Highlights
-
•
This ESMO Clinical Practice Guideline provides key recommendations for managing Merkel-cell carcinoma (MCC).
-
•
Recommendations are based on available scientific data and the multidisciplinary group of experts’ collective opinion.
-
•
The guideline covers clinical and pathological diagnosis, staging and risk assessment, treatment and follow-up.
-
•
Algorithms for the management of locoregional and inoperable/metastatic disease are provided.
-
•
A multidisciplinary team with a high level of expertise in MCC should diagnose and make decisions about therapy.
Key words: clinical practice guideline, diagnosis, follow-up, Merkel-cell carcinoma, treatment recommendation
Introduction
Merkel-cell carcinoma (MCC) is a rare primary neuroendocrine carcinoma of the skin. It affects predominantly older, fair-skinned Caucasians and exhibits aggressive behaviour with a high recurrence rate and a propensity for early metastasis.1 Despite new advances in therapies for MCC, the prognosis remains poor. With the incidence of MCC increasing rapidly across Europe, prompt diagnosis and effective and harmonised management are imperative for improving patient care.2
The European Parliament and the European Commission recommend treating patients with rare cancers in centres linked to the European Reference Network for Rare Adult Solid Cancers (EURACAN). In these referral centres with a high volume of MCC patients, the clinical experience of a multidisciplinary team (MDT) specialising in skin cancers guarantees better treatment outcomes and access to clinical trials. Therefore, the objective of this guideline is to provide a comprehensive reference for MCC, which is based on a critical evaluation of current evidence and opinion of European Society for Medical Oncology (ESMO) experts in partnership with EURACAN.
Incidence and epidemiology
The global incidence rates of MCC are difficult to calculate because of its rarity, geographic and demographic variability and lack of large epidemiological studies.3 Since its first description in 1972, MCC incidence rates have steadily increased, likely due to both the refinements in diagnostic capabilities and the progressively ageing population.4,5 The Surveillance of Rare Cancers in Europe (RARECARE) database reported the crude incidence estimate as 0.13 per 100000 in 1995-2002.6 The incidence rate reported over time in the recent analysis of the Surveillance, Epidemiology and End Results (SEER) database showed that in 1986, incidence and mortality rates per 100000 were 0.22 and 0.03, respectively; these rates increased to 0.79 and 0.43, respectively, in 2011.7 The highest incidence rates of MCC are in Australia, where the age-adjusted incidence rate reported in Queensland was 1.6 per 100000.8
MCC affects elderly (median age 76 years) Caucasian males eight times more frequently than people of colour and twice as often as females.9 MCC is correlated to infection with the Merkel-cell polyomavirus (MCPyV), exposure to ultraviolet (UV) light and/or immunosuppression [10% of patients are organ transplant recipients, patients with haematological malignancies or human immunodeficiency virus (HIV) infection].10,11 In Europe and North America, 80% of cases are caused by the integration of MCPyV into the host genome, with the remaining 20% caused by extensive UV-mediated damage. In Australia, this is the opposite, with 80% UV- and 20% MCPyV-related.3
Diagnosis and pathology/molecular biology
Clinical diagnosis
The clinical examination must include a physical examination with the assessment of locoregional lymph nodes (LNs), comorbid conditions and patient life expectancy. MCC classically presents as a pink or red-violaceous, painless, firm, rapidly growing, dome-shaped or red plaque skin lesion, ranging in size from 1 to 2 cm. It typically occurs in older patients in sun-exposed areas of their bodies. The predominant sites of MCC localisation include the head and neck (45%), upper limbs (24%), lower limbs (10%) and trunk or other sites (<10%), while in 11% of cases, there is no identifiable primary lesion. Ulceration may occur in more advanced MCC lesions. MCC enlarges rapidly, metastasises in transit to the skin and/or to first-echelon LNs (26% of cases at initial presentation) and then to higher-order nodal regions and distant sites such as bones, liver and brain (8% of cases at initial presentation).12 MCC may be misdiagnosed as squamous- or basal-cell carcinoma, amelanotic melanoma, adnexal tumour, primary cutaneous B-cell lymphoma or skin disorders such as pyogenic granuloma and inflammatory disorders.13 Since MCC has been observed contiguous to, or intermingled with, other skin cancers, the detection of polymorphous vessels and/or milky-red areas by dermatoscopy enhances the differential diagnostic accuracy, especially for patients with multiple skin lesions.14
Aetiology and molecular biology
Despite major advances in understanding MCC carcinogenesis, the cellular origin of MCC is still unclear. MCC has been hypothesised to originate from dermal fibroblasts, pre-/pro-B cells, Merkel-cell precursors potentially derived from epidermal stem cells and hair follicle stem cells.15 MCC carcinogenesis can be initiated in the cell of origin either by UV-mediated DNA damage caused by chronic exposure to sunlight or by integration of the MCPyV into the host genome.16,17 MCPyV is a ubiquitous virus usually acquired during childhood, as indicated by frequent seropositivity of antibodies against the capsid protein VP1 in the blood.18 However, primary infection with MCPyV does not cause any discernible signs or symptoms. Despite the high prevalence of MCPyV infection, very few people develop MCC. An essential feature of MCPyV-associated MCC is that the tumour cells express specific products of the viral early genes, i.e. small T antigen (ST) and a truncated version of large T antigen (LT). LT and ST have also been referred to as viral oncoproteins, and their capacity to interact with multiple cellular proteins, thereby altering their function, has been demonstrated.19,20 While MCPyV-associated MCCs are characterised by very low frequencies of somatic mutations, virus-negative MCCs are among the tumours with the highest mutational load, typically displaying UV signatures.21 Among the aberrations found in MCPyV-negative MCC, mutations disrupting the gene encoding the retinoblastoma-associated protein RB1 (the key protein controlling cell cycle entry) are almost always present. Analogously, RB1 function in virus-associated MCC is inhibited by binding the LXCXE motif of LT to RB1; in either case, cells are unable to arrest in the G1 phase of the cell cycle.21 Interestingly, MCPyV is not found in cases of MCC associated with cutaneous squamous-cell carcinoma, indicating that it does not play a part in these combined tumours.22,23
Histopathology
Histopathological features of MCC are those of a small-blue-round-cell tumour with a vesicular nucleus and scant cytoplasm.24 However, several different histopathological patterns exist, including trabecular, intermediate and small-cell variants. Neoplastic cells can also be large and may present with a pleomorphic morphology. The nucleoli are multiple and usually not prominent. Mitotic and apoptotic rates are frequently high. The tumour regularly infiltrates the reticular dermis and subcutis. The epidermis, papillary dermis and adnexal structures are usually spared, although epidermotropism is observed in up to 10% of cases. The presence of intra-lymphatic emboli and isolated tumour cells close to the surgical margins may explain the high rate of local recurrences.24,25 As the histomorphology of MCC on haematoxylin–eosin (H&E) sections is rather nonspecific, the definitive diagnosis of MCC requires immunohistochemical staining to rule out other tumours that display a small-blue-round-cell morphology (e.g. basal-cell carcinoma; metastatic small-cell carcinoma, particularly from the lung; cutaneous lymphoma; anaplastic sweat gland carcinoma; melanoma; Ewing’s sarcoma; neuroblastoma and rhabdomyosarcoma).24
MCC cells express several types of cytoskeletal keratins (CKs), particularly CK20 (membranous and/or paranuclear dot-like), CK8, CK18 and CK19. A small subset of MCCs (<10%) are negative for CK20; these cases are characterised by a high mutational burden and are generally not associated with MCPyV. In addition to CKs, neoplastic cells also express chromogranin A, synaptophysin, cluster of differentiation (CD)56, neuron-specific enolase (NSE) and huntingtin-interacting protein 1. MCC is usually negative for thyroid transcription factor 1 (TTF1), leukocyte common antigen, melan A, mammalian achaete-scute homologue 1, vimentin, protein S100 and CK7.23,24,26 However, rare cases of MCC can be positive for TTF1 or CK7, and so interpretation of the staining patterns of these two antigens should be carried out with caution. These markers should be included in an immunopanel for MCC confirmation and exclusion of other diagnostic considerations.
No histological marker has been reliably associated with the selective identification of either virus- or UV-associated MCC: while positive staining for MCPyV LT strongly suggests an MCPyV-associated MCC, negative staining does not necessarily rule it out.27,28 Although all of these markers are helpful and essential for diagnosis, particularly in the presence of artefacts, no convincing evidence supports their use to predict prognosis or response to therapy. Concerning the latter, variable numbers of tumour-infiltrating cytotoxic T lymphocytes (not identified, brisk, non-brisk) are found in MCC tumours, and their presence is associated with a better prognosis, which is particularly favourable if their T-cell receptor repertoire is characterised by clonal diversity.29
The definitive diagnosis of MCC requires histopathological examination of tissue obtained by incisional/excisional biopsy.4 The histology report after excisional biopsy should include tumour size; the involvement of other tissues such as fascia, muscle, cartilage or bones; surgical margins; tumour depth; lymphovascular invasion; intratumoural lymphocyte infiltration; immunohistochemical profile; MCPyV status and mitotic rate.
Recommendations
-
•
The clinical examination must include a physical examination with the assessment of locoregional LNs, comorbid conditions and patient life expectancy [III, A].
-
•
Histopathological MCC confirmation should include H&E with the dedicated immunopanel [III, A].
-
•
The histology report after excisional biopsy should include tumour size; the involvement of other structures such as fascia, muscle, cartilage or bones; surgical margins; tumour depth; lymphovascular invasion; intratumoural lymphocyte infiltration; immunohistochemical profile; MCPyV status and mitotic rate [III, A].
-
•
Dermatoscopy may enhance differential diagnostic accuracy, especially for patients with multiple skin lesions [IV, B].
Staging and risk assessment
The preferred classification is the eighth version of the Union for International Cancer Control (UICC) TNM (tumour–node–metastasis) staging and classification system, which provides information for both management and prognosis of patients with MCC (see Supplementary Tables S1-S4, available at https://doi.org/10.1016/j.esmoop.2024.102977).30 This classification was developed based on data collected from 9387 patients with MCC in the National Cancer Database. Staging and risk assessment procedures are determined based on disease presentation at diagnosis.31
The initial evaluation in MCC should include a complete examination of the skin with particular attention to any suspicious cancerous skin lesions, tumour satellites, in-transit metastases, regional LNs and systemic metastases. The assessment of disease extension in all patients is mandatory: ultrasound of regional LNs for patients with clinical stage I-II disease and computed tomography (CT) of the chest, abdomen and pelvis (and head/neck for head/neck primaries).32 Positron emission tomography (PET)–CT appears more sensitive than CT alone based on published meta-analyses and other retrospective studies; it was shown that 16.8% of patients who underwent PET–CT had their disease upstaged compared with 6.9% of those who underwent CT scans only.33,34 Therefore, if PET and/or PET–CT with [18F]2-fluoro-2-deoxy-d-glucose (FDG) are available, they are the preferred cross-sectional imaging methods to assess local and distant disease.16 If additional clinical symptoms are present, detailed imaging studies using magnetic resonance imaging (MRI) can be carried out.
