Abstract
We describe the cloning and expression of a nonreceptor tyrosine kinase, cymric (Uro-1), a HTK16-like (HydraTyrosineKinase-16) gene, identified in a subtractive screen for maternal ascidian cDNAs in Molgula oculata, an ascidian species with a tadpole larva. Cymric encodes a 4 kb mRNA expressed in gonads, eggs, and embryos in the tailed M. oculata, but is not detected in eggs or embryos of the closely related tailless species, M. occulta. There is a large insertion in cymric in the M. occulta genome, as shown by transcriptome and genome analyses, resulting in it becoming a pseudogene. The cymric amino acid sequence encodes a non-receptor tyrosine kinase (TK) with an N-terminal region containing two SH2 domains and five ankyrin repeats, similar to the HTK-16-like gene found in other ascidians. Thus, the ascidian cymric genes are members of the SHARK (Src-homology ankyrin-repeat containing tyrosine kinase) family of non-receptor TKs, which have been found throughout invertebrates. We show that cymric is lacking the tyrosine kinase domain in the tailless M. occulta, although the truncated mRNA is still expressed in transcriptome data. This maternal and zygotic HTK-16-like tyrosine kinase is another described pseudogene from M. occulta and appears to not be necessary for adult development.
Keywords: Evolution and development, pseudogenes, signaling, subtractive cloning, tyrosine kinases
1. Introduction
Ascidians are invertebrate chordates with a larval notochord, dorsal hollow nerve tube, muscle cells in their tails, and pharyngeal slits as adults (Swalla, 2004 a, b; Fodor et al. 2021 a). Ascidian larvae exhibit the simplest chordate body plan and gastrulate early in development, after the 110-cell stage (Swalla, 2004b). Cleavage is determinate and invariant with a known cell lineage in all solitary ascidians that have been studied, and there is great interest in understanding ascidian embryonic development for clues to the evolution of the chordate body plan (Swalla, 2004b; Zeng and Swalla, 2005) Two closely related ascidian species with completely different larvae were used for the studies described here – Molgula oculata, which has a tailed (urodele) larva exhibiting typical chordate features, and Molgula occulta, which develops into a tailless (anural) larva, lacking chordate features (Fig. 1) (Berrill, 1931; Swalla and Jeffery 1990). Hybrids can be made between the two species, if the tailed M. oculata egg is used then the resulting larva is always tailed, if the tailless M. occulta egg is fertilized with a tailed M. oculata sperm, then it results in a larva with a non-functional shortened tail (Swalla and Jeffery, 1990).
Figure 1.
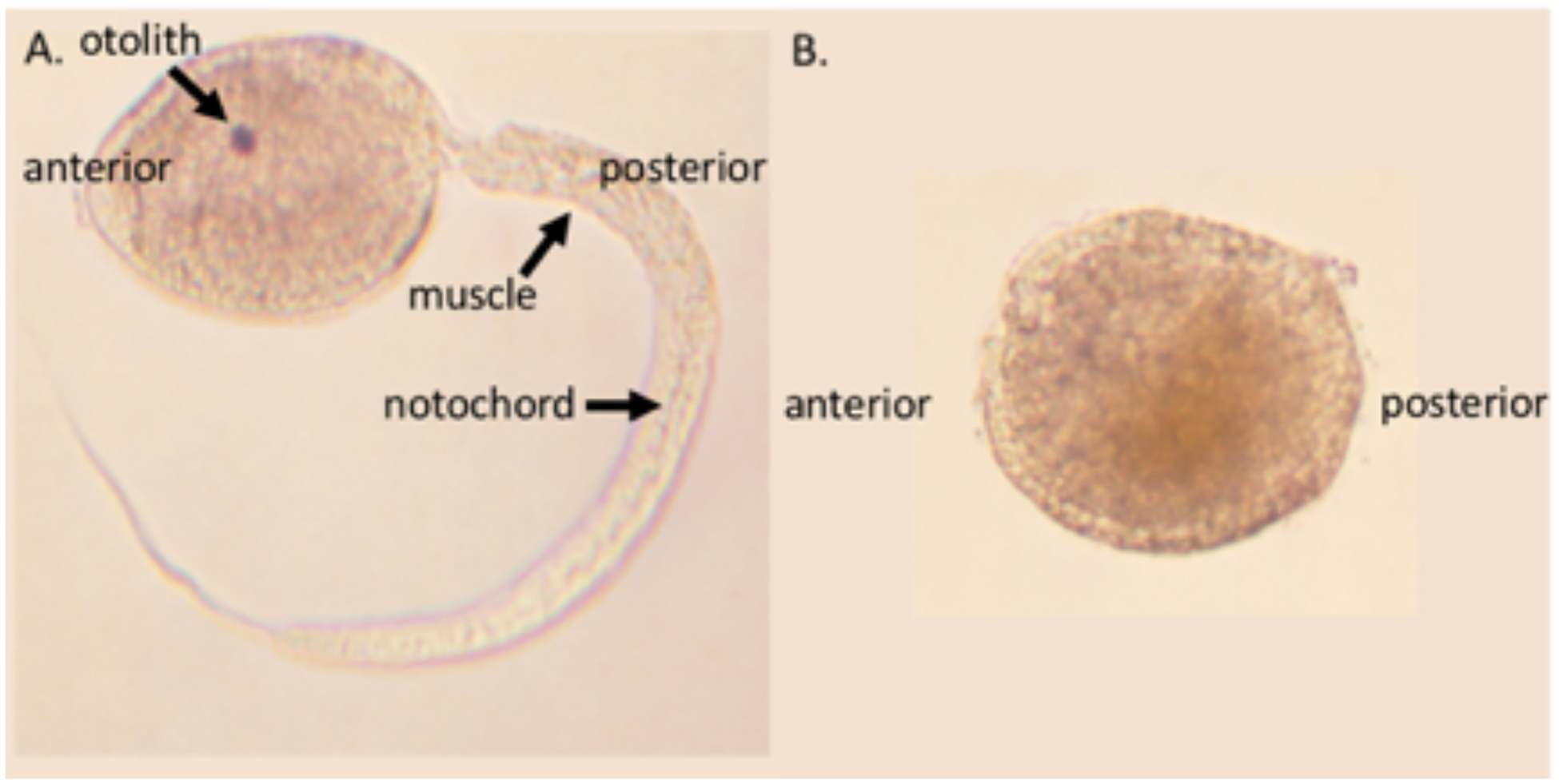
Photographs of A. Molgula oculata and B. Molgula occulta larvae. M. oculata is a tailed ascidian larva with arrows pointing to notochord and muscle cells in the tail, and a pigmented sensory organ (the otolith) in the head. In contrast, M. occulta is tailless, lacking a sensory otolith, notochord, and muscle cells (Swalla and Jeffery 1990; Berrill, 1931).
