Abstract
Background
Oxidative stress plays a significant role in the pathogenesis of many retinal diseases. However, only a few systematic bibliometric studies have been conducted. This study aims to visualize research hotspots and developmental trends in oxidative stress in the retina from 2013 to 2023 by analyzing bibliometric data.
Methods
We retrieved papers on oxidative stress in the retina published between 2013 and 2023 from the Web of Science Core Collection. The data were visually analyzed using CiteSpace and VOSviewer software.
Results
The total number of 2100 publications were included in the analysis. An overall increasing trend in the number of publications is observed between 2013 and 2023. Chinese publications were the most contributive, but United States publications were the most influential. Shanghai Jiao Tong University was the most active and prolific institution. Antioxidants was the most productive journal, while Oxidative Medicine and Cellular Longevity were the journals with the most-cited articles. Kaarniranta K, from Finland, was the most productive and influential author. Examination of co-cited references revealed that researchers in the field are primarily focused on investigating the molecular mechanisms, preventive strategies, and utilization of antioxidants to address retinal oxidative damage. Diabetic retinopathy, endothelial growth factor, retinitis pigmentosa, retinal degeneration, antioxidant response, retinal ganglion cells, and genes are the research hotspots in this field. Metabolism, sodium iodate, and system are at the forefront of research in this field.
Conclusion
Attention toward retinal oxidative stress has increased over the past decade. Current research focuses on the mechanisms of retinal diseases related to oxidative stress and the experimental study of antioxidants in retinal diseases, which may continue to be a trend in the future.
Keywords: Retina, Oxidative stress, Bibliometric analysis, Diabetic retinopathy, Age-related macular degeneration, Retinitis pigmentosa
1. Introduction
Retinal disease is one of the leading causes of visual impairment or loss worldwide and manifests as visual impairment, color vision impairment, or visual dysfunction [1,2]. The high incidence rate, variety, and complex pathogenesis of retinal diseases make it difficult to fully cure them, seriously impacting the quality of life [3]. At present, studies have shown a specific correlation between oxidative stress and retinal diseases, such as diabetic retinopathy (DR) [4], age-related macular degeneration (AMD) [5], and retinitis pigmentosa (RP) [6], etc. Despite differences in the causes and pathogenesis of various retinal diseases, the cell damage morphology and retinal tissue dysfunction are similar [7].
Oxidative stress occurs when oxidation and antioxidation are imbalanced within the body due to an imbalance between reactive oxygen species (ROS) production and clearance [8]. When the retinal tissue is stimulated by oxidative stress, pathological changes occur, leading to retinal cell damage and visual dysfunction [9]. The retina of an organism is one of the most metabolically active tissues with a high oxygen consumption rate. Optical and visual signal transduction processes can produce a considerable amount of ROS, which exposes retinal cells to high ROS levels for an extended period [10]. The endogenous antioxidant system typically removes excess ROS to maintain the balance. However, a disease state inhibits this steady-state mechanism, breaking the balance between the pro-oxidation and antioxidant systems and causing ROS to accumulate in the body [11]. Furthermore, phospholipid bilayers are present in the retinal tissue membrane, which maintains retinal tissue stability and normal function. Polyunsaturated fatty acids (PUFA) are abundant in phospholipid bilayers, and PUFA is sensitive to ROS, which can cause lipid peroxidation, retinal barrier damage, and tissue damage [12]. Furthermore, ROS reduces the activity of endogenous antioxidant enzymes, such as superoxide dismutase (SOD) and glutathione peroxidase (GPX), resulting in a decline in antioxidant defense ability, which further exacerbates the oxidant-antioxidant imbalance and increases oxidative damage [13]. Consequently, maintaining stable ROS levels in vivo is essential to delay the progression of retinal diseases.
Studies in preclinical cells and animals have demonstrated that many antioxidants nourish retinal cells, improve the symptoms of visual impairment and visual field defects, and delay disease progression [14]. As an effective antioxidant, vitamin D enhances the antioxidant defense capabilities of human retinal cells. It allows them to remove excess free radicals, protect them from oxidative stress-induced damage, and limit apoptosis [15]. Curcumin, a natural polyphenol compound extracted from plants, is an effective antioxidant. The curcumin-mediated Nrf2 signaling pathway activates antioxidant enzymes, plays an antioxidant role, and protects retinal cells in DR mice [16]. Furthermore, curcumin can improve retinal ultrastructure changes in DR rats by maintaining the stability of Nrf2, such as thinning of the retina and severe vacuolization of the inner core layer, etc [17]. Therefore, research on antioxidants as a potential cure for retinal diseases is crucial, especially considering the correlation between oxidative stress and the pathogenesis of these diseases.
In recent years, advancements in research on retinal diseases associated with oxidative stress have led to a substantial increase in academic literature in this area. Given the proliferation of publications, conventional analytical approaches are no longer adequate to comprehensively assess the evolution of this research domain. Bibliometrics is a discipline that uses mathematical and statistical methods to conduct a quantitative analysis of information in the literature. It helps scholars master development status and research hotspots in this field and predict future research trends [18,19]. In this study, we conducted a bibliometric analysis of the literature from the Web of Science Core Collection (WoSCC) database on retinal diseases associated with oxidative stress between 2013 and 2022. This research focused on integrating and evaluating research achievements in this field, including the quality of academic achievements of relevant countries, institutions, researchers, and publications, and provides new references for researchers interested in this field.
2. Methods
2.1. Database selection and retrieval strategy
The WoSCC database was used as the data source for the bibliometric analysis because it is one of the world's most authoritative and influential databases [20]. The search period from January 1, 2013, to June 25, 2023, was covered in the WoSCC database. Multiple pre-checks were performed before obtaining the final search results to continuously improve the search formula and to achieve more accurate and comprehensive results. The final retrieval strategy was TS = (“retinal disease” or retinopathy or retinal or epiretinal) and TS = (“oxidative stress” or “ros” or “reactive oxygen species” or “oxidative damage”). This study chose English as the publication language and article or review as the publication type.
2.2. Inclusion and exclusion criteria of literature
The relevant publications included in this study met the following criteria: (1) Papers published between January 1, 2013 and June 25, 2023 in the WoSCC database. (2) The manuscript is written in English. (3) The paper is an article or review. (4) Original papers related to retinal diseases associated with oxidative stress, including research on the pathogenesis, therapeutic drugs, clinical efficacy, etc.
Documents were excluded if they met the following conditions: (1) Non-English publications. (2) The paper was a correction, meeting abstract, retracted publication, letter, editorial, or a book chapter. (3) There was no apparent correlation between paper content and retinal diseases associated with oxidative stress. (4) Repeatedly and unofficially published papers.
2.3. Data extraction and analysis
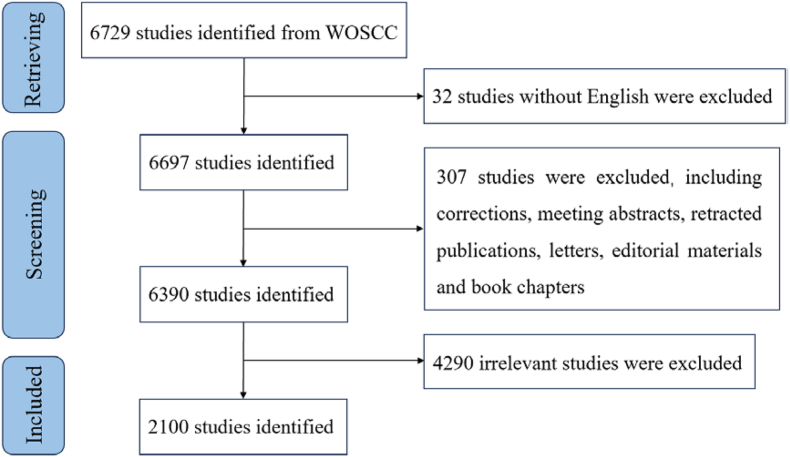
The flow chart of the literature screening portion of this study is shown in Fig. 1. A two-person screening process was conducted according to the criteria established by two researchers. A third party was consulted regarding controversial documents. After obtaining an undisputed result, the documents that met the inclusion criteria were exported to plain text in the form of “fully recorded and quoted references” using the export function of the WoSCC database. We extracted and statistically analyzed indicators, such as the number of annual publications, countries/regions, institutions, journals, authors, keywords, and references. In addition, the impact factor (IF) and journal citation reports (JCR) were used to evaluate the overall quality of the journal and predict its future scientific achievements [21,22].
