Abstract
» New knowledge about the molecular biology of fracture-healing provides opportunities for intervention and reduction of risk for specific phases that are affected by disease and medications.
» Modifiable and nonmodifiable risk factors can prolong healing, and the informed clinician should optimize each patient to provide the best chance for union.
» Techniques to monitor progression of fracture-healing have not changed substantially over time; new objective modalities are needed.
Background and Epidemiology
Skeletal injuries comprise an important socioeconomic burden in the United States, with 12 to 15 million fractures annually, resulting in lost wages and functional impairment until healing occurs1. While many fractures will heal successfully, up to 10% proceed to nonunion, a clinical state that causes additional morbidity, prolonged recovery, and expenses to the medical system2,3. Establishing a diagnosis of nonunion is difficult because the essence of nonunion is an inappropriate biologic response to pathology over the time course of normal fracture-healing. Prolonging interventions for nonunion can result in additional pain, disability, and uncertainty that imparts a burden on a patient’s quality of life.
Fracture care employs fundamental principles of stabilization to support mobilization and encourage immediate weight-bearing with many lower-extremity fractures. Fractures heal through 2 primary pathways, with the contribution of each depending on the strain profile and the biologic microenvironment at the fracture site. Primary bone-healing, or intramembranous ossification, occurs along the periosteal surface adjacent to the fracture and across minimal fracture gaps when absolute stability of <2% of mechanical strain is achieved4. The process of intramembranous ossification was originally described by Robert Danis as “self-welding”5 to describe haversian remodeling and migration of lamellar bone across these compressed stable fractures with a minimal gap6. Secondary bone-healing, or endochondral ossification, is the predominant form of healing in the majority of diaphyseal fractures. While intramembranous repair still occurs to seal off the ends of the bone and along the periosteal surfaces of the bone adjacent to the gap, a cartilaginous callus forms within the fracture gap, and the process of endochondral ossification describes mineralization and remodeling of the cartilage into bone. The cartilaginous callus thus aims to provide intermediate stability across the fracture gap during bone healing; John Charnley stated that “nature has thus done its own internal fixation.”7 Multiple microenvironmental factors drive osteochondral progenitors to a chondrogenic fate, including increased micromotion at the fracture site associated with larger gap size and mechanical strains between 2% and 10%, decreased oxygen tension, and lack of vascularization8,9. In order for bone to successfully heal, a series of properly timed molecular interactions must occur at the fracture site and in concert with systemic biology. Understanding biologic contributions and how they are affected by various risk factors can help guide individual treatment plans for patients and better predict the risk of nonunion. This review will focus on diaphyseal endochondral ossification and describe the phases of healing along with the perturbations and the opportunities for augmentation of each phase.
Phases of Bone-Healing
Inflammatory Phase
The inflammatory phase is classically considered the first phase of fracture-healing. At the time of fracture, intracortical, endosteal, and periosteal vessels are sheared, producing a fibrin-rich hematoma. Evacuation of the hematoma at 4 to 7 days after fracture or repeated irrigation and debridement of the fracture site can delay bone-healing, but is often necessary to debride devitalized tissue in order to reduce the risk of infection10,11. Chemokines that are released from activated platelets within the hematoma promote the migration of macrophages and neutrophils to the fracture12 (Fig. 1). These cells debride devitalized tissue and promote the recruitment of inflammatory cells through the release of proinflammatory cytokines that include tumor necrosis factor-alpha (TNF-α) and interleukins-1 and 6 (IL-1 and IL-6)13–16 (Fig. 2). This proinflammatory phase is important for the recruitment of progenitor cells that originate from the periosteum, the bone marrow, the soft tissue, and the systemic circulation. Proliferation and differentiation of these progenitor cells is modulated by the release of growth factors that include bone morphogenetic proteins (BMPs), transforming growth factor-beta (TGF-β), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and insulin-like growth factor (IGF) from the inflammatory cells13–16.
Fig. 1.
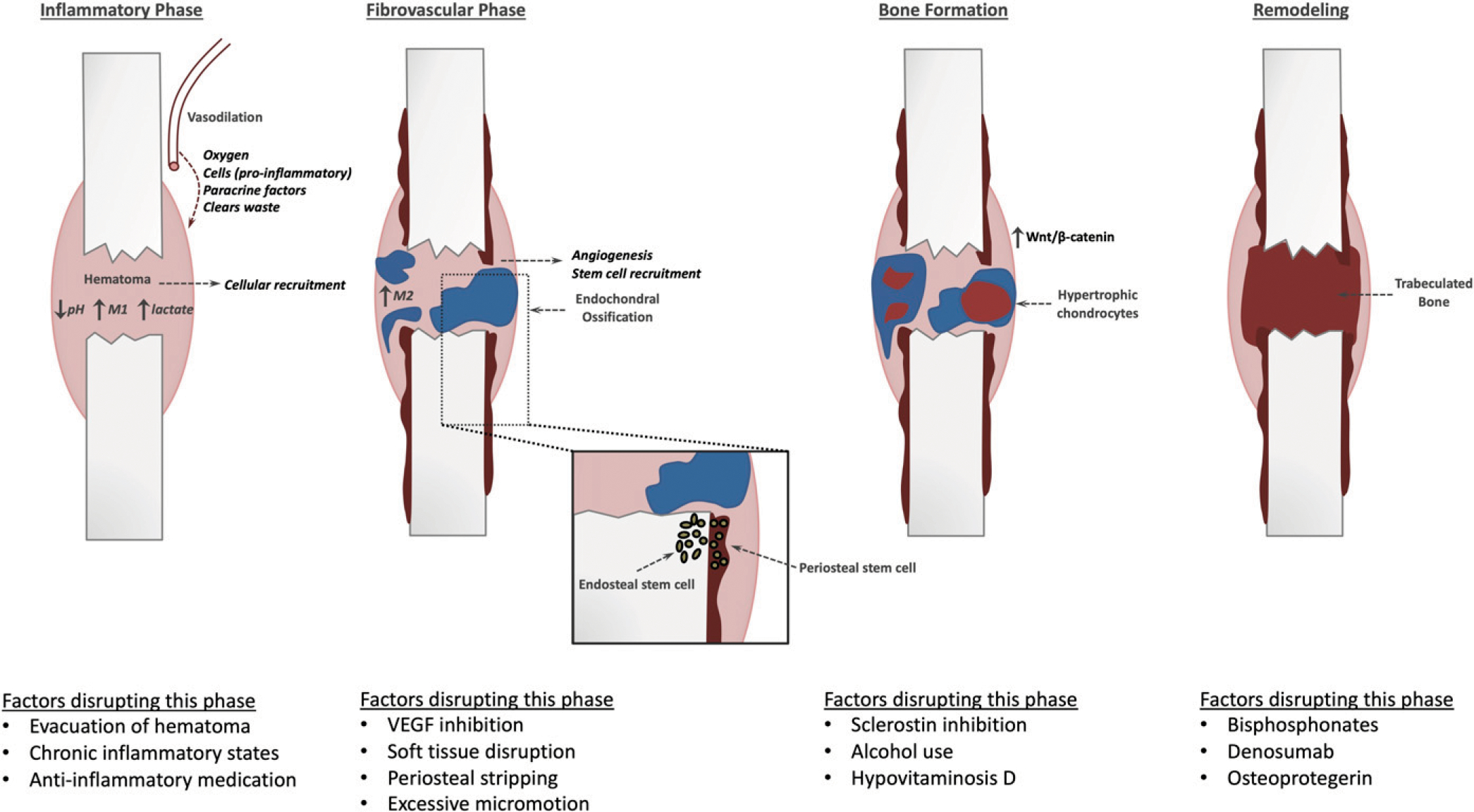
The key steps of endochondral ossification during fracture-healing and examples of common perturbations that disrupt the respective phases. VEGF 5 vascular endothelial growth factor. (Reproduced, with modification, from: Bahney CS, Hu DP, Miclau T III, Marcucio RS. The multifaceted role of the vasculature in endochondral fracture repair. Front Endocrinol [Lausanne]. 2015;6:4, under Open Access License CC BY 4.0.)
Fig. 2.
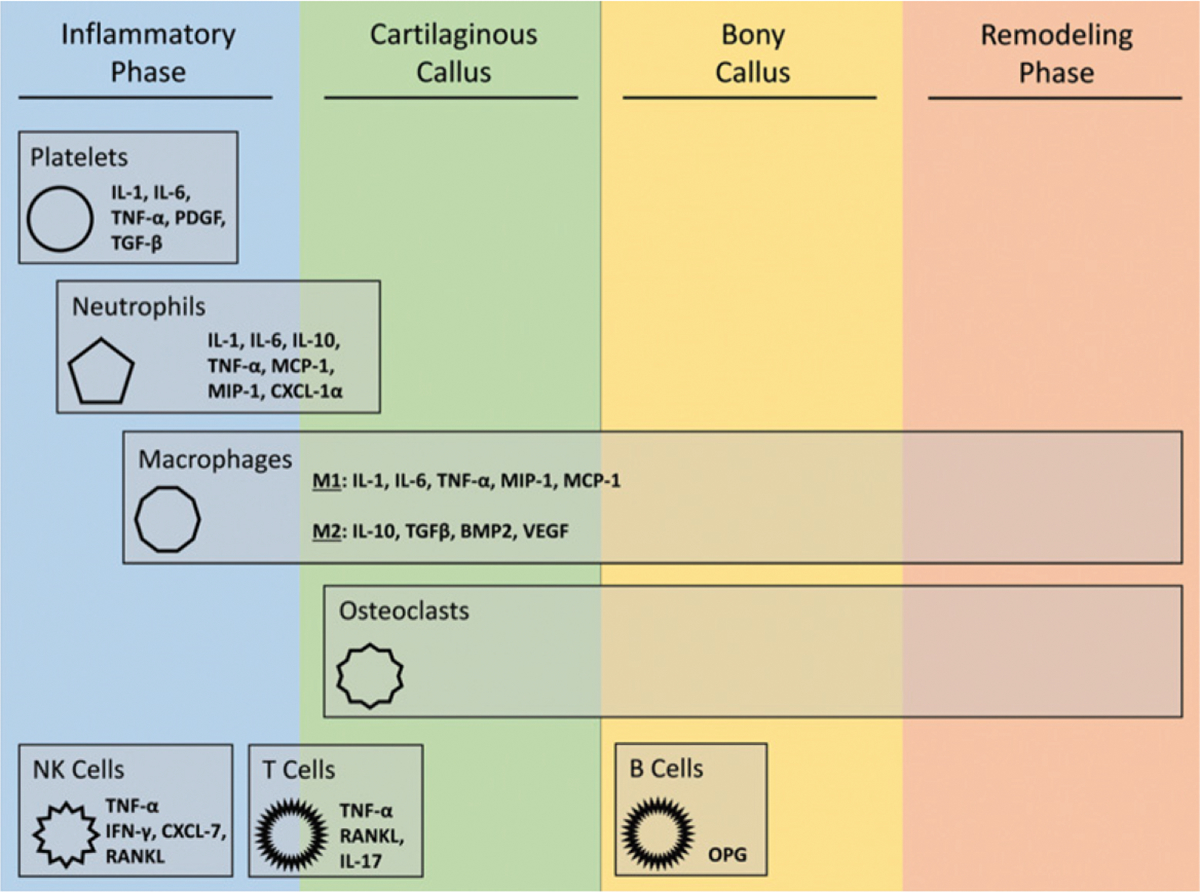
The role of immune cells during fracture repair14. Bone fracture-healing can be viewed as a 4-stage process. Immune cells play important roles throughout this process; however, a majority of their activity occurs during the early stages of fracture-healing. IL = interleukin, TNF-α = tumor necrosis factor-alpha, PDGF = platelet-derived growth factor, TGF-β = transforming growth factor-beta, MCP1 = monocyte chemoattractant protein-1, MIP-1 = macrophage inflammatory protein-1, CXCL = C-terminal crosslinking telopeptide of type-I collagen, BMP2 = bone morphogenetic protein-2, VEGF = vascular endothelial growth factor, NK = natural killer, IFN-γ = interferon gamma, RANKL = receptor activator of nuclear factor-κB ligand, and OPG = osteo-protegerin. (Reproduced from: Baht GS, Vi L, Alman BA. The role of the immune cells in fracture healing. Curr Osteoporos Rep. 2018 Apr;16[2]:138–45, under Creative Commons Attribution 4.0 International License.)
Careful cellular resolution of the inflammatory phase, through a shift from a proinflammatory macrophage (M1)-dominant state to the pro-reparative anti-inflammatory macrophage phenotype (M2), is critical to ensure proper healing17–19. This process is regulated through a complex positive feedback loop such that depletion of the macrophages or disruption of the initial proinflammatory signaling pathways will delay proper healing9. However, subsequent modulation of the inflammatory state to promote an M2 phenotype may be a potential target for augmentation of bone-healing. Stem cells are known to have a powerful anti-inflammatory secretome that likely plays a role in the resolution of inflammation. The biochemical milieu also influences macrophage polarity, and recent work suggests that endogenous or exogenous BMPs at the fracture site can promote M2 differentiation20–22.
