Abstract
To express the function encoded in its genome, the herpes simplex virus 1 capsid-tegument structure released by deenvelopment during entry into cells must be transported retrograde to the nuclear pore where viral DNA is released into the nucleus. This path is essential in the case of virus entering axons of dorsal root ganglia. The objective of the study was to identify the viral proteins that may be involved in the transport. We report the following findings. (i) The neuronal isoform of the intermediate chain (IC-1a) of the dynein complex pulled down, from lysates of [35S]methionine-labeled infected cells, two viral proteins identified as the products of UL34 and UL31 open reading frames, respectively. UL34 protein is a virion protein associated with cellular membranes and phosphorylated by the viral kinase US3. UL31 protein is a largely insoluble, evenly dispersed nuclear phosphoprotein required for optimal processing and packaging of viral DNA into preformed capsids. Reciprocal pulldown experiments verified the interaction of IC-1a and UL34 protein. In similar experiments, UL34 protein was found to interact with UL31 protein and the major capsid protein ICP5. (ii) To determine whether UL34 protein is transported to the nuclear membrane, a requirement if it is involved in transport, the UL34 protein was inserted into a baculovirus vector under the cytomegalovirus major early promoter. Cells infected with the recombinant baculovirus expressed UL34 protein in a dose-dependent manner, and the UL34 protein localized primarily in the nuclear membrane. An unexpected finding was that UL34-expressing cells showed a dissociation of the inner and outer nuclear membranes reminiscent of the morphologic changes seen in cells productively infected with herpes simplex virus 1. UL34, like many other viral proteins, may have multiple functions expressed both early and late in infection.
The herpes simplex virion contains four structural elements arranged in a concentric fashion. These are the DNA core, the capsid, a set of proteins surrounding the capsid known as the tegument, and an envelope. To initiate infection, herpes simplex virus 1 (HSV-1) and HSV-2 must attach to and penetrate the infected cell. In the process, the viral envelope fuses with the plasma membrane and the capsid-tegument structure is transported to the nuclear pore where it remains docked for at least several hours (3, 32). Viral gene expression necessary to initiate viral replication takes place after viral DNA is released from the docked capsids into the nucleus. The conclusion that at least some of the tegument protein docks with the capsid is based on studies of HSV-1(HFEM)tsB7. At the nonpermissive temperature, the capsids docked at the nuclear pore retain their DNA, whereas at the permissive temperature, the DNA is released in the nucleus and viral gene expression ensues (3, 17). The mutation which leads to the retention of the DNA in the docked capsid maps in the infected cell protein 1-2 (ICP1-2), a tegument protein encoded by the UL36 open reading frame (ORF) (3).
Among the many unresolved issues regarding initiation of infection is the mechanism by which the virus is transported from the site of entry—the junction between the plasma membrane rendered contiguous to the envelope and cytoplasm—to the nuclear pore. Recent studies have focused on the microtubular network and path and dynein as the motor that transports the capsid-tegument structure to the nuclear pore (2, 18, 28).
To define the mechanism of attachment of cytoplasmic dynein to herpesvirus, we tested the ability of viral proteins to interact with the neuronal isoform of the intermediate chain (IC) of cytoplasmic dynein. We report that the amino-terminal domain of the IC (IC-1a) of cytoplasmic dynein interacted in pulldown experiments with three viral proteins, the major capsid protein ICP5 and the products of the UL34 and UL31 genes. Since UL34 interacts with IC-1a and also independently with UL31 and the major capsid protein, it is likely that the primary interaction is between IC-1a and UL34 and that the latter pulled down the other viral proteins. Relevant to this report are the following observations.
(i) Cytoplasmic dynein is one of the major motor proteins involved in intracellular transport and is the only known retrograde motor in interphase cells (23). It is the largest and most complex of the motor proteins, consisting of four subunit classes: heavy chains responsible for force production, light intermediate chains and light chains of as yet uncertain function, and ICs involved in cargo attachment (22, 33, 34). A number of observations provide evidence for this role. The ICs have been found to reside at the base of the cytoplasmic dynein molecule by immunoelectron microscopy (30). A search for IC-interacting partners revealed a direct interaction though the amino-terminal domain of the ICs with the p150Glued subunit of another complex, dynactin (14, 35). Disruption of the dynactin complex by overexpression of one of its subunits in turn released both dynactin and cytoplasmic dynein from mitotic kinetochores. This result suggested linkage of dynein to the kinetochore through an IC-dynactin interaction (10), a model that has been supported by subsequent genetic studies (29). Antibodies directed against the amino-terminal portions of the ICs have been found to inhibit cytoplasmic dynein function when microinjected into cells (4, 12, 31). These antibodies also disrupt the IC-dynactin interaction, in further support of the IC-dynactin targeting model (31). Dynactin has been implicated in numerous cytoplasmic dynein functions (4), but recent evidence suggests that alternative mechanisms of cargo attachment also exist (25).
(ii) UL31 protein is a phosphoprotein distributed in a uniform fashion throughout the nucleus during infection. The protein is largely insoluble and partitions with the nuclear matrix during fractionation (5). In cells infected with UL31 null mutants, there is a significant decrease in the production of infectious virus, characterized by a decrease in the cleavage of viral DNA concatemers generated during synthesis of viral DNA into unit (mature)-length molecules and a decrease in the packaging of the viral DNA into preformed capsids (6). It has been suggested that the UL31 protein acts as a ligand to facilitate the packaging of viral DNA into preformed capsids and in the process renders the cleavage of concatemers more efficient. UL31 does not appear to be a component of the virion.
(iii) UL34 protein was initially identified as a substrate for the viral protein kinase encoded by US3 ORF (26). The protein has a hydrophilic amino-terminal domain but associates with membranes during viral replication (26). Inasmuch as UL34 protein is a component of the virion, it becomes an attractive candidate as the virion protein capable of anchoring the capsid-tegument protein to the dynein motor. In an attempt to define further the function of UL34 in infected cells, we inserted the UL34 ORF driven by a human cytomegalovirus (CMV) promoter into the baculovirus. UL34 protein expressed in a variety of human and primate cells accumulated in the perinuclear space and appeared to be somewhat toxic to the cell. The striking feature of the cells expressing the UL34 protein was the separation of the inner and outer nuclear membranes characteristic of productively infected cells. The apparent involvement of UL34 in the maturation and egress of the virus from the infected cell does not preclude it from performing functions associated with initiation of infection, since many of the viral proteins examined to date appear to perform multiple functions.
MATERIALS AND METHODS
Cells and viruses.
The limited-in vitro-passaged HSV-1 strain F [HSV-1(F)] and HSV-2 strain G [HSV-2(G)] are the prototype HSV-1 and HSV-2 strains used in this laboratory (11). The intertypic (HSV-1 × HSV-2) recombinants designated R7015, RS1G25, RS1G31, RH1G7, RH1G8, RH1G13, RH1G44, and RH1G48 were described elsewhere (1, 8). The crossover sites are shown in Fig. 1B. The sources and procedures for the cultivation of Vero, HEp-2, HeLa, 143TK−, and rabbit skin cells were described elsewhere (5, 6). Sf9 insect cells were purchased from Novagen, Inc. (Madison, Wis.), and maintained in TNM-FH medium (Pharmingen, San Diego, Calif.) supplemented with 10% fetal bovine serum.
