Abstract
Metastasis causes the majority of cancer-related deaths, however the efficacy of anti-metastatic drugs is limited by the incomplete understanding of the biological mechanisms driving metastasis. Focusing on the mechanics of metastasis, we propose that the ability of tumour cells to survive the metastatic process is enhanced by mechanical stresses in the primary tumour microenvironment that select for well adapted cells. In this Perspective, we suggest that biophysical adaptations favourable for metastasis are retained via mechanical memory, such that the extent of memory is influenced by both the magnitude and duration of the mechanical stress. Among the mechanical cues present in the primary tumour microenvironment, we focus on high matrix stiffness to illustrate how it alters tumour cell proliferation, survival, secretion of molecular factors, force generation, deformability, migration, and invasion. We particularly center our discussion on potential mechanisms of mechanical memory formation and retention via mechanotransduction and persistent epigenetic changes. Indeed, we propose that the biophysical adaptations that are induced by this process are retained throughout the metastatic process to improve tumour cell extravasation, survival, and colonization in the distant organ. Deciphering mechanical memory mechanisms will be key to discovering a new class of anti-metastatic drugs.
Introduction
Inhibiting metastasis is a longstanding and important clinical goal, and as such, the genetic signatures responsible for disease progression have become the target of intense investigation 1. However, therapies that specifically target the metastatic process have failed to improve patient survival 1, as the mechanisms by which primary tumour cells induce metastatic disease remain elusive. Focusing solely on genetic signatures ignores the fact that metastasis is fundamentally a mechanical process 2,3 requiring specific biophysical properties. Tumour cells endure similar mechanical stresses during the dissemination process independently of the organ of origin. Yet, metastases originate from different organs. This suggests that metastatic ability is acquired through a common underlying basis. The development of solid tumours is often associated with aberrant extracellular matrix (ECM) deposition and remodelling of the primary tumour microenvironment (TME), which increases the mechanical stresses sensed by tumour cells 4. Linking mechanical changes within the primary TME to metastatic outcomes at the secondary site could reveal new pathways for therapeutic interventions.
In this Perspective, we propose a novel mechanism by which mechanical cues experienced in the primary TME continue to drive cancer cells to metastasize even once they are removed from the primary tumour, through cell mechanical memory. The term “mechanical memory” was coined by Balestrini and colleagues when the phenomenon was first discovered in fibroblasts in 2012 5. The concept of cell mechanical memory postulates that cells change their phenotype in response to a certain physical microenvironment and that this phenotype is maintained even after withdrawal of the original physical stimulus and exposure to a new microenvironment. This phenomenon has also been studied in mesenchymal stem cells (MSCs) 6–12 and epithelial cells 13, mostly with high matrix stiffness as a physical stimulus Values of matrix stiffness will refer to the elastic modulus throughout this manuscript, unless otherwise noted. Mechanical conditioning on stiff substrates mimicking fibrosis (100 kPa) for 3 weeks activated fibroblasts towards the myofibroblast phenotype even after a subsequent 2-week exposure to soft substrates more similar to healthy tissue (5 kPa) 5. Human MSCs that were initially cultured on stiff tissue-culture plastic (~3 GPa) for a longer duration (5 and 10 days versus 1 day) and subsequently on a 2 kPa hydrogel for 7 days preferentially expressed osteogenic markers rather than adipogenic ones 6. Furthermore, culture of MSCs on stiff substrates (100 kPa) for 3 weeks was shown to induce fibrogenesis and increased levels of microRNA-21, which were retained over 2 weeks via mechanical memory 10. However, the investigation of cell mechanical memory in the cancer field is currently at its infancy with very few published studies. Given that primary tumour cells are primed by matrix stiffening and adapt by enhancing proliferation 14–16, drug resistance 15,17,18, secretion of factors 19–23, force generation 24–27, changes in mechanical properties 26–30, and migration 16,31–33, we hypothesize that these biophysical adaptations allow tumour cells to overcome environmental barriers and survive the metastatic process. Traits that emerge during this ‘biophysical evolution’ are retained via mechanical memory, and thus play a major role in determining the outcome in the later stages of metastasis, such as extravasation, dormancy, and colonization. We believe this hypothesis is novel and unifying. It attributes key adaptations required for metastatic progression to matrix stiffening, a phenotype common to solid tumours and a marker of worse prognosis 34. Furthermore, it is mechanistic, as we propose that mechanical cues in the primary TME induce epigenetic changes of variable persistence based on the magnitude and duration of the mechanical stress. Persistent epigenetic changes, in turn, encode mechanical memory and thus retention of cellular biophysical adaptations at the distant site. This cascade of events, provides a mechanistic explanation for different metastatic outcomes.
This Perspective focuses on matrix stiffness in the primary tumour and its ability to imprint mechanical memory to influence metastasis at the distant site. However, matrix stiffness should not be understood as the sole mechanical cue able to imprint mechanical memory, but rather as an illustrative example as we do not discount that other mechanical cues present in the primary TME may also imprint memory. Significant additional research is required to validate the veracity of this proposition. However, if true, this hypothesis will redefine our understanding of metastasis by establishing its mechanical basis.
Mechanical cues in the primary tumour
Matrix stiffness
Solid malignant tumours exhibit increased stiffness relative to healthy tissues or even benign tumours, as noted in breast 34–37, pancreas 38, liver 39, and prostate 40 cancers. This characteristic can be used as a diagnostic marker 36,39,41, as well as a predictor of cancer risk 37,42–44, survival 45, and recurrence 46. Different techniques can be used in clinics to measure tumour stiffness relative to the stiffness of the surrounding tissue or a healthy control tissue. Values of tumour stiffness vary widely based on measurement methods, however, relative differences in stiffness between tumour tissue and normal tissue can be observed irrespective of measurement technique. For example, in breast tissue, the mean stiffness of malignant tumours was 153 kPa (compared to 40–64 kPa for benign lesions) when measured at the macroscopic level with shear wave elastography 36. Nanoscale mechanical profiling using atomic force microscopy (AFM) of frozen and thawed human breast tissue sections yielded heterogenous stiffness distributions including values >5 kPa (and up to 20 kPa in freshly measured samples 47) for malignant breast tumours, and values <0.5 kPa for non-tumour tissue 34. These differences in stiffness values exist because elastography measures stiffness at much higher frequencies and much larger length scales than AFM, which in turn has a higher spatial resolution 47. Elastography has the advantage of being non-invasive and measuring the stiffness of tissues in their natural environment. On the other hand, AFM of tissue biopsies, either measured freshly on the same day of the surgery 47 or frozen and thawed to preserve the mechanical properties of the tissue 34,48, has a higher stiffness sensitivity, which makes it a better diagnostic tool 47. Importantly, tumour stiffness varies between tissue types, based on the stiffness of the normal associated tissue, and ranges from 1 kPa in the brain to 70 kPa in the bile ducts 4. It is also noteworthy that the TME, like other tissues, exhibits complex mechanical behaviours such as viscoelasticity (load relaxation over time) 49 and mechanical plasticity (irreversible deformation due to load) 50, which were shown to influence tumour cell spreading and migration, respectively.
Changes in mechanical properties of the TME result from ECM remodelling by tumour cells or activated stromal cells 51–53. These stromal cells often secrete transforming growth factor β (TGF-β), resulting in excessive deposition and cross-linking of ECM components such as collagen, fibronectin, and hyaluronic acid 54–58. Together, increased ECM deposition and cross-linking result in elevated ECM stiffness that affects tumour cell phenotypes 4. Increased stiffness also activates fibroblasts and promotes alpha-smooth muscle actin (α-SMA) positive myofibroblast phenotypes. This generates a positive feedback loop where increased myofibroblast activation stimulates ECM production, ECM crosslinking and myofibroblast contractility, thus promoting further stiffening 59. Myofibroblast-like cancer-associated fibroblasts (CAFs) are often considered tumour-promoting 60,61, but studies have shown that they can be tumour-restraining 62,63 depending on fibroblast type and tissue origin, exemplified by a study that found CAF-secreted hyaluronan was pro-tumorigenic, while CAF-secreted collagen I was tumour-restraining 64. ECM composition and stiffness can also impact immune cells. 65Collagen-rich stiff ECM impairs T cell infiltration 66 and reduces their cytotoxic activity 67. However, it is unclear whether high ECM stiffness promotes M1 (pro-inflammatory, tumor-suppressing) or M2 (tumor-promoting) macrophage polarization. Elevated stiffness was shown to support M1 phenotypes on two-dimensional (2D) substrates 68, while M2 polarization was shown on 2D substrates 69 and in three-dimensional (3D) collagen hydrogels 70. Interestingly, high matrix stiffness was associated with the accumulation of M2 macrophages in mouse breast tumours 23. Additionally, matrix stiffening reduces vascular growth and integrity 71, and alterations in endothelial cell mechanosensing through the Rho pathway may contribute to the dysfunctional vasculature present in the TME 72.
Solid and fluid stresses
Changes in ECM composition and stiffness, as well as tumour growth, result in physical forces or pressures imposed on tumour cells through both cell–cell and cell–matrix interactions 73,74. These forces exerted by non-fluid tumour components are called solid stresses. Rapid tumour cell proliferation strains the TME and the expanding stiffer tumour pushes and displaces the surrounding normal tissue, which in turn constrains tumour expansion 73. As a result, tumour cells in the tumour core experience predominantly compressive stresses in all directions, while tumour cells at the tumour periphery experience both tensile stresses in the circumferential direction and compressive stress in the radial direction 73. At the cellular scale, tensile stresses are also created by contractile cells which realign collagen fibres 75–77, and further increase ECM stiffness via strain-stiffening 78. Solid stresses in tumours range from <0.1 to 10 kPa as measured in excised and in situ tumours by quantifying tissue displacement after disruption of the constraining tissue structures 74.
Fluid stresses, or the physical forces exerted by fluid tumour components, stem from growth-induced and surrounding tissue-induced solid stresses that cause the collapse or impairment of blood and lymphatic vessels within and surrounding the tumour 79. This produces elevated interstitial fluid pressure (IFP) within the tumour 73,80,81. IFP is uniform in the core of the tumour, varying between 2 mmHg (0.3 kPa) and 29 mmHg (3.9 kPa) 82–85, and drops to normal values of about 0 mmHg (0 kPa) 84 at the tumour periphery or in the surrounding normal tissue 86. This IFP gradient results in increased interstitial fluid flow away from the tumour 81,87, which applies fluid shear stress and pressure gradients across the tumour cells in the periphery of the tumour 88, thus sustaining tumour cell proliferation 89, and promoting migration and invasion 90. Additionally, some studies have recently reported enhanced tumour cell migration due to exposure to high extracellular fluid viscosity in vitro 91–93. These studies were based on the assumption that the interstitial fluid viscosity in the TME is high due to degradation of the ECM and expression of mucins by epithelial cells 91–93. However, while it was shown that intratumor blood viscosity might be higher than in normal tissues 94,95, there is currently no empirical evidence that the viscosity of the interstitial fluid is significantly elevated in the TME. Extracellular fluid viscosity, like fluid and solid stresses, is challenging to measure in the TME. Future measurement techniques will hopefully be able to provide reliable values for these mechanical cues so that future studies can model them in a physiologically relevant manner to explore their effect on tumour and stromal cell behaviour.
Cellular biophysical adaptations
Although we hypothesize that solid and fluid stresses can imprint mechanical memory onto tumour cells, there is more evidence of matrix stiffness influencing tumour cell behaviours. This section describes the biophysical adaptations tumour cells undergo in response to high matrix stiffness in the primary TME (Fig. 1) and that can potentially be retained via mechanical memory to promote metastasis.
Figure 1: Tumour cell biophysical adaptations in response to the increase in matrix stiffness during primary tumour development.
