Abstract
Stuttering affects approximately 1 in 100 adults and can result in significant communication problems and social anxiety. It most often occurs as a developmental disorder but can also be caused by focal brain damage. These latter cases may lend unique insight into the brain regions causing stuttering.
Here, we investigated the neuroanatomical substrate of stuttering using three independent datasets: (i) case reports from the published literature of acquired neurogenic stuttering following stroke (n = 20, 14 males/six females, 16–77 years); (ii) a clinical single study cohort with acquired neurogenic stuttering following stroke (n = 20, 13 males/seven females, 45–87 years); and (iii) adults with persistent developmental stuttering (n = 20, 14 males/six females, 18–43 years). We used the first two datasets and lesion network mapping to test whether lesions causing acquired stuttering map to a common brain network. We then used the third dataset to test whether this lesion-based network was relevant to developmental stuttering.
In our literature dataset, we found that lesions causing stuttering occurred in multiple heterogeneous brain regions, but these lesion locations were all functionally connected to a common network centred around the left putamen, including the claustrum, amygdalostriatal transition area and other adjacent areas. This finding was shown to be specific for stuttering (PFWE < 0.05) and reproducible in our independent clinical cohort of patients with stroke-induced stuttering (PFWE < 0.05), resulting in a common acquired stuttering network across both stroke datasets. Within the common acquired stuttering network, we found a significant association between grey matter volume and stuttering impact for adults with persistent developmental stuttering in the left posteroventral putamen, extending into the adjacent claustrum and amygdalostriatal transition area (PFWE < 0.05).
We conclude that lesions causing acquired neurogenic stuttering map to a common brain network, centred to the left putamen, claustrum and amygdalostriatal transition area. The association of this lesion-based network with symptom severity in developmental stuttering suggests a shared neuroanatomy across aetiologies.
Keywords: acquired neurogenic stuttering, persistent developmental stuttering, lesion network mapping, putamen, amygdala, claustrum
By studying the locations of lesions causing stroke-induced stuttering, Theys, Jaakkola et al. identify a common acquired stuttering network, centred around the left posterior putamen. This network is also associated with the severity of developmental stuttering, providing support for a shared neural basis across aetiologies.
Introduction
Stuttering is characterized by the occurrence of sound and syllable repetitions, prolongations and blocks in speech. Associated behaviours (e.g. facial grimacing, word avoidance) and negative emotions and attitudes (e.g. social anxiety) are also important components of stuttering1,2 and can have a profound negative impact on people’s lives.3 Behavioural treatments can help, but results may be challenging to maintain4 and there are currently no effective pharmacological5 or neuromodulation6 treatment options for stuttering.
The most typical form of stuttering is developmental, which occurs in 5%–11% of pre-schoolers and persists into adulthood for almost 1% of the population.7,8 A large number of neuroimaging studies have investigated developmental stuttering, but results have been largely inconsistent, and the neuroanatomical basis of stuttering remains unclear.9 For example, studies have reported abnormalities in cortical motor, somatosensory and auditory regions, the anterior cingulate, frontal operculum, basal ganglia and cerebellum, among other regions, with no clear convergence.10-12
Stuttered speech disfluencies can also emerge following acquired brain injuries or diseases, in individuals who previously spoke without overt stuttering. Stroke is the most prevalent cause of acquired neurogenic stuttering, accounting for half of the reported cases in the literature.13 In a study focusing on incidence of acquired stuttering following stroke, 5% of patients met the criteria for diagnosis of neurogenic stuttering.14 People with acquired neurogenic stuttering may also present with associated behaviours such as fist clenching and word avoidance, and negative emotions such as frustration.13 Studying focal brain lesions can establish a causal neurobehavioural relationship—a link between brain damage and specific symptoms.15,16 However, lesions causing acquired neurogenic stuttering occur in highly heterogeneous brain areas, leaving its localization unclear.17,18 A recently developed method, lesion network mapping, extends the use of focal brain lesion location information by studying the network connections common to a group of lesions.15,19 Lesion network mapping has successfully been applied to localize multiple symptoms and signs,15,20 including movement disorders,21-25 neuropsychiatric disorders,26-28 consciousness29,30 and other behavioural changes.31,32 Importantly, the networks identified using lesion network mapping seem to consistently generalize across primary brain disorders causing similar symptoms, suggesting a shared neural substrate for a symptom despite distinct aetiologies.21,23 Finally, investigating lesion connectivity has been shown to identify efficacious neurosurgical treatment targets, suggesting relevance for treatment.23,24,33
Our first goal was to test if brain lesions causing acquired neurogenic stuttering connect to a common brain network that could lend insight into the neural substrates of stuttering. To accomplish this, we applied lesion network mapping to two independent datasets of acquired neurogenic stuttering following stroke. We hypothesized that lesion network mapping findings across both acquired neurogenic stuttering datasets would converge onto similar brain areas, resulting in a common neurogenic stuttering network. Our second aim was to test if the identified acquired neurogenic stuttering network might be relevant across stuttering aetiologies. We hypothesized that, in a third independent cohort comprised of adults with persistent developmental stuttering, stuttering severity would be associated with structural grey matter changes within the identified neurogenic stuttering network.
Materials and methods
Participants
Acquired neurogenic stuttering following stroke: literature cohort
Case reports of new-onset stuttering following acute onset brain lesions were searched from PubMed on 30 November 2021 using search terms ‘(stuttering OR stammering) AND (lesion OR stroke OR hemorrhage OR haemorrhage OR infarct OR bleeding OR bleed) AND (case report OR case series)’. The search resulted in 132 papers, which were evaluated for agreement with our inclusion/exclusion criteria. The inclusion criteria were: (i) new-onset stuttering caused by an acute onset lesion; (ii) article showing an image of the brain lesion with sufficient quality to delineate the lesion; and (iii) patient aged 16 years or older. The exclusion criteria were: (i) one or more earlier lesions in other locations; (ii) history of developmental or acquired stuttering; and (iii) additional traumatic haemorrhage, such as subarachnoid haemorrhage or subdural haematoma. The search identified 20 case reports (16–77 years, six females, 14 males), which are described in Supplementary Table 1.
To control for typical stroke lesion locations, 169 stroke patients (27–92 years, 64 females, 105 males) from Turku University Hospital were included as a control group to test the specificity of the findings to neurogenic stuttering.
