Abstract
Objectives
For lung segmentectomy of small lung cancers, we used a microwave surgical instrument for lung parenchymal dissection mainly at the pulmonary hilum, which is difficult to handle with surgical staplers. This technique facilitated the insertion of staples.
Methods
In total, 116 patients with cStage 0-1A3 non–small cell lung cancer who underwent lung segmentectomy were included in this study. We compared the perioperative factors of the group in which a microwave surgical instrument was used for dissection procedures, including lung parenchymal dissection at the pulmonary hilum, and peripheral intersegmental dissection was performed with surgical staplers (group M+S: N = 69), with those of the group in which parenchymal dissection was performed mainly with surgical staplers without using the microwave surgical instrument (group S: N = 47).
Results
Although more complex segmentectomies were performed in the M+S group (P = .001), the number of staple cartridges (7 staple cartridges vs 8 staple cartridges, P = .005), the surgical times (179 vs 221 minutes, P < .0001), and the blood loss (5 mL vs 30 mL, P = .012) were significantly lower in the M+S group. The duration of chest tube placement was significantly shorter in the M+S group (P = .019), and postoperative complications of grade 2 or greater were significantly lower in the M+S group (P = .049).
Conclusions
The effective use of the microwave surgical instrument combined with surgical staplers can simplify pulmonary hilar and intersegmental plane dissections not only for simple segmentectomy but also for complex segmentectomy, leading to favorable intraoperative and postoperative outcomes.
Key Words: lung segmentectomy, microwave surgical instrument, non–small cell lung cancer, surgical stapler
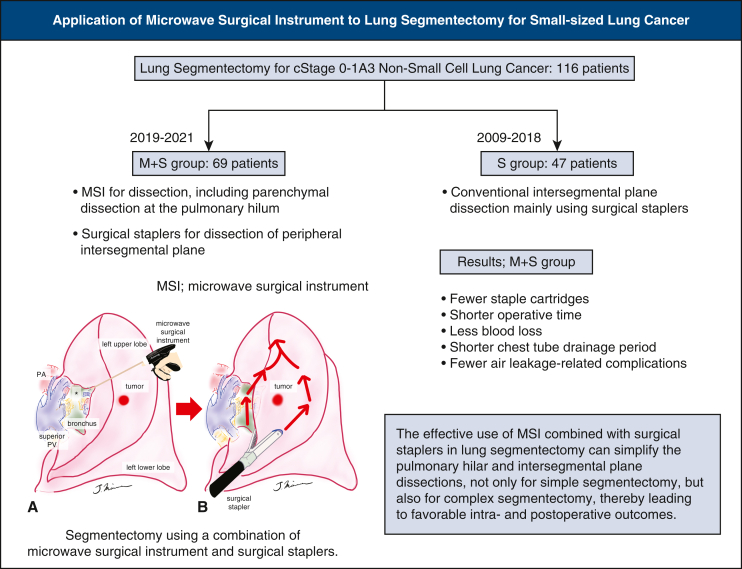
Graphical abstract
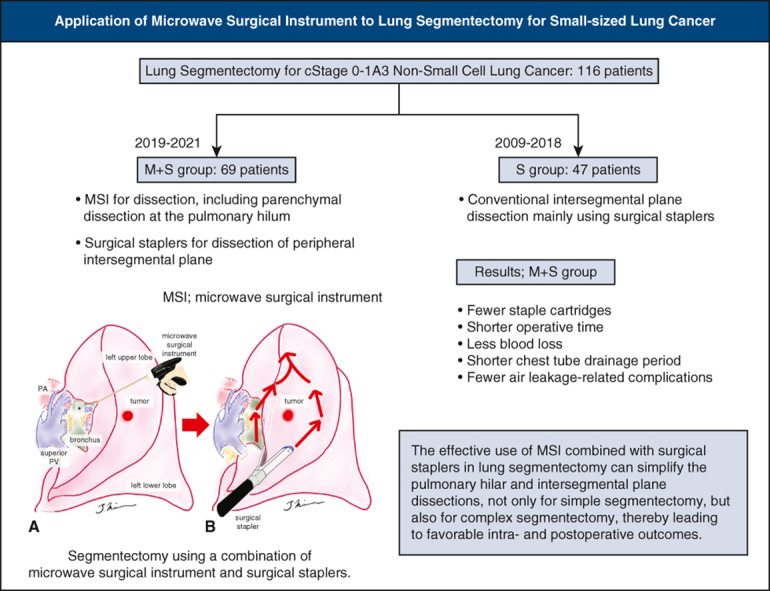
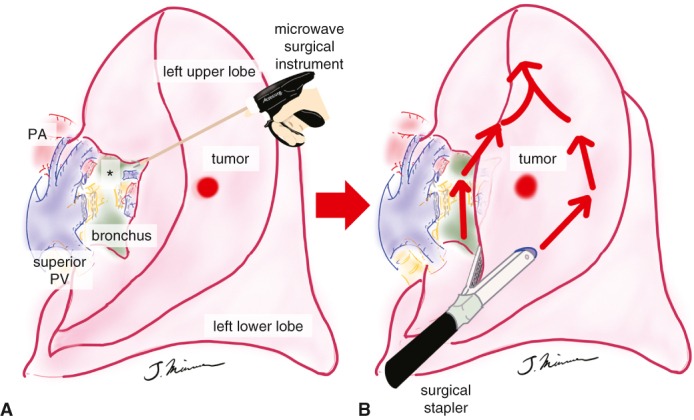
Segmentectomy using a combination of an MSI and staplers.