Subtractive hybridization between RNA isolated from gonads of the two species allowed the isolation of three RNAs expressed in the tailed species but not in the tailless species (Swalla et al. 1993; Swalla 1996). Two of those RNAs correspond to genes present in the genome of both species but were differentially expressed in the tailed M. oculata species (Swalla et al. 1993). One of these genes, manx, is found in a multigene complex with a differentially expressed RNA helicase, bobcat, p68, or DDX5/17 (Swalla et al. 1999; Fodor et al. 2021a). Expression of manx and bobcat is necessary for the formation of the larval tail in M. oculata, as shown by antisense oligo treatment (Swalla and Jeffery 1996). In contrast, the gene reported here, a non-receptor tyrosine kinase, cymric, is altered in the tailless M. occulta genome. It was isolated in the original screen because the M. occulta transcript lacks the 3’noncoding region and the tyrosine kinase domain, which is present in M. oculata, but missing in M. occulta’s disrupted kinase. The mutations to the cymric gene are not suspected to be the main cause of tail loss in M. occulta because a complete cymric gene is present in other ascidian species with tailless larvae (unpublished data). Instead, it is thought that cymric has become a pseudogene due lack of evolutionary time to complete purifying selection for larval genes. Since the loss of function of cymric in M. occulta does not seem to have affected the adult animal, we suspect that cymric is necessary for the development of the tadpole and has little, or a reduced role, in the adult.
Cymric (previously called Uro-1) belongs to the HTK-16-like SHARK family of non-receptor tyrosine kinases. These are unusual tyrosine kinases with five ankyrin repeats flanked by two SH2 domains at the 5’end (Ferrante et al. 1995). The first gene isolated from this family was described in hydra (Chan et al. 1994) and later also found in Drosophila melanogaster (Ferrante et al. 1995). In Drosophila, SHARK is present on the apical surface of ectodermal cells, and genetic evidence suggests that it may be involved in signaling by crumbs, but the exact function is still unknown (Ferrante et al. 1995). SHARK tyrosine kinase has been shown to be critical in signaling the migration of leading-edge cells to form the dorsal closure in Drosophila (Fernandez et al. 2000). Leading-edge cells express dpp, a TGFβ superfamily member, and this leads to the migration of the lateral ectoderm toward the dorsal midline. Drosophila SHARK is located upstream of dpp and downstream of Bullwinkle (bwk) in the Jun-kinase signaling pathway, regulating embryonic epidermal morphogenesis (Fernandez et al. 2000, Tran and Berg 2003). In Drosophila embryos, bwk regulates cell migration during the formation of dorsal appendages (Tran and Berg 2003). It has been shown in Drosophila that SHARK will restore bwk mutants to the wild phenotype, but the precise interaction at the protein level is not yet known (Tran and Berg 2003).
SHARK is also important in Drosophila for the recruitment of inflammatory macrophages via Src42A-Draper-Shark signaling in response to damage induced by H2O2. In Drosophila, Src42A is a homologue of zebrafish lyn. Src42A detects H2O2 in response to a wound and interacts with Draper to recruit SHARK, possibly equivalent to the SFK-ITAM-Syk pathway used by vertebrates in adaptive immune response (Evans et al. 2015). It has been demonstrated that SHARK mutant embryos fail to recruit macrophage cells and re-expression of SHARK in the mutants rescues the phenotype (Evans et al. 2015). SHARK has also been shown to be necessary in signaling Draper to initiate glial phagocytosis in C. elegans and axon-injury-induced changes. Knocking down SHARK in the glia complex of C. elegans causes a failure of phagocytosis and axonal healing (Stanley et al. 2008).
Ankyrin repeats have been identified in diverse proteins and are important in protein-protein interactions (Michaely and Bennett 1992). Ankyrin is a critical component of the ascidian egg myoplasm, which is necessary for axis determination and formation (Swalla 1992) and is frequently missing or altered in the tailless species (Jeffery and Swalla 1992; Jeffery and Swalla 1993; Swalla et al. 1993; Jeffery et al. 1999). It is intriguing that a specific tyrosine kinase might be anchored in the egg cortex of tailed species and necessary for the initial events of axis formation to occur during the first cell cycle, which will in turn lead to the proper movements during gastrulation (Swalla 1992, 2004b). In this paper, we describe the cloning and expression of cymric RNA in M. oculata and M. occulta ascidians. We present genomic analyses that cymric is intact in M. oculata and M. occidentalis, two tailed Molgula species, but is disrupted in the tailless M. occulta.
2. Materials and Methods
2.1. Biological materials
Molgula oculata and Molgula occulta were collected by dredging sand flats at Point de Bloscon near Roscoff, France. Gonads were dissected from gravid adults to obtain sperm and eggs. Procedures for obtaining gametes, fertilization, and culturing embryos have been described previously (Swalla and Jeffery 1990).
2.2. Isolation of DNA and RNA
Genomic DNA was prepared by homogenizing isolated testes in 1 mg/ml Proteinase K, incubating the mixture at 65°C for 15 min and then at 37°C overnight, followed by phenol:chloroform extraction (Davis et al. 1986). RNA was prepared from gonads or embryos by the guanidine isothiocyanate method (March et al. 1985).
2.3. Isolation of cDNA clones by subtractive hybridization
DNA was prepared from M. oculata gonad poly (A)+ RNA and a subtracted probe was prepared using M. occulta cDNA as described previously (Swalla et al. 1993). The subtracted cDNA was amplified by PCR and used to screen an M. oculata gonad cDNA library for positive clones. Probes made from the positive clones were then used to screen an M. occulta cDNA library. Clones that screened positive in the M. oculata cDNA library and negative in the M. occulta cDNA library were retained. The resulting M. oculata clones were then subjected to RNA slot blot hybridization (Swalla et al. 1993) to distinguish those encoding differentially expressed RNAs of the tailed and tailless species from the background. A complete description of the subtraction procedure is available elsewhere (Swalla 1996). One of the cDNA clones isolated in the subtractive hybridization was Urodele 1 (Uro 1), which encodes the 3’ region of the cymric mRNA (Fig. 2A). A probe made from the Uro-1 insert was used to screen the M. oculata cDNA library for longer clones. This screen resulted in the isolation of the overlapping clones, from which the cymric cDNA sequence of M. oculata was derived.
Figure 2.
