Key Points
Question
Is stepped care, with palliative care visits occurring only at key points in patients’ cancer trajectories and using a decrement in quality of life (QOL) to trigger more intensive palliative care, an effective model for delivering palliative care to patients with advanced cancer?
Findings
In this randomized trial of 507 adults with advanced lung cancer, patients assigned to stepped palliative care had significantly fewer palliative care visits and reported QOL scores at week 24 that were noninferior (adjusted mean, 100.6 vs 97.8; P < .001) to the QOL scores of patients assigned to an early palliative care model with monthly visits after diagnosis.
Meaning
Stepped palliative care is an effective and more scalable means to deliver palliative care to improve QOL for patients with advanced lung cancer.
Abstract
Importance
Despite the evidence for early palliative care improving outcomes, it has not been widely implemented in part due to palliative care workforce limitations.
Objective
To evaluate a stepped-care model to deliver less resource-intensive and more patient-centered palliative care for patients with advanced cancer.
Design, Setting, and Participants
Randomized, nonblinded, noninferiority trial of stepped vs early palliative care conducted between February 12, 2018, and December 15, 2022, at 3 academic medical centers in Boston, Massachusetts, Philadelphia, Pennsylvania, and Durham, North Carolina, among 507 patients who had been diagnosed with advanced lung cancer within the past 12 weeks.
Intervention
Step 1 of the intervention was an initial palliative care visit within 4 weeks of enrollment and subsequent visits only at the time of a change in cancer treatment or after a hospitalization. During step 1, patients completed a measure of quality of life (QOL; Functional Assessment of Cancer Therapy–Lung [FACT-L]; range, 0-136, with higher scores indicating better QOL) every 6 weeks, and those with a 10-point or greater decrease from baseline were stepped up to meet with the palliative care clinician every 4 weeks (intervention step 2). Patients assigned to early palliative care had palliative care visits every 4 weeks after enrollment.
Main Outcomes and Measures
Noninferiority (margin = −4.5) of the effect of stepped vs early palliative care on patient-reported QOL on the FACT-L at week 24.
Results
The sample (n = 507) mostly included patients with advanced non–small cell lung cancer (78.3%; mean age, 66.5 years; 51.4% female; 84.6% White). The mean number of palliative care visits by week 24 was 2.4 for stepped palliative care and 4.7 for early palliative care (adjusted mean difference, −2.3; P < .001). FACT-L scores at week 24 for the stepped palliative care group were noninferior to scores among those receiving early palliative care (adjusted FACT-L mean score, 100.6 vs 97.8, respectively; difference, 2.9; lower 1-sided 95% confidence limit, −0.1; P < .001 for noninferiority). Although the rate of end-of-life care communication was also noninferior between groups, noninferiority was not demonstrated for days in hospice (adjusted mean, 19.5 with stepped palliative care vs 34.6 with early palliative care; P = .91).
Conclusions and Relevance
A stepped-care model, with palliative care visits occurring only at key points in patients’ cancer trajectories and using a decrement in QOL to trigger more intensive palliative care exposure, resulted in fewer palliative care visits without diminishing the benefits for patients’ QOL. While stepped palliative care was associated with fewer days in hospice, it is a more scalable way to deliver early palliative care to enhance patient-reported outcomes.
Trial Registration
ClinicalTrials.gov Identifier: NCT03337399
This randomized clinical trial assesses whether a stepped-care model of palliative care is noninferior to early integrated palliative care based on patient-reported quality of life among patients with advanced lung cancer.
Introduction
More than a decade ago, national guidelines began to recommend delivery of palliative care by specialty-trained clinicians for patients with advanced cancer, either at the time of diagnosis or when their estimated life expectancy is limited.1,2 These guidelines were based on multiple trials demonstrating the benefits of early palliative care for improving key outcomes in both patients and caregivers, including patient-reported quality of life (QOL) and mood, as well as receipt of high-quality end-of-life care.3,4,5,6,7,8,9,10 Despite the evidence, this efficacious care model has not been widely implemented, due in part to the shortage of palliative care clinicians, especially in community and limited-resource settings.11,12,13,14,15,16 Moreover, in the years since most early palliative care trials were conducted, treatment options for patients with many historically poor-prognosis advanced cancers have expanded, affording patients more effective therapies that prolong survival.17,18 The lack of palliative care resources and shifting patient needs due to improvements in cancer therapeutics highlight the need for less resource-intensive and more patient-centered palliative care models.19 Moreover, the historical model of a referral system that relies on oncologists to identify patients with cancer who may benefit from early palliative care remains inadequate.20,21
We developed a stepped palliative care intervention based on evidence-based care models that improve access to health care for specialties with an insufficient number of trained clinicians.22,23,24,25 In stepped care, all patients receive care for their condition, but with a minimum of required contact with a specialty-trained clinician. More intensive treatment with the clinician is reserved for patients who do not benefit sufficiently from the less intensive therapies. A key element of this model is that patients must be monitored systematically and “stepped up” to more intensive treatment if less exposure to the clinician does not achieve sufficient benefit. In addition to using fewer resources by requiring minimum contact for patients who do not need more frequent visits, applying a stepped-care model is also a means to deliver more patient-centered care, tailoring palliative care to patients’ clinical needs.
We therefore conducted a multisite trial with a primary aim of examining the noninferiority of stepped vs early palliative care on patient-reported QOL among patients with advanced lung cancer based on the strong evidence base that established early palliative care as the standard of care in this population. We selected a noninferiority margin for QOL based on intervention effects observed in prior studies of early palliative care compared with usual oncology care and below the threshold for a clinically meaningful difference.5,26,27 Secondary and exploratory outcomes included the impact of the stepped-care model on utilization of palliative care and hospice services and other patient-reported outcomes.
Methods
Trial Design and Participants
We conducted a prospective, nonblinded, randomized noninferiority trial of stepped vs early palliative care among patients with a new diagnosis of incurable lung cancer at 3 US sites (Massachusetts General Hospital [MGH], Boston; Duke Cancer Institute, Durham, North Carolina; and the University of Pennsylvania, Philadelphia). The institutional review boards at the participating sites approved the protocol. Because this was a minimal-risk supportive care study in an advanced cancer population in which hospitalizations and deaths are expected due to disease worsening and given prior early palliative care trials demonstrating no risk of harm, we did not monitor serious adverse events.28,29 Thus, the MGH investigative team served as the data monitoring committee to monitor intervention delivery and data collection only. We amended the protocol to increase the sample size to account for missing data and to allow remote consenting and video visits after the onset of the COVID-19 pandemic. The protocol was published previously,30 and the trial protocol and statistical analysis plan are available in Supplement 1 and Supplement 2.
Eligible patients were adults (aged ≥18 years) receiving care at a participating site and diagnosed with advanced lung cancer (non–small cell lung cancer; small cell lung cancer) or mesothelioma within the prior 12 weeks that was not being treated with curative intent; had a documented Eastern Cooperative Oncology Group performance status of 0 (fully active with no restrictions) to 2 (unable to work and in bed <50% of the day); and could read and respond to questions in English or Spanish. Patients were not eligible if they were already receiving outpatient palliative care, were enrolled in hospice, or had cognitive or psychiatric conditions prohibiting study consent or participation, as determined by the treating oncologists. Participants were scheduled for study visits for a minimum of 12 months.
