Abstract
Introduction
We previously reported the significant upregulation of eight circulating exosomal microRNAs (miRNAs) in patients with diabetic kidney disease (DKD). However, their specific roles and molecular mechanisms in the kidney remain unknown. Among the eight miRNAs, we evaluated the effects of miR-5010-5p on renal tubular epithelial cells under diabetic conditions in this study.
Research design and methods
We transfected the renal tubular epithelial cell line, HK-2, with an miR-5010-5p mimic using recombinant plasmids. The target gene of hsa-miR-5010-5p was identified using a dual-luciferase assay. Cell viability was assessed via the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay. Moreover, mRNA and protein expression levels were determined via real-time PCR and western blotting, respectively.
Results
High glucose levels did not significantly affect the intracellular expression of miR-5010-5p in HK-2 cells. Transfection of the miR-5010-5p mimic caused no change in cell viability. However, miR-5010-5p-transfected HK-2 cells exhibited significantly decreased expression levels of inflammatory cytokines, such as the monocyte chemoattractant protein-1, interleukin-1β, and tumor necrosis factor-ɑ, under high-glucose conditions. These changes were accompanied by the restored expression of phosphorylated AMP-activated protein kinase (AMPK) and decreased phosphorylation of nuclear factor-kappa B. Dual-luciferase assay revealed that miR-5010-5p targeted the gene, protein phosphatase 2 regulatory subunit B delta (PPP2R2D), a subunit of protein phosphatase 2A, which modulates AMPK phosphorylation.
Conclusions
Our findings suggest that increased miR-5010-5p expression reduces high glucose-induced inflammatory responses in renal tubular epithelial cells via the regulation of the target gene, PPP2R2D, which modulates AMPK phosphorylation. Therefore, miR-5010-5p may be a promising therapeutic target for DKD.
Keywords: Inflammation, Kidney
WHAT IS ALREADY KNOWN ON THIS TOPIC
We previously reported the significant upregulation of eight circulating exosomal microRNAs in patients with diabetic kidney disease (DKD); however, their specific roles and molecular mechanisms in the kidney remain unknown.
WHAT THIS STUDY ADDS
Among the eight miRNAs, miR-5010-5p-transfected HK-2 cells exhibited significantly decreased expression levels of inflammatory cytokines. miR-5010-5p targeted the PPP2R2D (protein phosphatase 2 regulatory subunit B delta) gene, a regulatory subunit of protein phosphatase 2A, which was responsible for the decreased phosphorylation of AMP-activated protein kinase.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE, OR POLICY
These results showed that miR-5010-5p had a protective effect on renal tubular epithelial cells in diabetic conditions. Therefore, miR-5010-5p can be a therapeutic target in DKD.
Introduction
Chronic kidney disease (CKD) is a public health concern that affects approximately one-tenth of the global population.1 Diabetes mellitus is the leading cause of CKD and is responsible for the progression of kidney failure with replacement therapy.2 Recently, several medications have exhibited promising effects in the management of diabetic kidney disease (DKD).3 4 Despite the advances in treatment, some patients still progress to kidney failure, emphasizing the need to explore the unknown pathophysiology and develop novel treatment approaches for DKD.
MicroRNAs (miRNAs) are small single-stranded non-coding RNAs involved in the regulation of gene expression via post-transcriptional mechanisms. miRNAs regulate at least 60% of the human protein-coding genes.5 Therefore, miRNAs are rapidly emerging as targets for kidney disease research. Several miRNAs are associated with intrarenal inflammation and fibrosis in DKD.6–9 We previously reported circulating miRNAs in the exosomes of patients with DKD.10 In that study, we identified eight miRNAs that were uniquely upregulated in patients with DKD than in the healthy volunteers and patients with diabetes without kidney disease. Recently, a Chinese study validated one of our identified miRNAs, miR-4449, and reported that it regulated the inflammatory cytokine expression, reactive oxygen species levels, and pyroptosis by modulating the hypermethylation of cancer 1 gene.11 However, the specific genes and molecular mechanisms associated with the other miRNAs remain unknown.
In this study, we investigated the molecular mechanism underlying the upregulation of miR-5010-5p expression in patients with DKD. While several studies have documented alterations in the circulating levels of miR-5010-5p across different diseases,12–14 the molecular pathways involving this miRNA have not yet been identified, particularly in DKD. Here, we found that miR-5010-5p mitigated the inflammatory responses of tubular epithelial cells under diabetic conditions by modulating the expression of protein phosphatase 2 regulatory subunit B delta (PPP2R2D).
Methods
Cell culture
Human proximal tubule cells, HK-2 (American Type Culture Collection), were seeded at a density of 2×105 cells/well in a 6-well plate and cultured in the Dulbecco’s modified Eagle’s medium with F12 containing 10% fetal bovine serum (Gibco, Waltham, MA). Cells were cultured at 37°C in a 5% CO2 atmosphere. HK-2 cells were treated with 30 mM d-glucose (Sigma-Aldrich, St. Louis, MO) for 24 hours.
miRNA transfection
HK-2 cells were transfected with the miR-5010-5p mimic or negative control (mirVana; Invitrogen, Waltham, MA) at a concentration of 100 pmol using the Lipofectamine RNAiMAX transfection reagent (Invitrogen) in the Opti-MEM (Gibco) media, according to the manufacturers’ instructions. Cells were transfected for 24 hours, replaced with the serum-free medium, and stimulated with d-glucose the following day.
