Abstract
Objectives:
The VISION trial showed durable activity of tepotinib in MET exon 14 (METex14) skipping non-small cell lung cancer (NSCLC). We analyzed health state utilities using patient-reported outcomes from VISION.
Methods:
EQ-5D-5L and European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-Core 30 responses were collected at baseline, every 6–12 weeks during treatment, and at end-of-treatment and safety follow-up. EQ-5D-5L and EORTC Quality of Life Utility Measure-Core 10 Dimensions (QLU-C10D) utilities were derived using US, Canada, UK and Taiwan value sets, where available. Utilities were analyzed with linear mixed models including covariates for progression or time-to-death (TTD).
Results:
Utilities were derived for 273/291 patients (EQ-5D-5L, 1545 observations; QLU-C10D, 1546 observations). Mean (± standard deviation) US EQ-5D-5L utilities increased after tepotinib initiation, from 0.687 ± 0.287 at baseline to 0.754 ± 0.250 before independently assessed progression, and decreased post-progression (0.704 ± 0.288). US QLU-C10D utilities showed similar trends (0.705 ± 0.215, 0.753 ± 0.195, and 0.708 ± 0.209, respectively). Progression-based models demonstrated a statistically significant impact of progression on utilities and predicted higher utilities pre- versus post-progression. TTD-based models showed statistically significant associations of TTD with utilities and predicted declining utilities as TTD decreased. Prior treatment (yes/no) did not significantly predict utilities in progression- or TTD-based models. Utilities for Canada, UK and Taiwan showed comparable trends.
Conclusions:
In this first analysis of health state utilities in patients with METex14 skipping NSCLC, who received tepotinib, utilities were significantly associated with progression and TTD, but not prior treatment.
Introduction
Lung cancer ranks as the second most common malignancy and the leading cause of cancer death worldwide.1 Approximately 80–85% of all lung cancer cases are non-small cell lung cancer (NSCLC), which is usually diagnosed at an advanced stage.2 For many advanced NSCLCs, the management strategy is determined by testing for certain actionable oncogenic driver alterations that predict response to specific targeted therapies.3 A recent addition to the list of targetable alterations is MET exon 14 (METex14) skipping, a primary oncogenic driver that causes sustained activation of the MET receptor in approximately 3–4% of NSCLC tumors.4–6
Unlike other oncogenic drivers, METex14 skipping predominantly affects elderly patients (median age: 72 years) and occurs relatively evenly between males and females, and between smokers and non-smokers.6–8 Brain metastases affect up to a third of patients with METex14 skipping and most have adenocarcinoma tumor histology.8,9 Overall, the symptom burden of patients with METex14 skipping – as reflected in European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Lung Cancer 13 scores for chest pain, cough and dyspnea – is similar to that of the general population of patients with advanced NSCLC.10,11 Although METex14 skipping is associated with poor response to standard-of-care therapies and short overall survival,12,13 management of these tumors has been transformed by the development of MET inhibitors that target the underlying oncogenic abnormality to elicit meaningful clinical responses.4,14
Tepotinib is a potent, highly selective, oral, once-daily MET inhibitor.15 Following its first approval in Japan in March 2020 for treatment of advanced NSCLC harboring METex14 skipping,16 tepotinib has been approved by multiple regulatory authorities worldwide and is recommended for eligible patients with MET alterations in NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) and European Society for Medical Oncology (ESMO) guidelines.3,17,18 Pivotal clinical data were provided by the single-arm, multicenter Phase II VISION trial, in which tepotinib demonstrated durable clinical activity in patients with METex14 skipping identified in tissue and/or liquid biopsy samples.19,20 Based on independent review committee (IRC) assessment, the objective response rate was 49% and median duration of response was 13.8 months (data cut-off: February 1, 2021), with consistent efficacy observed irrespective of age or treatment history.20–22 Treatment-related adverse events were manageable and mostly mild-to-moderate.20 In secondary endpoint analyses, patient-reported outcomes (PROs) indicated stability of overall health-related quality of life (HRQL) during tepotinib treatment.21
Following the approval of tepotinib, cost-effectiveness analyses are being undertaken to inform healthcare decision making by payers and policy-makers. The recommended methodology for economic evaluations in many countries is the cost-utility analysis, which employs health utilities as a standardized measure of HRQL.23 Health utilities are preference-based metrics that are expressed on a scale on which 0 represents HRQL equivalent to being dead and 1 represents full health.23,24 Utilities can be derived from PROs assessing subjective health status. Based on these analyses, utilities can be assigned to specific health states and factored into economic models.23 Common approaches for modeling health states in patients with advanced cancer are based on either progression status or time-to-death (TTD).23
There are currently no available data on health state utilities in patients with advanced NSCLC harboring METex14 skipping or other MET alterations (e.g., MET amplification). Furthermore, given increasing use of liquid biopsy in clinical practice and evidence that liquid biopsy may identify patients with a poorer prognosis than conventional tissue biopsy,25 there is a need to understand how health utilities compare between patients identified by these two methods. Data on the impact of histologic subtype on health state utilities in NSCLC are also limited. To address these data gaps and to complement the clinical findings of VISION, we used PRO data collected in the trial to evaluate utilities in patients with METex14 skipping NSCLC, who were treated with tepotinib.
Methods
Data source
Analyses were based on data collected from Cohorts A and C of the international, multicohort, single-arm, open-label, Phase II VISION trial (NCT02864992) (Appendix Figure 1). As reported elsewhere,19,20 adults with advanced epidermal growth factor receptor (EGFR)/anaplastic lymphoma kinase (ALK) wild-type NSCLC with METex14 skipping, who were treatment-naïve or who had received up to two prior lines of therapy, were enrolled from September 2016. METex14 skipping was centrally evaluated in tissue biopsy and/or liquid biopsy (i.e., plasma) samples using next-generation sequencing-based assays. Patients received tepotinib until disease progression according to investigator (INV) assessment, intolerable toxicity or consent withdrawal, and were evaluated for objective response by IRC and INV. Treatment was not continued beyond INV-assessed progression. After tepotinib discontinuation, patients could receive standard-of-care therapy according to local practice.
HRQL was evaluated as a secondary endpoint of VISION using the EQ-5D-5L and EORTC Quality of Life Questionnaire-Core 30 (QLQ-C30) questionnaires (Appendix Figure 2). Questionnaires were administered to all patients on Day 1, then every 6 weeks for 9 months, and then every 12 weeks during treatment, as well as at the end of treatment and safety follow-up visits. The EQ-5D-5L is a generic instrument including five descriptive dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) rated on a five-point scale, and a visual analog scale (0–100) assessing overall health status.26 Better HRQL corresponds to lower scores on the descriptive dimensions and higher scores on the visual analog scale. The EORTC QLQ-C30 is a cancer-specific instrument comprising 30 items relating to functional domains (e.g., physical, role and emotional), common cancer-related symptoms (e.g., dyspnea, fatigue, and nausea/vomiting), and global health status.27 Responses are provided on four- or seven-point scales, with better HRQL represented by higher scores for functional domains and global health status, and lower scores for symptom scales.
