Abstract
Measles virus (MV) infection causes acute childhood disease, associated in certain cases with infection of the central nervous system (CNS) and development of neurological disease. To develop a murine model of MV-induced pathology, we generated several lines of transgenic mice ubiquitously expressing as the MV receptor a human CD46 molecule with either a Cyt1 or Cyt2 cytoplasmic tail. All transgenic lines expressed CD46 protein in the brain. Newborn transgenic mice, in contrast to nontransgenic controls, were highly sensitive to intracerebral infection by the MV Edmonston strain. Signs of clinical illness (lack of mobility, tremors, and weight loss) appeared within 5 to 7 days after infection, followed by seizures, paralysis, and death of the infected animals. Virus replication was detected in neurons from infected mice, and virus was reproducibly isolated from transgenic brain tissue. MV-induced apoptosis observed in different brain regions preceded the death of infected animals. Similar results were obtained with mice expressing either a Cyt1 or Cyt2 cytoplasmic tail, demonstrating the ability of different isoforms of CD46 to function as MV receptors in vivo. In addition, maternally transferred immunity delayed death of offspring given a lethal dose of MV. These results document a novel CD46 transgenic murine model where MV neuronal infection is associated with the production of infectious virus, similarly to progressive infectious measles encephalitis seen in immunocompromised patients, and provide a new means to study pathogenesis of MV infection in the CNS.
Measles virus (MV) infection is one of the leading causes of infant death in developing countries, and sporadic outbreaks of acute measles still occur in industrialized countries despite vaccination (52). This virus causes acute respiratory infection in children, which can be followed in certain cases by invasion of the central nervous system (CNS) and development of three different forms of measles encephalitis (27). Acute postinfectious encephalomyelitis occurs during or shortly after acute measles and is characterized by perivascular inflammation in the brain and demyelinization. Virus replication cannot be detected in the brains of affected patients, and this encephalitis seems to be associated with autoimmune pathogenesis. In contrast to acute encephalitis, subacute sclerosing panencephalitis (SSPE) presents a late complication of measles, with an incubation time of 1 to 10 years. It is based on the persistent MV infection of brain cells, where virus has been found to be only cell associated, presenting numerous mutations in its genome (10). This fatal disease occurs in the presence of a competent immune response and is followed by general destruction of the brain tissue, causing a progressive dementia, seizures, and ataxia. The third form of MV-induced CNS disease, progressive infectious encephalitis (also known as a measles inclusion body encephalitis) occurs in immunosuppressed patients 1 to 6 months following measles infection. Seizures, motor and sensory deficits, and lethargy are common, and the disease runs an acute or subacute fatal course. Nonrestricted virus replication due to absent or decreased immune response results in cytolytic viral infection of the brain tissue. Histologically, this progressive infectious encephalitis is characterized by (i) the presence of intracellular inclusion bodies which contain paramyxovirus nucleocapsids and (ii) sparseness of brain inflammation (43). Although measles vaccination significantly decreased the number of cases of the first two forms of MV-induced encephalitis, the third form remains problematic in an increasing population of immunocompromised patients (16, 37, 40) and has reemerged particularly in children infected with human immunodeficiency virus (HIV) (6, 35, 44).
Appropriate animal models are needed to analyze MV-induced pathology. Initially, rodents were shown to be susceptible to MV brain infection (30). Neuroadapted MV strains have been used for successful infection in a murine model, but these strains have several genetic changes, particularly in the sequence of the receptor binding protein hemagglutinin (H) (13). Identification of the human CD46 molecule, known as a membrane cofactor protein and inhibitor of complement activation, as a cellular receptor for MV (12, 41) opened new perspectives in developing animal models with which to study MV pathogenesis. Several lines of transgenic mice and rats were developed but subsequently shown to be resistant to intranasal or intraperitoneal inoculation of MV (3, 23, 42, 50) unless they were crossed in the genetic background with inactivated alpha/beta interferon receptor (39). Nevertheless, newborn transgenic mice expressing the BC-Cyt1 isoform of CD46 specifically in neurons and infected intracerebrally by MV developed nonproductive fatal MV brain infection resembling human SSPE (45). However, selective expression of the C-Cyt2 CD46 isoform was demonstrated in the human brain (4, 25), and CD46 was shown to be expressed on neurons as well as on other cell types (astrocytes and oligodendrocytes) (38). To adapt the murine model to the situation seen in humans, we generated transgenic mice ubiquitously expressing the CD46 C-Cyt2 isoform and compared their sensitivity to intracerebral (i.c.) MV infection with that of mice expressing the CD46 C-Cyt1 isoform. In the present study, we demonstrate that both isoforms of CD46 could function as MV receptors in vivo and render newborn transgenic mice highly sensitive to MV brain infection. MV replication in these mice is followed by the production of MV infectious particles and associated with widely spread neuronal lesions and apoptosis, leading to death of all infected animals. The observed pathology mimics the progressive infectious measles encephalitis seen in immunosuppressed patients. These mice present a new transgenic model of MV brain infection which may allow further dissection of pathogenic process in human measles encephalitis.
