Abstract
Sooty mangabeys naturally infected with simian immunodeficiency virus (SIV) remain healthy though they harbor viral loads comparable to those in rhesus macaques that progress to AIDS. To assess the immunologic basis of disease resistance in mangabeys, we compared the effect of SIV infection on T-cell regeneration in both monkey species. Measurement of the proliferation marker Ki-67 by flow cytometry showed that mangabeys harbored proliferating T cells at a level of 3 to 4% in peripheral blood irrespective of their infection status. In contrast, rhesus macaques demonstrated a naturally high fraction of proliferating T cells (7%) that increased two- to threefold following SIV infection. Ki-67+ T cells were predominantly CD45RA−, indicating increased proliferation of memory cells in macaques. Quantitation of an episomal DNA product of T-cell receptor α rearrangement (termed α1 circle) showed that the concentration of recent thymic emigrants in blood decreased with age over a 2-log unit range in both monkey species, consistent with age-related thymic involution. SIV infection caused a limited decrease of α1 circle numbers in mangabeys as well as in macaques. Dilution of α1 circles by T-cell proliferation likely contributed to this decrease, since α1 circle numbers and Ki-67+ fractions correlated negatively. These findings are compatible with immune exhaustion mediated by abnormal T-cell proliferation, rather than with early thymic failure, in SIV-infected macaques. Normal T-cell turnover in SIV-infected mangabeys provides an explanation for the long-term maintenance of a functional immune system in these hosts.
Simian immunodeficiency virus (SIV) infects a variety of Old World monkeys and apes in Africa without causing disease in these species (24, 28, 36, 52, 60). Phylogenetic evidence suggests that SIVsm, the virus isolated from naturally infected sooty mangabeys (Cercocebus atys), is the recent ancestor of two pathogenic lentiviruses that induce an immunodeficiency syndrome in their hosts, human immunodeficiency virus type 2 (HIV-2) and SIVmac (10, 26, 27, 37, 58). Transmission experiments have confirmed that SIVsm causes a disease very similar to AIDS in inoculated macaques, with progressive depletion of circulating CD4+ T lymphocytes and development of opportunistic infections (4, 22). The comparative study of SIV infection in species susceptible and resistant to disease, such as macaques and mangabeys, has proven to be a valuable system to analyze pathogenic mechanisms of AIDS (9, 80). An unexpected finding has been that the level of viral persistence is not sufficient to account for disease progression. Members of our group and others have shown that naturally infected sooty mangabeys harbor high viral loads and sustain active viral replication without developing disease (10, 13, 22, 41, 66). Viral loads of 105 to 107 SIV RNA copies per ml of plasma, which are commonly found in sooty mangabeys, are above the threshold values associated with disease progression in macaques (49, 76) and are associated with rapid progression to AIDS in humans (55). Since the viral loads are similar in macaques and mangabeys, differences are likely to lie in the susceptibility of T cells to be functionally impaired or to be killed, either directly or indirectly, by the virus.
HIV-1 and SIV infections are known to perturb T-cell homeostasis in susceptible hosts. HIV-1-infected patients undergo a progressive depletion of all CD4+ T-cell subsets and of naive CD8+ T cells in the periphery (68). The propensity of T lymphocytes to die, as evidenced by spontaneous apoptosis, is increased in HIV-1-infected patients and in SIV-infected macaques (2, 18, 31, 56). Several lines of evidence suggest that increased T-cell death triggers a compensatory mechanism that accelerates T-cell proliferation. Measurements of virus and T-lymphocyte dynamics during antiretroviral therapy have revealed a massive production of HIV particles that is paralleled by a rapid turnover of CD4+ T lymphocytes (38, 64, 83). The respective roles of T-cell production and T-cell redistribution on CD4+ T-cell recovery after therapy have been debated (30, 62, 82, 84). Most, but not all (20), studies of T-cell turnover during the chronic stage of infection indicate that HIV and SIV infections accelerate the peripheral T-cell regeneration process (35, 57, 69, 70, 88). The fraction of proliferating T lymphocytes, as measured by the percentage of cells expressing the marker Ki-67, is increased in both CD4+ and CD8+ subsets by HIV infection (70, 88). Analysis of newly synthesized DNA in HIV-positive individuals infused with 2H-glucose shows an increase in the T-cell replacement rate, which translates into an absolute increase in the production of CD8+ T cells but not CD4+ T cells (35). The kinetics of bromodeoxyuridine (BrdU) incorporation in macaques demonstrates that SIV infection accelerates both the renewal rate and the death rate of all lymphocyte subsets (57, 69). An “open tap-open drain” model of HIV pathogenesis has been proposed to describe the dynamic equilibrium between a high level of proliferation and a high level of destruction of T cells (38). In this view, homeostatic mechanisms that drive T-cell proliferation progressively fail to compensate for an increasingly high level of T-cell death, which ultimately leads to exhaustion of the regenerative capacity of the immune system.
A complementary mechanism by which HIV and SIV may impair T-cell regeneration is thymic damage. The thymic architecture is disrupted in AIDS patients (33, 54) and in most SIV-infected macaques (3, 85). Alteration of thymopoiesis would limit the production of naive T lymphocytes, which is consistent with the preferential depletion of these cells in HIV-infected patients (68). Thymocytes can be infected with both HIV and SIV in vitro and are depleted by HIV infection in severe combined immunodeficiency mice implanted with human fetal thymus (7, 40, 73). The percentage of CD34+ thymic progenitors was found to drop during primary SIVmac infection and to rebound after the peak of viremia, suggesting transient suppression of thymic function during intense viral replication (85). Using a PCR-based technique to detect DNA circles generated by T-cell receptor (TCR) rearrangement, Douek et al. showed that the number of recent thymic emigrants (RTE) in blood was decreased in HIV-infected patients and could be restored upon antiviral therapy (16). Our recent analysis of a large cohort of patients showed that RTE numbers were reduced in a subset of but certainly not in all HIV-infected adults, while they were reduced in nearly all HIV-infected children (87). Since cases of normal or slow disease progression in thymectomized adults infected with HIV have been reported (32), the extent to which thymus dysfunction contributes to the development of AIDS in adults remains unclear. Thymic output naturally decreases with age and drops sharply after early adulthood (42, 54, 72). Several studies suggest that the T-cell pool in adults is maintained predominantly by the proliferation of mature T-cells in the periphery rather than by the differentiation of thymic progenitors (51, 82). However, the adult thymus retains the capacity to generate fully functional T cells (39). Thymopoiesis is critical to the restoration of normal T-cell numbers after profound T-cell depletion, as is seen following bone marrow transplantion (51). Thus, HIV-induced thymic damage may impair T-cell regeneration in adult patients with severely depleted numbers of T cells.
The resistance of mangabeys to the pathogenic effects of SIV may be the consequence of a particularly high regenerative capacity of their immune system, which would be able to sustain a high lymphocyte turnover for years without functional impairment. A sufficient supply of lymphocytes could be obtained either through high thymic output or through rapid proliferation in the periphery. Alternatively, the resistance of mangabeys may result from a limited killing of infected cells or of bystander cells by the virus, which would translate into normal T-cell regeneration and a low turnover of lymphocytes. To distinguish between these possibilities, we evaluated the fraction of proliferating T lymphocytes and the numbers of RTE in the blood of SIV-infected and uninfected sooty mangabeys and compared these values to those found for rhesus macaques. Proliferating cells were identified by the expression of the Ki-67 marker, which is a nuclear antigen of short half-life expressed during the G1, S, G2, and M phases of the cell cycle, but not during G0 (8, 29, 71). Ki-67 is detected specifically in proliferating cells and is a widely used clinical marker for tumorigenesis (17). The number of RTE was assessed by measuring the concentration of DNA episomes produced during TCR rearrangement in peripheral T cells (21). These episomes, termed TCR excisional circles (TREC), are stable and do not replicate during mitosis (50). Since TREC are diluted with each cell division, they can serve as markers of RTE, which are defined as T cells that have undergone no more than a few cellular divisions since leaving the site of TCR rearrangement (42, 43). We detected the by-product of a predominant TCR rearrangement which occurs in about 70% of all human αβ T cells and which corresponds to the deletion of the δ locus embedded within the α locus (15, 79). The resulting episome, termed α1 circle, was quantified using a real-time PCR assay with a molecular beacon detection system (44, 45, 78).
This study demonstrates that sooty mangabeys maintain a normal T-cell turnover despite active SIV infection. In contrast, SIV infection accelerates peripheral T-cell proliferation in rhesus macaques. The concentration of α1 circles did not differ markedly between the two species. These findings emphasize the role of increased T-cell turnover, as opposed to blocked thymic production, in the mechanisms of AIDS pathogenesis.
MATERIALS AND METHODS
Animals.
Blood samples were obtained from SIVsm-positive sooty mangabeys (Cercocebus atys) housed at the Yerkes and the Tulane Regional Primate Research Centers. The majority of mangabeys from the Yerkes breeding colony acquire SIVsm infection when they become older juveniles or young adults (2 to 5 years old), probably via sexual transmission (23). The SIV-infected sooty mangabeys included in our study, which were between 4 and 23 years old, had probably been infected for most of their adult lives. However, none of these animals showed signs of SIV-induced disease. The colony of sooty mangabeys at Tulane was originally derived from the Yerkes colony. Mangabeys from both centers harbor closely related SIVsm viral strains, as indicated by the phylogenetic proximity of the Yerkes isolates SIVsmm9 and SIVsmmPBj14 to the Tulane isolates SIVsmB670 and SIVsmH4 (1, 10, 12, 37). Control blood samples were obtained from distinct groups of SIV-negative sooty mangabeys that were tested regularly for lack of serologic reactivity to SIV antigens. The absence of detectable SIV infection was confirmed by nested PCR (66). Two mangabeys that were found to be PCR positive and antibody negative were excluded from the study. Rhesus macaque (Macaca mulatta) blood samples were obtained from the Tulane primate center. SIV-positive macaques were infected with one of the following pathogenic viruses: SIVmac239, SIVmac251 (11), or SIVsmB670 (1). The macaques had been inoculated with SIV between 3 months and 3 years prior to blood sampling and were regularly monitored for viral load and the number of CD4+ T cells. The disease course was comparable for the three viral strains used. The protocols used in this study were reviewed and approved by Institutional Animal Care and Use Committees of both institutions.
Plasma viral RNA.
The concentration of SIV RNA in plasma was measured at Chiron Corporation (Emeryville, Calif.) by the branched DNA (bDNA) signal amplification assay (61). The target probes used in the bDNA assay were designed to hybridize with the pol region of SIVmac251 (14). It has been demonstrated that the SIV bDNA assay can equivalently quantify SIVmac and SIVsm strains (SIVmac32H and SIVsmPBj14-6.6, respectively) (J. Booth, P. J. Dailey, C. Wingfield, L. Sawyer, and P. Sheridan, Abstr. 16th Annu. Symp. Nonhum. Primate Models AIDS, abstr. 113, 1998; and P. J. Dailey, personal communication). SIV RNA associated with viral particles was measured in material pelleted from 1 ml of plasma collected on EDTA. The lower limit of detection was 1,500 SIV RNA copy equivalents per ml.
CFSE staining.
