Abstract
Background:
Anterior cruciate ligament (ACL) injuries often occur when an athlete experiences an unexpected disruption, or perturbation, during sports. ACL injury rates may also be influenced by the menstrual cycle.
Purpose:
To determine whether training adaptations to knee control and muscle activity during a perturbed single-leg squatting (SLS) task depend on menstrual cycle phase in female athletes.
Study Design:
Controlled laboratory study.
Methods:
A total of 21 healthy female collegiate athletes (current or former [<3 years]) who competed in 9 different sports performed an SLS task in which they attempted to match their knee position (user signal) to a target signal. The protocol consisted of a 9-condition pretest, 5 sets of 3 training trials, and a 9-condition posttest. One perturbation was delivered in each condition by altering the resistance of the device. Sagittal knee control (absolute error between the target signal and user signal) was assessed using a potentiometer. Muscle activity during perturbed squat cycles was normalized to maximal activation and to corresponding muscle activity during unperturbed squat cycles (%unperturbed) within the same test condition. Athletes performed the protocol during a distinct menstrual cycle phase (early follicular [EF], late follicular [LF], midluteal [ML]). Two-way mixed analysis of variance was used to determine the effects of the menstrual cycle and training on knee control and muscle activity during task performance. Venous blood was collected for hormonal analysis, and a series of health questionnaires and anthropometric measures were also assessed to determine differences among the menstrual cycle groups.
Results:
After training, athletes demonstrated better knee control during the perturbed squat cycles (lower absolute error, P < .001) and greater soleus feedback responses to the perturbation (%unperturbed, P = .035). Better knee control was demonstrated in the ML phase versus the EF phase during unperturbed and perturbed squat cycles (P < .039 for both). Quadriceps activation was greater in the ML phase compared with the EF and LF phases, both immediately before and after the perturbation (P < .001 for all).
Conclusion:
Athletes learned to improve knee control during the perturbed performance regardless of menstrual cycle phase. The best knee control and greatest quadriceps activation during the perturbed squatting task was found in the ML phase.
Clinical Relevance:
These findings may correspond to a lower incidence of ACL injury in the luteal phase and alterations in exercise performance across the menstrual cycle.
Keywords: menstrual cycle, athlete, perturbation, knee, motor control, soleus
The enactment of Title IX of the Education Amendments of 1972 in the United States 52 stimulated exponential growth in the number of female athletes competing at all levels of sport.48,58 Accordingly, the inclusion of female participants in sports medicine research has increased, yet they remain vastly underrepresented.9,10 A common justification for excluding female participants is to minimize the confounding effects of the menstrual cycle. 33 However, the menstrual cycle may be important to consider when studying female athlete performance 40 and injury.21,22,46 Among those surveyed, most female athletes (73%) report that their performance is affected by the menstrual cycle or hormonal contraception. 16 This perception coincides with trivial decrements in exercise outcomes across the menstrual cycle 40 as well as with the use of oral contraceptives. 17 It is unknown whether these differences are clinically meaningful due to a lack of robust research in this area.17,40 Perhaps more alarming is the fluctuation in anterior cruciate ligament (ACL) injury rates across phases of the menstrual cycle.21,22,46 Taken together, there is a pressing need in sports medicine research for greater inclusion of female athletes in various hormonal milieus to inform factors that may influence performance and injury.
Female athletes have disproportionately higher rates of ACL injury in the follicular phases of the menstrual cycle compared to the luteal phase.21,22,46 Most of these injuries occur without physical contact to the knee (noncontact), 1 which implicates the athlete's own movement strategy in the mechanism of injury. Although the cause of an ACL injury is likely multifactorial, 19 these findings support a potential hormone-mediated influence on female motor control injury.
Motor control is governed by the central nervous system and comprises anticipatory (feedforward) and response (feedback) strategies. When a feedforward strategy is unexpectedly disrupted, or perturbed, feedback adjustments are required to maintain performance. 43 The feedback responses that are triggered by a perturbation progress from involuntary (short-latency reflex) to voluntary (long-latency reflex, volitional reaction), depending on the time it takes for sensory signals to be processed by supraspinal centers. 27 In the absence of sporting performance, a person can respond to a perturbation simply by arresting movement (co-contract to avoid falling). During sport, however, an athlete attempts to respond to perturbations while maintaining high-velocity performance, which increases the potential for tissue overload. Unsurprisingly, noncontact ACL injuries often occur during a perturbed performance. 5
The motor control strategies people use to navigate perturbations differ between the sexes24,25,57 and between healthy and injured populations.7,36 When perturbations were delivered during the performance of a single-leg squatting (SLS) task, healthy female athletes demonstrated better sagittal plane knee control and greater quadriceps activation during feedforward and feedback strategies than those with a history of ACL reconstruction. 36 Other work with the same SLS task showed that people can learn to improve feedforward and feedback knee control by training. 42
To understand any potential hormonal effects on female motor control, a preclinical trial that focused on healthy, elite female athletes was conducted. The purpose of this study was to determine whether training adaptations to knee control and muscle activity during a perturbed SLS task depended on the phase of the menstrual cycle in female collegiate athletes. The secondary aim was to determine whether other pertinent factors to motor control, such as hormonal factors (testosterone, C-reactive protein, cortisol, prolactin), physical measures (anthropometrics, knee torque), and subjective measures (cognition, affect, fatigue, sleep, anxiety), were associated with task performance as these measures may fluctuate across the menstrual cycle. It was hypothesized that athletes in the midluteal (ML) phase of the menstrual cycle would demonstrate the best knee control.21,22,46
Methods
Participants
The protocol for this study received institutional review board approval. All participants signed informed consent in accordance with the institutional review board approval. Naturally menstruating (cycle length, 21-35 days) 18 female collegiate athletes (current or former within 3 years of competing) were eligible for participation. Athletes with a diagnosis that impaired natural hormone fluctuations (polycystic ovary syndrome, oligomenorrhea, etc), athletes using hormonal contraceptives ≤6 months before enrollment, and athletes with a history of lower extremity/lumbar surgery or a recent lower extremity/lumbar injury ≤6 months before enrollment were excluded. Athletes were recruited via university email and by word of mouth to local collegiate athletic staff. Based on the influence of the menstrual cycle on training performance of an upper extremity visuomotor task ( = 0.174) 26 , it was estimated that 15 participants (5 per menstrual cycle phase) were needed for an 80% power to detect an interaction effect in this study. To account for potential dropouts or exclusion based on abnormal hormone levels, a total of 21 participants were consented and enrolled between August 2021 and February 2022. The participants in this study competed in 9 different collegiate sports; 45% of participants were currently competing, and 25% of participants finished competing within 1 semester before study participation.
Menstrual Cycle Phase Determination
Participants completed a single-test visit during 1 of 3 hormonally distinct phases of the menstrual cycle: early follicular (EF; low estradiol, low progesterone); late follicular (LF; high estradiol, low progesterone); or ML (high estradiol, high progesterone). Participants were assigned to a menstrual cycle phase upon enrollment. Determination of menstrual cycle phases followed best practice recommendations by incorporating calendar-based counting of cycle days, ovulation testing, and serum hormone assessment.18,28 Testing in the EF phase occurred between cycle days 1 and 5, with the start of menstruation indicating cycle day 1. Testing in the LF and ML phases was determined by urinary ovulation test kits (Clearblue Advanced Digital Ovulation Test; Swiss Precision Diagnostics), which participants began using near cycle day 9. The LF test visit occurred after the participant received a “high” or “peak” ovulation test result, indicating increasing estradiol levels or a surge in luteinizing hormone, respectively. The ML test visit occurred 7 to 9 days after a “peak” ovulation test result.
