Abstract
Introduction
Lymphoedema is a chronic condition caused by lymphatic insufficiency. It leads to swelling of the limb/midline region and an increased risk of infection. Lymphoedema is often associated with mental and physical problems limiting quality of life. The first choice of treatment is a conservative treatment, consisting of exercises, skin care, lymph drainage and compression. Reconstructive lymphatic surgery is also often performed, that is, lymphovenous anastomoses, lymph node transfer or a combination. However, robust evidence on the effectiveness of reconstructive lymphatic surgery is missing. Therefore, the objective of this trial is to investigate the added value of reconstructive lymphatic surgery to the conservative treatment in patients with lymphoedema.
Methods and analysis
A multicentre randomised controlled and pragmatic trial was started in March 2022 in three Belgian university hospitals. 90 patients with arm lymphoedema and 90 patients with leg lymphoedema will be included. All patients are randomised between conservative treatment alone (control group) or conservative treatment with reconstructive lymphatic surgery (intervention group). Assessments are performed at baseline and at 1, 3, 6, 12, 18, 24 and 36 months. The primary outcome is lymphoedema-specific quality of life at 18 months. Key secondary outcomes are limb volume and duration of wearing the compression garment at 18 months. The approach of reconstructive lymphatic surgery is based on presurgical investigations including clinical examination, lymphofluoroscopy, lymphoscintigraphy, lymph MRI or CT angiography (if needed). All patients receive conservative treatment during 36 months, which is applied by the patient’s own physical therapist and by the patient self. From months 7 to 12, the hours a day of wearing the compression garment are gradually decreased.
Ethics and dissemination
The study has been approved by the ethical committees of University Hospitals Leuven, Ghent University Hospital and CHU UCL Namur. Results will be disseminated via peer-reviewed journals and presentations.
Trial registration number
Keywords: Vascular medicine, Plastic & reconstructive surgery, VASCULAR SURGERY
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This trial is stratified and powered for the effect of reconstructive lymphatic surgery in both arm and leg lymphoedema and will permit a conclusion regarding the effect of reconstructive lymphatic surgery in both groups.
As independent experts in reconstructive lymphatic surgery have trained the surgeons of the three study centres and advanced imaging techniques (ie, lymphofluoroscopy, lymph MRI, lymphoscintigraphy and CT angiography) are used to prepare the surgical procedure, high-qualitative reconstructive surgery procedures are guaranteed.
A comprehensive evaluation of the participants with lymphoedema will be performed by assessing lymphoedema-specific quality of life, which is a self-reported outcome, and by determining limb volume and duration of wearing the compression garment, which are objective outcomes.
If reconstructive lymphatic surgery is found effective, a detailed inventory of cost and quality of life will permit a cost-effectiveness analysis.
In addition to a statistical plan, also a monitoring plan, data management plan, communication plan and risk assessment plan have been set in place.
Introduction
Lymphoedema is a chronic and debilitating condition caused by lymphatic insufficiency. It leads to swelling of the limb/midline region and an increased risk of infection. It can be classified as primary (congenital) or secondary (acquired) lymphoedema. Lymphoedema is very burdensome for the patient, often causing mental problems such as frustration and stress.1 In addition, due to the increase in the volume of the limb, patients may develop physical problems, such as pain, heaviness, loss of strength, as well as functional problems with household, mobility or social activities.2 These mental, physical and functional problems have a negative impact on the quality of life (QoL) and the ability to work.3
There is a consensus that the first choice of treatment of lymphoedema is a conservative treatment, also called decongestive lymphatic therapy (DLT).4 5 In the case of pitting oedema, this consists of an intensive daily treatment to maximally reduce the oedema volume. This phase consists of skin care, manual lymph drainage (MLD), multilayer bandaging and exercise therapy. Once sufficient reduction of the pitting is obtained (ie, there is no or minimal pitting) and the patients receive education to improve their self-management skills, the maintenance phase starts, which aims at stabilising the results obtained in the previous phase. During the maintenance phase, skin care, exercises and lymph drainage are continued but bandaging is replaced by low-stretch compression garments. Professional’s involvement can be minimised in this phase.
Reconstructive lymphatic surgery is another treatment option, consisting of either lymphovenous anastomoses (LVA), lymph node transfer (LNT) or a combination of both. The choice can be based on the surgeons clinical judgement or on local algorithms, such as the barcelona lymphoedema algorithm.6 The objective of LVA is to redirect the lymph to the venous stream directly, bypassing areas of obstruction and without going through the thoracic duct. LVA is applied if functional lymphatics can be localised, primarily by lymphofluoroscopy (using Indocyanine Green or ICG) and lymph MRI.7 With LNT, orthotopically placed lymph nodes act as a sponge to absorb lymphatic fluid and direct it into the vascular network. The transferred nodes may also induce lymphangiogenesis and if they are placed in the site of lymphadenectomy, scar tissue and adhesions are removed, which may lower the pressure on the vein.8 The lymphangiogenesis and the increase of the diameter of the vein as well may improve vascularisation.5 9 Indications for LNT are a total occlusion of lymphatic transport visualised through lymphoscintigraphy and a stage 2 lymphoedema with repeated episodes of erysipelas. Only subjects who had a history of at least 6–12 months of conservative treatment with good decongestion of the limb are candidates for reconstructive lymphatic surgery.7
Our hypothesis is that reconstructive lymphatic surgery partially restores the lymphatic transport, which leads to a decrease of the lymphoedema volume and as a result lowers the need for a compression garment. This will probably improve lymphoedema-specific QoL.
Robust evidence on the effectiveness of reconstructive lymphatic surgery for lymphoedema has so far not been procured. In 2019, a Cochrane systematic review of Markkula et al revealed that there is not enough high-quality research investigating the effect of reconstructive lymphatic surgery on lymphoedema.10 Only one RCT so far evaluated the effect of LNT. Dionyssiou et al randomised 36 patients with breast cancer-related arm lymphoedema.11 After surgery/no surgery, all patients first received for 6 months DLT and DLT was discontinued for the next 12 months. At 18 months follow-up, mean limb volume reduction was superior in the group with LNT compared with no LNT (57% vs 18%, p<0.01). In the group with LNT infections were less frequent and subjective symptoms improved. An RCT evaluating the effect of LVA has not been performed yet.