FIG. 1.
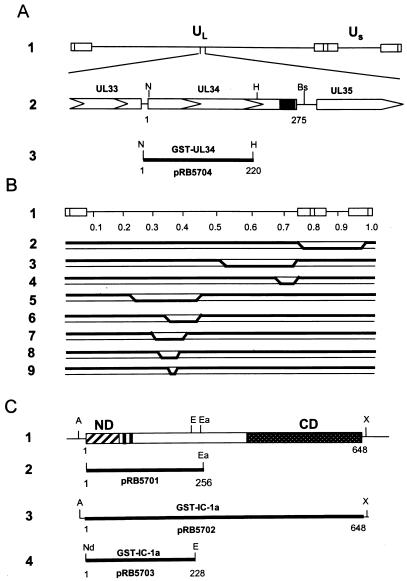
Sequence arrangements and relevant maps. (A) Schematic diagram of the HSV-1 DNA sequence arrangement and of the location of the UL31 to UL35 ORFs. Line 1, linear representation of the HSV-1 genome. The rectangles represent the inverted repeats flanking the unique sequences (UL and US, represented by thin lines). Line 2, enlarged portion of the fragment of HSV-1(F) containing the UL34 gene. The arrowheads indicate the direction of transcription; the solid box indicates the putative transmembrane domain of the UL34 gene product. Line 3, region of UL34 (codons 1 to 230) fused to GST to generate GST-UL34 fusion protein. (B) DNA sequence arrangements of HSV-1 × HSV-2 intertypic recombinants. Line 1, genome arrangement of HSV showing map units. The DNA sequence arrangements of the recombinants are as follows: R7015, lines 2; RS1G25, lines 3, RS1G31, lines 4; RH1G7, lines 5, RH1G8, lines 6; RH1G13, lines 7, RH1G44, lines 8; and RH1G48, lines 9. The boldface line segments identify HSV-2 sequences present in these genomes, with the approximate crossover points falling within the indicated diagonal regions. (C) Schematic representation of the distinct domains of the IC of cytoplasmic dynein motor protein and of the location of the fragments used for generation of GST–IC-1a fusion protein and for in vitro transcription-translation. Line 1 represents the sequence of the IC of cytoplasmic dynein (IC-1a), which was mapped to the base of the motor complex and implicated in targeting the motor to the transported organelle. Shown diagrammatically are the amino-terminal domain containing a predicted coiled-coil structure (ND) thought to bind the transported organelle and the conserved carboxyl-terminal region (CD), which appears to bind to the heavy chain of dynein. The serine-rich cluster located between the two regions of alternative splicing (filled rectangle) is also highlighted. Lines 2 and 3, PCR-amplified fragment of IC-1a (line 2) and whole IC-1a (line 3) cloned in pGEM5Zf(+) for in vitro transcription-translation. Line 4, fragment used for GST–IC-1a chimeric protein construction. An NdeI restriction site was introduced at the start codon by PCR. Abbreviations: A, ApaI; Bs, BspEI; E, EcoRI; Ea, EagI; Nd, NdeI; H, HincII; N, NcoI; X, XbaI.
Antibodies.
The polyclonal antibody to IC-1a, the IC of dynein, was described elsewhere (35). The production and properties of the polyclonal antibodies to UL31 and UL34 proteins were described elsewhere (5, 26). Monoclonal anti-α-tubulin antibody is from Sigma (St. Louis, Mo.). For immunofluorescence studies, the antibody to UL34 was purified from polyclonal rabbit anti-UL34 protein by chromatography on a protein A AffinityPak column (Pierce, Rockford, Ill.) and used at a 1:200 dilution.
Production and purification of glutathione S-transferase (GST)–dynein and GST-UL34 chimeric proteins.
The cDNA clone encoding the IC of dynein (IC-1a) was described elsewhere (21). For the studies described here, the cDNA clone of IC-1a was amplified by PCR and an NdeI restriction site was introduced at its translation start codon. The amplified fragment was digested with NdeI/EagI and inserted into pGEM5Zf(+) (Promega) that had been digested with NdeI/EagI. The resulting plasmid (pRB5701) was then cut with NdeI, blunt ended, and further digested with EcoRI. The NdeI/EcoRI N-terminal fragment of IC-1a (codons 1 to 228) was ligated into pGEX 4T-1 (Pharmacia Biotech, Piscataway, N.J.) to generate pRB5703 for GST–IC-1a fusion protein. To generate pRB5704 for GST-UL34 fusion protein, a 0.7-kbp fragment from pRB4164 encoding codons 1 to 230 of UL34 was excised as an NcoI-HincII fragment, blunt ended, and subcloned into the SmaI site of pGEX 4T-1. Escherichia coli BL21 cells were then transformed with the resulting plasmids, and the expression and purification of the GST fusion proteins were done according to the manufacturer's instructions (Pharmacia Biotech). The fusion proteins bound to the beads were examined by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and quantified by Coomassie blue staining.
Preparation of [35S]methionine-labeled or unlabeled infected cell lysates.
Vero or HEp-2 cells were exposed to 10 PFU of HSV-1(F) or of HSV-2(G) per cell and maintained in medium 199V consisting of mixture 199 supplemented with 1% calf serum. The cells were labeled with 200 μCi of [35S]methionine (Amersham; >1,000 μCi/nmol) for 3 h in the same medium but without methionine. The cells were then harvested, rinsed once with phosphate-buffered saline (PBS), and lysed in 1 ml HEPES buffer (50 mM HEPES [pH 7.4], 250 mM NaCl, 10 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, 0.1 mM TLCK [Nα-p-tosyl-l-lysine chloromethyl ketone], 0.1 mM TPCK [tolylsulfonyl phenylalanyl chloromethyl ketone], 1% Triton X-100). Cell debris was removed by centrifugation, and the supernatants were stored at 4°C until they were used.
Affinity precipitation with GST–IC-1a or GST-UL34 fusion proteins.
Two hundred microliters of infected or mock-infected cell lysate corresponding to 4 × 106 cells was reacted at 4°C for 12 h with GST or GST–IC-1a fusion protein bound to glutathione-agarose beads. After the beads were rinsed four times with HEPES buffer, the bound protein complexes were solubilized, subjected to electrophoresis in a denaturing polyacrylamide gel, and transferred to a nitrocellulose sheet for autoradiography (labeled cell lysate) or immunoblotting (unlabeled cell lysate).
In vitro transcription-translation of IC-1a.
