Abstract
Expression of either Epstein-Barr virus (EBV) immediate-early protein BZLF1 (Z) or BRLF1 (R) is sufficient to convert EBV infection from the latent to lytic form. Disruption of viral latency requires transcriptional activation of the Z and R promoters. The Z and R proteins are transcriptional activators, and each immediate-early protein activates expression of the other immediate-early protein. Z activates the R promoter through a direct binding mechanism. However, R does not bind directly to the Z promoter. In this study, we demonstrate that the ZII element (a cyclic AMP response element site) in the Z promoter is required for efficient activation by R. The ZII element has been shown to be important for induction of lytic EBV infection by tetradecanoyl phorbol acetate and surface immunoglobulin cross-linking and is activated by Z through an indirect mechanism. We demonstrate that both R and Z activate the cellular stress mitogen-activated protein (MAP) kinases, p38 and JNK, resulting in phosphorylation (and activation) of the cellular transcription factor ATF2. Furthermore, we show that the ability of R to induce lytic EBV infection in latently infected cells is significantly reduced by inhibition of either the p38 kinase or JNK pathways. In contrast, inhibition of stress MAP kinase pathways does not impair the ability of Z expression vectors to disrupt viral latency, presumably because expression of Z under the control of a strong heterologous promoter bypasses the need to activate Z transcription. Thus, both R and Z can activate the Z promoter indirectly by inducing ATF2 phosphorylation, and this activity appears to be important for R-induced disruption of viral latency.
Epstein-Barr virus (EBV) is a member of the human herpesvirus family of viruses and is the causative agent of infectious mononucleosis (61). EBV has also been found in association with a variety of cancers, including Burkitt's lymphoma and nasopharyngeal carcinoma (61, 82). Upon primary infection, EBV infects epithelial cells, where it undergoes lytic replication, and B cells, where it usually remains latent (37, 45, 61, 67). However, in a small percentage of B cells, latent EBV can become reactivated and undergo lytic replication. This viral reactivation is initiated by the two EBV immediate-early proteins, BZLF1 and BRLF1 (5, 8, 36, 57, 62, 63, 69, 77, 80).
The BZLF1 (Z) and BRLF1 (R) proteins function as transcriptional activators of the EBV early genes (9, 20, 28–30, 35, 46, 56, 74), and the expression of either Z or R is sufficient to induce lytic replication in both latently infected epithelial cells and B cells (5, 8, 57, 69, 77, 80). Regardless of which immediate-early gene is initially transcribed, expression of one immediate-early protein leads to the expression of the other (80), and full activation of early genes requires the presence of both immediate-early proteins (1, 80). Z activates the R promoter by directly binding to Z-response elements (ZREs) (1, 66). However, the mechanism by which R activates Z expression remains unknown. Although the R protein binds directly to a GC-rich motif present in enhancer elements upstream of several EBV early promoters (24, 25, 56), there are no known R binding sites within the Z promoter (25). Thus, R could potentially activate the Z promoter through an indirect mechanism involving modulation of a cellular transcription factor.
The ZII element in the Z promoter functions as an important positive regulator of Z transcription (16). ZII is required for both efficient tetradecanoyl phorbol acetate induction of Z transcription (16) and activation induced by surface immunoglobulin (Ig) cross-linking (10). In addition, human herpesvirus 6 (HHV-6) infection has been shown to activate the Z promoter through this site (14). Mutant forms of Z unable to bind DNA have also been shown to activate transcription dependent upon the ZII element (15), although the exact mechanism for this effect remains unknown. Previous reports, as well as data from our own laboratory, have shown that the cellular factors binding to the ZII element include CREB, ATF1, ATF2, and c-Jun (49, 76). Of these proteins, CREB and ATF1 have been reported to activate the Z promoter in reporter gene assays (49, 76).
CREB, ATF1, and ATF2 are all members of the CREB/ATF family of transcription factors that bind directly to DNA via cyclic AMP-response elements (CREs), and each of these proteins requires phosphorylation in order to function efficiently as transcriptional activators (22, 26, 51, 58, 60, 75). Activation of the CREB and ATF1 proteins occurs primarily through phosphorylation by protein kinase A (PKA) (22, 60), whereas ATF2 is activated after phosphorylation by either the p38 or c-Jun–N-terminal (JNK) stress kinases (26, 58, 75). JNK-mediated phosphorylation is also required for activation of the c-Jun transcription factor (11, 42), which can heterodimerize with ATF2 (27). Thus, activation of Z transcription through the ZII element could potentially be mediated through phosphorylation of the CRE family and/or c-Jun transcription factors.
Here we have studied the effects of R and Z expression upon the cellular transcription factors binding to the ZII motif. We demonstrate that although neither R nor Z expression significantly affects the levels of CREB, ATF1, ATF2, or c-Jun binding to ZII, R and Z both induce phosphorylation of p38 kinase as well as JNK. Furthermore, we find that the ability of R to disrupt viral latency efficiently requires activation of the stress mitogen-activated protein (MAP) kinase cascade. These results suggest that a key initial step in the disruption of viral latency by R is the activation of JNK and p38 kinase, thereby allowing R to induce Z transcription through phosphorylation of the ATF2 and c-Jun transcription factors.
MATERIALS AND METHODS
Cell lines.
DG75 is an EBV-negative Burkitt's lymphoma cell line. Akata (68) and Raji are EBV-positive Burkitt's lymphoma cell lines. B95-8 is an EBV-positive marmoset B-cell line. HeLa is a cervical carcinoma cell line. NIH 3T3 is a mouse fibroblast cell line. NPC-KT is an EBV-positive epithelial cell line derived from the fusion of a human adenoidal epithelial cell line and a primary EBV-positive nasopharyngeal carcinoma (70). D98/HE-R-1 is an epithelial cell line formed by fusion of a HeLa cell subclone (D98) with the EBV-positive Burkitt's lymphoma cell line P3HR/1 (21). All lymphoid cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum. Epithelial cell lines were maintained in Dulbecco's modified Eagle's medium H supplemented with 10% fetal calf serum.
Adenovirus construction and infection.
The BZLF1 and BRLF1 cDNAs were cloned into a shuttle vector (under the control of the cytomegalovirus [CMV] promoter) which contains a Lox P site, the left adenovirus terminal repeat, and a packaging signal. These vectors were recombined (in a cell line expressing the phage P1 Cre protein) into the Lox P site of an adenovirus lacking the E1 and E3 genes, to create adenovirus-Z and adenovirus-R, as previously described (77). A control vector (adenovirus-LacZ) containing the lacZ gene was made in the same manner.
HeLa cells were plated at a cell density of 3 × 106 cells per 140 by 20 mm plate. Cells were infected with no adenovirus (mock), adenovirus-LacZ, adenovirus-Z, or adenovirus-R at a multiplicity of infection of 50. The cells were harvested 24 to 48 h postinfection.
Plasmids.
EApBS-CAT contains the early EBV BMRF1 promoter sequences from −331 to +1 linked to the chloramphenicol acetyltransferase (CAT) gene (56). Gal4-E1b-CAT (gift of Michael Green) contains five copies of the Gal4 DNA binding site upstream of the E1B minimal TATA element and the CAT gene (47). ZpBS-CAT contains the immediate-early EBV BZLF1 promoter sequences from −552 to +12 linked to the CAT gene (1). −221 Zp-CAT contains the immediate-early EBV BZLF1 promoter sequences from −221 to +12 linked to the CAT gene, while −221 ZpZIIm-CAT contains a mutated ZII element (AAACGAATTCATCACAGAG) (16) (gifts of Erik Flemington).