Derivation of utility values
Patient-level data were extracted from the trial database (data cut-off: February 1, 2021). The assembled dataset included information on EQ-5D-5L and EORTC QLQ-C30 responses, time of progression per IRC and INV, time of death, prior treatment status (i.e., treatment-naïve or previously treated), method of METex14 skipping detection (i.e., tissue and/or liquid biopsy), and tumor histology.
EQ-5D-5L responses were used to derive EQ-5D-5L utilities based on value sets for the US,28 Canada,29 and Taiwan30 using the ‘eq5d’ package31 in the statistical software R (version 4.0.3; R Project for Statistical Computing).32 UK EQ-5D utilities were derived by first mapping EQ-5D-5L data to EQ-5D-3L responses using a crosswalk algorithm,33 and then applying the value set for EQ-5D-3L-derived weights for the UK.34 EORTC QLQ-C30 responses were used to derive EORTC Quality of Life Utility Measure-Core 10 Dimensions (QLU-C10D) utilities based on value sets for the US, Canada, and UK using published algorithms.35–37 Derivation of EQ-5D-5L and EORTC QLU-C10D utilities required complete responses for all dimensions of the corresponding questionnaire.
Utility summary analyses
Each utility observation was classified as occurring pre- or post-progression based on its timing relative to any recorded progression event for that patient. If no progression event was recorded during follow-up, all observations were classified as occurring pre-progression. Separate analyses were conducted for IRC- and INV-assessed progression. Given the treatment administration schedule and timing of HRQL assessments (Appendix Figure 2), post-progression (per INV) utility observations were collected at the at the end of treatment and safety follow-up visits in patients discontinuing due to INV-assessed progression. Patients discontinuing tepotinib for other reasons, including adverse events, were expected to contribute mostly pre-progression utility observations; however, these patients could also contribute post-progression (per INV) utility observations if INV-assessed progression occurred subsequent to discontinuation, but before the final HRQL assessment at the safety follow-up. Data on utility after IRC-assessed progression were provided by patients who had IRC-assessed progression before the safety follow-up visit, irrespective of the reason for treatment discontinuation.
The number of patients, number of observations and empirical mean (± standard deviation [SD]) utility values were summarized overall, at baseline, and according to IRC- or INV-assessed progression status (i.e., for the pre- and post-progression health states). Empirical means for the pre- and post-progression health states reflect all observations collected before and after the date of progression, respectively. Summaries were prepared for the overall population, and for the treatment-naïve and previously treated subgroups.
Regression analyses
To account for correlations between repeated measurements within patients over time, utilities were analyzed with linear mixed models (LMMs), using the ‘lme4’ package in R. Two sets of models were fitted: progression-based models (which include progression status as a covariate) and TTD models (which include different time periods prior to death as covariates) (Appendix Figure 3). Model fit was evaluated using the Akaike information criterion (AIC) and Bayesian information criterion (BIC). Separate analyses were conducted for EQ-5D-5L and EORTC QLU-C10D utilities derived using weights for each of the analyzed countries.
The progression-based models included a random intercept and fixed effects for either progression status alone (Model 1a) or both progression status and prior treatment status (Model 1b). Separate models were fitted based on IRC- or INV-assessed progression. The models were used to estimate mean utilities (with standard errors [SEs]), for the pre- and post-progression health states, overall (Model 1a) and by prior treatment status (Model 1b). Versions of these models were also evaluated for the subgroups of patients with METex14 skipping identified in tissue or liquid biopsy samples. In exploratory analyses, progression-based models were used to assess the impact of adenocarcinoma or squamous histology.
The TTD models included a random intercept and fixed effects for TTD, which was analyzed as a categorical variable according to two different sets of arbitrary cut-offs taken or adapted from previous analyses.38,39 Model 2a categorized TTD as >30, >15 to ≤30, >5 to ≤15, or ≤5 weeks, whereas Model 3a categorized TTD as ≥364, ≥182 to <364, ≥28 to <182, or <28 days. Versions of these models including fixed effects for prior treatment status were also constructed (Models 2b and 3b, respectively). For patients still alive at the data cut-off, the date of the last survival follow-up was used as the time of death. The models were used to predict mean utilities (with SEs) for each TTD time period, overall (Models 2a and 3a), and according to prior treatment status (Models 2b and 3b).
Results
Patient population
The study population comprised 291 patients with advanced METex14 skipping NSCLC who were treated with tepotinib for a median duration of 6.3 months (range: <0.1, 50.6). Of these patients, 273 provided EQ-5D-5L and EORTC QLQ-C30 responses (Appendix Table 1). The median age was 72.0 years (range: 41–94), 138 patients (50.5%) were female, and 130 patients (47.6%) had a history of smoking. Overall, 135 patients (49.5%) were treatment-naïve and 138 (50.5%) had received prior treatment. The majority of patients had adenocarcinoma tumor histology (n=219; 80.2%), while 24 patients (8.8%) had squamous histology. METex14 skipping was detected by tissue biopsy in 177 patients (64.8%) and by liquid biopsy in 158 patients (57.9%), with 62 patients (22.7%) testing positive by both methods.
US EQ-5D-5L and EORTC QLU-C10D utility summary analysis
Of 1677 responses from 273 patients, 1545 were complete and enabled derivation of utility EQ-5D-5L values. Based on IRC progression status, 1202 utility observations from 268 patients were recorded pre-progression and 343 observations from 185 patients were recorded post-progression (Appendix Figure 4A). Mean (± SD) US EQ-5D-5L utilities in the overall population increased after tepotinib initiation, from 0.687 (± 0.287) at baseline to 0.754 (± 0.250) before progression and decreased after progression to 0.704 (± 0.288). Comparable trends were observed in the subgroups of treatment-naïve patients (135 patients; 724 observations) and previously treated patients (138 patients; 821 observations) (Figure 1A).
Figure 1. Summary statistics for US EQ-5D-5L (A) and US EORTC QLU-C10D (B) utilities, by baseline and IRC progression status, for the overall population and according to prior treatment status.