Objectives
The main objective of this study is to investigate the added value of reconstructive lymphatic surgery to DLT (usual care) in patients with lymphoedema of the upper limb or lower limb in terms of lymphoedema-specific QoL (primary outcome), limb volume and duration of wearing the compression garment (key secondary outcomes) at 18 months and of other outcomes at 1, 3, 6, 12, 18, 24 and 36 months postbaseline (secondary outcomes; see table 1 for the outcomes).
Table 1.
Overview of the primary and secondary outcomes and the assessment method at each time interval
| Outcome | Method | A1 Baseline |
A2, 3, 4 1, 3, 6 months |
A5 12 months |
A6 18 months |
A7 24 months |
A8 36 months |
| Primary outcome | |||||||
| Lymphoedema-specific QoL | Lymphoedema Functiong, Disability and Health (Lymph-ICF) questionnaire for upper or lower limb lymphoedema13 19 20 27 | X | X | ||||
| Secondary outcomes | |||||||
| Self-reported questionnaire | |||||||
| Lymphoedema-specific QoL | See primary outcome | X | X | X | X | X | |
| Duration (key secondary outcome) and experience of wearing compression garment | Compression questionnaire developed by the International Compression Club (or ICC compression questionnaire)28 | X | X | X | X | X | X |
| Health-related QoL | EuroQol-5D-5L which is a health status measure developed by the EuroQol group containing 5 dimensions and 5 levels29 | X | X | X | X | X | X |
| Work capacity and ability | Work Productivity and Activity Impairment questionnaire30; QuickScan 1831 | X | X | X | X | X | X |
| Physical activity level | International Physical Activity Questionnaire32 | X | X | X | X | X | X |
| Costs related to lymphoedema and its treatment* | Study-specific questionnaire | X | X | X | X | X | |
| Usual care and self-management*†, including need for intensive treatment | Study-specific questionnaire | X | X | X | X | X | |
| Assessments | |||||||
| Limb volume (key secondary outcome) | Circumference measurements every 4 cm with perimeter16 33–35 | X | X | X | X | X | X |
| Hand/foot volume | Water displacement method of hand or foot34 36 | X | X | X | X | X | X |
| Failure to reduce hours a day of wearing compression garment | Based on change in limb volume | X | X | X | X | ||
| Body weight | Scale | X | X | X | X | X | X |
| Infection previous 18 months | Interview | X | X | X | |||
| Recurrence of cancer | Interview and medical file | X | X | ||||
| Adverse events and complications of surgery | Interview and medical file | X | X | X | X | X | |
| Lymphatic transport | Lymphofluoroscopy using Indocyanine Green (or ICG fluoroscopy)37; lymphoscintigraphy38 39 | X | X |
*Information is collected on a monthly basis.
†No secondary outcome.
QoL, quality of life.
A secondary objective is to verify whether the rate of complications in participants receiving reconstructive lymphatic surgery is acceptable and if so, whether these complications are reversible. We also verify in patients with lymphoedema due to cancer treatment, if reconstructive lymphatic surgery causes higher cancer recurrence rates.
The first exploratory objective is to compare the added value of the reconstructive surgery between different subgroups (stage 1 vs stage 2; normal weight vs overweight; combination of LVA and LNT vs one method). A second exploratory objective is to investigate predictive variables for lymphoedema-specific QoL at 36 months.
Methods and analysis
The manuscript adheres to the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) guidelines.12
Trial design and study setting
A multicentre, pragmatic randomised controlled trial is performed at three university hospitals in Belgium: University Hospitals Leuven (UZ Leuven), Ghent University Hospital (UZ Gent) and CHU UCL Namur.
The general flow, starting from screening for eligibility, is shown in figure 1.
Figure 1.
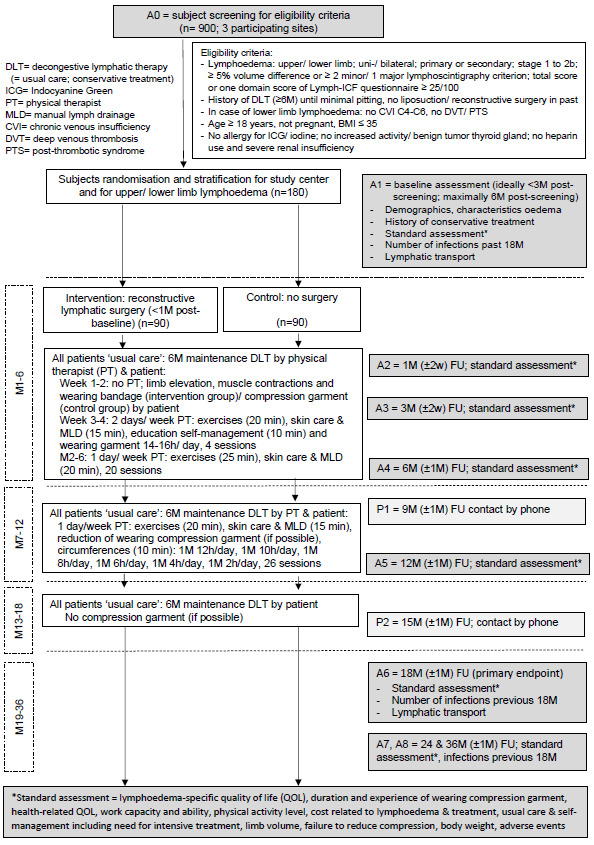
Flow diagram of the SurLym trial. BMI, body mass index.
Before the real screening (A0), a fast eligibility check is performed and Informed Consent Form is signed. If the patient is eligible and confirms participation, he/she is randomised. The interval between screening (A0) and baseline assessment (A1) is ideally less than 3 months but may be up to 6 months. The baseline assessments have to be performed shortly before the surgery, with a maximal interval of 1 month.
Patient and public involvement in the trial design
Four patients with arm lymphoedema and three patients with leg lymphoedema from the centre for lymphoedema of UZ Leuven have completed a questionnaire about the study design and feasibility of the SurLym study. All but one patient found the primary outcome, assessment of lymphoedema-specific QoL, a relevant and very important outcome. This patient preferred arm volume (which is a key secondary outcome) as outcome measure. None of the patients objected to a technical examination using an injection in the hand/foot of the affected side (for imaging of the lymphatic system). All patients found it feasible to come to the hospital for eight study visits during 36 months, well aware that two of the visits take up to 6 hours. Three of seven patients were not keen to undergo surgery on the affected limb. All patients declared having little problems performing usual care: only one patient considered self-management difficult and another patient was afraid to reduce the hours of wearing the compression garment.