Test Day Protocol
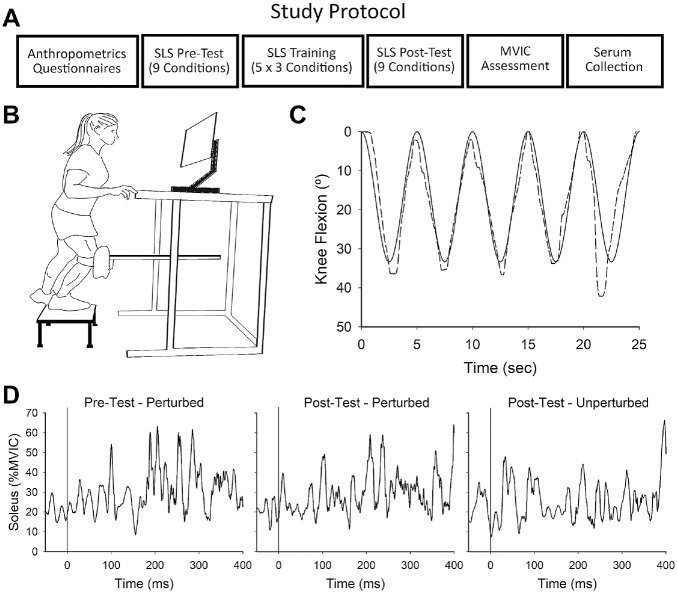
At the beginning of the visit, participants completed a series of health questionnaires followed by anthropometric assessments. Next, wireless EMG sensors (Trigno; Delsys) were secured to the participant's test limb (preferred kicking leg) over the vastus medialis, vastus lateralis, medial hamstrings, lateral hamstrings, and soleus muscles according to SENIAM guidelines. 20 Participants then performed an SLS task protocol that consisted of a pretest, a training session, and a posttest, each separated by a 5-minute seated rest. 42 Upon completion of SLS testing, maximum volitional involuntary contraction (MVIC) testing was performed in the following order: isometric knee flexion; isometric knee extension; eccentric knee extension; and isometric plantarflexion. Serum was collected by a venous blood draw after the MVIC assessments. The assessments were conducted in the same order for each participant as follows: questionnaires; anthropometric testing; EMG sensor placement; SLS testing; MVIC assessment; and venous blood draw (Figure 1A). Testing was carried out at the same time of day for each participant.
Figure 1.
SLS task. (A) Single-day study protocol. (B) Schematic of the SLS device. (C) Visual display during the SLS task. Participants attempted to track their knee position (dashed line) to a sinusoid target signal (solid line) by squatting down and up. Five single-leg squats were performed in each SLS condition, and 1 perturbation was randomly delivered during the descending phase of 1 of the squat cycles (during squat cycle 5 in this figure). (D) A representative example of a participant's soleus muscle activity (normalized to during a single SLS condition in the pretest and the posttest). Time zero indicates the time the perturbation was delivered (perturbed cycles) or would have been delivered (unperturbed cycle) at a corresponding point within the same SLS condition. SLS, single-leg squatting; MVIC, maximum volitional involuntary contraction.
Questionnaires
The Patient-Reported Outcomes Measurement Information System (PROMIS) questionnaires (US National Institutes of Health) were collected via a secure online platform (REDCap) to assess participant anxiety (Item Bank v1.0: Emotional Distress–Anxiety–Short Form 8a), cognitive functional abilities (Item Bank v2.0: Cognitive Function–Abilities Subset–Short Form 6a), fatigue (Item Bank v1.0: Fatigue–Short Form 6a), positive affect (Item Bank v1.0: Positive Affect–Short Form 15a), and sleep disturbance (Item Bank v1.0: Sleep Disturbance–Short Form 4a). Total T-scores were used for data analysis. Higher scores reflect greater magnitude of the tested construct and a score of 50 represents the average level of the construct in the US population. Physical activity status was determined using the International Physical Activity Questionnaire (IPAQ) Short Form, with the cumulative score used for data analysis and presented as the weekly metabolic equivalent.
Anthropometric Assessment
Participant height and weight were measured followed by an assessment of body composition using bioelectric impedance (InBody S10; Biospace). 30 Generalized joint laxity, which is a risk factor for ACL injury, 47 was measured by the Beighton criteria. 4
SLS Task
The SLS task assesses visuomotor performance during dynamic weightbearing movement.29,35,36,42,53 During the task, participants stood atop a stool on a single limb (test limb) with the knee strapped to a knee pad at the end of a rack and pinion system. The contralateral leg remained suspended off the ground (Figure 1B). Custom software (Labview v12.0f3; National Instruments) generated a sinusoid signal (target signal) on a computer monitor along with the superimposed position of the participant's test knee (user signal). Before testing, participants were informed that the position of their test knee (user signal) would appear on the computer monitor along with a target signal that traversed across the screen in a sinusoidal wave pattern. Participants were instructed that the goal of the task was to match their knee position (user signal) to the target signal as closely as possible by squatting down and up, a distance that approximated 0° to 33.3° knee flexion (Figure 1C).
Participants performed a pretest, training session, and posttest according to a previous protocol. 42 The pretest and posttest consisted of 9 distinct test conditions that varied by resistance (5%, 10%, and 15% bodyweight) and velocity of the target signal (0.2, 0.4, and 0.6 Hz) that corresponded to ~13.3, 26.6, and 40 deg/s knee joint flexion/extension velocity. The training session consisted of 5 sets of 3 test conditions (10% bodyweight at 0.2, 0.4, and 0.6 Hz). Resistance was controlled by an electromagnetic brake attached to the rack and pinion system. In each condition, 5 single-leg squats were performed and 1 perturbation was randomly delivered at 30% of the descending phase of 1 of the squat cycles (never cycle 1). The perturbation was induced by temporary release of resistance (400, 250, and 200 ms) in accordance with the target velocity (0.2, 0.4, and 0.6 Hz). Participants were informed that the velocity of the target signal and the resistance level provided by the device would change throughout the squatting trials. Prior work with the device showed that delivery of the perturbation in each test condition triggers reflex responses in the muscles of the test limb. 53 Linear knee displacement was measured by a potentiometer mounted to the rack and pinion system. Pilot work showed linear knee displacement strongly approximates angular range of motion during the task as measured by a motion capture system (R2 = 0.97).36,44
All signals were collected at 2000 Hz using an analog to digital data acquisition board (PCI-6221; National Instruments). Recording the knee position at 2000 Hz allowed for detection of ~0.04° change in sagittal knee motion during the fastest SLS condition. Muscle activity was recorded at 2000 Hz and normalized to maximum volitional isometric contraction (%MVIC). All participants were tested by the same researcher (K.A.J.). Motor performance and peak muscle activity during the SLS task were computed using a custom algorithm (Diadem 2012; National Instruments).
