Abstract
Aim:
To evaluate all-cause and liver-associated healthcare resource utilization (HCRU) and costs among patients with alpha-1 antitrypsin deficiency (AATD) with liver disease (LD) and/or lung disease (LgD).
Materials & methods:
This was a retrospective analysis of linked administrative claims data from the IQVIA PharMetrics® Plus and the IQVIA Ambulatory Electronic Medical Records (AEMR) databases from 1 July 2021 to 31 January 2022. Patients with AATD in the IQVIA PharMetrics Plus database were included with ≥1 inpatient or ≥2 outpatient medical claims ≥90 days apart with a diagnosis of AATD, or with records indicating a protease inhibitor (Pi)*ZZ/Pi*MZ genotype in the IQVIA AEMR database with linkage to IQVIA PharMetrics Plus. For a patient's identified continuous enrollment period, patient time was assigned to health states based on the initial encounter with an LD/LgD diagnosis. A unique index date was defined for each health state, and HCRU and costs were calculated per person-year (PPY).
Results:
Overall, 5136 adult and pediatric patients from the IQVIA PharMetrics Plus and IQVIA AEMR databases were analyzed. All-cause and liver-associated HCRU and costs were substantially higher following onset of LD/LgD. All-cause cost PPY ranged from US $11,877 in the absence of either LD/LgD to US $74,015 in the presence of both LD and LgD. Among liver transplant recipients in the AATD with LD health state, liver-associated total costs PPY were US $87,329 1-year pre-transplantation and US $461,752 1-year post-transplantation. In the AATD with LgD and AATD with LD and LgD health states, patients who received augmentation therapy were associated with higher all-cause total costs PPY and lower liver-associated total costs PPY than their counterparts who did not receive augmentation therapy.
Conclusion:
Patients with AATD had increased HCRU and healthcare costs in the presence of LD and/or LgD. HCRU and healthcare costs were highest in the AATD with LD and LgD health state.
Keywords: alpha-1 antitrypsin deficiency, claims data, costs, healthcare resource utilization, liver disease, lung disease
Alpha-1 antitrypsin deficiency (AATD) is an autosomal co-dominant genetic disease that is characterized by low levels of serum alpha-1 antitrypsin (AAT), leading to lung disease (including chronic obstructive pulmonary disease and emphysema) and accumulation of misfolded AAT in hepatocytes, which can result in liver disease [1,2].
Worldwide, more than 190 million people are estimated to carry at least one of the S or Z SERPINA1 alleles associated with AATD, of whom approximately 5.5 million carry two of these alleles (protease inhibitor [Pi]*SS, Pi*SZ or Pi*ZZ) [3]. The Pi*ZZ genotype is associated with severe AATD and increased risk of liver disease, which is frequently undetected before cirrhosis or hepatocellular carcinoma are evident [4,5]. The heterozygous Pi*MZ genotype is primarily considered a disease-modifying factor in patients with chronic liver disease and is associated with higher levels of serum transaminases, more AAT liver inclusions and higher liver stiffness measurement than adults without the Pi*Z variant (although lower than the Pi*ZZ genotype) [5]. Currently, there are no licensed pharmacological treatments available for patients with AATD-associated liver disease, often leading to liver transplantation in patients with advanced liver cirrhosis or failure [6].
Although health-related quality of life (HRQoL) data for AATD-associated liver/lung disease are limited, the disease is associated with an underappreciated HRQoL burden. Many aspects contribute to this burden, including delayed diagnosis, high costs of treatment and guilt about passing deficiency genes from one generation to the next [7]. Anxiety and depression are also common and underappreciated comorbidities in patients with AATD-associated liver/lung disease [7].
Economic data on AATD-associated liver disease are sparse. However, these data are an important contributor to the costs of hospitalization associated with metabolic dysfunction-associated genetic liver disease, and the considerable costs associated with transplantation with life-long immunosuppression [8–10].
The aim of this study was to evaluate all-cause and liver-associated healthcare resource utilization (HCRU) and healthcare costs among patients with AATD with liver and/or lung disease.
Methods
Study design
This was a non-interventional, retrospective, observational analysis of linked administrative claims data and electronic medical records from the IQVIA PharMetrics® Plus and IQVIA Ambulatory Electronic Medical Records (AEMR) databases over the study period (1 July 2011–31 January 2022).
Data source
The aggregated IQVIA PharMetrics Plus database comprises adjudicated medical and pharmacy claims for more than 190 million unique patients with commercially managed health insurance across the USA. The data are longitudinal, with approximately 20 million patients who have both medical and pharmacy coverage with 3 or more years of continuous enrollment. The IQVIA AEMR database comprises approximately 75 million patient records that are sourced from an ‘opt-in’ provider research network. The aggregated database comprises records collected across 40,000 physicians from large practices and physician networks.
Population
Patient data from each database were identified separately from the two databases and then combined (Figure 1A). All patients eligible for inclusion were required to have linkage to the IQVIA PharMetrics Plus database. Linkage to the IQVIA AEMR database was not required. Patients could be tracked across IQVIA PharMetrics Plus or IQVIA AEMR databases using a unique de-identified patient ID. HCRU and healthcare cost assessments were conducted only in the IQVIA PharMetrics Plus database to avoid ‘double counting’. For the IQVIA PharMetrics Plus database, patients were included if they had at least one inpatient or at least two outpatient medical claims at least 90 days apart with a diagnosis of AATD. Linkage to the IQVIA AEMR database was not required; however, patients from the IQVIA AEMR database were also included if they had at least one Systematized Nomenclature of Medicine (SNOMED)/International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM)/International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis code for AATD, with a problem name, laboratory test or SNOMED code indicating a Pi*ZZ/Pi*MZ genotype and linkage to the IQVIA PharMetrics Plus database. Genotype data were not recorded in the IQVIA PharMetrics Plus database and could only be obtained, if documented, through linkage to the IQVIA AEMR database.
Figure 1. . Study design, patient attrition, and example health state time contributions.
