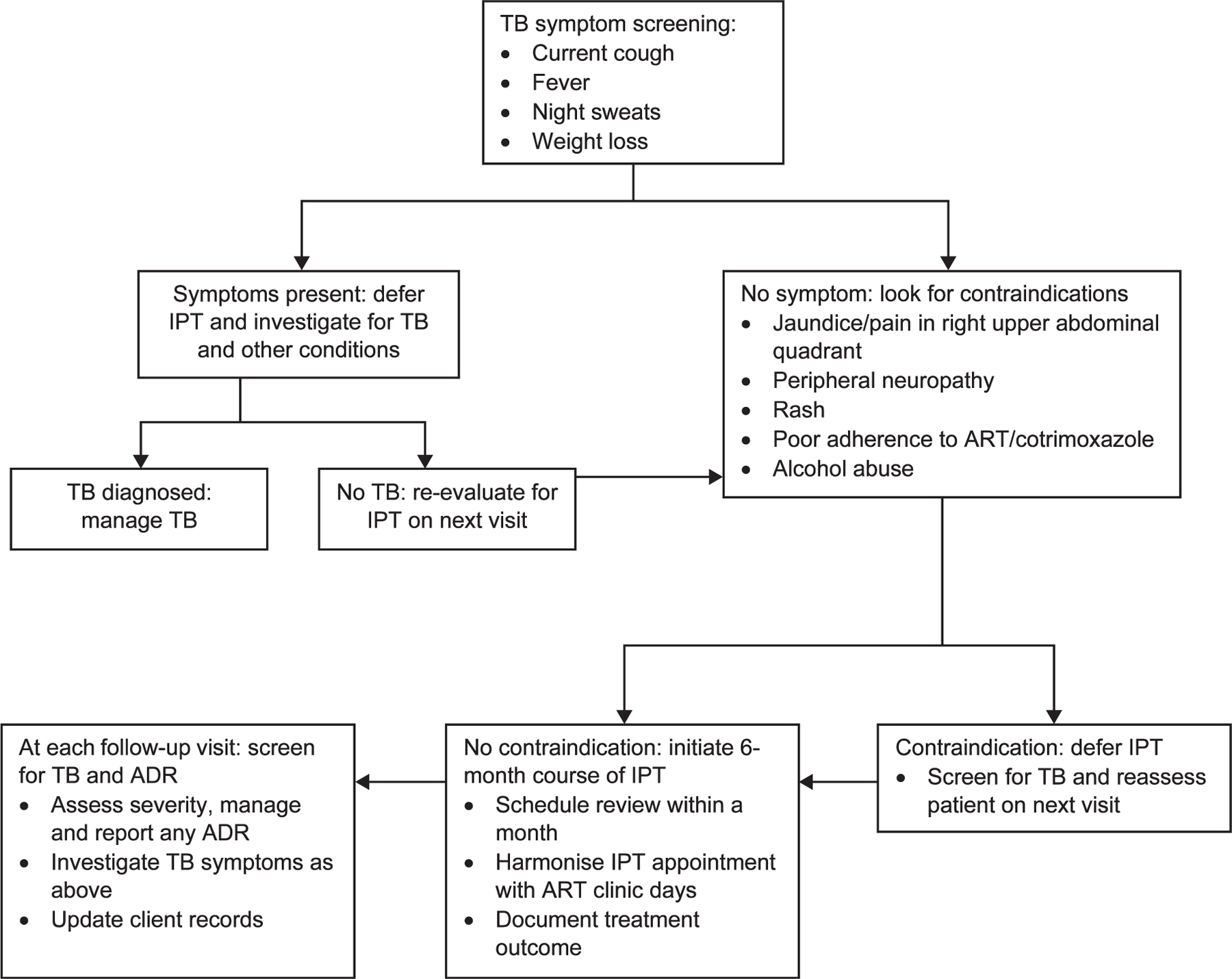
Algorithm for clinical processes for IPT initiation and monitoring among adults. ART = antiretroviral therapy; ADR = adverse drug reactions; IPT = isoniazid preventive therapy.