Peripheral blood mononuclear cells (PBMC) were washed twice in phosphate-buffered saline and resuspended in 1 ml of a 10 μM solution of 5(6)-carboxyfluorescein diacetate–succinimidyl ester (CFSE) that was made extemporaneously from a concentrated stock in dimethyl sulfoxide. Cells were incubated in the dark for 8 min at room temperature (RT), as described by Bird et al. (6). The labeling reaction was stopped by the addition of an equal volume of fetal calf serum (FCS), and the mixture was washed three times in RPMI medium with 10% FCS. The PBMC were resuspended in RPMI-FCS at a concentration of 3 × 106 PBMC/ml. Stimulated cultures were supplemented with interleukin 2 (20 U/ml) and concanavalin A (10 μg/ml). Stimulated and unstimulated cells were analyzed by flow cytometry 3 days after labeling.
Immunophenotyping.
CD4+ and CD8+ T-cell numbers were evaluated by flow cytometry with the following fluorochrome-labeled monoclonal antibodies: anti-rhesus monkey CD3-fluorescein isothiocyanate (FITC) (clone FN18; Biosource International, Camarillo, Calif.), anti-human CD4-phycoerythrin (PE) (clone OKT4; Ortho Diagnostic Systems, Raritan, N.J.), and anti-human CD8-peridinin chlorophyll protein (PerCP) (clone Leu2a; Becton Dickinson Immunocytometry Systems [BDIS], San Jose, Calif.). Antibodies were added to 50 μl of whole blood collected on EDTA and incubated for 15 min at RT, after which erythrocytes were lysed by the addition of 450 μl of fluorescence-activated cell sorter lysis solution (BDIS). The numbers of CD4+ and CD8+ T cells per microliter of blood were determined relative to a standard count of fluorescent beads used for calibration. To determine CD45RA subsets, four-color flow cytometry was performed with the following combination of antibodies: CD3-FITC, CD4-allophycocyanin (APC) (Exalpha, Boston, Ma.), CD8-PerCP, and CD45RA-PE (clone 2H4; Coulter, Miami, Fla.). The blood samples were incubated for 15 min with the antibody cocktail, lysed as described above, washed once in PBA buffer (phosphate-buffered saline–1% bovine serum albumin–10 mM NaN3), and resuspended in PBA with 1% paraformaldehyde (PBA-PF). Anti-human-CD45RO antibodies could not be used since they lack cross-reactivity with monkey antigens. Two additional CD45RA antibodies, Leu18 (BDIS) and MEM-56 (Caltag Laboratories, Burlingame, Calif.), were used to verify that the difference between macaque and mangabey CD45RA subsets was not associated with a particular antibody clone.
Intracellular Ki-67 staining.
For intracellular staining, 100 μl of whole blood was incubated with the antibodies to surface antigens CD3-PE (clone SP34; Pharmingen), CD4-APC, and CD8-PerCP for 15 min, after which they were fixed and permeabilized for 40 min at RT with 2 ml of the Permeafix reagent (Ortho Diagnostic Systems). The samples were washed once in PBA with 0.05% saponin (PBA-Sap), incubated at RT for 10 min to allow the completion of erythrocyte lysis, and washed a second time in PBA-Sap. The samples were then incubated for 30 min with the Ki-67-FITC antibody (clone MIB-1; Coulter), washed twice in PBA-Sap, and suspended in PBA-PF. To combine Ki-67 and CD45RA staining, blood samples were incubated with the antibodies to CD3-biotin (clone FN18; Biosource International), CD45RA-PE (clone 2H4; Coulter), and either CD4-APC or CD8-APC (Exalpha). After fixation with Permeafix and two washes in PBA-Sap, the samples were incubated with the Ki-67-FITC antibody and the secondary reagent streptavidine-PerCP, washed two more times, and suspended in PBA-PF. To detect Ki-67 expression in cells labeled with the dye CFSE, which is a derivative of fluorescein, we used a Ki-67 antibody conjugated to PE (clone R0840; DAKO, Carpinteria, Calif.).
Flow cytometry.
All analyses were performed with a FACScalibur flow cytometer with the Cellquest software (BDIS). The lymphocyte gate was defined with forward and side scatter parameters that were chosen to be large enough so that lymphocyte blasts were included in the analysis. For intracellular staining, an isotypic control incubated with a mouse immunoglobulin G1 (IgG1)-FITC antibody instead of Ki-67-FITC was processed in parallel for each sample and was used to set the gate for Ki-67+ cells.
Cloning and sequencing of α1 circle signal joints from monkeys.
Genomic DNA was extracted from mangabey and macaque PBMC with the DNA/RNA isolation kit (Amersham Life Science, Cleveland, Ohio) according to the manufacturer's instructions. The signal joint region from monkey α1 circles was amplified with primers derived from human sequences, with standard PCR conditions (66). Primers DREC-A (5′-CAG AGT GTG TTT TCG GCC GTG ATT CG-3′) and PJ-A (5′-ACA CTC TGC TCT CTC CTA TCT CTG C-3′) were used for the first round of amplification. The product was reamplified using heminested PCR with primers DREC-A and PJ-B (5′-CTG TCA ACA AAG GTG ATG CCA CAT CC-3′) or DREC-B (5′-GGA TGG AAA ACA CAG TGT GAC ATG G-3′) and PJ-A, which yielded fragments of 273 and 305 bp, respectively. The products were cloned in the TA vector pGEM-T Easy (Promega, Madison, Wis.) and sequenced in both orientations with an automated 373A sequencer (PE Applied Biosystems, Foster City, Calif.).
α1 circle quantitation.
To detect α1 circles, a molecular beacon was used in combination with real-time PCR, as previously described (44, 45, 78, 87). Each 50-μl reaction contained 5 μl of genomic DNA, and the final concentration of each component was as follows: 1× Taqman buffer A (Perkin-Elmer, Norwalk, Conn.), 3.5 mM MgCl2, 0.4 pmol of molecular beacon per μl, 0.4 pmol of each primer per μl, and 1.25 U of AmpliTaq Gold DNA polymerase (Perkin-Elmer). The primers were designed to hybridize to sequences conserved in both rhesus macaques and sooty mangabeys. The primers used, DREC-D (sense, 5′-CAG TGT GAC ATG GAG GGC TGA A-3′) and PJ-D (antisense, 5′-GTG TCT CTG TCA ACA AAG TTG ATG CC-3′), amplified a 206-bp product. The molecular beacon was designed to recognize a region upstream from the signal joint, as described in the assay for human α1 circles (87). The sequence of the beacon was 5′-CGG C(GT CTG CTC TTC ATT CAC CAT TCT CAC G)CC G-3′, the region complementary to the target recognition sequence being indicated in parentheses. A fluorophore (FAM [6-carboxyfluorescein]) was attached to the 5′ arm of the beacon, and a quencher (DABCYL [4-dimethylaminophenylazobenzoic acid]) was attached to the 3′ arm. One cycle of denaturation (95°C for 10 min) was performed, followed by 45 cycles of amplification (94°C for 30 s, 60°C for 30 s, and 72°C for 30 s). PCR was carried out in a spectrofluorometric thermal cycler (ABI PRISM 7700; PE Applied Biosystems) that monitors changes in the fluorescence spectrum of each reaction tube during the annealing phase while simultaneously carrying out programmed temperature cycles. For each run, a standard curve was generated from serial dilutions of purified PCR-generated product. The input copy number ranged from 106 to 101 copies. Copy numbers were calculated by interpolation of the experimentally determined threshold cycle as previously described (34, 47, 75). To normalize for cell equivalents in the input DNA, we used a separate real-time PCR with a molecular beacon assay to quantify the CCR5 coding sequence, since it is known that this gene is present at only 2 copies per cell; i.e., no pseudogenes are present (45).
Statistical analysis.
Statistical analysis was performed with the Prism version 2.0 software (GraphPad Software Inc.). The results were expressed as the mean ± 1 standard error of the mean. Statistical significance between SIV-infected and uninfected monkey groups was analyzed with the nonparametric Mann-Whitney U test. Significant linear correlations were analyzed with the Spearman test.
Nucleotide sequence accession numbers.
The nucleotide sequences of α-circle signal joints for seven mangabey clones and six macaque clones have been deposited in GenBank. The sequence data are available under accession no. AF148469 to AF148481.
RESULTS
Sooty mangabeys harbor a high viral load but exhibit a limited depletion of CD4+ T lymphocytes.
We compared immunologic and virologic parameters in sooty mangabeys naturally infected with SIVsm to those in rhesus macaques infected with pathogenic SIVmac isolates. SIV infection in mangabeys had a moderate, but significant, effect on the number of circulating CD4+ T lymphocytes, with a mean count of 1,051 CD4+ T cells/mm3 in SIV-infected animals (n = 19) versus 1,618 CD4+ T cells/mm3 in uninfected animals (n = 20) (Table 1). The decrease in circulating CD4+ T lymphocytes was less marked than in SIV-infected macaques (n = 21), which harbored a mean count of 518 CD4+ T cells/mm3 compared with 1,220 CD4+ T cells/mm3 in uninfected macaques (n = 17). The mean number of CD8+ T lymphocytes was not significantly increased by SIV infection in either species (Table 1).
TABLE 1.
Comparison of T-lymphocyte subsets and proliferation rates in sooty mangabeys and rhesus macaques
| Test condition and concn measured | Results fora:
|
|||||
|---|---|---|---|---|---|---|
| Mangabeys
|
Macaques
|
|||||
| SIV negative | SIV positive | P | SIV negative | SIV positive | P | |
| No. of subjects | 19 | 20 | 17 | 21 | ||
| CD4+ T cells/mm3 | 1618 ± 156 | 1051 ± 113 | 0.0086 | 1220 ± 105 | 518 ± 64 | <0.0001 |
| CD8+ T cells/mm3 | 1446 ± 197 | 2156 ± 296 | NS | 870 ± 94 | 862 ± 115 | NS |
| % of Ki-67+ CD4+ cells | 3.5 ± 0.2 | 4.2 ± 0.4 | NS | 7.0 ± 0.5 | 12.0 ± 1.1 | 0.0003 |
| % of Ki-67+ CD8+ cells | 3.6 ± 0.3 | 4.2 ± 0.3 | NS | 7.8 ± 0.9 | 19.0 ± 2.7 | <0.0001 |
| Ki-67+ CD4+ cells/mm3 | 54.4 ± 5.1 | 41.5 ± 6.1 | NS | 99.0 ± 14.6 | 56.9 ± 8.0 | 0.0091 |
| Ki-67+ CD8+ cells/mm3 | 57.2 ± 12.4 | 92.0 ± 14.7 | 0.0255 | 69.7 ± 11.1 | 165.2 ± 33.0 | 0.0100 |
| No. of subjects | 17 | 17 | 20 | 21 | ||
| % of CD45RA+ CD4+ cells | 22.8 ± 2.9 | 20.8 ± 2.9 | NS | 52.4 ± 4.3 | 65.6 ± 3.9 | 0.02 |
| % of CD45RA+ CD8+ cells | 50.4 ± 3.4 | 45.9 ± 3.8 | NS | 73.7 ± 3.3 | 66.1 ± 2.54 | NS |
| No. of subjects | 25 | 24 | 25 | 23 | ||
| % of CD3+ PBMC | 66.50 ± 1.4 | 70.27 ± 1.9 | NS | 64.13 ± 1.9 | 44.89 ± 2.6 | <0.0001 |
Data are means ± standard errors of the means. The statistical comparisons were done with the Mann-Whitney U test. NS, not significant.
