In cases of IOL dislocation associated with AD, LEC degeneration and lens capsule fragility were more frequently observed than in cases without AD.
Abstract
Purpose:
To explore lens capsule pathological characteristics in intraocular lens (IOL) dislocation after cataract surgery in patients with atopic dermatitis (AD).
Setting:
University hospital department of ophthalmology.
Design:
Case series with clinicopathological correlations.
Methods:
Lens capsules and surrounding tissues excised during surgery from eyes with AD (AD group) and eyes without AD (non-AD group) with IOL dislocation were histologically evaluated. Hematoxylin and eosin staining was used to assess abnormal changes in lens epithelial cells (LECs). Masson trichrome staining distinguished the fibrous metaplasia around the lens capsule into high-density and low-density fibrosis. Capsular splitting (thinning) was identified in both stained preparations.
Results:
The IOL dislocation morphology in the AD group (10 eyes of 10 patients) included 7 cases of capsular bag dislocation (CBD) and 3 cases of dead bag syndrome (DBS), with an average duration to IOL dislocation of 11.5 ± 5.6 years. All patients in the non-AD group (12 eyes of 12 patients) had CBD, averaging 10.2 ± 5.7 years to dislocation. Abnormal LECs, low-density fibrosis, and capsular splitting were observed in 9 (90), 9 (90), and 6 (60) of the patients in the AD group compared with 6 (50), 3 (25), and 2 (18), respectively, in the non-AD group (total n [%]).
Conclusions:
Compared with the non-AD group, the AD group exhibited higher frequencies of morphological changes in LECs, low-density fibrosis around the lens capsule, and capsular splitting characteristics of DBS. These results suggest LEC degeneration and increased lens capsule fragility occurred in patients with AD.
Intraocular lens (IOL) dislocation after cataract surgery with lens capsule IOL insertion can necessitate extensive surgery and cause significant distress for patients. IOL dislocation can occur several months or years after cataract surgery. Annual incidence rates range from 0.0% to 0.05%, with cumulative rates of 0.1% to 3% over 10 to 25 years.1 Owing to advancements in cataract surgery, even previously challenging cases, such as those with zonular weakness, can now have lens capsule IOL insertions.2 This suggests a potential rise in IOL dislocation cases in the future. IOL dislocation can be broadly categorized into capsular bag dislocation (CBD) due to zonular dialysis and dead bag syndrome (DBS) due to a very clear bag many years postoperatively, without fibrotic changes or proliferative material within the bag.3 Notably, DBS is characterized by maintained lens capsule transparency over long periods of post-IOL insertion, capsular splitting, loss of lens epithelial cells (LECs), and the absence of Soemmering ring formation.3 The cause of DBS remains unknown. Risk factors for IOL dislocation include a history of ocular trauma, vitrectomy, pseudoexfoliation syndrome, retinitis pigmentosa, high myopia, uveitis, and cataract surgery complicated by atopic dermatitis (AD).1 AD prevalence has doubled or even tripled over the past few decades in industrialized countries.4 AD can lead to a variety of ocular complications such as cataracts, retinal detachment, glaucoma, conjunctivitis, and keratoconus,5,6 which can significantly impair vision at young ages. In particular, IOL dislocation in patients with AD attributes not only to CBD, believed to be caused by eye scratching, but also to DBS.7,8 The reasons for these differences in dislocation forms remain elusive, and the true pathogenesis is unknown.
This study investigated lens capsule pathological characteristics in patients with AD-complicated IOL dislocation (AD group) and compared them with those in patients without AD-complicated IOL dislocation (non-AD group).
METHODS
Ethical Approval
This case series was approved by the Institutional Review Board of Jikei University School of Medicine and conducted in accordance with the tenets of the Declaration of Helsinki (approval number: 35-057[11680]). This study did not involve any invasive or interventional procedures and only used existing samples and information. Therefore, informed consent from the research participants was obtained using an opt-out method in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects (Japanese Ministry of Health, Labor, and Welfare). The documents approved by the Institutional Review Board of the Jikei University School of Medicine were posted on the university's website, and the information for this research was announced on the bulletin board of the hospital. All participants had the right to refuse to participate at any time (opt-out method). Furthermore, the transmission electron microscopy evaluation for this study was conducted under the authorization of the Fujita Medical University Ethics Committee, bearing the approval number 07-005.
