Abstract
Lung disease encompasses acute, infectious processes and chronic, non-infectious processes such as chronic obstructive pulmonary disease, asthma and lung cancer. People living with HIV are at increased risk of both acute and chronic lung diseases. Although the use of effective antiretroviral therapy has diminished the burden of infectious lung disease, people living with HIV experience growing morbidity and mortality from chronic lung diseases. A key risk factor for HIV-associated lung disease is cigarette smoking, which is more prevalent in people living with HIV than in uninfected people. Other risk factors include older age, history of bacterial pneumonia, Pneumocystis pneumonia, pulmonary tuberculosis and immunosuppression. Mechanistic investigations support roles for aberrant innate and adaptive immunity, local and systemic inflammation, oxidative stress, altered lung and gut microbiota, and environmental exposures such as biomass fuel burning in the development of HIV-associated lung disease. Assessment, prevention and treatment strategies are largely extrapolated from data from HIV-uninfected people. Smoking cessation is essential. Data on the long-term consequences of HIV-associated lung disease are limited. Efforts to continue quantifying the effects of HIV infection on the lung, especially in low-income and middle-income countries, are essential to advance our knowledge and optimize respiratory care in people living with HIV.
Introduction
The lung has historically been one of the organs most impacted by the immune deficits caused by infection with the human immunodeficiency virus (HIV). The first reports of an unusual pneumonia caused by the fungus Pneumocystis jirovecii reported among gay men heralded the start of the AIDS epidemic, and opportunistic pneumonias and malignancies quickly became a leading cause of morbidity and mortality in HIV infection1. With the development of effective antiretroviral therapy (ART), the incidence of lung infections and malignancies declined dramatically2. For example, the incidence of P. jirovecii pneumonia (PCP) decreased from 4.9 cases per 100 person-years to 0.3 cases per 100 person-years in the EuroSIDA cohort and from 3.1 cases per 100 person-years to 0.3 cases per 100-person years in the Multicenter AIDS Cohort Study following the widespread use of ART3,4. However, lung disease still remains a considerable problem in people living with HIV. Individuals who do not have access to ART, are unaware of their HIV infection or are not able to successfully take ART remain at high risk of pulmonary complications. In addition, despite ART, people living with HIV remain at elevated risk of certain chronic and acute lung conditions.
HIV is associated with both infectious and non-infectious pulmonary diseases. Opportunistic infections include PCP and mycobacterial pneumonia, and AIDS-associated malignancies include Kaposi sarcoma, lymphoma and primary lung cancer. Individuals with viral suppression on ART remain at elevated risk of bacterial pneumonia and tuberculosis (TB) and certain malignancies including lung cancer5. HIV infection also seems to be a risk factor for more severe COVID-19 pneumonia6. People living with HIV are at elevated risk of chronic obstructive pulmonary disease (COPD)7. Asthma, a common obstructive lung disease in the non-HIV population, is also seen in people living with HIV, and whether it has a different presentation and severity has been debated8,9. These diseases continue to have a considerable effect on the quality of life of people living with HIV, but few therapies have been studied in this population and the specific impacts of immunodeficiency, environmental exposures, direct viral effects, other aspects of chronic infection and ART remain under investigation.
In this Primer, we first review the current epidemiology of and risk factors for HIV-associated lung disease. We then explore the mechanisms underlying lung disease in HIV, including the impact of HIV on specific lung and immune cell populations, the role of oxidative stress, interactions with exposures such as cigarette smoke or environmental pollutants, and the interplay of HIV, the microbiota and lung disease. We also provide an overview of the current state of screening, diagnosis, prevention and management of common lung diseases in adults and children living with HIV, including pneumonia, COPD, asthma and lung cancer. Finally, we highlight gaps in knowledge and future areas for research.
Epidemiology
An estimated 38 million people currently live with HIV worldwide, over two-thirds of whom are in Africa10. HIV infection is associated with increased risks of pneumonia from bacteria (including mycobacteria), viruses, fungi and parasites, at all CD4+ T cell counts, although the risks increase with decreasing CD4+ T cell count4,11 (Fig. 1). For example, the risk of PCP in people living with HIV who have a CD4+ T cell count <200/μl is fivefold to eightfold higher than in people living with HIV who have a CD4+ T cell count >200/μl12,13. Mycobacterium tuberculosis and other bacteria are frequent causes of pneumonia across all levels of immunosuppression. The estimated annual risk of reactivating TB is up to 10% in people living with HIV compared with a lifetime risk of 5–10% for HIV-uninfected people14–18. Risks are mitigated by ART initiation with subsequent viral suppression (that is, undetectable HIV viral load) and immune recovery and with antimicrobial prophylaxis, but remain higher among people living with HIV than among HIV-uninfected people. Bacterial pneumonia is the most common pulmonary infection among people living with HIV in high-income countries (HICs), followed by PCP and TB. In people living with HIV, TB is the predominant pulmonary infection in low-income and middle-income countries (LMICs) and a leading cause of death globally19. Importantly, the range of pathogens causing pulmonary infections varies geographically, reflecting local factors and specific pathogen endemicity, which is particularly important in LMIC settings where pathogen identification can be challenging owing to limited resources. For example, melioidosis (caused by the bacterium Burkholderia pseudomallei) and talaromycosis (caused by the fungus Talaromyces marneffei) should be considered as aetiologies of pneumonia in Asia, whereas histoplasmosis (caused by the fungus Histoplasma capsulatum) should be considered an aetiology of pneumonia in temperate regions in Central, South and North America, Africa and Asia. Common risk factors for bacterial pneumonia, PCP and TB include decreased CD4+ T cell count11,20–29, detectable HIV viral load11,22,23,29 and lack of ART11,21,24,28–32. Injection of illicit drugs11,22,24, smoking21–23,25,33, and extremes of age and comorbidities are additional risk factors for bacterial pneumonia (Box 1).
Fig. 1 |. Risk of HIV-associated lung diseases.
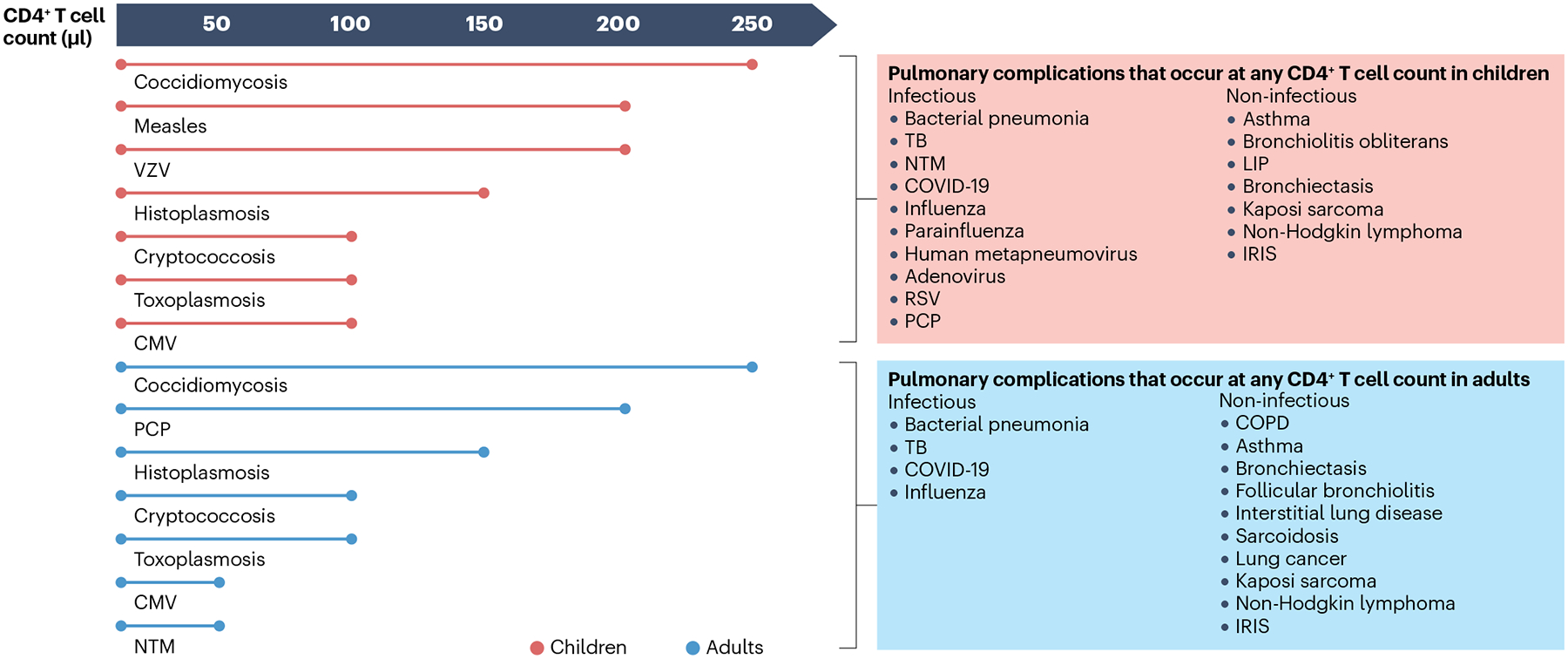
The risk of infectious and non-infectious pulmonary complications of HIV may vary between children and adults and by CD4+ T cell count. For several infectious lung diseases, specific CD4+ T cell count thresholds exist below which the risk is substantially increased (for example, the risk of Pneumocystis jirovecii pneumonia (PCP) is significantly increased in individuals with CD4+ T cell counts <200/μl). By contrast, no specific thresholds exist for infections such as bacterial pneumonia, tuberculosis (TB), and COVID-19, and non-infectious complications, such as Kaposi sarcoma, non-Hodgkin lymphoma and immune reconstitution inflammatory syndrome (IRIS), although the risk generally increases with lower CD4+ T cell count. CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease; LIP, lymphocytic interstitial pneumonitis; NTM, non-tuberculous mycobacteria; RSV, respiratory syncytial virus; VZV, varicella zoster virus. Adapted from figure courtesy of S. Pipavath.
Box 1. Risk factors for acute and chronic lung diseases in people living with HIV.
Infection (bacterial, viral, fungal or parasitic)
Older age, infancy, cigarette smoking, low CD4+ T cell count, neutropenia, high HIV viral load, history of AIDS-defining illness, history of bacterial pneumonia, co-infection with hepatitis C, lack of antiretroviral therapy, lack of appropriate Pneumocystis jirovecii pneumonia prophylaxis, lack of pneumococcal and influenza vaccinations, injection of illicit drugs, alcohol abuse, malnutrition, comorbidities.
Chronic obstructive pulmonary disease
Older age, cigarette smoking and more pack-years smoking, low BMI, low socioeconomic status, biomass fuel burning, injection of illicit drugs, low CD4+ T cell count, low nadir CD4+ T cell count, high HIV viral load, history of bacterial pneumonia, history of P. jirovecii pneumonia, history of pulmonary tuberculosis, co-infection with hepatitis C, antiretroviral therapy usea, alcohol abusea.
Asthma
Parental history of asthma, obesity, history of bacterial pneumonia, female sexa, older agea, high BMIa, CD4+ T cell counta, high HIV viral loada.
Lung cancer
Older age, cigarette smoking and more pack-years smoking, low CD4+ T cell count, high HIV viral load, history of chronic obstructive pulmonary disease, history of bacterial pneumonia, history of P. jirovecii pneumonia, oncogenicity of HIV itselfa, injection of drugsa.
aInconsistent or conflicting evidence regarding the association between these putative risk factors and corresponding lung disease.
Smoking is more prevalent among people living with HIV than in uninfected people in HICs and LMICs, although rates vary depending on the specific characteristics of the population and the years being studied34–40. For example, the prevalence of current smoking has previously been estimated as 40% in adults living with HIV compared with 21% in the general population, using nationally representative cross-sectional surveys in the USA34. Nevertheless, HIV infection is associated with COPD even after adjusting for smoking41. COPD prevalence among people living with HIV is in the range 3–38% in HICs42–52, compared with 3–22% in LMICs53–58. Key risk factors for COPD in people living with HIV include smoking43,44,46,52,59–65, older age45,46,52,59–61,63–66, lower BMI45,46,56,59,64,67,68 and history of pulmonary TB55,56,67,68. Lower nadir CD4+ T cell count and greater HIV viral load have also been identified as unique COPD risk factors in people living with HIV48,59,66,69 (Fig. 1). Additional non-tobacco-related risk factors for COPD in both the general population and in people living with HIV include socioeconomic disadvantage70,71, biomass fuel burning72 and chronic exposure to airborne particulate matter73 (Box 1). There is conflicting evidence regarding the association between HIV infection and incident COPD4,11,74, although data show that HIV is associated with more rapid lung function decline75,76. People living with HIV are at higher risk of COPD exacerbations (which are defined as episodes of acutely worsened respiratory symptoms requiring additional therapy) compared with HIV-uninfected people77,78. Among people living with HIV, prevalent and incident COPD is an independent risk factor for increased mortality79–81.
Variability in asthma definition in epidemiological studies — including definitions based on self-report, the presence of variable airflow obstruction, and the combination of spirometry and symptoms — limits robust conclusions about the association between HIV infection and asthma. Asthma prevalence does not differ by HIV status in most studies and is in the range 5‒38% in HICs9,42,44,82–84, compared with 1–16% in LMICs53,55,85–88. There is conflicting evidence regarding the association between female sex, older age, smoking, higher BMI and HIV-related characteristics (such as nadir CD4+ T cell count, HIV viral load and ART use) and asthma prevalence8,9,62,84,89,90 (Fig. 1 and Box 1), and the association between asthma and symptom burden and respiratory health status9,91. HIV infection is associated with worse respiratory symptom burden among men with asthma but not among women with asthma9. Limited data suggest that asthma is not associated with increased mortality risk in people living with HIV79.
Lung cancer is a leading non-AIDS-defining malignancy92–95 (cancers that are more likely to occur in people infected with HIV than in those who are not infected) and the most frequent cause of mortality from cancer in people living with HIV95–97. HIV infection is independently associated with lung cancer94,97–105. Although higher smoking prevalence among people living with HIV is the greatest contributor to lung cancer risk104–106, data support an association of older age98,105,107, pneumonia105,108, low CD4+ T cell count and HIV viral load with lung cancer risk98–100,102,107 (Fig. 1 and Box 1). Lung cancer is typically diagnosed at a similar stage109–111 but at a younger age in people living with HIV than in uninfected persons101,111. HIV infection is associated with poorer survival in patients with lung cancer after adjusting for stage99,104,109–112. Data on the association between HIV infection and lung cancer incidence outside North America and Europe are limited113,114.