The R expression vector (RTS15) contains the R genomic sequence under the control of the simian virus 40 early promoter (a gift from Diane Hayward). The wild-type Z expression vector contains the BZLF1 cDNA, downstream of the CMV immediate-early promoter, in the pHD1013 vector (56). The ZΔLZ vector has a deletion of the Z leucine zipper from amino acid 196 to amino acid 228. The Z311 vector contains a mutation in the DNA binding domain of Z in which amino acid 185 is altered (abolishing DNA binding) (20, 34). Z-CT (previously referred to as RAZΔR), has a deletion of Z amino acids 2 to 86 (a gift of Joseph Pagano) (18). The SG424 vector contains the GAL4 DNA binding domain alone (amino acids 1 to 147), whereas the Gal4-CREB, GAL4-ATF1, and GAL4-ATF2 constructs contain the indicated cDNAs linked in frame to GAL4 (gifts of Michael Green) (39, 64). GAL4–c-Jun contains the c-Jun cDNA fused to the Gal4 DNA binding domain in the SG424 vector (a gift of Al Baldwin). RSV (Rous sarcoma virus)-ATF2 contains the ATF2 cDNA in the RSV-pECE vector, and RSV-ATF1 contains the ATF1 cDNA in the RSV-pECE vector (gifts of Michael Green) (48). RSV-CREB contains the CREB cDNA (gift of Michael Green) (22). CMV-p38 contains the human p38 cDNA (a gift of Roger Davis) in the pcDNA3 vector. CMV-JNK contains the JNK cDNA in the pcDNA3B vector, and CMV-cdc42 contains the cdc42QL cDNA in the pcDNA3B vector (gifts from Lynn Vitale Cross). The PKA expression vector contains the catalytic subunit of PKA (mouse), under the mouse metallothionein 1 promoter (gift from Stanley McKnight) (73). Dominant-negative MKK3 contains a mutation at amino acid 193 of threonine to alanine, under the RSV promoter, in the pRc/RSV vector, and dominant-negative MKK6 contains a mutation at amino acid 82 of lysine to alanine, under the CMV promoter, in the pcDNA3 vector (59). GST (glutathione S-transferase)–c-Jun contains the first 79 amino acids of c-Jun linked to GST (a gift from Shelley Earp). The JIP-1 expression vector (12) is a gift from Roger Davis.
DNA purification.
Plasmid DNA was purified through QIAGEN columns as described by the manufacturer.
DNA transfection.
DNA (5 to 10 μg) was transfected into cells by electroporation with a Zapper electroporation unit (Medical Electronics Shop, University of Wisconsin) at 1,500 V as described previously (71). All cells were resuspended in RPMI 1640 medium prior to electroporation.
NIH 3T3 cells were transfected with Lipofectamine (Gibco BRL) as per the manufacturer's instructions.
CAT assays.
Cell extracts were prepared 48 h posttransfection and incubated at 37°C with [14C]chloramphenicol in the presence of acetyl coenzyme A as described previously (23). The percent acetylation of chloramphenicol was quantitated by thin-layer chromatography followed by PhosphorImager screening (Molecular Dynamics).
Immunoblot analysis.
Immunoblot analysis was performed for the detection of total ATF-2, CREB, p38, MKK3, MKK6, BMRF1, Z, and R proteins as follows. Briefly, 10 to 100 μg of protein was loaded in each lane, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed. The proteins were transferred overnight onto nitrocellulose (Protran), blocked in 1× phosphate-buffered saline (PBS)–5% milk–0.1% Tween 20, and incubated in primary antibody for 1 h at room temperature (ATF2 and CREB [1:500] from Santa Cruz, p38 [1:1,000] from New England Biolabs, MKK3 and MKK6 [1:1,000] from New England Biolabs, BMRF1 [1:100] from Capricorn, anti-EBV ZEBRA [1:100] from Argene or Z125 [1:50], from Alain Sergeant, and R [1:100] from Argene). The membrane was washed in PBS–0.1% Tween 20, incubated in secondary antibody for 1 h at room temperature {goat anti-mouse-kappa conjugated with horseradish peroxidase (GAM-kappa–HRP [1:2,000]) from Southern Biotechnology, goat anti-rabbit–HRP [1:10,000] from Promega} and washed, and the results were visualized with the ECL enhanced chemiluminescence kit (Amersham) according to the manufacturer's instructions.
The detection of phosphorylated ATF2, CREB/ATF1, p38, MKK3, and MKK6 proteins differed as follows: membranes were blocked in 1× Tris-buffered saline (TBS)–0.1% Tween 20–5% milk and washed with TBST (1× TBS–0.1% Tween 20). The primary antibody was diluted in 1× TBS–0.1% Tween 20–5% bovine serum albumin (BSA) and was incubated overnight at 4°C (phospho-ATF2, phospho-CREB/ATF1, phospho-MKK3, phospho-MKK6, and phospho-p38 [1:1,000] from New England Biolabs). The secondary antibody was diluted in blocking solution and was incubated for 1 h at room temperature.
Protein preparation.
B95-8 cells were washed twice with PBS, resuspended in 200 μl of extraction buffer (50 mM HEPES [pH 7], 250 mM NaCl, 0.1% NP-40, 5 mM EDTA, 5 mM dithiothreitol [DTT], protease inhibitors), freeze-thawed twice, and centrifuged. The supernatant was used for electrophoretic mobility shift assays (EMSAs).
Adenovirus-infected HeLa cells were resuspended in low-salt buffer (150 mM NaCl, 20 mM Tris [pH 7.5], 5 mM EDTA, 1% Triton X-100, 50 mM NaF, 10% glycerol, 1 mM NaVO4, protease inhibitors). Cell lysates were tumbled for 15 min at 4°C and centrifuged. The supernatant was used for Western blots.
Transfected or adenovirus-infected NPC-KT cells or D98/HE-R-1 cells were resuspended in 50 μl of buffer (0.25 M NaCl, 0.1% NP-40, 0.05 M HEPES [pH 7], 5 mM EDTA, protease inhibitors), freeze-thawed twice, and centrifuged. The supernatant was used for Western blots.
Cells treated with the p38 kinase inhibitor SB 202190 (Calbiochem) were incubated in medium containing a final concentration of 10 μM inhibitor for 24 h.
GST–c-Jun was induced, and cells were sonicated and centrifuged. The supernatant was incubated with glutathione-agarose beads for 30 min at room temperature, and the beads were washed three times with PBS.
Kinase assays.
HeLa cells were infected with adenovirus-LacZ, adenovirus-Z, or adenovirus-R for 24 h, washed twice with PBS, and lysed in 0.9 ml of low-salt buffer (150 mM NaCl, 20 mM Tris [pH 7.5], 5 mM EDTA, 1% Triton X-100, 50 mM NaF, 10% glycerol, 1 mM NaVO4, protease inhibitors). Cell lysates were tumbled for 15 min at 4°C and centrifuged. One hundred fifty micrograms of protein was incubated with GST–c-Jun–beads, tumbled for 2 h at 4°C, and centrifuged. The beads were washed three times with low-salt buffer and then washed one time with kinase buffer (7× buffer = 140 mM HEPES [pH 7.5], 70 mM MgCl2, 350 mM NaCl, 7 mM DTT, 35 mM B-glycerophosphate, 10.5 mM EGTA). The beads were incubated in 35 μl of reaction mix (per reaction, 5 μl of 7× kinase buffer, 10 mM ATP, 1 μl of [γ-32P]ATP, distilled water to 35 μl) for 10 min at 30°C and centrifuged, and the beads were loaded onto an SDS-PAGE (12% polyacrylamide) gel. After electrophoresis, the gel was dried and exposed to X-ray film.
EMSAs.
EMSAs were performed as previously described (19). The synthetic double-stranded oligonucleotide used in the binding reactions was 5′-end labeled with 32P by using the Klenow reaction. The ZII double-stranded oligonucleotide consists of the single-stranded oligonucleotides 5′ AGCTTCCATGACATCACAGAGGAGGCTGGGATC 3′ and 5′ GATCCCAGCCTCCTCTGTGATGTCATGGAAGCT 3′. The binding reactions were conducted in a buffer consisting of 60 mM potassium chloride, 12 mM HEPES (pH 7.9), 4 mM Tris-HCl (pH 7.9), 10% glycerol, 1 mM EDTA, and 1 mM DTT, with 4 μg of poly(dI-dC)–poly(dI-dC) (Pharmacia). Twenty micrograms of B95-8 cell extract was added, and the mixture was incubated at room temperature for 10 min prior to the addition of labeled probe (20,000 cpm). For supershifts, the protein extract was incubated with 3 μg of each antibody (ATF1, ATF2, CREB, or c-Jun from Santa Cruz) for 45 min prior to the binding reaction. The sequences of the competitors are as follows: SP1, 5′-ATTCGATCGGGGCGGGGCGAGC-3′ and 3′-TAAGCTAGCCCCGCCCCGCTCG-5′; and CREB, 5′-AGAGATTGCCTGACGTCAGAGAGCTAG-3′ and 3′-TCTCTAACGGACTGCAGTCTCTCGATC-5′. Each competitor was used at 200-fold excess over the probe. After addition of the probe, the reaction mixtures were incubated for 30 min at room temperature and then loaded onto a 4% polyacrylamide gel and run in 1× Tris-glycine-EDTA (TGE) at room temperature.
