Abstract
Background:
The COVID-19 pandemic affected cancer screening, diagnosis and treatments. Many surgeries were substituted with bridging therapies during the initial lockdown, yet consideration of treatment side effects and their management was not a priority.
Objectives:
To examine how the changing social restrictions imposed by the pandemic affected incidence and trends of endocrine treatment prescriptions in newly diagnosed (incident) breast and prostate cancer patients and, secondarily, endocrine treatment-related outcomes (including bisphosphonate prescriptions, osteopenia and osteoporosis), in UK clinical practice from March 2020 to June 2022.
Design:
Population-based cohort study using UK primary care Clinical Practice Research Datalink GOLD database.
Methods:
There were 13,701 newly diagnosed breast cancer patients and 12,221 prostate cancer patients with ⩾1-year data availability since diagnosis between January 2017 and June 2022. Incidence rates (IR) and incidence rate ratios (IRR) were calculated across multiple time periods before and after lockdown to examine the impact of changing social restrictions on endocrine treatments and treatment-related outcomes, including osteopenia, osteoporosis and bisphosphonate prescriptions.
Results:
In breast cancer patients, aromatase inhibitor (AI) prescriptions increased during lockdown versus pre-pandemic [IRR: 1.22 (95% confidence interval (CI): 1.11–1.34)], followed by a decrease post-first lockdown [IRR: 0.79 (95% CI: 0.69–0.89)]. In prostate cancer patients, first-generation antiandrogen prescriptions increased versus pre-pandemic [IRR: 1.23 (95% CI: 1.08–1.4)]. For breast cancer patients on AIs, diagnoses of osteopenia, osteoporosis and bisphosphonate prescriptions were reduced across all lockdown periods versus pre-pandemic (IRR range: 0.31–0.62).
Conclusion:
During the first 2 years of the pandemic, newly diagnosed breast and prostate cancer patients were prescribed more endocrine treatments compared to pre-pandemic due to restrictions on hospital procedures replacing surgeries with bridging therapies. But breast cancer patients had fewer diagnoses of osteopenia and osteoporosis and bisphosphonate prescriptions. These patients should be followed up in the coming years for signs of bone thinning. Evidence of poorer management of treatment-related side effects will help assess resource allocation for patients at high risk for bone-related complications.
Keywords: adjuvent therapy, breast cancer, COVID-19, endocrine therapy < hormone therapy, pandemic, prostate cancer
Plain language summary
Effects of the COVID-19 pandemic on hormone treatments for breast and prostate cancer in the UK: implications for bone health
The COVID-19 pandemic has had a big impact on health, going beyond just causing illness. One area it has influenced is how patients with breast cancer or prostate cancer are treated. Surgeries and radiotherapies were delayed from the first lockdown as hospitals reduced non-covid related procedures. Some patients with breast or prostate cancer were instead given some medications to help stop their cancers from growing until they were able to have surgery or radiotherapy. These medications (called endocrine treatments) have important side effects, such as conditions that affect the bones. Patients on these medications should be monitored by doctors for signs of bone thinning and should, in some cases, be given other medications to help stop this happening. This study used doctors’ records from more than 5 million people to find out whether the pandemic affected the number of endocrine medications being prescribed in patients with breast or prostate cancer, and also looked at the number of these patients that were diagnosed with conditions that affect their bones and whether they were given medications that could protect their bone health. We found that during the first lockdown, patients with breast cancer or prostate cancer had more of some types of endocrine treatments compared to before the lockdown. However, they had fewer diagnoses of conditions related to bone health and fewer medications to protect their bones. It is possible that appointments and tests that are usually carried out to diagnose conditions relating to bone health were not performed in the months after the first lockdown, and so these conditions were underdiagnosed. The use of medications to protect their bones was also reduced, likely because this was not considered a priority during the pandemic. This highlights that such patients should be followed up in the coming years for signs of bone thinning, given the relatively poorer management of these side effects in these people after the pandemic.
Introduction
The COVID-19 pandemic affected healthcare beyond the immediate effects of the virus. The collateral impact of lockdown affected cancer screening, diagnosis and treatment pathways, ultimately decreasing cancer survival. 1 Indeed, recent reports highlight that screening tests for breast cancer and visits to breast surgeons were delayed in the initial months following lockdown and up to at least June 2022 in the United Kingdom (UK).2,3 Furthermore, breast and prostate cancer were underdiagnosed between March 2020 and June 2022.2,4
Because healthcare staff were redeployed to care for COVID-19 patients, and many hospital beds were allocated to such patients, treatments for cancers were altered. 4 New guidelines were introduced in Europe for the management of cancer patients during the pandemic, including the recommendation to postpone surgery/radiotherapy and instead provide neoadjuvant endocrine therapy for some breast cancer patients during the waiting period (though not specifically in the UK). 5 Similar recommendations were implemented for prostate cancer. Patients with intermediate or high-risk prostate cancer were recommended to delay radiotherapy or surgical treatment for 3–6 months and instead be administered androgen deprivation therapy (ADT) during this waiting period in some European countries. 5
Increases in the use of endocrine therapies during the initial phases of the pandemic enabled access to treatment amidst a period of turmoil. Nevertheless, consideration of the side effects of these treatments and how such side effects were managed during the pandemic cannot be neglected. Indeed, well-known side effects of endocrine therapies such as aromatase inhibitors (AIs) for breast cancer, gonadotropin-releasing hormone (GnRH) analogues for breast and prostate cancer and ADT for prostate cancer include hot flushes, night sweats, vaginal dryness (in women); as well as mood changes, headaches, reduced sex drive and fatigue. In addition, some people experience musculoskeletal problems such as bone density loss, osteopenia and osteoporosis, increasing the risk of bone fractures in patients exposed to such drugs.6,7 Common preventive and treatment strategies to improve bone health in such patients include the administration of anti-osteoporotic treatments. However, the assessment of endocrine therapy side effects such as osteopenia and osteoporosis was conceivably not a priority during the COVID-19 pandemic. Thus, subsequent diagnosis and treatment of treatment-related conditions due to these therapies may have decreased during the COVID-19 pandemic. Despite this hypothesis, there is no available data on the pandemic’s impact on secondary diagnoses and anti-osteoporotic treatment prescriptions in breast and prostate cancer patients.
The primary aim of this study is to examine how the changing social restrictions imposed by the pandemic affected incidence and trends of endocrine treatment prescriptions in newly diagnosed (incident) breast and prostate cancer patients and, secondarily, endocrine treatment-related outcomes relating to bone health (including bisphosphonate prescriptions, osteopenia and osteoporosis), in UK clinical practice from March 2020 to June 2022. Evidence of poorer management of treatment-related side effects will allow us to determine whether there is a need to better allocate resources to patients at high risk for bone-related complications.
Methods
Study design and participants
This is a population-based cohort study using routinely collected electronic health records from UK Clinical Practice Research Datalink (CPRD) GOLD. CPRD GOLD contains pseudo-anonymized patient-level demographics, lifestyle data, clinical diagnoses, prescriptions and preventive care contributed by general practitioners (GP) from the UK. 8 The use of CPRD data for this study was approved by the Independent Scientific Advisory Committee (22_002331). This database was mapped to the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM). 9 The protocol for this research was approved by the Research Data Governance (RDG) Board of the Medicine and Healthcare Products Regulatory Agency database research (protocol number 22_002331).
People were eligible if they were registered between January 2017 and June 2022 with at least 1 year of prior history in the database before their cancer diagnosis. The incident breast and prostate cancer cohorts excluded individuals diagnosed with the same cancer at any time in clinical history and those with metastases as we were interested in the pandemic’s effect on cancer patients who had not previously been under cancer management pathways. All endocrine treatments and treatment-related outcomes were first-ever events in clinical history.