Research assistants screened patients presenting to the thoracic oncology clinic by reviewing the electronic health record to determine the cancer diagnosis, stage, and treatment goal. Research assistants obtained permission from the oncology team to approach their patients for study participation. With clinician approval, the research assistants approached patients to review study procedures and obtain consent in person before the COVID-19 pandemic and either in person or remotely (via telephone) after the start of the pandemic. Spanish-speaking patients provided consent through a Spanish-speaking research assistant or with the assistance of a hospital interpreter. Patients who consented were required to complete baseline self-report measures within 2 weeks of consent to be enrolled in the trial.
Randomization and Study Procedures
Registered patients were randomized 1:1 using computer-generated block randomization, stratified by study site and lung cancer diagnosis (non–small cell lung cancer vs other). Research staff not affiliated with the protocol conducted randomization centrally at MGH for all sites.
Research assistants informed patients of their group assignment and scheduled the initial palliative care visit within 4 weeks of enrollment. Before the COVID-19 pandemic, palliative care visits were scheduled in person, with phone calls used for missed visits that could not be rescheduled within the protocol-required time frame. After the onset of the pandemic, palliative care visits could be conducted using secure videoconferencing. Participants were permitted to defer and reinitiate palliative care visits at their or their clinicians’ request. Similarly, participants or their oncology and palliative care clinicians could request more frequent palliative care visits than required per the study protocol. Trained palliative care physicians and advanced practice practitioners (n = 34) conducted study visits. To ensure intervention fidelity, clinicians received an intervention guide and underwent comprehensive training in the principles of early palliative care delivery with a 6-hour in-person or recorded training session. Palliative care clinicians cared for patients assigned to both study groups and completed a standardized electronic survey after each study visit to document topics addressed during the encounter, although this survey was optional for telephone calls. Finally, the entire study team met monthly to support participant recruitment, intervention delivery, and data collection.
Patients assigned to early palliative care followed identical study procedures as in our prior trials.5,26 Specifically, patients were scheduled for palliative care visits every 4 weeks and were seen by the inpatient palliative care team during hospital admissions throughout their study participation.
Patients assigned to stepped palliative care started step 1 of the intervention, which included an initial palliative care visit within 4 weeks of enrollment, with subsequent palliative care visits scheduled only at the time of a change in cancer treatment (due to cancer progression, treatment toxicity, or discontinuation of therapy) or after a hospitalization. Patients in step 1 were not required to be seen by the inpatient palliative care team during hospital admissions. Patients in step 1 completed a QOL measure (Functional Assessment of Cancer Therapy–Lung [FACT-L]; range, 0-136, with higher scores indicating better QOL) every 6 weeks for up to 18 months after enrollment. Those with a 10-point or greater decrease from baseline in their score were stepped up to step 2, in which they were scheduled to meet with the palliative care clinician every 4 weeks (eFigure 1 in Supplement 3) and also were seen by the inpatient palliative care team during hospital admissions for the remainder of the study period. A 10-point change in the FACT-L is clinically meaningful and correlates with outcomes such as disease progression.27
Outcomes
Participants completed all study self-reported measures at baseline (including reporting of gender, race, and ethnicity) before randomization and every 12 weeks for a total of 48 weeks, except for the demographic questionnaire and Self-Administered Comorbidity Questionnaire (range, 0-36, with higher scores indicating greater comorbidity), which were administered only at baseline. Self-reported measures were described previously and included a sociodemographic questionnaire and validated measures scored in accordance with published guidelines (eAppendix 1 in Supplement 3).30,31,32,33,34,35,36,37 Participants could complete self-report measures on paper in person or via mail, electronically, or over the telephone at designated time points with a window of ±21 days. Measures that were not publicly available in Spanish were translated forward and backward by a Spanish-speaking clinician.
The primary outcome was patient QOL on the FACT-L. Secondary outcomes included (1) palliative care utilization, as measured by the number of palliative care visits; (2) patient-reported communication about end-of-life care preferences, as measured by an item on the Prognosis and Treatment Perceptions Questionnaire (PTPQ), scored dichotomously; and (3) length of stay in hospice, as measured from the date of hospice enrollment until death among patients who died.
There were 3 exploratory outcomes. Depression symptoms were measured by the Patient Health Questionnaire 9, with a range of 0 to 27 and higher scores indicating worse depression. Use of coping strategies were measured by the Brief Coping Orientation to Problems Experienced Inventory, including scales for approach-oriented coping (range, 6-24) and avoidant coping (range, 4-16), with higher scores indicating greater use of each strategy. Perception of prognosis and goal of therapy were measured by 2 items on the PTPQ, scored dichotomously.
Statistical Analyses
We designed the study to demonstrate the noninferiority of stepped vs early palliative care with a margin of −4.5 points on the FACT-L at week 24.26 This margin is a little more than half the QOL benefit we observed in our prior trial of early palliative care vs standard care in patients with advanced lung cancer on the FACT-L (7.5 points) and is also below the established threshold of a clinically meaningful difference on this measure (6 points).26,27 A sample size of 188 patients per group would achieve 80% power to detect noninferiority based on a 1-sided 5% significance level t test against the margin of −4.5 points, assuming a standard deviation of 17.5 points and an actual mean difference of zero.26,38 Given an anticipated 36% rate of missing data at week 24 due to loss to follow-up, withdrawal, or death, we planned to enroll 255 per group.
All regression models included main effects for intervention group and randomization stratification factors (study site and lung cancer type); additional terms are specified below. We report model-based estimates of within-group adjusted means (or proportions) with standard errors and between-group differences with either lower 1-sided 95% confidence limits for noninferiority analyses (which corresponds to the primary 1-sided 5% significance level test for noninferiority)39 or 2-sided 95% confidence intervals for superiority analyses. Primary comparisons of patient-reported outcomes were made among participants who survived through week 24 (ie, a survivors analysis), given that this was the comparison of primary scientific interest and similar death rates were anticipated across groups.40,41
The primary outcome was patient-reported QOL at week 24, measured by FACT-L. The difference in week 24 means between groups was estimated using a linear regression model adjusted for baseline FACT-L score. Noninferiority of stepped palliative care was established if the lower 1-sided 95% confidence limit for the estimated difference in means was greater than the prespecified margin of −4.5 points, which corresponds to the 1-sided 5% significance level test against this margin.39
We used a false discovery rate control approach to interpret the results of significance tests of the 3 secondary outcomes with a false discovery rate of 0.15.42,43 The difference between groups in the mean number of outpatient palliative care visits per patient by week 24 was assessed using linear regression and a 2-sided superiority test. Noninferiority of stepped palliative care in the proportion reporting patient-clinician communication about end-of-life care at each patient’s final follow-up assessment was evaluated using a binomial generalized linear model with identity link and a 1-sided test against the prespecified margin of −10%. Among patients who died, noninferiority of stepped palliative care in the mean length of stay in hospice was assessed using linear regression and a 1-sided test against the prespecified margin of −7 days, based on published quality metrics.44,45,46
Exploratory outcomes included patient-reported prognostic understanding, depression symptoms, and coping strategies at week 24. Proportions with prognostic understanding based on PTPQ items eliciting patients’ goals of cancer care (“to cure my cancer” vs any other option) and patients’ assessment of curability (yes vs no) were compared using binomial generalized linear models with identity link. Patient-reported depression symptoms (assessed by the Patient Health Questionnaire 9) and coping strategies (assessed by the Brief Coping Orientation to Problems Experienced Inventory) at week 24 were compared using linear regression models adjusted for baseline outcome scores. Exploratory outcomes are reported as estimates with 2-sided 95% CIs and are not adjusted for multiple comparisons.
