Abstract
Digital approaches to support advance care planning (ACP) documentation and sharing are increasingly being used, with a lack of research to characterise their design, content, and use. This study aimed to characterise how digital approaches are being used to support ACP documentation and sharing internationally. A scoping review was performed in accordance with the JBI (formerly Joanna Briggs Institute) guidelines and the PRISMA 2020 checklist, prospectively registered on Open Science Framework (https://osf.io/xnrg3). MEDLINE, EMBASE, PsycINFO, ACM Digital, IEEE Xplore and CINAHL were searched in February 2023. Only publications in English, published from 2008 onwards were considered. Eligibility criteria included a focus on ACP and electronic systems. Out of 2,393 records, 34 reports were included, predominantly from the USA (76.5%). ACP documentation is typically stored in electronic health records (EHRs) (67.6%), with a third (32.4%) enabling limited patient access. Non-standard approaches (n = 15;44.1%) were the commonest study design of included reports, with outcome measures focusing on the influence of systems on the documentation (i.e. creation, quantity, quality, frequency or timing) of ACP information (n = 23;67.6%). Digital approaches to support ACP are being implemented and researched internationally with an evidence base dominated by non-standard study designs. Future research is needed to extend outcome measurement to consider aspects of care quality and explore whether the content of existing systems aligns with aspects of care that are valued by patients.
Keywords: Advance care planning, digital technology, Resuscitation Orders, Resuscitation Decisions, Electronic Health Records, Health Information Exchange
Introduction
Palliative care aims to alleviate suffering for people living with progressive, life-limiting illnesses and improve quality of life through a holistic, person-centred, and multidisciplinary approach [1]. A means of facilitating person-centeredness in the delivery of care is through advance care planning (ACP). ACP can be characterised as a process that helps people understand and express their personal values, life goals and preferences about their future medical treatment. This process is connected to the ambition of ensuring that people with serious and chronic illnesses receive medical care that is consistent with their values, wishes, and preferences [2]. It is typically an ongoing, iterative process involving the elicitation of a patient’s goal and values for care often involving their families and healthcare providers [3]. By determining goals, values, wishes, and preferences for care in advance, it is intended that there is concordance in the care and treatments that are subsequently received [2]. There is evidence that ACP helps to ensure that end-of-life care wishes are known to healthcare providers and are more likely to be followed [4]. In addition, family members can benefit from documentation of end-of-life care preferences, including improved outcomes relating to stress, anxiety, and depression [4]. A lack of documented ACP can, in contrast, lead to medical interventions that are not in line with patients’ wishes [5].
To enable the implementation of documented care preferences, it is crucial to make them accessible to healthcare providers so that they can readily and easily access ACP information when necessary. This accessibility can be achieved, for example, through digital approaches such as storing ACP documentation in an electronic health record (EHR). Across multiple countries, there has been an evolution of digital approaches to support the collection, documentation, and sharing of ACP information between healthcare services and settings [6]. Internationally, this approach has been developing in countries including the USA [7, 8], Australia [9], and England [6]. Whilst there have been multiple examples cited for digital approaches, it is unclear how these have been adapted for different health systems, which information is collected and shared, and how data sharing is coordinated across multidisciplinary teams involved in the delivery of palliative and end-of-life care.
This Scoping Review aims to characterise how ACP documentation is currently integrated into electronic systems. We consider a scoping review to be an appropriate procedure, as heterogeneous studies and findings can be assumed due to the diverse approaches to storing ACP documentation in different countries and their respective healthcare systems.
Three research questions (RQs) are addressed:
RQ 1: What are the characteristics and structure of systems being used to support electronic documentation and sharing of ACP preferences?
RQ 2: What is the focus and type of existing evidence on the use of electronic documentation and sharing of ACP preferences?
RQ3: How are systems for electronic documentation and sharing of ACP preferences being evaluated?
Methods
This project was a collaborative project between the University of Leeds, UK, and the Brandenburg Medical School, Germany. A team approach was adopted in the conduct of the scoping review. The team met regularly throughout the review process, including data extraction, analysis and presentation [10]. This scoping review followed the updated JBI (formerly Joanna Briggs Institute) guide for scoping reviews [10]. In addition, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist was used [11].
Protocol and registration
This scoping review is registered on Open Science Framework (https://osf.io/xnrg3). An amendment was made to the inclusion criteria, which is detailed below.
Eligibility criteria
Inclusion criteria
Focus on advance care planning
Digital health technologies
Articles in English
Articles available in full-text
Articles published from 2008 onwards
Exclusion criteria
No full text available
Other languages than English
We only included studies published from 2008 onwards, as ACP supported by digital health technologies has only been developing in England since 2008 [12]. To our knowledge, England is the first country to address this topic. An inclusion criterion, removed between protocol and undertaking the review, was ‘People with chronic progressive illness’. We sought to adopt a broader focus on evidence that can inform digital advance care planning approaches, not confining the inclusion of studies limited to people with chronic, progressive illnesses.
