Abstract
Background
DESTINY-Breast03 is a randomized, multicenter, open-label, phase III study of trastuzumab deruxtecan (T-DXd) versus trastuzumab emtansine (T-DM1) in patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (mBC) previously treated with trastuzumab and a taxane. A statistically significant improvement in progression-free survival (PFS) versus T-DM1 was reported in the primary analysis. Here, we report exploratory efficacy data in patients with and without brain metastases (BMs) at baseline.
Patients and methods
Patients were randomly assigned 1 : 1 to receive T-DXd 5.4 mg/kg or T-DM1 3.6 mg/kg. Patients with clinically inactive/asymptomatic BMs were eligible. Lesions were measured as per modified RECIST, version 1.1. Outcomes included PFS by blinded independent central review (BICR), objective response rate (ORR), and intracranial ORR as per BICR.
Results
As of 21 May 2021, 43/261 patients randomized to T-DXd and 39/263 patients randomized to T-DM1 had BMs at baseline, as per investigator assessment. Among patients with baseline BMs, 20/43 in the T-DXd arm and 19/39 in the T-DM1 arm had not received prior local BM treatment. For patients with BMs, median PFS was 15.0 months [95% confidence interval (CI) 12.5-22.2 months] for T-DXd versus 3.0 months (95% CI 2.8-5.8 months) for T-DM1; hazard ratio (HR) 0.25 (95% CI 0.13-0.45). For patients without BMs, median PFS was not reached (95% CI 22.4 months-not estimable) for T-DXd versus 7.1 months (95% CI 5.6-9.7 months) for T-DM1; HR 0.30 (95% CI 0.22-0.40). Confirmed systemic ORR was 67.4% for T-DXd versus 20.5% for T-DM1 and 82.1% for T-DXd versus 36.6% for T-DM1 for patients with and without BMs, respectively. Intracranial ORR was 65.7% with T-DXd versus 34.3% with T-DM1.
Conclusions
Patients with HER2-positive mBC whose disease progressed after trastuzumab and a taxane achieved a substantial benefit from treatment with T-DXd compared with T-DM1, including those with baseline BMs.
Key words: HER2-positive, metastatic breast cancer, brain metastases, antibody–drug conjugates
Highlights
-
•
43/261 patients randomized to T-DXd and 39/263 patients randomized to T-DM1 had clinically inactive BMs at baseline.
-
•
For patients with BMs, median (95%CI) PFS was 15.0 (12.5-22.2) months for T-DXd vs 3.0 (95% CI, 2.8-5.8) months for T-DM1.
-
•
For patients with BMs, confirmed systemic ORR was 67.4% for T-DXd versus 20.5% for T-DM1.
-
•
Intracranial ORR was 65.7% with T-DXd versus 34.3% with T-DM1.
-
•
Patients with HER2+ mBC with and without BMs achieved greater benefit from treatment with T-DXd vs T-DM1.
Introduction
Although human epidermal growth factor receptor 2 (HER2)-targeted therapies have improved disease prognosis, most patients with advanced HER2-positive breast cancer will experience disease progression.1 Up to 50% of patients with advanced HER2-positive disease will develop brain metastases (BMs), and these patients have a poor prognosis.2 According to observational studies, for patients with HER2-positive metastatic breast cancer (mBC) with BM treated with HER2-targeted therapies, median overall survival (OS) ranges from ∼18 to 30 months and median progression-free survival (PFS) ranges from ∼6 to 10 months.2, 3, 4 Approximately 70%-90% of patients described in these studies had received prior local BM therapy and most had received at least one prior line of systemic anti-HER2 therapy, including trastuzumab.2,4 Initial treatment of BMs typically involves locally directed therapies, including resection with post-operative radiation, stereotactic radiosurgery, and/or whole-brain radiotherapy, depending on metastasis size, resectability, and symptoms.5,6 However, patients often experience intracranial disease progression within 6-12 months with these therapies.6 Therefore, additional treatment options are needed for patients with brain lesions.
Several anti-HER2 therapies have been investigated for the treatment of BMs in HER2-positive mBC. The HER2CLIMB trial enrolled a proportion of patients with BMs, including patients with BMs that were untreated, treated and stable, or treated and progressing. The trial evaluated tucatinib in combination with trastuzumab and capecitabine versus placebo in combination with trastuzumab and capecitabine in patients with HER2-positive locally advanced or metastatic mBC who had previously received trastuzumab, pertuzumab, and trastuzumab emtansine (T-DM1); patients were excluded from HER2CLIMB if they had been previously treated with neratinib, afatinib, or any investigational HER2/epidermal growth factor receptor or HER2 tyrosine kinase inhibitors (TKIs). However, patients who had received lapatinib at least 1 year before enrolling were eligible. Patients in the overall study population had received a median of three prior lines of therapy in the metastatic setting. The confirmed intracranial objective response rate (ORR) was 47.3% [95% confidence interval (CI) 33.7% to 61.2%] in the tucatinib arm and 20.0% (95% CI 5.7% to 43.7%) in the placebo arm.6,7 In a subgroup analysis of the single-arm KAMILLA trial of T-DM1 in patients with HER2-positive advanced breast cancer who had received prior HER2-targeted therapy and chemotherapy, median PFS was 5.5 months (95% CI 5.3-5.6 months) for patients with untreated, asymptomatic, or controlled BMs at baseline and 7.7 months (95% CI 6.8-8.1 months) for patients without BMs. In patients with BMs, a best overall response rate, defined as complete response (CR) or partial response (PR), of 21.4% (27/126; 95% CI 14.6% to 29.6%) was reported. A ≥30% reduction in the sum of largest diameters of brain lesions was observed in 42.9% (54/126; 95% CI 34.1% to 52.0%) of patients, including 49.3% (33/67; 95% CI 36.9% to 61.8%) of patients without prior radiotherapy to the brain.8 In a retrospective subgroup analysis of the EMILIA trial, median PFS was 5.9 months with T-DM1 versus 5.7 months with capecitabine–lapatinib in patients with advanced HER2-positive mBC with previously treated and stable central nervous system (CNS) metastases; hazard ratio (HR) 1.0 (95% CI 0.54-1.84).9
Before DESTINY-Breast03, T-DM1 was the standard-of-care treatment for patients with HER2-positive mBC whose disease progressed after trastuzumab and a taxane. Approval was based on the results of the phase III EMILIA trial, in which median PFS was 9.6 months with T-DM1 versus 6.4 months with lapatinib–capecitabine; HR 0.65 (95% CI 0.55-0.77), P < 0.001.10
Trastuzumab deruxtecan (T-DXd) is an antibody–drug conjugate (ADC) comprising a humanized monoclonal anti-HER2 antibody designed to deliver an optimal antitumor effect.11,12 A cytotoxic topoisomerase I inhibitor payload is attached via a cleavable linker.11,12 Release of the membrane-permeable payload occurs after internalization of T-DXd, causing a bystander antitumor effect, resulting in death of the cell that internalized T-DXd as well as adjacent cells, regardless of HER2 expression.13
T-DXd has previously demonstrated systemic efficacy in patients with BMs. In a subgroup analysis of DESTINY-Breast01, the systemic ORR and median PFS with T-DXd in patients with a history of BMs (n = 24) were 58.3% (95% CI 36.6% to 77.9%) and 18.1 months (95% CI 6.7-18.1 months), respectively, and were 61.3% (95% CI 53.2% to 68.8%) and 16.4 months (95% CI 12.7 months-nonevaluable), respectively, in patients without a history of BMs.14