FACS analysis.
Anti-IgG-treated Akata cells (100 μg of antihuman IgG per ml [Sigma] for 4 h), in the absence or presence of 10 μM p38 inhibitor SB 202190 (Calbiochem), were washed twice with PBS, fixed with 60% acetone for 10 min on ice, washed three times with PBS–0.5% BSA and incubated with a 1:100 dilution of the primary antibody (BMRF1 [Capricorn], Z [Argene], or R [Argene]) for 1 h at room temperature. The cells were washed three times and then incubated in the secondary antibody (GAM fluorescein isothiocyanate [1:100], from Sigma) for 1 h at room temperature. The cells were washed three times and resuspended in 0.5 ml of PBS. The percentage of immunofluorescent cells was determined with a FACS machine (Becton Dickinson).
RESULTS
The ZII element is an important component for R-mediated activation of the Z promoter.
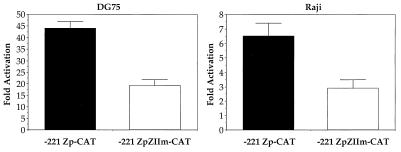
Although the R protein has been previously shown to activate the Z promoter (80), there are no known R-binding sites in the Z promoter. The Z promoter contains an upstream regulatory element, referred to as ZII, that has been previously shown to be an important positive regulator of Z transcription (10, 14, 16, 65). To examine whether the ZII element plays a role in R activation of Z expression, we examined the effect of R on Z promoter activity by using Z promoter constructs that contain either a wild-type (−221 Zp-CAT) or mutant (−221 ZpZIIm-CAT) ZII element, in DG75 (EBV-negative) and Raji (EBV-positive) cell lines (Fig. 1). In both cell lines, R activation of the wild-type promoter was significantly higher than activation of the promoter containing a mutant ZII site. Therefore the ZII element plays a major role in R activation of the Z promoter.
FIG. 1.
R activates the Z promoter through the ZII element. DG75 or Raji cells were transfected with 5 μg of promoter construct (−221 Zp-CAT or −221 ZpZIIm-CAT) and 1 μg of vector control DNA or R expression plasmid. CAT assays were performed. The fold activation, relative to vector alone, is shown. Error bars indicate range.
The ZII element of the Z promoter binds ATF1, ATF2, CREB, and c-Jun.
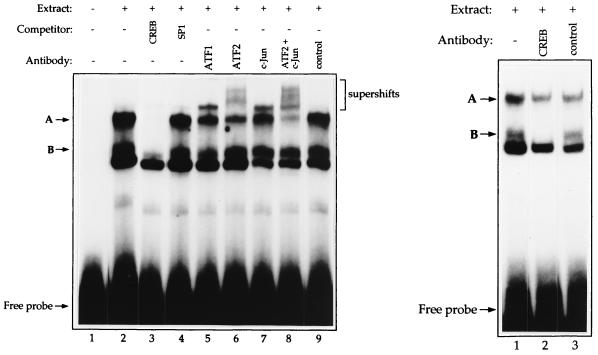
ZII was originally identified as an AP1 site (16), but has since been shown to be a CRE site (4, 49, 76). We performed EMSAs with an oligonucleotide probe spanning the ZII element and B95-8 cell extracts. As shown in Fig. 2 (left panel), a CRE competitor oligonucleotide competed the top two binding complexes (labeled A and B), while a control competitor oligonucleotide (Sp1) did not. An anti-ATF1 antibody supershifted protein, although it is difficult to determine which complex is being supershifted. Anti-ATF2 and anti-c-Jun antibodies both supershifted proteins from the upper CRE complex (left panel), while an anti-CREB antibody blocked formation of the lower CRE complex (right panel). These results suggest that the A complex contains predominantly ATF2 and c-Jun, while the B complex contains predominantly CREB (and possibly ATF1). The finding that ATF1, ATF2, CREB, and c-Jun bind to the ZII element has recently been obtained by two other laboratories (14, 49, 76).
FIG. 2.
ATF1, ATF2, CREB, and c-Jun bind to the ZII element of the Z promoter. EMSAs were performed with a 32P-labeled ZII probe and B95-8 protein extracts. (Left panel) Twenty micrograms of protein extract was incubated in the absence (lane 2) or presence of a CRE consensus DNA competitor (lane 3) or an Sp1 consensus DNA competitor (lane 4); in the presence of antibodies directed against ATF1 (lane 5), ATF2 (lane 6), c-Jun (lane 7), or ATF2 plus c-Jun (lane 8); or a control antibody (lane 9). (Right panel) Twenty micrograms of protein extract was incubated in the absence (lane 1) or presence of antibodies directed against CREB (lane 2) or a control antibody (lane 3). A and B indicate the two pertinent complexes. The antibodies alone did not induce supershifts in the absence of cellular extract (data not shown).
CREB, ATF1, ATF2, and c-Jun activate the Z promoter.
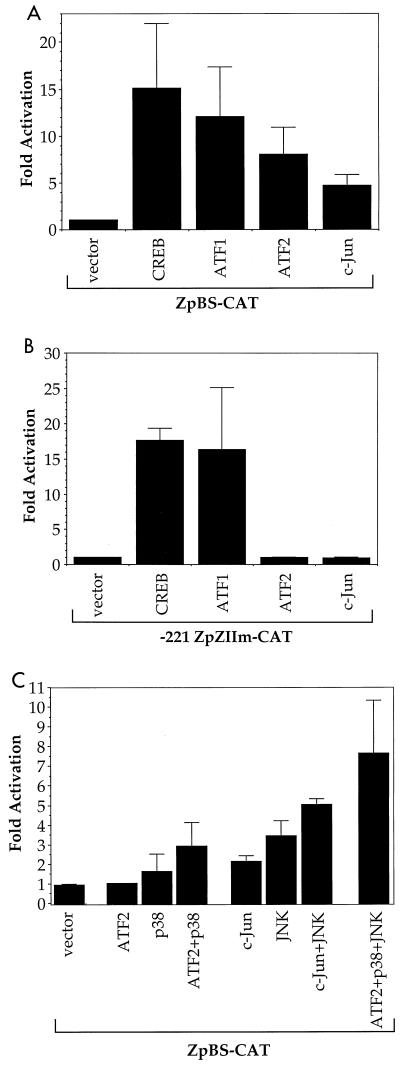
The ZII regulatory element is a positive regulator of the Z promoter, suggesting that factors which bind to ZII may activate the Z promoter. To examine whether CREB, ATF1, ATF2, or c-Jun is able to activate Zp, we cotransfected a Z promoter construct (linked to the CAT gene) with expression vectors for either CREB, ATF1, ATF2, or c-Jun, plus the kinases known to activate each of these transcription factors (Fig. 3A). CREB, ATF1, ATF2, and c-Jun each activate the intact Z promoter in the presence of the appropriate kinases. Similar results were previously obtained for the ATF1/CREB factors (14, 76); this is the first demonstration that phosphorylated ATF2 also activates the Z promoter. While the ATF2 and c-Jun transcription factors require the ZII motif for activation of the Z promoter, CREB and ATF1 both retained the ability to activate the mutant Z promoter construct. Therefore, CREB and ATF1 may also activate the Z promoter through motifs other than ZII. To confirm that efficient ATF2 and c-Jun activation of the Z promoter requires p38 kinase and/or JNK, we compared the effect of ATF2 and c-Jun with or without p38 kinase or JNK (Fig. 3C). As expected, maximal activation of the Z promoter required cotransfection with both a transactivator (ATF2 or c-Jun) and a kinase. JNK and p38 kinase alone, however, also induced some activation of the Z promoter, likely reflecting the ability of these kinases to activate endogenous ATF2 and c-Jun.
FIG. 3.