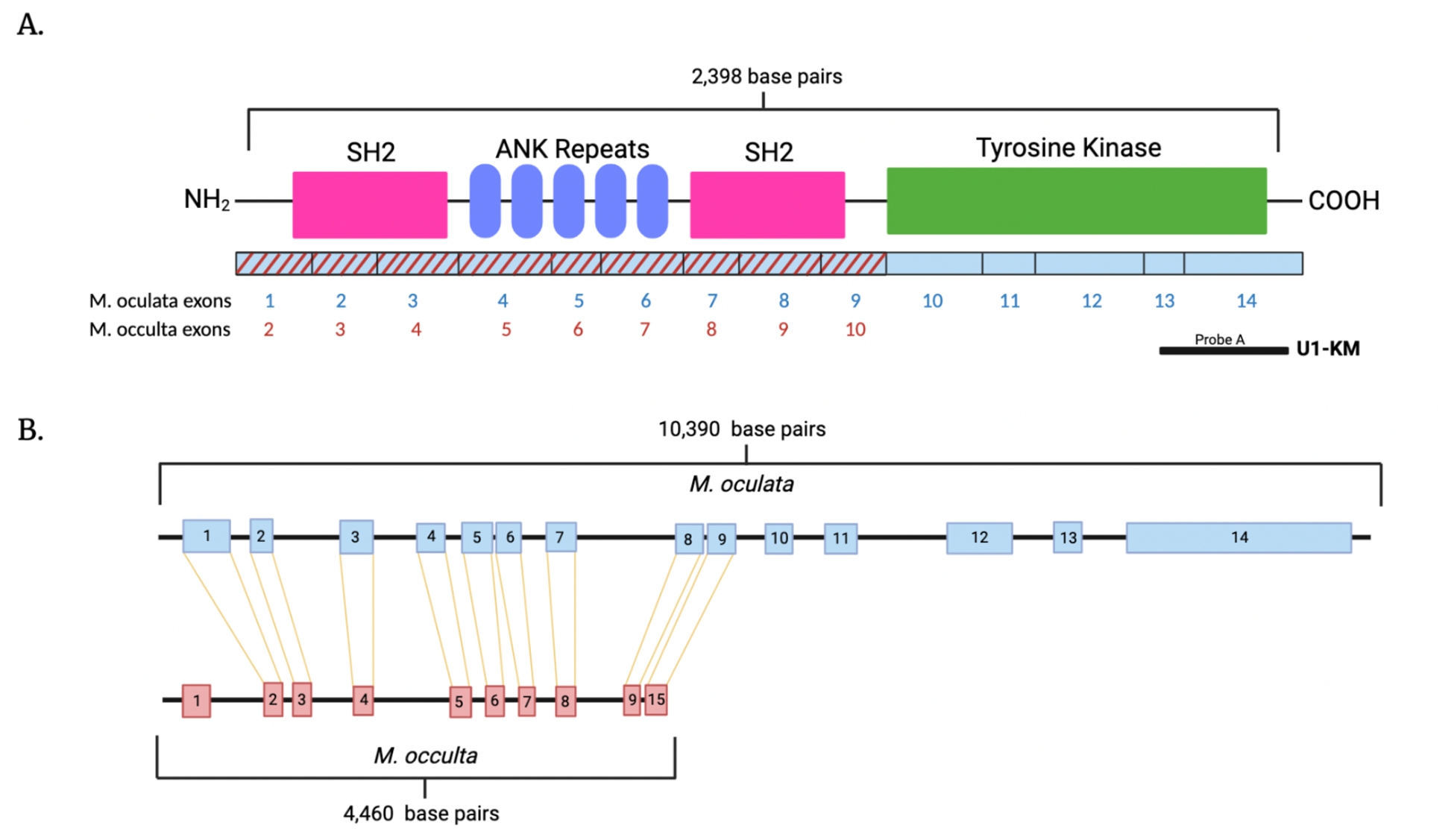
A. A schematic diagram of the putative M. oculata cymric protein. The amino and carboxy termini are labeled, the locations of SH2 domains are in pink, ankyrin repeats are in blue, and the tyrosine kinase catalytic domain is in green. Below the protein diagram are the corresponding exons from M. oculata in blue and M. occulta in red that encode the cymric protein. Shown below is the position of cDNA clone (probe A) used for identifying the cymric amino acid sequence and as probes for in situ hybridizations. B. Intron and exon organization of M. oculata and M. occulta SHARK tyrosine kinase transcripts compared to the M. oculata and M. occulta genomes. The M. occulta genome is missing the last 5 exons transcribed from the M. oculata genome (exons 10–14) and has an extra exon at the 5’ end of the transcript (not shown). The M. occulta cymric transcript is truncated missing the tyrosine kinase domain.
We have since sequenced the transcriptomes of M. oculata, M. occulta and the hybrid embryos (made with M. occulta eggs, fertilized by M. oculata sperm) at three different time points: 3 hours after fertilization (F+3; gastrulation), 4 hours after fertilization (F+4; neurulation), and 5 hours after fertilization (F+5; early tailbud) (Lowe et al. 2014). De novo assembly of the transcriptome of these three stages yielded about 16,000 genes in M. oculata and M. occulta. Next, we sequenced the genomes of both species of molgulid ascidians, with a third species, the tailed Molgula occidentalis, that is available all year from Gulf Specimen Marine Laboratory Inc. in Panacea, Florida, USA. Genomes were sequenced in collaboration with the lab of Lionel Christiaen at New York University (Stolfi et al. 2014). The genomic sequences for M. oculata, M. occulta, and M. occidentalis were deposited into ANISEED (Ascidian Network for In Situ Expression and Embryological Database) (Brozovic et al. 2017; Tassy et al. 2010) to facilitate sharing of these unique genomic resources.
2.4. Sequencing and computer analysis
The original Uro-1 cDNA clones were Sanger sequenced (old school) on both strands using [35S]-dATP (800 Ci/mmole; New England Nuclear, Boston, MA) by the dideoxy chain termination method (Sanger et al. 1977) with Sequenase (United States Biochemical Corp., Cleveland, OH). Oligonucleotide primers were made on a Pharmacia LKB Gene Assembler Plus (Pharmacia Biosystems, Inc.; Piscataway, NJ) to create overlapping sequence information. Sequences were read and compared with the MacVector, Inc. Program (MacVector, Inc.; Apex, NC). The genomic sequences for cymric in M. oculata and M. occulta as well as the other ascidian species were retrieved from ANISEED by BLAST searching, using the sequenced cDNA clones as a query. The resulting sequences were then used to search the ascidian transcriptomes. The deduced protein sequences were compared with MacVector, Inc. along with sequences present in the National Biomedical Research Foundation protein database and the on-line GenBank protein sequences available through the National Library of Medicine. The accession number for M. oculata cymric is OQ445879, M. occulta (OQ589864), M. occidentalis (OQ589865), C. intestinalis (XP_002121112), C. savignyi (OQ589867), H. roretzi (OQ589866), P. mammillata (CAB3267847) and S. clava (XP_039270686).