Intracranial efficacy of T-DXd has also been reported in both preclinical and clinical studies. Kabraji et al. reported that T-DXd reduced tumor size and prolonged survival in orthotopic HER2-positive breast cancer BM-derived xenograft mice compared with vehicle control (67-78 days versus 154-156 days).15 In the prospective, single-center, single-arm, phase II TUXEDO-1 trial, T-DXd demonstrated efficacy and safety in patients with HER2-positive mBC with newly diagnosed, untreated, or progressing BMs. Intracranial response was observed in 11/15 patients [73.3% (95% CI 48.1% to 89.1%)].16 In DEBBRAH, a multicenter, open-label, multicohort, phase II trial in patients with advanced HER2-positive or HER2-low mBC with BMs, T-DXd demonstrated preliminary intracranial efficacy in patients with pretreated HER2-positive stable, untreated, or progressing BMs. Intracranial objective response was reported in 2/4 patients [50.0% (95% CI 6.7% to 93.2%)] with asymptomatic untreated BMs and 4/9 patients [44.4% (95% CI 13.7% to 78.8%)] with treated and progressing BMs.17
In the phase III DESTINY-Breast03 trial, T-DXd demonstrated a clinically meaningful and statistically significant improvement in PFS versus T-DM1 in patients with HER2-positive mBC: not reached (NR) [95% CI 18.5 months-not estimable (NE)] versus 6.8 months (95% CI 5.6-8.2 months); HR 0.284 (95% CI 0.217-0.373, P < 0.0001). The 12-month PFS was 75.8% for T-DXd (95% CI 69.8% to 80.7%) versus 34.1% (95% CI 27.7% to 40.5%) for T-DM1.18 Based on the strength of DESTINY-Breast03 efficacy and safety data, the United States Food and Drug Administration (FDA) approved T-DXd for the treatment of patients with unresectable or advanced HER2-positive breast cancer who have received prior anti-HER2 therapy in the metastatic setting or in the adjuvant/neoadjuvant setting and have progressed within 6 months.19 We report subgroup analyses for patients from DESTINY-Breast03 with and without BMs at baseline.
Patients and methods
Trial design
DESTINY-Breast03 is an open-label, multicenter, phase III study in patients with HER2-positive breast cancer designed to investigate the efficacy and safety of T-DXd versus T-DM1 in patients with HER2-positive, unresectable, and/or metastatic BC whose disease has progressed on or after trastuzumab and a taxane in the advanced/metastatic setting or whose disease recurred within 6 months after neoadjuvant or adjuvant treatment involving trastuzumab and a taxane. The trial design, study site information, and primary results have previously been published.18
All participants were assessed as per investigator at baseline for the presence of BMs. In this study, patients with clinically inactive/asymptomatic BM were allowed if they did not require treatment with corticosteroids or anticonvulsants. Concomitant continuous use of corticosteroids was prohibited during the study. The initial version of the protocol allowed patients with previously locally untreated BMs to be enrolled. Following the protocol amendment, prior local therapy to BM became mandatory. Patients with treated BMs were eligible if they were stable and not symptomatic. Patients with BMs must have recovered from the acute toxic effect of radiotherapy. At least 2 weeks must have elapsed since the receipt of whole-brain radiotherapy or stereotactic radiation therapy, and radiotherapy during the treatment period was prohibited.
Patients were excluded if they had a history of noninfectious interstitial lung disease (ILD)/pneumonitis that required glucocorticoids or had suspected ILD/pneumonitis that could not be ruled out by imaging at screening. Full inclusion and exclusion criteria are included with the trial protocol and have previously been published.18
Randomization and masking
Patients were randomly assigned 1 : 1 to T-DXd or T-DM1. T-DXd was administered intravenously every 3 weeks at 5.4 mg per kilogram of body weight, and T-DM1 was administered intravenously every 3 weeks at 3.6 mg per kilogram of body weight. Randomization was carried out with the use of an interactive web-based system. Patients and investigators were not masked to the treatment administered. Full randomization details have been previously published.18
Trial oversight
This study was sponsored and designed by Daiichi Sankyo and was funded by AstraZeneca and Daiichi Sankyo. The study was approved by the institutional review board at each site and conducted in adherence with the International Conference on Harmonization Good Clinical Practice, the Declaration of Helsinki, and local regulations on the conduct of clinical research. All patients provided written informed consent before study participation.
Assessment and classification of brain metastases in the primary trial
Brain lesions were considered nontarget lesions only and were not required to be measurable for the assessment of the primary trial efficacy outcome, as per modified RECIST, version 1.1 (mRECIST v1.1).20 Assessment of the primary outcome has been previously published.18 Baseline brain computed tomography (CT) or magnetic resonance imaging (MRI) was mandatory for all patients at screening. Regular brain imaging was continued at 6-week (±7 days) intervals from randomization in patients with baseline BMs as per investigator assessment, independent of treatment cycle. Patients without BMs at baseline as per investigator assessment did not require additional brain scans for tumor assessment unless clinically indicated. Imaging results were reviewed by an independent radiologic facility for patients with baseline BMs as identified by investigator.
Efficacy assessments
Preplanned efficacy assessments included PFS by blinded independent central review (BICR) and ORR by BICR, as assessed by mRECIST v1.1.20 For efficacy assessments, patients with BMs were evaluated regardless of prior local treatment to BMs.
Exploratory efficacy assessments
Assessment of intracranial response was not planned in the protocol. Measurement of brain lesions was carried out retrospectively by BICR. Exploratory analyses included percentage change in sum of diameters of intracranial lesions, intracranial ORR as per BICR, and sites of progression. Intracranial ORR was assessed as the proportion of patients with a best overall intracranial response of CR or PR as assessed by BICR as per mRECIST v1.1.20
Safety assessments
Safety results have been previously described.18
Primary statistical analyses
PFS was defined as the time from the date of randomization to the date of the first radiographic disease progression or death due to any cause, whichever came first. Median PFS was evaluated using Kaplan–Meier analysis; 95% CI was evaluated using the Brookmeyer–Crowley method. Estimates and 95% CI for PFS rate at the specified time point were from Kaplan–Meier analysis.
ORR and 95% CI were determined based on Clopper–Pearson method for single proportion, with the difference between treatment arms based on the difference of two proportions with continuity correction. The full statistical analysis plan has been previously published.18
Exploratory statistical analyses
Intracranial assessments were not prespecified in the protocol, and statistical analyses were not carried out. This study is registered with ClinicalTrials.gov, NCT03529110.