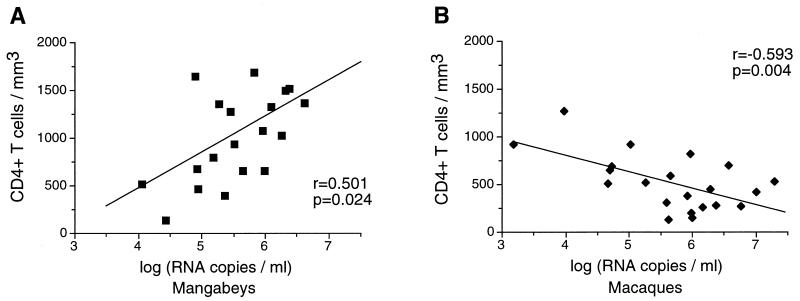
The number of viral RNA copies in plasma was measured by the bDNA technique, which has been shown to quantify SIVmac and SIVsm viral strains with equivalent sensitivity (J. Booth et al., Abstr. 16th Annu. Symp. Nonhum. Primate Models AIDS; and P. J. Dailey, personal communication). The viral load in plasma was found to be comparable for mangabeys and for macaques, with values ranging from 104 to 107 viral RNA copies/ml (Fig. 1). These data indicated that SIV could induce as high a viral load in its natural host as in a species susceptible to the development of AIDS. The viral load correlated inversely with the concentration of circulating CD4+ T lymphocytes in infected macaques (P = 0.004), which was consistent with a progressive depletion of CD4+ target cells in susceptible species. Interestingly, the viral load correlated positively with the CD4+ T-cell concentration in mangabeys (P = 0.02). The fact that mangabeys with a viral load ranging from 106 to 107 viral RNA copies/ml had more than 1,000 CD4+ T cells/mm3 highlighted fundamental differences in SIV-induced physiopathologic changes depending on host species.
FIG. 1.
Association between the plasma viral load and the concentration of peripheral CD4+ T cells. A positive correlation between the virus load and the CD4 T-cell number was observed in sooty mangabeys (A), while a negative correlation was observed in rhesus macaques (B). The viral load, which was measured with the SIV bDNA assay (Chiron Corporation), is expressed as the log of the equivalent number of viral RNA copies per milliliter of plasma.
Ki-67 is expressed specifically in proliferating monkey cells.
To evaluate the effect of SIV infection on T-cell turnover, we measured the expression of the proliferation marker Ki-67 in monkey T lymphocytes using four-color flow cytometry. The Ki-67 gene from rhesus macaques, which has been partially sequenced, shares 90% amino acid identity with its human homolog (71). Antibody cross-reactivity is ensured since the repeated motif recognized by the MIB-1 antibody is conserved in the macaque Ki-67 gene. We first performed in vitro experiments to verify that Ki-67 was a valid proliferation marker for both rhesus macaque and sooty mangabey T lymphocytes. In mangabey PBMC cultivated for 3 days in the absence of stimuli, less than 2% of the CD3+ CD4+ lymphocytes expressed Ki-67, as measured by flow cytometry. When the PBMC were stimulated with the mitogen concanavalin A and cultured in the presence of interleukin 2, 57% of the CD3+ CD4+ cells became Ki-67+. Similar percentages were observed in CD3+ CD8+ cells (unstimulated, 2% Ki-67+; stimulated, 56% Ki-67+). The marker Ki-67 was induced in a similar manner in stimulated macaque PBMC (from 1 to 2% to 60 to 62%).
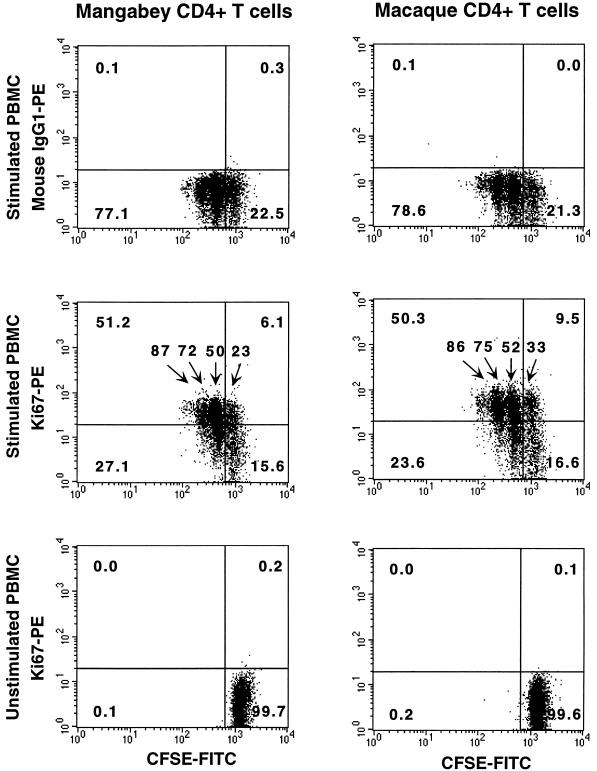
To verify that Ki-67 was specifically expressed in actively proliferating cells, we performed a cytofluorometric analysis on cells that had been labeled with CFSE, a dye that allows tracking of successive generations of dividing cells (6). Monkey PBMC were initially labeled with CFSE, a fluorescein derivative that reacts with amino groups as it enters the intracellular environment. The amount of dye per cell decreased at each cell division, so that each new generation could be distinguished by a decrease in fluorescence intensity in the FL1 channel. As shown in Fig. 2, Ki-67 was preferentially expressed in cells that had divided after 3 days in culture, the fraction of Ki-67+ cells being progressively higher in cells that had undergone more divisions. For instance, mangabey CD4+ T cells that had divided 0, 1, 2, and 3 times had Ki-67+ fractions of 23, 50, 72, and 87%, respectively. Unstimulated cells, which did not divide during the 3-day culture, remained Ki-67−. This experiment showed that the Ki-67 staining detected specifically actively dividing cells in both mangabey and macaque PBMC.
FIG. 2.
Specific expression of Ki-67 in proliferating monkey CD4+ T cells. Macaque and mangabey PBMC were labeled with the dye CFSE, which allows tracking of successive generations of dividing cells. The amount of dye per cell decreases at each cell division, which allowed identification of each new generation by its decreased fluorescence intensity in the FL1 channel (x axis). The CFSE-stained cells were cultivated for 3 days in stimulated (top and middle panels) or unstimulated (bottom panels) conditions and were subsequently labeled with the Ki-67-PE antibody or the control antibody immunoglobulin G1 (IgG1)-PE (y axis). Ki-67 was preferentially expressed in cells that had divided after 3 days in culture, the Ki-67+ fraction being increasingly higher in cells that had undergone more divisions. The percentage of Ki-67+ cells among CD4+ T lymphocytes is reported above each generation. The numbers in the corners of the plots indicate the percentage of CD4+ T cells in each quadrant.
Lymphocyte turnover is increased by pathogenic SIV infection, not by natural SIV infection.
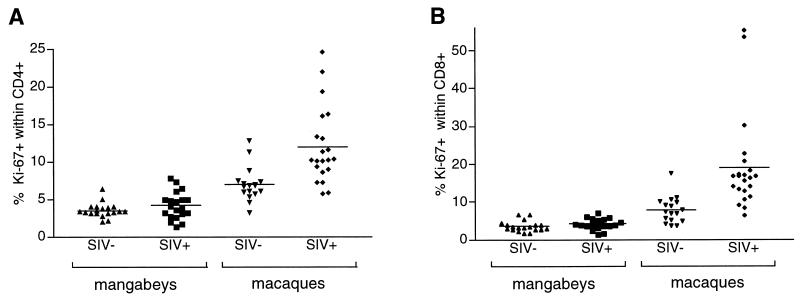
We next measured the Ki-67+ fraction in PBMC obtained from mangabeys and macaques. The Ki-67 labeling was performed on whole-blood samples to minimize the possible loss of more fragile activated cells. The mean percentage of Ki-67+ cells in CD4+ T lymphocytes was 3.5% in uninfected mangabeys and 4.2% in SIV-infected mangabeys (Fig. 3; difference not statistically significant). Isotype-matched controls processed in parallel for each sample yielded readings below 1% in all cases. Uninfected macaques had an intrinsically high Ki-67+ fraction in CD4+ T cells (7.0%), which was significantly increased in SIV-infected animals (12.0%, P < 0.001). Thus, SIV infection did not change the level of CD4+ lymphocyte proliferation in mangabeys, while it led to an enhanced turnover in macaques. A similar pattern was observed in CD8+ T lymphocytes, with mean levels of 3 to 4% of Ki-67+ cells in mangabey PBMC independently of the infection status, 7.8% in control macaques, and 19.0% in SIV-infected macaques (P = 0.001). Two of the infected macaques had more than half of their peripheral CD8+ cells expressing Ki-67, which indicated that SIV infection could induce states of intense lymphocyte proliferation in a susceptible species.
FIG. 3.
Percentage of Ki-67 expression in monkey CD4+ and CD8+ T cells. The percentage of Ki-67+ cells among the CD4+ T cells (A) and the CD8+ T cells (B) is plotted for each of the following four monkey populations: uninfected mangabeys (SIV−), SIV-positive mangabeys (SIV+), uninfected macaques (SIV−), and SIV-positive macaques (SIV+). The mean percentage of Ki-67+ T cells is indicated by a horizontal bar.
The absolute number of proliferating T cells was calculated by multiplying the Ki-67+ fraction by the number of CD4+ or CD8+ T lymphocytes per microliter of blood. The number of proliferating CD4+ cells was not changed by SIV infection in mangabeys but was significantly decreased in macaques (Table 1). Thus, while the fraction of proliferating cells was increased in macaques, this did not translate into an actual increase in the number of proliferating CD4+ T cells, due to the already low CD4+ T-cell numbers. This dichotomy was not observed for CD8+ T cells. In macaques, the increase in the proliferating fraction resulted in more than a doubling of the number of proliferating CD8+ T cells (from 69 to 167 Ki-67+ cells/mm3, P = 0.01). Interestingly, SIV infection also caused an increase in the number of proliferating CD8+ T cells in mangabeys (from 57 to 92 Ki-67+ cells/mm3, P = 0.02). Though the increase of the proliferating fraction was minimal in mangabeys, SIV infection still had a detectable impact on CD8+ lymphocyte numbers and thus could not be considered immunologically silent.
Positive correlation between proliferation in the CD4+ and the CD8+ T-cell subsets.