Acquired neurogenic stuttering following stroke: clinical case-control cohort
The second, independent dataset consisted of 20 patients with stroke-related (ischaemic stroke or intracerebral haemorrhage) acquired neurogenic stuttering (45–87 years, seven females, 13 males) and 17 matched controls who had a stroke but did not develop stuttering (50–83 years, six females, 11 males; Supplementary Table 2).34 Controls did not differ significantly from the neurogenic stuttering group with regard to age, sex, cognitive functioning and other speech-language symptoms caused by the stroke, including aphasia, dysarthria and apraxia of speech (all P > 0.3).34 To assess presence of stuttered disfluencies, speech samples during conversation, monologue and reading tasks were video-recorded, transcribed and analysed by a qualified speech-language therapist. Neurogenic stuttering was diagnosed if patients had >3% stuttered syllables (sound and syllable repetitions, monosyllabic word repetitions, prolongations and blocks) during at least one of the included speech tasks (conversation, monologue and/or reading), similar to previously published work.14,34,35 The control group was used to identify connectivity specific to neurogenic stuttering, by controlling for other stroke-induced speech-language symptoms.
Persistent developmental stuttering cohort
Twenty adults with developmental stuttering (18–43 years, six females, 14 males) were recruited for the third dataset. The study was conducted with ethical approval of the University of Canterbury and all participants provided written informed consent prior to their participation in accordance with the Declaration of Helsinki. Apart from developmental stuttering, the participants did not have a history of speech, language or neurological problems. All participants self-identified as having developmental stuttering. Diagnosis of stuttering was confirmed by a qualified speech-language therapist, based on clinical observation and analysis of stuttered disfluencies, associated behaviours (e.g. eye blinking, facial movements) and stuttering impact. For analysis of disfluencies, video-recorded conversation samples of at least 400 words were transcribed and the frequency of occurrence of stuttered disfluencies was calculated (in % stuttered syllables). The Overall Assessment of the Speaker’s Experience of Stuttering (OASES) was used to assess the impact of stuttering from the speaker’s perspective.36 These two measures—the objective speech measure calculated by the clinician and the subjective impact measure reported by the participant—were not highly correlated (Spearman’s rank correlation test rs = −0.02, P = 0.93), reflecting the discrepancy between clinician scores of observed speech behaviours and self-perceived impact, which is commonly reported in persistent developmental stuttering37 (Supplementary Table 2).
Brain imaging acquisition and analysis
Lesion network mapping in the literature cohort with acquired neurogenic stuttering
The methodology of lesion network mapping has been described in detail,19,31 including a full description of image processing.27 Briefly, the lesion locations shown in the 20 original case reports were first manually transferred to the Montreal Neurological Institute 152 (MNI152) template. This approach produced 2D cross-sections of lesions that capture only a portion of the entire 3D lesion. This could theoretically introduce bias; however, this approach has been validated previously, showing that 2D and 3D lesion connectivity profiles correspond very well (spatial correlation coefficients between connectivity maps calculated from 2D and 3D lesions are ∼0.9) and have little impact on lesion network mapping results.19,31,38 Second, whole brain connectivity maps were calculated for each of the lesion locations using a resting state functional connectivity MRI (rs-fcMRI) dataset from a large cohort of 1000 healthy volunteers (18–36 years, 500 females, 500 males). Details regarding this connectome dataset have been described previously and a processed version of this connectome dataset is publicly available (https://doi.org/10.7910/DVN/ILXIKS).39-41 Note that it is not possible to compute functional connectivity with the lesion location using functional connectivity data from the same stroke patient, as the tissue (and thus the functional MRI signal) at the lesion location is lost. As such, we use a large normative connectome as an approximation of the connectivity in each individual at the time of their stroke, consistent with prior lesion network mapping studies.19,23 Although rs-fcMRI can vary as a function of age, sex and disease, prior work has shown that these changes are small relative to the overall connectivity pattern and have little impact on network mapping results.15,19,27,42,43 Third, the connectivity maps were thresholded to T ≥ 5 [corresponding to the uncorrected P < 10−6 and whole brain family-wise error (FWE) corrected PFWE < 0.05] to define brain regions connected to these lesions (‘lesion networks’). Finally, the lesion networks of each case were combined and the amount of overlap was calculated for every brain voxel. Thresholding the resultant map at 80%–100% overlap identified brain regions consistently connected to most of the lesions causing stuttering, i.e. brain regions sensitive for stuttering.
The lesion network overlap analysis allowed determination of common brain regions connected to lesions causing neurogenic stuttering. To test whether connectivity to these regions was specific to stuttering and not due to the tendency of strokes to affect some brain regions more than others, the lesion network connectivity values were compared between the cases with stroke-related neurogenic stuttering and the group of 169 consecutive stroke controls with heterogeneous symptoms from Turku University Hospital. The group comparisons were conducted using FMRIB Software Library (FSL)’s tool for non-parametric permutation inference of neuroimaging data.44 Correction of multiple comparisons was conducted for the whole brain search volume, with FWE corrected P-values < 0.05 using threshold-free cluster enhancement (TFCE) considered significant.45 Cluster coordinates are reported for clusters including at least 10 voxels. A conjunction analysis with the areas identified as sensitive in this cohort (i.e. connected to lesion locations causing stuttering), resulted in the identification of areas both sensitive and specific to stuttering.23
Lesion network mapping in the clinical cohort with acquired neurogenic stuttering
Lesion network mapping in the 20 cases in the clinical cohort was performed as described for the literature cohort, with the exception that full 3D lesion maps in MNI152 space were used. As the clinical cohort was less heterogeneous than the literature cohort, an increased threshold of 90%–100% overlap was used. The thresholds for the literature and clinical cohort lesion network overlap analyses were data-driven and selected based on the maximal overlap in these datasets (higher threshold for less heterogeneous clinical cohort). However, to ensure this choice did not influence our results, we repeated our analyses using the same threshold in both datasets.
Specificity of the identified network for stuttering compared to other speech-language problems following stroke was assessed by comparing the 20 participants with acquired neurogenic stuttering and the 17 matched controls. Similarly to the literature cohort, voxel-wise non-parametric permutation tests with TFCE and FWE corrected P-values < 0.05 were considered significant. Again, a conjunction analysis with the areas identified as sensitive in this cohort was performed to identify areas both sensitive and specific to stuttering.
Next, a final common acquired neurogenic stuttering network was created by identifying the areas of overlap across the sensitivity and specificity analyses in both datasets.