Central Message.
The combined use of an MSI and surgical staplers simplifies pulmonary hilar and intersegmental plane dissection, even in complex lung segmentectomy.
Perspective.
The effective use of an MSI combined with surgical staplers in lung segmentectomy can simplify pulmonary hilar and intersegmental plane dissection for simple segmentectomy and complex segmentectomy, leading to favorable intraoperative and postoperative outcomes.
Based on the results of JCOG0802/WJOG4607L, JCOG1211, and CALGB140503, which are prospective studies on the efficacy of segmentectomy for small-sized lung cancers, lung segmentectomy is expected to be increasingly applied in the near future.1, 2, 3 However, although simple lung segmentectomy may be less complicated than lobectomy, complex segmentectomy procedures that cannot be performed with only surgical staplers for fissure creation are generally more technically demanding for thoracic surgeons, with concerns about increased operative time, air leaks that must be managed intraoperatively, and increased complications.4
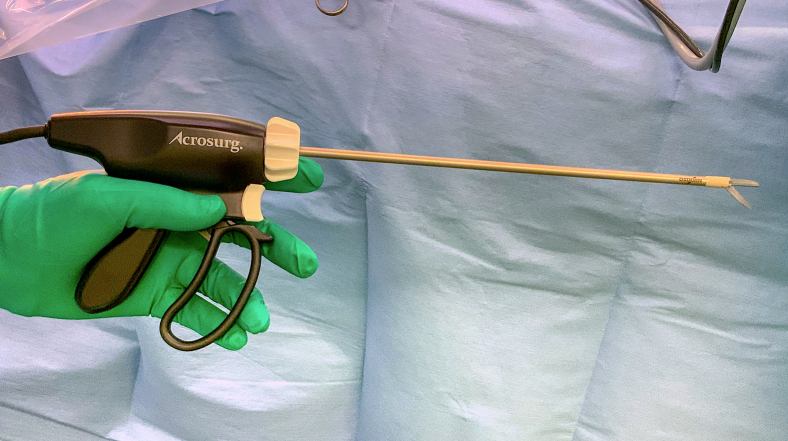
We clinically applied a microwave surgical instrument (MSI; Acrosurg, Nikkiso Co, Ltd) and found that the MSI is useful, especially in lung parenchymal dissection.5, 6, 7 The microwaves generated between the cutting edges of the instrument heat the tissue by causing water molecules in the tissue to vibrate 2.45 billion times per second, thus forming a uniform coagulation layer. The MSI facilitates a series of processes, such as tissue dissection, coagulation, hemostasis, and vascular sealing, by freely advancing the ceramic cutting edge. Considering that using surgical staplers for intersegmental plane dissection is common worldwide in lung segmentectomies,8,9 we used the MSI for lung parenchymal dissection, mainly at the pulmonary hilum, which is difficult to handle with surgical staplers, thereby facilitating the insertion of surgical staplers for the intersegmental plane dissection. In this study, we retrospectively compared lung segmentectomy for small non–small cell lung cancer (NSCLC) using the MSI and surgical staplers with the conventional technique performed mainly with staplers.
Material and Methods
Patients
This retrospective study was approved by the Institutional Review Board of National Hospital Organization Kure Medical Center and Chugoku Cancer Center (Registration Number: 2021-66; January 18, 2022), and the requirement for informed consent was waived. This study included patients with NSCLC with clinical stages 0 to 1A3, from January 2019 to December 2022, in whom the pulmonary hilum was treated mainly with the MSI, and peripheral intersegmental plane dissection was performed with surgical staplers (M+S group). We compared these patients with those who underwent lung parenchymal dissection, mainly using surgical staplers and without the MSI, from April 2009 to December 2018 (S group). The patients who underwent almost all intersegmental plane dissections by electrocautery with or without surgical staplers, those in a prospective validation study who underwent all intersegmental plane dissections with the MSI,5 those who had complete adhesions in the thoracic cavity, and those who underwent 2 segmentectomies were excluded. We categorized segmentectomy into 3 types: patients with peripheral small-sized NSCLC with a consolidation/tumor ratio of 0.25 or more (intentional segmentectomy), elderly patients or those with insufficient pulmonary reserve (compromised segmentectomy), and noninvasive ground-glass opacity nodules with a consolidation/tumor ratio of 0.25 or less, which is deeper than the pleural surface.