The study conformed to the Strengthening the Reporting of Observational Studies in Epidemiology checklist, which is used to improve the quality of observational studies.9
Study Setting and Data Sources
This study was conducted between November 2022 and June 2023 at the Department of Ophthalmology, Tokyo Jikei Medical University Hospital. This study included patients who were diagnosed with IOL dislocation and underwent IOL extraction surgery. Only cases in which the extracted lens bag was appropriately processed as a specimen were included. Based on slitlamp examination and intraoperative findings, IOL dislocations were classified as CBD or DBS. Specifically, dislocations where the IOL remained within the capsular bag, forming a complex with the capsule, were classified as CBD. Cases where the crystalline lens capsule was intact at the time of the initial cataract surgery but subsequently ruptured, leading to dislocation of the IOL without the accompanying capsular bag, were classified as DBS. Patient medical records were reviewed to collect data on age, sex, history of prior intraocular surgeries, eye axial length, AD, history of trauma, pseudoexfoliation syndrome, uveitis, and the presence or absence of retinitis pigmentosa. The participants were divided into AD group with 10 eyes (10 patients) and non-AD group with 12 eyes (12 patients) for evaluation.
Surgical Technique
The surgeries were performed by 7 surgeons (2 authors of this study). To extract the acrylic IOL, a small incision was made, and the IOL was either removed as a whole using IOL extraction forceps or fragmented using an IOL cutter and then extracted. An L-shaped scleral tunnel incision was made for the polymethyl methacrylate IOL. In CBD cases, the lens bag was simultaneously extracted with the IOL. In DBS cases, after IOL extraction, the zonular fibers were incised using a vitreous cutter and then extracted.
Pathology Specimen Preparation
The lens capsule, which was extracted during surgery, was fixed in 10% neutral buffered formalin. After dehydration, it was embedded in paraffin, sectioned into slices of 3 μm thickness, and then stained with hematoxylin and eosin and Masson trichrome. Pathological evaluations were conducted by 2 ophthalmologists (2 authors) and 3 pathologists (3 authors). Hematoxylin–eosin staining was primarily used to evaluate the LECs. The cells were assessed for quantity (abundant/scarce/absent), disorderly arrangement (present/absent), clear abundant cytoplasmic (CAC) LECs (cells that were enlarged with a pale, abundant cytoplasm) (present/not present), and flat LECs (present/not present). Masson trichrome staining was used to evaluate the fibrous metaplasia around the lens capsule. Cases with fibrosis were further categorized as “high-density” for substantial fibrosis and “low-density” for minimal fibrosis. In addition, the presence or absence of capsular splitting and a Soemmering ring were evaluated.
Transmission Electron Microscopy Evaluation
We conducted an electron microscopic evaluation of the anterior lens capsule samples because the pathological features of AD cataracts may offer some insights into this study.
Patient Sample for Transmission Electron Microscopy Evaluation
During the surgical procedures for 10 cases of age-related cataracts and 8 cases of atopic cataracts (no eye complications other than cataracts), LECs adhering to the anterior capsule were removed by anterior capsulotomy. The anterior capsule and adherent LECs were removed by anterior capsulotomy.
Methods of Transmission Electron Microscopy
The anterior capsule and adherent LECs isolated during surgery for either age-related or atopic cataract were immersion fixed in half Karnovsky fixative (2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M phosphate-buffered saline, pH 7.4) at 4 °C overnight, postfixed with 1% osmium tetroxide in 0.1 M phosphate-buffered saline (pH 7.4) at 4 °C for 30 minutes, dehydrated through a graded ethanol series, transferred to n-butyl glycidyl ether (QY-1, Nisshin EM Co., Ltd.), and embedded in epoxy resin (TAAB EPON812, Nisshin EM Co., Ltd.). Ultrathin sections (100 nm thickness) were cut using an ultramicrotome (EM UC7i, Leica Microsystems GmbH), placed on a copper grid (VECO, No. 2503, Nisshin EM Co., Ltd.), stained with uranyl acetate for 7 min and lead citrate for 3 min, and examined using a transmission electron microscope (TEM, JEM-1010, JEOL Ltd.).