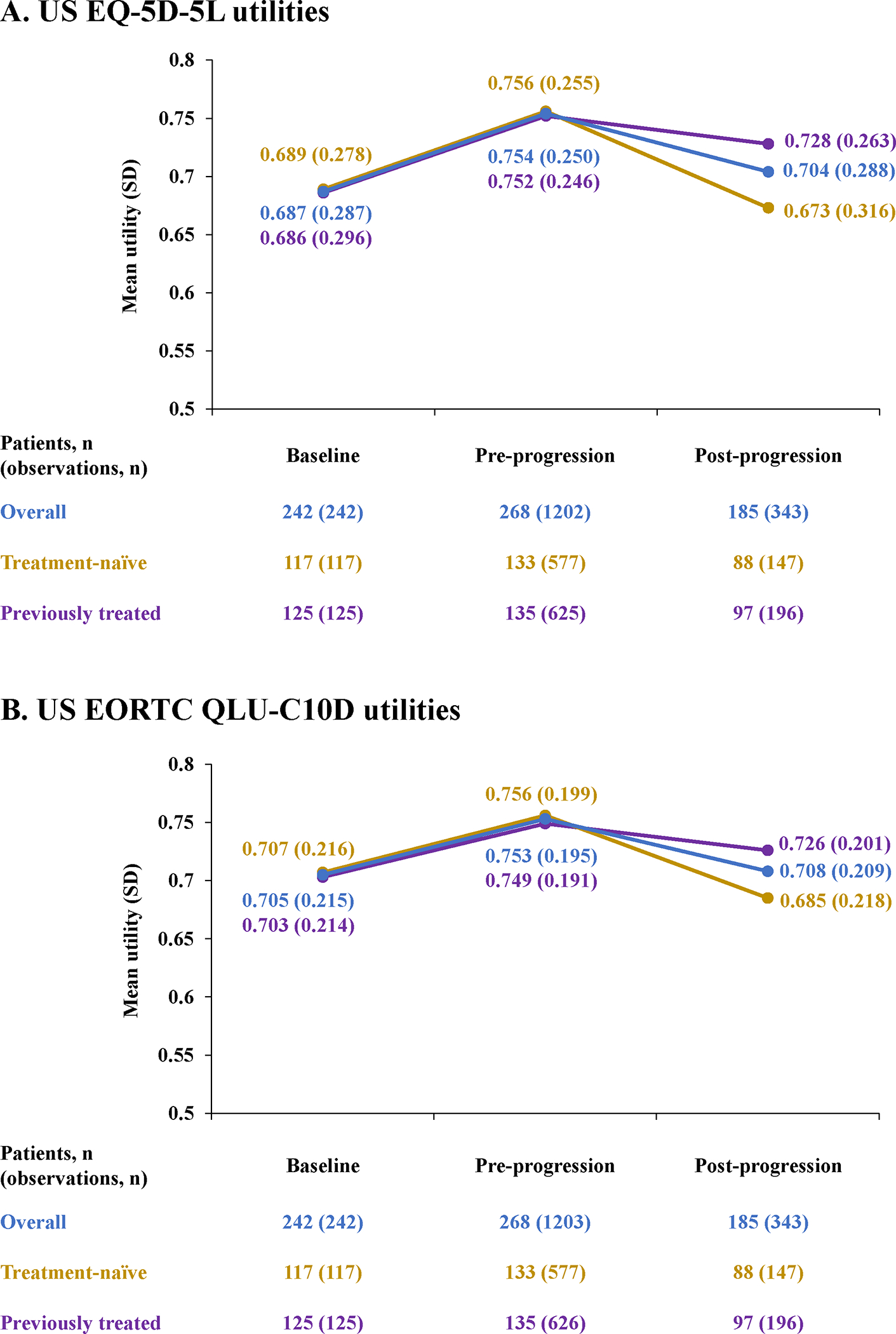
EORTC indicates European Organisation for the Research and Treatment of Cancer; EQ-5D-5L, 5-level version of EQ-5D; IRC, independent review committee; QLU-C10D, Quality of Life Utility Measure Core 10 Dimensions.
Of 1677 available EORTC QLQ-C30 responses from 273 patients, 1546 responses were complete and were used to derive EORTC QLU-C10D utilities (Appendix Figure 4B). The numbers of observations from the IRC pre- and post-progression health states, respectively, were 1203 (from 268 patients) and 343 (from 185 patients). Mean (± SD) US EORTC QLU-C10D utilities increased after tepotinib initiation, from 0.705 (± 0.215) at baseline to 0.753 (± 0.195) pre-progression and decreased after progression to 0.708 (± 0.209). Trends were similar in treatment-naïve patients (135 patients; 724 observations) and previously treated patients (138 patients; 822 observations) (Figure 1B).
US EQ-5D-5L utilities and US EORTC QLU-C10D utilities from data collection time points matched to within 7 days were highly correlated (Pearson’s correlation coefficient: 0.728) (Appendix Figure 5).
US EQ-5D-5L utilities regression analyses
Parameters for the IRC progression-based models for US EQ-5D-5L utilities are shown in Table 1. IRC progression status had a significant impact on utility irrespective of whether prior treatment status was included as a covariate (P<0.001). In contrast, prior treatment status was not significantly associated with utility (P=0.458), and its inclusion slightly worsened the statistical goodness-of-fit of the model to the data (Appendix Table 2). Mean US EQ-5D-5L utilities estimated using these models showed lower utility values post-progression compared with the pre-progression health state, both in the overall population and in treatment-naïve and previously treated patients (Figure 2A, 2B). Analyses based on INV-assessed progression were similar (data not shown).
Table 1.
US EQ-5D-5L and EORTC QLU-C10D utility linear mixed model estimated coefficients for progression-based Models 1a and 1b, by IRC progression status
| EQ-5D-5L | EORTC QLU-C10D | |||
|---|---|---|---|---|
| Model 1a* | Model 1b† | Model 1a* | Model 1b† | |
| Intercept‡ (SE) | 0.7271 (0.0142) | 0.7166 (0.0201) | 0.7271 (0.0107) | 0.7211 (0.0151) |
| Post-progression (SE) | −0.0682 (0.0112) | −0.0683 (0.0112) | −0.0441 (0.0085) | −0.0442 (0.0085) |
| Previously treated (SE) | – | 0.0207 (0.0279) | – | 0.0117 (0.0211) |
Coefficients for significant covariates are shown in bold.
Includes a random intercept and fixed effect for progression status.
Includes a random intercept and fixed effects for progression status and prior treatment status.
Corresponds to pre-progression utilities overall (Model 1a) or pre-progression utilities in treatment-naïve patients (Model 1b).
EORTC, European Organisation for Research and Treatment of Cancer; IRC, independent review committee; QLU-C10D, Quality of Life Utility Measure Core 10 Dimensions; SE, standard error.
Figure 2. Estimated mean (SE) US EQ-5D-5L and EORTC QLU-C10D health state utilities using IRC progression-based Models 1a (A) and 1b (B), and TTD models 2a (C), 2b (D), 3a (E) and 3b (F).