Pulmonary diseases are also common in HIV-infected children, encompassing acute infection and chronic disease115 (Fig. 1). Furthermore, HIV-exposed but uninfected infants, who are HIV-uninfected but born to an HIV-infected mother, have an increased risk of respiratory illness compared with unexposed infants, a finding that may be at least partly explained by in utero and postnatal exposures leading to immune dysfunction106. Early diagnosis and use of ART have reduced the burden and severity of acute illness in HIV-infected children. With improved survival of perinatally infected children into adulthood, chronic respiratory disease contributes to morbidity116. Although the incidence and severity of pneumonia have decreased considerably with ART and improved preventive strategies including pneumococcal conjugate vaccine (PCV), vaccination against Haemophilus influenzae type b, and co-trimoxazole prophylaxis, pneumonia remains common115. Bacterial, viral or fungal pathogens can occur, with co-infections in severe AIDS. Bacterial pneumonia and TB are the most frequent respiratory infections in both ART-naive and ART-exposed children infected with HIV. In the Pneumonia Etiology Research for Child Health (PERCH) study, the most common pathogens in HIV-infected children hospitalized with severe or very severe pneumonia were Staphylococcus aureus, Streptococcus pneumoniae and M. tuberculosis117,118. HIV-infected children on ART have a higher risk of TB than HIV-uninfected children119. Bordetella pertussis may cause pneumonia in young HIV-infected or HIV-exposed infants, particularly if these children are incompletely immunized117. Viruses (especially respiratory syncytial virus) are increasingly reported as a cause of pneumonia in the context of high immunization coverage with PCV and H. influenzae type b vaccine120. In children undiagnosed with HIV or those not on ART, opportunistic infections such as PCP or cytomegalovirus (CMV) can cause severe pneumonia. P. jirovecii accounted for 23–25% of radiologically confirmed cases of pneumonia in HIV-infected African children in the PERCH study in South Africa and Zambia, although ART coverage was low117,118. HIV-exposed but uninfected infants are at higher risk of pneumonia than HIV-unexposed children, and have worse outcomes121.
Mechanisms/pathophysiology
HIV
HIV is a member of the Lentivirus genus of the Retroviridae family122. HIV is a single-stranded, positive-sense, enveloped RNA virus. There are two species, HIV-1 and HIV-2. HIV-1 is more virulent and infectious than HIV-2 and by far the more common species. HIV-1 has given rise to the global epidemic of HIV/AIDS, with an estimated 38 million infected individuals living today, whereas HIV-2 is rare outside West Africa123,124.
The HIV viral envelope trimeric glycoprotein (gp160 spike) binds to the CD4 receptor and either CC-chemokine receptor 5 (CCR5) or CXC-chemokine receptor 4 (CXCR4) of CD4+ T cells, macrophages, microglia and dendritic cells. On binding, the HIV virion uncoats and fuses with the cell membrane. The viral RNA is injected into the cytoplasm together with several viral enzymes that are required for completion of the virus life cycle intracellularly. One enzyme, reverse transcriptase, reverse transcribes viral RNA into complementary DNA. Two complementary DNAs form a double-stranded viral DNA that translocates to the nucleus and integrates into the host genome by the action of the integrase enzyme, where it can lie dormant for years or immediately be transcribed to viral mRNA, transported to the cytoplasm, translated to an HIV polypeptide that is cleaved by a virally encoded serine protease, assembled, and released as progeny viral particles. These virions may infect other cells and restart the replication cycle. Virion fusion with the target cell, co-receptor binding, reverse transcription, integration and protease cleavage are the main targets of antiretroviral treatment. HIV is highly genetically varied owing to its fast replication cycle (leading to the daily generation of 1010 virions), its high mutation rate (3 × 10−5 mutations per nucleotide base per cycle of replication), and its recombination ability. These properties give rise to immune escape and antiretroviral drug resistance. The development of novel antiretroviral drugs has largely diverted the issues of drug resistance, while viral integration, dormancy and immune escape are the largest obstacles to a cure and the development of vaccines.
Structural damage — cilia, epithelial cells
The surface of the airways interfaces with the environment through a continuous epithelial sheet containing distinct morphological characteristics based on location in the airway. In the proximal airway, the columnar epithelium includes mucus-secreting goblet cells along with ciliated epithelium responsible for mucus transport. Progressing from the distal airways to the alveolar epithelium is associated with a transition from columnar and ciliated epithelium to type I and type II alveolar epithelial cells. Data exist for specific impacts of HIV viral infection along the course of the human airway (Figs. 2 and 3). E-cadherin is an adhesion molecule produced by airway epithelial cells that plays a vital part in maintaining the intercellular epithelial barrier125. Disruption of this barrier can lead to increased epithelial permeability to harmful inhalants as well as altered mucosal immunity, both of which are mechanisms associated with chronic lung disease. Infection of human airway epithelial cells with HIV in vitro leads to decreased expression of E-cadherin and increased intercellular permeability126. Disruption of mucosal immunity can lead to alterations in the level and function of antimicrobial peptides. Reduced blood levels of the antimicrobial peptide cathelicidin are correlated with reduced lung function among people living with HIV127. It remains unclear whether HIV infection directly alters lung cathelicidin levels or function. Impairments in mucociliary clearance are a hallmark of chronic bronchitis and predispose individuals to recurrent respiratory symptoms and infections. HIV infection impairs mucociliary clearance through multiple mechanisms. Specifically, exposure of the bronchial epithelium in vitro to HIV Tat impairs airway cellular differentiation, cystic fibrosis transmembrane conductance regulator (CFTR) function and ciliogenesis128. The mechanisms for such alterations might involve HIV infection-related induction of transforming growth factor-β1 (TGFβ1) expression129, which is implicated as a putative mechanism for COPD development in the general population130.
Fig. 2 |. Factors implicated in the pathogenesis of acute and chronic lung disease in people living with HIV.

There is a complex interplay between structural and immunological alterations and environmental and microbiological exposures. The degree to which these mechanisms are unique to HIV infection or whether their effect is modified by HIV infection is unknown. ART, antiretroviral therapy; HBV, hepatitis B virus; HCV, hepatitis C virus.
Fig. 3 |. Effects of HIV on the structure and function of alveoli and the airway epithelium.
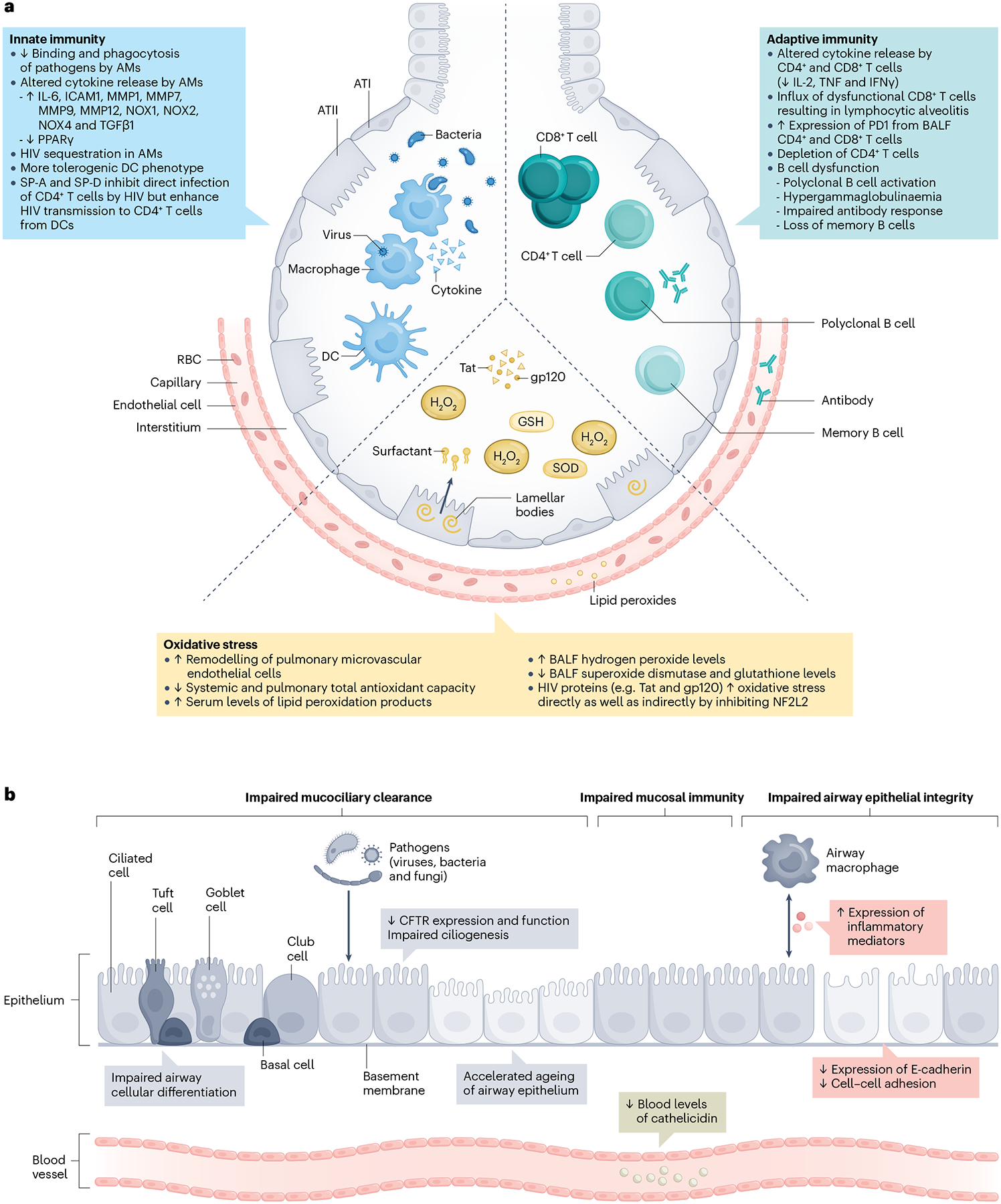
a, Innate immune effects of HIV include impaired alveolar macrophage (AM) binding and phagocytosis of pathogens134,135,151 and altered cytokine release149,175,177, as well as sequestration of HIV138. Dendritic cells (DCs) display a more tolerogenic phenotype139, and surfactant protein A (SP-A) and SP-D inhibit direct infection of CD4+ T cells by HIV but enhance transmission of HIV from DCs to CD4+ T cells140,141. Adaptive immunity effects include depletion of CD4+ T cells142, altered cytokine release by CD4+ and CD8+ T cells136,137, influx of dysfunctional CD8+ T cells (resulting in lymphocytic alveolitis143) and increased expression of PD1 by bronchoalveolar lavage fluid (BALF) CD4+ T cells and CD8+ T cells143. Furthermore, polyclonal B cell activation, hypergammaglobulinaemia, an impaired antibody response and loss of memory B cells result in B cell dysfunction144. HIV also increases oxidative stress by decreasing systemic and pulmonary total antioxidant capacity147,151,157, decreasing superoxide dismutase (SOD) and glutathione (GSH) levels145,148,156,158 and increasing hydrogen peroxide levels156 in BALF, and increasing serum levels of lipid peroxidation products146. HIV proteins (such as Tat and gp120) directly and indirectly inhibit nuclear factor erythroid 2-related factor 2 (NF2L2)150. HIV also increases remodelling of pulmonary microvascular endothelial cells153–155. b, HIV infection impairs airway epithelial integrity126,150 by decreasing E-cadherin expression, increasing inflammatory mediator expression, and reducing cell–cell adhesion. Mucociliary clearance is also impaired128,132 owing to impaired ciliogenesis and airway cellular differentiation, accelerated airway epithelium ageing, and decreased cystic fibrosis transmembrane conductance regulator (CFTR) expression and function. Mucosal immunity is disrupted by reduced levels of cathelicidin in blood127. ATI, alveolar epithelial type I cell; ATII, alveolar epithelial type II cell; RBC, red blood cell; TGFβ1, transforming growth factor-β1. Part a adapted from ref. 248, Springer Nature Limited. Part b adapted from ref. 249, Springer Nature Limited.
Immune deficits
The lungs are constantly exposed to inhaled pathogens (for example, bacteria, viruses and Pneumocystis), allergens (for example, pollen, dust mites and animal dander) and toxins (for example, tobacco, illicit drugs and air pollutants) (Fig. 2). The innate and adaptive immune systems in the lungs respond to these stimuli. HIV infection affects both the innate and adaptive immune systems at multiple key points (Fig. 3). The resultant impaired or dysregulated responses result in opportunistic infections and chronic lung diseases such as COPD131.
Similar to other mucosal surfaces (such as the skin and gastrointestinal tract), the epithelial surface of the lungs presents a barrier to pathogen entry. However, HIV can infect bronchial epithelial cells, as they express CD4, CCR5 and CXCR4. HIV infection can alter the function of bronchial epithelial cells through impaired cell–cell adhesion and increased expression of inflammatory mediators126 as well as suppressed tracheobronchial mucociliary clearance128. Cigarette smoking can potentiate HIV infection of bronchial epithelial cells through upregulation of CD4 and CCR5 expression. HIV and cigarette smoking additively suppress CFTR mRNA transcription and function132. HIV can also infect alveolar macrophages — the primary innate immune cells in the lung — through contact of these cells with infected CD4+ T cells133, disrupting alveolar macrophage phagocytosis and altering their release of cytokines134–137. Alveolar macrophages also represent a potential HIV reservoir138. Finally, HIV can infect dendritic cells, the main antigen-presenting cells in the lung. Dendritic cells exposed to HIV and suppressive T cells develop a more tolerogenic phenotype and express multiple co-inhibitory molecules that can result in suppressed immune responses139. Although surfactant proteins D and A can inhibit the ability of HIV to infect CD4+ T cells, they can also stimulate HIV transfer from dendritic cells to CD4+ T cells140,141.
During acute HIV infection, there is systemic depletion of CD4+ T cells from the gut mucosa and release of pro-inflammatory cytokines142. Subsequently, the systemic immune activation from chronic HIV infection leads to progressive depletion of CD4+ T cells, including in the lungs. HIV causes an influx of CD8+ T cells into the lungs, with a resulting lymphocytic alveolitis. These CD8+ T cells target HIV-infected cells and opportunistic pathogens such as CMV and P. jirovecii. HIV-1-specific CD4+ T cells and CD8+ T cells in bronchoalveolar lavage fluid (BALF) demonstrate decreased proliferative capacity and increased expression of the immune checkpoint protein PD1 (ref. 143). HIV infection is also associated with B cell dysfunction, including polyclonal B cell activation, hypergammaglobulinaemia, impaired antibody response and loss of memory B cells144.
Oxidative stress
HIV infection exacerbates oxidative stress within the alveolar space, thereby contributing to increased susceptibility to pulmonary infections and chronic pulmonary diseases (Figs. 2 and 3). People living with HIV have decreased systemic and pulmonary total antioxidant capacity and elevated serum levels of lipid peroxidation products145,146. Greater oxidative stress can persist despite ART147, although ART and the concomitant increase in CD4+ T cell count are beneficial, as higher levels of oxidative stress correlate with lower CD4+ T cell counts146. For example, healthy people living with HIV who are not on ART have decreased glutathione levels in BALF compared with people living with HIV who are on ART148. Furthermore, healthy people living with HIV who are on ART have altered critical mediators of alveolar macrophage oxidative stress and phagocytic function, with abnormalities including increased hydrogen peroxide in BALF, decreased peroxisome proliferator-activated receptor-γ and increased NADPH oxidase isoforms NOX1, NOX2 and NOX4, and TGFβ1, contributing to immune dysfunction that increases the risk of pulmonary infection149.