RESULTS
In the AD group, which consisted of 10 patients, the average age was 47.0 ± 10.3 years (Table 1). One patient had a history of trauma, 2 had a history of vitreous surgery, and 1 had a history of uveitis. Regarding the IOL dislocation type, 7 cases were CBD and 3 were DBS (Figure 1, A and B). The average duration until IOL dislocation was 11.5 ± 5.6 years. The non-AD group consisted of 12 patients, with an average age of 64.0 ± 13.8 years (Table 2). Six patients had a history of trauma, 5 had a history of vitreous surgery, and 3 had unknown causes. All the patients in the non-AD group had CBD-type IOL dislocations. The average period until IOL dislocation was 10.2 ± 5.7 years. The average axial length was 25.5 ± 1.4 mm in the AD group and 25.6 ± 1.5 mm in the non-AD group.
Table 1.
Atopic dermatitis case characteristics
| Parameter | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Average ± SD |
| Classification of IOL dislocation | DBS | DBS | DBS | CBD | CBD | CBD | CBD | CBD | CBD | CBD | |
| Explanting surgeon | YM | YM | YM | IN | KK | SO | TW | YM | YM | KK | |
| Age at explantation (y) | 55 | 39 | 46 | 46 | 47 | 59 | 47 | 26 | 62 | 43 | 47.0 ± 10.3 |
| Sex | M | M | M | M | M | F | M | M | M | M | |
| Eye | L | R | L | L | R | R | R | L | R | L | |
| Length of implantation (y) | 17 | 14 | 3 | 5 | 22 | 13 | 12 | 8 | 10 | 11 | 11.5 ± 5.6 |
| Axial length (mm) | 25.0 | 25.5 | 24.3 | 26.3 | 25.5 | 24.2 | 27.0 | 23.1 | 27.6 | 26.4 | 25.5 ± 1.4 |
| Causes of IOL dislocation | AD, trauma | AD | AD | AD | AD | AD, Bechet disease | AD, vitrectomy | AD | AD | AD, vitrectomy | |
| History of ophthalmic surgical treatments | |||||||||||
| In-the-bag IOL | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | |
| Vitrectomy | (+) | (+) | Total n (%) | ||||||||
| LECs | |||||||||||
| Quantity | Abundant | Abundant | Abundant | Absent | Abundant | Scarce | Abundant | Absent | Abundant | Scarce | 4 (40) |
| Disorderly arrangement | (−) | (−) | (−) | NA | (−) | (+) | (−) | NA | (−) | (−) | 1 (13) |
| CAC LECs | (+) | (+) | (−) | (+) | (−) | (+) | (+) | (−) | 5 (63) | ||
| Flat LECs | (+) | (+) | (−) | (−) | (+) | (−) | (−) | (+) | 4 (50) | ||
| Fibrous metaplasia around the lens capsule | |||||||||||
| High-density fibrosis | (−) | (−) | (−) | (+) | (−) | (−) | (−) | (−) | (−) | (+) | 2 (20) |
| Low-density fibrosis | (+) | (+) | (+) | (+) | (+) | (−) | (+) | (+) | (+) | (+) | 9 (90) |
| Capsular splitting | (+) | (+) | (−) | (−) | (+) | (−) | (+) | (−) | (+) | (+) | 6 (60) |
| Soemmering ring | (+) | (+) | (+) | (+) | (+) | (−) | (+) | (−) | (+) | (−) | 7 (70) |
AD = atopic dermatitis; CAC = clear abundant cytoplasm; CBD = capsular bag dislocation; CTR = capsular tension ring; DBS = dead bag syndrome; LEC = lens epithelial cell; NA = not applicable
Figure 1.
