Although lifesaving, mechanical ventilation is also associated with iatrogenic complications. Perhaps the best-known examples are ventilator-induced lung injury and ventilator-induced diaphragm dysfunction, but other forms of ventilator-associated iatrogenesis may exist and must be understood to improve outcomes for mechanically ventilated patients. Here, we argue that ventilator-associated brain injury (VABI) merits urgent attention to establish whether brain-protective ventilation strategies could improve outcomes for critically ill patients.
Defining VABI
It is widely appreciated that mechanical ventilation can cause secondary brain injury in patients with established primary brain injury (i.e., stroke or traumatic brain injury). Emerging evidence suggests that mechanical ventilation can also be a primary cause of brain injury in patients without antecedent brain injury, a separate and distinct entity referred to as “VABI.” We propose to define VABI as de novo brain injury or dysfunction directly resulting from the application of positive pressure mechanical ventilation and not attributable to cointerventions or confounding factors such as sedation.
Evidence for VABI in Preclinical Studies
Preclinical studies have shown that positive pressure ventilation per se can cause acute inflammation and cellular apoptosis in multiple brain regions. In animals without antecedent brain injury, positive pressure ventilation impairs blood–brain barrier function and induces neuroinflammation and hippocampal apoptosis (1, 2). The resulting neuropathology is similar to that of Alzheimer’s disease (1).
VABI Results from Injurious Lung Stress and Strain
In murine and porcine models, VABI develops in proportion to the “dose” and duration of mechanical lung stress and strain from positive pressure ventilation, increasing neuronal activity in a dose-dependent manner (3). This neuronal activity inactivates prosurvival pathways and triggers an intrinsic apoptotic cascade involving acute neuroinflammation and neuronal injury (4). Compared with usual Vt (6–8 ml/kg), very high Vt (20–30 ml/kg) increases neuronal apoptosis in the hippocampus, retrosplenial cortex, thalamus, amygdala, paraventricular nuclei, and supraoptic nuclei (3). On the other hand, very low Vt (2–3 ml/kg) significantly lowers cerebral proinflammatory cytokine concentrations in comparison with usual Vt (8–9 ml/kg) (5). Higher driving pressure and mechanical power also increase neuroinflammation and apoptosis. The resulting brain injury causes cognitive impairment proportional to the duration of ventilation: In one study, mice ventilated for 3 hours had worse cognitive scores than mice ventilated for 1 hour, and the impairment in cognition persisted for up to 3 days after extubation (6).
Observational Evidence of VABI in Humans
Circumstantial evidence suggests a link between injurious mechanical ventilation and long-term neurological outcomes (7). In a retrospective observational study (n = 256) of patients with out-of-hospital cardiac arrest, lower Vt (<8 ml/kg) over the first 48 hours after hospital admission were associated with a better cerebral performance category at hospital discharge (odds ratio for good neurological outcome, 1.6; 95% confidence interval, 1.1–2.3; per 1-ml/kg decrease in Vt) (7). A systematic review identified a consistent association between the duration of mechanical ventilation and the risk of delirium (8). Delirium is, in turn, an important risk factor for long-term neurocognitive impairment (8). Long-term neurocognitive impairment is highly prevalent among patients who survive mechanical ventilation, affecting up to one-third of patients 1 year after their index illness (9).
Potential Therapeutic Strategies for VABI
Preclinical studies suggest multiple potential approaches to mitigating VABI. First, avoiding high driving pressures may attenuate VABI by limiting lung stress and strain from positive pressure ventilation. Second, pharmacologic or neuromodulatory approaches have been proposed, including pulmonary stretch receptor antagonism (e.g., by nebulized lidocaine) (10), pulmonary purinergic receptor blockade (e.g., achieved by intratracheal administration of an experimental drug, iso-PPADS-tetrasodium, in preclinical models) (10), olfactory bulb stimulation (11, 12), or diaphragm neurostimulation (13, 14), all of which might be evaluated in future randomized trials. In a clinical study, olfactory bulb stimulation led to activation of the default mode network in patients with coma from opioid overdose; activation of the default mode network has been linked to better cognition (11). The mechanisms by which olfactory bulb stimulation and diaphragm neurostimulation prevent VABI are unclear. These findings would also suggest that spontaneous breathing and/or olfactory bulb stimulation under assisted ventilation may protect against VABI.