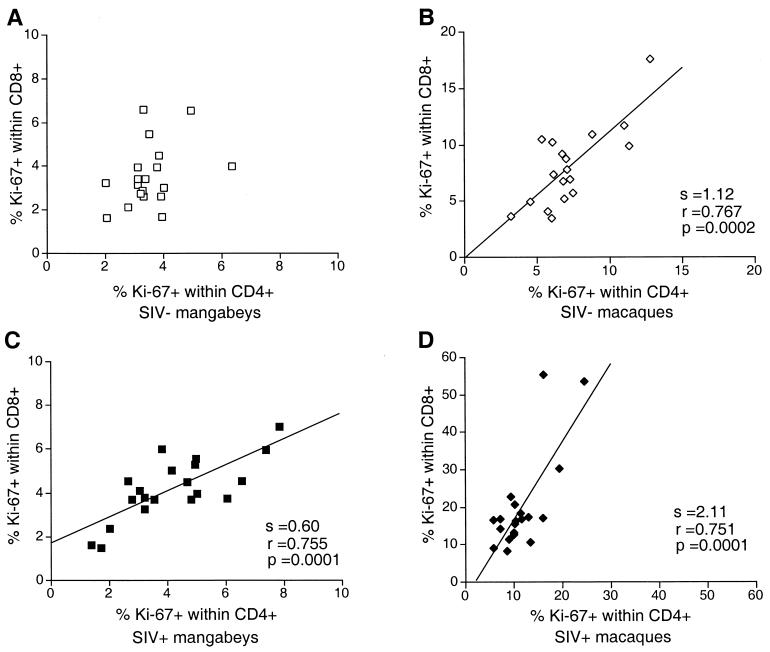
A strong correlation was observed between the Ki-67+ fractions within CD4+ T cells and CD8+ T cells in infected macaques (P = 0.0001; Fig. 4), suggesting that similar mechanisms may drive T-cell proliferation in both subsets. However, the slope of the regression line was 2.1, which indicated that proliferation levels were consistently higher in the CD8+ subset than in the CD4+ subset. Uninfected macaques had equivalent Ki-67+ fractions in both T-lymphocyte subsets, the slope of the regression line being 1.1 (P = 0.0002). Although the overall proliferation levels were lower in mangabeys, a positive correlation was still observed between the CD4+ Ki-67+ and CD8+ Ki-67+ fractions in infected animals (P = 0.0001). The slope was 0.6, indicating that proliferation in the CD8+ subset was limited. No significant correlation was observed in uninfected mangabeys. Thus, a proportionally higher fraction of proliferating cells in CD8+ T lymphocytes was characteristic of pathogenic SIV infection.
FIG. 4.
Positive correlation between the percentage of Ki-67+ cells within the CD4+ and CD8+ T-cell subsets. (A) Uninfected mangabeys; (B) uninfected macaques; (C) SIV-positive mangabeys; (D) SIV-positive macaques. The slope (s), the correlation coefficient (r), and the P value (p) associated with the regression lines are indicated. SIV−, SIV-negative; SIV+, SIV-positive.
SIV infection specifically increases the turnover of memory cells in macaques.
The CD45RA marker is expressed by naive T lymphocytes. It is down-regulated from the surface of memory lymphocytes and can be reexpressed in a fraction of the memory population (5). We did not observe a major effect of SIV infection on CD45RA expression in T lymphocytes of either monkey species (Table 1). The differences observed were intrinsic to the species tested, as uninfected macaques harbored a higher fraction of CD45RA+ T cells (52 and 74% in CD4+ and CD8+ subsets) than did mangabeys (23 and 50%). Similar percentages were obtained with three distinct CD45RA antibodies (data not shown), making it unlikely that epitope differences accounted for the observed phenotype.
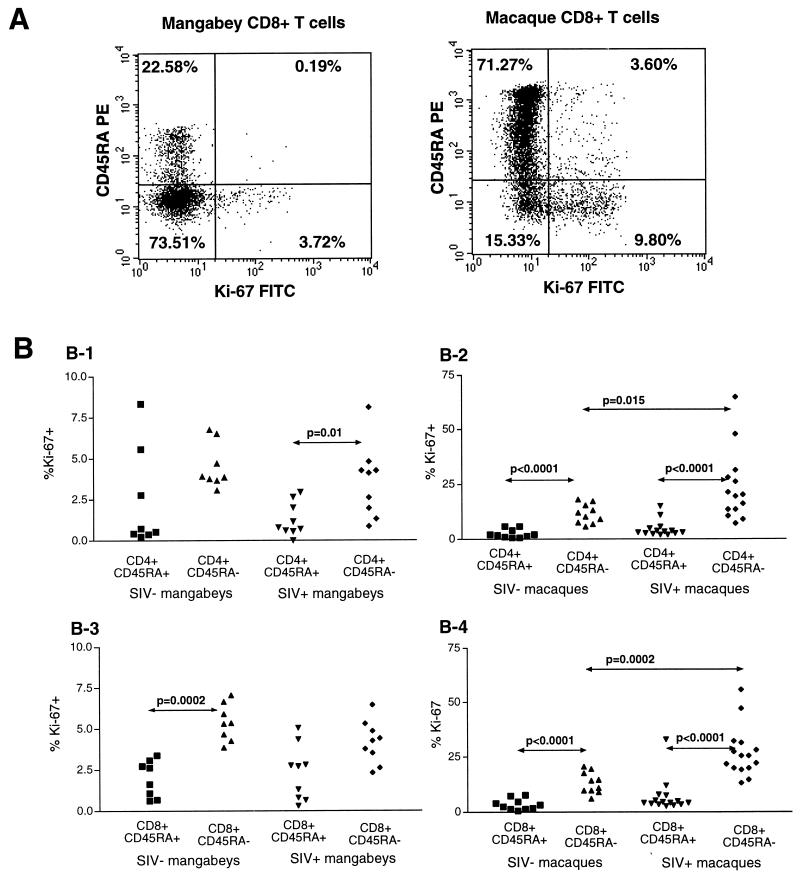
CD45RA and Ki-67 detection were combined using a two-step labeling protocol on whole-blood samples. Analysis of the Ki-67+ fractions revealed that proliferating T cells belonged predominantly to the CD45RA− subset and thus were of the memory phenotype (Fig. 5). The differences in the Ki-67+ fractions between the CD45RA+ and CD45RA− subsets were highly significant in infected macaques (P was < 0.0001 for both CD4+ and CD8+ T cells; Fig. 5B). Significant differences were also observed in uninfected macaques, which confirmed that proliferation is characteristic of memory lymphocytes in normal physiological conditions. The trend of higher Ki-67 fractions in the CD45RA− subset was observed for mangabeys, though the overall proliferation levels were lower. SIV infection caused a doubling of the proliferating fraction within the CD45RA− T-cell subset in macaques (mean Ki-67 values within CD4+ RA− T cells were 13% in SIV-negative animals and 26% in SIV-positive animals) (Fig. 5B). No increase was detected in mangabeys (mean Ki-67 values within CD4+ RA− T cells were 4.5% in SIV-negative animals and 3.5% in SIV-positive animals). These results emphasized the different effects of SIV infection in the two species.
FIG. 5.
Preferential expression of Ki-67 in the CD45RA− subset of T lymphocytes. (A) Combined analysis of Ki-67 and CD45RA expression by flow cytometry. Dot plots showing the staining for Ki-67 (x axis) and CD45RA (y axis) in the CD3+ CD8+ T-cell population of two representative animals. Ki-67 is expressed preferentially in the CD45RA− memory T-cell subset, both in an infected mangabey (left panel) and in an infected macaque (right panel). The numbers in the corners of the plots indicate the percentage of CD3+ CD8+ T cells in each quadrant. (B) Percentage of Ki-67+ cells within CD45RA+ and CD45RA− T-cell subsets. Graphs show results for four types of cells, as follows: B-1, mangabey CD4+ T cells; B-2, macaque CD4+ T cells; B-3, mangabey CD8+ T cells; B-4, macaque CD8+ T cells. Only the P values lower than 0.05 in the nonparametric Mann-Whitney test are reported. SIV−, SIV-negative; SIV+, SIV-positive.
Relation between Ki-67 expression and CD4 T-cell count.
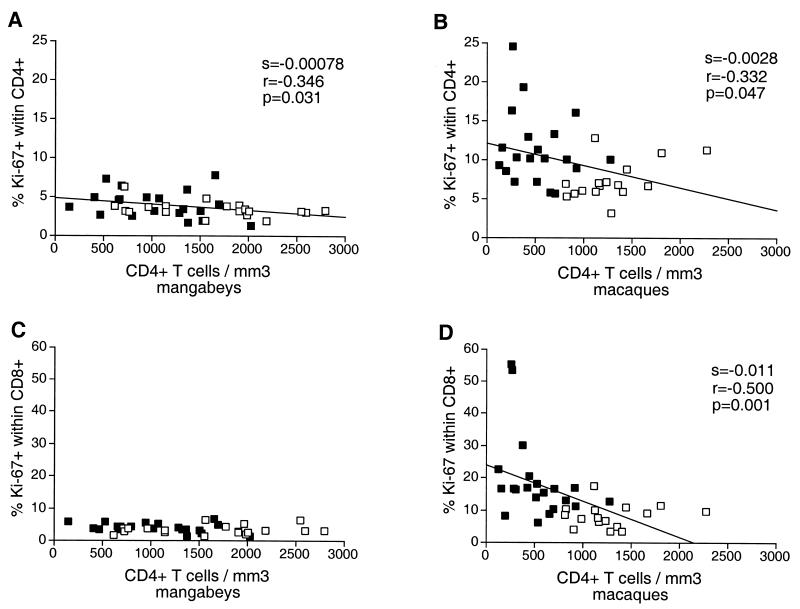
A negative correlation was observed between the fraction of proliferating CD4+ T cells and the CD4+ T-cell number in macaques (P = 0.047) (Fig. 6), suggesting that homeostatic mechanisms were activated to compensate for CD4+ T-cell loss. The fraction of proliferating CD8+ T cells did not correlate with the absolute count of CD8+ T cells (data not shown) but was inversely correlated to the CD4+ T-cell count in macaques (P = 0.001). These findings indicated that the depletion of CD4+ T cells in macaques was associated with a general activation of T-cell proliferation. Although the proliferation levels were lower in mangabeys, an inverse correlation could still be distinguished between the fraction of proliferating CD4+ T cells and the CD4+ T-cell count (P = 0.031). This suggested that homeostatic mechanisms that regulate CD4+ T-cell numbers were active in both species. The downslope of the regression line was higher in macaques than in mangabeys (2.8 × 10−3 versus 7.8 × 10−4). The ratio of the slopes was 3.5, indicating that for a given CD4+ T-cell decrease, the increase in proliferating CD4+ T cells would be 3.5 times higher in macaques than in mangabeys. This finding highlighted the more active CD4+ T-cell regeneration process in the species susceptible to SIV-induced disease.
FIG. 6.
Association between the CD4+ T-cell concentration and the expression of Ki-67 in T cells. A negative correlation between the CD4+ T-cell count and the percentage of Ki67+ CD4+ T cells was observed in mangabeys (A) as well as in macaques (B). The absolute value of the slope is higher for macaques (28 × 10−4) than for mangabeys (7.8 × 10−4), underscoring the more active T-cell renewal process in the former species. A negative correlation between the percentage of Ki67+ CD8+ T cells and the CD4+ T cell count is observed in macaques (D) but not in mangabeys (C). Open squares, uninfected animals; solid squares, SIV-infected animals. The slope (s), the correlation coefficient (r), and the P value (p) associated with the regression lines are indicated.
Age-dependent decline of α1 circle numbers in macaques and mangabeys.