Luciferase assay
We used TargetScan Human (V.8.0; https://targetscan.org) to identify the target genes of miR-5010-5p. To confirm the targets of several candidate genes, we performed a luciferase assay using the Gaussia luciferase/secreted alkaline phosphatase (GLuc/SEAP) dual-reporter vector (Secrete-Pair Dual Luminescence Assay; GeneCopoeia, Rockville, MD). Target clone for the 3′-untranslated region (UTR) of PPP2R2D gene or control clone was inserted into the vector. After 24 hours of transfection, the miR-5010-5p mimic and negative control were cotransfected for another 24 hours. Luminescence intensities of GLuc and SEAP were measured in the culture media, and SEAP was used as an internal control to compare the relative intensities of GLuc.
Cell viability assay
HK-2 cells were plated in a 96-well plate at a density of 1×104 cells/well at 37°C and 5% CO2 for 48 hours, and 50 µL of 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent (Abcam, Cambridge, UK) was added to each well. After 3 hours of incubation at 37°C, absorbance was measured at an optical density (OD) of 590 nm. The percentage of cell viability was calculated as follows: cell viability (%) = (ODsample–ODblank)/(ODcontrol–ODblank)×100.
Single-nucleus RNA sequencing data analysis
The Seurat object was obtained from the Kidney Precision Medicine Project (KPMP) data repository (https://atlas.kpmp.org/repository). We downloaded the dataset file (WashU-UCSD_KPMP-Biopsy_10X-R_05142021.h5Seurat), for which the KPMP team performed quality control and cluster annotation already. Seurat package (V.4.3.1) in R (V.4.3.0; The R Foundation for Statistical Computing, Vienna, Austria) was used for analysis. The DimPlot function was used for the Uniform Manifold Approximation and Projection plot. The FeaturePlot and VlnPlot functions were used to examine the gene expression of PPP2R2D in each cell cluster. The DotPlot function was used to compare gene expression patterns of regulatory subunits of protein phosphatase 2A (PP2A) in cell clusters.
Enzyme activity of PP2A
PP2A activity was measured using the serine/threonine phosphatase assay kit (Millipore, Massachusetts, USA), according to the manufacturer’s protocol. Briefly, the total protein concentration of the cell lysate was measured using the Bicinchoninic acid (BCA) reagent for normalization. Samples were mixed with the serine/threonine assay buffer (50 mM Tris–HCl, pH 7.0, and 100 µM CaCl2) and 25 µL of the mixture was added to each well of a 96-well plate, followed by the addition of 100 µL of malachite green solution and incubation for 15 min at room temperature for color development. Absorbance was measured at OD 650 nm, and phosphate release was calculated using a phosphate standard curve generated using a phosphate standard (KH2PO4).
Reverse transcription (RT)-PCR
RT-PCR was performed to determine changes in mRNA and miRNA expression levels. Total RNA was extracted from the cells using the TRIzol Reagent (Life Technologies, Carlsbad, California, USA) and reverse-transcribed to cDNA using the StepOne Plus Real-Time PCR System (Applied Biosystems, Massachusetts, USA). TaqMan Assays-on-Demand Gene Expression Product (Applied Biosystems) was used for mRNAs (interleukin-1β (IL-1β), Hs01555410_m1; monocyte chemoattractant protein-1 (MCP-1), Hs00234140_m1; tumor necrosis factor-ɑ (TNF-α), Hs00174128_m1; PPP2R2D, Hs00908762_g1) and miRNAs (hsa-miR-5010-5p, 476643_mat; hsa-miR-4449, 463005_mat; hsa-miR-1246, 462575_mat; hsa-miR-642a-3p, 474715_mat; hsa-let-7c, 000379; hsa-miR-1255B, 002801; hsa-let-7i, 002172). Glyceraldehyde 3-phosphate dehydrogenase was used as a housekeeping gene for the internal control for mRNA, whereas RNU48 was used for miRNAs. Relative mRNA expression changes were determined using the ΔΔCt method.
Western blotting
Cells were lysed using the radioimmunoprecipitation assay buffer (CureBio, Seoul, Korea) and a protease inhibitor cocktail (Sigma-Aldrich). Protein quantification was performed using a BCA reagent. Lysates mixed with the sodium dodecyl sulfate–polyacrylamide gel electrophoresis buffer run in gels (Bio-Rad, California, USA), transferred to membranes, and incubated with the following primary antibodies: PPP2R2D (1:1000; Invitrogen), total AMP-activated kinase (AMPK; 1:1000; Cell Signaling, MA, USA), phosphorylated AMPK (pAMPK) (1:1000, Cell Signaling), total nuclear factor-kappa B (NF-κB; p65; 1:1000, Cell Signaling), and phosphorylated NF-κB (pNF-κB; 1:1000; Cell Signaling). After incubating with the secondary antibodies (antirabbit 1:5000; Cell Signaling), a chemiluminescent reagent (Thermo Scientific, Illinois, USA) was added to the membranes, and the density of the bands was measured using ImageQuant LAS 500 (Cytiva, Massachusetts, USA).
