Abstract
Copanlisib is a pan-class I phosphoinositide 3-kinase (PI3K) inhibitor with activity against all four PI3K class I isoforms (PI3Kα, PI3Kβ, PI3Kγ, and PI3Kδ). Whole genome and RNA sequencing data have revealed several PI3K aberrations in osteosarcoma tumor samples. The in vivo anticancer effects of copanlisib were assessed in a panel of 6 osteosarcoma models. Copanlisib induced prolonged event-free survival in 5/6 osteosarcoma models, however, all models demonstrated progressive disease suggesting minimal activity. While copanlisib did not result in tumor regression, more data are needed to fully explore the role of the PI3K pathway in the pathogenesis of osteosarcoma.
Introduction
The outcomes for pediatric patients diagnosed with metastatic and/or refractory osteosarcoma continue to be poor and have not changed in several decades despite extensive preclinical and clinical investigation. The 5 year event-free-survival (EFS) for patients with newly diagnosed metastatic osteosarcoma is approximately 20% with current treatment regimens.1–3 Patients who recur following frontline conventional chemotherapy and surgical resection fare even worse with a 4 month EFS below 20%, although in some studies EFS has been shown to be as low as 12%.4,5 Novel therapies are desperately needed to drive improvements in outcomes for this very high risk patient population.
Copanlisib is a pan-class I phosphoinositide 3-kinase (PI3K) inhibitor with activity against all four PI3K class I isoforms (PI3Kα, PI3Kβ, PI3Kγ, and PI3Kδ). Copanlisib received accelerated approval by the Food and Drug Administration in 2017 for the treatment of adult patients with relapsed follicular lymphoma who have received at least two prior systemic therapies. The NCI-MATCH phase 2 trial of copanlisib for patients with PIK3CA mutations met its primary endpoint with an objective response rate of 16%.6
Preclinical data have suggested that deregulation of the PI3K pathway may be a key driver of multiple malignancies, including osteosarcoma.7–10 The relevance of this pathway in osteosarcoma tumorigenesis remains a subject of debate and active research, but whole genome and RNA sequencing data have revealed aberrations in genes affecting the PI3K pathway in osteosarcoma tumor samples.7,9,10 One study reported PI3K/mTOR pathway alterations in 24% of osteosarcoma tumors evaluated.9 Patient derived xenograft (PDX) models are commonly utilized to assess the potential activity of anti-cancer agents in osteosarcoma. In this study, the in vivo efficacy of copanlisib were assessed in osteosarcoma PDX models by the Pediatric Preclinical Testing Consortium (PPTC).
Materials and Methods
The in vivo anticancer effects of copanlisib were assessed in a panel of 6 osteosarcoma models (OS2, OS9, OS31, OS33, OS36, OS60). None of the models exhibited evidence of PI3KCA alteration based on whole exome sequencing, although a PTEN-DGKB fusion was identified in OS33. PDX models were heterotopically injected into the flanks of CB17SC scid−/− female mice (Envigo, Indianapolis, IN). Copanlisib was administered at a dose of 10 mg/kg/dose administered by intravenous bolus (IV), dosed two days on, 5 days off, and repeated weekly x 4 weeks to PDX cohorts (n=10). Tumor volumes were measured as previously described, and a control cohort of mice (n=10) that was administered vehicle was utilized for each PDX model.9 During testing, all mice were maintained under barrier conditions, and experiments were conducted using protocols approved by the Institutional Animal Care and Use Committees at MD Anderson Cancer Center. Copanlisib’s activity was evaluated using standard PPTC measures, including time to event (EFS T/C), tumor growth delay (tumor volume T/C), and objective responses as previously described.9 The exact long-rank test (Proc StatXaxt for SAS®) was used to compare EFS distributions between treatment and control groups. P-values were two-sided and were considered statistically significant if <0.05.
Results
Copanlisib was well tolerated in vivo in the osteosarcoma PDX models with minimal weight loss and no treatment related mortality as compared to controls. Copanlisib induced prolonged EFS in 5/6 osteosarcoma models (p<0.05, Gehan-Wilcoxon) (Figure 1). Tumor regression (mean minimum attained relative tumor volume (minRTV) < 1.0) was not observed for any of the tested models. 3/6 models exhibited lower min RTV compared to untreated controls (p < 0.05, Wilcoxon rank sum). All osteosarcoma models showed “Progressive Disease 1 (PD1)” as their objective response measure (< 50% tumor regression, > 25% tumor growth, time-to-event < 200%, the KM median in control) (Table 1).
Figure 1. Event-free Survival in response to Copanlisib as a single agent across all osteosarcoma tumor models.