HIV also increases oxidative stress through the effects of HIV proteins, resulting in impaired alveolar macrophage function and alveolar epithelial barrier cell function. In HIV transgenic rats, chronic expression of gp120 and Tat is associated with considerable oxidative stress and glutathione depletion in the lung150. gp120 and Tat inhibit nuclear factor erythroid 2-related factor 2 (NF2L2), the master transcription factor that regulates antioxidant defences within alveolar macrophages, and alveolar epithelial cell barrier function via increased expression of microRNA-144 in the alveolar epithelium151,152.
The increased oxidative stress in people living with HIV can place them at greater risk of lung injury from other factors that also cause oxidative stress. The HIV proteins Nef, Tat and gp120 are implicated in oxidative stress-mediated apoptosis of endothelial cells153,154, and HIV acts synergistically with other exposures, such as cocaine and opioids, to increase oxidative stress and augment remodelling of pulmonary microvascular endothelial cells, including medial hypertrophy, intimal proliferation and complex lesion formation, in non-human primate models155. Susceptibility to lung injury in the setting of infection can also be enhanced by increased oxidative stress. For example, endotoxin administration in an HIV transgenic mouse model resulted in significantly greater lung oxidative and nitrosative stress, with elevated nitric oxide metabolites and decreased glutathione levels in BALF156.
Other oxidative stressors include cigarette smoke exposure, hypoxia and air pollution. Cigarette smoking is more common among people living with HIV than in uninfected populations across different regions of the world and country income categories34–40 and is a major risk factor for pulmonary infections and chronic pulmonary diseases. Plasma glutathione levels are significantly lower in people living with HIV who are smokers than in those who are non-smokers157, and over time, glutathione levels in BALF decrease to a greater extent in people living with HIV who are smokers than in those who are non-smokers158. Taken together, these effects of chronic HIV infection that result in increased oxidative stress contribute to the pathogenesis of acute and chronic pulmonary diseases in people living with HIV.
Microbiota
The effects of HIV have been studied primarily in the gut microbiota, but given the immune defects caused by HIV, alterations in the lung microbiota in people living with HIV are possible (Fig. 2). In healthy individuals, the lung microbiota typically has a very low biomass, and most detectable bacteria originate primarily from the oral cavity159,160. Lung bacteria in healthy people living with HIV are essentially indistinguishable from those of HIV-uninfected people161,162. People with advanced HIV not on ART have decreased α diversity (within sample diversity) and increased β diversity (between sample diversity) in BALF, with differential abundance of several bacterial taxa, including increased Streptococcus161. Differences between people living with HIV and HIV-uninfected people decrease after ART initiation but do not completely resolve161. Tropheryma whipplei (the causative agent of Whipple disease) has been detected in the lungs of people living with HIV and in non-human primate models of HIV-like immunodeficiency, but its significance is unknown163,164. The fungal microbiome has also been investigated in people living with HIV, with differences identified in fungal communities between people living with HIV and HIV-uninfected people162. In particular, P. jirovecii is disproportionately found in people living with HIV regardless of CD4+ T cell count.
Alterations in the microbiota of various body sites might play a direct or indirect part in the pathogenesis of COPD in HIV. A study in people with advanced HIV found a correlation of certain bacterial taxa with lung function and inflammation165, but other studies in people living with HIV on ART did not find differences in the lung bacterial communities despite lung function differences161,162. By contrast, oral bacterial communities are altered in people living with HIV and COPD. A study of the oral microbiota in people living with HIV found a relationship between alterations in the oral microbiome and airway obstruction, impaired diffusing capacity and systemic inflammation, suggesting that aspiration of oral microbial communities may contribute to COPD166. In addition, detection of P. jirovecii in the lung is associated with COPD in people living with HIV and in non-human primate models162,164.
The gut microbiota might also influence lung disease via production of metabolic products and/or influencing systemic immune response. HIV is linked to disruptions in the translocation of bacterial or fungal organisms or products, which might result in an aberrant immune response and inflammation. The microbiota can gain access to the circulation through an impaired epithelial barrier in people living with HIV, leading to systemic inflammation and immune activation even in those on ART167–169. Several lines of evidence show that HIV increases gut epithelial permeability through direct disruption of tight junctions, inflammatory damage to the epithelium and loss of lymphoid-associated tissue167–169. While investigations have generally focused on bacterial translocation from the gut, translocation of fungal organisms or parts of the fungal cell wall occurs in people living with HIV and COPD. Serum levels of β-d-glucan (BDG) are used as a marker of clinical fungal infection but can also indicate fungal translocation in people without active infection170. People living with HIV have detectable BDG in the peripheral blood that is associated with systemic inflammation and COPD171; however, detection of BDG as a marker of translocation is not clinically applicable currently.
CMV seropositivity is also associated with worse lung function in adolescents living with HIV172, raising questions about the role of viral co-infections and the virome on lung health in people living with HIV. CMV seropositivity has been associated with higher COPD mortality173 and more rapid lung function decline in adults without HIV174, but data in adults living with HIV are currently lacking.
Smoking and interactions with HIV
Cigarette smoking is common among people living with HIV, and might serve as a ‘second hit’ with HIV to enhance susceptibility to lung diseases (Fig. 2). Smoking has multiple harmful consequences including immunosuppression, inflammation, oxidative stress and tissue injury. Smoking is associated with greater systemic levels of inflammatory and endothelial biomarkers in people living with HIV175. In a study of Nepalese people living with HIV, longer duration and greater intensity of smoking, as measured by pack-years or number of daily cigarettes, correlated with higher levels of C-reactive protein (defined as >3 mg/l)176.
Smoking also causes perturbations within the alveolar space that might be unique in people living with HIV. HIV infection together with cigarette smoking favour a pro-inflammatory macrophage phenotype and increased expression of inflammatory mediators177. Cigarette smoking is associated with decreased CD8+ T cells in BALF of both people living with HIV and HIV-uninfected people, but with increased mucosal CD8+ T cells in people living with HIV who smoke178. Greater numbers of mucosal CD8+ T cells are inversely correlated with mean lung aeration on chest CT scan, suggesting that mucosal CD8+ T cells might be associated with lung inflammation and remodelling. Cigarette smoke exposure also increases the concentration of matrix metalloproteinase 9 in the alveolar space, but this response is greater in the presence of HIV proteins, demonstrating the synergy between HIV and cigarette smoke in promoting lung disease179.
People living with HIV who smoke are at risk of accelerated progression of HIV180 and impaired response to ART181–185. Although some of these associations may be due to persistent residual confounding, current smoking and intensity of smoking correlate with higher HIV DNA and cell-associated RNA levels in peripheral blood mononuclear cells in people living with HIV on long-term ART and virally suppressed for over 2 years186.
Within the lungs, smoking is associated with increased detection of HIV in BALF and might enhance HIV viral entry and replication187. Exposure to smoke results in upregulation of CCR5 expression on bronchial epithelial cells via a TGFβ pathway as well as suppression of the expression and function of CFTR, resulting in enhanced HIV viral entry and viral replication132. In laboratory-based studies, benzo(a) pyrene, a carcinogenic component of cigarettes, enhances HIV replication in alveolar macrophages through a cytochrome p450 pathway188. The increase in HIV viral replication may contribute to a feedback loop, further driving lung inflammation and tissue injury.
Diagnosis, screening and prevention
Adults
Infections.
Signs and symptoms of bacterial pneumonia, PCP and pulmonary TB may be useful to distinguish PCP from the other infections, while signs and symptoms of bacterial pneumonia and pulmonary TB overlap. The symptoms of PCP in people living with HIV typically develop over weeks and include shortness of breath, a non-productive cough, fever, fatigue and weight loss. Oral thrush caused by candidiasis is often present, and is indicative of immunodeficiency. Bacterial pneumonia characteristically develops over days, leading to acute onset of symptoms that include a cough (often productive but may be dry), shortness of breath, fever and chest pain. Symptoms of pulmonary TB are typically more prolonged than those of bacterial pneumonia and develop over weeks, with a cough (usually productive and may contain blood in more advanced stages), fever, night sweats, difficulty breathing, weight loss and fatigue. Chest radiographs are often non-specific due to overlapping findings. However, PCP is characterized by diffuse bilateral infiltrates, bacterial pneumonia by lobar infiltrates, and pulmonary TB by upper lobe infiltrates and cavitation, although such TB findings are less likely with more severe immunodeficiency (Fig. 4). Plasma lactate dehydrogenase levels are often elevated with PCP, while plasma C-reactive protein typically is elevated with bacterial pneumonia.
Fig. 4 |. Manifestations of HIV-associated lung disease.
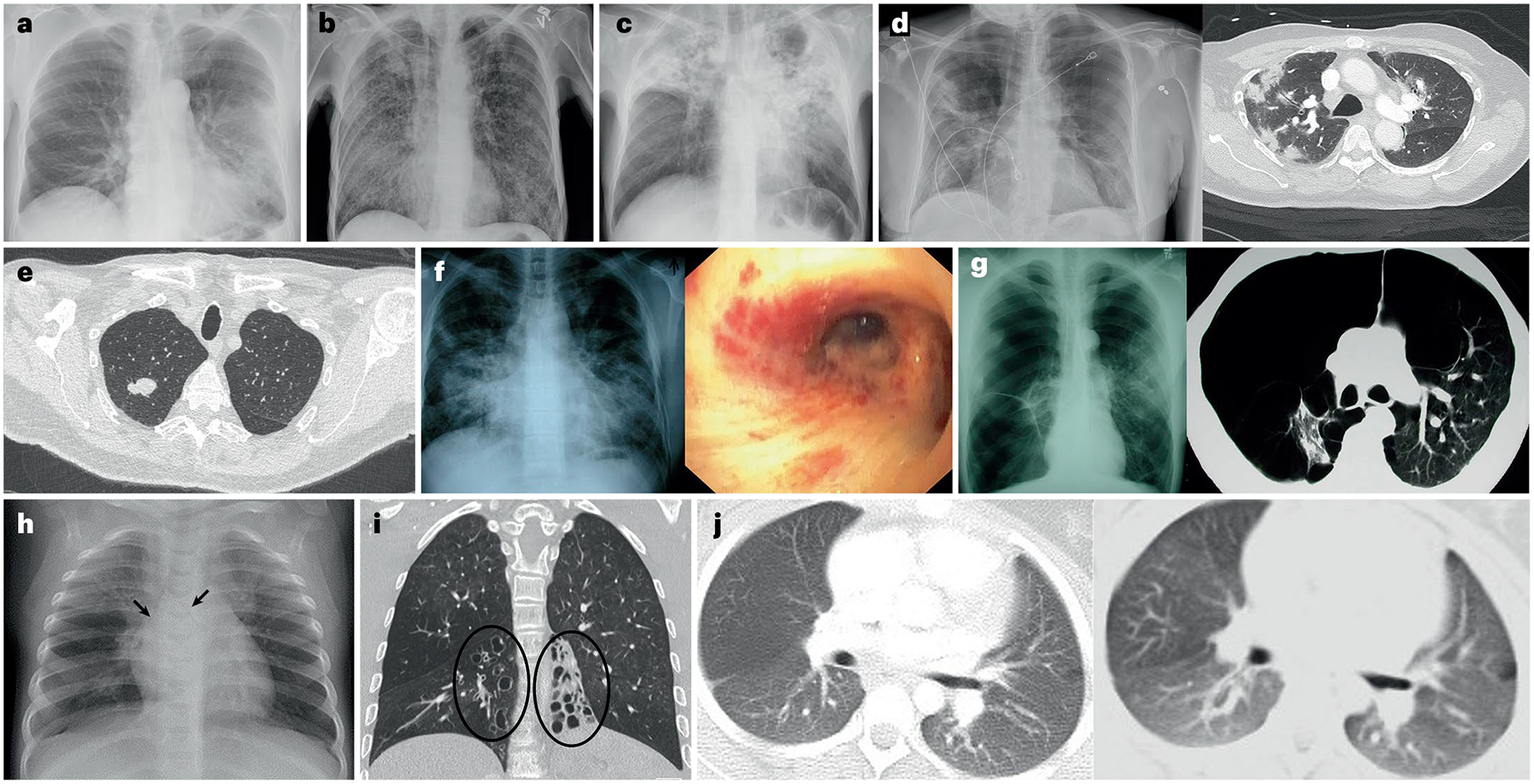
In adults, different manifestations include bacterial pneumonia (part a; caused by Streptococcus pneumoniae), Pneumocystis jirovecii pneumonia (part b), pulmonary tuberculosis (part c), COVID-19 pneumonia (part d), primary lung cancer (part e), Kaposi sarcoma (part f) and emphysema (part g). In children, manifestations include pulmonary tuberculosis (part h; arrows show bilateral compression of the bronchi from lymphadenopathy due to pulmonary tuberculosis), bronchiectasis (part i; ellipses) and bronchiolitis obliterans (part j). Parts i and j courtesy of A.-M. du Plessis.
The diagnosis of Pneumocystis is ideally made by visualizing cysts of trophozoites by microscopy after immunostaining of BALF, but is more often guided by either molecular detection of PCP DNA in an airway specimen or by detection of BDG in serum. Bacterial pneumonia and pulmonary TB are diagnosed by microscopy and culture of a specimen from the lower respiratory tract. While bacterial infections rarely are diagnosed by molecular methods, the diagnosis of pulmonary TB increasingly relies on rapid molecular detection, which is also able to detect rifampin resistance. Testing of urine for lipoarabinomannan might be useful to diagnose TB in severely immunocompromised individuals. CMV pneumonia remains a diagnostic challenge. Demonstration of CMV inclusion bodies in a lung tissue specimen is highly suggestive, while detection of CMV in serum, sputum or BALF by culture, PCR, immunohistochemistry or in situ hybridization might be suggestive in severely immunocompromised individuals. Respiratory viruses can be detected by molecular (DNA or RNA) or immunological testing (antigen) of either a nasopharyngeal or an oropharyngeal swab specimen. Broad diagnostic work-up is often indicated, as the presence of more than one pathogen is more common in immunosuppressed individuals.