Drug utilization
The study focused on prescriptions of AIs, AIs with GnRH agonists/antagonists, Tamoxifen and Tamoxifen with GnRH agonists/antagonists in breast cancer patients and first-generation ADT, GnRH agonists, GnRH agonists with first-generation ADT, GnRH antagonists and second-generation ADT in prostate cancer patients. Endocrine treatment-related side effects in breast and prostate cancer patients included bisphosphonate prescriptions, osteopenia and osteoporosis.
All cancer diagnoses and medications were defined based on Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT)/RxNorm codes (as appropriate), in the OMOP-mapped data. Diagnostic codes indicative of either non-malignant cancer or metastasis were excluded. The cancer diagnosis definitions and endocrine treatments were reviewed with the aid of the CohortDiagnostics R package. 10 This package was used to identify additional codes of interest and to remove those highlighted as irrelevant based on feedback from clinicians with oncology expertise through an iterative process during the initial stages of analysis. A list of all codes used to define the population and each outcome can be found in our GitHub repository: https://github.com/oxford-pharmacoepi/CancerCovidEndocrineTx/tree/main/Concept%20Sets.
Public health restrictions
The ‘exposures’ were the periods of the changing social restrictions due to the pandemic in the UK. Our observation period was dissected into seven time periods as follows: pre-pandemic (January 2017–February 2020), first lockdown (March 2020–June 2020), post-first lockdown (July 2020–October 2020), second lockdown (November 2020–December 2020), third lockdown (January 2021–March 2021), easing of restrictions (April 2021–June 2021) and legal restrictions removed (July 2021–June 2022). We also examined the period covering all lockdown periods from March 2020 to June 2022 to make comparisons with the pre-pandemic period.
Statistical analyses
Characterization
Patients with incident endocrine treatment prescriptions were characterized on age at index date (date of incident outcome), sex, comorbidities (based on SNOMED codes) at any time in patient history and medication use (based on RxNorm codes) within the 90 days prior to their first endocrine prescription to gain an understanding of their clinical profile. Continuous variables were summarized as means and standard deviations, medians and interquartile ranges, and categorical variables as counts and percentages. Frequency counts of less than five were censored to enhance patient/practice confidentiality.
Incidence rates and incidence rate ratios
Incidence rates (IR) with 95% confidence intervals (CIs) were calculated for all endocrine treatments and treatment-related outcomes monthly and within the pre-pandemic, lockdown and post-lockdown periods across the entire observation period using the IncidencePrevalence R package. 11 Patients with breast cancer or prostate cancer who were diagnosed within the observation period contributed time-at-risk and, as such, contributed to the ‘denominator population’, until the earliest of a record of the endocrine treatment/treatment-related outcome, transfer out of the database, end of the study period or death. Incidence rate ratios (IRR) with 95% CI were calculated using the IR estimates across the post-lockdown periods divided by the IR estimates before lockdown. All statistical code can be found in our GitHub repository: https://github.com/oxford-pharmacoepi/CancerCovidEndocrineTx.
Study reporting
The reporting of this study conforms to ‘The Strengthening the Reporting of Observational Studies in Epidemiology’ Statement: guidelines for reporting observational studies from the EQUATOR network 12 (Supplemental Table S17).
Results
Characterizations of breast and prostate cancer patients
Overall, there were 13,760 incident breast cancer patients and 8805 incident prostate cancer patients included in the denominator populations from January 2017 to June 2022. Of those, there were 8805 breast cancer patients and 8591 prostate cancer patients on endocrine treatments in the year after diagnosis. These patients may have been prescribed more than one endocrine treatment during this period after diagnosis. Attrition tables showing how the study cohorts were derived are shown in Supplemental Tables S1 and S2.
Demographic characteristics, comorbidities and comedications in breast and prostate cancer patients are shown in Tables 1 and 2, and in breast and prostate cancer patients on different endocrine treatments are shown in Supplemental Tables S3 and S4. Breast cancer patients on AIs were older and had a greater proportion of comorbidities and comedications compared to the other breast cancer patient groups. There were no patterns in the comorbidities or comedications of prostate cancer patients as a function of their endocrine treatment.
