Abstract
Objective
In select patients with borderline ventricular hypoplasia, we adopted a strategy of initial single-ventricle palliation followed by staged or direct biventricular conversion by 2 years of age.
Methods
Between 2018 and 2023, 14 newborns with borderline hypoplastic heart disease deemed high risk for primary biventricular repair underwent palliative procedures as a neonate/infant, followed by staged or direct biventricular conversion.
Results
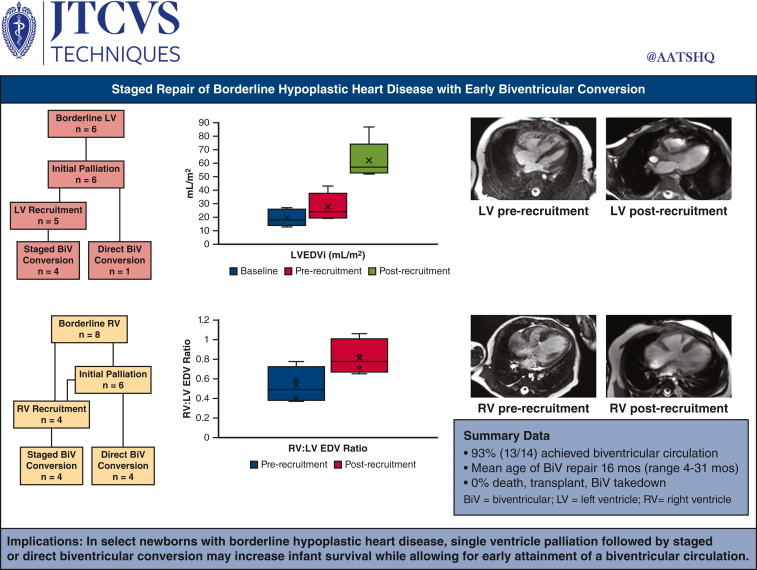
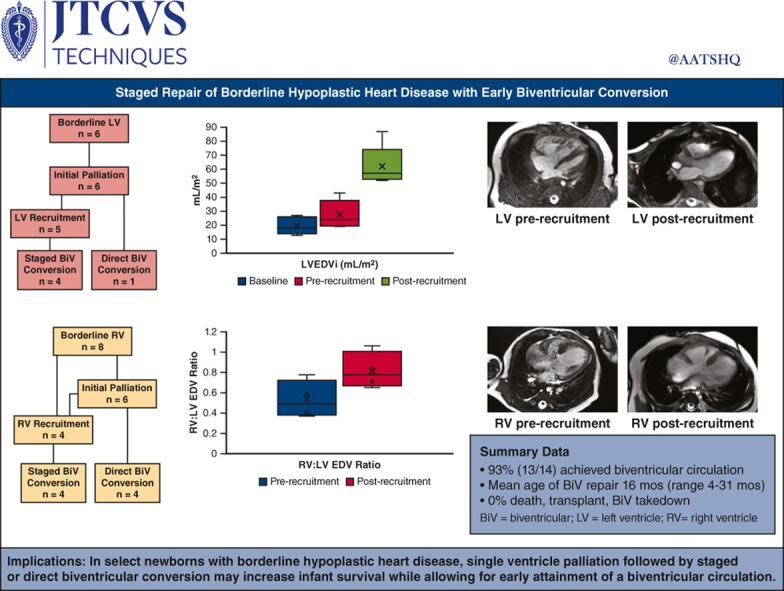
Of the 14 patients, 6 had borderline left ventricles and 8 had borderline right ventricles. Index neonatal operations were performed in 12 patients and included the Norwood operation (n = 5), pulmonary artery band (n = 3), ductal stent (n = 3), and hybrid Norwood (n = 1). Five patients underwent direct biventricular conversion, and the remaining 9 patients underwent staged ventricular recruitment operations at a mean age of 6 months (range, 3-11 months). Ventricular recruitment operations included atrial septation with or without ventricular rehabilitation, atrioventricular valve repair, or outflow tract operations. At a mean duration of 8 months (range, 4-10 months) after ventricular recruitment, there was a significant increase in chamber volume, aortic valve, and mitral valve size in patients with borderline left ventricles, and a normalization of the right ventricle:left ventricle end-diastolic volume ratio in patients with borderline right ventricles. To date, 13 of 14 patients have undergone successful biventricular conversion at a mean age of 16 months (range, 4-31 months).
Conclusions
In select newborns with borderline hypoplastic heart disease, single-ventricle palliation followed by staged or direct biventricular conversion may increase infant survival while allowing for early attainment of a biventricular circulation.
Key Words: atrioventricular septal defect, biventricular conversion, borderline ventricle, hypoplastic left heart syndrome, ventricular recruitment
Graphical Abstract
Normalization of borderline ventricle size after ventricular recruitment.
Central Message.
Staged or direct biventricular conversion safely achieved an early biventricular circulation in select newborns with borderline hypoplastic heart disease.
Perspective.
In select newborns with borderline ventricles, we found single-ventricle palliation followed by early pursuit of a biventricular circulation to be successful. This strategy allows for a conservative approach in the high-risk newborn phase, followed by controlled progression to a biventricular circulation during the infant period when the heart remains amenable to rebound developmental growth.
Inappropriate pursuit of primary biventricular repair in newborns with borderline hypoplastic heart disease has been associated with increased mortality.1 To reduce the risk of neonatal/infant mortality in select patients with borderline ventricular hypoplasia, we adopted a strategy of initial single-ventricle palliation followed by early pursuit of ventricular recruitment and biventricular conversion. This strategy allows for a conservative approach in the highest risk newborn phase, followed by controlled progression to a biventricular circulation during the infant period when the heart remains amenable to rebound developmental growth.2 The rationale is to avoid the early morbidity/mortality associated with high-risk neonatal biventricular repair, while later avoiding long-term complications of single-ventricle physiology.3
Our management approach has been influenced by several new techniques described for ventricular recruitment, aimed at preparing borderline ventricles for safe biventricular repair.4, 5, 6, 7 However, unique elements of our approach include exclusion of patients with endocardial fibroelastosis (EFE), preferential use of Sano shunts during interstage recruitment periods, and earlier age of ventricular recruitment and biventricular conversion, allowing for attainment of a biventricular circulation by 2 years of age or younger in the majority of cases. This article describes decision-making, management, and outcomes of 14 diverse patients with borderline ventricles followed from birth who were intended for biventricular repair after initial palliative procedures.