Information sources
We searched the following seven bibliographic databases: MEDLINE, EMBASE, PsycINFO, ACM Digital, IEEE Xplore and CINAHL. Grey literature was searched on greylit.org and government websites. We also searched Google Scholar and we manually searched for additional studies by cross-checking the reference lists of all included studies. In addition, backward citation screening was performed for all included studies. For the conference abstracts we found, we tried to obtain the full text through additional searches or by contacting the study authors. We contacted one author and did not receive a response, but a full-text version was subsequently obtained. Reminders were not sent.
Search strategy
The search strategy was developed by the research team in collaboration with a librarian. It is based on the Peer Review of Electronic Search Strategies (PRESS) guideline [13]. The search strategy includes general terminology and names of specific systems that we are aware of or that have been referenced in a previous review [14]. For all databases, the literature search was conducted in February 2023. However, the grey literature was searched in June 2023. The search strategy is presented in Appendix 1.
Source of evidence selection
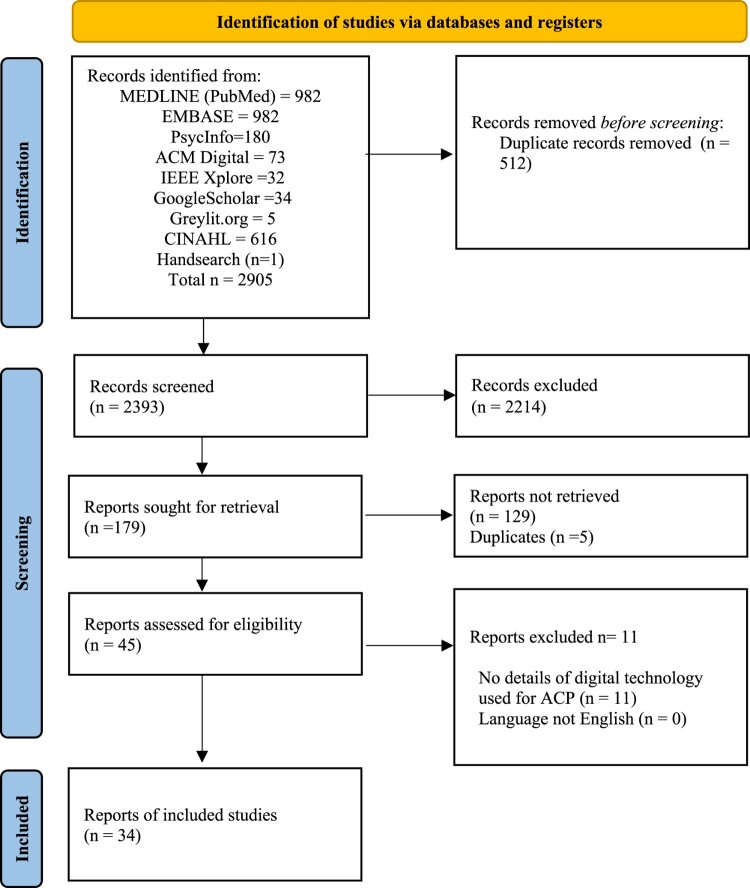
After compiling the search results, all citations were imported into the bibliographic manager, EndNote. All duplicates were removed by using an automated function in EndNote [15]. Subsequently, title and abstract screening was conducted by two independent reviewers (HSC and FM) against our defined eligibility criteria, via Rayyan – a web and mobile application for systematic reviews [15]. Any disagreements were solved by consensus or by the decision of a third reviewer (MA). Full texts were obtained for references that were deemed potentially relevant. Each full text was screened independently by two reviewers from a group of three reviewers in total (HSC, CM, MA) using our eligibility criteria. The reasons for the exclusion of full texts were documented and entered in the flow chart, provided in Figure 1. In all steps, disagreements were resolved by discussion and consensus between the two reviewers or by involving a third reviewer in case of doubt. The list of included studies is provided in Table 1.
Figure 1.
PRISMA flowchart for identification, screening, and inclusion of studies.
Table 1.
Summary of included studies.