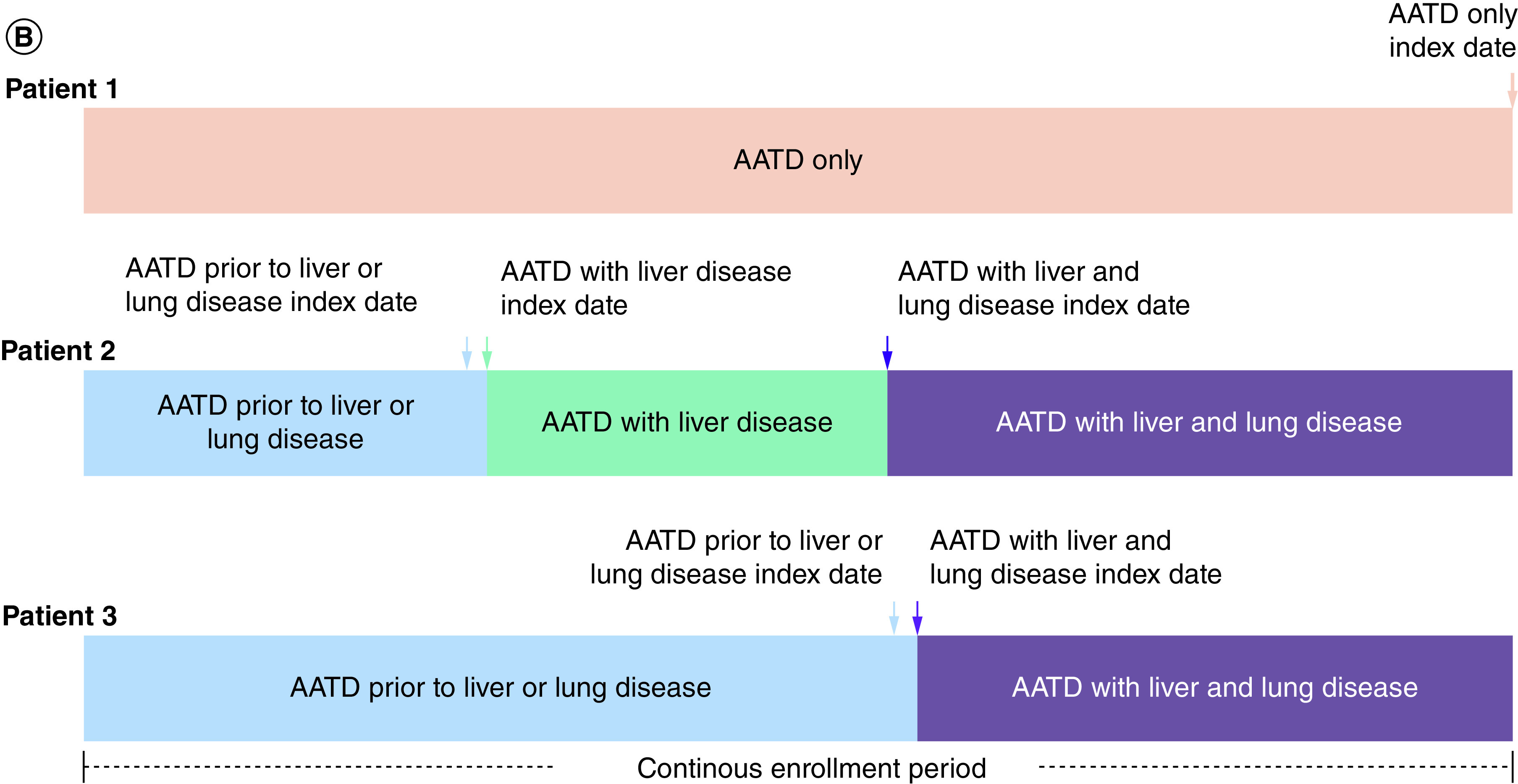

(A) Study design and patient attrition. (B) Examples of time contributions to different health states during the continuous enrollment period. aPatients aged under 1 year at index date do not require 180 days of continuous enrollment pre-index. bPatients without a liver or lung disease diagnosis do not require 90 days of continuous enrollment post-index. cExcludes patients with incomplete data or data quality issues (Medicare Cost coverage or enrollment in State Children's Health Insurance Program [owing to incomplete data coverage]; invalid/missing year of birth or health plan enrollment dates; duplicate de-identified unique patient ID matches from the IQVIA AEMR database to the PharMetrics IQVIA PharMetrics Plus database; n = 89).
AATD: Alpha-1 antitrypsin deficiency; AEMR: Ambulatory electronic medical record; ICD-9/10-CM: International Classification of Disease, Ninth or Tenth Revision, Clinical Modification; Pi: Protease inhibitor; SNOMED: Systematized nomenclature of medicine.
A person–time approach was taken to maximize the use of patient data. At the patient level, for a patient's identified continuous enrollment period in the IQVIA PharMetrics Plus database, patient time was assigned to different health states based on the presence of a liver or lung disease diagnosis code: AATD only (no liver or lung disease diagnosis code), AATD prior to liver or lung disease, AATD with liver disease, AATD with lung disease, and AATD with liver and lung disease (Table 1 & Figure 1B). Patients could contribute time to more than one health state during their continuous enrollment period. The date of first liver or lung disease diagnosis was termed the index date for patients within the AATD with liver disease health state and AATD with lung disease health state, respectively. For patients assigned to the AATD with liver and lung disease health state, the date of first lung disease diagnosis (after a liver disease diagnosis) or date of first liver disease diagnosis (after lung disease diagnosis) was termed the index date; if a patient had a first liver disease diagnosis and first lung disease diagnosis code within 90 days of each other, the index date was the date of the first occurring liver or lung disease diagnosis.
Table 1. . Dates for time in health state and index date definitions.
| Health state | Index date for baseline characteristics assessment | Start of time in health state | End of time in health state |
|---|---|---|---|
| Patients with AATD and no liver or lung disease during the continuous enrollment period | |||
| AATD only | Last day of continuous enrollment | First day of continuous enrollment | Last day of continuous enrollment |
| Patients with AATD and liver and/or lung disease during the continuous enrollment period | |||
| AATD prior to liver or lung disease | Day before first liver or lung disease diagnosis | First day of continuous enrollment | Day before first liver or lung disease diagnosis |
| AATD with liver disease | Day of first liver disease diagnosis | Day of first liver disease diagnosis | Day before lung disease diagnosis or last day of continuous enrollment, whichever occurred first |
| AATD with lung disease | Day of first lung disease diagnosis | Day of first lung disease diagnosis | Day before liver disease diagnosis or last day of continuous enrollment, whichever occurred first |
| AATD with liver and lung disease | Day of first lung disease diagnosis (if liver disease diagnosed first), day of first liver disease diagnosis (if lung disease diagnosed first), or day of first liver and lung disease diagnosis (if diagnosed on the same day)† | Day of first lung disease diagnosis (if liver disease diagnosed first), day of first liver disease diagnosis (if lung disease diagnosed first), or day of first liver and lung disease diagnosis (if diagnosed on the same day)† | Last day of continuous enrollment |
If a patient had (1) a first liver disease diagnosis and (2) a first lung disease diagnosis code within 90 days, the patient was considered to be in the ‘AATD with liver and lung disease health state’ at the date of the first occurring disease diagnosis code.