Test Conditions
Performance outcomes were participant knee control, which was quantified by the absolute error (AE), calculated as the peak difference between the target signal (goal knee position) and user signal (actual knee position), during test conditions: (1) perturbed squat cycles; (2) unperturbed squat cycles; and (3) the feedforward and feedback time bins surrounding the perturbation. The time immediately before perturbation delivery was considered the feedforward time bin (–50 to 0 ms), while the feedback responses to the perturbation were separated into the short-latency reflex (1-50 ms), long-latency reflex (51-200 ms), and volitional response (201-400 ms). Peak muscle activity was also measured during each of these time bins. A representative example of soleus muscle activity from a single participant is depicted in Figure 1D. Due to their motor equivalence and similar perturbation responses, muscle activity of vastus medialis and vastus lateralis and of medial hamstrings and lateral hamstrings were averaged together into the quadriceps and hamstrings, respectively. To better understand the effect of training on perturbation-induced muscle activity, the long-latency reflex was normalized to muscle activity at the corresponding position of an unperturbed squat cycle within the same task condition (%unperturbed). It was previously shown that this normalized response differs between female participants who have undergone ACL reconstruction and healthy female participants. 36
Maximum Volitional Isometric Contraction Assessment
Strength testing was conducted on the limb used during the SLS task. Participants were seated on an isokinetic dynamometer (KinCom; Chattecx) with the hip flexed at 80° and the knee flexed at 30° and 60° during knee flexion and knee extension MVIC testing, respectively. The lower leg was secured to the device via a strap placed proximal to the malleoli. Eccentric knee extension torque was tested at 30 deg/s from 20° to 90° of knee flexion. Peak torque (N·m/kg) was used for data analysis. Participants performed plantarflexion MVIC testing seated in a chair with the knee flexed at 90° and the ankle in neutral (0°). A strap was placed over the thigh and anchored to a platform under the foot to secure the limb. 30 After 3 to 5 submaximal warm-up contractions, 3 MVICs were performed for 5 seconds with a 60-second rest between repetitions. Four maximal eccentric knee extensor contractions were performed with a 120-second rest between repetitions. The tester provided verbal encouragement during all trials.
Serum Collection and Hormone Quantification
Venous blood was collected in 6-mL serum collection tubes (BD 367814 Vacutainer; Becton, Dickinson and Company) from the antecubital region. The collected sample remained upright for at least 15 minutes to coagulate before being centrifuged at 3000 rpm at room temperature for 10 minutes. Serum supernatant was separated into 3 aliquots and stored at –80°C until hormone analysis was conducted. Extracted serum samples were analyzed using standard immunoassays with detection limits of 5 pg/mL for estradiol, 0.05 ng/mL for progesterone, and 0.025 ng/mL for testosterone.
Statistical Analysis
Two-way mixed analysis of variance was used to determine the effects of the menstrual cycle and training on SLS task performance (AE) and muscle activity (%MVIC, %unperturbed). The model included the between-subject factor (cycle phase), within-subject factor (test session), and their interaction (cycle phase × test session). Significance was set at P≤ .05.
Post hoc analyses were performed using a Bonferroni adjustment for the number of comparisons. Pearson correlation coefficients and Spearman rank tests were used to determine the relationships between SLS performance and hormonal, physical, and subjective measures. These factors were compared among menstrual cycle groups using 1-way analysis of variance.
Results
A total of 21 female collegiate athletes completed the study protocol. Data from 1 participant in the LF phase were excluded. Due to her participation in a separate study, hormonal data for this participant were available from multiple menstrual cycle phases and indicated that her estradiol levels in the LF phase did not exceed those in the EF phase; therefore, her data were excluded from this study, and the analysis was performed on the remaining 20 participants. Knee control (AE) and muscle activity (%MVIC) during the squatting task did not depend on the participant's level of sport as determined by the activity level classification 41 (P > .101 for all) or on the participant's athletic status (current vs former) (P > .066 for all).
Characteristics of the Menstrual Cycle Groups
Menstrual cycle groups differed in their levels of estradiol (P = .004) and progesterone (P < .001) as expected (Table 1). Menstrual cycle groups were similar in all other assessed hormonal levels (Table 1); anthropometrics and knee torque (Table 2); and subjective measures of physical, emotional, and cognitive status (Table 3). The sport played by the participants as well as the National Collegiate Athletic Association level of competition and years of collegiate athletic experience are reported in Table 3.
Table 1.
Hormone Levels by Menstrual Cycle Group a
| Early Follicular (n = 7) | Late Follicular (n = 6) | Midluteal (n = 7) | P b | |
|---|---|---|---|---|
| Day of menstrual cycle | 3.3 ± 1.4 | 13.3 ± 3.0 | 23.0 ± 3.4 | <.001 c , d , e |
| Estradiol, pg/mL | 40.2 ± 16.6 | 128.1 ± 92.8 | 137.4 ± 45.0 | .004 d , e |
| Progesterone, ng/mL | 0.15 ± 0.07 | 0.52 ± 0.59 | 13.04 ± 4.62 | <.001 c , d |
| E/P ratio | 0.31 ± 0.18 | 0.39 ± 0.24 | 0.01 ± 0.01 | .001 f , g |
| Testosterone, ng/mL | 0.24 ± 0.12 | 0.27 ± 0.07 | 0.23 ± 0.06 | .749 |
| Cortisol, ng/mL | 64.2 ± 35.5 | 55.5 ± 9.1 | 78.1 ± 35.9 | .417 |
| C-reactive protein, mg/L | 0.85 ± 1.20 | 0.38 ± 0.21 | 0.57 ± 0.35 | .7 |
| Prolactin, ng/mL | 9.1 ± 3.2 | 11.6 ± 6.5 | 9.6 ± 3.6 | .578 |
Data are reported as mean ± SD. Boldface P values indicate a statistically significant difference between groups (P≤ .05). E/P ratio, estradiol-progesterone ratio.
Post hoc indicators: c midluteal > late follicular; d midluteal > early follicular; e late follicular > early follicular; f early follicular > midluteal; g late follicular > midluteal.
Table 2.
Anthropometrics and Knee Torque by Menstrual Cycle Group a
| Early Follicular (n = 7) | Late Follicular (n = 6) | Midluteal (n = 7) | P | |
|---|---|---|---|---|
| Age, y | 23.3 ± 2.1 | 21.5 ± 1.1 | 22.0 ± 1.7 | .251 |
| Weight, kg | 67.4 ± 12.3 | 69.2 ± 11.4 | 68.0 ± 10.0 | .957 |
| Height, cm | 170.2 ± 11.9 | 169.7 ± 7.4 | 172.0 ± 5.8 | .86 |
| Beighton score | 2.3 ± 1.9 | 2.3 ± 2.2 | 3.0 ± 1.5 | .752 |
| KF MVIC, N·m/kg | 2.08 ± 0.37 | 2.14 ± 0.44 | 2.11 ± 0.35 | .967 |
| KE MVIC, N·m/kg | 3.52 ± 0.96 | 3.22 ± 0.79 | 3.38 ± 0.85 | .822 |
| KE MVEC, N·m/kg | 4.23 ± 0.92 | 3.43 ± 0.58 | 3.81 ± 0.64 | .177 |
Data are reported as mean ± SD. KE, knee extension; KF, knee flexion; MVEC, maximum volitional eccentric contraction; MVIC, maximum volitional isometric contraction.