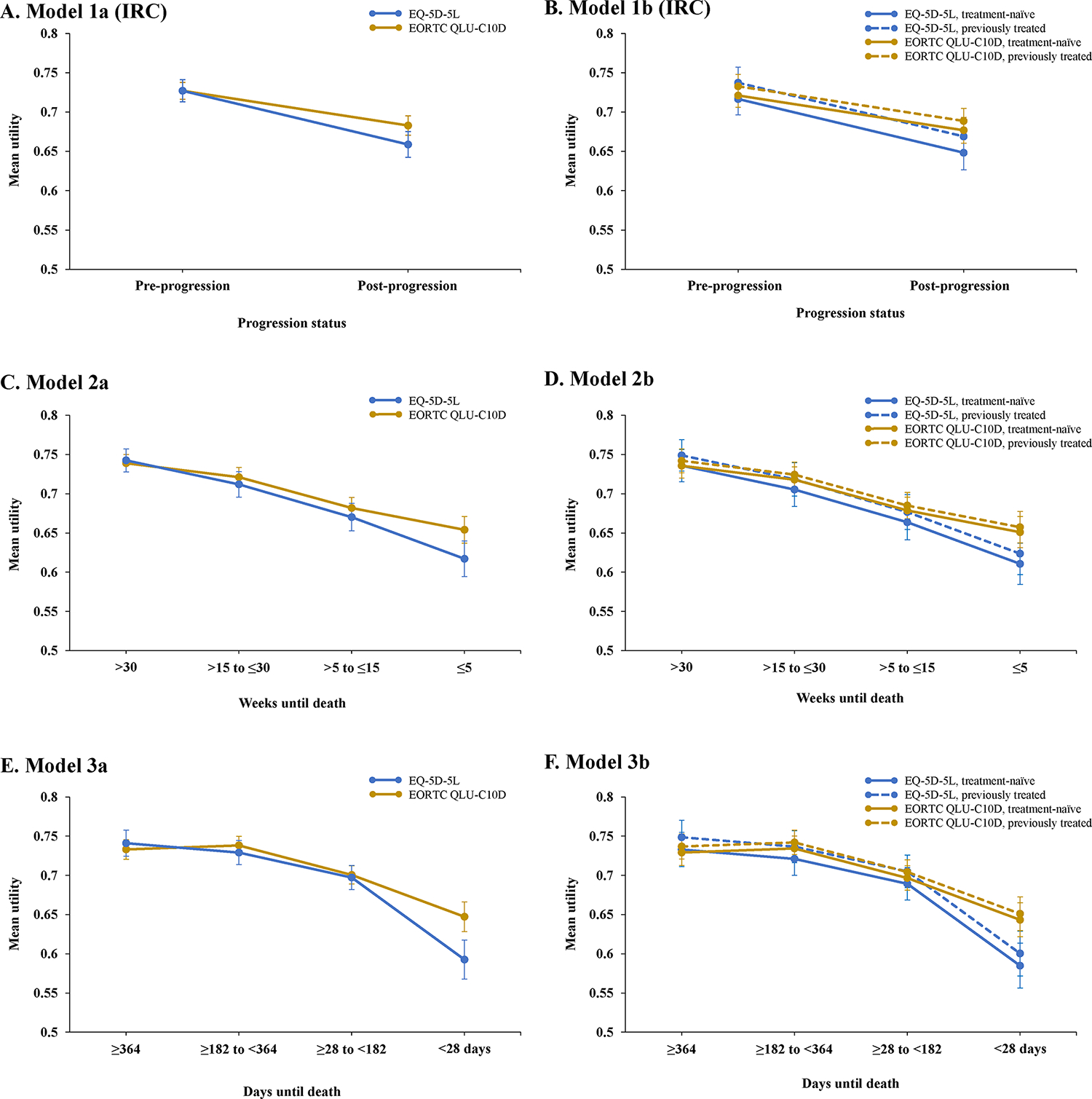
EORTC indicates European Organisation for the Research and Treatment of Cancer; EQ-5D-5L, 5-level version of EQ-5D; IRC, independent review committee; QLU-C10D, Quality of Life Utility Measure Core 10 Dimensions; SD, standard deviation.
Progression status was also a significant covariate in separate models fitted for the tissue biopsy- and liquid biopsy-positive subgroups (P<0.001; Appendix Tables 3 and 4). Mean utilities estimated using these models were higher in tissue biopsy- versus liquid biopsy-positive patients (Appendix Figure 6), which is consistent with the better prognosis of patients enrolled by tissue compared with liquid biopsy.25 Although prior treatment status did not significantly predict utility, treatment-naïve and liquid biopsy-positive patients had the lowest estimated pre-progression health state utility. In exploratory analyses using IRC progression-based models, utilities were not significantly predicted by adenocarcinoma (P=0.400) or squamous histology (P=0.689).
TTD models for US EQ-5D-5L utilities showed a significant effect (P<0.05) of all TTD categories included in Model 2a (>30, >15 to ≤30, and >5 to ≤15 weeks) or Model 3a (≥364, ≥182 to <364, and ≥28 to <182 days) (Tables 2 and 3). When incorporated into each regression model, prior treatment status did not significantly predict utility (P=0.635 and P=0.572, respectively) and slightly reduced the goodness-of-fit (Models 2b and 3b) (Appendix Table 5). Mean health state utility estimates derived using these models showed that utilities decreased as patients approached death (Figure 2C, 2E). Consistent trends were seen in the treatment-naïve and previously treated subgroups (Figure 2D, 2F). Although prior treatment status did not significantly predict utility in the TTD models, mean health state utility was numerically slightly higher in previously treated versus treatment-naïve patients.
Table 2.
US EQ-5D-5L and EORTC QLU-C10D utility linear mixed model estimated coefficients for TTD-based Models 2a and 2b
| EQ-5D-5L | EORTC QLU-C10D | |||
|---|---|---|---|---|
| Model 2a* | Model 2b† | Model 2a* | Model 2b† | |
| Intercept‡ (SE) | 0.6171 (0.0227) | 0.6107 (0.0264) | 0.6541 (0.0171) | 0.6510 (0.0199) |
| >5 to ≤15 weeks to death (SE) | 0.0531 (0.0220) | 0.0530 (0.0220) | 0.0277 (0.0165) | 0.0277 (0.0165) |
| >15 to ≤30 weeks to death (SE) | 0.0949 (0.0212) | 0.0947 (0.0212) | 0.0671 (0.0160) | 0.0670 (0.0160) |
| >30 weeks to death (SE) | 0.1255 (0.0207) | 0.1252 (0.0207) | 0.0848 (0.0156) | 0.0847 (0.0156) |
| Previously treated (SE) | – | 0.0130 (0.0274) | 0.0064 (0.0207) | |
Coefficients for significant covariates are shown in bold.
Includes a random intercept and fixed effects for TTD categories.
Includes a random intercept and fixed effects for TTD categories and prior treatment status.
Corresponds to utilities at ≤5 weeks to death overall (Model 2a) or utilities at ≤5 weeks to death in treatment-naïve patients (Model 2b).
EORTC, European Organisation for Research and Treatment of Cancer; QLU-C10D, Quality of Life Utility Measure Core 10 Dimensions; SE, standard error; TTD, time-to-death.
Table 3.