Table 1.
Characterizations of breast cancer patients on endocrine treatments.
| Variable | Aromatase inhibitors | Aromatase inhibitors with GnRH agonists or antagonists | Tamoxifen | Tamoxifen with GnRH agonists or antagonists |
|---|---|---|---|---|
| N | 6634 | 253 | 2887 | 132 |
| Mean (SD) age | 68.2 (11.97) | 44.86 (6.27) | 54.69 (12.19) | 42.94 (6.94) |
| Median (IQR) age | 68 (59–77) | 46 (40–50) | 52 (47–62) | 44 (38.75–48) |
| Median prior history (years) | 16.06 (11.1–18.85) | 14.47 (6.65–18.23) | 15.15 (9.48–17.95) | 13.86 (6.66–17.46) |
| Mean Charlson Index | 0 | 0 | 0 | 0 |
| Median Charlson Index | 0 | 0 | 0 | 0 |
| N male | 0 | 0 | 0 | 0 |
| N Female | 6634 | 253 | 2887 | 132 |
| % Female | 100 | 100 | 100 | 100 |
| Comorbidities [n (%)] | ||||
| Atrial fibrillation | 390 (5.88) | 0 (0) | 40 (1.39) | 0 (0) |
| Cerebrovascular disease | 306 (4.61) | <5 (~1) | 35 (1.21) | <5 (~1) |
| Chronic liver disease | 40 (0.6) | <5 (~0) | 8 (0.28) | 0 (0) |
| Chronic obstructive lung disease | 348 (5.25) | <5 (~0) | 80 (2.77) | 0 (0) |
| Coronary arteriosclerosis | 38 (0.57) | 0 (0) | 13 (0.45) | 0 (0) |
| Crohn’s disease | 18 (0.27) | 0 (0) | 8 (0.28) | 0 (0) |
| Dementia | 127 (1.91) | 0 (0) | 10 (0.35) | 0 (0) |
| Depressive disorder | 1195 (18.01) | 58 (22.92) | 540 (18.7) | 29 (21.97) |
| Diabetes mellitus | 731 (11.02) | 11 (4.35) | 127 (4.4) | <5 (~3) |
| Helicobacter pylori infection | 20 (0.3) | <5 (~0) | <5 (~0) | 0 (0) |
| Heart disease | 925 (13.94) | 5 (1.98) | 172 (5.96) | <5 (~1) |
| Heart failure | 129 (1.94) | 0 (0) | 14 (0.48) | 0 (0) |
| Hepatitis C | <5 (~0) | 0 (0) | <5 (~1) | 0 (0) |
| HIV | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hyperlipidaemia | 615 (9.27) | <5 (~1) | 109 (3.78) | <5 (~1) |
| Hypertension | 1835 (27.66) | 26 (10.28) | 442 (15.31) | 10 (7.58) |
| Ischaemic heart disease | 324 (4.88) | <5 (~1) | 62 (2.15) | 0 (0) |
| Lesion liver | 87 (1.31) | 9 (3.56) | 16 (0.55) | 0 (0) |
| Obesity | 277 (4.18) | 6 (2.37) | 78 (2.7) | <5 (~3) |
| Osteoarthritis | 1532 (23.09) | <5 (~1) | 328 (11.36) | <5 (~2) |
| Peripheral vascular disease | 81 (1.22) | 0 (0) | 7 (0.24) | 0 (0) |
| Pneumonia | 142 (2.14) | <5 (~1) | 36 (1.25) | 0 (0) |
| Psoriasis | 245 (3.69) | 11 (4.35) | 98 (3.39) | 5 (3.79) |
| Pulmonary embolism | 119 (1.79) | <5 (~1) | 12 (0.42) | 0 (0) |
| Renal impairment | 811 (12.22) | <5 (~1) | 124 (4.3) | 0 (0) |
| Rheumatoid arthritis | 66 (0.99) | <5 (~0) | 30 (1.04) | <5 (~1) |
| Schizophrenia | 21 (0.32) | <5 (~0) | 5 (0.17) | 0 (0) |
| Ulcerative colitis | 29 (0.44) | 0 (0) | 9 (0.31) | <5 (~1) |
| Urinary Tract Infection | 1055 (15.9) | 33 (13.04) | 375 (12.99) | 10 (7.58) |
| Venous thrombosis | 418 (6.3) | 15 (5.93) | 93 (3.22) | <5 (~3) |
| Visual system disorder | 2437 (36.74) | 48 (18.97) | 731 (25.32) | 20 (15.15) |
| Comedications [n (%)] | ||||
| Antidepressants | 3529 (53.2) | 146 (57.71) | 1468 (50.85) | 58 (43.94) |
| Antiepileptics | 1214 (18.3) | 32 (12.65) | 451 (15.62) | 17 (12.88) |
| Antiinflammatory/antirheumatic | 4489 (67.67) | 155 (61.26) | 1930 (66.85) | 76 (57.58) |
| Antineoplastics | 426 (6.42) | <5 (~1) | 122 (4.23) | 5 (3.79) |
| Antipsoriatics | 147 (2.22) | 9 (3.56) | 77 (2.67) | <5 (~3) |
| Antipsychotics | 2020 (30.45) | 77 (30.43) | 785 (27.19) | 37 (28.03) |
| Antithrombotics | 1256 (18.93) | 32 (12.65) | 218 (7.55) | 13 (9.85) |
| Anxiolytics | 2115 (31.88) | 86 (33.99) | 888 (30.76) | 40 (30.3) |
| Beta-blockers | 2055 (30.98) | 68 (26.88) | 725 (25.11) | 34 (25.76) |
| Calcium channel blockers | 2092 (31.53) | 18 (7.11) | 421 (14.58) | 7 (5.3) |
| Diuretics | 2267 (34.17) | 18 (7.11) | 449 (15.55) | 6 (4.55) |
| Drugs for acid-related disorders | 4496 (67.77) | 141 (55.73) | 1707 (59.13) | 67 (50.76) |
| Drugs for diabetes | 660 (9.95) | 13 (5.14) | 134 (4.64) | <5 (~3) |
| Drugs for obstructive airway diseases | 3239 (48.82) | 126 (49.8) | 1350 (46.76) | 60 (45.45) |
| Hypnotics/sedatives | 1686 (25.41) | 77 (30.43) | 778 (26.95) | 39 (29.55) |
| Immunosuppressants | 128 (1.93) | <5 (~1) | 51 (1.77) | <5 (~1) |
| Opioids | 4517 (68.09) | 160 (63.24) | 1798 (62.28) | 79 (59.85) |
| Psychostimulants | <5 (~1) | 0 (0) | <5 (~0) | 0 (0) |
Counts < 5 masked and proportions rounded to nearest 1% in order for patients to remain masked.
IQR, interquartile range; SD, standard deviation.
Table 2.
Characterizations of prostate cancer patients on endocrine treatments.
| Variable | First-generation antiandrogens | GnRH agonists | GnRH agonists with first-generation ADT | GnRH/LHRH antagonists | Second-generation antiandrogens |
|---|---|---|---|---|---|
| N | 3215 | 5281 | 2669 | 499 | 20 |
| Mean (SD) age | 73.23 (8.15) | 73.48 (8.06) | 73.45 (7.89) | 73.92 (9.09) | 72.3 (6.75) |