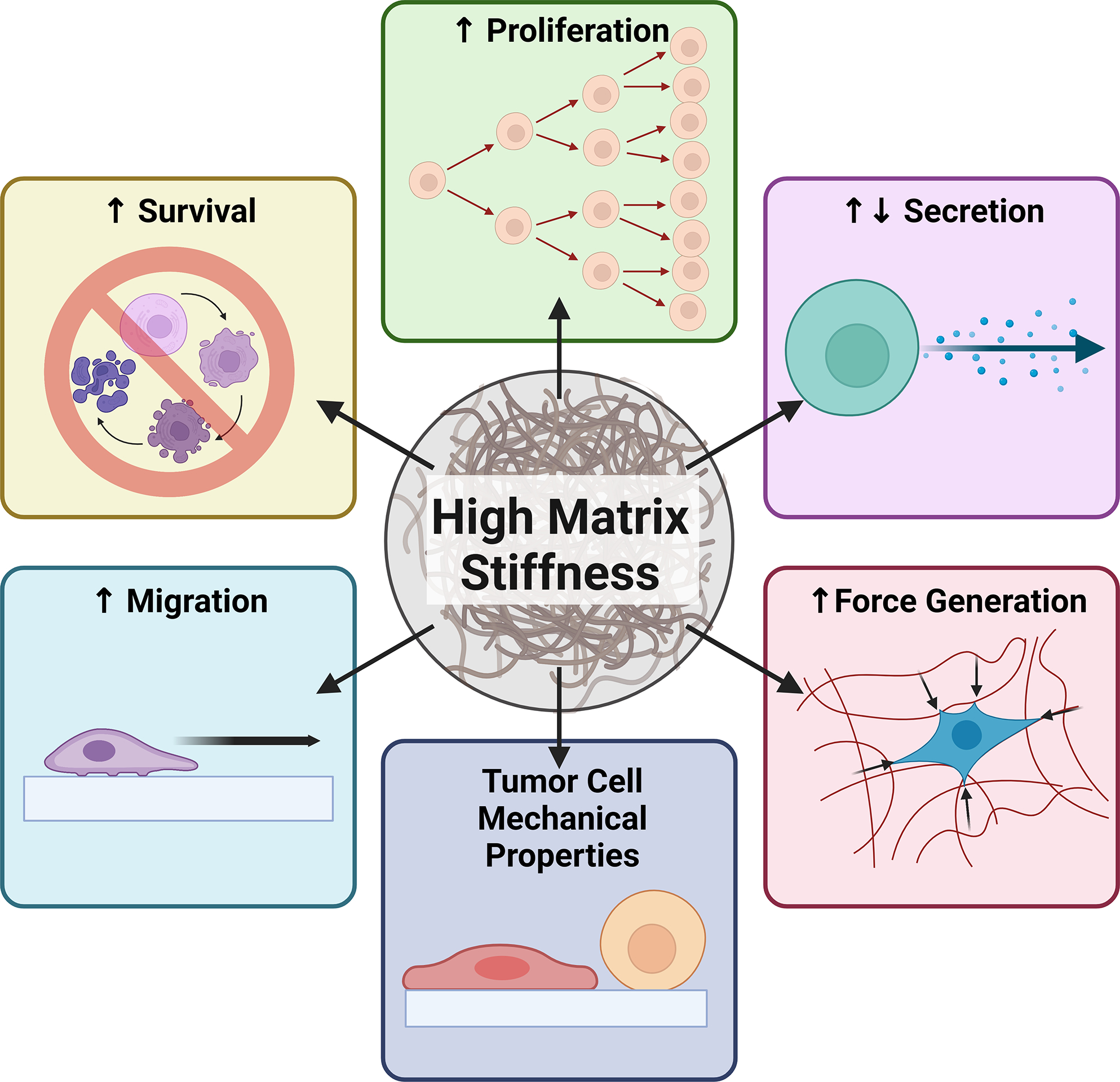
As solid tumours grow, invading tumour cells and activated stromal cells remodel the extracellular matrix via an excessive deposition and cross-linking of extracellular matrix proteins such as collagen, fibronectin, and hyaluronic acid. Together, increased matrix deposition and cross-linking result in elevated matrix stiffness. High matrix stiffness induces rapid proliferation of tumour cells and enhances tumour cell stemness and therefore survival. These anti-apoptotic and self-renewal capabilities contribute to increased tumour drug resistance. Elevated matrix stiffness also promotes tumour cell secretion of pro-tumourigenic soluble factors, including matrix-modifying enzymes, pro-angiogenic factors and cytokines. Tumour cells also adapt mechanically to high matrix stiffness by increasing the traction forces exerted on the extracellular matrix, thus resulting in its deformation. Altered force generation further accompanies changes in the deformability of tumour cells in response to elevated matrix stiffness. Moreover, high matrix stiffness primes cancer cells to migrate faster and enhance their invasion into surrounding tissues. We propose that tumour cells that adapt to mechanical stresses in the primary tumour, particularly in response to high matrix stiffness, increase their fitness to successfully navigate the subsequent stresses of the metastatic process.
Proliferation
Tumour cell proliferation is enhanced on stiffer 2D matrices compared to soft matrices. Glioma cell lines (U373-MG, U87-MG, U251-MG, SNB19, C6) were shown to divide faster and spread more on stiff 2D matrices (119 kPa) compared to the same cells grown on soft matrices (0.08 and 0.8 kPa) 16. Similarly, the proliferation of hepatocellular carcinoma cell lines (Huh7 and HepG2) was higher on hydrogels at 12 kPa (shear modulus) versus 1 kPa, and this effect was regulated by integrin-β1 and focal adhesion kinase (FAK) 15. While the relationship between stiffness and cell proliferation in 2D is clear, the influence of matrix stiffness on tumour cells grown in 3D is more difficult to assess. In natural hydrogels, the increased stiffness is often accomplished by an increase in hydrogel concentration, which is inherently coupled to an increase in adhesion ligand density and a reduction in pore size 96. High adhesion ligand density can promote cell proliferation 97 while reduced pore size can restrict it 98. Although mechanical and biochemical cues can be uncoupled in synthetic hydrogels, a difference in pore size often persists between soft and stiff conditions. This was the case in a study of breast tumour spheroid growth within stiff (~20 kPa) and soft (~2kPa) 3D polyethylene glycol-heparin hydrogels. Spheroids cultured in stiff hydrogels were smaller, more compact and showed attenuated tumour cell proliferation, a higher fraction of cells in the G0/G1 phase, and an increase in the expression of p21 compared to spheroids cultured in soft hydrogels 99. While these effects were attributed mainly to the difference in hydrogel stiffness, differences in pore size between the hydrogels could have also influenced the findings. Moreover, tumour cell proliferation could also have been affected by radial compressive stresses of up to 2 kPa generated by spheroid growth in stiff hydrogels but not in soft ones 99. In a mouse model of mammary fat pad fibrosis, orthotopically injected mammary tumour cells produced larger and more proliferative tumours compared to controls 14. While tissue stiffness was considerably increased in this model, other elements such as altered fibroblast secretome could have also contributed to these findings 14. Therefore, tumour cells can increase their proliferation in response to high matrix stiffness, however other physical constraints within the TME can limit this.
Survival and drug resistance
In addition to enhancing cell cycle programs and proliferation, high matrix stiffness also promotes anti-apoptotic capabilities that contribute to increased tumour cell survival and drug resistance 15,17,18,100. Hepatocellular carcinoma cell lines (Huh7 and HepG2) not only display enhanced proliferation on stiff 2D synthetic gels (12 kPa, shear modulus) compared to soft 2D gels (1 kPa) but also reduced apoptosis in response to cisplatin treatment 15. Stiffness-induced resistance to sorafenib was also shown in human breast cancer cell lines cultured on stiff (400 kPa) 2D synthetic hydrogels coated with ECM proteins 17. Chemoresistance to paclitaxel, but not to gemcitabine, was further reported in in human pancreatic cancer cell lines (BxPC-3, AsPC-1, Suit2–007) cultured on stiff 2D synthetic hydrogels (4 kPa) but not on soft hydrogels (1 kPa) 18. The hydrogels matched the stiffness of the pancreatic tissue of control mice (maximum 1 kPa) and mice with pre-malignant (maximum 2 kPa) and malignant (maximum 4 kPa) pancreatic cancer 18. Stiffness-dependent chemoresistance was recently shown to occur via an increase in cancer stemness 100. Growing breast cancer cell lines (MCF-7 and BT-474) on stiff 2D polyacrylamide substrates (9 kPa) increased TAZ nuclear translocation and nuclear compartmentalization of TAZ and NANOG in comparison to those grown on soft substrates (0.5 kPa) 100. This promoted the transcription of stem cell markers SOX2 and OCT4 and an overall increase in the proportion of cancer stem cells 100. Targeting NANOG or TAZ reduced cancer stemness and chemoresistance in a mouse xenograft model of breast cancer 100. This highlights the role of matrix stiffness in tumour cell chemosensitivity and its influence on drug screenings.
Secretion of molecular factors
To further promote a microenvironment more conducive to tumour growth and evading therapeutics, tumour cells can adaptively secrete factors to alter stromal, endothelial, and immune cell phenotypes. Matrix metalloproteinases (MMPs), which degrade ECM proteins, promoting tumour growth, and invasion 101, are a key example of this. Exposure to stiffer 2D collagen substrates (2 kPa versus 0.05 kPa) increased MMP activity in pancreatic cancer cell line Panc-1 but decreased it in BxPC-3 and AsPC-1 cells 19. Stiffness-induced MMP activity in Panc-1 cells was not dependent on ligand density, as comparison of stiffer cross-linked collagen gels with softer uncross-linked ones of the same collagen concentration showed higher MMP activity on cross-linked gels relative to uncross-linked ones 19. Human colorectal cancer cells (T84 cell line) increased MMP7 expression in response to higher matrix stiffness (126 kPa versus 2 kPa) on 2D polyacrylamide gels 20. This was shown to be mediated by signalling through integrin-β1, integrin-α2, epidermal growth factor receptor (EGFR), myosin light chain 2 (MLC2), and yes-associated protein (YAP) 20. High substrate stiffness (16 kPa versus 10 kPa and 6 kPa) was also shown to increase secretion of ECM crosslinking enzyme, lysyl oxidase-like 2 (LOXL2) in hepatocellular carcinoma cells (MHCC97H and Hep3B) cultured on 2D polyacrylamide gels 21. Moreover, tumour cell-secreted LOXL2 promoted C-X-C motif chemokine ligand 12 (CXCL12) gene expression and production of MMP9 and fibronectin in lung fibroblasts, as well as enhanced motility and invasion of bone marrow-derived cells in vitro 21.
Increased matrix stiffness can further promote angiogenesis directly through mechanosensitive pathways involving MMP activity in endothelial cells 71. Moreover, angiogenesis can be regulated by high matrix stiffness indirectly through the secretion of pro-angiogenic factors such as vascular endothelial growth factor (VEGF) by tumour cells, which then affect endothelial cell behaviour 22,102. Increased matrix stiffness in liver cancer rat models was shown to upregulate expression of the Piezo1 mechanosensor and angiogenesis 22. In the same study, human hepatocellular carcinoma cell lines cultured on stiff 2D hydrogels (16 kPa and 10 kPa versus 6 kPa) showed increased Piezo1 activation, which subsequently promoted the secretion of pro-angiogenic factors VEGF, CXCL16, and insulin-like growth factor binding protein 2 (IGFBP2) 22. However, in neuroblastoma clinical samples, a negative correlation was observed between tissue stiffness and blood vessel length 102. To investigate this finding, a neuroblastoma cell line (SK-N-SH) was cultured on 2D polyacrylamide gels of different stiffnesses 102. Elevated matrix stiffness (30 kPa versus 1 kPa and 8 kPa) inhibited the secretion of VEGF by SK-N-SH cells via the YAP–Runt-related transcription factor 2 (RUNX2)–Serine/Arginine Splicing Factor 1 (SRSF1) axis 102. Nevertheless, the stiffness of the neuroblastoma tissues ranged between ~0.1 kPa and ~8 kPa 102 and the 30 kPa in vitro stiffness might thus not be physiologically relevant. More research is needed to elucidate if stiffness-induced angiogenesis is promoted at particular stiffnesses and if these stiffnesses depend on tissue type.