Our primary analysis excluded participants with missing FACT-L scores at week 24. Sensitivity analyses incorporating additional longitudinal data and using multiple imputation for nonresponse (but not truncation due to death) were conducted to evaluate the impact of missing data on results for the primary outcome (eAppendix 2 in Supplement 3). All analyses used SAS version 9.4 (SAS Institute Inc) or R version 4.3.2 (R Core Team).
Results
Patient Participants
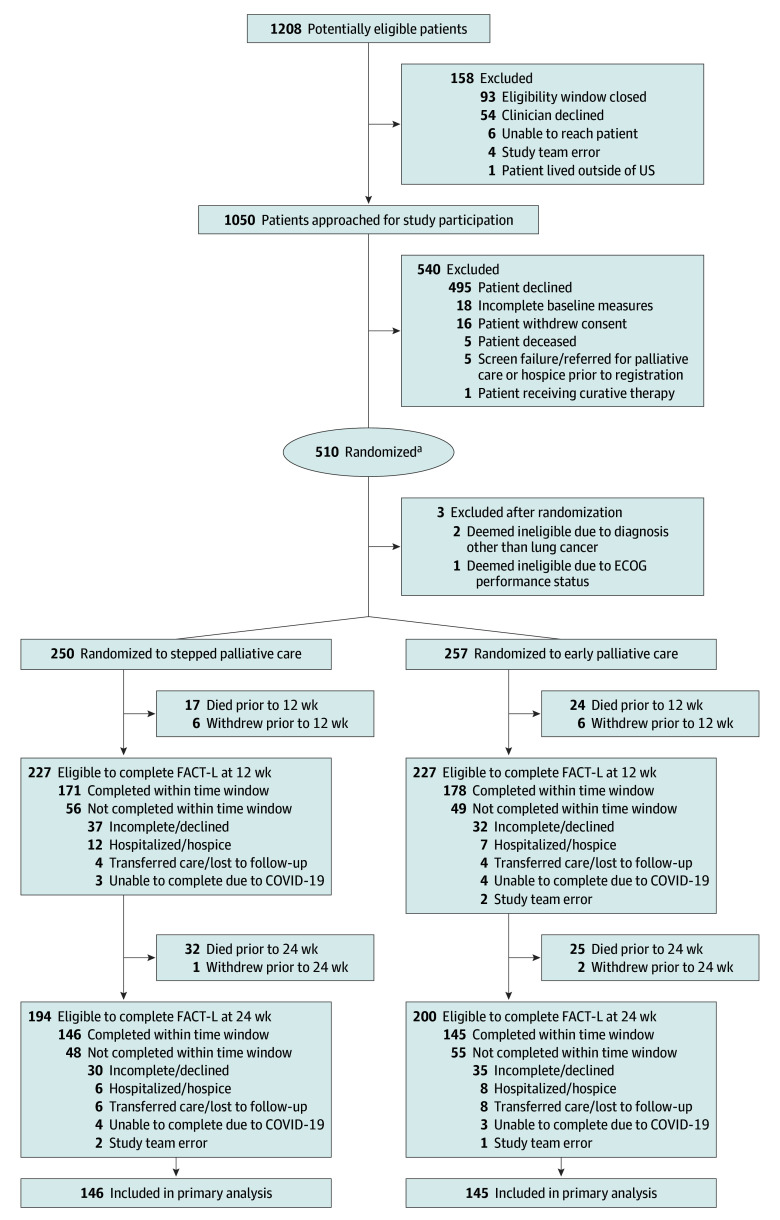
Between February 12, 2018, and December 15, 2022, 510 patients were randomized to stepped palliative care (n = 250) or early palliative care (n = 257); 3 patients were deemed ineligible after enrollment (Figure 1). At 24 weeks, 291 (73.9% of eligible) participants completed the FACT-L. Rates of FACT-L completion at weeks 36 and 48 are shown in eTable 1 in Supplement 3. As shown in Table 1, participants’ mean age was 66.5 years, and 260 (51.4%) were women. Fifty-seven participants (11.3%) self-identified as African American or Black and 427 (84.6%) as White. Most participants had non–small cell lung cancer (78.3%) and an Eastern Cooperative Oncology Group performance status of 1 or 2 (75.3%) and were married/partnered (65.7%). Study groups were generally balanced with respect to clinical characteristics. Baseline characteristics of participants who did vs did not complete the FACT-L at week 24 are shown in eTable 2 in Supplement 3.
Figure 1. Flow of Participants Through a Trial of Stepped vs Early Palliative Care for Patient With Advanced Lung Cancer.
ECOG indicates Eastern Cooperative Oncology Group; FACT-L, Functional Assessment of Cancer Therapy–Lung.
aA total of 397 participants had diagnoses of non–small cell lung cancer, 100 had small cell lung cancer, and 10 had mesothelioma.
Table 1. Baseline Participant Characteristics.