| Author(s) | Year | Country | Study design | Population and sample size (n) | Description of system(s) involved | Name of system being described |
|---|---|---|---|---|---|---|
| Auret et al. | 2019 | Australia | Quantitative (observational) | N = 73 (Physicians (n = 26), nurses (n = 15) other HCPs (n = 12), audit of patients (n = 20)) | EHR | NA |
| Brungardt et al. | 2019 | USA | Quantitative (observational) | N = 105 (patients) | EHR | My Health Connection |
| Fin et al. | 2016 | USA | Quantitative (observational) | N = 900 (people who were users of the MyDirectives platform) | Standalone system not involving an EHR | MyDirectives |
| Goodwin et al. | 2021 | New Zealand | Other | N = 3,238 (The findings were obtained from an analysis of a database of 3238 people who had finalised an AC Plan between December 2013 and December 2019.) | EHR | eAC Plans |
| Hall et al. | 2012 | UK (Scotland) | Qualitative | N = 22 (purposive sampling of practice nurses (3 interviews), GPs (12 interviews), a practice manager (1 interview) from practices using different computing software systems and patients and/or carers (6 interviews for whom an ePCS had been completed)) | EHR | Electronic Palliative Care Summaries (ePCSs) |
| Halpert et al. | 2021 | USA | Quantitative (observational) | N = 426 (patients aged 75 years or older who had an upcoming appointment with their provider within 2 weeks and who had no documentation of ACP in their chart) | EHR | Epic EMR, which includes an ACP activity tab |
| Harrington et al. | 2020 | UK (England) | Mixed | N = 159 (data on gerontology inpatients (n = 133) with electronic do not attempt cardiopulmonary resuscitation (e-DNACPR) decisions and survey data from staff (n = 26) including senior and junior doctors, and medical trainees) | EHR | NA |
| Holt et al. | 2019 | USA | Other | NA | Standalone system not involving an EHR | Oregon’s online registry of out-of-hospital DNR orders |
| Jordan et al. | 2019 | USA | Qualitative | N = 46 (patients, mean age: 49, 63% female) | EHR | My Health Connection |
| Klugman et al. | 2013 | USA | Quantitative (observational) | N = 371 (platform users) | Standalone system not involving an EHR | TexasLivingWill.org (TLW), NVLivingWill.com (NVLW) |
| McDarby et al. | 2021 | USA | Other | NA | Other | ACP Tools (iOS and Android) BIDMC Health Care Contingency Plan – Personal MedStar CR My Dot Mediq My Directives My Health Proxy My Living Will Paper Health |
| Millington-Sanders et al. | 2012 | UK | Quantitative (observational) | N = 597 (patients) | Standalone system not involving an EHR | Coordinate My Care (CMC) |
| Mills et al. | 2021 | Australia | Mixed | N = 170 (Medical, nursing, and allied health practitioners working in palliative care) | Standalone system not involving an EHR | MyHealth Record |
| Moses et al. | 2020 | USA | Other | NA | EHR | NA |
| Nakagawa et al. | 2014 | USA | Quantitative (observational) | N = 93 (patients) | EHR | NA |
| Neubauer et al. | 2015 | USA | Quantitative (observational) | N = 5,467 (patients) | EHR | My Choices, My Wishes |
| Obel et al. | 2014 | USA | Quantitative (observational) | N = 48 (patients) | EHR | NA |
| Portz et al. | 2020 | USA | Quantitative (observational) | N = 3292 (UCHealth patients who interacted with the My Health Connection patient portal, advance care planning tools, specifically by completing an electronic MDPOA form or sending an electronic message to the advance care planning support team) | EHR | My Health Connection |
| Portz et al. | 2020 | USA | Qualitative | N = 24 (participants from the KPCO EMR with inclusion criteria included: ≥65 years of age, KPCO member for ≥ 1 year, presence of multiple chronic conditions (Charlson Comorbidity Index > 2), and a patient with one of the participating KPCO study clinics) | EHR | My Health Manager (Kaiser Permanente Colorado’s (KPCO) patient portal) |
| Reidy et al. | 2017 | USA | Mixed | N = 27 (hospitalists, residents, and nurse practitioners on conducting goals-of-care conversations – although other survey work also outlined) | EHR | Luminat |
| Riley et al. | 2013 | UK (England) | Other | NA | Standalone system not involving an EHR | Coordinate My Care |
| Rolnick et al. | 2021 | USA | Quantitative (experimental) | N = 91 (Adults with gastrointestinal and lung malignancies were recruited from the Penn Medicine Infusion Center. 46 randomly allocated to the web AD and 45 to paper) | EHR Plus | Our Care Wishes |
| Saiki et al. | 2017 | USA | Other | NA | EHR | Goals of Care template, as part of TEAM approach (Time, Education, Assessment, and Management) |
| Seecof et al. | 2022 | USA | Other | N = 50 (Patients at the Center for Healthy Aging aged 65 or older without documented ACP in the EMR) | EHR | NA |
| Serrano-Eanelli et al. | 2019 | USA | Other | N = 331 (medical records of patients) | EHR Plus | New York State eMOLST (electronic Medical Order for Life-Sustaining Treatment form) |
| Seuli Bose-Brill et al. | 2016 | USA | Quantitative (experimental) | N = 50 (patients) | EHR Plus | MyChart |
| Shervin Esfahani et al. | 2020 | USA | Other | N = 365 (Retrospective record review of all ICU deaths across the system in 1 year) | EHR | SmartPhrase (a tool in the Epic EMR system) |
| Stepan et al. | 2019 | USA | Other | N = ∼15,600 (patient records of all new patients with a diagnosis of advanced or metastatic cancer who had a documented advance directive and/or ACP conversations with an ambulatory care provider (physicians, fellows, residents, physician assistants, nurse practitioners, nurses, social work counsellors, and chaplains) by their third office visit) | EHR | NA |