| Median (IQR) age | 73 (68–78) | 74 (68–79) | 74 (68–78) | 74 (68–80) | 74 (67.5–78.2) |
| Median prior history (years) | 16.14 (12.6–18.53) | 16.11 (12.68–18.72) | 16.08 (12.84–18.44) | 16,23 (13.8–18.52) | 17.98 (14.77–19.9) |
| Mean Charlson index | 0 | 0 | 0 | 0 | 0 |
| Median Charlson index | 0 | 0 | 0 | 0 | 0 |
| N male | 3215 | 5281 | 2669 | 499 | 20 |
| N Female | 0 | 0 | 0 | 0 | 0 |
| % Male | 0% | 0% | 0% | 0% | 0% |
| Comorbidities [n (%)] | |||||
| Atrial fibrillation | 286 (8.9) | 505 (9.56) | 242 (9.07) | 69 (13.83) | <5 (~5) |
| Cerebrovascular disease | 233 (7.25) | 372 (7.04) | 196 (7.34) | 52 (10.42) | 0 (0) |
| Chronic liver disease | 12 (0.37) | 24 (0.45) | 10 (0.37) | 7 (1.4) | 0 (0) |
| Chronic obstructive lung disease | 268 (8.34) | 413 (7.82) | 216 (8.09) | 45 (9.02) | <5 (~5) |
| Coronary arteriosclerosis | 61 (1.9) | 105 (1.99) | 53 (1.99) | 21 (4.21) | 0 (0) |
| Crohn’s disease | 11 (0.34) | 11 (0.21) | 8 (0.3) | 0 (0) | 0 (0) |
| Dementia | 41 (1.28) | 71 (1.34) | 35 (1.31) | 8 (1.6) | 0 (0) |
| Depressive disorder | 264 (8.21) | 446 (8.45) | 205 (7.68) | 55 (11.02) | <5 (~10) |
| Diabetes mellitus | 437 (13.59) | 747 (14.15) | 351 (13.15) | 98 (19.64) | <5 (~20) |
| Helicobacter pylori infection | 12 (0.37) | 18 (0.34) | 10 (0.37) | 0 (0) | 0 (0) |
| Heart disease | 798 (24.82) | 1295 (24.52) | 650 (24.35) | 185 (37.07) | 0 (0) |
| Heart failure | 103 (3.2) | 180 (3.41) | 80 (3) | 29 (5.81) | 0 (0) |
| Hepatitis C | <5 (~0) | <5 (~0) | <5 (~0) | <5 (~0) | 0 (0) |
| HIV | <5 (~0) | <5 (~0) | <5 (~0) | 0 (0) | 0 (0) |
| Hyperlipidaemia | 331 (10.3) | 577 (10.93) | 270 (10.12) | 47 (9.42) | <5 (~5) |
| Hypertension | 1065 (33.13) | 1741 (32.97) | 873 (32.71) | 162 (32.46) | 9 (45) |
| Ischaemic heart disease | 402 (12.5) | 618 (11.7) | 332 (12.44) | 109 (21.84) | <5 (~15) |
| Lesion liver | 38 (1.18) | 63 (1.19) | 31 (1.16) | 13 (2.61) | <5 (~10) |
| Obesity | 87 (2.71) | 134 (2.54) | 72 (2.7) | 13 (2.61) | 0 (0) |
| Osteoarthritis | 731 (22.74) | 1217 (23.04) | 607 (22.74) | 102 (20.44) | <5 (~20) |
| Peripheral vascular disease | 74 (2.3) | 114 (2.16) | 63 (2.36) | 15 (3.01) | <5 (~5) |
| Pneumonia | 81 (2.52) | 134 (2.54) | 66 (2.47) | 18 (3.61) | 0 (0) |
| Psoriasis | 93 (2.89) | 161 (3.05) | 72 (2.7) | 12 (2.4) | <5 (~5) |
| Pulmonary embolism | 36 (1.12) | 69 (1.31) | 32 (1.2) | 13 (2.61) | 0 (0) |
| Renal impairment | 453 (14.09) | 789 (14.94) | 363 (13.6) | 116 (23.25) | <5 (~10) |
| Rheumatoid arthritis | 36 (1.12) | 56 (1.06) | 32 (1.2) | 10 (2) | 0 (0) |
| Schizophrenia | 5 (0.16) | 7 (0.13) | <5 (~0) | <5 (~0) | 0 (0) |
| Ulcerative colitis | 9 (0.28) | 19 (0.36) | 7 (0.26) | <5 (~1) | 0 (0) |
| Urinary Tract Infection | 265 (8.24) | 417 (7.9) | 211 (7.91) | 44 (8.82) | <5 (~5) |
| Venous thrombosis | 176 (5.47) | 291 (5.51) | 148 (5.55) | 28 (5.61) | <5 (~10) |
| Visual system disorder | 1147 (35.68) | 2012 (38.1) | 929 (34.81) | 160 (32.06) | 6 (30) |
| Comedications [n (%)] | |||||
| Antidepressants | 1098 (34.15) | 1789 (33.88) | 906 (33.95) | 169 (33.87) | 8 (40) |
| Antiepileptics | 485 (15.09) | 706 (13.37) | 386 (14.46) | 81 (16.23) | <5 (~10) |
| Antiinflammatory/antirheumatic | 2106 (65.51) | 3397 (64.32) | 1747 (65.46) | 316 (63.33) | 14 (70) |
| Antineoplastics | 188 (5.85) | 249 (4.72) | 145 (5.43) | 26 (5.21) | <5 (~5) |
| Antipsoriatics | 88 (2.74) | 138 (2.61) | 73 (2.74) | 19 (3.81) | <5 (~5) |
| Antipsychotics | 600 (18.66) | 932 (17.65) | 497 (18.62) | 84 (16.83) | <5 (~15) |
| Antithrombotics | 969 (30.14) | 1517 (28.73) | 816 (30.57) | 205 (41.08) | <5 (~10) |
| Anxiolytics | 711 (22.12) | 1025 (19.41) | 585 (21.92) | 108 (21.64) | <5 (~15) |
| Beta-blockers | 1130 (35.15) | 1774 (33.59) | 923 (34.58) | 239 (47.9) | 8 (40) |
| Calcium channel blockers | 1310 (40.75) | 2145 (40.62) | 1078 (40.39) | 223 (44.69) | 7 (35) |
| Diuretics | 1030 (32.04) | 1693 (32.06) | 841 (31.51) | 205 (41.08) | 7 (35) |
| Drugs for acid-related disorders | 2175 (67.65) | 3446 (65.25) | 1788 (66.99) | 346 (69.34) | 14 (70) |
| Drugs for diabetes | 417 (12.97) | 708 (13.41) | 342 (12.81) | 92 (18.44) | <5 (~20) |
| Drugs for obstructive airway diseases | 1496 (46.53) | 2365 (44.78) | 1243 (46.57) | 230 (46.09) | 12 (60) |
| Hypnotics/sedatives | 604 (18.79) | 912 (17.27) | 498 (18.66) | 112 (22.44) | 6 (30) |
| Immunosuppressants | 80 (2.49) | 114 (2.16) | 60 (2.25) | 18 (3.61) | 0 (0) |
| Opioids | 2117 (65.85) | 3372 (63.85) | 1747 (65.46) | 374 (74.95) | 17 (85) |
| Psychostimulants | <5 (~1) | <5 (~1) | <5 (~1) | <5 (~0) | 0 (0) |
Counts < 5 masked and proportions rounded to nearest 1% in order for patients to remain masked.
ADT, androgen deprivation therapy; GnRH, gonadotropin-releasing hormone; IQR, interquartile range; LHRH, leutinizing hormonre releasing hormone; SD, standard deviation.
IRs of endocrine treatments and treatment-related outcomes in breast and prostate cancer patients