Table 3.
Athletic History and Physical, Cognitive, and Emotional Status by Menstrual Cycle Group a
| Early Follicular (n = 7) | Late Follicular (n = 6) | Midluteal (n = 7) | P | |
|---|---|---|---|---|
| Sport played | Volleyball, softball, rowing, field hockey, swimming, track, basketball | Tennis, softball, track, rowing (n = 2), basketball | Field hockey, softball, rowing, track (n = 2), soccer, basketball | .869 |
| NCAA collegiate level | DI (n = 6); DII/DIII (n = 1) | DI (n = 4); DII/DIII (n = 2) | DI (n = 5); DII/DIII (n = 2) | .705 |
| Collegiate experience, y | 4.3 ± 0.8 | 3.6 ± 1.2 | 3.1 ± 1.2 | .145 |
| IPAQ, MET/wk | 2973 ± 2072 | 4447 ± 3407 | 5093 ± 2876 | .291 |
| PROMIS T-score | ||||
| Fatigue | 52.7 ± 7.0 | 44.3 ± 6.6 | 49.0 ± 4.3 | .065 |
| Sleep disturbance | 46.5 ± 9.3 | 48.2 ± 2.5 | 47.6 ± 7.2 | .904 |
| Cognitive function | 47.3 ± 4.1 | 53.1 ± 6.2 | 51.4 ± 6.3 | .111 |
| Anxiety | 56.5 ± 3.7 | 52.1 ± 9.2 | 52.4 ± 9.2 | .4 |
| Positive affect | 46.8 ± 4.8 | 47.2 ± 5.5 | 48.0 ± 5.0 | .905 |
Data are reported as mean ± SD unless otherwise indicated. DI, Division I; DII, Division II; DIII, Division III; IPAQ, International Physical Activity Questionnaire; MET, metabolic equivalent; NCAA, National Collegiate Athletic Association; PROMIS, Patient-Reported Outcomes Measurement Information System.
SLS Task Performance
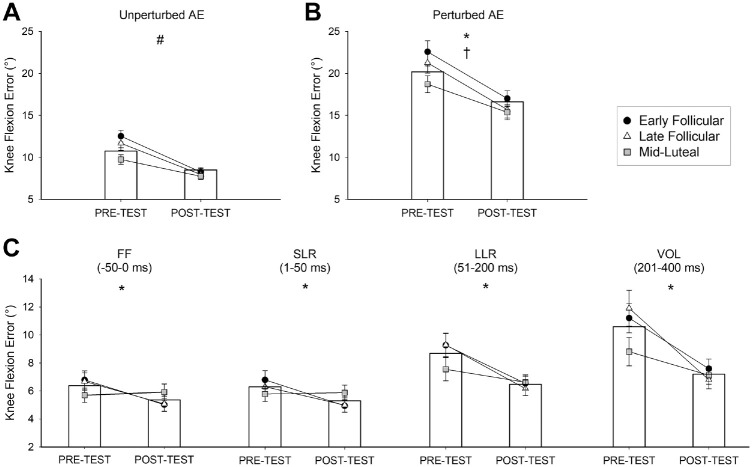
There was an interaction effect on knee control during unperturbed squat cycles (P = .009) in which AE decreased after training by an average of 3.4° among athletes in all menstrual cycle phases (P < .001 for all), but athletes in the ML phase demonstrated an AE 3.0° lower compared to athletes in the EF phase during the pretest (P = .005) (Figure 2A). There was no interaction effect on knee control during perturbed squat cycles (P = .200), but there were main effects for the test session (P < .001) and menstrual cycle phase (P = .044) (Figure 2B) in which AE decreased from the pretest to the posttest by 5.1° and athletes in the ML phase demonstrated an 3.1° lower than athletes in the EF, respectively. Training also improved knee control in the feedforward and feedback time bins surrounding the perturbation (test session, P < .012 for all) (Figure 2C).
Figure 2.
SLS task performance. Knee flexion AE during the (A) unperturbed squat cycles, (B) perturbed squat cycles, and (C) the time bins immediately surrounding the perturbation separated into the FF and feedback responses. Bar graphs depict mean AE values for the entire cohort. #An interaction effect (cycle phase × test session), with post hoc tests revealing lower AE in posttest vs pretest for all menstrual cycle groups and lower AE in ML vs EF group during pretest. *A main effect for test session, with post hoc tests revealing lower AE during the posttest vs pretest. †A main effect for menstrual cycle, with post hoc tests revealing lower AE in the ML vs EF group. SLS, single-leg squatting; AE, absolute error; FF, feedforward; LLR, long-latency reflex; SLR, short-latency reflex; VOL, volitional reaction.
Muscle Activity During Perturbed Squat Cycles
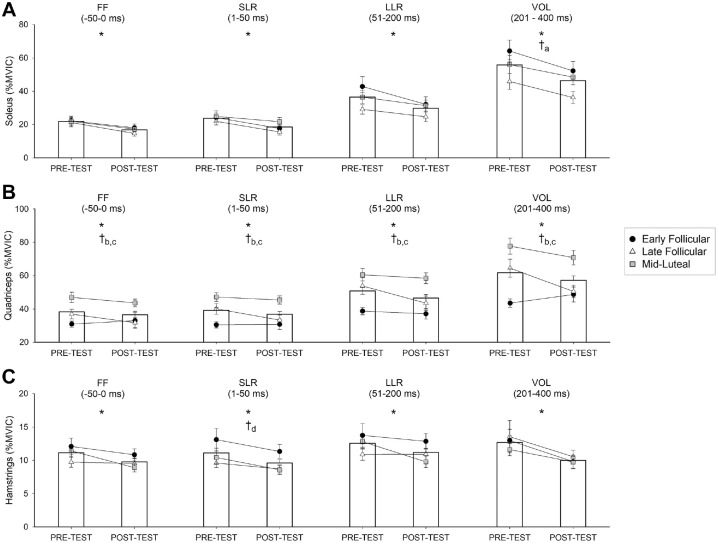
There were no interaction effects on muscle activity during the feedforward or feedback time bins surrounding the perturbation but there were main effects for the test session and menstrual cycle phase. Soleus activity decreased after training by 5% to 9% (%MVIC) across all feedforward and feedback time bins (test session, P < .030 for all) (Figure 3A). Athletes in the EF phase activated their soleus 18% (%MVIC) more than athletes in the LF phase during the volitional response (cycle phase, P = .041).
Figure 3.
Muscle activity during perturbed squat cycles of the single-leg squatting (SLS) task for (A) the soleus, (B) quadriceps, and (C) hamstrings. Bar graphs depict mean normalized maximum volitional isometric contraction (%MVIC) values for entire cohort. *A main effect for test session. Post hoc tests revealed muscle activity (%MVIC) was lower in the posttest than the pretest. †A main effect for menstrual cycle group. Post hoc test indicators: aearly follicular > late follicular; bmidluteal > early follicular; cmidluteal > late follicular; and dno significant differences after correction for multiple comparisons. MVIC, maximum volitional isometric contraction; FF, feedforward; LLR, long-latency reflex; SLR, short-latency reflex; VOL, volitional reaction.