To determine whether thymic function differed in the two monkey species, we evaluated the number of RTE as measured by the concentration of α1 circles. These 89-kb episomes are by-products of the δRec-ΨJα rearrangement, which occurs in a majority of human αβ T cells (79). α1 circles can be specifically detected by using PCR to amplify a fragment spanning the signal joint generated by the δRec-ΨJα rearrangement (16, 87). To adapt the technique for use with monkey DNA, we sequenced a 333-bp fragment encompassing the amplified α1 circle region in four sooty mangabeys and two rhesus macaques. The fragment contained the appropriate motifs for recombination signal sequences (21, 50, 79) and shared 94% (mangabeys) to 95% (macaques) nucleotide identity with the homologous human sequence (see Material and Methods for database accession numbers). A feature characteristic of both monkey species was the presence of small nucleotide insertions and deletions at the signal joint site. This property may be specific to monkeys, since the signal joint is precise in humans and mice (48). Two primers and a probe (the molecular beacon) were designed in regions conserved between mangabeys and macaques to amplify a 206-bp DNA fragment. Quantification of α1 circles in PBMC DNA was achieved by real-time detection of the fluorescence emitted by the molecular beacon. This detection system, which allows quantitation of PCR products over a wide dynamic range, has been described in detail elsewhere (45, 87). The specificity of the assay was evaluated by testing monkey tissues of various origins. α1 circles were detected at high levels in the thymus, lymph nodes, and PBMC of macaques and mangabeys (up to 106 copies/106 cells) but were undetectable in immortalized cell lines of monkey origin (<102 copies/106 cells).
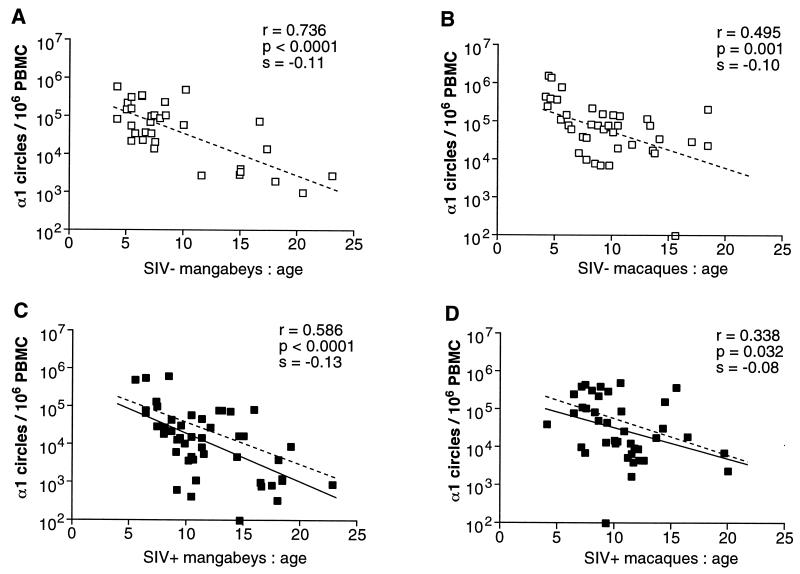
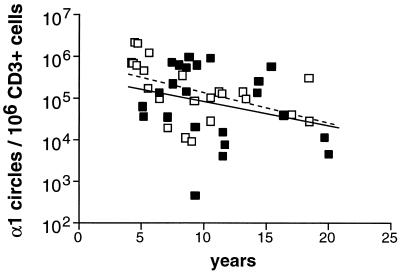
The number of α1 circles in PBMC was assessed in a total of 145 animals divided into four groups as follows: SIV-negative mangabeys (n = 33), SIV-positive mangabeys (n = 42), SIV-negative macaques (n = 32), and SIV-positive macaques (n = 38). The animals tested were aged between 4 and 23 years; i.e., they ranged from early adulthood to old age. As shown in Fig. 7, the α1 circle concentration decreased with age in the four groups tested (P = 0.03 to P < 0.0001). The progressive loss of RTE in monkeys was consistent with the notion of age-dependent thymic involution (16, 54, 74). The decrease spanned a 2-log unit range, from approximately 105 to 103 α1 circles/106 PBMC, which was very similar to the values observed for humans (87). The age-related α1 circle decrease occurred within a shorter time scale in monkeys than in humans, with downslopes of approximately 0.1/year in monkeys, as compared to 0.03/year in humans. However, these rates could be considered equivalent if one took into account the relative life spans of monkeys and humans. The decrease in α1 circles was comparable in uninfected mangabeys and in uninfected macaques, with downslopes of 0.11 and 0.10/year, respectively. Thus, the levels of RTE did not appear naturally elevated in mangabeys.
FIG. 7.
Age-dependent decline of α1 circle numbers in PBMC. (A) Uninfected mangabeys; (B) uninfected macaques; (C) SIV-infected mangabeys; (D) SIV-infected macaques. The correlation coefficient (r), the P value (p), and the slope (s) associated with the regression lines are indicated. The regression lines in panels A and B (dashed line) were reported in panels C and D, respectively, to allow the comparison of α1 circle numbers between infected and uninfected animals. SIV−, SIV-negative; SIV+, SIV-positive.
Impact of SIV infection on α1 circle numbers.
Comparison of the regression lines for infected and uninfected animals showed a moderate impact of SIV infection on α1 circle numbers (Fig. 7C and D). The α1 circle values tended to be lower in infected animals of both species, though it should be noted that the normal variation of α1 circles numbers for monkeys of a given age was large (1 to 2 log units). Comparison between species revealed that SIV-positive mangabeys had equivalent or slightly lower α1 circle numbers than SIV-positive macaques, with downslopes of 0.13 and 0.08/year, respectively. This observation suggested that an SIV-induced decrease of α1 circles was not sufficient to account for the impairment of immune function in macaques.
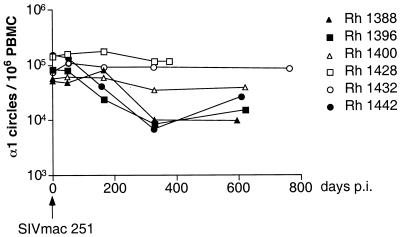
To further analyze the effect of SIV infection, we performed a longitudinal analysis of α1 circles in six rhesus macaques that had been inoculated intravaginally with SIVmac251 (53). Three of the animals showed a decrease of about 1 log unit in α1 circle numbers at 1 year postinfection (Fig. 8). The other three animals maintained constant α1 circle levels over a 1- to 2-year period. Neither the plasma viral load nor the percentage of CD4+ T cells correlated with the α1 circle number measured at 1 year postinfection (data not shown). The finding that SIV infection decreased α1 circle numbers in only a subset of rhesus macaques was consistent with findings in HIV-1-infected humans (87).
FIG. 8.
Longitudinal analysis of α1 circle numbers in six infected macaques. The concentration of α1 circles in PBMC was measured over a 2-year period following SIVmac251 inoculation. p.i., postinoculation.
Since α1 circles were quantified per million PBMC but were generated only in the T-cell population, α1 circle numbers were dependent on the fraction of T cells within PBMC. The percentage of CD3+ T cells was equivalent in uninfected mangabeys and macaques (Table 1). This percentage remained unchanged in SIV-positive mangabeys but decreased from 64 to 48% in SIV-positive macaques. To verify whether SIV infection had an impact on α1 circles within the T-cell population, α1 circles were expressed per million CD3+ T cells for the subset of macaques for which flow cytometry data were available (Fig. 9). The regression line was slightly lower for infected animals, which confirmed that SIV infection caused a limited but detectable decrease of α1 circles within T cells.
FIG. 9.
α1 circle numbers expressed in macaques. The number of α1 circles per million CD3+ T cells is reported as a function of the age of the animals (in years). Solid squares, SIV-positive macaques; open squares, uninfected macaques. The dashed line and the solid line correspond to regression lines obtained for uninfected and infected macaques, respectively. The slope of the regression line was s = −0.073 for uninfected macaques (P = 0.02). The slope did not significantly differ from 0 for SIV-positive macaques.
Relation between α1 circle numbers and proliferation rates.
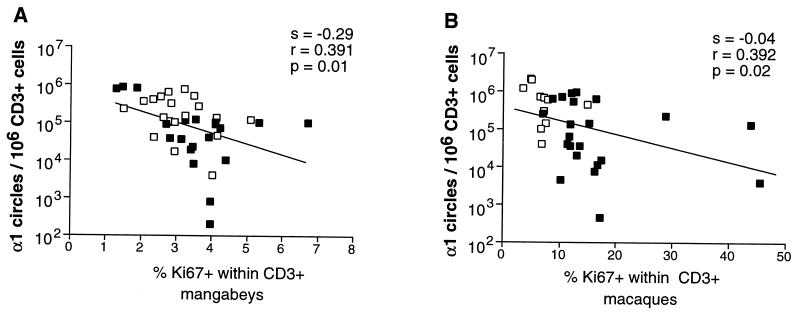
The concentration of α1 circles within PBMC depends not only on thymic output but also on the extent of peripheral proliferation, which leads to the dilution of circles in expanded T-cell populations. In addition, α1 circles may be lost due to the death of T cells. We investigated the relation between α1 circle concentration and peripheral T-cell proliferation as measured by Ki-67 fractions. Figure 10 shows that the number of α1 circles correlated inversely with the Ki-67+ percentage within CD3+ T cells in mangabeys and macaques (P = 0.01 and P = 0.03, respectively). These results suggested that dilution by proliferation had a significant impact on α1 circle numbers. It followed that the decreased α1 circle numbers observed in SIV-positive animals could be accounted for, at least in part, by increased peripheral expansion of T cells.
FIG. 10.
Inverse correlation between α1 circle numbers and T-cell proliferation rates. (A) Mangabeys; (B) macaques. The slope (s), correlation coefficient (r), and the P value (p) associated with the regression lines are indicated. Solid squares, SIV-positive animals; open squares, uninfected animals.
DISCUSSION
This study identified characteristic properties of SIV infection in a natural host. Infected sooty mangabeys were healthy, harbored a high viral load, had a slightly lower peripheral CD4+ T-cell count than uninfected controls, and showed a limited decrease of α1 circles in peripheral T cells. SIV did not increase the T-cell turnover in its natural host, since mangabeys harbored 3 to 4% Ki-67+ T cells in the periphery irrespective of their infection status. It was striking that the plasma viral load in mangabeys spanned the same range as that in rhesus macaques (104 to 107 viral RNA copies/ml) while the impact of SIV infection on the immune system differed markedly for the two species. Macaques, which progressed to AIDS, exhibited a severe depletion of CD4+ T cells and high T-cell proliferation levels (12% in CD4+ T cells, 19% in CD8+ T cells) that were increased two- to threefold compared to the levels for uninfected animals. SIV infection induced a moderate decrease of α1 circle numbers in macaques, which reflected either thymic impairment or, more likely, loss of RTE through rapid proliferation. Thus, the key factor that distinguished the two monkey species was the extent of peripheral T-cell proliferation induced upon infection. SIV did not perturb the T-cell turnover in mangabeys, which could explain the long-term persistence of a functional immune system in these hosts.
It cannot be said that SIV infection in mangabeys is silent. The reduced CD4+ T-cell numbers in these animals may result from a cytopathic effect of the virus or from a homing of activated CD4+ T cells within lymphoid organs. SIV infection also induces a limited increase in the absolute number of proliferating CD8+ T cells in mangabeys, which may indicate an ongoing antiviral immune response. Infected mangabeys do seroconvert and have the capacity to mount a cytotoxic T-lymphocyte response to SIV, even though the intensities of both the humoral and the cytotoxic T-lymphocyte responses are low in natural SIV infection (25, 41, 65, 81). The limited decrease of α1 circle numbers in infected mangabeys may reflect a dilution associated with the higher number of proliferating CD8+ T cells, though we cannot rule out a moderate impairment of thymic function. Taken together, these observations suggest that SIV perturbs the mangabey immune system to some extent. SIV induces a functional antiviral immune response in this species, but it does so without triggering an excessive T-cell activation that would impair the immune system in the long term.