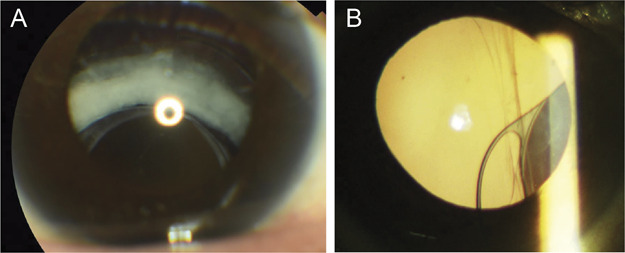
Slitlamp photographs of the anterior segment. A: Shows capsular bag dislocation. The complex of the capsular bag and IOL is displaced. A Soemmering ring is also observed. B: Indicates dead bag syndrome. There is a crack in the capsular bag, although its transparency is maintained. A: Case 5 and (B) case 2.
Table 2.
Nonatopic dermatitis case characteristics
| Parameter | Case 11 | Case 12 | Case 13 | Case 14 | Case 15 | Case 16 | Case 17 | Case 18 | Case 19 | Case 20 | Case 21 | Case 22 | Average ± SD |
| Classification of IOL dislocation | CBD | CBD | CBD | CBD | CBD | CBD | CBD | CBD | CBD | CBD | CBD | CBD | |
| Explanting surgeon | KK | IN | KKon/AW | YM | TW | IN | KKon/AW | SO | YM | KK | IN | KKon/TW | |
| Age at explantation (y) | 57 | 67 | 78 | 66 | 67 | 33 | 51 | 55 | 63 | 76 | 70 | 85 | 64.0 ± 13.8 |
| Sex | M | M | M | M | M | M | M | M | M | M | F | F | |
| Eye | R | L | R | R | L | L | L | L | L | L | R | L | |
| Length of implantation (y) | 12 | 8 | 10 | 19 | 8 | 13 | 23 | 6 | 6 | 6 | 7 | 4 | 10.2 ± 5.7 |
| Axial length (mm) | 24.5 | 25.0 | 24.2 | 25.5 | 28.2 | 26.7 | 24.3 | 26.6 | 25.9 | 27.9 | 24.8 | 23.9 | 25.6 ± 1.5 |
| Causes of IOL dislocation | Trauma, vitrectomy | Trauma, vitrectomy | Vitrectomy | Unknown | Trauma | Trauma | Trauma | Vitrectomy | Trauma | Unknown | Unknown | Vitrectomy | |
| History of ophthalmic surgical treatments | |||||||||||||
| In-the-bag IOL | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | |
| Vitrectomy | (+) | (+) | (+) | (+) | (+) | Total n (%) | |||||||
| LECs | |||||||||||||
| Quantity | Abundant | Abundant | Scarce | Abundant | Abundant | Abundant | Abundant | Abundant | Scarce | Absent | Abundant | Abundant | 3 (25) |
| Disorderly arrangement | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (+) | (−) | (−) | 1 (8) |
| CAC LECs | (+) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (+) | (−) | (−) | (+) | 3 (25) |
| Flat LECs | (−) | (−) | (+) | (−) | (−) | (−) | (+) | (−) | (+) | (−) | (−) | (+) | 4 (33) |
| Fibrous metaplasia around the lens capsule | |||||||||||||
| High-density fibrosis | (+) | (+) | (+) | (−) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | 11 (92) |
| Low-density fibrosis | (−) | (−) | (−) | (+) | (−) | (+) | (−) | (−) | (−) | (+) | (−) | (−) | 3 (25) |
| Capsular splitting | (−) | (−) | (−) | (+) | (−) | NA | (+) | (−) | (−) | (−) | (−) | (−) | 2 (18) |
| Soemmering ring | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | 12 (100) |
CAC = clear abundant cytoplasm; CBD = capsular bag dislocation; LEC = lens epithelial cell; NA = not applicable; PVR = proliferative vitreoretinopathy
Concerning LEC morphology, in the AD group, of 10 cases, 2 had LECs that were absent. Of the 8 evaluable cases, 5 had CAC LECs and 4 had flat LECs (Table 1, Figure 2, B and C). Only 1 patient had no abnormalities in the LEC evaluation criteria. Of the 12 patients in the non-AD group, 3 had LECs that were scarce or absent, 3 had CAC LECs, and 4 had flat LECs (Table 2). Six cases had no abnormalities in the LEC evaluation criteria. Of these, 9 (90%) of the AD group and 6 (50%) of the non-AD group exhibited abnormal LECs.