Fig. 1.
Flow chart of retrieving literature on oxidative stress in the retina from 2013 to 2023 in WoSCC database.
2.4. Visualized analysis
CiteSpace, a visual analysis software developed by Chen Chaomei, tracks research hotspots within a particular field and predicts development trends [23]. In this study, the CiteSpace (6.2.R4) produces a dual-map overlay, timeline view, and citation burst. In the visual analysis atlas, each node represents a research project, and its size represents its frequency of occurrence. Thereby, nodes with connections indicate a correlation between them, and connection thickness indicates the tightness of the connection.
VOSviewer is a Java-based software designed for bibliometric analysis [24]. Its strong ability to handle large datasets and draw graphics makes it ideal for large-scale analyses [25]. In this study, the co-citations of references and the co-occurrence of keywords were analyzed using VOSviewer (1.6.18). The radius of the nodes is determined based on the weight of the research project, and the link strength can be used to determine the correlation between the nodes. Optical networks are analyzed by clustering all the nodes, and nodes with similar colors exhibit similar characteristics [26].
Bioinformatics was used to draw a world map and heat map of the distribution of publications. Additionally, Microsoft Excel 2021 was used to collect data from various sources and create related tables.
3. Results
3.1. Analysis of articles by publication year
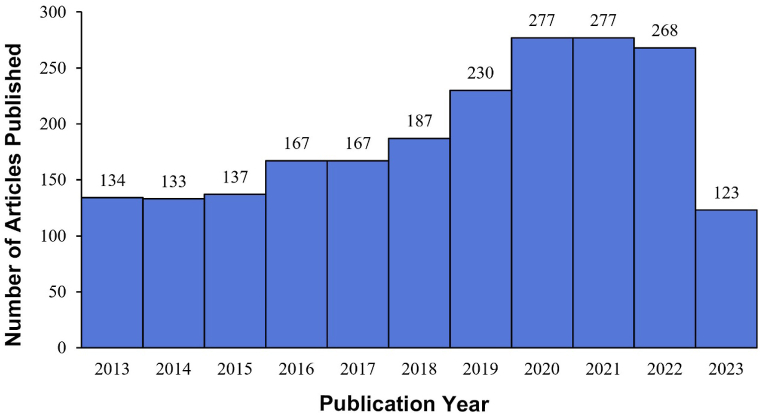
A total of 2100 publications were retrieved from the WoSCC database between 2013 and 2023 that met the retrieval criteria for this study, including 1793 articles (85.4 %) and 307 reviews (14.6 %). As shown in Fig. 2, the overall number of publications from 2013 to 2023 shows an upward trend. Notably, the annual production peaked in 2020 and 2021 at 277. This indicates that research on oxidative stress in the retina has reached a relatively mature stage.
Fig. 2.
Annual publication statistics for oxidative stress in the retina from 2013 to 2023.
3.2. Analysis of countries/regions
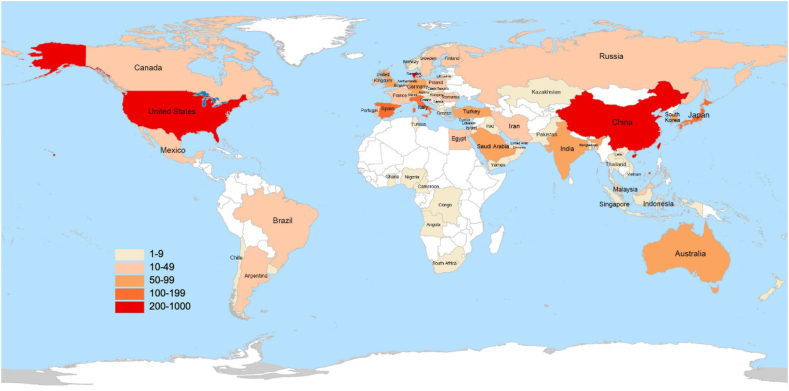
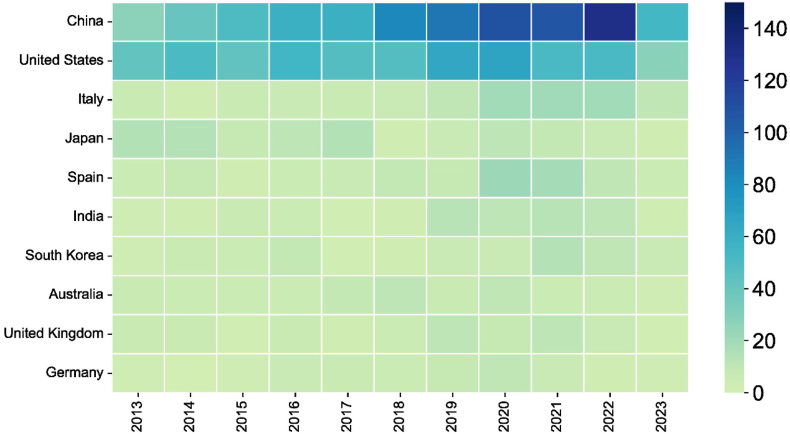
The 2100 publications come from 338 countries/regions. The distribution map of the relevant publications indicates that the global contribution in this field is uneven (Fig. 3). We ranked the top 10 countries/regions based on the number of publications. As shown in Table 1, China (811 publications, 38.62 %) had the most significant number of publications, followed by the United States (551 publications, 26.24 %) and Italy (116 publications, 5.52 %). Over half of the studies were conducted in China and the United States, indicating that researchers in these two countries were highly interested in studying oxidative stress in the retina. Studies from the United States (30), Australia (25.92), and the United Kingdom (25.63) had the highest average citations per item (ACI). This indicates that the research results of these countries were relatively mature and highly recognized. The United States has the highest centrality and total link strength (TLS), indicating its authoritative position in this field and maintaining good academic exchanges with other countries. The publication status of the literature in the top 10 countries/regions from 2013 to 2023 is shown in Fig. 4.
Fig. 3.
Global distribution map of publications on oxidative stress in the retina from 2013 to 2023.
Table 1.
Top 10 countries/regions with the highest productivity regarding the research on oxidative stress in the retina in 2013–2023.
| Rank | Country | TP | TC | ACI | Centrality | TLS |
|---|---|---|---|---|---|---|
| 1 | China | 811 | 13,551 | 16.71 | 0.25 | 181 |
| 2 | United States | 551 | 16,526 | 30 | 0.47 | 290 |
| 3 | Italy | 116 | 2323 | 20 | 0.1 | 72 |
| 4 | Japan | 103 | 2479 | 24.07 | 0.06 | 28 |
| 5 | Spain | 101 | 2197 | 21.75 | 0.17 | 66 |
| 6 | India | 74 | 1126 | 15.22 | 0.06 | 34 |
| 7 | South Korea | 74 | 908 | 12.27 | 0.03 | 14 |
| 8 | Australia | 71 | 1840 | 25.92 | 0.06 | 57 |
| 9 | United Kingdom | 67 | 1717 | 25.63 | 0.22 | 93 |
| 10 | Germany | 59 | 1232 | 20.88 | 0.1 | 48 |
TP, Total number of publications; TC, Total number of citations in total publications; ACI, Average citations per item; TLS, Total link strength.