Sentinel LN biopsy (SLNB) with an appropriate immunopanel is considered the most reliable staging procedure for identifying subclinical nodal involvement. As such, it is recommended for all patients with clinically node-negative disease who are fit for radical therapy. SLNB should be carried out alongside local surgical therapy of the primary tumour with special attention to drainage patterns. The observed 17.1% false-negative SLNB results may occur in immunocompromised patients or tumours localised in the head, neck or midline trunk region.35,36 SLNB enables the detection of micrometastases in approximately one-third of patients with clinically node-negative MCC, and its positivity rate is ∼20% for T1 and 40%-50% for T2 MCCs.37, 38, 39 In challenging locations for SLNB, single-photon emission computed tomography–CT techniques should be utilised.40
Access to a full pathological report with the number of involved LNs, the size of metastatic deposits and the status of the extracapsular extension is crucial for staging, prediction and decision making.41 Patients with clinically apparent nodal disease at presentation with an unknown primary should have a biopsy for histological confirmation of MCC.12,42
The 5-year overall survival (OS) in all MCC patients is between 48% and 63%: 64% in patients without metastases (stage I-II), 51% in those with regional LN involvement (stage III), 68% in those with an unknown primary tumour and 17%-29% in patients with distant metastases (stage IV).39,43, 44, 45
Retrospective data have shown that unfavourable clinical prognostic factors are the presence of regional and distant metastases, primary tumour diameter >2 cm and/or its extension beyond the dermis, location in the head/neck region, >75 years of age, male sex and presence of comorbidity, especially immunosuppression (e.g. HIV, chronic lymphocytic leukaemia).3,39,43,46 The poor prognosis has also been linked to histopathological futures such as positive margins after resection, a high mitotic rate, infiltrative (rather than circumscribed) growth pattern, lymphovascular infiltration and p63 expression, whereas LT and RB1 protein expression and intratumoural CD8+ T-lymphocyte infiltration correlate with a more favourable prognosis.10,12,24 However, there are no prospective data available in MCC.
Programmed death-ligand 1 (PD-L1) expression is frequently detected in MCC tumour cells and the tumour microenvironment. Nevertheless, its expression does not correlate with prognosis.47, 48, 49 Serum markers such as antibodies against MCPyV or NSE require further prospective validation.50,51
Recommendations
-
•
SLNB is indicated to improve prognostic staging, e.g. to rule out occult nodal disease, but the precision of SLNB is less reliable in immunocompromised patients and in patients whose tumours are located in the head, neck or midline trunk region and with aberrant LN drainage [III, A].
-
•
Mandatory imaging studies comprise CT scans of the chest, abdomen, pelvis and head/neck (for head/neck primaries). If available, whole-body FDG–PET/CT is preferable over contrast-enhanced CT scan; MRI imaging of specific organs should be carried out if clinically indicated [III, A].
-
•
The value of immunohistological markers, such as p63, PD-L1, NSE or CD200, is not fully established [IV, B].
Management of local/locoregional disease
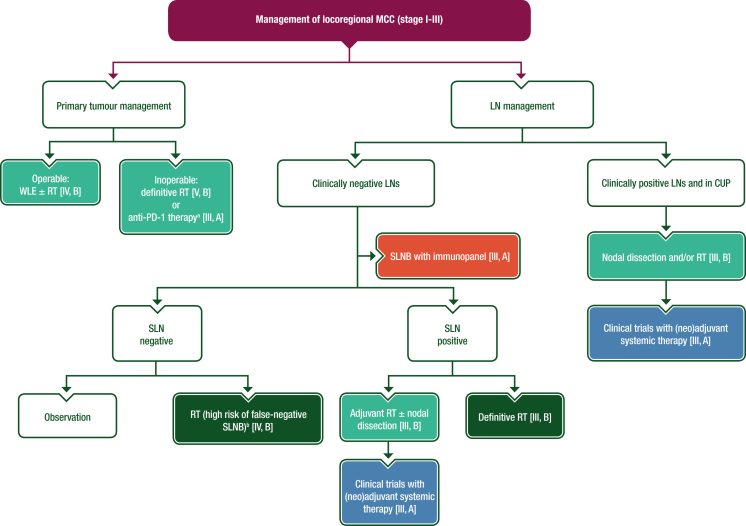
The mainstay of treatment for patients with localised MCC is wide local excision (WLE) followed by tumour bed radiotherapy (RT) and management of the nodal basin. A proposed algorithm for the management of locoregional MCC is shown in Figure 1.
Figure 1.
Management of locoregional MCC (stage I-III).
Purple: general categories or stratification; blue: systemic anticancer therapy; dark green: radiotherapy; turquoise: combination of treatments or other systemic treatments; red: surgery; white: other aspects of management.
CUP, cancer of unknown primary; LN, lymph node; MCC, Merkel-cell carcinoma; MDT, multidisciplinary team; N, node; PD-1, programmed cell death protein 1; RT, radiotherapy; SLN, sentinel lymph node; SLNB, sentinel lymph node biopsy; WLE, wide local excision.
aPatients with N0 disease eligible for immunotherapy should be preferably considered for anti-PD-1 therapy and reassessed for response and suitability for surgery by an MDT.
bFalse-negative SLNBs may be seen in patients (i) with profound immunosuppression, (ii) who are subject to anatomic compromise and (iii) with aberrant lymph node drainage and the presence of multiple SLN basins in head, neck or midline trunk MCC.
Since wide excision can potentially compromise lymphatic drainage, an SLNB should be carried out simultaneously with surgical treatment of the primary MCC. A precise surgical technique called Mohs surgery may be considered instead of WLE in selected cases based on results from meta-analyses of patients with stage I MCC, which showed similar recurrence rates for both surgical modalities: local recurrence rates were 6.8% for WLE versus 8.5% for Mohs surgery, and regional recurrence rates were similar at ∼15%, although no randomised trials comparing traditional WLE with Mohs surgery were included in these meta-analyses.52
After resection, there is a need to obtain a clear pathological margin, although the size of surgical margin is under debate.53 In one publication, a margin >2 cm was associated with a significant improvement in OS54; however, other data have not supported a correlation between margin size beyond 1 cm and additional clinical benefit. In a study of 6156 patients with localised MCC, resection margins >1 cm were associated with improvements in OS compared with margins of <1 cm; 5-year survival rates were 90% and 77% (P < 0.001), respectively.55
According to findings from a retrospective study comprising a cohort of patients who had clear margins following resection and received post-operative RT, surgical excision with margins of 0.5-1.0 cm versus >1.0 cm was not associated with any significant difference in terms of OS, any recurrence-free survival (RFS) or local RFS.56 Thus, surgical margins might be reduced to avoid reconstruction, especially for preserving cosmetics (face) or function (locations close to joints). Findings from this retrospective study also showed that excision, even with narrow margins (0.5-1.0 cm), was not associated with outcome. Cancer-specific survival was 76.8% versus 76.2% for patients with resection margins of 0.5-1.0 cm versus 1-2 cm, respectively. However, for patients with narrow resection margins, post-operative RT to the tumour bed is mandatory.56 In summary, there is a need to obtain surgical margins of 1-2 cm and histologically negative margins, if feasible, which must be followed by adjuvant RT regardless of the type of surgery.15,57, 58, 59
The benefit of adjuvant RT following excision of the primary tumour was shown in a systematic review and meta-analysis considering >17 000 patients with stages I-III MCC from 29 observational studies.60 A favourable OS benefit was associated with surgery plus adjuvant RT versus surgery alone [hazard ratio (HR) 0.81, 95% confidence interval (CI) 0.75-0.86, P < 0.001]. The study also reported a significant benefit in locoregional and local disease-free survival (DFS), but not distant DFS (HR 0.3, 95% CI 0.22-0.42; HR 0.21, 95% CI 0.14-0.33 and HR 0.79, 95% CI 0.49-1.14, respectively).60 The largest series to date to assess whether adjuvant therapy was associated with better survival included 6908 cases from the National Cancer Database.61 It showed that for localised MCC, surgery and adjuvant RT was associated with a statistically significant improvement in OS compared with surgery alone (stage I: HR 0.71, 95% CI 0.64-0.80; stage II: HR 0.77, 95% CI 0.66-0.89), but in patients with regional nodal metastases (stage III), neither the addition of adjuvant RT nor chemotherapy (ChT) had a significant impact on OS. These findings suggest that the addition of RT may benefit the local control of localised disease; however, it is reasonable to believe that survival in patients with more advanced disease may be driven by the presence of subclinical distant metastasis.61
It is worth noting that the MCC patient population contains elderly patients, which frequently causes clinicians to deviate from standard treatment protocols. This was recently illustrated in a study that investigated the concordance to adjuvant treatment guidelines in patients with stage I-II MCC.62 Of the 2330 patients in this study, 1858 had an indication for adjuvant RT [according to National Comprehensive Cancer Network (NCCN) guidelines criteria63] but only 57% of these patients received RT; those who received RT had a 5-year OS advantage over those who did not (76% versus 68%, P < 0.0003). Conversely, of the 472 patients without an indication for adjuvant RT (according to NCCN criteria), 43% received RT; this group did not show an OS benefit over those who did not receive RT (79% versus 75%, P = 0.48).62
The optimal adjuvant RT dose is unclear.64 In a retrospective study of 2093 patients who underwent surgery followed by adjuvant RT, four groups of patients receiving different doses of RT (30-40 Gy, 40-50 Gy, 50-55 Gy and 55-70 Gy) were analysed; the 3-year OS rates were 41.8%, 69.0%, 69.2% and 66.0%, respectively.65 An Australian study documenting a dose response showed that no patients with macroscopic MCC developed in-field relapses at doses >56 Gy.66 Furthermore, in a large population-based study of patients with head and neck-located MCC (N = 1625) undergoing adjuvant RT (85% with negative margins and 15% with residual MCC), a dose range of 50-55 Gy conferred a survival advantage compared with doses <50 Gy. There was no significant improvement by escalating doses beyond this range, which could reflect toxicity-related morbidity or death from competing risks in this mostly older patient cohort.65 Therefore, a radiation dose of 50-60 Gy is often recommended in MCC, achieving in-field control.
In some patients, surgical therapy is not feasible due to the extent of disease (technically inoperable) or the presence of significant (co)morbidity. Since MCC is radiosensitive, RT as a single modality is an alternative to surgery in this group. The natural history of patients treated with definitive RT is out-of-field relapse with in-field control achieved in most patients.38 In a systematic review, an almost 90% in-field control rate was documented following definitive RT with a mean dose delivered of just under 50 Gy.38 Recurrences occurred at 39/332 sites (13 local relapses, 26 regional) for a cumulative post-RT in-field recurrence rate of 11.7%. Of note, there was no association between RT dose and incidence of recurrence or non-recurrence. RT of the primary tumour can also be curative when excision is not possible due to severe comorbidity disqualifying the patient from surgery or in individuals who refuse surgical treatment.67 However, there is also a lack of consensus regarding the optimal dose/fractionation schedule. Independently, the European Consensus-based interdisciplinary guideline and NCCN guidelines propose doses of 60-66 Gy for definitive treatment of patients with grossly positive primary tumour resection margins and/or clinically evident lymphadenopathy.15,63
Management of early-stage MCC (stage I-II)
In the early stages of MCC (stage I-II), the standard treatment approach is WLE of the primary tumour.15,68 In T1-4 N0 M0 disease, adjuvant RT of the primary MCC site (50-60 Gy to the tumour bed) is recommended.15,68 In selected cases with very-low-risk MCC (T1 N0 M0; <1 cm) and no unfavourable prognostic factors, no additional RT may be needed after WLE, but the decision should be made by experts at high-volume referral centres.68 Extensive tissue movement and grafting should be avoided if adjuvant RT is planned.