The cornerstone of prevention of pneumonia is ART-induced immune recovery followed by smoking cessation and immunization against S. pneumoniae, influenza and SARS-CoV-2. Prophylaxis against PCP is indicated in individuals with a CD4+ T cell count <200/μl, a CD4+ T cell percentage <14% of total lymphocyte count, and a CD4+ T cell count in the range 200–300/μl if ART initiation is delayed and CD4+ T cell count monitoring is unfeasible, or following an episode of PCP. Prophylaxis continues until the CD4+ T cell count is >200/μl for at least 3 months while on ART18. Trimethoprim-sulfamethoxazole (TMP-SMX) is the recommended prophylactic drug. In individuals who cannot tolerate TMP-SMX, alternative regimens include dapsone, dapsone plus pyrimethamine plus leucovorin, aerosolized pentamidine, and atovaquone18. Individuals with latent TB can be treated with 12 weeks of once-weekly rifapentine plus isoniazid plus pyridoxine or daily rifampin plus isoniazid plus pyridoxine. Daily isoniazid plus pyridoxine for 6–9 months is an alternative regimen when drug–drug interactions between rifamycins and specific ART drugs limit the use of rifamycin-based regimens for latent TB18. Globally, the provision of TB-preventive treatment to people living with HIV has been a remarkable success, exceeding 2.5 million treatments annually19.
COPD.
The most common symptoms of COPD are dyspnoea (shortness of breath) on exertion and chronic bronchitis (classically defined as having cough and mucus production on most days for at least 3 months per year and for at least 2 years in a row). COPD symptoms in people living with HIV are similar to those in seronegative individuals, although the symptom burden in people living with HIV may be higher for the same degree of impairment in lung function48.
The high prevalence of COPD in people living with HIV has raised questions about optimal COPD screening and case-finding approaches in HIV clinics. The current diagnostic approach to COPD is the same in people with and without HIV (Fig. 4). The gold standard test for COPD diagnosis is spirometry, which is recommended in people with COPD symptoms and risk factors (such as tobacco smoking, biomass fuel burning exposure, history of TB, and HIV)189. However, screening for COPD with spirometry in adults at high risk who are asymptomatic is not recommended by organizations such as the US Preventive Services Task Force (USPSTF) and the Global Initiative for Chronic Obstructive Lung Disease189,190. Studies of COPD case-finding in people living with HIV have found very low spirometry completion rates of 30–50% in HICs191. In LMICs, where access to spirometry can be constrained, completion rates might be even lower, although we are unaware of COPD case-finding studies in this setting. Alternative COPD case-finding methods such as hand-held micro-spirometers are available and could be combined with newer COPD screening questionnaires, but these approaches require further study and validation before wide implementation192.
Prevention strategies in people living with HIV who smoke should focus on smoking cessation. Data demonstrate that people living with HIV who smoke are at higher risk for COPD74 and lose lung function faster than people living with HIV who do not smoke193. Among people living with HIV who do not smoke, COPD prevention measures should focus on optimizing indoor air quality and mitigating exposures to outdoor air pollution sources. In TB-endemic settings, public health efforts at TB control would also be expected to reduce the burden of COPD, given data that prior TB is associated with increased risk of COPD194. Further research is needed to identify effective HIV-specific COPD prevention strategies, targeting proposed mechanisms of COPD pathogenesis in people living with HIV, such as heightened inflammation, immune dysregulation and microbial dysbiosis.
Asthma.
Asthma is defined as a chronic disorder of the airways involving a complex interaction of variable and recurring respiratory symptoms, airflow obstruction, airway hyper-responsiveness and underlying inflammation195. The common symptoms of asthma include wheezing, dyspnoea, chest tightness and cough. These symptoms vary over time and in intensity. The physiological signs of asthma include variable expiratory airflow limitation, bronchodilator reversibility and airways hyper-responsiveness measured through spirometry testing. The diagnosis of asthma requires a history of asthma symptoms together with variable expiratory airflow limitation195. The diagnostic approach to asthma in people living with HIV is similar to that in the general population195.
Because the penetrance of symptoms and airflow abnormalities are variable in asthma, multiple definitions of asthma have been used in clinical studies196. Self-reported asthma has good agreement with physician diagnosis in reports from the general population, with 92% agreement and 94% specificity197. Studies of asthma and HIV typically define asthma using self-reported data, but incorporating respiratory symptoms, lung function and treatment for asthma is likely to provide a more robust approach to diagnosing asthma among people living with HIV. In the most recent analysis of the MACS/WIHS Combined Cohort Study, asthma prevalence was found to be twofold to threefold higher when using self-report than when using robust criteria9. While HIV was not associated with increased asthma prevalence, regardless of the definition used, there was increased respiratory burden among men with HIV and asthma than among seronegative men with asthma.
Lung cancer.
The presentation, radiographic findings and diagnosis of lung cancer are similar in people living with HIV to those in HIV-uninfected people (Fig. 4). Adenocarcinoma is the most frequent cancer type, followed by squamous cell carcinoma98,105. Lung cancer tends to be diagnosed at a younger age in people living with HIV than in the general population198,199. Considering demographic differences and access to care, stage of lung cancer at presentation is generally similar by HIV status, with approximately 70% of all patients diagnosed at stage III or stage IV in the absence of screening105,198,200. Bronchoscopic biopsy, lymph node sampling with endobronchial ultrasonography, and transthoracic needle aspiration or sampling of extra-pulmonary sites that may represent metastatic disease should be pursued to obtain a tissue diagnosis when feasible; genetic sequencing should be performed to identify targetable mutations that will affect the treatment regimen.
Smoking cessation is of primary importance in decreasing the risk of lung cancer among people living with HIV, although sustained smoking cessation is difficult to achieve despite investigations combining behavioural and pharmacological cessation therapies. Although less than optimal, harm reduction may also be a beneficial goal201. In the context of smoking cessation, harm reduction is defined as decreasing the negative effects of smoking without complete cessation. In people living with HIV, this can be most safely achieved by reducing the daily number of cigarettes smoked, as the safety and efficacy of substituting alternative forms of tobacco and nicotine products, including smokeless tobacco and electronic cigarettes, respectively, for harm reduction has not been extensively studied in this population.
Eligible people living with HIV should be offered lung cancer screening. In the National Lung Cancer Screening Trial, individuals with well-controlled HIV derived a similar mortality benefit from lung cancer screening as older, HIV-uninfected people with a heavy smoking history202,203. There are no lung cancer screening recommendations tailored for people living with HIV despite their high risk of lung cancer. In general, the USPSTF recommends screening for lung cancer annually with low-dose CT in adults at high risk (that is, smokers) aged 50–80 years who have a smoking history of ≥20 pack-years and currently smoke or formerly smoked but quit within the past 15 years204. An analysis of MACS/WIHS Combined Cohort Study data that investigated the application of the 2021 USPSTF screening recommendations to people living with HIV found that these lung cancer screening criteria would have detected lung cancer in only 44% of women with HIV and 63% of men with HIV205. These findings also suggest that decreasing the age and pack-year history eligibility requirements would increase the sensitivity and detect additional lung cancer cases in women; further work is needed to understand how to tailor lung cancer screening eligibility for people living with HIV to maximize benefits and minimize harms.
Children
Infections.
PCP in infants and children is usually acute with rapidly progressive hypoxia, in contrast to its more subacute and indolent course in adults. Compared with adults, the signs and symptoms of bacterial and viral pneumonia in infants and children can be more acute, and cough is frequently non-productive. Key signs are age-specific tachypnoea (rapid breathing) and lower chest indrawing. Pneumonia, especially severe disease, is frequently due to mixed infections, and clinical signs are unreliable for distinguishing bacterial from viral illness. TB in children may similarly present with acute illness (Fig. 4). In infants and young children, a sample from the lower respiratory tract can be obtained using sputum induction. Molecular testing or culture methods are similar to those used in adults.
Effective specific preventive options include immunization with PCV and H. influenzae type b according to national immunization schedules. Current WHO recommendations are to provide a booster PCV dose in the second year in HIV-infected children who received their three-dose primary PCV series in infancy206. The use of effective ART is crucial to prevent opportunistic infections and optimize health. Long-term prophylaxis with co-trimoxazole (a combination of trimethoprim and sulfamethoxazole) is recommended in settings with a high burden of malaria or bacterial infections206. Prophylaxis can be stopped in children older than 5 years who are clinically stable, established on ART and virally suppressed in other settings206. Primary isoniazid prophylaxis in areas of high TB prevalence might reduce mortality and TB incidence207 and WHO recommends its use in these areas in children older than 1 year for up to 3 years208. The Bacillus Calmette–Guérin (BCG) vaccine should be given to neonates born to women of unknown HIV status and to neonates with unknown HIV status born to HIV-infected women if they have no suggestion of HIV infection, as the benefits of BCG vaccination outweigh the risks208. In neonates with confirmed HIV infection, BCG should be delayed until ART has been instituted and the infant is clinically and immunologically stable208.
Chronic lung disease.
Chronic respiratory disease is also common among children and adolescents living with HIV. Symptoms include chronic cough, dyspnoea, reduced exercise tolerance, increased work of breathing and impaired lung function. The spectrum of chronic lung disease includes bronchiolitis obliterans and bronchiectasis, chronic infection, lymphocytic interstitial pneumonitis (LIP), asthma, immune reconstitution inflammatory syndrome (IRIS) and malignancy115 (Fig. 4). Before the widespread use of ART, chronic lung disease was dominated by LIP and chronic or recurrent infections with bronchiectasis. In children stable on ART, high-resolution CT scans indicate that bronchiolitis obliterans or bronchiectasis predominate183,185; these conditions are associated with prior TB or pneumonia.
Impairment in lung function has been found in children living with HIV, especially in those with delayed access to ART116. However, in a study of perinatally HIV-infected South African adolescents diagnosed in early childhood and stable on ART for several years, mild lung function impairment occurred in approximately 25%209. Lung function abnormalities included reductions in spirometry that were not bronchodilator-responsive, greater respiratory system resistance, and decreased compliance and alveolar gas diffusion compared with lung function in healthy age-matched controls209. Lung function impairment was associated with previous severe lower respiratory tract illness or pulmonary TB. Reassuringly, a small study found that if ART was initiated in infancy, most children had normal spirometry by school age200.
Management
Adults
Infections.
The treatment of choice for PCP is trimethoprim plus sulfamethoxazole210. Alternative regimens include pentamidine and the combination of clindamycin and primaquine. Adjunctive corticosteroid therapy improves outcomes in severe PCP. Treatment of community-acquired pneumonia and TB follows general recommendations. For community-acquired pneumonia, recommendations for empiric treatment depend on the severity of illness and usually include a β-lactam antimicrobial plus a macrolide or doxycycline, or a fluoroquinolone. It is recommended that antimicrobials are narrowed according to the causative pathogen and antimicrobial susceptibility pattern, if these data are available18. Treatment of drug-susceptible pulmonary TB is with a four-drug regimen using a rifamycin (rifampin, rifabutin or rifapentine) and isoniazid for 6–9 months together with pyrazinamide plus ethambutol for the first 2 months18,211. A trial found that a 4-month regimen of isoniazid, rifapentine, ethambutol and moxifloxacin was non-inferior to a standard 6-month regimen212. Drug–drug interactions between rifamycins and specific ART drugs should be considered in selection and dosing (Box 2). Influenza is treated with oseltamivir or baloxavir. People living with HIV with COVID-19 might be considered at risk of progression to severe COVID-19, particularly if they are unvaccinated or older. Treatment strategies to reduce this risk include nirmatrelvir and ritonavir, remdesivir and molnupiravir. Treatment for severe COVID-19 consists of remdesivir and dexamethasone with the addition of anti-IL-6 monoclonal antibodies and baricitinib, depending on disease severity. Overall, treatment of COVID-19 in people living with HIV is the same as in the general population, with awareness of drug–drug interactions and overlapping toxicities between COVID-19 treatments and ART drugs210.
Box 2. Special considerations for management of HIV-associated lung diseases.
Infections
Be aware of potential increased risk of progression to severe COVID-19 pneumonia
Consider drug–drug interactions and overlapping toxicities between COVID-19 and TB treatments and ART
COPD
Employ more intensive smoking cessation interventions given low quit rates among people living with HIV
Consider risk of ICS use given potential for bacterial pneumonia and TB risk among people living with HIV
Monitor for hypercortisolism with co-administration of ICSs and CYP3A4 inhibitors (for example, ritonavir and cobicistat)
Asthma
Use beclomethasone as preferred ICS among people living with HIV using protease inhibitors
Avoid budesonide, ciclesonide, fluticasone, mometasone, betamethasone, and budesonide in individuals receiving protease inhibitors
Avoid salmeterol in individuals receiving protease inhibitors or elvitegravir/cobicistat
Consider total IgE and testing for environmental sensitivities among people living with HIV
Lung cancer
Be aware of increased risk of opportunistic infections in people living with HIV with lung cancer
Monitor for increased HIV viral load with use of immune checkpoint inhibitors
Follow lung cancer screening guidelines for the general population
Children
Start TB treatment before ART initiation 2 weeks later in ART-naive children living with HIV and TB
Consider weekly azithromycin in adolescents with HIV-associated chronic lung disease
Screen children for TB and CMV before ART initiation given the risk of IRIS
Consider alternative diagnosis to asthma in children living with HIV (for example, bronchiolitis obliterans, endobronchial obstruction due to TB, or HIV-associated airway inflammation)
ART, antiretroviral therapy; CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease; CYP34A, cytochrome P450 3A4; ICS, inhaled corticosteroid; IRIS, immune reconstitution inflammatory syndrome; TB, tuberculosis.
COPD.
The goals of therapy for stable COPD are to improve survival, reduce symptoms, improve quality of life and functional status, and reduce the frequency and severity of exacerbations. The cornerstones of management are: smoking cessation; influenza, pneumococcal and SARS-CoV-2 vaccinations; pharmacotherapies; oxygen supplementation and non-invasive positive pressure ventilation in appropriately selected patients; and pulmonary rehabilitation189. Compared with HIV-uninfected smokers, people living with HIV who smoke are just as likely or more likely than HIV-uninfected people to initiate cessation interventions213. However, people living with HIV have low smoking quit rates with the usual behavioural and pharmacological interventions employed in HIV-uninfected people214. Promising smoking cessation strategies in people living with HIV include mobile health approaches215 and clinic-level interventions rather than only provider-level or patient-level interventions216.
Pharmacological therapies for COPD consist of inhaled bronchodilators (short-acting β2 agonists (SABAs), long-acting β2 agonists (LABAs), short-acting muscarinic antagonists (SAMAs) and long-acting muscarinic antagonists (LAMAs)), inhaled corticosteroids (ICSs), and combinations of these therapies (SABA/SAMA, LABA/LAMA, LABA/ICS, LABA/LAMA/ICS). Changes in the most recent COPD guidelines focus on the use of long-acting bronchodilators, with minimization of ICS use unless patients have asthma features or peripheral eosinophilia, which are predictors of more favourable responses to ICSs189,217,218. ICS use in COPD is associated with an increased risk of pneumonia of approximately 40%219 and possibly an increased risk of TB220. Given the underlying impact of HIV infection on pneumonia and TB risk, preventing further increases in pneumonia and TB risk from unnecessary ICS use in people living with HIV with COPD is clinically important (Box 2). ICSs also have well-described interactions with ART regimens that contain CYP3A4 inhibitors (such as ritonavir and cobicistat) that can lead to hypercortisolism. Due to the narrow indications for ICS use in COPD and the special risks of ICS use in people living with HIV with COPD, providers are encouraged to only prescribe ICSs in people living with HIV with COPD with asthma features or peripheral eosinophilia and to consider ICS de-escalation when these individuals do not meet these criteria and are clinically stable221,222. In people living with HIV who require both ICSs and CYP3A4 inhibitors, inhaled beclomethasone dipropionate is the preferred ICS due to minimal interactions with CYP3A4 inhibitors223.