AATD: Alpha-1 antitrypsin deficiency.
Outcomes
Baseline demographics were collected at the index date and included age, gender, geographic region and payer type (commercial [primarily employer based; the health plan assumes the risk of insuring the enrolled member], Medicaid, Medicare risk, self-insured [specific subset of commercial plans in which the employer assumes the risk of insuring the enrolled members], other/unknown). Clinical characteristics (Charlson comorbidity index [CCI] score and comorbidities) were collected during the 6 months before the index date (baseline period).
All-cause HCRU and costs were assessed during the follow-up time for each health state. Liver-associated HCRU and costs were also assessed for the AATD with liver disease health state and the AATD with liver and lung disease health state. Owing to the varying length of time in each health state, HCRU and costs were calculated per person-year (PPY; total number of services or costs in a health state divided by the total length of follow-up in a health state).
Liver-associated HCRU and costs consisted of three measures (liver disease-specific, liver-related clinical event and all other liver disease-related). Liver disease-specific HCRU and costs were based on diagnosis codes used to identify health states associated with liver disease. Liver disease-specific HCRU and costs also included the costs of medications used in the treatment of hepatitis and metabolic dysfunction-associated steatohepatitis/metabolic dysfunction-associated steatotic liver disease and only liver disease-specific costs were considered for liver-associated pharmacy costs. HCRU and costs for liver-related clinical events were identified with diagnosis and/or procedure codes for the following decompensated events among patients with liver cirrhosis: liver transplantation, ascites, gastrointestinal bleed, spontaneous bacterial peritonitis, hepatocellular carcinoma and hepatic encephalopathy. These liver-related clinical events were not considered for liver disease health state identification. All other liver disease-related HCRU and costs were based on diagnosis codes specific to the liver that were not considered for liver disease health state identification or for liver-related clinical events.
Data analysis
The number of services and costs, respectively, were summed over the contributed patient time in each health state and divided by total patient time contributed in the health state. Standard error and 95% confidence intervals were estimated using the bootstrap method with 1000 replicated samples. No statistical comparisons or methods for adjustment of p values were determined. Data were analyzed by health state, and among the overall cohort with all ages and stratified by age group (<1, 1–5, 6–17, ≥18 years). All-cause and liver-associated HCRU and costs were further stratified for patients in the AATD with lung disease health state and the AATD with liver and lung disease health state based on use of augmentation therapy (e.g., alpha-1 proteinase inhibitor) during the respective time in each health state. HCRU and costs PPY were reported for mutually exclusive healthcare services: outpatient pharmacy prescription (prescription fill and total pharmacy cost), inpatient hospitalizations (number of hospitalizations, days hospitalized and total inpatient cost) and outpatient medical care. Outpatient medical care included the mutually exclusive healthcare categories of physician office visits, emergency room visits, laboratory/pathology tests, radiology exams, outpatient surgical visits and ancillary services.
Results
Patient demographics and baseline characteristics
Overall, 5136 (48.2%) of the 10,660 patients with AATD from the IQVIA PharMetrics Plus and IQVIA AEMR databases were included in the study (Figure 1A). There were 1804 patients who contributed time to the AATD only health state, 3291 patients who contributed time to the AATD prior to liver or lung disease health state, 877 patients who contributed time to the AATD with liver disease health state, 2343 patients who contributed time to the AATD with lung disease health state and 566 patients who contributed time to the AATD with liver and lung disease health state. Among the overall cohort, 7 (0.1%) and 55 (1.1%) patients had a reported Pi*ZZ or Pi*MZ genotype, respectively, in the IQVIA AEMR database. The majority of patients in the overall cohort (98.8%) had no documented genotype.
Across health states, median age ranged from 44 to 54 years, the proportion of pediatric patients (aged under 18 years) ranged from 4.4% to 12.9%, the most frequent comorbidities were hypertension and hyperlipidemia, the most common payer type was commercial, and the median CCI ranged from 0 to 1 (Table 2). Median duration in health state was 39 months for the AATD only health state, 16 months for the AATD prior to liver or lung disease health state, 23 months for the AATD with liver disease health state, 31 months for the AATD with lung disease health state, and 21 months for the AATD with liver and lung disease health state (Table 2).
Table 2. . Baseline participant characteristics in overall population (N = 5136).
| AATD only health state (n = 1804) | AATD prior to liver or lung disease health state (n = 3291) | AATD with liver disease health state (n = 877) | AATD with lung disease health state (n = 2343) | AATD with liver and lung disease health state (n = 566) | |
|---|---|---|---|---|---|
| Age, years, median (IQR) | 44 (27–57) | 52 (40–59) | 46 (33–56) | 53 (42–59) | 54 (44–61) |
| Gender, n (%) Male Female |
826 (45.8) 978 (54.2) |
1596 (48.5) 1695 (51.5) |
514 (58.6) 363 (41.4) |
1042 (44.5) 1301 (55.5) |
290 (51.2) 276 (48.8) |
| Pediatric patients aged under 18 years, n (%) | 232 (12.9) | 197 (6.0) | 106 (12.1) | 125 (5.3) | 25 (4.4) |
| Time in health state, months, median (IQR)† | 39 (21–64) | 16 (10–30) | 23 (11–42) | 31 (15–54) | 21 (10–38) |
| Most common comorbidities, n (%)‡ Anemia Asthma COPD Diabetes mellitus Hyperlipidemia Hypertension Liver/gall bladder/pancreatic disease Obesity Sleep disorders Liver cirrhosis Study liver disease§ Study lung disease§ |
46 (2.5) 220 (12.2) 0 103 (5.7) 241 (13.4) 252 (14.0) 38 (2.1) 162 (9.0) 164 (9.1) 6 (0.3) 0 0 |
133 (4.0) 463 (14.1) 0 296 (9.0) 630 (19.1) 740 (22.5) 122 (3.7) 331 (10.1) 321 (9.8) 19 (0.6) 0 0 |
46 (5.2) 65 (7.4) 0 108 (12.3) 210 (23.9) 223 (25.4) 81 (9.2) 131 (14.9) 97 (11.1) 15 (1.7) 0 0 |
73 (3.1) 384 (16.4) 0 173 (7.4) 397 (16.9) 485 (20.7) 35 (1.5) 184 (7.9) 210 (9.0) 3 (0.1) 0 0 |
65 (11.5) 97 (17.1) 87 (15.4) 108 (19.1) 168 (29.7) 219 (38.7) 135 (23.9) 132 (23.3) 103 (18.2) 61 (10.8) 96 (17.0) 132 (23.3) |
| Charlson comorbidity index, median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 1 (0–2) |
| Payer type, n (%) Commercial Medicaid Medicare risk Self-insured Other/unknown |
1219 (67.6) 46 (2.5) 39 (2.2) 485 (26.9) 15 (0.8) |
2197 (66.8) 72 (2.2) 132 (4.0) 879 (26.7) 11 (0.3) |
578 (65.9) 22 (2.5) 21 (2.4) 253 (28.8) 3 (0.3) |
1561 (66.6) 53 (2.3) 108 (4.6) 613 (26.2) 8 (0.3) |
372 (65.7) 8 (1.4) 25 (4.4) 157 (27.7) 4 (0.7) |
| Geographic region within the USA Northeast Midwest South West Unknown/missing |
372 (20.6) 507 (28.1) 636 (35.3) 258 (14.3) 31 (1.7) |
564 (17.1) 873 (26.5) 1416 (43.0) 395 (12.0) 43 (1.3) |
166 (18.9) 220 (25.1) 379 (43.2) 101 (11.5) 11 (1.3) |
394 (16.8) 642 (27.4) 989 (42.2) 288 (12.3) 30 (1.3) |
81 (14.3) 135 (23.9) 284 (50.2) 59 (10.4) 7 (1.2) |
Difference in time from first liver disease to the earlier of first lung disease or end of continuous enrollment period.