After histologically confirmed negative SLNB, the decision between observation and adjuvant RT to the nodal basin must be made by the referral centre MDT after considering the experience of the surgeon and confidence regarding the SLNB procedure.15 The purpose of SLNB is to avoid unnecessary adjuvant treatment. In a review of 29 studies that included patients with stage I-II MCC, no significant difference in regional recurrence rate was demonstrated with versus without adjuvant RT (14.3% versus 4.6%, P = 0.31).38 Furthermore, similar regional nodal recurrence and OS rates were reported in a cohort of 240 patients who underwent SLNB or elective LN dissection without prior pathological nodal staging (P = 0.056).69 However, in selected clinical situations, there is value in adjuvant regional RT. It is recommended in patients with profound immunosuppression, if SLNB is not carried out or its accuracy is questionable [i.e. anatomic compromise, aberrant LN drainage or the presence of multiple sentinel LN (SLN) basins which is typical in head, neck or midline trunk MCC].70
Management of locoregional MCC (stage III)
Patients with stage III MCC have a competing risk of systemic recurrence. On the other hand, in elderly patients, severe comorbidity may lead to increased risk for intra-/post-operative complications and higher morbidity related to local therapy. Each decision on adjuvant therapy should ideally be made within the confines of an MDT. In this situation, there is a recommendation to include patients in clinical trials for neoadjuvant or adjuvant therapy when available.
In the presence of microscopic metastasis detected on SLNB or during node dissection [pathological stage IIIA: T1-4, N1a, N1a(sn), M0], adjuvant RT alone or in combination with complete LN dissection (CLND) may be considered and requires an individualised approach. In a prospective study, 163 patients with SLN metastasis only underwent CLND or RT, and there were no significant differences in survival outcomes (5-year OS: 71% versus 64%, P = 1.0; DFS: 52% versus 61%, P = 0.8; nodal RFS: 76% versus 91%, P = 0.3 or distant relapse-free survival: 65% versus 75%, P = 0.3, respectively). With sufficient in-field control of macroscopic MCC, in selected fragile patients who cannot tolerate nodal excision under local anaesthesia, RT as monotherapy should be considered.71 Another retrospective study of 447 patients with MCC and a positive SLNB collected in the National Cancer Database showed that adjuvant RT ± CLND led to a survival benefit compared with CLND alone or observation.72 Therefore, in younger patients (<75 years of age) with fewer comorbidities, a multidisciplinary approach with adjuvant RT and CLND may be beneficial. However, the potential benefit may be decreased by the potential complications of CLND, such as lymphoedema, post-operative wound infection, skin necrosis and wound dehiscence, which is higher in the inguinal basin than in axillary dissection (26% versus 9%, respectively).73
No randomised trials have evaluated the efficacy of adjuvant ChT or chemoradiotherapy (CRT) in patients with MCC. Therefore, adjuvant ChT is not routinely indicated. The data supporting CRT are derived from a retrospective study of 4815 patients with head and neck MCC.74 In male patients with positive margins and a tumour size of >3 cm, post-operative CRT and RT both provided a survival benefit compared with surgery alone (HR 0.62, 95% CI 0.47-0.81; HR 0.80, 95% CI 0.70-0.92, respectively).74 In a study of 6908 patients in the National Cancer Database, there was no benefit (or detriment) of giving adjuvant ChT to patients with high-risk disease.61 In this fragile population, the risk of serious side-effects is significantly higher due to existing comorbidities, and ChT-related mortality is between 4% and 8%. There is also a concern about the immunosuppressive effects of ChT, which may lead to the development and progression of MCC.75, 76, 77
A limited number of published case reports and series have addressed MCC of unknown primary (pathological stage IIIA: T0, N1b, M0). This group has a better outcome than patients with known primary and synchronous nodal metastases.12,42 The recommendation in this group is to carry out a biopsy for pathological confirmation of MCC, FDG–PET–CT to rule out distant metastatic disease and management of the nodal lesions similar to that proposed for stage IIIB MCC.
For patients with clinically positive nodal disease (pathological stage IIIB, T1-4, N1b-3, M0), cross-sectional imaging is advised before surgery. There have been no prospective trials evaluating the appropriate extent of CLND clearance for MCC; in the absence of such evidence, it is recommended to follow the ESMO Clinical Practice Guideline (CPG) for cutaneous melanoma.78 An analysis of the SEER database incorporated propensity scoring and matched-pair analysis and reported no difference in MCC-specific survival in patients who received RT after CLND versus observation.79,80 In another study, Lewis et al. carried out a database analysis encompassing 1254 patients and found reductions in local and regional recurrences with similar rates of distant metastases, but no statistical difference in terms of OS and MCC-specific survival.81 Therefore, patients with node-positive MCC should be considered for clinical trials with (neo)adjuvant systemic therapy because neither adjuvant RT nor ChT has been associated with a statistically significant impact on OS.61
Satellite or in-transit metastases (pathological stage IIIB, T1-4, N2-3, M0) at post-operative histology are caused by the cutaneous or subcutaneous intra-lymphatic spread. The frequency is unclear but it is always associated with poor survival.42,82,83 In bulky, multiple and/or frequently recurrent locoregional metastases, isolated limb perfusion may be a safe and effective option.84 Overall and complete response (CR) rates are ∼80% and 50%, respectively.84 The recommended treatment for these locoregional metastases in the absence of distant disease should consist of surgery and/or RT or clinical trials, whereas adjuvant ChT is not recommended.
The role of neoadjuvant or adjuvant immunotherapy in the management of patients with MCC is currently under investigation. In a phase I-II study (CheckMate 358) of nivolumab in the neoadjuvant setting, surgical resection was conducted after two doses of nivolumab 240 mg given 2 weeks apart.85 Among the 36 patients who underwent surgery, 17 had a confirmed pathological CR (pCR) and 18 had a partial response (PR) by radiological assessment. At a median follow-up of 20 months, no patient with a pCR experienced disease relapse. Additional studies are required to confirm the role of neoadjuvant immunotherapy and the extent of surgery or post-operative RT in patients with a pCR.85 In the adjuvant setting, although initial reports failed to show a therapeutic benefit with ipilimumab as adjuvant monotherapy, interim results from a prospective randomised study suggest a benefit in terms of DFS for the programmed cell death protein 1 (PD-1) inhibitor, nivolumab. However, final results from prospective randomised trials are required to determine the true benefits of adjuvant PD-(L)1 blockade.86
Recommendations
-
•
An MDT meeting comprising experts with significant MCC experience should be convened to diagnose and make decisions about therapy; the preferred option is participation in clinical trials [III, A].
Management of local/locoregional disease (stage I-III)
-
•
After excisional biopsy, WLE with a margin of 1-2 cm is considered adequate; if a resection margin of 1-2 cm is not technically achievable, a narrower margin (0.5-1.0 cm) with adjuvant RT may also be acceptable [IV, B].
-
•
Adjuvant RT with 50-60 Gy to the tumour bed is recommended for tumours of ≥1 cm in diameter and/or with negative prognostic features (stage ≥IB) [IV, A].
-
•
In patients at very low risk of locoregional recurrence (stage IA), clinical observation may be an alternative but such a decision should only be made at referral centres [V, A].
-
•
When WLE is not feasible, definitive RT of the primary tumour is an alternative approach [V, B].
Management of early-stage MCC (stage I-II; T1-4 N0 M0 disease)
-
•
SLNB should be carried out during local surgical therapy of the primary tumour with special attention to drainage patterns [III, A].
-
•
In patients with a negative SLNB, observation is an option, but in case of a risk of false negativity of SLNB or when SLNB is not carried out, adjuvant RT to the primary site and nodal basin may be considered. This decision must only be made at referral centres [IV, B].
Management of locoregional MCC (stage III; T1-4 N1-3 M0 disease)
-
•
After a positive SLNB, adjuvant RT alone or in combination with CLND is recommended after an MDT discussion [IV, B].
-
•
In clinically positive LNs, multiple nodal involvements or extra-nodal extension of the SLN, regional LN dissection with post-operative RT is recommended (or definitive RT in inoperable patients) [III, B].
-
•
Adjuvant ChT is not recommended [IV, D]; patients should be considered for clinical trials with (neo)adjuvant systemic therapy of modern immunotherapies [III, A].
Management of advanced/metastatic disease
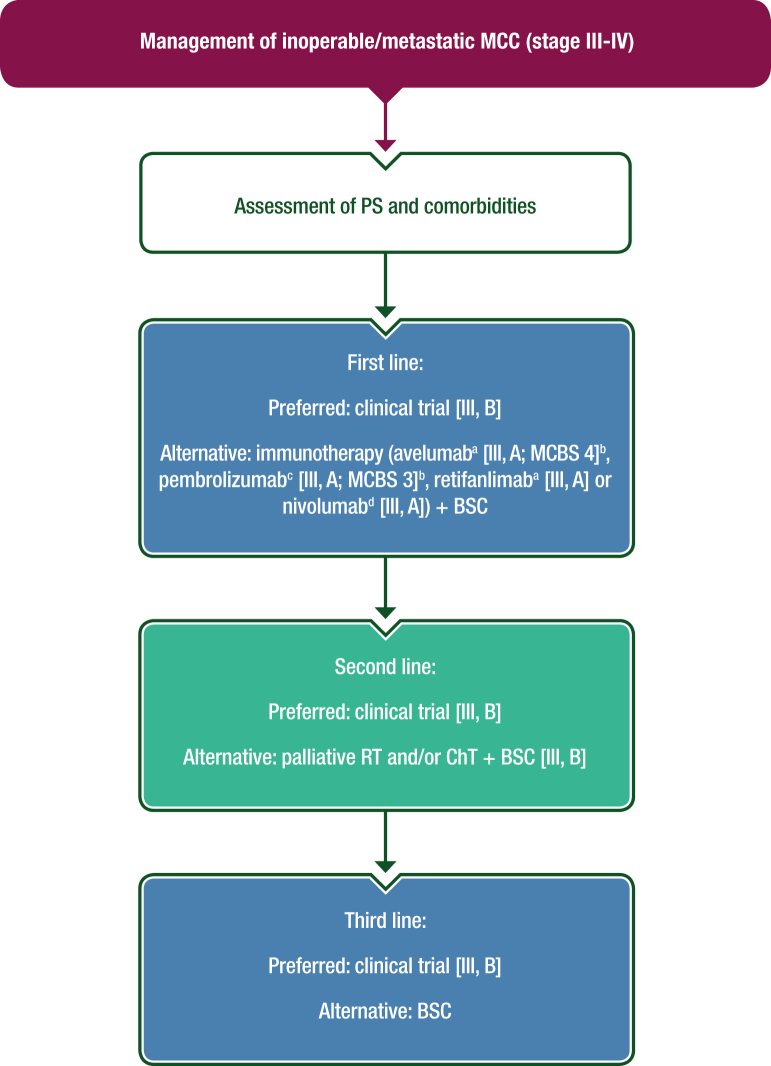
Despite the recent advances in diagnosis and treatment, inoperable stage III and IV MCC remain incurable. Administration of immunotherapy in the first-/second-line settings is recommended (if there is no contraindication for immunotherapy).87,88 ChT, palliative RT, best supportive care or participation in clinical trials should be considered based on the clinical situation. In patients with recurrent oligometastatic and resectable disease, surgical removal or stereotactic irradiation of metastases can be considered if systemic immunotherapy is contraindicated or the disease is refractory. A proposed algorithm for the management of inoperable stage III and IV MCC is shown in Figure 2.