Statistical analyses
All experiments were repeated at least thrice. Data are expressed as the mean±SD, and differences between groups were analyzed using a one-way analysis of variance. Post-hoc analysis between groups was performed using Tukey’s test. Statistical significance was set at p<0.05. All statistical analyses were performed using SPSS (V.22.0; IBM, Armonk, New York, USA).
Results
Expression levels of DKD-associated miRNAs are not increased in HK-2 cells
We previously reported that the levels of eight miRNAs (miR-4449, miR-1246, miR-642a-3p, let-7c-5p, miR-1255b-5p, let-7i-3p, miR-5010-5p, and miR-150–3 p) were significantly upregulated in the circulating exosomes of patients with DKD.10 Therefore, to determine whether this upregulation of miRNA expression is due to their increased expression in the kidneys, we determined their expression levels in HK-2 cells under high glucose conditions in this study. We observed no significant differences in the expression levels of the eight miRNAs on treatment with 30 mM of d-glucose in HK-2 cells (online supplemental figure S1). These findings suggest that the increased levels of circulating miRNAs in patients were not due to increased secretion by the kidney tubules.
bmjdrc-2023-003784supp001.pdf (39.2KB, pdf)
Transfection of miR-5010-5p ameliorates the inflammatory response in HK-2 cells under high glucose conditions
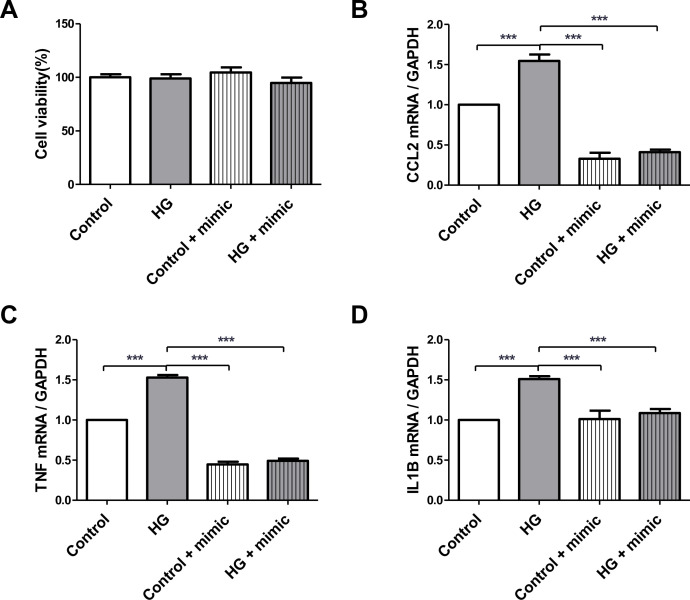
To identify the effects of high miRNA concentrations on HK-2 cells, we transfected HK-2 cells with an miRNA mimic. HK-2 cells were transfected with the miR-5010-5p mimic and subsequently treated with 30 mM d-glucose for 24 hours. MTT assay showed that the cell viability remained high in all groups (figure 1A). However, RT-PCR revealed that the expression levels of proinflammatory cytokines, such as the MCP-1, TNF-α, and IL-1β, which were increased by high glucose stimulation, were significantly attenuated by miR-5010-5p transfection (figure 1B–D). These results suggest that miR-5010-5p exerts a protective effect by reducing the inflammatory response in renal tubular epithelial cells under diabetic conditions.
Figure 1.
Transfection of miR-5010-5p mimic maintains the cell viability and decreases the expression levels of proinflammatory cytokines in HK-2 cells. (A) MTT assay was used to determine the cell viability after transfection. RT-PCR was used to determine the mRNA expression levels of the (B) RT-PCR for the mRNA expressions of MCP-1, (C) TNF-α, and (D) IL-1β. HK-2 cells were transfected with 100 pmol of miR-5010-5p mimic and treated with 30 mM of d-glucose. ***p<0.001. IL-1β, interleukin-1β; MCP-1, monocyte chemoattractant protein-1; MTT, 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide; RT, reverse transcription; TNF-α, tumor necrosis factor-α; HG, high glucose; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase; CCL2, chemokine (C-C motif) ligand 2.