From the patients willing to be part of the trial’s patient board (n=5), two patients were selected: one patient with arm lymphoedema and one with leg lymphoedema. They are both members of the trial steering committee. The rationale and design of the trial were thoroughly discussed with them. They will be invited to further participate during future meetings of the trial steering committee, to advise us during the course of the trial and for the dissemination of the project results.
Eligibility criteria
Patients eligible for inclusion in the trial have to meet all of the following criteria:
Unilateral or bilateral, primary or secondary lymphoedema of the upper or lower limb.
If cancer-related lymphoedema, approval for participation from the multidisciplinary oncological board; participation only if estimated cancer-related survival is ≥3 years and no concerns on oncological safety are raised.
Lymphoedema stages 1–2 (according to staging 1–3 of International Society of Lymphology).5
Objective diagnosis of lymphoedema: ≥5% vol difference OR≥2 minor/1 major criteria on lymphoscintigraphy OR presence of ICG dermal backflow.
Total score or one of domain scores on Lymph-ICF questionnaire at screening: ≥25/100 (=moderate level of problems in functioning related to the development of lymphoedema).13
History of at least 6 months of DLT until minimal pitting (sustained thumb pressure on the skin is performed for 5 s; after removing the thumb, indentation of tissue is evaluated and is scored as 0=no clinical pitting, 1=slight/doubtful pitting and 2=noticeably pitting; a patient with score 2 may not participate).14
Age ≥18 years.
Following persons are excluded:
Persons with a history of liposuction, LVA or LNT.
Persons who are pregnant or plan to become pregnant in the next 18 months.
Severely obese participants: body mass index (BMI) >35 kg/m2.
In case of lower limb lymphoedema: the presence of chronic venous insufficiency C4, C5, C6; deep venous thrombosis; post-thrombotic syndrome.
Allergy for ICG, iodine; increased activity of thyroid gland; benign tumour in thyroid gland; heparin use and severe renal insufficiency.
Recruitment, participant screening and consent
The recruitment of patients started in March 2022. 180 patients have to be recruited by the 3 hospitals. Initially, a recruitment period of 24 months (=7.5 pts/month) was planned, however, difficulties in accessing operating theatres linked to COVID-19 have caused delays. To make the recruitment period as short as possible, a competitive recruitment is applied. We estimate that around 20% of the patients screened for eligibility (A0, n=900) can be accepted for participation.
Identification of eligible patients will be performed by the (sub)investigators of the lymphoedema centres of the three hospitals (ST, BB-H, A-KH and ND for UZ Leuven; CM, CR, TD, VVB and MDS for UZ Gent and TD, JF, MS, AB and PF for CHU UCL Namur), supported by the study coordinators. The consultation lists of the lymphoedema centres are screened before the consultation and the possible patients eligible for the trial are marked.
During the lymphoedema consultation, the clinician checks the eligibility criteria for which a measurement is not necessary; if the patient seems eligible and he/she is interested to receive information about the trial, the trial is discussed using a study-specific recruitment document: this is a concise and well-organised document that clarifies the design of the study and provides information about side effects, costs and potential benefits and harms of participation. If a patient is interested to participate, he/she receives the informed consent form and the ‘study at a glance (summary)’ document. In addition, the patient receives an appointment for the screening (A0). Some patients are informed about the trial through another way, for example, by their oncologist. In that case, the patient contacts the study coordinator by phone, who performs the fast eligibility check and discusses the study during the phone call. If the patient is interested to participate, the informed consent form and the ‘study at a glance’ document are sent. In addition, the patient receives an appointment for the screening (A0).
During the screening appointment (A0), patients receive all information and explanation they request or need before signing the informed consent form. Thereafter, the complete screening procedure is executed to verify whether the participant fulfils all eligibility criteria.
In order to optimally recruit patients with lymphoedema, the study is presented inside (at other departments) as well as outside the hospitals of the study centres through lectures, posters and mailing. Potential candidates with lymphoedema as well as their treating physicians, physical therapists and other healthcare providers are informed about the trial (through social media, publication in local journals and on websites).
Allocation and randomisation
Given the nature of the trial, blinding participants and care providers (surgeon/physical therapist/compression specialist) is not feasible. Because the participants fill out different questionnaires to determine the primary outcome and some of the secondary outcomes, detection bias may be a potential risk. However, bias of the participants will be limited as much as possible because the study will be explained by a neutral person (physical therapists ND, A-KH, VVB, MDS and JF or physical medicine and rehabilitation physician TD (hospital of Ghent), TD (hospital of CHU UCL)).
The randomisation is computer generated. To obtain concealment of allocation, the randomisation list is prepared by the trial’s statistician (SF) and is incorporated in the data management tool ‘REDCap’. Randomisation is performed by using varying block sizes. A 1:1 allocation ratio is applied. A stratification is applied for the study centre (UZ Leuven vs UZ gent vs CHU-UCL Namur) and for region of lymphoedema (upper limb vs lower limb, with a ratio 1:1). At each participating site, only the chief investigator (ND) and trial manager (TDV), investigators and study coordinators have access to the randomisation tool in REDCap. After randomisation, the study coordinator of the specific study centre plans the intervention if applicable (surgery), as well as the usual care and the follow-up assessments.
After all patients have finished the trial and the database is locked to analyse the data, the randomisation code will be broken.
Intervention
All participants are randomised to the intervention or control group. The intervention group is treated with reconstructive lymphatic surgery in addition to conservative DLT (DLT; usual care). In the control group, patients only receive conservative DLT (usual care) without surgery (see figure 1).
The researchers will follow the protocol as strictly as possible. However, since the pragmatic nature of the trial, a deviation of the protocol is allowed if necessary. This protocol deviation has to be registered in the protocol deviation log.
Reconstructive lymphatic surgery
The intervention treatment is reconstructive lymphatic surgery and is performed by the team of vascular and/or plastic surgeons from each study centre (ST and KS of UZ Leuven; BDP and LD of Ghent University Hospital; and MS, AB and PF of CHU UCL Namur). As reconstructive technique, an LVA, LNT or a combination of both is applied. The choice of the technique is determined by the surgeons of the study centre. See table 2 for the overview of the preparation and for the technical description of the reconstructive procedure (which is based on Chang et al).7 In table 3, the aftercare is discussed.