Preoperative Examination
Chest computed tomography (CT), whole-body positron emission tomography with [18F]-fluoro-2-deoxy-D-glucose, brain magnetic resonance imaging, and pulmonary function tests were performed preoperatively to determine the clinical stage and treatment strategies. The clinical stage was determined on the basis of the 8th edition of the TNM classification of malignant tumors.10 For lung segmentectomy, 3-dimensional chest CT scanning using the SYNAPSE VINCENT imaging software program (Fujifilm Medical) was performed to identify anatomic variations. The surgical method for lung segmentectomy was categorized as simple or complex. Simple segmentectomy was defined as resection of the right or left superior segment of the lower lobe (S6), right basal segment (S7+S8+S9+S10), left upper division segment (S1+2+S3), left lingular segment (S4+S5), and left basal segment (S8+S9+S10), whereas complex segmentectomy was defined as resection of segments other than those resected in simple segmentectomy.4
Surgical Procedure
The surgical approach was performed completely thoracoscopically with a 4-port access or the hybrid approach with minithoracotomy. In both groups, bipolar scissors (POWERSTAR 280 mm, Ethicon Endo-Surgery) were used for dissection to expose the structures at the pulmonary hilum. The vessels were dissected using a surgical stapler or ligation, and the bronchi were secured and dissected. In the S group, the line of intersegmental plane was identified by the method described below, and the scheduled segment was dissected with surgical staplers. In the M+S group, the lung parenchyma between the segments, which often follows the pulmonary vein, was dissected approximately 2 to 3 cm with the MSI (Acrosurg Revo; Nikkiso Co, Ltd) (Figure 1). In patients with moderate or severe emphysema, sealing may be inadequate because of the lack of cells and collagen to coagulate the tissue. Therefore, the blade of the MSI should be run more slowly than usual to firmly seal the lung parenchymal dissection surface (Figure 2, A-D, and Video 1). In addition, the lung parenchyma located posterior to the bronchial stump was firmly dissected with the MSI, so that the segment to be resected was completely floating, which is called the “eave” formation (Figure 3, A). This technique allowed easy insertion of the cartridge and anvil of the surgical stapler. Afterward, the line of the intersegmental plane was established by the inflation-deflation line with the jet ventilation or by the administration of indocyanine green. If detection of the intersegmental plane was not sufficient, it was established with reference to the location of the tumor and the lung parenchyma dissected by the MSI at the pulmonary hilum. Thereafter, the segment to be resected was divided using surgical staplers along the line of the intersegmental plane, taking maximum care not to entrap the peripheral bronchial stump in the surgical stapler (Figure 3, B). The excised specimen was confirmed to have no problems with the surgical margin using an intraoperative frozen diagnosis. A water-sealing test was performed to confirm the absence of air leaks. If air leaks existed, they were closed with soft coagulation11 and further reinforced with polyglycolic acid sheets and fibrin glue. When the patient was at risk of steroid administration, diabetes mellitus, or severe emphysema, the bronchial stump was covered with a pericardial fat pad. Chest tube drainage was managed with a 20F nylon tube or 19F silicone tube placed in the thoracic cavity and a digital suction system with −7 cmH2O. The postoperative complications were evaluated according to the Clavien-Dindo classification.12
Figure 1.
MSI with a 25-cm–long shaft (Acrosurg Revo, Nikkiso Co Ltd).
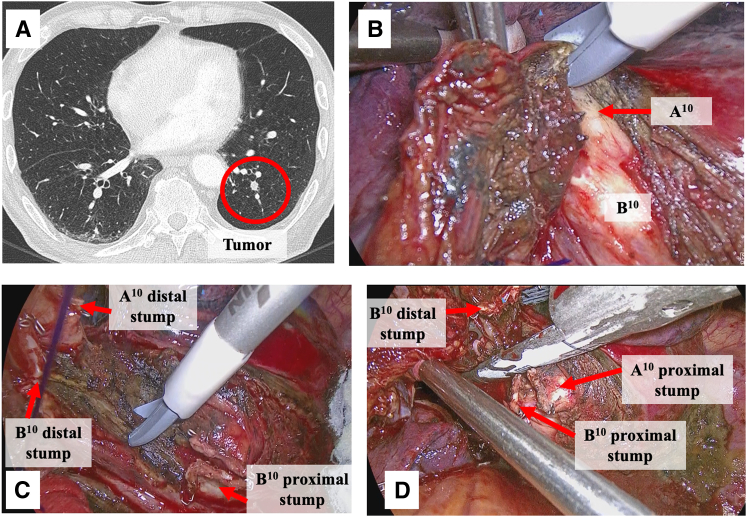
Figure 2.
Representative case of a complex segmentectomy showing left S10 (posterior basal segment) segmentectomy (Video 1). A, CT shows an intrapulmonary nodule located deeper than the pleural surface, with severe emphysema. B, Lung parenchymal dissection is performed from the dorsal side of the left lower lobe with an MSI along the inferior pulmonary vein. B10 and A10 in the deeper areas are exposed. C, The lung parenchyma located posterior to the bronchial stump is firmly dissected with MSI (“eave” formation). D, The “eave” formation facilitates the insertion of a surgical stapler for the dissection of the intersegmental plane.
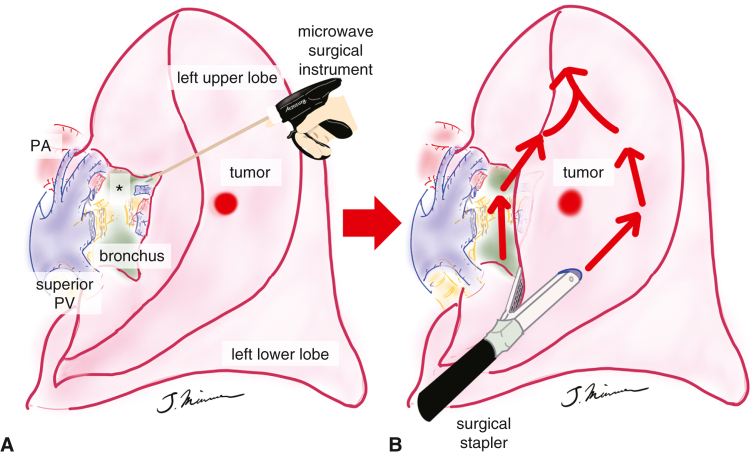
Figure 3.