miR-1050-5p inhibits the expression of PPP2R2D
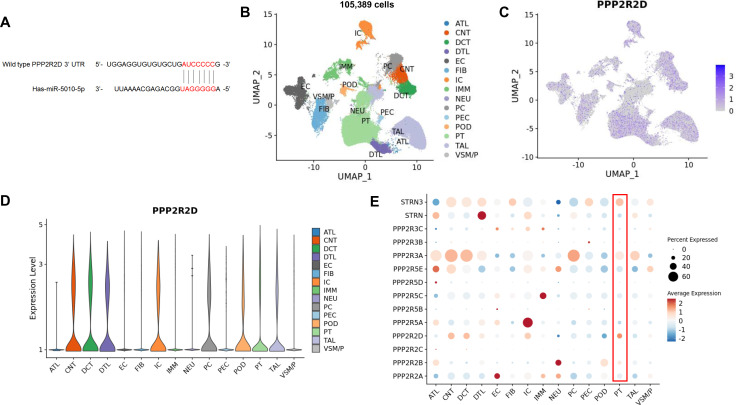
To gain further insight into the molecular pathways involved in the changes in HK-2 cells following miR-5010-5p transfection, we examined the target genes of miR-5010-5p using the TargetScan database. Among several candidate genes, we identified 3′-UTR of PPP2R2D as having a potential binding site of miR-5010-5p (figure 2A). To identify whether PPP2R2D gene is expressed in human kidney tubule, we used the transcriptome of 105 389 cells from 19 human kidney samples (3 healthy controls, 6 cases of acute kidney injury, and 10 cases of CKD) obtained from the KPMP data repository (https://atlas.kpmp.org/repository). Among the 15 clusters of kidney cells (figure 2B), we found PPP2R2D gene was generally expressed in all types of tubular clusters, from proximal tubular epithelial cells to collecting duct cells (figure 2C,D). Among the several regulatory subunits of PP2A, we observed that PPP2R2D is one of the highly expressed subunits in proximal tubular epithelial cells (figure 2E). In addition, when we further examined the protein expression of PPP2R2D, we found that PPP2R2D was exclusively expressed in tubular epithelial cells, but not in glomerular cells, in the pathology data from the Human Protein Atlas (https://www.proteinatlas.org/ENSG00000175470-PPP2R2D/tissue/kidney). Taken together, these data established the presence of both gene and protein expressions of PPP2R2D within human proximal tubular epithelial cells.
Figure 2.
PPP2R2D is expressed in human renal tubular epithelial cells. (A) Candidate sequence of wild-type PPP2R2D complementary to miR-5010-5p. Candidate target genes complementary to miR-5010-5p were searched using TargetScan (https://targetscan.org). (B) UMAP plot of 105 389 kidney cells from the KPMP data (https://atlas.kpmp.org/repository). (C) Feature plot of the expression of PPP2R2D in clusters. (D) Violin plot of the expression of PPP2R2D in clusters. (E) Dot plot of the expression pattern of regulatory subunits of PP2A in clusters. ATL, ascending thin limb; CNT, connecting tubule; DCT, distal convoluted tubule; DTL, descending thin limb; EC, endothelial cell; FIB, fibroblast; IC, intercalated cell; IMM, immune cell; NEU, neural cell; PC, principal cell; PEC, parietal epithelial cell; POD, podocyte; PT, proximal tubule epithelial cell; TAL, thick ascending limb; VSM/P, vascular smooth muscle cell/pericyte. The results here in a part are based on data generated by KPMP: DK133081, DK133091, DK133092, DK133093, DK133095, DK1330971, DK114866, DK114908, DK133090, DK133113, DK133766, DK133768, DK114907, DK114920, DK114923, DK114933, DK114886. https://www.kpmp.org. Data were downloaded on July 10, 2023. KPMP, Kidney Precision Medicine Project; PP2A, protein phosphatase 2A; PPP2R2D, protein phosphatase 2 regulatory subunit B delta; UMAP, Uniform Manifold Approximation and Projection.
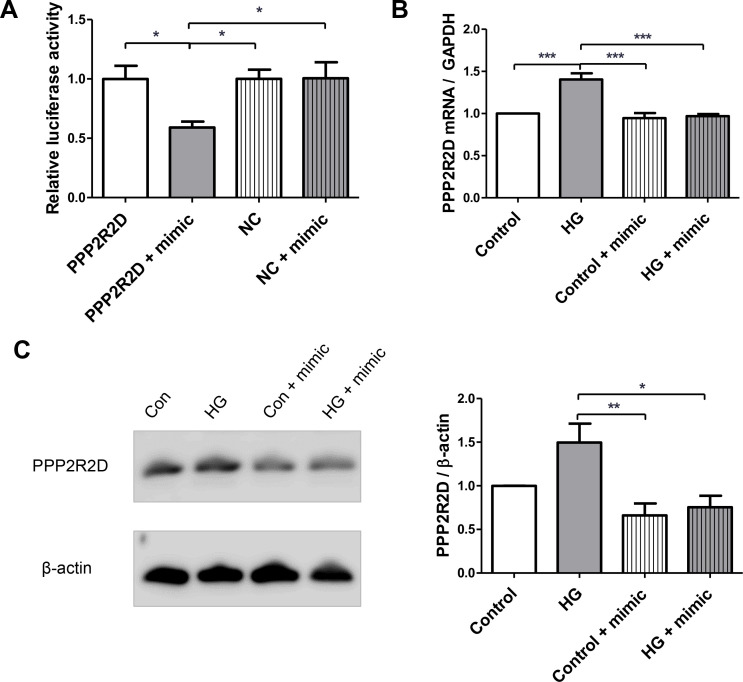
Therefore, to further validate the interaction between miR-5010-5p and PPP2R2D gene, clones targeting 3′-UTR of PPP2R2D containing complementary sequence of miR-5010-5p were inserted into reporter vectors, and we transfected them into HK-2 cells. When we cotransfected the miR-5010-5p mimic with the reporter vectors, we observed a significant decrease in relative luciferase activity, whereas no difference was observed when the mimic was cotransfected with the control vectors (figure 3A). Furthermore, RT-PCR revealed that the mRNA expression level of PPP2R2D was significantly increased under high glucose stimulation, but this increase was attenuated by miR-5010-5p transfection in HK-2 cells (figure 3B). Consistent with mRNA expression, the significantly increased protein expression of PPP2R2D induced by high glucose was decreased by transfection with miR-5010-5p (figure 3C).