Fig. 4.
Bubble chart of publications on oxidative stress in the retina from 2013 to 2023 in the top 10 countries/regions.
3.3. Analysis of institutions
The 2100 publications come from 6380 institutions. Table 2 lists the top 10 institutions for the total number of publications (TP). The results show that most of the top ten institutions are in China and the United States. Shanghai Jiao Tong University (54 papers) published the most research papers, followed by Sun Yat-Sen University (50 papers) and Xi An Jiao Tong University (45 papers). Interestingly, although the number of publications was small, the ACI and TLS of the University of Eastern Finland and Kuopio University Hospital in Finland were the highest.
Table 2.
Top 10 institutions with the highest productivity regarding the research on oxidative stress in the retina in 2013–2023.
| Rank | Institution | Country | TP | TC | ACI | TLS |
|---|---|---|---|---|---|---|
| 1 | Shanghai Jiao Tong University | China | 54 | 1099 | 20.35 | 59 |
| 2 | Sun Yat-Sen University | China | 50 | 824 | 16.48 | 42 |
| 3 | Xi An Jiao Tong University | China | 45 | 706 | 15.69 | 16 |
| 4 | Wayne State University | United States | 42 | 1743 | 41.5 | 6 |
| 5 | University of Eastern Finland | Finland | 39 | 1940 | 49.74 | 88 |
| 6 | Nanjing Medical University | China | 36 | 1095 | 30.42 | 31 |
| 7 | Kuopio University Hospital | Finland | 34 | 1853 | 54.5 | 83 |
| 8 | University of Oklahoma | United States | 33 | 1127 | 34.15 | 28 |
| 9 | Augusta University | United States | 31 | 551 | 17.77 | 32 |
| 10 | Case Western Reserve University | United States | 30 | 1254 | 41.8 | 31 |
3.4. Analysis of journals
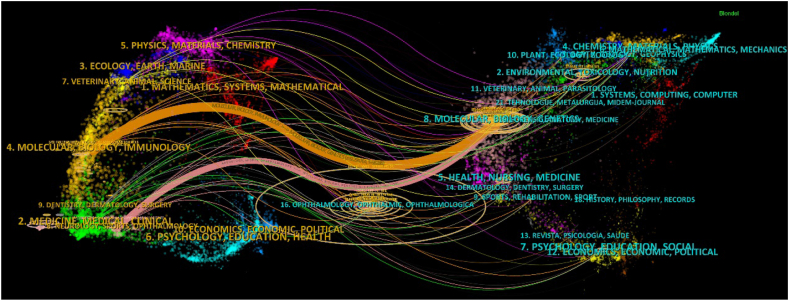
The 2100 publications were obtained from 528 journals. As shown in Table 3, the top 10 journals of TP published 640 publications, accounting for 30.48 % of the total publications. Antioxidants was the most productive journal, with 118 publications, followed by Investigative Ophthalmology & Visual Science (94) and Experimental Eye Research (87). In Table 4, Oxidative Medicine and Cellular Longevity are the journals with the highest total number of citations (TC), with 3272 citations, followed by Investigative Ophthalmology & Visual Science (2,620) and Experimental Eye Research (1,859). Although Progress in Retinal and Eye Research has only published eight articles, its IF and ACI are the highest, indicating that the quality of the articles in this journal is high and widely recognized. A dual-map overlay of the journals is shown in Fig. 5. The color curves represent the citation paths between the left and right nodes in the figure. The left nodes are the citing journals, and the right nodes are the cited journals. The orange and pink paths represent reference relationships between highly active research fields. Most of the papers were published in the fields of “NEUROLOGY, SPORTS, and OPHTHALMOLOGY” and “MOLECULAR, BIOLOGY, and IMMUNOLOGY,” and these were mainly influenced by the field of “MOLECULAR, BIOLOGY, and GENETICS.”
Table 3.
Top 10 journals with the highest productivity regarding the research on oxidative stress in the retina in 2013–2023.
| Rank | Journal | TP | TC | ACI | JCR | IF (2022) |
|---|---|---|---|---|---|---|
| 1 | Antioxidants | 118 | 1416 | 12 | Q1 | 7 |
| 2 | Investigative Ophthalmology & Visual Science | 94 | 2620 | 27.87 | Q1 | 4.4 |
| 3 | Experimental Eye Research | 87 | 1859 | 21.37 | Q2 | 3.4 |
| 4 | Oxidative Medicine and Cellular Longevity | 83 | 3272 | 39.42 | Q2 | 7.31 |
| 5 | International Journal of Molecular Sciences | 73 | 1128 | 15.45 | Q1 | 5.6 |
| 6 | PLoS One | 46 | 1599 | 34.76 | Q2 | 3.7 |
| 7 | Scientific Reports | 42 | 1079 | 25.69 | Q2 | 4.6 |
| 8 | Free Radical Biology and Medicine | 41 | 1228 | 29.95 | Q1 | 7.4 |
| 9 | Redox Biology | 30 | 1130 | 37.67 | Q1 | 11.4 |
| 10 | Nutrients | 26 | 512 | 19.69 | Q1 | 5.9 |
Table 4.
Top 10 journals with the most citations regarding the research on oxidative stress in the retina in 2013–2023.
| Rank | Journal | TP | TC | ACI | JCR | IF (2022) |
|---|---|---|---|---|---|---|
| 1 | Oxidative Medicine and Cellular Longevity | 83 | 3272 | 39.42 | Q2 | 7.31 |
| 2 | Investigative Ophthalmology & Visual Science | 94 | 2620 | 27.87 | Q1 | 4.4 |
| 3 | Experimental Eye Research | 87 | 1859 | 21.37 | Q2 | 3.4 |
| 4 | Cell Death & Disease | 24 | 1730 | 72.08 | Q1 | 9 |
| 5 | PLoS One | 46 | 1599 | 34.76 | Q2 | 3.7 |
| 6 | Antioxidants | 118 | 1416 | 12 | Q1 | 7 |
| 7 | Free Radical Biology and Medicine | 41 | 1228 | 29.95 | Q1 | 7.4 |
| 8 | Progress in Retinal and Eye Research | 8 | 1215 | 151.88 | Q1 | 17.8 |
| 9 | Redox Biology | 30 | 1130 | 37.67 | Q1 | 11.4 |
| 10 | International Journal of Molecular Sciences | 73 | 1128 | 15.45 | Q1 | 5.6 |
Fig. 5.
Dual-map overlay of journals involved in studies of oxidative stress in the retina in 2013–2023.
3.5. Analysis of authors
The 2100 publications come from 9450 authors. As shown in Table 5, Kaarniranta K from the University of Eastern Finland ranked first with 35 papers, Kowluru RA from Wayne State University with 23 papers, and Hara H from Gifu Pharmaceutical University with 22 papers. Among the most-cited authors, Kaarniranta K tops the list with 1844 citations, followed by Kowluru RA (1,304) and Blasiak J from the University of Lodz (1,253).
Table 5.