Table 2.
Overview of the preparation and procedure of lymphovenous anastomosis (LVA) and lymph node transfer (LNT)
| Timing | LVA | LNT | |
| Before surgery | Clinical reasoning based on presurgical investigations | Presence of suitable lymphatic vessel(s), visualised through ICG (Indocyanine Green) lymphofluoroscopy and/or lymph MRI. | Presence of fibrosis or adhesions due to surgery, lymph node dissection and/or radiotherapy, known through inspection and visualisation of interruption of lymphatic transport by lymphoscintigraphy. Presence of a well-vascularised donor flap (CT angiography is performed if needed). |
| Week before surgery | Compression garment | Measured by the team of compression specialists of the specific centre; Choice of the type of compression garment is made pragmatically, as performed in the real clinical situation. So, length, options, compression class, type (flat/round-knitted, standard/custom-made) of the compression garment are determined patient-specific. |
|
| Registration of compression garment | Compression specialist registers each time after delivery the type of compression material and cost for patient/health insurance. | ||
| Surgery | Material | Microsurgical equipment to make anastomoses of vessels with diameter of 0.3–0.8 mm (suture size 11 or 12), supermicro clips, fine bipolar. | Microsurgical equipment to perform vascularised lymph-tissue transfer, suturing vein and artery with suture size 9 or 10, micro clips, fine bipolar. |
| Preparation | ICG is injected interdigitally and lymph transport is designed on skin and location(s) of anastomosis is indicated (confirmed by lymph MRI). | To check for the safety of not developing limb oedema due to the dissection of lymph nodes, 99mTc nanocolloids or ICG are injected in first web of both hands (in case the donor site is the axilla) or feet (in case the donor site is the groin). | |
| Anaesthesia | General or if wish of patient local | General | |
| Procedure |
|
|
|
| Registration |
|
||
Table 3.
Overview of the after care in the hospital and at home following lymphovenous anastomosis (LVA) and lymph node transfer (LNT)
| Timing | LVA | LNT | |
| Aftercare in hospital | Number of days | 1 day or longer if necessary | 2 days or longer if necessary |
| Medication | To prevent thrombosis, to stimulate vasodilation, to reduce pain, to prevent infection | ||
| Inelastic bandage | In most of the patients (if risk of damaging LVA/LNT by putting on compression garment; First tubular bandage and cotton wool covering whole limb, then non-elastic bandages, finally other tubular bandage over bandages (to keep everything together); keep it day and night |
||
| Advise | As much as possible limb elevation and regularly muscle contractions | ||
| Registration |
|
||
| Aftercare at home | Wound control | Once a week, inelastic bandage is removed, wound is cared and bandage is reapplied | |
| Advise | As long as wound is not closed, as much as possible limb elevation and regularly muscle contractions | ||
| Compression garment | If wound is healed, new compression garment is applied and usual care protocol is started | ||
| Registration |
|
||
To obtain standardisation and to ascertain the quality of the reconstructive lymphatic surgery, all surgeons received training in the Reconstructive Microsurgery European School (by JMA and GP) in May 2021. Moreover, to improve standardisation of the patient selection and the reconstructive lymphatic procedure between the surgeons and between the centres, every patient that is planned for surgery in the trial is discussed during a monthly meeting with at least one surgeon per centre attending. A final quality control measure is that the first 10 surgical procedures are discussed with the whole surgical team including the independent experts JMA, GP, SS and KVL.
Usual care
All patients receive usual care. The patient’s own (regular) physical therapist performs the usual care in a pragmatic way consisting of exercises and skin care and MLD (ie, the maintenance phase of decongestive lymphatic therapy (DLT)). Nevertheless, MLD is not an evidence-based treatment modality for lymphoedema, it was added to the usual care as it stimulates lymph flow through functional lymphatics.15 Many surgeons specialised in performing reconstructive lymphatic surgery believe that performing MLD after LVA is important to keep the anastomosis open. Moreover, the physical therapist educates the patient to perform self-management, that is, self-exercises, self-skin care, self-MLD, self-bandaging and putting on and removing the compression garment. In all patients (of intervention and control group), a new compression garment is measured by the compression specialist at baseline. The schematic overview of the usual care is given in figure 1 and is divided into four periods:
Months 1–6: From week 3 (or in the intervention group, after healing of the wounds), the patient sees the home physical therapist twice per week and from week 5 once a week. The patient also performs self-management.
Months 7–12: The patient sees the own physical therapist once a week. The compression garment use is gradually reduced from 16 hours/d (end of 6th month) to 0 hour/day (end of 12th month). The own physical therapist performs circumference measurements of the limb weekly (ie, with a perimeter provided by the study team) to control for changes in the limb volume.16 The patient completes a digital scoring form in REDCap weekly. The study investigator of the centre checks the change in limb volume every week: if the limb volume increases ≥5% compared with baseline, the patient is planned for an intermediate check-up in the study centre. The study investigator decides whether the hours a day of wearing the compression garment has to be increased again.
Months 13–18: The patient only performs self-management and does not see the own physical therapist anymore. If possible, the patient does not wear the compression garment.
Months 19–36: The patient may choose whether he/she visits the own physical therapist or performs self-management or a combination.
This scheme of usual care has to be followed as strictly as possible, except when the patient’s clinical situation deteriorates or risks to deteriorate. For example, a patient may visit the physical therapist more often in case of more lymphoedema-related complaints due to warm weather. Or, if during the follow-up, the clinical situation of the lymphoedema deteriorates unacceptably (eg, there is the presence of pitting oedema in the limb or there is a wound), the study investigator may advise the patient and physical therapist to perform an intensive treatment of the lymphoedema with bandaging. This information has to be registered by the patient in the usual care questionnaire.
To obtain standardisation of the usual care, the physical therapist of the patient receives training before the start of the study. During this training, instructions about the study protocol are given orally. In addition, the physical therapist receives an informative leaflet explaining the aim and design of the trial, the treatment in the intervention/control group and the assessment of the patient. It also clarifies what the study investigators expect from the patient’s physical therapist and vice-versa. Following information regarding the patient’s physical therapist is collected: age and gender, education level and experience with treating lymphoedema (number of years of experience and in which modalities, type of lymphoedema education).