Figure 2.
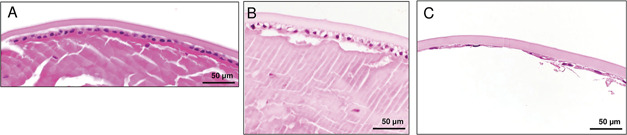
Hematoxylin and eosin staining of the LECs. A: A case of non-AD. LECs show preserved alignment, abundant cell quantity, and a square cell shape. B: A case of AD. Numerous clear and abundant cytoplasmic LECs are observed. C: Another AD case. The LEC observations reveal a lack of aligned structures, reduced cell quantity, varied cell sizes, and the presence of flat LECs. A: Case 15, (B) case 5, and (C) case 1. AD = atopic dermatitis; LEC = lens epithelial cell
Regarding fibrous metaplasia around the lens capsule, the AD group had 2 cases of high-density fibrosis and 9 had low-density fibrosis, including 2 exhibiting both high-density and low-density fibrosis (Table 1, Figure 3, A). By contrast, the non-AD group had high-density fibrosis in 11 of 12 cases and 3 had low-density fibrosis, including 2 cases exhibiting both high-density and low-density fibrosis (Table 2, Figure 3, B). Low-density fibrosis was detected in 9 (90%) of the AD group and 3 (25%) of the non-AD group.
Figure 3.
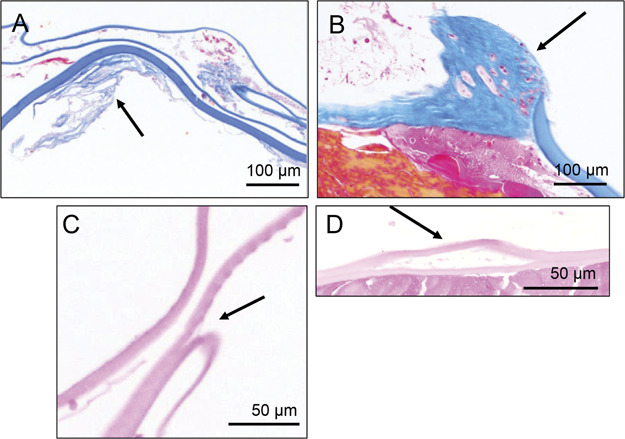
Masson trichrome staining of fibrous metaplasia around the lens capsule showing AD with low-density fibrosis (A, arrow) and non-AD with high-density fibrosis (B, arrow). Hematoxylin and eosin staining of sections from the lens–capsular bags in AD. The arrows in C and D indicate sites of capsular splitting. A: Case 1, (B) case 15, (C) case 10, and (D) case 5. AD = atopic dermatitis
In the AD group, 2 of the 3 DBS cases and 4 of the 7 CBD cases exhibited capsular splitting (Table 1, Figure 3, C and D). By contrast, in the non-AD group, 2 of 11 patients exhibited capsular splitting (Table 2). In summary, 6 (60%) of the patients in the AD group and 2 (18%) of the patients in the non-AD group exhibited capsular splitting, with a higher prevalence observed in the AD group.
Figure 4 presents the TEM findings. The anterior capsule structure of age-related cataracts was uniform, and the LECs adhered to the anterior capsule. In atopic cataracts, the anterior capsule was slightly thinner than that in age-related cataracts and cracks were observed, particularly along the surface of the capsule. Small cracks were also observed in the central capsule. Numerous vacuoles were observed in the cytoplasm of LECs in atopic cataracts, and the shape of the cell nuclei was deformed. Cracks were observed in all 8 cases of atopic cataracts that were examined but were not found in any of the 10 cases of age-related cataracts.
Figure 4.