The finding of high Ki-67 expression in infected macaques is consistent with previous studies that demonstrated enhanced lymphocyte turnover rates in SIV-infected macaques as measured by BrdU incorporation (57, 69). Proliferation rates computed from BrdU incorporation are very low in uninfected macaques (0.1%), while they can rise to 3% in animals with a high viral load (57). The low proliferating fractions found in these studies compared to those obtained by Ki-67 labeling may result from several factors, including incomplete BrdU uptake in proliferating cells (67) and Ki-67 expression in cells that have entered the late G1 phase of the cells cycle but that have not yet duplicated their DNA (8, 29). Measurements made by using BrdU and Ki-67 are consistent in that both demonstrate a mean two- to threefold increase in the T-cell turnover rate of infected macaques (69). The phenomenon of increased Ki-67 expression is also a hallmark of HIV infection in humans. Sachsensberg et al. reported that the Ki-67+ fractions, which were naturally low in human peripheral CD4+ and CD8+ T cells (mean, 1%), increased to a mean of 4 to 6% in HIV-positive individuals, with values up to 20% in patients with low CD4+ T-cell counts (70). Using immunohistochemistry, Zhang et al. detected a threefold increase in the fraction of Ki-67+ CD4+ T cells from tonsil biopsies of HIV-infected patients, the proliferation levels returning to normal when the patients received antiretroviral therapy (88). In one study of HIV-infected subjects in early disease, increased Ki-67 expression was detected only in the CD8+ T-cell subset, which suggests that abnormal proliferation of the CD4+ T cells occurs predominantly in advanced disease (20). The higher Ki-67+ percentages observed in SIV-positive macaques (12 to 19%) compared to HIV-positive humans (4 to 6%) may account for the more rapid course to disease in monkeys, as SIV infection usually leads to AIDS in 6 months to 3 years in macaques versus a mean progression time of 10 years in untreated HIV-1-infected humans (19). It is interesting that uninfected macaques exhibit a higher fraction of proliferating T cells than uninfected mangabeys (7 versus 3% of Ki-67+ T cells, P < 0.001). This observation raises the possibility that monkey species with an intrinsically high lymphocyte turnover are more prone to lentivirus-induced disease. However, humans, who have a spontaneously low T-cell turnover (1% of Ki-67+ T cells), do develop AIDS, while mangabeys do not. This comparison indicates that the intrinsic turnover of T lymphocytes is not the sole determinant of the susceptibility to lentiviral disease.
Other groups have suggested that the T-cell production measured by Ki-67 labeling or by 2H-glucose incorporation is blocked, rather than increased, in HIV infection (20, 35). These differences result in part from emphasizing that the absolute number of proliferating CD4+ T cells is reduced in HIV or SIV infection, rather than considering that the percentage of proliferating CD4+ T cells is increased. In our view, high proliferation rates accompanied by low absolute numbers of proliferating CD4+ T cells are indicative of immune exhaustion, i.e., increased T-cell production that fails to compensate for T-cell destruction. The failure of homeostatic mechanisms in maintaining normal CD4+ T-cell numbers would further drive proliferation. Homeostatic regulation is active in mangabeys as well as in macaques, since a negative correlation between the CD4+ T-cell count and the fraction of proliferating CD4+ T cells is observed in both species. However, the compensatory changes in T-cell proliferation for a given CD4+ T-cell decrease are 3.5 times higher in macaques than in mangabeys, which provides further evidence for exacerbated T-cell production in the susceptible species.
Thymic impairment has been proposed as a mechanism contributing to the development of AIDS (16, 54, 73). To determine whether thymic function differed between the susceptible and the resistant monkey species, we used a recently described technique designed to evaluate the concentration of RTE in peripheral blood (16, 87). Quantitation of α1 circles by real-time PCR indicated that these episomes were present at high concentrations in both monkey species, with values ranging approximately from 5 × 105 to 103 circles per million PBMC. Thus, α1 circles represent major products of TCR α rearrangement in monkeys, as is the case for humans (79). The numbers of α1 circles per million PBMC exhibited an age-dependent decline that had similar kinetics in uninfected mangabeys and macaques. These data suggested that the progressive decrease of thymic output, which parallels the involution of the thymus (42, 43), occurred at the same rate in the two monkey species. The numbers of α1 circles were equivalent or slightly lower in mangabeys than in macaques of the same age group. Thus, mangabeys were not characterized by a particularly high RTE concentration that could have accounted for their resistant phenotype.
The impact of SIV infection on α1 circle numbers was detectable but limited. Both macaques and mangabeys showed a decrease inferior or equal to 0.3 log units in cross-sectional analyses (Fig. 7). Since α1 circle numbers were equivalent in the susceptible and the resistant monkey species, this parameter did not appear to correlate with disease progression. However, the longitudinal analysis of α1 circle in macaques revealed a marked individual variability (Fig. 8). Out of six animals, three showed an appreciable decrease of α1 circle numbers (1 log unit) following SIVmac infection, while the other three maintained constant α1 circle numbers for up to 2 years. Longitudinal follow-up studies of a larger number of animals may be required to assess the predictive value of α1 circle concentrations.
Three parameters need to be taken into account to interpret a decrease in α1 circle numbers, since these episomes are produced in the thymus, diluted by cell proliferation, and lost by cell death. The decrease of α1 circles seen in infected animals can reflect a low thymic output or a dilution of α1 circles through T-cell proliferation, or both. The possibility that the decrease reflects a higher death rate in RTE (rich in circles) than in cycling cells (poor in circles) can also be considered, though this is unlikely. The literature indicates that cycling T cells have a shorter half-life and are more susceptible to apoptosis than resting T cells, especially in HIV-infected patients (2, 18, 31, 56, 77). In view of both the negative correlation between α1 circles and proliferation and the active T-cell proliferation characteristic of SIV-positive macaques, most of the α1 circle decrease in this species may be accounted for by proliferation.
A major conclusion can be drawn from α1 circle data, despite the fact that this parameter does not give a direct measurement of thymic function. The persistence of relatively high levels of α1 circles in infected animals indicates that SIV infection does not entirely block thymopoiesis. A loss of thymic function would cause a dramatic decrease of α1 circles, since the thymus is most likely the sole source of these episomes. The combined effects of thymic failure and increased proliferation in macaques should lead to an almost complete loss of α1 circles in peripheral T cells, which is not observed. Thus, our data are consistent with immune exhaustion through T-cell proliferation, rather than with thymic failure, in AIDS-susceptible species.
The scale of the α1 circle decrease in SIV-positive macaques was comparable to that seen in HIV-infected patients (87). Also similar was the fact that only half of HIV-positive patients monitored longitudinally exhibited an appreciable decline of α1 circle numbers following seroconversion (87). Taken together, these results suggested that HIV and SIV do not dramatically affect thymic function in adult individuals. The fact that the few known cases of thymectomized HIV-positive patients exhibited normal rates of progression to AIDS and did not show a precipitous CD4+ T-cell loss supports the idea that the adult immune system does not rely predominantly on an active thymus (32). It is noteworthy that lymphoid hyperplasia develops during the early stage of HIV or SIV infection. The fact that there are obviously more, not fewer, T lymphocytes within lymphoid tissues at an early stage does not fit with the block in T-cell production hypothesis. This does not mean that a role for thymic dysfunction should be excluded in late-stage disease. Histopathologic lesions in the thymus of AIDS patients point to some degree of thymic dysfunction (33, 54), which may result from the emergence of X4 viruses that target thymic progenitors (63). Also, HIV may significantly impair thymopoiesis in young hosts, as suggested by the more prominent decrease of α1 circles in infected children than in adults (87). Signs similar to those of severe congenital thymic defect have been observed in HIV-infected infants that progress rapidly to disease (46, 59), suggesting that the mechanism of T-cell depletion is in part thymus dependent in young hosts.
It was intriguing that α1 circle numbers were not lower in SIV-infected macaques, considering the extent of peripheral T-cell proliferation in these animals. The downslope reflecting α1 circle loss as a function of T-cell proliferation was low in macaques and paradoxically higher in mangabeys (0.04 versus 0.29; Fig. 10). In particular, the extremely high proliferating fractions seen in three SIV-positive macaques (30 to 45% of Ki-67+ CD3+ cells; Fig. 10) did not lead to very low α1 circle numbers in these animals (105 to 4 × 103 copies/106 T cells). These results may be explained by a high death rate of cycling macaque T cells. A higher death rate in cycling cells than in RTE would enrich the T-cell population in RTE and hence artificially increase the concentration of α1 circles. Thus, the limited impact of SIV infection on α1 circles may point to the rapid death of proliferating T cells.
The normal T-cell turnover observed in infected mangabeys suggests that SIV does not significantly increase the T-cell death rate in this species. It is possible that the virus does not efficiently kill mangabey CD4+ T cells, which has been proposed by some but not all studies that evaluated SIV cytopathogenicity for cultured mangabey PBMC (22, 86). The fact that SIV infection in macaques increases the turnover of CD8+ T cells more than it does that of CD4+ T cells (57, 69) implies that indirect cell killing is a dominant component of the T-cell death rate. Thus, the major difference between natural and pathogenic SIV infection may lie in the amount of indirect cell killing that results from abnormal T-cell activation and apoptosis.
In conclusion, our findings demonstrate that natural SIV infection does not increase the turnover of T lymphocytes in sooty mangabeys, while this process is exacerbated by pathogenic SIV infection in macaques. The concentration of RTE in peripheral blood did not differ markedly between the two species. These findings are compatible with immune exhaustion mediated by increased peripheral proliferation rather than by early thymic failure in AIDS-susceptible species.
ACKNOWLEDGMENTS
We thank Fred Lee for technical assistance, Simon Monard and Jeremy Segal for help with flow cytometry, and Yong Guo for help with sequencing. We also thank James Blanchard for assistance with animal studies at the Tulane Regional Primate Research Center; Harold McClure for providing mangabey specimens from the Yerkes Regional Primate Research Center; Ruth Connor, Gary Baskin, and Xia Jin for macaque specimens; and Peter Dailey at Chiron Corporation for viral load determination. We are grateful to Janet Harouse for critical reading of the manuscript.
This work was funded by NIH grants to P.A.M. (R01 AI38573), to C.C.-M. (R01 AI41945), and to D.D.H. (R01 AI40387; U0-1 AI42848); by the Pasteur Institute (Paris, France); and by the Aaron Diamond Foundation. S.R.L. is supported by a C. J. Martin Fellowship from the National Health and Medical Research Council of Australia. L.N.M. acknowledges support from the NIH (contract N01-AI-65310) and from a Tulane Regional Primate Research Center base grant (RR-00164). Some of the specimens used in the study were obtained from the Yerkes Regional Primate Research Center (base grant RR-00165).