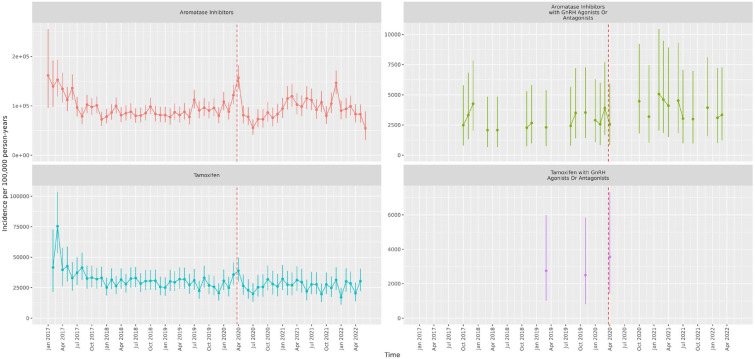
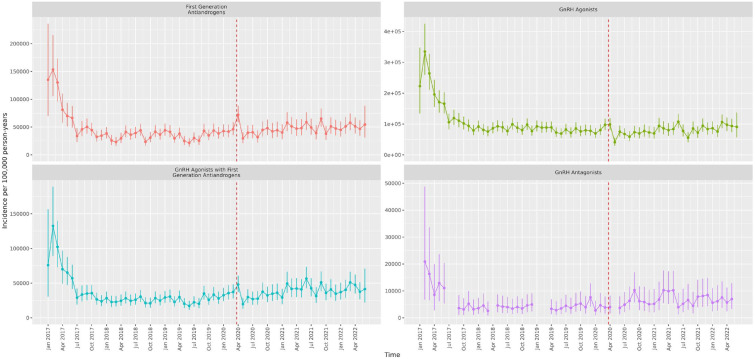
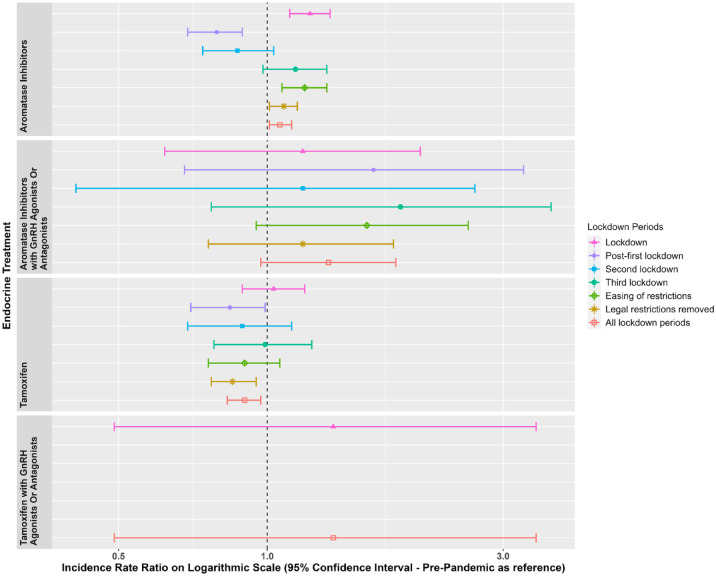
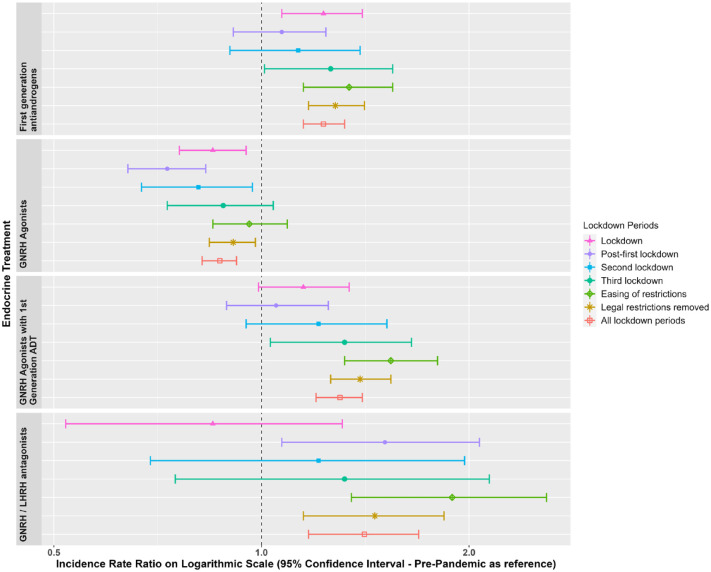
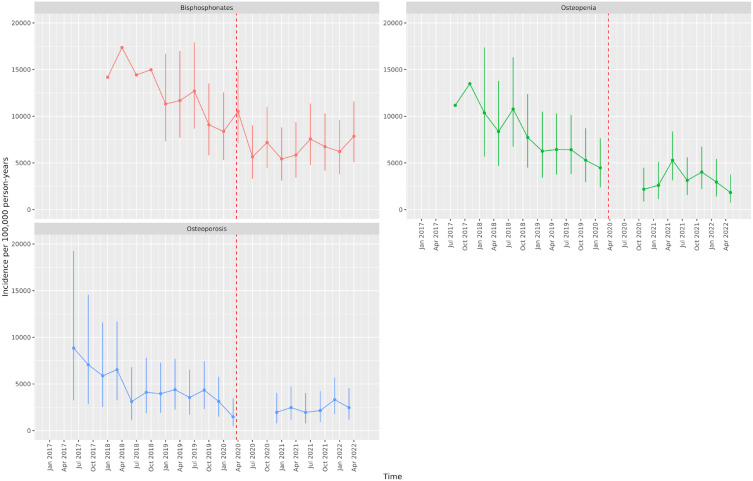
Figures 1 and 2 show the IRs for the endocrine treatment prescriptions in breast and prostate cancer patients over the whole observation period. It is evident that prescriptions of AIs, tamoxifen, first-generation ADT and GnRH agonists with first-generation ADT initially increased at the time of the first lockdown and then sharply reduced in the immediate period thereafter. GnRH agonists sharply reduced during the first lockdown. Figures 3 and 4 show the IRR of endocrine treatment prescriptions in breast and prostate cancer patients during the lockdown and post-lockdown periods compared to pre-pandemic rates. In patients with breast cancer, during the initial lockdown, prescriptions of AIs increased compared to the pre-pandemic period [IRR: 1.22 (95% CI: 1.11–1.34)] and remained elevated across the majority of the post-lockdown periods. In patients with prostate cancer, during the initial lockdown, there was an increase in prescriptions of first-generation ADT compared to pre-lockdown [IRR: 1.23 (95% CI: 1.08–1.4)] which remained elevated across the majority of the post-lockdown periods, and at the same time a decrease in prescriptions of GnRH agonists [IRR: 0.85 (95% CI: 0.76–0.95)]. Rates remained below pre-pandemic rates for GnRH agonists until the third lockdown. First-generation ADT and GnRH agonists/antagonists, singularly or in combination, were more frequently prescribed from March 2021 onwards.
Figure 1.
Incidence rates (and 95% confidence intervals) of endocrine treatments in breast cancer patients.
Dashed line indicates start of pandemic. Gaps between values indicate absence of data for the corresponding months.
Figure 2.
Incidence rates (and 95% confidence intervals) of endocrine treatments in prostate cancer patients.
Dashed line indicates start of pandemic. Gaps between values indicate absence of data for the corresponding months.
Figure 3.
Incidence rate ratios (and 95% confidence intervals) of endocrine treatments in breast cancer patients in the post-lockdown periods compared to pre-pandemic rates.
Dashed line indicates start of pandemic. Lockdown periods defined as: lockdown (March 2020–June 2020); post-first lockdown (July 2020–October 2020); second lockdown (November 2020–December 2020); third lockdown (January 2021–March 2021); easing of restrictions (April 2021–June 2021) and most legal restrictions removed (July 2021–June 2022).
Figure 4.
Incidence rate ratios (and 95% confidence intervals) of endocrine treatments in prostate cancer patients in the post-lockdown periods compared to pre-pandemic rates.
Dashed line indicates start of pandemic. Lockdown periods defined as: lockdown (March 2020–June 2020); post-first lockdown (July 2020–October 2020); second lockdown (November 2020–December 2020); third lockdown (January 2021–March 2021); easing of restrictions (April 2021–June 2021) and most legal restrictions removed (July 2021–June 2022).
IRR, number of events and IR, which show the data used to derive Figures 1–4 are included in Supplemental Tables S5–S7 for breast cancer and Supplemental Tables S8–S10 for prostate cancer.
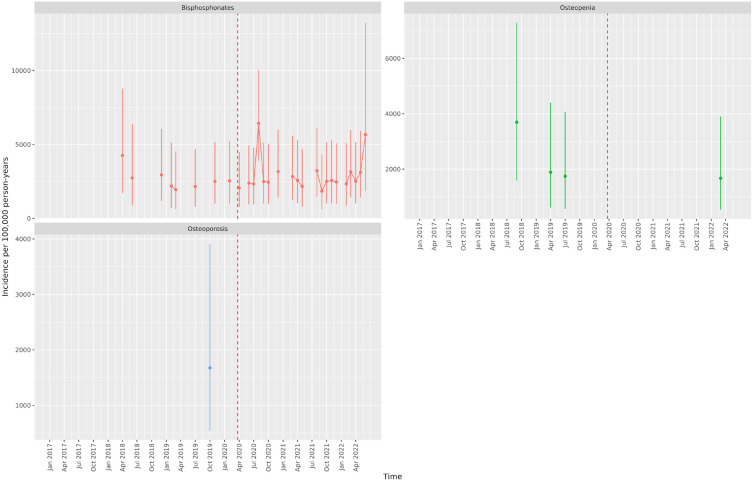
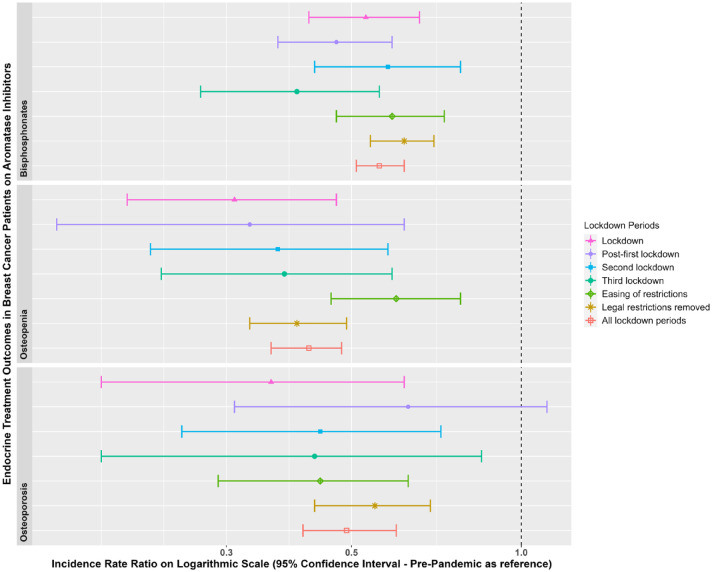
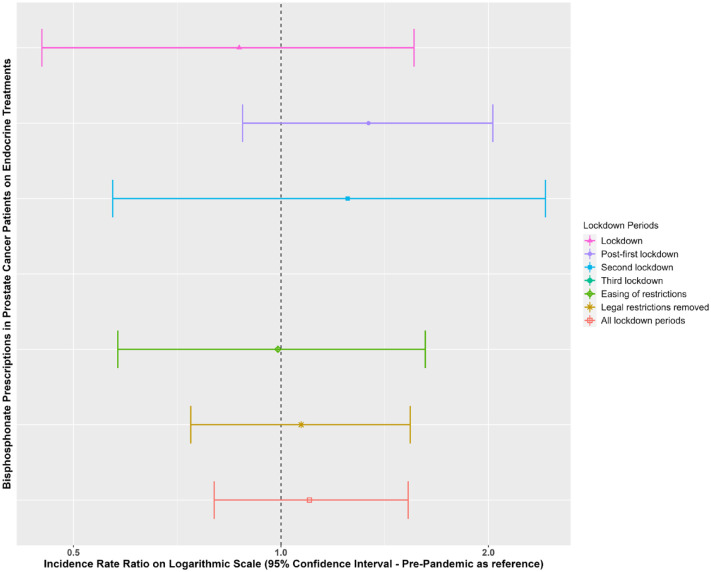
Figures 5 and 6 show the IR of endocrine treatment-related outcomes in breast cancer patients on AIs and prostate cancer patients on any endocrine treatment over the whole observation period. It is evident that in breast cancer patients on AIs, diagnoses of osteopenia and osteoporosis were not being made immediately following the first lockdown. There were no clear patterns for prostate cancer patients, largely due to a small number of events. Figures 7 and 8 show the IRR of endocrine treatment-related outcomes in breast cancer patients on AIs and prostate cancer patients on any endocrine treatment during the lockdown and post-lockdown periods compared to pre-pandemic rates. Bisphosphonate prescriptions were significantly reduced across all lockdown periods between March 2020 and June 2022 (IRR range: 0.40–0.62) for breast cancer patients on AIs, as were diagnoses of osteopenia (IRR range: 0.31–0.6) and osteoporosis (all except for the post-first lockdown period) (IRR range: 0.36–0.55). For breast cancer patients on tamoxifen, monthly counts of all treatment-related outcomes were too small to be included in IR analyses (counts per month < 5). For prostate cancer patients on any endocrine treatments, IR was no different pre-pandemic compared to after March 2020 for bisphosphonates, and monthly counts too small for osteopenia and osteoporosis.