Quadriceps activity also decreased after training by 4% to 6% (%MVIC) across all feedforward and feedback time bins (test session, P > .023 for all) and differed by menstrual cycle phase (cycle phase, P < .001 for all) in which athletes in the ML phase activated their quadriceps 10% to 29% (%MVIC) more than athletes in the EF and LF phases during all assessed time bins (Figure 3B).
Hamstring activity decreased after training by 2% to 3% (%MVIC) during all feedforward and feedback time bins (test session, P < .030 for all) (Figure 3C). There was a menstrual cycle effect on the hamstring short-latency reflex (P = .043) showing a trend for greater activation in the EF phase than in the LF and ML phases, but these differences did not reach significance after post hoc correction for multiple comparisons (P > .059 for all). When long-latency reflexes were normalized to the unperturbed squat cycle (%unperturbed), training induced a 30% increase in the soleus perturbation response (test session, P = .035). Training did not alter the quadriceps’ or hamstrings’ normalized responses (P > .229 for all).
Correlation With Knee Control During the SLS Task
The hormonal factors significantly related to knee control included estradiol, progesterone, cortisol, and C-reactive protein (r = –0.664 to 0.003; P≤ .05 for all). The associations between each assessed factor and knee control are shown in Table 4.
Table 4.
Associations of Hormonal Factors, Knee Torque, and Questionnaire Data With Single-Leg Squatting Performance a
| Pretest AE | Posttest AE | Difference in AE b | ||||
|---|---|---|---|---|---|---|
| Unperturbed | Perturbed | Unperturbed | Perturbed | Unperturbed | Perturbed | |
| Estradiol | −0.322 | −0.310 | −0.086 | −0.423 | −0.442 | −0.048 |
| (0.163) | (0.180) | (0.714) | (0.062) | (0.050) | (0.836) | |
| Progesterone | −0.450 | −0.501 | −0.160 | −0.312 | −0.489 | −0.317 |
| (0.046) | (0.024) | (0.496) | (0.176) | (0.029) | (0.169) | |
| E/P ratio | 0.417 | 0.456 | 0.164 | 0.238 | 0.423 | 0.346 |
| (0.067) | (0.043) | (0.484) | (0.308) | (0.062) | (0.132) | |
| Testosterone | 0.083 | −0.093 | 0.197 | 0.134 | −0.016 | −0.101 |
| (0.729) | (0.691) | (0.406) | (0.573) | (0.947) | (0.673) | |
| Prolactin | −0.023 | −0.275 | 0.211 | 0.004 | −0.157 | −0.211 |
| (0.923) | (0.236) | (0.372) | (0.985) | (0.509) | (0.371) | |
| Cortisol | −0.029 | −0.487 | 0.412 | 0.520 | −0.335 | −0.608 |
| (0.901) | (0.029) | (0.070) | (0.019) | (0.145) | (0.005) | |
| C-reactive protein | 0.173 | 0.315 | 0.152 | −0.003 | 0.143 | 0.444 |
| (0.460) | (0.171) | (0.517) | (0.987) | (0.542) | (0.049) | |
| KF MVIC | −0.550 | −0.368 | −0.664 | −0.254 | −0.283 | −0.178 |
| (0.012) | (0.108) | (0.001) | (0.280) | (0.227) | (0.453) | |
| KE MVIC | −0.348 | −0.292 | −0.438 | −0.069 | −0.169 | −0.126 |
| (0.133) | (0.208) | (0.054) | (0.773) | (0.476) | (0.598) | |
| KE MVEC | −0.120 | −0.144 | −0.132 | −0.009 | −0.070 | −0.142 |
| (0.613) | (0.538) | (0.579) | (0.969) | (0.769) | (0.549) | |
| IPAQ score | −0.122 | −0.496 | −0.075 | 0.209 | −0.090 | −0.511 |
| (0.603) | (0.026) | (0.748) | (0.371) | (0.700) | (0.021) | |
| PROMIS–Fatigue | 0.063 | −0.239 | 0.125 | 0.361 | 0.003 | −0.286 |
| (0.791) | (0.305) | (0.598) | (0.117) | (0.991) | (0.221) | |
| PROMIS–Sleep Disturbance | −0.146 | −0.093 | 0.059 | −0.197 | −0.218 | −0.120 |
| (0.539) | (0.691) | (0.806) | (0.406) | (0.357) | (0.613) | |
| PROMIS–Cognitive Function | −0.131 | 0.028 | 0.304 | −0.098 | −0.347 | 0.015 |
| (0.582) | (0.901) | (0.192) | (0.681) | (0.134) | (0.952) | |
| PROMIS–Anxiety | −0.032 | 0.400 | 0.268 | 0.180 | −0.203 | 0.224 |
| (0.892) | (0.079) | (0.253) | (0.448) | (0.391) | (0.343) | |
| PROMIS–Positive Affect | −0.299 | −0.190 | −0.071 | −0.188 | −0.330 | 0.006 |
| (0.201) | (0.414) | (0.767) | (0.428) | (0.156) | (0.980) | |
Data are reported as r value (P value). Boldface P values indicate statistical significance (P≤ .05). AE, absolute error; E/P ratio, estradiol-progesterone ratio; IPAQ, International Physical Activity Questionnaire; KE, knee extension; KF, knee flexion; MVEC, maximum volitional eccentric contraction; MVIC, maximum volitional isometric contraction; PROMIS, Patient-Reported Outcomes Measurement Information System.
Difference in AE = pretest AE – posttest AE; hence, a positive relationship indicates higher levels of hormone/construct associate with a greater reduction in AE from pretest to posttest.
Discussion
The purpose of this study was to determine whether training adaptations to knee control and muscle activity during a perturbed SLS task depended on the phase of menstrual cycle in female collegiate athletes. Secondarily, we aimed to establish the relationships between sex hormones and motor proficiency. There were several key findings: (1) athletes learned to improve knee control during perturbed performance by training regardless of menstrual cycle phase; (2) athletes in the ML phase demonstrated better knee control and greater quadriceps activation compared to athletes in the EF phase; (3) training enhanced the relative soleus response to perturbed performance; and (4) sex and stress hormones were associated with knee control. The most compelling discovery is that training was effective for improving athletes’ knee control during perturbed performance regardless of their menstrual cycle phase.