Figure 2.
Management of inoperable/metastatic MCC (stage III-IV).
Purple: general categories or stratification; blue: systemic anticancer therapy; turquoise: combination of treatments or other systemic treatments; white: other aspects of management. BSC, best supportive care; ChT, chemotherapy; EMA, European Medicines Agency; FDA, Food and Drug Administration; MCBS, ESMO-Magnitude of Clinical Benefit Scale; MCC, Merkel-cell carcinoma; PS, performance status; RT, radiotherapy.
aEMA and FDA approved.
bESMO-MCBS v1.1120 was used to calculate scores for new therapies/indications approved by the EMA or FDA. The scores have been calculated and validated by the ESMO-MCBS Working Group and reviewed by the authors (https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-evaluation-forms).
cFDA approved, not EMA approved.
dNot EMA or FDA approved.
Immunotherapy
PD-(L)1 checkpoint inhibitors are effective in the treatment of metastatic MCC.89 These drugs allow the reactivation of T lymphocytes and the triggering of the adaptive immune system by blocking the interaction between PD-L1 of the tumour microenvironment and PD-1 expressed on lymphocyte surfaces. Blocking PD-1/PD-L1 restores the antitumour activity of effector T cells and the function of exhausted T cells.89
Despite their proven efficacy, resistance to immune checkpoint inhibitors (ICIs) can occur due to different mechanisms: intrinsic resistance in patients who do not respond to PD-1/PD-L1 blockade or acquired resistance in patients whose tumours progress after the initial response.90, 91, 92 Open questions regarding the immunogenic characteristics of both MCPyV-positive and -negative Merkel-cell tumours and the lack of predictive biomarkers of response to treatment are still under evaluation. Clinical benefit was not associated with any other biomarker evaluated to date, such as PD-L1, MCPyV status, tumour mutational burden or CD8+ T-lymphocyte infiltration.90, 91, 92
Immunotherapeutic agents, such as avelumab [ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS) v1.1 score: 4; Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved], pembrolizumab [ESMO-MCBS v1.1 score: 3; FDA approved, not EMA approved], retifanlimab (FDA and approved) and nivolumab (not EMA or FDA approved), have shown high response rates both as first- and second-line treatments, with long response durations and greater long-term benefits, when indirectly compared with ChT. Therefore, immunotherapy is recommended in this setting if no contraindications exist.
The ideal duration of ICI therapy is currently unknown. One retrospective analysis of data collected at a single institute showed that among 65 patients treated with avelumab, 25 had an FDG–PET–CT-confirmed CR. The 12-month RFS rate in these patients was 88% (95% CI 0.74-1.0). Reasons for stopping treatment included completion of 1 year of treatment (13 patients), toxicity (5 patients) and patient preference (7 patients).93 Prospective data from larger patient cohorts and with longer follow-up are therefore required to confirm the ideal duration of ICI therapy in patients with MCC.
Avelumab
The efficacy and safety of avelumab, an anti-PD-L1 inhibitor, were analysed in the JAVELIN Merkel 200 study, which was divided into parts A and B.94,95 Part A included 88 patients with metastatic MCC who progressed after at least one line of ChT. After >2 years of follow-up, the objective response rate (ORR) was 33.0% (11.4% CR), median duration of response (DoR) was 40.5 months and the DoR was >2 years in 67.0% of responders.90,94,95 In part B, patients who had not received prior systemic treatment for metastatic disease were enrolled; the ORR was 39.7% (16.4% CR) with 30.2% having a response lasting >6 months; the median OS was 20.3 months. First-line avelumab treatment was generally well-tolerated, and no treatment-related deaths or grade 4 adverse events (AEs) occurred. In the pretreated population, grade 3-4 treatment-related AEs (TRAEs) were observed in 11.4% of patients. From December 2015 to March 2019, 494 patients received avelumab as a first- or second-line treatment for MCC within an expanded access programme (EAP); the ORR was 46.7% (22.9% CR) and the disease control rate was 71.2%. Avelumab was also associated with long-lasting disease control and a positive effect on OS.90,94,95 Emerging real-world data suggest that outcomes with avelumab in clinical practice are in line with those seen in clinical trials.96 Recently published global data from EAPs and retrospective studies reported ORRs ranging from 29.1% to 72.1% [CR 15.8%-37.2%; PR 18.2%-42.1% and stable disease (SD) 7.1%-30.9%], with median progression-free survival (PFS) ranging from 8.1 to 24.1 months. Importantly, immunosuppressed patients also benefited from avelumab, with response rates equivalent to the general population. The best outcomes were achieved when avelumab was given as first-line therapy. The most common AEs were fatigue and infusion-related reactions; autoimmune hepatitis and thyroiditis were also observed.97
Pembrolizumab
Pembrolizumab, a humanised immunoglobulin G4 (IgG4) monoclonal antibody that selectively binds PD-1 on the surface of T lymphocytes, has been tested as a first-line treatment in patients with MCC.98,99 In a phase II clinical trial (KEYNOTE-017), 50 patients received pembrolizumab for up to 2 years. TRAEs of any grade occurred in 49 of 50 patients (98%) and 15 patients (30%) had grade ≥3 TRAEs with one death due to pericardial and pleural effusion. The ORR was 58%, with 30% of patients achieving a CR. At 3 years, the PFS was 39.1% with a median PFS of 16.8 months; the 3-year OS rate was 59.4%, with a median OS not reached.99 Age, gender, baseline tumour burden, anatomic sites of metastasis and tumour PD-L1 expression and viral status expression did not correlate with ORR, PFS or OS.98,99
Nivolumab
Nivolumab, a fully human anti-PD-1 IgG4 monoclonal antibody, was evaluated in two prospective studies in combination with ipilimumab. A randomised, open-label, phase II trial showed that in 50 patients [including 26 who had received prior anti-PD-(L)1 therapy], nivolumab–ipilimumab [with half of the patients also receiving stereotactic body RT (SBRT) (24 Gy in three fractions)] was associated with a high response rate and an expected safety profile.100 A CR was achieved in 41% of ICI-naive patients and in 15% of ICI-exposed patients; however, SBRT did not influence ORR. Although the effectiveness of nivolumab–ipilimumab looks promising, data are immature and limited to a small sample size. It is also important to consider the significant risk of severe AEs in the elderly MCC population. Findings from the phase I/II CheckMate 358 trial also suggest that nivolumab is active as monotherapy in patients with advanced MCC. Among 25 treated patients (22 assessable), the ORR was 68% and the 3-month PFS and OS rates were 82% and 92%, respectively.77
Retifanlimab
Retifanlimab is a humanised, hinge-stabilised IgG4κ, anti-PD-1 antibody that is EMA and FDA approved for the treatment of metastatic or recurrent locally advanced MCC not amenable to curative surgery or RT based on data from the phase II POD1UM-201 trial. In this trial, retifanlimab treatment resulted in an ORR of 52% (95% CI 40% to 65%) in ChT-naive patients (N = 65; CR 18% and PR 34%) with a median DoR between 1.1 months and >24.9 months. Twenty-two percent of patients experienced serious TRAEs, including fatigue, arrhythmia and pneumonitis, and 11% discontinued therapy due to AEs (the most common AEs were fatigue, musculoskeletal pain, pruritus, diarrhoea, rash, pyrexia and nausea).101
ChT
As there are no randomised trials in patients with MCC and distant metastases, the impact of systemic ChT on survival is unclear. To date, available data are insufficient to determine which of the different ChT regimens ensures the best PFS and OS in patients with metastatic MCC.
ChT regimens that have been used to treat patients with MCC include taxanes, topotecan, a combination of etoposide–cisplatin, etoposide–carboplatin or cyclophosphamide–doxorubicin–vincristine.102 Response rates ranged from 20% to 75%, with higher response rates in the first-line setting (53%-61%) versus the second-line setting (23%-45%); among responders, the median DoRs were ∼2-9 months.76,103,104 Unfortunately, in this fragile, elderly population, the occurrence of toxic deaths limits ChT usage; toxic deaths were observed in 3%-10% of patients.76,103,104 However, ChT might be proposed after failure on immunotherapy or when immunotherapy is contraindicated.
Targeted therapies
In the UKMCC-01 trial, the efficacy of pazopanib was assessed in 16 patients with MCC and a median age of 73 years (range 56-90 years).105 A clinical benefit was reported in nine patients (three had a PR and six had SD) with a median duration of 8.0 weeks (range 1.3-38.4 weeks). The trial was stopped due to slow accrual.105 A lack of activity and limited tolerance were also shown in a phase II trial with cabozantinib in patients with recurrent/metastatic MCC after platinum failure.106 Based on published results, the use of targeted therapy is not recommended.
With regard to radioligand therapy, although MCC frequently expresses somatostatin receptors, Ga68-DOTATOC and Lu177-DOTATATE have only been studied in a few patients and further clinical trials are needed to determine their role in MCC.102,107 As such, they should not currently be considered as a standard of care.
Palliative RT
Palliative RT has mainly been assessed retrospectively. In a study applying an 8-Gy single fraction, a CR with almost 80% of in-field lesion control was documented in 45% of patients.87 Another study comparing 8 Gy as a single or multiple fractions (3 × 8 Gy) documented a CR in 10% versus 48% and a recurrence rate of 41% versus 5% (P = 0.04), respectively.108 Patients of poor performance status should be considered for a lower dose hypofractionation schedule (e.g. 20 Gy in 5 fractions or 30 Gy in 10 fractions), which can still achieve tumour regression.
Recommendations
-
•
Patients should receive individualised multimodality treatment in referral centres for rare skin cancers with access to clinical trials [III, B].
-
•
In oligometastatic disease progression, surgical removal or stereotactic irradiation of operable locoregional recurrence or a single distant metastasis should be considered in fit patients, although systemic immunotherapy should be considered as the first step [V, B].