US EQ-5D-5L and EORTC QLU-C10D utility linear mixed model estimated coefficients for TTD-based Models 3a and 3b
| EQ-5D-5L | EORTC QLU-C10D | |||
|---|---|---|---|---|
| Model 3a* | Model 3b† | Model 3a* | Model 3b† | |
| Intercept‡ (SE) | 0.5926 (0.0250) | 0.5848 (0.0286) | 0.6472 (0.0189) | 0.6433 (0.0216) |
| ≥28 to <182 days to death (SE) | 0.1045 (0.0228) | 0.1045 (0.0228) | 0.0533 (0.0172) | 0.0533 (0.0172) |
| ≥182 to <364 days to death (SE) | 0.1364 (0.0235) | 0.1362 (0.0235) | 0.0910 (0.0177) | 0.0909 (0.0177) |
| ≥364 days to death (SE) | 0.1484 (0.0246) | 0.1482 (0.0246) | 0.0859 (0.0186) | 0.0858 (0.0186) |
| Previously treated (SE) | – | 0.0156 (0.0276) | – | 0.0077 (0.0208) |
Coefficients for significant covariates are shown in bold.
Includes a random intercept and fixed effects for TTD categories.
Includes a random intercept and fixed effects for TTD categories and prior treatment status.
Corresponds to utilities at <28 days to death overall (Model 3a) or utilities at <28 days to death in treatment-naïve patients (Model 3b).
EORTC, European Organisation for Research and Treatment of Cancer; QLU-C10D, Quality of Life Utility Measure Core 10 Dimensions; SE, standard error; TTD, time-to-death.
US EORTC QLU-C10D regression analyses
In the progression-based models, progression status significantly predicted US EORTC QLU-C10D utilities (P<0.001) in both Model 1a (excludes prior treatment) and Model 1b (includes prior treatment) (Table 1). Prior treatment status had no significant impact on utility (P=0.579) and slightly reduced the goodness-of-fit (Appendix Table 6). Mean utilities predicted using these models showed a decrease from the pre- to the post-progression state in the overall population, and the treatment-naïve and previously treated subgroups (Figure 2A, 2B). Although prior treatment did not predict utility, estimated US EORTC QLU-C10D utilities were numerically higher in previously treated versus treatment-naïve patients. Analyses based on INV-assessed progression yielded comparable findings (data not shown).
In the TTD models, all TTD categories had a significant impact on utility except for the >5 to ≤15 weeks category (P=0.094 in Model 2a; P=0.095 in Model 2b) (Tables 2 and 3). Prior treatment status did not significantly impact on utility (Model 2b, P=0.758; Model 3b, P=0.712) and worsened model fit when included as a covariate (Appendix Table 7). Mean US EORTC QLU-C10D utilities estimated using Model 2 decreased progressively with decreasing TTD (Figure 2C). Estimated using Model 3, utility was highest for the TTD category of ≥182 to <364 days and decreased thereafter as patients approached death (Figure 2E). Estimated health state utilities were numerically marginally greater in previously treated versus treatment-naïve patients at each TTD time period in both models (Figure 2D, 2F).
Analyses of utilities for Canada, the UK, and Taiwan
Summary analyses of utilities for Canada (EQ-5D-5L and EORTC QLU-C10D), the UK (EQ-5D crosswalk and EORTC QLU-C10D) and Taiwan (EQ-5D-5L) showed increases in utilities after tepotinib initiation from baseline until progression (Appendix Figure 7). In linear mixed models, both progression status and TTD were significant predictors of utilities (data not shown). As for US utilities, mean estimated health state utilities for Canada, the UK, and Taiwan were lower in the post- versus pre-progression state, and progressively decreased as patients approached death (Appendix Figures 8–10).
Discussion
To our knowledge, this analysis of PRO data from VISION provides the first information on health state utilities in patients with METex14 skipping NSCLC. Summary statistics showed that mean utilities increased from baseline during tepotinib therapy until progression in the overall population, and in the treatment-naïve and previously treated subgroups. Mean utilities decreased after progression, but remained above the baseline level in the overall population (for EQ-5D-5L utilities) and previously treated subgroup (for both EQ-5D-5L and EORTC QLU-C10D utilities), which may favor the use of further lines of therapy. Although the empirical means do not control for repeated measurements and must therefore be interpreted with caution, they are in line with the stability in overall HRQL, as well as the durable efficacy and manageable safety of tepotinib, which were observed in the VISION trial.19,20,40
US EQ-5D-5L utilities were estimated at 0.727 and 0.659 for the pre- and post-progression states, respectively. Using TTD models, US EQ-5D-5L utilities were estimated to range between 0.743 (TTD >30 weeks) and 0.617 (TTD ≤5 weeks), and between 0.741 (TTD ≥364 days) and 0.593 (TTD <28 days). These model-estimated health state utility values for tepotinib-treated METex14 skipping NSCLC fall broadly within the same range as prior estimates for other advanced NSCLC populations,41–44 including programmed death ligand-1 (PD-L1)-expressing tumors.45,46 However, they appear slightly lower than previously reported for NSCLC with EGFR mutations or ALK rearrangements treated with targeted therapies,47–49 which could reflect age-related comorbidities and functional decline in the elderly METex14 skipping population.50 Of note, targeted therapy has been associated with higher health utilities compared with chemotherapy in patients with other oncogene-driven NSCLC subtypes.49,51
While summary statistics show an increase in health state utilities from baseline after tepotinib initiation until progression, the modeling analyses collectively suggest that health state utilities and HRQL decline as the disease progresses and TTD decreases. The progression- and TTD-based analyses illustrate two valid methods to modeling health state utilities that are commonly applied in oncology.23 While progression-based models are more closely aligned with standard clinical trial endpoints, TTD models may be valuable where HRQL is not tightly linked to progression status.52 Since the determinants of HRQL in this setting are not fully understood, the implementation of alternative model structures overcomes the limitations of any single approach and, given the consistency of the results, can increase confidence in the study findings. Furthermore, utilities derived from responses to two independent PRO questionnaires (EQ-5D-5L and EORTC QLQ-C30) collected at the same time points (±7 days) were highly correlated (Pearson’s correlation coefficient: 0.728) and their respective analyses can be considered mutually supportive.
Whether assessed by IRC or INV, disease progression was a statistically significant predictor of utility in all progression-based models tested, with lower HRQL consistently observed in the post- versus pre-progression states. One factor that may have contributed to poor post-progression HRQL is the use of subsequent therapies in less than half of patients with METex14 skipping in VISION.20,53 Limited use of multiple treatment lines has also been documented in clinical practice,8 and may reflect the older age of the METex14 skipping population. Our findings are in agreement with a recent large cohort study in which progression was associated with significant and clinically relevant decrements in HRQL across a variety of metastatic tumor types, with the greatest deterioration seen in lung cancer.54 In patients with advanced NSCLC, decreases in health utilities upon progression have been reported in biomarker-unselected populations,41,42 as well as in patients with EGFR-mutant47 or PD-L1-expressing tumors.45 Overall, the present data support the use of separate utility values for the pre- and post-progression health states.