Results
Patients
Between 30 July 2018 and 23 June 2020, 524 patients at 169 study centers in Asia, Australia, Europe, North America, and South America were enrolled and randomly assigned to receive either T-DXd (n = 261) or T-DM1 (n = 263) (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.102924). At data cut-off (21 May 2021), 125 patients (48.6%) who received T-DXd and 214 patients (82.0%) who received T-DM1 had discontinued treatment. Baseline characteristics and prior therapies were balanced across treatment arms and in patients with and without BMs (Table 1). In the T-DXd arm, 62 patients (23.8%) had a history of BMs compared with 52 patients (19.8%) in the T-DM1 arm.18 In the T-DXd arm, 43 patients (16.5%) had BMs at baseline, compared with 39 patients (14.8%) in the T-DM1 arm. There were 20/43 patients (46.5%) randomly assigned to T-DXd and 19/39 patients (48.7%) randomly assigned to T-DM1 who had not received any prior BM treatment (radiotherapy or surgery). In the T-DXd arm, 22/43 patients (51.2%) with BMs had received prior radiotherapy to the brain and 21/43 patients (48.8%) had not. In the T-DM1 arm, 18/39 patients (46.2%) with BMs had received prior radiotherapy to the brain and 21/39 patients (53.8%) had not.
Table 1.
Patient characteristics and prior therapies
| T-DXd (n = 261) |
T-DM1 (n = 263) |
|||
|---|---|---|---|---|
| Patients with BMs (n = 43) | Patients without BMs (n = 218) | Patients with BMs (n = 39) | Patients without BMs (n = 224) | |
| Age, median (range), years | 52.8 (29.2-76.2) | 54.4 (27.9-83.1) | 51.8 (26.0-78.2) | 54.8 (20.2-83.0) |
| Female, n (%) | 43 (100) | 217 (99.5) | 38 (97.4) | 224 (100) |
| Region, n (%) | ||||
| Europe | 10 (23.3) | 44 (20.2) | 4 (10.3) | 46 (20.5) |
| Asia | 24 (55.8) | 125 (57.3) | 30 (76.9) | 130 (58.0) |
| North America | 1 (2.3) | 16 (7.3) | 1 (2.6) | 16 (7.1) |
| Rest of the worlda | 8 (18.6) | 33 (15.1) | 4 (10.3) | 32 (14.3) |
| HER2 status (IHC), n (%) | ||||
| 3+ | 39 (90.7) | 195 (89.4) | 38 (97.4) | 194 (86.6) |
| 2+ | 4 (9.3) | 21 (9.6) | 1 (2.6) | 29 (12.9) |
| 1+/not evaluable | 0/0 | 1 (0.5)/1 (0.5) | 0/0 | 0/1 (0.4) |
| ECOG PS, n (%) | ||||
| 0/1 | 20 (46.5)/23 (53.5) | 134 (61.5)/83 (38.1) | 21 (53.8)/18 (46.2) | 154 (68.8)/69 (30.8) |
| Hormone receptor, n (%) | ||||
| Positive/negative | 23 (53.5)/20 (46.5) | 108 (49.5)/110 (50.5) | 19 (48.7)/20 (51.3) | 115 (51.3)/109 (48.7) |
| Visceral disease, n (%) | ||||
| Yes/no | 34 (79.1)/9 (20.9) | 150 (68.8)/68 (31.2) | 33 (84.6)/6 (15.4) | 152 (67.9)/72 (32.1) |
| Prior treatment for mBC, n (%) | 43 (100) | 217 (99.5) | 39 (100) | 223 (99.6) |
| Prior lines of therapy in the metastatic setting, n (%) | ||||
| 0-1 | 15 (34.9) | 94 (43.1) | 12 (30.8) | 91 (40.6) |
| ≥2 | 28 (65.1) | 124 (56.9) | 27 (69.2) | 133 (59.4) |
| Prior anti-HER2 therapy, n (%) | ||||
| Trastuzumab | 43 (100) | 217 (99.5) | 39 (100) | 223 (99.6) |
| Pertuzumab | 26 (60.5) | 136 (62.4) | 21 (53.8) | 137 (61.2) |
| Prior HER2 TKI therapy, n (%) | 10 (23.3) | 32 (14.7) | 12 (30.8) | 24 (10.7) |
| Prior treatment for BMs, n (%) | ||||
| None | 20 (46.5) | — | 19 (48.7) | — |
| RT alone | 16 (37.2) | 15 (38.5) | ||
| Surgery alone | 1 (2.3) | 2 (5.1) | ||
| RT and surgery | 6 (14.0) | 3 (7.7) | ||
| Time since prior RT to the brain, median (range), months | 1.6 (0.5-45.2) | — | 3.4 (0.5-80.1) | — |
BMs, brain metastases; ECOG PS, Eastern Cooperative Oncology Group performance status; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ISH, in situ hybridization; mBC, metastatic breast cancer; RT, radiotherapy; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan, TKI, tyrosine kinase inhibitor.
Includes South America and Australia.
Efficacy
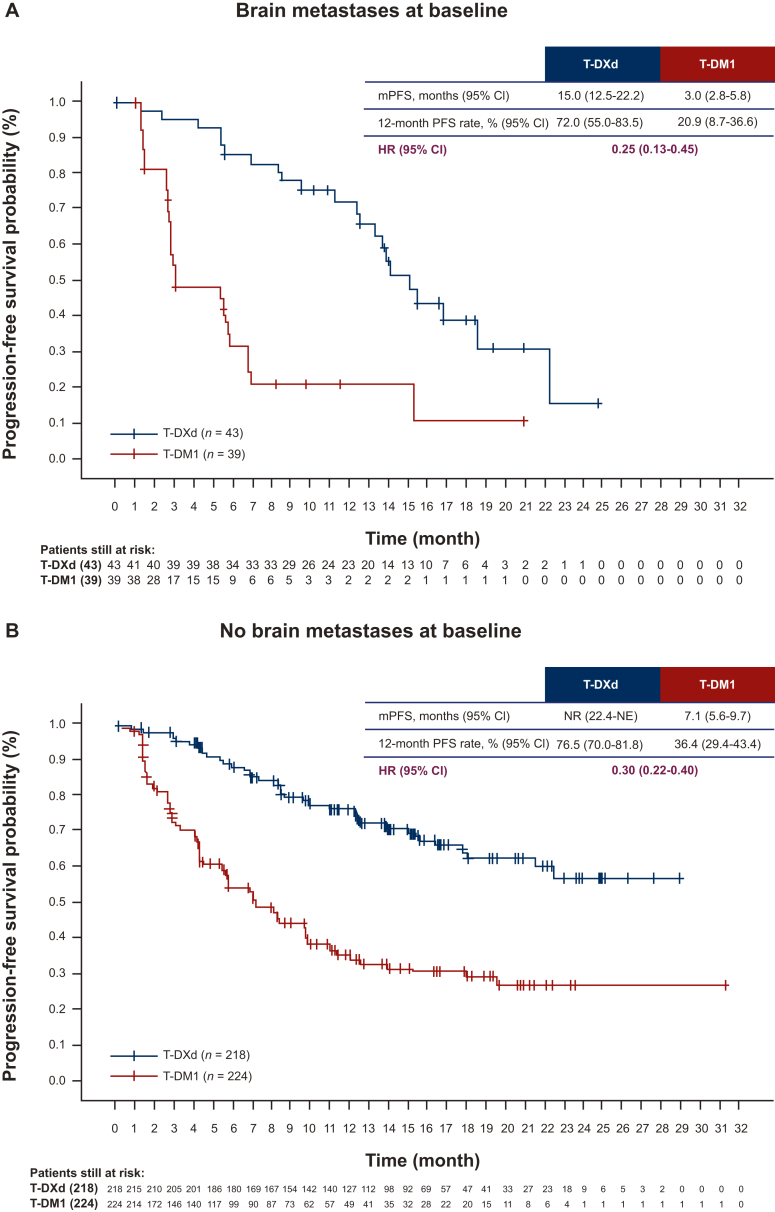
Median PFS for patients with BMs at baseline was 15.0 months (95% CI 12.5-22.2 months) for T-DXd versus 3.0 months (95% CI 2.8-5.8 months) for T-DM1 (HR 0.25, 95% CI 0.13-0.45) (Figure 1, Table 2). The 12-month PFS rate for patients with BMs was 72.0% (95% CI 55.0% to 83.5%) for T-DXd versus 20.9% (95% CI 8.7% to 36.6%) for T-DM1.