Present in at least 10% of patients in at least one of the health states.
Clinician determined specific liver/lung disease diagnosis codes as AATD-specific; separately reported comorbidities identified by the IQVIA clinical coding may include broader definitions (e.g., alcoholic liver cirrhosis).
AATD: Alpha-1 antitrypsin deficiency; COPD: Chronic obstructive pulmonary disease; IQR: Interquartile range.
Healthcare resource utilization
The numbers of all-cause services PPY for emergency room visits, outpatient surgery visits, outpatient prescription fills, radiology exams, inpatient hospitalizations and inpatient hospitalization days were higher in the AATD with liver disease health state, AATD with lung disease health state and AATD with liver and lung disease health state than in the AATD only or AATD prior to liver or lung disease health states (Figure 2). For both all-cause and liver-associated HCRU, the number of services PPY was uniformly highest in the AATD with liver and lung disease health state (Figure 2).
Figure 2. . All-cause and liver-associated healthcare resource utilization.
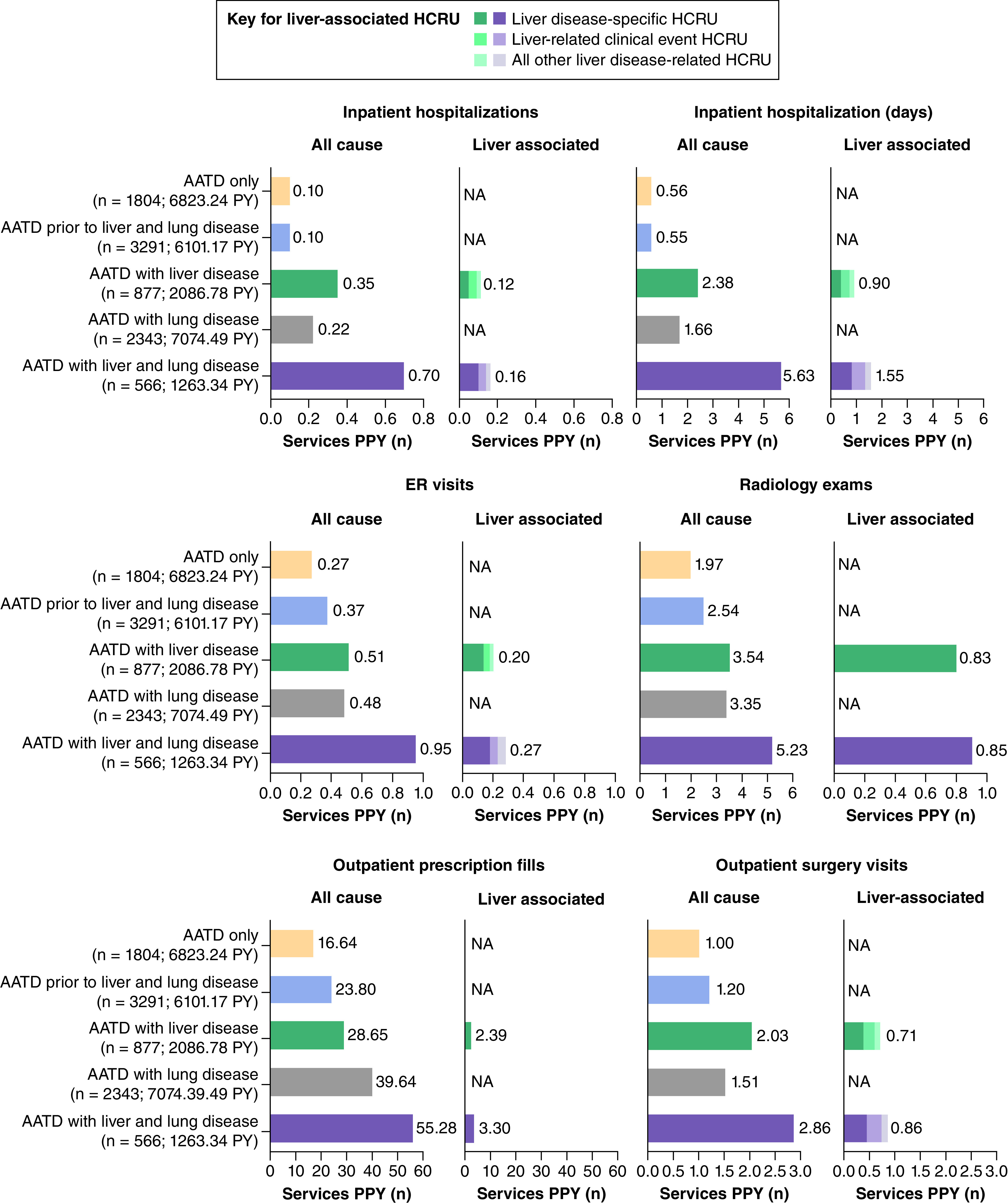
Only liver disease-specific costs were available for liver-associated outpatient prescription fills and radiology exams.
AATD: Alpha-1 antitrypsin deficiency; ER: Emergency room; HCRU: Healthcare resource utilization; NA: Not applicable; PPY: Per person-year; PY: Person-years.