Outcomes
The outcome measures were chosen based on input from patients with lymphoedema (see the ‘Patient and public involvement’ section) and on input from the investigators of this trial who have experience in evaluating and treating patients with lymphoedema. Patient-reported outcomes provide essential information about the patient experience with the intervention that cannot be reliably captured in another way and are necessary for the complete evaluations of risks and benefits and the value of the intervention. As a consequence, the trial’s primary outcome is a patient-reported outcome.17 Moreover, recently, Chang et al stated in their systematic review and meta-analysis about the surgical treatment of lymphoedema that better designed studies are necessary: with objective reporting of outcomes using quantitative methods for measuring fluid and both physiologic and immunological function during longer follow-up.18
Assessments are performed at baseline (A1) and at 1 month (A2), 3 months (A3), 6 months (A4), 12 months (A5), 18 months (A6), 24 months (A7) and 36 months (A8) postbaseline. However, to limit the burden for the patients, not all outcomes are assessed at each time interval. See table 1 for the overview of the outcomes per time interval and see the online supplemental appendix for the assessment method and the description of the assessment per variable and outcome. Figure 1 gives an overview of the timing of the baseline assessment related to the screening and to the surgery, and of the foreseen windows for the follow-up assessments.
bmjopen-2023-078114supp001.pdf (86.9KB, pdf)
At baseline, patient’s demographics and information about the characteristics of the lymphoedema and its treatment are collected.
The primary outcome is lymphoedema-specific QoL (=problems in functioning related to development of lymphoedema) at 18 months, evaluated with the Dutch or French version of the Lymphoedema Functioning, Disability and Health (Lymph-ICF) questionnaire for upper or lower limb lymphoedema.13 19–21 In addition to this patient-reported outcome, the trial contains also two key secondary outcomes at 18 months that are objective outcomes. These are limb volume and failure to reduce the hours a day of wearing the compression garment. In addition, these outcomes will be investigated at other time points in the short term (1, 3, 6, 12 months) and longer term (24 and 36 months) as a secondary outcome parameter. The outcome limb volume is determined differently in participants with upper and lower limb lymphoedema. Since most of the patients with upper limb lymphoedema have unilateral lymphoedema, limb volume is determined as the relative excessive arm volume. As too many patients with lower limb lymphoedema have bilateral lymphoedema, limb volume is determined as the leg volume.
Other secondary outcomes are duration of wearing the compression garment during 1 week and experience of the compression garment, health-related QoL, work capacity and ability, physical activity level, costs related to lymphoedema and its treatment, need for intensive treatment, hand/foot volume, failure to reduce the hours a day of wearing the compression garment, body weight, episodes of infection previous 18 months, recurrence of cancer (in patients with a history of cancer), adverse events and lymphatic transport.
Complications of surgery (in the intervention group) and information regarding usual care and self-management are collected during the trial period as well.
There is also a follow-up contact by phone at 9 months and 15 months, respectively. During the phone call, information is further collected about adverse events and complications of the surgery, about the usual care and self-management (to check for the adherence of the patient) and about the costs related to lymphoedema and its treatment.
To guarantee standardisation of the assessments, all assessors are trained before the start of the trial.
Sample size
The sample size is calculated to have at least 90% power to detect a difference between the intervention group receiving reconstructive surgery and the control group without surgery, on lymphoedema-specific QoL at 18 months, separately within patients with upper limb lymphoedema and within patients with lower limb lymphoedema. Both comparisons are considered as separate trials and therefore alpha has been set equal to 0.05.
The planned analysis to compare the groups is a constrained longitudinal data analysis (cLDA),22 using the baseline measurement and the follow-up measurements after 1, 3, 6, 12 and 18 months as outcome. The primary analysis refers to the comparison after 18 months (based on a two-sided test with alpha=0.05). The approach is similar in spirit as an analysis of covariance but does not exclude subjects with one or more missing measurements. The calculation of the required sample size is based on an approach presented by Stroup.23 Information with respect to variability of the lymphoedema-specific QoL score and the correlation between the time points was obtained from two retrospective series (130 patients with arm oedema and 83 patients with leg oedema).
The following assumptions have been made for the comparison of the lymphoedema-specific QoL:
SD of the lymphoedema-specific QOL equal to 20.
Correlation between the baseline and each of the follow-up measurements equal to 0.50.
Drop-out of 5%, 10%, 15% and 20% after 1 and 3 months, 6 months, 12 months and 18, 24 and 36 months, respectively
To detect a difference of 15 points, which is a clinically important difference,13 20 36 subjects are required per group (2×2×36=144 subjects in total for the two trials) to have at least 90% power. If the number of subjects is reached before the end of the planned recruitment period of 24 months, recruitment will continue up to 45 subjects per group (180 subjects for the whole study) to obtain more precise information, especially on the set of secondary outcomes. If the number is not attained, the recruitment period will be prolonged.
The sample size estimation heavily depends on estimates of the variability of the lymphoedema-specific QoL and the correlation with the baseline measurement. Therefore, after inclusion of 40 subjects per group the already available information will be used to verify if the assumptions were plausible (note however that there will be no information yet at the moment of the primary endpoint). If the observed SD and correlations deviate from the assumed values such that the desired power level of 90% is not guaranteed anymore, an increase of the planned sample size will be considered (if feasible). At the moment of this blinded interim analysis for sample size reestimation, the assumed drop-out rates will also be verified. No interim analyses are planned to stop the study earlier for efficacy or futility, this is to avoid loss of information on the secondary endpoints.
Data analyses
Statistical analysis will comply with the Consolidated Standards of Reporting Trials guidelines. The analysis will be conducted in a blinded way. The continuous data will be summarised using mean and SD and median and range values. Different analysis sets will be defined. The intention-to-treat analysis set (ITT) contains all randomised patients, grouped according to the allocated treatment. The modified ITT contains all randomised patients grouped according to the allocated treatment, but excluding patients who have withdrawn their consent to the randomised procedure. The as-treated analysis set also contains all randomised patients but grouping the patients according to their received treatment. The per-protocol analysis set contains all randomised patients who received the allocated treatment. The main analyses will be performed on the ITT analysis set. Results on the other analysis sets will be reported additionally.
Primary outcome
Lymphoedema-specific QoL.