Figure 5.
Incidence rates (and 95% confidence intervals) of endocrine treatment-related outcomes in breast cancer patients on aromatase inhibitors.
Dashed line indicates start of pandemic. Gaps between values indicate absence of data for the corresponding months.
Figure 6.
Incidence rates (and 95% confidence intervals) of endocrine treatment-related outcomes in prostate cancer patients on endocrine treatments.
Dashed line indicates start of pandemic. Gaps between values indicate absence of data for the corresponding months.
Figure 7.
Incidence rate ratios (and 95% confidence intervals) of endocrine treatment-related outcomes in breast cancer patients on aromatase inhibitors in the post-lockdown periods compared to pre-pandemic rates.
Dashed line indicates start of pandemic. Lockdown periods defined as: lockdown (March 2020–June 2020); post-first lockdown (July 2020–October 2020); second lockdown (November 2020–December 2020); third lockdown (January 2021–March 2021); easing of restrictions (April 2021–June 2021) and most legal restrictions removed (July 2021–June 2022).
Figure 8.
Incidence rate ratios (and 95% confidence intervals) of endocrine treatment-related outcomes (bisphosphonates) in prostate cancer patients on endocrine treatments in the post-lockdown periods compared to pre-pandemic rates.
Dashed line indicates start of pandemic. Lockdown periods defined as: lockdown (March 2020–June 2020); post-first lockdown (July 2020–October 2020); second lockdown (November 2020–December 2020); third lockdown (January 2021–March 2021); easing of restrictions (April 2021–June 2021) and most legal restrictions removed (July 2021–June 2022).
IRR, number of events and IR, which show the data used to derive Figures 5–8 are included in Supplemental Tables S11–S13 for breast cancer patients on AIs and Supplemental Tables S14–S16 for prostate cancer patients on any endocrine treatment.
Discussion
In this study, we examined the impact of the changing social restrictions imposed by the COVID-19 pandemic on the incidence and trends of endocrine treatments, and secondarily endocrine treatment-related outcomes of osteopenia and osteoporosis, and bisphosphonate prescriptions in breast and prostate cancer patients on endocrine treatments in the UK from January 2017 to June 2022.
In the months immediately following the first lockdown, incidence of prescriptions of AIs in breast cancer patients and first-generation ADT in prostate cancer patients increased compared to pre-pandemic rates and remained elevated across the majority of the post-lockdown period between March 2020 and June 2022. This mirrors recommendations by some European guidelines for the management of breast and prostate cancer patients diagnosed during the early pandemic: delaying surgery or radiotherapy in the first 3–6 months of the pandemic and instead prescribing endocrine therapy. 5 While delaying surgery or radiotherapy for breast or prostate cancer was not an official change to UK management guidelines, the results presented here demonstrate that approaches that limited in-patient hospital time appear to have been implemented in the UK during the pandemic (though it should be acknowledged that our results from primary care do not allow us to examine reductions in surgery or radiotherapy). This is in line with other research from the UK and worldwide. Indeed, one UK study demonstrated that alterations to breast cancer management were implemented in nearly 60% of patients, and many surgical interventions were substituted with ‘bridging’ endocrine therapy. 13 In the Netherlands neoadjuvant endocrine therapies for breast cancer increased by 339% during lockdown. 14 As well as reduced availability of surgical resources, radiotherapies and hospital beds, concern that chemotherapy-induced immunosuppression would increase risk for COVID-19 complications may have influenced clinicians’ decisions to switch patients to alternative therapies. 15 An international survey of breast cancer management strategies indicated that 51% of clinician respondents reported modifications to chemotherapy treatments during the pandemic and that 68% considered postponing surgery and administering endocrine treatments to patients with luminal A disease during the pandemic. 15
With regards to changes to prostate cancer management, it is perhaps no surprise that prescriptions of GnRH analogues were reduced across the pandemic as these drugs are typically injected by a clinician, whereas the first-generation ADT therapies can be administered orally. That said, initial concerns about ADT increasing SARS-Cov-2 infection risk, COVID-19 complications requiring hospitalization and mortality 16 might have led clinicians to be cautious about prescribing such medications in the early pandemic. Despite contradictory evidence, several systematic reviews and meta-analyses have now demonstrated no association between ADT and COVID-19 complications.17 –20
While endocrine therapies can be effective in neoadjuvant settings for breast and prostate cancer, the use of some endocrine therapies has been associated with poor bone health. One study demonstrated that AIs exhibit a significantly increased relative risk of 1.3 for bone loss (including osteopenia and osteoporosis) compared to patients not treated with AIs. 21 Likewise, the use of endocrine therapy in the treatment of prostate cancer has been shown to be associated with around 4.6% bone loss per year in men treated with GnRH analogues compared to a typical rate of 0.5% per year in healthy men. 22 In a small study of 105 patients treated with ADT for prostate cancer, prevalence of osteoporosis increased from around 10% at the beginning of the study to 22% at 2-year follow-up. 23
Given the increased use of AIs in breast cancer patients and ADT in prostate cancer patients across the pandemic, a secondary aim of this study was to investigate the rate of diagnosis of secondary diseases such as osteoporosis before and after lockdown and the possibility that such diagnoses may have been missed due to poorer treatment evaluation during the pandemic for these two therapies. Our results indicate that diagnoses of osteopenia and osteoporosis were reduced across the pandemic compared to the pre-pandemic era for new AI users. It is possible that this is at least partially driven by delayed assessments and bone scans during the pandemic. Selective channelling of patients and reductions in unnecessary testing may have occurred to reduce social contact during the pandemic, leading to fewer bone-related diagnoses than expected. Indeed, in a worldwide survey to primarily medical oncologists, 64% of respondents reported reduced frequency of DEXA scans in the first 4 months of the pandemic and difficulties with access to GP or hospital-administered treatments such as intravenous bisphosphonates or subcutaneous denosumab. 24 Sixty-six percent of respondents reported that adjuvant intravenous bisphosphonate use had been impacted by the pandemic in terms of delayed treatment, missed appointments and reduced clinical capacity, requiring a switch from intravenous to oral administration, while nearly a quarter of respondents reported decreased use of oral bisphosphonates. This is in line with our results, which show that bisphosphonate prescriptions were indeed reduced across the pandemic for breast cancer patients on AIs (though it should be noted that this pattern is seen in other populations, not limited to cancer patients 25 ).