Figure 3.
miR-5010-5p targets the PPP2R2D. (A) Dual luciferase assay was used to identify the target genes of miR-5010-5p. Both GLuc and SEAP reporters targeted the 3′ -untranslated region of PPP2R2D or negative control. HK-2 cells were transfected with the miR-5010-5p mimic for 24 hours. Relative activities of GLuc/SEAP were compared between groups. (B) Reverse transcription-PCR was used to determine the mRNA expression of PPP2R2D. (C) Western blotting was used to determine the protein expression of PPP2R2D. HK-2 cells were transfected with 100 pmol of miR-5010-5p mimic and treated with 30 mM of d-glucose. ***p<0.001, **p<0.01, and *p<0.05. GLuc, Gaussia luciferase; PPP2R2D, protein phosphatase 2 regulatory subunit B delta; SEAP, secreted alkaline phosphatase; NC, negative control.
Transfection of miR-5010-5p modulates the phosphorylation of AMPK and NF-κB
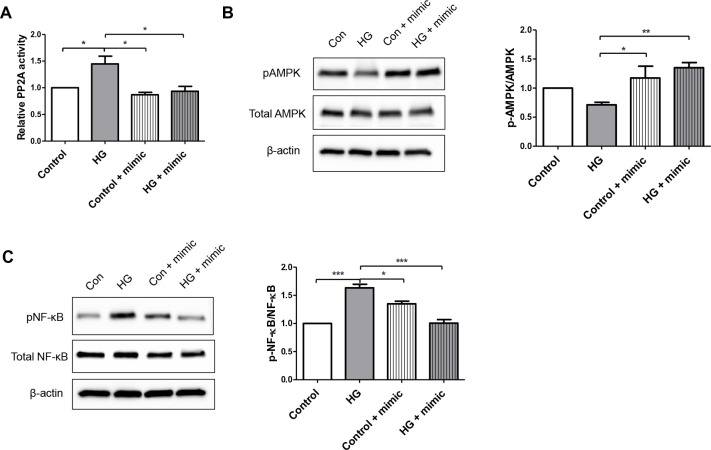
Since PPP2R2D is a regulatory subunit of PP2A, we examined the effect of inhibition of miR-5010-5p for the expression of PPP2R2D on the enzyme activity of PP2A. We observed that the increased phosphatase activity of PP2A induced by high glucose stimulation was mitigated by transfection of miR-5010-5p in HK-2 cells (figure 4A). Furthermore, previous studies have reported that PP2A along with PPP2R2D directly interacts with AMPK and decreases its kinase activity through dephosphorylation.15 16 Western blotting analysis showed that the protein expression of pAMPK tended to be lower in HK-2 cells stimulated with high glucose, but transfection with miR-5010-5p significantly increased the expression of pAMPK (figure 4B). AMPK is also known to inhibit NF-κB signaling and inflammation via several pathways.17 Therefore, we could also observe that the increased expression of pNF-κB induced by high glucose in HK-2 cells was ameliorated by the transfection of miR-5010-5p, which likely contributed to the decreased expression of pro-inflammatory cytokines (figure 4C).
Figure 4.
Transfection with miR-5010-5p mimic changes the PP2A activity and protein expression levels of AMPK and NF-κB. (A) Serine/threonine phosphatase assay was used to determine the activity of PP2A. Western blotting was used to determine the protein expression levels of total and phosphorylated (B) AMPK and (C) NF-κB (p65). HK-2 cells were transfected with 100 pmol of miR-5010-5p mimic and treated with 30 mM of d-glucose. ***p<0.001, **p<0.01, and *p<0.05. AMPK, AMP-activated protein kinase; NF-κB, nuclear factor-kappa B; pAMPK, phosphorylated AMPK; pNF-κB, phosphorylated NF-κB; PP2A, protein phosphatase 2A; HG, high glucose.