A cLDA22 using the baseline measurement and the follow-up measurements after 1 month, 3 months, 6 months, 12 months and 18 months as outcomes will be used to compare the mean lymphoedema-specific QoL after 18 months based on a two-sided test with alpha=0.05. The choice of the covariance structure for the five measurements will be based on the Aikake criterion.24 Study site is added as a fixed factor in this model. For patients with a recurrence of cancer in the root of the limb, only observations before the recurrence are included.
Since the analysis is only valid under the missing at random (MAR) assumption (the probability of a missing lymphoedema-specific QoL measurement does not depend on the unobserved value), sensitivity analyses will be performed allowing a non-MAR mechanism. More specifically, starting from the MAR model, a jump-to-reference and tipping-point analysis will be applied.25
Key secondary outcomes
Change in limb volume:
For the arm/hand volume, ratios of the volume of the ipsilateral versus the contralateral side will be calculated. A multivariate model for the longitudinal measured ratios (seven time points) will be used to compare (changes in) log-transformed ratios between both groups. A log transformation for the ratios is used since intervals between units are not equidistant. For the leg/foot volume, the same model will be used but on the original measurements of the (most) affected limb instead of on the (log-transformed) ratios versus the contralateral side (since also patients with bilateral leg volume are included).
Duration of wearing the compression garment:
The same modelling approach will be used for the primary outcome.
Other secondary outcomes
Continuous outcomes will be analysed in a similar way as the primary outcome. Categorical (binary) data will be analysed using stratified χ² test and logistic regression models with general estimating equations for repeatedly measured binary data. Adverse events and complications after surgery will be reported descriptively.
This study has been designed to permit economic analysis in a later phase. If reconstructive surgery is deemed superior to no surgery (ie, is clinically effective), the next step is to investigate its cost-effectiveness by determining the incremental cost-effectiveness ratio (ICER). To determine the ICER, the costs from a healthcare payer’s perspective and from a societal perspective will be considered, as well as the effectiveness by using the EQ-5D-5L questionnaire. If reconstructive surgery is proven cost-effective, the budget impact will be calculated from a reimburse perspective.
Exploratory analyses
Subgroup analyses for the primary outcome will be considered as a function of the stage (stage 1 vs 2 a/2b), primary versus secondary lymphoedema, weight (normal weight (BMI≤25 kg/m2) vs overweight (BMI>25)) and combination of reconstructive techniques (combination of LVA/LNT vs only LVA or only LNT).
Moreover, a multivariable model will be constructed to predict the lymphoedema-specific QoL at 36 months based on 14 baseline variables. For subjects with a missing lymphoedema-specific QoL at 36 months, values will be imputed based on a multivariate longitudinal model for the lymphoedema-specific QoL measurements. A model reduction will be performed on a stacked dataset consisting of the multiple imputed data (at least 10 imputations), using a weighting scheme to account for the fraction of missing data in each covariate.26 Considering the drop-outs at 36 months, data for lymphoedema-specific QoL of 144 patients will be available.
Data security and management
A study-specific data management plan has been developed by the data management team. Participant data are stored on a secure database in accordance with the General Data Protection Regulations (2018). Data are deidentified and a unique trial identification number is used on all source documents. These source documents are being checked for completeness and congruity before data entry into REDCap. All trial documentation and data will be archived for at least 20 years after completion of the trial.
A risk assessment plan has also been made with a summary of the concerns in the trial, how they were mitigated, the probability that this will occur and its impact. This finally leads to a risk score (low, medium, high and critical). The concerns with the highest risks are discussed during the meeting of the trial steering committee (during recruitment period: once each 6 months; thereafter: once a year).
Trial monitoring
A separate monitoring plan has been constructed and will be conducted periodically by trial monitors (independent from trial staff). The first monitoring visit at each site will be conducted within 4–8 weeks following the baseline visit of the first study subject at that site. Thereafter, monitoring visits will be organised at mean intervals of 6 months during the recruitment, and mean intervals of 12 months thereafter. The participating site will provide direct access to the trial data and to the corresponding source data and documents. The trial will be monitored to ensure that it is being conducted in compliance with GCP and current legislation, that written informed consent has been obtained correctly, that the trial procedures have been followed as shown in the protocol, and that the data have been recorded, for which the source data will be compared with the data recorded in REDCap.
Ethics and dissemination
The SurLym trial will be conducted in compliance with the principles of the Declaration of Helsinki, the principles of GCP and in accordance with all applicable regulatory requirements. Approval has been obtained for the study protocol, the informed consent forms and other related documents by the main Ethical Committee of UZ Leuven (S631212) and the local Ethical Committees of UZ Gent and CHU UCL Namur. Any subsequent protocol amendments will be submitted to the ethical committee. Furthermore, the study is approved by the Federal Agency for Medicines and Health Products (EudraCT: 2021-000397-29).
Dissemination of results
The results of the study owned by the sponsor shall be disseminated as soon as possible after the end of the trial, by disclosing them to the public by appropriate means, including publications in peer-reviewed scientific journals and presentations at congresses and events. Open access will be ensured to all peer-reviewed scientific publications relating to the results of the study.