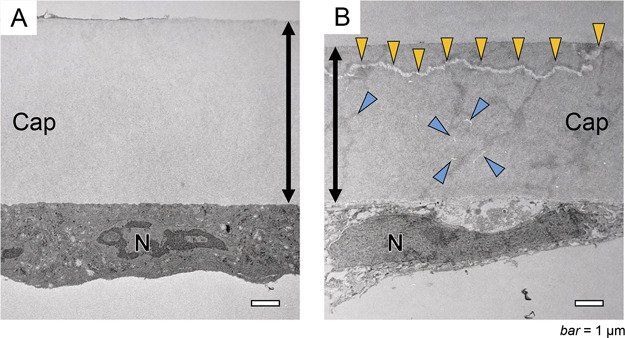
Transmission electron microscope images at the anterior capsule of age-related cataract and atopic cataract. A: Anterior capsule of an age-related cataract. B: Anterior capsule of an atopic cataract. The anterior capsule of the atopic cataract is thinner than that of age-related cataract. Cracks (yellow arrowheads) are observed along the surface of the capsule. Small cracks (blue arrowheads) are also observed in the middle of the capsule. Cap = anterior capsule; N = cell nucleus of lens epithelial cell
DISCUSSION
To our knowledge, this is the first study to investigate the pathological characteristics of the lens capsule in patients with IOL dislocations associated with AD. In the AD group, we observed a high prevalence of abnormal LECs and a low prevalence of high-density fibrosis. By contrast, low-density fibrosis was observed at a high rate, suggesting abnormalities in the LEC morphology and weak fibrosis of the lens capsule. Furthermore, capsular splitting, which is considered a characteristic pathological finding of DBS, was observed relatively frequently in the AD group, irrespective of the DBS or CBD presence. Typical DBS is reported not to show the Soemmering ring, but AD combined with DBS did show the Soemmering ring. In addition, TEM of the anterior capsule removed during cataract surgery in patients with AD revealed abnormalities in LEC morphology and existing cracks within the capsule. These findings suggest that the AD group may have had a unique vulnerability to the lens capsule, which may have progressed even after cataract surgery.
Yamamoto et al. reported that major basic protein (MBP), a toxin released from eosinophils present in the anterior chamber in AD, damages LECs and contributes to cataract development.10 MBP adheres to and invades cell membranes, leading to membrane disruption due to lysosome accumulation, causing cytotoxicity.11,12 Patients with AD have elevated serum levels of MBP, which, when the blood–aqueous barrier is compromised, increases its concentration in the aqueous humor, damaging the LECs and leading to the development of specific cataracts at a young age.13 Iida et al. also mentioned the possibility that the thinning associated with AD-related cataracts might be due to LEC damage from toxins such as MBP, resulting in lens protein leakage and incomplete lens fiber formation.14 We hypothesized that toxins such as MBP present in the anterior chamber fluid in AD cases continue to damage the remaining LECs after cataract surgery, potentially contributing to the dislocation of the IOL. Our hypotheses suggest 2 potential mechanisms: (1) damage to LECs by agents such as MBP may reduce fibrosis through LEC metaplasia after cataract surgery. This could further exacerbate the preexisting vulnerability of the lens capsule, possibly leading to DBS. (2) MBP may also harm ciliary epithelial cells, contributing to the progression of zonular ligament rupture and potentially leading to CBD.
Furthermore, as traditionally believed, AD may increase the risk of damage to the zonular ligaments due to mechanical irritation caused by itching and rubbing of the eyelids, and additionally, rubbing of the eyeball can induce failure of the blood–aqueous barrier and potentially increase the concentration of MBP in the aqueous humor.10
The severity of AD varies among cases, leading to individual differences in the risk and pathology of IOL dislocation. In other words, owing to the varying concentrations of MBP in the anterior chamber fluid and different degrees of mechanical irritation from rubbing, a stronger zonular ligament defect may result in CBD formation and a stronger lens capsule fragility may result in DBS formation.15 Consequently, IOL dislocation in conjunction with AD is considered to involve 2 factors: damage to the lens capsule itself by anterior chamber toxins and zonular ligament damage by both anterior chamber toxins and mechanical irritation. To prevent IOL dislocation, it is considered necessary to lower the concentration of anterior chamber toxins, avoid mechanical irritation from rubbing, and consider initial cataract surgery methods that prevent IOL insertion within the capsule. Interestingly, although atopic cataracts show fibrous formation similar to that seen in anterior subcapsular cataracts, in this study, the AD group tended to show a lower prevalence of high-density fibrosis. We consider that the fibrous hyperplasia under the anterior capsule during cataract formation and the low fibrosis across the entire lens capsule after cataract surgery in the AD group may have formed through different mechanisms, although the detailed mechanisms remain unclear.