| Characteristics | Stepped palliative care (n = 250) | Early integrated palliative care (n = 257) |
|---|---|---|
| Age, mean (SD), y | 66.8 (9.2) | 66.1 (11.1) |
| No. (%) >75 | 43 (17.2) | 58 (22.6) |
| Women, No./total (%)a | 130/250 (52.0) | 130/256 (50.8) |
| Race, No. (%)b | n = 250 | n = 255 |
| African American or Black | 29 (11.6) | 28 (11.0) |
| American Indian or Alaska Native | 2 (0.8) | 2 (0.8) |
| Asian | 3 (1.2) | 5 (2.0) |
| Native Hawaiian or Pacific Islander | 0 | 0 |
| White | 215 (86.0) | 212 (83.1) |
| Otherc | 3 (1.2) | 5 (2.0) |
| Hispanic or Latino ethnicity, No./total (%) | 3/243 (1.2) | 5/250 (2.0) |
| Religion, No. (%) | n = 246 | n = 249 |
| Catholic | 98 (39.8) | 91 (36.5) |
| Other Christian (eg, Protestant) | 97 (39.4) | 106 (42.6) |
| Jewish | 15 (6.1) | 13 (5.2) |
| Atheist | 0 | 3 (1.2) |
| Muslim | 0 | 2 (0.8) |
| None | 24 (9.8) | 28 (11.2) |
| Other | 12 (4.9) | 6 (2.4) |
| Relationship status, No. (%) | n = 247 | n = 252 |
| Married/partnered | 152 (61.5) | 176 (69.8) |
| Divorced/separated | 48 (19.4) | 25 (9.9) |
| Widowed/loss of partner | 30 (12.1) | 34 (13.5) |
| Single | 17 (6.9) | 17 (6.7) |
| Education, No. (%) | n = 244 | n = 248 |
| High school graduate or lower | 80 (32.8) | 78 (31.5) |
| Associate degree/technical school | 66 (27.0) | 59 (23.8) |
| College degree | 52 (21.3) | 55 (22.2) |
| Master’s, professional, or doctoral degree | 46 (18.9) | 56 (22.6) |
| Annual income, No. (%), $ | n = 221 | n = 225 |
| <25 000 | 42 (19.0) | 38 (16.9) |
| 25 000-49 999 | 49 (22.2) | 52 (23.1) |
| 50 000-99 999 | 63 (28.5) | 56 (24.9) |
| 100 000-149 999 | 27 (12.2) | 33 (14.7) |
| ≥150 000 | 40 (18.1) | 46 (20.4) |
| Smoking status, No. (%) | n = 232 | n = 228 |
| Current or former | 168 (72.4) | 146 (64.0) |
| Never or <10 pack-years | 64 (27.6) | 82 (36.0) |
| Cancer type, No. (%) | ||
| Non–small cell lung cancer | 194 (77.6) | 203 (79.0) |
| Small cell lung cancer | 53 (21.2) | 47 (18.3) |
| Mesothelioma | 3 (1.2) | 7 (2.7) |
| Cancer treatment, No. (%) | ||
| Platinum-based doublet chemotherapy | 128 (51.2) | 116 (45.1) |
| Radiation | 54 (21.6) | 38 (14.8) |
| Oral targeted chemotherapy | 40 (16.0) | 50 (19.5) |
| Immunotherapy | 22 (8.8) | 39 (15.2) |
| Single-agent intravenous chemotherapy | 3 (1.2) | 9 (3.5) |
| No treatment | 2 (0.8) | 4 (1.6) |
| Combined radiation and chemotherapy | 0 | 1 (0.4) |
| Other treatment | 1 (0.4) | 0 |
| Cancer gene variant status, No. (%) | ||
| EGFR | 37 (14.8) | 39 (15.2) |
| ALK | 11 (4.4) | 10 (3.9) |
| ROS | 3 (1.2) | 2 (0.8) |
| RET | 1 (0.4) | 2 (0.8) |
| Other or no gene variant | 198 (79.2) | 204 (79.4) |
| Eastern Cooperative Oncology Group performance status, No. (%) | ||
| 0 (Fully active with no restrictions) | 61 (24.4) | 64 (24.9) |
| 1 (Able to do light work) | 153 (61.2) | 153 (59.5) |
| 2 (Unable to work and in bed <50% of the day) | 36 (14.4) | 40 (15.6) |
| Medical comorbidity (SCQ score), mean (SD)d | 8.6 (4.4) [n = 240] | 7.7 (4.0) [n = 247] |
| Quality of life (FACT-L score), mean (SD)e | 93.6 (19.4) [n = 250] | 95.7 (19.7) [n = 256] |
| Depression symptoms (PHQ-9 score), mean (SD)f | 6.6 (5.1) [n = 240] | 6.0 (4.9) [n = 246] |
| Coping skills (Brief COPE score), mean (SD)g | ||
| Approach-oriented coping | 17.7 (3.9) [n = 216] | 18.2 (3.7) [n = 220] |
| Avoidant coping | 6.0 (2.3) [n = 225] | 6.4 (2.6) [n = 228] |
| Perceptions of prognosis, No./total (%) | ||
| Perceives goal of therapy is to cure cancer | 80/237 (33.8) | 74/235 (31.5) |
| Perceives cancer is curable | 59/218 (27.1) | 54/224 (24.1) |
| End-of-life care communication, No./total (%) | 37/232 (15.9) | 23/239 (9.6) |
Gender was collected by self-report that allowed a single selection from a predetermined list: man, woman, or other/write-in. No participants selected other.
Race was collected by self-report that allowed multiple selections from a predetermined list, including an other/write-in option. Sum of percentages may exceed 100%.
Indicates participant selected other race. See eAppendix 3 in Supplement 3 for itemization.
Self-Administered Comorbidity Questionnaire (SCQ) score range, 0-36, with higher scores indicating greater comorbidity.
Functional Assessment of Cancer Therapy–Lung Scale (FACT-L) score range, 0-136, with higher scores indicating better quality of life; minimum clinically important difference = 6.
Patient Health Questionnaire 9 (PHQ-9) score range, 0-27, with higher scores indicating more significant depression symptoms.
Brief Coping Orientation to Problems Experienced Inventory (Brief COPE) scores: approach-oriented coping score range, 6-24, with higher scores indicating greater use of approach-oriented coping strategies; avoidant coping score range, 4-16, with higher scores indicating greater use of avoidant coping strategies.
Intervention Delivery
eTable 3 in Supplement 3 shows the proportions of eligible patients in the stepped palliative care group (alive and not hospitalized or in hospice) in step 1 of the intervention who completed the FACT-L at each 6-week interval to determine whether they needed to move to step 2. Sixty-six patients (26.4%) assigned to stepped palliative care were stepped up to step 2 by 24 weeks at a median of 70 days, and 91 (36.4%) were stepped up to step 2 by 48 weeks. Palliative care clinicians completed the survey documenting the visit content for 1180 of 1251 (94.3%) and 1812 of 1927 (94.0%) face-to-face visits by weeks 24 and 48, respectively. Intervention fidelity by study group assignment up to weeks 24 and 48 is shown in eFigure 2 in Supplement 3.
Primary Outcome
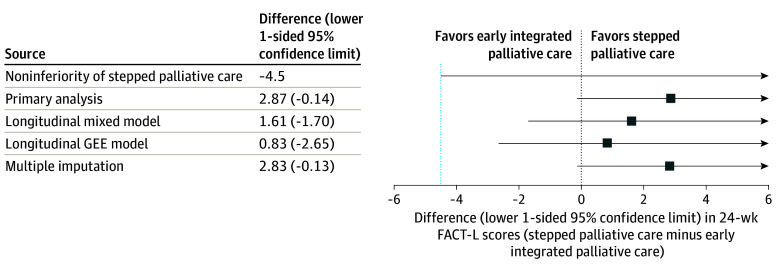
Quality-of-life scores on the FACT-L at week 24 for patients assigned to stepped palliative care were noninferior to those assigned to early palliative care (adjusted mean score, 100.6 vs 97.8; difference, 2.9; lower 1-sided 95% confidence limit, −0.1; P < .001) (Table 2 and Figure 2). In accordance with a more stringent 1-sided 2.5% significance level test for noninferiority, we also calculated a post hoc lower 1-sided 97.5% confidence limit for the primary QOL outcome (difference, 2.9; lower 1-sided 97.5% confidence limit, −0.7), which also supported noninferiority of stepped palliative care. Figure 3A shows the distribution of FACT-L scores up to week 24 by study group, and eFigure 3 in Supplement 3 shows score distribution up to week 48. Sensitivity analyses assessing the impact of missing data supported noninferiority of stepped palliative care (Figure 2; eTable 4 in Supplement 3).