After training, athletes improved control of sagittal knee motion during the squatting task by approximately 3° to 5°, which is consistent with the improvement demonstrated by a mixed-sex, nonathletic cohort. 42 The implications of greater sagittal knee control by only a few degrees remain to be shown. Knee flexion angles differ by <5° when performing distinctly different jumping tasks, 13 supporting the potential relevance of minor changes in sagittal knee position to athletic performance. Improved sagittal knee control during perturbed performance is likely pertinent to ACL injury as sagittal plane forces are the primary generator of ACL strain 3 and ruptures often occur during perturbed performance. 5 Although a 5° improvement in knee control might not deter an acute overload of tissue, it may help to mitigate the less common occurrence of ACL rupture by repetitive overloading. 3
Athletes who performed the SLS task during distinct menstrual cycle phases demonstrated similar training improvements in knee control during perturbed performance. However, athletes’ training improvement in knee control during unperturbed squatting performance was dependent on their menstrual cycle phase. This finding is similar to nonathletes’ performance of an upper extremity visuomotor task in which training improvements depended on participants’ menstrual cycle phase. 26 Interestingly, that finding appeared to be driven by differences in initial task performance in which female participants in the luteal phase performed the best, which also corresponds to the findings from this study in which athletes in the ML phase performed better than athletes in the EF phase. Again, the difference in knee accuracy between athletes in these 2 phases was only 3°, a result that coincides with small reductions in exercise performance in the EF phase. 40
The finding of greater knee control among athletes who were in the ML phase may be congruent with a lower occurrence of ACL injuries in this phase of the menstrual cycle.21,22,46 Attribution of these results exclusively to a menstrual cycle influence is precluded due to the cross-sectional study design. It is noteworthy, however, that all of the assessed factors (knee strength, anthropometrics, athletic experience, subjective health measures) were similar between the menstrual cycle groups except for the levels of estradiol and progesterone. The hormonal cause of ACL injuries is still unknown. The predominant theory is that elevated levels of estradiol increase soft tissue laxity and make the ACL more prone to rupture. 6 However, the time of the menstrual cycle when knee joint laxity is greatest does not match the time when ACL injuries are most frequent, 46 suggesting a hormonal influence on injury by factors other than laxity alone. Sex hormones exert pleiotropic effects beyond the reproductive system, 32 which creates the potential for various hormone-mediated injury mechanisms, including altered neural excitability2,23,45 and specific force of muscle. 34
Despite an approximately 5% (%MVIC) reduction in quadriceps activity after training, the perturbation consistently triggered a marked increase in quadriceps activity. High levels of quadriceps activation are common during the SLS task29,53 and replicated by the athletes in this study who engaged 50% to 75% of the muscle. Athletes in the ML phase activated their quadriceps the most and performed the best on the task, another consistent pattern in SLS performance.35,36 The similarities in Beighton scores and knee muscle torque among these athletes suggests that neither generalized joint laxity nor strength capacity accounted for the difference in muscle activation strategies during perturbed performance. One potential explanation for the athletes’ varied quadriceps activity is an alteration in quadriceps motor cortex excitability 2 . Intracortical inhibition (ICI) is a measure of motor cortex communication, and it is greatest in the luteal phase of the menstrual cycle.2,45 Although ICI has been inversely related to quadriceps strength in people after ACL reconstruction, 11 in healthy people, ICI can vary while quadriceps torque remains stable. 2 Furthermore, ICI is increased by balance training and associated with greater stance stability during platform perturbations.31,49 It is believed that the motor cortex plays a minimal role in postural stability and, therefore, its inhibition during perturbation responses may reflect the central nervous system shifting operations to subcortical regions, 50 the neurological command center for motor control. Determining central nervous system excitability was beyond the scope of this study, but it is possible that the state of cortical excitability when athletes are in the ML phase primes them for greater motor control during perturbed performance.
Generally, athletes’ muscle activity decreased as they became more proficient in the task. Reduced hamstrings activity is expected among those who perform better on the SLS task.29,35 This strategy may be counterproductive to injury mitigation as co-contraction of knee musculature helps to stabilize the knee joint when it is perturbed. 57 One way to potentially upregulate protective hamstrings forces during perturbations is to flex the trunk/hip with the knee.29,56 In practice, however, athletes may avoid excessive co-contraction of knee musculature as it would impede performance. Perhaps another way to maintain performance and protect the knee joint when navigating perturbations is to upregulate soleus muscle activity.
The soleus muscle is a key force contributor to running mechanics. 14 It is also the muscle that generates the greatest posterior shear force at the knee, even more than individual hamstrings muscles, during unanticipated cutting 38 and single-leg landing. 37 Soleus activity may even minimize frontal plane knee motion. 12 At the time of ACL injury, the foot is usually in a flat position, 5 likely impairing the soleus from generating protective forces at the knee joint. The findings from this study indicate that training decreased soleus activity during perturbed performance by 5% to 9% (%MVIC), but training also increased its relative response to the perturbation. In other words, training induced a 30% increase in athletes’ soleus response to the perturbation compared to the baseline soleus activity required to perform the task. This response was not observed in knee musculature, possibly due to their invariance from feedforward strategies. 29 These findings also indicate that soleus activity may be augmented in different hormonal milieus as athletes in the EF phase responded to the perturbation with greater soleus activity than athletes in the LF phase. This finding coincides with the alteration in soleus presynaptic inhibition (gating of the spinal reflex) that occurs across these phases. 23 It also aligns with the evidence that female athletes with a greater capacity to upregulate soleus excitability have lower estradiol levels. 30 Taken together, the soleus may be a key player when learning to navigate perturbed performance.
Estradiol was inversely associated with the training improvement in knee control during unperturbed squats, an opposite relationship to the one shown between estradiol and training improvements in hand control during an upper extremity task. 26 Progesterone was associated with less training improvement in knee control during perturbed and unperturbed squats. These hormonal relationships may have been driven by athletes in the ML phase who performed well on the task and experienced elevated levels of estradiol and progesterone. Cortisol demonstrated the strongest hormonal relationship to knee control, with higher levels associated with better initial knee control during perturbed squats. The body releases cortisol in response to psychological and physical stressors, an arousal mechanism leveraged by athletes in preparation for performance. 54 Accordingly, athletes in this study who showed elevated cortisol levels may have optimized the physiological arousal necessary for perturbed performance.
These findings support the implementation of perturbation training during dynamic performance during any phase of the menstrual cycle to improve knee joint control and lower limb muscle responses (soleus) in female athletes to unexpected events. These findings also suggest female athletes’ motor control may be optimal in the ML phase of the menstrual cycle, but caution is advised when interpreting these results. The body of evidence indicating a potential influence of the menstrual cycle on ACL injury,21,22,46 exercise performance, 40 and adaptations to resistance training 51 is growing but remains limited by the low quality and low quantity of research in these areas. Thus, current evidence does not support adapting training to an athlete's menstrual cycle 8 or altering an athlete's menstrual cycle using hormonal contraception to mitigate injury. 55 This collective work does, however, compel larger-scale prospective studies to determine the magnitude and clinical relevance of hormonal influences on female athlete performance and injury.
Limitations
There are several limitations to this study. The participants in this study were collegiate, eumenorrheic athletes, which limits generalizability of these findings to lower-level athletes, athletes using hormonal contraception, or young female athletes with poor motor control. Some of the participants in this study (30%) had not competed in collegiate athletics for >6 months before study participation; however, none of the athletes were >3 years removed from high-level competition and participant athletic status (current/former) did not influence the effect of training on study outcomes. The inclusion of high-level and elite athletes in this study likely improved the ability to detect small differences in knee motion by reducing the variability in motor proficiency expected in less-skilled populations. Female collegiate athletes are also at considerable risk for ACL injury, 15 highlighting the importance of studying precision knee control capabilities in this group. Approximately 50% of elite athletes use hormonal contraceptives, 39 making them an important group for future study. Overall, this study followed best practice recommendations to accurately determine menstrual cycle phases,18,28 and it contributes new knowledge to an area 40 and population9,10 with great need.