Relevance of lesion network mapping results for developmental stuttering
For participants with persistent developmental stuttering, MRI was performed with a 3 T scanner (Siemens Skyra) using a 64-channel head and neck coil. It included a 3D T1-weighted sequence [magnetization prepared rapid gradient echo; MPRAGE, echo time/repetition time/inversion time = 2.85/2000/880 ms, flip angle = 8°, Field of view = 256 mm, acquisition matrix = 256 × 256, sagittal acquisition with 208 slices, phase acceleration generalized autocalibrating partially parallel acquisitions (GRAPPA) = 2, bandwidth = 240 Hz/pixel, voxel size = 1 × 1 × 1 mm3]. The Computational Anatomy Toolbox (CAT12) (r1450, http://www.neuro.uni-jena.de/cat/), a toolbox of Statistical Parametric Mapping (SPM12; v7487, http://www.fil.ion.ucl.ac.uk/spm/), running in MATLAB 9.6 (R2019a), was used to segment and normalize T1-weighted structural images. Images were bias corrected, spatially normalized via SHOOT (geodesic shooting, using the MNI-registered template provided within CAT12), modulated to compensate for the effect of spatial normalization, and classified into grey matter, white matter and CSF, all within the same generative model.46,47 Modulated, normalized grey matter segments were smoothed with an 8 mm full-width at half-maximum (FWHM) Gaussian kernel. Intracranial volume (ICV) was calculated using the ‘reverse brain mask method’, in which the MNI ICV mask (provided with SPM) was warped into each participant’s native space using the inverse deformation fields produced during the structural processing.48
To identify relevance of the acquired stuttering findings to developmental stuttering, multiple linear regression models were used to test the positive and negative associations between regional grey matter volume and both OASES scores and frequency of syllables stuttered in participants with persistent developmental stuttering. All models included age, sex and intracranial volume as covariates. Comparisons were performed using FSL’s randomize within the common acquired stuttering network, i.e. regions identified to be both sensitive and specific in both of the lesion datasets. For each contrast, the null distribution was generated over 10 000 permutations and the voxel-wise alpha level set at TFCE-corrected PFWE < 0.05.44
Results
Neurogenic stuttering in the literature cohort
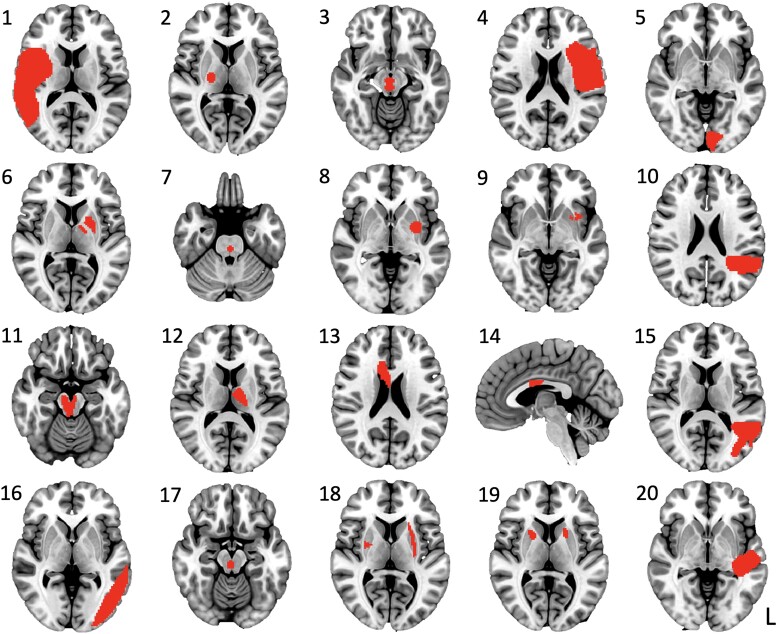
In the dataset of 20 published cases of neurogenic stuttering caused by a focal stroke, the location of the brain lesions was highly heterogeneous, with little overlap between lesions (Fig. 1). Three cases presented with right-sided, six with bilateral and 11 with left-sided lesions. Lesions were present in the frontal, temporal, parietal and occipital lobes; thalamus, basal ganglia and brainstem; as well as in the corona radiata and corpus callosum.
Figure 1.
Lesion maps of the literature cohort. Numbers correspond to cases listed in Supplementary Table 1 and represent the 20 cases with stroke-induced neurogenic stuttering identified in the published literature.
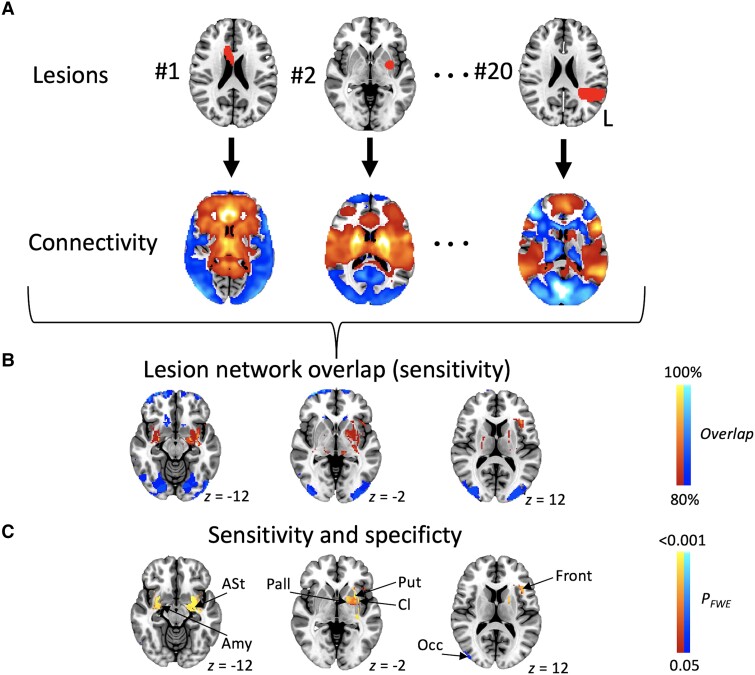
Next, we applied lesion network mapping to these cases. Lesions causing neurogenic stuttering were connected to a common set of brain regions (Fig. 2A and B). We also compared the neurogenic stuttering data with findings from 169 stroke controls to understand which brain areas were specific to neurogenic stuttering rather than associated with brain regions commonly affected by stroke. Compared to lesion locations of controls, lesions causing neurogenic stuttering were significantly more positively connected to brain regions centred around the bilateral putamen and left inferior frontal gyrus. The two most prominent clusters with negative associations were located in the occipital lobes (Fig. 2C and Supplementary Table 3).
Figure 2.
Lesion network mapping in the literature cohort. (A) Lesion locations for each of the 20 cases served as the input for the lesion network analysis. For each case, an individual lesion network map was created using a normative connectome, resulting in a map of brain networks typically connected to the focal brain lesion location. Individual lesion network maps were thresholded at T > 5 corresponding to PFWE < 0.05. Positive associations are shown in red-yellow, negative associations in blue-light blue. (B) Lesion network maps of the 20 cases were overlaid and thresholded at ≥80% overlap to show regions connected to most of the lesion locations (i.e. regions sensitive to stuttering). (C) To identify regions specific for stuttering, the 20 acquired stuttering cases and 169 stroke controls were compared (whole brain PFWE < 0.05), followed by a conjunction analysis with B to identify areas both sensitive and specific for stuttering. Amy = amygdala; ASt = amygdalostriatal transition area; Cl = claustrum; Front = frontal; FWE = family-wise error; Occ = occipital; Pall = Pallidum; Put = putamen.