Patients and Methods
Data Source and Patient Population
Between 2018 and 2023, 14 newborns with borderline hypoplastic heart disease deemed high risk for primary biventricular repair underwent palliative procedures as a neonate/infant, followed by staged or direct biventricular conversion. All operations were performed at Duke University Medical Center, Durham, North Carolina. Management decisions were made by the Pediatric and Congenital Heart Center using a collaborative team-based approach. Patients with 2 normal-sized ventricles but complex intracardiac anatomy undergoing staged biventricular repair were excluded from the study. Two recent patients who underwent left ventricular (LV) recruitment procedures but have not yet had follow-up imaging were further excluded. Baseline assessment of anatomy, including valve measurements and estimated ventricular volumes, was obtained from transthoracic echocardiography (TTE). Cardiac magnetic resonance imaging (cMRI) was used to assess ventricular volume, morphology, and estimated cardiac index during surveillance. Demographic, anatomic, operative, and clinical details were identified from the electronic medical record. The study was approved by the Duke University Medical Center Institutional Review Board, and the need for informed patient consent was waived given the retrospective study design (Pro00101549, approved January 2019).
The general management approach has been described previously3 but also evolved during the study period. Ventricular recruitment operations aimed to promote growth of the borderline ventricle and heart valves through a combination of volume loading, surgical ventricular enlargement (deemed ventricular rehabilitation), and valve repair maneuvers.3 Recruitment procedures involved atrial septation with or without ventricular rehabilitation or papillary muscle mobilization, atrioventricular valve repair, or outflow tract operations. Patients were considered candidates for biventricular conversion on a case-by-case basis, but general target parameters included normalization of ventricular volume and ventricular morphology, well-functioning valves with Z-scores of −2 or greater, ventricular end-diastolic pressure less than 13 mm Hg (with atrial septal defect [ASD] test occlusion when present), and normal predicted cardiac index by cMRI.3,8,9
Statistical Analysis
Categorical variables are presented as percent (number), and continuous variables are presented as mean (range). The paired t test was used for statistical comparisons. Calculations were performed using STATA 11.1 (StataCorp, LLC).
Results
Borderline Left Ventricle Patients
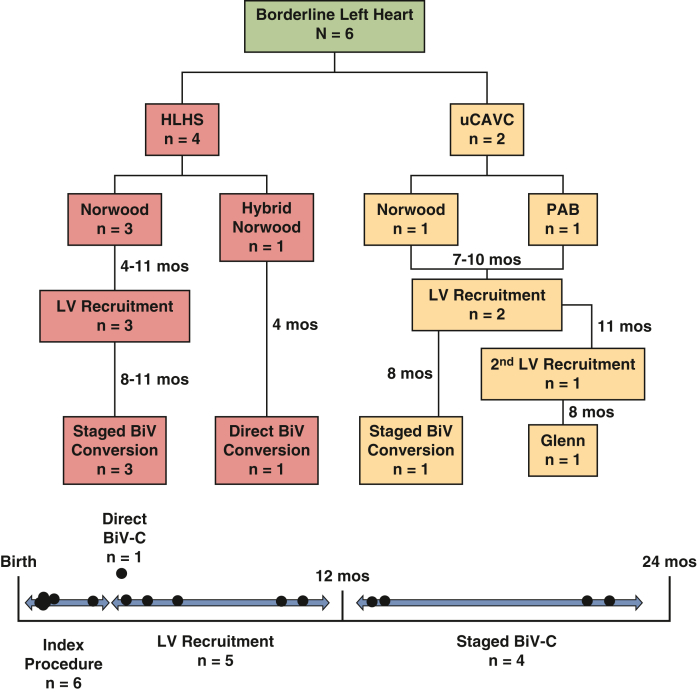
Six patients with borderline LV hypoplasia deemed high risk for primary biventricular repair were included in the study (Figure 1 and Table 1). Diagnoses included hypoplastic left heart syndrome (n = 4) and right dominant unbalanced complete atrioventricular canal (n = 2). Of note, no patients had a suggestion of EFE on neonatal TTE or surveillance cMRI. Initial operations performed included Norwood operation (n = 4), hybrid Norwood (n = 1), and pulmonary artery band (PAB, n = 1).
Figure 1.
Flowchart of patient management for 6 patients with borderline left heart disease. HLHS, Hypoplastic left heart syndrome; uCAVC, unbalanced complete atrioventricular canal; PAB, pulmonary artery band; LV, left ventricle; BiV, biventricular; BiV-C, biventricular conversion.
Table 1.
Patients with borderline left ventricle
| No. | Baseline anatomy | Reason staged | Index procedures | Interval imaging | Recruitment procedure | Interval imaging | Biventricular conversion | Follow-up status |
|---|---|---|---|---|---|---|---|---|
| 1 | HLHS (MS/AS) – 2.8 kg Non–apex forming LV Parachute MV, no MR LVEDVi 12-25 mL/m2 AV 5 mm (Z – 2.5) MV 8.2 mm (Z – 1.9) no EFE |
Borderline LH Parachute MV |
DOL 6 Norwood/Sano No atrial septectomy PLOS 19 d |
2 mo LVEDV 5 mL/20 mL/m2 LV CI 1.8 L/min/m2 LV:RV EDV 0.45:1 AV 6 mm (Z – 2.4) MV 10 mm (Z – 1.5) Small ASD |
4 mo, 6.3 kg Glenn No septectomy PLOS 16 d |
8 mo LVEDV 19 mL/54 mL/m2 LV CI 3.4 L/min/m2 LV:RV EDV 1.05:1 AV 9 mm (Z – 0.6) MV 15 mm (Z + 0.8) LVEDP 11 mm Hg Intact atrial septum |
13 mo, 9 kg DKS TD Glenn TD 4-mm ASD PLOS 23 d |
5 y 2 mo Trace MR No MS No AI/AS Normal BiV size and function LVEDVi 60 mL/m2 LVEDP 11 mm Hg PVR 0.87 WUi |
| 2 | HLHS (MS/AS) – 3.8 kg Co-apex forming LV LVEDVi 8-21 mL/m2 AV 5.3 mm (Z – 2.8) MV 8.6 mm (Z – 2.2) ASD LTR 6-14 mm Hg no EFE |
Borderline LH Large left MCA infarct |
DOL 4 Atrial septostomy DOL 10 Hybrid Norwood PLOS 29 d |