Systemic inflammatory conditions, such as diabetes mellitus and rheumatoid arthritis, exhibit prolonged healing responses and elevation of inflammatory cytokines at the fracture site, suggesting that excessive inflammation, at least partially, contributes to delayed repair23–25. Failure to resolve inflammation in these states may impair later healing processes, including angiogenesis, osteoclast recruitment, and deposition of bone; however, the exact mechanisms of chronic inflammation that cause delayed bone-healing are not completely understood26.
Debate exists regarding the effects of anti-inflammatory medication on fracture-healing. Prostaglandins released from inflammatory cells have a multitude of positive effects on subsequent phases of fracture-healing to promote bone formation27. Animal studies have demonstrated that cyclooxygenase-2 (COX-2) inhibition with nonsteroidal anti-inflammatory drugs (NSAIDs) or gene knockout will impair healing. Decreased expression of COX-2 has been observed in the fractures of older mice, which may partially explain prolonged healing with aging28. Limited human data suggest that there may be a dose-dependent relationship with prolonged NSAID use, but data currently remain inconclusive29–32. Similarly, long-term corticosteroid use can delay bone-healing, but through more complex mechanisms than just the anti-inflammatory effects33–35. There exists a growing body of literature that NSAID use at clinically relevant dosing likely does not impair fracture-healing, and this supports the use of NSAIDs in the perioperative period without conferring additional nonunion risk36–40. NSAIDs should be used judiciously in patients with suspected risk factors for nonunion to avoid a potential compounding effect41.
Fibrovascular Phase
The next phase of healing is directed toward angiogenesis, stem cell recruitment, proliferation, and differentiation. The process of angiogenesis is critical during fracture repair as it provides a source of cells, oxygen, nutrients, and waste removal for the healing tissue. Angiogenesis relies on multiple signaling molecules and their receptors, most notably vascular endothelial growth factor (VEGF)9,42. Many animal studies have implicated hypoxia and poor blood flow as direct causes of delayed union, and blockade of the VEGF receptor with bevacizumab has directly delayed healing43–45. Diseases associated with delayed healing, including diabetes mellitus, demonstrate decreased levels of VEGF and angiogenesis, which can be reversed by treatment with TNF-α inhibitors46. Interestingly, atrophic nonunion tissue has a similar content of VEGF expression and vascularity to normal healing tissue; thus, it is likely that an unbalanced ratio between vasculogenic and osteogenic factors contributes to impaired healing47,48.
The osteochondral progenitor cells that give rise to the fracture callus are recruited locally from the bone (the endosteum and the periosteum), the surrounding soft tissue, the bone marrow, and the circulation49–52. Periosteal and endosteal stem cells within the bone both make a contribution to the fracture callus53,54. As such, the functional consequence of surgical techniques that disrupt either of these populations should be considered. Aggressive soft-tissue handling, including periosteal stripping and muscular manipulation, impairs fracture-healing by altering blood supply, but it is likely that evacuation of stem cells is at least partially responsible as well51,55. Although intramedullary reaming has been shown to disrupt valuable endosteal blood supply, the Study to Prospectively Evaluate Reamed Intramedullary Nails with Tibial Fractures (SPRINT) trial prospectively demonstrated that reaming for tibial nails may instead decrease the rate of nonunion56,57. Interestingly, intramedullary reaming also increases marrow progenitor cells at the fracture site and reduces inflammatory cytokines, effectively pushing the progression of healing to the fibrovascular phase on postoperative day zero58. In the setting of hypertrophic nonunion, undifferentiated stem cells contained within the fracture callus maintain their ability to differentiate to osteogenic or chondrogenic cells, which may offer a target for nonunion intervention59.
Bone Formation
In addition to the direct differentiation of progenitor cells to bone during intramembranous bone repair, chondrocytes within the fracture gap give rise directly to bone through endochondral repair60 (Fig. 3). Conversion of cartilage to bone begins with proliferation and hypertrophic maturation of the chondrocytes in order to create a temporary bridge across the bone gap61. These hypertrophic chondrocytes are highly bioactive and secrete angiogenic factors, including VEGF, PDGF, and placental growth factor (PlGF), along with nerve growth factor (NGF), to recruit the neurovascular bundle into the avascular, aneural cartilage anlagen62,63. Vascular invasion causes mineralization of the cartilage matrix through secretion of the osteogenic promoters BMP and Wnt60,64–67. At this point, the unique cellular and mechanical microenvironment seems to support phenotypic plasticity within the chondrocytes so that they can either become osteoblasts that form the new hard callus, undergo apoptosis to form the marrow cavity, or potentially dedifferentiate to give rise to osteochondral progenitors in the bone-lining tissue through a conserved process that recently has been defined as palingenesis60,68,69.
Fig. 3.
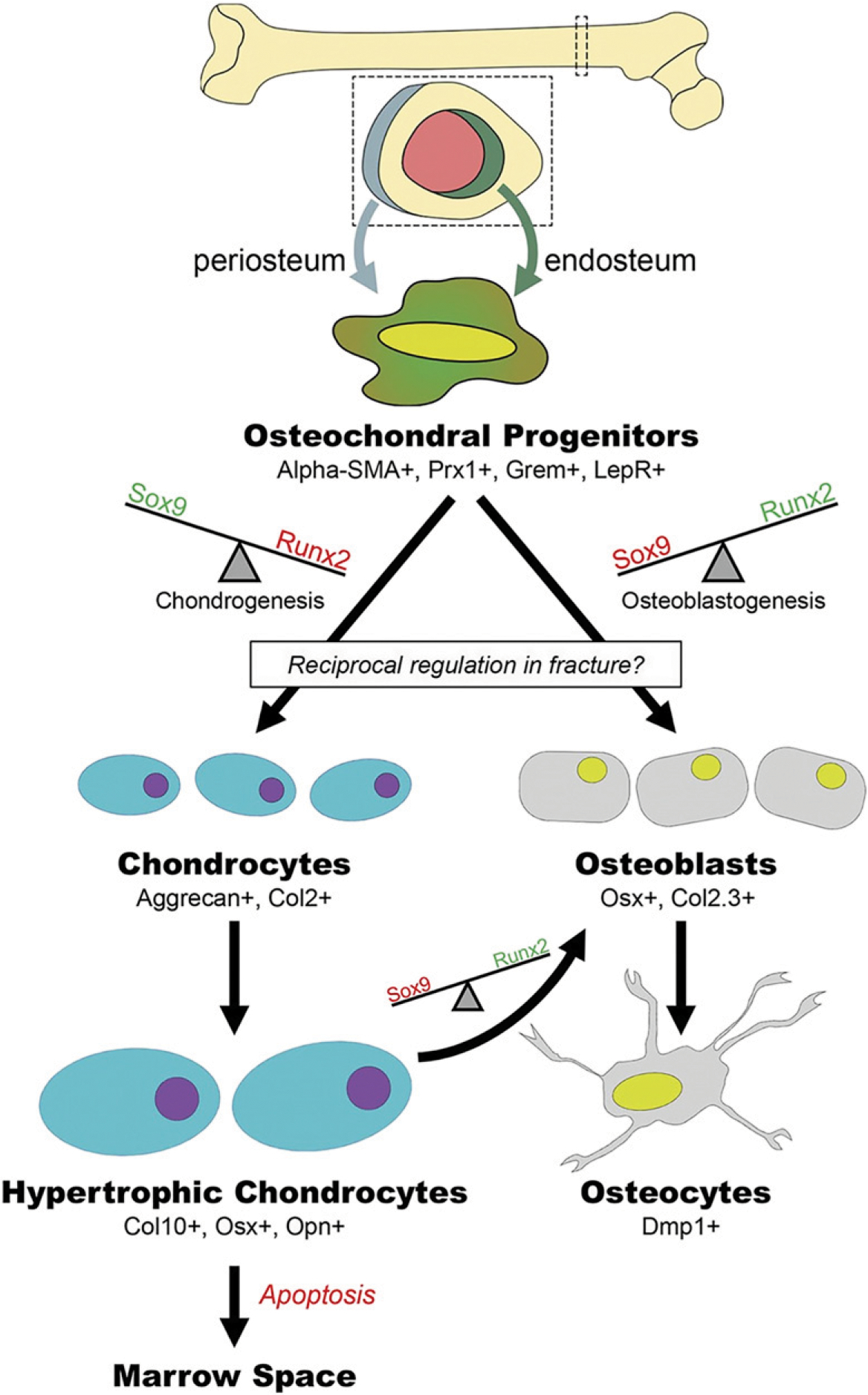
Chondrocyte to osteoblast transformation. Alpha-SMA = alpha smooth muscle actin. (Reproduced from: Bahney CS, Zondervan RL, Allison P, Theologis A, Ashley JW, Ahn J, Miclau T, Marcucio RS, Hankenson KD. Cellular biology of fracture healing. J Orthop Res. 2019 Jan;37[1]:35–50.)
Clinical opportunities to augment fracture-healing have focused on 3 critical molecular pathways that are known to regulate chondrogenesis and osteogenesis at various phases of repair: BMPs, Wnt/β-catenin, and parathyroid hormone (PTH) derivatives. BMPs are canonical osteogenic proteins that are required for effective fracture repair and are expressed by inflammatory cells, vascular endothelial cells, and muscle stem cells to modulate to fracture repair65,66,70–72. Interestingly, BMPs play an important role in both intramembranous and endochondral fracture-healing. This potent osteogenic effect has led to the development of BMP-2 as a therapeutic agent. While predominantly approved for use in spinal fusion, BMP-2 does have U.S. Food and Drug Administration (FDA) approval for the narrow indication window of surgical implantation involving acute open tibial shaft fractures that are stabilized with an intramedullary nail and treated within 14 days of the initial injury. Off-label application has been used to treat other types of fractures, and clinical evidence for efficacy was nicely summarized by Nauth et al.73 (Fig. 4). This success is balanced by the high product costs and reports of severe side effects, including heterotopic ossification and cancer74.
Fig. 4.
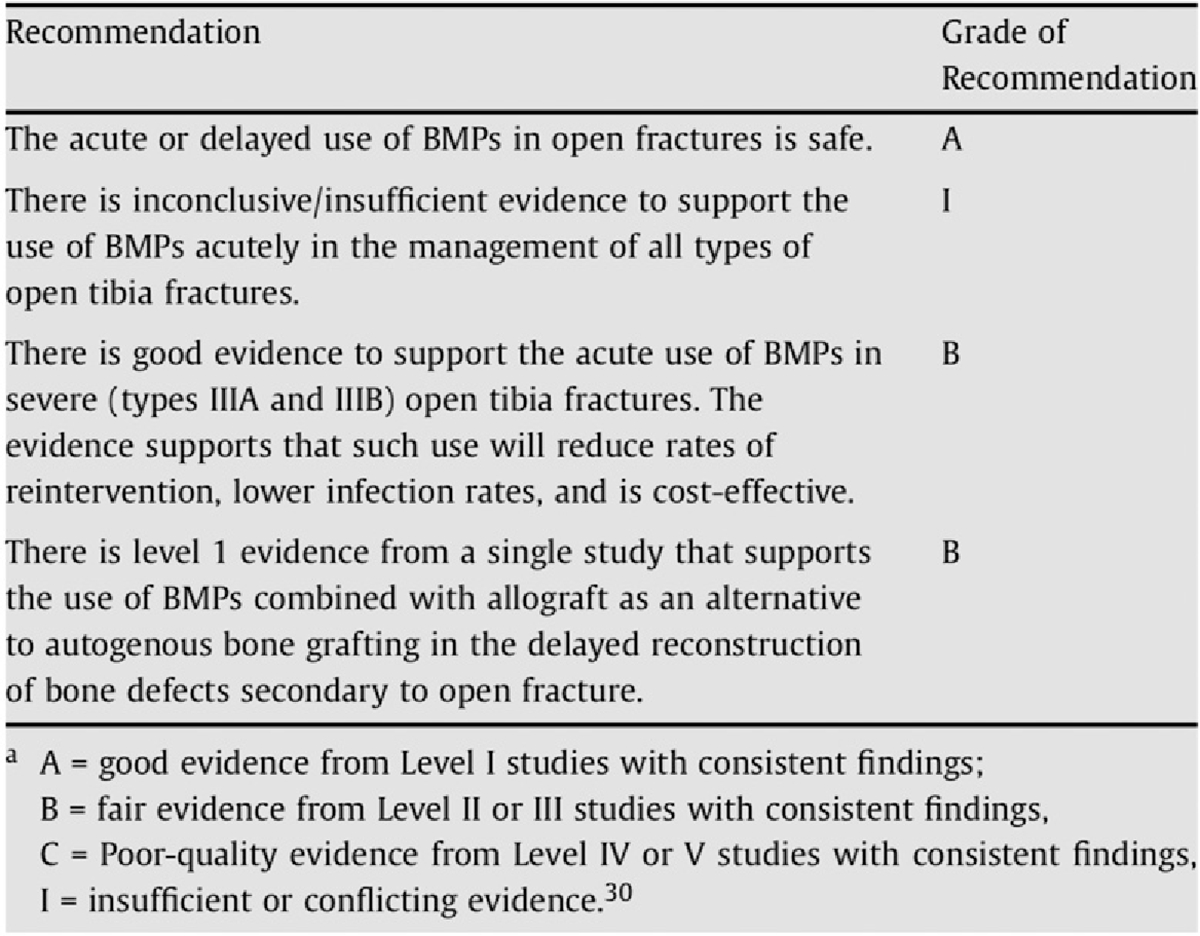
Summary of grades of recommendation for BMP use in open fractures73. (Reprinted from: Injury 40[Suppl 3], Nauth A, Ristiniemi J, McKee MD, Schemitsch EH. Bone morphogenetic proteins in open fractures: past, present, and future, p S27–31, Copyright 2009, with permission from Elsevier.)