-
•
Immunotherapeutic agents, such as avelumab (III, A; ESMO-MCBS v1.1 score: 4; FDA and EMA approved), pembrolizumab (III, A; ESMO-MCBS v1.1 score: 3; FDA approved, not EMA approved), retifanlimab (III, A; FDA and EMA approved) and nivolumab (III, A; not EMA or FDA approved), are recommended as first- and second-line treatments if no contraindications exist.
-
•
In cases of inoperable/disseminated disease, first-line treatment with an anti-PD-(L)1 antibody is suggested as it is more effective and safer than ChT [II, B].
-
•
In patients with contraindications or after failure of immunotherapy, palliative RT and/or ChT may be options, but their impact on OS is uncertain [III, B].
Populations of special consideration
Since MCC is present mainly in elderly patients, use of geriatric scales and concomitant comorbidity assessments are important for decision making to achieve disease control and assure an acceptable quality of life. For patients who are not medical candidates for surgery, RT alone may be considered. However, in patients with inoperable/metastatic disease, given the relatively good tolerability of ICIs, these agents are the preferred choice of systemic therapy for this subset of patients.
Recommendations
-
•
Use of geriatric scales and concomitant comorbidity assessments are important for decision making [V, A].
-
•
RT alone may be considered in patients not suitable for surgery [IV, A].
-
•
In elderly patients with inoperable/metastatic disease, ICIs are the preferred choice of systemic therapy [IV, A].
Follow-up, long-term implications and survivorship
After primary surgical excision, local recurrence develops in 27%-60% of patients, regional nodal involvement is reported in 45%-91% of patients and distant metastases are found in 18%-52%.42,109,110 Risk factors for recurrence include immunosuppression, advancing age, advancing stage of disease (stage II-IV), individuals assigned male at birth, non-SLN metastases, MCPyV-negative status, as well as additional factors as determined by the treating physicians. No formal clinical trials have evaluated the optimal surveillance schedule in MCC, either in terms of frequency or type of diagnostic examinations. As the risk of relapse is higher in the first 2-3 years after initial treatment (40%-50% nodal and 33% distant metastases),15 more intense follow-up should be applied in this period. Follow-up examinations are recommended every 3-6 months for the first 3 years, and then every 6 months until year 5. After 5 years of observation, a general physical examination is recommended every 12 months lifelong, including a complete skin check-up. Physical examination should include total-body skin examination and LN assessment with particular attention to the scar region, including the primary site, in-transit region and regional LN basin. Distant metastases may develop in a wide range of anatomical locations; thus, routine cross-sectional imaging may be proposed in higher-risk patients.
The radiological examination may be either a diagnostic CT of the thorax/abdomen/pelvis (and head/neck for patients with head and neck primaries) or whole-body PET–CT, undertaken every 6-12 months for the first 3 years, with ultrasound scans used for local LN assessment. After year 3, imaging studies should be carried out as clinically indicated. Patients with MCC have a higher risk for developing another skin cancer9,15,39,111,112; therefore, education regarding self-examination of the whole skin surface is valuable. The role of MCPyV oncoprotein antibody testing is uncertain but it may be utilised in patients who are seropositive at baseline.50,113,114 The early detection of a locoregional relapse may be cured by surgery. However, inoperable/metastatic relapse should be treated as metastatic disease, as described in the previous section.115, 116, 117 Therapy should be coordinated by an MDT at the referral centre.87,118,119
Recommendations
-
•
Follow-up examinations are recommended in radically treated patients every 3-6 months for the first 3 years, and then every 6 months up to year 5. After 5 years of observation, a general physical examination is recommended every 12 months lifelong, including a complete skin check-up [IV, A].
-
•
Patient education regarding self-examination of the whole skin is valuable as patients with MCC have a higher risk of developing another skin cancer [IV, A].
-
•
The role of MCPyV oncoprotein antibody testing is uncertain, but it may be utilised in patients who are seropositive at baseline [V, A].
-
•
Routine cross-sectional imaging may be proposed in higher-risk patients [IV, A].
Methodology
This CPG has been developed by ESMO in partnership with EURACAN, in accordance with the ESMO standard operating procedures for CPG development (http://www.esmo.org/Guidelines/ESMO-GuidelinesMethodology). The relevant literature has been selected by the expert authors. The guideline is conceived to provide a standard approach to diagnosis, treatment and survivorship of MCC. Due to the rarity of MCC incidence, prospective and randomised studies are limited; therefore, the basis of this guideline was mainly data from retrospective, observational cohort studies, both institutional and cancer registry-based, often with heterogeneous results. These studies have many limitations, such as selection bias, confounding factors, limited and missing data and lack of randomisation. Recommended interventions are intended to correspond to the ‘standard’ approaches according to current consensus among the European multidisciplinary MCC community of experts. These are represented by the members of the ESMO Melanoma Faculty and experts appointed by all institutions belonging to the Rare Skin Cancer domain of EURACAN. Experimental interventions considered to be beneficial are labelled as ‘investigational’. Other non-standard approaches may be proposed to the single patient as ‘options’ for a shared patient–physician decision in conditions of uncertainty as long as some supporting evidence (though not conclusive) is available. Algorithms accompany the text, covering the main typical presentations of disease, and are meant to guide the user throughout the text. An ESMO-MCBS table with ESMO-MCBS scores is included in Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2024.102977. ESMO-MCBS v1.1120 was used to calculate scores for new therapies/indications approved by the EMA and/or the FDA (https://www.esmo.org/Guidelines/ESMO-MCBS). The scores have been calculated and validated by the ESMO-MCBS Working Group and reviewed by the authors. The FDA/EMA or other regulatory body approval status of new therapies/indications is reported at the time of writing this CPG. Levels of evidence and grades of recommendation have been applied using the system shown in Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2024.102977.121 Statements without grading were considered justified standard clinical practice by the authors. For future updates to this CPG, including Living Guidelines, please see the ESMO Guidelines website: https://www.esmo.org/guidelines/guidelines-by-topic/endocrine-and-neuroendocrine-cancers.
Acknowledgements
Manuscript editing support was provided by Fraser Simpson, Claire Bramley and Jennifer Lamarre (ESMO Guidelines staff) and Angela Corstorphine of Kstorfin Medical Communications Ltd (KMC); this support was funded by ESMO. Nathan Cherny, member of the ESMO-MCBS Working Group, and Urania Dafni, Giota Zygoura, Georgia Dimopoulou and Tereza Dellaporta of Frontier Science Foundation Hellas provided review and validation of the ESMO-MCBS scores. Nicola Latino (ESMO Scientific Affairs staff) provided coordination and support of the ESMO-MCBS scores and Angela Corstorphine and Sian-Marie Lucas of KMC provided medical writing and editing support in the preparation of the ESMO-MCBS table; this support was funded by ESMO.
Funding
No external funding has been received for the preparation of this guideline. Production costs have been covered by ESMO from central funds.
Disclosure
IL reports personal fees for writing engagements for ESMO and Roche; personal and institutional fees as coordinating principal investigator (PI) for Agenus, Amgen, AstraZeneca, Bristol Myers Squibb (BMS), Celon, Incyte, Janssen, Menarini, MSD, Pfizer, Rhizen, Roche, RyVu and Siropa; institutional research grants from Agenus and Roche; non-financial interests as project lead for MSCI and board member for OECI; spouse has co-ownership of Clininote. JCB reports personal fees as a data safety monitoring board member for 4SC; personal fees as an advisory board member for Almirall, Amgen, Boehringer, InProTher, Merck, Recordati and Sanofi; institutional research grants from Alcedis, IQVIA and Merck; non-financial interests for receipt of product samples from 4SC. PAA reports personal fees for consultancy roles for 4SC, AstraZeneca, Bio-AI Health, BMS, Boehringer Ingelheim, Daiichi Sankyo, Idera, Immunocore, Italfarmaco, iTeos, Lunaphone, Medicenna, Merck Serono, MSD, Nektar, Nouscom, Novartis, Oncosec, Pfizer, Pfizer/Array, Pierre Fabre, Regeneron, Replimmune, Roche Genentech, Sandoz, Sanofi, Sun Pharma and ValoTx; personal fees for advisory roles for 4SC, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Eisai, Idera, Immunocore, iTeos, Merck Serono, MSD, Nektar, Novartis, Pfizer/Array, Pierre Fabre, Regeneron, Replimmune, Roche Genentech, Sandoz, Sanofi, Seagen, Sun Pharma and ValoTx; personal fees as an advisory board member from Erasca, iTeos, Replimmune and ValoTx; personal fees as travel support from Bio-AI-Health, Pfizer/Array and Replimmune; institutional funding from BMS, Pfizer/Array, Roche Genentech and Sanofi; non-financial interests as President of SCITO (Campania Society of ImmunoTherapy of Cancer) and Fondazione Melanoma Onlus Italy; non-financial interests as a member of the board of directors of the Society for Immunotherapy of Cancer (SITC; November 2017-December 2021) and a member of the steering committee for the Society for Melanoma Research (SMR); non-financial interests as a member of the Associazione Italiana di Oncologia Medica (AIOM), American Society of Clinical Oncology (ASCO), the European Organisation for Research and Treatment of Cancer (EORTC), Melanoma Cooperative Group, SITC and SMR. MV reports no potential conflicts of interests. ABl reports personal fees for advisory board membership from Merck (consultant for revision of approval of avelumab by French authorities). CL reports personal fees as an advisory board member from Amgen, BMS, Merck Serono, MSD, Novartis, Pierre Fabre, Roche and Sanofi; funding from BMS and Roche; non-financial interests for advisory roles for Amgen, BMS, Merck Serono, MSD, Novartis, Pierre Fabre, Roche and Sanofi; honoraria from Amgen, BMS, Incyte, MSD, Novartis, Pfizer, Pierre Fabre and Roche; travel/accommodation expenses from Avantis Medical Systems, BMS, Jazz Pharmaceuticals, MSD, Novartis, Pierre Fabre and Sanofi; research funding from BMS and Roche; participation on a data safety monitoring board/advisory board for InfalRx. EM reports no potential conflicts of interests. OH-V reports no potential conflicts of interests. MG reports personal fees for expert testimony from Almirall; personal fees as an advisory board member for GSK, Leo Pharma and Pfizer; personal fees as an invited speaker from GSK, Janssen, Lilly and Novartis; personal stocks/shares in BioNTech, Novo Nordisk and Siemens Healthineers; institutional fees as coordinating PI for Argenx and as local PI for Boehringer Ingelheim, Galderma, Janssen, Novartis and UCB Pharma; non-financial interests as Treasurer of the Deutsche Dermatologische Gesellschaft (DDG) and member of the board of directors of University Hospital Würzburg. HK reports no potential conflicts of interests. PN reports personal fees as an advisory board member for 4SC, BMS, IDEAYA Biosciences, Immunocore, Merck, Novartis and Pfizer; personal fees as an invited speaker for Novartis; institutional research support from Immunocore; non-financial interests as a steering committee member (personal) for 4SC and coordinating PI (institutional) for BMS, Immunocore, Ipsen, Merck, Novartis and Pfizer. PR reports personal fees as an invited speaker for AstraZeneca, BMS, Merck, MSD, Novartis, Pierre Fabre and Sanofi; personal fees as an advisory board member for Blueprint Medicines, BMS, Merck, MSD, Philogen, Pierre Fabre and Sanofi; institutional research grants from BMS and Pfizer; non-financial interests as President of the Polish Oncological Society and a member of the board of directors of the Polish Society of Surgical Oncology. MS reports personal fees as an invited speaker for Aristo-Pharma, Medac, Novartis and Takeda; personal fees as an advisory board member for Novartis and Takeda; personal fees as a PI for Amgen, Dermira, Eli Lilly and Company, Galderma, Parexel International and Regeneron; personal fees as a sub-investigator (SI) for OBWF NIO-PIB, Eli Lilly and Takeda. DS reports personal fees as an invited speaker for BMS, Merck Serono, MSD, Novartis, Roche and Sanofi; personal fees as an advisory board member for BMS, Immunocore, MSD, Neracare, Novartis, Pfizer, Philogen, Pierre Fabre and Sanofi/Regeneron; personal fees as a steering committee member for BMS, MSD and Novartis; institutional research grants from BMS and MSD; institutional fees as coordinating PI for BMS, MSD, Novartis and Pierre Fabre; institutional fees as local PI for Philogen and Sanofi; a non-financial interest as a member of the board of directors of EORTC-MG. JMP reports personal fees as an advisory board member for Astellas, AstraZeneca, BeiGene, BMS, Janssen, MSD, Roche and VCN Biosciences; personal and institutional research grants from BeiGene, BMS, Janssen, Mirati and Pfizer. FP reports no potential conflicts of interests. ACJvA reports institutional fees as an advisory board member for 4SC, Amgen, BMS, Merck–Pfizer, MSD–Merck, Novartis, Pierre Fabre, Provectus, Sanofi and Sirius Medical; institutional research grants from Amgen and Merck–Pfizer. ABe reports personal fees as an invited speaker for Amgen and HRA; personal fees as an advisory board member for Amgen, Astellas, Ipsen and Janssen; institutional funding from Astellas and Janssen; non-financial interests for receipt of product samples from Novartis and Sanofi.