Neurogenic stuttering in the clinical cohort
Similar to the literature cohort, the locations of lesions causing neurogenic stuttering in our clinical cohort were highly heterogeneous (Supplementary Fig. 1A). Fifteen lesions were left-lateralized, four were restricted to the right hemisphere and one was bilateral. Lesions were present in the cerebral cortex in all four lobes, thalamus, basal ganglia, brainstem and cerebellum.
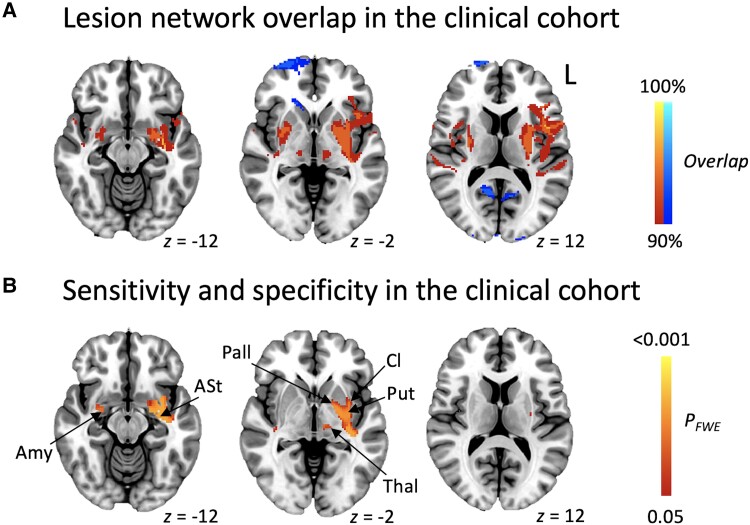
Using lesion network mapping, lesion locations were connected to a common set of brain regions (Fig. 3A), converging with the lesion network mapping results in the literature cohort. Specificity of the findings to stuttering versus other speech-language disorders following stroke was assessed by comparing the neurogenic stuttering cases in the clinical cohort to their matched controls. Only significant positive associations resulted from this analysis, with the largest cluster centred around the left putamen (Fig. 3B and Supplementary Table 4).
Figure 3.
Lesion network mapping in the clinical cohort. (A) Lesion network maps of the 20 cases were overlaid, and thresholded at ≥90% overlap to show regions connected to most of the lesion locations (i.e. regions sensitive to stuttering). (B) To identify regions specific for stuttering, the 20 cases with neurogenic stuttering and 17 matched controls were compared (whole brain PFWE < 0.05), followed by a conjunction analysis with B to identify areas both sensitive and specific for stuttering. Positive associations are shown in red-yellow, negative in blue-light blue. Amy = amygdala; ASt = amygdalostriatal transition area; Cl = claustrum; FWE = family-wise error; Pall = Pallidum; Put = putamen; Th = thalamus.
Common acquired neurogenic stuttering network
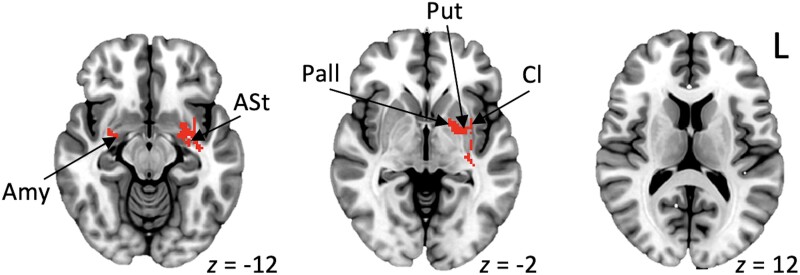
In the conjunction analysis of the two cohorts, the common network of brain areas linked with acquired stuttering across both the literature and clinical cohorts included the putamen, extending to the claustrum and amygdala bilaterally, and to the pallidum in the left hemisphere (Fig. 4 and Supplementary Table 5).
Figure 4.
Common acquired neurogenic stuttering network. Common areas that were sensitive and specific across both neurogenic stuttering cohorts. Amy = amygdala; ASt = amygdalostriatal transition area; Cl = claustrum; Pall = Pallidum; Put = putamen.
Relevance to developmental stuttering
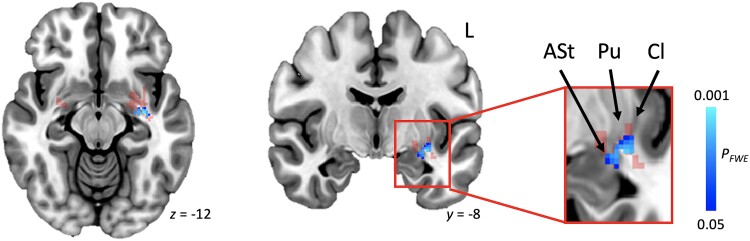
Next, we set out to investigate relevance of the identified data-driven acquired neurogenic stuttering network to persistent developmental stuttering. More severe impact of stuttering was significantly associated with increased grey matter volume in one cluster of voxels consisting of the left-sided posteroventral putamen, ventral claustrum and amygdalostriatal transition area [PFWE = 0.01, size = 105 voxels, centre of gravity (COG) = −32.8 −11.4 −10.5, Fig. 5]. No such relationship was present with the frequency of occurrence of stuttered disfluencies, an objective measure of severity recorded by the clinician.
Figure 5.
Association between stuttering impact and grey matter volume in developmental stuttering. Regression analyses within the identified common acquired neurogenic stuttering network (from Fig. 4, transparent red in current figure) showed that more negative experiences with stuttering (OASES scores) were associated with increased grey matter volume in participants with persistent developmental stuttering (PFWE < 0.05, shown in blue). ASt = amygdalostriatal transition area; Cl = claustrum FWE = family-wise error; OASES = Overall Assessment of the Speaker’s Experience of Stuttering; Pu = putamen.
Robustness to methodological variation
To ensure that our results are not driven by the choice of the lesion network overlap thresholds, all analyses were repeated using different threshold combinations and the significance of the main results and final localization did not change (Supplementary Figs 2 and 3).
Discussion
This study reveals several important findings. First, although the lesions causing acquired stuttering described in the literature occurred in heterogeneous locations, they were connected to a common brain network, centred around the putamen. Second, this stuttering network was reproducible in an independent clinical cohort with lesion-induced stuttering and shown to be specific for stuttering versus other communication changes following stroke. Finally, in adults with persistent developmental stuttering, grey matter volumes within the identified common neurogenic stuttering connectivity network were associated with stuttering impact, confirming the involvement of the posteroventral putamen, posteroventral claustrum and amygdalostriatal transition area in the left hemisphere in developmental stuttering.