Research Agenda for VABI
We emphasize that the concept of VABI currently remains a hypothesis and that its clinical significance is uncertain. The precise mechanisms linking positive pressure ventilation to brain injury remain to be delineated. The concept of VABI also requires further definition and validation in clinical settings. Such validation could be accomplished by assessing the relationships between injurious mechanical ventilation settings and physiology or imaging-based measures of brain inflammation, injury, and dysfunction, particularly within randomized trials of protective mechanical ventilation strategies. The confounding effects of cointerventions such as sedation will require careful investigation.
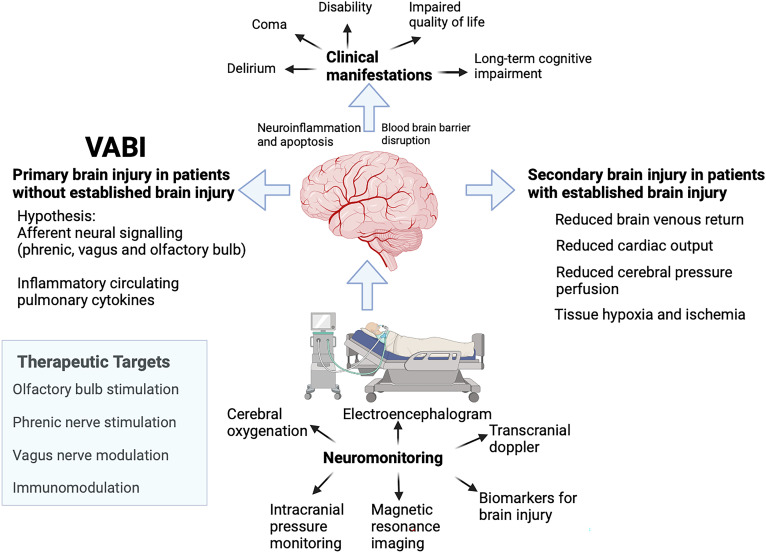
The development of assays (biomarkers, imaging) to detect primarily VABI would substantially facilitate clinical investigation. There are several candidate biomarkers, including S100β, glial fibrillary acid protein, ubiquitin C-terminal hydrolase L1, and neurofilament light chain (13). Electrophysiological monitoring or functional imaging techniques might also be means to detect VABI (Figure 1). The relationship between these assays and VABI requires careful delineation, given many confounding factors.
Figure 1.
Brain injury from mechanical ventilation. Mechanical ventilation may cause brain injury secondarily in patients with established acute brain injury by potentiating ischemia, particularly when cerebral autoregulation is impaired and intracranial pressure is increased. Mechanical ventilation may also cause primary brain injury in the absence of established brain injury by various possible mechanisms. In both forms of injury, neuromonitoring techniques might be useful to detect and monitor such injury. Possible therapeutic targets could be phrenic nerve stimulation, olfactory bulb stimulation, and vagus and immune modulation. VABI = ventilator-associated brain injury. Figure created with BioRender.
If a working clinical definition of VABI can be validated, then the clinical manifestations of VABI, associated risk factors, and long-term outcomes could be systematically characterized. Ultimately, strategies to prevent VABI during mechanical ventilation (“brain-protective ventilation”) should be developed and rigorously evaluated.
VABI: A New Frontier in Acute Respiratory Failure?
We suggest that the concept of VABI currently stands where the concept of ventilator-induced lung injury stood in the 1970s after the seminal publication by Webb and Tierney or where the concept of ventilator-induced diaphragm dysfunction stood in the first decade of the 21st century: an intriguing hypothesis of uncertain clinical significance. If validated, VABI and associated brain-protective ventilation strategies could be a new frontier for alleviating long-term disability and improving the quality of life in survivors of mechanical ventilation. Given the priority of long-term neurological function for critically ill patients who survive respiratory failure, VABI merits urgent attention and investigation.
Footnotes
Supported by an Early Career Investigator Award from the National Sanitarium Association (E.C.G.) and Doctoral Award from the Canadian Institutes of Health Research (S.T.).