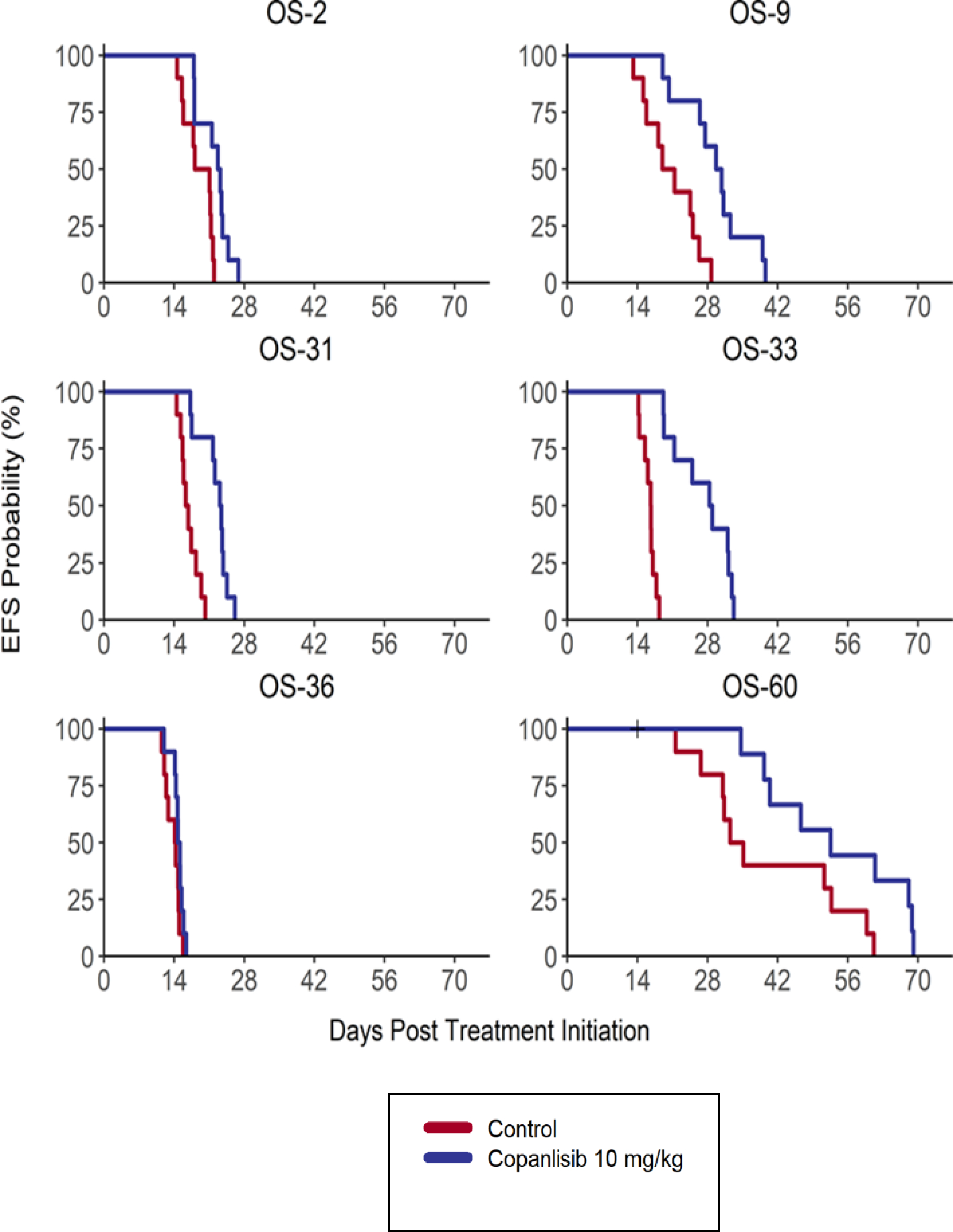
Treatment groups are as described in the text and as shown in the legend.
Table 1: Copanlisib as single agent vs. control (N=10/agent).
Tumor regression (mean minimum attained relative tumor volume (minRTV) < 1.0) was not observed for any of the tested models. 3/6 models exhibited lower min RTV compared to untreated controls (p < 0.05, Wilcoxon rank sum). All osteosarcoma models showed Progressive Disease 1 (PD1) as their objective response measure.