Top 10 prolific authors and cited authors regarding the research on oxidative stress in the retina in 2013–2023.
| Rank | Productive author | Institution | TP | Cited author | Institution | TC |
|---|---|---|---|---|---|---|
| 1 | Kaarniranta K | University of Eastern Finland | 35 | Kaarniranta K | University of Eastern Finland | 1844 |
| 2 | Kowluru RA | Wayne State University | 23 | Kowluru RA | Wayne State University | 1304 |
| 3 | Hara H | Gifu Pharmaceutical University | 22 | Blasiak J | University of Lodz | 1253 |
| 4 | Shimazawa M | Gifu Pharmaceutical University | 19 | Handa JT | Johns Hopkins School of Medicine | 1241 |
| 5 | Wang J | Augusta University | 18 | Cano M | Emory University School of Medicine | 1022 |
| 6 | Blasiak J | University of Lodz | 17 | Mishra MK | Bhabha Atomic Research Centre | 832 |
| 7 | Zhou FF | The University of Sydney | 17 | Wang L | Johns Hopkins School of Medicine | 779 |
| 8 | Handa JT | Johns Hopkins School of Medicine | 16 | Kauppinen A | University of Eastern Finland | 759 |
| 9 | Yang CH | National Taiwan University | 16 | Hara H | Gifu Pharmaceutical University | 752 |
| 10 | Zhu L | The University of Sydney | 16 | Shimazawa M | Gifu Pharmaceutical University | 682 |
3.6. Analysis of references
The number of citations reflects the degree of recognition of the research results in the field of oxidative stress in the retina. This is an important indicator for testing the quality of the study. Table 6 provides information on the top 10 cited references. The paper with the highest TC was written by Asmat U, published in Saudi Pharmaceutical Journal in 2016, followed by the paper written by Nita M and published in Oxidative Medicine and Cellular Longevity in 2016, and the paper written by Datta S, published in Progress in retinal and eye research in 2017.
Table 6.
Top 10 cited references regarding the research on oxidative stress in the retina in 2013–2023.
| Rank | PMID number | Year | Journal | Author | TC |
|---|---|---|---|---|---|
| 1 | 27752226 | 2016 | Saudi Pharmaceutical Journal | Asmat U | 788 |
| 2 | 26881021 | 2016 | Oxidative Medicine and Cellular Longevity | Nita M | 661 |
| 3 | 28336424 | 2017 | Progress in Retinal and Eye Research | Datta S | 377 |
| 4 | 25484094 | 2014 | Autophagy | Mitter SK | 308 |
| 5 | 30030554 | 2018 | Diabetologia | Simó R | 248 |
| 6 | 23590900 | 2013 | Autophagy | Kaarniranta K | 237 |
| 7 | 29988039 | 2018 | Cell Death & Disease | Sun Y | 228 |
| 8 | 24067647 | 2013 | Proceedings of the National Academy of Sciences of the United States of America | Du Y | 222 |
| 9 | 25975734 | 2015 | Progress in Retinal and Eye Research | Kowluru RA | 216 |
| 10 | 28055007 | 2017 | Cell Death & Disease | Golestaneh N | 208 |
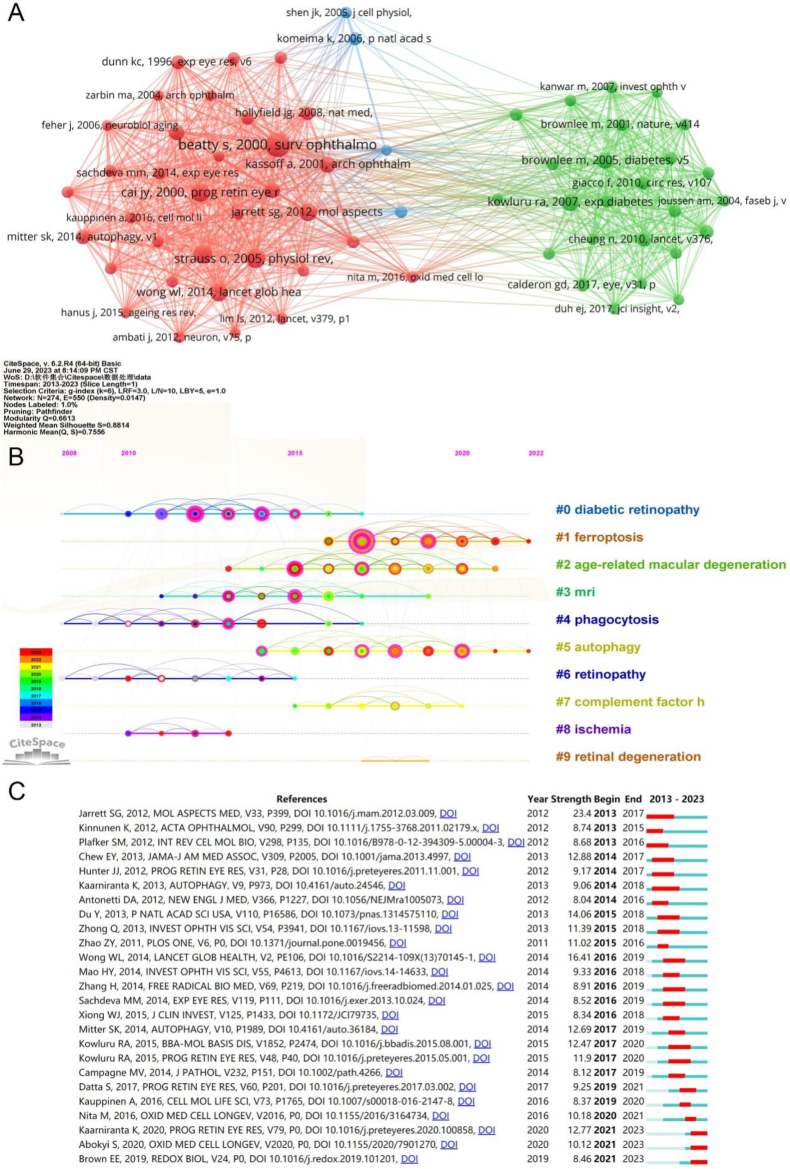
A co-citation relationship between two publications occurs when they are cited simultaneously in another document. Reference co-citation analysis is a valuable method for constructing the fundamental knowledge base of a research field. As shown in Table 7, the paper with the most significant number of co-citations was written by Beatty S and published in the Survey of Ophthalmology in 2000, followed by the paper written by Strauss O, published in Physiological Reviews in 2005, and the paper written by Datta S, published in Progress in Retinal and Eye Research in 2017. A visualization of the co-citation relationships among the references is shown in Fig. 6A, which contains 63 items organized into three clusters. As shown in Fig. 6B, the ten significant clusters of co-cited references were diabetic retinopathy, ferroptosis, age-related macular degeneration, MRI, phagocytosis, autophagy, retinopathy, complement factor h, ischemia, and retinal degeneration. A list of the top 25 references with the most vigorous citation bursts is presented in Fig. 6C. The most significant citation burst was for a paper written by Jarrett SG in 2012 [27]. This paper has an intensity of 23.4.
Table 7.
Top 10 co-cited references regarding the research on oxidative stress in the retina in 2013–2023.
| Rank | PMID number | Year | Journal | Author | TC |
|---|---|---|---|---|---|
| 1 | 11033038 | 2000 | Survey of Ophthalmology | Beatty S | 261 |
| 2 | 15987797 | 2005 | Physiological Reviews | Strauss O | 159 |
| 3 | 28336424 | 2017 | Progress in Retinal and Eye Research | Datta S | 153 |
| 4 | 10674708 | 2000 | Progress in Retinal and Eye Research | Cai JY | 148 |
| 5 | 22510306 | 2012 | Molecular Aspects of Medicine | Jarrett SG | 137 |
| 6 | 25104651 | 2014 | Lancet Global Health | Wong WL | 132 |
| 7 | 17641741 | 2007 | Experimental Diabetes Research | Kowluru RA | 131 |
| 8 | 11594942 | 2001 | Archives of Ophthalmology | Kassoff A | 119 |
| 9 | 15919781 | 2005 | Diabetes | Brownlee M | 117 |
| 10 | 12634104 | 2003 | Experimental Eye Research | Liang FQ | 105 |
Fig. 6.