Tumour cells can also secrete molecular factors that recruit immune cells in response to high matrix stiffness 23,34,103. In human breast tumours, the highest numbers of macrophages were measured at the invasive front, where the stroma was the stiffest and TGF-β signalling was the highest 34. Similarly, high collagen density in mouse breast tumours induced high cyclooxygenase-2 (COX-2) expression and high levels of secreted cytokines, which increased macrophage and neutrophil recruitment 103. Moreover, a breast cancer cell line (MDA-MB-231) cultured on stiffer 2D synthetic hydrogels (10 kPa versus 1 kPa) was shown to express higher levels of colony stimulating factor 1 (CSF1), which enhanced the recruitment of macrophages 23. These results confirmed observations that elevated matrix stiffness enhances M2 macrophage recruitment in mouse breast tumours 23.
Force generation
To complement their altered growth and secretion of molecular factors, tumour cells further display irregular traction forces relative to normal cells. For example, a normal fibroblast cell line, NIH-3T3, on 2D hydrogels showed spatial patterns characterized by traction forces concentrated at the cell periphery and orientation towards the cell body 104. However, malignant transformation caused traction forces of uncoordinated directions and weaker magnitude 105. Moreover, tumour cell force generation on 2D hydrogels can either increase 24,106 or decrease 107 with increasing metastatic potential. At this time, it is not definitely known how metastatic potential influences tumour cell force generation. These studies all had the advantage of measuring force generation in isogenic cell lines. The conflicting results thus likely stem from the variations in cell lines, substrate stiffnesses, surface coatings, and analysis algorithms used in the studies. Substrate stiffness in particular might be a crucial experimental parameter, as a recent study of non-isogenic prostate cell lines found that cells of higher metastatic potential had higher traction forces than cells of lower metastatic potential on stiffer 2D substrates (3 and 12 kPa) but the opposite was found on softer substrates (1 kPa) 27.
Interestingly, traction forces of both normal 104,108–112 and cancerous 24–27 cells, grown as single cells or small clusters on 2D substrates consistently show increased traction forces in response to elevated substrate stiffness, independent of the measurement technique. In 3D, high stiffness has also been shown to increase traction forces of NIH-3T3 fibroblast cell line 113 and MDA-MB-231 breast cancer cells 114. Mechanistically, force generation by tumour cells in response to high ECM stiffness is driven by integrin clustering and signalling through EGFR, which activates extracellular signal-regulated kinase 1/2 (ERK) and increases RHO–Rho-associated kinase (ROCK)-mediated actomyosin contractility 25,108. If enhanced force generation and activation of its related pathways are maintained, at least in part, via mechanical memory, they could aid metastasis, notably during tumour cell extravasation at the distant site.
Deformability
Altered contractility further accompanies changes in the deformability of cells. Micropipette aspiration measurements showed that SV40-transformed dermal fibroblasts were more deformable and less contractile than normal fibroblasts 115. Similarly, AFM measurements showed that metastatic tumour cells obtained from body (pleural) fluids of patients with suspected lung, breast and pancreas cancer were significantly softer than normal cells that line the body cavity 116. While some studies on breast 117–119, ovary 120, prostate 118, and bladder 118 cancer cell lines have shown that tumour cell softness increases with metastatic potential, others have found that in prostate 27, skin 118, and kidney 29,118 cancer cell lines, this is not the case. In this context, the study by Coughlin et al. 118 is particularly informative as the measurements were performed on weakly and strongly metastatic cancer isogenic cell line pairs originating from human cancers of the skin (A375P and A375SM cells), kidney (SN12C and SN12PM6 cells), prostate (PC3M and PC3MLN4 cells), and bladder (253J and 253JB5 cells) instead of cell lines with genetically different lineages. This study found that strongly metastatic PC3MLN4 cells were more fluid-like compared to the weakly metastatic PC3M cells 118. However, another study found the opposite when comparing cell lines of increasing metastatic potential of different lineages (22RV1, LNCaP, DU145, and PC3) 27. The choice of cell lines could thus influence this outcome. Indeed, tumour cell softness or stiffness is unique for every tumour and is likely dependent on a host of factors, such as tissue of origin, spatial localization, oncogenic drivers, or site of metastasis. It is hypothesized that tumour cells only adopt a softer phenotype after extravasation 118,119. This is partially supported by recent studies measuring tumour cell stiffness before and after migration through confined spaces 121 or across a layer of endothelial cells 122 In these models representative of the stresses imposed on tumour cells during extravasation, tumour cells were shown to soften after migration 121,122. However, it has also been reported that invading cells exhibit dorsoventral (top-to-bottom) polarity and that the nucleus stiffens when cells migrate along a surface and are confined in the vertical (along the dorsoventral polarity axis) as opposed to lateral (perpendicularly to the dorsoventral polarity axis) direction 123. Whether cells soften upon migration in confined spaces might thus depend on cell polarity and the geometry of the migration track.
Additionally, the relationship between metastatic potential and tumour cell deformability depends on substrate stiffness. In a study of MCF10A breast epithelial cells cultured in 3D collagen gels as single cells, ErbB2-transformed cells stiffened in response to increased, albeit very low, matrix stiffness (391 Pa versus 104 Pa, storage modulus), but the stiffness of non-transformed cells remained constant 28. Moreover, several studies have shown that invasive tumour cells can adjust their mechanical properties in response to ECM stiffness, contrary to non-invasive tumour cells or normal cells 26,29,30. Remarkably, some invasive tumour cells become stiffer in response to elevated ECM stiffness [breast (MDA-MB-231) 26,30, pancreas KPR172HC) 30, and prostate (PC3) 27 cell lines], while others become softer [kidney (ACHN) 29, breast (4T1) 30, and colon (SW620) 30 cell lines]. In these studies, cell stiffness was measured on 2D substrates, except for the study by Wullkopf et al., where it was measured in 3D collagen gels 30. Interestingly, the stiffness of a lowly metastatic prostate cancer cell line on 2D substrates ranging from 1 to 50 kPa increased from 1 to 12 kPa and then decreased from 12 to 50 kPa 27. Moreover, in a 3D invasion assay with mouse breast cancer spheroids (4T1) in a 1.2 kPa collagen gel, those cancer cells leading the invasion into the surrounding matrix were found to be softer than the invasive cancer cells following, or the tumour cells still in the centre of the spheroids 30. In breast cancer spheroids cultured in stiff hydrogels (~20 kPa), the stiffness of the individual cells and overall spheroid increased via the stiffness-induced activation of ROCK and expression of F-actin at the rim of the spheroid 99. Retention of strong cell mechanical properties acquired in the primary TME via mechanical memory could help the tumour cells to resist the various mechanical stresses encountered during the metastatic process.
Migration
The relationship between substrate stiffness and tumour cell migration is related to the metastatic potential of the tumour cells, as defined by a mesenchymal or epithelial phenotype 33. A highly invasive oral squamous cell carcinoma cell line (SCC25) migrated faster, both collectively and individually, on stiffer substrates (20 kPa versus 0.48 kPa), while a less invasive cell line (Cal27) was insensitive to substrate stiffness 33. In this study, invasiveness was quantified by measuring the invasion depth of cells seeded on a collagen hydrogel containing primary fibroblasts and was complemented by the analysis of epithelial-to-mesenchymal transition (EMT) marker expression, as well as the analysis of the E-cadherin to N-cadherin ratio. Prolonged exposure to stiff substrates led to EMT of less invasive tumour cells, which then acquired stiffness-dependent migration 33. Indeed, matrix stiffness and the resulting forces applied to the nucleus promote the translocation of transcription factors involved in the EMT process, which play a key role in tumour cell dissemination 124–127.
Several studies have reported that tumour cells migrate faster on stiffer 2D substrates relative to softer substrates 16,32,33. However, some metrics of migration, such as speed 128,129, velocity 130, or distance 128,130,131, were shown to be maximized at particular substrate stiffnesses. For example, U-251MG glioma cells migrated towards areas with stiffness of ~8 kPa where traction forces were maximized when seeded on fibronectin-coated polyacrylamide hydrogels having a continuous stiffness gradient of ~0.5 to 22 kPa 131. On the other hand, breast tumour cells cultured on 2D hydrogel substrates ranging from 10 kPa to 57 kPa showed enhanced migration distance and velocity at 38 kPa 130.
Similar phenomena also occur in more physiologically relevant 3D matrices, where the migration speed of DU-145 human prostate carcinoma cells in Matrigel hydrogels of varying density (50% to 75%, ~10 to ~32 Pa, storage modulus) was shown to peak at an intermediate Matrigel density of 67% that balances traction and adhesion forces 132. However, when integrin binding was inhibited, speed was higher in hydrogels of lower densities 132. Indeed, besides substrate stiffness, migration of cells embedded in 3D matrices might be influenced by physical variables (reviewed in 133), such as adhesion 132, confinement through matrix pores 132,134, and surface topology including constrictions points that challenge the movement of the nucleus 135. Engineered models consisting of microfabricated channels overcome these confounding factors by providing defined migratory tracks with prescribed substrate stiffness 31,114,136,137. Remarkably, U373-MG glioma cells confined to narrow channels (10 μm) migrated concordantly faster when substrate stiffness increased (120 kPa versus 10 kPa versus 0.4 kPa), while unconfined tumour cells (in 20 and 40 μm channels) exhibited a migration speed that increased between 0.4 kPa and 10 kPa and decreased between 10 kPa and 120 kPa 31. More research is needed to understand how matrix stiffness and its interaction with other physical variables affect tumour cell migration in 3D. Retention of stiffness-induced fast tumour cell migration via mechanical memory could facilitate metastasis, particularly extravasation, at the distant site.
Mechanical memory and epigenetics
Our hypothesis that extravasation, dormancy, and metastatic colonization of tumour cells are influenced by the past mechanical experiences of the tumour cells in the primary TME not only assumes that tumour cells sense and react to mechanical signals, but that they also retain the biophysical adaptations induced by these physical stresses after they move to a new environment. In this section, we discuss cell mechanical memory and persistent epigenetic modifications as molecular mechanisms that enable tumour cells to retain the biophysical adaptations acquired in the primary tumour even after their dissemination (Fig. 2).
Figure 2: Tumour cell mechanosensing, mechanotransduction, and mechanical memory of matrix stiffness imprinted through persistent epigenetic changes.