| Cancer Type | Model | Agent | KM med (days) | EFS T - C (days) | EFS T/C | p-value Gehan-Wilcoxon | Area Over Curve | minRTV mean±SD | minRTV p-value | Objective Response Measure+ |
|---|---|---|---|---|---|---|---|---|---|---|
| Osteosarcoma | OS-2 | Control | 19.6 | 8.2 | 1.735±0.127 | PD | ||||
| Copanlisib | 23.0 | 3.4 | 1.17 | p = 0.020 | 11.3 | 1.334±0.202 | p < 0.001 | PD1 | ||
| OS-9 | Control | 20.2 | 10.0 | 1.531±0.539 | PD | |||||
| Copanlisib | 30.2 | 10.1 | 1.5 | p = 0.002 | 14.8 | 1.249±0.337 | p = 0.143 | PD1 | ||
| OS-31 | Control | 16.5 | 7.7 | 1.621±0.187 | PD | |||||
| Copanlisib | 23.3 | 6.7 | 1.41 | p < 0.001 | 9.8 | 1.446±0.182 | p = 0.052 | PD1 | ||
| OS-33 | Control | 16.6 | 7.6 | 1.757±0.201 | PD | |||||
| Copanlisib | 28.6 | 12.0 | 1.72 | p < 0.001 | 13.7 | 1.357±0.117 | p < 0.001 | PD1 | ||
| OS-36 | Control | 14.2 | 5.5 | 2.353±0.207 | PD | |||||
| Copanlisib | 14.9 | 0.7 | 1.05 | p = 0.109 | 6.0 | 2.264±0.163 | p = 0.123 | PD1 | ||
| OS-60 | Control | 33.8 | 14.7 | 1.361±0.178 | PD | |||||
| Copanlisib | 52.5 | 18.7 | 1.55 | p = 0.039 | 27.0 | 1.017±0.123 | p < 0.001 | PD1 |
Conclusion
The outcomes for recurrent or refractory osteosarcoma remain poor despite extensive in vitro, in vivo, and clinical evaluation of multiple novel agents without improvement in outcome over several decades. In vivo testing in pediatric preclinical sarcoma models remains an efficient approach to rapidly screen agents of interest and generate data to support the development of vitally needed clinical trials for this patient population. Copanlisib held promise as a potential novel agent to evaluate in osteosarcoma due to preclinical data from whole genome and RNA sequencing data identifying multiple PI3K pathway aberrations in osteosarcoma tumor samples.68 In this study from the Pediatric Preclinical Testing Consortium, copanlisib induced prolonged EFS in 5/6 osteosarcoma models, but did not yield tumor regression in any of the osteosarcoma PDX models as compared to controls, with all models developing progressive disease with only modest slowing of tumor growth, suggesting copanlisib has minimal activity in osteosarcoma. The modest slowing of tumor growth observed with copanlisib treatment is similar to the preclinical results observed for osteosarcoma models using other PI3K inhibitors,11,12 AKT inhibitors,13,14 rapamycin,15 and MTOR kinase inhibitors.16 Interestingly, the most profound treatment effect against osteosarcoma preclinical models among these agents targeting the PI3K pathway appeared to be for rapamycin.15
Genomic alterations in the PI3K pathway are present in multiple malignancies, including osteosarcoma, for which the pathway is thought to drive tumorigenesis, metastasis, and chemoresistance in a subset of tumors through the upregulation of downstream oncogenic drivers such as mTORC1 and mTORC2, c-Myc, MDM2, RB, TP53, among others.9,10 mTORC1 inhibitors such as the rapalogues, sirolimus and temsirolimus, were initially thought to hold promise as potentially well tolerated agents that could block downstream signaling of the AKT pathway in osteosarcoma by inhibiting the major downstream target, mTORC1. However, subsequent work suggested that the impact of rapalogues may be attenuated because when mTORC1 is inhibited by a rapalogue, feedback inhibition then drives increased AKT signaling and subsequent activation of mTORC2, possibly counteracting the anticancer effect.17,18 Potential options to counterbalance this include evaluation of pan-PI3K inhibitors such as copanlisib to down regulate the signaling pathway. Alternatively, dual mTORC1/2 inhibitors such as sapanisertib (MLN0128, TAK228) have been investigated to determine whether they can provide more effective inhibition of PI3K pathway signaling. Disappointingly, in a randomized phase 2 trial for patients with advanced sarcoma comparing sapanisertib to pazopanib, patients receiving sapanisertib had a median PFS of only 2.0 months (compared to 2.1 months for pazopanib) and at 4 months only 5.4% of patients receiving sapanisertib were responding or had stable disease (compared to 13.8% for pazopanib).19
While copanlisib did not result in tumor regression, further study is needed to fully explore the role of the PI3K pathway in the pathogenesis of osteosarcoma and its potential utility as a therapeutic target. One weakness of this current study is that the PDXs evaluated were not selected based on specific PI3KCA mutations, and as such, it remains a possibility that enhanced activity could be seen in OS PDXs that exhibit specific PI3KCA activating mutations. While one PDX model – OS33 – was noted to have a PTEN-DGKB fusion, none exhibited PIK3CA alterations on whole exome sequencing. As none of the 178 osteosarcoma specimens profiled on the PeCan Data Portal have activating mutations in PIK3CA that predict response to PI3K inhibitors,20 both preclinical and clinical evaluation of this line of research will be challenging. Osteosarcoma’s inherent genomic complexity may limit the impact of signaling pathway inhibitors or require multi-targeted approaches that simultaneously inhibit more than one aberrantly activated pathway in order to elicit a response. Further research is required to determine whether either the single or multiple pathway targeting strategies can achieve a favorable therapeutic index.