Asthma.
The management of asthma among people living with HIV aligns with the published guidelines for the general population195, although specific considerations are necessary (Box 2). The main-stay of therapy consists of step-up treatment starting with either as-needed low-dose ICS and formoterol or as-needed ICS and SABA. With inadequate symptom control, treatment should be increased to regular ICS combined with a LABA. For severe disease, the addition of a LAMA and referral to a specialist are appropriate. Given the potential effect of HIV on atopy, obtaining serological testing for IgE and environmental sensitivities might be informative in individuals with uncontrolled asthma. Potential drug–drug interactions should be considered as described above. Current HIV treatment guidelines recommend against co-administration of the ICSs budesonide, ciclesonide, fluticasone, mometasone, betamethasone, and budesonide in individuals receiving protease inhibitors224. Beclomethasone can be used as an alternative ICS in this situation. The effects of the LABA salmeterol may be potentiated in people living with HIV who are on regimens that include a protease inhibitor or elvitegravir/cobicistat, raising concerns for QTc prolongation; no clinically significant drug–drug interactions have been reported for other LABAs. There are no reports of interactions between HIV-specific therapies and LAMAs.
Lung cancer.
Treatment of lung cancer in people living with HIV is generally similar to that in HIV-uninfected people. The risk of poor short-term outcomes following lung cancer surgery is similar in people living with HIV and in uninfected people, with pneumonia being the most common complication in both groups225. People living with HIV and malignancy have an increased risk of opportunistic infections, although the risk is similar in those with a CD4+ T cell count >200/μl and suppressed HIV viral load to that in uninfected people226 (Box 2). In terms of immunotherapies, large-scale studies on the safety and efficacy of immune checkpoint inhibitors (ICIs) among people living with HIV are limited. However, initial data suggest benefits and acceptable adverse effect profiles227. People living with HIV who are treated with ICIs might be more likely to have an increase in HIV viral load in the year after treatment, so close monitoring is warranted228. The effect of HIV infection on survival in patients with lung cancer is inconsistent, with some studies showing increased mortality in people living with HIV and others demonstrating similar outcomes198,200. Among people living with HIV, additional risk factors, such as a history of AIDS-defining illness at or before lung cancer diagnosis, can decrease survival229.
Children
Infections.
Case management guidelines for pneumonia, as described in the WHO Integrated Management of Childhood Illness programme, are associated with decreased pneumonia and all-cause mortality230 but should be adapted in areas with high HIV prevalence231. These management guidelines include ampicillin and gentamicin or ceftriaxone as first-line therapy for severe pneumonia; co-trimoxazole should be added in infants. Empiric therapy for TB traditionally included four drugs (isoniazid, rifampin or rifabutin, pyrazinamide and ethambutol) daily for 2 months followed by a minimum of 4 months of daily isoniazid and rifampin (or rifabutin), but the SHINE trial found that for non-severe TB, 4 months of treatment was non-inferior to 6 months232. Revised WHO guidelines now recommend 4 months of treatment for non-severe TB, depending on the degree of immunosuppression and ART status, and provided that there is no suspicion or evidence of multidrug-resistant or rifampin-resistant TB208. Adjunctive corticosteroids can be used for bronchial obstruction. Adjustment of ART and TB therapy might be needed to provide optimal therapy and minimize toxicity and drug interactions (Box 2). If TB is diagnosed in a child who has not yet started ART, TB treatment should be started first, followed by ART within 2 weeks to reduce the risk of IRIS. Co-trimoxazole is the treatment of choice for PCP. In severe PCP, corticosteroids might be beneficial224. Ganciclovir should be used for treatment of CMV pneumonia, with a switch to oral valganciclovir with clinical improvement (or in older children or those with mild illness)224. According to WHO recommendations, TB preventive treatment should be given to all children and adolescents who are household contacts of people with bacteriologically confirmed pulmonary TB and after TB disease has been excluded208. Options include 6 or 9 months of daily isoniazid, 3 months of weekly rifapentine plus isoniazid, 3 months of daily rifampin plus isoniazid, 1 month of daily rifapentine plus isoniazid, or 4 months of daily rifampin208. Children aged ≥12 months living with HIV who are unlikely to have TB disease should be offered TB preventive treatment as part of a comprehensive package of HIV prevention and care if they live in a setting with high TB transmission, regardless of contact with TB208. In high TB transmission settings, adolescents living with HIV who have an unknown or a positive TB infection test and are unlikely to have TB disease should receive at least 36 months of daily isoniazid preventive therapy, regardless of ART status and degree of immunosuppression208.
Chronic lung disease.
Management includes ART, optimizing nutrition, vaccination against respiratory pathogens (including PCV and influenza), avoidance of exposure to tobacco smoke and air pollution, regular screening and prophylaxis for TB in endemic areas, and airway clearance techniques. Intercurrent infective exacerbations should be treated early. Azithromycin given weekly decreases acute respiratory exacerbations in adolescents with HIV-associated chronic lung disease but does not improve lung function233. Treatment of LIP includes ART, inhaled bronchodilators and oxygen for hypoxaemia. Although treatment of LIP with oral corticosteroids might improve hypoxaemia234, there are no controlled clinical trials. To minimize the risk of IRIS, children should be carefully screened for TB and CMV before ART initiation. TB treatment should be initiated first followed by ART as soon as possible and within 2 weeks. Oral corticosteroids might be beneficial in ameliorating IRIS, but there are no controlled trials in children233. Asthma should be treated with inhaled bronchodilators and ICSs, as for HIV-uninfected children, depending on phenotype and severity, with the same considerations for potential drug–drug interactions as described above. However, bronchodilator responsiveness should be evaluated, as wheezing may be due to bronchiolitis obliterans, endobronchial obstruction due to TB, or HIV-associated airway inflammation, none of which may be responsive to bronchodilators.
Quality of life
Respiratory-related quality of life is frequently suboptimal in people living with HIV, with many studies investigating respiratory symptoms such as cough and breathlessness. The presence of respiratory symptoms in people living with HIV is associated with more depressive symptoms and worse quality of life scores235, highlighting the clinical relevance of these symptoms. A systematic review and meta-analysis of 24 publications found that, compared with HIV-uninfected controls, people living with HIV were more likely to report cough and breathlessness, in both resource-limited and resource-rich settings236. The difference in symptom burden seemed larger in people living with HIV who did not have access to ART compared with those with ART access, suggesting that ART might improve respiratory symptoms in people living with HIV. Potential benefits of ART on respiratory symptoms are also suggested by data showing that lower nadir CD4+ T cell counts are associated with worse self-reported respiratory health status91, along with results from the pulmonary substudy of the randomized START trial in which immediate ART initiation in early HIV was found to result in better respiratory symptoms than deferred ART initiation, but only in current smokers237. However, an analysis of men living with HIV showed that protease inhibitor use was associated with worse dyspnoea than the use of other classes of ART238. Ultimately, the effect of ART and specific ART regimens on respiratory symptoms currently remains unclear and requires further study.
In addition to self-reported symptoms, another method to assess respiratory health is functional testing such as the 6-min walk distance (6MWD) test. Two studies that measured 6MWD in both people living with HIV and in HIV-uninfected people found worse 6MWD in people living with HIV than in controls239,240. Both studies found no relationship between 6MWD and HIV variables such as CD4+ T cell count, HIV viral load or ART use, suggesting that other non-viral mechanisms might be responsible for the impaired physical function seen in people living with HIV. Factors that have been associated with worse 6MWD in people living with HIV include respiratory disease markers such as impaired lung function240,241 and increased radiographic emphysema242, as well as non-respiratory factors such as older age, higher BMI and cigarette smoking239,241. 6MWD can also be affected by heart diseases such as heart failure, which is more common in people living with HIV than in HIV-uninfected people243, but studies are lacking more comprehensive and concurrent assessments of 6MWD with measures of lung function, heart function and other factors that can affect 6MWD, such as sarcopenia, peripheral vascular disease and metabolic derangements.
Outlook
Unless an effective vaccine against HIV is developed and until there is a cure for people already infected with HIV, the estimated 38 million people living with HIV and those who will become HIV infected worldwide will continue to experience substantial morbidity and mortality from lung diseases. The tremendous success of ART in reducing new HIV transmission, HIV-associated opportunistic infections and malignancies, and AIDS-associated mortality has altered the epidemiology of AIDS and led to an ageing population of people living with HIV and the dominance of age-associated and non-communicable pulmonary conditions such as COPD. Similarly, the prevalence of chronic lung disease has increased in children and adolescents living with HIV. The long-term consequences of childhood respiratory infections, HIV-associated opportunistic pneumonias and chronic lung disease in these individuals as they transition into adulthood and especially late adulthood remain unknown. The ongoing scourges of inhaled tobacco products, illicit drug use, and air pollution, both indoor and outdoor, will continue to pose multiple threats to lung health worldwide. Thus, redoubled multifaceted efforts at tobacco and drug use cessation and improved air quality are crucial to mitigate additional morbidity and mortality. Given the impact of these scourges on cardiovascular disease and health in general, these efforts will have benefits beyond enhanced respiratory health. In addition, new dangers from pathogens both recent (for example, SARS-CoV-2) and centuries-old (for example, M. tuberculosis) and from emerging perils from global climate change will add to these enduring threats in unpredictable but ominous ways. Humanitarian crises from global pandemics, climate change and human conflict bring with them the potential to overwhelm our public health and medical systems and divert attention and essential resources away from vulnerable populations such as those living with HIV. As such, continued investigation into the effects of HIV on the lung is essential to expand our knowledge base and to ensure continued provision of the best clinical care to these millions of individuals (Box 3).
Box 3. Future directions for research in HIV-associated lung disease.
Elucidate the mechanistic role of perturbed innate and adaptive immunity, local and systemic inflammation, imbalance of lung proteases and antiproteases, endothelial activation, oxidative stress and accelerated cell senescence in the pathogenesis of HIV-associated lung disease
Examine the impact of air pollution, electronic cigarettes, and inhaled illicit drugs on the development of chronic lung disease
Assess the longitudinal disease course of HIV-associated chronic lung diseases
Expand the evidence basis regarding prevalence of HIV-associated chronic lung diseases in low-income and middle-income countries
Develop and deploy rapid, point-of-care tests to accurately detect HIV-associated respiratory pathogens
Improve diagnostics for obstructive lung diseases that can be readily deployed in low-income and middle-income countries
Develop HIV-specific screening strategies for chronic lung diseases
Test interventions to prevent and treat chronic lung disease in people living with HIV at the patient, provider and systems levels
Examine the impact of HIV infection on functional outcomes, exacerbations and mortality of chronic lung disease
Evaluate biomarkers to predict lung disease development and outcomes in people living with HIV
As detailed above, there have been considerable advances in our understanding of the often synergistic and deleterious effects of HIV infection and tobacco use on the lung. However, the chronic effects of the inhalation from electronic cigarettes (e-cigarettes) on the lungs of people living with HIV are unknown. In addition, although a wide range of acute pulmonary complications of illicit drugs that are injected or inhaled has been described previously244, comparatively less is known about the long-term complications from chronic use of illicit drugs. With the longer life expectancy among people living with HIV, studying the chronic pulmonary complications of illicit drugs is needed. However, as the specific drugs that are used will undoubtedly change over time, flexibility to pivot and study new illicit drugs of choice will also be required.
Substantial advances have been made in the diagnosis of HIV-associated opportunistic pneumonias, especially pneumonia associated with M. tuberculosis, the major pulmonary pathogen in people living with HIV worldwide. Currently, the WHO recommends rapid molecular tests as the initial diagnostic test for suspected TB, supplanting smear microscopy from expectorated sputum. However, the requirement for an adequate sputum sample remains an important challenge to TB diagnosis in young children and infants and the continued study of alternative, non-sputum samples for diagnosis of TB is critical. Although molecular tests have been developed for respiratory viral and bacterial pathogens, current guidelines from the American Thoracic Society and the Infectious Diseases Society of America continue to recommend sputum Gram stain and culture and blood cultures as the initial diagnostic tests for inpatients hospitalized with suspected community-acquired pneumonia245. These TB and community-acquired pneumonia guidelines also apply to people living with HIV224. The development, validation and widespread clinical application of rapid, point-of-care tests that can accurately detect a panel of the most frequent HIV-associated opportunistic pathogens, using readily collected samples and deployed in a range of clinical settings throughout the world, remains an aspirational goal as it would be a game-changing addition to our clinical toolbox.
Compared to advances in the diagnosis of the major HIV-associated opportunistic pneumonias (especially TB), diagnosis of the major HIV-associated non-communicable lung diseases, including but not limited to COPD and asthma, urgently needs similar advances. Spirometry is used to diagnose these obstructive lung diseases; this lung function test can be performed in medical (that is, hospital and clinic) settings, and portable equipment allows its clinical application in community settings, including in LMIC and resource-limited settings where the majority of people living with HIV reside. However, the most frequent lung function abnormality in people living with HIV is a reduction in the diffusing capacity of the lungs for carbon monoxide (DLCO). This test requires specialized equipment and a source of 0.3% carbon monoxide, and therefore is unavailable in community settings and is largely unavailable in LMIC settings. The presence of abnormal DLCO with normal spirometry (“iso↓DLCO”) is a particularly novel, HIV-specific lung function phenotype246. Until these equipment and gas mixture limitations can be overcome, further advances in our understanding of this important, potentially HIV-specific lung function abnormality and its prevalence worldwide will be hampered. In addition, improved diagnosis and treatment of TB has led to an increasing appreciation for the substantial burden of post-TB lung disease and its impact in people living with HIV.
As noted, there are no HIV-specific guidelines for screening for non-communicable lung diseases, such as COPD and lung cancer, which are seen at increased frequency in people living with HIV. For COPD, alternative case-finding approaches and screening questionnaires described above require further study and validation. Moreover, given the results of two large randomized clinical trials that demonstrated that screening with low-dose chest CT reduces lung cancer mortality in high-risk patients202,247, lung cancer screening should be undertaken in people living with HIV who meet USPSTF guideline criteria, particularly those with well-controlled HIV; additional guidance is needed to determine whether screening regimens or eligibility criteria should be tailored further for people living with HIV.