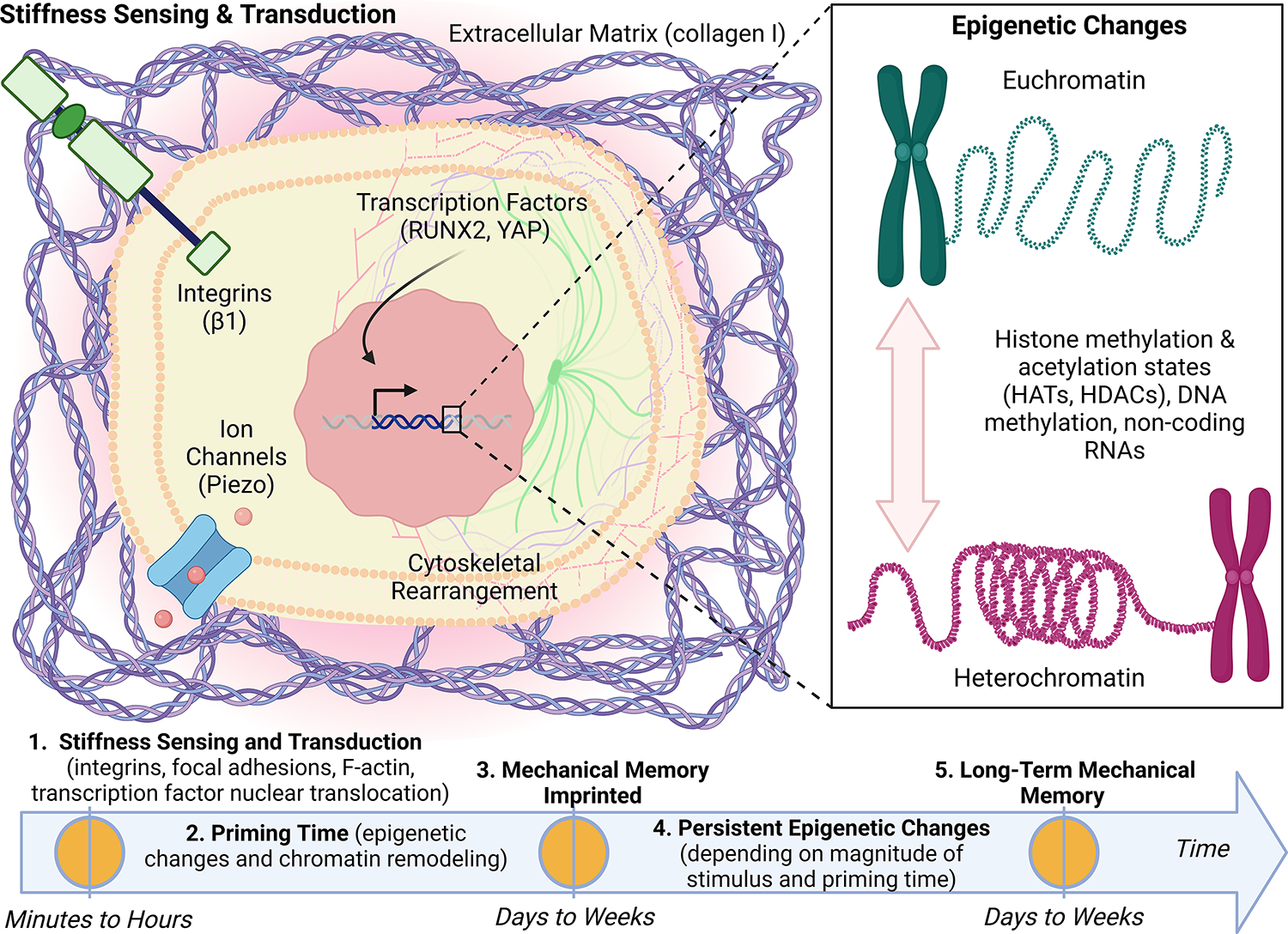
Tumour cells sense high matrix stiffness via the activation of integrins which promotes cytoskeletal rearrangement that in turn promote nuclear translocation of mechanotransducive transcription factors, runt-related transcription factor 2 (RUNX2) and yes-associated protein (YAP). These mechanosensing and mechanotransduction mechanisms occur within minutes to hours of exposure to stiff matrix. On a longer timescale, mechanotransduction can lead to epigenetic changes including histone modification through histone acetyltransferases (HATs) and histone deacetylases (HDACs), DNA methylation, and non-coding RNAs. If the magnitude of the mechanical stress and priming time are sufficient (days to weeks), we hypothesize that the phenotypic adaptations will be maintained via tumour cell mechanical memory encoded by persistent epigenetic changes. We propose that mechanical memory mechanisms play a fundamental role in the metastatic process by directly linking tumour cell biophysical adaptations acquired in the primary tumour to the adaptations that drive disease progression in secondary sites.
Epithelial cells (both non-tumorigenic cell line MCF10A and tumorigenic cell lines MCF7 and A431) were shown to undergo collective migration faster and in a more coordinated fashion, when they were first grown (primed) for 3 days on a 2D polyacrylamide gel at 50 kPa stiffness versus 0.5 kPa, even when they were subsequently exposed to a soft gel for 2 days 13. This cell mechanical memory was retained through nuclear translocation of the mechanosensitive transcription factor YAP, as cells primed on stiff substrates retained nuclear YAP even after arriving on a soft secondary gel 13. This also regulated actomyosin expression and focal adhesion formation 13. Interestingly, in YAP-knockdown cells (where YAP expression and thus nuclear translocation were significantly reduced), mechanical memory-dependent migration was abrogated, but mechanosensitivity to stiffness was conserved via focal adhesions 13. Recently, mechanical conditioning of various breast cancer cell lines and patient-derived xenograft primary cells on a 2D stiff polyacrylamide hydrogel (8 kPa) for 7 days induced enhanced cytoskeletal dynamics and invasiveness that were maintained after transfer to a softer hydrogel (0.5 kPa) for 1 day 138. Mechanically conditioned cells that were injected into mouse models were able to promote osteolytic bone metastasis through the nuclear translocation of the transcription factor RUNX2 138. Stiffness-induced cytoskeletal changes and ERK phosphorylation induced RUNX2 activation, and further delayed chromatin remodelling by preserving chromatin accessibility at loci of genes implicated in bone remodelling 138. Upon transfer from a 2D stiff to a soft substrate, the expression of RUNX2 target genes was stable for 2 days but significantly reduced 7 days after the transfer, while the expression of YAP target genes was lost 2 days after the transfer 138. While mechanical memory studies involving cancer cells are only recently emerging, they support the findings of other studies involving non-cancer cells 5–12 by showing that the magnitude of the stiffness and priming time need to reach a cell type-dependent threshold (>8 kPa for days/weeks) to imprint mechanical memory that can be maintained for days to weeks (Fig. 2). Further extensive research is required to validate these findings comprehensively. Future studies should aim at imprinting mechanical memory in a physiologically relevant way, by prioritizing 3D in vitro models when possible. Additionally, these studies should monitor the persistence of genetic and epigenetic programs over sufficiently long timeframes and study their influence on different stages of metastasis both in engineered in vitro systems and in vivo.
Despite the identification of two transcription factors, YAP and RUNX2, little is known about how mechanical memory is molecularly retained. Recent results from a computational model suggest that mechanical memory is acquired and stored via a positive feedback loop between mechanical signalling and gene transcription 139. An example of such mechanism is stiffness-induced integrin signalling increasing F-actin polymerization, which frees transcription factors such as YAP, which in turn translocates to the nucleus and regulates F-actin via the Rho pathway, thus closing the loop 139. In early studies, it was hypothesized that long-term mechanical memory might be encoded by persistent epigenetic changes 5 including histone methylation, histone acetylation (regulated by (de-)methylases, histone acetyltransferases (HATs), and histone deacetylases (HDACs) enzymes), as well as DNA methylation and non-coding RNAs. It is well-known that mechanical cues alter chromatin accessibility 140 and transcriptional ability 141 in normal epithelial cells and studies covering this aspect in the cancer field are starting to emerge as well. Both the restrictive and permissive states of chromatin might influence cancer progression, as a restrictive state could hinder tumour suppressor programs, and a permissive state could favour oncogenic changes 142. It was recently shown that increased matrix stiffness can induce tumorigenesis in MCF10A breast epithelial cells via changes in chromatin remodelling 143. When cells were embedded in stiffer (2 kPa) 3D hydrogels for 14 days, they displayed more wrinkled nuclei (a sign of chromatin remodelling), increased lamina-associated chromatin, and more accessible chromatin sites binding to the specificity protein 1 (Sp1) transcription factor than cells embedded in soft (0.1 kPa) 3D hydrogels 143. Although a direct interaction was not established, HDACs 3 and 8 were found to act with Sp1 to promote stiffness-induced tumorigenicity 143. While this study is a prime example of mechanically induced epigenetic changes relevant to cancer, it does not investigate their persistence after withdrawal of the mechanical stimulus, which is required to encode mechanical memory.
Persistent epigenetic changes that potentially form mechanical memory have not yet been identified in cancer cells but have been shown in non-cancer cells 8,10,11. In MSCs, exposure to dynamic tensile loading 8 or stiff microenvironments 11 has led to persistent chromatin remodelling. Increasing tensile strains and number of loading cycles led to a higher level of chromatin condensation that was sustained for up to 5 days after the end of loading 8. This was abolished by the inhibition of class I and II HDACs or the histone-lysine N-methyltransferase Enhancer of Zeste Homolog 2 (EZH2) 8. Exposure to stiff 2D synthetic hydrogels (32.7 kPa) induced histone acetylation, which correlated with both YAP nuclear localization and changes in HAT1 and HDACs 1, 2, and 3 mRNA expression 11. However, causality was not tested as experiments involving YAP, HAT, or HDAC modulation were not conducted in this study. After substrate softening (reduced to 5.5 kPa), the reversibility of histone acetylation and chromatin organization depended on the duration of stiff substrate priming, with irreversible changes induced after 10 days 11. Besides histone modifications, non-coding RNAs have also been shown to preserve mechanical memory 10. Exposure of MSCs to stiff silicone substrates (100 kPa) for 3 weeks increased levels of microRNA-21, which were retained over 2 weeks after transfer to soft substrates (5 kPa) and regulated the transcription of genes implicated in fibrogenesis 10. MicroRNA-21 knockdown erased mechanical memory and sensitized MSCs to subsequent exposure to soft substrates 10.
While these aforementioned studies were focused on persistent epigenetic changes in non-cancer cells 8,10,11, it is still largely unknown whether mechanical signals present in the primary TME regulate the metastatic potential of cancer cells via persistent epigenetic changes and chromatin remodelling. However, this hypothesis is very plausible given that mechanical cues in the TME were shown to induce tumorigenicity of non-malignant cells via histone modification 143 and that molecular pathways that cause tumorigenesis can also endow tumour cells with metastatic properties 1. Moreover, tumour cells have been shown to form mechanical memory in response to high matrix stiffness 13,138 similarly to fibroblasts 5 or MSCs 6,10,11. It is thus likely that they can keep long-term mechanical memory via persistent epigenetic changes like those observed in MSCs 8,10,11. Interestingly, microRNA-21, which keeps long-term mechanical memory in MSCs, was shown to be upregulated in several cancer types 144 and to promote both tumour growth and metastasis in breast cancer 145,146. This suggests that microRNA-21 upregulation could persist along the metastatic process. Although specific persistent epigenetic changes in response to mechanical cues have not been identified in tumour cells, maintenance of chromatin accessibility has been shown as described earlier 138 and it would be reasonable to infer that it was induced by persistent epigenetic changes. Persistent epigenetic changes induced by mechanical cues in the primary TME might thus represent a novel paradigm that complements well-known genetic signatures in cancer and could open new possibilities to develop epigenetic therapies to prevent and treat metastasis.
Metastatic biophysical evolution
Genetic instability and unregulated proliferation lead to heterogeneous primary tumour cell populations 147. Environmental barriers, such as immune surveillance, hypoxia, or mechanical stresses, then select primary tumour cells that exhibit the specific traits that allow them to overcome these barriers and advance through the metastatic process 147. Focusing on mechanical barriers, we argue that the successful specific traits can not only occur randomly due to genetic instability, but also emerge through the acquisition of persistent epigenetic changes giving rise to cellular biophysical adaptations in response to mechanical stresses. We term this ‘biophysical evolution’. Many of the same mechanical barriers present at the primary tumour also exist at the secondary site 147, suggesting that selection of tumour cell populations at the primary tumour may determine outcomes in the later stages of metastasis at the secondary site (Fig. 3).
Figure 3: Mechanically induced tumour cell biophysical adaptations acquired in the primary tumour and retained in the distant organ foster extravasation, avoidance of dormancy, and secondary site colonization.