Pharmacovigilance
Patients were educated on potential side effects of IPT, and what to do if these occurred. At each visit, patients were assessed for active TB, adherence and ADRs. Patients with TB symptoms were tested using Xpert, and those with suspected hepatitis subjected to liver enzyme testing per national guidelines. ADRs were reported to the Pharmacy and Poisons Board (Nairobi, Kenya) using already existing systems. ADRs trends were routinely analyzed and assessed for accuracy based on historical trends.
The rapid IPT scale-up and training of patients and healthcare workers to recognize and report ADRs was associated with an increase in reporting of IPT-related ADRs. This observation was jointly investigated by the Pharmacy and Poisons Board and HIV-TB programs to determine the cause. Subsequently, the MOH communicated to health facilities explaining and reinforcing positive messaging on benefits of IPT to enhance enrollment.
Monitoring and evaluation
IPT was rapidly rolled out following the national launch in 2015. However, despite availability of facility-level recording and reporting tools, data were not initially reported to the national system due to a lack of data aggregation tools. This was, in part, due to the need for consensus between NASCOP and NTP on which indicators to report nationally, and the balance between need for details and burden of reporting. An interim reporting tool was developed in 2015 by the MOH and used to summarize facility-level IPT data in DHIS2. By 2017, both IPT enrollment and completion indicators were captured in standard MOH monthly reporting tools.
Throughout the 100-day IPT scale-up in 2016, county weekly reports were submitted to NASCOP, summarized and shared with the Director of Medical Services. Performance was tracked using a quarterly IPT reporting tool that captured cumulative IPT coverage for PLHIV currently in care, quarterly increase in uptake, and treatment outcomes for patient cohorts initiated 12 months earlier. These indicators were reported during quarterly PEPFAR partner review meetings to guide continuous program improvement. In addition, quarterly MOH, implementing and development partners meetings were held to discuss progress and challenges, providing solutions that informed IPT program implementation and data flow.