Figure 1.
Kaplan–Meier curves of PFS in patients (A) with and (B) without BMs.
BMs, brain metastases; CI, confidence interval; HR, hazard ratio; mPFS, median progression-free survival; NE, not estimable; NR, not reached; PFS, progression-free survival; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan.
Table 2.
Progression-free survival summary
| Patients with BMs (n = 82) |
Patient without BMs (n = 442) |
|||
|---|---|---|---|---|
| T-DXd (n = 43) | T-DM1 (n = 39) | T-DXd (n = 218) | T-DM1 (n = 224) | |
| Median PFS (95% CI), months | 15.0 (12.5-22.2) | 3.0 (2.8-5.8) | NR (22.4-NE) | 7.1 (5.6-9.7) |
| HR 0.25 (95% CI 0.13-0.45) | HR 0.30 (95% CI 0.22-0.40) | |||
| 12-month PFS rate (95% CI), % | 72.0 (55.0-83.5) | 20.9 (8.7-36.6) | 76.5 (70.0-81.8) | 36.4 (29.4-43.4) |
| Median PFS, patients without prior BM therapy (95% CI), months | 16.8 (12.4-NE) (n = 20) |
5.6 (2.8-5.8) (n = 19) |
— | — |
| HR 0.16 (95% CI 0.06-0.44) | — | — | ||
| Median PFS, patients with prior BM therapy (95% CI), months | 14.1 (8.5-18.5) (n = 23) |
2.8 (2.6-NE) (n = 20) |
— | — |
| HR 0.35 (95% CI 0.15-0.79) | — | — | ||
BMs, brain metastases; CI, confidence interval; HR, hazard ratio; NE, not estimable; NR, not reached; PFS, progression-free survival; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan.
Median PFS for patients without BMs was NR (95% CI 22.4 months-NE) for T-DXd versus 7.1 months (95% CI 5.6-9.7 months) for T-DM1 (HR 0.30, 95% CI 0.22-0.40). Twelve-month PFS rate for patients without BMs was 76.5% (95% CI 70.0% to 81.8%) for T-DXd versus 36.4% (95% CI 29.4% to 43.4%) for T-DM1.
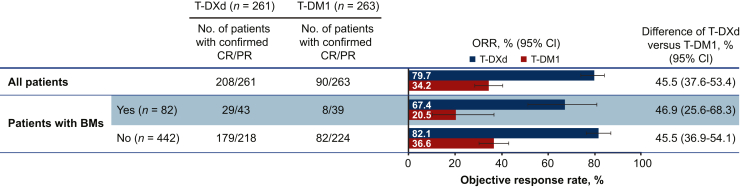
Confirmed systemic objective response was achieved by 208 patients (79.7%) in the T-DXd arm (95% CI 74.3% to 84.4%) versus 90 patients (34.2%) in the T-DM1 arm (95% CI 28.5% to 40.3%) (Figure 2, Table 3). For patients with BMs, the confirmed systemic ORR was 67.4% (29/43; 95% CI 51.5% to 80.9%) for T-DXd versus 20.5% (8/39; 95% CI 9.3% to 36.5%) for T-DM1. For patients without BMs, confirmed systemic ORR was 82.1% (179/218; 95% CI 76.4% to 87.0%) for T-DXd versus 36.6% (82/224; 95% CI 30.3% to 43.3%) for T-DM1.
Figure 2.
Confirmed systemic ORR in patients with and without BMs.
BMs, brain metastases; CR, complete response; ORR, objective response rate; PR, partial response; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan.
Table 3.
Confirmed systemic and intracranial objective response rate and best overall response
| Confirmed systemic objective response rate and best overall response | ||||||
|---|---|---|---|---|---|---|
| T-DXd |
T-DM1 |
|||||
| Overall population (n= 261) | Patients with BMs (n = 43) | Patients without BMs (n = 218) | Overall population (n = 263) | Patients with BMs (n = 39) | Patients without BMs (n = 224) | |
| Confirmed ORR,an [% (95% CI)] | 208 [79.7 (74.3-84.4)] | 29 [67.4 (51.5-80.9)] | 179 [82.1 (76.4-87.0)] | 90 [34.2 (28.5-40.3)] | 8 [20.5 (9.3-36.5)] | 82 [36.6 (30.3-43.3)] |
| CR | 42 (16.1) | 2 (4.7) | 40 (18.3) | 23 (8.7) | 0 | 23 (10.3) |
| PR | 166 (63.6) | 27 (62.8) | 139 (63.8) | 67 (25.5) | 8 (20.5) | 59 (26.3) |
| SD | 44 (16.9) | 11 (25.6) | 33 (15.1) | 112 (42.6) | 22 (56.4) | 90 (40.2) |
| PD | 3 (1.1) | 1 (2.3) | 2 (0.9) | 46 (17.5) | 7 (17.9) | 39 (17.4) |
| Not evaluable | 6 (2.3) | 2 (4.7) | 4 (1.8) | 15 (5.7) | 2 (5.1) | 13 (5.8) |
| CR + PR + SD (DCR) | 252 (96.6) | 40 (93.0) | 212 (97.2) | 202 (76.8) | 30 (76.9) | 172 (76.8) |
| mDOR (95% CI), months | Not evaluable (20.3-not evaluable) | 12.9 (8.5-not evaluable) | Not evaluable (20.3-not evaluable) | Not evaluable (12.6-not evaluable) | 7.2 (2.8-not evaluable) | Not evaluable (12.6-not evaluable) |
|
Intracranial response as per blinded independent central reviewb | ||||||
|---|---|---|---|---|---|---|
| T-DXd (n = 35) | T-DM1 (n = 35) | |||||
| Best overall response,an (%) | ||||||
| Patients with objective response of CR or PR, n | 23 | 12 | ||||
| CR | 10 (28.6) | 1 (2.9) | ||||
| PR | 13 (37.1) | 11 (31.4) | ||||
| Non-CR/non-PD | 6 (17.1) | 7 (20.0) | ||||
| SD | 4 (11.4) | 7 (20.0) | ||||
| PD | 0 | 7 (20.0) | ||||
| Not evaluable | 0 | 1 (2.9) | ||||
| Missing | 2 (5.7) | 1 (2.9) | ||||
BICR, blinded independent central review; BMs, brain metastases; CI, confidence interval; CR, complete response; DCR, disease control rate; mDOR, median duration of response; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan.