Table 2. Regression Model Estimates of Study Group Effects on 24-Week Outcome Measures.
| Outcome measures | Estimated mean or proportion (SE)a | Difference (lower 1-sided 95% confidence limit) or difference (95% CI)b | P valuec | |
|---|---|---|---|---|
| Stepped palliative care (n = 250) | Early integrated palliative care (n = 257) | |||
| Primary outcome test of noninferiority | ||||
| Quality of life, FACT-L, mean scored | 100.6 (1.3) [n = 146] | 97.8 (1.3) [n = 145] | 2.9 (−0.1) | <.001e |
| Secondary outcome tests of noninferiority | ||||
| Discussed end-of-life care with clinician, %f | 30.4 (3.3) [n = 190] | 33.0 (3.4) [n = 187] | −2.6 (−10.4) | .09e |
| Length of hospice stay, mean, dg | 19.5 (4.3) [n = 159] | 34.6 (4.2) [n = 161] | −15.2 (−25.1) | .91e |
| Secondary outcome test of superiority | ||||
| Palliative care visits, mean No. per patient | 2.4 (0.2) [n = 250] | 4.7 (0.1) [n = 257] | −2.3 (−2.7 to −1.8) | <.001 |
| Exploratory outcome tests of superiority | ||||
| Depression symptoms, PHQ-9, mean score | 5.0 (0.3) [n = 139] | 5.3 (0.3) [n = 142] | −0.4 (−1.3 to 0.5) | |
| Coping skills, Brief COPE | ||||
| Approach-oriented coping, mean score | 17.7 (0.3) [n = 116] | 17.5 (0.3) [n = 115] | 0.2 (−0.7 to 1.0) | |
| Avoidant coping, mean score | 5.7 (0.2) [n = 126] | 5.7 (0.2) [n = 124] | 0.0 (−0.5 to 0.5) | |
| Perceptions of prognosis | ||||
| Perceives goal of therapy is to cure cancer, % | 27.9 (3.3) [n = 181] | 25.6 (3.2) [n = 184] | 2.3 (−6.7 to 11.3) | |
| Perceives cancer is curable, % | 22.2 (3.0) [n = 182] | 23.8 (3.1) [n = 176] | −1.6 (−10.0 to 6.8) | |
Abbreviations: Brief COPE, Brief Coping Orientation to Problems Experienced Inventory; FACT-L, Functional Assessment of Cancer Therapy–Lung; PHQ-9, Patient Health Questionnaire 9.
All estimates are adjusted for study site and cancer type (non–small cell lung cancer vs small cell lung cancer or mesothelioma). Estimates for FACT-L, PHQ-9, and coping skills are additionally adjusted for baseline scores of outcome variable. Numbers in brackets reflect the number of patients in each group whose data were included in the model.
Comparing the lower 1-sided 95% confidence limit with the noninferiority margin corresponds to the primary 1-sided 5% significance level test for noninferiority. Confidence intervals for secondary and exploratory outcomes are not adjusted for multiple testing.
P values for secondary outcomes are adjusted for multiple testing using the false discovery rate approach. P values are not reported for exploratory outcomes.
Prespecified noninferiority margin, −4.5 points.
P value for noninferiority.
Prespecified noninferiority margin, −10 percentage points.
Prespecified noninferiority margin, −7 days.
Figure 2. Primary and Sensitivity Analysis Model Estimates of Study Group Effects on the Primary 24-Week Outcome Measure of FACT-L Scores.
FACT-L indicates Functional Assessment of Cancer Therapy–Lung; GEE, generalized estimating equation. Points indicate model estimates of the mean between-group difference in FACT-L scores at week 24. All estimates are adjusted for study site and cancer type. Whiskers extend to the lower 1-sided 95% confidence limit for each estimate. Comparing the lower 1-sided 95% confidence limit with the noninferiority margin corresponds to the primary 1-sided 5% significance level test for noninferiority. Sensitivity analysis methods are described in eAppendix 2 in Supplement 3.
Figure 3. Longitudinal Patient-Reported Outcome Measures Up to 24 Weeks.
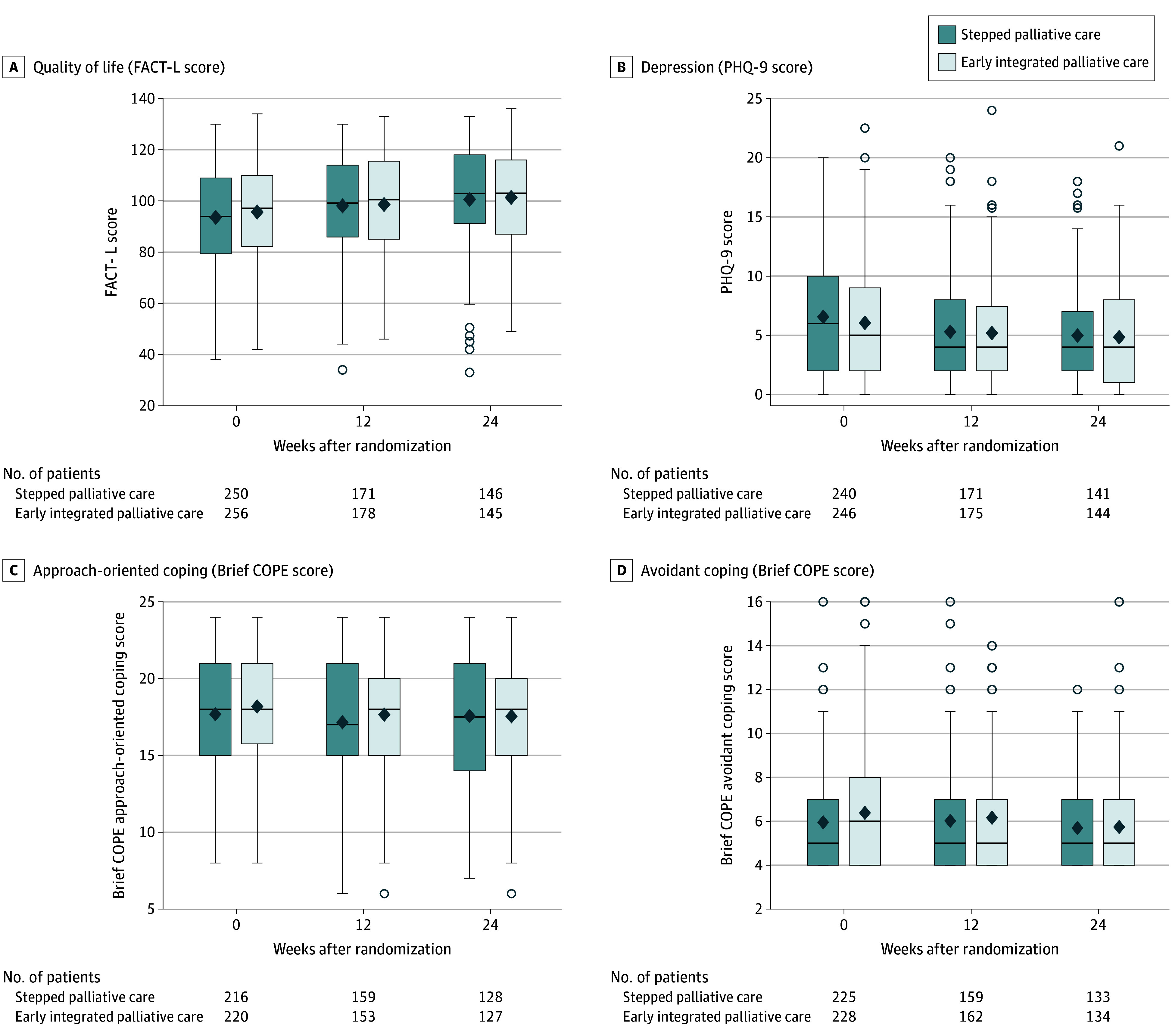
Brief COPE indicates Brief Coping Orientation to Problems Experienced Inventory; FACT-L, Functional Assessment of Cancer Therapy–Lung; PHQ-9, Patient Health Questionnaire 9. On the box plots, the tops and bottoms of the boxes indicate IQRs; center horizontal lines, medians; and diamonds, means. Whiskers extend to the highest and lowest values within 1.5 times the IQR, and dots beyond the whiskers reflect outlying data. The numbers beneath the box plots show the number of patients in each group who completed the patient-reported assessment.