CREB, ATF1, ATF2, and c-Jun activate the Z promoter. (A) DG75 cells were transfected with 5 μg of ZpBS-CAT and 1 μg of transactivator plasmid (vector alone, CREB, ATF1, ATF2, or c-Jun), along with 1 μg of the appropriate kinase expression vectors to activate each transactivator (PKA for CREB and ATF1; p38, JNK, and cdc42 for ATF2; and JNK and cdc42 for c-Jun). (B) DG75 cells were transfected with 5 μg of −221 ZpZIIm-CAT and 1 μg of transactivator plasmid (vector alone, CREB, ATF1, ATF2, or c-Jun), along with 1 μg of the appropriate kinase expression vectors to activate each transactivator (PKA for CREB and ATF1; p38, JNK, and cdc42 for ATF2; and JNK and cdc42 for c-Jun). (C) DG75 cells were transfected with 5 μg of ZpBS-CAT, along with either 1 μg of transactivator plasmid (vector alone, ATF2 or c-Jun), 1 μg of kinase (p38 or JNK and cdc42), or a combination of these plasmids as indicated. CAT assays were performed as described. The average fold activation (and range), relative to vector alone, is presented.
Z and R increase the level of phosphorylated ATF2.
Since CREB, ATF1, ATF2, and c-Jun activate the Z promoter, we examined whether the expression of Z or R affects Z transcription by altering the levels of protein binding to ZII. Electromobility gel shift assays were performed with a ZII probe and extracts of HeLa cells infected with adenovirus-LacZ, adenovirus-Z, or adenovirus-R (data not shown). The expression of either Z or R in the HeLa cells had a minimal effect upon the level of protein binding to ZII. Therefore, neither Z nor R activates the Z promoter by increasing the levels of cellular transactivators binding to the ZII element.
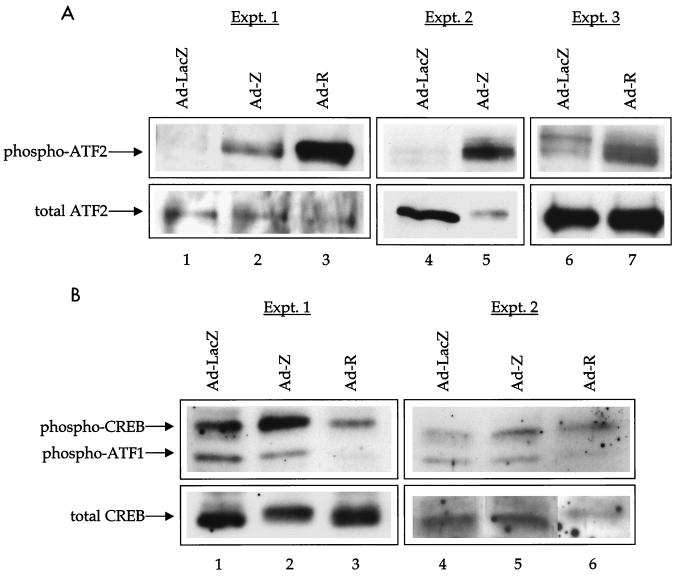
The CREB, ATF1, and ATF2 transcription factors require phosphorylation by PKA (CREB and ATF1), p38 (ATF2), or JNK (c-Jun and ATF2) for efficient transactivation function. Therefore, we examined whether Z or R increases the levels of phosphorylated ATF2, CREB, or ATF1. In extracts of HeLa cells that had been infected with adenovirus-LacZ, adenovirus-Z, or adenovirus-R (Fig. 4A), total ATF2 levels were unchanged after expression of either Z or R. In the same extracts, however, by using an antibody that specifically recognizes only the transcriptionally active (phosphorylated) form of ATF2, both Z and R were found to significantly increase the phosphorylation of ATF2. In contrast, R and Z did not consistently alter the levels of phosphorylated CREB or phosphorylated ATF1 (Fig. 4B).
FIG. 4.
Z and R increase phosphorylation of ATF2. HeLa cells were infected with adenovirus (Ad)-LacZ, adenovirus-Z, or adenovirus-R as indicated. Immunoblot analysis was performed with antibodies directed against total ATF2 and phosphorylated ATF2 (A) or total CREB and phosphorylated CREB/ATF1 (B). Both phosphorylated CREB and phosphorylated ATF1 are recognized by a single antibody. At least two separate experiments are presented for each antibody. Infection of cells with adenovirus-LacZ did not affect ATF2, ATF1, or CREB phosphorylation in comparison to levels in mock-infected cells (data not shown).
Z activates ATF2 and c-Jun transactivator functions in reporter gene assays.
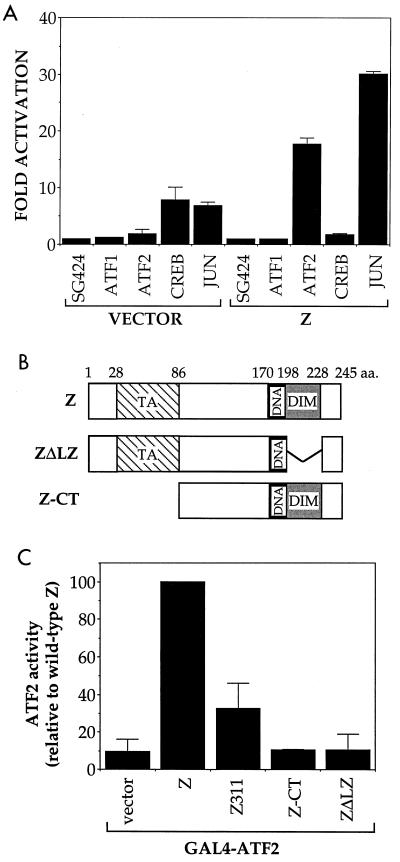
The finding that adenovirus-Z and adenovirus-R infections induce the activity of ATF2 by phosphorylation suggests that Z and R could indirectly activate the ZII site by inducing the transcriptional function of ATF2. We examined the ability of the CREB, ATF1, ATF2, and c-Jun proteins (fused to the yeast GAL4 DNA-binding domain) to activate a reporter construct containing five copies of the GAL4 DNA-binding site upstream of the adenovirus E1B TATA box, in the presence or absence of Z (Fig. 5A). Although Z clearly enhanced the transcriptional activator function of ATF2 and c-Jun, Z did not increase ATF1 transactivator function and decreased CREB transactivator function. Thus, Z primarily activates ATF2 and c-Jun (rather than ATF1 or CREB) transactivation function. These reporter gene assays also confirm that Z-mediated activation of ATF2 is not dependent upon any adenovirus-encoded proteins. We were unable to perform similar reporter gene assays with R, since R activated the GAL4-E1B-CAT reporter construct alone (in the absence of transactivators) to a high level (data not shown).
FIG. 5.
Z activates ATF2 and c-Jun transactivator functions. (A) DG75 cells were transfected with 5 μg of GAL4-E1B-CAT reporter construct and 1 μg of SG424 (GAL4 DNA-binding domain alone), GAL4-ATF1, GAL4-ATF2, GAL4-CREB, or GAL4-c-Jun, with or without the Z expression vector. CAT assays were performed as described in the text. The average fold activation (and range) relative to SG424 alone is presented. (B) Construction of Z deletion mutants. The location of the Z transactivator (TA) and DNA binding (DNA) and dimerization (DIM) domains is indicated. The Z311 construct is mutated at amino acid (a.a.) 185 (alanine to lysine) in the Z DNA binding domain and is defective in DNA binding. (C) DG75 cells were transfected with 5 μg of GAL4-E1B-CAT reporter construct, 1 μg of GAL4-ATF2, and 1 μg of either control vector, Z, Z311, Z-CT, or ZΔLZ expression vectors. The ATF2 activation induced by each Z construct (relative to the activation induced by wild-type Z, set at 100%) is shown.
To examine the domains of Z required for activation of ATF2 transcriptional function, a series of Z mutants (Fig. 5B) were cotransfected with GAL4-ATF2 and the GAL4-E1B-CAT reporter. As shown in Fig. 5C, both the transactivation domain (Z-CT) and the leucine zipper dimerization domain (ZΔLZ) of Z are required to activate ATF2. A mutation within the DNA binding domain of Z (Z311), which renders the protein unable to bind directly to Z-response elements or AP1 motifs, still activates ATF2 to some extent.
Z and R increase the activity of p38 and Jun kinases.