MATERIALS AND METHODS
Production of transgenic mice.
Human CD46 cDNA of the C-Cyt1 isoform, containing exons 1 to 6, 9 to 12, and 13, was under the control of the promoter for the ubiquitously expressed hydroxymethylglutaryl coenzyme A reductase (HMGCR) gene (15). The construct was microinjected into the pronuclei of B6DBA mouse ovocytes, and transgenic mice were generated by a previously described procedure (22). Seven founding transgenic mice and their initial offspring were identified by dot blot analysis of tail DNA as described elsewhere (28). Two lines, named MCP-8 and MCP-10, were chosen for further analysis, crossed with BALB/c mice, and used as heterozygotes in all experiments. Production of C-Cyt2 CD46 transgenic mice (lines MCP-3 and MCP-7) was described previously (23). These mice were crossed in the BALB/c background and used as homozygotes in all experiments.
Cytofluorometry analysis.
Brain structures (frontal cortex, hippocampus, and cerebellum) were isolated from ice-cold phosphate-buffered saline (PBS)-perfused transgenic and nontransgenic mice and gently dissociated by pipetting. Resulting cell suspensions were passed through a 100-μm-pore-size cell strainer (Becton Dickinson) to remove cell clumps, washed, and incubated with rabbit polyclonal anti-CD46 serum R1839-C (generous gift from B. Loveland, Heidelberg, Germany). Suspensions were washed again and incubated with a goat anti-rabbit immunoglobulin G (IgG)-fluorescein isothiocyanate (FITC) conjugate, washed thoroughly, and then analyzed. All incubations were carried out in Dulbecco modified Eagle medium (DMEM) containing 10% fetal calf serum (FCS) on ice for 30 min. Cytofluorometry analyses were performed on a FACScan (Becton Dickinson). At least 10,000 events were collected, and dead cells were excluded from further analysis by propidium iodide staining. Cells from nontransgenic mice and CD46 transgenic mice incubated with normal rabbit serum served as negative controls.
Virus.
MV Edmonston strain (ATCC VR-24) was used in most experiments. Two other strains, Hallé (vaccinal MV strain) and TT (initially described as a wild-type strain of MV [49]), able to downregulate CD46 (2) were used in several experiments for comparative studies. Edmonston and Hallé were propagated on Vero fibroblasts, and TT was grown on Jurkat cells. Virus was harvested from infected cells when a strong cytopathic effect developed. Suspensions containing virus were submitted to a freezing-thawing cycle, clarified by centrifugation, and stored at −70°C until use. Some experiments were performed with MV-UV, i.e., MV inactivated by 30-min exposure at 4°C to UV irradiation from a 254-nm UV lamp. A mock preparation contained virus-free supernatant from Vero cells prepared in same way as virus-infected cells.
Inoculation of mice and virus titration.
Suckling mice (1 to 3 days after birth) of transgenic and control lines were inoculated i.c. with 30 μl of appropriate dilution of MV, MV-UV, or mock preparation. Animals were observed for clinical signs and weighed daily for 3 weeks and later on a weekly basis.
In some experiments, animals were sacrificed at indicated times and brain homogenates (20 mg/ml in DMEM–2% FCS) were prepared. Following freezing-thawing, serial 10-fold dilutions were incubated with Vero cell monolayer cultures for 4 days in DMEM supplemented with 2% FCS. Cells were further fixed in 10% formaldehyde and stained with methylene blue. The median tissue culture infective dose (TCID50) was calculated as described previously (47).
DNA fragmentation analysis.
Different regions of the brain were dissected and lysed in 0.5 ml of extraction buffer (0.5% Triton X-100, 5 mM Tris [pH 7.5], 20 mM EDTA, 100 μg of proteinase K per ml) for 20 min on ice. The fraction containing low-molecular-weight (low-MW) DNA was isolated by centrifugation at 13,000 × g for 20 min, phenol-chloroform extracted three times, and ethanol precipitated. DNA was then resuspended in Tris-EDTA (pH 8.0), containing 20 μg of RNase A per ml and incubated at 37°C for 2 h. Finally, DNA was run on a 1.5% agarose gel and visualized by ethidium bromide staining.
Histopathology and TUNEL staining.