Based on BICR.
Table includes target and nontarget lesions.
Intracranial response
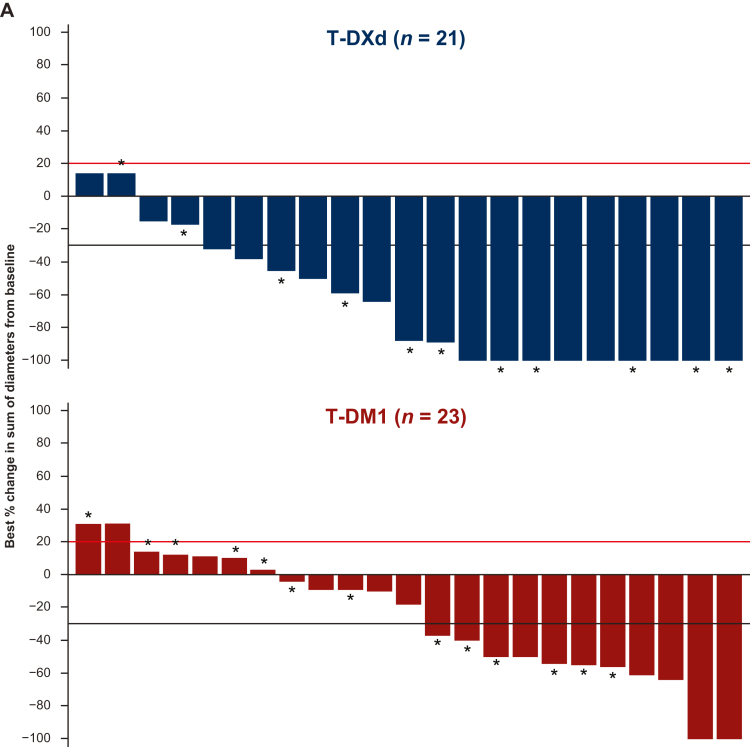
Although there were 82 patients with baseline BMs as assessed by investigator, 70 patients had imaging-detectable BMs (35 in each arm) as assessed by independent central review at baseline. In the T-DXd arm, 11 patients (31.4%) had BMs detected by CT scan and 24 (68.6%) by MRI at baseline. In the T-DM1 arm, 12 patients (34.4%) had BMs detected by CT scan and 23 (65.7%) by MRI at baseline. Of the 70 patients with imaging-detectable BMs, 23 patients in the T-DXd arm and 24 patients in the T-DM1 arm had at least one measurable lesion (a minimum size of 10 mm by CT/MRI scan) at baseline; the remaining patients had nontarget lesions only.
The best percentage change in sum of diameters of intracranial lesions from baseline is shown in Figure 3A for patients with BMs. Only patients with measurable BM lesion assessments and at least one post-baseline scan were eligible for inclusion in the waterfall plot. Of the patients in the waterfall plot, 10/21 (47.6%) randomly assigned to T-DXd and 10/23 (43.5%) randomly assigned to T-DM1 had received prior radiotherapy to the brain.
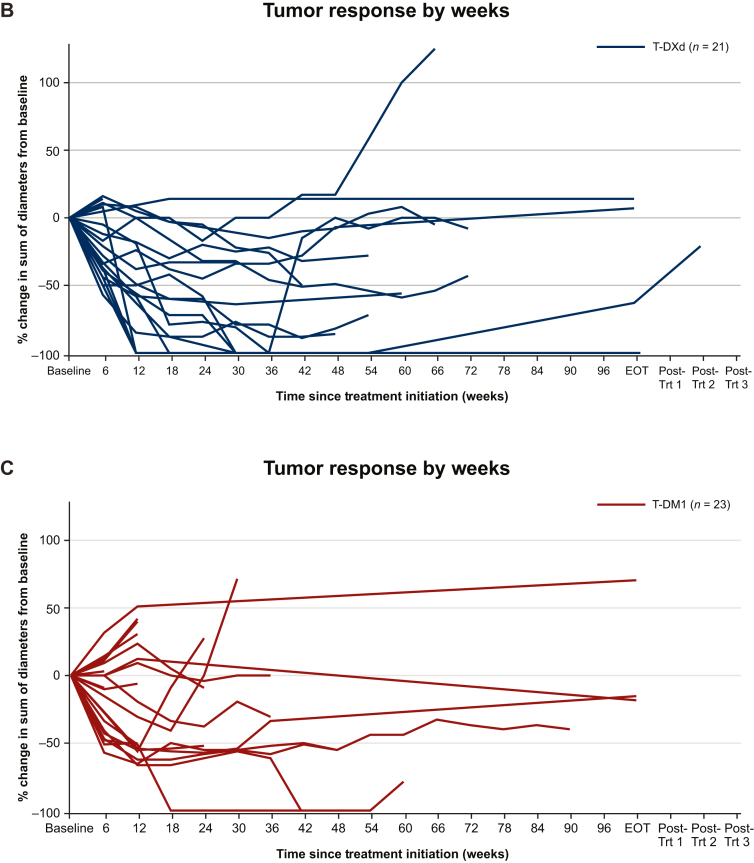
Figure 3.
Waterfall and spider plots of brain lesion measurements. (A) Best percentage change in sum of diameters of measurable intracranial lesions from baseline in patients with BMs. The red line at 20% indicates PD; the black line at −30% indicates PR. ∗Patients without prior radiation therapy to the brain. Brain lesion measurements over time in (B) the T-DXd subgroup and (C) the T-DM1 subgroup.
BM, brain metastases; EOT, end of treatment; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan; Trt, treatment.
An objective intracranial response of CR or PR was experienced by 23/35 patients (65.7%) randomized to T-DXd, compared with 12/35 patients (34.3%) randomized to T-DM1 (Table 3). Intracranial CR was experienced by 28.6% (10/35) of patients with T-DXd compared with 2.9% (1/35) with T-DM1. Intracranial PR was experienced by 37.1% (13/35) of patients with T-DXd versus 31.4% (11/35) with T-DM1. Intracranial progressive disease (PD) was not reported in any of the 35 patients with BMs in the T-DXd arm compared with 7/35 patients (20.0%) in the T-DM1 arm. No patients in the T-DXd arm and one patient (2.9%) in the T-DM1 arm had an intracranial response that was not evaluable.
BM lesion size over time is shown in Figure 3B for patients randomly assigned to T-DXd and in Figure 3C for patients randomly assigned to T-DM1.