OUTCOMES
Overall, IPT initiation among 1,050,000 PLHIV reported in DHIS2 nationally increased 75-fold from 9981 to 749,890 between December 2014–2018 (Figure 2). By December 2018, an estimated 85% of PLHIV in care had received IPT. An initial 49% increase in initiation during the first quarter (Q1) of 2014 coincided with pilot projects. This declined to 6% in Q4 but rose to 54% in Q1 after the national launch, and then progressively increased to peak at 158% in Q1 2016, coinciding with the national HIV treatment acceleration initiative. Quarterly IPT initiation subsequently declined as Kenya focused on initiating IPT only for the PLHIV newly enrolled in care, as the majority of current ART clients had already completed a course (Figure 3).
Figure 2.
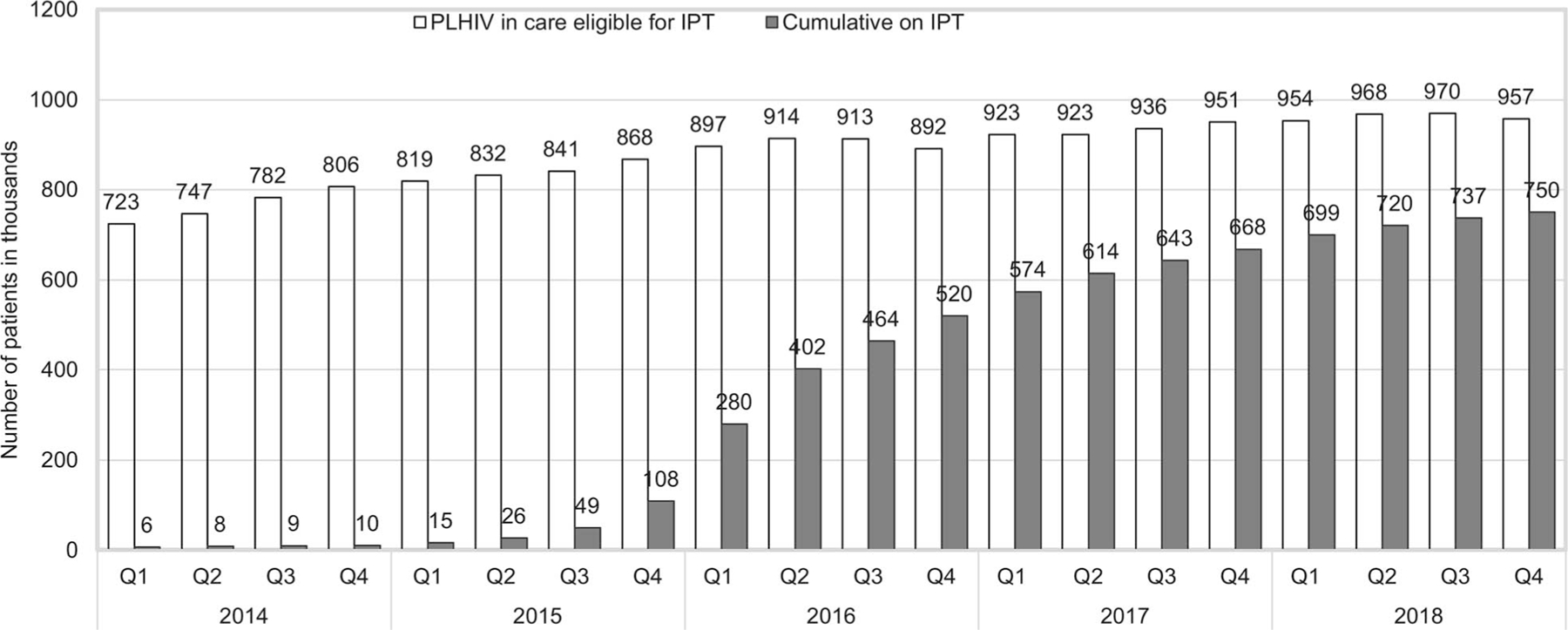
Trends in cumulative number of eligible PLHIV on IPT, Kenya, 2014–2018. PLHIV = people living with HIV; IPT = isoniazid preventive therapy; Q = quarterly.
Figure 3.
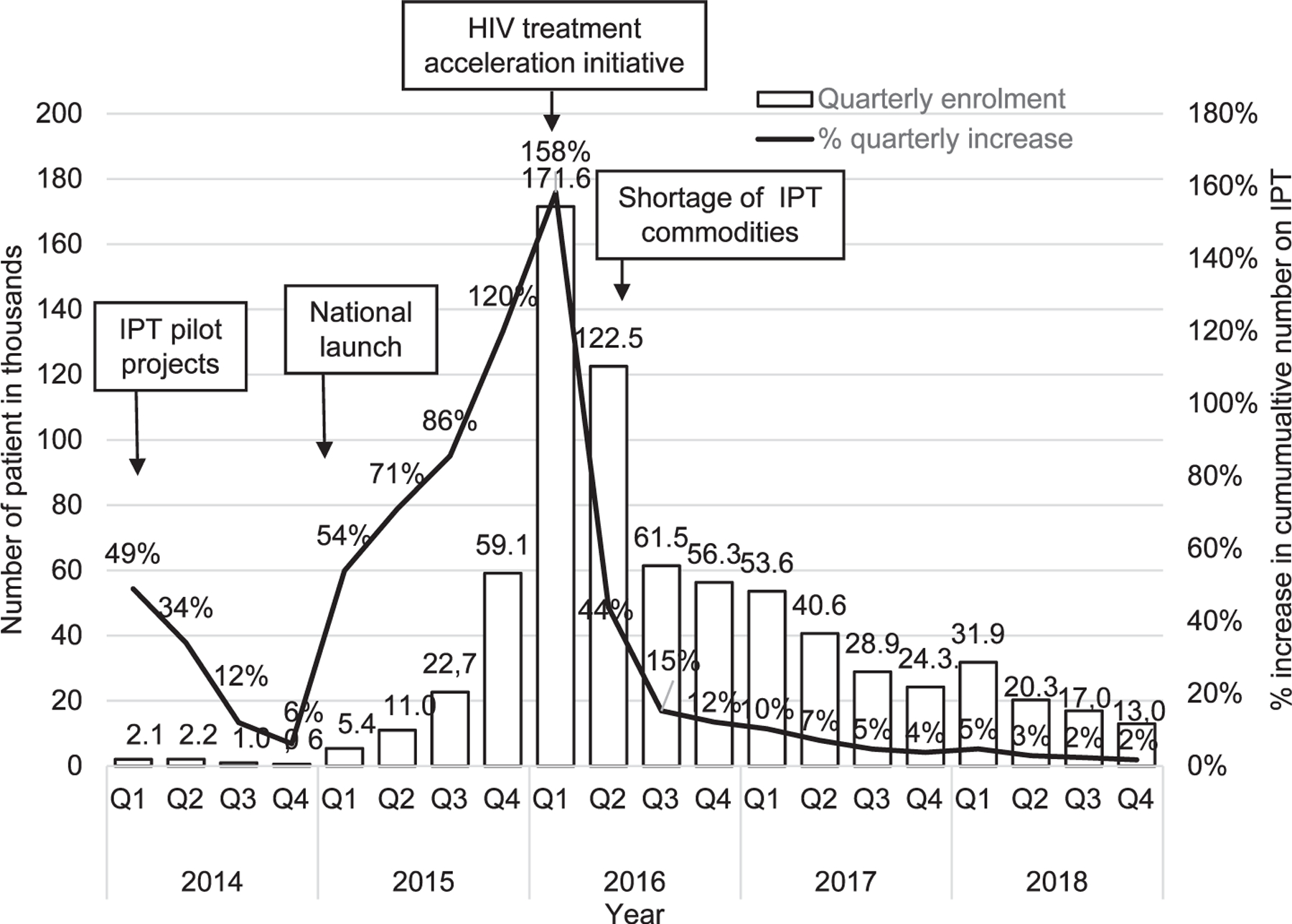
Enrolment and quarterly increase in cumulative number of PLHIV on IPT Kenya, 2014– 2018. IPT = isoniazid preventive therapy; PLHIV = people living with HIV; Q = quarterly.
Among 250,069 patients initiated on IPT between 2015 and 2017 by the MOH, CDC-supported sites in Nairobi, Central, Eastern and Western Kenya regions, 243,722 (97.5%) completed treatment, 420 (0.2%) died, 1954 (0.8%) were lost to follow-up, 2406 (1.0%) were not evaluated and 1567 (0.6%) were discontinued. Among those who discontinued, poor adherence accounted for 1076 (68.7%), while active TB and ADRs accounted for 223 (14.2%) and 268 (17.1%), respectively (Table). There were no regional differences in outcomes. Drug resistance data were unavailable for the persons who developed active TB.
Table.