Supplementary data
References
- 1.Song Y., Azari F.S., Tang R., et al. Patterns of metastasis in Merkel cell carcinoma. Ann Surg Oncol. 2021;28(1):519–529. doi: 10.1245/s10434-020-08587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schadendorf D., Lebbe C., Zur Hausen A., et al. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:53–69. doi: 10.1016/j.ejca.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Stang A., Becker J.C., Nghiem P., et al. The association between geographic location and incidence of Merkel cell carcinoma in comparison to melanoma: an international assessment. Eur J Cancer. 2018;94:47–60. doi: 10.1016/j.ejca.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coggshall K., Tello T.L., North J.P., et al. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78(3):433–442. doi: 10.1016/j.jaad.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105(1):107–110. [PubMed] [Google Scholar]
- 6.van der Zwan J.M., Trama A., Otter R., et al. Rare neuroendocrine tumours: results of the surveillance of rare cancers in Europe project. Eur J Cancer. 2013;49(11):2565–2578. doi: 10.1016/j.ejca.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald T.L., Dennis S., Kachare S.D., et al. Dramatic increase in the incidence and mortality from Merkel cell carcinoma in the United States. Am Surg. 2015;81(8):802–806. doi: 10.1177/000313481508100819. [DOI] [PubMed] [Google Scholar]
- 8.Youlden D.R., Soyer H.P., Youl P.H., et al. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993-2010. JAMA Dermatol. 2014;150(8):864–872. doi: 10.1001/jamadermatol.2014.124. [DOI] [PubMed] [Google Scholar]
- 9.Albores-Saavedra J., Batich K., Chable-Montero F., et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37(1):20–27. doi: 10.1111/j.1600-0560.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 10.Clarke C.A., Robbins H.A., Tatalovich Z., et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107(2) doi: 10.1093/jnci/dju382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Arcy M.E., Castenson D., Lynch C.F., et al. Risk of rare cancers among solid organ transplant recipients. J Natl Cancer Inst. 2021;113(2):199–207. doi: 10.1093/jnci/djaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asgari M.M., Sokil M.M., Warton E.M., et al. Effect of host, tumor, diagnostic, and treatment variables on outcomes in a large cohort with Merkel cell carcinoma. JAMA Dermatol. 2014;150(7):716–723. doi: 10.1001/jamadermatol.2013.8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo T., Piccolo V., Lallas A., et al. Dermoscopy of malignant skin tumours: what’s new? Dermatology. 2017;233(1):64–73. doi: 10.1159/000472253. [DOI] [PubMed] [Google Scholar]
- 14.Dalle S., Parmentier L., Moscarella E., et al. Dermoscopy of Merkel cell carcinoma. Dermatology. 2012;224(2):140–144. doi: 10.1159/000337411. [DOI] [PubMed] [Google Scholar]
- 15.Gauci M.L., Aristei C., Becker J.C., et al. Diagnosis and treatment of Merkel cell carcinoma: European consensus-based interdisciplinary guideline - Update 2022. Eur J Cancer. 2022;171:203–231. doi: 10.1016/j.ejca.2022.03.043. [DOI] [PubMed] [Google Scholar]
- 16.Sauer C.M., Haugg A.M., Chteinberg E., et al. Reviewing the current evidence supporting early B-cells as the cellular origin of Merkel cell carcinoma. Crit Rev Oncol Hematol. 2017;116:99–105. doi: 10.1016/j.critrevonc.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Sunshine J.C., Jahchan N.S., Sage J., et al. Are there multiple cells of origin of Merkel cell carcinoma? Oncogene. 2018;37(11):1409–1416. doi: 10.1038/s41388-017-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tolstov Y.L., Pastrana D.V., Feng H., et al. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125(6):1250–1256. doi: 10.1002/ijc.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harms P.W., Harms K.L., Moore P.S., et al. The biology and treatment of Merkel cell carcinoma: current understanding and research priorities. Nat Rev Clin Oncol. 2018;15(12):763–776. doi: 10.1038/s41571-018-0103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houben R., Shuda M., Weinkam R., et al. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J Virol. 2010;84(14):7064–7072. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knepper T.C., Montesion M., Russell J.S., et al. The genomic landscape of Merkel cell carcinoma and clinicogenomic biomarkers of response to immune checkpoint inhibitor therapy. Clin Cancer Res. 2019;25(19):5961–5971. doi: 10.1158/1078-0432.CCR-18-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reisinger D.M., Shiffer J.D., Cognetta A.B., Jr., et al. Lack of evidence for basal or squamous cell carcinoma infection with Merkel cell polyomavirus in immunocompetent patients with Merkel cell carcinoma. J Am Acad Dermatol. 2010;63(3):400–403. doi: 10.1016/j.jaad.2009.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeCaprio J.A. Molecular pathogenesis of Merkel cell carcinoma. Annu Rev Pathol. 2021;16:69–91. doi: 10.1146/annurev-pathmechdis-012419-032817. [DOI] [PubMed] [Google Scholar]
- 24.Tetzlaff M.T., Harms P.W. Danger is only skin deep: aggressive epidermal carcinomas. An overview of the diagnosis, demographics, molecular-genetics, staging, prognostic biomarkers, and therapeutic advances in Merkel cell carcinoma. Mod Pathol. 2020;33(suppl 1):42–55. doi: 10.1038/s41379-019-0394-6. [DOI] [PubMed] [Google Scholar]
- 25.Harary M., Kavouridis V.K., Thakuria M., et al. Predictors of survival in neurometastatic Merkel cell carcinoma. Eur J Cancer. 2018;101:152–159. doi: 10.1016/j.ejca.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Marghalani S., Feller J.K., Mahalingam M., et al. Huntingtin interacting protein 1 as a histopathologic adjunct in the diagnosis of Merkel cell carcinoma. Int J Dermatol. 2015;54(6):640–647. doi: 10.1111/ijd.12454. [DOI] [PubMed] [Google Scholar]
- 27.Kervarrec T., Tallet A., Miquelestorena-Standley E., et al. Diagnostic accuracy of a panel of immunohistochemical and molecular markers to distinguish Merkel cell carcinoma from other neuroendocrine carcinomas. Mod Pathol. 2019;32(4):499–510. doi: 10.1038/s41379-018-0155-y. [DOI] [PubMed] [Google Scholar]
- 28.Pasternak S., Carter M.D., Ly T.Y., et al. Immunohistochemical profiles of different subsets of Merkel cell carcinoma. Hum Pathol. 2018;82:232–238. doi: 10.1016/j.humpath.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 29.Farah M., Reuben A., Spassova I., et al. T-cell repertoire in combination with T-cell density predicts clinical outcomes in patients with Merkel cell carcinoma. J Invest Dermatol. 2020;140(11):2146–2156.e2144. doi: 10.1016/j.jid.2020.02.031. [DOI] [PubMed] [Google Scholar]
- 30.Brierley J.D., Gospodarowicz M.K., Wittekind C., editors. TNM Classification of Malignant Tumours. 8th ed. John Wiley & Sons, Inc; Oxford, UK: 2016. [Google Scholar]
- 31.O’Sullivan B., Brierley J., Byrd D., et al. The TNM classification of malignant tumours-towards common understanding and reasonable expectations. Lancet Oncol. 2017;18(7):849–851. doi: 10.1016/S1470-2045(17)30438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zijlker L.P., Bakker M., van der Hiel B., et al. Baseline ultrasound and FDG-PET/CT imaging in Merkel cell carcinoma. J Surg Oncol. 2023;127(5):841–847. doi: 10.1002/jso.27193. [DOI] [PubMed] [Google Scholar]
- 33.Treglia G., Kakhki V.R., Giovanella L., et al. Diagnostic performance of fluorine-18-fluorodeoxyglucose positron emission tomography in patients with Merkel cell carcinoma: a systematic review and meta-analysis. Am J Clin Dermatol. 2013;14(6):437–447. doi: 10.1007/s40257-013-0040-x. [DOI] [PubMed] [Google Scholar]
- 34.Singh N., Alexander N.A., Lachance K., et al. Clinical benefit of baseline imaging in Merkel cell carcinoma: analysis of 584 patients. J Am Acad Dermatol. 2021;84(2):330–339. doi: 10.1016/j.jaad.2020.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straker R.J., 3rd, Carr M.J., Sinnamon A.J., et al. Predictors of false negative sentinel lymph node biopsy in clinically localized Merkel cell carcinoma. Ann Surg Oncol. 2021;28(12):6995–7003. doi: 10.1245/s10434-021-10031-z. [DOI] [PubMed] [Google Scholar]
- 36.Song Y., Zheng C., Shannon A.B., et al. Sentinel lymph node positivity and overall survival in immunosuppressed patients with Merkel cell carcinoma: a national cohort study. Br J Dermatol. 2020;183(3):569–571. doi: 10.1111/bjd.19021. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues L.K., Leong S.P., Kashani-Sabet M., et al. Early experience with sentinel lymph node mapping for Merkel cell carcinoma. J Am Acad Dermatol. 2001;45(2):303–308. doi: 10.1067/mjd.2001.114749. [DOI] [PubMed] [Google Scholar]
- 38.Gunaratne D.A., Howle J.R., Veness M.J. Sentinel lymph node biopsy in Merkel cell carcinoma: a 15-year institutional experience and statistical analysis of 721 reported cases. Br J Dermatol. 2016;174(2):273–281. doi: 10.1111/bjd.14240. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez M.R., Bryce-Alberti M., Portmann-Baracco A., et al. Treatment and survival outcomes in metastatic Merkel cell carcinoma: analysis of 2010 patients from the SEER database. Cancer Treat Res Commun. 2022;33 doi: 10.1016/j.ctarc.2022.100665. [DOI] [PubMed] [Google Scholar]