Costs
All-cause and disease-specific costs
The all-cause costs PPY for total outpatient medical, inpatient and total costs were higher in the AATD with liver disease health state, AATD with lung disease health state, and AATD with liver and lung disease health state than in the AATD only or AATD prior to liver or lung disease health states (Figure 3). However, the all-cause costs PPY for outpatient pharmacy costs were higher only in the AATD with lung disease and AATD with liver and lung disease health states than in the AATD only or AATD prior to liver or lung disease health states (Figure 3). Liver disease-specific costs PPY represented 25% and 22% of all-cause inpatient costs PPY and 22% and 14% of all-cause total costs PPY in the AATD with liver disease and AATD with liver and lung disease health states, respectively (Figure 3).
Figure 3. . All-cause and liver-associated costs.
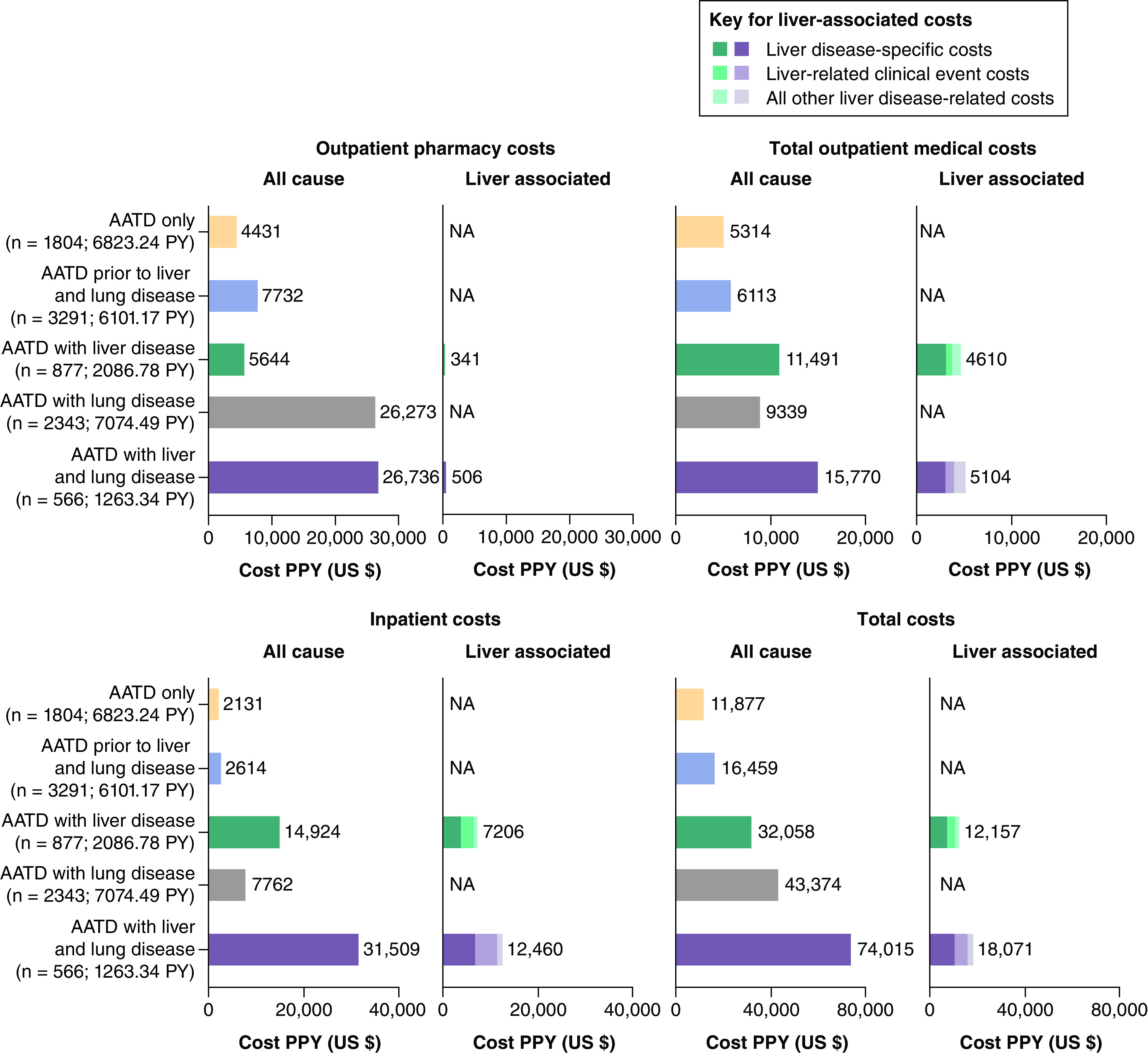
Only liver disease-specific costs were available for liver-associated pharmacy costs. Liver disease-specific costs included the costs of medications used in the treatment of hepatitis and MASH/MASLD.
AATD: Alpha-1 antitrypsin deficiency; MASH: Metabolic dysfunction-associated steatohepatitis; MASLD: Metabolic dysfunction-associated steatotic liver disease; NA: Not applicable; PPY: Per person-year; PY: Person-years.
Augmentation therapy
All-cause total cost PPY in the AATD with lung disease health state was higher in patients who received augmentation therapy (US $124,545; n = 443; 1398.89 person-years) than in those who did not receive augmentation therapy (US $23,367; n = 1900; 5675.60 person-years). Similarly, all-cause total cost PPY in the AATD with liver and lung disease health state was higher in patients who received augmentation therapy (US $150,981; n = 76; 172.83 person-years) than in those who did not receive augmentation therapy (US $61,817; n = 490; 1090.52 person-years). For the AATD with lung disease health state and AATD with liver and lung disease health state, the higher all-cause total cost PPY in patients who received augmentation therapy compared with patients who did not receive augmentation therapy was driven by high all-cause total pharmacy cost (more than US $100,000 in patients who received augmentation therapy vs less than US $15,000 in patients who did not receive augmentation therapy, in both health states; Tables 3 & 4). Liver-associated total cost PPY in the AATD with liver and lung disease health state was lower in patients who received augmentation therapy (US $10,883) than in patients who did not receive augmentation therapy (US $19,210) owing to lower total inpatient costs in patients who received augmentation therapy (US $5497 vs US $13,564; Table 4).