4 mo LVEDV 8 mL/28 mL/m2 LV CI 2.6 L/min/m2 LV:RV EDV 0.33:1 AV 7.4 mm (Z – 1.8) MV 10 mm (Z – 2.1) LVEDP 9 mm Hg Small ASD |
None | 4 mo, 7.4 kg Arch repair PAB removal PLOS 22 d |
3 y 11 mo No MR/MS No AI/AS Normal BiV size and function |
|
| 3 | HLHS (MS/AS) – 3.8 kg Co-apex forming LV Severe MR LVEDVi 25 mL/m2 AV 5 mm (Z – 2.9) MV 8.2 mm (Z – 2.6) ASD LTR 9-11 mm Hg no EFE |
Borderline LH Arcade MV with severe MR, ? need for oversewing |
DOL 3 Norwood/Sano Atrial septectomy PLOS 32 d |
4 mo LVEDV 6 mL/19 mL/m2 LV CI 1.6 L/min/m2 LV:RV EDV 0.21:1 AV 5.9 mm (Z – 3.3) MV 9.9 mm (Z – 2.3) LVEDP 8 mm Hg Mild MR/MS |
6 mo, 8.2 kg Sano upsize 8 mm 4-mm ASD PLOS 4 d |
14 mo LVEDV 23 mL/57 mL/m2 LV CI 3.3 L/min/m2 LV:RV EDV 0.65:1 AV 10 mm (Z + 0.2) MV 13 mm (Z – 1.2) LVEDP 7 mm Hg Intact atrial septum |
14 mo, 9.7 kg DKS TD Sano TD PLOS 6 d |
3 y 1 mo Mild MR/MS Severe AS/no AI Normal BiV size and function Moderate branch PS LVEDP 12 mm Hg RVp 60/9 PVR 1.4 WUi |
| 4 | HLHS (MS/AS) – 2.3 kg Non–apex forming LV LVEDVi 15 mL/m2 AV 4 mm (Z – 3.9) MV 7 mm (Z – 2.8) Small membranous VSD no EFE |
Borderline LH | DOL 6 Norwood/Sano No atrial septectomy PLOS 67 d |
5 mo LVEDV 8 mL/32 mL/m2LV CI 3.1 L/min/m2 LV:RV EDV 0.46:1 AV 6 mm (Z – 2.4) MV 10 mm (Z – 2.0) LVEDP 11 mm Hg Small ASD and VSD |
11 mo, 6.4 kg Sano upsize 8 mm 4-mm ASD Supra mitral membrane resection PLOS 11 d |
21 mo LVEDV 22 mL/52 mL/m2 LV CI 2.9 L/min/m2 LV:RV EDV 0.52:1 AV 8 mm (Z – 1.9) MV 14 (Z – 0.5) LVEDP 11 mm Hg Small ASD |
22 mo, 8.6 kg DKS TD Sano TD 4-mm ASD Supra mitral membrane resection VSD closure PLOS 29 d |
2 y 6 mo Mild-moderate MS Mild MR Trivial AS Mild AI Normal BiV size and function ASD LTR |
| 5 | uCAVC – 4.3 kg Moderate LV hypoplasia Single LV papillary muscle Arch hypoplasia LVEDVi 13 mL/m2 AV 4.3 mm (Z – 4.5) LAVV 5.8 mm (Z – 4.4) Left AVVi 0.33 Trivial CAVV regurgitation |
Borderline LH | DOL 5 Norwood/Sano No septectomy PLOS 29 d |
6 mo LVEDV 9 mL/24 mL/m2 LV CI 1.9 L/min/m2 LV:RV EDV 0.23:1 Left AVVi 0.31 AV 7 mm (Z – 2.6) LAVV 12 mm (Z – 1.4) |
7 mo, 8.1 kg CAVV septation 4-mm ASD Sano upsize 8 mm PLOS 36 d |
14 mo LVEDV 27 mL/61 mL/m2 LV CI 3.6 L/min/m2 LV:RV EDV 0.80:1 AV 9 mm (Z – 1.1) LAVV 15 mm (Z – 0.2) LVEDP 13 mm Hg Subaortic tunnel with 30 mm Hg gradient |
Second Staging Procedure: 19 mo, 10.2 kg Sub AS resection LAVV cleft closure ASD closure |
Aborted BiV Conversion: 27 mo, 11.8 kg AV 8 mm on direct inspection Bilateral Glenn |
| 6 | uCAVC – 3.9 kg Heterotaxy DORV with subaortic tunnel Interrupted IVC Azygous continuation to LSVC to LA Left hepatics Moderate LV hypoplasia LVEDVi 27 mL/m2 Left AVVi 0.46 LAVV 7.3 mm (Z – 3.1) AV 9.2 mm (Z + 1.9) |
Borderline LH Anatomic complexity COVID-19 infection |
3 mo, 5.6 kg PAB PLOS 23 d |
9 mo LVEDV 17 mL/43 mL/m2 LV CI 4.3 L/min/m2 LV:RV EDV 0.38:1 AV 10 mm (Z + 0) LAVV 12 mm (Z – 1.6) LVOT tunnel with 21 mm Hg gradient LVEDP 12 mm Hg |
10 mo, 8.5 kg Sub AS resection LV papillary muscle splitting and resection CAVV septation Complex atrial septation (L hepatics and LSVC) 4-mm ASD PAB loosening PLOS 10 d |
17 mo LVEDV 39 mL/87 mL/m2 LV CI 5.7 L/min/m2 LV:RV EDV 0.49:1 LAVV 14 mm (Z – 1.0) AV 13 mm (Z + 2.8) LVOT tunnel with 23 mm Hg gradient LVEDP 12 mm Hg |
18 mo, 10.9 kg LVOT tunnel resection LV to Ao baffle MV repair PAB takedown 4-mm ASD PLOS 10 d |
2 y 2 mo No LVOTO Mild AI Mild-Moderate MR Mild TR Mild MS/TS No VSD Normal biventricular size and function ASD LTR |
HLHS, Hypoplastic left heart syndrome; MS, mitral stenosis; AS, aortic stenosis; LV, left ventricle; MV, mitral valve; MR, mitral regurgitation; LVEDVi, left ventricle end-diastolic volume index; EFE, endocardial fibroelastosis; LH, left heart; DOL, day of life; PLOS, postoperative length of stay; CI, cardiac index; RV, right ventricle; EDV, end-diastolic volume; ASD, atrial septal defect; AI, aortic insufficiency; BiV, biventricular; LVEDP, left ventricle end-diastolic pressure; PVR, pulmonary vascular resistance; WUi, Woods units index; AV, aortic valve; LTR, left to right; MCA, middle cerebral artery; VSD, ventricular septal defect; DKS, Damus-Kaye-Stansel; TD, takedown; PS, pulmonary stenosis; RVp, right ventricle pressure; uCAVC, unbalanced complete atrioventricular canal; LAVV, left atrioventricular valve; AVVi, atrioventricular valve index; CAVV, common atrioventricular valve; IVC, inferior vena cava; LSVC, left super vena cava; PAB, pulmonary artery band; LVOTO, left ventricle outflow tract obstruction; DORV, double-outlet right ventricle; LA, left atrium; LVOT, left ventricle outflow tract; Ao, aorta; TR, tricuspid regurgitation; TS, tricuspid stenosis; D-TGA, D-transposition of the great arteries.