Similarly, Wnt/β-catenin signaling has a well-established osteogenic role and is important during both the intramembranous and endochondral phases of fracture-healing75. Dysregulation of this pathway can contribute to impaired fracture-healing and has been associated with age-related delayed healing76. Binge alcohol exposure prior to fracture has been shown to decrease β-catenin levels in mice and may contribute to poor healing outcomes that are associated with alcohol abuse77. Conversely, therapeutic activation is being explored as a novel pathway to promote healing. Interestingly, activation of Wnt/β-catenin by low-dose lithium administration improves callus mineralization and torsional strength and can attenuate the effects of alcohol exposure78,79. More recently, there has been clinical interest in the use of the romosozumab antibody that blocks the Wnt/β-catenin inhibitor sclerostin in order to indirectly increase signaling. While early evidence showed enhanced bone mass and strength after fracture in animal studies, follow-up studies have not demonstrated the same efficacy in large human trials80–85.
Teriparatide and abaloparatide are promising PTH and PTH-related protein (PTHrP) analog drugs that may be able to enhance fracture-healing86. PTH and PTHrP function to maintain calcium homeostasis via a negative feedback loop and promote normal cartilage maturation during endochondral ossification. Animal studies clearly show that intermittent PTH therapy promotes fracture-healing and suggest a strong therapeutic potential of PTH87. Mechanistically, PTH therapies result in proliferation of the fracture callus progenitors and enhanced soft callus formation that ultimately lead to accelerated union rates and increased mechanical strength due to improved bone mineral quality88–93. Clinical studies offer a less certain view of efficacy, partly due to variability in doses, fracture sites, and patient age, with much more limited data comparing the efficacy of teriparatide and abaloparatide86,87,94. Future studies will be necessary to further delineate the therapeutic potential of PTH/PTHrP analogs for fracture-healing in humans.
Widespread prevalence of hypovitaminosis D raises concern for delays in fracture-healing95. Vitamin D has been implicated in every stage of fracture-healing, but most importantly during the mineralization phase96. Although deficiency is associated with nonunion, decreased bone turnover, and increased fracture risk, there is mixed evidence as to the clinical importance of vitamin D as a causal factor for nonunion97,98. Supplementation in fracture cases can increase bone mineral density and callus area; however, no universal guidelines for vitamin D supplementation exist99. A recent survey among members of the Orthopaedic Trauma Association (OTA) and the Canadian Orthopaedic Trauma Association (COTA) established that the most common dosing strategies in fracture patients were 1,000 IU daily (14.6%) and 2,000 IU daily (13.4%) among fellowship-trained traumatologists, demonstrating little to no agreement99. A systematic review concluded that while vitamin D supplementation clearly increases 25-hydroxyvitamin D (25[OH]D) serum levels, no studies exist that definitively demonstrate that this impacts fracture-healing100.
Remodeling
Hard callus remodeling is the final phase of fracturere repair and can continue for several years. Haversian remodeling is orchestrated in part by osteoclasts to exchange woven to lamellar bone. Osteoporosis medications, such as bisphosphonates or the RANKL (receptor activator of nuclear factor-κB ligand) inhibitors denosumab and osteoprotegerin, delay remodeling in animal studies and cause changes in the material properties of fracture callus, including increased strength and decreased ultimate stress101,102. Meta-analyses of human studies have not demonstrated a delay in healing with bisphosphonate use; thus, many recommend the continuation of osteoporosis medication after a fracture because the risk of secondary fracture outweighs the potential consequence for fracture-healing103–105. There is a known phenomenon with prolonged bisphosphonate use that results in deranged remodeling, leading to osteonecrosis of the jaw and stress fractures, most notably in the subtrochanteric femoral region. These occur in <5% of bisphosphonate users and have proven difficult to heal106. The American Society for Bone and Mineral Research recommends reevaluation after 3 to 5 years of use of bisphosphonates to minimize these complications107.
Defining Nonunion
Disruption or failure of the normal cascade of fracture-healing will result in bone nonunion. The FDA defines a nonunion as any fracture that is 9 months old and has not shown evidence of fracture-healing in 3 months108. Clinicians utilize subjective patient reports, serial physical examinations, and radiographic evidence of mineralization across the fracture site to help determine union; however, the fracture-healing timeline and definitions are not universally agreed upon in the orthopaedic community109,110. This variability also exists in the European community, with even less consensus between orthopaedic and trauma surgeons who both treat fracture nonunions111.
Nonunions can be classified as hypertrophic, atrophic, or oligotrophic. Hypertrophic nonunions form a soft callus that fails to convert to bone due to excessive micromotion at the fracture site from lack of proper stabilization. The fracture callus is typically enlarged on radiographs and is unstable to weight-bearing. Atrophic nonunions present because of inadequate formation of bone in the callus, and often are attributable to inadequate micromotion at the fracture site and/or a suboptimal local or systemic healing environment112. Atrophic nonunions were originally thought to be formed by poor vascular flow to the fracture site. Recent studies have shown that atrophic non-union tissue contains similar vascular density to hypertrophic nonunions and normally healing bone, although the role of vascular contribution is not completely understood113. Oligotrophic nonunions fit neither criterion and are not hypertrophic in their tissue response, nor are they avascular; they often occur with inappropriate alignment and proximity of the fracture fragments. Both atrophic and hypertrophic tissue retain the ability to differentiate into osteogenic, chondrogenic, and adipogenic cells in vitro; however, atrophic tissue has much less osteogenic potential and greater senescence, and it can impair the differentiation of surrounding cells59,114.
Risk Factors for Developing Nonunion
Nonmodifiable Risk Factors
Injury severity is the strongest predictor of nonunion. In a nationwide study of 309,330 patients, the number of fractures healing at 1 time point was most predictive for nonunion, with an odds ratio of 2.6540. A systematic review by Santolini et al. found that an open fracture and a need for open reduction were the variables with the highest level of evidence115. Aside from osseous involvement, soft-tissue injury produces a compromised local environment that impairs bone-healing116,117. Soft-tissue injury that is severe enough to warrant flap coverage or fasciotomy for compartment syndrome can increase the risk of nonunion up to 20%118,119. Additional wound complications, including infections, can compromise fracture-healing120, although an in-depth discussion of septic nonunion is outside the scope of this article. Concomitant thoracic and hemorrhagic injuries are common in polytrauma fracture cases. Well-controlled animal studies have demonstrated an enhanced inflammatory response and impaired bone and callus formation after fracture with hemorrhagic shock121–123. Subsequent resuscitation produces a larger callus size and a prolonged remodeling phase, suggesting that resuscitation may confer additional benefits for trauma patients in these cases124. Interestingly, traumatic brain injury is observed to enhance fracture-healing in patients with polytrauma, although the neuro-inflammatory mechanisms of this phenomenon are not completely understood125. Recent controlled animal studies demonstrated that healing responses and serum inflammatory profiles were more robust when fractures occurred contralateral to brain injury, suggesting the importance of neuronal crossover in modulating healing126.
The impact of age on fracture-healing is not well understood. Elderly patients have an elevated risk of sustaining a fracture, and healing may progress more slowly than in younger patients, perhaps because of osteoporosis127. Age has been demonstrated as an independent risk factor for clavicle nonunion in patients between the ages of 20 and 46 years128. However, examination of >47,000 Medicare patients revealed that more elderly (>75-year-old) patients have a surprisingly lower rate of nonunion compared with their younger (<69-year-old) counterparts, suggesting that other factors such as mobility or injury severity may have primacy129. Although there are no remarkable differences in the relative expression of genes or the ratio of cartilage to bone in fracture callus of elderly mice, overall callus size is decreased130. These findings are likely due to inflammatory response derangement, decreased vascularity, and poor proliferation and differentiation of cells131–134. Although the molecular and cellular mechanisms for delayed bone-healing in older patients are not fully understood, additional research has begun to address this complex issue135–138.
Modifiable Risk Factors
Systemic factors impact bone-healing, and patients must optimize modifiable behaviors to decrease the risk of non-union (Fig. 5). Obesity alone has not been shown to be an independent risk factor for fracture-healing in human studies, but animal studies have demonstrated that obesity increases risk of delay in union and is associated with an exaggerated inflammatory response after fracture139–142. Diabetes is a common comorbidity of obesity and prolongs bone-healing through impaired vascularization, immune dysregulation, and poor callus mineralization143. Patients with diabetes have demonstrated higher rates of nonunion after ankle fractures, whether treated operatively or non-operatively, and this phenomenon remains uninvestigated in other injuries144,145. In animal studies, administration of insulin locally can help rescue this phenotype and return fractures to a normal healing trajectory139,140,146–148. Interestingly, obese patients can be malnourished, resulting in metabolic derangements that delay fracture-healing149. The correction of metabolic or endocrine abnormalities should be addressed in every patient as it can be sufficient to promote union in patients with nonunion150,151.
Fig. 5.
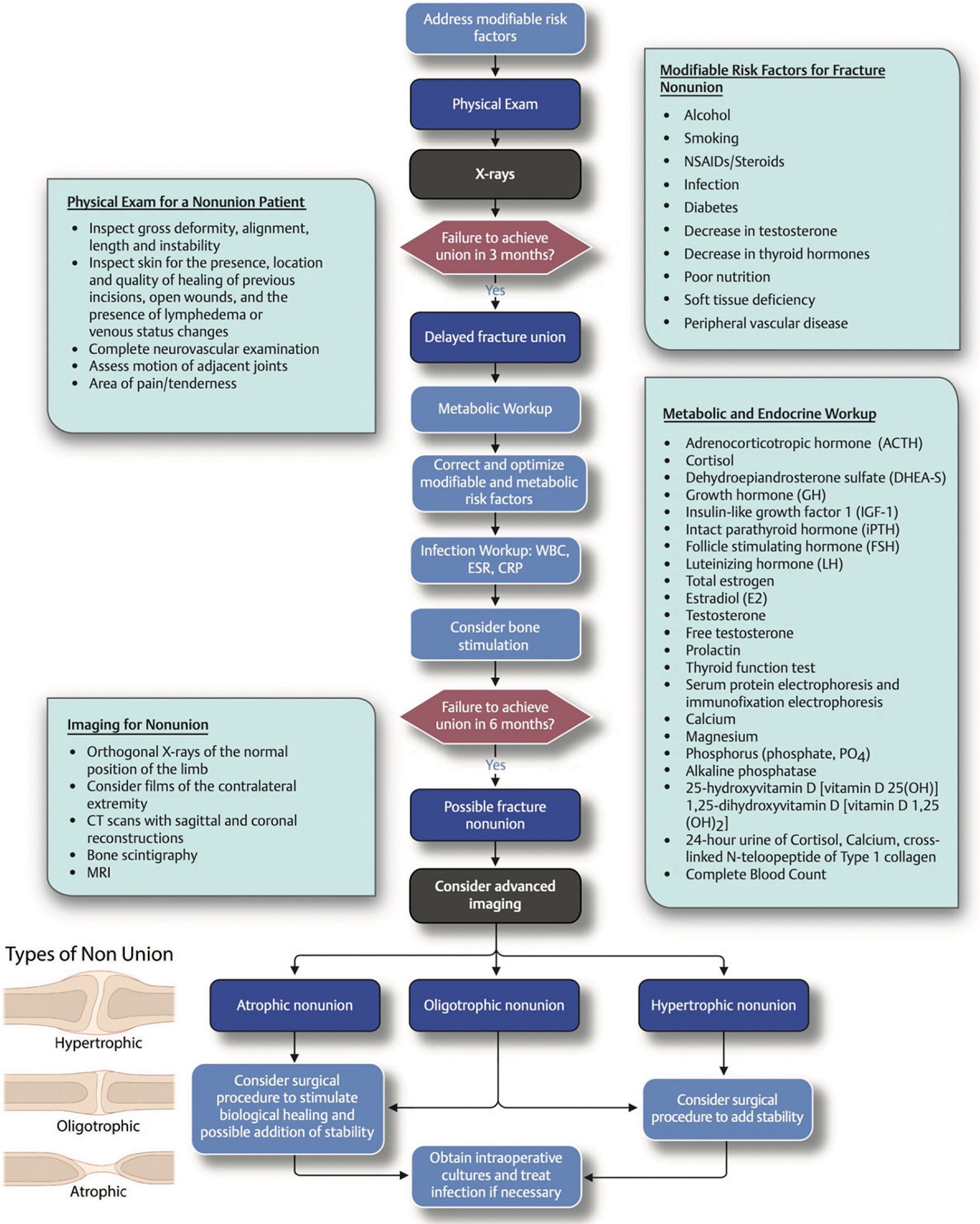
Modifiable and nonmodifiable risk factors that may lead to the development of nonunion. WBC = white blood-cell count, ESR = erythrocyte sedimentation rate, CRP = C-reactive protein level, CT = computed tomography, MRI = magnetic resonance imaging, and NSAIDs = nonsteroidal anti-inflammatory drugs. (Reproduced, with modification, with permission of Thieme Publishers, from: Miclau T. Fracture delayed and nonunion. In: Marmor MT. Decision making in orthopaedic trauma. Thieme NY; 2017. p 154–5.)