With the exception of PCP, there are limited data from large randomized controlled trials to guide the treatment of the major HIV-associated opportunistic pneumonias. Given the decreased incidence of these opportunistic pneumonias, it is unlikely that there will ever be such an evidence base from randomized controlled trials. There is an even greater paucity of data to guide management of COPD and asthma in people living with HIV. Instead, the findings of studies in people without HIV have been extrapolated to those living with HIV, in general successfully. The development of additional, potent ICSs — for use either as single medications or in combination with long-acting bronchodilators — which can be co-administered with current antiretroviral medications would be an important addition to the medical management of people with these obstructive lung diseases who benefit from this therapy.
Tremendous advances have been made in our understanding of HIV’s effects on the lung and in the diagnosis, screening and prevention, and the treatment and management of lung diseases in people living with HIV over the past >40 years, but major gaps remain. The magnitude of HIV/AIDS worldwide and the current absence of a vaccine against HIV and a cure for those already infected with HIV demands continued clinical focus and research in order to ensure provision of the best clinical care.
Footnotes
Competing interests
K.M.K. reports personal fees for Data and Safety Monitoring Board activities (Nuvaira, Organicell) and consulting (Allergan/AbbVie) outside the published work. T.B. reports grants (Novo Nordisk Foundation, Lundbeck Foundation, Simonsen Foundation, GSK, Pfizer, Gilead, Kai Hansen Foundation and Erik and Susanna Olesen’s Charitable Fund) and personal fees (GSK, Pfizer, Bavarian Nordic, Boehringer Ingelheim, Gilead, MSD, Pentabase ApS, Becton Dickinson, Janssen and Astra Zeneca) outside the published work. The other authors declare no competing interests.
Peer review information Nature Reviews Disease Primers thanks K. A. Norris, M. C. I. Lipman, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Disclaimer The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Masur H et al. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N. Engl. J. Med 305, 1431–1438 (1981). [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med 338, 853–860 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Weverling GJ et al. Discontinuation of Pneumocystis carinii pneumonia prophylaxis after start of highly active antiretroviral therapy in HIV-1 infection. Lancet 353, 1293–1298 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Gingo MR et al. The impact of HAART on the respiratory complications of HIV infection: longitudinal trends in the MACS and WIHS cohorts. PLoS ONE 8, e58812 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segal LN et al. HIV-1 and bacterial pneumonia in the era of antiretroviral therapy. Proc. Am. Thorac. Soc 8, 282–287 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SeyedAlinaghi S et al. COVID-19 mortality in patients with immunodeficiency and its predictors: a systematic review. Eur. J. Med. Res 27, 195 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gingo MR, Morris A & Crothers K Human immunodeficiency virus-associated obstructive lung diseases. Clin. Chest Med 34, 273–282 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gingo MR et al. Asthma diagnosis and airway bronchodilator response in HIV-infected patients. J. Allergy Clin. Immunol 129, 708–714.e8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drummond MB et al. Association between HIV and prevalence and manifestations of asthma: analysis of the Multicenter AIDS Cohort Study and Women’s Interagency HIV Study. J. Acquir. Immune Defic. Syndr 10.1097/QAI.0000000000003088 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. World Health Statistics 2022: Monitoring Health for the SDGs, Sustainable Development Goals (WHO, 2022). [Google Scholar]
- 11.Crothers K et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am. J. Respir. Crit. Care Med 183, 388–395 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phair J et al. The risk of Pneumocystis carinii pneumonia among men infected with human immunodeficiency virus type 1. N. Engl. J. Med 322, 161–165 (1990). [DOI] [PubMed] [Google Scholar]
- 13.Stansell JD et al. Predictors of Pneumocystis carinii pneumonia in HIV-infected persons. Pulmonary complications of HIV Infection Study Group. Am. J. Respir. Crit. Care Med 155, 60–66 (1997). [DOI] [PubMed] [Google Scholar]
- 14.Horsburgh CR Priorities for the treatment of latent tuberculosis infection in the United States. N. Engl. J. Med 350, 2060–2067 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Andrews JR et al. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin. Infect. Dis 54, 784–791 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sloot RS, van der Loeff MF, Kouw PM & Borgdorff MW Risk of tuberculosis after recent exposure. A 10-year follow-up study of contacts in Amsterdam. Am. J. Respir. Crit. Care Med 190, 1044–1052 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Menzies NA et al. Time since infection and risks of future disease for individuals with Mycobacterium tuberculosis infection in the United States. Epidemiology 32, 70–78 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. Clinical Info HIV.gov https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection (2023). [PubMed] [Google Scholar]
- 19.World Health Organization. Global tuberculosis report 2022. WHO https://www.who.int/publications/i/item/9789240061729 (2022). [Google Scholar]
- 20.Hirschtick RE et al. Bacterial pneumonia in persons infected with the human immunodeficiency virus. N. Engl. J. Med 333, 845–851 (1995). [DOI] [PubMed] [Google Scholar]
- 21.Kohli R et al. Bacterial pneumonia, HIV therapy, and disease progression among HIV-infected women in the HIV Epidemiologic Research (HER) study. Clin. Infect. Dis 43, 90–98 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Balakrishna S et al. Decreasing incidence and determinants of bacterial pneumonia in people with HIV: the Swiss HIV Cohort Study. J. Infect. Dis 10.1093/infdis/jiab573 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Mussini C et al. Incidence, timing, and determinants of bacterial pneumonia among HIV-infected patients: data from the ICONA Foundation Cohort. J. Acquir. Immune Defic. Syndr 63, 339–345 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Sullivan JH, Moore RD, Keruly JC & Chaisson RE Effect of antiretroviral therapy on the incidence of bacterial pneumonia in patients with advanced HIV infection. Am. J. Respir. Crit. Care Med 162, 64–67 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Bénard A et al. Bacterial pneumonia among HIV-infected patients: decreased risk after tobacco smoking cessation. ANRS CO3 Aquitaine Cohort, 2000–2007. PLoS ONE 5, e8896 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan JE, Hanson DL, Navin TR & Jones JL Risk factors for primary Pneumocystis carinii pneumonia in human immunodeficiency virus-infected adolescents and adults in the United States: reassessment of indications for chemoprophylaxis. J. Infect. Dis 178, 1126–1132 (1998). [DOI] [PubMed] [Google Scholar]
- 27.Wood R, Maartens G & Lombard CJ Risk factors for developing tuberculosis in HIV-1-infected adults from communities with a low or very high incidence of tuberculosis. J. Acquir. Immune Defic. Syndr 23, 75–80 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Girardi E et al. Impact of combination antiretroviral therapy on the risk of tuberculosis among persons with HIV infection. AIDS 14, 1985–1991 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Girardi E et al. Incidence of tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin. Infect. Dis 41, 1772–1782 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Severe P et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N. Engl. J. Med 363, 257–265 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badri M, Wilson D & Wood R Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet 359, 2059–2064 (2002). [DOI] [PubMed] [Google Scholar]
- 32.del Amo J et al. Impact of antiretroviral therapy on tuberculosis incidence among HIV-positive patients in high-income countries. Clin. Infect. Dis 54, 1364–1372 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordin FM et al. Pneumonia in HIV-infected persons: increased risk with cigarette smoking and treatment interruption. Am. J. Respir. Crit. Care Med 178, 630–636 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mdodo R et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann. Intern. Med 162, 335–344 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Mdege ND, Shah S, Ayo-Yusuf OA, Hakim J & Siddiqi K Tobacco use among people living with HIV: analysis of data from demographic and health surveys from 28 low-income and middle-income countries. Lancet Glob. Health 5, e578–e592 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pacek LR & Crum RM A review of the literature concerning HIV and cigarette smoking: morbidity and mortality, associations with individual- and social-level characteristics, and smoking cessation efforts. Addict. Res. Theory 23, 10–23 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Helleberg M et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin. Infect. Dis 56, 727–734 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Cornelius ME, Wang TW, Jamal A, Loretan CG & Neff LJ Tobacco product use among adults – United States, 2019. Morb. Mortal. Wkly Rep 69, 1736–1742 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asfar T et al. National estimates of prevalence, time-trend, and correlates of smoking in US people living with HIV (NHANES 1999–2016). Nicotine Tob. Res 23, 1308–1317 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy JD, Liu B & Parascandola M Smoking and HIV in sub-Saharan Africa: a 25-country analysis of the demographic health surveys. Nicotine Tob. Res 21, 1093–1102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bigna JJ, Kenne AM, Asangbeh SL & Sibetcheu AT Prevalence of chronic obstructive pulmonary disease in the global population with HIV: a systematic review and meta-analysis. Lancet Glob. Health 6, e193–e202 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Brown J et al. Respiratory health status is impaired in UK HIV-positive adults with virologically suppressed HIV infection. HIV Med. 18, 604–612 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Madeddu G et al. Chronic obstructive pulmonary disease: an emerging comorbidity in HIV-infected patients in the HAART era? Infection 41, 347–353 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Ronit A et al. Airflow limitation in people living with HIV and matched uninfected controls. Thorax 73, 431–438 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Makinson A et al. HIV is associated with airway obstruction: a matched controlled study. AIDS 32, 227–232 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Verboeket SO et al. Reduced forced vital capacity among human immunodeficiency virus-infected middle-aged individuals. J. Infect. Dis 219, 1274–1284 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Cui Q et al. Effect of smoking on lung function, respiratory symptoms and respiratory diseases amongst HIV-positive subjects: a cross-sectional study. AIDS Res. Ther 7, 6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crothers K et al. HIV infection is associated with reduced pulmonary diffusing capacity. J. Acquir. Immune Defic. Syndr 64, 271–278 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fitzpatrick ME et al. Novel relationships of markers of monocyte activation and endothelial dysfunction with pulmonary dysfunction in HIV-infected persons. AIDS 30, 1327–1339 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang RJ et al. Lung function in women with and without HIV. Clin. Infect. Dis 10.1093/cid/ciac391 (2022). [DOI] [Google Scholar]
- 51.Kunisaki KM et al. Lung function in men with and without HIV. AIDS 34, 1227–1235 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura H et al. The prevalence of airway obstruction among Japanese HIV-positive male patients compared with general population; a case-control study of single center analysis. J. Infect. Chemother 20, 361–364 (2014). [DOI] [PubMed] [Google Scholar]
- 53.van den Berg OE et al. The influence of HIV infection and antiretroviral treatment on pulmonary function in individuals in an urban setting in sub-Saharan Africa. South. Afr. J. HIV Med 22, 1312 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varkila MRJ et al. The association between HIV infection and pulmonary function in a rural African population. PLoS ONE 14, e0210573 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zifodya JS et al. HIV, pulmonary infections, and risk of chronic lung disease among Kenyan adults. Ann. Am. Thorac. Soc 18, 2090–2093 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pefura-Yone EW, Fodjeu G, Kengne AP, Roche N & Kuaban C Prevalence and determinants of chronic obstructive pulmonary disease in HIV infected patients in an African country with low level of tobacco smoking. Respir. Med 109, 247–254 (2015). [DOI] [PubMed] [Google Scholar]
- 57.van Gemert F et al. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): a prospective cross-sectional observational study. Lancet Glob. Health 3, e44–e51 (2015). [DOI] [PubMed] [Google Scholar]
- 58.North CM et al. HIV infection, pulmonary tuberculosis, and COPD in rural Uganda: a cross-sectional study. Lung 196, 49–57 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Risso K et al. COPD in HIV-infected patients: CD4 cell count highly correlated. PLoS ONE 12, e0169359 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sampériz G et al. Prevalence of and risk factors for pulmonary abnormalities in HIV-infected patients treated with antiretroviral therapy. HIV Med. 15, 321–329 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Costiniuk CT et al. Prevalence and predictors of airflow obstruction in an HIV tertiary care clinic in Montreal, Canada: a cross-sectional study. HIV Med. 20, 192–201 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gingo MR et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am. J. Respir. Crit. Care Med 182, 790–796 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drummond MB et al. Factors associated with abnormal spirometry among HIV-infected individuals. AIDS 29, 1691–1700 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drummond MB et al. Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax 67, 309–314 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gupte AN et al. Factors associated with pulmonary impairment in HIV-infected South African adults. PLoS ONE 12, e0184530 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akanbi MO et al. HIV associated chronic obstructive pulmonary disease in Nigeria. J. AIDS Clin. Res 6, 453 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kayongo A et al. Chronic obstructive pulmonary disease prevalence and associated factors in a setting of well-controlled HIV, a cross-sectional study. J. COPD 17, 297–305 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ddungu A et al. Chronic obstructive pulmonary disease prevalence and associated factors in an urban HIV clinic in a low income country. PLoS ONE 16, e0256121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drummond MB et al. The effect of HIV infection on longitudinal lung function decline among IDUs: a prospective cohort. AIDS 27, 1303–1311 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grigsby M et al. Socioeconomic status and COPD among low- and middle-income countries. Int. J. Chron. Obstruct. Pulmon. Dis 11, 2497–2507 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gershon AS, Dolmage TE, Stephenson A & Jackson B Chronic obstructive pulmonary disease and socioeconomic status: a systematic review. J. COPD 9, 216–226 (2012). [DOI] [PubMed] [Google Scholar]
- 72.Pathak U, Gupta NC & Suri JC Risk of COPD due to indoor air pollution from biomass cooking fuel: a systematic review and meta-analysis. Int. J. Environ. Health Res 30, 75–88 (2020). [DOI] [PubMed] [Google Scholar]
- 73.Park J, Kim H-J, Lee C-H, Lee CH & Lee HW Impact of long-term exposure to ambient air pollution on the incidence of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Environ. Res 194, 110703 (2021). [DOI] [PubMed] [Google Scholar]
- 74.Ronit A et al. Incidence of chronic obstructive pulmonary disease in people with HIV, their parents and siblings in Denmark. J. Infect. Dis 10.1093/infdis/jiab369 (2021). [DOI] [PubMed] [Google Scholar]
- 75.Verboeket SO et al. Changes in lung function among treated HIV-positive and HIV-negative individuals: analysis of the prospective AGEhIV cohort study. Lancet Healthy Longev. 2, e202–e211 (2021). [DOI] [PubMed] [Google Scholar]