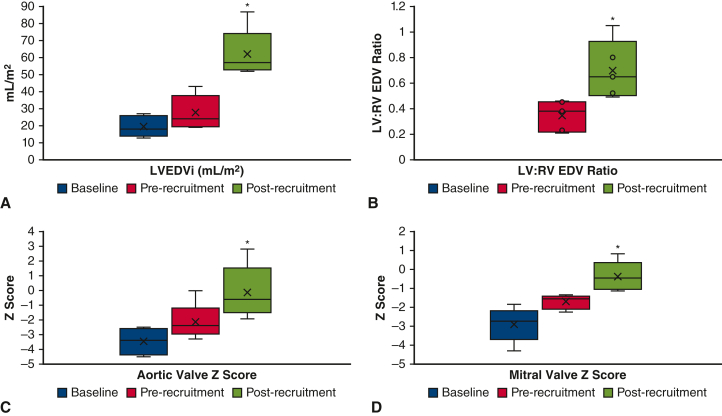
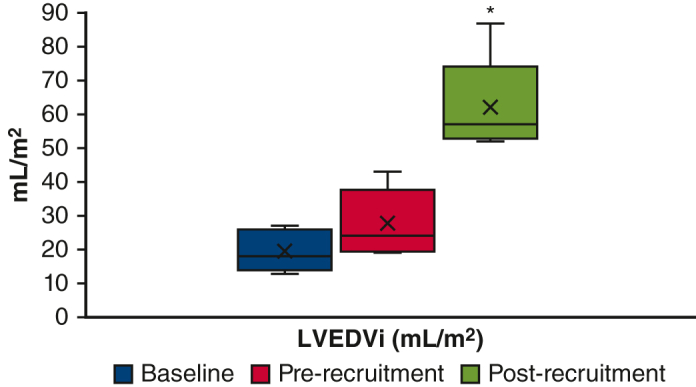
One patient underwent direct biventricular conversion at 4 months of age after hybrid Norwood operation. The remaining 5 patients underwent LV recruitment operations between 4 and 11 months of age, involving atrial septal restriction and valvar procedures. Pulmonary blood flow (PBF) was managed by Sano shunt upsizing in 3 patients, conversion to a Glenn in 1 patient, and PAB loosening in 1 patient. Mean postoperative length of stay (PLOS) after ventricular recruitment was 15 days (range, 4-36 days). At a mean duration of 7 months (range, 4-10 months) after ventricular recruitment, there was a significant increase in LV chamber volume, aortic valve size, and mitral valve size in patients with borderline LVs. LV end-diastolic volume index increased from 28 mL/m2 to 62 mL/m2 after LV recruitment (P = .002, Figures 2, A, and 3, A and B), and LV end-diastolic pressure ranged from 7 to 13 mm Hg. To date, 4 of 5 patients have undergone successful staged biventricular conversion at a mean age of 17 months (range, 13-22 months). Mean PLOS after biventricular conversion was 18 days (range, 6-29 days). The remaining patient (#5) underwent 2 staging procedures followed by an attempt at biventricular conversion at 27 months of age, but was found to have a prohibitively small aortic valve. After aortic valve inspection, the patient instead underwent bilateral bidirectional Glenn placement, with plans to consider biventricular conversion with a Ross operation in the future.
Figure 2.
A, LVEDVi; (B) LV:RV EDV ratio; (C) aortic valve Z score; (D) mitral valve Z zcore. Changes in left heart parameters before and after LV recruitment procedures in 5 patients. LVEDVi, Left ventricle end-diastolic volume index; LV, left ventricle; RV, right ventricle; EDV, end-diastolic volume. ∗P < .05.
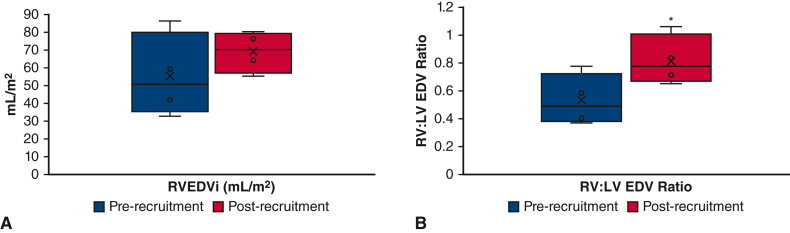
Figure 5.
A, RVEDVi; (B) RV:LV EDV ratio. Changes in right heart parameters before and after RV recruitment procedures in 4 patients. RVEDVi, Right ventricle end-diastolic volume index; RV, right ventricle; LV, left ventricle; EDV, end-diastolic volume. ∗P < .05.
Of the 5 patients who underwent successful biventricular conversion, mean age at latest follow-up is 3.4 years (range, 2.2-5.2 years). No patients have experienced death, transplant, or takedown of the biventricular circulation. Two patients (#1 and #3) have required catheter reintervention for branch pulmonary artery stenosis after Damus-Kaye-Stansel takedown with pulmonary artery reconstruction. One patient (#3) has developed severe stenosis of a bicuspid aortic valve 18 months after biventricular conversion and is being evaluated for catheter intervention versus Ross procedure.
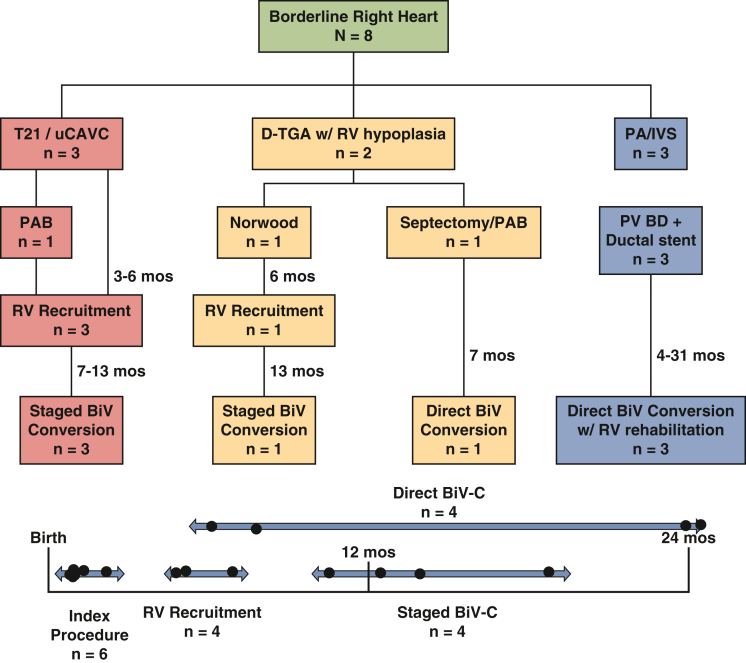
Borderline Right Ventricle Patients
Eight patients with borderline right ventricle (RV) hypoplasia deemed high risk for primary biventricular repair were included in the study (Figure 4 and Table 2). Diagnoses included Trisomy 21 with left dominant unbalanced complete atrioventricular canal (n = 3), D-transposition of the great arteries with ventricular septal defect and RV hypoplasia (n = 2), and pulmonary atresia with intact ventricular septum (PA/IVS, n = 3). Initial operations performed included pulmonary valve balloon dilation with ductal stent placement (n = 3), PAB (n = 2), and Norwood operation (n = 1).