Smoking status is the lifestyle factor with the greatest amount of evidence that is correlated with risk of nonunion115. In a systematic review, Pearson et al. found that smokers have a 2.2-fold higher risk of developing a nonunion and display prolonged healing times after nonunion surgery152. Others have correlated this latter finding with greater amounts of pain as well as disability153. Among a variety of other perturbations in the healing cascade, inhibition of stem cell migration by nicotine is at least partially responsible for this delay154. Smoking cessation is arguably the most important intervention a patient can undergo to decrease the risk of nonunion after fracture. In smokers, cessation for the first 6 weeks after fracture surgery has been shown to significantly decrease the risk of postoperative complications155.
Many medications interact with fracture biology; however, few are more widely utilized in the fracture setting for pain management than opioids and NSAIDs. The effects of NSAIDs have long been investigated, but few studies have investigated the risk of nonunion with opioid use. The epidemic of opioid abuse has brought to light the dangers of overprescribing; however, opioids are still an effective pain medication156. In a retrospective review of 309,330 fractures, opioid use was associated with prolonged healing, even when controlling for age, sex, number of fractures, and smoking status, and the risk of nonunion was almost double for chronic opioid users157. Animal studies demonstrated that those that were treated with opioids had smaller callus volume and delayed maturation of callus after 8 weeks compared with healthy controls158. There are little human data on the causal relationship between opioid use and nonunion; perhaps increased cultural acceptance of multimodal analgesia will allow for comparative studies.
Military Extremity Trauma
Ongoing military conflicts have led to an increased incidence of combat-related traumatic injuries159,160. Over 75% of modern war injuries involve the extremities, with contaminated open fractures and bone and tissue loss from explosive devices160,161. Complications in fracture-healing, such as delayed union or nonunion, are estimated to occur in approximately 10% to 20% of normal civilian injuries. In contrast, nonunion rates as high as 50% have been reported at 1 year after injury for open tibial fractures that were sustained during combat, in part due to an increased occurrence of infection162,163. Unfortunately, a return-to-duty rate of only 22% is reported for American soldiers with isolated type-III open tibial fractures, which is less than half of the return-to-work rate for similarly severe injuries in the civilian population164,165. These injuries also lead to emotional and psychiatric dysfunction, and less than half of soldiers sustaining high-energy extremity trauma ever resume civilian employment161.
Monitoring Fracture-Healing
The measurement of fracture-healing remains an unsolved clinical problem; there is no universal consensus on the best method to make this assessment in a quantitative fashion166–176.
Clinical Assessment
Clinical assessment is commonly relied upon as a gross measurement of fracture-healing, despite poor reliability and subjectivity177. The most common assessment methods are presence of pain, tenderness with palpation, and ability to bear weight on the affected limb178. Patient-reported scoring systems reflect functional capabilities and restrictions but are unable to directly assess fracture biology. In low-resource settings, such as in low and middle-income countries, clinical judgment is sometimes the only available method179. New and simple clinical instruments, such as the squat-and-smile test, may provide outcome predictions with strong interrater reliability180.
Imaging
Serial radiography is the most common method to track fracture-healing over time, but this method is subjective and fallible. Experts blinded to other clinical information struggle to gauge healing status and can change their opinion in 40% of cases when unblinded181–183. Opinion varies between radiologists and orthopaedic surgeons analyzing radiographs; the use of standardized scoring systems can help decrease this discrepancy109,181. The Radiographic Union Score for Tibial Fractures (RUST) was developed in 2010 to standardize qualitative radiographic scoring166. The RUST score grades callus progression on 4 cortices and correlates with callus strength and rigidity. In a large validation study, 90% of orthopaedic traumatologists agreed that a score of 10 on the RUST and 13 on the modified RUST should correlate with union167. The ability to objectively and qualitatively measure fracture-healing is an improvement over subjective opinion, increasing intraobserver reliability. Similar methodology can be used in metadiaphyseal tibial, hip, and distal radial fractures168,184. Evidence of cortical bridging and use of these scoring systems demonstrate strong interrater reliability169,185,186. The Nonunion Risk Determination tool is a predictive model that is based on 7 factors that are associated with tibial nonunion, primarily depending on a strong association of patient health and the severity of injury with nonunion170.
Radiographic scoring systems remain indirect assessments of fracture biology and are limited in their efficacy. Assessment is difficult during the initial phases of fracture-healing before mineralization, as well as when implants obscure the view of the fracture site171. New computerized algorithms can predict stages of healing and mechanical strength based on radiographs, which may help improve reliability187. Other imaging modalities can be helpful to determine if a fracture has failed to heal, including computed tomography (CT), ultrasound, and nuclear medicine; however, these modalities carry their own risks and are not commonly used to monitor early fracture-healing172,188. Implantable smart devices, such as plates and intramedullary nails, are being developed to provide telemetric information of strain through a fracture189,190. These devices allow for live assessment of biomechanical properties during the formation of soft callus and conversion to bone and are correlated with histologic and micro-CT data of bone-healing in preclinical models191.
Serologic Markers
A serum biomarker could allow for the quantification of fracture-healing. Identification of a marker with stable basal levels in circulation and perturbation specific to only fracture-healing has yet to be definitively established. Many have been investigated but are nonspecific and are involved in immunologic cascades, growth factors, markers of bone turnover, and cellular signaling molecules173–175. Many vary by age, sex, and metabolic or endocrine derangement, which is found in up to 84% of patients with nonunion, limiting their use as a specific biomarker150. Unsuccessful attempts have been made to correlate TGF-β with fracture-healing192. Proteins associated with osteoclast activity such as tartrate-resistant acid phosphatase 5b and C-terminal cross-linking telopeptide of type-I collagen have shown greater promise to evaluate nonunion, but they are not useful in the early phases to predict a healing trajectory193. The use of many biomarkers consecutively in a predictive algorithm is potentially more optimal. A recent proteomic study demonstrated time-dependent changes in 850 proteins, which could be clustered throughout phases of fracture-healing to yield 50 candidate biomarkers194. At this time, it is not feasible to characterize the proteome of every patient with a fracture at every visit, but these data provide a map to further investigate combinations that can guide a predictive model of fracture-healing.
One promising candidate that is specific for cartilage to bone conversion has emerged: collagen X. This extracellular matrix protein is synthesized by hypertrophic chondrocytes during endochondral fracture-healing and is associated with vascularization and mineralization of the cartilaginous callus195. Recently, a repeatable and reliable assay has been validated for the measurement of a collagen X degradation fragment from serum (“CXM”)196. This biomarker was tested primarily in the skeletally immature in order to predict growth trajectories, but a small series with 3 patients showed strong correlation between CXM and progression of fracture-healing196. Preclinical data demonstrated that this collagen X biomarker correlates with normal fracture-healing in male and female mice and that the kinetics of the biomarker appropriately correlated with gene expression and histomorphometric quantification of fracture callus composition176. Clinically, this promising avenue remains in development.
Overview
Current definitions and assessment of union are inconsistent and vary based on expert opinion. There exists a clinical need for a superior assessment method to quantify fracture-healing and define union. The biology of fracture-healing compels us to optimize modifiable patient factors to provide the best opportunity for union, which is best achieved through local and systemic biology working in conjunction with biomechanical stability. Circulating biomarkers are appealing future targets for clinical development to track progress, but to date, none have been validated or are specific for fractures. Additional research is required to identify an accurate and reliable measurement of union.
Footnotes
Disclosure: The authors indicated that no external funding was received for any aspect of this work. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work; “yes” to indicate that the author had a patent and/or copyright, planned, pending, or issued, broadly relevant to this work; and “yes” to indicate that the author had other relationships or activities that could be perceived to influence, or have the potential to influence, what was written in this work (http://links.lww.com/JBJSREV/A629).
References
- 1. https://www.boneandjointburden.org/2014-report/via23/fracture-trends .