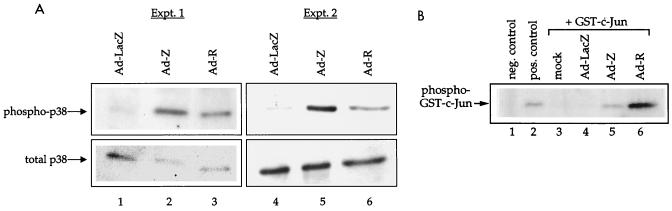
Activation of ATF2 transcription function results from phosphorylation at Ser 69/71 by either p38 kinase or c-Jun–N-terminal kinase (JNK) (26, 58, 75). Since Z and R each increase the level of phosphorylated ATF2, we investigated whether Z and R increase the activities of p38 kinase and/or JNK. Extracts of HeLa cells that had been infected with adenovirus-LacZ, adenovirus-Z, or adenovirus-R were examined for activation of p38 kinase and JNK. p38 kinase activity was examined by immunoblot analysis with an antibody which specifically recognizes the active (phosphorylated) form of p38 kinase, or an antibody which recognizes both inactive and active (total) forms of p38 kinase (Fig. 6A). Although neither Z nor R expression increased the level of total p38 protein, Z and R both increased the level of phosphorylated p38. The level of active JNK was quantitated by comparing the phosphorylation of a GST versus a GST–c-Jun fusion protein (containing the amino-terminal region of c-Jun that is known to be phosphorylated by JNK), as described in Materials and Methods (Fig. 6B). R and Z both significantly increased JNK activity. Taken together, these results indicate that both R and Z activate ATF2 transactivator function by increasing the levels of active forms of p38 kinase and JNK.
FIG. 6.
Z and R increase the levels of activated p38 kinase and JNK. (A) HeLa cells were infected with adenovirus (Ad)-LacZ, adenovirus-Z, or adenovirus-R as indicated. Immunoblot analysis was performed with antibodies directed against either total p38 or phosphorylated p38. The results from two separate experiments are presented. (B) Kinase assays were performed as described in the text with extracts from mock-, adenovirus-LacZ-, adenovirus-Z-, or adenovirus-R-infected HeLa cells. The negative and positive controls are extracts from HEL cells that were either UV irradiated (positive) or unirradiated (negative). Similar results were obtained in a second experiment (data not shown). Infection of cells with adenovirus-LacZ did not affect p38 kinase or JNK phosphorylation in comparison to levels in mock-infected cells (data not shown).
We considered the possibility that Z and R activation of the stress MAP kinases could be due to an irrelevant stress response induced by nonphysiologic overexpression of these proteins. However, by using immunofluorescence (FACS analysis) to quantitate the level of Z and R expression in the adenovirus-infected HeLa cells versus that in TPA-induced B95-8 cells, we found similar levels of cellular Z and R expression (data not shown). Thus, the levels of Z and R produced in HeLa cells infected by the Z and R adenoviruses are comparable to physiological levels of Z and R produced by the endogenous EBV genome.
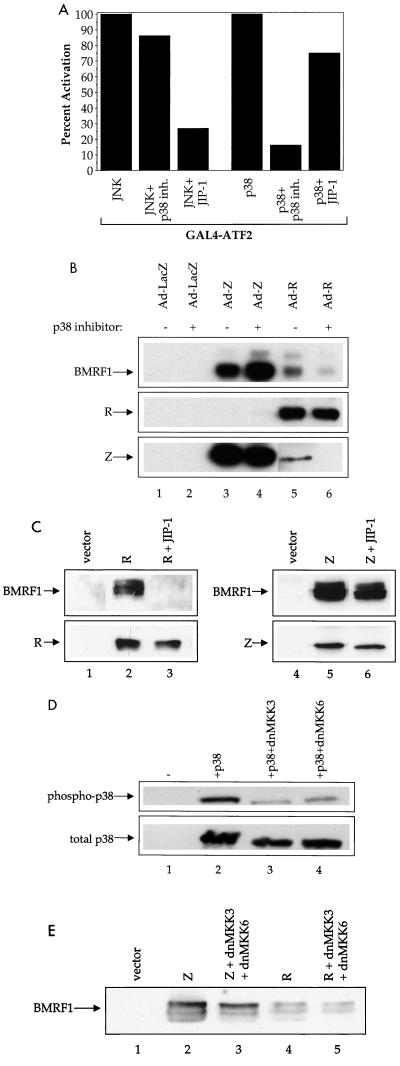
The disruption of EBV viral latency by R is prevented by inhibitors of p38 kinase or JNK.
JNK is activated by phosphorylation via SKK1/SKK4, while p38 kinase is activated (phosphorylated) by either SKK1/SKK4 or MKK3/MKK6 (11, 43, 64a). To determine which signal transduction pathway (p38 versus JNK) is important for the Z and R effects and whether activation of the stress MAP kinases is required for disruption of viral latency, we examined the ability of the R and Z adenovirus vectors to induce expression of an early lytic EBV gene, BMRF1, in NPC-KT (EBV-positive) cells in the absence or presence of the p38 inhibitor SB 202190 or JIP-1 (which inhibits JNK activity) (12). The specificities of these inhibitors are shown in Fig. 7A. SB 202190, which predominantly inhibits p38 kinase activity, significantly reduced the level of BMRF1 produced by adenovirus-R infection, while not affecting the level of BMRF1 produced by adenovirus-Z infection (Fig. 7B). The level of Z produced by adenovirus-R infection was also reduced by the p38 inhibitor, although the level of R in the adenovirus-R-infected cells was not affected. These data suggest that p38 kinase activation is important for the disruption of viral latency by R and, more specifically, for the ability of R to induce Z expression. Similarly, overexpression of the scaffolding JIP-1 protein (which inhibits JNK activity) also prevented the disruption of latency by R, but did not affect the ability of Z to disrupt latency (Fig. 7C).
FIG. 7.
p38 kinase and JNK are necessary for R to disrupt EBV viral latency. (A) DG75 cells were transfected with 5 μg of GAL4-E1B-CAT reporter construct, and 1 μg of SG424 or GAL4-ATF2, along with 1 μg of p38 or JNK expression plasmids, with or without the p38 inhibitor SB 202190 (10 μM) or 2 μg of JIP-1 expression plasmid. CAT assays were performed as described in the text. The ATF2 activation induced (relative to the activation induced by JNK alone or p38 kinase alone, set at 100%) is shown. (B) NPC-KT cells were infected with adenovirus-R or adenovirus-Z in the absence or presence of the p38 kinase inhibitor SB 202190 (10 μM). Immunoblot analysis was performed with antibodies directed against BMRF1, Z or R. Longer exposures indicated that the p38 inhibitor did not affect the level of R protein induced by adenovirus-Z. (C) D98/HE-R-1 cells were transfected with vector alone, R, Z, R plus JIP-1, or Z plus JIP-1 as indicated. Immunoblot analysis was performed with antibodies directed against BMRF1, R, or Z. (D) NIH 3T3 cells were transfected with p38 kinase or a combination of p38 kinase and the dominant-negative MKK3 or MKK6 expression plasmids (dnMKK3 or dnMKK6). Immunoblot analysis was performed with antibodies directed against phosphorylated p38 or total p38. (E) D98/HE-R-1 cells were transfected with vector alone, R, Z, R plus dnMKK3/dnMKK6, or Z plus dnMKK3/dnMKK6 as indicated. Immunoblot analysis was performed with antibodies directed against BMRF1.
To examine the effects of inhibition of upstream activators of p38 kinase, we transfected D98/HE-R-1 (EBV-positive) cells with expression plasmids for Z or R, with or without expression plasmids for dominant-negative forms of MKK3 and MKK6. As expected, both the MKK3 and MKK6 dominant-negatives inhibited phosphorylation of p38 (Fig. 7D). However, inhibition of MKK3 and MKK6 activities had little effect upon induction of BMRF1 expression by Z or R (Fig. 7E). Therefore, R activation of p38 kinase is unlikely to be mediated through MKK3 or MKK6. Taken together, these results suggest that activation of both JNK and p38 kinase is required for R-induced disruption of viral latency.
p38 kinase activation is also necessary for efficient disruption of viral latency by surface Ig cross-linking.