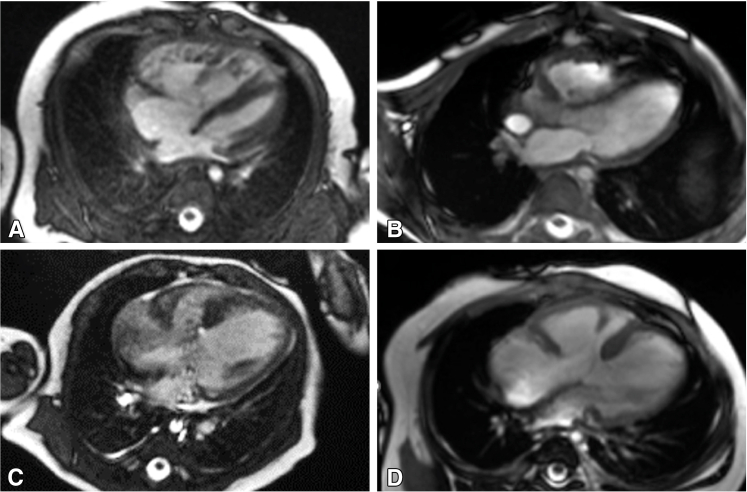
Figure 3.
Representative cMRI findings before (A) and after (B) LV recruitment, demonstrating normalization of LV chamber size and morphology 8 months after atrial septal restriction. Representative cMRI findings before (C) and after (D) RV recruitment, demonstrating normalization of RV size and ventricular balance, and liberation of the anterior papillary muscle from the ventricular septum, 6 months after atrial septation with RV rehabilitation. cMRI, Cardiac magnetic resonance imaging; LV, left ventricle; RV, right ventricle.
Table 2.
Patients with borderline right ventricle
| No. | Baseline anatomy | Reason staged | Index procedure | Interval imaging | Recruitment procedure | Interval imaging | Biventricular conversion | Follow-up status |
|---|---|---|---|---|---|---|---|---|
| 1 | uCAVC – 2.1 kg Trisomy 21 Moderate RV hypoplasia Right AVVi 0.25:1 |
Borderline RV | No neonatal intervention | 3 mo RVEDV 9 mL/33 mL/m2 RV CI 3.1 L/min/m2 RV:LV EDV 0.37:1 RAVV 12 mm (Z – 0.8) Right AVVi 0.5:1 RVEDP 12 mm Hg PVR 5.0 WUi |
3 mo, 5 kg CAVV septation 4-mm ASD RV rehabilitation PAB PLOS 28 d |
13 mo RVEDV 24 mL/64 mL/m2 RV CI 1.9 L/min/m2 RV:LV EDV 0.71:1 RAVV 12 mm (Z – 1.6) RVEDP 10 mm Hg severe RAVV regurgitation |
16 mo, 8.9 kg VSD closure TVR with 16-mm Melody valve PAB takedown PLOS 10 d |
4 y 2 mo Mild MR/no MS No TR/Mild TS No VSD/ASD No RVOTO No PS Normal BiV size and function |
| 2 | uCAVC – 3 kg Trisomy 21 Moderate-severe RV hypoplasia Moderate-severe common AVV regurgitation Right AVVi 0.4:1 |
Borderline RV | No neonatal intervention | 6 wk RVEDV 13 mL/59 mL/m2 RV CI 4.8 L/min/m2 RV:LV EDV 0.4:1 RAVV 12 mm (Z + 0.3) Right AVVi 0.58:1 RVEDP 8 mm Hg PVR 7.5 WUi Severe CAVV regurgitation |
3 mo, 5.5 kg CAVV septation 4-mm ASD RV rehabilitation PAB PLOS 20 d |
9 mo RVEDV 22 mL/55 mL/m2 RV CI 2.5 L/min/m2 RV:LV EDV 0.65:1 RAVV 16 mm (Z + 0.2) RVEDP 8 mm Hg PVR 2.3 WUi Mod-severe RAVV regurgitation |
10 mo, 10 kg VSD closure TV repair MV repair PAB takedown PLOS 9 d |
2 y 11 mo Mild MR/no MS Mild TR/no TS No VSD/ASD No RVOTO No PS Normal BiV size and function |
| 3 | uCAVC – 3.4 kg Trisomy 21 Moderate RV hypoplasia Moderate common AVV regurgitation |
Borderline RV | 6 wk, 4 kg PAB (performed at outside hospital) |
5 mo RVEDV 17 mL/86 mL/m2 RV CI 6.9 L/min/m2 RV:LV EDV 0.58:1 RAVV 12 mm (Z + 0) RVEDP 10 mm Hg PVR 1.8 WUi Moderate CAVV regurgitation |
6 mo, 6.8 kg CAVV septation ASD closure RV rehabilitation PAB adjustment PLOS 12 d |
13 mo RVEDV 30 mL/76 mL/m2 RV CI 4.2 L/min/m2 RV:LV EDV 0.83:1 RAVV 16 mm (Z + 0.4) RVEDP 8 mm Hg PVR 4 WUi Moderate RAVV regurgitation |
13 mo, 10 kg VSD closure TV repair PAB takedown PLOS 18 d PPM for CHB |
1 y 9 mo Mild TR/TS Mild MR/MS No RVOTO No VSD Normal BiV size and function Normal sinus rhythm |
| 4 | D-TGA/VSD/Coarctation – 3 kg Moderate RV hypoplasia Large inlet VSD Arch hypoplasia Subaortic muscle bundles TV 8.4 mm (Z – 1.7) |
Borderline RV | DOL 3 Norwood/Sano No septectomy PLOS 45 d |
4 mo RVEDV 11 mL/42 mL/m2 RV CI 3.1 L/min/m2 RV:LV EDV 0.77:1 TV 13 mm (Z + 0.3) RVEDP 10 mm Hg |
6 mo, 5.9 kg RV rehabilitation ASD closure Sano upsize 8 mm PLOS 24 d |
15 mo RVEDV 33/80 mL/m2 RV CI 3.8 L/min/m2 RV:LV EDV 1.06:1 TV 1.7 mm (Z+0.7) RVEDP 9 mm Hg PVR 2.1 WUi VSD × 2 (inlet + apical) |
19 mo, 10 kg Inlet VSD patch closure Apical VSD device closure DKS takedown Arterial switch with Lecompte Sano takedown Sub PS resection PLOS 21 d PPM for CHB |
2 y 11 mo No VSD No ASD No AI/AS No PI/PS No TR/TS Mild branch PS Normal BiV size and function |
| 5 | D-TGA/VSD/sub PS/RV hypoplasia – 3.5 kg Mild RV hypoplasia Aneurysmal atrial septum obstructing TV inflow TV annulus (8 mm Z – 2) with effective orifice 4 mm (Z – 5) Sub PS from TV chordal attachments |
Borderline RV TV obstruction |
DOL 11 Atrial septectomy PDA ligation Sub PS resection PAB PLOS 15 d |
7 mo RVEDV 17 mL/49 mL/m2 RV CI 3.1 L/min/m2 RV:LV EDV 0.69:1 TV 13 mm (Z – 0.8) RVEDP 9 mm Hg |
None | None | 7 mo, 7.6 kg Arterial switch VSD closure Sub as resection PAB takedown 4-mm ASD PLOS 13 d |
1 y 5 mo Trivial TR Mild TS No VSD No LVOTO No RVOTO Normal BiV size and function |
| 6 | PA/IVS – 3.4 kg Mild RV hypoplasia Moderate RV dysfunction TV 11 mm (Z – 0.3) Moderate TR PV 6 mm (Z – 2) No RVDCC RV Sinusoids present |
Borderline RV/ductal dependent | DOL 8 PV RF perforation and balloon dilation 5 wk PDA stent PV balloon dilation PLOS 74 d |
2 y RVEDV 20 mL/40 mL/m2 RV CI 2.7 L/min/m2 RV:LV EDV 0.56:1 TV 13 mm (Z – 2) Mild TR PV 13 mm (Z – 0.8) RVEDP 11 mm Hg Severe PR |
None | None | 2.5 y, 13.5 kg RV rehabilitation DS takedown 4-mm ASD PLOS 8 d |
6 y 8 mo Moderate TR No TS No RVOTO Severe PR No ASD Normal BiV size and function |
| 7 | PA/IVS – 1.8 kg 34 wk GA Moderate RV hypoplasia Severe RV dysfunction TV 7 mm (Z – 1.7) No TR Unicommissural PV with severe stenosis No RVDCC or sinusoids |