This study had several limitations. First, it was conducted at a single institution with a limited sample size. Particularly in DBS cases, due to the fragility of the extracted lens capsule, there was a shortage of specimens, which made it difficult to increase the number of cases. Second, information regarding the presence or absence of AD in each case was obtained from the medical records, which included self-reports. Consequently, there may be cases in which a diagnosis of AD or non-AD was not accurate. To investigate the detailed morphological features of the mechanism of IOL dislocation in AD, further studies are required to confirm the relationship between IOL dislocation in AD and MBP with a larger number of cases and using standardized specimen extraction techniques. Furthermore, the involvement of myofibroblasts in CBD has been reported in pseudoexfoliation syndrome.16 In this study, we observed low-density fibrosis in the AD group and a qualitative comparison of myofibroblasts in the capsules of the AD and non-AD groups may be useful.
To our knowledge, this is the first study to investigate the lens capsule pathological characteristics in patients with IOL dislocation and AD. Compared with the non-AD group, the AD group had a higher incidence of LEC morphological changes, low-density fibrosis around the lens capsule, and capsular splitting, which are characteristic of DBS. AD may possibly cause pathological changes in the lens capsule and surrounding tissues that are not caused by other background factors.
WHAT WAS KNOWN
IOL dislocation associated with atopic dermatitis (AD) is considered to result from the progression of zonular dialysis due to ocular itching and rubbing; capsular bag dislocation and dead bag syndrome-induced IOL dislocations have been reported.
The true pathogenesis and pathological characteristics of AD-associated IOL dislocation morphologies have not been elucidated.
WHAT THIS PAPER ADDS
Compared with the non-AD group, the AD-associated IOL dislocation group frequently exhibited changes in lens epithelial cell morphology, low-density fibrosis around the lens capsule, and capsular splitting characteristics of dead bag syndrome, regardless of the dislocation morphology.
AD-associated IOL dislocation may involve pathological changes in the lens capsule and its surrounding tissues that are not present in other background factors.
Acknowledgments
We thank the patients for their participation in this study. We express our deepest gratitude to Dr. Akira Watanabe, Dr. Shumpei Ogawa, Dr. Tomoyuki Watanabe, Dr. Euido Nishijima, and Dr. Kokoro Konuma for their cooperation in providing surgical specimens. We also express our deepest gratitude to Dr. Liliana Werner and Dr. Nick Mamalis for their invaluable guidance and expertise in DBS diagnosis for Case 3.
Footnotes
Presented at The 62nd Annual Meeting of the Japanese Society for Cataract Research/The 49th Annual Meeting of the Japanese Society for Crystalline Lens Research, Morioka, Japan, June 2023; and The 38th Annual Meeting of the Japanese Society of Cataract and Reflective surgery (JSCRS), Sapporo, Japan, July 2023.
Disclosures: None of the authors have any financial or proprietary interest in any material or method mentioned.

First author:
Koji Komatsu, MD, MBA
Department of Ophthalmology, The Jikei University School of Medicine, Minato-ku, Tokyo, Japan
Contributor Information
Koji Komatsu, Email: koji.0406.kk0000@gmail.com.
Ai Iwauchi, Email: ai.iwauchi@gmail.com.
Hoshiho Kubota, Email: utsubotto@gmail.com.
Masanobu Iida, Email: jmasa94@gmail.com.
Kosuke Ichihara, Email: kosuke.violin@gmail.com.