- 2.Antonova E, Le TK, Burge R, Mershon J. Tibia shaft fractures: costly burden of nonunions. BMC Musculoskelet Disord. 2013. Jan 26;14(42):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calori GM, Mazza E, Colombo M, Ripamonti C, Tagliabue L. Treatment of long bone non-unions with polytherapy: indications and clinical results. Injury. 2011. Jun;42(6):587–90. Epub 2011 Apr 27. [DOI] [PubMed] [Google Scholar]
- 4.Elliott DS, Newman KJ, Forward DP, Hahn DM, Ollivere B, Kojima K, Handley R, Rossiter ND, Wixted JJ, Smith RM, Moran CG. A unified theory of bone healing and nonunion: BHN theory. Bone Joint J. 2016. Jul;98-B(7):884–91. [DOI] [PubMed] [Google Scholar]
- 5.Danis R. Théorie et Pratique de lʼostéosynthése. Masson; 1949. [Google Scholar]
- 6.Thiel A, Reumann MK, Boskey A, Wischmann J, von Eisenhart-Rothe R, Mayer-Kuckuk P. Osteoblast migration in vertebrate bone. Biol Rev Camb Philos Soc. 2018. Feb;93(1):350–63. Epub 2017 Jun 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charnley J. The closed treatment of common fractures. 4th ed. Cambridge University Press; 2003. [Google Scholar]
- 8.Augat P, Margevicius K, Simon J, Wolf S, Suger G, Claes L. Local tissue properties in bone healing: influence of size and stability of the osteotomy gap. J Orthop Res. 1998. Jul;16(4):475–81. [DOI] [PubMed] [Google Scholar]
- 9.Bahney CS, Zondervan RL, Allison P, Theologis A, Ashley JW, Ahn J, Miclau T, Marcucio RS, Hankenson KD. Cellular biology of fracture healing. J Orthop Res. 2019. Jan;37(1):35–50. Epub 2018 Nov 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schell H, Duda GN, Peters A, Tsitsilonis S, Johnson KA, Schmidt-Bleek K. The haematoma and its role in bone healing. J Exp Orthop. 2017. Dec;4(1):5. Epub 2017 Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SH, Silva M, Bahk WJ, McKellop H, Lieberman JR. Effect of repeated irrigation and debridement on fracture healing in an animal model. J Orthop Res. 2002. Nov;20(6):1197–204. [DOI] [PubMed] [Google Scholar]
- 12.Bolander ME. Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med. 1992. Jun;200(2):165–70. [DOI] [PubMed] [Google Scholar]
- 13.Loi F, Córdova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone. 2016. May;86:119–30. Epub 2016 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baht GS, Vi L, Alman BA. The role of the immune cells in fracture healing. Curr Osteoporos Rep. 2018. Apr;16(2):138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolar P, Schmidt-Bleek K, Schell H, Gaber T, Toben D, Schmidmaier G, Perka C, Buttgereit F, Duda GN. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev. 2010. Aug;16(4):427–34. [DOI] [PubMed] [Google Scholar]
- 16.Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008. Oct;19(5):459–66. Epub 2008 Jul 25. [DOI] [PubMed] [Google Scholar]
- 17.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003. Apr 1;88(5):873–84. [DOI] [PubMed] [Google Scholar]
- 18.Raggatt LJ, Wullschleger ME, Alexander KA, Wu AC, Millard SM, Kaur S, Maugham ML, Gregory LS, Steck R, Pettit AR. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am J Pathol. 2014. Dec;184(12):3192–204. Epub 2014 Oct 5. [DOI] [PubMed] [Google Scholar]
- 19.Schlundt C, El Khassawna T, Serra A, Dienelt A, Wendler S, Schell H, van Rooijen N, Radbruch A, Lucius R, Hartmann S, Duda GN, Schmidt-Bleek K. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone. 2018. Jan;106:78–89. Epub 2015 Oct 31. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Lee GT, Woo SH, Ha YS, Kwon SJ, Kim WJ, Kim IY. BMP-6 in renal cell carcinoma promotes tumor proliferation through IL-10-dependent M2 polarization of tumor-associated macrophages. Cancer Res. 2013. Jun 15;73(12):3604–14. Epub 2013 Apr 30. [DOI] [PubMed] [Google Scholar]
- 21.Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, Miyake T, Matsushita K, Okazaki T, Saitoh T, Honma K, Matsuyama T, Yui K, Tsujimura T, Standley DM, Nakanishi K, Nakai K, Akira S. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010. Oct;11(10):936–44. Epub 2010 Aug 22. [DOI] [PubMed] [Google Scholar]
- 22.Singla DK, Singla R, Wang J. BMP-7 treatment increases m2 macrophage differentiation and reduces inflammation and plaque formation in Apo E−/− mice. PLoS One. 2016. Jan 29;11(1):e0147897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoff P, Gaber T, Strehl C, Jakstadt M, Hoff H, Schmidt-Bleek K, Lang A, Röhner E, Huscher D, Matziolis G, Burmester GR, Schmidmaier G, Perka C, Duda GN, Buttgereit F. A pronounced inflammatory activity characterizes the early fracture healing phase in immunologically restricted patients. Int J Mol Sci. 2017. Mar 8;18(3):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012. Jan 31;8(3):133–43. [DOI] [PubMed] [Google Scholar]
- 25.Schneider PS, Sandman E, Martineau PA. Osteoimmunology: effects of standard orthopaedic interventions on inflammatory response and early fracture healing. J Am Acad Orthop Surg. 2018. May 15;26(10):343–52. [DOI] [PubMed] [Google Scholar]
- 26.Abou-Khalil R, Yang F, Mortreux M, Lieu S, Yu YY, Wurmser M, Pereira C, Relaix F, Miclau T, Marcucio RS, Colnot C. Delayed bone regeneration is linked to chronic inflammation in murine muscular dystrophy. J Bone Miner Res. 2014. Feb;29(2):304–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pountos I, Georgouli T, Calori GM, Giannoudis PV. Do nonsteroidal anti-inflammatory drugs affect bone healing? A critical analysis. ScientificWorldJournal. 2012;2012:606404. Epub 2012 Jan 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naik AA, Xie C, Zuscik MJ, Kingsley P, Schwarz EM, Awad H, Guldberg R, Drissi H, Puzas JE, Boyce B, Zhang X, O’Keefe RJ. Reduced COX-2 expression in aged mice is associated with impaired fracture healing. J Bone Miner Res. 2009. Feb;24(2):251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O’Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002. Jun;109(11):1405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerstenfeld LC, Al-Ghawas M, Alkhiary YM, Cullinane DM, Krall EA, Fitch JL, Webb EG, Thiede MA, Einhorn TA. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing. Reversibility of effects after short-term treatment. J Bone Joint Surg Am. 2007. Jan;89(1):114–25. [DOI] [PubMed] [Google Scholar]
- 31.Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br. 2003. Jul;85(5):700–5. [PubMed] [Google Scholar]
- 32.Sagi HC, Jordan CJ, Barei DP, Serrano-Riera R, Steverson B. Indomethacin prophylaxis for heterotopic ossification after acetabular fracture surgery increases the risk for nonunion of the posterior wall. J Orthop Trauma. 2014. Jul;28(7):377–83. [DOI] [PubMed] [Google Scholar]
- 33.Liu YZ, Akhter MP, Gao X, Wang XY, Wang XB, Zhao G, Wei X, Wu HJ, Chen H, Wang D, Cui L. Glucocorticoid-induced delayed fracture healing and impaired bone biomechanical properties in mice. Clin Interv Aging. 2018. Aug 24;13:1465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hachemi Y, Rapp AE, Picke AK, Weidinger G, Ignatius A, Tuckermann J. Molecular mechanisms of glucocorticoids on skeleton and bone regeneration after fracture. J Mol Endocrinol. 2018. Jul;61(1):R75–90. Epub 2018 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bissinger O, Kreutzer K, Götz C, Hapfelmeier A, Pautke C, Vogt S, Wexel G, Wolff KD, Tischer T, Prodinger PM. A biomechanical, micro-computertomographic and histological analysis of the influence of diclofenac and prednisolone on fracture healing in vivo. BMC Musculoskelet Disord. 2016. Sep 5;17(1):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borgeat A, Ofner C, Saporito A, Farshad M, Aguirre J. The effect of nonsteroidal anti-inflammatory drugs on bone healing in humans: A qualitative, systematic review. J Clin Anesth. 2018. Sep;49:92–100. Epub 2018 Jun 15. [DOI] [PubMed] [Google Scholar]
- 37.Marquez-Lara A, Hutchinson ID, Nuñez F Jr, Smith TL, Miller AN. Nonsteroidal anti-inflammatory drugs and bone-healing: a systematic review of research quality. JBJS Rev. 2016. Mar 15;4(3):e41–414. [DOI] [PubMed] [Google Scholar]
- 38.Donohue D, Sanders D, Serrano-Riera R, Jordan C, Gaskins R, Sanders R, Sagi HC. Ketorolac administered in the recovery room for acute pain management does not affect healing rates of femoral and tibial fractures. J Orthop Trauma. 2016. Sep;30(9):479–82. [DOI] [PubMed] [Google Scholar]
- 39.Fader L, Whitaker J, Lopez M, Vivace B, Parra M, Carlson J, Zamora R. Tibia fractures and NSAIDs. Does it make a difference? A multicenter retrospective study. Injury. 2018. Dec;49(12):2290–4. Epub 2018 Sep 18. [DOI] [PubMed] [Google Scholar]
- 40.Zura R, Xiong Z, Einhorn T, Watson JT, Ostrum RF, Prayson MJ, Della Rocca GJ, Mehta S, McKinley T, Wang Z, Steen RG. Epidemiology of fracture nonunion in 18 human bones. JAMA Surg. 2016. Nov 16;151(11):e162775. Epub 2016 Nov 16. [DOI] [PubMed] [Google Scholar]
- 41.Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol. 2009. Oct;22(5):588–93. [DOI] [PubMed] [Google Scholar]
- 42.Miclau KR, Brazina SA, Bahney CS, Hankenson KD, Hunt TK, Marcucio RS, Miclau T. Stimulating fracture healing in ischemic environments: does oxygen direct stem cell fate during fracture healing? Front Cell Dev Biol. 2017. May 4;5(45):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu C, Saless N, Wang X, Sinha A, Decker S, Kazakia G, Hou H, Williams B, Swartz HM, Hunt TK, Miclau T, Marcucio RS. The role of oxygen during fracture healing. Bone. 2013. Jan;52(1):220–9. Epub 2012 Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bahney CS, Hu DP, Miclau T 3rd, Marcucio RS. The multifaceted role of the vasculature in endochondral fracture repair. Front Endocrinol (Lausanne). 2015. Feb 5;6(4):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu C, Miclau T, Hu D, Marcucio RS. Ischemia leads to delayed union during fracture healing: a mouse model. J Orthop Res. 2007. Jan;25(1):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim JC, Ko KI, Mattos M, Fang M, Zhang C, Feinberg D, Sindi H, Li S, Alblowi J, Kayal RA, Einhorn TA, Gerstenfeld LC, Graves DT. TNFα contributes to diabetes impaired angiogenesis in fracture healing. Bone. 2017. Jun;99:26–38. Epub 2017 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia P, Pieruschka A, Klein M, Tami A, Histing T, Holstein JH, Scheuer C, Pohlemann T, Menger MD. Temporal and spatial vascularization patterns of unions and nonunions: role of vascular endothelial growth factor and bone morphogenetic proteins. J Bone Joint Surg Am. 2012. Jan 4;94(1):49–58. [DOI] [PubMed] [Google Scholar]
- 48.Li B, Wang H, Qiu G, Su X, Wu Z. Synergistic effects of vascular endothelial growth factor on bone morphogenetic proteins induced bone formation invivo: influencing factors and future research directions. Biomed Res Int. 2016;2016:2869572. Epub 2016 Dec 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bragdon BC, Bahney CS. Origin of reparative stem cells in fracture healing. Curr Osteoporos Rep. 2018. Aug;16(4):490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuroda R, Matsumoto T, Kawakami Y, Fukui T, Mifune Y, Kurosaka M. Clinical impact of circulating CD34-positive cells on bone regeneration and healing. Tissue Eng Part B Rev. 2014. Jun;20(3):190–9. Epub 2014 Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah K, Majeed Z, Jonason J, O’Keefe RJ. The role of muscle in bone repair: the cells, signals, and tissue responses to injury. Curr Osteoporos Rep. 2013. Jun;11(2):130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hadjiargyrou M, O’Keefe RJ. The convergence of fracture repair and stem cells: interplay of genes, aging, environmental factors and disease. J Bone Miner Res. 2014. Nov; 29(11):2307–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colnot C, Zhang X, Knothe Tate ML. Current insights on the regenerative potential of the periosteum: molecular, cellular, and endogenous engineering approaches. J Orthop Res. 2012. Dec;30(12):1869–78. Epub 2012 Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roberts SJ, van Gastel N, Carmeliet G, Luyten FP. Uncovering the periosteum for skeletal regeneration: the stem cell that lies beneath. Bone. 2015. Jan;70:10–8. Epub 2014 Sep 2. [DOI] [PubMed] [Google Scholar]