Borderline RV/ductal dependent | 2 mo PV balloon dilation 5 mo PDA stent PLOS 194 d |
2.75 y RVEDV 19 mL/37 mL/m2 RV CI 1.6 L/min/m2 RV:LV EDV 0.41:1 TV 11 mm (Z – 2.8) PV 10 mm (Z – 2.0) Moderate sub PS RVEDP 10 mm Hg |
None | None | 2.9 y, 12.1 kg RV rehabilitation PV repair Subvalvar PS resection DS takedown 4-mm ASD PLOS 7 d |
6 y 9 mo Trace PI No RVOTO Trace TR No TS Tiny ASD Mild RV hypoplasia Normal BiV function |
| 8 | PA/IVS – 2.8 kg Mild RV hypoplasia Moderate RV dysfunction TV 9.9 mm (Z – 0.4) Mild TR Membranous PA PV 5.5 mm (Z – 2.8) No RVDCC or sinusoids |
Borderline RV/ductal dependent | DOL 5 PV balloon dilation 4 wk PDA stent PLOS 38 d |
4 mo RVEDV 6 mL/20 mL/m2 RV CI 1.8 L/min/m2 RV:LV EDV 0.33:1 TV 12 mm (Z – 0.9) Trivial TR PV 9 mm (Z – 0.7) Moderate PS RVEDP 9 mm Hg |
None | None | 5 mo, 6 kg RV rehabilitation Limited transannular patch DS takedown 4-mm ASD PLOS 15 d |
2 y 7 mo Free PI No RVOTO Trace TR no TS No ASD Mild RV hypoplasia Normal BiV function |
uCAVC, Unbalanced complete atrioventricular canal; RV, right ventricle; AVVi, atrioventricular valve index; CI, cardiac index; LV, left ventricle; EDV, end-diastolic volume; RAVV, right atrioventricular valve; PVR, pulmonary vascular resistance; CAVV, common atrioventricular valve; ASD, atrial septal defect; PAB, pulmonary artery band; PLOS, postoperative length of stay; VSD, ventricular septal defect; TVR, tricuspid valve replacement; MR, mitral regurgitation; MS, mitral stenosis; MV, mitral valve; TR, tricuspid regurgitation; TS, tricuspid stenosis; RVOTO, right ventricle outflow tract obstruction; PS, pulmonary stenosis; PPM, permanent pacemaker; CHB, complete heart block; D-TGA, D-transposition of the great arteries; TV, tricuspid valve; WUi, Woods units index; AI, Aortic insufficiency; AS, aortic stenosis; DOL, day of life; DKS, Damus-Kaye-Stansel; PDA, patent ductus arteriosus; PA/IVS, pulmonary atresia with intact ventricular septum; PV, pulmonary valve; RF, radiofrequency; DS, ductal stent; RVDCC, right ventricle–dependent coronary circulation; BD, balloon dilation; DORV, double-outlet right ventricle; TD, takedown.
The first 4 patients underwent RV recruitment operations between 3 and 6 months of age. The rationale for ventricular recruitment in these patients was the finding of abnormal RV morphology generally manifest as tethering apical trabeculations, anterior papillary muscle fusion to the septum, and restricted RV inflow with deficient sinus portion. Recruitment procedures involved atrial septation with RV rehabilitation to allow for improved preload and growth of the RV chamber. PBF was managed by PAB placement in 3 patients and Sano shunt upsizing in 1 patient. Mean PLOS after ventricular recruitment was 21 days (range, 12-28 days).
At a mean duration of 8 months (range, 6-10 months) after ventricular recruitment, there was no significant change in the right ventricle end-diastolic volume index (RVEDVi; 55 mL/m2 vs 69 mL/m2, P = .3, Figure 5, A). However, the ventricular mass appeared more balanced with normalization of the RV:LV end-diastolic volume ratio (0.50 ± 0.2:1 to 0.8 ± 0.2:1, P = .001, Figure 3, B-D), and all 4 patients proceeded with biventricular conversion at a mean age of 15 months (range, 10-19 months).
Figure 4.
Flowchart of patient management for 8 patients with borderline right heart disease. T21, Trisomy 21; uCAVC, unbalanced complete atrioventricular canal defect; D-TGA, D-transposition of the great arteries; RV, right ventricle; PA/IVS, pulmonary atresia with intact ventricular septum; PAB, pulmonary artery band; PV BD, pulmonary valve balloon dilation; BiV, biventricular; BiV-C, biventricular conversion.
The remaining 4 patients (#5-8) underwent direct biventricular conversion without an intervening RV recruitment procedure. The first patient (#5) had D-transposition of the great arteries with ventricular septal defect with a small tricuspid valve (TV) and RV, likely as a result of a highly aneurysmal atrial septum that occluded TV inflow during development. Initial palliation was atrial septectomy with PAB placement with resection of obstructing TV chordal attachments from beneath the pulmonary valve. The patient then underwent successful biventricular conversion at age 7 months after repeat imaging demonstrated improvement in RV size and morphology. Three patients with PA/IVS (#6-8) initially palliated with percutaneous RV decompression and ductal stent placement were all considered candidates for direct biventricular conversion with concomitant RV rehabilitation performed between 4 and 31 months of age. The mean RVEDVi for these patients was 32 mL/m2 (range, 20-40 mL/m2). Mean PLOS after biventricular conversion for all patients was 13 days (range, 7-21 days).