In contrast, no differences in bone-related treatment outcomes across lockdown periods were observed for new prostate cancer ADT users. This could be explained by the fact that bone health assessments are less common in the male population compared to females (whose risk for bone-related complications, particularly after menopause, is higher than males 26 ). Alternative explanations include the fact that first-generation ADT used as monotherapy (such as bicalutamide) preserves bone mineral density, reducing the likelihood of bone-related complications. In contrast, GnRH agonists do affect bone health, and the decreased prescriptions observed during the pandemic may have consequently reduced any pandemic-related effects on bone-related outcomes in this population.
Strengths and weaknesses of the study
This study benefits from the strengths of CPRD GOLD, known for its extensive UK population coverage and comprehensive healthcare records, 8 facilitating thorough phenotyping of cancers and endocrine treatments. The longitudinal nature of the database enabled an examination of the trend in endocrine prescriptions over a period of nearly 2 years beyond the start of the pandemic. However, this study also has some limitations. First, as these data are derived from primary care and not linked to cancer registry or in-patient data, we were unable to investigate the hypothesis that endocrine treatments may have increased in use across the pandemic because of delays in surgery, radiotherapy and other hospital-initiated treatments. Furthermore, our assessment of rates of endocrine therapies that may be administered in secondary care (e.g. GnRH analogues) may be underestimated, given our focus on primary care data. Further research on secondary care, hospital settings and cancer registries is therefore needed. Second, the generalizability of findings is predominantly limited to England and Scotland, with less representation from Wales and Northern Ireland. That said, the composition of patients and practices in the database has changed over time. The advent of the CPRD AURUM database saw some practices transferred from GOLD to AURUM. Across our observation period, practices from England and Northern Ireland reduced, while practices from Scotland and Wales increased. Reassuringly, the IR of the endocrine treatments in the breast and prostate cancer populations across time and region were largely equivalent, except for smaller counts of AIs with GnRH and Tamoxifen with GnRH in England and Northern Ireland post-lockdown and slightly higher rates of GnRH agonists with first-generation ADT post-lockdown, likely reflecting the change in population composition (see Supplemental Figures S1 and S2). Finally, the focus of our manuscript was on endocrine treatment outcomes related to bone health. We acknowledge that there is a multitude of potential side effects of these drugs [including hot flushes, night sweats, vaginal dryness (in women), reduced sex drive, mood changes and fatigue] and that further research should shed light on the impact of the pandemic on these other outcomes.
Conclusion
During the early months of the pandemic, newly diagnosed breast cancer or prostate cancer patients were more likely to be prescribed AIs (for breast) or first-generation antiandrogens (for prostate cancer) compared to before the pandemic. This is likely driven by delays in surgery, radiotherapy or other treatments requiring hospital visits, and endocrine therapy being prescribed as a neoadjuvant/bridging therapy. These changes to routine practice (delays in surgery and radiotherapy) have far-reaching impacts not only for the patient journey but health economy. Delays in surgery and radiotherapy are likely to result in higher healthcare costs in the long term, as delayed treatment can lead to more advanced disease progression, requiring more intensive and costly interventions such as chemotherapy, long hospital stays and palliative care. Indirect economic costs include reduced workforce productivity, as well as management of psychological distress associated with delayed provision of cancer care during the pandemic.27,28 Furthermore, prolonged exposure to these endocrine drugs may lead to adverse effects such as cardiovascular complications, osteoporosis and metabolic disorders, 29 impacting the overall health and quality of life of cancer patients.
Despite this initial increased prescribing of AIs in breast cancer patients, it appears these patients received fewer bisphosphonate prescriptions to protect against bone thinning as a result of endocrine exposure. At the same time, diagnosis rates of osteopenia and osteoporosis were reduced compared to pre-pandemic, potentially due to the lack of diagnostic testing for these conditions during the pandemic. These results highlight the need to follow-up with breast cancer patients on AIs in the coming years for signs of bone thinning, given the relatively poorer management of endocrine treatment-related side effects in this population during the pandemic.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359241253115 for Collateral effects of the COVID-19 pandemic on endocrine treatments for breast and prostate cancer in the UK: a cohort study by Nicola L. Barclay, Marti Català, Annika M. Jödicke, Daniel Prieto-Alhambra, Danielle Newby, Antonella Delmestri, Wai Yi Man, Àlvar Roselló Serrano and Marta Pineda Moncusí, The OPTIMA Consortium in Therapeutic Advances in Medical Oncology
Acknowledgments
None.
Footnotes
ORCID iD: Nicola L. Barclay  https://orcid.org/0000-0002-6500-9909
https://orcid.org/0000-0002-6500-9909
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Nicola L. Barclay, Pharmaco- and Device Epidemiology, Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK
Marti Català, Pharmaco- and Device Epidemiology, Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK.
Annika M. Jödicke, Pharmaco- and Device Epidemiology, Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK
Daniel Prieto-Alhambra, Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, Botnar Research Centre, University of Oxford, Windmill Road, Oxford OX3 7LD, UK; Department of Medical Informatics, Erasmus Medical Center University, Rotterdam, The Netherlands.
Danielle Newby, Pharmaco- and Device Epidemiology, Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK.
Antonella Delmestri, Pharmaco- and Device Epidemiology, Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK.
Wai Yi Man, Pharmaco- and Device Epidemiology, Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK.
Àlvar Roselló Serrano, Institut Català d’Oncologia, Hospital Universitari Dr Josep Trueta, Girona, Catalonia, Spain.
Marta Pineda Moncusí, Pharmaco- and Device Epidemiology, Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK.
Declarations
Ethics approval and consent to participate: The protocol for this research was approved by the Research Data Governance (RDG) Board of the Medicine and Healthcare Products Regulatory Agency database research (protocol number 22_002331). The data is provided by patients to their GPs and collected by the NHS as part of their care and support, so consent is provided to GPs prior to inclusion in this study.
Consent for publication: All authors gave their consent for publication.
Author contributions: Nicola L. Barclay: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Visualization; Writing – original draft.
Marti Català: Conceptualization; Methodology; Supervision; Writing – review & editing.
Annika M. Jödicke: Methodology; Writing – review & editing.
Daniel Prieto-Alhambra: Conceptualization; Funding acquisition; Writing – review & editing.
Danielle Newby: Writing – review & editing.
Antonella Delmestri: Data curation; Writing – review & editing.
Wai Yi Man: Data curation; Writing – review & editing.
Àlvar Roselló Serrano: Methodology; Writing – review & editing.
Marta Pineda Moncusí: Conceptualization; Formal analysis; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This research was partially funded by the European Health Data and Evidence Network (EHDEN) (grant number 806968), the Optimal Treatment for Patients with Solid Tumours in Europe through Artificial Intelligence (OPTIMA) initiative (grant number 101034347) and the Oxford NIHR Biomedical Research Centre. OPTIMA is funded through the IMI2 Joint Undertaking and is listed under grant agreement No. 101034347. IMI2 receives support from the European Union’s Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries and Associations (EFPIA). IMI supports collaborative research projects and builds networks of industrial and academic experts in order to boost pharmaceutical innovation in Europe. The views communicated within are those of OPTIMA. Neither the IMI nor the European Union, EFPIA or any Associated Partners are responsible for any use that may be made of the information contained herein. The study funders had no role in the conceptualization, design, data collection, analysis, interpretation of data, decision to publish or preparation of the manuscript.