Table 3. . Total healthcare resource utilization and costs stratified by receipt of augmentation therapy in the alpha-1 antitrypsin deficiency with lung disease health state.
| AATD with lung disease health state | ||
|---|---|---|
| All-cause costs | ||
| With augmentation therapy (n = 443; 1398.9 person-years) | Without augmentation therapy (n = 1900; 5676.6 person-years) | |
| Total pharmacy cost PPY, US $ (SE) | 103,734 (3802) | 7181 (550) |
| Total outpatient medical cost PPY, US $ (SE) Physician office visits Emergency room only visits Laboratory/pathology Radiology Outpatient surgery Ancillary services |
12,999 (627) 1709 (83) 544 (62) 631 (53) 1040 (68) 1290 (117) 7785 (523) |
8436 (272) 1775 (48) 673 (47) 670 (26) 1225 (67) 1315 (61) 2779 (157) |
| Total inpatient cost PPY, US $ (SE) | 7812 (1959) | 7750 (1064) |
| Total cost PPY, US $ (SE) | 124,545 (4189) | 23,367 (1383) |
AATD: Alpha-1 antitrypsin deficiency; SE: Standard error.
Table 4. . Total healthcare resource utilization and costs stratified by receipt of augmentation therapy in the alpha-1 antitrypsin deficiency with liver and lung disease health state.
| AATD with liver and lung disease health state | ||||||||
|---|---|---|---|---|---|---|---|---|
| All-cause | Liver disease-specific | Liver disease-related clinical events | All other liver disease-related | |||||
| With augmentation therapy (n = 76; 172.8 person-years) | Without augmentation therapy (n = 490; 1090.5 person-years) | With augmentation therapy (n = 76; 172.8 person-years) | Without augmentation therapy (n = 490; 1090.5 person-years) | With augmentation therapy (n = 76; 172.8 person-years) | Without augmentation therapy (n = 490; 1090.5 person-years) | With augmentation therapy (n = 76; 172.8 person-years) | Without augmentation therapy (n = 490; 1090.5 person-years) | |
| Total pharmacy cost PPY, US $ (SE) | 121,651 (10 309) | 11,694 (1274) | 722 (619) | 472 (214) | – | – | – | – |
| Total outpatient medical cost PPY, US $ (SE) Physician office visits Emergency room only visits Laboratory/pathology Radiology Outpatient surgery Ancillary services |
17,439 (2320) 2091 (242) 1132 (324) 1248 (208) 2149 (356) 1948 (324) 8869 (1667) |
15,505 (1128) 2709 (158) 1297 (197) 1455 (124) 2119 (182) 3287 (574) 4637 (459) |
3062 (1050) 318 (79) 156 (86) 297 (80) 563 (155) 473 (126) 1254 (817) |
3004 (402) 336 (33) 264 (43) 265 (26) 518 (64) 820 (215) 801 (226) |
608 (281) – – – – – – |
959 (215) – – – – – – |
995 (451) – – – – – – |
1212 (233) – – – – – – |
| Total inpatient cost PPY, US $ (SE) | 11,891 (3494) | 34,618 (3957) | 3561 (2414) | 7294 (1551) | 1935 (1691) | 4992 (1596) | 0 | 1278 (552) |
| Total cost PPY, US $ (SE) | 151,981 (10 747) | 61,817 (5360) | 7345 (2892) | 10,770 (1692) | 2543 (1939) | 5951 (1662) | 995 (451) | 2489 (623) |
Outpatient medical costs may not sum to the total owing to rounding.
AATD: Alpha-1 antitrypsin deficiency; SE: Standard error.
Costs associated with liver transplantation
Among liver transplant patients in the AATD with liver disease health state, liver-associated total cost PPY was higher in patients 1-year post-transplantation (US $461,752) than 1-year pre-transplantation (US $87,329), which was driven by high inpatients costs 1-year post-transplantation (US $428,492 vs $35,231). These figures were considerably higher than those observed among patients who did not receive a liver transplant (total cost PPY: US $6845; inpatient cost: US $2780; Figure 4).
Figure 4. . Liver-associated total costs among patients with alpha-1 antitrypsin deficiency and liver disease considering liver transplantation.
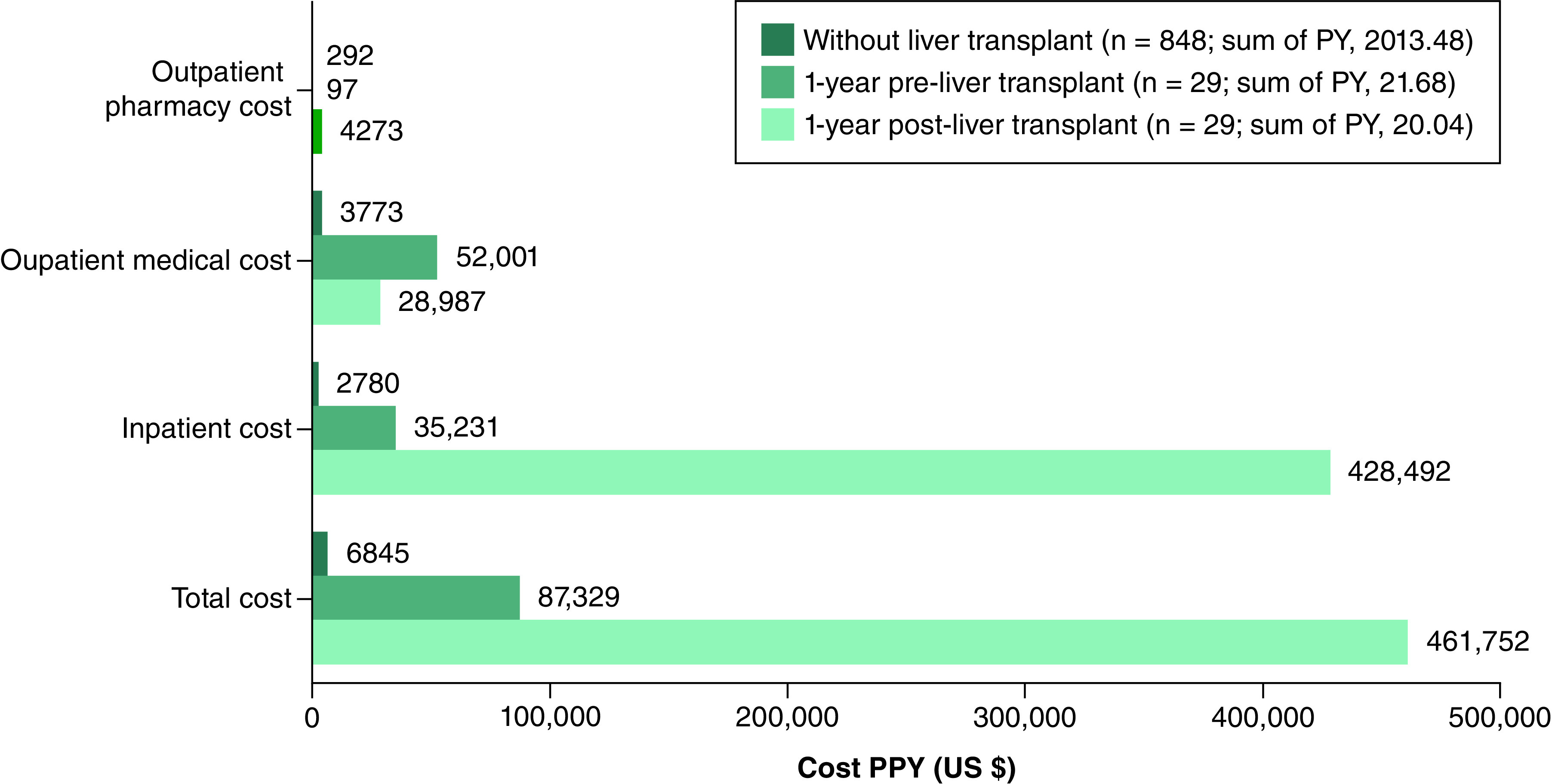
AATD: Alpha-1 antitrypsin deficiency; PPY: Per person-year.