- 55.Utvåg SE, Grundnes O, Reikeraos O. Effects of periosteal stripping on healing of segmental fractures in rats. J Orthop Trauma. 1996;10(4):279–84. [DOI] [PubMed] [Google Scholar]
- 56.Bhandari M, Guyatt G, Tornetta P 3rd, Schemitsch EH, Swiontkowski M, Sanders D, Walter SD; Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures Investigators. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008. Dec;90(12):2567–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kessler SB, Hallfeldt KK, Perren SM, Schweiberer L. The effects of reaming and intramedullary nailing on fracture healing. Clin Orthop Relat Res. 1986. Nov;(212):18–25. [PubMed] [Google Scholar]
- 58.Rocha LR, Sartore RC, Leal AC, Dias RB, Duarte MEL, Guimarães JAM, Bonfim DC. Bone intramedullary reaming grafts the fracture site with CD146+ skeletal progenitors and downmodulates the inflammatory environment. Injury. 2017. Oct;48(Suppl 4):S41–9. [DOI] [PubMed] [Google Scholar]
- 59.Iwakura T, Miwa M, Sakai Y, Niikura T, Lee SY, Oe K, Hasegawa T, Kuroda R, Fujioka H, Doita M, Kurosaka M. Human hypertrophic nonunion tissue contains mesenchymal progenitor cells with multilineage capacity in vitro. J Orthop Res. 2009. Feb;27(2):208–15. [DOI] [PubMed] [Google Scholar]
- 60.Hu DP, Ferro F, Yang F, Taylor AJ, Chang W, Miclau T, Marcucio RS, Bahney CS. Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development. 2017. Jan 15;144(2):221–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cooper KL, Oh S, Sung Y, Dasari RR, Kirschner MW, Tabin CJ. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature. 2013. Mar 21;495(7441):375–8. Epub 2013 Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asaumi K, Nakanishi T, Asahara H, Inoue H, Takigawa M. Expression of neurotrophins and their receptors (TRK) during fracture healing. Bone. 2000. Jun;26(6):625–33. [DOI] [PubMed] [Google Scholar]
- 63.Zhang R, Liang Y, Wei S. The expressions of NGF and VEGF in the fracture tissues are closely associated with accelerated clavicle fracture healing in patients with traumatic brain injury. Ther Clin Risk Manag. 2018. Nov 21;14:2315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bahney CS, Hu DP, Taylor AJ, Ferro F, Britz HM, Hallgrimsson B, Johnstone B, Miclau T, Marcucio RS. Stem cell-derived endochondral cartilage stimulates bone healing by tissue transformation. J Bone Miner Res. 2014;29(5):1269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu YY, Lieu S, Lu C, Miclau T, Marcucio RS, Colnot C. Immunolocalization of BMPs, BMP antagonists, receptors, and effectors during fracture repair. Bone. 2010. Mar;46(3):841–51. Epub 2009 Nov 11. [DOI] [PubMed] [Google Scholar]
- 66.Matsubara H, Hogan DE, Morgan EF, Mortlock DP, Einhorn TA, Gerstenfeld LC. Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone. 2012. Jul;51(1):168–80. Epub 2012 Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morgan EF, Pittman J, DeGiacomo A, Cusher D, de Bakker CM, Mroszczyk KA, Grinstaff MW, Gerstenfeld LC. BMPR1A antagonist differentially affects cartilage and bone formation during fracture healing. J Orthop Res. 2016. Dec;34(12):2096–105. Epub 2016 Apr 6. [DOI] [PubMed] [Google Scholar]
- 68.Houben A, Kostanova-Poliakova D, Weissenböck M, Graf J, Teufel S, von der Mark K, Hartmann C. β-catenin activity in late hypertrophic chondrocytes locally orchestrates osteoblastogenesis and osteoclastogenesis. Development. 2016. Oct 15;143(20):3826–38. Epub 2016 Sep 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong SA, Rivera KO, Miclau T 3rd, Alsberg E, Marcucio RS, Bahney CS. Microenvironmental regulation of chondrocyte plasticity in endochondral repair-a new frontier for developmental engineering. Front Bioeng Biotechnol. 2018. May 15;6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006. Dec;38(12):1424–9. Epub 2006 Nov 12. [DOI] [PubMed] [Google Scholar]
- 71.Dumic-Cule I, Peric M, Kucko L, Grgurevic L, Pecina M, Vukicevic S. Bone morphogenetic proteins infracture repair. Int Orthop. 2018. Nov;42(11):2619–26. Epub 2018 Sep 15. [DOI] [PubMed] [Google Scholar]
- 72.Abou-Khalil R, Yang F, Lieu S, Julien A, Perry J, Pereira C, Relaix F, Miclau T, Marcucio R, Colnot C. Role of muscle stem cells during skeletal regeneration. Stem Cells. 2015. May;33(5):1501–11. [DOI] [PubMed] [Google Scholar]
- 73.Nauth A, Ristiniemi J, McKee MD, Schemitsch EH. Bone morphogenetic proteins in open fractures: past, present, and future. Injury. 2009. Dec;40(Suppl 3):S27–31. [DOI] [PubMed] [Google Scholar]
- 74.Lowery JW, Rosen V. Bone morphogenetic protein-based therapeutic approaches. Cold Spring Harb Perspect Biol. 2018. Apr 2;10(4):a022327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Secreto FJ, Hoeppner LH, Westendorf JJ. Wnt signaling during fracture repair. Curr Osteoporos Rep. 2009. Jul;7(2):64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baht GS, Silkstone D, Vi L, Nadesan P, Amani Y, Whetstone H, Wei Q, Alman BA. Exposure to a youthful circulaton rejuvenates bone repair through modulation of β-catenin [Erratum in: Nat Commun. 2015;6:7761]. Nat Commun. 2015. May 19;6:7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lauing KL, Sundaramurthy S, Nauer RK, Callaci JJ. Exogenous activation of Wnt/β-catenin signaling attenuates binge alcoholinduced deficient bone fracture healing. Alcohol Alcohol. 2014. Jul-Aug;49(4):399–408. Epub 2014 Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bernick J, Wang Y, Sigal IA, Alman BA, Whyne CM, Nam D. Parameters for lithium treatment are critical in its enhancement of fracture-healing in rodents. J Bone Joint Surg Am. 2014. Dec 3;96(23):1990–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vachhani K, Whyne C, Wang Y, Burns DM, Nam D. Low-dose lithium regimen enhances endochondral fracture healing in osteoporotic rodent bone. J Orthop Res. 2018. Jun;36(6):1783–9. Epub 2017 Nov 28. [DOI] [PubMed] [Google Scholar]
- 80.Agholme F, Li X, Isaksson H, Ke HZ, Aspenberg P. Sclerostin antibody treatment enhances metaphyseal bone healing in rats. J Bone Miner Res. 2010. Nov;25(11):2412–8. [DOI] [PubMed] [Google Scholar]
- 81.Suen PK, Qin L. Sclerostin, an emerging therapeutic target for treating osteoporosis and osteoporotic fracture: A general review. J Orthop Translat. 2015. Sep 12;4:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ominsky MS, Li C, Li X, Tan HL, Lee E, Barrero M, Asuncion FJ, Dwyer D, Han CY, Vlasseros F, Samadfam R, Jolette J, Smith SY, Stolina M, Lacey DL, Simonet WS, Paszty C, Li G, Ke HZ. Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res. 2011. May;26(5):1012–21. [DOI] [PubMed] [Google Scholar]
- 83.Amgen. Study to assess FRacTure healing with SclerosTin Antibody - Hip (STARTT-Hip). 2019. May 2. Accessed 2020 Jun 18. https://clinicaltrials.gov/ct2/show/results/NCT01081678?view5results [Google Scholar]
- 84.Amgen. Study of romosozumab (AMG 785) in tibial diaphyseal fractures status post intramedullary nailing (STARTT). 2019. May 1. Accessed 2020 Jun 18. https://clinicaltrials.gov/ct2/show/NCT00907296 [Google Scholar]
- 85.Schemitsch EH, Miclau T, Karachalios T, Nowak LL, Sancheti P, Poolman RW, Caminis J, Daizadeh N, Dent-Acosta RE, Egbuna O, Chines A, Maddox J, Grauer A, Bhandari M. A randomized, placebo-controlled study of romosozumab for the treatment of hip fractures. J Bone Joint Surg Am. 2020. Apr 15;102(8):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Silverman SL, Kupperman ES, Bukata SV; Members of IOF Fracture Working Group. Fracture healing: a consensus report from the International Osteoporosis Foundation Fracture Working Group. Osteoporos Int. 2016. Jul;27(7):2197–206. Epub 2016 Apr 25. [DOI] [PubMed] [Google Scholar]
- 87.Yamashita J, McCauley LK. Effects of intermittent administration of parathyroid hormone and parathyroid hormone-related protein on fracture healing: a narrative review of animal and human studies. JBMR Plus. 2019. Nov 22;3(12):e10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alkhiary YM, Gerstenfeld LC, Krall E, Westmore M, Sato M, Mitlak BH, Einhorn TA. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1–34). J Bone Joint Surg Am. 2005. Apr;87(4):731–41. [DOI] [PubMed] [Google Scholar]
- 89.Lin EA, Liu CJ, Monroy A, Khurana S, Egol KA. Prevention of atrophic nonunion by the systemic administration of parathyroid hormone (PTH 1–34) in an experimental animal model. J Orthop Trauma. 2012. Dec;26(12):719–23. [DOI] [PubMed] [Google Scholar]
- 90.Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1–34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res. 1999. Jun;14(6):960–8. [DOI] [PubMed] [Google Scholar]
- 91.Nakajima A, Shimoji N, Shiomi K, Shimizu S, Moriya H, Einhorn TA, Yamazaki M. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1–34). J Bone Miner Res. 2002. Nov;17(11):2038–47. [DOI] [PubMed] [Google Scholar]
- 92.Nakazawa T, Nakajima A, Shiomi K, Moriya H, Einhorn TA, Yamazaki M. Effects of low-dose, intermittent treatment with recombinant human parathyroid hormone (1–34) on chondrogenesis in a model of experimental fracture healing. Bone. 2005. Nov;37(5):711–9.Epub 2005 Sep 6. [DOI] [PubMed] [Google Scholar]
- 93.Lanske B, Chandler H, Pierce A, Brown J, Ominsky M, Kostenuik P, Hattersley G. Abaloparatide, a PTH receptor agonist with homology to PTHrP, enhances callus bridging and biomechanical properties in rats with femoral fracture. J Orthop Res. 2019. Apr;37(4):812–20. Epub 2019 Mar 21. [DOI] [PubMed] [Google Scholar]
- 94.Sleeman A, Clements JN. Abaloparatide: A new pharmacological option for osteoporosis. Am J Health Syst Pharm. 2019. Jan 25;76(3):130–5. [DOI] [PubMed] [Google Scholar]
- 95.Nino S, Soin SP, Avilucea FR. Vitamin D and metabolic supplementation in orthopedic trauma. Orthop Clin North Am. 2019. Apr;50(2):171–9. [DOI] [PubMed] [Google Scholar]
- 96.Gorter EA, Hamdy NAT, Appelman-Dijkstra NM, Schipper IB. The role of vitamin D in human fracture healing: a systematic review of the literature. Bone. 2014. Jul;64:288–97. Epub 2014 May 2. [DOI] [PubMed] [Google Scholar]
- 97.Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, Meyer HE, Pfeifer M, Sanders KM, Stähelin HB, Theiler R, Dawson-Hughes B. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012. Jul 5;367(1):40–9. [DOI] [PubMed] [Google Scholar]
- 98.Stewart CC, O’Hara NN, Orwig D, Hochberg MC, Sprague S, Magaziner J, Slobogean GP. Serum 25(OH)D is associated with an altered bone turnover marker response after a hip fracture. J Orthop Res. 2019. Mar;37(3):535–40. Epub 2019 Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sprague S, Bhandari M, Devji T, Scott T, Petrisor B, McKay P, Slobogean GP. Prescription of vitamin D to fracture patients: a lack of consensus and evidence. J Orthop Trauma. 2016. Feb;30(2):e64–9. [DOI] [PubMed] [Google Scholar]
- 100.Sprague S, Petrisor B, Scott T, Devji T, Phillips M, Spurr H, Bhandari M, Slobogean GP. What is the role of vitamin D supplementation in acute fracture patients? A systematic review and meta-analysis of the prevalence of hypovitaminosis D and supplementation efficacy. J Orthop Trauma. 2016. Feb;30(2):53–63. [DOI] [PubMed] [Google Scholar]
- 101.Ulrich-Vinther M, Andreassen TT. Osteoprotegerin treatment impairs remodeling and apparent material properties of callus tissue without influencing structural fracture strength. Calcif Tissue Int. 2005. Apr; 76(4):280–6. Epub 2005 Apr 11. [DOI] [PubMed] [Google Scholar]
- 102.Gerstenfeld LC, Sacks DJ, Pelis M, Mason ZD, Graves DT, Barrero M, Ominsky MS, Kostenuik PJ, Morgan EF, Einhorn TA. Comparison of effects of the bisphosphonate alendronate versus the RANKL inhibitor denosumab on murine fracture healing. J Bone Miner Res. 2009. Feb;24(2):196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kates SL, Ackert-Bicknell CL. How do bisphosphonates affect fracture healing? Injury. 2016. Jan;47(Suppl 1):S65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xue D, Li F, Chen G, Yan S, Pan Z. Do bisphosphonates affect bone healing? A meta-analysis of randomized controlled trials. J Orthop Surg Res. 2014. Jun 5;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hak DJ. The biology of fracture healing in osteoporosis and in the presence of anti-osteoporotic drugs. Injury. 2018. Aug;49(8):1461–5. Epub 2018 Apr 20. [DOI] [PubMed] [Google Scholar]