2.5. In situ hybridizations
Whole mounts of eggs and embryos were subjected to in situ hybridization with DIG labeled probes as described by Swalla et al. (1994). Embryos were dechorionated manually after fixation or by treatment with Sodium thioglycolate and Pronase E (Sigma Chemical Corp., St. Louis, MO) before fixation. Proteinase K treament was carried out at a concentration of 10μg/ml for 30min at 37°C. The hybridization and washing temperatures were 47°C and the final wash was done in 0.5XSSC, 50 % formamide at 47°C. The whole mounts were cleared in 1:2 benzyl alcohol: benzyl benzoate and photographed.
2.6. Gene Tree Analyses
Protein sequences were deduced from M. oculata and M. occulta transcriptome data using MacVector. Other species’ protein sequences were found by Blast searching using M. oculata protein sequences as a query in NCBI and ANISEED (Brozovic et al. 2017). Proteins were then aligned in MacVector using a Muscle alignment.
3. Results
3.1. Isolation and characterization of cymric cDNA clones
The cymric cDNA clone was obtained in a subtractive screen designed to identify maternal genes expressed in the tailed ascidian species, Molgula oculata, but not expressed (or down regulated) in the tailless ascidian species, Molgula occulta (Fig. 1) (Swalla et al. 1993). Three different clones, Uro-1, Uro-2, and Uro-11, were isolated in this screen. The full-length Uro-2 (lynx) and Uro-11 (manx) cDNAs encode leucine zipper and zinc finger proteins respectively, which are expressed preferentially in the tailed species (Swalla et al. 1993). The initial cymric clone was incomplete, although it contained an open reading frame corresponding to the C-terminal region of a protein tyrosine kinase (Figure 2; (Swalla 1996). To obtain longer cDNA clones, the M. oculata cDNA library was re screened with the cymric insert, and five additional overlapping clones were isolated and sequenced. Further screening failed to produce longer cDNA clones. The longest clone, Uro-1–2, consists of a 2073 nucleotide (nt) coding region and a 1215 nt 3’ UTR, which terminates in a polyadenylation site and a poly (A) tail (Figure 2). The gene corresponding to the Uro-1 cDNA compiled sequence was designated cymric, consistent with the practice of naming ascidian Uro genes after tailless cats (Swalla 1996). The translated amino acid sequence shows that cymric encodes a HTK-16-like tyrosine kinase of the SHARK family (Figure 2). The Genbank accession number for M. oculata cymric is OQ445879.
3.2. The cymric gene encodes a SHARK family protein tyrosine kinase
There was high sequence identity between Cymric and proteins associated with the HTK-16 and SHARK families of tyrosine kinases (Chan et al. 1994, Ferrante et al. 1995). Figure 3 shows an alignment of tailed and tailless Cymric to other metazoans including M. occidentalis (OQ589865), Ciona intestinalis (XP_002121112.2), Strongylocentrotus purpuratus (XP_001180232.2), Aplysia californica (XP_005102068.1), Hydra vulgaris (NP_00129668.1) (Chan et al. 1994), Amphimedon queenslandica (BAA81720) (Suga et al. 1999), and Drosophila melanogaster (NP_524743.2) (Ferrante et al. 1995). All metazoan HTK-16 genes share 2 SH2 domains (Fig. 3 pink) flanking a series of ankyrin repeats (Fig. 3 blue), and all except for the tailless ascidian, Molgula occulta, have a very conserved tyrosine kinase domain (Fig. 3 green). The SHARK domains share a high similarity between sponges and the distantly related ascidians, indicating that this protein was present in the metazoan ancestor and has been highly conserved. Blast searches of ascidian SHARK proteins did not yield any vertebrate homologues, indicating that this gene was lost in the vertebrate lineage prior to whole genome duplications.
Figure 3.
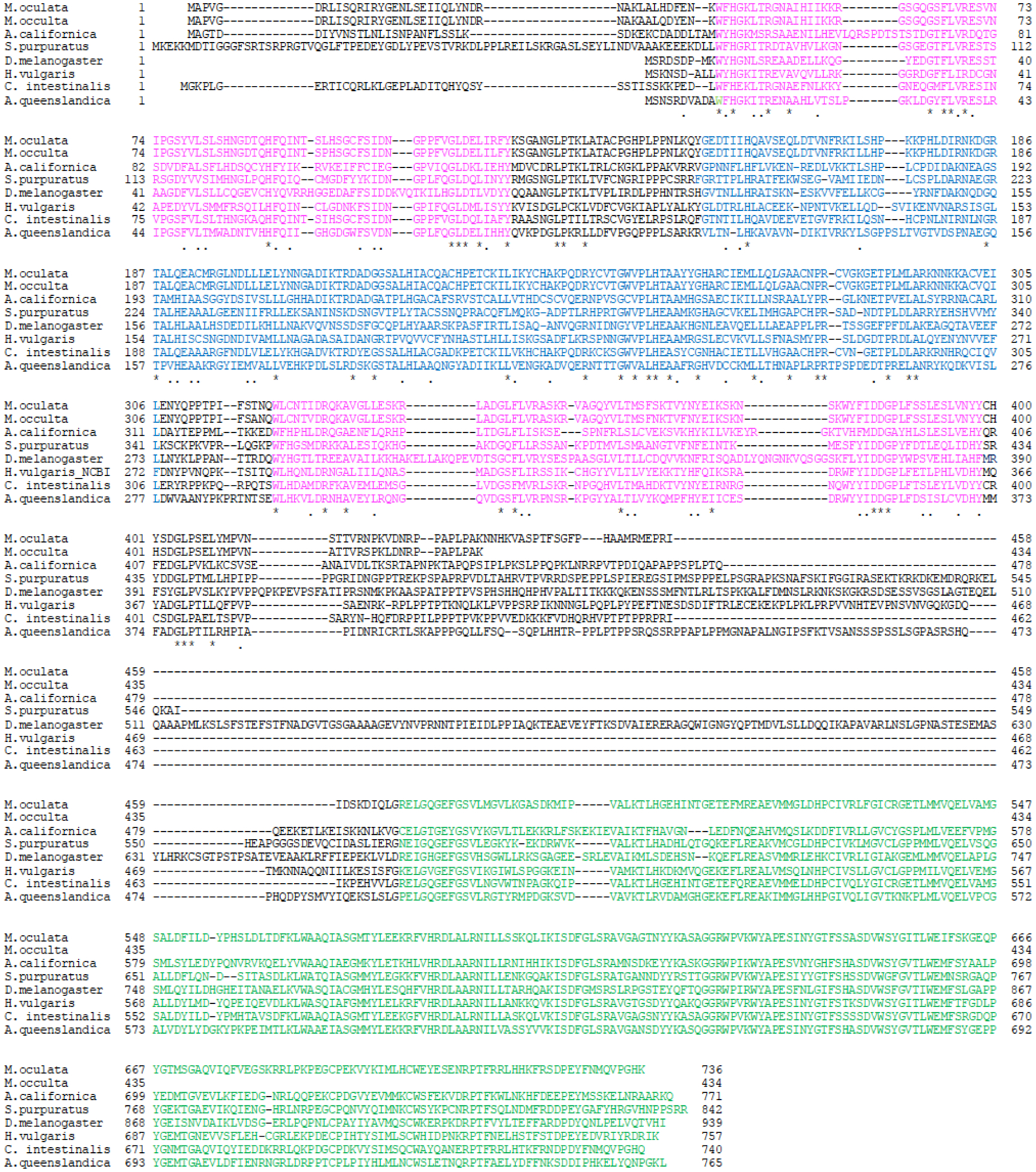