- 76.Thudium RF et al. Faster lung function decline in people living with HIV despite adequate treatment: a longitudinal matched cohort study. Thorax 10.1136/thorax-2022-218910 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Depp TB et al. Risk factors associated with acute exacerbation of chronic obstructive pulmonary disease in HIV-infected and uninfected patients. AIDS 30, 455–463 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lambert AA et al. HIV infection is associated with increased risk for acute exacerbation of COPD. J. Acquir. Immune Defic. Syndr 69, 68–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gingo MR et al. Decreased lung function and all-cause mortality in HIV-infected individuals. Ann. Am. Thorac. Soc 15, 192–199 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Triplette M et al. Markers of chronic obstructive pulmonary disease are associated with mortality in people living with HIV. AIDS 32, 487–493 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kalmin MM et al. Incident obstructive lung disease and mortality among HIV-positive and negative persons with a history of injecting drugs. AIDS 10.1097/QAD.0000000000002914 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Petoumenos K et al. Prevalence of self-reported comorbidities in HIV positive and HIV negative men who have sex with men over 55 years – the Australian Positive & Peers Longevity Evaluation Study (APPLES). PLoS ONE 12, e0184583 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosen MJ et al. Pulmonary function tests in HIV-infected patients without AIDS. Pulmonary Complications of HIV Infection Study Group. Am. J. Respir. Crit. Care Med 152, 738–745 (1995). [DOI] [PubMed] [Google Scholar]
- 84.Yano C et al. Airway hyperresponsiveness and inflammation in Japanese patients with human immunodeficiency virus 1 infection. J. Infect. Chemother 28, 426–433 (2022). [DOI] [PubMed] [Google Scholar]
- 85.Kheaw-on N et al. Bronchial hyperresponsiveness in HIV patients with CD4 count less than 500 cells/μL. Asian Biomed. 3, 255–260 (2009). [Google Scholar]
- 86.Kummerow M et al. Unexpected low frequency of respiratory symptoms in an HIV-positive urban sub-Saharan population compared to an HIV-negative control group. South. Afr. J. HIV Med 20, 1010 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kirenga BJ et al. The impact of HIV on the prevalence of asthma in Uganda: a general population survey. Respir. Res 19, 184 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Munyati SS et al. Chronic cough in primary health care attendees, Harare, Zimbabwe: diagnosis and impact of HIV infection. Clin. Infect. Dis 40, 1818–1827 (2005). [DOI] [PubMed] [Google Scholar]
- 89.Barton JH et al. Adiposity influences airway wall thickness and the asthma phenotype of HIV-associated obstructive lung disease: a cross-sectional study. BMC Pulm. Med 16, 111 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gingo MR et al. Pulmonary symptoms and diagnoses are associated with HIV in the MACS and WIHS cohorts. BMC Pulm. Med 14, 75 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leung JM et al. The determinants of poor respiratory health status in adults living with human immunodeficiency virus infection. AIDS Patient Care STDS 28, 240–247 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Robbins HA et al. Excess cancers among HIV-infected people in the United States. J. Natl Cancer Inst 107, dju503 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Worm SW et al. Non-AIDS defining cancers in the D:A:D Study – time trends and predictors of survival: a cohort study. BMC Infect. Dis 13, 471 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hernández-Ramírez RU, Shiels MS, Dubrow R & Engels EA Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV 4, e495–e504 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vandenhende M-A et al. Cancer-related causes of death among HIV-infected patients in France in 2010: evolution since 2000. PLoS ONE 10, e0129550 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Simard EP & Engels EA Cancer as a cause of death among people with AIDS in the United States. Clin. Infect. Dis 51, 957–962 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Phelps RM et al. Cancer incidence in women with or at risk for HIV. Int. J. Cancer 94, 753–757 (2001). [DOI] [PubMed] [Google Scholar]
- 98.Engels EA et al. Elevated incidence of lung cancer among HIV-infected individuals. J. Clin. Oncol 24, 1383–1388 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Engsig FN et al. Lung cancer in HIV patients and their parents: a Danish cohort study. BMC Cancer 11, 272 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clifford GM et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J. Natl Cancer Inst 97, 425–432 (2005). [DOI] [PubMed] [Google Scholar]
- 101.Hleyhel M et al. Risk of non-AIDS-defining cancers among HIV-1-infected individuals in France between 1997 and 2009: results from a French cohort. AIDS 28, 2109–2118 (2014). [DOI] [PubMed] [Google Scholar]
- 102.Shiels MS, Cole SR, Kirk GD & Poole C A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J. Acquir. Immune Defic. Syndr 52, 611–622 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grulich AE, van Leeuwen MT, Falster MO & Vajdic CM Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 370, 59–67 (2007). [DOI] [PubMed] [Google Scholar]
- 104.Shiels MS, Cole SR, Mehta SH & Kirk GD Lung cancer incidence and mortality among HIV-infected and HIV-uninfected injection drug users. J. Acquir. Immune Defic. Syndr 55, 510–515 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sigel K et al. HIV as an independent risk factor for incident lung cancer. AIDS 26, 1017–1025 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Park LS, Hernández-Ramírez RU, Silverberg MJ, Crothers K & Dubrow R Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis. AIDS 30, 273–291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hessol NA et al. Lung cancer incidence and survival among HIV-infected and uninfected women and men. AIDS 29, 1183–1193 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shebl FM, Engels EA, Goedert JJ & Chaturvedi AK Pulmonary infections and risk of lung cancer among persons with AIDS. J. Acquir. Immune Defic. Syndr 55, 375–379 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sigel K et al. Prognosis in HIV-infected patients with non-small cell lung cancer. Br. J. Cancer 109, 1974–1980 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hysell K et al. Decreased overall survival in HIV-associated non-small-cell lung cancer. Clin. Lung Cancer 22, e498–e505 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marcus JL et al. Survival among HIV-infected and HIV-uninfected individuals with common non-AIDS-defining cancers. Cancer Epidemiol. Biomark. Prev 24, 1167–1173 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y-H & Shen X-D Human immunodeficiency virus infection and mortality risk among lung cancer patients: a systematic review and meta-analysis. Medicine 97, e0361 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stein L et al. The spectrum of human immunodeficiency virus-associated cancers in a South African black population: results from a case-control study, 1995–2004. Int. J. Cancer 122, 2260–2265 (2008). [DOI] [PubMed] [Google Scholar]
- 114.Jaquet A et al. Cancer and HIV infection in referral hospitals from four West African countries. Cancer Epidemiol. 39, 1060–1065 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Githinji L & Zar HJ Respiratory complications in children and adolescents with human immunodeficiency virus. Pediatr. Clin. North Am 68, 131–145 (2021). [DOI] [PubMed] [Google Scholar]
- 116.Frigati LJ et al. Chronic comorbidities in children and adolescents with perinatally acquired HIV infection in sub-Saharan Africa in the era of antiretroviral therapy. Lancet Child Adolesc. Health 4, 688–698 (2020). [DOI] [PubMed] [Google Scholar]
- 117.Moore DP et al. The etiology of pneumonia in HIV-1-infected South African children in the era of antiretroviral treatment: findings from the Pneumonia Etiology Research for Child Health (PERCH) study. Pediatr. Infect. Dis. J 40, S69–S78 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Seidenberg P et al. The etiology of pneumonia in HIV-infected Zambian children: findings from the Pneumonia Etiology Research for Child Health (PERCH) study. Pediatr. Infect. Dis. J 40, S50–S58 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frigati LJ et al. Tuberculosis infection and disease in South African adolescents with perinatally acquired HIV on antiretroviral therapy: a cohort study. J. Int. AIDS Soc 24, e25671 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marangu D & Zar HJ Childhood pneumonia in low-and-middle-income countries: an update. Paediatr. Respir. Rev 32, 3–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Evans C, Jones CE & Prendergast AJ HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. Lancet Infect. Dis 16, e92–e107 (2016). [DOI] [PubMed] [Google Scholar]
- 122.Deeks SG, Overbaugh J, Phillips A & Buchbinder S HIV infection. Nat. Rev. Dis. Primers 1, 15035 (2015). [DOI] [PubMed] [Google Scholar]
- 123.Visseaux B, Damond F, Matheron S, Descamps D & Charpentier C Hiv-2 molecular epidemiology. Infect. Genet. Evol 46, 233–240 (2016). [DOI] [PubMed] [Google Scholar]
- 124.Campbell-Yesufu OT & Gandhi RT Update on human immunodeficiency virus (HIV)-2 infection. Clin. Infect. Dis 52, 780–787 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yuksel H, Ocalan M & Yilmaz O E-Cadherin: an important functional molecule at respiratory barrier between defence and dysfunction. Front. Physiol 12, 720227 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brune KA et al. HIV impairs lung epithelial integrity and enters the epithelium to promote chronic lung inflammation. PLoS ONE 11, e0149679 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lambert AA et al. A cross sectional analysis of the role of the antimicrobial peptide cathelicidin in lung function impairment within the ALIVE cohort. PLoS ONE 9, e95099 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chinnapaiyan S et al. HIV infects bronchial epithelium and suppresses components of the mucociliary clearance apparatus. PLoS ONE 12, e0169161 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Unwalla HJ, Ivonnet P, Dennis JS, Conner GE & Salathe M Transforming growth factor-β1 and cigarette smoke inhibit the ability of β2-agonists to enhance epithelial permeability. Am. J. Respir. Cell Mol. Biol 52, 65–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aschner Y & Downey GP Transforming growth factor-β: master regulator of the respiratory system in health and disease. Am. J. Respir. Cell Mol. Biol 54, 647–655 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cribbs SK, Crothers K & Morris A Pathogenesis of HIV-related lung disease: immunity, infection, and inflammation. Physiol. Rev 100, 603–632 (2020). [DOI] [PubMed] [Google Scholar]
- 132.Chinnapaiyan S et al. Cigarette smoke promotes HIV infection of primary bronchial epithelium and additively suppresses CFTR function. Sci. Rep 8, 7984 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schiff AE et al. T cell-tropic HIV efficiently infects alveolar macrophages through contact with infected CD4+ T cells. Sci. Rep 11, 3890 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Joshi PC, Raynor R, Fan X & Guidot DM HIV-1-transgene expression in rats decreases alveolar macrophage zinc levels and phagocytosis. Am. J. Respir. Cell Mol. Biol 39, 218–226 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Akata K et al. Altered polarization and impaired phagocytic activity of lung macrophages in people with human immunodeficiency virus and chronic obstructive pulmonary disease. J. Infect. Dis 225, 862–867 (2022). [DOI] [PubMed] [Google Scholar]
- 136.Tachado SD, Zhang J, Zhu J, Patel N & Koziel H HIV impairs TNF-α release in response to Toll-like receptor 4 stimulation in human macrophages in vitro. Am. J. Respir. Cell Mol. Biol 33, 610–621 (2005). [DOI] [PubMed] [Google Scholar]
- 137.Alexandrova Y, Costiniuk CT & Jenabian M-A Pulmonary immune dysregulation and viral persistence during HIV infection. Front. Immunol 12, 808722 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cribbs SK, Lennox J, Caliendo AM, Brown LA & Guidot DM Healthy HIV-1-infected individuals on highly active antiretroviral therapy harbor HIV-1 in their alveolar macrophages. AIDS Res. Hum. Retrovir 31, 64–70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Svanberg C et al. HIV-1 induction of tolerogenic dendritic cells is mediated by cellular interaction with suppressive T cells. Front. Immunol 13, 790276 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gaiha GD et al. Surfactant protein A binds to HIV and inhibits direct infection of CD4+ cells, but enhances dendritic cell-mediated viral transfer. J. Immunol 181, 601–609 (2008). [DOI] [PubMed] [Google Scholar]
- 141.Madsen J et al. Surfactant protein D modulates HIV infection of both T-cells and dendritic cells. PLoS ONE 8, e59047 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Doitsh G et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505, 509–514 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Neff CP et al. Lymphocytic alveolitis is associated with the accumulation of functionally impaired HIV-specific T cells in the lung of antiretroviral therapy-naive subjects. Am. J. Respir. Crit. Care Med 191, 464–473 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moir S & Fauci AS B cells in HIV infection and disease. Nat. Rev. Immunol 9, 235–245 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morris D et al. Unveiling the mechanisms for decreased glutathione in individuals with HIV infection. Clin. Dev. Immunol 2012, 734125 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ivanov AV et al. Oxidative stress during HIV infection: mechanisms and consequences. Oxid. Med. Cell. Longev 2016, 8910396 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mandas A et al. Oxidative imbalance in HIV-1 infected patients treated with antiretroviral therapy. J. Biomed. Biotechnol 2009, 749575 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cribbs SK, Guidot DM, Martin GS, Lennox J & Brown LA Anti-retroviral therapy is associated with decreased alveolar glutathione levels even in healthy HIV-infected individuals. PLoS ONE 9, e88630 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yeligar SM et al. Dysregulation of alveolar macrophage PPARγ, NADPH oxidases, and TGFβ1 in otherwise healthy HIV-infected individuals. AIDS Res. Hum. Retrovir 33, 1018–1026 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lassiter C et al. HIV-1 transgene expression in rats causes oxidant stress and alveolar epithelial barrier dysfunction. AIDS Res. Ther 6, 1 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Staitieh BS et al. HIV-1 decreases Nrf2/ARE activity and phagocytic function in alveolar macrophages. J. Leukoc. Biol 102, 517–525 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kukoyi AT et al. MiR-144 mediates Nrf2 inhibition and alveolar epithelial dysfunction in HIV-1 transgenic rats. Am. J. Physiol., Cell Physiol 317, C390–C397 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang T et al. Transfer of intracellular HIV Nef to endothelium causes endothelial dysfunction. PLoS ONE 9, e91063 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kanmogne GD, Primeaux C & Grammas P Induction of apoptosis and endothelin-1 secretion in primary human lung endothelial cells by HIV-1 gp120 proteins. Biochem. Biophys. Res. Commun 333, 1107–1115 (2005). [DOI] [PubMed] [Google Scholar]
- 155.Spikes L et al. Enhanced pulmonary arteriopathy in simian immunodeficiency virus-infected macaques exposed to morphine. Am. J. Respir. Crit. Care Med 185, 1235–1243 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jacob BA et al. HIV-1-induced pulmonary oxidative and nitrosative stress: exacerbated response to endotoxin administration in HIV-1 transgenic mouse model. Am. J. Physiol. Lung Cell. Mol. Physiol 291, L811–L819 (2006). [DOI] [PubMed] [Google Scholar]
- 157.Cole SB et al. Oxidative stress and antioxidant capacity in smoking and nonsmoking men with HIV/acquired immunodeficiency syndrome. Nutr. Clin. Pract 20, 662–667 (2005). [DOI] [PubMed] [Google Scholar]
- 158.Pacht ER, Diaz P, Clanton T, Hart J & Gadek JE Alveolar fluid glutathione decreases in asymptomatic HIV-seropositive subjects over time. Chest 112, 785–788 (1997). [DOI] [PubMed] [Google Scholar]