REFERENCES
- 1.Amedee A M, Lacour N, Gierman J L, Martin L N, Clements J E, Bohm R, Jr, Harrison R M, Murphey-Corb M. Genotypic selection of simian immunodeficiency virus in macaque infants infected transplacentally. J Virol. 1995;69:7982–7990. doi: 10.1128/jvi.69.12.7982-7990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ameisen J C, Capron A. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol Today. 1991;12:102–105. doi: 10.1016/0167-5699(91)90092-8. [DOI] [PubMed] [Google Scholar]
- 3.Baskin G, Murphey-Corb M, Martin L, Davison-Fairburn B, Hu F-S, Kuebler D. Thymus in simian immunodeficiency virus-infected rhesus monkeys. Lab Investig. 1991;65:400–407. [PubMed] [Google Scholar]
- 4.Baskin G B, Murphey-Corb M, Watson E A, Martin L N. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/delta. Vet Pathol. 1988;25:456–467. doi: 10.1177/030098588802500609. [DOI] [PubMed] [Google Scholar]
- 5.Bell E B, Sparshott S M, Bunce C. CD4+ T-cell memory, CD45R subsets and the persistence of antigen—a unifying concept. Immunol Today. 1998;19:60–64. doi: 10.1016/s0167-5699(97)01211-5. [DOI] [PubMed] [Google Scholar]
- 6.Bird J J, Brown D R, Mullen A C, Moskowitz N H, Mahowald M A, Sider J R, Gajewski T F, Wang C R, Reiner S L. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 7.Bonyhadi M L, Rabin L, Salimi S, Brown D A, Kosek J, McCune J M, Kaneshima H. HIV induces thymus depletion in vivo. Nature. 1993;363:728–732. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 8.Bruno S, Darzynkiewicz Z. Cell cycle dependent expression and stability of the nuclear protein detected by Ki-67 antibody in HL-60 cells. Cell Prolif. 1992;25:31–40. doi: 10.1111/j.1365-2184.1992.tb01435.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Kwon D, Jin Z, Monard S, Telfer P, Jones M, Lu C, Aguilar R, Ho D, Marx P. Natural infection of a homozygous δ24 CCR5 red-capped mangabey with an R2b-tropic simian immunodeficiency virus. J Exp Med. 1998;188:2057–2065. doi: 10.1084/jem.188.11.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Telfer P, Gettie A, Reed P, Zhang L, Ho D D, Marx P A. Genetic characterization of new West African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J Virol. 1996;70:3617–3627. doi: 10.1128/jvi.70.6.3617-3627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor R I, Montefiori D C, Binley J M, Moore J P, Bonhoeffer S, Gettie A, Fenamore E A, Sheridan K E, Ho D D, Dailey P J, Marx P A. Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J Virol. 1998;72:7501–7509. doi: 10.1128/jvi.72.9.7501-7509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courgnaud V, Laure F, Fultz P N, Montagnier L, Brechot C, Sonigo P. Genetic differences accounting for evolution and pathogenicity of simian immunodeficiency virus from a sooty mangabey monkey after cross-species transmission to a pig-tailed macaque. J Virol. 1992;66:414–419. doi: 10.1128/jvi.66.1.414-419.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courgnaud V, Saurin W, Villinger F, Sonigo P. Different evolution of simian immunodeficiency virus in a natural host and a new host. Virology. 1998;247:41–50. doi: 10.1006/viro.1998.9217. [DOI] [PubMed] [Google Scholar]
- 14.Dailey P J, Zamroud M, Kelso R, Kolberg J, Urdea M. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected macaques using a branched DNA (bDNA) signal amplification assay. J Med Primatol. 1995;95:209. [Google Scholar]
- 15.De Villartay J-P, Hockett R, Coran D, Korsmeyer S, Cohen D. Deletion of the human T-cell receptor δ-gene by a site-specific recombination. Nature. 1988;335:170–174. doi: 10.1038/335170a0. [DOI] [PubMed] [Google Scholar]
- 16.Douek D C, McFarland R D, Keiser P H, Gage E A, Massey J M, Haynes B F, Polis M A, Haase A T, Feinberg M B, Sullivan J L, Jamieson B D, Zack J A, Picker L J, Koup R A. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 17.Duchrow M, Schluter C, Key G, Kubbutat M H, Wohlenberg C, Flad H D, Gerdes J. Cell proliferation-associated nuclear antigen defined by antibody Ki-67: a new kind of cell cycle-maintaining proteins. Arch Immunol Ther Exp. 1995;43:117–121. [PubMed] [Google Scholar]
- 18.Estaquier J, Idziorek T, de Bels F, Barre-Sinoussi F, Hurtrel B, Aubertin A M, Venet A, Mehtali M, Muchmore E, Michel P, Mouton Y, Girard M, Ameisen J C. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc Natl Acad Sci USA. 1994;91:9431–9435. doi: 10.1073/pnas.91.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fauci A S. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 20.Fleury S, de Boer R J, Rizzardi G P, Wolthers K C, Otto S A, Welbon C C, Graziosi C, Knabenhans C, Soudeyns H, Bart P A, Gallant S, Corpataux J M, Gillet M, Meylan P, Schnyder P, Meuwly J Y, Spreen W, Glauser M P, Miedema F, Pantaleo G. Limited CD4+ T-cell renewal in early HIV-1 infection: effect of highly active antiretroviral therapy. Nat Med. 1998;4:794–801. doi: 10.1038/nm0798-794. [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto S, Yamagashi H. Isolation of an excision product of T-cell receptor α-chain gene rearrangements. Nature. 1987;327:242–243. doi: 10.1038/327242a0. [DOI] [PubMed] [Google Scholar]
- 22.Fultz P N, Anderson D C, McClure H M, Dewhurst S, Mullins J I. SIVsmm infection of macaque and mangabey monkeys: correlation between in vivo and in vitro properties of different isolates. Dev Biol Stand. 1990;72:253–258. [PubMed] [Google Scholar]
- 23.Fultz P N, Gordon T P, Anderson D C, McClure H M. Prevalence of natural infection with simian immunodeficiency virus and simian T-cell leukemia virus type I in a breeding colony of sooty mangabey monkeys. AIDS. 1990;4:619–625. doi: 10.1097/00002030-199007000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Fultz P N, McClure H M, Anderson D C, Swenson R B, Anand R, Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys) Proc Natl Acad Sci USA. 1986;83:5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fultz P N, Stricker R B, McClure H M, Anderson D C, Switzer W M, Horaist C. Humoral response to SIV/SMM infection in macaque and mangabey monkeys. J Acquir Immune Defic Syndr. 1990;3:319–329. [PubMed] [Google Scholar]
- 26.Gao F, Yue L, White A T, Pappas P G, Barchue J, Hanson A P, Greene B M, Sharp P M, Shaw G M, Hahn B H. Human infection by genetically diverse SIVsm-related HIV-2 in West Africa. Nature. 1992;358:495–499. doi: 10.1038/358495a0. [DOI] [PubMed] [Google Scholar]
- 27.Gardner M B. The history of simian AIDS. J Med Primatol. 1996;25:148–157. doi: 10.1111/j.1600-0684.1996.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 28.Georges-Courbot M C, Lu C Y, Makuwa M, Telfer P, Onanga R, Dubreuil G, Chen Z, Smith S M, Georges A, Gao F, Hahn B H, Marx P A. Natural infection of a household pet red-capped mangabey (Cercocebus torquatus torquatus) with a new simian immunodeficiency virus. J Virol. 1998;72:600–608. doi: 10.1128/jvi.72.1.600-608.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerdes J, Lemke H, Baisch H, Wacker H H, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 30.Gorochov G, Neumann A U, Kereveur A, Parizot C, Li T, Katlama C, Karmochkine M, Raguin G, Autran B, Debre P. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat Med. 1998;4:215–221. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- 31.Gougeon M L, Montagnier L. Apoptosis in AIDS. Science. 1993;260:1269–1270. doi: 10.1126/science.8098552. [DOI] [PubMed] [Google Scholar]
- 32.Haynes B, Hale L, Weinhold K, Patel D, Liao H-X, Bressler P, Jones D, Demarest J, Gebhard-Mitchell K, Haase A, Bartlett J. Analysis of the adult thymus in reconstitution of T lymphocytes in HIV-1 infection. J Clin Investig. 1999;103:453–460. doi: 10.1172/JCI5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haynes B F, Hale L P. The human thymus. A chimeric organ comprised of central and peripheral lymphoid components. Immunol Res. 1998;18:175–192. doi: 10.1007/BF02788778. [DOI] [PubMed] [Google Scholar]
- 34.Heid C A, Stevens J, Livak K J, Williams P M. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 35.Hellerstein M, Hanley M B, Cesar D, Siler S, Papageorgopoulos C, Wieder E, Schmidt D, Hoh R, Neese R, Macallan D, Deeks S, McCune J M. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5:83–89. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch V M, Campbell B J, Bailes E, Goeken R, Brown C, Elkins W R, Axthelm M, Murphey-Corb M, Sharp P M. Characterization of a novel simian immunodeficiency virus (SIV) from L'Hoest monkeys (Cercopithecus l'hoesti): implications for the origins of SIVmnd and other primate lentiviruses. J Virol. 1999;73:1036–1045. doi: 10.1128/jvi.73.2.1036-1045.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirsch V M, Olmsted R A, Murphey-Corb M, Purcell R H, Johnson P R. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989;339:389–391. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- 38.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 39.Jamieson B D, Douek D C, Killian S, Hultin L E, Scripture-Adams D D, Giorgi J V, Marelli D, Koup R A, Zack J A. Generation of functional thymocytes in the human adult. Immunity. 1999;10:569–575. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- 40.Jamieson B D, Uittenbogaart C H, Schmid I, Zack J A. High viral burden and rapid CD4+ cell depletion in human immunodeficiency virus type 1-infected SCID-hu mice suggest direct viral killing of thymocytes in vivo. J Virol. 1997;71:8245–8253. doi: 10.1128/jvi.71.11.8245-8253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur A, Grant R, Means R, McClure H, Feinberg M, Johnson R. Diverse host responses and outcomes following simian immunodeficiency virus SIVmac239 infection in sooty mangabeys and rhesus macaques. J Virol. 1998;72:9597–9611. doi: 10.1128/jvi.72.12.9597-9611.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong F-K, Chen C-L, Cooper M. Thymic function can be accurately monitored by the level of recent T cell emigrants in the circulation. Immunity. 1998;8:97–104. doi: 10.1016/s1074-7613(00)80462-8. [DOI] [PubMed] [Google Scholar]
- 43.Kong F-K, Chen C-L, Six A, Hockett R, Cooper M. T cell receptor gene deletion circles identify recent thymic emigrants in the peripheral T cell pool. Proc Natl Acad Sci USA. 1999;96:1536–1540. doi: 10.1073/pnas.96.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kostrikis L, Huang Y, Moore J, Wolinsky S, Zhang L, Guo Y, Deutsch L, Phair J, Neumann A, Ho D. A chemokine receptor CCR2 allele delays HIV-1 disease progression and is associated with a CCR5 promoter mutation. Nat Med. 1998;4:350–353. doi: 10.1038/nm0398-350. [DOI] [PubMed] [Google Scholar]
- 45.Kostrikis L G, Tyagi S, Mhlanga M M, Ho D D, Kramer F R. Spectral genotyping of human alleles. Science. 1998;279:1228–1229. doi: 10.1126/science.279.5354.1228. [DOI] [PubMed] [Google Scholar]