| Tieu et al. | 2017 | USA | Quantitative (observational) | N = 200 (patients within the Division of Primary Care Internal Medicine, aged 65 and older, with access to the secure PEM system and without an AD on file, were included for randomization) | EHR Plus | Mayo Clinic Patient Online Services system |
| Walker et al. | 2018 | USA | Quantitative (observational) | N = 414 (primary care patients at the San Francisco Veterans Affairs Medical Center, were $60 years old, and had $2 chronic/serious health conditions) | EHR | Prepare for your care (www.prepareforyourcare.org) |
| Wilson et al. | 2020 | USA | Other | NA | EHR | Epic Systems EMR |
| Wu et al. | 2022 | USA | Quantitative (observational) | N = 187,690 (unique patients 65 years and older with at least one clinical encounter at an SHC outpatient clinic over the study period) | EHR | Epic Systems |
| Wye et al. | 2016 | UK | Mixed | N = 3,594 (Data from 1,022 North Somerset and 2,572 Somerset palliative patients; interview participants = 101 professionals, 49 by telephone, 29 face to face and 23 informally) | EHR | Adastra |
| Zive et al. | 2016 | USA | Other | NA. Intended for those nearing end of life. | EHR | ePOLST |
Key: EHR = Electronic Health Record; EMR = Electronic Medical Record; NA = Not applicable.
Data extraction
The standardised data extraction form offered by JBI [16] was used as a basis and adapted to the research questions of this scoping review. The spreadsheet software Microsoft Excel was used for this purpose. A pilot test of the data extraction form was conducted on a sample of five publications by three reviewers (MA, HSC, CM) independently to assess completeness and applicability. The results of the pilot testing were discussed with the whole review team and necessary changes were made to the extraction form. Disagreements were resolved through discussion. Data extraction was completed by team members (MA, HSC, CM) with all extracted data checked by a second reviewer (MA, HSC, CM) to ensure accuracy and completeness.
Data items
Data extraction included the following elements:
Authors
Title
Year
Country (in the first author’s affiliation)
Aim
Study type
Methodology
Publication type
Population and sample size
Description of system(s) involved (i.e. EHR system, EHR and other system(s), or standalone system not involving an EHR)
Name / title of the system being described
Settings (care or universal access) involved in introducing the system
Description of the ways that different healthcare providers interact with the system and responsibilities of providers across different care settings when interacting with the system
Processes for interacting with the system (healthcare provider and/or patient) or receiving communication from the system (e.g. healthcare provider access via EHR template, patient access via patient portal)
Content of the system (i.e. details of types of data recorded by system)
Outcomes evaluated (study outcomes)
Authors’ conclusion
Source of funding
Study risk of bias assessment
As this is a scoping review, no risk of bias assessment was performed. Critical appraisal or risk of bias assessment is generally not recommended for scoping reviews. Rather, the aim is to capture the available evidence [17].
Synthesis of results
The results were synthesised using qualitative content analysis as suggested by the JBI Scoping Review Methodology Group [18]. This included some form of categorisation to simplify the findings for the reader, particularly through the use of thematic networks [19]. CM has developed a first draft of the code system which was developed through discussion with the research team. Appendix 2 includes the definitions used to guide the categorisation of extracted data. The software MAXQDA® Software (Verbi Software Ltd, Berlin, Germany), version 2020, was used for the data analysis. The codes developed were discussed and adapted in regular meetings of MA, HSC and CM. A quality appraisal of the included studies was not performed.
Stakeholder involvement
This Scoping Review was developed with the support of patient organisations in the UK that specialise in the development and dissemination of patient-centred material to support decision-making for people with life-limiting or life-threatening conditions. The organisations contributed to the focus of the Scoping Review and interpretation of the findings. They also supported the development of a plain English summary of project findings that was used as part of a public dissemination event and to increase the reach of study findings for lay audiences and people with life-limiting or life-threatening conditions and their care partners.
Results
Selection of sources of evidence
We identified 2393 records on the title and abstract level after removing the duplicates. A total of 34 reports could be included. One of the 34 reports was identified through a hand search by checking the references of the included studies. We received no full texts for 129 reports. The vast majority were conference abstracts for which no full texts were available. The PRISMA 2020 flow chart, as shown in Figure 1, provides an overview of the included and excluded publications and illustrates the reasons for exclusion. A summary of coded extracted data can be found in Table 1, with a comprehensive summary table and definitions of coding in Appendix 2.