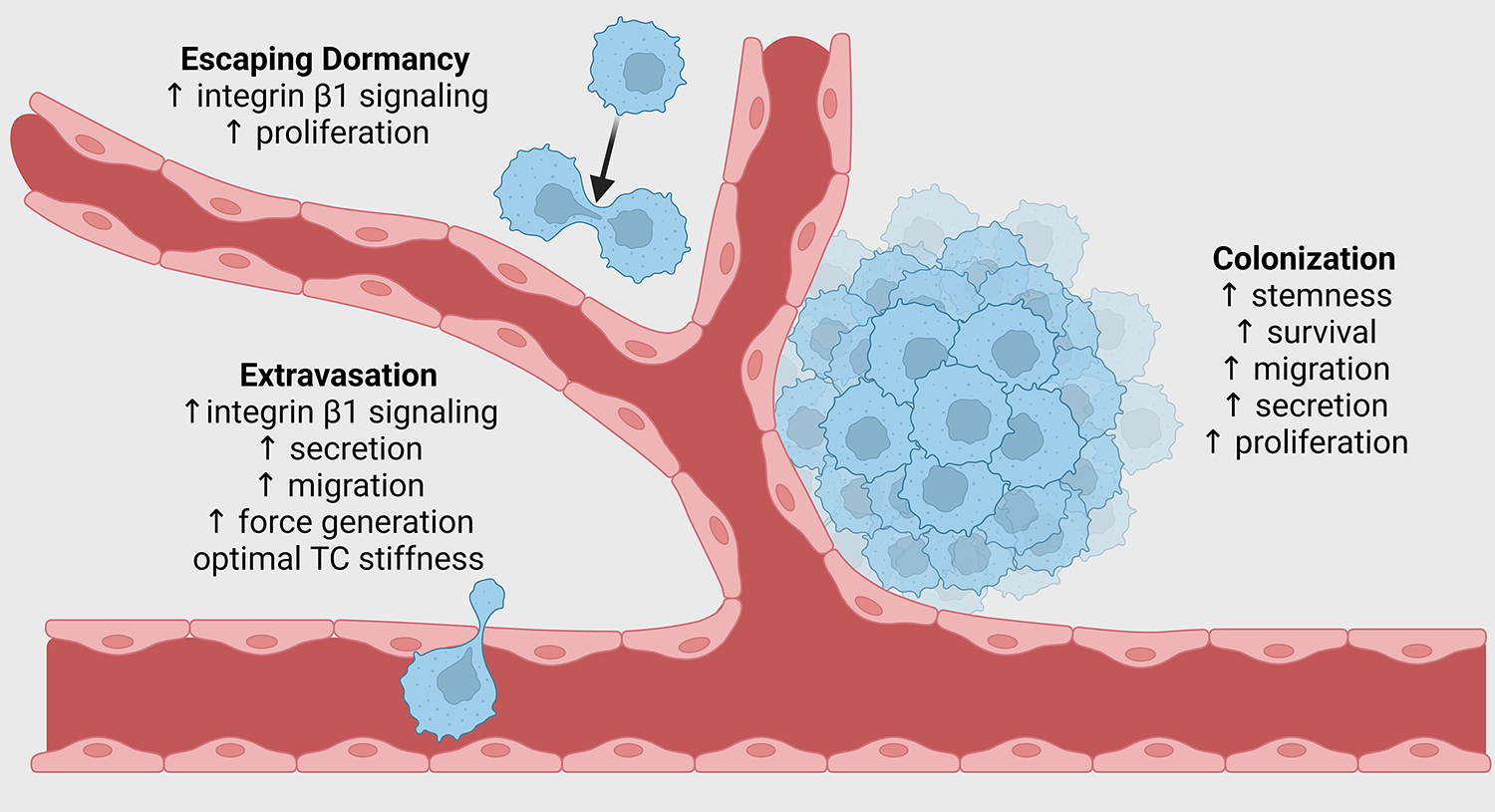
We propose that biophysical adaptations acquired by tumour cells in the primary site are retained at the secondary site via mechanical memory and facilitate the later steps of metastasis. Extravasation can be enhanced by memory-induced retention of increased integrin β1 signalling, secretion of matrix-modifying enzymes and pro-angiogenic factors, fast migration, high traction forces, and optimal tumour cell stiffness. Dormancy avoidance can be facilitated by maintenance of increased integrin β1 signalling, promoting faster proliferation. Colonization can be enhanced by retention of increased tumour cell stemness, resistance to apoptosis, secretion of growth factors, cytokines, and matrix-remodelling enzymes, and increased proliferation.
Extravasation
Among the biophysical adaptations acquired in the primary tumour, we hypothesize that several are retained via tumour cell mechanical memory and can enhance extravasation of circulating tumour cells at the secondary site. Tumour cells generate increased intracellular pressure via rear cortex contraction to facilitate nuclear transit through constrictions such as matrix pores and the intercellular space between endothelial cells 148,149. They also require the engagement of integrin-β1 when they send protrusions between endothelial cells to bind to the basement membrane and then extravasate 150. As previously described, elevated ECM stiffness activates integrin β1 signalling 15,20,25,108,130, and therefore we hypothesize that retention of activated integrin β1 via mechanical memory, even if partial, is likely to enhance extravasation. While maintenance of integrin β1 activation was not directly quantified in mechanical memory studies, maintenance of increased focal adhesion area, actin alignment, and MLC phosphorylation was shown in MCF10A cells migrating on soft (0.5 kPa) polyacrylamide hydrogels 2 days after exposure to stiff (50 kPa) hydrogels 13.
Extravasation is further promoted by remodelling of the vasculature through tumour cell secretion of angiogenic factors and MMPs 151–153. Interestingly, breast tumour cells cultured on stiffer 2D silicone substrates (1.1 MPa and 14.3 kPa versus 5.2 kPa) showed subsequent higher transendothelial migration in a soft 3D hydrogel in vitro, which was accompanied by increased MMP9 secretion, suggesting that enhanced migration in response to stiffness was retained and potentially mediated by MMP9 154. Moreover, tumour cells upregulate the production of VEGF in response to primary tumour stiffness, as shown in liver cancer rat models and human hepatocellular carcinoma cell lines cultured on stiff 2D hydrogels 22. This could also increase the permeability of the vascular barrier, thus increasing extravasation.
Stiffness-induced enhanced migration was shown to be retained via mechanical memory involving the regulation of actomyosin and focal adhesions in MCF10A, MCF7, and A431 cell lines grown on 2D polyacrylamide hydrogels 13. As extravasation is by definition transendothelial migration, retention of an enhanced migratory phenotype likely benefits extravasation. Additionally, force generation, a necessity of migration, is especially important for tumour cell extravasation 155. Force generation impaired by the inhibition of MLC kinase (MLCK) and small GTPases, including ROCK, in breast tumour cells was shown to reduce transendothelial migration 156. In a mechanical memory study, fibroblasts cultured on 100 kPa substrates and then transferred to 5 kPa substrates showed enhanced contractility compared to fibroblasts exclusively cultured on 5 kPa substrates 5. Moreover, enhanced MLC phosphorylation was retained in MCF10A cells transferred from 50 kPa to 0.5 kPa hydrogels 13. Increased cell contractility can thus be maintained and is likely to enhance extravasation if these findings extend to tumour cells. Moreover, retention of an optimal tumour cell stiffness would allow the tumour cell to simultaneously resist the high fluid shear stresses in the vessel and to extrude across endothelial cell junctions without fatal nuclear damage. Maintenance of cell stiffness after priming on stiff substrates has not been shown yet. It would be important to test this in tumour cells to understand if stiffness-induced cell mechanical properties can influence metastasis at the distant site. We hypothesize that although tumour cell stiffness will tend to adapt to the stiffness of the new microenvironment, it can partially be memorized.
Dormancy
We suggest that tumour cell biophysical adaptations acquired in the primary tumour and retained via mechanical memory could also regulate cellular dormancy at the secondary sites. Tumour cells arriving in the distant organ become dormant due to failure to engage integrin-β1 and activate FAK 150,157, as well as hostile biochemical and mechanical cues at the secondary site. Interestingly, higher matrix stiffness (~1 kPa versus 0.09 kPa) was shown to induce dormancy in various tumour cell types in 3D fibrin hydrogels, which was maintained via an epigenetic program that downregulated integrin-β3 158. These stiffnesses are too low to mimic tumour stiffness and were rather chosen by the authors of this study to match cell stiffness. Nevertheless, even a marginal increase in hydrogel stiffness could induce dormancy. While these results demonstrate that dormancy can be epigenetically maintained, stiffness was modulated by increasing fibrinogen concentration in the hydrogels, and thus simultaneously increasing adhesion ligand density, so dormancy cannot only be attributed to mechanical cues. Indeed, the findings could not be replicated in collagen I with the same cells in the same model 158. In a mouse model of pulmonary fibrosis, increased deposition of collagen I in the lungs was shown to trigger the proliferation of tail vein-injected dormant mouse breast cancer cells (D2.0R) via integrin-β1 signalling and downstream activation of proto-oncogene tyrosine-protein kinase (Src), FAK, ERK, and MLCK 159. These pathways are commonly activated in response to collagen deposition and elevated matrix stiffness in the primary TME 15,20,25,108,130, as described earlier. Therefore, retention of these mechanisms via mechanical memory might prevent tumour cells from entering dormancy in the first place upon arrival at the metastatic site. To test this, non-dormant cells that were mechanically primed on soft or stiff substrates should be injected in mice and their proliferation (or the lack thereof) should be monitored upon arrival in a metastatic site that is prone to induce dormancy, such as the bone marrow 160.
Colonization
We propose that biophysical adaptations mechanically induced in the primary TME and retained via mechanical memory can also influence colonization of the distant organ. Importantly, our theory complies with the three prerequisites of successful colonization: i) re-initiation of tumour growth via cancer stem cell self-renewal, ii) adaption and establishment of organ-specific colonization programs, and iii) establishment of a microenvironment that supports metastatic colonization 161. As described in earlier sections, tumour cells enhance their survival 15,17,18,100, stemness 100, and proliferation 14–16 in response to elevated matrix stiffness in the primary TME. If retained at the secondary site, increased cancer stemness can confer tumour cells the ability to re-initiate tumour growth (Prerequisite i). Moreover, retention of an enhanced survival phenotype could help tumour cells to endure the hostile microenvironment of the secondary organ. Studies are needed to test if stiffness-induced survival and stemness can be retained from the primary TME to the secondary site. Adaptation to the specific microenvironment and cell populations in the secondary organ and establishment of organ-specific colonization programs (Prerequisite ii) can also be supported by persistence of mechanically induced phenotypes. In particular, stiffness-induced secretion of osteolytic factors was shown to promote colonization of the bone both in vitro 162 and in vivo 138. A breast cancer cell line cultured then dissociated from stiffer 3D alginate-gelatin hydrogels (6–10 kPa versus ~2 kPa) showed increased secretion of osteolytic factors when cultured in 3D-printed bone-mimicking scaffolds 162. Moreover, stiffness-induced activation of RUNX2 in breast cancer cell lines was retained after cell injection into mice and promoted osteolytic bone colonization and metastasis 138. Furthermore, given that substrate stiffness can enhance soluble factor secretion 19–23 and that secretion of growth factors, cytokines, and matrix-remodelling enzymes has been shown to co-opt stromal cells such as fibroblasts at the secondary site to facilitate metastatic colonization 101,163, it is likely that mechanically-induced secretion of various soluble factors could promote metastatic colonization (Prerequisite iii). Additional research is needed to study if and with which intensity and duration biophysical adaptations acquired in the primary TME persist in the distant site. If these hypotheses are validated, tumour cell mechanical memory may well be a key mechanism for successful metastatic colonization.
Conclusions and perspective
The primary TME is characterized by a diverse array of harsh mechanical cues, including increased matrix stiffness, solid, and fluid stresses. Tumour cell biophysical adaptations acquired in response to these cues have been widely explored in the primary tumour. However, questions remain as to whether tumour cells retain a memory of these biophysical adaptations and whether they influence later steps of metastasis, including extravasation, dormancy, and colonization. Piecing together evidence from different landscapes, we propose that: i) the selection of tumour cells adapted to mechanical cues in the primary tumour increases their fitness; ii) the magnitude and duration of mechanical stress alter the phenotype of the selected cells, which is retained via mechanical memory; iii) the biophysical adaptations are retained throughout metastasis influencing tumour cell extravasation, dormancy, and colonization at distant sites.
While matrix stiffness has been shown to imprint mechanical memory in cancerous 13,138 and non-cancerous cells 5–12, we believe other mechanical cues within the TME can imprint mechanical memory provided that the magnitude of the stimulus is sufficiently high and the duration is sufficiently long. For example, exposure to high extracellular fluid viscosity in vitro for 6 days was shown to imprint mechanical memory in a breast cancer cell line, which led to increased migration in zebrafish, extravasation in chick embryos, and lung colonization in mice93. Moreover, several mechanical cues in the TME, such as solid stresses and fluid stresses, could act simultaneously and synergistically on tumour cells to form memory. We speculate that the primary tumour is the ideal environment for tumour cells to form mechanical memory because this is where mechanical cues are present with the longest priming time during tumour development and dissemination and can thus reprogram tumour cells with persistent epigenetic changes. Nevertheless, mechanical stimuli of very high intensity such as fluid shear stress in the vasculature 164 and solid stresses during intravasation 2 and extravasation 2 could potentially also imprint mechanical memory even if their duration is considerably shorter. These mechanical cues were shown to alter tumour cell behaviours 164,165, but the persistence of these changes is so far unexplored. Furthermore, our hypothesis is also compatible with the concept of mechanical adaptability 3. We believe that tumour cells can still adapt to the mechanical cues occurring outside the primary tumour, but the level of adaptation might depend on the level of mechanical memory (i.e. the reversibility and persistence of epigenetic changes) previously acquired in the primary tumour as well as the strength and duration of the subsequent mechanical stimuli.