The N-terminal fragment of IC-1a (codons 1 to 256) or the whole IC-1a chain was produced as a fusion protein by in vitro transcription-translation from pRB5701 or pRB5702 by using the TNT Coupled Reticulocyte Lysate system (Promega). The 35S-labeled reaction products were affinity precipitated by the GST-UL34 fusion protein bound to glutathione beads. The protein complex was then electrophoretically separated on an SDS-polyacrylamide gel, transferred to a nitrocellulose filter, and subjected to autoradiography or immunoblotting as described in Results.
Generation of recombinant baculovirus expressing UL34.
Recombinant baculovirus expressing UL34 was constructed by using shuttle vectors derived from pFastBac1 (Life Technologies, Grand Island, N.Y.). Plasmid DNA was digested with Bst1107I and EcoRI to remove the baculovirus polyhedron gene promoter sequences. A 0.8-kbp NruI/EcoRI fragment from pcDNA3.1(+) (Invitrogen), which contains the CMV immediate-early promoter, was inserted into the pFastBac1 backbone (pFastBac2) to yield pRB5705. To construct the shuttle plasmid pFastBac34 (pRB5706), a 0.85-kbp NcoI/BspEI fragment which contains the UL34 gene was excised from pRB4164, blunt ended with T4 DNA polymerase, and inserted into pFastBac2 at the HindIII site which had been blunt ended.
Recombinant baculovirus (RB34) was generated by using the Bac-to-Bac system (Life Technologies). The shuttle plasmid DNA (pRB5706) was transformed into DH10Bac competent cells for transposition into the bacmid, and the white colonies with recombinant bacmid were further verified by using PCR. The positive colonies were grown in liquid culture, and the recombinant bacmid DNA was isolated by using the Qiagen plasmid purification kit (Qiagen, Chatsworth, Calif.). Sf9 (Spodoptera frugiperda) insect cells were transfected with the recombinant bacmid DNA, and the virus was further amplified by propagation in Sf9 cells maintained in TNM-FH medium. Stocks of virus were concentrated by centrifugation at 105 × g (24,000 rpm in a Beckman SW28 rotor) for 60 min, and the pelleted virus was resuspended in TNM-FH mediun. Virus titers were determined by plaque assay on Sf9 cells (20).
Transduction of mammalian cells by RB34 virus.
Cells were seeded in six-well culture dishes (35-mm diameter) at 500,000 cells per well (for immunoblots and electron microscopy) or in four-well glass slides at 50,000 cells per well (for immunofluorescence studies). Cells were mock infected or infected with wild-type baculovirus or RB34 viruses at indicated multiplicities of infection. After incubation for 1 h at 37°C, the virus inoculum was replaced with medium 199V (mixture 199 plus 1% calf serum) supplemented with 5 mM sodium butyrate. Incubation was continued at 37°C.
Immunoblotting of lysates of cells transduced by RB34 recombinant baculovirus.
Vero cells seeded in six-well culture plates (35-mm diameter) were mock infected or exposed to 30, 100, or 200 PFU of RB34 virus per cell. The infected cells were collected 24 h after infection, rinsed once with PBS, resuspended in disruption buffer (50 mM Tris-HCl [pH 6.8], 100 mM dithiothreitol, 2% SDS, 10% glycerol, 0.1% bromphenol blue), subjected to electrophoresis in denaturing polyacrylamide gels, transferred to nitrocellulose membranes, and reacted with anti-UL34 antibody by standard methods.
Immunofluorescence studies.
Approximately 5 × 104 cells were seeded onto glass slides (Cell-line Inc., Newfield, N.J.) and allowed to attach for 2 h and then mock infected or exposed to 200 PFU of RB34 virus per cell. At the time after infection indicated in Results, the cultures were blocked in PBS containing 20% human serum for 1 h at room temperature, rinsed, reacted for 4 h at room temperature with primary antibody diluted in PBS supplemented with 10% human serum, rinsed extensively with PBS, reacted for 1 h with anti-rabbit immunoglobulin G conjugated to fluorescein isothiocyanate, rinsed extensively, and mounted in 90% glycerol in PBS containing 1 mg of p-phenylenediamine per ml. The slides were examined in a Zeiss confocal fluorescence microscope.
Recombinant baculovirus expressing UL34 (RB34) infection and drug treatment.
Nocodazole purchased from Sigma was dissolved in dimethyl sulfoxide as a 20 mM stock solution. Cells grown on glass slides (5 × 104 cells/well) were exposed to RB34 and incubated at 37°C. After 1 h, the inoculum was removed and replaced with fresh medium supplemented with 5 mM sodium butyrate and 10 μM nocodazole. Cells were then fixed with cold methanol at times indicated in the results, reacted with antibodies to UL34 and α-tubulin, and examined in a Zeiss confocal microscope.
Electron microscopy.
Vero cells were infected with RB34 virus or wild-type baculovirus at a multiplicity of infection of 200. The infected cells were collected 24 h after infection, and electron microscopic examination was done in a Siemens 102 microscope. The procedures for staining and fixation were the same as previously described.
RESULTS
The IC 74 (IC-1a) of dynein interacts with UL34 and UL31 proteins.
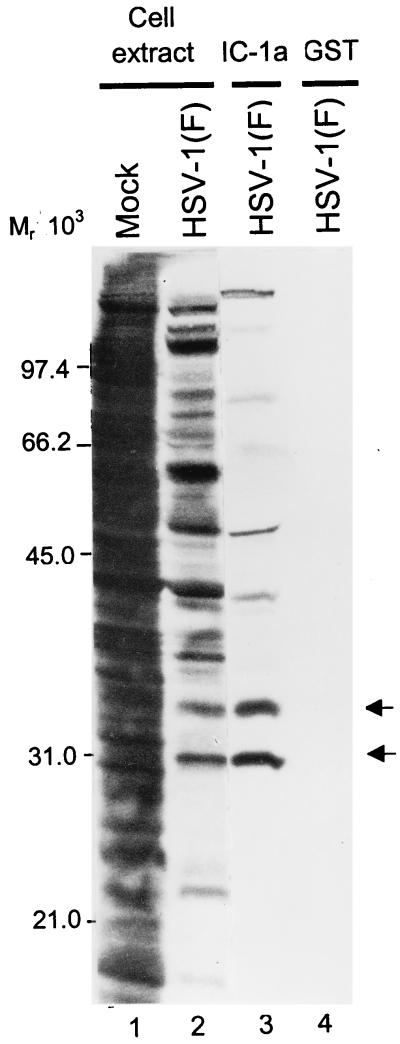
The purpose of this series of experiments was to identify the component of the capsid-tegument structure of HSV that interacts with the component of dynein responsible for the retrograde movement of the capsid-tegument structure in the entry process. For this reason, we fused the N-terminal fragment of IC-1a, a neuronal IC isoform (24), to GST (Fig. 1C, line 4). The GST–IC-1a fusion protein or GST bound to glutathione beads was reacted with lysates of infected cells as described in Materials and Methods. The bound proteins were rinsed, solubilized, electrophoretically separated in a denaturing gel, transferred to a nitrocellulose sheet, and subjected to autoradiography. As shown in Fig. 2, IC-1a pulled down several proteins, of which two marked by arrows were particularly prominent and reproducible and one, the top band, was readily recognizable on the basis of its electrophoretic mobility as the major capsid protein ICP5.