Alignment of SHARK tyrosine kinases including ascidian (M. oculata, M. occulta, M. occidentalis, and C. intestinalis (XP_002121112.2)) cymric, H. vulgaris HTK16 (NP_00129668.1) (Chan et al., 1994), A. queenslandica (sponge) SHARK (BAA81720) (Suga et al. 1999), D. melanogaster SHARK (NP_524743.2) (Ferrante et al., 1995), S. purpuratus SHARK (XP_001180232.2), and A. californica SHARK (XP_005102068.1) tyrosine kinases. The amino acid positions are numbered at the beginning and end of each row. The gaps in the alignment are indicated by dashes. The SH2 domains are in pink, the ankyrin repeats are blue, and the tyrosine kinase domain is green. There is very high sequence similarity across metazoans, suggesting that cymric was present in the metazoan common ancestor and has been very conserved.
The Cymric protein also exhibits a long proline-rich region linking the N-terminal SH2 domain and ankyrin repeats with the C-terminal catalytic domain (Ferrante et al. 1995). The HTK-16, sponge SHARK and ascidian Cymric proteins have truncated proline-rich regions linking their N- and C-terminal domains. Cymric is a new member of the SHARK family of protein tyrosine kinases; and the fact that SHARK proteins are found in many invertebrate metazoan groups, including cnidarians, bugs, beetles and bivalves suggests that it is a very ancient and conserved protein in the evolution of metazoans. However, there were no cymric gene sequences reported for vertebrates, which suggests that it was lost in a long ago vertebrate ancestor.
3.3. The single-copy cymric gene is disrupted in the tailless M. occulta
A single copy of intact Cymric was found in the genomes of the two tailed species M. occidentalis and M. oculata, and a partial copy, lacking the tyrosine kinase domain, was found in the tailless species, M. occulta (Fig. 2A). When looking at the genomic organization, M. oculata cymric contains 14 exons which code for the entire protein. However, M. occulta cymric has 10 exons, the first exon in the 5’ noncoding region is unique and not expressed in the tailed species; the next 9 coding exons share high homology with the first 9 exons of M. oculata (Fig. 2A). There is an interruption after the 10th exon in the tailless M. occulta genome and the rest of the gene is truncated before the tyrosine kinase domain (Fig. 2B). All of the other ascidian species’ genomes in ANISEED had a complete cymric that contained all of the critical domains whereas the cymric gene from M. occulta was truncated before reaching the tyrosine kinase domain (Fig. 2).
Transcriptomes for 3 different stages of development (gastrula, neurula, and tailbud) are available for three Molgulid species, M. oculata, M. occulta, and M. occidentalis. The transcripts in each stage were then combined into a “developmental transcriptome” which we used to search for the cymric transcript. The transcriptome data confirms the genomic findings. Both M. oculata and M. occulta cymric are found expressed in the transcriptome; the tailed M. oculata transcript encodes a full-length protein, while the tailless M. occulta’s transcript is truncated, missing the tyrosine kinase domain (Fig. 2A). To summarize, the M. occulta Cymric protein lacks a tyrosine kinase domain, making it nonfunctional, and the cymric gene is now a pseudogene.
3.4. Cymric is expressed in oocytes and embryos of the tailed species, M. oculata
The accumulation of cymric transcripts during development of the tailed M. oculata was examined by in situ hybridization, since earlier studies showed that cymric is expressed in M. oculata gonads and not M. occulta gonads (Swalla et al. 1993). As shown in Figure 4, cymric mRNA was observed in the vegetal pole region of the oocytes of the tailed species (Fig. 4A). Expression remains in the vegetal and later the posterior poles of the embryo where the larval muscle cells are determined (Fig. 4B–C), then was seen posteriorly in the muscle cell precursors during gastrulation and neurulation (Fig. 4D–E), and expression was lost in the tailbud stage (Fig. 4F).
Figure 4.