Characteristics of sources of evidence
The first report was published in 2012, and the most recent one is from 2022. A total of 55.8% (19/34) of the reports were published in 2019 or later. All publications appeared in English. Of these, 76.5% (26/34) were from the USA, 14.7% (5/34) were from the UK, 5.9% (2/34) were from Australia, and 2.9% (1/34) were from New Zealand. 47.1% of the reports (n = 16/34) were funded by different sources, with 52.9% (18/34) conducted without financial support. Across all studies, 223,550 (range 6–187,690) participants were involved, including patients (n = 221,880), healthcare providers (n = 399) and ‘Other’ (n = 1,271) that included users of online platforms. Most systems were only featured in one report, except for multiple reports relating to MyHealthConnection [20–22], Coordinate My Care [23, 24] and the use of the Epic electronic health record [25–27].
System characteristics and content (RQ1)
The characteristics and structure of systems described in reports varied. Most systems (n = 23;67.7%) were contained within a single EHR system. The remaining systems involved a standalone online platform that was embedded in an EHR (n = 6;17.7%), EHRs that enable viewing or editing of content by patients (n = 4;11.8%), and ‘other’ (i.e. a mobile phone application-based intervention) (n = 1;3.0%).
Reports described systems that enabled recording and sharing of ACP information across different settings. The most common setting with access to systems outlined in reports was the hospital (n = 15;44.1%), alongside systems that enabled access to providers across multiple healthcare settings (n = 10;29.4%). Systems also included those providing access to providers in outpatient (non-hospital) settings (n = 5;14.7%), palliative care providers (n = 4;11.8%), preclinical emergency care (n = 2;5.9), nursing (N = 2;5.9%) and care homes (n = 1;2.9%), and ‘other’ care organisations (n = 1;2.9%).
Healthcare providers interacted with systems outlined in reports via one of three approaches; access via an EHR system (n = 27;79.4%), via a web-based platform designed for healthcare providers that can be accessed by a provider in any care setting (n = 6;17.6%), or an online platform enabling professional access to a patient-facing platform where preferences for care are recorded (n = 1;2.9%). There was variation in how systems facilitated access between patients and their healthcare providers. These included reports (n = 15;44.1%) detailing systems that only enabled healthcare providers’ access to ACP information, either via an EHR system (n = 12;35.3%) or a standalone online platform (n = 3;8.8%). Around one-third of reports (n = 11;32.4%) detailed systems that enabled patients to access and edit records of the ACP information, accessed either via an online platform (n = 8;23.5%) or a patient portal linked to an EHR (n = 3;8.8%). The content of systems varied across reports, although most systems contained information relating to a patient’s care preferences and healthcare choices (e.g., preferred place of care and death, and advance directives) (n = 27;79.4%). Information relating to the identification of advocates and nomination of people to support decision-making (i.e., lasting power of attorney, surrogate decision makers) were present in systems (n = 15;44.1%). Also reported to be included in the content of systems were diagnosis and disease status information (n = 10;29.4%), personal and social needs (including religious and spiritual preferences) (n = 6;17.6%), educational content for patients to support ACP (n = 5;14.7%), and medical, nursing, and psychological needs (n = 1;2.9%).
Type of existing studies and assessing outcomes of systems (RQ2)
In 35.3% (12/34) of the reports, a quantitative observational study design was used. Other study designs included qualitative design (n = 3;8.8%), quantitative experimental design (n = 2;5.9%), and mixed method approaches (n = 2;5.9%). The most common study design tended to be ‘other’ non-standard approaches (n = 15;44.1%), including quality improvement project reports, commentaries, case reports, and service evaluations.
Evaluation of systems for electronic documentation and sharing of advance care planning (RQ3)
Across the included studies, multiple types of outcomes were being used to evaluate how systems were supporting and influencing care delivery. The most common type of outcome measures was those focusing on the influence of systems on the documentation of ACP information (n = 23;67.6%), particularly relating to the creation, quantity, quality, frequency, or timing of ACP records. Other types of outcome measures included those focusing on the impact of systems of healthcare provider practice (e.g. confidence in undertaking, or number of, ACP conversations) (n = 9;26.5%), the impact of systems on health service cost or service utilisation by patients (n = 6;17.6%), patient and healthcare providers’ experience and perceptions of the use of a system (n = 3;8.8%), satisfaction with documented end-of-life care plans (n = 1;2.9%) and the sharing of documentation with surrogates (n = 1;2.9%).
Discussion
Main findings
This scoping review highlights the characteristics and structure of digital ACP systems currently reported across the international research literature. Most digital ACP approaches were hosted and contained within a single EHR system, either within a single hospital site or intended to facilitate information sharing across multiple settings involved in palliative and end-of-life care delivery. Most digital ACP systems sought to record and share information relating to a patient’s care preferences and healthcare choices, with nearly half able to record an identified advocate or nominate people to support decision-making on behalf of a patient. Included records also reflected the capabilities of systems to support patient access to and editing of their own ACP as recorded on a digital system. The existing evidence reporting digital ACP systems was dominated by US studies, with varied study designs used. Most studies used non-standard designs (e.g. quality improvement project reports, commentaries, and case reports). Where study designs included the assessment of outcomes, these were typically focused on the influence of digital ACP systems on documentation (e.g. quantifying the creation, completeness, frequency, or timing of ACP records). There was a deficit in research exploring outcomes relating to the experience of system users, including both patients and healthcare providers, alongside limited measurement of satisfaction with ACP documented through digital systems.