Schema of lung segmentectomy, for example, left S3 (anterior segment) segmentectomy. A, The MSI is used to dissect the lung parenchyma between the segments along the vessels at the pulmonary hilum, and the lung parenchyma located posterior to the bronchial stump is firmly dissected with MSI (“eave” formation, asterisk). B, The segment to be resected is divided using surgical staplers along the line of the intersegmental plane. PA, Pulmonary artery; PV, pulmonary vein.
Statistical Analysis
The results are presented as median and interquartile range (IQR) for continuous variables, and numbers (n) and percentages for categorical variables. For continuous variables, the differences were evaluated using a 2-sided Student t test. For categorical variables, statistical differences between the groups were evaluated using the chi-square test or Fisher exact test. The multivariate analysis using a logistic regression model was performed to assess the independent predictive factors for early postoperative complications of grade 2 or higher. A P value <.05 was considered statistically significant. For statistical analysis, JMP 13 (SAS Institute Inc) was used.
Results
The preoperative patient characteristics of the M+S group (N = 69) and the S group (N = 47) are shown in Table 1. Age was significantly higher in the M+S group, whereas sex, smoking history, Charlson Comorbidity Index, respiratory function, clinical stage, tumor size, and tumor location were well balanced and not significantly different. The intraoperative and postoperative factors are shown in Table 2. There was no difference in the indications for segmentectomy for small-sized lung cancer between the 2 groups, and segmentectomy was significantly more complex in the M+S group (N = 45 vs N = 16, P = .001, Table E1). The degree of incomplete interlobar fissure affecting the procedure was severe in a greater number of patients in the M+S group; however, the number of staple cartridges used was significantly lower in the M+S group (median, 7 staple cartridges; IQR, 6-8 staple cartridges vs 8 staple cartridges; IQR, 7-9 staple cartridges; P = .005). In the M+S group, all procedures were accomplished under complete video-assisted thoracoscopic surgery, and there was no conversion to open thoracotomy. Both operation times (median, 179 minutes; IQR, 158-207 minutes vs 221 minutes; IQR, 197-249, P < .0001) and blood loss (median, 5 mL; IQR, 5-50 mL vs 30 mL; 5-195 mL, P = .012) were significantly lower in the M+S group. Postoperatively, the duration of chest tube placement was significantly shorter (median, 2 days; IQR, 1-2 days, vs 2 days; IQR, 2-3 days, P = .019), and postoperative complications of grade 2 or higher were significantly lower in the M+S group (M+S group: acute exacerbation of interstitial pneumonia (N = 1), pleurodesis associated with prolonged air leak (N = 1), and difficulty in sputum expectoration (N = 1); S group: empyema (N = 2), prolonged air leak (N = 4), bronchopulmonary fistula (N = 1), P = .049, Table E2). There were no differences in the histological diagnosis and pathological stage between the 2 groups. The multivariate analysis of the predictors of grade 2 or above showed that the procedure of lung parenchymal dissection was a favorable predictor (odds ratio, 0.17; 95% CI, 0.04-0.81; P = .025) (Table 3).
Table 1.
Patient characteristics
| Variables | M+S group (N = 69) | S group (N = 47) | Total (N = 116) | P value |
|---|---|---|---|---|
| Age (y) | .013 | |||
| Mean (SD) | 74.4 (8.2) | 70.5 (8.1) | 72.8 (8.4) | |
| Median (IQR) | 74 (70-80) | 70 (66-76) | 74 (68-78) | |
| Sex | .648 | |||
| Men | 44 (63.8%) | 28 (59.6%) | 72 (62.1%) | |
| Women | 25 (36.2%) | 19 (40.4%) | 44 (37.9%) | |
| Smoking history | .631 | |||
| Yes | 47 (68.1%) | 30 (63.8%) | 77 (63.4%) | |
| No | 22 (31.9%) | 17 (36.2%) | 39 (33.6%) | |
| Charlson Comorbidity Index | .541 | |||
| Mean (SD) | 1.6 (1.6) | 1.5 (1.3) | 1.6 (1.5) | |
| Median (IQR) | 1 (0-2) | 1 (0-2) | 1 (0-2) | |
| %VC (%) | .065 | |||
| Mean (SD) | 91.8 (21.1) | 99.6 (23.8) | 95.0 (22.5) | |
| Median (IQR) | 91.5 (79.8-103.8) | 95.3 (84.9-107.8) | 93.7 (82.9-105.7) | |
| FEV1.0% (%) | .396 | |||
| Mean (SD) | 73.9 (11.4) | 75.6 (9.1) | 74.6 (10.5) | |
| Median (IQR) | 77.5 (67.3-81.7) | 76.9 (71.6-82.0) | 77.3 (69.0-82.0) | |
| Clinical stage | .972 | |||
| 0 | 3 (4.4%) | 2 (4.2%) | 5 (4.3%) | |
| 1A1 | 30 (43.5%) | 21 (44.7%) | 51 (44.0%) | |
| 1A2 | 25 (36.2%) | 18 (38.3%) | 43 (37.1%) | |
| 1A3 | 11 (15.9%) | 6 (12.8%) | 17 (14.7%) | |
| Maximum tumor diameter (cm) | .540 | |||
| Mean (SD) | 18.1 (8.5) | 17.8 (6.8) | 18.0 (7.9) | |
| Median (IQR) | 16 (13-21) | 17 (12-23) | 16 (12-22) | |
| Location of tumor | .666 | |||
| Right upper lobe | 19 (27.5%) | 9 (19.2%) | 28 (24.1%) | |
| Right lower lobe | 10 (14.5%) | 7 (14.9%) | 17 (14.7%) | |
| Left upper lobe | 30 (43.5%) | 21 (44.7%) | 51 (44.0%) | |
| Left lower lobe | 10 (14.5%) | 10 (21.3%) | 20 (17.2%) |
SD, Standard deviation; IQR, interquartile range; VC, vital capacity; FEV1.0, forced expiratory volume in 1 second.