| Primary registry and trial identifying number | ClinicalTrials.gov identifier: NCT05064176 |
| Date of registration in primary registry | 24-8-2021 |
| Secondary identifying numbers | Ethical Committee UZ Leuven: S63212; EudraCT: 2021-000397-29 |
| Source of monetary and material support | Belgian Healthcare Knowledge Centre |
| Sponsor | University Hospitals Leuven, Clinical Trial centre, Herestraat 49, 3000 Leuven, Belgium |
| Contact for public and scientific queries | Nele.devoogdt@uzleuven.be |
| Public title | Added value of reconstructive lymphatic surgery to usual care in lymphoedema |
| Scientific title | Comparison of reconstructive lymphatic surgery vs no surgery, additional to decongestive lymphatic therapy (usual care), for the treatment of lymphoedema, through a multicenter, pragmatic andomized controlled trial |
| Acronym | SurLym-trial |
| Protocol version | V3.0 19-4-2022 |
| Country of recruitment | Belgium |
| Health condition studied | Primary or secondary upper or lower limb lymphoedema stage 1 to 2b |
| Intervention | Intervention group: Reconstructive lymphatic surgery (ie, LVA or LNT or combination), added to usual care Control group: Only usual care (no surgery) |
| Key inclusion and exclusion criteria | -Lymphoedema: upper/ lower limb; uni-/ bilateral; primary or secondary; stage 1 to 2b; ≥ 5% vol difference or≥2 minor/ 1 major lymphoscintigraphy criterion; total score or one domain score of Lymph-ICF questionnaire≥25/100 -History of DLT (≥6M) until minimal pitting, no liposuction/ reconstructive surgery in past -In case of lower limb lymphoedema: no CVI C4-C6, no DVT/ PTS -Age≥18 years, not pregnant, BMI≤35 No allergy for ICG/ iodine; no increased activity/ benign tumour thyroid gland; no heparin use and severe renal insufficiency |
| Study type | Multicentre, pragmatic randomised controlled trial |
| Date of first enrolment | March 2022 |
| Target sample size | 180 |
| Recruitment status | Recruiting |
| Primary endpoint | Lymphoedema-specific QOL, at 18 months post-baseline |
| Key secondary endpoints | Limb volume, at 18 months post-baseline Duration of wearing the compression garment, at 18 months post-baseline |
| Treatment duration | 18 months (usual care) |
| Follow-up duration | 36 months |
Supplementary Material
Acknowledgments
We are grateful to all medical doctors for referring potential participants for inclusion in the trial. We also want to thank the data management team and the representative of the clinical trial centre of UZ Leuven for their support. Finally, we are thankful to the patients of the advisory board and to the independent expert for their valuable advises in the preparation phase and during the course of the trial.
Footnotes
@DevoogdtNele
IF and ST contributed equally.
Contributors: ND is the chief investigator of the SurLym trial. TDV is the trial manager. ND, CR and TD are the principal investigators of the three study sites. SF is the statistician. LG is expert in occupational medicine and will supervise the economic analysis (if executed). ND, A-KH, ST, BB-H, IF, VVB, TDecorte, MDS, CR, CM, JF and T Deltombe will perform the recruitment of patients. ST, KS, PF, MS, AB, BDP and LD will perform the surgical procedures and follow-up. A-KH, JF, VVB, TDecorte and MDS are responsible for the clinical assessments (including lymphofluoroscopy and lymphoscintigraphy). GM, FK, AF and DD are the radiologists responsible for the lymph MRI an BK is a nuclear medicine physician responsible for the lymphoscintigraphy. JMA, SS, GP, KVL are the independent experts in reconstructive lymphatic surgery and will verify the quality of the surgical procedures. ND drafted the manuscript. All authors contributed to the establishment of the protocol, revised the manuscript and provided input according to their area of expertise.
Funding: This study (KCE19-1245) is an independent research study funded by the Belgian Health Care Knowledge Centre under the KCE Trials Programme.
Disclaimer: The views expressed in this publication are those of the author(s) and not necessarily those of Belgian Health Care Knowledge Centre.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: TDV is a postdoctoral research fellow of the Research Foundation–Flanders (FWO).
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Fu MR, Ridner SH, Hu SH, et al. Psychosocial impact of lymphedema: a systematic review of literature from 2004 to 2011. Psychooncology 2013;22:1466–84. 10.1002/pon.3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mercier G, Pastor J, Moffatt C, et al. LIMPRINT: health-related quality of life in adult patients with chronic edema. Lymphat Res Biol 2019;17:163–7. 10.1089/lrb.2018.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neubauer M, Schoberwalter D, Cenik F, et al. Lymphedema and employability - review and results of a survey of Austrian experts. Wien Klin Wochenschr 2017;129:186–91. 10.1007/s00508-017-1167-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Damstra RJ, Halk A-B, Dutch Working Group on Lymphedema . The dutch lymphedema guidelines based on the International classification of functioning, disability, and health and the chronic care model. J Vasc Surg Venous Lymphat Disord 2017;5:756–65. 10.1016/j.jvsv.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 5. Executive committee of the International society of L . The diagnosis and treatment of peripheral lymphedema: 2020 consensus document of the International society of lymphology. Lymphology 2020;53:3–19. 10.2458/lymph.4649 [DOI] [PubMed] [Google Scholar]
- 6. Kung TA, Champaneria MC, Maki JH, et al. Current concepts in the surgical management of lymphedema. Plast Reconstr Surg 2017;139:1003e–13e. 10.1097/PRS.0000000000003218 [DOI] [PubMed] [Google Scholar]
- 7. Chang DW, Masia J, Garza R, et al. Lymphedema: surgical and medical therapy. Plast Reconstr Surg 2016;138:209S–218S. 10.1097/PRS.0000000000002683 [DOI] [PubMed] [Google Scholar]
- 8. Jeong HH, Yoon IA, Al-Shomer FM, et al. Decompression of Axillary vein - an essential adjunct for advanced lymphedema. Plast Reconstr Surg 2023. 10.1097/PRS.0000000000011032 [DOI] [PubMed] [Google Scholar]
- 9. Gould DJ, Mehrara BJ, Neligan P, et al. Lymph node transplantation for the treatment of lymphedema. J Surg Oncol 2018;118:736–42. 10.1002/jso.25180 [DOI] [PubMed] [Google Scholar]