Discussion
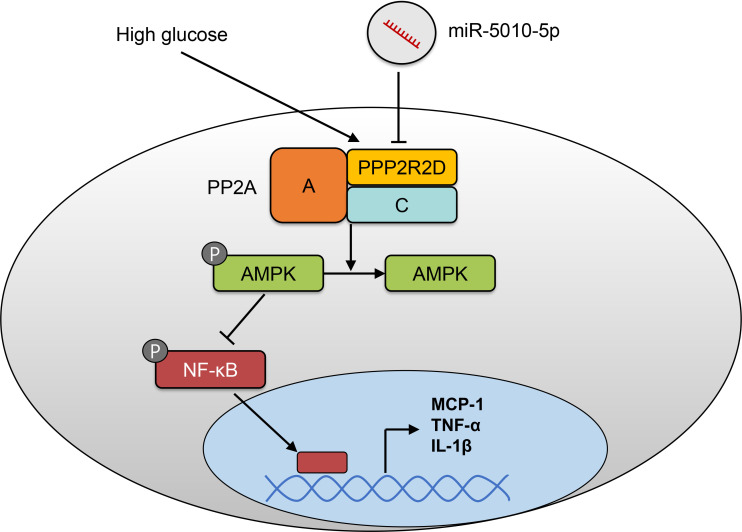
In this study, we demonstrated that miR-5010-5p is associated with the inflammatory response in high glucose-stimulated HK-2 cells under high glucose stimulation. Overexpression of miR-5010-5p in HK-2 cells significantly attenuated the increased expression of proinflammatory cytokines induced by high glucose. Luciferase reporter assay revealed that miR-5010-5p directly targets and inhibits the gene expression of PPP2R2D, which is a regulatory subunit of PP2A. In addition, transfection with miR-5010-5p decreased the phosphatase activity of PP2A, subsequently increasing the expression of pAMPK and decreasing the expression of pNF-κB, which might result in decreased expression of pro-inflammatory cytokines (figure 5).
Figure 5.
Schematic of the molecular pathway underlying the effects of miR-5010-5p. AMPK, AMP-activated protein kinase; IL-1β, interleukin-1β; MCP-1, monocyte chemoattractant protein-1; TNF-α, tumor necrosis factor-α; NF-κB, nuclear factor-kappa B; PP2A, protein phosphatase 2A; PPP2R2D, protein phosphatase 2 regulatory subunit B delta.
Numerous miRNAs have been reported to be altered during the pathogenesis of DKD, and the underlying molecular mechanisms have been identified.18 In general, miRNAs upregulated in DKD are associated with increased fibrosis and inflammatory responses in the kidneys, whereas downregulated miRNAs have a protective effect in the kidneys. However, miR-5010-5p was one of the miRNAs that was upregulated in DKD but exhibited a protective effect. Another miRNA we discovered to be upregulated in DKD, Let-7c family, also showed a beneficial effect by attenuating fibrosis in the kidney via the transforming growth factor-β pathway.19 These increased levels of miRNAs could counteract the increased renal fibrosis and inflammation in DKD, making them promising therapeutic targets for the treatment of DKD.
PP2A is a major class of serine/threonine protein phosphatase consisting of three subunits: A (scaffolding subunit), B (regulatory subunit), and C (catalytic subunit). PPP2R2D is a regulatory subunit of PP2A that modulates the phosphatase activity of the catalytic subunit of PP2A. It was initially recognized for its involvement in mitosis by modulating the phosphorylation of cell cycle proteins.20 In addition, PPP2R2D has been linked to the proliferation and apoptosis of T cells and pathogenesis of cancer and autoimmune diseases.21 22 Moreover, PPP2R2D regulated AMPK activity. PP2A inhibits AMPK activation.23 Among several regulatory subunits of PP2A, Joseph et al reported that PP2A with PPP2R2D directly interacted with AMPK by dephosphorylation of Thr-172 in both rodent and human vascular smooth muscle cells.15 Furthermore, increased PPP2R2D expression was correlated with elevated blood lipid levels. Kim et al reported that PPP2R2D-dependent dephosphorylation of AMPK inhibited autophagy and induced mitochondrial dysfunction in a rodent model of alcoholic liver disease.16 However, to the best of our knowledge, no studies have investigated the role of PPP2R2D in DKD.
The role of PP2A in diabetes is complex, and contradictory results have been reported. Previous studies have reported that PP2A activity is increased in glucotoxic or lipotoxic conditions in various cell types, including hepatocytes, cardiomyocytes, muscle cells, retinal endothelial cells, and pancreatic β-cells.24 In addition, the dysregulation of cellular function under these conditions was alleviated by pharmacological inhibition of PP2A. For example, Du et al reported that increased PP2A activity led to increased NF-κB activation and cell death in bovine aortic endothelial cells under high glucose conditions.25 Moreover, the inhibition of PP2A by benfotiamine inhibited high-glucose-induced NF-κB activation and cell death. On the contrary, metformin reduced apoptosis and NF-κB pathway in diabetic cardiomyocytes by activating PP2A.26 Regarding DKD, an adiponectin receptor agonist ameliorated lipotoxicity, oxidative stress, and apoptosis in the kidneys of a DKD mouse model by activating AMPK phosphorylation and downregulating PP2A.27 However, contradictory results also reported that arctigenin reduced proteinuria in a diabetes mouse model by decreasing NF-κB mediated inflammatory response in podocytes via enhancing PP2A activity.28 Therefore, it is likely that the PP2A is involved in a variety of cell signaling processes in DKD, many of which remain to be elucidated.