Table 2.
Factors related to surgery and pathology
| Variables | M+S group (N = 69) | S group (N = 47) | Total (N = 116) | P value |
|---|---|---|---|---|
| Reason for segmentectomy | ||||
| Intentional segmentectomy Compromised segmentectomy | 28 (40.6%) 36 (52.2%) |
16 (34.0%) 30 (63.8%) |
44 (37.9%) 66 (56.9%) |
.301 |
| Noninvasive GGN (C/T ratio ≤0.25) located deeper from visceral pleura | 5 (7.3%) | 1 (2.1%) | 6 (5.2%) | |
| Type of segmentectomy | .001 | |||
| Simple | 24 (34.8%) | 31 (66.0%) | 55 (47.4%) | |
| Complex | 45 (65.2%) | 16 (34.0%) | 61 (52.6%) | |
| Surgical approach | <.0001 | |||
| Complete VATS | 69 (100%) | 31 (66.0%) | 100 (86.2%) | |
| Hybrid VATS | 0 (0%) | 16 (34.0%)∗ | 16 (13.8%) | |
| Operation time (min) | <.0001 | |||
| Mean (SD) | 185 (35) | 224 (52) | 200 (47) | |
| Median (IQR) | 179 (158-207) | 221 (197-249) | 197 (167-229) | |
| Intraoperative blood loss (mL) | .012 | |||
| Mean (SD) | 27 (38) | 53 (71) | 37 (55) | |
| Median (IQR) | 5 (5-50) | 30 (5-95) | 10 (5-50) | |
| No. of staple cartridges used | .005 | |||
| Mean (SD) | 6.9 (1.7) | 7.9 (1.9) | 7.3 (1.8) | |
| Median (IQR) | 7 (6-8) | 8 (7-9) | 7 (6-8) | |
| Degree of incomplete lobar fissure affecting the procedure | .015 | |||
| None | 48 (71.6%) | 36 (94.7%) | 84 (80.0%) | |
| Moderate | 13 (19.4%) | 2 (5.3%) | 15 (14.3%) | |
| Severe | 6 (9.0%) | 0 (0%) | 6 (5.7%) | |
| Duration of chest tube placement (d) | .019 | |||
| Mean (SD) | 2.2 (1.3) | 3.1 (2.6) | 2.6 (2.0) | |
| Median (IQR) | 2 (1-2) | 2 (2-3) | 2 (2-3) | |
| Length of stay (d) | .501 | |||
| Mean (SD) | 11.4 (16) | 9.7 (5.3) | 10.7 (12.9) | |
| Median (IQR) | 8 (7-10) | 8 (7-10) | 8 (7-10) | |
| No. of patients with postoperative complication grade ≥2 | 3 (4.4%) | 7 (14.9%) | 10 (8.6%) | .049 |
| Histologic diagnosis on surgical specimen | .953 | |||
| Adenocarcinoma | 59 (85.5%) | 40 (85.1%) | 99 (85.3%) | |
| Squamous cell carcinoma | 8 (11.6%) | 6 (12.8%) | 14 (12.1%) | |
| Other | 2 (2.9%) | 1 (2.1%) | 3 (2.6%) | |
| Pathological stage | .542 | |||
| 0 | 9 (13.0%) | 3 (6.4%) | 12 (10.3%) | |
| 1A1 | 34 (49.3%) | 24 (51.1%) | 58 (50.0%) | |
| 1A2 | 16 (23.2%) | 10 (21.3%) | 26 (22.4%) | |
| 1A3 | 6 (8.7%) | 4 (8.5%) | 10 (8.6%) | |
| IB | 3 (4.4%) | 5 (10.6%) | 8 (6.9%) | |
| IIB | 0 (0%) | 1 (2.1%) | 1 (0.9%) | |
| IIIA | 1 (1.5%) | 0 (0%) | 1 (0.9%) |
GGN, Ground-glass opacity nodule; C/T, consolidation/tumor; VATS, video-assisted thoracoscopic surgery; SD, standard deviation; IQR, interquartile range.
N = 1: conversion to open thoracotomy.
Table 3.