TPT treatment outcomes 12 months after initiation at CDC-supported MOH sites in Nairobi, Central Eastern and Western Kenya, 2015–2017 (n =250,069)
| Outcome | n | % |
|---|---|---|
| Treatment completed | 243,722 | 97.5 |
| Dead | 420 | 0.2 |
| Lost to follow-up | 1954 | 0.8 |
| Not evaluated | 2406 | 1.0 |
| Discontinued (n = 1567) | 0.6 | |
| Poor adherence | 1076 | 0.4 |
| Diagnosed with TB | 223 | 0.1 |
| Adverse drug reactions | 268 | 0.1 |
| Total | 250,069 | 100 |
TPT = TB preventive therapy; CDC = Centers of Disease Control and Prevention; MOH = Ministry of Health.
DISCUSSION
Kenya made substantial progress in scaling up TPT among PLHIV.10 While challenges were encountered initially, IPT was scaled up rapidly with high treatment completion rates. Elements critical to success included a favorable policy environment at the global and national level, high-level MOH commitment, ambitious target setting, strong collaboration and joint accountability between NTP and NASCOP, close involvement of partners in programing, capacity building and service delivery design, and engagement of civil societies to create and sustain IPT demand.
Dramatic increases in the number and rate of IPT enrollment following initiatives including pilot projects, national launch and HIV treatment acceleration, demonstrated feasibility of rapid national scale-up. While policies and guidelines are available in many countries, attention must be paid to every step of their operationalization. Among other factors, countries should carefully consider scale-up targets and global availability of drugs to effectively plan for commodity security, consider integrating IPT within existing HIV and TB program structures for sustainability, and include initiatives that rapidly increase TPT uptake in program design. Such initiatives should preferably be led by high-ranking health officials with the capacity to engage all stakeholders, mobilize resources and ensure accountability at all levels. We recommend that strategies for communication, data flow, M&E, pharmacovigilance and ADRs monitoring be built into overall program planning.