- 40.Doepker M.P., Yamamoto M., Applebaum M.A., et al. Comparison of single-photon emission computed tomography-computed tomography (SPECT/CT) and conventional planar lymphoscintigraphy for sentinel node localization in patients with cutaneous malignancies. Ann Surg Oncol. 2017;24(2):355–361. doi: 10.1245/s10434-016-5590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grabowski J., Saltzstein S.L., Sadler G.R., et al. A comparison of Merkel cell carcinoma and melanoma: results from the California cancer registry. Clin Med Oncol. 2008;2:327–333. doi: 10.4137/cmo.s423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harms K.L., Healy M.A., Nghiem P., et al. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol. 2016;23(11):3564–3571. doi: 10.1245/s10434-016-5266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silling S., Kreuter A., Gambichler T., et al. Epidemiology of Merkel cell polyomavirus infection and Merkel cell carcinoma. Cancers (Basel) 2022;14(24):6176. doi: 10.3390/cancers14246176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tam M., Luu M., Barker C.A., et al. Improved survival in women versus men with Merkel cell carcinoma. J Am Acad Dermatol. 2021;84(2):321–329. doi: 10.1016/j.jaad.2020.02.034. [DOI] [PubMed] [Google Scholar]
- 45.Fazio N., Maisonneuve P., Spada F., et al. Nodal Merkel cell carcinoma with unknown primary site and no distant metastasis: a single-center series. Cancers (Basel) 2022;14(19):4777. doi: 10.3390/cancers14194777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yaghi M., Benedetto P., Greskovich J., et al. Merkel cell carcinoma: epidemiology, disease presentation, and current clinical practice outcomes. JAAD Int. 2022;9:128–136. doi: 10.1016/j.jdin.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paulson K.G., Iyer J.G., Tegeder A.R., et al. Transcriptome-wide studies of Merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J Clin Oncol. 2011;29(12):1539–1546. doi: 10.1200/JCO.2010.30.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sihto H., Kukko H., Koljonen V., et al. Merkel cell polyomavirus infection, large T antigen, retinoblastoma protein and outcome in Merkel cell carcinoma. Clin Cancer Res. 2011;17(14):4806–4813. doi: 10.1158/1078-0432.CCR-10-3363. [DOI] [PubMed] [Google Scholar]
- 49.Stetsenko G.Y., Malekirad J., Paulson K.G., et al. p63 expression in Merkel cell carcinoma predicts poorer survival yet may have limited clinical utility. Am J Clin Pathol. 2013;140(6):838–844. doi: 10.1309/AJCPE4PK6CTBNQJY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paulson K.G., Lewis C.W., Redman M.W., et al. Viral oncoprotein antibodies as a marker for recurrence of Merkel cell carcinoma: a prospective validation study. Cancer. 2017;123(8):1464–1474. doi: 10.1002/cncr.30475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Veenendaal L.M., Bertolli E., Korse C.M., et al. The clinical utility of neuron-specific enolase (NSE) serum levels as a biomarker for Merkel cell carcinoma (MCC) Ann Surg Oncol. 2021;28(2):1019–1028. doi: 10.1245/s10434-020-08656-7. [DOI] [PubMed] [Google Scholar]
- 52.Carrasquillo O.Y., Cancel-Artau K.J., Ramos-Rodriguez A.J., et al. Mohs micrographic surgery versus wide local excision in the treatment of Merkel cell carcinoma: a systematic review. Dermatol Surg. 2022;48(2):176–180. doi: 10.1097/DSS.0000000000003331. [DOI] [PubMed] [Google Scholar]
- 53.Uitentuis S.E., Bambach C., Elshot Y.S., et al. Merkel cell carcinoma, the impact of clinical excision margins and Mohs micrographic surgery on recurrence and survival: a systematic review. Dermatol Surg. 2022;48(4):387–394. doi: 10.1097/DSS.0000000000003402. [DOI] [PubMed] [Google Scholar]
- 54.Yan L., Sun L., Guan Z., et al. Analysis of cutaneous Merkel cell carcinoma outcomes after different surgical interventions. J Am Acad Dermatol. 2020;82(6):1422–1434. doi: 10.1016/j.jaad.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Andruska N., Fischer-Valuck B.W., Mahapatra L., et al. Association between surgical margins larger than 1 cm and overall survival in patients with Merkel cell carcinoma. JAMA Dermatol. 2021;157(5):540–548. doi: 10.1001/jamadermatol.2021.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jaouen F., Kervarrec T., Caille A., et al. Narrow resection margins are not associated with mortality or recurrence in patients with Merkel cell carcinoma: a retrospective study. J Am Acad Dermatol. 2021;84(4):921–929. doi: 10.1016/j.jaad.2020.11.038. [DOI] [PubMed] [Google Scholar]
- 57.Gillenwater A.M., Hessel A.C., Morrison W.H., et al. Merkel cell carcinoma of the head and neck: effect of surgical excision and radiation on recurrence and survival. Arch Otolaryngol Head Neck Surg. 2001;127(2):149–154. doi: 10.1001/archotol.127.2.149. [DOI] [PubMed] [Google Scholar]
- 58.Poulsen M. Merkel-cell carcinoma of the skin. Lancet Oncol. 2004;5(10):593–599. doi: 10.1016/S1470-2045(04)01593-1. [DOI] [PubMed] [Google Scholar]
- 59.Levy S., Blankenstein S.A., Grünhagen D.J., et al. Postoperative radiotherapy in stage I-III Merkel cell carcinoma. Radiother Oncol. 2022;166:203–211. doi: 10.1016/j.radonc.2021.11.017. [DOI] [PubMed] [Google Scholar]
- 60.Petrelli F., Ghidini A., Torchio M., et al. Adjuvant radiotherapy for Merkel cell carcinoma: a systematic review and meta-analysis. Radiother Oncol. 2019;134:211–219. doi: 10.1016/j.radonc.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 61.Bhatia S., Storer B.E., Iyer J.G., et al. Adjuvant radiation therapy and chemotherapy in Merkel cell carcinoma: survival analyses of 6908 cases from the national cancer data base. J Natl Cancer Inst. 2016;108(9) doi: 10.1093/jnci/djw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wong W.G., Stahl K., Olecki E.J., et al. Survival benefit of guideline-concordant postoperative radiation for local Merkel cell carcinoma. J Surg Res. 2021;266:168–179. doi: 10.1016/j.jss.2021.03.062. [DOI] [PubMed] [Google Scholar]
- 63.NCCN Clinical Practice Guidelines in Oncology Merkel cell carcinoma version 2.2022. 2022. https://www.nccn.org/professionals/physician_gls/pdf/mcc.pdf Available at.
- 64.Sundaresan P., Hruby G., Hamilton A., et al. Definitive radiotherapy or chemoradiotherapy in the treatment of Merkel cell carcinoma. Clin Oncol (R Coll Radiol) 2012;24(9):e131–e136. doi: 10.1016/j.clon.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Patel S.A., Qureshi M.M., Sahni D., et al. Identifying an optimal adjuvant radiotherapy dose for extremity and trunk Merkel cell carcinoma following resection: an analysis of the national cancer database. JAMA Dermatol. 2017;153(10):1007–1014. doi: 10.1001/jamadermatol.2017.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Veness M., Foote M., Gebski V., et al. The role of radiotherapy alone in patients with Merkel cell carcinoma: reporting the Australian experience of 43 patients. Int J Radiat Oncol Biol Phys. 2010;78(3):703–709. doi: 10.1016/j.ijrobp.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 67.Harrington C., Kwan W. Outcomes of Merkel cell carcinoma treated with radiotherapy without radical surgical excision. Ann Surg Oncol. 2014;21(11):3401–3405. doi: 10.1245/s10434-014-3757-8. [DOI] [PubMed] [Google Scholar]
- 68.Migliano E., Monarca C., Rizzo M.I., et al. Merkel cell carcinoma: our therapeutic algorithm: treatment of Merkel cell carcinoma. Ann Surg Oncol. 2009;16(11):3211–3213. doi: 10.1245/s10434-009-0596-0. [DOI] [PubMed] [Google Scholar]
- 69.Tarantola T.I., Vallow L.A., Halyard M.Y., et al. Prognostic factors in Merkel cell carcinoma: analysis of 240 cases. J Am Acad Dermatol. 2013;68(3):425–432. doi: 10.1016/j.jaad.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 70.Mattavelli I., Patuzzo R., Torri V., et al. Prognostic factors in Merkel cell carcinoma patients undergoing sentinel node biopsy. Eur J Surg Oncol. 2017;43(8):1536–1541. doi: 10.1016/j.ejso.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 71.Lee J.S., Durham A.B., Bichakjian C.K., et al. Completion lymph node dissection or radiation therapy for sentinel node metastasis in Merkel cell carcinoma. Ann Surg Oncol. 2019;26(2):386–394. doi: 10.1245/s10434-018-7072-7. [DOI] [PubMed] [Google Scholar]
- 72.Cramer J.D., Suresh K., Sridharan S. Completion lymph node dissection for Merkel cell carcinoma. Am J Surg. 2020;220(4):982–986. doi: 10.1016/j.amjsurg.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 73.Morton D.L., Cochran A.J., Thompson J.F., et al. Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg. 2005;242(3):302–311. doi: 10.1097/01.sla.0000181092.50141.fa. discussion 311-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen M.M., Roman S.A., Sosa J.A., et al. The role of adjuvant therapy in the management of head and neck Merkel cell carcinoma: an analysis of 4815 patients. JAMA Otolaryngol Head Neck Surg. 2015;141(2):137–141. doi: 10.1001/jamaoto.2014.3052. [DOI] [PubMed] [Google Scholar]
- 75.Tai P., Yu E., Assouline A., et al. Multimodality management for 145 cases of Merkel cell carcinoma. Med Oncol. 2010;27(4):1260–1266. doi: 10.1007/s12032-009-9369-7. [DOI] [PubMed] [Google Scholar]
- 76.Voog E., Biron P., Martin J.P., et al. Chemotherapy for patients with locally advanced or metastatic Merkel cell carcinoma. Cancer. 1999;85(12):2589–2595. doi: 10.1002/(sici)1097-0142(19990615)85:12<2589::aid-cncr15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 77.Topalian S.L., Bhatia S., Hollebecque A., et al. Abstract CT074: Non-comparative, open-label, multiple cohort, phase 1/2 study to evaluate nivolumab (NIVO) in patients with virus-associated tumors (CheckMate 358): efficacy and safety in Merkel cell carcinoma (MCC) Cancer Res. 2017;77(13_Supplement):CT074. [Google Scholar]
- 78.Michielin O., van Akkooi A.C.J., Ascierto P.A., et al. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(12):1884–1901. doi: 10.1093/annonc/mdz411. [DOI] [PubMed] [Google Scholar]
- 79.Fang L.C., Lemos B., Douglas J., et al. Radiation monotherapy as regional treatment for lymph node-positive Merkel cell carcinoma. Cancer. 2010;116(7):1783–1790. doi: 10.1002/cncr.24919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim J.A., Choi A.H. Effect of radiation therapy on survival in patients with resected Merkel cell carcinoma: a propensity score surveillance, epidemiology, and end results database analysis. JAMA Dermatol. 2013;149(7):831–838. doi: 10.1001/jamadermatol.2013.409. [DOI] [PubMed] [Google Scholar]