Predictor of early postoperative complications of grade 2 or more
| Predictor | No. per event/total, % | Univariable |
Multivariable |
||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| Sex | .888 | .289 | |||
| Men | 6/72 (8.3) | 1 | 1 | ||
| Women | 4/44 (9.1) | 1.1 (0.29-4.14) | 3.13 (0.29-25.8) | ||
| Age (y) | .343 | .138 | |||
| ≤74 | 4/63 (6.4) | 1 | 1 | ||
| >75 | 6/53 (11.3) | 1.88 (0.13-2.00) | 3.16 (0.69-14.5) | ||
| Smoking history | .798 | .343 | |||
| Yes | 7/77 (9.1) | 1.2 (0.31-5.82) | 2.84 (0.33-24.7) | ||
| No | 3/39 (7.7) | 1 | 1 | ||
| %VC | .421 | .226 | |||
| ≥80 | 7/92 (7.5) | 1 | 1 | ||
| <80 | 3/23 (13.1) | 1.84 (0.37-7.29) | 2.78 (0.53-14.5) | ||
| FEV1.0% | .910 | .834 | |||
| ≥70 | 7/83 (8.4) | 1 | 1 | ||
| <70 | 3/33 (9.1) | 1.09 (0.22-4.19) | 1.19 (0.24-5.85) | ||
| Maximum tumor diameter (cm) | .910 | .940 | |||
| ≤20 | 7/83 (8.4) | 1 | 1 | ||
| >20 | 3/33 (9.1) | 1.09 (0.22-4.19) | 1.06 (0.24-4.64) | ||
| Procedure of lung parenchymal dissection | .049 | .025 | |||
| MSI + surgical stapler | 3/69 (4.4) | 0.26 (0.05-0.99) | 0.17 (0.04-0.81) | ||
| Only surgical stapler | 7/47 (14.9) | 1 | 1 | ||
CI, Confidence interval; VC, vital capacity; FEV1.0, forced expiratory volume in 1 second; MSI, microwave surgical instrument.
Discussion
In this study, we demonstrated that effective use of the MSI in combination with surgical staplers can simplify pulmonary hilar and intersegmental plane dissections for simple and complex segmentectomies. Application of this technique resulted in fewer staple cartridges, shorter operative time, less blood loss, shorter chest tube drainage period, and fewer air leakage-related complications, even in more complex lung segmentectomies.
According to the recently published results of 3 large clinical trials, JCOG0802/WJOG4607L, JCOG1211, and CALGB140503, the indications for lung segmentectomy for peripheral small lung cancer are expected to increase.1, 2, 3 The extent of lung segmentectomy has become complex with the development of preoperative 3-dimensional CT. We consider lung segmentectomy to be specific to each patient depending on their anatomy, similar to precision medicine. Occasionally, the surgical margin between the tumor and staple line may be very short when performing intersegmental plane dissection using surgical staplers. Furthermore, the more complex the segmentectomy, the more difficult it is to insert a surgical stapler into thicker lung parenchyma.
In addition, intraoperative or postoperative air leaks are among the most frequent complications of segmentectomy. JCOG0802/WJOG4607L reported leak-related complications in 6.5% of patients, although this was a very mature, selective population.4 In contrast, in a large prospective observational study, Mimae and colleagues13 reported that 24% of patients undergoing sublobar resection had pleurodesis, and prolonged air leakage is a common complication in clinical practice. Kagimoto and colleagues14 reported that patients who underwent postoperative pleurodesis had a significantly greater decline in vital capacity 6 and 12 months after lung segmentectomy than those who did not. Therefore, eliminating prolonged air leaks after lung resection is mandatory for all surgeries performed by general thoracic surgeons.
In a prospective study of 30 patients who underwent the lung parenchymal dissection using the MSI alone, we found that the procedure was successfully completed with no pleural suturing and few intraoperative and postoperative air leaks.5 The MSI is useful in various lung resection scenarios, such as partial resection of centrally located tumors, division of incomplete interlobar fissures, and even dissection of adhesions.7 The newly released Acrosurg Revo, which is currently used in most surgeries, can be used without difficulty in video-assisted thoracoscopic surgery.6 However, the use of surgical staplers, electrocautery, or other energy devices for dissection of the intersegmental plane remains controversial.15, 16, 17, 18, 19 In a previous prospective study, the degree of pulmonary function preservation did not change in a comparison of intersegmental dissection using surgical staplers or electrocautery, and the surgical stapler group required more staple cartridges. However, the medical costs remained the same because of increased complications in the electrocautery group.17 In many previous reports, lung segmentectomy with surgical staplers seems to be the mainstream worldwide,8,9,17, 18, 19 and surgical staplers are inevitably used because of the desire of general thoracic surgeons to reduce air leak–related complications. Asakura and colleauges20 proved in a pig model that segmentectomy using a combination of scissors and a surgical stapler can preserve the pulmonary function, similar to segmentectomy using scissors alone.
In our procedure, when using an MSI for the lung parenchymal dissection at the pulmonary hilum, which requires careful handling, intersegmental plane dissection with surgical staplers can be achieved without difficulty, even for complex segmentectomies. Of particular importance is that the distal bronchial end is firmly lifted, and the posterior lung parenchyma is dissected with the MSI (known as “eave” formation among Japanese general thoracic surgeons; the eave is exactly the edge of a roof that sticks out over the top of a wall) so that insertion of a surgical stapler is easier. The more complex the segmentectomy, the more effective it is. The MSI, which can freely perform a sharp lung parenchymal dissection in the pulmonary hilum, is useful, although it gives a perception of performing a manual procedure that requires getting used to. In this regard, we believe that other conventional energy devices are not suitable for pulmonary parenchymal dissection because of their mechanism of activation; the tissue is first clamped by the jaws of the device and then sealed, and parenchymal tissue can tear at the jaw clamping stage. On the other hand, the MSI can be used for pulmonary parenchymal dissection because the tissue can be dissected and sealed at the same time. In particular, the hilum is the most suitable site for lung parenchymal dissection using the MSI because, unlike the peripheral lung surface, the thickness of the tissue here is enough for cutting at the same time when it is clamped by the jaws.