Despite Kenya’s gains, TPT outcomes need improvement by addressing data quality, adherence to treatment and ADRs. The high IPT completion rates could be explained by an initial focus on initiating IPT among PLHIV with good adherence to ART, a group that was likely to complete IPT and may differ from patients newly enrolling on ART. In addition, deliberate efforts were made to educate patients on the benefits of IPT, make a 6-month supply available for individual patients, and enhanced patient support to ensure they complete the full course of treatment. All-form TB notifications increased from 89,276 in 2014 to 96,562 in 2018, but HIV-TB declined from 30,164 to 25,535. These trends should be interpreted in the context of improved TB case-finding, molecular testing and ART coverage.
Our analysis is subject to some limitations. National reporting was initiated several months after IPT rollout and may be an underestimate of the actual initial IPT coverage. Although a remarkable 97.5% of PLHIV completed IPT, this may have been initially explained by focus on PLHIV with good ART adherence, a group which may differ from new ART enrollees. However, deliberate efforts to educate patients on IPT benefits, availability of a 6-month supply, and enhanced patient support were likely contributing factors. Reported ADRs was limited to those discontinuing treatment and might have been underestimated. Outcomes analyzed for 250,069 PLHIV may differ from the rest of the population. Finally, IPT was scaled up before widespread differentiated HIV service delivery models using multi-month ART dispensing. Therefore, we are unable to comment on optimal IPT delivery in such settings.
Nevertheless, we have demonstrated that IPT can be dramatically and effectively scaled up nationwide. As other country programs seek to treat all eligible ART clients with potentially life-saving IPT/TPT, important lessons learned from the Kenya experience can help inform their program design and rapid response to encountered challenges.
Acknowledgements
This publication has been supported (in part) by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Centers for Disease Control and Prevention (CDC) under the terms of NU2GGH001949, NU2GGH001965, NU2GGH001961 and PS001917–05S2 and GH 13–1307.
Footnotes
Disclaimer: The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the funding agencies.
Conflict of interests: none declared.
References
- 1.World Health Organization.Global tuberculosis report, 2019 Geneva, Switzerland: WHO, 2019. [Google Scholar]
- 2.Dye C, et al. Prospects for tuberculosis elimination. Annu Rev Public Health 2013; 34: 271–286. [DOI] [PubMed] [Google Scholar]
- 3.De Cock K. The new tuberculosis. Africa Health 1994; 16(3): 8. [PubMed] [Google Scholar]
- 4.Dye C, Williams BG. Antiretroviral therapy is the principal cause of tuberculosis decline in southern and eastern Africa. BioRxiv 2018; 10.1101/477158. [DOI] [Google Scholar]
- 5.Harries AD, et al. The HIV-associated tuberculosis epidemic — when will we act? Lancet 2010; 375(9729): 1906–1919. [DOI] [PubMed] [Google Scholar]
- 6.Badje A, et al. Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: long-term follow-up of the Temprano ANRS 12136 trial. Lancet Glob Health 2017; 5(11): e1080–e1089. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventative therapy for people living with HIV in resource-constrained settings Geneva, Switzerland: WHO, 2011.https://apps.who.int/iris/handle/10665/44472. [Google Scholar]
- 8.UNAIDS. UNAIDS Data, 2019 Geneva, Switzerland: UNAIDS, 2019. http://www.unaids.org/en/resources/documents/2018/unaids-data-2018. [Google Scholar]
- 9.Masini E,. Sitienei J, Weyenga H. Outcomes of isoniazid prophylaxis among HIV-infected children attending routine HIV care in Kenya. Public Health Action 2013; 3(3): 204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melgar M. Tuberculosis preventive treatment scale-up among antiretroviral therapy patients — 16 countries supported by the US President’s Emergency Plan for AIDS Relief, 2017–2019. MMWR Morb Mortal Wkly Rep 2020; 69(12): 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]