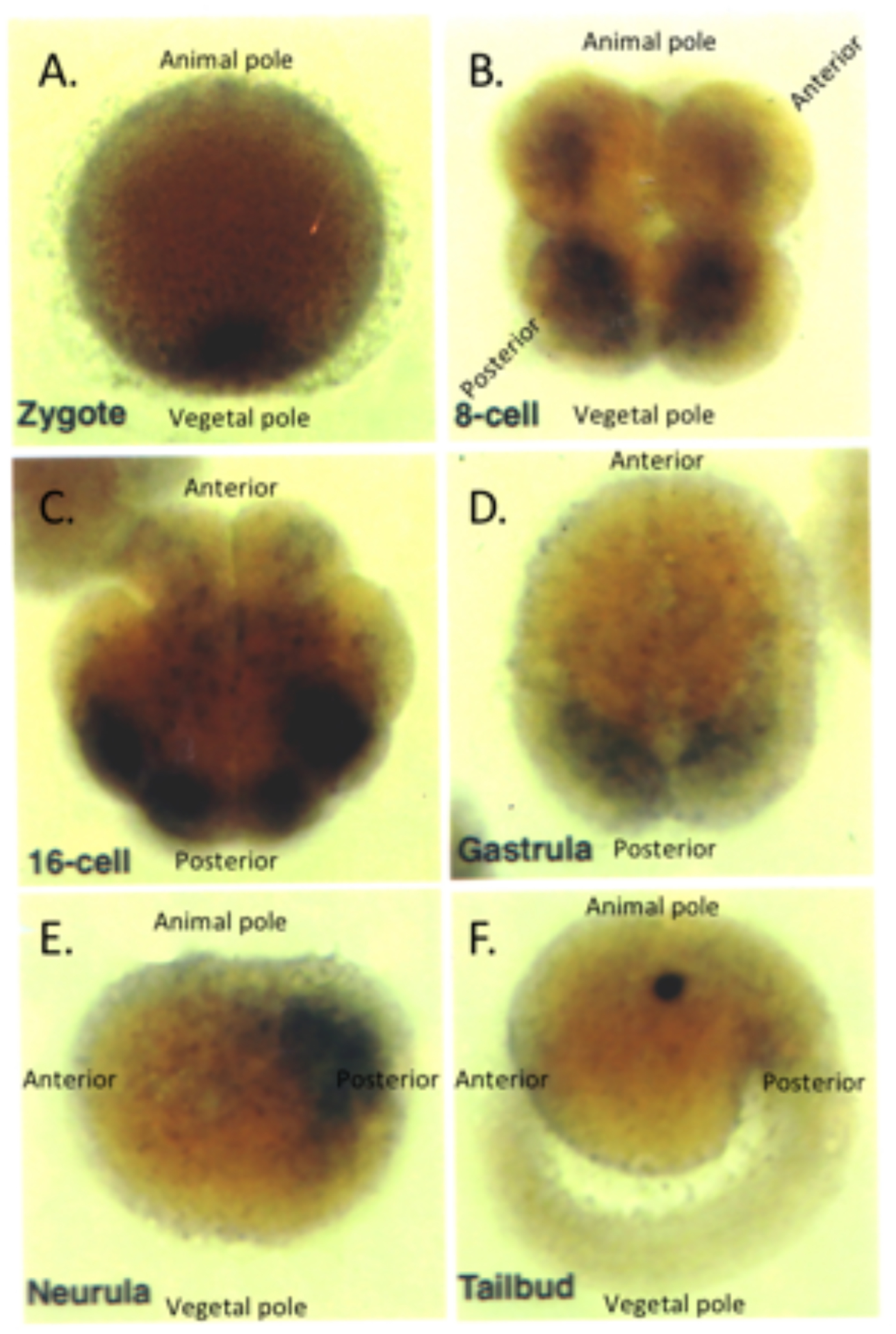
Distribution of cymric transcripts throughout the tailed Molgula oculata development as determined by in situ hybridization. A. Oocyte at first ooplasmic segregation with animal pole up and vegetal pole down, the cymric transcript is expressed in the vegetal pole. B. 8-cell with animal pole up and vegetal pole down. Cymric expression accumulates in the blastomeres on the vegetal and posterior poles. C. 16-cell embryo in a vegetal view. Cymric transcripts are sequestered into the 4 tail muscle cells at the posterior of the embryo. D. A vegetal view of a gastrula showing cymric accumulation in all the larval tail muscle cells. E. Neurula with the animal pole up, the vegetal pole down, the anterior left and posterior right; cymric transcripts still remain expressed in the tail muscle cells. G. Tailbud stage with animal pole up, vegetal down, anterior left, and posterior right. Cymric expression is no longer detectable in the larva.
4. Discussion
Subtractive hybridization between two molgulid ascidian species, Molgula occulta and Molgula oculata, with different developmental modes (Swalla et al. 1993), allowed the isolation of cymric, an HTK-16 SHARK family non-receptor tyrosine kinase. Lynx (Uro-2) and manx (Uro-11), two other genes from the same screen, have been previously published (Swalla et al. 1993). From the results shown here of the genome and transcriptome analyses, we conclude that the full length cymric transcript is present in M. occidentalis and M. oculata, two molgulid species that make tadpole larvae, but the tailless M. occulta transcript is missing the tyrosine kinase domain, making cymric non-functional at phosphorylation of other proteins.
The role of cymric in ascidian development is still not clear, but it is intriguing that a protein containing ankyrin repeats has been implicated in protein-protein interactions. Ascidian eggs contain a special cortical cytoskeletal domain, the myoplasm, which is known to be important in later tail muscle cell development (Swalla 1992, 1993, 2004b). The cortex contains ankyrin (Jeffery and Swalla 1993) and p58 (Swalla et al. 1991) in all species that develop into tailed tadpole larvae. Cymric may be anchored in the cortical egg myoplasm and be important in signaling during muscle cell specification in ascidian embryos.
The myoplasm, an egg cytoplasmic region that is segregated to muscle lineages in ascidian embryos (Swalla 1992), is modified in the eggs of anural species (Swalla et al. 1991; Jeffery and Swalla 1992b. The modification occurs during oogenesis, after vitellogenesis begins (Swalla et al. 1991). Later, this cortical cytoplasm is inherited by tail muscle cells, which then undergo myogenesis (Swalla 1992, 2004b). In contrast, Molgula occulta and other tailless species’ eggs contained p58, but failed to initiate and maintain cortical localization of the protein (Swalla et al. 1991), which was correlated with the development of embryos lacking muscle cells (Swalla and Jeffery 1990; Jeffery and Swalla 1992a). The mechanism(s) of the changes in oogenesis are not fully understood, but it has also been shown that all of the investigated tailless species in the family Molgulidae lack an ankyrin-like protein in their eggs and embryos (Jeffery and Swalla 1993). This raises the interesting possibility that the Molgulid ancestor modified the ankyrin-like protein, possibly being a pre-adaptation to the further changes during oogenesis seen in all the tailless Molgulid species. This indicates that although the truncated cymric transcript is present in the tailless M. occulta, if translated from the mRNA, it would be a truncated nonfuctional protein, lacking the tyrosine kinase. Further research will focus on how changes in oogenesis and gene expression result in the morphological changes seen in the tadpole larvae in other closely related Molgula species.
There have been several genes critical for larval development that have become pseudogenes in the tailless Molgula occulta, including the larval muscle actin genes (Kusakabe et al. 1996) and tyrosinase, a gene vital for forming the ocellus (a pigmented light sensing organ) which is absent in M. occulta (Racioppi et al. 2017). Cymric has become a pseudogene in the tailless Molgula occulta as well, where the mRNA with the truncated tyrosine kinase domain is still expressed in the transcriptome, but it no longer produces a protein with a tyrosine kinase domain. This indicates that the tail loss is a fairly recent development in the evolutionary history of M. occulta and that these pseudogenizations are likely a secondary effect of other mutations that are the primary cause of the tail loss.