Secondary Outcomes
The mean number of palliative care visits by week 24 for patients assigned to stepped palliative care was significantly lower than for those assigned to early palliative care (2.4 vs 4.7 visits; adjusted mean difference, −2.3; 95% CI, −2.7 to −1.8; P < .001) (Table 2). The mean number of visits by week 48 was also lower for those assigned to stepped palliative care (3.8 vs 7.7 visits; adjusted mean difference, −3.9; 95% CI, −4.7 to −3.1). Twenty-seven patients assigned to early palliative care and 11 assigned to stepped palliative care had no palliative care visits, primarily due to death, withdrawal, or transfer of care.
The proportion of patients assigned to stepped palliative care who reported discussing their end-of-life care preferences was noninferior to those in the early palliative care group (adjusted proportion, 30.4% vs 33.0%; difference, −2.6%; lower 1-sided 95% confidence limit, −10.4%; P = .09). Among deceased participants, 115 of 160 (71.9%) patients in the stepped palliative care group and 125 of 162 (77.2%) patients in the early palliative care group received hospice services before death, and hospice length of stay was shorter for patients assigned to stepped palliative care (adjusted mean, 19.5 vs 34.6 days; difference, −15.2 days; lower 1-sided 95% confidence limit, −25.1; P = .91 for noninferiority).
Exploratory Outcomes
The 2 groups did not differ in prognostic understanding at week 24 with respect to the proportion of patients who reported that the goal of therapy was to cure their cancer or who reported that their cancer was curable, as noted in Table 2. Depression symptoms and use of approach-oriented and avoidant coping at week 24 were not different between those assigned to stepped vs early palliative care (Table 2 and Figure 3).
Discussion
Stepped care is an effective way to deliver early, integrated palliative and oncology care to improve patients’ QOL. Patients assigned to stepped palliative care participated in significantly fewer palliative care visits, thus using substantially fewer palliative care resources, but had noninferior QOL at week 24 compared with patients participating in monthly visits with a palliative care clinician. Compared with patients assigned to early integrated palliative care, those assigned to stepped palliative care had 49.5% fewer palliative care visits through week 24. Notably, other salient patient-reported outcomes, including depression symptoms, coping, prognostic understanding, and end-of-life care communication, were also not different between study groups. In future research, we plan to evaluate the cost-effectiveness of stepped palliative care compared with early palliative care. To our knowledge, this is the first randomized trial to establish the noninferiority of a patient-centered model tailored to a patient’s needs by triggering more intensive palliative care services based on patient-reported QOL compared with the efficacious yet resource-intensive early palliative care model.
The stepped-care model ensures that patients are introduced to palliative care at the time of diagnosis but then triages further palliative care delivery based on their illness trajectory and QOL needs. With integration of palliative care early in the illness course, patients are less likely to equate palliative care with hospice or end-of-life care while still being enabled to establish a rapport with a palliative care clinician over time.47,48 To ensure continued integration of palliative and oncology care, the stepped-care model entailed visits at key clinical turning points in patients’ illness courses. However, many patients who are not hospitalized or experiencing a change in cancer treatment still struggle with living with an incurable illness, so monitoring QOL allows for identification of patients who would benefit from more intensive palliative care.49,50 Although this model holds promise as a patient-centered strategy to deliver early palliative care using fewer palliative care resources, barriers to implementing it include required monitoring of electronic health records for changes in cancer therapy and hospitalizations as well as frequent administration of patient-reported outcomes. However, developing automated queries from electronic health records and more widespread implementation of standard patient-reported outcome measure collection will facilitate using these approaches to deliver palliative care services.51,52
Although stepped palliative care was noninferior to early palliative care for QOL, and we detected no differences in patient-reported communication about end-of-life care, hospice length of stay was significantly shorter with stepped palliative care vs early palliative care. Thus, although both groups did meet quality metrics for length of stay in hospice on average (ie, >7 days), more frequent contact with palliative care, especially in the months before death, may facilitate earlier referrals for hospice.53,54 However, because patients with metastatic cancer, including lung cancer, are living longer, early palliative care starting at the time of diagnosis may no longer be a feasible model to ensure the delivery of high-quality care at the end of life. Integrating additional methods of prognostication, such as predictive modeling to identify patients at risk of death, with novel palliative care delivery models, such as stepped palliative care, warrants further investigation.55
Limitations
This study had several notable limitations. First, although the overall sample was representative of the US population with respect to the proportion of Black participants, few patients self-identified as other than Black or White or as Hispanic or Latino. While our study protocol included procedures for enrolling Spanish-speaking participants and patient-reported outcomes were available in Spanish, additional strategies are needed to ensure an ethnically diverse study sample. Second, as the participating institutions needed to have a sufficient palliative care clinician workforce to deliver monthly palliative care services to study participants, we conducted this trial at 3 academic medical centers that are likely not representative of the community settings where most patients with cancer receive health care. Our study sample included only patients with advanced lung cancer and mesothelioma, and therefore, the generalizability of the findings to patients diagnosed with other advanced cancers, including those with more indolent disease, requires further investigation. Third, while the eligibility criteria in the current study were similar to our single-site palliative care trials in patients with advanced lung cancer, our rate of missing data was slightly higher than in prior trials, likely due to the heterogeneity of the population and cancer care practices when enrolling patients at multiple sites. The COVID-19 pandemic also likely contributed to participant nonresponse by leading to delays in cancer diagnoses and challenges with in-person data collection.56,57 The higher rate of missing data may have reduced the precision of our outcome estimates and potentially introduced bias. Fourth, during and after the COVID-19 pandemic, palliative care visits were permitted via secure video despite a lack of available data demonstrating that video-delivered palliative care is equivalent to in-person palliative care.