Our hypothesis is unifying and mechanistic because it traces mechanosensing in the primary tumour to the induction of persistent epigenetic changes (similarly to epigenetically driven EMT) and their retention throughout the metastatic process via mechanical memory. These modifications ensure the selection of tumour cells that can overcome the physical barriers of the metastatic process and improve early tumour cell survival, adaptation, and colonization or dormancy at the secondary site. By designing interventions to reverse mechanical memory and the induced persistent epigenetic changes in the primary TME, biophysical adaptations that are carried from the primary tumour over to the secondary site would be abrogated, thus effectively inhibiting metastasis. While therapies targeting epigenetic changes have already been approved to treat cancer (reviewed in 166) and many ongoing clinical trials are promising 166, therapies targeting persistent epigenetic changes encoding mechanical memory would specifically inhibit the transcription or silencing of genes that are necessary for the survival of the tumour cells during the mechanical process of metastasis. As such, we believe that understanding the role of mechanical memory in metastasis will be key to designing novel anti-metastatic drugs and improve patient survival.
Acknowledgements
The authors would like to thank Jason Godfrey (MIT’s Koch Institute for Integrative Cancer Research) for his valuable and insightful comments that helped to improve this manuscript. Elena Cambria was supported by an Early Postdoc Mobility fellowship from the Swiss National Science Foundation and a postdoctoral fellowship from the Ludwig Center at MIT’s Koch Institute for Integrative Cancer Research. Giovanni S. Offeddu received funding from Amgen Inc., and an American Italian Cancer Foundation Postdoctoral Fellowship. Sarah Shelton was supported by fellowship K00CA212227 from the National Cancer Institute, National Institute of Health. This work was supported by a grant from the National Cancer Institute (U54-CA261694). Figures were created with BioRender.com.
Glossary
- Elastic Modulus
the slope of the stress-strain relationship that gives a quantitative measure of material stiffness
- Shear Wave Elastography
technique that uses sound to induce shear deformation in a material to quantify mechanical properties
- Atomic Force Microscopy
technique that imposes a small, local deformation to a sample to probe mechanical properties
- Compressive Stresses
normal component of the stress that pushes on a surface
- Tensile Stresses
normal component of the stress that pulls on a surface
- Strain-stiffening
a phenomenon where the elastic modulus of a material increases with deformation
- Traction forces
forces exerted tangential to a substrate to generate motion
Footnotes
Competing interests
R.D.K. is a co-founder of AIM Biotech, a company that markets microfluidic technologies, and receives research support from Amgen, Abbvie, Boehringer-Ingelheim, GSK, Novartis, Roche, Takeda, Eisai, EMD Serono, and Visterra. None of these activities are related to the content of this article.
References
- 1.Steeg PS Targeting metastasis. Nature Reviews Cancer 16, 201–218, doi: 10.1038/nrc.2016.25 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wirtz D, Konstantopoulos K & Searson PC The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer 11, 512–522, doi: 10.1038/nrc3080 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gensbittel V et al. Mechanical Adaptability of Tumor Cells in Metastasis. Dev Cell 56, 164–179, doi: 10.1016/j.devcel.2020.10.011 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Nia HT, Munn LL & Jain RK Physical traits of cancer. Science 370, doi: 10.1126/science.aaz0868 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A & Hinz B The mechanical memory of lung myofibroblasts. Integr Biol (Camb) 4, 410–421, doi: 10.1039/c2ib00149g (2012). [DOI] [PubMed] [Google Scholar]
- 6.Yang C, Tibbitt MW, Basta L & Anseth KS Mechanical memory and dosing influence stem cell fate. Nat Mater 13, 645–652, doi: 10.1038/nmat3889 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, Abdeen AA & Kilian KA Rewiring mesenchymal stem cell lineage specification by switching the biophysical microenvironment. Sci Rep 4, 5188, doi: 10.1038/srep05188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heo SJ et al. Biophysical Regulation of Chromatin Architecture Instills a Mechanical Memory in Mesenchymal Stem Cells. Sci Rep 5, 16895, doi: 10.1038/srep16895 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank V et al. Frequent mechanical stress suppresses proliferation of mesenchymal stem cells from human bone marrow without loss of multipotency. Scientific Reports 6, doi:ARTN 24264 10.1038/srep24264 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li CX et al. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat Mater 16, 379–389, doi: 10.1038/nmat4780 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Killaars AR et al. Extended Exposure to Stiff Microenvironments Leads to Persistent Chromatin Remodeling in Human Mesenchymal Stem Cells. Adv Sci (Weinh) 6, 1801483, doi: 10.1002/advs.201801483 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunham C, Havlioglu N, Chamberlain A, Lake S & Meyer G Adipose stem cells exhibit mechanical memory and reduce fibrotic contracture in a rat elbow injury model. FASEB J 34, 12976–12990, doi: 10.1096/fj.202001274R (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasrollahi S et al. Past matrix stiffness primes epithelial cells and regulates their future collective migration through a mechanical memory. Biomaterials 146, 146–155, doi: 10.1016/j.biomaterials.2017.09.012 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammer AM et al. Stromal PDGFR-α Activation Enhances Matrix Stiffness, Impedes Mammary Ductal Development, and Accelerates Tumor Growth. Neoplasia (New York, N.Y.) 19, 496–508, doi: 10.1016/j.neo.2017.04.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrader J et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology (Baltimore, Md.) 53, 1192–1205, doi: 10.1002/hep.24108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulrich TA, de Juan Pardo EM & Kumar S The Mechanical Rigidity of the Extracellular Matrix Regulates the Structure, Motility, and Proliferation of Glioma Cells. Cancer Research 69, 4167–4174, doi: 10.1158/0008-5472.can-08-4859 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen TV, Sleiman M, Moriarty T, Herrick WG & Peyton SR Sorafenib resistance and JNK signaling in carcinoma during extracellular matrix stiffening. Biomaterials 35, 5749–5759, doi: 10.1016/j.biomaterials.2014.03.058 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Rice AJ et al. Matrix stiffness induces epithelial-mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis 6, e352, doi: 10.1038/oncsis.2017.54 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haage A & Schneider IC Cellular contractility and extracellular matrix stiffness regulate matrix metalloproteinase activity in pancreatic cancer cells. FASEB J 28, 3589–3599, doi: 10.1096/fj.13-245613 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Nukuda A et al. Stiff substrates increase YAP-signaling-mediated matrix metalloproteinase-7 expression. Oncogenesis 4, e165, doi: 10.1038/oncsis.2015.24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S et al. Matrix stiffness-upregulated LOXL2 promotes fibronectin production, MMP9 and CXCL12 expression and BMDCs recruitment to assist pre-metastatic niche formation. Journal of experimental & clinical cancer research : CR 37, 99, doi: 10.1186/s13046-018-0761-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M et al. Activation of Piezo1 contributes to matrix stiffness-induced angiogenesis in hepatocellular carcinoma. Cancer communications (London, England) 42, 1162–1184, doi: 10.1002/cac2.12364 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taufalele PV et al. Matrix stiffness enhances cancer-macrophage interactions and M2-like macrophage accumulation in the breast tumor microenvironment. Acta Biomater, doi: 10.1016/j.actbio.2022.04.031 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraning-Rush CM, Califano JP & Reinhart-King CA Cellular traction stresses increase with increasing metastatic potential. PloS one 7, e32572, doi: 10.1371/journal.pone.0032572 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grasset EM et al. Matrix Stiffening and EGFR Cooperate to Promote the Collective Invasion of Cancer Cells. Cancer Res 78, 5229–5242, doi: 10.1158/0008-5472.can-18-0601 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Tian F et al. Mechanical Responses of Breast Cancer Cells to Substrates of Varying Stiffness Revealed by Single-Cell Measurements. The journal of physical chemistry letters 11, 7643–7649, doi: 10.1021/acs.jpclett.0c02065 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Molter CW et al. Prostate cancer cells of increasing metastatic potential exhibit diverse contractile forces, cell stiffness, and motility in a microenvironment stiffness-dependent manner. Frontiers in cell and developmental biology 10, 932510, doi: 10.3389/fcell.2022.932510 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker EL, Lu J, Yu D, Bonnecaze RT & Zaman MH Cancer cell stiffness: integrated roles of three-dimensional matrix stiffness and transforming potential. Biophys J 99, 2048–2057, doi: 10.1016/j.bpj.2010.07.051 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rianna C & Radmacher M Influence of microenvironment topography and stiffness on the mechanics and motility of normal and cancer renal cells. Nanoscale 9, 11222–11230, doi: 10.1039/c7nr02940c (2017). [DOI] [PubMed] [Google Scholar]
- 30.Wullkopf L et al. Cancer cells’ ability to mechanically adjust to extracellular matrix stiffness correlates with their invasive potential. Mol Biol Cell 29, 2378–2385, doi: 10.1091/mbc.E18-05-0319 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pathak A & Kumar S Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc Natl Acad Sci U S A 109, 10334–10339, doi: 10.1073/pnas.1118073109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pogoda K et al. Soft Substrates Containing Hyaluronan Mimic the Effects of Increased Stiffness on Morphology, Motility, and Proliferation of Glioma Cells. Biomacromolecules 18, 3040–3051, doi: 10.1021/acs.biomac.7b00324 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matte BF et al. Matrix stiffness mechanically conditions EMT and migratory behavior of oral squamous cell carcinoma. J Cell Sci 132, doi: 10.1242/jcs.224360 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acerbi I et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol (Camb) 7, 1120–1134, doi: 10.1039/c5ib00040h (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinkus R et al. MR elastography of breast lesions: understanding the solid/liquid duality can improve the specificity of contrast-enhanced MR mammography. Magnetic resonance in medicine 58, 1135–1144, doi: 10.1002/mrm.21404 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Evans A et al. Differentiating benign from malignant solid breast masses: value of shear wave elastography according to lesion stiffness combined with greyscale ultrasound according to BI-RADS classification. British Journal of Cancer 107, 224–229, doi: 10.1038/bjc.2012.253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyd NF et al. Evidence That Breast Tissue Stiffness Is Associated with Risk of Breast Cancer. Plos One 9, doi:ARTN e100937 10.1371/journal.pone.0100937 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carrara S et al. EUS elastography (strain ratio) and fractal-based quantitative analysis for the diagnosis of solid pancreatic lesions. Gastrointest Endosc 87, 1464–1473, doi: 10.1016/j.gie.2017.12.031 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Shahryari M et al. Tomoelastography Distinguishes Noninvasively between Benign and Malignant Liver Lesions. Cancer Res 79, 5704–5710, doi: 10.1158/0008-5472.CAN-19-2150 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Rouvière O et al. Stiffness of benign and malignant prostate tissue measured by shear-wave elastography: a preliminary study. European Radiology 27, 1858–1866, doi: 10.1007/s00330-016-4534-9 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Cochlin DL, Ganatra RH & Griffiths DF Elastography in the detection of prostatic cancer. Clin Radiol 57, 1014–1020, doi: 10.1053/crad.2002.0989 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Singh S et al. Liver Stiffness Is Associated With Risk of Decompensation, Liver Cancer, and Death in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol H 11, 1573–U1237, doi: 10.1016/j.cgh.2013.07.034 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ichikawa S, Motosugi U, Enomoto N & Onishi H Magnetic resonance elastography can predict development of hepatocellular carcinoma with longitudinally acquired two-point data. European Radiology 29, 1013–1021, doi: 10.1007/s00330-018-5640-7 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Northey JJ et al. Stiff stroma increases breast cancer risk by inducing the oncogene ZNF217. The Journal of Clinical Investigation 130, 5721–5737, doi: 10.1172/JCI129249 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maskarinec G et al. Mammographic density as a predictor of breast cancer survival: the Multiethnic Cohort. Breast Cancer Res 15, R7, doi: 10.1186/bcr3378 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J et al. 3D MR Elastography of Hepatocellular Carcinomas as a Potential Biomarker for Predicting Tumor Recurrence. J Magn Reson Imaging 49, 719–730, doi: 10.1002/jmri.26250 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zanetti-Dällenbach R et al. Length Scale Matters: Real-Time Elastography versus Nanomechanical Profiling by Atomic Force Microscopy for the Diagnosis of Breast Lesions. BioMed Research International 2018, 3840597, doi: 10.1155/2018/3840597 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen Y, Schmidt T & Diz-Muñoz A Protocol on Tissue Preparation and Measurement of Tumor Stiffness in Primary and Metastatic Colorectal Cancer Samples with an Atomic Force Microscope. STAR protocols 1, 100167, doi: 10.1016/j.xpro.2020.100167 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaudhuri O et al. Substrate stress relaxation regulates cell spreading. Nat Commun 6, 6364, doi: 10.1038/ncomms7365 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wisdom KM et al. Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat Commun 9, 4144, doi: 10.1038/s41467-018-06641-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalluri R & Zeisberg M Fibroblasts in cancer. Nat Rev Cancer 6, 392–401, doi: 10.1038/nrc1877 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Deligne C & Midwood KS Macrophages and Extracellular Matrix in Breast Cancer: Partners in Crime or Protective Allies? Front Oncol 11, 620773, doi: 10.3389/fonc.2021.620773 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Offeddu GS et al. Personalized models of breast cancer desmoplasia reveal biomechanical determinants of drug penetration. bioRxiv, 2021.2012.2012.472296, doi: 10.1101/2021.12.12.472296 (2023). [DOI] [Google Scholar]
- 54.Casey TM et al. Cancer associated fibroblasts stimulated by transforming growth factor beta1 (TGF-beta 1) increase invasion rate of tumor cells: a population study. Breast Cancer Res Treat 110, 39–49, doi: 10.1007/s10549-007-9684-7 (2008). [DOI] [PubMed] [Google Scholar]
- 55.Costea DE et al. Identification of two distinct carcinoma-associated fibroblast subtypes with differential tumor-promoting abilities in oral squamous cell carcinoma. Cancer Res 73, 3888–3901, doi: 10.1158/0008-5472.CAN-12-4150 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Papageorgis P & Stylianopoulos T Role of TGFbeta in regulation of the tumor microenvironment and drug delivery (review). Int J Oncol 46, 933–943, doi: 10.3892/ijo.2015.2816 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pankova D et al. Cancer-Associated Fibroblasts Induce a Collagen Cross-link Switch in Tumor Stroma. Mol Cancer Res 14, 287–295, doi: 10.1158/1541-7786.MCR-15-0307 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Afik R et al. Tumor macrophages are pivotal constructors of tumor collagenous matrix. J Exp Med 213, 2315–2331, doi: 10.1084/jem.20151193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C & Brown RA Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature Reviews Molecular Cell Biology 3, 349–363, doi: 10.1038/nrm809 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Erez N, Truitt M, Olson P & Hanahan D Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-κB-Dependent Manner. Cancer cell 17, 135–147, 10.1016/j.ccr.2009.12.041 (2010). [DOI] [PubMed] [Google Scholar]
- 61.Su S et al. CD10+GPR77+ Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell 172, 841–856.e816, doi: 10.1016/j.cell.2018.01.009 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Özdemir Berna C. et al. Depletion of Carcinoma-Associated Fibroblasts and Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Reduced Survival. Cancer cell 25, 719–734, doi: 10.1016/j.ccr.2014.04.005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhim Andrew D. et al. Stromal Elements Act to Restrain, Rather Than Support, Pancreatic Ductal Adenocarcinoma. Cancer cell 25, 735–747, doi: 10.1016/j.ccr.2014.04.021 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhattacharjee S et al. Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. The Journal of Clinical Investigation 131, doi: 10.1172/JCI146987 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei J et al. The role of matrix stiffness in cancer stromal cell fate and targeting therapeutic strategies. Acta Biomaterialia 150, 34–47, doi: 10.1016/j.actbio.2022.08.005 (2022). [DOI] [PubMed] [Google Scholar]
- 66.Nicolas-Boluda A et al. Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. eLife 10, e58688, doi: 10.7554/eLife.58688 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuczek DE et al. Collagen density regulates the activity of tumor-infiltrating T cells. J Immunother Cancer 7, 68, doi: 10.1186/s40425-019-0556-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sridharan R, Cavanagh B, Cameron AR, Kelly DJ & O’Brien FJ Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomaterialia 89, 47–59, doi: 10.1016/j.actbio.2019.02.048 (2019). [DOI] [PubMed] [Google Scholar]
- 69.Xing X et al. Matrix stiffness-mediated effects on macrophages polarization and their LOXL2 expression. The FEBS journal 288, 3465–3477, doi: 10.1111/febs.15566 (2021). [DOI] [PubMed] [Google Scholar]
- 70.Friedemann M et al. Instructing Human Macrophage Polarization by Stiffness and Glycosaminoglycan Functionalization in 3D Collagen Networks. Adv Healthc Mater 6, doi: 10.1002/adhm.201600967 (2017). [DOI] [PubMed] [Google Scholar]
- 71.Bordeleau F et al. Matrix stiffening promotes a tumor vasculature phenotype. Proceedings of the National Academy of Sciences 114, 492–497, doi:doi: 10.1073/pnas.1613855114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghosh K et al. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proceedings of the National Academy of Sciences 105, 11305–11310, doi: 10.1073/pnas.0800835105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jain RK, Martin JD & Stylianopoulos T The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng 16, 321–346, doi: 10.1146/annurev-bioeng-071813-105259 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nia HT et al. Solid stress and elastic energy as measures of tumour mechanopathology. Nat Biomed Eng 1, doi: 10.1038/s41551-016-0004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS & Sahai E ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol 16, 1515–1523, doi: 10.1016/j.cub.2006.05.065 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Kopanska KS, Alcheikh Y, Staneva R, Vignjevic D & Betz T Tensile Forces Originating from Cancer Spheroids Facilitate Tumor Invasion. PLoS One 11, e0156442, doi: 10.1371/journal.pone.0156442 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sahai E et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer 20, 174–186, doi: 10.1038/s41568-019-0238-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han YL et al. Cell contraction induces long-ranged stress stiffening in the extracellular matrix. Proc Natl Acad Sci U S A 115, 4075–4080, doi: 10.1073/pnas.1722619115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Padera TP et al. Pathology: cancer cells compress intratumour vessels. Nature 427, 695, doi: 10.1038/427695a (2004). [DOI] [PubMed] [Google Scholar]
- 80.Baxter LT & Jain RK Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvasc Res 37, 77–104, doi: 10.1016/0026-2862(89)90074-5 (1989). [DOI] [PubMed] [Google Scholar]
- 81.Jain RK & Baxter LT Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res 48, 7022–7032 (1988). [PubMed] [Google Scholar]
- 82.Boucher Y, Kirkwood JM, Opacic D, Desantis M & Jain RK Interstitial hypertension in superficial metastatic melanomas in humans. Cancer Res 51, 6691–6694 (1991). [PubMed] [Google Scholar]
- 83.Gutmann R et al. Interstitial hypertension in head and neck tumors in patients: correlation with tumor size. Cancer Res 52, 1993–1995 (1992). [PubMed] [Google Scholar]
- 84.Nathanson SD & Nelson L Interstitial fluid pressure in breast cancer, benign breast conditions, and breast parenchyma. Annals of surgical oncology 1, 333–338, doi: 10.1007/bf03187139 (1994). [DOI] [PubMed] [Google Scholar]
- 85.Boucher Y, Salehi H, Witwer B, Harsh G. R. t. & Jain RK Interstitial fluid pressure in intracranial tumours in patients and in rodents. Br J Cancer 75, 829–836, doi: 10.1038/bjc.1997.148 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boucher Y, Baxter LT & Jain RK Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res 50, 4478–4484 (1990). [PubMed] [Google Scholar]
- 87.Butler TP, Grantham FH & Gullino PM Bulk transfer of fluid in the interstitial compartment of mammary tumors. Cancer Res 35, 3084–3088 (1975). [PubMed] [Google Scholar]
- 88.Pedersen JA, Lichter S & Swartz MA Cells in 3D matrices under interstitial flow: effects of extracellular matrix alignment on cell shear stress and drag forces. J Biomech 43, 900–905, doi: 10.1016/j.jbiomech.2009.11.007 (2010). [DOI] [PubMed] [Google Scholar]
- 89.Hofmann M et al. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia (New York, N.Y.) 8, 89–95, doi: 10.1593/neo.05469 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shields JD et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer cell 11, 526–538, doi: 10.1016/j.ccr.2007.04.020 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Gonzalez-Molina J et al. Extracellular fluid viscosity enhances liver cancer cell mechanosensing and migration. Biomaterials 177, 113–124, doi: 10.1016/j.biomaterials.2018.05.058 (2018). [DOI] [PubMed] [Google Scholar]
- 92.Pittman M et al. Membrane ruffling is a mechanosensor of extracellular fluid viscosity. Nature Physics 18, 1112–1121, doi: 10.1038/s41567-022-01676-y (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bera K et al. Extracellular fluid viscosity enhances cell migration and cancer dissemination. Nature, doi: 10.1038/s41586-022-05394-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sevick EM & Jain RK Viscous resistance to blood flow in solid tumors: effect of hematocrit on intratumor blood viscosity. Cancer Res 49, 3513–3519 (1989). [PubMed] [Google Scholar]
- 95.Yin J, Kong X & Lin W Noninvasive Cancer Diagnosis In Vivo Based on a Viscosity-Activated Near-Infrared Fluorescent Probe. Analytical Chemistry 93, 2072–2081, doi: 10.1021/acs.analchem.0c03803 (2021). [DOI] [PubMed] [Google Scholar]
- 96.Vining KH & Mooney DJ Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol 18, 728–742, doi: 10.1038/nrm.2017.108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Farino Reyes CJ, Pradhan S & Slater JH The Influence of Ligand Density and Degradability on Hydrogel Induced Breast Cancer Dormancy and Reactivation. Adv Healthc Mater 10, e2002227, doi: 10.1002/adhm.202002227 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murphy CM, Haugh MG & O’Brien FJ The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 31, 461–466, doi: 10.1016/j.biomaterials.2009.09.063 (2010). [DOI] [PubMed] [Google Scholar]
- 99.Taubenberger AV et al. 3D Microenvironment Stiffness Regulates Tumor Spheroid Growth and Mechanics via p21 and ROCK. Advanced biosystems 3, e1900128, doi: 10.1002/adbi.201900128 (2019). [DOI] [PubMed] [Google Scholar]
- 100.Liu X et al. Niche stiffness sustains cancer stemness via TAZ and NANOG phase separation. Nat Commun 14, 238, doi: 10.1038/s41467-023-35856-y (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kessenbrock K, Plaks V & Werb Z Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67, doi: 10.1016/j.cell.2010.03.015 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bao M et al. Extracellular matrix stiffness controls VEGF(165) secretion and neuroblastoma angiogenesis via the YAP/RUNX2/SRSF1 axis. Angiogenesis 25, 71–86, doi: 10.1007/s10456-021-09804-7 (2022). [DOI] [PubMed] [Google Scholar]
- 103.Esbona K et al. COX-2 modulates mammary tumor progression in response to collagen density. Breast Cancer Research 18, 35, doi: 10.1186/s13058-016-0695-3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lo CM, Wang HB, Dembo M & Wang YL Cell movement is guided by the rigidity of the substrate. Biophysical journal 79, 144–152, doi:Doi 10.1016/S0006-3495(00)76279-5 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Munevar S, Wang Y & Dembo M Traction force microscopy of migrating normal and H-ras transformed 3T3 fibroblasts. Biophysical journal 80, 1744–1757, doi: 10.1016/s0006-3495(01)76145-0 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rosel D et al. Up-regulation of Rho/ROCK signaling in sarcoma cells drives invasion and increased generation of protrusive forces. Mol Cancer Res 6, 1410–1420, doi: 10.1158/1541-7786.MCR-07-2174 (2008). [DOI] [PubMed] [Google Scholar]