- 46.Kourtis A P, Ibegbu C, Nahmias A J, Lee F K, Clark W S, Sawyer M K, Nesheim S. Early progression of disease in HIV-infected infants with thymic dysfunction. N Engl J Med. 1996;335:1431–1436. doi: 10.1056/NEJM199611073351904. [DOI] [PubMed] [Google Scholar]
- 47.Lewin S R, Vesanen M, Kostrikis L, Hurley A, Duran M, Zhang L, Ho D D, Markowitz M. Use of real-time PCR and molecular beacons to detect virus replication in human immunodeficiency virus type 1-infected individuals on prolonged effective antiretroviral therapy. J Virol. 1999;73:6099–6103. doi: 10.1128/jvi.73.7.6099-6103.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis S M. The mechanism of V(D)J joining: lessons from molecular, immunological, and comparative analyses. Adv Immunol. 1994;56:27–150. doi: 10.1016/s0065-2776(08)60450-2. [DOI] [PubMed] [Google Scholar]
- 49.Lifson J D, Nowak M A, Goldstein S, Rossio J L, Kinter A, Vasquez G, Wiltrout T A, Brown C, Schneider D, Wahl L, Lloyd A L, Williams J, Elkins W R, Fauci A S, Hirsch V M. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol. 1997;71:9508–9514. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Livak F, Schatz D. T-cell receptor α locus V(D)J recombination by-products are abundant in thymocytes and mature T cells. Mol Cell Biol. 1996;16:609–618. doi: 10.1128/mcb.16.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mackall C L, Hakim F T, Gress R E. Restoration of T-cell homeostasis after T-cell depletion. Semin Immunol. 1997;9:339–346. doi: 10.1006/smim.1997.0091. [DOI] [PubMed] [Google Scholar]
- 52.Marx P A, Li Y, Lerche N W, Sutjipto S, Gettie A, Yee J A, Brotman B H, Prince A M, Hanson A, Webster R G, Desrosiers R C. Isolation of a simian immunodeficiency virus related to human immunodeficiency virus type 2 from a West African pet sooty mangabey. J Virol. 1991;65:4480–4485. doi: 10.1128/jvi.65.8.4480-4485.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marx P A, Spira A I, Gettie A, Dailey P J, Veazey R S, Lackner A A, Mahoney C J, Miller C J, Claypool L E, Ho D D, Alexander N J. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 54.McCune J M. Thymic function in HIV-1 disease. Semin Immunol. 1997;9:397–404. doi: 10.1006/smim.1997.0098. [DOI] [PubMed] [Google Scholar]
- 55.Mellors J W, Rinaldo C, Jr, Gupta P, White R M, Todd J A, Kingsley L A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 56.Meyaard L, Otto S A, Keet I P, Roos M T, Miedema F. Programmed death of T cells in human immunodeficiency virus infection. No correlation with progression to disease. J Clin Investig. 1994;93:982–988. doi: 10.1172/JCI117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohri H, Bonhoeffer S, Monard S, Perelson A S, Ho D D. Rapid turnover of T lymphocytes in SIV-infected rhesus macaques. Science. 1998;279:1223–1227. doi: 10.1126/science.279.5354.1223. [DOI] [PubMed] [Google Scholar]
- 58.Murphey-Corb M, Martin L N, Rangan S R, Baskin G B, Gormus B J, Wolf R H, Andes W A, West M, Montelaro R C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986;321:435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- 59.Nahmias A, Clark W, Kourtis A, Lee F, Cotsonis G, Ibegbu C, Thea D, Palumbo P, Vink P, Simonds R, Nesheim S. Thymic dysfunction and time of infection predict mortality in human immunodeficiency virus-infected infants. J Infect Dis. 1998;178:680–685. doi: 10.1086/515368. [DOI] [PubMed] [Google Scholar]
- 60.Norley S G. SIVagm infection of its natural African green monkey host. Immunol Lett. 1996;51:53–58. doi: 10.1016/0165-2478(96)02555-2. [DOI] [PubMed] [Google Scholar]
- 61.Pachl C, Todd J A, Kern D G, Sheridan P J, Fong S J, Stempien M, Hoo B, Besemer D, Yeghiazarian T, Irvine B, et al. Rapid and precise quantification of HIV-1 RNA in plasma using a branched DNA signal amplification assay. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:446–454. doi: 10.1097/00042560-199504120-00003. [DOI] [PubMed] [Google Scholar]
- 62.Pakker N G, Notermans D W, de Boer R J, Roos M T, de Wolf F, Hill A, Leonard J M, Danner S A, Miedema F, Schellekens P T. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–214. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 63.Pedroza-Martins L, Gurney K B, Torbett B E, Uittenbogaart C H. Differential tropism and replication kinetics of human immunodeficiency virus type 1 isolates in thymocytes: coreceptor expression allows viral entry, but productive infection of distinct subsets is determined at the postentry level. J Virol. 1998;72:9441–9452. doi: 10.1128/jvi.72.12.9441-9452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perelson A S, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho D D. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 65.Powell J D, Bednarik D P, Folks T M, Jehuda-Cohen T, Villinger F, Sell K W, Ansari A A. Inhibition of cellular activation of retroviral replication by CD8+ T cells derived from non-human primates. Clin Exp Immunol. 1993;91:473–481. doi: 10.1111/j.1365-2249.1993.tb05927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rey-Cuille M A, Berthier J L, Bomsel-Demontoy M C, Chaduc Y, Montagnier L, Hovanessian A G, Chakrabarti L A. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J Virol. 1998;72:3872–3886. doi: 10.1128/jvi.72.5.3872-3886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rocha B, Penit C, Baron C, Vasseur F, Dautigny N, Freitas A A. Accumulation of bromodeoxyuridine-labeled cells in central and peripheral lymphoid organs: minimal estimates of production and turnover rates of mature lymphocytes. Eur J Immunol. 1990;20:1697–1708. doi: 10.1002/eji.1830200812. [DOI] [PubMed] [Google Scholar]
- 68.Roederer M, De Rosa S C, Watanabe N, Herzenberg L A. Dynamics of fine T-cell subsets during HIV disease and after thymic ablation by mediastinal irradiation. Semin Immunol. 1997;9:389–396. doi: 10.1006/smim.1997.0097. [DOI] [PubMed] [Google Scholar]
- 69.Rosenzweig M, DeMaria M A, Harper D M, Friedrich S, Jain R K, Johnson R P. Increased rates of CD4(+) and CD8(+) T lymphocyte turnover in simian immunodeficiency virus-infected macaques. Proc Natl Acad Sci USA. 1998;95:6388–6393. doi: 10.1073/pnas.95.11.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sachsenberg N, Perelson A S, Yerly S, Schockmel G A, Leduc D, Hirschel B, Perrin L. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J Exp Med. 1998;187:1295–1303. doi: 10.1084/jem.187.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schluter C, Duchrow M, Wohlenberg C, Becker M H, Key G, Flad H D, Gerdes J. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol. 1993;123:513–522. doi: 10.1083/jcb.123.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scollay R G, Butcher E C, Weissman I L. Thymus cell migration: quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 73.Stanley S K, McCune J M, Kaneshima H, Justement J S, Sullivan M, Boone E, Baseler M, Adelsberger J, Bonyhadi M, Orenstein J, Fox C H, Fauci A S. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178:1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steinmann G. Changes in the human thymus during aging. Curr Top Pathol. 1986;75:43–80. doi: 10.1007/978-3-642-82480-7_2. [DOI] [PubMed] [Google Scholar]
- 75.Suryanarayana K, Wiltrout T A, Vasquez G M, Hirsch V M, Lifson J D. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res Hum Retrovir. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- 76.Ten Haaft P, Verstrepen V, Überla K, Rosenwirth B, Heeney J. A pathogenic threshold of virus load defined in simian immunodeficiency virus- or simian-human immunodeficiency virus-infected macaques. J Virol. 1998;72:10281–10285. doi: 10.1128/jvi.72.12.10281-10285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tough D F, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tyagi S, Kramer F R. Molecular beacons: probes that fluoresce upon hybridization. Nat Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 79.Verschuren M, Wolvers-Tettro I, Breit T, Noordzij J, van Wering E, van Dongen J. Preferential rearrangements of the T cell receptor-δ-deletion elements in human T cells. J Immunol. 1997;158:1208–1216. [PubMed] [Google Scholar]
- 80.Villinger F, Folks T M, Lauro S, Powell J D, Sundstrom J B, Mayne A, Ansari A A. Immunological and virological studies of natural SIV infection of disease-resistant non-human primates. Immunol Lett. 1996;51:59–68. doi: 10.1016/0165-2478(96)02556-4. [DOI] [PubMed] [Google Scholar]
- 81.Villinger F, Powell J D, Jehuda-Cohen T, Neckelmann N, Vuchetich M, De B, Folks T M, McClure H M, Ansari A A. Detection of occult simian immunodeficiency virus SIVsmm infection in asymptomatic seronegative nonhuman primates and evidence for variation in SIV gag sequence between in vivo- and in vitro-propagated virus. J Virol. 1991;65:1855–1862. doi: 10.1128/jvi.65.4.1855-1862.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walker R E, Carter C S, Muul L, Natarajan V, Herpin B R, Leitman S F, Klein H G, Mullen C A, Metcalf J A, Baseler M, Falloon J, Davey R T, Jr, Kovacs J A, Polis M A, Masur H, Blaese R M, Lane H C. Peripheral expansion of pre-existing mature T cells is an important means of CD4+ T-cell regeneration HIV-infected adults. Nat Med. 1998;4:852–856. doi: 10.1038/nm0798-852. [DOI] [PubMed] [Google Scholar]
- 83.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 84.Wolthers K C, Schuitemaker H, Miedema F. Rapid CD4+ T-cell turnover in HIV-1 infection: a paradigm revisited. Immunol Today. 1998;19:44–48. doi: 10.1016/s0167-5699(97)01188-2. [DOI] [PubMed] [Google Scholar]
- 85.Wykrzykowska J J, Rosenzweig M, Veazey R S, Simon M A, Halvorsen K, Desrosiers R C, Johnson R P, Lackner A A. Early regeneration of thymic progenitors in rhesus macaques infected with simian immunodeficiency virus. J Exp Med. 1998;187:1767–1778. doi: 10.1084/jem.187.11.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yehuda-Cohen T, Powell J D, Villinger F, McClure H M, Sell K W, Ahmed-Ansari A. Comparison of SIV/SMM replication in CD4+ T cell and monocyte/macrophage cultures from rhesus macaques and sooty mangabeys. J Med Primatol. 1990;19:251–267. [PubMed] [Google Scholar]
- 87.Zhang L, Lewin S R, Markowitz M, Lin H H, Skulsky E, Karanicolas R, He Y, Xia J, Tuttleton S, Vesanen M, Spiegel H, Kost R, Wolinsky S, Borkowsky W, Palumbo P, Kostrikis L G, Ho D D. Measuring recent thymic emigrants in blood of normal persons and HIV-1-infected patients before and after effective therapy. J Exp Med. 1999;190:725–732. doi: 10.1084/jem.190.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Z Q, Notermans D W, Sedgewick G, Cavert W, Wietgrefe S, Zupancic M, Gebhard K, Henry K, Boies L, Chen Z, Jenkins M, Mills R, McDade H, Goodwin C, Schuwirth C M, Danner S A, Haase A T. Kinetics of CD4+ T cell repopulation of lymphoid tissues after treatment of HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:1154–1159. doi: 10.1073/pnas.95.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]