The number of staple cartridges used in the M+S group was 1 less than in the S group; this result was achieved despite the fact that the M+S group had significantly more complex segmentectomies. This means that the number of staple cartridges used in the M+S group was significantly reduced by the application of the MSI to the pulmonary hilum, even though more staple cartridges normally would be required for complex segmentectomy. Moreover, despite a significant increase in the number of complex segmentectomies compared with previous cases, the combination of MSI dissection at the pulmonary hilum and surgical stapler dissection of the intersegmental plane resulted in shorter operative time, less blood loss, and fewer complications.
Study Limitations
The limitations of this study were as follows. (1) This was a single-center retrospective study and only included a moderate number of patients. (2) The study was not randomized and was a comparison with the past as a historical control. (3) New modalities, such as 3-dimensional CT, were introduced in the current method. However, preoperative factors were equally distributed in the population, except for age, which was higher in the MS group than in the S group. However, despite a population with more complex segmentectomies, the novel procedure produced favorable results.
Conclusions
For lung segmentectomies, which are increasingly indicated, the effective use of the MSI in combination with surgical staplers in pulmonary hilar and intersegmental plane dissections can simplify the procedure for simple and complex segmentectomies, thereby leading to favorable intraoperative and postoperative outcomes (Figure 4).
Figure 4.
Graphical abstract summarizing results. MSI, Microwave surgical instrument; PA, pulmonary artery; PV, pulmonary vein.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
The authors thank Editage (www.editage.com) for the English language editing.
Appendix E1
Table E1.
Details of resected lung segments
| Resected lung segments | M+S group (N = 69) | S group (N = 47) | Total (N = 116) |
|---|---|---|---|
| Site of segmentectomies, Right lung/left lung, n (%) | 30 (43.5)/39 (56.5) | 16 (34.0)/31 (66.0) | 46 (39.7)/70 (60.3) |
| Simple segmentectomy, right lung | 5 | 4 | 9 |
| S6 | 4 | 3 | 7 |
| S7+S8+S9+S10 (basal segmentectomy) | 1 | 1 | 2 |
| Complex segmentectomy, right lung | 25 | 12 | 37 |
| S1 | 7 | 5 | 12 |
| S1+S2a | 1 | 0 | 1 |
| S1+S2 | 1 | 2 | 3 |
| S1a+S2 | 3 | 0 | 3 |
| S1b+S3 | 2 | 0 | 2 |
| S2 | 5 | 1 | 6 |
| S2+S6 | 1 | 0 | 1 |
| S2a | 1 | 0 | 1 |
| S2b | 0 | 1 | 1 |
| S6+ S∗ | 1 | 0 | 1 |
| S7b | 1 | 0 | 1 |
| S8a | 1 | 0 | 1 |
| S9+S10 | 0 | 3 | 3 |
| S∗+S9+S10 | 1 | 0 | 1 |
| Simple segmentectomy, left lung | 20 | 26 | 45 |
| S1+2+S3 (upper division segmentectomy) | 8 | 14 | 22 |
| S4+S5 (lingulectomy) | 5 | 4 | 9 |
| S6 | 7 | 7 | 14 |
| S8+S9+S10 (basal segmentectomy) | 0 | 1 | 1 |
| Complex segmentectomy, left lung | 19 | 5 | 24 |
| S1+2 | 9 | 1 | 10 |
| S1+2+S3c | 1 | 0 | 1 |
| S1+2+S3a | 2 | 0 | 2 |
| S3 | 2 | 1 | 3 |
| S3a+b | 1 | 0 | 1 |
| S3b+c+S4 | 1 | 0 | 1 |
| S5 | 0 | 1 | 1 |
| S6+S9a | 1 | 0 | 1 |
| S8 | 0 | 1 | 1 |
| S9+S10 | 0 | 1 | 1 |
| S10 | 1 | 0 | 1 |
| S10c | 1 | 0 | 1 |
S6, Superior segment; S7, medial basal segment; S8, anterior basal segment; S9, lateral basal segment; S10, posterior basal segment; S1, apical segment; S2, posterior segment; S∗, subsuperior segment; S1+2, apico-posterior segment; S3, anterior segment; S4, superior segment; S5, inferior segment.
Table E2.
Early postoperative complications of grade 2 or more
| Complication | M+S group (N = 69) | S group (N = 47) | P value |
|---|---|---|---|
| Pneumonia | 1 | 0 | |
| Prolonged air leak | 0 | 4 | |
| Pleurodesis associated with prolonged air leak | 1 | 0 | |
| Difficulty in sputum expectoration | 1 | 0 | |
| Empyema | 0 | 2 | |
| Bronchopulmonary fistula | 0 | 1 | |
| Total postoperative complications | 3 (4.4%) | 7 (14.9%) | .049 |
Supplementary Data
Representative case of complex left S10 (posterior basal segment) segmentectomy. Video available at: https://www.jtcvs.org/article/S2666-2507(24)00057-9/fulltext.