Sites of progression
Sites of first progression by BICR are shown in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.102924. Overall, any PD was experienced by 84 patients (32.2%) randomly assigned to T-DXd versus 155 (58.9%) to T-DM1 at the time of data cut-off. For patients with BMs, 48.8% (21/43) had PD with T-DXd versus 69.2% (27/39) with T-DM1. For patients without BMs, 28.9% (63/218) had PD with T-DXd versus 57.1% (128/224) with T-DM1. For patients with BMs, the most common first site of progression with T-DXd was the brain [9 patients (20.9%)], followed by lung, breast, and liver [3 patients, each (7.0%)]; with T-DM1, the most common site was the brain [11 patients (28.2%)], followed by lung [10 patients (25.6%)], breast [6 patients (15.4%)], and liver [5 patients (12.8%)]. For patients without BMs, the most common sites of first progression with T-DXd were the liver and lung [16 patients, each (7.3%)], followed by breast [7 patients (3.2%)], and axillary lymph nodes [5 patients (2.3%)]; with T-DM1, the most common site was the lung [41 patients (18.2%)], followed by breast [32 patients (14.3%)], liver [19 patients (8.5%)], and axillary lymph nodes [17 patients (7.6%)]. For patients without BMs, the rate of disease progression in the brain as the first site was 1.8% (4/218) for T-DXd versus 0.4% (1/224) for T-DM1. Radiation necrosis in the brain occurred in one patient (0.4%) in the T-DXd arm and no patients in the T-DM1 arm.
Discussion
In this exploratory analysis, consistent systemic disease control and efficacy benefit were observed with T-DXd versus T-DM1 in patients with HER2-positive mBC with and without BMs. Median PFS was numerically longer with T-DXd than with T-DM1 for patients with BMs (15.0 versus 3.0 months) and estimated to be numerically longer for patients without BMs (NR versus 7.1 months). A higher proportion of patients randomly assigned to T-DXd exhibited confirmed systemic objective response relative to those randomly assigned to T-DM1, including in patients with baseline BMs. T-DXd treatment was associated with substantial intracranial response [23/35 patients (65.7%) versus 12/35 patients (34.3%)] and reduction in CNS disease. Intracranial PD was observed in fewer patients randomized to T-DXd compared with T-DM1 [0/35 patients (0%) versus 7/35 patients (20.0%)]. For patients with BMs at baseline, those randomized to T-DXd experienced fewer PD events versus T-DM1 (48.8% versus 69.2%).
Patients with advanced HER2-positive mBC with BMs have a poor prognosis and limited treatment options.2 The current treatment landscape for these patients includes T-DM1 in addition to the small-molecule HER2 TKIs lapatinib, neratinib, and tucatinib.21 Although cross-trial comparisons should be made with caution, prior trials evaluating the efficacy of these TKIs in patients with HER2-positive mBC and BMs have reported numerically lower median PFS and ORRs compared with the efficacy observed with T-DXd in DESTINY-Breast03 (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.102924). It should be noted that some of these trials included patients with previously treated progressive BMs.
TKIs are hypothesized to more readily penetrate the blood–brain barrier compared with larger molecules, such as trastuzumab. However, early data showed that the use of large molecules, including ADCs, can lead to intracranial responses, and preclinical studies have reported the accumulation of ADCs in brain lesions.22 Data reported here demonstrate intracranial efficacy for patients treated with T-DXd, providing evidence that T-DXd may penetrate the blood–brain barrier to elicit response. Even as research is ongoing, data suggest that intracranial efficacy of ADCs may result from the disruption of the blood–brain barrier by decreased expression of tight junction proteins, loss of astrocyte pedicles, and reduction in pericyte coverage in brain tumors. Moreover, neoangiogenesis in early BM formation has been shown to lead to leaky vasculature.23 Other potential mechanisms of ADC CNS delivery include exposure to prior radiation and/or local surgery and a blood–tumor barrier with heterogeneous permeability.23,24
Indeed, evidence for efficacy of T-DM1 in patients with HER2-positive mBC with BMs has been reported.25,26 In the EMILIA trial, patients with treated, asymptomatic BMs at baseline had significantly improved OS with T-DM1 compared with capecitabine–lapatinib (26.8 versus 12.9 months).9 In subgroup analyses from the KAMILLA trial, intracranial response was observed in 42.9% of patients.8 Median PFS and ORR values reported for T-DXd in the current analysis are numerically higher than values reported for T-DM1 in KAMILLA and EMILIA.8,9
Intracranial activity of T-DXd has also been reported in clinical trials in patients with HER2-positive mBC and BMs, including those with active BMs, including untreated and treated progressing BMs (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.102924).14, 15, 16, 17 Results from the DESTINY-Breast03 study provide an additional, larger dataset of T-DXd activity in patients with BMs.
DESTINY-Breast03 initially enrolled patients with active BMs. In July 2020, the FDA issued guidelines for the inclusion of patients with BMs in clinical trials. Guidance was given for including patients with treated/stable BMs (patients who have received previous CNS-targeted therapy and their CNS disease is stable), active BMs (new or progressive BMs that have not been treated with CNS-directed therapy since documented progression), or leptomeningeal metastases. The eligibility criteria were amended to no longer include patients with untreated BMs.
The population of patients with baseline BMs randomized in DESTINY-Breast03 was represented by patients with asymptomatic CNS disease. Nearly half of the randomly assigned population had not received any local therapy for their BMs, which would classify them as having active BMs as per FDA criteria.27 The other half of patients with baseline BMs represents a subgroup with treated/stable BMs as per FDA criteria. In this analysis, the outcomes in patients with active and stable BMs were not assessed separately, which represents a limitation. Because clinical trials often exclude patients with progressive/active BMs, it is not clear if HER2-directed therapy is effective in these patients, and further studies are therefore needed.3 The outcomes of patients with active and stable BMs treated with T-DXd will be assessed in further publications, which will consider longer follow-up and larger sample size. Furthermore, the authors acknowledge that Response Assessment in Neuro-Oncology BM (RANO-BM) criteria might be considered as more applicable to assess efficacy outcomes in patients with brain tumors in prospective clinical trials.28 However, our exploratory analysis was a retrospective one and the parent protocol included patients with and without BMs; therefore, RANO-BM criteria could not be applied. We agree that this might be considered as a limitation. However, other similar trials, including HER2CLIMB and KAMILLA, assessed intracranial response using mRECIST.6,8,29 Additionally, as RANO-BM considers symptomatic changes in assessment, and as these were not collected, it could not therefore be utilized. Additionally, quality of life, neurocognitive, and neurological function measures were not assessed, which is a limitation of this retrospective study. The authors note that these important parameters should be included in future prospective studies in patients with BMs.
The ongoing DESTINY-Breast07 (NCT04538742; includes patients with active or stable, untreated, or previously treated and progressive BMs), DESTINY-Breast09 (NCT04784715; includes patients with inactive or treated and asymptomatic BMs), DESTINY-Breast12 (NCT04739761; includes patients with untreated, or previously treated and stable, or progressive BMs), DEBBRAH (NCT04420598; includes patients with pretreated asymptomatic, untreated, or progressive BMs), and TUXEDO-1 (NCT04752059; includes patients with newly diagnosed, untreated, or progressive BMs) trials continue to evaluate T-DXd efficacy in patients with HER2-expressing mBC and BMs, either as monotherapy or in combination with other therapies. Additional limitations of this report include that intracranial response assessment was carried out as a retrospective analysis and outcomes related to CNS were not prespecified in the protocol. In addition, as with other trials that have included subgroups of patients with BMs, (HER2CLIMB, KAMILLA), brain scans were mandated only during study treatment for patients with BMs at baseline or with suspected progression in the brain. This limits our ability to report on rates of progression in the brain for participants without BMs at baseline. Furthermore, it could not be ruled out that the response in some patients was the result of previous local therapy, including radiotherapy. A minimum of 2 weeks must have elapsed between the end of whole-brain radiotherapy and study enrollment; however, this washout period may not have been sufficient to eliminate the possible confounding of treatment effects. In addition, the number of patients with BMs in each treatment arm was small, and therefore any analyses in these patients, or any further subgroups by treatment status, should be interpreted with caution.