However, the role of the regulatory B subunit of PP2A in DKD pathogenesis remains largely unexplored. These regulatory B subunits bind to the core dimer (composed of subunits A and C) in a mutually exclusive manner, determining the substrate specificity and subcellular localization of PP2A holoenzymes.29 In addition, the expression patterns of the PP2A subunits vary among organs.29 In this study, we identified that PPP2R2D was expressed in human proximal tubular epithelial cells using the KPMP and Human Protein Atlas data. Therefore, using HK-2 cells, we observed that the high glucose-induced increase in PPP2R2D expression and PP2A activity was attenuated by miR-5010-5p. Moreover, involvement of the PP2A/AMPK axis in tubular epithelial cell injury has been demonstrated in an ischemia/reperfusion kidney model.30 In addition, as aforementioned, a direct interaction between PPP2R2D and AMPK was reported in other organs.15 16 In this study, we showed that the PP2A/AMPK axis, which is mediated by PPP2R2D, also affects HK-2 cells under diabetic conditions. Furthermore, AMPK acts as a repressor of inflammation, particularly by inhibiting NF-κB signaling via several pathways, including peroxisome proliferator-activated receptor γ coactivator 1α, Forkhead box O family, and sirtuin 1.17 Therefore, in our study, the restoration of AMPK expression by miR-5010-5p may have resulted in reduced NF-κB signaling and decreased expression of pro-inflammatory cytokines.
This study has several limitations. In a previous study, we discovered increased expression of miR-5010-5p in the circulating exosomes of patients with DKD. However, we did not observe a similar increase in miR-5010-5p expression in HK-2 cells following high-glucose stimulation. Therefore, we must assume that this miRNA originates from other tissues; however, its exact source has not yet been identified. Second, the conservation of miR-5010-5p across species has not yet been established. Therefore, although we used human renal tubular epithelial cells in this study, we could not validate the molecular pathways associated with miR-5010-5p expression in vivo. Finally, we could not determine whether pharmacological changes in the activity of PP2A could impact the protective effect of miR-5010-5p transfection in HK-2 cells.
Conclusion
In conclusion, our findings demonstrated that miR-5010-5p exerted anti-inflammatory effects on high-glucose-stimulated HK-2 cells. miR-5010-5p directly inhibits the expression of PPP2R2D and reduces the phosphatase activity of PP2A, resulting in the increased phosphorylation of AMPK. This PP2A/AMPK axis may be responsible for the decreased expression of NF-κB and subsequent reduction in the levels of proinflammatory cytokines by miR-5010-5p. However, because the increased expression of miR-5010-5p did not originate in HK-2 cells, further studies are needed to identify the potential miRNA-mediated crosstalk among tissues in the pathogenesis of DKD.
Footnotes
SC and MKS contributed equally.
Contributors: SC contributed to experiments and drafted the original manuscript. MKS and MRY conducted experiments. HL, SHK, JSJ, and HN researched the data and wrote the revised manuscript. HK contributed to the concept of the study and drafted the original manuscript. All authors contributed to the discussion and approved the final version of the manuscript. HK is a guarantor responsible for the overall content.
Funding: This research was supportive by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2019RIG1A1100498) and Soonchunhyang University Research Fund.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Because this study only used cell line purchased from the American Type Culture Collection, not animal or human tissue, this study was exempt from review by the Institutional Review Board of Soonchunhyang University Hospital.
References
- 1. Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011) 2022;12:7–11. 10.1016/j.kisu.2021.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hong YA, Ban TH, Kang C-Y, et al. Trends in epidemiologic characteristics of end-stage renal disease from 2019 Korean renal data system (KORDS). Kidney Res Clin Pract 2021;40:52–61. 10.23876/j.krcp.20.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nuffield Department of Population Health Renal Studies Group, SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium . Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet 2022;400:1788–801. 10.1016/S0140-6736(22)02074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219–29. 10.1056/NEJMoa2025845 [DOI] [PubMed] [Google Scholar]