- 106.Bogdan Y, Tornetta P 3rd, Einhorn TA, Guy P, Leveille L, Robinson J, Bosse MJ, Haines N, Horwitz D, Jones C, Schemitsch E, Sagi C, Thomas B, Stahl D, Ricci W, Brady M, Sanders D, Kain M, Higgins TF, Collinge C, Kottmeier S, Friess D. Healing time and complications in operatively treated atypical femur fractures associated with bisphosphonate use: a multicenter retrospective cohort. J Orthop Trauma. 2016. Apr;30(4):177–81. [DOI] [PubMed] [Google Scholar]
- 107.Conley RB, Adib G, Adler RA, Åkesson KE, Alexander IM, Amenta KC, Blank RD, Brox WT, Carmody EE, Chapman-Novakofski K, Clarke BL, Cody KM, Cooper C, Crandall CJ, Dirschl DR, Eagen TJ, Elderkin AL, Fujita M, Greenspan SL, Halbout P, Hochberg MC, Javaid M, Jeray KJ, Kearns AE, King T, Koinis TF, Koontz JS, Kužma M, Lindsey C, Lorentzon M, Lyritis GP, Michaud LB, Miciano A, Morin SN, Mujahid N, Napoli N, Olenginski TP, Puzas JE, Rizou S, Rosen CJ, Saag K, Thompson E, Tosi LL, Tracer H, Khosla S, Kiel DP. Secondary fracture prevention: consensus clinical recommendations from a multistakeholder coalition. J Bone Miner Res. 2020. Jan;35(1):36–52. Epub 2019 Dec 1. [DOI] [PubMed] [Google Scholar]
- 108.United States Food and Drug Administration. Guidance document for the preparation of investigational device exemptions and pre-market approval applications for bone growth stimulator devices. Rockville: United States Food and Drug Administration; 1993. https://www.fda.gov/media/72463/download [Google Scholar]
- 109.Corrales LA, Morshed S, Bhandari M, Miclau T 3rd. Variability in the assessment of fracture-healing in orthopaedic trauma studies. J Bone Joint Surg Am. 2008. Sep;90(9):1862–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhandari M, Guyatt GH, Swiontkowski MF, Tornetta P 3rd, Sprague S, Schemitsch EH. A lack of consensus in the assessment of fracture healing among orthopaedic surgeons. J Orthop Trauma. 2002. Sep;16(8):562–6. [DOI] [PubMed] [Google Scholar]
- 111.Özkan S, Nolte PA, van den Bekerom MPJ, Bloemers FW. Diagnosis and management of long-bone nonunions: a nationwide survey. Eur J Trauma Emerg Surg. 2019. Feb;45(1):3–11. Epub 2018 Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Megas P. Classification of non-union. Injury. 2005. Nov;36(Suppl 4):S30–7. [DOI] [PubMed] [Google Scholar]
- 113.Reed AA, Joyner CJ, Brownlow HC, Simpson AHRW. Human atrophic fracture non-unions are not avascular. J Orthop Res. 2002. May;20(3):593–9. [DOI] [PubMed] [Google Scholar]
- 114.Bajada S, Marshall MJ, Wright KT, Richardson JB, Johnson WEB. Decreased osteogenesis, increased cell senescence and elevated Dickkopf-1 secretion in human fracture non union stromal cells. Bone. 2009. Oct; 45(4):726–35. Epub 2009 Jun 18. [DOI] [PubMed] [Google Scholar]
- 115.Santolini E, West R, Giannoudis PV. Risk factors for long bone fracture non-union: a stratification approach based on the level of the existing scientific evidence. Injury. 2015. Dec;46(Suppl 8):S8–19. [DOI] [PubMed] [Google Scholar]
- 116.Mangum LH, Avila JJ, Hurtgen BJ, Lofgren AL, Wenke JC. Burn and thoracic trauma alters fracture healing, systemic inflammation, and leukocyte kinetics in a rat model of polytrauma. J Orthop Surg Res. 2019. Feb 19;14(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hurtgen BJ, Ward CL, Garg K, Pollot BE, Goldman SM, McKinley TO, Wenke JC, Corona BT. Severe muscle trauma triggers heightened and prolonged local musculoskeletal inflammation and impairs adjacent tibia fracture healing. J Musculoskelet Neuronal Interact. 2016. Jun 1;16(2):122–34. [PMC free article] [PubMed] [Google Scholar]
- 118.O’Halloran K, Coale M, Costales T, Zerhusen T Jr, Castillo RC, Nascone JW, O’Toole RV. Will my tibial fracture heal? Predicting nonunion at the time of definitive fixation based on commonly available variables. Clin Orthop Relat Res. 2016. Jun;474(6):1385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Blair JA, Stoops TK, Doarn MC, Kemper D, Erdogan M, Griffing R, Sagi HC. Infection and nonunion after fasciotomy for compartment syndrome associated with tibia fractures: a matched cohort comparison. J Orthop Trauma. 2016. Jul;30(7):392–6. [DOI] [PubMed] [Google Scholar]
- 120.Metsemakers WJ, Handojo K, Reynders P, Sermon A, Vanderschot P, Nijs S. Individual risk factors for deep infection and compromised fracture healing after intramedullary nailing of tibial shaft fractures: a single centre experience of 480 patients. Injury. 2015. Apr;46(4):740–5. Epub 2014 Dec 27. [DOI] [PubMed] [Google Scholar]
- 121.Bundkirchen K, Macke C, Angrisani N, Schäck LM, Noack S, Fehr M, Krettek C, Neunaber C. Hemorrhagic shock alters fracture callus composition and activates the IL6 and RANKL/OPG pathway in mice. J Trauma Acute Care Surg. 2018. Aug;85(2):359–66. [DOI] [PubMed] [Google Scholar]
- 122.Bundkirchen K, Macke C, Reifenrath J, Schäck LM, Noack S, Relja B, Naber P, Welke B, Fehr M, Krettek C, Neunaber C. Severe hemorrhagic shock leads to a delayed fracture healing and decreased bone callus strength in a mouse model. Clin Orthop Relat Res. 2017. Nov; 475(11):2783–94. Epub 2017 Aug 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lichte P, Kobbe P, Pfeifer R, Campbell GC, Beckmann R, Tohidnezhad M, Bergmann C, Kadyrov M, Fischer H, Glüer CC, Hildebrand F, Pape HC, Pufe T. Impaired fracture healing after hemorrhagic shock. Mediators Inflamm. 2015; 2015:132451. Epub 2015 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brady J, Hardy BM, Yoshino O, Buxton A, Quail A, Balogh ZJ. The effect of haemorrhagic shock and resuscitation on fracture healing in a rabbit model: an animal study. Bone Joint J. 2018. Sep;100-B(9):1234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hofman M, Koopmans G, Kobbe P, Poeze M, Andruszkow H, Brink PR, Pape HC. Improved fracture healing in patients with concomitant traumatic braininjury:proven ornot? Mediators Inflamm. 2015;2015:204842. Epub 2015 Mar 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Morioka K, Marmor Y, Sacramento JA, Lin A, Shao T, Miclau KR, Clark DR, Beattie MS, Marcucio RS, Miclau T 3rd, Ferguson AR, Bresnahan JC, Bahney CS. Differential fracture response to traumatic brain injury suggests dominance of neuroinflammatory response in polytrauma. Sci Rep. 2019. Aug 21;9(1):12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cheung WH, Miclau T, Chow SKH, Yang FF, Alt V. Fracture healing in osteoporotic bone. Injury. 2016. Jun;47(Suppl 2):S21–6. [DOI] [PubMed] [Google Scholar]
- 128.Robinson CM, Court-Brown CM, McQueen MM, Wakefield AE. Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture. J Bone Joint Surg Am. 2004. Jul;86(7):1359–65. [DOI] [PubMed] [Google Scholar]
- 129.Zura R, Braid-Forbes MJ, Jeray K, Mehta S, Einhorn TA, Watson JT, Della Rocca GJ, Forbes K, Steen RG. Bone fracture nonunion rate decreases with increasing age: A prospective inception cohort study. Bone. 2017. Feb;95:26–32. Epub 2016 Nov 9. [DOI] [PubMed] [Google Scholar]
- 130.Lopas LA, Belkin NS, Mutyaba PL, Gray CF, Hankenson KD, Ahn J. Fractures in geriatric mice show decreased callus expansion and bone volume. Clin Orthop Relat Res. 2014. Nov;472(11):3523–32. Epub 2014 Aug 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hebb JH, Ashley JW, McDaniel L, Lopas LA, Tobias J, Hankenson KD, Ahn J. Bone healing in an aged murine fracture model is characterized by sustained callus inflammation and decreased cell proliferation. J Orthop Res. 2018. Jan;36(1):149–58. Epub 2017 Oct 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Clark D, Nakamura M, Miclau T, Marcucio R. Effects of aging on fracture healing. Curr Osteoporos Rep. 2017. Dec;15(6):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ahmed ASI, Sheng MH, Wasnik S, Baylink DJ, Lau KW. Effect of aging on stem cells. World J Exp Med. 2017. Feb 20;7(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lu C, Hansen E, Sapozhnikova A, Hu D, Miclau T, Marcucio RS. Effect of age on vascularization during fracture repair. J Orthop Res. 2008. Oct;26(10):1384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.He B, Zhang ZK, Liu J, He YX, Tang T, Li J, Guo BS, Lu AP, Zhang BT, Zhang G. Bioinformatics and microarray analysis of miRNAs in aged female mice model implied new molecular mechanisms for impaired fracture healing. Int J Mol Sci. 2016. Aug 3;17(8):E1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gruber R, Koch H, Doll BA, Tegtmeier F, Einhorn TA, Hollinger JO. Fracture healing in the elderly patient. Exp Gerontol. 2006. Nov;41(11):1080–93. Epub 2006 Nov 7. [DOI] [PubMed] [Google Scholar]
- 137.Zhao SJ, Kong FQ, Fan J, Chen Y, Zhou S, Xue MX, Yin GY. Bioinformatics analysis of the molecular mechanism of aging on fracture healing. Biomed Res Int. 2018. Dec 16;2018:7530653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McCoy TH Jr, Fragomen AT, Hart KL, Pellegrini AM, Raskin KA, Perlis RH. Genomewide association study of fracture nonunion using electronic health records.JBMR Plus. 2018. Jun 20;3(1):23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gao F, Lv TR, Zhou JC, Qin XD. Effects of obesity on the healing of bone fracture in mice. J Orthop Surg Res. 2018. Jun 8;13(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Histing T, Andonyan A, Klein M, Scheuer C, Stenger D, Holstein JH, Veith NT, Pohlemann T, Menger MD. Obesity does not affect the healing of femur fractures in mice. Injury. 2016. Jul;47(7):1435–44. Epub 2016 Apr 23. [DOI] [PubMed] [Google Scholar]
- 141.Thorud JC, Mortensen S, Thorud JL, Shibuya N, Maldonado YM, Jupiter DC. Effect of obesity on bone healing after foot and ankle long bone fractures. J Foot Ankle Surg. 2017Mar - Apr;56(2):258–62. Epub 2017 Jan 18. [DOI] [PubMed] [Google Scholar]
- 142.Li Y, James C, Byl N, Sessel J, Caird MS, Farley FA, Robbins C. Obese children have different forearm fracture characteristics compared with normal-weight children. J Pediatr Orthop. 2020. Feb;40(2):e127–30. [DOI] [PubMed] [Google Scholar]
- 143.Liuni FM, Rugiero C, Feola M, Rao C, Pistillo P, Terracciano C, Giganti MG, Tarantino U. Impaired healing of fragility fractures in type 2 diabetes: clinical and radiographic assessments and serum cytokine levels. Aging Clin Exp Res. 2015. Oct;27(Suppl 1):S37–44. Epub 2015 Jul 22. [DOI] [PubMed] [Google Scholar]
- 144.Liu J, Ludwig T, Ebraheim NA. Effect of the blood HbA1c level on surgical treatment outcomes of diabetics with ankle fractures. Orthop Surg. 2013. Aug;5(3):203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lovy AJ, Dowdell J, Keswani A, Koehler S, Kim J, Weinfeld S, Joseph D. Nonoperative versus operative treatment of displaced ankle fractures in diabetics. Foot Ankle Int. 2017. Mar; 38(3):255–60. Epub 2016 Nov 14. [DOI] [PubMed] [Google Scholar]
- 146.Gandhi A, Beam HA, O’Connor JP, Parsons JR, Lin SS. The effects of local insulin delivery on diabetic fracture healing. Bone. 2005. Oct;37(4):482–90. [DOI] [PubMed] [Google Scholar]
- 147.Park AG, Paglia DN, Al-Zube L, Hreha J, Vaidya S, Breitbart E, Benevenia J, O’Connor JP, Lin SS. Local insulin therapy affects fracture healing in a rat model. J Orthop Res. 2013. May;31(5):776–82. Epub 2012 Dec 13. [DOI] [PubMed] [Google Scholar]
- 148.Paglia DN, Wey A, Breitbart EA, Faiwiszewski J, Mehta SK, Al-Zube L, Vaidya S, Cottrell JA, Graves D, Benevenia J, O’Connor JP, Lin SS. Effects of local insulin delivery on subperiosteal angiogenesis and mineralized tissue formation during fracture healing. J Orthop Res. 2013. May;31(5):783–91. Epub 2012 Dec 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Robinson MK, Mogensen KM, Casey JD, McKane CK, Moromizato T, Rawn JD, Christopher KB. The relationship among obesity, nutritional status, and mortality in the critically ill. Crit Care Med. 2015. Jan;43(1):87–100. [DOI] [PubMed] [Google Scholar]