FIG. 2.
Autoradiographic images of electrophoretically separated infected cell proteins bound to GST or to the GST–IC-1a fusion protein. HEp-2 cells were mock infected or exposed to 10 PFU of HSV-1(F) per cell in medium 199V and labeled with [35S]methionine between 12 and 16 h after infection. Lysates of infected cells were reacted with GST or GST–IC-1a fusion protein bound to glutathione-agarose beads. After extensive rinsing, the protein complexes bound to beads were subjected to electrophoresis on an SDS–12% polyacrylamide gel, transferred to a nitrocellulose sheet, and subjected to autoradiography. Lanes 1 and 2, whole-cell extracts from mock-infected or HSV-1(F)-infected HEp-2 cells, respectively; lanes 3 and 4, HSV-1(F)-infected cell proteins bound to GST–IC-1a and to GST, respectively. Arrows indicate two prominent bands of infected cell proteins specifically precipitated by GST–IC-1a.
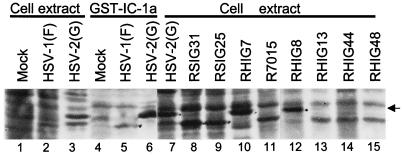
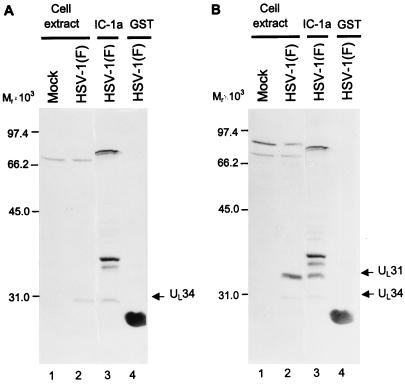
Preliminary experiments (data not shown) indicated that the HSV-1(F) and HSV-2(G) proteins bound to GST–IC-1a differed with respect to electrophoretic mobility in denaturing gels. To identify the two proteins, we subjected the eluted proteins to electrophoresis in a denaturing polyacrylamide gel along with lysates of cells infected with a set of well-characterized intertypic (HSV-1 × HSV-2) recombinants designated R7015, RS1G25, RS1G31, RH1G7, RH1G8, RH1G13, RH1G44, and RH1G48 (Fig. 1B). The results shown in Fig. 3 suggested that the fast-migrating prominent band could be UL34 on the basis of size and map position since the HSV-2 UL34 would be expected to be present in RH1G7 and RH1G8 but not in lysates of other recombinants. A tentative identification of the UL31 protein was based on its size relative to that of UL34. To verify this conclusion, a second experiment was done as illustrated in Fig. 4. In this instance, the proteins electrophoretically transferred to a nitrocellulose sheet were first reacted with the polyclonal antibody to UL34 (Fig. 4A), and after photography, the same blot was reacted with the polyclonal antibody to UL31 (Fig. 4B). As shown in that figure, the two infected cell proteins specifically precipitated by IC-1a were the products of the UL31 and UL34 genes, respectively.
FIG. 3.
Autoradiographic images of electrophoretically separated infected cell proteins from lysates of cells infected with HSV-1, HSV-2, or intertypic (HSV-1 × HSV-2) recombinant viruses. Lanes 1 to 3, whole-cell extracts from mock-infected cells or cells infected with HSV-1(F) or HSV-2(G). Lanes 4 to 6, viral proteins pulled down from mock-infected cells or cells infected with HSV-1(F) or HSV-2(G) with GST–IC-1a fusion protein. Lanes 7 to 15, lysates of cells infected with individual intertypic recombinants. The arrow indicates the labeled protein band in intertypic recombinant viruses that corresponds to the protein band precipitated by GST–IC-1a in HSV-2(G)-infected cell extract.
FIG. 4.
Photograph of immunoblots of infected cell proteins bound to GST or GST–IC-1a fusion proteins, electrophoretically separated in a denatured polyacrylamide gel and reacted with a polyclonal antibody to UL34 (A) or with polyclonal antibodies to UL34 and UL31 (B). Lysates of infected HEp-2 cells were reacted with GST or GST–IC-1a fusion protein bound to glutathione-agarose beads. After extensive rinsing with binding buffer, the beads were subjected to electrophoresis on an SDS–12% polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with the UL34 antibody or with UL34 and UL31 antibodies. Lanes 1 and 2, lysates of HEp-2 cells mock infected and HSV-1(F) infected, respectively; lanes 3 and 4, HSV-1-infected cell proteins bound to GST–IC-1a and GST, respectively. The UL34- and UL31-specific bands are indicated to the right of each panel. Molecular weights of marker proteins (in thousands) are shown to the left of each panel.
IC-1a is pulled down by UL34 fused to GST.
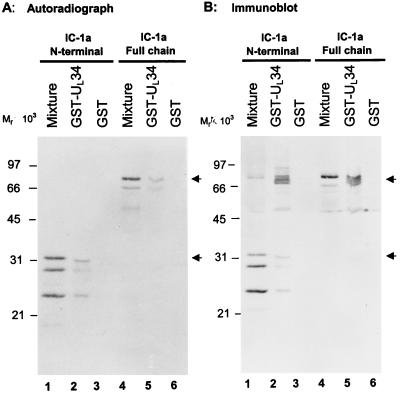
To further verify the interaction of IC-1a with the UL34 protein, a portion of the UL34 ORF (codons 1 to 230) was fused to GST in frame, and the fusion protein product was prepared as described in Materials and Methods. An amino-terminal fragment of IC-1a which was shown to interact with UL34 and UL31 in affinity precipitation experiments, as well as the whole IC-1a clone, was inserted into pGEM5Zf(+) (Fig. 1C, line 2 and 3) and subjected to in vitro transcription-translation as described in Materials and Methods. The in vitro-translated proteins were reacted with GST-UL34 bound to glutathione beads. After extensive rinsing, the bound protein complex was eluted and subjected to electrophoresis in a denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, and subjected to autoradiography. The results were as follows.
(i) Figure 5A, lanes 1 and 4, shows that the in vitro-translated products corresponded to the full size of the cloned fragment and truncated polypeptides migrating faster. All these bands reacted with the anti-IC-1a antibody (Fig. 5B, lanes 1 and 4).
FIG. 5.