Digital approaches to the documentation and sharing of patient information and preferences are increasingly being used to support the delivery of high-quality end-of-life care internationally [7–9]. The prominence of non-standard study designs and the recency of records included in the review may reflect the research exploring their impact and implementation as an emerging research area. This research is required to understand how to optimally implement digital ACP systems and crucially to determine if and how they can be useful tools to support the delivery of palliative and end-of-life care [6]. Systems to support healthcare provider documentation and sharing of ACP information in palliative care need to account for the multi-setting and multidisciplinary nature of care delivery [28]. In countries, such as the UK, where systems typically seek to coordinate information sharing across multiple settings, implementation has been fragmented and sub-optimal [29]. For example, most were not able to share information across all providers, often excluding care homes and social care providers from information exchange [29]. Understanding how to optimise the implementation of digital ACP systems is a priority for future research [30].
An emerging capability that was reported in a third of records was the ability of patients to access and edit their own ACP information within EHR systems. This aligns with an increasing recognition of the value of patient access to their records, viewed as an important way of empowering patients in their decision-making related to their health and care [31]. Current content is largely focused on care preferences and healthcare choices, such as place of care and place of death which are viewed as important aspects of care from a patient’s perspective [32]. However, additional factors related to quality of life are known to be important to people receiving palliative care relating to multiple domains including personal autonomy and emotional, social, and spiritual factors [33]. Content relating to these elements was present in fewer than a fifth of the studies included in the review. Patient and carer engagement in the development of digital ACP systems is currently lacking [30] and may be an important next step to explore how well the content of existing ACP systems aligns with aspects of care that are valued by patients. Patient engagement research that accompanies increasing patient access to their record for ACP documentation may also provide opportunities to explore disparities (e.g. those relating to age, disease, and deprivation) that have been identified previously with patient portals for access to medical records [34]. Irrespective of the route developed to facilitate patient access to their own ACP information, research is required to ensure they do not exacerbate known disparities in access to and use of palliative care services [35, 36].
Strengths and limitations
Although this scoping review provides a detailed insight into the possibilities of digital documentation of ACP preferences, there are some limitations. Firstly, our study was restricted solely to publications written in English. There may be relevant publications from other countries written in the respective language that are not included in our scoping review. Secondly, another limitation is that the primary aim of the included studies was not necessarily to report on the systems used, but mostly to answer other research questions. For example, the most common outcome measure focused on the impact of the systems on the documentation of ACP information. Other types of outcome measures related to the impact of the systems on health providers’ practice (e.g. confidence in conducting ACP conversations). The information relevant to answering the research questions for this scoping review was not always explicitly addressed in the included primary studies. Therefore, misinterpretations cannot be ruled out. However, to avoid misinterpretation as far as possible, the data extraction was carried out by one person and checked by another. Any ambiguities were discussed with another reviewer and the relevant text passages were discussed. Despite these limitations, this scoping review contributes to mapping the available evidence on digital documentation of ACP preferences.
Implications for practice
Across the research literature, interventions are being reported that may influence ACP before and after the documentation and sharing of APC information using digital systems. These include interventions to increase a person’s willingness and readiness for ACP conversations and decision-making (e.g. [37]). Interventions are also being developed to improve the quality of ACP discussions (e.g. [38]). Following documentation and sharing of ACP documentation, there is an exploration of the review and realisation of care that is concordant with patient wishes (e.g. [39]). Future work may be required across these different elements and emerging interventions to understand any interdependencies. Such research could explore how the different activities influence the engagement of providers and patients with the ACP process and the quality of information that is recorded and shared on digital ACP systems. This may also need to take account of situations in which, irrespective of documentation, challenges exist in providing care in line with documented wishes (e.g. a conflict between honouring preference for comfort care and extending life, changes in patient preferences not being possible to reflect, a lack of resources, and challenges with retrieving ACP documents) [40]. Such research will require the development of more nuanced outcome measures for understanding the impact of digital ACP systems. Within the review, where used, outcomes largely focused on documentation as an endpoint. Future research could seek to understand outcomes relating to, for example, timeliness of access to and use of ACP information accessed via digital systems, or wider implementation outcomes (e.g. appraisal of success in alignment with strategic fit and priority and integration into communications and workflows) [41]. Factors influencing such proximal outcomes relating to the use of systems and their implementation may be a necessary first step before exploring the impact of digital ACP systems on care delivery and patient outcomes.