Analysis of co-cited references on studies of oxidative stress in the retina in 2013–2023. (A) Network map of co-cited references. (B) A timeline view of co-cited references. (C) Top 25 co-cited references with the strongest citation burst.
3.7 Keywords analysis of research hotspots.
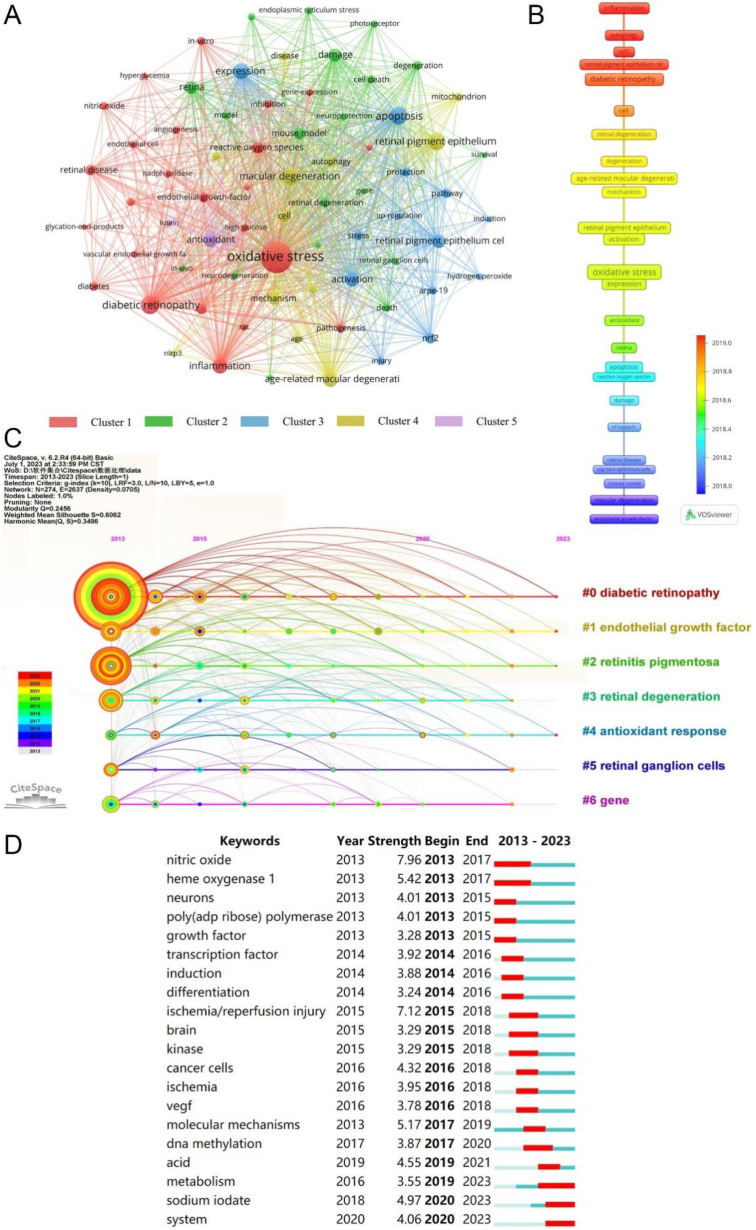
Keyword co-occurrence analysis based on VOSviewer can reflect the oxidative stress in the retina field's research direction and hot issues. As shown in Fig. 7A, a network map of co-occurring keywords illustrating the distribution of hotspots in this field was created based on 70 keywords with more than 50 co-occurrence times, which were divided into five clusters, with each color representing a cluster. The red cluster, with 24 keywords, was mainly related to the pathogenesis of DR, including diabetic retinopathy, oxidative stress, reactive oxygen species, and inflammatory responses. The green cluster, with 19 keywords, was mainly involved in the pathogenesis of RP, including retinitis pigmentosa, mouse model, damage, and degeneration. The blue cluster with 13 keywords was primarily associated with the mechanism of Nrf2 in retinal pigment epithelium (RPE) cells, including retinal pigment epithelium cells, Nrf2 apoptosis, expression, and activation. The yellow cluster with 12 keywords was mainly related to the mechanisms of age-related macular degeneration, including age-related macular degeneration, retinal pigment epithelium, autophagy, and mitochondria. The purple cluster with two keywords focused on the application of antioxidants and lutein. Fig. 7B shows the average year of emergence of the top 25 keywords, highlighting inflammation, autophagy, and Nrf2 as prominent research areas in recent times. Fig. 7C illustrates the researchers' enduring interest in diabetic retinopathy, endothelial growth factor, retinitis pigmentosa, retinal degeneration, antioxidant response, retinal ganglion cell, and genes. In Fig. 7D, we report the top 20 keywords with citation bursts in the field of oxidative stress in the retina over the past ten years. Nitric oxide (7.96) ranked highest in outburst strength, followed by ischemia/reperfusion injury (7.12) and heme oxygenase 1 (5.42). Recent research has focused on metabolism, sodium iodate, and system.
Fig. 7.
Analysis of keywords on studies of oxidative stress in the retina in 2013–2023. (A) Network map of keywords. (B) The average year in which keywords with high frequency appear. (C) A timeline view of keywords. (D) Top 20 keywords with the strongest citation burst.
4. Discussion
4.1. General information
Through the statistical analysis of 2100 articles from the WoSCC database over the past ten years, it was found that the annual changes in publications can be divided into three stages: slow growth period (2013–2018), rapid growth period (2018–2020), and stable development period (2020–2023). Studies of oxidative stress in the retina have maintained an overall growth trend, indicating that there is still significant interest in this research field.
Only five of the 338 countries/regions have published over 100 papers on oxidative stress in the retina, indicating an imbalance in global publications across countries/regions. China has the highest publishing volume, and the top three institutions in terms of total publishing volume are all from China, demonstrating that China has been in a rapid development stage in this field over the past decade, with a high degree of attention and a great deal of scientific research. The United States not only has the second-highest number of publications but also has the highest TC, ACI, TLS, and centrality. Consequently, this indicates that the United States enjoys a strong academic influence in the research field of oxidative stress in the retina. This advantage is probably because the United States is the center of the global cooperation network, maintains close contact with other developed countries, and facilitates resource sharing. Thus, international academic exchanges must be broken down to learn from each other and progress. It is worth noting that the top 10 countries/regions and institutions are mainly distributed in developed countries and have strong centrality, probably because of the muscular economic strength and scientific research level of developed countries, causing the research level in China to still lag behind the international level. Therefore, in addition to strengthening cooperation and exchanges with foreign institutions, Chinese researchers should accelerate the training of scientific research talent and increase support for improving Chinese research in the future.
Shanghai Jiao Tong University, which is located in China, has the highest TP and has contributed significantly to the active development of this field. The central university with the highest ACI is the University of Eastern Finland, which not only contributes to high-quality achievements in this field but also plays a bridging role in the global cooperation network. Interestingly, the most fruitful and recognized author was from the University of Eastern Finland. Kaarniranta K published 35 papers on oxidative stress in the retina and received 1844 citations. Based on his highest-cited article, he found that oxidative stress-induced RPE damage was the leading risk factor for AMD and that mitochondria were an essential source of ROS increase in RPE cells, thus reducing mitochondrial DNA damage, which plays an essential role in alleviating AMD [28]. In another highly cited paper, he studied the mechanism of mitochondrial dysfunction in AMD and provided a new research direction for follow-up clinical experiments on AMD [29]. Kowluru RA from Wayne State University had the second-largest number of publications and citations. One of his most frequently cited studies found that oxidative stress is closely related to DR, which has important practical implications for developing antioxidant-controlled trials [30]. In the field of oxidative stress in the retina, these institutions and authors have enjoyed significant academic influence and contributed significantly to its development.