Conclusions
In conclusion, stepped palliative care holds considerable promise to increase the scalability of integrated palliative and oncology care by maintaining the effect of early palliative care on patients’ QOL and other salient patient-reported outcomes with fewer palliative care visits. As patients with metastatic cancers are living longer due to improvements in cancer therapeutics, they may have different palliative care needs while living with their cancer vs at the end of life. Identifying additional triggers to intensify palliative care near death is a potential next step to further optimize the stepped palliative care model.
Trial Protocol
Statistical Analysis Plan
eAppendix 1. Self-Report Assessments
eAppendix 2. Sensitivity Analysis Methods for Primary FACT-L 24-Week Outcome Measure
eAppendix 3. Participant Race Selected as Other
eTable 1. FACT-L Completion by Study Group at Weeks 36 and 48
eTable 2. Baseline Characteristics by Week 24 FACT-L Completion
eTable 3. FACT-L Completion Among Stepped PC Patients on Step 1 at All Study Timepoints
eTable 4. Sensitivity Analysis Model Estimates of Study Group Effects on Primary FACT-L 24-Week Outcome Measure
eFigure 1. Stepped PC Study Procedures
eFigure 2. Intervention Delivery Up to 24 and 48 Weeks
eFigure 3. Patient-Reported Quality of Life Over 48 Weeks
Data Sharing Statement
References
- 1.Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30(8):880-887. doi: 10.1200/JCO.2011.38.5161 [DOI] [PubMed] [Google Scholar]
- 2.Levy MH, Smith T, Alvarez-Perez A, et al. Palliative care, version 1.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2014;12(10):1379-1388. doi: 10.6004/jnccn.2014.0136 [DOI] [PubMed] [Google Scholar]
- 3.Rabow MW, Dibble SL, Pantilat SZ, McPhee SJ. The comprehensive care team: a controlled trial of outpatient palliative medicine consultation. Arch Intern Med. 2004;164(1):83-91. doi: 10.1001/archinte.164.1.83 [DOI] [PubMed] [Google Scholar]
- 4.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749. doi: 10.1001/jama.2009.1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742. doi: 10.1056/NEJMoa1000678 [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730. doi: 10.1016/S0140-6736(13)62416-2 [DOI] [PubMed] [Google Scholar]
- 7.Bakitas MA, Tosteson TD, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33(13):1438-1445. doi: 10.1200/JCO.2014.58.6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA. 2016;316(20):2104-2114. doi: 10.1001/jama.2016.16840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grudzen CR, Richardson LD, Johnson PN, et al. Emergency department–initiated palliative care in advanced cancer: a randomized clinical trial. JAMA Oncol. 2016;2(5):591-598. doi: 10.1001/jamaoncol.2015.5252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hui D, Bruera E. Integrating palliative care into the trajectory of cancer care. Nat Rev Clin Oncol. 2016;13(3):159-171. doi: 10.1038/nrclinonc.2015.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui D, Elsayem A, De la Cruz M, et al. Availability and integration of palliative care at US cancer centers. JAMA. 2010;303(11):1054-1061. doi: 10.1001/jama.2010.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lupu D. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40(6):899-911. doi: 10.1016/j.jpainsymman.2010.07.004 [DOI] [PubMed] [Google Scholar]
- 13.Lupu D, Quigley L, Mehfoud N, Salsberg ES. The growing demand for hospice and palliative medicine physicians: will the supply keep up? J Pain Symptom Manage. 2018;55(4):1216-1223. doi: 10.1016/j.jpainsymman.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 14.Kayastha N, LeBlanc TW. Why are we failing to do what works? musings on outpatient palliative care integration in cancer care. JCO Oncol Pract. 2022;18(4):255-257. doi: 10.1200/OP.21.00794 [DOI] [PubMed] [Google Scholar]
- 15.Hui D, Kim SH, Kwon JH, et al. Access to palliative care among patients treated at a comprehensive cancer center. Oncologist. 2012;17(12):1574-1580. doi: 10.1634/theoncologist.2012-0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aldridge MD, Hasselaar J, Garralda E, et al. Education, implementation, and policy barriers to greater integration of palliative care: a literature review. Palliat Med. 2016;30(3):224-239. doi: 10.1177/0269216315606645 [DOI] [PubMed] [Google Scholar]
- 17.Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med. 2017;377(9):849-861. doi: 10.1056/NEJMra1703413 [DOI] [PubMed] [Google Scholar]
- 18.Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640-649. doi: 10.1056/NEJMoa1916623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bekelman DB, Rabin BA, Nowels CT, et al. Barriers and facilitators to scaling up outpatient palliative care. J Palliat Med. 2016;19(4):456-459. doi: 10.1089/jpm.2015.0280 [DOI] [PubMed] [Google Scholar]
- 20.Hui D, Meng YC, Bruera S, et al. Referral criteria for outpatient palliative cancer care: a systematic review. Oncologist. 2016;21(7):895-901. doi: 10.1634/theoncologist.2016-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan RI, Allsop MJ, ElMokhallalati Y, et al. Duration of palliative care before death in international routine practice: a systematic review and meta-analysis. BMC Med. 2020;18(1):368. doi: 10.1186/s12916-020-01829-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bower P, Gilbody S. Stepped care in psychological therapies: access, effectiveness and efficiency. Br J Psychiatry. 2005;186:11-17. doi: 10.1192/bjp.186.1.11 [DOI] [PubMed] [Google Scholar]
- 23.Jakicic JM, Tate DF, Lang W, et al. Effect of a stepped-care intervention approach on weight loss in adults: a randomized clinical trial. JAMA. 2012;307(24):2617-2626. doi: 10.1001/jama.2012.6866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroenke K, Krebs EE, Wu J, et al. Telecare collaborative management of chronic pain in primary care: a randomized clinical trial. JAMA. 2014;312(3):240-248. doi: 10.1001/jama.2014.7689 [DOI] [PubMed] [Google Scholar]
- 25.Aspvall K, Andersson E, Melin K, et al. Effect of an internet-delivered stepped-care program vs in-person cognitive behavioral therapy on obsessive-compulsive disorder symptoms in children and adolescents: a randomized clinical trial. JAMA. 2021;325(18):1863-1873. doi: 10.1001/jama.2021.3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834-841. doi: 10.1200/JCO.2016.70.5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cella D, Eton DT, Fairclough DL, et al. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) Questionnaire? results from Eastern Cooperative Oncology Group (ECOG) Study 5592. J Clin Epidemiol. 2002;55(3):285-295. doi: 10.1016/S0895-4356(01)00477-2 [DOI] [PubMed] [Google Scholar]