TTD had a significant impact on health utilities when categorized according to the Model 2 and Model 3 cut-offs (except for TTD >5 to ≤15 weeks for US EORTC QLU-C10D), which is expected given the strong association between HRQL and survival in lung cancer and other tumor types.55,56 Similar trends have been reported in TTD analyses of pembrolizumab NSCLC trial participants43–46 and patients with NSCLC from the general South Korean population.57 Overall, we observed a progressive decline in utilities with decreasing TTD, with the sharpest fall observed as patients transitioned into the health state closest to death (TTD ≤5 weeks in Model 2 and <28 days in Model 3). In this final TTD period, estimated utilities were lower in Model 3 than Model 2, which likely reflects the narrower final TTD period in Model 3, in the context of rapidly declining HRQL during the last weeks of life. Conversely, the two models produced very similar utility estimates for the time periods furthest from death (>30 weeks in Model 2 and ≥364 days in Model 3) despite the different time ranges covered, suggesting stability in HRQL at longer TTD. Overall, these models indicate that different utility values should be assigned for each TTD health state.
Although prior treatment status was not significantly associated with utilities in either the progression- or TTD-based models, health state utility estimates were slightly higher in previously treated versus treatment-naïve patients. Interestingly, a real-world cross-sectional survey of patients with advanced NSCLC reported marginally greater pre-progression utilities in the second- versus first-line setting.41 This counterintuitive observation could reflect stronger recollection of premorbid health status in treatment-naïve patients and/or psychological adaptation to the disease in previously treated patients. In VISION, there was no indication that differences in baseline characteristics were an explanatory factor and treatment efficacy was comparable between the two subgroups.20
A novel aspect of our analysis is the estimation of separate health state utility values for patients, in which the same oncogenic driver was detected in either tissue or liquid biopsy samples. Compared with patients with METex14 skipping positivity by tissue biopsy, those with METex14 skipping positivity by liquid biopsy had lower utilities, which accords with the worse baseline HRQL and higher prevalence of poor prognostic factors in this subgroup.25 Since larger or more proliferative tumors are more likely to shed ctDNA at detectable levels,58,59 liquid biopsy appears to select a subgroup with greater tumor burden and therefore worse health status.
Study limitations include the single-arm design, which prevented comparison of health state utilities with tepotinib versus other treatments, and the lack of HRQL assessments beyond the 30-day safety visit of the trial, which limited the number of utility observations available post-progression. Additionally, the TTD analyses should be interpreted with caution, due to the potential impact of censoring of overall survival for patients alive at the time of the analysis. Exploratory analyses of histology may have been underpowered due to the low number of patients with non-adenocarcinoma subtypes. Although brain metastases have a strong negative impact on HRQL,60 patients with symptomatic brain lesions were excluded from VISION, as is standard for trials in this setting.61 Further research is required to understand health state utilities in patients with brain metastases and other clinically important subgroups that may be underrepresented in clinical trials.
Conclusions
These analyses provide the first data on health state utilities in patients with NSCLC harboring METex14 skipping. They highlight the different approaches that can be adopted for the estimation of utilities based on the same data. Compared with any single method, these multiple approaches can increase flexibility for economic modeling and thereby facilitate adaptation according to local health technology assessment body preferences. In the evaluated statistical models, health state utilities increased from baseline during tepotinib treatment until progression, and were significantly associated with both progression status and TTD.
Supplementary Material
Highlights.
Tepotinib is approved for treatment of advanced/metastatic MET exon 14 (METex14) skipping non-small cell lung cancer (NSCLC). Data on health state utilities in this population are lacking.
EQ-5D-5L and European Organisation for Research and Treatment of Cancer Quality of Life Utility Measure-Core 10 Dimensions (EORTC QLU-C10D) utilities, derived from VISION trial patient-reported outcomes, increased from baseline during tepotinib treatment until progression. Utilities were significantly predicted by progression and time-to-death.
These analyses provide the first health state utility estimates for patients with METex14 skipping NSCLC, which will populate cost-effectiveness models for tepotinib as a new treatment for these patients.
Acknowledgments:
The authors would like to acknowledge Thomas McLean (Health Economics and Outcomes Research Lead, Merck Serono Limited, Feltham, UK, an affiliate of Merck KGaA, Darmstadt, Germany) for his work on the UK utilities analyses. The authors would like to thank patients and their families, investigators, co-investigators, and the study teams at all participating centers, as well as the healthcare business of Merck KGaA, Darmstadt, Germany. Medical writing assistance (funded by the healthcare business of Merck KGaA, Darmstadt, Germany) was provided by Mark Dyson, DPhil (Berlin, Germany), on behalf of Syneos Health, UK.
Funding/Support:
The VISION trial and analysis of utilities were sponsored by the healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945).
Role of Funder/Sponsor:
Employees of the healthcare business of Merck KGaA, Darmstadt, Germany were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation and review of the manuscript. All authors approved the submitted manuscript.
Footnotes
Conflict of Interest Disclosures:
Mo Yang and Bruce R. Gaumond are employees at EMD Serono, the healthcare business of Merck KGaA, Darmstadt, Germany. Helene Vioix is an employee at the healthcare business of Merck KGaA, Darmstadt, Germany. Aurora O’Brate is an employee of Merck SL, Madrid, Spain, an affiliate of Merck KGaA, Darmstadt, Germany, where she also holds stocks. Emma Hook, Anthony Hatswell and Rachael Batteson are employees of Delta Hat, who were funded by the healthcare business of Merck KGaA, Darmstadt, Germany to conduct the analysis. Sanjay Popat reports personal fees from Amgen, AstraZeneca, Bayer, BeiGene, Blueprint, Bristol Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Guardant Health, Janssen, Lilly, the healthcare business of Merck KGaA, Darmstadt, Germany, Novartis, Roche, Takeda; non-financial support from ALK Positive UK, Lung Cancer Europe, International Association for the Study of Lung Cancer and Ruth Strauss Foundation, British Thoracic Oncology Group and European Thoracic Oncology Platform, European Society of Medical Oncology, Mesothelioma Applied Research Foundation, other from Merck & Co., Ariad, Celgene, Turning Point Therapeutics, GlaxoSmithKline, Trizel, outside the submitted work. Paul Paik reports personal fees from Takeda, EMD Serono, Mirati, Xencor, CrownBio, Novartis, outside the submitted work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):1–41. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Bareschino MA, Schettino C, Rossi A, et al. Treatment of advanced non small cell lung cancer. J Thorac Dis. 2011;3(2):122–133. doi: 10.3978/J.ISSN.2072-1439.2010.12.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V.3.2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed March 16, 2022. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.. [Google Scholar]
- 4.Drilon A, Cappuzzo F, Ou SHI, Camidge DR. Targeting MET in Lung Cancer: Will Expectations Finally Be MET? J Thorac Oncol. 2017;12(1):15–26. doi: 10.1016/j.jtho.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong L, Zhang J, Heymach JV, Le X. Current and future treatment options for MET exon 14 skipping alterations in non-small cell lung cancer. Ther Adv Med Oncol. 2021;13:1758835921992976. doi: 10.1177/1758835921992976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le X, Heymach JV. New verse for a familiar song: Small molecule inhibitors for MET exon 14 skipping non‐small cell lung cancer. Oncologist. 2020;25(10):822–825. doi: 10.1634/theoncologist.2020-0760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazieres J, Cortot A, Gezin A, et al. 159P Clinical characteristics of patients with advanced NSCLC and MET exon 14 (METex14) skipping: A systematic literature review. J Thorac Oncol. 2021;16(4):S784–S785. doi: 10.1016/S1556-0864(21)02001-3 [DOI] [Google Scholar]
- 8.Bittoni M, Yang JCH, Shih JY, et al. Real-world insights into patients with advanced NSCLC and MET alterations. Lung Cancer. 2021;159:96–106. doi: 10.1016/j.lungcan.2021.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Offin M, Luo J, Guo R, et al. CNS metastases in patients with MET exon 14–altered lung cancers and outcomes with crizotinib. JCO Precis Oncol. 2020;4:871–876. doi: 10.1200/po.20.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paik P, Horn L, Kowalski DM, et al. Tepotinib in patients (pts) with NSCLC with MET exon 14 (MET ex14) skipping: Health-related quality of life (HRQoL). J Clin Oncol. 2020;38(15_Suppl):9575. doi: 10.1200/JCO.2020.38.15_suppl.9575 [DOI] [Google Scholar]
- 11.Scott NW, Fayers PM, Aaronson NK, et al. EORTC QLQ-C30 Reference Values. 2008.