The top 10 most prolific journals and cited journals all belong to Q1 or Q2, which implies that these journals have a high academic reputation in this field. Antioxidants have the highest TP and contribute significantly to the research frontier. Oxidative Medicine and Cellular Longevity are the most frequently cited journals and have been recognized by most researchers. Redox Biology and Progress in Retinal and Eye Research are prolific and highly cited journals with the highest IF, contributing to high-quality articles in this field. The most mature and influential research results in this field can be obtained by focusing on these journals. Moreover, the results of the dual-map overlay of journals showed that the current research on oxidative stress in the retina mainly focuses on the molecular level.
Although the number of citations does not fully reflect the quality of an article, owing to factors such as publication time, highly cited literature in a given period has a particularly leading role in this field. The most-cited literature has primarily focused on the molecular mechanisms underlying retinal diseases and oxidative stress. In the literature with the highest number of citations, Asmat U and his team explored the role and biomarkers of oxidative stress in the pathogenesis of diabetes and its complications and summarized the corresponding basic knowledge for reference by other researchers [31]. The top 10 co-cited studies investigated molecular mechanisms, preventive measures against oxidative damage in the retina, and the utilization of antioxidants. A publication authored by Beatty S in 2000, which appeared in the Survey of Ophthalmology, held the highest ranking in terms of co-citations. Beatty et al. provided a comprehensive summary of the molecular mechanisms linking AMD with oxidative stress, introduced a defense mechanism against oxidative stress in AMD, and explored the application of various antioxidants, including vitamins A, C, and E, and carotenoids in the treatment of AMD [32]. These results indicate that the research community in the field of retinal oxidative stress greatly emphasizes the study of molecular mechanisms and clinical prevention and treatment.
4.2. Research hotspots and frontiers
Based on visual maps of co-cited references and keywords, we summarized an overview of the field of oxidative stress in the retina, including the following: (1) DR, AMD, and RP are among the most prevalent retinal diseases related to oxidative stress [33]. (2) The dysfunction and death of RPE cells are mediated by oxidative stress and are related to inflammation, autophagy, and ferroptosis [[34], [35], [36]]. (3) The Sodium iodate-induced retinal degeneration model has been extensively employed in diverse fundamental and translational investigations because of its ability to effectively replicate features observed in cases of retinal degeneration [37]. Furthermore, this model can be utilized in wild-type and gene-knockout strains [38]. (4) As an antioxidant regulatory gene, Nrf2 has been shown to resist oxidative stress damage and inhibit retinal inflammation [39]. The efficacy of Nrf2 activators has been validated in several models of retinal pathological models [40]. (5) Quench-assisted MRI can measure the degree of free radical production in different retinal cell layers and evaluate their physiological and pathological statuses [41,42]. (6) The Y402H polymorphism of complement factor H was significantly associated with susceptibility to AMD [43,44]. (7) Ranibizumab, aflibercept, and conbercept, which are anti-vascular endothelial growth factor (VEGF) drugs, are extensively employed in the treatment of diverse vascular retinal diseases, including AMD and DR, owing to their advantageous properties in promoting neovascular regression, reducing leakage, and facilitating absorption [45,46]. (8) Prolonged utilization of antioxidants such as lutein and multivitamins can effectively decelerate the progression of AMD and RP [47,48]. Molecular biology, cell biology, and other research methodologies are used to elucidate the molecular mechanisms underlying the onset and progression of retinal diseases. Additionally, identifying biomarkers for disease diagnosis, ascertaining therapeutic targets, and subsequently informing clinical drug utilization represent the current focal point and cutting-edge pursuit within the domain of oxidative stress in the retina. In conjunction with the relevant literature, we analyze the following aspects.
4.2.1. DR and oxidative stress
As the most common microvascular complication of diabetes, the pathogenesis of DR has been proven to be related to the excessive production of ROS [49]. Under high glucose conditions, excessive glucose participates in the tricarboxylic acid cycle and decomposes to produce excessive ROS; intracellular lipids and proteins initiate chain reactions, thus inducing oxidative stress, leading to cell damage and even death [50]. Based on these findings, antioxidants have been widely used in preclinical trials as potential drugs for treating retinal diseases. Vitamin D3 can reduce high ROS levels caused by diabetes by activating the ROS/TXNIP/NLRP3 signaling pathway to achieve the purpose of preventing DR [51]. Anthocyanin extracts can delay the progression of diabetic retinopathy by preventing the oxidative damage caused by high glucose [52]. Several natural dietary compounds, including polyphenols and carotenoids, have been shown to resist DR retinal oxidative stress in vivo and in vitro [53].
4.2.2. AMD and oxidative stress
In addition to its high oxygen consumption and metabolic activity, the retina is exposed to light for long periods, making it highly susceptible to oxidative stress [54]. During aging, the density of RPE cells and photoreceptors gradually decreases, their antioxidant capacity decreases, and cellular ROS levels increase. ROS are primarily formed in the mitochondria of retinal photoreceptors and RPE cells. Excessive ROS damage DNA oxidatively and cause abnormal protein expression when exceeding the scavenging capacity of the antioxidant system [55,56]. Therefore, protecting mitochondrial DNA from oxidative stress damage is a new strategy for delaying AMD progression [57]. The first report on the mechanism of RPE cell death induced by light injury suggested that a disorder in mitochondrial dynamics might be involved, indicating that the regulation of mitochondrial dynamics is likely to be a new target for treating AMD [58]. Many antioxidants, including melatonin and resveratrol, have been extensively studied in recent years as potential AMD medications [59,60].
4.2.3. RP and oxidative stress
RPE cell dysfunction and the progressive loss of photoreceptors are pathological features of RP. The loss of photoreceptor cells starts from the rod cells that occupy most of the outer layer of the retina. The cone cells are then exposed to a high-oxygen environment, which causes oxidative damage [13]. According to both in vivo and in vitro studies, kaempferol treatment reduced the apoptosis of RPE cells and photoreceptors, significantly alleviated retinal dysfunction in RP, and improved vision in RP animals [61]. Another study found that ARPE-24 cells treated with gypenosides exhibited significantly increased activity, suggesting that gypenosides protect RPE cells from oxidative damage [62]. Consequently, oxidative stress is an important cause of RPE cell and photoreceptor death, and antioxidants may effectively alleviate its occurrence and development.
5. Strengths and limitations
This is the first study that used CiteSpace and VOSviewer software to perform quantitative analyses of documents on oxidative stress in the retina. Using this accurate and objective quantitative analysis, the ophthalmic research community can not only summarize the hotspots and developmental status in the field of oxidative stress in the retina but also determine the developmental trend. However, this study had some limitations. Firstly, the data sources were not sufficiently comprehensive. Despite being a high-quality and authoritative database, WoSCC does not contain documents available in PubMed, Cochrane, or other databases. Secondly, only documents written in English were included, which did not fully depict the global situation. Finally, the time difference between the publications of literature varies for various reasons; therefore, the perspective of this study may need to be more accurate. Nonetheless, this study attempts to present the overall situation and developmental trends of oxidative stress in the retina as comprehensively as possible.
6. Conclusion
This data visualization study through bibliometric research revealed the dynamic development process, hot frontier fields, and future development trends in the field of oxidative stress research in the retina over the past ten years. The current emphasis and forefront exploration in the realm of retinal oxidative stress involves utilizing molecular biology, cell biology, and other research methodologies to elucidate the molecular mechanisms underlying the onset and progression of retinal diseases, identify biomarkers for disease diagnosis, pinpoint therapeutic targets, and subsequently offer insights for clinical drug applications.