Conclusion
Athletes learned to improve knee control during perturbed performance regardless of menstrual cycle phase. The ML phase may be a time in the menstrual cycle for optimal motor control as athletes in this phase demonstrated the best knee control and the greatest quadriceps activation during the perturbed squatting task. These findings may correspond to the lower incidence of ACL injuries in the luteal phase and to the alteration in exercise performance across the menstrual cycle. Training alterations in soleus muscle activity might be important when learning to navigate perturbations during dynamic performance.
Acknowledgments
The authors acknowledge Michael A. Petrie, PhD, DC, for his assistance in serum collection and data analysis.
Footnotes
Final revision submitted October 26, 2023; accepted November 16, 2023.
One or more of the authors has declared the following potential conflict of interest or source of funding: Financial support for supplies, serum analysis, and participant reimbursement was provided by the National Center for Injury Prevention and Control (NCIPC) of the U.S. Centers for Disease Control and Prevention (grant R49CE003095). R.K.S. received grants from the National Institutes of Health (grants 5R01-HD082109-03 and 5R01-HD084645-12) and holds a patent for the single-leg squatting device, therapeutic exercise system, and method for a paralyzed and nonparalyzed neuromusculoskeletal training system (patent No. 60 7011 5). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto. Ethical approval for this study was obtained from The University of Iowa (ref No. 201904706).
ORCID iD: Kristin A. Johnson  https://orcid.org/0000-0002-7690-9777
https://orcid.org/0000-0002-7690-9777
References
- 1. Agel J, Rockwood T, Klossner D. Collegiate ACL injury rates across 15 sports: National Collegiate Athletic Association injury surveillance system data update (2004-2005 through 2012-2013). Clin J Sport Med. 2016;26(6):518-523. [DOI] [PubMed] [Google Scholar]
- 2. Ansdell P, Brownstein CG, Skarabot J, et al. Menstrual cycle-associated modulations in neuromuscular function and fatigability of the knee extensors in eumenorrheic women. J Appl Physiol (1985). 2019;126(6):1701-1712. [DOI] [PubMed] [Google Scholar]
- 3. Beaulieu ML, Ashton-Miller JA, Wojtys EM. Loading mechanisms of the anterior cruciate ligament. Sports Biomech. 2023;22(1):1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beighton P, Solomon L, Soskolne CL. Articular mobility in an African population. Ann Rheum Dis. 1973;32(5):413-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boden BP, Sheehan FT. Mechanism of non-contact ACL injury: OREF Clinical Research Award 2021. J Orthop Res. 2022;40(3):531-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chidi-Ogbolu N, Baar K. Effect of estrogen on musculoskeletal performance and injury risk. Front Physiol. 2018;9:1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chmielewski TL, Hurd WJ, Snyder-Mackler L. Elucidation of a potentially destabilizing control strategy in ACL deficient non-copers. J Electromyogr Kinesiol. 2005;15(1):83-92. [DOI] [PubMed] [Google Scholar]
- 8. Colenso-Semple LM, D’Souza AC, Elliott-Sale KJ, Phillips SM. Current evidence shows no influence of women's menstrual cycle phase on acute strength performance or adaptations to resistance exercise training. Front Sports Act Living. 2023:5:1054542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Costello JT, Bieuzen F, Bleakley CM. Where are all the female participants in sports and exercise medicine research? Eur J Sport Sci. 2014;14(8):847-851. [DOI] [PubMed] [Google Scholar]
- 10. Cowley ES, Olenick AA, McNulty KL, Ross EZ. “Invisible sportswomen”: the sex data gap in sport and exercise science research. Women Sport Phys Act J. 2021;29(2):146-151. [Google Scholar]
- 11. Criss CR, Melton MS, Ulloa SA, et al. Rupture, reconstruction, and rehabilitation: a multi-disciplinary review of mechanisms for central nervous system adaptations following anterior cruciate ligament injury. Knee. 2021;30:78-89. [DOI] [PubMed] [Google Scholar]
- 12. Dadfar M, Soltani M, Novinzad MB, Raahemifar K. Lower extremity energy absorption strategies at different phases during single and double-leg landings with knee valgus in pubertal female athletes. Sci Rep. 2021;11(1):17516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dai B, Garrett WE, Gross MT, Padua DA, Queen RM, Yu B. The effect of performance demands on lower extremity biomechanics during landing and cutting tasks. J Sport Health Sci. 2019;8(3):228-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dorn TW, Schache AG, Pandy MG. Muscular strategy shift in human running: dependence of running speed on hip and ankle muscle performance. J Exp Biol. 2012;215(Pt 11):1944-1956. [DOI] [PubMed] [Google Scholar]
- 15. Dragoo JL, Castillo TN, Braun HJ, Ridley BA, Kennedy AC, Golish SR. Prospective correlation between serum relaxin concentration and anterior cruciate ligament tears among elite collegiate female athletes. Am J Sports Med. 2011;39(10):2175-2180. [DOI] [PubMed] [Google Scholar]
- 16. Ekenros L, von Rosen P, Solli GS, et al. Perceived impact of the menstrual cycle and hormonal contraceptives on physical exercise and performance in 1,086 athletes from 57 sports. Front Physiol. 2022;13:954760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elliott-Sale KJ, McNulty KL, Ansdell P, et al. The effects of oral contraceptives on exercise performance in women: a systematic review and meta-analysis. Sports Med. 2020;50(10):1785-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elliott-Sale KJ, Minahan CL, de Jonge X, et al. Methodological considerations for studies in sport and exercise science with women as participants: a working guide for standards of practice for research on women. Sports Med. 2021;51(5):843-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ellison TM, Flagstaff I, Johnson AE. Sexual dimorphisms in anterior cruciate ligament injury: a current concepts review. Orthop J Sports Med. 2021;9(12):23259671211025304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10(5):361-374. [DOI] [PubMed] [Google Scholar]
- 21. Herzberg SD, Motu’apuaka ML, Lambert W, Fu R, Brady J, Guise JM. The effect of menstrual cycle and contraceptives on ACL injuries and laxity: a systematic review and meta-analysis. Orthop J Sports Med. 2017;5(7):2325967117718781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hewett TE, Zazulak BT, Myer GD. Effects of the menstrual cycle on anterior cruciate ligament injury risk: a systematic review. Am J Sports Med. 2007;35(4):659-668. [DOI] [PubMed] [Google Scholar]
- 23. Hoffman MA, Doeringer JR, Norcross MF, Johnson ST, Chappell PE. Presynaptic inhibition decreases when estrogen level rises. Scand J Med Sci Sports. 2018;28(9):2009-2015. [DOI] [PubMed] [Google Scholar]
- 24. Hurd WJ, Chmielewski TL, Snyder-Mackler L. Perturbation-enhanced neuromuscular training alters muscle activity in female athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):60-69. [DOI] [PubMed] [Google Scholar]
- 25. Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24(4):427-436. [DOI] [PubMed] [Google Scholar]