A shared neuroanatomical substrate across developmental and acquired stuttering aetiologies
Previous studies have reported parallels in behavioural characteristics (i.e. stuttered speech disfluencies, associated non-speech and negative affective behaviours) across acquired neurogenic and persistent developmental stuttering.13 The current study provides evidence for similarities across stuttering aetiologies at a neuroanatomical level by showing that, within the common acquired neurogenic stuttering network, structural brain differences in adults with persistent developmental stuttering were associated with self-reported impact of stuttering. However, no such relationship was observed with the frequency of occurrence of stuttered disfluencies as measured by the clinician. One possible explanation may be that, while stuttering frequency can be used to identify the presence of stuttering, it may be an insufficient indicator of the extent of neuroanatomical variation between people who stutter, given the many factors that may influence the frequency of stuttered disfluencies, such as the inherent temporal variability of stuttering frequency, use of coping and avoidance behaviours, and fluency-enhancing skills acquired during treatment. Because measures such as the OASES can be thought of as capturing a combination of the experienced severity of disfluencies and their impact on the individual, this broad-based assessment may better reflect the neuroanatomical variation associated with stuttering.
The putamen as the core neuroanatomical substrate
Theoretical accounts suggesting a causal role for the basal ganglia in stuttering have been formulated previously,49 but lack comprehensive direct evidence.50 Our study provides support for an important role of the putamen in stuttering and extends previous theories by suggesting that specifically the posteroventral part of the putamen may be a key node in an extended network.
The putamen is generally responsible for internal timing and sequencing of complex motor movements in an integrated manner.51 It is organized somatotopically, with the posterior region identified in our study known to be responsible for motor regulation of the face area, including lip movements.52,53 Deficiencies in internal timing networks have been proposed to result in developmental stuttering, based on observations, such as the decrease in stuttered speech disfluencies during fluency-inducing conditions (e.g. singing and rhythmic speech).49,54 While these theories focused on involvement of wider networks, including the basal ganglia and supplementary motor area in stuttering, our results suggest that a specific alteration in left posterior putaminal function may be a key contributor to differences in internal timing networks. In addition, the left posterior putamen exhibits a response profile that is linked to habituation and learning in humans.55,56 This is consistent with observations that developmental stuttering typically has an onset in the preschool years during periods of rapid development when speech production needs to become more automated to cope with language growth.57 Findings on treatment-induced changes in adults with persistent developmental stuttering further support a key role of the putamen, as this area has consistently been reported to respond to successful behavioural stuttering treatments.58-60
In our cohort of adults with persistent developmental stuttering, increased left posterior grey matter putaminal volume was associated with a more severe impact of stuttering. A similar increase in grey matter volume in the left putamen has previously been identified in adults with persistent developmental stuttering, supporting our findings.61 However, children with developmental stuttering seem to have decreased putaminal volumes.62 Contrasting findings between children and adults with developmental stuttering have previously been reported, and could be linked to divergent trajectories of brain development between children who later recover from stuttering, compared to those whose stuttering persists into adulthood.63-65 Mapping the developmental trajectories in grey matter volume, specifically in the posteroventral part of the left putamen, is an important avenue for future research.
Our findings are also supported by observations from patients with stuttering onset following other acquired neurological aetiologies. For example, 7 of 10 patients in a study on acquired stuttering following penetrating brain injuries presented with lesions in the lentiform nuclei (putamen and pallidum).66 In addition, these regions are known to be affected in parkinsonism,23,55,67 which may explain the high (up to 60%)13 prevalence of neurogenic stuttering following onset of Parkinson’s disease. Interestingly, pallidal (GPi) deep brain stimulation in Parkinson’s disease has been reported to cause new-onset stuttering,68 providing further evidence for the causal role of the basal ganglia motor circuits in stuttering and suggesting relevance of our findings for neuromodulation treatments.
Together, these observations on putaminal involvement suggest that deficiencies in automation of internal timing mechanisms for sequencing of complex motor movements of the articulators may lead to speech disfluencies across both acquired neurogenic and developmental stuttering aetiologies.
The claustrum and amydalostriatal transition area as new areas of interest
In addition to the posteroventral putamen, our analyses highlighted involvement of the claustrum and amygdalostriatal transition area across all three independent datasets. The claustrum is typically not reported as a separate area in studies and models of speech production and its function is still poorly understood.69-71 However, our results included the claustrum, which is clearly differentiated from the putamen and insular regions. The claustrum has extensive connections with cortical and subcortical brain areas, and has been hypothesized to integrate sensory information and play a role in neural homeostasis.72 In speech, emerging evidence suggests involvement in motor planning as well as execution, including in the coordination of movements in articulatory subsystems.73 The claustrum also plays a role in social punishment in humans74 and in controlling anxiety-related responses in mice,75 suggesting that the positive relationship between stuttering impact and grey matter volume in our cohort with persistent developmental stuttering could reflect motor as well as associated emotional consequences of stuttering.
To date, the claustrum has not been considered important for stuttering, despite two previous studies reporting a positive relationship between claustrum activation and severity of developmental stuttering.76,77 Of note, one of the treatment studies reporting putaminal changes had a cluster peak in the left claustrum, with pretreatment claustrum overactivation normalizing in those who successfully completed treatment.58 As the claustrum is a very small structure—its maximal thickness only a few millimetres72—future studies should consider making a priori decisions on including it as a region of interest in their analyses and further assessing its contributions to speech motor changes and associated affective behaviours in stuttering.
The significant left-sided findings across all three datasets also extended into the amygdalostriatal transition area. As a small structure positioned between the posteroventral putamen and amygdala, it is ideally suited for evaluating threats and providing motor responses to those threats.78 A recent study in mice indicated that its neuronal activity is responsible for driving freezing and avoidance behaviour,78 showing direct relevance to stuttering as both freezing, for example blocks in speech, and speech avoidance are key components of stuttering.1,2
Of clinical relevance, the acquired stuttering network areas that showed relevance to persistent developmental stuttering were identified when stuttering severity was assessed from the perspective of the speaker, taking all components of stuttering into account. The lack of significant findings based on observable speech disfluencies alone emphasizes the importance of also considering speaker experiences, including socio-emotional responses, in assessment and management of stuttering. In fact, it is possible that the identified brain regions are primarily associated with multiple factors contributing to self-perceived impact of developmental stuttering. Therefore, treatments targeting these areas may have a broader impact on stuttering, such as reducing anxiety and mitigating visible signs of distress associated with stuttering episodes.
Limitations
There are some limitations that should be acknowledged. The literature cohort was developed following a systematic literature search. We therefore cannot exclude publication bias, as lesion locations previously reported to be linked to stuttering may be more likely to be reported; although the competing novelty bias might mitigate this. In addition, relying on diagnoses of acquired stuttering from prior publications leaves a certain degree of uncertainty in the causal link between the lesion and symptoms. The control group for the literature-based cases also included unselected stroke patients and was not matched to the literature cohort stuttering sample. However, these issues would mainly add noise to our analyses, biasing us against the current findings. Furthermore, the clinical cohort with full 3D lesion maps, systematic assessment of stuttering symptoms, and clear temporal relationship between the stroke and onset of stuttering alleviates these limitations and demonstrates consistent results with the literature cohort.