Cumulative evidence suggests that gene pseudogenization is common in the tailless Molgulids. Not only have cymric, tyrosinase, and the muscle actin genes all become pseudogenes in M. occulta (Kusakabe et al. 1996, Racioppi et al. 2017), but muscle actin has also become a pseudogene in both of the tailless species Molgula bleizi and Molgula tectiformis (Gyoja et al. 2007, Kusakabe et al. 1996). Recent studies have suggested that pseudogenes may play a role in regulation and development, and they can arise in various ways in genomes (Li et al 2013). The Molgulid family offers a unique system to study what some of those effects may be while we investigate upstream factors for the primary change causing the tailless phenotype in Molgula occulta.
Acknowledgments:
The Molgula oculata, M. occulta and M. occidentalis genomes are available on Aniseed. Cymric mRNAs in M. occulta and M. oculata are available in Genbank as well. We thank Barbara Baldwin, Ruth Tower, Margaret Just, Jana Machula, David Martasian, and Jennifer Reardon who worked on this project in W.R. Jeffery’s laboratory at Bodega Marine Lab at the University of California at Davis in the early 1990’s. Kristen Suling and Allison Hatmaker worked on this project as undergraduates doing independent study in B.J. Swalla’s lab at Vanderbilt University from 1994–1997. We are also indebted to Nicole Sanseau, Dr. Andre Toulmond, Dr. Laurent Meijer, and the collection staff at Station Biologique, Roscoff for their support of this work.
Data Availability Statement:
Sequences for the Cymric gene were retrieved from ANISEED (http://www.aniseed.cnrs.fr/), a public database containing the genomes of various tunicate species. These sequences are also available in greater detail in the NCIB (https://www.ncbi.nlm.nih.gov/). The accession numbers are as follows: Styela clava XP_039270686.1, Phallusia mammillata CAB3267847.1, Ciona intestinalis XP_002121112.2, Molgula oculata OQ445879, Molgula occulta OQ445879, Molgula occidentalis OQ589865, Halocynthia rorezi OQ589866, Ciona savigny OQ589867.
Literature Cited
- Berrill NJ 1931. Studies in tunicate development, Part II. Abbreviation of development in the Molgulidae. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 219: 281–346. [Google Scholar]
- Brozovic M, Dantec C, Dardaillon J, Dauga D, Faure E, Gineste M, Louis A, Naville M, Nitta KR, Piette J, Reeves W, Scornavacca C, Simion P, Vincentelli R, Bellec M, Ben Aicha S, Fagotto M, Guéroult-Bellone M, Haeussler M, … Lamaire P 2017. ANISEED.
- Chan TA, Chu CA, Rauen KA, Kroiher M, Tatarewicz SM, and Steele RE 1994. Identification of a gene encoding a novel protein-tyrosine kinase containing SH2 domains and ankyrin-like repeats. Oncogene. 9(4): 1253–1259. [PubMed] [Google Scholar]
- Davis LG 1986. Basic methods in molecular biology. Elsevier. [Google Scholar]
- Evans IR, Rodrigues FS, Armitage EL, and Wood W 2015. Draper/CED-1 Mediates an Ancient Damage Response to Control Inflammatory Blood Cell Migration In Vivo. Current Biology. 25(12): 1606–1612. 10.1016/j.cub.2015.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez R, Takahashi F, Liu Z, Steward R, Stein D, and Stanley ER 2000. The Drosophila shark tyrosine kinase is required for embryonic dorsal closure. Genes and Development. 14(5): 604–614. 10.1101/gad.14.5.604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante AW, Reinke R, and Stanley ER 1995. Shark, a Src homology 2, ankyrin repeat, tyrosine kinase, is expressed on the apical surfaces of ectodermal epithelia. Proceedings of the National Academy of Sciences – PNAS. 92(6): 1911–1915. 10.1073/pnas.92.6.1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor ACA, Powers MM, Andrykovich K, Liu J, Lowe EK, Brown CT, Di Gregorio A, Stolfi A, and Swalla BJ 2021. The Degenerate Tale of Ascidian Tails. Integrative and Comparative Biology. 61:(2), 358–369. 10.1093/icb/icab022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor A, Liu J, Turner L, and Swalla BJ 2021. Transitional chordates and vertebrate origins: Tunicates. Current Topics in Developmental Biology. 141: 149–171. 10.1016/bs.ctdb.2020.10.001 [DOI] [PubMed] [Google Scholar]
- Gyoja F, Satou Y, Shin-i T, Kohara Y, Swalla BJ, and Satoh N 2007. Analysis of large scale expression sequenced tags (ESTs) from the anural ascidian, Molgula tectiformis. Developmental Biology. 307:(2), 460–482. 10.1016/j.ydbio.2007.03.035 [DOI] [PubMed] [Google Scholar]
- Higgins DG, and Sharp PM 1988. CLUSTAL: A package for performing multiple sequence alignment on a microcomputer. Gene. 73(1): 237–244. 10.1016/0378-1119(88)90330-7 [DOI] [PubMed] [Google Scholar]
- Jeffery WR, and Swalla BJ 1992a. Evolution of alternate modes of development in ascidians. BioEssays. 14(4): 219–226. 10.1002/bies.950140404 [DOI] [PubMed] [Google Scholar]
- Jeffery WR, and Swalla BJ 1992b. Factors necessary for restoring an evolutionary change in an anural ascidian embryo. Developmental Biology. 153(2): 194–205. 10.1016/0012-1606(92)90105-P [DOI] [PubMed] [Google Scholar]
- Jeffery WR, and Swalla BJ 1993. An ankryin-like protein in ascidian eggs and its role in the evolution of direct development. Zygote (Cambridge). 1(3): 197–208. 10.1017/S0967199400001477 [DOI] [PubMed] [Google Scholar]
- Jeffery WR, Swalla BJ, Ewing N, and Kusakabe T 1999. Evolution of the ascidian anural larva: Evidence from embryos and molecules. Molecular Biology and Evolution. 16(5): 646–654. 10.1093/oxfordjournals.molbev.a026147 [DOI] [PubMed] [Google Scholar]
- Kusakabe T, Swalla BJ, Satoh N, and Jeffery WR 1996. Mechanism of an Evolutionary Change in Muscle Cell Differentiation in Ascidians with Different Modes of Development. Developmental Biology. 174(2): 379–392. 10.1006/dbio.1996.0082 [DOI] [PubMed] [Google Scholar]
- Li W, Yang W, and Wang X-J 2013. Pseudogenes: Pseudo or Real Functional Elements. Journal of Genetics and Genomics. 40(4): 171–177. 10.1016/j.jgg.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Lowe EK, Swalla BJ, and Brown C Titus. 2014. Evaluating a lightweight transcriptome assembly pipeline on two closely related ascidian species. PeerJ Preprints. 10.7287/peerj.preprints.505v1 [DOI] [Google Scholar]