Of the 8 patients in the RV group who underwent biventricular conversion, the mean age at latest follow-up is 3.6 years (range, 1.4-6.8 years). No patients have experienced death, transplant, takedown of the biventricular circulation, or conversion to a 1.5 ventricle circulation with addition of a Glenn. One patient (#1) required TV replacement at the time of biventricular conversion. Two patients required permanent pacemaker placement for complete heart block, although 1 patient has now recovered a normal sinus rhythm and the pacemaker has been deactivated.
Discussion
This case series describes a comprehensive approach to the management of newborns with borderline hypoplastic heart disease at a regional center, aimed at achieving a biventricular circulation in an expedient but judicious fashion. Although the patient population was small and heterogenous, we aimed to provide a holistic overview of our management of borderline ventricle heart disease in all its varieties. Results demonstrated 100% survival for all patients in the neonatal/infant period, as well as after all ventricular recruitment and biventricular conversion procedures. Procedural morbidity was low, and hospital length of stay averaged 2 to 3 weeks after biventricular repair procedures. To date, 93% of selected patients have achieved a biventricular circulation. We believe these outcomes demonstrate the merits of a conservative approach in the newborn period, while progressing toward a biventricular circulation in a controlled stepwise fashion after neonatal recovery.
The largest contemporary experience of ventricular recruitment and biventricular repair for patients with borderline ventricles comes from Boston Children's Hospital,4, 5, 6, 7 which heavily influenced our surgical philosophy and clinical protocols. However, there are some unique differences in our approach that warrant mention. First, our patients tended to be younger at the time of ventricular recruitment (mean age 6 months vs 1-2 years). This is likely because our patients were all born at our center, and we had control of patient management from birth, which differs from a referral center where patients are often referred from other institutions at a later age and stage. However, our experience demonstrates that ventricular recruitment can be performed at an earlier age, and we suspect this may be advantageous by harnessing the developmental capacity of the infant heart to respond to changes in hemodynamic conditions with rebound growth.2 We observed marked improvement in the size and morphology of hypoplastic ventricles in most patients within 6 to 8 months after recruitment. Valve size also increased, consistent with developmental biology studies showing a requirement for transvalvar blood flow for normal valve growth and development.10,11 Early application of ventricular recruitment then allowed for attainment of a biventricular circulation by 18 to 24 months of age in most patients, significantly earlier than the age of Fontan completion.
Another unique element of our approach included the use of upsized Sano shunts for PBF throughout the staging process in patients who were initially palliated with a Norwood/Sano operation.12 Our intent was to avoid performing Glenn connections during the recruitment phase to simplify the biventricular conversion operation, because a Glenn takedown was not required. However, the benefit of a Glenn is that it is a more stable source of PBF that grows with the patient, which may be useful for patients who are thought to require a longer period of ventricular recruitment. We found the upsized Sano shunt to serve as a stable source of PBF for up to 12 months, but for longer recruitment periods other considerations such as adding a Glenn or converting to a systemic-pulmonary artery shunt could be required.
The ventricular recruitment maneuvers were predicated primarily on increasing preload to the developing ventricle through atrial septal restriction6 and were found to be highly effective at improving the size/morphology of the hypoplastic ventricle. For borderline LVs, our strategy to achieve appropriate volume loading evolved to placement of an 8-mm Sano shunt + 4-mm ASD fenestration when patients were between 6 and 8 kg in size. We found that this combination achieved adequate saturations (>80%) with an initial Qp:Qs ratio of 1.5 to 2 and was effective at promoting growth of the ventricle and maintaining oxygen saturations for up to 12 months after placement. In patients managed with a PAB, Qp:Qs was similarly calibrated at 1.5 to 2 in the operating room. In patients with borderline RVs, the maximum volume loading that can be achieved is a Qp:Qs of 1:1 with the ASD completely closed. Although this yields a lower volume load compared with a borderline LV where a supraphysiologic Qp:Qs can be achieved, the combination of atrial restriction + surgical RV chamber enlargement was shown to be effective at promoting growth of the borderline RV. In most patients with a borderline RV, a 4-mm atrial fenestration was placed, although in 2 patients with borderline RV with ventricular septal defect and favorable TV annulus the ASD was completely closed to drive a full cardiac output across the growing valve and ventricle.
Patient surveillance during the interstage period is important to assess for signs left or right atrial hypertension, congestive heart failure, or alternatively inadequate volume load leading to failure of ventricular growth. In our program, ventricular recruitment patients are followed closely by our single-ventricle interstage monitoring program. They receive surveillance TTE at least once a month with attention paid to the ASD gradient as well as right atrial and hepatic vein dilation in patients with a borderline RV. In general, an ASD gradient above 8 mm Hg or clinical signs of right atrial hypertension would warrant cardiac catheterization for consideration of ballooning the atrial septum. However, no patients in our series required ASD ballooning during surveillance.
When comparing our data on patients with borderline LVs to other centers, it is important to note that no patients in our series harbored EFE. We intentionally avoided ventricular recruitment efforts in patients with borderline LVs and EFE given the suboptimal results in prior studies,8 suggesting that hearts with EFE possess intrinsically abnormal myocardium that may not be a suitable substrate for recruitment. However, the ventricular end-diastolic volume numbers for our patients before and after LV recruitment (baseline 28 mL/m2 increasing to 62 mL/m2) are similar to those reported by the Boston program.4, 5, 6,8,9 Although anecdotally an LV end-diastolic volume index of 20 mL/m2 has been described as a cutoff for primary biventricular repair or biventricular conversion, this represents a significantly hypoplastic ventricle (Z score between −3 and −5) yielding a high-risk biventricular repair in our opinion. We support the higher threshold of 45 mL/m2 described in a recent editorial, particularly for patients with borderline LVs.13
Of the patients in our series, the neonatal management of PA/IVS is the least controversial. However, techniques for RV rehabilitation (deemed “RV overhaul” by other authors) are poorly described and are paramount to achieving a successful long-term biventricular circulation in patients with PA/IVS with a borderline RV. In our experience, RV rehabilitation at the time of biventricular conversion involved complete relief of RV outflow obstruction through transannular patch or subvalvar muscle resection, division of tethering apical trabeculations, and complete delamination of the anterior papillary muscle from the ventricular septum. With these maneuvers, a previously masked apical chamber can often be developed and the RV free wall is released from the septum, which then allows for remarkable growth potential during follow-up in concert with volume loading through atrial level restriction. Using these techniques, we were able to achieve a well-functioning and durable 2-ventricle circulation in patients with PA/IVS with RVEDVi as low as 20 mL/m2 (mean 32 mL/m2). These results compare favorably to a recent publication from Boston Children's Hospital, where 5 patients with PA/IVS and borderline RV underwent biventricular conversion at a mean RVEDVi of 46 mL/m2 (minimum 27 mL/m2).14
In our experience, the assessment of a borderline RV with ventricular septal defect in early life was a nuanced decision and did not always correlate with ventricular volume estimates. In some patients we found that cMRI generated a normal RV volume measurement, despite a highly abnormal RV morphology and questionable size when viewed from the standpoint of the RV:LV volume ratio. It was also common to have a near-normal TV annular measurement, but with significantly restricted inflow due to fusion/restriction at the leaflet tips, creating additional hesitancy for primary biventricular repair. The discrepancy between the measured RV volume by cMRI and the clinical impression of a borderline ventricle may be due in part to the limitations of performing volumetric measurements in small children aged less than 3 months, as well as deciding on the LV to RV boundary in the setting of an unseptated ventricular mass. Nonetheless, significant improvements in ventricular morphology were observed after recruitment procedures in patients with borderline RV, best evidenced by the normalization of the RV:LV volume ratio. This suggests the RV:LV end-diastolic volume ratio may be another important metric to consider when evaluating the candidacy of a borderline RV for biventricular repair.