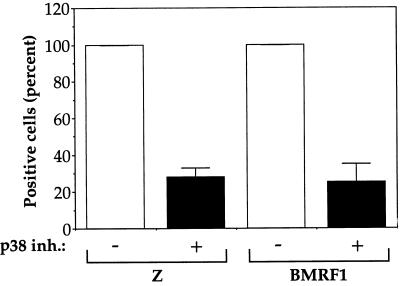
To determine if p38 kinase is important for the disruption of latency induced by surface Ig cross-linking, we induced Akata cells with anti-IgG either in the absence or presence of the p38 kinase inhibitor SB 202190. We examined expression of BZLF1 and BMRF1 by immunofluorescence (FACS analysis) (Fig. 8). In the presence of the p38 kinase inhibitor, the number of cells expressing BZLF1 or BMRF1 after anti-IgG induction was significantly decreased. Therefore, p38 kinase activation is also necessary to efficiently disrupt EBV viral latency from the endogenous viral genome in B cells by surface Ig cross-linking.
FIG. 8.
p38 kinase is important for the disruption of viral latency via anti-IgG cross-linking. Akata cells were treated with 100 μg of anti-IgG per ml for 24 h in the absence or presence of the p38 kinase inhibitor (inh.) SB 202190 (10 μM), and the percentage of cells positive for BZLF1 and BMRF1 expression was determined by BZLF1 and BMRF1 antibody staining and FACS analysis. Results are normalized such that the number of BZLF1- or BMRF1-positive cells for each experiment in the absence of drug is set at 100%. The average (and range) from two separate experiments is shown.
DISCUSSION
The EBV immediate-early proteins Z and R are important regulatory factors which together initiate lytic replication. Disruption of viral latency requires transcriptional activation of the Z and R promoters, and the ability of Z and R to activate one another's transcription appears to be a crucial aspect of this process (1, 80). Z activates the R promoter by a direct binding mechanism (1, 66). Our results here indicate that R activates Z transcription indirectly, by activating p38 kinase and JNK, thereby inducing phosphorylation of transcription factors (ATF2 and c-Jun) which bind to the ZII element of the Z promoter. Interestingly, members of the MEF2 transcription factor family, which bind to and activate the Z promoter through ZI motifs (3, 50), have also recently been shown to be phosphorylated (and at least in some cases activated) by p38 kinase (54, 78). Therefore, R-induced activation of p38 kinase and JNK could potentially induce BZLF1 transcription by a combined effect on several different transcription factors (ATF2, c-Jun, and MEF2) involving binding to both the ZI and ZII binding motifs. The finding that p38 kinase activation is required for efficient disruption of EBV latency by surface Ig cross-linking suggests that this pathway plays an important role in regulating the stringency of viral latency in vivo.
The ZII element is bound by CREB, ATF1, and an ATF2/c-Jun heterodimer. Potentially, phosphorylation of any one of these ZII-binding factors might be sufficient to activate the Z promoter, given our finding that each of these transcription factors activates the Z promoter in the presence of the appropriate activated kinase. Although it is possible that some stimuli (such as coinfection with HHV-6) induce lytic EBV infection through activation of CREB or ATF1, the effects of both R and Z on ZII-mediated transcription appear to be mediated through activation ATF-2 and c-Jun.
R and Z increase ATF2 phosphorylation through at least two different mechanisms. R and Z clearly increase the level of active (phosphorylated) p38 kinase, which is known to phosphorylate (and activate) ATF2. In addition, R and Z also induce JNK activity, which phosphorylates ATF2 at the same sites as p38 (26). Z activation of ATF2 requires the Z transactivation and leucine zipper domains of Z, but does not require Z DNA-binding activity. Therefore, Z likely modulates the p38 and JNK kinase pathways through protein-protein interactions. We have not yet defined the regions of R required for ATF2 phosphorylation.
Our finding that both of the EBV immediate-early proteins activate the stress MAP kinases suggests that activation of these kinases is important for efficient lytic EBV infection. Interestingly, another EBV protein, LMP-1, has also recently been shown to activate the JNK pathway (13, 38). The results from the p38 and JNK inhibitor studies suggest that R must activate both JNK and p38 kinase to disrupt viral latency in epithelial cells. The finding that efficient induction of lytic EBV infection through activation of the B-cell receptor (cross-linking of surface Ig) likewise requires p38 kinase activation suggests that this pathway is important for lytic EBV infection in B cells, as well.
However, even in the presence of the p38 kinase inhibitor, cross-linking of Ig produced some induction of lytic EBV infection (although greatly reduced). The residual induction of lytic EBV infection which occurs even in the absence of activated p38 kinase could be mediated through activated JNK. Alternatively, activation of the R promoter alone, since it directs transcription of a bicistronic R-Z message (53), is potentially sufficient to disrupt viral latency, albeit less efficiently.
Although both R and Z activate ATF-2 and c-Jun phosphorylation, only R has been shown to activate Z transcription from the endogenous EBV genome when transfected into latently infected cells (40, 44, 80). The inability of transfected Z to induce BZLF1 transcription from the endogenous viral genome may reflect the recent finding that Z interacts directly with CBP (CREB-binding protein) (2, 81). CREB transactivator function requires not only phosphorylation at amino acid 133, but also a direct interaction between CREB and CBP (6, 41). The amount of CBP in cells is limiting, and we have recently shown that Z inhibits CREB transcriptional function by competing for available CBP (2).
The ATF2 and c-Jun proteins also require direct interaction with CBP and/or p300 for optimal transactivator function (32, 33). Therefore, Z regulation of its own transcription likely reflects the balance between Z-induced stimulation (mediated through phosphorylation of ATF-2 and c-Jun, and possibly by direct Z binding to upstream ZRE sites [17]), versus Z-induced inhibition (mediated through competition for limiting amounts of cellular CBP). The overall effect of Z on its own transcription is likely dependent upon the availability of CBP and the amount of Z. We propose a model in which very low levels of Z protein initially activate BZLF1 transcription through phosphorylation of ATF-2 and/or c-Jun, but, as higher levels of Z protein accumulate, Z shuts down its own transcription through competition for limiting amounts of CBP and/or p300. In any event, the requirement for Z to stimulate its own transcription is clearly bypassed when Z is transfected into cells under the control of a strong heterologous promoter, and the predominant effect of such vectors instead may be competition for limiting amounts of CBP and/or p300 by Z.
A number of different types of cellular stresses (UV irradiation, osmotic shock, inflammatory cytokines, and growth factors, among others) induce activation of the cellular stress MAP kinases (7, 52, 72). Induction of the stress MAP kinase cascade has a multitude of phenotypic effects (including either induction of, or protection from, apoptosis), depending upon the cell type and circumstances. Activation of the stress MAP kinase cascade may be a common response of cells to infection by viruses, as well. Interestingly, in addition to our findings with EBV, CMV infection has been shown to activate p38 kinase (31), and HSV-1 infection has been shown to activate p38 kinase and JNK (55, 79), and activation of at least one of these kinases is likewise required for efficient lytic viral replication by both of these viruses. It is quite remarkable that three different herpesviruses (herpes simplex virus type 1, CMV, and EBV) take advantage of (and in fact, amplify) a normally protective response to cellular stress and use it to induce replication of their own genomes. Inhibition of p38 kinase and/or JNK activities may therefore prove to be an effective mechanism for preventing viral replication of a variety of herpesviruses.
ACKNOWLEDGMENTS
This work was supported by grants RO1-CA58853, RO1-CA66519, and PO1-CA19014 from the National Institutes of Health. R.A.J. is a Virology Training grant recipient (2T32 AI07419).
We thank Roger Davis, Erik Flemington, and Diane Hayward for plasmids and the UNC Gene Therapy Core (R. Jude Samulski and Douglas McCarty) for preparing adenovirus-LacZ, adenovirus-Z, and adenovirus-R.