- 12.Sabari JK, Leonardi GC, Shu CA, et al. PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann Oncol. 2018;29(10):2085–2091. doi: 10.1093/annonc/mdy334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong JH, Yeung SF, Chan AWH, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res. 2016;22(12):3048–3056. doi: 10.1158/1078-0432.CCR-15-2061 [DOI] [PubMed] [Google Scholar]
- 14.Paik P, Drilon A, Fan PDD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring met mutations causing exon 14 skipping. Cancer Discov. 2015;5(8):842–849. doi: 10.1158/2159-8290.CD-14-1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falchook GS, Kurzrock R, Amin HM, et al. First-in-man Phase I trial of the selective MET inhibitor tepotinib in patients with advanced solid tumors. Clin Cancer Res. 2020;26(6):1237–1246. doi: 10.1158/1078-0432.CCR-19-2860 [DOI] [PubMed] [Google Scholar]
- 16.Markham A Tepotinib: First Approval. Drugs. 2020;80(8):829–833. doi: 10.1007/s40265-020-01317-9 [DOI] [PubMed] [Google Scholar]
- 17.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237. doi: 10.1093/annonc/mdy275 [DOI] [PubMed] [Google Scholar]
- 18.ESMO. Clinical Practice Living Guidelines – Metastatic Non-small-cell Lung Cancer. https://www.esmo.org/guidelines/lung-and-chest-tumours/clinical-practice-living-guidelines-metastatic-non-small-cell-lung-cancer
- 19.Paik P, Felip E, Veillon R, et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383(10):931–943. doi: 10.1056/NEJMoa2004407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le X, Sakai H, Felip E, et al. Tepotinib efficacy and safety in patients with MET exon 14 skipping NSCLC: Outcomes in patient subgroups from the VISION study with relevance for clinical practice. Clin Cancer Res. 2022;28(6):1117–1126. doi: 10.1158/1078-0432.CCR-21-2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garassino MCC, Felip E, Sakai H, et al. 1254P Efficacy and safety of tepotinib in patients (pts) with advanced age: VISION subgroup analysis of pts with MET exon 14 (METex14) skipping NSCLC. Ann Oncol. 2021;32:S984–S985. doi: 10.1016/J.ANNONC.2021.08.1857 [DOI] [Google Scholar]
- 22.Morise M, Sakai H, Kato T, et al. Efficacy and intracranial activity of tepotinib in Japanese patients with MET exon 14 skipping (METex14) NSCLC (VISION). In: JSMO. ; 2022:Abstract 10481. [Google Scholar]
- 23.Hatswell AJ, Bullement A, Schlichting M, Bharmal M. What is the Impact of the Analysis Method Used for Health State Utility Values on QALYs in Oncology? A Simulation Study Comparing Progression-Based and Time-to-Death Approaches. Appl Health Econ Health Policy. 2021;19(3):389–401. doi: 10.1007/S40258-020-00620-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tosh JC, Longworth LJ, George E. Utility values in National Institute for Health and Clinical Excellence (NICE) Technology Appraisals. Value Health. 2011;14(1):102–109. doi: 10.1016/J.JVAL.2010.10.015 [DOI] [PubMed] [Google Scholar]
- 25.Felip E, Garassino MC, Sakai H, et al. P45.03 Tepotinib in patients with MET exon 14 skipping NSCLC as identified by liquid or tissue biopsy. J Thorac Oncol. 2021;16(10):S1085. doi: 10.1016/j.jtho.2021.08.471 [DOI] [Google Scholar]
- 26.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/S11136-011-9903-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 28.Pickard AS, Law EH, Jiang R, et al. United States Valuation of EQ-5D-5L Health States Using an International Protocol. Value Health. 2019;22(8):931–941. doi: 10.1016/J.JVAL.2019.02.009 [DOI] [PubMed] [Google Scholar]
- 29.Xie F, Pullenayegum E, Gaebel K, et al. A Time Trade-off-derived Value Set of the EQ-5D-5L for Canada. Med Care. 2016;54(1):98–105. doi: 10.1097/MLR.0000000000000447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin HW, Li CI, Lin FJ, et al. Valuation of the EQ-5D-5L in Taiwan. PLoS One. 2018;13(12). doi: 10.1371/JOURNAL.PONE.0209344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CRAN - Package eq5d. 2022. https://cran.r-project.org/web/packages/eq5d/index.html. Accessed January 25, 2022
- 32.R: The R Project for Statistical Computing. https://www.r-project.org/. Accessed January 25, 2022
- 33.Van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Heal. 2012;15(5):708–715. doi: 10.1016/J.JVAL.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 34.Dolan P Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–1108. doi: 10.1097/00005650-199711000-00002 [DOI] [PubMed] [Google Scholar]
- 35.Revicki DA, King MT, Viney R, et al. United States Utility Algorithm for the EORTC QLU-C10D, a Multiattribute Utility Instrument Based on a Cancer-Specific Quality-of-Life Instrument. Med Decis Making. 2021;41(4):485–501. doi: 10.1177/0272989X211003569 [DOI] [PubMed] [Google Scholar]
- 36.Norman R, Mercieca-Bebber R, Rowen D, et al. U.K. utility weights for the EORTC QLU-C10D. Health Econ. 2019;28(12):1385–1401. doi: 10.1002/HEC.3950 [DOI] [PubMed] [Google Scholar]
- 37.McTaggart-Cowan H, King MT, Norman R, et al. The EORTC QLU-C10D: The Canadian Valuation Study and Algorithm to Derive Cancer-Specific Utilities From the EORTC QLQ-C30. MDM Policy Pract. 2019;4(1):238146831984253. doi: 10.1177/2381468319842532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NICE. Atezolizumab for treating locally advanced or metastatic non-small-cell lung cancer after chemotherapy. 2018. https://www.nice.org.uk/guidance/ta520
- 39.NICE. Pembrolizumab for treating PD-L1-positive non-small-cell lung cancer after chemotherapy. 2017. https://www.nice.org.uk/guidance/ta428
- 40.Reinmuth N, Popat S, Paz-Ares L, et al. 1255P Health utility with tepotinib in patients (pts) with MET exon 14 (METex14) skipping non-small cell lung cancer (NSCLC). Ann Oncol. 2021;32:S985–S986. doi: 10.1016/J.ANNONC.2021.08.1858 [DOI] [Google Scholar]