DP-A’s research group has received research grants from the European Medicines Agency, the Innovative Medicines Initiative, Amgen, Chiesi and UCB Biopharma and consultancy or speaker fees from Astellas, Amgen and UCB Biopharma. DP-A receives funding from the UK National Institute for Health Research (NIHR) in the form of a senior research fellowship and the Oxford NIHR Biomedical Research Centre.
Availability of data and materials: This study is based on data from the Clinical Practice Research Datalink (CPRD) obtained under licence from the UK Medicines and Healthcare Products Regulatory Agency. The data is provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the author/s alone. Patient-level data used in this study was obtained through an approved application to the CPRD RDG process (application number 22_002331). Data is only available directly from CPRD following RDG approval. Details on how to apply for data access can be found at https://cprd.com/data-access. Analytical code and code lists for identifying the events are available in GitHub repositories: https://github.com/oxford-pharmacoepi/CancerCovidEndocrineTx.
References
- 1. Barclay NL, Burkard T, Burn E, et al. The impact of the COVID-19 pandemic on short-term cancer survival in the United Kingdom: a cohort analysis. MedRXiv, 2023. DOI: 10.1101/2023.09.14.23295563. [DOI] [Google Scholar]
- 2. Barclay NL, Pineda-Moncusí M, Jödicke AM, et al. The impact of the UK COVID-19 lockdown on the screening, diagnostics and incidence of breast, colorectal, lung and prostate cancer in the UK: a population-based cohort study. Frontiers in Oncology, 14, 2024. DOI: 10.3389/fonc.2024.1370862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gazivoda V, Greenbaum A, Roshal J, et al. Assessing the immediate impact of COVID-19 on surgical oncology practice: experience from an NCI-designated Comprehensive Cancer Center in the Northeastern United States. J Surg Oncol 2021; 124: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prodhan A, Islam DZ, Khandker SS, et al. Breast cancer management in the era of Covid-19; key issues, contemporary strategies, and future implications. Breast Cancer 2023; 15: 51–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belkacemi Y, Grellier N, Ghith S, et al. A review of the international early recommendations for departments organization and cancer management priorities during the global COVID-19 pandemic: applicability in low- and middle-income countries. Eur J Cancer 2020; 135: 130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bargiota A, Oeconomou A, Zachos I, et al. Adverse effects of androgen deprivation therapy in patients with prostate cancer: focus on muscle and bone health. J BUON 2020; 25: 1286–1294. [PubMed] [Google Scholar]
- 7. Song Y, Xu Y-l, Lin Y, et al. Fractures due to aromatase inhibitor therapy for breast cancer: a real-world analysis of FAERS data in the past 15 years. Oncol Res Treat 2020; 43: 96–102. [DOI] [PubMed] [Google Scholar]
- 8. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol 2015; 44: 827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Voss EA, Makadia R, Matcho A, et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J Am Med Inform Assoc 2015; 22: 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gilbert J, Rao G, Schuemie M, et al. CohortDiagnostics: diagnostics for OHDSI cohorts [Internet], https://ohdsi.github.io/CohortDiagnostics, https://github.com/OHDSI/CohortDiagnostics (2023, accessed 2 August 2023).
- 11. Raventós B, Català M, Du M, et al. IncidencePrevalence: an R package to calculate population-level incidence rates and prevalence using the OMOP common data model. Pharmacoepidemiol Drug Saf 2024; 33: e5717. [DOI] [PubMed] [Google Scholar]
- 12. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 13. Dave RV, Kim B, Courtney A, et al. Breast cancer management pathways during the COVID-19 pandemic: outcomes from the UK ‘Alert Level 4’ phase of the B-MaP-C study. Br J Cancer 2021; 124: 1785–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eijkelboom AH, de Munck L, Menke-van der Houven van Oordt CW, et al. Changes in breast cancer treatment during the COVID-19 pandemic: a Dutch population-based study. Breast Cancer Res Treat 2023; 197: 161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gasparri ML, Gentilini OD, Lueftner D, et al. Changes in breast cancer management during the corona virus disease 19 pandemic: an international survey of the European Breast Cancer Research Association of Surgical Trialists (EUBREAST). Breast 2020; 52: 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang YB, Li WL, Sun M, et al. Impacts of androgen deprivation therapy on the risks and outcomes of SARS-CoV-2 infection in patients with prostate cancer. Asian J Androl 2023; 25: 366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karimi A, Nowroozi A, Alilou S, et al. Effects of androgen deprivation therapy on COVID-19 in patients with prostate cancer: a systematic review and meta-analysis. Urol J 2021; 18: 577–584. [DOI] [PubMed] [Google Scholar]
- 18. Kim DK, Park JJ, Yang WJ, et al. Relationship between androgen deprivation therapy for prostate cancer and risk of SARS-CoV-2 infection: a systematic review and meta-analysis. J Korean Med Sci 2022; 37: e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manolache NG, Mjaess G, Diamand R, et al. Prostate cancer, androgen deprivation, and risk of COVID-19 infection : a systematic review and meta-analysis. Prog Urol 2022; 32: 1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sari Motlagh R, Abufaraj M, Karakiewicz PI, et al. Association between SARS-CoV-2 infection and disease severity among prostate cancer patients on androgen deprivation therapy: a systematic review and meta-analysis. World J Urol 2022; 40: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mincey BA, Duh MS, Thomas SK, et al. Risk of cancer treatment-associated bone loss and fractures among women with breast cancer receiving aromatase inhibitors. Clin Breast Cancer 2006; 7: 127–132. [DOI] [PubMed] [Google Scholar]
- 22. Rachner TD, Coleman R, Hadji P, et al. Bone health during endocrine therapy for cancer. Lancet Diabetes Endocrinol 2018; 6: 901–910. [DOI] [PubMed] [Google Scholar]
- 23. Poulsen MH, Frost M, Abrahamsen B, et al. Osteoporosis and prostate cancer; a 24-month prospective observational study during androgen deprivation therapy. Scand J Urol 2019; 53: 34–39. [DOI] [PubMed] [Google Scholar]
- 24. Brown J, Wood S, Confavreux C, et al. Management of bone metastasis and cancer treatment-induced bone loss during the COVID-19 pandemic: an international perspective and recommendations. J Bone Oncol 2021; 29: 100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tan EH, Robinson DE, Jödicke AM, et al. Drug utilization analysis of osteoporosis medications in seven European electronic health databases. Osteoporos Int 2023; 34: 1771–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cawthon PM. Gender differences in osteoporosis and fractures. Clin Orthorp Relat Res 2011; 469: 1900–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirby A, Drummond FJ, Lawlor A, et al. Counting the social, psychological, and economic costs of COVID-19 for cancer patients. Support Care Cancer 2022; 30: 8705–8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ 2020; 371: m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boszkiewicz K, Piwowar A, Petryszyn P. Aromatase inhibitors and risk of metabolic and cardiovascular adverse effects in breast cancer patients – a systematic review and meta-analysis. J Clin Med 2022; 11: 3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359241253115 for Collateral effects of the COVID-19 pandemic on endocrine treatments for breast and prostate cancer in the UK: a cohort study by Nicola L. Barclay, Marti Català, Annika M. Jödicke, Daniel Prieto-Alhambra, Danielle Newby, Antonella Delmestri, Wai Yi Man, Àlvar Roselló Serrano and Marta Pineda Moncusí, The OPTIMA Consortium in Therapeutic Advances in Medical Oncology