Discussion
To our knowledge, the estimated incremental costs of AATD, liver disease, lung disease or both liver and lung disease have not been published previously. In our study, HCRU and healthcare costs in patients with AATD were higher in the presence of liver and/or lung disease than in patients with AATD only or AATD prior to liver or lung disease.
Patients in the AATD with liver and lung disease health state had higher all-cause and liver-associated HCRU and healthcare costs than those in the other health states for all parameters examined. Total liver-associated healthcare costs were also higher in the AATD with liver and lung disease health state than in the AATD with liver disease health state, driven by higher inpatient costs. Overall, for patients in the AATD with liver disease and AATD with liver and lung disease health states, liver-associated costs accounted for only a moderate proportion of the all-cause total healthcare costs, which could be related to under-coding for liver disease diagnosis codes. Patients with liver fibrosis or cirrhosis were also not distinguished in this study owing to the lack of available claims data from the IQVIA PharMetrics Plus database and the small sample size of patients with linked laboratory data in the IQVIA AEMR database; patients with cirrhosis and decompensating events may drive higher HCRU and costs than patients with fibrosis who are more likely to be asymptomatic with lower HCRU and costs.
All-cause total costs PPY in the AATD with lung disease and AATD with liver and lung disease health states were higher in patients who received augmentation therapy than in those who did not receive augmentation therapy owing to high all-cause pharmacy costs PPY in patients who received augmentation therapy. In patients with AATD, liver-associated costs were lower in patients who received augmentation therapy than in those who did not receive augmentation therapy because of lower inpatient costs in patients who received augmentation therapy. Notably, the sample size of patients who received augmentation therapy was smaller than that of patients who did not receive augmentation therapy (n = 76 vs n = 490), and therefore larger studies are required to confirm these findings.
In the AATD with liver disease health state, patients undergoing liver transplantation had considerably higher inpatient and total healthcare costs 1-year post-transplantation than patients who did not undergo liver transplantation. These data are expected considering the substantial inpatient costs associated with liver transplantation, although they are based on a relatively small sample (n = 29 with liver transplant [sum of person-years, 20]; n = 848 without liver transplant [sum of person-years, 2013]). Further research on the long-term liver-associated costs among patients with AATD after liver transplantation is warranted.
Strengths and limitations
Strengths of this study included the characterization of 5136 patients, which represents a large sample of patients with AATD. In addition, a person–time approach was taken to maximize the use of patient data and to standardize reporting of outcomes for each health state given the variable time in each health state. The use of administrative claims data to assess periods with continuous enrollment allowed comprehensive capture of HCRU and costs submitted through the plan benefit. To the best of our knowledge, this study is the first to evaluate HCRU and costs across the entire disease history in patients with AATD, covering a period prior to and after liver and/or lung disease manifestations.
Limitations of this study included that these findings are from patients with commercially managed health insurance and may not be representative of uninsured populations and those with government-sponsored health insurance, or generalizable to the wider US population. The overall burden may also be underestimated, owing to under-representation of older (at least 65 years of age) patients in the IQVIA PharMetrics Plus database, who are more likely than younger patients to have AATD with liver disease and/or lung disease. Although a person–time approach was taken to standardize the reporting of outcomes, patients were still followed for variable amounts of time which could affect their respective healthcare costs and relate to unknown factors, inherent differences or biases. In addition, costs associated with liver disease can be challenging to capture and the identification of augmentation therapy among patients with lung disease could be underestimated if patients went through manufacturer assistance programs, rather than a typical plan benefit process through their insurance, which may have led to an underestimation of healthcare costs. Furthermore, as previously discussed, patients with liver fibrosis or cirrhosis were not distinguished in this study. It was also not possible to identify the fibrosis stage in patients with liver disease using ICD-9-CM or ICD-10-CM diagnosis codes. Last, the analysis of patients with AATD stratified by genotype was not possible because the ICD-9-CM and ICD-10-CM codes are not used to identify patients with AATD by Pi genotype; if documented, genotypes were only present in the IQVIA AEMR database and required linkage to the IQVIA PharMetrics Plus database. Among the overall cohort, most patients had an unknown genotype (unknown, 98.8%; Pi*ZZ, 0.1%; Pi*MZ, 1.1%), which restricted further analysis by genotype and subsequent interpretation of these data. Given the small sample size, the HCRU and costs associated with patients with AATD and a Pi*ZZ or Pi*MZ genotype are yet to be described.
Conclusion
In this study, patients with AATD had increased HCRU and all-cause costs in the presence of liver and/or lung disease. Both HCRU and healthcare costs were highest in the AATD with liver and lung disease health state. Among patients who received a liver transplant, inpatient and total healthcare costs were substantially higher 1 year after transplantation than among patients who did not undergo liver transplantation. All-cause total cost PPY was higher in patients who received augmentation therapy than in those who did not, largely owing to all-cause pharmacy costs. Limited genotype information in the current study restricted interpretation of HCRU and healthcare costs by genotype. To facilitate future studies, improved documentation of genotype and development of specific diagnosis codes for the identification of patients with AATD with lung and/or liver disease manifestations is required. Therefore, further research on the disease burden among AATD patients with a Pi*ZZ genotype is warranted.
Summary points
Alpha-1 antitrypsin deficiency (AATD) is an autosomal co-dominant genetic disease that is characterized by low levels of serum alpha-1 antitrypsin (AAT), leading to lung disease, and accumulation of misfolded AAT in hepatocytes, which can result in liver disease.