REFERENCES
- 1.Adamson A L, Kenney S C. Rescue of the Epstein-Barr virus BZLF1 mutant, Z(S186A), early gene activation defect by the BRLF1 gene product. Virology. 1998;251:187–197. doi: 10.1006/viro.1998.9396. [DOI] [PubMed] [Google Scholar]
- 2.Adamson A L, Kenney S. The Epstein-Barr virus BZLF1 protein interacts physically and functionally with the histone acetylase, CREB-binding protein. J Virol. 1999;73:6551–6558. doi: 10.1128/jvi.73.8.6551-6558.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borras A M, Strominger J L, Speck S H. Characterization of the ZI domains in the Epstein-Barr virus BZLF1 gene promoter: role in phorbol ester induction. J Virol. 1996;70:3894–3901. doi: 10.1128/jvi.70.6.3894-3901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cen H, McKnight J L C. EBV-immortalized isogenic human B-cell clones exhibit differences in DNA-protein complex formation on the BZLF1 and BRLF1 promoter regions among latent, lytic and TPA-activated cell lines. Virus Res. 1994;31:89–107. doi: 10.1016/0168-1702(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 5.Chevallier-Greco A, Manet E, Chavrier P, Mosnier C, Daillie J, Sergeant A. Both Epstein-Barr virus (EBV) encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an early EBV promoter. EMBO J. 1986;5:3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chrivia J C, Kwok R P S, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 7.Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7:353–361. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- 8.Countryman J, Miller G. Activation of expression of latent Epstein-Barr virus after gene transfer with a small cloned fragment of heterogeneous viral DNA. Proc Natl Acad Sci USA. 1985;82:4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox M A, Leahy J, Hardwick J M. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J Virol. 1990;64:313–321. doi: 10.1128/jvi.64.1.313-321.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daibata M, Speck S H, Mulder C, Sairenji T. Regulation of the BZLF1 promoter of Epstein-Barr virus by second messengers in anti-immunoglobulin-treated B cells. Virology. 1994;198:446–454. doi: 10.1006/viro.1994.1056. [DOI] [PubMed] [Google Scholar]
- 11.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 12.Dickens M, Rogers J S, Cavanagh J, Raitano A, Xia Z, Halpern J R, Greenberg M E, Sawyers C L, Davis R J. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277:693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- 13.Eliopoulos A G, Young L S. Activation of the c-Jun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) Oncogene. 1998;16:1731–1742. doi: 10.1038/sj.onc.1201694. [DOI] [PubMed] [Google Scholar]
- 14.Flamand L, Menezes J. Cyclic AMP-responsive element-dependent activation of Epstein-Barr virus Zebra promoter by human herpesvirus 6. J Virol. 1996;70:1784–1791. doi: 10.1128/jvi.70.3.1784-1791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flemington E K, Lytle J P, Cayrol C, Borras A M, Speck S H. DNA-binding-defective mutants of the Epstein-Barr virus lytic switch activator Zta transactivate with altered specificities. Mol Cell Biol. 1994;14:3041–3052. doi: 10.1128/mcb.14.5.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flemington E K, Speck S H. Identification of phorbol ester response elements in the promoter of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990;64:1217–1226. doi: 10.1128/jvi.64.3.1217-1226.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flemington E K, Speck S H. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990;64:1227–1232. doi: 10.1128/jvi.64.3.1227-1232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furnari F B, Zacny V, Quinlivan E B, Kenney S, Pagano J S. RAZ, an Epstein-Barr virus transdominant repressor that modulates the viral reactivation mechanism. J Virol. 1994;68:1827–1836. doi: 10.1128/jvi.68.3.1827-1836.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garner M, Rezvin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981;9:3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giot J-F, Mikaelian I, Buisson M, Manet E, Joab I, Nicolas J-C, Sergeant A. Transcriptional synergy and interference between the EBV transcription factors EB1 and R require both the basic region and the activation domains of EB1. Nucleic Acids Res. 1991;19:1251–1258. doi: 10.1093/nar/19.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glaser R, O'Neill F J. Hybridization of Burkitt lymphoblastoid cells. Science. 1972;176:1245–1247. doi: 10.1126/science.176.4040.1245. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez G A, Montminy M R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 23.Gorman C M, Moffat L F, Howard B H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruffat H, Duran N, Buisson M, Wild F, Buckland R, Sergeant A. Characterization of an R-binding site mediating the R-induced activation of the Epstein-Barr virus BMLF1 promoter. J Virol. 1992;66:46–52. doi: 10.1128/jvi.66.1.46-52.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruffat H, Sergeant A. Characterization of the DNA-binding site repertoire for the Epstein-Barr virus transcription factor R. Nucleic Acids Res. 1994;22:1172–1178. doi: 10.1093/nar/22.7.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta S, Campbell D, Derijard B, Davis R J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 27.Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardwick J M, Lieberman P M, Hayward S D. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J Virol. 1988;62:2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardwick J M, Tse L, Applegren N, Nicholas J, Veliuona M A. The Epstein-Barr virus R transactivator (Rta) contains a complex, potent activation domain with properties different from those of VP16. J Virol. 1992;66:5500–5508. doi: 10.1128/jvi.66.9.5500-5508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holley-Guthrie E A, Quinlivan E B, Mar E-C, Kenney S. The Epstein-Barr virus (EBV) promoter for early antigen (EA-D) is regulated by the EBV transactivators, BRLF1 and BZLF1, in a cell-specific manner. J Virol. 1990;64:3753–3759. doi: 10.1128/jvi.64.8.3753-3759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson R A, Huong S-M, Huang E-S. Inhibitory effect of 4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidizale (FHPI) on HCMV DNA replication and permissive infection. Antivir Res. 1999;41:101–111. doi: 10.1016/s0166-3542(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 32.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki H, Song J, Eckner R, Ugai H, Chiu R, Taira K, Shi Y, Jones N, Yokoyama K K. p300 and ATF-2 are components of the DRF complex, which regulates retinoic acid- and E1A-mediated transcription of the c-jun gene in F9 cells. Genes Dev. 1998;12:133–145. doi: 10.1101/gad.12.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenney S, Holley-Guthrie E, Quinlivan E B, Gutsch D, Zhang Q, Bender T, Giot J-F, Sergeant A. The cellular oncogene c-myb can interact synergistically with the Epstein-Barr virus BZLF1 transactivator in lymphoid cells. Mol Cell Biol. 1992;12:136–146. doi: 10.1128/mcb.12.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenney S, Holley-Guthrie E, Mar E-C, Smith M. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J Virol. 1989;63:3878–3883. doi: 10.1128/jvi.63.9.3878-3883.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenney S, Kamine J, Holley-Guthrie E, Lin J-C, Mar E-C, Pagano J. The Epstein-Barr virus (EBV) BZLF1 immediate-early gene product differentially affects latent versus productive EBV promoters. J Virol. 1989;63:1729–1736. doi: 10.1128/jvi.63.4.1729-1736.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2343–2396. [Google Scholar]
- 38.Kieser A, Kilger E, Gires O, Ueffing M, Kolch W, Hammerschmidt W. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 1997;16:6478–6485. doi: 10.1093/emboj/16.21.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S-J, Wagner S, Liu F, O'Reilly M A, Robbins P D, Green M R. Retinoblastoma gene product activates expression of the human TGF-β2 gene through transcription factor ATF-2. Nature. 1992;358:331–334. doi: 10.1038/358331a0. [DOI] [PubMed] [Google Scholar]
- 40.Kolman J L, Taylor N, Gradoville L, Countryman J, Miller G. Comparing transcriptional activation and autostimulation by ZEBRA and ZEBRA/c-Fos chimeras. J Virol. 1996;70:1493–1504. doi: 10.1128/jvi.70.3.1493-1504.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwok R P S, Lundblad J R, Chrivia J C, Richards J P, Bachinger H P, Brennan R G, Roberts S G E, Green M R, Goodman R H. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 42.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 43.Lawler S, Cuenda A, Goedert M, Cohen P. SKK4, a novel activator of stress-activated protein kinase-1 (SAPK1/JNK) FEBS Lett. 1997;414:153–158. doi: 10.1016/s0014-5793(97)00990-3. [DOI] [PubMed] [Google Scholar]