Author Contributions: T.B., S.T., T.D.G., C.R., and E.C.G. participated in the interpretation of the results. T.B., S.T., T.D.G., C.R., and E.C.G. critically revised the manuscript for intellectually important content and gave final approval of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202401-0069VP on March 25, 2024
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Lahiri S, Regis GC, Koronyo Y, Fuchs DT, Sheyn J, Kim EH, et al. Acute neuropathological consequences of short-term mechanical ventilation in wild-type and Alzheimer’s disease mice. Crit Care . 2019;23:63. doi: 10.1186/s13054-019-2356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bassi TG, Rohrs EC, Fernandez KC, Ornowska M, Nicholas M, Gani M, et al. Brain injury after 50 h of lung-protective mechanical ventilation in a preclinical model. Sci Rep . 2021;11:5105. doi: 10.1038/s41598-021-84440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quilez ME, Fuster G, Villar J, Flores C, Martí-Sistac O, Blanch L, et al. Injurious mechanical ventilation affects neuronal activation in ventilated rats. Crit Care . 2011;15:R124. doi: 10.1186/cc10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. González-López A, López-Alonso I, Aguirre A, Amado-Rodríguez L, Batalla-Solís E, Astudillo A, et al. Mechanical ventilation triggers hippocampal apoptosis by vagal and dopaminergic pathways. Am J Respir Crit Care Med . 2013;188:693–702. doi: 10.1164/rccm.201304-0691OC. [DOI] [PubMed] [Google Scholar]
- 5. Ruemmler R, Ziebart A, Moellmann C, Garcia-Bardon A, Kamuf J, Kuropka F, et al. Ultra-low tidal volume ventilation—a novel and effective ventilation strategy during experimental cardiopulmonary resuscitation. Resuscitation . 2018;132:56–62. doi: 10.1016/j.resuscitation.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 6. Chen C, Zhang Z, Chen T, Peng M, Xu X, Wang Y. Prolonged mechanical ventilation-induced neuroinflammation affects postoperative memory dysfunction in surgical mice. Crit Care . 2015;19:159. doi: 10.1186/s13054-015-0882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beitler JR, Ghafouri TB, Jinadasa SP, Mueller A, Hsu L, Anderson RJ, et al. Favorable neurocognitive outcome with low tidal volume ventilation after cardiac arrest. Am J Respir Crit Care Med . 2017;195:1198–1206. doi: 10.1164/rccm.201609-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bassi T, Rohrs E, Reynolds S. Systematic review on brain injury after mechanical ventilation. Crit Care . 2021;25:99. doi: 10.1186/s13054-021-03521-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. BRAIN-ICU Study Investigators Long-term cognitive impairment after critical illness. N Engl J Med . 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. González-López A, López-Alonso I, Pickerodt PA, von Haefen C, Amado-Rodríguez L, Reimann H, et al. Lung purinoceptor activation triggers ventilator-induced brain injury. Crit Care Med . 2019;47:e911–e918. doi: 10.1097/CCM.0000000000003977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salimi M, Javadi AH, Nazari M, Bamdad S, Tabasi F, Parsazadegan T, et al. Nasal air puff promotes default mode network activity in mechanically ventilated comatose patients: a noninvasive brain stimulation approach. Neuromodulation . 2022;25:1351–1363. doi: 10.1016/j.neurom.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 12. Ghazvineh S, Salimi M, Nazari M, Garousi M, Tabasi F, Dehdar K, et al. Rhythmic air-puff into nasal cavity modulates activity across multiple brain areas: a non-invasive brain stimulation method to reduce ventilator-induced memory impairment. Respir Physiol Neurobiol . 2021;287:103627. doi: 10.1016/j.resp.2021.103627. [DOI] [PubMed] [Google Scholar]
- 13. Bassi TG, Rohrs EC, Fernandez KC, Ornowska M, Nicholas M, Gani M, et al. Transvenous diaphragm neurostimulation mitigates ventilation-associated brain injury. Am J Respir Crit Care Med . 2021;204:1391–1402. doi: 10.1164/rccm.202101-0076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parfait M, Rohrs E, Joussellin V, Mayaux J, Decavèle M, Reynolds S, et al. An initial investigation of diaphragm neurostimulation in patients with acute respiratory distress syndrome. Anesthesiology . 2024;140:483–494. doi: 10.1097/ALN.0000000000004873. [DOI] [PubMed] [Google Scholar]