Economic data on AATD-associated liver disease are sparse, with the disease contributing to the costs of hospitalization related to metabolic dysfunction-associated genetic liver disease, and the considerable costs associated with transplantation with life-long immunosuppression.
This was a non-interventional, retrospective, observational analysis of linked administrative claims data and electronic medical records from the IQVIA PharMetrics Plus and IQVIA Ambulatory Electronic Medical Records databases that aimed to evaluate all-cause and liver-associated healthcare resource utilization (HCRU) and healthcare costs among patients with AATD with liver and/or lung disease.
Patient follow-up time was assigned to the following health states: AATD only (n = 1804), AATD prior to liver or lung disease (n = 3291), AATD with liver disease (n = 877), AATD with lung disease (n = 2343), and AATD with liver and lung disease (n = 566).
Patients with AATD had increased HCRU and healthcare costs per person-year (PPY) in the presence of liver and/or lung disease.
Both HCRU and healthcare costs PPY were highest in the AATD with liver and lung disease health state.
In the AATD with lung disease and AATD with liver and lung disease health states, patients who received augmentation therapy had higher all-cause total costs PPY than patients who did not receive augmentation therapy.
In the AATD with liver and lung disease health state, patients who received augmentation therapy had lower liver-associated total costs PPY than patients who did not receive augmentation therapy.
Among patients in the AATD with liver disease health state, inpatient and total healthcare costs were substantially higher 1 year after liver transplantation (US $428,492 and US $461,752, respectively) than in patients who did not undergo liver transplantation (US $2780 and US $6845, respectively).
Further research in a population with a confirmed protease inhibitor (Pi) genotype and focused on fibrosis and cirrhosis is warranted to investigate the impact of Pi genotype (Pi*ZZ or Pi*MZ) on disease burden.
Footnotes
Author contributions
M Hagiwara, M Delegge, EG Marins and C Strange were responsible for conceptualization of the study. M Hagiwara was responsible for study investigation and validation. M Hagiwara, V Divino, S Munnangi, M Delegge, S Park, K Ren and C Strange were responsible for study methodology. M Hagiwara, V Divino, S Munnangi and S Park were responsible for project administration. M Hagiwara and K Ren were responsible for study supervision. M Hagiwara, S Park, EG Marins and K Ren were responsible for study visualization. M Hagiwara was responsible for original draft preparation and all authors were responsible for review and editing.
Financial disclosure
This study was funded by Takeda Development Center Americas, Inc. Medical writing support was provided by E Sugrue of Oxford PharmaGenesis, Oxford, UK and funded by Takeda Development Center Americas, Inc. M Hagiwara, S Park, E Marins and K Ren are employees and stockholders of Takeda Development Center Americas, Inc. V Divino, S Munnangi and M Delegge are employees of IQVIA, which received funding for this study from Takeda Development Center Americas, Inc. C Strange is an employee of AlphaNet, has grants paid to the Medical University of South Carolina from Adverum, Arrowhead Pharmaceuticals, AstraZeneca, CSA Medical, Grifols, National Institutes of Health, Nuvaira, Takeda and Vertex Pharmaceuticals, and has consulted for Bronchus, CSL Behring, Dicerna Pharmaceuticals, GlaxoSmithKline, PulManage and Vertex Pharmaceuticals for alpha-1 and/or chronic obstructive pulmonary disease. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interest disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
Medical writing support was provided by E Sugrue of Oxford PharmaGenesis (Oxford, UK) and funded by Takeda Development Center Americas, Inc.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Jezela-Stanek A, Chorostowska-Wynimko J. SERPINA1 and more? A putative genetic contributor to pulmonary dysfunction in alpha-1 antitrypsin deficiency. J. Clin. Med. 12(5), 1708–1720 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strnad P, McElvaney N, Lomas D. Alpha1-antitrypsin deficiency. N. Engl. J. Med. 382(15), 1443–1455 (2020). [DOI] [PubMed] [Google Scholar]; •• This article reviews the pathophysiology and clinical features of alpha-1 antitrypsin deficiency (AATD)-associated liver and lung disease.
- 3.de Serres FJ, Blanco I. Prevalence of alpha1-antitrypsin deficiency alleles PI*S and PI*Z worldwide and effective screening for each of the five phenotypic classes PI*MS, PI*MZ, PI*SS, PI*SZ, and PI*ZZ: a comprehensive review. Ther. Adv. Respir. Dis. 6(5), 277–295 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Abboud R, Nelson T, Jung B, Mattman A. Alpha1-antitrypsin deficiency: a clinical-genetic overview. Appl. Clin. Genet. 4, 55–65 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This review article outlines clinical features of AATD, AAT augmentation therapy and the biochemical/molecular genetic components of AATD.
- 5.Schneider CV, Hamesch K, Gross A et al. Liver phenotypes of European adults heterozygous or homozygous for Pi*Z variant of AAT (Pi*MZ vs Pi*ZZ genotype) and noncarriers. Gastroenterology 159(2), 534–548.e11 (2020). [DOI] [PubMed] [Google Scholar]; • This article reports an analysis of the European Alpha-1 Liver Cohort, which found that adults with a Pi*MZ genotype had lower levels of serum transaminases, fewer AAT inclusions in the liver and lower liver stiffness than adults with the Pi*ZZ genotype.
- 6.Patel D, Teckman J. Liver disease with unknown etiology – have you ruled out alpha-1 antitrypsin deficiency? Ther. Adv. Chronic Dis. 12(Suppl.), 2040622321995684 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This review article discusses clinical manifestations, genetics and therapies for AATD-associated liver disease.
- 7.Beiko T, Strange C. Anxiety and depression in patients with alpha-1 antitrypsin deficiency: current insights and impact on quality of life. Ther. Clin. Risk Manag. 15, 959–964 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This article discusses the burden of anxiety and depression in patients with AATD.
- 8.Sieloff EM, Rutledge B, Huffman C, Vos D, Melgar T. National trends and outcomes of genetically inherited non-alcoholic chronic liver disease in the USA: estimates from the National Inpatient Sample (NIS) database. Gastroenterol. Rep. (Oxf.) 9(1), 38–48 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arya G, Balistreri W. Pediatric liver disease in the United States: epidemiology and impact. J. Gastroenterol. Hepatol. 17(5), 521–525 (2002). [DOI] [PubMed] [Google Scholar]; • This review article describes the burden of liver disease in pediatric patients and discusses approaches for intervention.
- 10.Udompap P, Kim D, Kim W. Current and future burden of chronic nonmalignant liver disease. Clin. Gastroenterol. Hepatol. 13(12), 2031–2041 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