Funding
This study was funded by grants from the Science and Technology Innovation Program of Hunan Province (No. 2021RC3101), the National Natural Science Foundation of China (No. 82004427, 82374429), the Guidance Project of Academician Liu Liang's Expert Workstation (No. 22YS003), the Natural Science Foundation of Hunan Province (No. 2023JJ30460, 2023JJ40474), and the Open Disciple Construction Project of Hunan University of Chinese Medicine (No. 22JBZ027).
Data availability
Relevant data from this study will be provided as required.
CRediT authorship contribution statement
Meng Xiong: Writing – original draft, Visualization, Conceptualization. Chang Yu: Visualization. Baoping Ren: Investigation. Meiqi Zhong: Investigation. Jing Lu: Data curation. Chengzhi Yuan: Software. Qifang Sun: Software. Qinghua Peng: Supervision, Conceptualization. Meiyan Zeng: Writing – review & editing, Funding acquisition, Conceptualization. Houpan Song: Writing – review & editing, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Meiyan Zeng, Email: 004324@hnucm.edu.cn.
Houpan Song, Email: songhp@hnucm.edu.cn.
References
- 1.Tauscher R.G., Simon S.S., Volpe N.J. Retinal disease in the neurology clinic. Curr. Opin. Neurol. 2021;34:122–132. doi: 10.1097/WCO.0000000000000883. [DOI] [PubMed] [Google Scholar]
- 2.Alfonsetti M., Castelli V., d'Angelo M., Benedetti E., Allegretti M., Barboni B., Cimini A. Looking for in vitro models for retinal diseases. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms221910334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardue M.T., Allen R.S. Neuroprotective strategies for retinal disease. Prog. Retin. Eye Res. 2018;65:50–76. doi: 10.1016/j.preteyeres.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammes H.-P. Diabetic retinopathy: hyperglycaemia, oxidative stress and beyond. Diabetologia. 2018;61:29–38. doi: 10.1007/s00125-017-4435-8. [DOI] [PubMed] [Google Scholar]
- 5.Ruan Y., Jiang S., Gericke A. Age-related macular degeneration: role of oxidative stress and blood vessels. Int. J. Mol. Sci. 2021;22:1296. doi: 10.3390/ijms22031296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallenga C.E., Lonardi M., Pacetti S., Violanti S.S., Tassinari P., Di Virgilio F., Tognon M., Perri P. Molecular mechanisms related to oxidative stress in retinitis pigmentosa. Antioxidants. 2021;10:848. doi: 10.3390/antiox10060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuenca N., Fernández-Sánchez L., Campello L., Maneu V., De la Villa P., Lax P., Pinilla I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 2014;43:17–75. doi: 10.1016/j.preteyeres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Nathan C., Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat. Rev. Immunol. 2013;13:349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura Y., Hara H., Kondo M., Hong S., Matsugi T. Oxidative stress in retinal diseases. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/4076518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright A.F., Chakarova C.F., Abd El-Aziz M.M., Bhattacharya S.S. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat. Rev. Genet. 2010;11:273–284. doi: 10.1038/nrg2717. [DOI] [PubMed] [Google Scholar]
- 11.Pinilla I., Maneu V. Oxidative stress as a main contributor of retinal degenerative diseases. Antioxidants. 2022;11:1190. doi: 10.3390/antiox11061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres-Cuevas I., Millán I., Asensi M., Vento M., Oger C., Galano J.-M., Durand T., Ortega Á.L. Analysis of lipid peroxidation by UPLC-MS/MS and retinoprotective effects of the natural polyphenol pterostilbene. Antioxidants. 2021;10:168. doi: 10.3390/antiox10020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campochiaro P.A., Mir T.A. The mechanism of cone cell death in Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018;62:24–37. doi: 10.1016/j.preteyeres.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Miranda M., Romero F.J. Antioxidants and retinal diseases. Antioxidants. 2019;8:604. doi: 10.3390/antiox8120604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-Robredo P., González-Zamora J., Recalde S., Bilbao-Malavé V., Bezunartea J., Hernandez M., Garcia-Layana A. Vitamin D protects against oxidative stress and inflammation in human retinal cells. Antioxidants. 2020;9:838. doi: 10.3390/antiox9090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong Q., Mishra M., Kowluru R.A. Transcription factor Nrf2-mediated antioxidant defense system in the development of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2013;54:3941–3948. doi: 10.1167/iovs.13-11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie T., Chen X., Chen W., Huang S., Peng X., Tian L., Wu X., Huang Y. Curcumin is a potential adjuvant to alleviates diabetic retinal injury via reducing oxidative stress and maintaining Nrf2 pathway homeostasis. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.796565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson D.F., Walker C.K. A descriptive and historical review of bibliometrics with applications to medical sciences. Pharmacotherapy. 2015;35:551–559. doi: 10.1002/phar.1586. [DOI] [PubMed] [Google Scholar]
- 19.Hicks D., Wouters P., Waltman L., de Rijcke S., Rafols I. Bibliometrics: the leiden manifesto for research metrics. Nature. 2015;520:429–431. doi: 10.1038/520429a. [DOI] [PubMed] [Google Scholar]
- 20.Merigó J.M., Núñez A. Influential journals in health research: a bibliometric study. Glob. Health. 2016;12:46. doi: 10.1186/s12992-016-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X.-F., Fu Q., Rousseau R. On indexing in the Web of Science and predicting journal impact factor. J. Zhejiang Univ. - Sci. B. 2008;9:582–590. doi: 10.1631/jzus.B0840001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sims J.L., McGhee C.N.J. Citation analysis and journal impact factors in ophthalmology and vision science journals. Clin. Exp. Ophthalmol. 2003;31:14–22. doi: 10.1046/j.1442-9071.2003.00610.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. 2004;101(Suppl 1):5303–5310. doi: 10.1073/pnas.0307513100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang F., Zhu G., Chen J., Zhang J., Dong S., Cheng S. Current status and trends in the modernization of pulse diagnosis research: a bibliometric analysis based on Citespace and VOSviewer. Digital Chinese Medicine. 2023;6:405–415. [Google Scholar]
- 25.van Eck N.J., Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leydesdorff L., Bornmann L., Wagner C.S. Generating clustered journal maps: an automated system for hierarchical classification. Scientometrics. 2017;110:1601–1614. doi: 10.1007/s11192-016-2226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarrett S.G., Boulton M.E. Consequences of oxidative stress in age-related macular degeneration. Mol Aspects Med. 2012;33:399–417. doi: 10.1016/j.mam.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaarniranta K., Pawlowska E., Szczepanska J., Jablkowska A., Blasiak J. Role of mitochondrial DNA damage in ROS-mediated pathogenesis of age-related macular degeneration (AMD) Int. J. Mol. Sci. 2019;20:2374. doi: 10.3390/ijms20102374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaarniranta K., Uusitalo H., Blasiak J., Felszeghy S., Kannan R., Kauppinen A., Salminen A., Sinha D., Ferrington D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020;79 doi: 10.1016/j.preteyeres.2020.100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kowluru R.A., Chan P.-S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007;2007 doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asmat U., Abad K., Ismail K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm J. 2016;24:547–553. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beatty S., Koh H., Phil M., Henson D., Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 33.Wang J., Li M., Geng Z., Khattak S., Ji X., Wu D., Dang Y. Role of oxidative stress in retinal disease and the early intervention strategies: a review. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/7836828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dehdashtian E., Mehrzadi S., Yousefi B., Hosseinzadeh A., Reiter R.J., Safa M., Ghaznavi H., Naseripour M. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci. 2018;193:20–33. doi: 10.1016/j.lfs.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Trachsel-Moncho L., Benlloch-Navarro S., Fernández-Carbonell Á., Ramírez-Lamelas D.T., Olivar T., Silvestre D., Poch E., Miranda M. Oxidative stress and autophagy-related changes during retinal degeneration and development. Cell Death Dis. 2018;9:812. doi: 10.1038/s41419-018-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Totsuka K., Ueta T., Uchida T., Roggia M.F., Nakagawa S., Vavvas D.G., Honjo M., Aihara M. Oxidative stress induces ferroptotic cell death in retinal pigment epithelial cells. Exp. Eye Res. 2019;181:316–324. doi: 10.1016/j.exer.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koh A.E.-H., Alsaeedi H.A., Rashid M.B.A., Lam C., Harun M.H.N., Saleh M.F.B.M., Luu C.D., Kumar S.S., Ng M.H., Isa H.M., Leow S.N., Then K.Y., Bastion M.-L.C., Ali Khan M.S., Mok P.L. Retinal degeneration rat model: a study on the structural and functional changes in the retina following injection of sodium iodate. J. Photochem. Photobiol., B. 2019;196 doi: 10.1016/j.jphotobiol.2019.111514. [DOI] [PubMed] [Google Scholar]
- 38.Kannan R., Hinton D.R. Sodium iodate induced retinal degeneration: new insights from an old model. Neural Regen Res. 2014;9:2044–2045. doi: 10.4103/1673-5374.147927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tu Y., Li L., Zhu L., Guo Y., Du S., Zhang Y., Wang Z., Zhang Y., Zhu M. Geniposide attenuates hyperglycemia-induced oxidative stress and inflammation by activating the Nrf2 signaling pathway in experimental diabetic retinopathy. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/9247947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakagami Y. Nrf2 is an attractive therapeutic target for retinal diseases. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/7469326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berkowitz B.A., Lewin A.S., Biswal M.R., Bredell B.X., Davis C., Roberts R. MRI of retinal free radical production with laminar resolution in vivo. Invest. Ophthalmol. Vis. Sci. 2016;57:577–585. doi: 10.1167/iovs.15-18972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ba B., Rh P., J L., N K., C T., Jy W., Am B., K D., K S., R R. Sodium iodate produces a strain-dependent retinal oxidative stress response measured in vivo using QUEST MRI. Invest. Ophthalmol. Vis. Sci. 2017;58 doi: 10.1167/iovs.17-21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toomey C.B., Johnson L.V., Bowes Rickman C. Complement factor H in AMD: bridging genetic associations and pathobiology. Prog. Retin. Eye Res. 2018;62:38–57. doi: 10.1016/j.preteyeres.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klein R.J., Zeiss C., Chew E.Y., Tsai J.-Y., Sackler R.S., Haynes C., Henning A.K., SanGiovanni J.P., Mane S.M., Mayne S.T., Bracken M.B., Ferris F.L., Ott J., Barnstable C., Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown D.M., Wykoff C.C., Boyer D., Heier J.S., Clark W.L., Emanuelli A., Higgins P.M., Singer M., Weinreich D.M., Yancopoulos G.D., Berliner A.J., Chu K., Reed K., Cheng Y., Vitti R. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 2021;139:946–955. doi: 10.1001/jamaophthalmol.2021.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L., Zhang C., Hua R. Clinical effectiveness of ranibizumab and conbercept for neovascular age-related macular degeneration: a meta-analysis. Drug Des Devel Ther. 2018;12:3625–3633. doi: 10.2147/DDDT.S176021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pameijer E.M., Heus P., Damen J.A.A., Spijker R., Hooft L., Ringens P.J., Imhof S.M., van Leeuwen R. What did we learn in 35 years of research on nutrition and supplements for age-related macular degeneration: a systematic review. Acta Ophthalmol. 2022;100:e1541–e1552. doi: 10.1111/aos.15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H.-J., Liu X.-B., Chen X.-M., Kong Q.-H., Liu Y.-S., So K.-F., Chen J.-S., Xu Y., Mi X.-S., Tang S.-B. Lutein delays photoreceptor degeneration in a mouse model of retinitis pigmentosa. Neural Regen Res. 2022;17:1596–1603. doi: 10.4103/1673-5374.330622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cecilia O.-M., José Alberto C.-G., José N.-P., Ernesto Germán C.-M., Ana Karen L.-C., Luis Miguel R.-P., Ricardo Raúl R.-R., Adolfo Daniel R.-C. Oxidative stress as the main target in diabetic retinopathy pathophysiology. J. Diabetes Res. 2019;2019 doi: 10.1155/2019/8562408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang Q., Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu L., Lu Q., Chen W., Li J., Li C., Zheng Z. Vitamin D3 protects against diabetic retinopathy by inhibiting high-glucose-induced activation of the ROS/TXNIP/NLRP3 inflammasome pathway. J. Diabetes Res. 2018;2018 doi: 10.1155/2018/8193523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang W., Yan Z., Li D., Ma Y., Zhou J., Sui Z. Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/1862462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rossino M.G., Casini G. Nutraceuticals for the treatment of diabetic retinopathy. Nutrients. 2019;11:771. doi: 10.3390/nu11040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernández-Zimbrón L.F., Zamora-Alvarado R., Ochoa-De la Paz L., Velez-Montoya R., Zenteno E., Gulias-Cañizo R., Quiroz-Mercado H., Gonzalez-Salinas R. Age-related macular degeneration: new paradigms for treatment and management of AMD. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/8374647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu M.-Y., Yiang G.-T., Lai T.-T., Li C.-J. The oxidative stress and mitochondrial dysfunction during the pathogenesis of diabetic retinopathy. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/3420187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown E.E., DeWeerd A.J., Ildefonso C.J., Lewin A.S., Ash J.D. Mitochondrial oxidative stress in the retinal pigment epithelium (RPE) led to metabolic dysfunction in both the RPE and retinal photoreceptors. Redox Biol. 2019;24 doi: 10.1016/j.redox.2019.101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eells J.T. Mitochondrial dysfunction in the aging retina. Biology. 2019;8:31. doi: 10.3390/biology8020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alaimo A., Liñares G.G., Bujjamer J.M., Gorojod R.M., Alcon S.P., Martínez J.H., Baldessari A., Grecco H.E., Kotler M.L. Toxicity of blue led light and A2E is associated to mitochondrial dynamics impairment in ARPE-19 cells: implications for age-related macular degeneration. Arch. Toxicol. 2019;93:1401–1415. doi: 10.1007/s00204-019-02409-6. [DOI] [PubMed] [Google Scholar]
- 59.Chang C.-C., Huang T.-Y., Chen H.-Y., Huang T.-C., Lin L.-C., Chang Y.-J., Hsia S.-M. Protective effect of melatonin against oxidative stress-induced apoptosis and enhanced autophagy in human retinal pigment epithelium cells. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/9015765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maugeri A., Barchitta M., Mazzone M.G., Giuliano F., Basile G., Agodi A. Resveratrol modulates SIRT1 and DNMT functions and restores LINE-1 methylation levels in ARPE-19 cells under oxidative stress and inflammation. Int. J. Mol. Sci. 2018;19:2118. doi: 10.3390/ijms19072118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du W., An Y., He X., Zhang D., He W. Protection of kaempferol on oxidative stress-induced retinal pigment epithelial cell damage. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/1610751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alhasani R.H., Biswas L., Tohari A.M., Zhou X., Reilly J., He J.-F., Shu X. Gypenosides protect retinal pigment epithelium cells from oxidative stress. Food Chem. Toxicol. 2018;112:76–85. doi: 10.1016/j.fct.2017.12.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Relevant data from this study will be provided as required.