Reciprocal affinity precipitation of dynein IC-1a by GST-UL34 fusion protein. (A) Autoradiographic images of electrophoretically separated, [35S]methionine-labeled proteins from the in vitro translation reaction mixture of IC-1a bound to GST or GST-UL34 fusion proteins. In vitro-translated IC-1a whole chain or its N-terminal domain was reacted with GST or GST-UL34 fusion protein bound to glutathione-agarose beads. After being rinsed with binding buffer, the proteins bound to the beads were solubilized, subjected to electrophoresis on an SDS–12% polyacrylamide gel, transferred to a nitrocellulose sheet, and exposed to X-ray film. (B) Photograph of an immunoblot of proteins in vitro translated from IC-1a and bound to GST or GST-UL34 fusion proteins. The same blot from panel A, after being exposed to X-ray film, was reacted with polyclonal antibodies to IC-1a. Lanes 1 and 4, in vitro transcription-translation reaction mixtures of the N-terminal domain and the whole chain of IC-1a, respectively; lanes 2 and 5, in vitro-translated proteins bound to GST-UL34; lanes 3 and 6, in vitro-translated proteins bound to GST.
(ii) As shown in Fig. 5A, the labeled bands pulled down by the GST-UL34 protein corresponded in electrophoretic mobility to the in vitro-synthesized product. Moreover, the anti-IC-1a antibody reacted with both N-terminal and the full-length IC-1a polypeptide pulled down by the GST-UL34 chimeric beads from the in vitro-translated mixtures (Fig. 5A, lanes 2 and 4, and 5B, lanes 2 and 4).
(iii) The GST beads did not bind any in vitro-translated proteins.
(iv) Figure 5B also shows that several unlabeled protein bands that reacted with polyclonal antibody to IC-1a were pulled down by GST-UL34. Although we cannot exclude the possibility that antibody reacted with an unrelated protein, given the very close electrophoretic mobility of these bands to that of the full-length IC-1a protein, it is conceivable that these unlabeled bands represent cellular IC-1a present in the lysate for in vitro transcription-translation. We conclude that the reciprocal pulldown experiment affirms the association of UL34 protein with the IC-1a protein.
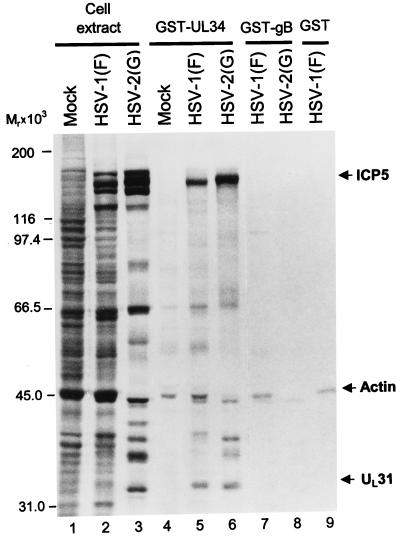
UL34 forms a complex with UL31 and also associates with ICP5, the major capsid protein.
This series of experiments was done to characterize the association of UL34 protein with other viral gene products. GST-UL34 chimeric protein or GST bound to glutathione agarose beads was reacted with lysates of HSV-1(F)- or HSV-2(G)-infected cells labeled with [35S]methionine as described in Materials and Methods. After extensive rinsing of the glutathione-agarose beads, the bound proteins were electrophoretically separated in a denaturing gel, transferred to a nitrocellulose sheet, and subjected to autoradiography or immunoblotting as described in Materials and Methods. The results shown in Fig. 6 indicate that GST-UL34 chimeric protein pulled down three prominent sets of proteins specific for infected cells. One of these, with an apparent Mr of 45,000, was also pulled down by GST-gB and therefore was not specific for UL34. The other two were identified as UL31 protein and ICP5, on the basis of their electrophoretic mobility and immune reactivity (data not shown). We conclude from this series of experiments that UL34 interacts with UL31 and ICP5 in addition to the IC-1a protein and that it is likely that, in the experiment shown in Fig. 2, ICP5 and UL31 protein were pulled down by UL34 rather than by IC-1a.
FIG. 6.
Autoradiographic images of electrophoretically separated infected cell proteins bound to GST or to GST-UL34 fusion protein. HEp-2 cells were infected and labeled with radioactive methionine as described in the legend to Fig. 2. The protein complexes bound to beads were subjected to electrophoresis on an SDS–8% polyacrylamide gel, transferred to a nitrocellulose membrane, and subjected to autoradiography. Lanes 1 to 3, lysates of mock-infected cells or cells infected with HSV-1(F) or HSV-2(G), respectively; lanes 4 to 6, proteins pulled down by GST-UL34 chimeric protein from cells mock infected or infected with HSV-1(F) or HSV-2(G), respectively; lanes 7 to 9, proteins pulled down by GST-gB fusion protein (lanes 7 and 8) or just GST (lane 9). Arrows indicate the infected cell proteins specifically precipitated by GST-UL34. The identities of UL31 and ICP5 were verified by immunoblotting.
Expression of UL34 from recombinant baculovirus-transduced cells.
If UL34 acts as an anchor for transport of capsid-tegument structures to the nuclear pore, its destination after expression by itself, in the absence of other HSV-1 proteins, should be the nuclear membrane. Earlier studies have shown that the UL34 gene product is a virion component associated with cellular membranes (26). The purpose of the experiments described in this section was to determine the localization of UL34 and the phenotype of cells expressing the UL34 protein.
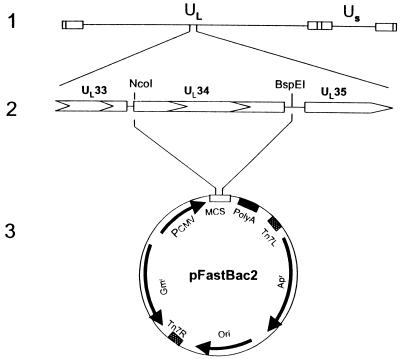
To carry out this study, we constructed a recombinant baculovirus carrying the UL34 ORF driven by the human CMV major immediate-early promoter as described in Materials and Methods. Recent studies have shown that baculovirus can infect mammalian cells and that genes directed by a mammalian promoter can be expressed in mammalian cells whereas the baculovirus genes are not expressed under these conditions (7). The recombinant baculovirus carrying the UL34 gene driven by the CMV promoter was constructed as described in Materials and Methods and graphically illustrated in Fig. 7.
FIG. 7.
Schematic representation of the DNA sequence arrangements in the genomes of HSV-1(F) and the shuttle vector used to construct RB34 baculovirus. Line 1, sequence arrangement of the HSV-1 genome. Line 2, ORFs encoded within the HSV-1(F) DNA fragment in pRB4164. Line 3, shuttle vector for RB34 construction. The entire UL34 ORF was released as an NcoI/BspEI fragment from pRB4164, blunt ended, and inserted into pFastBac2 as described in Materials and Methods.
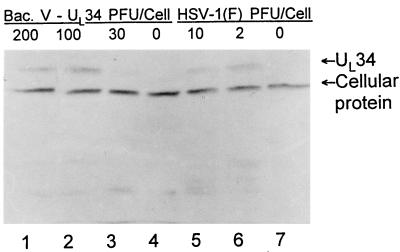
In the first series of experiments, we examined the expression of UL34 in Vero cells. As shown in Fig. 8, UL34 was readily detected in cells exposed to 30 or more PFU of the recombinant baculovirus per cell. At 100 or 200 PFU of recombinant baculovirus per cell, the UL34 protein levels were comparable to or higher than those obtained in cells infected with wild-type virus (compare lanes 1 and 2 with lane 5 in Fig. 8). In the immunoblot shown, a cellular protein that reacted with the UL34 polyclonal antibody served as a loading control in this experiment.