It should also be noted that lesion network mapping is based on normative functional connectivity data, leaving some uncertainty to the causal relationships and warranting independent confirmation.79 Nevertheless, multiple lines of evidence, for example from lesions following other acquired stuttering aetiologies66 and deep brain stimulation of the basal ganglia motor circuits,68 support the causal role of the brain regions identified here in stuttering.
Conclusion
Lesion locations causing neurogenic stuttering map to a common brain network, which includes brain areas that also demonstrate relevance for persistent developmental stuttering, suggesting a shared neural substrate across aetiologies. Findings overlapped in the left-sided posteroventral putamen, including the ventral claustrum and amygdalostriatal transition area. Together, these neuroanatomical findings provide links with brain functions supporting stuttering characteristics.
Of the many theoretical accounts of stuttering proposed previously, our data provide support for a crucial role of the basal ganglia. Importantly, our findings propose a specific and biologically plausible neuroanatomical circuit for stuttering centred on the posteroventral part of the left putamen, which is responsible for automation and internal timing for sequencing of complex motor movements of the lips and other articulators. The additional findings implicating the ventral claustrum and amygdalostriatal transition area in stuttering provide an important new direction in mapping the neural basis of stuttering, and ensuring the best possible diagnostic and treatment approaches can be developed.
Supplementary Material
Acknowledgements
We thank our participants for their contribution to this research.
Contributor Information
Catherine Theys, School of Psychology, Speech and Hearing, University of Canterbury, 8140 Christchurch, New Zealand; New Zealand Institute of Language, Brain and Behaviour, University of Canterbury, 8140 Christchurch, New Zealand; New Zealand Brain Research Institute, 8011 Christchurch, New Zealand.
Elina Jaakkola, Turku Brain and Mind Center, Clinical Neurosciences, University of Turku, 20014 Turku, Finland; Department of Psychiatry, University of Helsinki and Helsinki University Hospital, 00014 Helsinki, Finland.
Tracy R Melzer, School of Psychology, Speech and Hearing, University of Canterbury, 8140 Christchurch, New Zealand; New Zealand Brain Research Institute, 8011 Christchurch, New Zealand; Department of Medicine, University of Otago, 8011 Christchurch, New Zealand; RHCNZ—Pacific Radiology Canterbury, 8031 Christchurch, New Zealand.
Luc F De Nil, Department of Speech-Language Pathology, University of Toronto, Toronto, ON M5G 1V7, Canada; Rehabilitation Sciences Institute, University of Toronto, Toronto, ON M5G 1V7, Canada.
Frank H Guenther, Departments of Speech, Language and Hearing Sciences and Biomedical Engineering, Boston University, Boston, MA 02215, USA; The Picower Institute for Learning and Memory, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Alexander L Cohen, Department of Neurology, Boston Children’s Hospital, Boston, MA 02115, USA; Center for Brain Circuit Therapeutics, Brigham and Women’s Hospital, Boston, MA 02115, USA; Department of Neurology, Harvard Medical School, Boston, MA 02115, USA.
Michael D Fox, Center for Brain Circuit Therapeutics, Brigham and Women’s Hospital, Boston, MA 02115, USA; Department of Neurology, Harvard Medical School, Boston, MA 02115, USA.
Juho Joutsa, Turku Brain and Mind Center, Clinical Neurosciences, University of Turku, 20014 Turku, Finland; Turku PET Centre, Neurocenter, Turku University Hospital, 20014 Turku, Finland.
Data availability
The authors confirm that data supporting the findings of this study are available within the article and in the Supplementary material. Additional data supporting the findings are available from the corresponding authors upon reasonable request, subject to national legislation and institutional regulations where the dataset was collected.
Funding
C.T., T.R.M. and F.H.G. received funding from the Royal Society of New Zealand Marsden Fund (M1180). E.J. reports research funding from the Finnish Medical Foundation and the Finnish Parkinson Foundation. T.R.M. also received funding from a Health Research Council of New Zealand Charles Hercus Career Development Fellowship. F.H.G. received funding from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health (R01 DC007683). L.F.D. received funding from the Natural Sciences and Engineering Research Council. A.C. received funding from the NIMH (K23MH120510) and the Simons Foundation Autism Research Initiative. M.D.F. was supported by grants from the NIH (R01MH113929, R21MH126271, R56AG069086, R21NS123813, R01NS127892), the Kaye Family Research Endowment, the Ellison/Baszucki Family Foundation, and the Manley Family. J.J. received funding from the Finnish Medical Foundation, Sigrid Juselius Foundation, Instrumentarium Research Foundation, University of Turku, and Turku University Hospital.