- 10. Markkula SP, Leung N, Allen VB, et al. Surgical interventions for the prevention or treatment of lymphoedema after breast cancer treatment. Cochrane Database Syst Rev 2019;2:CD011433. 10.1002/14651858.CD011433.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dionyssiou D, Demiri E, Tsimponis A, et al. A randomized control study of treating secondary stage II breast cancer-related lymphoedema with free lymph node transfer. Breast Cancer Res Treat 2016;156:73–9. 10.1007/s10549-016-3716-0 [DOI] [PubMed] [Google Scholar]
- 12. Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Vrieze T, Vos L, Gebruers N, et al. Revision of the lymphedema functioning, disability and health questionnaire for upper limb lymphedema (lymph-ICF-UL): reliability and validity. Lymphat Res Biol 2019;17:347–55. 10.1089/lrb.2018.0025 [DOI] [PubMed] [Google Scholar]
- 14. De Vrieze T, Gebruers N, Nevelsteen I, et al. Reliability of the Moisturemeterd compact device and the Pitting test to evaluate local tissue water in subjects with breast cancer-related Lymphedema. Lymphat Res Biol 2020;18:116–28. 10.1089/lrb.2019.0013 [DOI] [PubMed] [Google Scholar]
- 15. Tan I-C, Maus EA, Rasmussen JC, et al. Assessment of lymphatic contractile function after manual lymphatic drainage using near-infrared fluorescence imaging. Arch Phys Med Rehabil 2011;92:756–64. 10.1016/j.apmr.2010.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Devoogdt N, Lemkens H, Geraerts I, et al. A new device to measure upper limb circumferences: reliability and validity. Int Angiol 2010;29:401–7. [PubMed] [Google Scholar]
- 17. Atkinson TM, Wagner JS, Basch E. Trustworthiness of patient-reported outcomes in Unblinded cancer clinical trials. JAMA Oncol 2017;3:738–9. 10.1001/jamaoncol.2016.3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang DW, Dayan J, Greene AK, et al. Surgical treatment of Lymphedema: A systematic review and meta-analysis of controlled trials. Plast Reconstr Surg 2021;147:975–93. 10.1097/PRS.0000000000007783 [DOI] [PubMed] [Google Scholar]
- 19. De Vrieze T, Gebruers N, Nevelsteen I, et al. Responsiveness of the lymphedema functioning, disability, and health questionnaire for upper limb lymphedema in patients with breast cancer-related lymphedema. Lymphat Res Biol 2020;18:365–73. 10.1089/lrb.2019.0073 [DOI] [PubMed] [Google Scholar]
- 20. Devoogdt N, De Groef A, Hendrickx A, et al. Lymphedema functioning, disability and health questionnaire for lower limb lymphedema (lymph-ICF-LL): reliability and validity. Phys Ther 2014;94:705–21. 10.2522/ptj.20130285 [DOI] [PubMed] [Google Scholar]
- 21. Devoogdt N, Van Kampen M, Geraerts I, et al. Lymphoedema functioning, disability and health questionnaire (lymph-ICF): Reliability and validity. Phys Ther 2011;91:944–57. 10.2522/ptj.20100087 [DOI] [PubMed] [Google Scholar]
- 22. Liang KY, Zeger S. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Sankhya: Indian J Stat 2000;Series B 62:134–48. [Google Scholar]
- 23. Stroup WW. Mixed model procedures to assess power, precision, and sample size in the design of experiments. Proc Biopharmaceutical Section Am Stat Assoc 1999. [Google Scholar]
- 24. Fitzmaurice GM. Longitudinal Data Analysis. xiv. Boca Raton: CRC Press, 2009:618. [Google Scholar]
- 25. Molenberghs G, Fitzmaurice GM, Kenward MG, et al. Handbook of Missing Data Methodology. xxiv. Boca Raton: CRC Press, Taylor & Francis Group, 2015:574. [Google Scholar]
- 26. Wood AM, White IR, Royston P. How should variable selection be performed with multiply imputed data Stat Med 2008;27:3227–46. 10.1002/sim.3177 [DOI] [PubMed] [Google Scholar]
- 27. De Vrieze T, Frippiat J, Deltombe T, et al. Cross-cultural validation of the French version of the lymphedema functioning, disability and health questionnaire for upper limb lymphedema (lymph-ICF-UL). Disabil Rehabil 2021;43:2797–804. 10.1080/09638288.2020.1716271 [DOI] [PubMed] [Google Scholar]
- 28. Devoogdt N, Partsch H, Heroes A-K, et al. The ICC compression questionnaire: a comprehensive tool to evaluate compression materials or devices applied in subjects with lymphedema or chronic venous disease. Lymphat Res Biol 2022;20:191–202. 10.1089/lrb.2020.0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 30. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993;4:353–65. 10.2165/00019053-199304050-00006 [DOI] [PubMed] [Google Scholar]
- 31. Goorts K, Vandenbroeck S, Vander Elst T, et al. Quickscan assesses risk factors of long-term sickness absence: a cross-sectional (factorial) construct validation study. PLoS One 2019;14:e0210359. 10.1371/journal.pone.0210359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ainsworth BE, Macera CA, Jones DA, et al. Comparison of the 2001 BRFSS and the IPAQ physical activity questionnaires. Med Sci Sports Exerc 2006;38:1584–92. 10.1249/01.mss.0000229457.73333.9a [DOI] [PubMed] [Google Scholar]
- 33. De Vrieze T, Gebruers N, Tjalma WA, et al. What is the best method to determine excessive arm volume in patients with breast cancer-related Lymphoedema in clinical practice? Reliability, time efficiency and clinical feasibility of five different methods. Clin Rehabil 2019;33:1221–32. 10.1177/0269215519835907 [DOI] [PubMed] [Google Scholar]
- 34. Devoogdt N, Cavaggion C, Van der Gucht E, et al. Reliability, validity, and feasibility of water displacement method, figure-of-eight method, and circumference measurements in determination of ankle and foot edema. Lymphat Res Biol 2019;17:531–6. 10.1089/lrb.2018.0045 [DOI] [PubMed] [Google Scholar]
- 35. Hidding JT, Viehoff PB, Beurskens CHG, et al. Measurement properties of instruments for measuring of lymphedema. Phys Ther 2016;96:1965–81. 10.2522/ptj.20150412 [DOI] [PubMed] [Google Scholar]
- 36. Farrell K, Johnson A, Duncan H, et al. The intertester and intratester reliability of hand volumetrics. J Hand Ther 2003;16:292–9. 10.1197/s0894-1130(03)00153-4 [DOI] [PubMed] [Google Scholar]
- 37. Thomis S, Dams L, Fourneau I, et al. Correlation between clinical assessment and lymphofluoroscopy in patients with breast cancer-related lymphedema: a study of concurrent validity. Lymphat Res Biol 2020;18:539–48. 10.1089/lrb.2019.0090 [DOI] [PubMed] [Google Scholar]
- 38. Devoogdt N, Van den Wyngaert T, Bourgeois P, et al. Reproducibility of lymphoscintigraphic evaluation of the upper limb. Lymphat Res Biol 2014;12:175–84. 10.1089/lrb.2013.0034 [DOI] [PubMed] [Google Scholar]
- 39. Villa G, Campisi CC, Ryan M, et al. Procedural recommendations for lymphoscintigraphy in the diagnosis of peripheral lymphedema: the genoa protocol. Nucl Med Mol Imaging 2019;53:47–56. 10.1007/s13139-018-0565-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-078114supp001.pdf (86.9KB, pdf)