Conclusion
Digital ACP approaches are an emerging area for palliative and end-of-life care research, with a predominance of literature from the USA, UK, Australia, and New Zealand. Reported digital ACP approaches use EHR platforms or stand-alone digital platforms, typically supporting documentation in hospitals, nursing homes and outpatient facilities. A fifth of reports detail systems that enable patient access to their records. Future patient and healthcare provider engagement is required to understand the experience of using digital ACP systems and ensure the alignment of system content with what matters to patients. Irrespective of system design, there will be a need to monitor disparities in the use and impact of digital ACP systems and to refine outcome measures to understand their implementation.
Supplementary Material
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The review was conducted using data that were already available in the public domain, with appropriate references provided throughout. The full search strategy used in the present review can be found in the supplementary material.
Author contribution statements
MJA developed the initial idea for the study. MJA, FM, HSC, CM, JB, AP, and DP designed the scope and planned the methodological approach. DW and AP coordinated the scoping review process and development of the scoping review protocol, completed the Open Science Framework registration, and extracted the data for further analysis. DP and AP with MJA defined the search strings, executed the search, exported the results, and removed duplicate records. FM, HCS, CM and MJA screened the abstracts and texts for the scoping review and extracted relevant data from the articles. MJA and CM wrote the first draft of the manuscript and all authors contributed to critically revising the manuscript. MJA is the study guarantor. HSC and CM contributed equally to this study. All authors had full access to all the data in the study, and the corresponding author had final responsibility for the decision to submit for publication. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09699260.2024.2339106.
References
- 1.Payne A. Introducing palliative care, Fourth Edn. Robert Twycross. Radcliffe Medical Press, 2003. 190pp. paperback. ISBN: 1-85775-915-X. Psycho-Oncology 2005;14(1):80–80. [Google Scholar]
- 2.Sudore RL, Lum HD, You JJ, et al. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage 2017 May;53(5):821–32 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jimenez G, Tan WS, Virk AK, et al. Overview of systematic reviews of advance care planning: summary of evidence and global lessons. J Pain Symptom Manage 2018 Sep;56(3):436–459 e25. [DOI] [PubMed] [Google Scholar]
- 4.Detering KM, Hancock AD, Reade MC, Silvester W.. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ 2010 Mar 23;340:c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houben CHM, Spruit MA, Groenen MTJ, et al. Efficacy of advance care planning: a systematic review and meta-analysis. J Am Med Dir Assoc 2014 Jul;15(7):477–89. [DOI] [PubMed] [Google Scholar]
- 6.Allsop MJ, Chumbley K, Birtwistle J, et al. Building on sand: digital technologies for care coordination and advance care planning. BMJ Support Palliat Care 2022 Jun;12(2):194–7. [DOI] [PubMed] [Google Scholar]
- 7.Huber MT, Highland JD, Krishnamoorthi VR, Tang JW.. Utilizing the electronic health record to improve advance care planning:a systematic review. Am J Hosp Palliat Care 2018 Mar;35(3):532–41. [DOI] [PubMed] [Google Scholar]
- 8.Lamas D, Panariello N, Henrich N, et al. Advance care planning documentation in electronic health records: current challenges and recommendations for change. J Palliat Med 2018 Apr;21(4):522–8. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy S, Meredith J, Bryant L, Hemsley B.. Legal and ethical issues surrounding advance care directives in Australia: implications for the advance care planning document in the Australian My health record. J Law Med 2017 Nov;25(1):136–49. [PubMed] [Google Scholar]
- 10.Tricco AC, Tetzlaff J, Moher D.. The art and science of knowledge synthesis. J Clin Epidemiol 2011 Jan;64(1):11–20. [DOI] [PubMed] [Google Scholar]
- 11.Tricco AC, Lillie E, Zarin W, et al. Prisma extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018 Oct 2;169(7):467–73. [DOI] [PubMed] [Google Scholar]
- 12.(NHS) NHS. End of Life Care Strategy . 2008. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/136431/End_of_life_strategy.pdf.