Masami Iwamoto, Email: iwmt.m33@gmail.com.
Kenji Kawai, Email: k-kawai@is.icc.u-tokai.ac.jp.
Naoki Yamamoto, Email: naokiy@fujita-hu.ac.jp.
Masayuki Shimoda, Email: shimoda@keio.jp.
Tadashi Nakano, Email: tnakano@ca2.so-net.ne.jp.
REFERENCES
- 1.Kristianslund O, Dalby M, Drolsum L. Late in-the-bag intraocular lens dislocation. J Cataract Refract Surg 2021;47:942–954 [DOI] [PubMed] [Google Scholar]
- 2.Darian-Smith E, Safran SG, Coroneo MT. Zonular and capsular bag disorders: a hypothetical perspective based on recent pathophysiological insights. J Cataract Refract Surg 2023;49:207–212 [DOI] [PubMed] [Google Scholar]
- 3.Culp C, Qu P, Jones J, Fram N, Ogawa G, Masket S, Mamalis N, Werner L. Clinical and histopathological findings in the dead bag syndrome. J Cataract Refract Surg 2022;48:177–184 [DOI] [PubMed] [Google Scholar]
- 4.Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab 2015;66:8–16 [DOI] [PubMed] [Google Scholar]
- 5.Hsu JI, Pflugfelder SC, Kim SJ. Ocular complications of atopic dermatitis. Cutis 2019;104:189–193 [PubMed] [Google Scholar]
- 6.Pietruszyńska M, Zawadzka-Krajewska A, Duda P, Rogowska M, Grabska-Liberek I, Kulus M. Ophthalmic manifestations of atopic dermatitis. Postepy Dermatol Alergol 2020;37:174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamazaki S, Nakamura K, Kurosaka D. Intraocular lens subluxation in a patient with facial atopic dermatitis. J Cataract Refract Surg 2001;27:337–338 [DOI] [PubMed] [Google Scholar]
- 8.Bassily R, Lencova A, Rajan MS. Bilateral rupture of the posterior capsule and intraocular lens dislocation from excessive eye rubbing. J Cataract Refract Surg 2016;42:329–331 [DOI] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–1499 [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto N, Hiramatsu N, Isogai S, Kondo M, Imaizumi K, Horiguchi M. Mechanism of atopic cataract caused by eosinophil granule major basic protein. Med Mol Morphol 2020;53:94–103 [DOI] [PubMed] [Google Scholar]
- 11.Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol 1986;39:177–253 [DOI] [PubMed] [Google Scholar]
- 12.Abu-Ghazaleh RI, Gleich GJ, Prendergast FG. Interaction of eosinophil granule major basic protein with synthetic lipid bilayers: a mechanism for toxicity. J Membr Biol 1992;128:153–164 [DOI] [PubMed] [Google Scholar]
- 13.Morita H, Yamamoto K, Kitano Y. Elevation of serum major basic protein in patients with atopic dermatitis. J Dermatol Sci 1995;9:165–168 [DOI] [PubMed] [Google Scholar]
- 14.Iida M, Masuda Y, Sano K, Ichihara K, Komatsu K, Shiba T, Iwaki H, Oki K, Tatemichi M, Nakano T. Lens thickness in atopic cataract: case-control study. J Cataract Refract Surg 2023;49:853–857 [DOI] [PubMed] [Google Scholar]
- 15.Yokoi N, Hirano S, Okamoto S, Matsumoto Y, Yokoi K, Ikeda T, Kinoshita S, Katoh N, Yasuno H. Association of eosinophil granule major basic protein with atopic cataract. Am J Ophthalmol 1996;122:825–829 [DOI] [PubMed] [Google Scholar]
- 16.Bisevac J, Anisimova NS, Nagymihály R, Kristianslund O, Katta K, Noer A, Sharafetdinov IH, Drolsum L, Moe MC, Malyugin BE, Petrovski G. Long-term myofibroblast persistence in the capsular bag contributes to the late spontaneous in-the-bag intraocular lens dislocation. Sci Rep 2020;10:20532. [DOI] [PMC free article] [PubMed] [Google Scholar]