- 150.Brinker MR, O’Connor DP, Monla YT, Earthman TP. Metabolic and endocrine abnormalities in patients with nonunions. J Orthop Trauma. 2007. Sep;21(8):557–70. [DOI] [PubMed] [Google Scholar]
- 151.Nauth A, Lee M, Gardner MJ, Brinker MR, Warner SJ, Tornetta P 3rd, Leucht P. Principles of nonunion management: state of the art. J Orthop Trauma. 2018. Mar;32(Suppl 1):S52–7. [DOI] [PubMed] [Google Scholar]
- 152.Pearson RG, Clement RGE, Edwards KL, Scammell BE. Do smokers have greater risk of delayed and non-union after fracture, osteotomy and arthrodesis? A systematic review with meta-analysis. BMJ Open. 2016. Nov 14;6(11):e010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Christiano AV, Pean CA, Konda SR, Egol KA. Predictors of patient reported pain after lower extremity nonunion surgery: the nicotine effect. Iowa Orthop J. 2016;36:53–8. [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang J, Wan Q, Yu X, Cheng G, Ni Y, Li Z. Low-dose nicotine reduces the homing ability of murine BMSCs during fracture healing. Am J Transl Res. 2018. Sep 15;10(9):2796–809. [PMC free article] [PubMed] [Google Scholar]
- 155.Nåsell H, Adami J, Samnegård E, Tønnesen H, Ponzer S. Effect of smoking cessation intervention on results of acute fracture surgery: a randomized controlled trial. J Bone Joint Surg Am. 2010. Jun;92(6):1335–42. [DOI] [PubMed] [Google Scholar]
- 156.Koehler RM, Okoroafor UC, Cannada LK. A systematic review of opioid use after extremity trauma in orthopedic surgery. Injury. 2018. Jun; 49(6):1003–7. Epub 2018 Apr 12. [DOI] [PubMed] [Google Scholar]
- 157.Buchheit T, Zura R, Wang Z, Mehta S, Della Rocca GJ, Steen RG. Opioid exposure is associated with nonunion risk in a traumatically injured population: An inception cohort study. Injury. 2018. Jul;49(7):1266–71. Epub 2018 May 21. [DOI] [PubMed] [Google Scholar]
- 158.Chrastil J, Sampson C, Jones KB, Higgins TF. Postoperative opioid administration inhibits bone healing in an animal model. Clin Orthop Relat Res. 2013. Dec;471 (12):4076–81.Epub 2013 Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rush RM Jr, Kjorstad R, Starnes BW, Arrington E, Devine JD, Andersen CA. Application of the Mangled Extremity Severity Score in a combat setting. Mil Med. 2007. Jul; 172(7):777–81. [DOI] [PubMed] [Google Scholar]
- 160.Fischer H. A guide to U.S. military casualty statistics: Operation Freedom’s Sentinel, Operation Inherent Resolve, Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. 2015. Aug 7. Accessed 2020 Jun 18. https://fas.org/sgp/crs/natsec/RS22452.pdf [Google Scholar]
- 161.Doukas WC, Hayda RA, Frisch HM, Andersen RC, Mazurek MT, Ficke JR, Keeling JJ, Pasquina PF, Wain HJ, Carlini AR, MacKenzie EJ. The Military Extremity Trauma Amputation/Limb Salvage (METALS) study: outcomes of amputation versus limb salvage following major lower-extremity trauma. J Bone Joint Surg Am. 2013. Jan 16;95(2):138–45. [DOI] [PubMed] [Google Scholar]
- 162.Penn-Barwell JG, Bennett PM, Fries CA, Kendrew JM, Midwinter MJ, Rickard RF. Severe open tibial fractures in combat trauma: management and preliminary outcomes. Bone Joint J. 2013. Jan;95-B(1):101–5. [DOI] [PubMed] [Google Scholar]
- 163.Tribble DR, Krauss MR, Murray CK, Warkentien TE, Lloyd BA, Ganesan A, Greenberg L, Xu J, Li P, Carson ML, Bradley W, Weintrob AC. Epidemiology of trauma-related infections among a combat casualty cohort after initial hospitalization: the Trauma Infectious Disease Outcomes Study. Surg Infect (Larchmt). 2018. Jul;19(5):494–503. Epub 2018 May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cross JD, Stinner DJ, Burns TC, Wenke JC, Hsu JR; Skeletal Trauma Research Consortium (STReC). Return to duty after type III open tibia fracture. J Orthop Trauma. 2012. Jan;26(1):43–7. [DOI] [PubMed] [Google Scholar]
- 165.Bosse MJ, MacKenzie EJ, Kellam JF, Burgess AR, Webb LX, Swiontkowski MF, Sanders RW, Jones AL, McAndrew MP, Patterson BM, McCarthy ML, Travison TG, Castillo RC. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. N Engl J Med. 2002. Dec 12;347(24):1924–31. [DOI] [PubMed] [Google Scholar]
- 166.Whelan DB, Bhandari M, Stephen D, Kreder H, McKee MD, Zdero R, Schemitsch EH. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J Trauma. 2010. Mar;68(3):629–32. [DOI] [PubMed] [Google Scholar]
- 167.Cooke ME, Hussein AI, Lybrand KE, Wulff A, Simmons E, Choi JH, Litrenta J, Ricci WM, Nascone JW, O’Toole RV, Morgan EF, Gerstenfeld LC, Tornetta P 3rd. Correlation between RUST assessments of fracture healing to structural and biomechanical properties. J Orthop Res. 2018. Mar; 36(3):945–53. Epub 2017 Sep 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Litrenta J, Tornetta P 3rd, Mehta S, Jones C, OʼToole RV, Bhandari M, Kottmeier S, Ostrum R, Egol K, Ricci W, Schemitsch E, Horwitz D. Determination of Radiographic Healing: An Assessment of Consistency Using RUST and Modified RUST in Metadiaphyseal Fractures. J Orthop Trauma. 2015. Nov;29(11):516–20. [DOI] [PubMed] [Google Scholar]
- 169.Kooistra BW, Dijkman BG, Busse JW, Sprague S, Schemitsch EH, Bhandari M. The radiographic union scale in tibial fractures: reliability and validity. J Orthop Trauma. 2010. Mar;24(Suppl 1):S81–6. [DOI] [PubMed] [Google Scholar]
- 170.Ross KA, O’Halloran K, Castillo RC, Coale M, Fowler J, Nascone JW, Sciadini MF, LeBrun CT, Manson TT, Carlini AR, Jolissaint JE, O’Toole RV. Prediction of tibial nonunion at the 6-week time point. Injury. 2018. Nov;49(11):2075–82. Epub 2018 Jul 29. [DOI] [PubMed] [Google Scholar]
- 171.Mitchell SL, Obremskey WT, Luly J, Bosse MJ, Frey KP, Hsu JR, MacKenzie EJ, Morshed S, OʼToole RV, Scharfstein DO, Tornetta P 3rd; Major Extremity Trauma Rehabilitation Consortium (METRC). Inter-rater reliability of the modified radiographic union score for diaphyseal tibial fractures with bone defects. J Orthop Trauma. 2019. Jun;33(6):301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Morshed S. Current options for determining fracture union. Adv Med. 2014; 2014:708574. Epub 2014 Sep 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pountos I, Georgouli T, Pneumaticos S, Giannoudis PV. Fracture non-union: Can biomarkers predict outcome? Injury. 2013. Dec; 44(12):1725–32. Epub 2013 Sep 13. [DOI] [PubMed] [Google Scholar]
- 174.Cunningham BP, Brazina S, Morshed S, Miclau T 3rd. Fracture healing: A review of clinical, imaging and laboratory diagnostic options. Injury. 2017. Jun;48(Suppl 1):S69–75. Epub 2017 May 5. [DOI] [PubMed] [Google Scholar]
- 175.Chaverri D, Vives J. Toward the clinical use of circulating biomarkers predictive of bone union. Biomark Med. 2017. Dec;11(12):1125–33. Epub 2017 Nov 28. [DOI] [PubMed] [Google Scholar]
- 176.Working ZM, Morris ER, Chang JC, Coghlan RF, Johnstone B, Miclau T, Horton WA, Bahney CS. A quantitative serum biomarker of circulating collagen X effectively correlates with endochondral fracture healing. J Orthop Res. 2020. Jun 13. Epub 2020 Jun 13. [DOI] [PubMed] [Google Scholar]
- 177.Morshed S, Corrales L, Genant H, Miclau T 3rd. Outcome assessment in clinical trials of fracture-healing. J Bone Joint Surg Am. 2008. Feb;90(Suppl 1):62–7. [DOI] [PubMed] [Google Scholar]
- 178.Webb J, Herling G, Gardner T, Kenwright J, Simpson AH. Manual assessment of fracture stiffness. Injury. 1996. Jun;27(5):319–20. [DOI] [PubMed] [Google Scholar]
- 179.Miclau T, Hoogervorst P, Shearer DW, El Naga AN, Working ZM, Martin C, Pesántez R, Huttl T, Kojima KE, Schütz M; International Orthopaedic Trauma Study Consortium. Current status of musculoskeletal trauma care systems worldwide. J Orthop Trauma. 2018. Oct; 32(Suppl 7):S64–70. [DOI] [PubMed] [Google Scholar]
- 180.Wu HH, Liu M, Challa ST, Morshed S, Eliezer EN, Haonga BT, Zirkle L, Shearer DW. Development of squat-and-smile test as proxy for femoral shaft fracture-healing in patients in Dar es Salaam, Tanzania. J Bone Joint Surg Am. 2019. Feb 20;101(4):353–9. [DOI] [PubMed] [Google Scholar]
- 181.Bhandari M, Chiavaras M, Ayeni O, Chakraverrty R, Parasu N, Choudur H, Bains S, Sprague S, Petrisor B; Assessment Group for Radiographic Evaluation and Evidence (AGREE) Study Group (AGREE Investigators Writing Committee). Assessment of radiographic fracture healing in patients with operatively treated femoral neck fractures. J Orthop Trauma. 2013. Sep;27(9):e213–9. [DOI] [PubMed] [Google Scholar]
- 182.Davis BJ, Roberts PJ, Moorcroft CI, Brown MF, Thomas PB, Wade RH. Reliability of radiographs in defining union of internally fixed fractures. Injury. 2004. Jun;35(6):557–61. [DOI] [PubMed] [Google Scholar]
- 183.Dijkman BG, Busse JW, Walter SD, Bhandari M; TRUST Investigators. The impact of clinical data on the evaluation of tibial fracture healing. Trials. 2011. Nov 3;12:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Patel SP, Anthony SG, Zurakowski D, Didolkar MM, Kim PS, Wu JS, Kung JW, Dolan M, Rozental TD. Radiographic scoring system to evaluate union of distal radius fractures. J Hand Surg Am. 2014. Aug;39(8):1471–9. Epub 2014 Jul 2. [DOI] [PubMed] [Google Scholar]
- 185.Lack WD, Starman JS, Seymour R, Bosse M, Karunakar M, Sims S, Kellam J. Any cortical bridging predicts healing of tibial shaft fractures. J Bone Joint Surg Am. 2014. Jul 2; 96(13):1066–72. Epub 2014 Jul 2. [DOI] [PubMed] [Google Scholar]
- 186.Frank T, Osterhoff G, Sprague S, Garibaldi A, Bhandari M, Slobogean GP; FAITH Investigators. The Radiographic Union Score for Hip (RUSH) identifies radiographic nonunion of femoral neck fractures. Clin Orthop Relat Res. 2016. Jun;474(6):1396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Duryea J, Evans C, Glatt V. Image analysis software as a strategy to improve the radiographic determination of fracture healing. J Orthop Trauma. 2018. Sep;32(9):e354–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fisher JS, Kazam JJ, Fufa D, Bartolotta RJ. Radiologic evaluation of fracture healing. Skeletal Radiol. 2019. Mar;48(3):349–61. Epub 2018 Sep 21. [DOI] [PubMed] [Google Scholar]
- 189.Seide K, Aljudaibi M, Weinrich N, Kowald B, Jürgens C, Müller J, Faschingbauer M. Telemetric assessment of bone healing with an instrumented internal fixator: a preliminary study. J Bone Joint Surg Br. 2012. Mar;94(3):398–404. [DOI] [PubMed] [Google Scholar]
- 190.Wolynski JG, Sutherland CJ, Demir HV, Unal E, Alipour A, Puttlitz CM, McGilvray KC. Utilizing multiple BioMEMS sensors to monitor orthopaedic strain and predict bone fracture healing. J Orthop Res. 2019. Sep;37(9):1873–80. Epub 2019 May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lin MC, Hu D, Marmor M, Herfat ST, Bahney CS, Maharbiz MM. Smart bone plates can monitor fracture healing. Sci Rep. 2019. Feb 14;9(1):2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sarahrudi K, Thomas A, Mousavi M, Kaiser G, Köttstorfer J, Kecht M, Hajdu S, Aharinejad S. Elevated transforming growth factor-beta 1 (TGF-β1) levels in human fracture healing. Injury. 2011. Aug;42(8):833–7. Epub 2011 May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Moghaddam A, Müller U, Roth HJ, Wentzensen A, Grützner PA, Zimmermann G. TRACP 5b and CTX as osteological markers of delayed fracture healing.Injury. 2011Aug;42(8):758–64. Epub 2010 Dec 17. [DOI] [PubMed] [Google Scholar]
- 194.Hussein AI, Mancini C, Lybrand KE, Cooke ME, Matheny HE, Hogue BL, Tornetta P 3rd, Gerstenfeld LC. Serum proteomic assessment of the progression of fracture healing. J Orthop Res. 2018. Apr;36(4):1153–63. Epub 2017 Nov 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Grant WT, Wang GJ, Balian G. Type X collagen synthesis during endochondral ossification in fracture repair. J Biol Chem. 1987. Jul 15;262(20):9844–9. [PubMed] [Google Scholar]
- 196.Coghlan RF, Oberdorf JA, Sienko S, Aiona MD, Boston BA, Connelly KJ, Bahney C, LaRouche J, Almubarak SM, Coleman DT, Girkontaite I, von der Mark K, Lunstrum GP, Horton WA. A degradation fragment of type X collagen is a real-time marker for bone growth velocity. Sci Transl Med. 2017. Dec 6;9(419):eaan4669. [DOI] [PMC free article] [PubMed] [Google Scholar]