- 5. Kato M, Natarajan R. Micrornas in diabetic nephropathy: functions, biomarkers, and therapeutic targets. Ann N Y Acad Sci 2015;1353:72–88. 10.1111/nyas.12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu S, Wu W, Liao J, et al. Microrna-21: a critical pathogenic factor of diabetic nephropathy. Front Endocrinol (Lausanne) 2022;13:895010. 10.3389/fendo.2022.895010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conserva F, Barozzino M, Pesce F, et al. Urinary miRNA-27B-3P and miRNA-1228-3p correlate with the progression of kidney fibrosis in diabetic nephropathy. Sci Rep 2019;9:11357. 10.1038/s41598-019-47778-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang ZH, Tang YZ, Song HN, et al. miRNA-342 suppresses renal interstitial fibrosis in diabetic nephropathy by targeting SOX6. Int J Mol Med 2020;45:45–52. 10.3892/ijmm.2019.4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kato M, Zhang J, Wang M, et al. Microrna-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A 2007;104:3432–7. 10.1073/pnas.0611192104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim H, Bae Y-U, Jeon JS, et al. The circulating exosomal microRNAs related to albuminuria in patients with diabetic nephropathy. J Transl Med 2019;17:236. 10.1186/s12967-019-1983-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao C, Wang B, Chen Q, et al. Serum exosomes from diabetic kidney disease patients promote pyroptosis and oxidative stress through the miR-4449/HIC1 pathway. Nutr Diabetes 2021;11:33. 10.1038/s41387-021-00175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen X, Jin Y, Feng Y. Evaluation of plasma extracellular vesicle microrna signatures for lung adenocarcinoma and granuloma with monte-carlo feature selection method. Front Genet 2019;10:367. 10.3389/fgene.2019.00367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hashemi Sheikhshabani S, Amini-Farsani Z, Modarres P, et al. In silico identification of potential miRNAs -mRNA inflammatory networks implicated in the pathogenesis of COVID-19. Hum Gene (Amst) 2023;36:201172. 10.1016/j.humgen.2023.201172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu JX, Li W, Li JT, et al. Screening key long non-coding RNAs in early-stage colon adenocarcinoma by RNA-sequencing. Epigenomics 2018;10:1215–28. 10.2217/epi-2017-0155 [DOI] [PubMed] [Google Scholar]
- 15. Joseph BK, Liu H-Y, Francisco J, et al. Inhibition of AMP kinase by the protein phosphatase 2A heterotrimer, PP2APpp2R2d. J Biol Chem 2015;290:10588–98. 10.1074/jbc.M114.626259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim YS, Ko B, Kim DJ, et al. Induction of the hepatic aryl hydrocarbon receptor by alcohol dysregulates autophagy and phospholipid metabolism via PPP2R2D. Nat Commun 2022;13:6080. 10.1038/s41467-022-33749-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salminen A, Hyttinen JMT, Kaarniranta K. AMP-activated protein kinase inhibits NF-ΚB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl) 2011;89:667–76. 10.1007/s00109-011-0748-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang J, Yao D, Yan H, et al. The role of micrornas in the pathogenesis of diabetic nephropathy. Int J Endocrinol 2019;2019:8719060. 10.1155/2019/8719060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang B, Yao K, Huuskes BM, et al. Mesenchymal stem cells deliver exogenous Microrna-let7C via exosomes to attenuate renal fibrosis. Mol Ther 2016;24:1290–301. 10.1038/mt.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barr FA, Elliott PR, Gruneberg U. Protein phosphatases and the regulation of mitosis. J Cell Sci 2011;124:2323–34. 10.1242/jcs.087106 [DOI] [PubMed] [Google Scholar]
- 21. Zhou P, Shaffer DR, Alvarez Arias DA, et al. In vivo discovery of Immunotherapy targets in the tumour microenvironment. Nature 2014;506:52–7. 10.1038/nature12988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pan W, Sharabi A, Ferretti A, et al. PPP2R2D suppresses IL-2 production and treg function. JCI Insight 2020;5:19. 10.1172/jci.insight.138215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salminen A, Kaarniranta K, Kauppinen A. Age-related changes in AMPK activation: role for AMPK phosphatases and inhibitory phosphorylation by upstream signaling pathways. Ageing Res Rev 2016;28:15–26. 10.1016/j.arr.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 24. Kowluru A, Matti A. Hyperactivation of protein phosphatase 2A in models of glucolipotoxicity and diabetes: potential mechanisms and functional consequences. Biochem Pharmacol 2012;84:591–7. 10.1016/j.bcp.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 25. Du Y, Kowluru A, Kern TS. PP2A contributes to endothelial death in high glucose: inhibition by benfotiamine. Am J Physiol Regul Integr Comp Physiol 2010;299:R1610–7. 10.1152/ajpregu.00676.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng G, Li L. High-glucose-induced apoptosis, ROS production and pro-inflammatory response in cardiomyocytes is attenuated by metformin treatment via PP2A activation. J Biosci 2020;45:126. [PubMed] [Google Scholar]
- 27. Choi SR, Lim JH, Kim MY, et al. Adiponectin receptor agonist adiporon decreased ceramide, and lipotoxicity, and ameliorated diabetic nephropathy. Metabolism 2018;85:348–60. 10.1016/j.metabol.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 28. Zhong Y, Lee K, Deng Y, et al. Arctigenin attenuates diabetic kidney disease through the activation of PP2A in podocytes. Nat Commun 2019;10:4523. 10.1038/s41467-019-12433-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reynhout S, Janssens V. Physiologic functions of PP2A: lessons from genetically modified mice. Biochim Biophys Acta Mol Cell Res 2019;1866:31–50. 10.1016/j.bbamcr.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 30. Ma H, Guo X, Cui S, et al. Dephosphorylation of AMP-activated protein kinase exacerbates ischemia/reperfusion-induced acute kidney injury via mitochondrial dysfunction. Kidney Int 2022;101:315–30. 10.1016/j.kint.2021.10.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2023-003784supp001.pdf (39.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request.