Abbreviations
- OS
Osteosarcoma
- PPTC
Pediatric Preclinical Testing Consortium
- PDX
Patient Derived Xenograft
- EFS
Event Free Survival
Footnotes
Conflict of Interest: The authors have no conflicts
References:
- 1.Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment - where do we stand? A state of the art review. Cancer treatment reviews. 2014;40(4):523–532. [DOI] [PubMed] [Google Scholar]
- 2.Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert review of anticancer therapy. 2018;18(1):39–50. [DOI] [PubMed] [Google Scholar]
- 3.Goorin AM, Harris MB, Bernstein M, et al. Phase II/III trial of etoposide and high-dose ifosfamide in newly diagnosed metastatic osteosarcoma: a pediatric oncology group trial. J Clin Oncol. 2002;20(2):426–433. [DOI] [PubMed] [Google Scholar]
- 4.Kempf-Bielack B, Bielack SS, Jurgens H, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol. 2005;23(3):559–568. [DOI] [PubMed] [Google Scholar]
- 5.Lagmay JP, Krailo MD, Dang H, et al. Outcome of Patients With Recurrent Osteosarcoma Enrolled in Seven Phase II Trials Through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: Learning From the Past to Move Forward. J Clin Oncol. 2016;34(25):3031–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damodaran S, Zhao F, Deming DA, et al. Phase II Study of Copanlisib in Patients With Tumors With PIK3CA Mutations: Results From the NCI-MATCH ECOG-ACRIN Trial (EAY131) Subprotocol Z1F. J Clin Oncol. 2022:JCO2101648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sayles LC, Breese MR, Koehne AL, et al. Genome-Informed Targeted Therapy for Osteosarcoma. Cancer discovery. 2019;9(1):46–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol. 2018;15(5):273–291. [DOI] [PubMed] [Google Scholar]
- 9.Perry JA, Kiezun A, Tonzi P, et al. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(51):E5564–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Yu X-H, Yan Y-G, Wang C, Wang W-J. PI3K/Akt signaling in osteosarcoma. Clinica Chimica Acta. 2015;444:182–192. [DOI] [PubMed] [Google Scholar]
- 11.Gobin B, Battaglia S, Lanel R, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, inhibits osteosarcoma cell proliferation and tumor development in vivo with an improved survival rate. Cancer letters. 2014;344(2):291–298. [DOI] [PubMed] [Google Scholar]
- 12.Gobin B, Huin MB, Lamoureux F, et al. BYL719, a new alpha-specific PI3K inhibitor: single administration and in combination with conventional chemotherapy for the treatment of osteosarcoma. International journal of cancer. 2015;136(4):784–796. [DOI] [PubMed] [Google Scholar]
- 13.Carol H, Morton CL, Gorlick R, et al. Initial testing (stage 1) of the Akt inhibitor GSK690693 by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2010;55(7):1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houghton PJ, Gorlick R, Kolb EA, et al. Initial testing (stage 1) of the mTOR kinase inhibitor AZD8055 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2012;58(2):191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houghton PJ, Morton CL, Kolb EA, et al. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2008;50(4):799–805. [DOI] [PubMed] [Google Scholar]
- 16.Kang MH, Reynolds CP, Maris JM, et al. Initial testing (stage 1) of the investigational mTOR kinase inhibitor MLN0128 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2014;61(8):1486–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slotkin EK, Patwardhan PP, Vasudeva SD, de Stanchina E, Tap WD, Schwartz GK. MLN0128, an ATP-competitive mTOR kinase inhibitor with potent in vitro and in vivo antitumor activity, as potential therapy for bone and soft-tissue sarcoma. Molecular cancer therapeutics. 2015;14(2):395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer research. 2006;66(3):1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingham M, Mahoney MR, Remotti F, et al. A randomized phase II study of MLN0128 (M) versus pazopanib (P) in patients (pt) with advanced sarcoma (Alliance A091304). Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020;38(15_suppl):11562–11562. [Google Scholar]
- 20.St. Jude Cloud PeCan 2022. https://pecan.stjude.cloud/. Accessed February 21, 2022.