Results from this exploratory analysis show that T-DXd provides meaningful efficacy benefit for patients with HER2-positive mBC either with or without clinically inactive/asymptomatic BMs in the second-line setting. These findings also further support the continued investigation of T-DXd in patients with BMs, including active BMs, a population for whom treatment options are currently limited.
Acknowledgements
This study was sponsored and designed by Daiichi Sankyo in collaboration with AstraZeneca. We thank the patients and their families for their participation and the study site staff for their contributions. We acknowledge Antonio Cagnazzo for his assistance with statistical analysis and data generation. Medical writing support was provided by Laura Halvorson, PhD, and Marianna B. Johnson, PhD (ApotheCom), and was funded by Daiichi Sankyo, Inc.
Funding
This study was supported by Daiichi Sankyo and AstraZeneca (no grant number).
Data collection and study design
For data collection and analysis, the study was designed and led by Daiichi Sankyo. AstraZeneca entered into a collaboration agreement with Daiichi Sankyo Co., Ltd., for T-DXd in March 2019. Daiichi Sankyo and AstraZeneca were both involved in study oversight and data collection. All authors and sponsors assisted in data interpretation, writing the report, and reviewing the manuscript. All authors had full access to all data in the study and provided final approval to submit the manuscript for publication.
Disclosure
SAH reports contracted paid research from Ambrx, Amgen, AstraZeneca, Arvinas, Bayer, CytomX, Daiichi Sankyo, Dignitana, Genentech/Roche, Gilead, GSK, Immunomedics, Eli Lilly, Macrogenics, Novartis, Pfizer, OBI Pharma, Orinove, Pieris, PUMA, Radius, Sanofi, Seagen, Zymeworks, and Phoenix Molecular Designs, Ltd; institutional grants from Ambrx and Samumed; research funding as national/international principal investigator from Novartis, Daiichi Sankyo, Seagen, and GNE/Roche; is a steering committee member of Novartis, Lilly, Daiichi Sankyo/AstraZeneca, GNE/Roche, and Sanofi; travel expenses paid for by Lilly (2019); and uncompensated consulting/advisory board memberships with 4DPharma, Ambrx, Amgen, Artios, Arvinas, Daiichi Sankyo, Dantari, Genentech/Roche, Immunomedics, Macrogenics, Eli Lilly, Novartis, NK Max, Pieris, Pyxis, Seagen, and Biotheranostics. SBK reports institutional research grants from Novartis, Sanofi Aventis, and Dongkook Pharmaceutical Company; consulting fees from Novartis, AstraZeneca, Lilly Dae Hwa Pharmaceutical Co, Ltd, IUS Abxis, and Daiichi Sankyo; participation on an advisory board with Lilly Dae Hwa Pharmaceutical Co, Ltd, IUS Abxis, Daiichi Sankyo, and Beigene; leadership or fiduciary role with ESMO Breast 2021-2023; and stocks or stock options from Genopeak and NeoGene TC. WPC reports payment or honoraria for lectures and presentations from Amgen, AstraZeneca, Chugai Pharma, Daiichi Sankyo, Eli Lilly, Everest Medicine, Foundation Medicine Kyowa Kirin, Novartis, Pfizer, Roche, Sanofi, and Takeda; travel support from Amgen, AstraZeneca, Pfizer, and Roche; participation on data safety monitoring or advisory board with Amgen, AstraZeneca, Daiichi Sankyo, Everest Medicine, Eli Lilly, IMPACT Therapeutics, MSD, Novartis, Pfizer, Roche, and Sanofi. SAI reports grants or contracts from AstraZeneca, Boryung Pharm, Daewoong Pharmaceutical Co. Ltd, Eisai, Pfizer, and Roche; and consulting fees from AstraZeneca, Hanmi, Idience, Lilly, MSD, GSK, Pfizer, Novartis, Roche, and Daiichi Sankyo. YHP reports grants or contracts from AstraZeneca, Roche, Pfizer, Gencurix, and Novartis; consulting fees from Pfizer, AstraZeneca, Roche, MSD, Eisai, Novartis, Daiichi Sankyo, and Lilly; payment or honoraria from Pfizer, Roche, MSD, and Novartis; participation on data safety monitoring or advisory board with Roche, AstraZeneca, Eisai, MSD, Daiichi Sankyo, and Novartis. VP reports payment or honoraria from Daiichi Sankyo, Novartis, Pfizer, and AstraZeneca; travel support from Pfizer, Mundipharma, Daiichi Sankyo, and Lilly; and participation on an advisory board with Daiichi Sankyo. CFC reports participation on a data safety monitoring or advisory board with Roche, Pfizer, and Daiichi Sankyo. HI reports consulting fees from Daichi Sankyo, Chugai, AstraZeneca, Sanofi, Lilly, MSD, Pfizer, and Novartis; and payment or honoraria from Daiichi Sankyo, Chugai, AstraZeneca, Lilly, MSD, and Pfizer. EH reports institutional research and consulting fees for the present manuscript from Daiichi Sankyo and AstraZeneca; other institutional research grants from AbbVie, Acerta Pharma, ADC Therapeutics, AKESOBIO Australia, Amgen, Aravive, ArQule, Arvinas AtlasMedX, Black Diamond, Boehringer Ingelheim, Clovis, Compugen, Curis, CytomX, Dana Farber Cancer Institute, Deciphera, eFFECTOR Therapeutics, Ellipses Pharma, EMD Serono, Fochon, FujiFilm, G1 Therapeutics, H3 Biomedicine, Harpoon, Hutchinson MediPharma, Immunogen, Immunomedics, Incyte, InvestisBio, Jacobio, Karyopharm, Leap Therapeutics, Lilly, Lycera, Mabspace Biosciences, Macrogenics, MedImmune, Merck, Mersana, Merus, Millenium, Molecular Templates, Myriad Genetic Labs, Novartis, Nucana, Olema, OncoMed, Onconova Therapeutics, ORIC Pharmaceuticals, Orinove, Pfizer, PharmaMar, Pieris Pharmaceuticals, Pionyr Immunotherapeutics, Plexxikon, Radius Health, Regeneron, Repertoire Immune Medicine, Rgenix, Roche/Genentech, Seagen, Sermonix Pharmaceuticals, Shattuck Labs, Silverback, StemCentRx, Sutro, Syndax, Syros, Taiho, TapImmune, Tesaro, Treadwell Therapeutics, Verastem, Vincerx Pharma, Zenith Epigenetics, and Zymeworks; and consulting fees from Arcus, Arvinas, Black Diamond, Boehringer Ingelheim, CytomX, Dantari, Deciphera Pharmaceuticals, Eisai, H3 Biomedicine, iTeos, Janssen, Lilly, Loxo, Merck, Mersana, Novartis, Pfizer, Puma Biotechnology, Relay Therapeutics, Roche/Genentech, Seagen, and Silverback Therapeutics. GC reports support for the current manuscript from AstraZeneca and Daichii Sankyo; grants or contracts from Merck; consulting fees from Bristol Myers Squibb, Roche, Pfizer, Novartis, Lilly, AstraZeneca, Daichii Sankyo, Merck, Seagen, and Ellipsis; payments or honoraria from Pfizer and Lilly; and travel support from Roche and Lilly. BX reports consulting fees from AstraZeneca and Novartis and payments or honoraria for lectures from AstraZeneca, Pfizer, and Roche. AE reports stocks/stock options from and is an employee of Daiichi Sankyo. YL reports stocks/stock options from and was an employee of Daiichi Sankyo at the time of the study. JC is an employee of Daiichi Sankyo. EB was an employee of Daiichi Sankyo at the time of the study. KT is an employee of Daiichi Sankyo. SV reports stocks/stock options from and is an employee of AstraZeneca. JC reports support for the current work from Daiichi Sankyo and AstraZeneca; consulting fees from AstraZeneca, Athenex, Bioasis, BioInvent, Boehringer Ingelheim, Celgene, Cellestia, Clovis Oncology, Daiichi Sankyo, Ellipses, Erytech, GEMoaB, Gilead, GSK, Hibercell, Leuko, Lilly, Menarini, Merck Sharp & Dohme, Polyphor, Roche, Seagen, Zymeworks; payment or honoraria from Celegene, Daiichi Sankyo, Eisai, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Samsung Bioepis; travel support from AstraZeneca, Daiichi Sankyo, Eisai, Novartis, Pfizer, and Roche; stocks/stock options from Leuko (relative), MedSIR, and Nektar Pharmaceuticals. All other authors have declared no conflicts of interest.