- 41.Chouaid C, Agulnik J, Goker E, et al. Health-related quality of life and utility in patients with advanced non-small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol. 2013;8(8):997–1003. doi: 10.1097/JTO.0B013E318299243B [DOI] [PubMed] [Google Scholar]
- 42.Chevalier J, Lay K Le, Pouvourville G de. Health State Utility Values in Advanced Non-Small Cell Lung Cancer Patients. Value Heal. 2013;16(7):A419. doi: 10.1016/J.JVAL.2013.08.550 [DOI] [Google Scholar]
- 43.Insinga RP, Vanness DJ, Feliciano JL, Vandormael K, Traore S, Burke T. Cost-effectiveness of pembrolizumab in combination with chemotherapy in the 1st line treatment of non-squamous NSCLC in the US. J Med Econ. 2018;21(12):1191–1205. doi: 10.1080/13696998.2018.1521416 [DOI] [PubMed] [Google Scholar]
- 44.Insinga RP, Vanness DJ, Feliciano JL, et al. Cost-effectiveness of pembrolizumab in combination with chemotherapy versus chemotherapy and pembrolizumab monotherapy in the first-line treatment of squamous non-small-cell lung cancer in the US. Curr Med Res Opin. 2019;35(7):1241–1256. doi: 10.1080/03007995.2019.1571297 [DOI] [PubMed] [Google Scholar]
- 45.Huang M, Chandwani S, Insinga R, Burke T, Pellissier J, Pickard AS. Health state utilities in metastatic NSCLC: A study of multiple immuno-oncology trials. Value Heal. 2018;21:S72–S73. doi: 10.1016/J.JVAL.2018.09.427 [DOI] [Google Scholar]
- 46.Huang M, Lou Y, Pellissier J, et al. Cost Effectiveness of Pembrolizumab vs. Standard-of-Care Chemotherapy as First-Line Treatment for Metastatic NSCLC that Expresses High Levels of PD-L1 in the United States. Pharmacoeconomics. 2017;35(8):831–844. doi: 10.1007/S40273-017-0527-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shor A, Forsythe A, Li S, Galaznik A. Systematic Literature Review (SLR) Of Health State Utility Values (HSUV) In Epidermal Growth Factor Receptor Mutant (Egfr-Mutant) Non-Small Cell Lung Cancer (NSCLC) Patients Previously Treated With Targeted Therapies. Value Heal. 2018;21:S35–S36. doi: 10.1016/J.JVAL.2018.04.300 [DOI] [Google Scholar]
- 48.Bodnar C, Ryan J, Green M. Health state utility measured by EQ-5D-5L for EGFRm T790M NSCLC patients treated with osimertinib. Ann Oncol. 2016;27:vi357. doi: 10.1093/ANNONC/MDW377.21 [DOI] [Google Scholar]
- 49.Blackhall F, Kim DW, Besse B, et al. Patient-reported outcomes and quality of life in PROFILE 1007: a randomized trial of crizotinib compared with chemotherapy in previously treated patients with ALK-positive advanced non-small-cell lung cancer. J Thorac Oncol. 2014;9(11):1625–1633. doi: 10.1097/JTO.0000000000000318 [DOI] [PubMed] [Google Scholar]
- 50.Meregaglia M, Cairns J. A systematic literature review of health state utility values in head and neck cancer. Health Qual Life Outcomes. 2017;15(1):1–13. doi: 10.1186/S12955-017-0748-Z/TABLES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang SX, Walton RN, Hueniken K, et al. Real-world health utility scores and toxicities to tyrosine kinase inhibitors in epidermal growth factor receptor mutated advanced non-small cell lung cancer. Cancer Med. 2019;8(18):7542–7555. doi: 10.1002/CAM4.2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hatswell AJ, Pennington B, Pericleous L, Rowen D, Lebmeier M, Lee D. Patient-reported utilities in advanced or metastatic melanoma, including analysis of utilities by time to death. Health Qual Life Outcomes. 2014;12(1):140. doi: 10.1186/S12955-014-0140-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smit EF, Garassino MC, Felip E, et al. 985P Tepotinib outcomes according to prior therapies in patients with MET exon 14 (METex14) skipping NSCLC. Ann Oncol. 2022;33(Suppl 7):S1002–S1003. doi: 10.1016/j.annonc.2022.07.1112 [DOI] [Google Scholar]
- 54.Marschner N, Zacharias S, Lordick F, et al. Association of Disease Progression With Health-Related Quality of Life Among Adults With Breast, Lung, Pancreatic, and Colorectal Cancer. JAMA Netw Open. 2020;3(3):e200643–e200643. doi: 10.1001/JAMANETWORKOPEN.2020.0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ben Bouazza Y, Chiairi I, El Kharbouchi O, et al. Patient-reported outcome measures (PROMs) in the management of lung cancer: A systematic review. Lung Cancer. 2017;113:140–151. doi: 10.1016/J.LUNGCAN.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 56.Sloan JA, Zhao X, Novotny PJ, et al. Relationship between deficits in overall quality of life and non-small-cell lung cancer survival. J Clin Oncol. 2012;30(13):1498–1504. doi: 10.1200/JCO.2010.33.4631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang C, Park S, CHOI Y, et al. Measurement of Utilities by Time to Death Related to Advanced Non-Small Cell Lung Cancer in South Korea. Value Heal. 2016;19(7):A744. doi: 10.1016/J.JVAL.2016.09.2276 [DOI] [Google Scholar]
- 58.Abbosh C, Birkbak NJ, Swanton C. Early stage NSCLC - challenges to implementing ctDNA-based screening and MRD detection. Nat Rev Clin Oncol. 2018;15(9):577–586. doi: 10.1038/S41571-018-0058-3 [DOI] [PubMed] [Google Scholar]
- 59.Rolfo C, Mack PC, Scagliotti GV, et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J Thorac Oncol. 2018;13(9):1248–1268. doi: 10.1016/J.JTHO.2018.05.030 [DOI] [PubMed] [Google Scholar]
- 60.Chamberlain MC, Baik CS, Gadi VK, Bhatia S, Chow LQM. Systemic therapy of brain metastases: Non-small cell lung cancer, breast cancer, and melanoma. Neuro Oncol. 2017;19(1):i1–i24. doi: 10.1093/neuonc/now197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Camidge DR, Lee EQ, Lin NU, et al. Clinical trial design for systemic agents in patients with brain metastases from solid tumours: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol. 2018;19(1):e20–e32. doi: 10.1016/S1470-2045(17)30693-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