- 44.Le Roux F, Sergeant A, Corbo L. Epstein-Barr virus (EBV) EB1/Zta protein provided in trans and competent for the activation of productive cycle genes does not activate the BZLF1 gene in the EBV genome. J Gen Virol. 1996;77:501–509. doi: 10.1099/0022-1317-77-3-501. [DOI] [PubMed] [Google Scholar]
- 45.Li Q X, Young L S, Niedobitek G, Dawson C W, Birkenbach M, Wang F, Rickinson A B. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature. 1992;356:347–350. doi: 10.1038/356347a0. [DOI] [PubMed] [Google Scholar]
- 46.Lieberman P M, Hardwick J M, Hayward S D. Responsiveness of the Epstein-Barr virus NotI repeat promoter to the Z transactivator is mediated in a cell-type-specific manner by two independent signal regions. J Virol. 1989;63:3040–3050. doi: 10.1128/jvi.63.7.3040-3050.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lillie J, Green M. Transcriptional activation by the adenovirus E1a protein. Nature. 1989;338:39–44. doi: 10.1038/338039a0. [DOI] [PubMed] [Google Scholar]
- 48.Liu F, Green M R. A specific member of the ATF transcription factor family can mediate transcription activation by the adenovirus E1a protein. Cell. 1990;61:1217–1224. doi: 10.1016/0092-8674(90)90686-9. [DOI] [PubMed] [Google Scholar]
- 49.Liu P, Liu S, Speck S H. Identification of a negative cis element within the ZII domain of the Epstein-Barr virus lytic switch BZLF1 gene promoter. J Virol. 1998;72:8230–8239. doi: 10.1128/jvi.72.10.8230-8239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu S, Liu P, Borras A, Chatila T, Speck S H. Cyclosporin A-sensitive induction of the Epstein-Barr virus lytic switch is mediated via a novel pathway involving a MEF2 family member. EMBO J. 1997;16:143–153. doi: 10.1093/emboj/16.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Livingstone C, Patel G, Jones N. ATF-2 contains a phosphorylation-dependent transcriptional activation domain. EMBO J. 1995;14:1785–1797. doi: 10.1002/j.1460-2075.1995.tb07167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madhani H D, Fink G R. The riddle of MAP kinase signalling. Trends Genet. 1998;14:151–155. doi: 10.1016/s0168-9525(98)01425-5. [DOI] [PubMed] [Google Scholar]
- 53.Manet E, Gruffat H, Trescol-Biemont M C, Moreno I, Chambard P, Giot J F, Sergeant A. Epstein-Barr virus bicistronic mRNAs generated by facultative splicing code for two transcriptional trans-activators. EMBO J. 1989;8:1819–1826. doi: 10.1002/j.1460-2075.1989.tb03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marinissen M J, Chiariello M, Pallante M, Gutkind J S. A network of mitogen-activated protein kinases links G protein-coupled receptors to the c-jun promoter: a role for c-Jun NH2-terminal kinase, p38s, and extracellular signal-regulated kinase 5. Mol Cell Biol. 1999;19:4289–4301. doi: 10.1128/mcb.19.6.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLean T I, Bachenheimer S L. Activation of cJUN N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J Virol. 1999;73:8415–8426. doi: 10.1128/jvi.73.10.8415-8426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quinlivan E, Holley-Guthrie E, Norris M, Gutsch D, Bachenheimer S, Kenney S. Direct BRLF1 binding is required for cooperative BZLF1/BRLF1 activation of the Epstein-Barr virus early promoter, BMRF1. Nucleic Acids Res. 1993;21:1999–2007. doi: 10.1093/nar/21.8.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ragoczy T, Heston L, Miller G. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. 1998. J Virol. 1998;72:7978–7984. doi: 10.1128/jvi.72.10.7978-7984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raingeaud J, Gupta S, Rogers J, Dickens M, Han J, Ulevitch R J, Davis R J. Pro-inflammatory cytokines and environmental stress cause p38 MAP kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 59.Raingeaud J, Whitmarsh A J, Barrett T, Dérijard B, Davis R J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rehfuss R P, Walton K M, Loriaux M M, Goodman R H. The c-AMP-regulated enhancer-binding protein ATF-1 activates transcription in response to cAMP-dependent protein kinase A. J Biol Chem. 1991;266:18431. [PubMed] [Google Scholar]
- 61.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- 62.Rooney C, Taylor N, Countryman J, Jenson H, Kolman J, Miller G. Genome rearrangements activate the Epstein-Barr virus gene whose product disrupts latency. Proc Natl Acad Sci USA. 1988;85:9801–9805. doi: 10.1073/pnas.85.24.9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rooney C M, Rowe D T, Ragot T, Farrell P J. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J Virol. 1989;63:3109–3116. doi: 10.1128/jvi.63.7.3109-3116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sadowski I, Ptashne M. A vector for expressing GAL4(1-147) fusions in mammalian cells. Nucleic Acids Res. 1989;17:7539. doi: 10.1093/nar/17.18.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64a.Sanchez I, Hughes R T, Mayer B J, Yee K, Woodgett J R, Avruch J, Kyriakis J M, Zon L I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–800. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 65.Shimizu N, Takada K. Analysis of the BZLF1 promoter of Epstein-Barr virus: identification of an anti-immunoglobulin response sequence. J Virol. 1993;67:3240–3245. doi: 10.1128/jvi.67.6.3240-3245.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sinclair A J, Brimmell M, Shanahan F, Farrell P J. Pathways of activation of the Epstein-Barr virus productive cycle. J Virol. 1991;65:2237–2244. doi: 10.1128/jvi.65.5.2237-2244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sixby J W, Nedrud J G, Raab-Traub N, Hanes R A, Pagano J S. Epstein-Barr virus replication in oropharyngeal epithelial cells. N Engl J Med. 1984;310:1225–1230. doi: 10.1056/NEJM198405103101905. [DOI] [PubMed] [Google Scholar]
- 68.Takada K, Ono Y. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J Virol. 1989;63:445–449. doi: 10.1128/jvi.63.1.445-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takada K, Shimizu N, Sakuma S, Ono Y. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J Virol. 1986;57:1016–1022. doi: 10.1128/jvi.57.3.1016-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takimoto T, Kamide M, Umeda R. Establishment of Epstein-Barr virus (EBV)-associated nuclear antigen (EBNA)-positive nasopharyngeal carcinoma hybrid cell line (NPC-KT) Arch Otorhinolaryngol. 1984;239:87–92. doi: 10.1007/BF00454266. [DOI] [PubMed] [Google Scholar]
- 71.Tonneguzzo F, Hayday A C, Keating A. Electric field-mediated DNA transfer: transient and stable gene expression in human and mouse lymphoid cells. Mol Cell Biol. 1986;6:703–706. doi: 10.1128/mcb.6.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 73.Uhler M D, McKnight G S. Expression of cDNAs for two isoforms of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1987;262:15202–15207. [PubMed] [Google Scholar]
- 74.Urier G, Buisson M, Chambard P, Sergeant A. The Epstein-Barr virus early protein EB1 activates transcription from different responsive elements including AP-1 binding sites. EMBO J. 1989;8:1447–1453. doi: 10.1002/j.1460-2075.1989.tb03527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Dam H, Wilhelm D, Herr I, Steffen A, Herrlich P, Angel P. ATF2 is preferentially activated by stress-activated protein kinases to mediate c-Jun induction in response to genotoxic agents. EMBO J. 1995;14:1798–1811. doi: 10.1002/j.1460-2075.1995.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y-C, Huang J-M, Montalvo E A. Characterization of proteins binding to the ZII element in the Epstein-Barr virus BZLF1 promoter: transactivation by ATF1. Virology. 1997;227:323–330. doi: 10.1006/viro.1996.8326. [DOI] [PubMed] [Google Scholar]
- 77.Westphal E-M, Mauser A, Swenson J, Davis M G, Talarico C L, Kenney S C. Induction of lytic Epstein-Barr virus (EBV) infection in EBV-associated malignancies using adenovirus vectors in vitro and in vivo. Cancer Res. 1999;59:1485–1491. [PubMed] [Google Scholar]
- 78.Yang S-H, Galanis A, Sharrocks A D. Targeting of p38 mitogen-activated protein kinases to MEF2 transcription factors. Mol Cell Biol. 1999;19:4028–4038. doi: 10.1128/mcb.19.6.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zachos G, Clements B, Conner J. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J Biol Chem. 1999;274:5097–5103. doi: 10.1074/jbc.274.8.5097. [DOI] [PubMed] [Google Scholar]
- 80.Zalani S, Holley-Guthrie E, Kenney S. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc Natl Acad Sci USA. 1996;93:9194–9199. doi: 10.1073/pnas.93.17.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zerby D, Chen C-J, Poon E, Lee D, Shiekhattar R, Lieberman P M. The amino-terminal C/H1 domain of CREB binding protein mediates Zta transcriptional activation of latent Epstein-Barr virus. Mol Cell Biol. 1999;19:1617–1626. doi: 10.1128/mcb.19.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zur Hausen H, Schulte-Holthauzen H, Klein G, Henle G, Henle W, Clifford P, Santesson L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970;228:1956–1958. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]