Although we observed normalization of ventricular size and morphology in our series, residual valvular disease appears to represent the greatest source for long-term reintervention. This inevitably poses the question of how hard to push for a biventricular circulation in patients with multivalve disease. For instance, is a complex staged biventricular repair with hypoplastic aortic and mitral valves, followed by several future valve repair or replacement procedures and possibly anticoagulation, better than a Fontan circulation? Although the long-term performance of the Fontan circulation is known to be poor, there is likely a threshold at which the number of procedures required to avoid a Fontan becomes excessive. Conversely, the 100% survival in our series is notable and suggests we could be more aggressive with patient selection and direct a larger proportion of patients toward biventricular repair.
Study Limitations
Our study represents a single-institution retrospective case series with descriptive data. Management decisions evolved over the study period. The sample size was small, and the patients were heterogeneous. Some patients in the study harbored mild ventricular hypoplasia and may have been managed successfully with primary biventricular repair or staged repair without ventricular recruitment at experienced centers.
Conclusions
In select newborns with borderline hypoplastic heart disease, we found single-ventricle palliation followed by early pursuit of a biventricular circulation to be a successful strategy (Figure 6). In our program, these techniques represented an important avenue for decreasing neonatal mortality in high-risk patients, while also reducing the burden of Fontan disease in our patients and program. Multi-institutional efforts to better understand the balance between long-term circulatory performance and procedural morbidity will be important to refine patient selection and determine which patients are best served with single-ventricle palliation, heart transplantation, or biventricular repair.
Figure 6.
Staged repair of borderline hypoplastic heart disease was associated with 100% survival and 93% of patients reaching a biventricular circulation by 2 years of age. LVEDVi, Left ventricle end-diastolic volume index; EDV, end-diastolic volume.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Hickey E.J., Caldarone C.A., Blackstone E.H., et al. Critical left ventricular outflow tract obstruction: the disproportionate impact of biventricular repair in borderline cases. J Thorac Cardiovasc Surg. 2007;134(6):1429–1436. doi: 10.1016/j.jtcvs.2007.07.052. discussion 1436-1427. [DOI] [PubMed] [Google Scholar]
- 2.Di Donato R.M., Fujii A.M., Jonas R.A., Castaneda A.R. Age-dependent ventricular response to pressure overload. Considerations for the arterial switch operation. J Thorac Cardiovasc Surg. 1992;104(3):713–722. [PubMed] [Google Scholar]
- 3.Andersen N.D., Scherba J.C., Turek J.W. Biventricular conversion in the borderline hypoplastic heart. Curr Cardiol Rep. 2020;22(10):115. doi: 10.1007/s11886-020-01363-5. [DOI] [PubMed] [Google Scholar]
- 4.Emani S.M., McElhinney D.B., Tworetzky W., et al. Staged left ventricular recruitment after single-ventricle palliation in patients with borderline left heart hypoplasia. J Am Coll Cardiol. 2012;60(19):1966–1974. doi: 10.1016/j.jacc.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 5.Oladunjoye O.O., Piekarski B., Banka P., et al. Staged ventricular recruitment in patients with borderline ventricles and large ventricular septal defects. J Thorac Cardiovasc Surg. 2018;156(1):254–264. doi: 10.1016/j.jtcvs.2018.03.111. [DOI] [PubMed] [Google Scholar]
- 6.Kwak J.G., Del Nido P.J., Piekarski B., Marx G., Emani S.M. Restriction of atrial septal defect leads to growth of hypoplastic ventricle in patients with borderline right or left heart. Semin Thorac Cardiovasc Surg. 2022;34(1):215–223. doi: 10.1053/j.semtcvs.2021.03.039. [DOI] [PubMed] [Google Scholar]
- 7.Oh N.A., Doulamis I.P., Guariento A., et al. Staged ventricular recruitment and biventricular conversion following single-ventricle palliation in unbalanced atrioventricular canal defects. JTCVS Open. 2023;13:278–291. doi: 10.1016/j.xjon.2022.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beattie M.J., Sleeper L.A., Lu M., et al. Factors associated with morbidity, mortality, and hemodynamic failure after biventricular conversion in borderline hypoplastic left hearts. J Thorac Cardiovasc Surg. 2023;166(3):933–942.e933. doi: 10.1016/j.jtcvs.2023.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Herrin M.A., Zurakowski D., Baird C.W., et al. Hemodynamic parameters predict adverse outcomes following biventricular conversion with single-ventricle palliation takedown. J Thorac Cardiovasc Surg. 2017;154(2):572–582. doi: 10.1016/j.jtcvs.2017.02.070. [DOI] [PubMed] [Google Scholar]
- 10.Bartman T., Walsh E.C., Wen K.K., et al. Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2004;2(5) doi: 10.1371/journal.pbio.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hove J.R., Koster R.W., Forouhar A.S., Acevedo-Bolton G., Fraser S.E., Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421(6919):172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 12.Prabhu N.K., Overbey D.M., Iranmanesh A.M., Campbell M.J., Andersen N.D., Turek J.W. Staged left ventricular recruitment facilitated by Sano conduit upsizing. JTCVS Tech. 2022;13:171–173. doi: 10.1016/j.xjtc.2022.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emani S.M. Biventricular repair in patients with borderline left heart-the "growing" experience. World J Pediatr Congenit Heart Surg. 2019;10(1):18–19. doi: 10.1177/2150135118819418. [DOI] [PubMed] [Google Scholar]
- 14.Prasad D., Romanowicz J., Banka P., et al. Cardiac magnetic resonance parameters associated with successful conversion from a single ventricular to a one-and-a-half or biventricular circulation in patients with a hypoplastic right ventricle. J Cardiovasc Magn Reson. 2023;25(1):51. doi: 10.1186/s12968-023-00965-6. [DOI] [PMC free article] [PubMed] [Google Scholar]