Competing interests
M.D.F. has intellectual property on the use of brain connectivity imaging to analyse lesions and guide brain stimulation and is a consultant for Magnus Medical, Soterix, Abbott, and Boston Scientific. J.J. has received conference travel support from Abbvie, Abbott and Insightec, lecturer honoraria from Lundbeck and Novartis, and consultation fees from Summaryx and Adamant Health.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Craig A, Tran Y. Trait and social anxiety in adults with chronic stuttering: Conclusions following meta-analysis. J Fluency Disord. 2014;40:35–43. [DOI] [PubMed] [Google Scholar]
- 2. Iverach L, Rapee RM, Wong QJ, Lowe R. Maintenance of social anxiety in stuttering: A cognitive-behavioral model. Am J Speech Lang Pathol. 2017;26:540–556. [DOI] [PubMed] [Google Scholar]
- 3. Matrone G. Moving past my stutter. Science. 2022;378:106. [DOI] [PubMed] [Google Scholar]
- 4. Baxter S, Johnson M, Blank L, et al. Non-pharmacological treatments for stuttering in children and adults: A systematic review and evaluation of clinical effectiveness, and exploration of barriers to successful outcomes. Health Technol Assess (Rockv). 2016;20:1–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maguire GA, Nguyen DL, Simonson KC, Kurz TL. The pharmacologic treatment of stuttering and its neuropharmacologic basis. Front Neurosci. 2020;14:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wiltshire CE, Watkins KE. Failure of tDCS to modulate motor excitability and speech motor learning. Neuropsychologia. 2020;146:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reilly S, Onslow M, Packman A, et al. Natural history of stuttering to 4 years of age: A prospective community-based study. Pediatrics. 2013;132:460–467. [DOI] [PubMed] [Google Scholar]
- 8. Craig A, Hancock K, Tran Y, Craig M, Peters K. Epidemiology of stuttering in the community across the entire life span. J Speech Lang Hear Res. 2002;45:1097–1105. [DOI] [PubMed] [Google Scholar]
- 9. Belyk M, Kraft SJ, Brown S. Stuttering as a trait or a state revisited: Motor system involvement in persistent developmental stuttering. Eur J Neurosci. 2017;45:622–624. [DOI] [PubMed] [Google Scholar]
- 10. Craig-McQuaide A, Akram H, Zrinzo L, Tripoliti E. A review of brain circuitries involved in stuttering. Front Hum Neurosci. 2014;8:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Etchell AC, Civier O, Ballard KJ, Sowman PF. A systematic literature review of neuroimaging research on developmental stuttering between 1995 and 2016. J Fluency Disord. 2018;55:6–45. [DOI] [PubMed] [Google Scholar]
- 12. Chang S-E, Garnett EO, Etchell A, Chow HM. Functional and neuroanatomical bases of developmental stuttering: Current insights. Neuroscientist. 2019;25:566–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Theys C, De Nil L. Acquired stuttering: Etiology, symptomatology, identification and treatment. In: Zebrowski P, Anderson J, Conture E, eds. Stuttering: Characteristics, assessment and treatment. 4th ed. Thieme Publishers; 2022:271–286. [Google Scholar]
- 14. Theys C, van Wieringen A, Sunaert S, Thijs V, De Nil LF. A one year prospective study of neurogenic stuttering following stroke: Incidence and co-occurring disorders. J Commun Disord. 2011;44:678–687. [DOI] [PubMed] [Google Scholar]
- 15. Fox MD. Mapping symptoms to brain networks with the human connectome. N Engl J Med. 2018;379:2237–2245. [DOI] [PubMed] [Google Scholar]
- 16. Damasio H, Damasio AR. Lesion analysis in neuropsychology. Oxford University Press; 1989. [Google Scholar]
- 17. Cruz C, Amorim H, Beça G, Nunes R. Neurogenic stuttering: A review of the literature. Revista de Neurologiá. 2018;66:59–64. [PubMed] [Google Scholar]
- 18. De Nil L, Theys C, Jokel R. Stroke-related acquired neurogenic stuttering. In: Coppens P, Patterson J, eds. Aphasia rehabilitation: Clinical challenges. Jones & Bartlett Learning; 2018:173–202. [Google Scholar]
- 19. Boes AD, Prasad S, Liu H, et al. Network localization of neurological symptoms from focal brain lesions. Brain. 2015;138:3061–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joutsa J, Fox MD. Lesion network mapping for symptom localization: Recent developments and future directions. Curr Opin Neurol. 2022;35:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corp DT, Joutsa J, Darby RR, et al. Network localization of cervical dystonia based on causal brain lesions. Brain. 2019;142:1660–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fasano A, Laganiere SE, Lam S, Fox MD. Lesions causing freezing of gait localize to a cerebellar functional network. Ann Neurol. 2017;81:129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joutsa J, Horn A, Hsu J, Fox MD. Localizing parkinsonism based on focal brain lesions. Brain. 2018;141:2445–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joutsa J, Shih LC, Fox MD. Mapping holmes tremor circuit using the human brain connectome. Ann Neurol. 2019;86:812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laganiere S, Boes AD, Fox MD. Network localization of hemichorea-hemiballismus. Neurology. 2016;86:2187–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Darby RR, Laganiere S, Pascual-Leone A, Prasad S, Fox MD. Finding the imposter: Brain connectivity of lesions causing delusional misidentifications. Brain. 2017;140:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joutsa J, Moussawi K, Siddiqi SH, et al. Brain lesions disrupting addiction map to a common human brain circuit. Nat Med. 2022;28:1249–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Padmanabhan JL, Cooke D, Joutsa J, et al. A human depression circuit derived from focal brain lesions. Biol Psychiatry. 2019;86:749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Snider SB, Hsu J, Darby RR, et al. Cortical lesions causing loss of consciousness are anticorrelated with the dorsal brainstem. Hum Brain Mapp. 2020;41:1520–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fischer DB, Boes AD, Demertzi A, et al. A human brain network derived from coma-causing brainstem lesions. Neurology. 2016;87:2427–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Darby RR, Horn A, Cushman F, Fox MD. Lesion network localization of criminal behavior. Proc Natl Acad Sci U S A. 2018;115:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Darby RR, Joutsa J, Burke MJ, Fox MD. Lesion network localization of free will. Proc Natl Acad Sci U S A. 2018;115:10792–10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Joutsa J, Shih LC, Horn A, et al. Identifying therapeutic targets from spontaneous beneficial brain lesions. Ann Neurol. 2018;84:153–157. [DOI] [PubMed] [Google Scholar]
- 34. Theys C, De Nil L, Thijs V, van Wieringen A, Sunaert S. A crucial role for the cortico-striato-cortical loop in the pathogenesis of stroke-related neurogenic stuttering. Hum Brain Mapp. 2013;34:2103–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Penttilä N, Korpijaakko-Huuhka A-M, Kent RD. Disfluency clusters in speakers with and without neurogenic stuttering following traumatic brain injury. J Fluency Disord. 2019;59:33–51. [DOI] [PubMed] [Google Scholar]
- 36. Yaruss JS, Quesal RW. Overall assessment of the speaker's experience of stuttering (OASES): Documenting multiple outcomes in stuttering treatment. J Fluency Disord. 2006;31:90–115. [DOI] [PubMed] [Google Scholar]
- 37. Ward D, Miller R, Nikolaev A. Evaluating three stuttering assessments through network analysis, random forests and cluster analysis. J Fluency Disord. 2021;67:1–11. [DOI] [PubMed] [Google Scholar]
- 38. Cohen AL, Soussand L, Corrow SL, Martinaud O, Barton JJ, Fox MD. Looking beyond the face area: Lesion network mapping of prosopagnosia. Brain. 2019;142:3975–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cohen A, Soussand L, McManus P, Fox M.. GSP1000 preprocessed connectome. Harvard Dataverse, V3; 2020. https://doi:10.7910/DVN/ILXIKS