- 26. Ikarashi K, Sato D, Iguchi K, Baba Y, Yamashiro K. Menstrual cycle modulates motor learning and memory consolidation in humans. Brain Sci. 2020;10(10):696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm (Vienna). 2007;114(10):1339-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Janse DEJX, Thompson B, Han A. Methodological recommendations for menstrual cycle research in sports and exercise. Med Sci Sports Exerc. 2019;51(12):2610-2617. [DOI] [PubMed] [Google Scholar]
- 29. Johnson KA, Nozu S, Shields RK. Trunk angle modulates feedforward and feedback control during single-limb squatting. J Funct Morphol Kinesiol. 2021;6(4):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson KA, Petrie MA, Shields RK. Biomarkers for rapid H-reflex operant conditioning among females. J Neurophysiol. 2023;129(3):685-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lauber B, Gollhofer A, Taube W. What to train first: balance or explosive strength? Impact on performance and intracortical inhibition. Scand J Med Sci Sports. 2021;31(6):1301-1312. [DOI] [PubMed] [Google Scholar]
- 32. Legerlotz K, Nobis T. Insights in the effect of fluctuating female hormones on injury risk-challenge and chance. Front Physiol. 2022;13:827726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lew LA, Williams JS, Stone JC, Au AKW, Pyke KE, MacDonald MJ. Examination of sex-specific participant inclusion in exercise physiology endothelial function research: a systematic review. Front Sports Act Living. 2022;4:860356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lowe DA, Baltgalvis KA, Greising SM. Mechanisms behind estrogen's beneficial effect on muscle strength in females. Exerc Sport Sci Rev. 2010;38(2):61-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Madhavan S, Shields RK. Movement accuracy changes muscle-activation strategies in female subjects during a novel single-leg weight-bearing task. PM R. 2009;1(4):319-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Madhavan S, Shields RK. Neuromuscular responses in individuals with anterior cruciate ligament repair. Clin Neurophysiol. 2011;122(5):997-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maniar N, Schache AG, Pizzolato C, Opar DA. Muscle contributions to tibiofemoral shear forces and valgus and rotational joint moments during single leg drop landing. Scand J Med Sci Sports. 2020;30(9):1664-1674. [DOI] [PubMed] [Google Scholar]
- 38. Maniar N, Schache AG, Sritharan P, Opar DA. Non-knee-spanning muscles contribute to tibiofemoral shear as well as valgus and rotational joint reaction moments during unanticipated sidestep cutting. Sci Rep. 2018;8(1):2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martin D, Sale C, Cooper SB, Elliott-Sale KJ. Period prevalence and perceived side effects of hormonal contraceptive use and the menstrual cycle in elite athletes. Int J Sports Physiol Perform. 2018;13(7):926-932. [DOI] [PubMed] [Google Scholar]
- 40. McNulty KL, Elliott-Sale KJ, Dolan E, et al. The effects of menstrual cycle phase on exercise performance in eumenorrheic women: a systematic review and meta-analysis. Sports Med. 2020;50(10):1813-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moksnes H, Snyder-Mackler L, Risberg MA. Individuals with an anterior cruciate ligament-deficient knee classified as noncopers may be candidates for nonsurgical rehabilitation. J Orthop Sports Phys Ther. 2008;38(10):586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Petrie M, Johnson K, McCue P, Shields RK. Neuromuscular electrical stimulation primes feedback control during a novel single leg task. J Mot Behav. 2021;53(4):409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scott SH, Cluff T, Lowrey CR, Takei T. Feedback control during voluntary motor actions. Curr Opin Neurobiol. 2015;33:85-94. [DOI] [PubMed] [Google Scholar]
- 44. Shields RK, Madhavan S, Gregg E, et al. Neuromuscular control of the knee during a resisted single-limb squat exercise. Am J Sports Med. 2005;33(10):1520-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith MJ, Keel JC, Greenberg BD, et al. Menstrual cycle effects on cortical excitability. Neurology. 1999;53(9):2069-2072. [DOI] [PubMed] [Google Scholar]
- 46. Somerson JS, Isby IJ, Hagen MS, Kweon CY, Gee AO. The menstrual cycle may affect anterior knee laxity and the rate of anterior cruciate ligament rupture: a systematic review and meta-analysis. JBJS Rev. 2019;7(9):e2. [DOI] [PubMed] [Google Scholar]
- 47. Sundemo D, Hamrin Senorski E, Karlsson L, et al. Generalised joint hypermobility increases ACL injury risk and is associated with inferior outcome after ACL reconstruction: a systematic review. BMJ Open Sport Exerc Med. 2019;5(1):e000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tanaka MJ, LiBrizzi CL, Rivenburgh DW, Jones LC. Changes in U.S. girls’ participation in high school sports: implications for injury awareness. Phys Sportsmed. 2021;49(4):450-454. [DOI] [PubMed] [Google Scholar]
- 49. Taube W, Gollhofer A, Lauber B. Training-, muscle- and task-specific up- and downregulation of cortical inhibitory processes. Eur J Neurosci. 2020;51(6):1428-1440. [DOI] [PubMed] [Google Scholar]
- 50. Taube W, Gruber M, Gollhofer A. Spinal and supraspinal adaptations associated with balance training and their functional relevance. Acta Physiol (Oxf). 2008;193(2):101-116. [DOI] [PubMed] [Google Scholar]
- 51. Thompson B, Almarjawi A, Sculley D, Janse de, Jonge X. The effect of the menstrual cycle and oral contraceptives on acute responses and chronic adaptations to resistance training: a systematic review of the literature. Sports Med. 2020;50(1):171-185. [DOI] [PubMed] [Google Scholar]
- 52. Title IX of the Education Amendments of 1972, 20 USC §1681-§1688. The United States Department of Justice. Updated November 13, 2000. Accessed April 23, 2024. https://www.justice.gov/crt/title-ix-education-amendments-1972
- 53. Tseng SC, Cole KR, Shaffer MA, Petrie MA, Yen CL, Shields RK. Speed, resistance, and unexpected accelerations modulate feed forward and feedback control during a novel weight bearing task. Gait Posture. 2017;52:345-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van Paridon KN, Timmis MA, Nevison CM, Bristow M. The anticipatory stress response to sport competition; a systematic review with meta-analysis of cortisol reactivity. BMJ Open Sport Exerc Med. 2017;3(1):e000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. White L, Losciale JM, Squier K, et al. Combined hormonal contraceptive use is not protective against musculoskeletal conditions or injuries: a systematic review with data from 5 million females. Br J Sports Med. 2023;57(18):1195-1202. [DOI] [PubMed] [Google Scholar]
- 56. Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. Effect of varying hamstring tension on anterior cruciate ligament strain during in vitro impulsive knee flexion and compression loading. J Bone Joint Surg Am. 2008;90(4):815-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wojtys EM, Ashton-Miller JA, Huston LJ. A gender-related difference in the contribution of the knee musculature to sagittal-plane shear stiffness in subjects with similar knee laxity. J Bone Joint Surg Am. 2002;84(1):10-16. [DOI] [PubMed] [Google Scholar]
- 58. Women in the Olympic Movement. International Olympic Committee. Updated December 09, 2021. Accessed January 31, 2023. https://stillmed.olympics.com/media/Documents/Olympic-Movement/Factsheets/Women-in-the-Olympic-Movement.pdf?_ga=2.247824004.2092933100.1637840588-1078385095.1637840588