- 107.Indra I et al. An in vitro correlation of mechanical forces and metastatic capacity. Phys Biol 8, 015015, doi: 10.1088/1478-3975/8/1/015015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paszek MJ et al. Tensional homeostasis and the malignant phenotype. Cancer cell 8, 241–254, doi: 10.1016/j.ccr.2005.08.010 (2005). [DOI] [PubMed] [Google Scholar]
- 109.Saez A, Buguin A, Silberzan P & Ladoux B Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophysical journal 89, L52–54, doi: 10.1529/biophysj.105.071217 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ghibaudo M et al. Traction forces and rigidity sensing regulate cell functions. Soft matter 4, 1836, doi: 10.1039/b804103b (2008). [DOI] [Google Scholar]
- 111.Califano JP & Reinhart-King CA Substrate Stiffness and Cell Area Predict Cellular Traction Stresses in Single Cells and Cells in Contact. Cell Mol Bioeng 3, 68–75, doi: 10.1007/s12195-010-0102-6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tee SY, Fu J, Chen CS & Janmey PA Cell shape and substrate rigidity both regulate cell stiffness. Biophysical journal 100, L25–27, doi: 10.1016/j.bpj.2010.12.3744 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Song D et al. Recovery of Tractions Exerted by Single Cells in Three-Dimensional Nonlinear Matrices. J Biomech Eng 142, doi: 10.1115/1.4046974 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Afthinos A et al. Migration and 3D Traction Force Measurements inside Compliant Microchannels. Nano letters 22, 7318–7327, doi: 10.1021/acs.nanolett.2c01261 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thoumine O & Ott A Comparison of the mechanical properties of normal and transformed fibroblasts. Biorheology 34, 309–326, doi: 10.1016/S0006-355X(98)00007-9 (1997). [DOI] [PubMed] [Google Scholar]
- 116.Cross SE, Jin YS, Rao J & Gimzewski JK Nanomechanical analysis of cells from cancer patients. Nat Nanotechnol 2, 780–783, doi: 10.1038/nnano.2007.388 (2007). [DOI] [PubMed] [Google Scholar]
- 117.Guck J et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophysical journal 88, 3689–3698, doi: 10.1529/biophysj.104.045476 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Coughlin MF et al. Cytoskeletal stiffness, friction, and fluidity of cancer cell lines with different metastatic potential. Clin Exp Metastasis 30, 237–250, doi: 10.1007/s10585-012-9531-z (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Z et al. Cancer cells display increased migration and deformability in pace with metastatic progression. FASEB J 34, 9307–9315, doi: 10.1096/fj.202000101RR (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Swaminathan V et al. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res 71, 5075–5080, doi: 10.1158/0008-5472.can-11-0247 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rianna C, Radmacher M & Kumar S Direct evidence that tumor cells soften when navigating confined spaces. Mol Biol Cell 31, 1726–1734, doi: 10.1091/mbc.E19-10-0588 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roberts AB et al. Tumor cell nuclei soften during transendothelial migration. J Biomech 121, 110400, doi: 10.1016/j.jbiomech.2021.110400 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wisniewski EO et al. Dorsoventral polarity directs cell responses to migration track geometries. Sci Adv 6, eaba6505, doi: 10.1126/sciadv.aba6505 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wei SC et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol 17, 678–688, doi: 10.1038/ncb3157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fattet L et al. Matrix Rigidity Controls Epithelial-Mesenchymal Plasticity and Tumor Metastasis via a Mechanoresponsive EPHA2/LYN Complex. Dev Cell 54, 302–316 e307, doi: 10.1016/j.devcel.2020.05.031 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nader GPF et al. Compromised nuclear envelope integrity drives TREX1-dependent DNA damage and tumor cell invasion. Cell 184, 5230–5246 e5222, doi: 10.1016/j.cell.2021.08.035 (2021). [DOI] [PubMed] [Google Scholar]
- 127.Andreu I et al. Mechanical force application to the nucleus regulates nucleocytoplasmic transport. Nat Cell Biol 24, 896–905, doi: 10.1038/s41556-022-00927-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ansardamavandi A, Tafazzoli-Shadpour M & Shokrgozar MA Behavioral remodeling of normal and cancerous epithelial cell lines with differing invasion potential induced by substrate elastic modulus. Cell Adh Migr 12, 472–488, doi: 10.1080/19336918.2018.1475803 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang J et al. Transfer of assembled collagen fibrils to flexible substrates for mechanically tunable contact guidance cues. Integr Biol (Camb) 10, 705–718, doi: 10.1039/c8ib00127h (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Peng Y et al. ROCK isoforms differentially modulate cancer cell motility by mechanosensing the substrate stiffness. Acta biomaterialia 88, 86–101, doi: 10.1016/j.actbio.2019.02.015 (2019). [DOI] [PubMed] [Google Scholar]
- 131.Isomursu A et al. Directed cell migration towards softer environments. Nat Mater 21, 1081–1090, doi: 10.1038/s41563-022-01294-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zaman MH et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proceedings of the National Academy of Sciences 103, 10889, doi: 10.1073/pnas.0604460103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Charras G & Sahai E Physical influences of the extracellular environment on cell migration. Nat Rev Mol Cell Biol 15, 813–824, doi: 10.1038/nrm3897 (2014). [DOI] [PubMed] [Google Scholar]
- 134.Ulrich TA, Jain A, Tanner K, MacKay JL & Kumar S Probing cellular mechanobiology in three-dimensional culture with collagen-agarose matrices. Biomaterials 31, 1875–1884, doi: 10.1016/j.biomaterials.2009.10.047 (2010). [DOI] [PubMed] [Google Scholar]
- 135.Petrie RJ, Koo H & Yamada KM Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science (New York, N.Y.) 345, 1062–1065, doi: 10.1126/science.1256965 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pathak A & Kumar S Transforming potential and matrix stiffness co-regulate confinement sensitivity of tumor cell migration. Integr Biol (Camb) 5, 1067–1075, doi: 10.1039/c3ib40017d (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zanotelli MR et al. Energetic costs regulated by cell mechanics and confinement are predictive of migration path during decision-making. Nature Communications 10, 4185, doi: 10.1038/s41467-019-12155-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Watson AW et al. Breast tumor stiffness instructs bone metastasis via maintenance of mechanical conditioning. Cell Rep 35, 109293, doi: 10.1016/j.celrep.2021.109293 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Price CC, Mathur J, Boerckel JD, Pathak A & Shenoy VB Dynamic self-reinforcement of gene expression determines acquisition of cellular mechanical memory. Biophys J 120, 5074–5089, doi: 10.1016/j.bpj.2021.10.006 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nava MM et al. Heterochromatin-Driven Nuclear Softening Protects the Genome against Mechanical Stress-Induced Damage. Cell 181, 800–817 e822, doi: 10.1016/j.cell.2020.03.052 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tajik A et al. Transcription upregulation via force-induced direct stretching of chromatin. Nat Mater 15, 1287–1296, doi: 10.1038/nmat4729 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Flavahan WA, Gaskell E & Bernstein BE Epigenetic plasticity and the hallmarks of cancer. Science 357, doi: 10.1126/science.aal2380 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stowers RS et al. Matrix stiffness induces a tumorigenic phenotype in mammary epithelium through changes in chromatin accessibility. Nat Biomed Eng 3, 1009–1019, doi: 10.1038/s41551-019-0420-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bica-Pop C et al. Overview upon miR-21 in lung cancer: focus on NSCLC. Cell Mol Life Sci 75, 3539–3551, doi: 10.1007/s00018-018-2877-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhu S et al. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res 18, 350–359, doi: 10.1038/cr.2008.24 (2008). [DOI] [PubMed] [Google Scholar]
- 146.Wang H et al. microRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer 19, 738, doi: 10.1186/s12885-019-5951-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gupta GP & Massague J Cancer metastasis: building a framework. Cell 127, 679–695, doi: 10.1016/j.cell.2006.11.001 (2006). [DOI] [PubMed] [Google Scholar]
- 148.Mistriotis P et al. Confinement hinders motility by inducing RhoA-mediated nuclear influx, volume expansion, and blebbing. J Cell Biol 218, 4093–4111, doi: 10.1083/jcb.201902057 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Keys J, Cheung BCH, Wu M & Lammerding J Rear cortex contraction aids in nuclear transit during confined migration by increasing pressure in the cell posterior. bioRxiv, 2022.2009.2010.507419, doi: 10.1101/2022.09.10.507419 (2022). [DOI] [Google Scholar]
- 150.Chen MB, Lamar JM, Li R, Hynes RO & Kamm RD Elucidation of the Roles of Tumor Integrin beta1 in the Extravasation Stage of the Metastasis Cascade. Cancer Res 76, 2513–2524, doi: 10.1158/0008-5472.CAN-15-1325 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gupta GP et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 446, 765–770, doi: 10.1038/nature05760 (2007). [DOI] [PubMed] [Google Scholar]
- 152.Padua D et al. TGFβ Primes Breast Tumors for Lung Metastasis Seeding through Angiopoietin-like 4. Cell 133, 66–77, doi: 10.1016/j.cell.2008.01.046 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Karreman MA et al. Active Remodeling of Capillary Endothelium via Cancer Cell-Derived MMP9 Promotes Metastatic Brain Colonization. Cancer Res 83, 1299–1314, doi: 10.1158/0008-5472.CAN-22-3964 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Azadi S, Tafazzoli Shadpour M & Warkiani ME Characterizing the effect of substrate stiffness on the extravasation potential of breast cancer cells using a 3D microfluidic model. Biotechnol Bioeng 118, 823–835, doi: 10.1002/bit.27612 (2021). [DOI] [PubMed] [Google Scholar]
- 155.Javanmardi Y et al. Endothelium and Subendothelial Matrix Mechanics Modulate Cancer Cell Transendothelial Migration. Adv Sci (Weinh), e2206554, doi: 10.1002/advs.202206554 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mierke CT Cancer Cells Regulate Biomechanical Properties of Human Microvascular Endothelial Cells*. Journal of Biological Chemistry 286, 40025–40037, doi: 10.1074/jbc.M111.256172 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shibue T & Weinberg RA Integrin β1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proceedings of the National Academy of Sciences 106, 10290–10295, doi: 10.1073/pnas.0904227106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu Y et al. Fibrin Stiffness Mediates Dormancy of Tumor-Repopulating Cells via a Cdc42-Driven Tet2 Epigenetic Program. Cancer Res 78, 3926–3937, doi: 10.1158/0008-5472.can-17-3719 (2018). [DOI] [PubMed] [Google Scholar]
- 159.Barkan D et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer research 70, 5706–5716, doi: 10.1158/0008-5472.CAN-09-2356 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Price TT et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Science translational medicine 8, 340ra373, doi: 10.1126/scitranslmed.aad4059 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lambert AW, Pattabiraman DR & Weinberg RA Emerging Biological Principles of Metastasis. Cell 168, 670–691, doi: 10.1016/j.cell.2016.11.037 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shah L, Latif A, Williams KJ, Mancuso E & Tirella A Invasion and Secondary Site Colonization as a Function of In Vitro Primary Tumor Matrix Stiffness: Breast to Bone Metastasis. Advanced healthcare materials 12, e2201898, doi: 10.1002/adhm.202201898 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kaplan RN et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827, doi: 10.1038/nature04186 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Follain G et al. Fluids and their mechanics in tumour transit: shaping metastasis. Nat Rev Cancer 20, 107–124, doi: 10.1038/s41568-019-0221-x (2020). [DOI] [PubMed] [Google Scholar]
- 165.Yankaskas CL et al. The fluid shear stress sensor TRPM7 regulates tumor cell intravasation. Sci Adv 7, doi: 10.1126/sciadv.abh3457 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cheng Y et al. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduction and Targeted Therapy 4, 62, doi: 10.1038/s41392-019-0095-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