- 40. Holmes AJ, Hollinshead MO, O’Keefe TM, et al. Brain genomics superstruct project initial data release with structural, functional, and behavioral measures. Sci Data. 2015;2:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Horn A, Reich M, Vorwerk J, et al. Connectivity predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol. 2017;82:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. NeuroImage. 2014;92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 47. Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 48. Hansen TI, Brezova V, Eikenes L, Håberg A, Vangberg TR. How does the accuracy of intracranial volume measurements affect normalized brain volumes? Sample size estimates based on 966 subjects from the HUNT MRI cohort. Am J Neuroradiol. 2015;36:1450–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alm PA. Stuttering and the basal ganglia circuits: A critical review of possible relations. J Commun Disord. 2004;37:325–369. [DOI] [PubMed] [Google Scholar]
- 50. Cler GJ, Krishnan S, Papp D, Wiltshire CE, Chesters J, Watkins KE. Elevated iron concentration in putamen and cortical speech motor network in developmental stuttering. Brain. 2021;144:2979–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bednark JG, Campbell ME, Cunnington R. Basal ganglia and cortical networks for sequential ordering and rhythm of complex movements. Front Hum Neurosci. 2015;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gerardin E, Lehéricy S, Pochon J-B, et al. Foot, hand, face and eye representation in the human striatum. Cerebral Cortex. 2003;13:162–169. [DOI] [PubMed] [Google Scholar]
- 53. Rodriguez-Oroz MC, Jahanshahi M, Krack P, et al. Initial clinical manifestations of Parkinson's disease: Features and pathophysiological mechanisms. Lancet Neurol. 2009;8:1128–1139. [DOI] [PubMed] [Google Scholar]
- 54. Etchell AC, Johnson BW, Sowman PF. Behavioral and multimodal neuroimaging evidence for a deficit in brain timing networks in stuttering: A hypothesis and theory. Front Hum Neurosci. 2014;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Redgrave P, Rodriguez M, Smith Y, et al. Goal-directed and habitual control in the basal ganglia: Implications for Parkinson's disease. Nat Rev Neurosci. 2010;11:760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tricomi E, Balleine BW, O’Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci. 2009;29:2225–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Anderson JD, Ntourou K, Wagovich S. Speech, language, and cognitive processes. In: Zebrowski PM, Anderson JD, Conture EG, eds. Stuttering and related disorders of fluency. 4th ed. Thieme; 2022:52–66. [Google Scholar]
- 58. Ingham RJ, Wang Y, Ingham JC, Bothe AK, Grafton ST. Regional brain activity change predicts responsiveness to treatment for stuttering in adults. Brain Lang. 2013;127:510–519. [DOI] [PubMed] [Google Scholar]
- 59. Neumann K, Euler HA, von Gudenberg AW, et al. The nature and treatment of stuttering as revealed by fMRI: A within-and between-group comparison. J Fluency Disord. 2003;28:381–410. [DOI] [PubMed] [Google Scholar]
- 60. Toyomura A, Fujii T, Kuriki S. Effect of an 8-week practice of externally triggered speech on basal ganglia activity of stuttering and fluent speakers. NeuroImage. 2015;109:458–468. [DOI] [PubMed] [Google Scholar]
- 61. Lu C, Peng D, Chen C, et al. Altered effective connectivity and anomalous anatomy in the basal ganglia-thalamocortical circuit of stuttering speakers. Cortex. 2010;46:49–67. [DOI] [PubMed] [Google Scholar]
- 62. Beal DS, Gracco VL, Brettschneider J, Kroll RM, De Nil LF. A voxel-based morphometry (VBM) analysis of regional grey and white matter volume abnormalities within the speech production network of children who stutter. Cortex. 2013;49:2151–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Garnett EO, Chow HM, Chang S-E. Neuroanatomical correlates of childhood stuttering: MRI indices of white and gray matter development that differentiate persistence versus recovery. J Speech Lang Hear Res. 2019;62:2986–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chow HM, Garnett EO, Koenraads SP, Chang S-E. Brain developmental trajectories associated with childhood stuttering persistence and recovery. Dev Cogn Neurosci. 2023;60:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Beal DS, Lerch JP, Cameron B, Henderson R, Gracco VL, De Nil LF. The trajectory of gray matter development in Broca’s area is abnormal in people who stutter. Front Hum Neurosci. 2015;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ludlow CL, Rosenberg J, Salazar A, Grafman J, Smutok M. Site of penetrating brain lesions causing chronic acquired stuttering. Ann Neurol. 1987;22:60–66. [DOI] [PubMed] [Google Scholar]
- 67. Kalaitzakis ME, Pearce RKB, Gentleman SM. Clinical correlates of pathology in the claustrum in Parkinson's disease and dementia with Lewy bodies. Neurosci Lett. 2009;461:12–15. [DOI] [PubMed] [Google Scholar]
- 68. Rusz J, Tykalová T, Fečíková A, Šťastná D, Urgošík D, Jech R. Dualistic effect of pallidal deep brain stimulation on motor speech disorders in dystonia. Brain Stimul. 2018;11:896–903. [DOI] [PubMed] [Google Scholar]
- 69. Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32:821–841. [DOI] [PubMed] [Google Scholar]
- 70. Civier O, Bullock D, Max L, Guenther FH. Computational modeling of stuttering caused by impairments in a basal ganglia thalamo-cortical circuit involved in syllable selection and initiation. Brain Lang. 2013;126:263–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Miller HE, Guenther FH. Modelling speech motor programming and apraxia of speech in the DIVA/GODIVA neurocomputational framework. Aphasiology. 2021;35:424–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nikolenko VN, Rizaeva NA, Beeraka NM, et al. The mystery of claustral neural circuits and recent updates on its role in neurodegenerative pathology. Behav Brain Funct. 2021;17:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pützer M, Moringlane JR, Reith W, Krick CM. A contribution to the role of the claustrum in speech motor control processes. SSRN. [Preprint] doi: 10.2139/ssrn.3972674 [DOI]
- 74. Zinchenko O. Brain responses to social punishment: A meta-analysis. Sci Rep. 2019;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Niu M, Kasai A, Tanuma M, et al. Claustrum mediates bidirectional and reversible control of stress-induced anxiety responses. Sci Adv. 2022;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fox PT, Ingham RJ, Ingham JC, et al. A PET study of the neural systems of stuttering. Nature. 1996;382:158–162. [DOI] [PubMed] [Google Scholar]
- 77. Ingham RJ, Grafton ST, Bothe AK, Ingham JC. Brain activity in adults who stutter: Similarities across speaking tasks and correlations with stuttering frequency and speaking rate. Brain Lang. 2012;122:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mills F, Lee CR, Howe JR, et al. Amygdalostriatal transition zone neurons encode sustained valence to direct conditioned behaviors. bioRxiv. [Preprint] doi:10.1101/2022.10.28.514263
- 79. Siddiqi SH, Kording KP, Parvizi J, Fox MD. Causal mapping of human brain function. Nat Rev Neurosci. 2022;23:361–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Cohen A, Soussand L, McManus P, Fox M.. GSP1000 preprocessed connectome. Harvard Dataverse, V3; 2020. https://doi:10.7910/DVN/ILXIKS
Supplementary Materials
Data Availability Statement
The authors confirm that data supporting the findings of this study are available within the article and in the Supplementary material. Additional data supporting the findings are available from the corresponding authors upon reasonable request, subject to national legislation and institutional regulations where the dataset was collected.