- 13.McGowan J, Sampson M, Salzwedel DM, et al. Press peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 2016 Jul;75:40–6. [DOI] [PubMed] [Google Scholar]
- 14.Dupont C, Smets T, Monnet F, et al. Publicly available, interactive web-based tools to support advance care planning. Systematic review. J Med Internet Res 2022 Apr 20;24(4):e33320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A.. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016 Dec 5;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters MDJ, Godfrey C, McInerney P, Munn Z, Tricco A, Khalil, H.. Chapter 11: scoping reviews. In: Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. 2020. Available from: https://synthesismanual.jbi.global [Google Scholar]
- 17.Peters MDJ, Marnie C, Tricco AC, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth 2020 Oct;18(10):2119–2126. [DOI] [PubMed] [Google Scholar]
- 18.Pollock D, Peters MDJ, Khalil H, et al. Recommendations for the extraction, analysis, and presentation of results in scoping reviews. JBI Evid Synth 2023 Mar 1;21(3):520–32. [DOI] [PubMed] [Google Scholar]
- 19.Attride-Stirling J. Thematic networks: an analytic tool for qualitative research. Qual Res 2001;1(3):385–405. [Google Scholar]
- 20.Portz JD, Brungardt A, Shanbhag P, et al. Advance care planning among users of a patient portal during the COVID-19 pandemic: retrospective observational study. J Med Internet Res 2020 Aug 11;22(8):e21385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brungardt A, Daddato AE, Parnes B, Lum HD.. Use of an ambulatory patient portal for advance care planning engagement. J Am Board Fam Med 2019 Nov-Dec;32(6):925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan SR, Brungardt A, Phimphasone-Brady P, Lum HD.. Patient perspectives on advance care planning via a patient portal. Am J Hospice Palliat Care 2019 Aug;36(8):682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riley J, Madill D.. Coordinate my care: a clinical approach underpinned by an electronic solution. Progr Palliat Care 2013;21(4):214–9. [Google Scholar]
- 24.Millington-Sanders C, Nadicksbernd JJ, O’Sullivan C, et al. Electronic palliative care co-ordination system: an electronic record that supports communication for end-of-life care - a pilot in Richmond, UK. Lond J Prim Care (Abingdon) 2012;5(1):130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson E, Bernacki R, Lakin JR, et al. Rapid adoption of a serious illness conversation electronic medical record template: lessons learned and future directions. J Palliat Med 2020 Feb;23(2):159–61. [DOI] [PubMed] [Google Scholar]
- 26.Wu A, Huang RJ, Colón GR, et al. Low rates of structured advance care planning documentation in electronic health records: results of a single-center observational study. BMC Palliat Care 2022 Nov 22;21(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halpert KD, Ward K, Sloane PD.. Improving advance care planning documentation using reminders to patients and physicians: a longitudinal study in primary care. Am J Hosp Palliat Care 2022 Jan;39(1):62–7. [DOI] [PubMed] [Google Scholar]
- 28.Fernando G, Hughes S.. Team approaches in palliative care: a review of the literature. Int J Palliat Nurs 2019 Sep 02;25(9):444–51. [DOI] [PubMed] [Google Scholar]
- 29.Birtwistle J, Millares-Martin P, Evans CJ, et al. Mapping and characterising electronic palliative care coordination systems and their intended impact: A national survey of end-of-life care commissioners. PLoS One 2022;17(10):e0275991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allsop MJ, Chumbley K, Birtwistle J, et al. Building on sand: digital technologies for care coordination and advance care planning. BMJ Support Palliat Care 2022;12(2):194–7. [DOI] [PubMed] [Google Scholar]
- 31.Maria H, Brian M, Robyn W, Charlotte B.. Patient empowerment through online access to health records. BMJ 2022;378:e071531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomes B, Calanzani N, Gysels M, et al. Heterogeneity and changes in preferences for dying at home: a systematic review. BMC Palliat Care 2013 Feb 15;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCaffrey N, Bradley S, Ratcliffe J, Currow DC.. What aspects of quality of life are important from palliative care patients’ perspectives? A systematic review of qualitative research. J Pain Symptom Manage 2016 Aug;52(2):318–28.e5. [DOI] [PubMed] [Google Scholar]
- 34.Grossman LV, Creber M, Benda RM, C N, et al. Interventions to increase patient portal use in vulnerable populations: a systematic review. J Am Med Inform Assoc 2019 Aug 1;26(8–9):855–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.French M, Keegan T, Anestis E, Preston N.. Exploring socioeconomic inequities in access to palliative and end-of-life care in the UK: a narrative synthesis. BMC Palliat Care 2021 Nov 21;20(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koffman J, Shapiro GK, Schulz-Quach C.. Enhancing equity and diversity in palliative care clinical practice, research and education. BMC Palliat Care 2023 2023/06/05;22(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lum HD, Barnes DE, Katen MT, et al. Improving a full range of advance care planning behavior change and action domains: the PREPARE randomized trial. J Pain Symptom Manage 2018 Oct 01/;56(4):575–81.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hafid A, Howard M, Guenter D, et al. Advance care planning conversations in primary care: a quality improvement project using the serious illness care program. BMC Palliat Care 2021 July 30;20(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanders JJ, Curtis JR, Tulsky JA.. Achieving goal-concordant care: a conceptual model and approach to measuring serious illness communication and its impact. J Palliat Med 2017 2018 Mar 1;21(S2):S-17–S-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malhotra C, Ramakrishnan C, Yue S-MG.. Challenges in providing end-of-life care consistent with documented patient preferences. Ann Palliat Med 2022;11(12):3610–3619. [DOI] [PubMed] [Google Scholar]
- 41.Wakefield DS, Mehr D, Keplinger L, et al. Issues and questions to consider in implementing secure electronic patient-provider web portal communications systems. Int J Med Inform 2010 Jul;79(7):469–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The review was conducted using data that were already available in the public domain, with appropriate references provided throughout. The full search strategy used in the present review can be found in the supplementary material.