- March CJ, Mosley B, Larsen A, Cerretti DP, Braedt G, Price V, Gillis S, Henney CS, Kronheim SR, Grabstein K, Conlon PJ, Hopp TP, and Cosman D 1985. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature (London). 315(6021): 41–647. 10.1038/315641a0 [DOI] [PubMed] [Google Scholar]
- Michaely P, and Bennett V 1992. The ANK repeat: A ubiquitous motif involved in macromolecular recognition. Trends in Cell Biology. 2(5): 127–129. 10.1016/0962-8924(92)90084-Z [DOI] [PubMed] [Google Scholar]
- Racioppi C, Valoroso MC, Coppola U, Lowe EK, Brown CT, Swalla BJ, Christiaen L, Stolfi A, and Ristoratore F 2017. Evolutionary loss of melanogenesis in the tunicate Molgula occulta. EvoDevo. 8(1): 11–11. 10.1186/s13227-017-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, and Coulson AR 1977. DNA sequencing with chain-terminating inhibitors. Proceedings of the National Academy of Sciences – PNAS. 74:(12), 5463–5467. 10.1073/pnas.74.12.5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley ER, Freeman MR, Ziegenfuss JS, Biswas R, Avery MA, Hong K, Sheehan AE, and Yeung Y-G 2008. Draper-dependent glial phagocytic activity is mediated by Src and Syk family kinase signalling. Nature. 453(7197): 935–939. 10.1038/nature06901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolfi A, Lowe EK, Racioppi C, Ristoratore F, Brown CT, Swalla BJ, and Christiaen L 2014. Divergent mechanisms regulate conserved cardiopharyngeal development and gene expression in distantly related ascidians. ELife. 3, e03728–e03728. 10.7554/eLife.03728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga H, Koyanagi M, Hoshiyama D, Ono K, Iwabe N, Kuma K, and Miyata T 1999. Extensive gene duplication in the early evolution of animals before the parazoan-eumetazoan split demonstrated by G proteins and protein tyrosine kinases from sponge and hydra. Journal of Molecular Evolution. 48(6): 646–653. 10.1007/PL00006508 [DOI] [PubMed] [Google Scholar]
- Swalla B 1996. Strategies for cloning developmental genes using closely related species. Pp. 197–208 in Molecular Zoology: Advances, Strategies and Protocols, Ferraris JD and Palumbi SR. Wiley-Liss, John Wiley and Sons, Inc. [Google Scholar]
- Swalla B 2004a. Procurement and Culture of Ascidian Embryos. Pp. 115–141 in Methods in Cell Biology: Experimental Analysis of the Development of Sea Urchins and Other Non-Vertebrate Deuterostomes, Ettenson C, Wessell G, and Wray G. Elsevier Science/Academic Press,. [DOI] [PubMed] [Google Scholar]
- Swalla B 2004b. Protochordate Gastrulation: Lancelets and Ascidians. Pp. 139–149 in C. Stern, Gastrulation. Cold Spring Harbor Press. [Google Scholar]
- Swalla BJ 1992. The role of maternal factors in ascidian larval muscle development. Seminars in Developmental Biology. 3(4): 287–295. [Google Scholar]
- Swalla BJ 1993. Mechanisms of gastrulation and tail formation in ascidians. Microscopy Research and Technique. 26(4): 274–284. 10.1002/jemt.1070260403 [DOI] [PubMed] [Google Scholar]
- Swalla BJ, Badgett MR, and Jeffery WR 1991. Identification of a cytoskeletal protein localized in the myoplasm of ascidian eggs: Localization is modified during anural development. Development (Cambridge). 111(2): 425–436. 10.1242/dev.111.2.425 [DOI] [PubMed] [Google Scholar]
- Swalla BJ, and Jeffery WR. 1990. Interspecific hybridization between an anural and urodele ascidian: Differential expression of urodele features suggests multiple mechanisms control anural development. Developmental Biology. 142(2): 319–334. 10.1016/0012-1606(90)90353-K [DOI] [PubMed] [Google Scholar]
- Swalla BJ, and Jeffery WR. 1996. Requirement of the Manx Gene for Expression of Chordate Features in a Tailless Ascidian Larva. Science (American Association for the Advancement of Science). 274: 1205–1208. 10.1126/science.274.5290.1205 [DOI] [PubMed] [Google Scholar]
- Swalla BJ, Just MA, Pederson EL, and Jeffery WR 1999. A multigene locus containing the Manx and bobcat genes is required for development of chordate features in the ascidian tadpole larva. Development (Cambridge). 126: 1643–1653. 10.1242/dev.126.8.1643 [DOI] [PubMed] [Google Scholar]
- Swalla BJ, Makabe KW, Satoh N, and Jeffery WR 1993. Novel genes expressed differentially in ascidians with alternate modes of development. Development (Cambridge). 119(2): 307–318. 10.1242/dev.119.2.307 [DOI] [PubMed] [Google Scholar]
- Swalla BJ, White ME, Zhou J, and Jeffery WR 1994. Heterochronic expression of an adult muscle actin gene during ascidian larval development. Developmental Genetics. 15(1): 51–63. 10.1002/dvg.1020150107 [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, and Kumar S 2011. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution. 28(10): 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassy O, Dauga D, Daian F, Sobral D, Robin F, Khoueiry P, Salgado D, Fox V, Caillol D, Schiappa R, Laporte B, Rios A, Luxardi G, Kusakabe T, Joly J-S, Darras S, Christiaen L, Contensin M, Auger H, … Lemaire P 2010. The ANISEED database: Digital representation, formalization, and elucidation of a chordate developmental program. Pp. 1459–1468 in Genome research 20(10). Cold Spring Harbor Laboratory Press. 10.1101/gr.108175.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran DH, and Berg CA 2003. Bullwinkle and shark regulate dorsal-appendage morphogenesis in Drosophila oogenesis. Development (Cambridge). 130(25): 6273–6282. 10.1242/dev.00854 [DOI] [PubMed] [Google Scholar]
- Zeng L, and Swalla BJ 2005. Molecular phylogeny of the protochordates: Chordate evolution. Canadian Journal of Zoology. 83(1): 24–33. 10.1139/z05-010 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequences for the Cymric gene were retrieved from ANISEED (http://www.aniseed.cnrs.fr/), a public database containing the genomes of various tunicate species. These sequences are also available in greater detail in the NCIB (https://www.ncbi.nlm.nih.gov/). The accession numbers are as follows: Styela clava XP_039270686.1, Phallusia mammillata CAB3267847.1, Ciona intestinalis XP_002121112.2, Molgula oculata OQ445879, Molgula occulta OQ445879, Molgula occidentalis OQ589865, Halocynthia rorezi OQ589866, Ciona savigny OQ589867.