FIG. 8.
Photograph of an immunoblot showing the expression of UL34 protein in HSV- or recombinant baculovirus-infected cells. Vero cells were exposed to recombinant baculovirus (lanes 1 to 3) or HSV-1 (lanes 5 and 6) at multiplicities of infection shown and harvested at 24 h after infection. Lanes 4 and 7, mock-infected cells. A cellular protein which cross-reacted with the antibody to UL34 protein served as the loading control.
The UL34 gene product is associated with the microtubular network and is primarily localized in the perinuclear region.
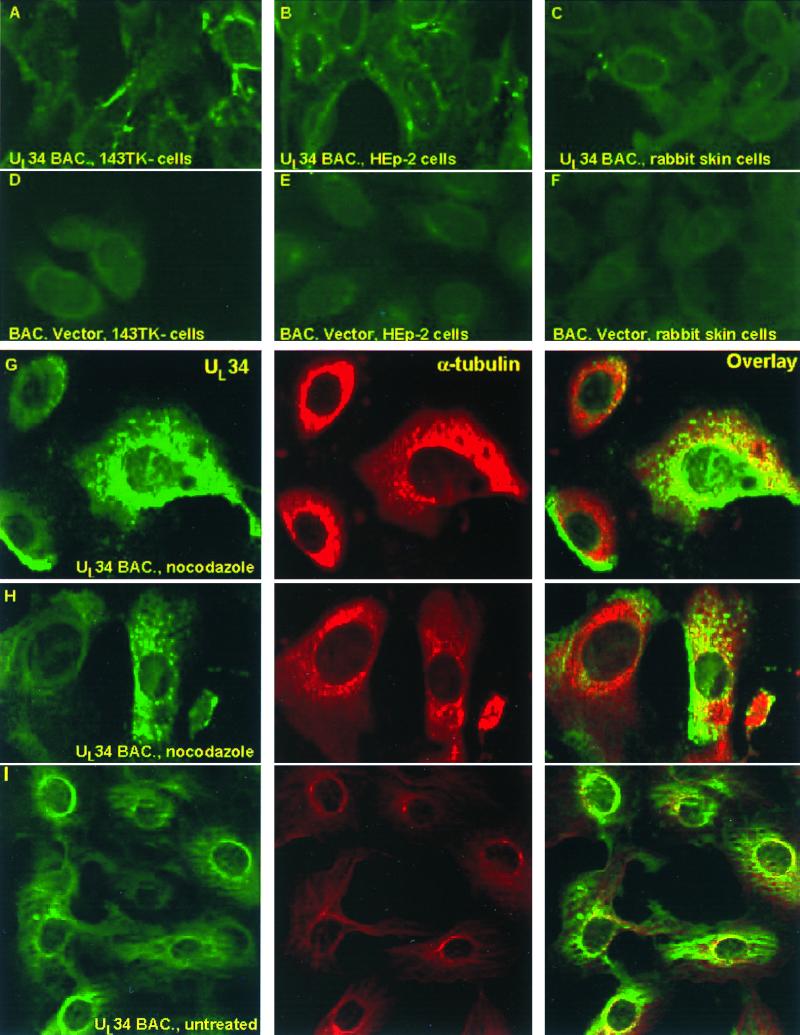
To examine the localization of UL34 in the infected cells, 143TK−, HEp-2, or rabbit skin cells were seeded on four-well glass slides and exposed to 200 PFU of the recombinant baculovirus per cell. The cells were maintained as described in Materials and Methods. At 24 h after infection, the cells were fixed in cold methanol and then reacted with antibody to UL34, as described in Materials and Methods. As shown in Fig. 9A to E, UL34 protein expressed in all three baculovirus-infected cell lines was primarily localized in the perinuclear region. In 143TK− and HEp-2 cells, UL34 was also detected in the cytoplasm.
FIG. 9.
Localization of UL34 protein in cells infected with recombinant baculovirus. (A to F) Confocal, digitized images of 143TK− (A and D), HEp-2 (B and E), or rabbit skin (C and F) cells infected with RB34 virus, maintained for 24 h, and fixed and stained with antibody to UL34 as described in Materials and Methods. (G and H) Vero cells were exposed to 30 PFU of RB34 recombinant baculovirus and then incubated for 17 h at 37°C in medium containing nocodazole and sodium butyrate as described in Materials and Methods and Results. Cells were stained with fluorescein isothiocyanate UL34 protein, Texas Red, and α-tubulin. (I) Vero cells were exposed to 100 PFU of RB34, incubated for 5 h in medium containing sodium butyrate only, and then fixed and stained with the same antibodies as described for panels G and H above. The images were collected with the aid of a Zeiss confocal microscope.
If UL34 protein is transported via the microtubular network to the nuclear membrane, it could be expected that depolymerization of the microtubular network by nocodazole would cause a dispersion of the UL34 protein in the cytoplasm and impede the concentration of the protein at the nuclear membrane. In these experiments, Vero cells grown in glass coverslips were exposed to 30 or 100 PFU of RB34 per cell. After 1 h, the cells exposed to 30 PFU of RB34 were rinsed and incubated in medium containing 5 mM sodium butyrate and 10 μM nocodazole. These cultures were fixed and stained with antibody to UL34 and α-tubulin at intervals between 5 and 17 h after infection. The cultures exposed to 100 PFU of RB34 per cell were incubated in the same medium but without the drug and fixed and stained 5 h after infection. The results shown in Fig. 9G and H indicate that even after 17 h in the presence of nocodazole a significant portion of the UL34 protein is dispersed in the cytoplasm. An unexpected observation was that UL34 protein was unevenly distributed in the cytoplasm of a relatively large fraction of nocodazole-treated cells. The untreated cells exposed to 100 PFU of RB34 and fixed 5 h after infection exhibited both perinuclear and cytoplasmic localization of UL34. In this instance, unlike that observed in 24-h-infected cells (Fig. 9A to C), the distribution of UL34 appeared to be very similar to that of the microtubular network (Fig. 9I).
Dissociation of inner and outer nuclear membranes in cells infected with the recombinant baculovirus expressing the UL34 protein.