Data sharing
Anonymized individual participant data (IPD) on completed studies and applicable supporting clinical study documents may be available upon request at https://vivli.org/. In cases where clinical study data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo Companies will continue to protect the privacy of company and our clinical study subjects. Details on data sharing criteria and the procedure for requesting access can be found at this web address: https://vivli.org/ourmember/daiichi-sankyo/.
Supplementary data
References
- 1.Harbeck N., Penault-Llorca F., Cortes J., et al. Breast cancer. Nat Rev Dis Primers. 2019;5(1):66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 2.Hurvitz S.A., O'Shaughnessy J., Mason G., et al. Central nervous system metastasis in patients with HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from SystHERs. Clin Cancer Res. 2019;25(8):2433–2441. doi: 10.1158/1078-0432.CCR-18-2366. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Alvarez A., Papakonstantinou A., Oliveira M. Brain metastases in HER2-positive breast cancer: current and novel treatment strategies. Cancers (Basel) 2021;13(12):2927. doi: 10.3390/cancers13122927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brufsky A.M., Mayer M., Rugo H.S., et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834–4843. doi: 10.1158/1078-0432.CCR-10-2962. [DOI] [PubMed] [Google Scholar]
- 5.Ramakrishna N., Anders C.K., Lin N.U., et al. Management of advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO guideline update. J Clin Oncol. 2022;40:2636–2655. doi: 10.1200/JCO.22.00520. [DOI] [PubMed] [Google Scholar]
- 6.Lin N.U., Borges V., Anders C., et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610–2619. doi: 10.1200/JCO.20.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murthy R.K., Loi S., Okines A., et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 8.Montemurro F., Delaloge S., Barrios C.H., et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Ann Oncol. 2020;31(10):1350–1358. doi: 10.1016/j.annonc.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Krop I.E., Lin N.U., Blackwell K., et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015;26(1):113–119. doi: 10.1093/annonc/mdu486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma S., Miles D., Gianni L., et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakada T., Sugihara K., Jikoh T., et al. The latest research and development into the antibody-drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem Pharm Bull. 2019;67(3):173–185. doi: 10.1248/cpb.c18-00744. [DOI] [PubMed] [Google Scholar]
- 12.Ogitani Y., Aida T., Hagihara K., et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22(20):5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 13.Ogitani Y., Hagihara K., Oitate M., et al. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039–1046. doi: 10.1111/cas.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerusalem G., Park Y.H., Yamashita T., et al. Trastuzumab deruxtecan in HER2-positive metastatic breast cancer patients with brain metastases: a DESTINY-Breast01 subgroup analysis. Cancer Discov. 2022;12(12):2754–2762. doi: 10.1158/2159-8290.CD-22-0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabraji S., Ni J., Sammons S., et al. Preclinical and clinical efficacy of trastuzumab deruxtecan in breast cancer brain metastases. Clin Cancer Res. 2022;29:174–182. doi: 10.1158/1078-0432.CCR-22-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartsch R., Berghoff A.S., Furtner J., et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med. 2022;28:1840–1847. doi: 10.1038/s41591-022-01935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez-García J.M., Batista M.V., Cortez P., et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: the DEBBRAH trial. Neuro Oncol. 2022;25:157–166. doi: 10.1093/neuonc/noac144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortés J., Kim S.B., Chung W.P., et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–1154. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 19.US Food and Drug Administration . Silver Spring. US Food and Drug Administration; MD: 2022. FDA grants regular approval to fam-trastuzumab deruxtecan-nxki for breast cancer. [Google Scholar]
- 20.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Hurvitz S.A., Saura C., Oliveira M., et al. Efficacy of neratinib plus capecitabine in the subgroup of patients with central nervous system involvement from the NALA Trial. Oncologist. 2021;26(8):e1327–e1338. doi: 10.1002/onco.13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venur V.A., Leone J.P. Targeted therapies for brain metastases from breast cancer. Int J Mol Sci. 2016;17(9):1543. doi: 10.3390/ijms17091543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mair M.J., Bartsch R., Le Rhun E., et al. Understanding the activity of antibody-drug conjugates in primary and secondary brain tumours. Nat Rev Clin Oncol. 2023;20(6):372–389. doi: 10.1038/s41571-023-00756-z. [DOI] [PubMed] [Google Scholar]
- 24.Askoxylakis V., Ferraro G.B., Kodack D.P., et al. Preclinical efficacy of ado-trastuzumab emtansine in the brain microenvironment. J Natl Cancer Inst. 2016;108(2):djv313. doi: 10.1093/jnci/djv313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartsch R., Berghoff A.S., Vogl U., et al. Activity of T-DM1 in Her2-positive breast cancer brain metastases. Clin Exp Metastasis. 2015;32(7):729–737. doi: 10.1007/s10585-015-9740-3. [DOI] [PubMed] [Google Scholar]
- 26.Jacot W., Pons E., Frenel J.S., et al. Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res Treat. 2016;157(2):307–318. doi: 10.1007/s10549-016-3828-6. [DOI] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services . US Department of Health and Human Services; 2020. Cancer clinical trial eligibility criteria: brain metastases guidance for industry. [Google Scholar]
- 28.Lin N.U., Lee E.Q., Aoyama H., et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270–e278. doi: 10.1016/S1470-2045(15)70057-4. [DOI] [PubMed] [Google Scholar]
- 29.Curigliano G., Mueller V., Borges V., et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol. 2022;33(3):321–329. doi: 10.1016/j.annonc.2021.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.