References
- 1.Saji H., Okada M., Tsuboi M., et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomized, controlled, non-inferiority trial. Lancet. 2022;399:1607–1617. doi: 10.1016/S0140-6736(21)02333-3. [DOI] [PubMed] [Google Scholar]
- 2.Altorki N., Wang X., Kozono D., et al. Lobar or sublobar resection for peripheral stage IA non-small-cell lung cancer. N Engl J Med. 2023;388:489–498. doi: 10.1056/NEJMoa2212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aokage K., Suzuki K., Saji H., et al. Segmentectomy for ground-glass-dominant lung cancer with a tumour diameter of 3 cm or less including ground-glass opacity (JCOG1211): a multicentre, single-arm, confirmatory, phase 3 trial. Lancet Respir Med. 2023;11:540–549. doi: 10.1016/S2213-2600(23)00041-3. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki K., Saji H., Aokage K., et al. Comparison of pulmonary segmentectomy and lobectomy: safety results of a randomized trial. J Thorac Cardiovasc Surg. 2019;158:895–907. doi: 10.1016/j.jtcvs.2019.03.090. [DOI] [PubMed] [Google Scholar]
- 5.Mimura T., Yamashita Y., Kagimoto A., et al. Safety of a novel microwave surgical instrument for lung parenchyma dissection during segmentectomy. Ann Thorac Surg. 2020;109:1692–1699. doi: 10.1016/j.athoracsur.2019.12.068. [DOI] [PubMed] [Google Scholar]
- 6.Mimura T., Kamigaichi A., Kagimoto A., Yamashita Y. Lung segmentectomy with novel microwave surgical instrument (Acrosurg. Revo) Asian J Endosc Surg. 2021;14:821–823. doi: 10.1111/ases.12921. [DOI] [PubMed] [Google Scholar]
- 7.Kamigaichi A., Mimura T., Yamashita Y. Novel microwave surgical instrument for use in various lung resection situations. J Cardiothorac Surg. 2021;16:305. doi: 10.1186/s13019-021-01692-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerfolio R.J., Watson C., Minnich D.J., Calloway S., Wei B. One hundred planned robotic segmentectomies: early results, technical details, and preferred port placement. Ann Thorac Surg. 2016;101:1089–1095. doi: 10.1016/j.athoracsur.2015.08.092. [DOI] [PubMed] [Google Scholar]
- 9.Yotsukura M., Okubo Y., Yoshida Y., Nakagawa K., Watanabe S. Indocyanine green imaging for pulmonary segmentectomy. JTCVS Tech. 2021;6:151–158. doi: 10.1016/j.xjtc.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UICC TNM Classification of Malignant Tumours. 8th ed. Wiley; 2016. [Google Scholar]
- 11.Deguchi H., Tomoyasu M., Shigeeda W., et al. Usefulness of a suction ball coagulation probe for hemostasis in complete VATS lobectomy for patients with non-small cell lung cancer. Surg Today. 2019;49:580–586. doi: 10.1007/s00595-019-1769-5. [DOI] [PubMed] [Google Scholar]
- 12.Clavien P.A., Barkun J., de Oliveira M.L., et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 13.Mimae T., Saji H., Nakamura H., et al. Sublobar resection for non-small cell lung cancer in octogenarians: a prospective, multicenter study. Ann Thorac Surg. 2023;116:543–551. doi: 10.1016/j.athoracsur.2023.02.061. [DOI] [PubMed] [Google Scholar]
- 14.Kagimoto A., Tsutani Y., Hirai Y., et al. Impact of postoperative pleurodesis on pulmonary function after lung segmentectomy. JTCVS Open. 2021;5:110–118. doi: 10.1016/j.xjon.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okada M., Mimura T., Ikegaki J., Katoh H., Itoh H., Tsubota N. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg. 2007;133:753–758. doi: 10.1016/j.jtcvs.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Kuroda H., Dejima H., Mizumo T., Sakakura N., Sakao Y. A new LigaSure technique for the formation of segmental plane by intravenous indocyanine green fluorescence during thoracoscopic anatomical segmentectomy. J Thorac Dis. 2016;8:1210–1216. doi: 10.21037/jtd.2016.04.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X., Jin R., Xiang J., et al. Methods for dissecting intersegmental planes in segmentectomy: a randomized controlled trial. Ann Thorac Surg. 2020;110:258–264. doi: 10.1016/j.athoracsur.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Yazawa T., Igai H., Numajiri K., Ohsawa F., Matsuura N., Kamiyoshihara M. Comparison of stapler and electrocautery for division of the intersegmental plane in lung segmentectomy. J Thorac Dis. 2021;13:6331–6342. doi: 10.21037/jtd-21-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu T., Zhang R., Jiang K., et al. Electrocautery vs. stapler in comparing safety for segmentectomy of lung cancer: a meta-analysis. Front Surg. 2021;8 doi: 10.3389/fsurg.2021.711685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asakura K., Izumi Y., Kohno M., et al. Effect of cutting technique at the intersegmental plane during segmentectomy on expansion of the preserved segment: comparison between staplers and scissors in ex vivo pig lung. Eur J Cardiothorac Surg. 2011;40:e34–e38. doi: 10.1016/j.ejcts.2011.02.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative case of complex left S10 (posterior basal segment) segmentectomy. Video available at: https://www.jtcvs.org/article/S2666-2507(24)00057-9/fulltext.