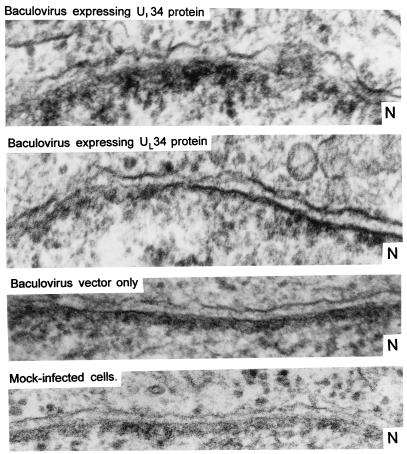
In contrast to cells exposed to the baculovirus vector, cells infected at high multiplicities with the recombinant baculovirus expressing UL34 exhibited morphologic changes characteristic of unhealthy cells. To examine the changes in more detail, we studied with the aid of an electron microscope thin sections of Vero cells exposed to 200 PFU of the recombinant baculovirus expressing UL34 or to the baculovirus vector and incubated for 24 h at 37°C. The striking feature of the cells infected with the baculovirus expressing UL34 was the separation of the inner and outer nuclear membranes (Fig. 10). In uninfected cells or cells infected with the baculovirus vector only, the two membranes were tightly juxtaposed to each other. In cells infected with the baculovirus expressing the UL34 protein, the outer nuclear membrane was dissociated from the inner membrane and formed arcs, giving it a wavy appearance.
FIG. 10.
Thin sections of Vero cells mock infected or exposed to 200 PFU of recombinant baculovirus or baculovirus vector. The images show the inner and outer nuclear membranes. In each image, the cytoplasmic domain is above the membranes whereas the nuclear domain is below the membranes. N, nucleus.
DISCUSSION
HSV penetrates cells by fusion of the envelope with the plasma membrane. Next, the HSV-1 capsid-tegument structure released by deenvelopment during entry into cells must be transported retrograde to the nuclear pore where viral DNA is released into the nucleus. The objective of the studies described in this report was to identify the virion components capable of anchoring the capsid-tegument structures to the dynein motor. We report two significant observations.
The first observation is that IC-1a, the neuronal isoform of the IC of the dynein complex, interacted in pulldown assays with two viral proteins, UL34 and UL31. The interaction with UL34 was reciprocal in the sense that this protein pulled down in vitro-synthesized IC-1a. The identification of the viral proteins is unambiguous: it was based on analyses of proteins made by known HSV-1 × HSV-2 recombinants and by the reactivity of the UL34 and UL31 proteins with the corresponding antibodies. Of the two, only the UL34 protein has been shown to be a virion component. It is conceivable that the pulldown of UL31 protein was due to its interaction with UL34 rather than a direct binding to IC-1a. Consistent with this hypothesis was the observation that the UL34 protein pulls down from lysates of labeled infected cells the UL31 protein and, in addition, ICP5.
The observation that UL34 pulled down a large fraction of available ICP5 provides a possible solution to a conundrum. The properties of the UL34 protein reported to date suggest that it is primarily a membrane protein. The strong interaction with ICP5 suggests that it is at least partly in the tegument anchored in or associated with the major capsid protein, and therefore UL34 could serve as the ligand to the dynein motor on deenvelopment. It should be noted that the definition of capsid-tegument structures reported from this laboratory earlier (19) is based on deenvelopment with detergents and may not correspond to the structures produced by deenvelopment of virions on entry into cells. Experiments are in progress to define the location and role of UL34 protein following entry of the virion into susceptible cells.
The second key observation arose from attempts to determine whether UL34 accumulated in nuclear membranes after synthesis. The rationale for the experiment was based on the hypothesis that, if UL34 is a membrane-associated protein, it would, after synthesis and in the absence of other viral proteins, accumulate largely in the cytoplasm, the cellular compartment rich in a variety of membranes. Such an accumulation would cast doubts on the hypothesis that UL34 is a dynein ligand or at the very least would require that UL34 protein must be modified or accompanied by a viral protein to act as a ligand. To this end, we constructed a recombinant baculovirus carrying the UL34 gene. The aim of the experiment was to make as much UL34 protein as there would be normally in cells infected with wild-type virus. Our results were consistent with that aim. The results of the immunofluorescence assays showed that the preponderance of UL34 protein colocalized with the nuclear membranes. The model was tested further. If its progression to the nucleus was dependent on the association with the dynein motor, nocodazole should depolymerize the microtubular network, disperse the UL34 protein, and impede its accumulation at the nuclear membrane. As shown in Fig. 9G and H, the UL34 protein was significantly more dispersed in nocodazole-treated cells than in untreated cells. An interesting observation documented in Fig. 9I was that the pattern of distribution of the UL34 protein in untreated cells was similar, but not totally identical, to that of microtubules.
An unexpected finding was that the cells infected with the recombinant baculovirus exhibited a separation of the inner and outer nuclear membranes. The outer membrane exhibited a wavy appearance that, together with the separation of inner and outer membranes, is the hallmark of cells productively infected with HSV-1. Thus, in productively infected cells, virions acquire an envelope at the inner nuclear membrane and accumulate in the space between the inner and outer membranes. The hypothesis that UL34 plays a role in the envelopment process is supported by recent studies showing that in cells infected with a UL34− mutant envelopment is severely curtailed (27).
Taken all together, the available data suggest that, after entry into cells, UL34 protein becomes exposed, interacts with the dynein motor, and uses the microtubular network for retrograde transport of the capsid-tegument structure to the nuclear pore. The involvement of IC of cytoplasmic dynein in binding the motor complex to membranous organelles has been reported previously (36). At the nuclear pore, an event associated with a change in conformation or structure of ICP1-2, another tegument protein, triggers the release of viral DNA into the nucleus. On the other end of the replicative cycle, the newly synthesized UL34 protein is transported to the nuclear membranes and forms two links. The first one is with UL31 protein. Given the known functions of the UL34 protein, we may speculate that UL34 anchors a network containing UL31 which enables efficient packaging of DNA into capsids. The second link is with ICP5 in capsids undergoing envelopment. It should be noted that the mechanism of adhesion of capsids to the inner nuclear membranes preceding envelopment is yet to be explained at the molecular level.
One of the major problems in the development of live HSV vectors for immunization in gene therapy is establishment of latent or acute central nervous system infections as a consequence of retrograde transport of virus from the portal of entry to the neuronal nucleus. Elimination of the dynein motor ligand through deletion or mutagenesis of the binding site would greatly facilitate construction of viral vectors specific for the intended task while at the same time eliminating or diminishing the risks associated with retrograde neuronal spread.
ACKNOWLEDGMENTS
These studies were aided by grants from the National Cancer Institute (CA47451, CA71933, and CA78766), United States Public Health Service.