- 81.Lewis K.G., Weinstock M.A., Weaver A.L., et al. Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol. 2006;142(6):693–700. doi: 10.1001/archderm.142.6.693. [DOI] [PubMed] [Google Scholar]
- 82.Grotz T.E., Tarantola T.I., Otley C.C., et al. Natural history of Merkel cell carcinoma following locoregional recurrence. Ann Surg Oncol. 2012;19(8):2556–2562. doi: 10.1245/s10434-011-2161-x. [DOI] [PubMed] [Google Scholar]
- 83.Poulsen M., Round C., Keller J., et al. Factors influencing relapse-free survival in Merkel cell carcinoma of the lower limb--a review of 60 cases. Int J Radiat Oncol Biol Phys. 2010;76(2):393–397. doi: 10.1016/j.ijrobp.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 84.van Veenendaal L.M., Madu M.F., Tesselaar M.E.T., et al. Efficacy of isolated limb perfusion (ILP) in patients with Merkel cell carcinoma (MCC): a multicenter experience. Eur J Surg Oncol. 2017;43(11):2157–2162. doi: 10.1016/j.ejso.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 85.Topalian S.L., Bhatia S., Amin A., et al. Neoadjuvant nivolumab for patients with resectable Merkel cell carcinoma in the CheckMate 358 trial. J Clin Oncol. 2020;38(22):2476–2487. doi: 10.1200/JCO.20.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Becker J.C., Ugurel S., Leiter-Stoppke U., et al. 787O - Adjuvant immunotherapy with nivolumab (NIVO) versus observation in completely resected Merkel cell carcinoma (MCC): disease-free survival (DFS) results from ADMEC-O, a randomized, open-label phase II trial. Ann Oncol. 2022;33(suppl 7):S356–S409. doi: 10.1016/S0140-6736(23)00769-9. [DOI] [PubMed] [Google Scholar]
- 87.Iyer J.G., Parvathaneni U., Gooley T., et al. Single-fraction radiation therapy in patients with metastatic Merkel cell carcinoma. Cancer Med. 2015;4(8):1161–1170. doi: 10.1002/cam4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zitvogel L., Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology. 2012;1(8):1223–1225. doi: 10.4161/onci.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boyerinas B., Jochems C., Fantini M., et al. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res. 2015;3(10):1148–1157. doi: 10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.D’Angelo S.P., Bhatia S., Brohl A.S., et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma: long-term data and biomarker analyses from the single-arm phase 2 JAVELIN Merkel 200 trial. J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2020-000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaufman H.L., Russell J., Hamid O., et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walker J.W., Lebbe C., Grignani G., et al. Efficacy and safety of avelumab treatment in patients with metastatic Merkel cell carcinoma: experience from a global expanded access program. J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zijlker L.P., Levy S., Wolters W., et al. Avelumab treatment for patients with metastatic Merkel cell carcinoma can be safely stopped after 1 year and a PET/CT-confirmed complete response. Cancer. 2024;130(3):433–438. doi: 10.1002/cncr.35050. [DOI] [PubMed] [Google Scholar]
- 94.D’Angelo S.P., Bhatia S., Brohl A.S., et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma (JAVELIN Merkel 200): updated overall survival data after >5 years of follow-up. ESMO Open. 2021;6(6) doi: 10.1016/j.esmoop.2021.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.D’Angelo S.P., Lebbé C., Mortier L., et al. First-line avelumab in a cohort of 116 patients with metastatic Merkel cell carcinoma (JAVELIN Merkel 200): primary and biomarker analyses of a phase II study. J Immunother Cancer. 2021;9(7) doi: 10.1136/jitc-2021-002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Levy S., Aarts M.J.B., Eskens F., et al. Avelumab for advanced Merkel cell carcinoma in the Netherlands: a real-world cohort. J Immunother Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lohray R., Verma K.K., Wang L.L., et al. Avelumab for advanced Merkel cell carcinoma: global real-world data on patient response and survival. Pragmat Obs Res. 2023;14:149–154. doi: 10.2147/POR.S398151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nghiem P., Bhatia S., Lipson E.J., et al. Durable tumor regression and overall survival (OS) in patients with advanced Merkel cell carcinoma (aMCC) receiving pembrolizumab as first-line therapy. J Clin Oncol. 2018;36(15_suppl):9506. doi: 10.1200/JCO.18.01896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nghiem P., Bhatia S., Lipson E.J., et al. Three-year survival, correlates and salvage therapies in patients receiving first-line pembrolizumab for advanced Merkel cell carcinoma. J Immunother Cancer. 2021;9(4) doi: 10.1136/jitc-2021-002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim S., Wuthrick E., Blakaj D., et al. Combined nivolumab and ipilimumab with or without stereotactic body radiation therapy for advanced Merkel cell carcinoma: a randomised, open label, phase 2 trial. Lancet. 2022;400(10357):1008–1019. doi: 10.1016/S0140-6736(22)01659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Food and Drug Administration Retifanlimab-dlwr prescribing information. Published 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761334s000lbl.pdf Available at.
- 102.Zaggana E., Konstantinou M.P., Krasagakis G.H., et al. Merkel cell carcinoma-update on diagnosis, management and future perspectives. Cancers (Basel) 2022;15(1):103. doi: 10.3390/cancers15010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nghiem P., Kaufman H.L., Bharmal M., et al. Systematic literature review of efficacy, safety and tolerability outcomes of chemotherapy regimens in patients with metastatic Merkel cell carcinoma. Future Oncol. 2017;13(14):1263–1279. doi: 10.2217/fon-2017-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tai P.T., Yu E., Winquist E., et al. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol. 2000;18(12):2493–2499. doi: 10.1200/JCO.2000.18.12.2493. [DOI] [PubMed] [Google Scholar]
- 105.Nathan P.D., Gaunt P., Wheatley K., et al. UKMCC-01: a phase II study of pazopanib (PAZ) in metastatic Merkel cell carcinoma. J Clin Oncol. 2016;34(15_suppl):9542. [Google Scholar]
- 106.Rabinowits G., Lezcano C., Catalano P.J., et al. Cabozantinib in patients with advanced Merkel cell carcinoma. Oncologist. 2018;23(7):814–821. doi: 10.1634/theoncologist.2017-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sachpekidis C., Sidiropoulou P., Hassel J.C., et al. Positron emission tomography in Merkel cell carcinoma. Cancers (Basel) 2020;12(10):2897. doi: 10.3390/cancers12102897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cimbak N., Barker C.A. Short-course radiation therapy for Merkel cell carcinoma: relative effectiveness in a “radiosensitive” tumor. Int J Radiat Oncol Biol Phys. 2016;96(2(suppl)) 2016;96[2(suppl)]:S160. [Google Scholar]
- 109.Kaae J., Hansen A.V., Biggar R.J., et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst. 2010;102(11):793–801. doi: 10.1093/jnci/djq120. [DOI] [PubMed] [Google Scholar]
- 110.Koljonen V., Kukko H., Tukiainen E., et al. Second cancers following the diagnosis of Merkel cell carcinoma: a nationwide cohort study. Cancer Epidemiol. 2010;34(1):62–65. doi: 10.1016/j.canep.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 111.Allen P.J., Bowne W.B., Jaques D.P., et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23(10):2300–2309. doi: 10.1200/JCO.2005.02.329. [DOI] [PubMed] [Google Scholar]
- 112.Farley C.R., Perez M.C., Soelling S.J., et al. Merkel cell carcinoma outcomes: does AJCC8 underestimate survival? Ann Surg Oncol. 2020;27(6):1978–1985. doi: 10.1245/s10434-019-08187-w. [DOI] [PubMed] [Google Scholar]
- 113.Paulson K.G., Carter J.J., Johnson L.G., et al. Antibodies to Merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in Merkel cell carcinoma patients. Cancer Res. 2010;70(21):8388–8397. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Samimi M., Molet L., Fleury M., et al. Prognostic value of antibodies to Merkel cell polyomavirus T antigens and VP1 protein in patients with Merkel cell carcinoma. Br J Dermatol. 2016;174(4):813–822. doi: 10.1111/bjd.14313. [DOI] [PubMed] [Google Scholar]
- 115.Bzhalava D., Bray F., Storm H., et al. Risk of second cancers after the diagnosis of Merkel cell carcinoma in Scandinavia. Br J Cancer. 2011;104(1):178–180. doi: 10.1038/sj.bjc.6605989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fields R.C., Busam K.J., Chou J.F., et al. Five hundred patients with Merkel cell carcinoma evaluated at a single institution. Ann Surg. 2011;254(3):465–473. doi: 10.1097/SLA.0b013e31822c5fc1. discussion 473-465. [DOI] [PubMed] [Google Scholar]
- 117.Naseri S., Steiniche T., Ladekarl M., et al. Management recommendations for Merkel cell carcinoma-a Danish perspective. Cancers (Basel) 2020;12(3):554. doi: 10.3390/cancers12030554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Poulsen M., Rischin D. Merkel cell carcinoma--current therapeutic options. Expert Opin Pharmacother. 2003;4(12):2187–2192. doi: 10.1517/14656566.4.12.2187. [DOI] [PubMed] [Google Scholar]
- 119.Tai P. A practical update of surgical management of Merkel cell carcinoma of the skin. ISRN Surg. 2013;2013 doi: 10.1155/2013/850797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cherny N.I., Dafni U., Bogaerts J., et al. ESMO-magnitude of clinical benefit scale version 1.1. Ann Oncol. 2017;28(10):2340–2366. doi: 10.1093/annonc/mdx310. [DOI] [PubMed] [Google Scholar]
- 121.Dykewicz C.A. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis. 2001;33(2):139–144. doi: 10.1086/321805. [Adapted from: Gross PA, Barrett TL, Dellinger EP, et al. Purpose of quality standards for infectious diseases. Clin Infect Dis.1994;18(3):421] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.