- 159.Morris A et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am. J. Respir. Crit. Care Med 187, 1067–1075 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Segal LN et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat. Microbiol 1, 16031 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Beck JM et al. Multicenter comparison of lung and oral microbiomes of HIV-infected and HIV-uninfected individuals. Am. J. Respir. Crit. Care Med 192, 1335–1344 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cui L et al. Topographic diversity of the respiratory tract mycobiome and alteration in HIV and lung disease. Am. J. Respir. Crit. Care Med 191, 932–942 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lozupone C et al. Widespread colonization of the lung by Tropheryma whipplei in HIV infection. Am. J. Respir. Crit. Care Med 187, 1110–1117 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Morris A et al. Longitudinal analysis of the lung microbiota of cynomolgous macaques during long-term SHIV infection. Microbiome 4, 38 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhou JJ et al. Supraglottic lung microbiome taxa are associated with pulmonary abnormalities in an HIV longitudinal cohort. Am. J. Respir. Crit. Care Med 202, 1727–1731 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yang L et al. Alterations in oral microbiota in HIV are related to decreased pulmonary function. Am. J. Respir. Crit. Care Med 201, 445–457 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nazli A et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 6, e1000852 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Estes JD et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 6, e1001052 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brenchley JM Mucosal immunity in human and simian immunodeficiency lentivirus infections. Mucosal Immunol. 6, 657–665 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.He S et al. A systematic review and meta-analysis of diagnostic accuracy of serum 1,3-β-d-glucan for invasive fungal infection: focus on cutoff levels. J. Microbiol. Immunol. Infect 48, 351–361 (2015). [DOI] [PubMed] [Google Scholar]
- 171.Morris A et al. Serum (1→3)-β-D-glucan levels in HIV-infected individuals are associated with immunosuppression, inflammation, and cardiopulmonary function. J. Acquir. Immune Defic. Syndr 61, 462–468 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hameiri Bowen D et al. Cytomegalovirus-specific immunoglobulin G is associated with chronic lung disease in children and adolescents from sub-Saharan Africa Living with perinatal human immunodeficiency virus. Clin. Infect. Dis 73, e264–e266 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nenna R et al. High cytomegalovirus serology and subsequent COPD-related mortality: a longitudinal study. ERJ Open Res. 6, 00062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nenna R et al. Cytomegalovirus serology in young to mid-adult life and decline of lung function. Clin. Respir. J 10.1111/crj.13600 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ahlström MG et al. Association between smoking status assessed with plasma-cotinine and inflammatory and endothelial biomarkers in HIV-positive and HIV-negative individuals. HIV Med. 19, 679–687 (2018). [DOI] [PubMed] [Google Scholar]
- 176.Poudel KC, Poudel-Tandukar K, Bertone-Johnson ER, Pekow P & Vidrine DJ Inflammation in relation to intensity and duration of cigarette smoking among people living with HIV. AIDS Behav. 25, 856–865 (2021). [DOI] [PubMed] [Google Scholar]
- 177.Neff CP et al. HIV infection is associated with loss of anti-inflammatory alveolar macrophages. J. Immunol 205, 2447–2455 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Corleis B et al. Smoking and human immunodeficiency virus 1 infection promote retention of CD8+ T cells in the airway mucosa. Am. J. Respir. Cell Mol. Biol 65, 513–520 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Staitieh BS et al. HIV increases the risk of cigarette smoke-induced emphysema via MMP-9. J. Acquir. Immune Defic. Syndr 10.1097/QAI.0000000000003125 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nieman RB, Fleming J, Coker RJ, Harris JR & Mitchell DM The effect of cigarette smoking on the development of AIDS in HIV-1-seropositive individuals. AIDS 7, 705–710 (1993). [DOI] [PubMed] [Google Scholar]
- 181.Feldman JG et al. Association of cigarette smoking with HIV prognosis among women in the HAART era: a report from the Women’s Interagency HIV study. Am. J. Public Health 96, 1060–1065 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Winhusen T et al. Baseline cigarette smoking status as a predictor of virologic suppression and CD4 cell count during one-year follow-up in substance users with uncontrolled HIV infection. AIDS Behav. 22, 2026–2032 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Brown JL et al. The association between cigarette smoking, virologic suppression, and CD4+ lymphocyte count in HIV-infected Russian women. AIDS Care 29, 1102–1106 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Akhtar-Khaleel WZ et al. Trends and predictors of cigarette smoking among HIV seropositive and seronegative men: the multicenter aids cohort study. AIDS Behav. 20, 622–632 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jones TPW et al. Alcohol, smoking, recreational drug use and association with virological outcomes among people living with HIV: cross-sectional and longitudinal analyses. HIV Med. 23, 209–226 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cyktor J et al. Associations of HIV persistence, cigarette smoking, inflammation, and pulmonary dysfunction in people with HIV on antiretroviral therapy. Medicine 101, e29264 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Clarke JR et al. The epidemiology of HIV-1 infection of the lung in AIDS patients. AIDS 7, 555–560 (1993). [DOI] [PubMed] [Google Scholar]
- 188.Ranjit S, Sinha N, Kodidela S & Kumar S Benzo(a)pyrene in cigarette smoke enhances HIV-1 replication through NF-κB activation via CYP-mediated oxidative stress pathway. Sci. Rep 8, 10394 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Global Initiative for Chronic Obstructive Lung Disease https://goldcopd.org/wp-content/uploads/2022/12/GOLD-2023-ver-1.1-2Dec2022_WMV.pdf (2023). [Google Scholar]
- 190.US Preventive Services Task Force. Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force reaffirmation recommendation statement. J. Am. Med. Assoc 327, 1806–1811 (2022). [DOI] [PubMed] [Google Scholar]
- 191.Lambert AA et al. Implementation of a COPD screening questionnaire in an outpatient HIV clinic. Chron. Obstruct. Pulmon. Dis 13, 767–772 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schnieders E et al. Performance of alternative COPD case-finding tools: a systematic review and meta-analysis. Eur. Respir. Rev 30, 200350 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.MacDonald DM et al. Smoking and accelerated lung function decline in HIV-positive individuals: a secondary analysis of the START pulmonary substudy. J. Acquir. Immune Defic. Syndr 79, e85–e92 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fan H et al. Pulmonary tuberculosis as a risk factor for chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ann. Transl Med 9, 390 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Global Initiative for Asthma. Global strategy for asthma management and prevention. Global Initiative for Asthma https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf (2022). [Google Scholar]
- 196.Pekkanen J & Pearce N Defining asthma in epidemiological studies. Eur. Respir. J 14, 951–957 (1999). [DOI] [PubMed] [Google Scholar]
- 197.Weakley J et al. Agreement between obstructive airways disease diagnoses from self-report questionnaires and medical records. Prev. Med 57, 38–42 (2013). [DOI] [PubMed] [Google Scholar]
- 198.Coghill AE et al. Advanced stage at diagnosis and elevated mortality among US patients with cancer infected with HIV in the National Cancer Data Base. Cancer 125, 2868–2876 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shiels MS, Pfeiffer RM & Engels EA Age at cancer diagnosis among persons with AIDS in the United States. Ann. Intern. Med 153, 452–460 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rengan R, Mitra N, Liao K, Armstrong K & Vachani A Effect of HIV on survival in patients with non-small-cell lung cancer in the era of highly active antiretroviral therapy: a population-based study. Lancet Oncol. 13, 1203–1209 (2012). [DOI] [PubMed] [Google Scholar]
- 201.Shuter J, Reddy KP, Hyle EP, Stanton CA & Rigotti NA Harm reduction for smokers living with HIV. Lancet HIV 8, e652–e658 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med 365, 395–409 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kong CY et al. Benefits and harms of lung cancer screening in HIV-infected individuals with CD4+ cell count at least 500 cells/μl. AIDS 32, 1333–1342 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. J. Am. Med. Assoc 325, 962–970 (2021). [Google Scholar]
- 205.Sellers SA et al. Optimal lung cancer screening criteria among persons living with HIV. J. Acquir. Immune Defic. Syndr 90, 184–192 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.World Health Organization. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach (WHO, 2021). [PubMed] [Google Scholar]
- 207.Frigati LJ et al. The impact of isoniazid preventive therapy and antiretroviral therapy on tuberculosis in children infected with HIV in a high tuberculosis incidence setting. Thorax 66, 496–501 (2011). [DOI] [PubMed] [Google Scholar]
- 208.World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Module 5: Management of Tuberculosis in Children and Adolescents (WHO, 2022). [PubMed] [Google Scholar]
- 209.Githinji LN, Gray DM, Hlengwa S, Myer L & Zar HJ Lung function in South African adolescents infected perinatally with HIV and treated long-term with antiretroviral therapy. Ann. Am. Thorac. Soc 14, 722–729 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.European AIDS Clinical Society. Guidelines, version 11.1. EACS https://www.eacsociety.org/media/guidelines-11.1_final_09-10.pdf (2022). [Google Scholar]
- 211.Nahid P et al. Executive summary: official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin. Infect. Dis 63, 853–867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dorman SE et al. Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N. Engl. J. Med 384, 1705–1718 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lam JO et al. Smoking and cessation treatment among persons with and without HIV in a US integrated health system. Drug Alcohol. Depend 213, 108128 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pool ERM, Dogar O, Lindsay RP, Weatherburn P & Siddiqi K Interventions for tobacco use cessation in people living with HIV and AIDS. Cochrane Database Syst. Rev 10.1002/14651858.CD011120.pub2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Uthman OA et al. Comparison of mHealth and face-to-face interventions for smoking cessation among people living with HIV: meta-analysis. JMIR Mhealth Uhealth 7, e203 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mann-Jackson L et al. A qualitative systematic review of cigarette smoking cessation interventions for persons living with HIV. J. Cancer Educ 34, 1045–1058 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Oshagbemi OA, Odiba JO, Daniel A & Yunusa I Absolute blood eosinophil counts to guide inhaled corticosteroids therapy among patients with COPD: systematic review and meta-analysis. Curr. Drug Targets 20, 1670–1679 (2019). [DOI] [PubMed] [Google Scholar]
- 218.Harries TH et al. Blood eosinophil count, a marker of inhaled corticosteroid effectiveness in preventing COPD exacerbations in post-hoc RCT and observational studies: systematic review and meta-analysis. Respir. Res 21, 3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Miravitlles M et al. Systematic review on long-term adverse effects of inhaled corticosteroids in the treatment of COPD. Eur. Respir. Rev 30, 210075 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dong YH et al. Use of inhaled corticosteroids in patients with COPD and the risk of TB and influenza: a systematic review and meta-analysis of randomized controlled trials. Chest 145, 1286–1297 (2014). [DOI] [PubMed] [Google Scholar]
- 221.Chapman KR et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am. J. Respir. Crit. Care Med 198, 329–339 (2018). [DOI] [PubMed] [Google Scholar]
- 222.Rogliani P, Ritondo BL, Gabriele M, Cazzola M & Calzetta L Optimizing de-escalation of inhaled corticosteroids in COPD: a systematic review of real-world findings. Expert Rev. Clin. Pharmacol 13, 977–990 (2020). [DOI] [PubMed] [Google Scholar]
- 223.Boyd SD et al. Influence of low-dose ritonavir with and without darunavir on the pharmacokinetics and pharmacodynamics of inhaled beclomethasone. J. Acquir. Immune Defic. Syndr 63, 355–361 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Clinical Info HIV.gov https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv (2023). [Google Scholar]
- 225.Sigel KM et al. Short-term outcomes for lung cancer resection surgery in HIV infection. AIDS 33, 1353–1360 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Makinson A et al. Risks of opportunistic infections in people with human immunodeficiency virus with cancers treated with chemotherapy. Open Forum Infect. Dis 8, ofab389 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Uldrick TS et al. Assessment of the safety of pembrolizumab in patients with HIV and advanced cancer – a phase 1 study. JAMA Oncol. 5, 1332–1339 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Peluso MJ et al. Outcomes of immunomodulatory and biologic therapy in people living with HIV. AIDS 34, 1171–1179 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Grover S et al. Reduced cancer survival among adults with HIV and AIDS-defining illnesses despite no difference in cancer stage at diagnosis. J. Acquir. Immune Defic. Syndr 79, 421–429 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.World Health Organization. Revised WHO classification and treatment of childhood pneumonia at health facilities: evidence summaries. WHO https://apps.who.int/iris/bitstream/handle/10665/137319/9789241507813_eng.pdf (2014). [PubMed] [Google Scholar]
- 231.Punpanich W, Groome M, Muhe L, Qazi SA & Madhi SA Systematic review on the etiology and antibiotic treatment of pneumonia in human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J 30, e192–202 (2011). [DOI] [PubMed] [Google Scholar]
- 232.Turkova A et al. Shorter treatment for non-severe tuberculosis in African and Indian children. N. Engl. J. Med 386, 911–922 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Price A et al. Effect of azithromycin on incidence of acute respiratory exacerbations in children with HIV taking antiretroviral therapy and co-morbid chronic lung disease: a secondary analysis of the BREATHE trial. eClinicalMedicine 42, 101195 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rubinstein A, Bernstein LJ, Charytan M, Krieger BZ & Ziprkowski M Corticosteroid treatment for pulmonary lymphoid hyperplasia in children with the acquired immune deficiency syndrome. Pediatr. Pulmonol 4, 13–17 (1988). [DOI] [PubMed] [Google Scholar]
- 235.Sabin CA et al. Respiratory symptoms and chronic bronchitis in people with and without HIV infection. HIV Med. 22, 11–21 (2021). [DOI] [PubMed] [Google Scholar]
- 236.Brown J et al. Respiratory symptoms in people living with HIV and the effect of antiretroviral therapy: a systematic review and meta-analysis. Thorax 72, 355–366 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kunisaki KM et al. Pulmonary effects of immediate versus deferred antiretroviral therapy in HIV-positive individuals: a nested substudy within the multicentre, international, randomised, controlled Strategic Timing of Antiretroviral Treatment (START) trial. Lancet Respir. Med 4, 980–989 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Terry C et al. Dyspnea and pulmonary function among participants in the multicenter AIDS cohort study using protease inhibitors: a cross-sectional study. AIDS Res. Hum. Retrovir 38, 143–151 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Khoury AL et al. Diminished physical function in older HIV-infected adults in the Southeastern U.S. despite successful antiretroviral therapy. PLoS ONE 12, e0179874 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Robertson TE et al. HIV infection is an independent risk factor for decreased 6-minute walk test distance. PLoS ONE 14, e0212975 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Campo M et al. Association of chronic cough and pulmonary function with 6-minute walk test performance in HIV infection. J. Acquir. Immune Defic. Syndr 65, 557–563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Triplette M et al. The differential impact of emphysema on respiratory symptoms and 6-minute walk distance in HIV infection. J. Acquir. Immune Defic. Syndr 74, e23–e29 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chen Y et al. Human immunodeficiency virus infection and incident heart failure: a meta-analysis of prospective studies. J. Acquir. Immune Defic. Syndr 87, 741–749 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wolff AJ & O’Donnell AE Pulmonary effects of illicit drug use. Clin. Chest Med 25, 203–216 (2004). [DOI] [PubMed] [Google Scholar]
- 245.Metlay JP et al. Diagnosis and treatment of adults with community-acquired pneumonia. an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med 200, e45–e67 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jan AK et al. Markers of inflammation and immune activation are associated with lung function in a multi-center cohort of persons with HIV. AIDS 35, 1031–1040 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.de Koning HJ et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med 382, 503–513 (2020). [DOI] [PubMed] [Google Scholar]
- 248.Matthay MA et al. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 5, 18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hewitt RJ & Lloyd CM Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol 21, 347–362 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