REFERENCES
- 1.Ackermann M, Longnecker R, Roizman B, Pereira L. Identification, properties, and gene location of a novel glycoprotein specified by herpes simplex virus 1. Virology. 1986;150:207–220. doi: 10.1016/0042-6822(86)90280-1. [DOI] [PubMed] [Google Scholar]
- 2.Aniento F, Emanns N, Griffiths G, Gruenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol. 1993;123:1373–1387. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batterson W, Furlong D, Roizman B. Molecular genetics of herpes simplex virus. VIII. Further characterization of a temperature-sensitive mutant defective in release of viral DNA and in other stages of the viral reproductive cycle. J Virol. 1983;45:397–407. doi: 10.1128/jvi.45.1.397-407.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkhardt J K, Echeverri C J, Nilsson I, Vallee R B. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang Y E, Roizman B. The product of the UL31 gene of herpes simplex virus 1 is a nuclear phosphoprotein which partitions with the nuclear matrix. J Virol. 1993;67:6348–6356. doi: 10.1128/jvi.67.11.6348-6356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Y E, Van Sant C, Roizman B. The null mutant of the UL31 gene of herpes simplex virus 1: construction and phenotype in infected cells. J Virol. 1997;71:8307–8315. doi: 10.1128/jvi.71.11.8307-8315.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condreay J P, Witherspoon S M, Clay W C, Kost T A. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc Natl Acad Sci USA. 1999;96:127–132. doi: 10.1073/pnas.96.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conley A J, Knipe D M, Jones P C, Roizman B. Molecular genetics of herpes simplex virus. VII. Characterization of a temperature-sensitive mutant produced by in vitro mutagenesis and defective in DNA synthesis and accumulation of γ polypeptides. J Virol. 1981;37:191–206. doi: 10.1128/jvi.37.1.191-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corthesy-Theulaz I, Pauloin A, Pfeffer S R. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J Cell Biol. 1992;118:1333–1345. doi: 10.1083/jcb.118.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Echeverri C J, Paschal B M, Vaughan K T, Vallee R B. Molecular characterization of the 50kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ejercito P M, Keiff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 12.Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- 13.Hughes S M, Herskovits J S, Vaughan K T, Vallee R B. Cloning and characterization of cytoplasmic dynein 53/55 and 57/59 subunits. Mol Biol Cell. 1993;4:47. . (Abstract.) [Google Scholar]
- 14.Karki S, Holzbaur E L F. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- 15.King S M, Witman G B. Localization of an intermediate chain of outer arm dynein by immunoelectron microscopy. J Biol Chem. 1990;269:5452–5457. [PubMed] [Google Scholar]
- 16.King S M, Wilkerson C G, Witman G B. The Mr 78,000 intermediate chain of Chlamydomonas outer arm dynein interacts with alpha-tubulin in situ. J Biol Chem. 1991;266:8401–8407. [PubMed] [Google Scholar]
- 17.Knipe D M, Batterson W, Nosal C, Roizman B, Buchan A. Molecular genetics of herpes simplex virus. VI. Characterization of a temperature-sensitive mutant defective in the expression of all early viral gene products. J Virol. 1981;38:539–547. doi: 10.1128/jvi.38.2.539-547.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kristensson K, Lycke E, Roytta M, Svennerholm B, Vahilne A. Neuritic transport of herpes simplex virus in rat sensory neurons in vitro. Effects of substances interacting with microtubular function and axonal flow. J Gen Virol. 1986;67:2023–2028. doi: 10.1099/0022-1317-67-9-2023. [DOI] [PubMed] [Google Scholar]
- 19.LeMaster S, Roizman B. Herpes simplex virus phosphoproteins. II. Characterization of the virion protein kinase and of the polypeptides phosphorylated in the virion. J Virol. 1980;35:798–811. doi: 10.1128/jvi.35.3.798-811.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy C I, Piwnica-Worms H, Grunwald S, Romanow W G. Current protocols in molecular biology, suppl. 38. New York, N.Y: John Wiley & Sons, Inc.; 1997. pp. 16.10.12–16.10.14. [Google Scholar]
- 21.Paschal B M, Mikami A, Pfister K K, Vallee R B. Homology of the 74-kD cytoplasmic dynein subunit with a flagella dynein polypeptide suggests an intracellular targeting function. J Cell Biol. 1992;118:1133–1143. doi: 10.1083/jcb.118.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paschal B M, Shpetner H S, Vallee R B. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J Cell Biol. 1987;105:1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paschal B M, Vallee R B. Retrograde transport by the microtubule associated protein MAP 1C. Nature. 1987;330:181–183. doi: 10.1038/330181a0. [DOI] [PubMed] [Google Scholar]
- 24.Pfister K K, Salata M W, Dillman J F, Vaughan K T, Vallee R B, Torre E, Lye R J. Differential expression and phosphorylation of the 74-kDa intermediate chains of cytoplasmic dynein in cultured neurons and glia. J Biol Chem. 1996;271:1687–1694. doi: 10.1074/jbc.271.3.1687. [DOI] [PubMed] [Google Scholar]
- 25.Purohit A, Tynan S H, Vallee R B, Doxsey S J. Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J Cell Biol. 1999;147:481–492. doi: 10.1083/jcb.147.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purves F C, Spector D, Roizman B. UL34, the target of the herpes simplex virus US3 protein kinase, is a membrane protein which in its unphosphorylated state associates with novel phosphoproteins. J Virol. 1992;66:4295–4303. doi: 10.1128/jvi.66.7.4295-4303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roller R J, Zhou Y, Schnetzer R, Ferguson J, DeSalvo D. Herpes simplex virus type 1 UL34 gene product is required for viral envelopment. J Virol. 2000;74:117–129. doi: 10.1128/jvi.74.1.117-129.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sodeik B, Ebersold M W, Helenius R. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136:1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starr D A, Williams B C, Hays T S, Goldberg M L. ZW10 helps recruit dynactin and dynein to the kinetochore. J Cell Biol. 1998;142:763–774. doi: 10.1083/jcb.142.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steffen W, Hodgkinson J L, Wiche G. Immunogold localization of the intermediate chain within the protein complex of cytoplasmic dynein. J Struct Biol. 1996;117:227–235. doi: 10.1006/jsbi.1996.0087. [DOI] [PubMed] [Google Scholar]
- 31.Steffen W, Karki S, Vaughan K T, Vallee R B, Holzbaur E L F, Weiss D G, Kuznetsov S A. The involvement of the intermediate chain of cytoplasmic dynein in binding the motor complex to membranous organelles of Xenopus oocytes. Mol Biol Cell. 1997;8:2077–2088. doi: 10.1091/mbc.8.10.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tognon M, Furlong D, Conley A J, Roizman B. Molecular genetics of herpes simplex virus. V. Characterization of a mutant defective in ability to form plaques at low temperatures and in a viral function which prevents accumulation of coreless capsids at nuclear pores late in infection. J Virol. 1981;40:870–880. doi: 10.1128/jvi.40.3.870-880.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vallee R B, Wall J S, Paschal B M, Shpetner H S. Microtubule associated protein 1C from brain is a two-headed cytosolic dynein. Nature. 1988;332:561–563. doi: 10.1038/332561a0. [DOI] [PubMed] [Google Scholar]
- 34.Vallee R B, Sheetz M P. Targeting of motor proteins. Science. 1996;271:1539–1544. doi: 10.1126/science.271.5255.1539. [DOI] [PubMed] [Google Scholar]
- 35.Vaughan K T, Vallee R B. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss D G, Kuznetsov S R. The involvement of the intermediate chain of cytoplasmic dynein in binding the motor complex to membranous organelles of Xenopus oocytes. Mol Biol Cell. 1997;8:2077–2088. doi: 10.1091/mbc.8.10.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]