- 28.Baim-Lance A, Ferreira KB, Cohen HJ, et al. Improving the approach to defining, classifying, reporting and monitoring adverse events in seriously ill older adults: recommendations from a multi-stakeholder convening. J Gen Intern Med. 2023;38(2):399-405. doi: 10.1007/s11606-022-07646-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112. doi: 10.1200/JCO.2016.70.1474 [DOI] [PubMed] [Google Scholar]
- 30.Post KE, Heuer LB, Kamal AH, et al. Study protocol for a randomised trial evaluating the noninferiority of stepped palliative care versus early integrated palliative care for patients with advanced lung cancer. BMJ Open. 2022;12(2):e057591. doi: 10.1136/bmjopen-2021-057591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sangha O, Stucki G, Liang MH, et al. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156-163. doi: 10.1002/art.10993 [DOI] [PubMed] [Google Scholar]
- 32.Cella D. The Functional Assessment of Cancer Therapy-Lung and Lung Cancer Subscale assess quality of life and meaningful symptom improvement in lung cancer. Semin Oncol. 2004;31(3)(suppl 9):11-15. doi: 10.1053/j.seminoncol.2004.04.008 [DOI] [PubMed] [Google Scholar]
- 33.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carver CS. You want to measure coping but your protocol’s too long: consider the Brief COPE. Int J Behav Med. 1997;4(1):92-100. doi: 10.1207/s15327558ijbm0401_6 [DOI] [PubMed] [Google Scholar]
- 35.Hagan TL, Fishbein JN, Nipp RD, et al. Coping in patients with incurable lung and gastrointestinal cancers: a validation study of the Brief COPE. J Pain Symptom Manage. 2017;53(1):131-138. doi: 10.1016/j.jpainsymman.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Jawahri A, Traeger L, Park ER, et al. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer. 2014;120(2):278-285. doi: 10.1002/cncr.28369 [DOI] [PubMed] [Google Scholar]
- 37.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673. doi: 10.1001/jama.300.14.1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Julious SA. Sample sizes for clinical trials with normal data. Stat Med. 2004;23(12):1921-1986. doi: 10.1002/sim.1783 [DOI] [PubMed] [Google Scholar]
- 39.Piaggio G, Elbourne DR, Pocock SJ, et al. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308(24):2594-2604. doi: 10.1001/jama.2012.87802 [DOI] [PubMed] [Google Scholar]
- 40.Kurland BF, Johnson LL, Egleston BL, Diehr PH. Longitudinal data with follow-up truncated by death: match the analysis method to research aims. Stat Sci. 2009;24(2):211. doi: 10.1214/09-STS293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colantuoni E, Scharfstein DO, Wang C, et al. Statistical methods to compare functional outcomes in randomized controlled trials with high mortality. BMJ. 2018;360:j5748. doi: 10.1136/bmj.j5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bender CM, Ergÿn FS, Rosenzweig MQ, et al. Symptom clusters in breast cancer across 3 phases of the disease. Cancer Nurs. 2005;28(3):219-225. doi: 10.1097/00002820-200505000-00011 [DOI] [PubMed] [Google Scholar]
- 43.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67(8):850-857. doi: 10.1016/j.jclinepi.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 44.Earle CC, Park ER, Lai B, et al. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol. 2003;21(6):1133-1138. doi: 10.1200/JCO.2003.03.059 [DOI] [PubMed] [Google Scholar]
- 45.Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol. 2008;26(23):3860-3866. doi: 10.1200/JCO.2007.15.8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCarthy EP, Burns RB, Ngo-Metzger Q, et al. Hospice use among Medicare managed care and fee-for-service patients dying with cancer. JAMA. 2003;289(17):2238-2245. doi: 10.1001/jama.289.17.2238 [DOI] [PubMed] [Google Scholar]
- 47.Yoong J, Park ER, Greer JA, et al. Early palliative care in advanced lung cancer: a qualitative study. JAMA Intern Med. 2013;173(4):283-290. doi: 10.1001/jamainternmed.2013.1874 [DOI] [PubMed] [Google Scholar]
- 48.Zimmermann C, Mathews J. Palliative care is the umbrella, not the rain—a metaphor to guide conversations in advanced cancer. JAMA Oncol. 2022;8(5):681-682. doi: 10.1001/jamaoncol.2021.8210 [DOI] [PubMed] [Google Scholar]
- 49.Sedhom R, Shulman LN, Parikh RB. Precision palliative care as a pragmatic solution for a care delivery problem. J Clin Oncol. 2023;41(16):2888-2892. doi: 10.1200/JCO.22.02532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Temel JS, Shaw AT, Greer JA. Challenge of prognostic uncertainty in the modern era of cancer therapeutics. J Clin Oncol. 2016;34(30):3605-3608. doi: 10.1200/JCO.2016.67.8573 [DOI] [PubMed] [Google Scholar]
- 51.Zimmermann C, Pope A, Hannon B, et al. Phase II trial of symptom screening with targeted early palliative care for patients with advanced cancer. J Natl Compr Canc Netw. 2021;20(4):361-370.e3. doi: 10.6004/jnccn.2020.7803 [DOI] [PubMed] [Google Scholar]
- 52.Zimmermann C, Pope A, Hannon B, et al. Symptom screening with targeted early palliative care (STEP) versus usual care for patients with advanced cancer: a mixed methods study. Support Care Cancer. 2023;31(7):404. doi: 10.1007/s00520-023-07870-9 [DOI] [PubMed] [Google Scholar]
- 53.Greer JA, Pirl WF, Jackson VA, et al. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol. 2012;30(4):394-400. doi: 10.1200/JCO.2011.35.7996 [DOI] [PubMed] [Google Scholar]
- 54.Dy SM, Kiley KB, Ast K, et al. Measuring what matters: top-ranked quality indicators for hospice and palliative care from the American Academy of Hospice and Palliative Medicine and Hospice and Palliative Nurses Association. J Pain Symptom Manage. 2015;49(4):773-781. doi: 10.1016/j.jpainsymman.2015.01.012 [DOI] [PubMed] [Google Scholar]
- 55.He JC, Moffat GT, Podolsky S, et al. Machine learning to allocate palliative care consultations during cancer treatment. J Clin Oncol. 2024;42(14):1625-1634. doi: 10.1200/JCO.23.01291 [DOI] [PubMed] [Google Scholar]
- 56.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023-1034. doi: 10.1016/S1470-2045(20)30388-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhalla S, Bakouny Z, Schmidt AL, et al. Care disruptions among patients with lung cancer: a COVID-19 and cancer outcomes study. Lung Cancer. 2021;160:78-83. doi: 10.1016/j.lungcan.2021.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eAppendix 1. Self-Report Assessments
eAppendix 2. Sensitivity Analysis Methods for Primary FACT-L 24-Week Outcome Measure
eAppendix 3. Participant Race Selected as Other
eTable 1. FACT-L Completion by Study Group at Weeks 36 and 48
eTable 2. Baseline Characteristics by Week 24 FACT-L Completion
eTable 3. FACT-L Completion Among Stepped PC Patients on Step 1 at All Study Timepoints
eTable 4. Sensitivity Analysis Model Estimates of Study Group Effects on Primary FACT-L 24-Week Outcome Measure
eFigure 1. Stepped PC Study Procedures
eFigure 2. Intervention Delivery Up to 24 and 48 Weeks
eFigure 3. Patient-Reported Quality of Life Over 48 Weeks
Data Sharing Statement