Animals were sacrificed, and brains were immediately dissected out, snap-frozen in isopentane at −40°C, and stored at −70°C. Serial sections (16 μm) were made with a cryomicrotome (Jung1800; Reichert), mounted on glass slides precoated with 0.05% poly-l-lysine (Sigma), and stored at −20°C. Brain histology was analyzed on frontal sections by using conventional cresyl violet staining. Detection of apoptotic cells was performed on alternate sections by the terminal deoxynucleotidyltransferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) method as previously described (26). Briefly, sections were fixed 30 min with 4% paraformaldehyde in PBS and rinsed. Slides were then incubated for 15 min at room temperature with 20 μg of proteinase K per ml in PBS, further treated with 2% H2O2 for 5 min, and rinsed. After a 5-min rinse in TdT buffer (30 mM Tris-HCl [pH 7.5], 140 mM sodium cacodylate, 1 mM cobalt chloride), slides were incubated with biotinylated dUTP (5 nmol/ml; Boehringer) and TdT (150 U/ml; Boehringer) in TdT buffer for 1 h at 37°C. Slides were then rinsed at room temperature in 300 mM sodium chloride–30 mM sodium citrate solution, further rinsed in PBS, and incubated for 10 min with 2% bovine serum albumin (Eurobio) in PBS. Slides were then incubated for 30 min with the horseradish peroxidase-coupled ABC system (Vector) as instructed by the manufacturer, rinsed, and reacted with 0.05% diaminobenzidine (Sigma) in 50 mM Tris-HCl (pH 7.5) containing 0.6% H2O2 and 0.03% NiCl. After 4 to 5 min of reaction at room temperature, slides were rinsed in cold buffer, dehydrated through graded ethanol and xylene, and coverslipped with Depex. In some experiments, slides were additionally counterstained with 1% phloxine. Sections were observed and photographed with a photonic microscope (Leitz Axioplan).
Immunohistochemical analysis.
Cryostat brain sections were fixed for 30 min with 4% paraformaldehyde in PBS, and nonspecific binding was blocked by incubation with 2% normal goat serum in PBS only or in the presence of 0.1% Triton X-100 if staining required cell permeabilization. Sections were incubated overnight at 4°C with an appropriate dilution of biotinylated antinucleoprotein (anti-NP) mouse monoclonal antibody (Cl.120 IgG2a) (20), anti-H mouse monoclonal antibody (Cl.55 IgG2b) (20), antineurofilament (anti-NF) rabbit polyclonal serum (Sigma), anti-glial fibrillary acid protein (GFAP) rabbit polyclonal serum (Sigma), biotinylated anti-mouse CD11b (Mac-1; PharMingen), FITC-conjugated hamster anti-mouse CD3 (2C11), or phycoerythrin-conjugated rat anti-mouse B220 (RA3-6B2; Sigma) and thoroughly washed. When necessary, secondary reagents (rhodamine-conjugated streptavidin, FITC-conjugated streptavidin [Jackson ImmunoResearch], goat anti-mouse IgG-FITC conjugate [Sigma] or goat anti-rabbit IgG-FITC conjugate [Nordic Immunology]) were added for 2 h at room temperature. Sections were washed three times in PBS and mounted in Fluoromount (BDH). Staining with irrelevant primary antibodies served as negative controls. Confocal microscopy was performed on a model 510 laser scanning microscope (Carl Zeiss Inc.) using LSM image processing software.
RESULTS
Transgenic mice express CD46 in the brain.
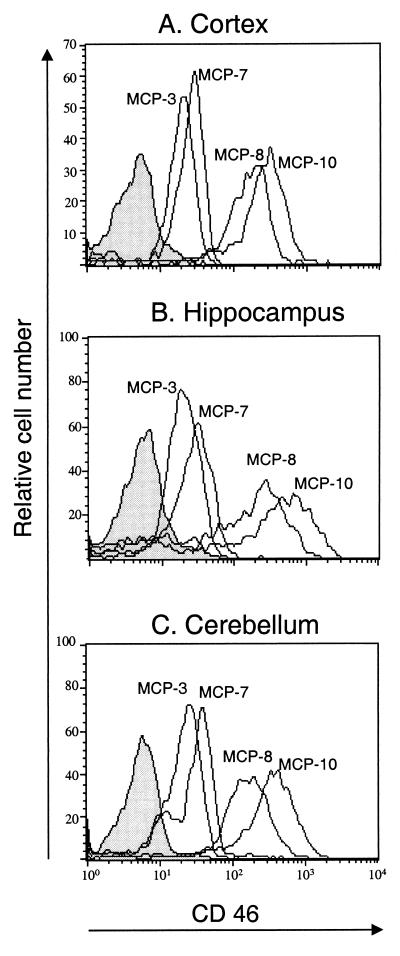
Transgenic mice containing CD46 cDNA under the control of the ubiquitously expressed HMGCR promoter were analyzed for CD46 expression in the brain. Transgenic lines containing the CD46 C-Cyt1 isoform (lines MCP-8 and MCP-10) or C-Cyt2 isoform (lines MCP-3 and MCP-7) were used. Expression of CD46-specific RNA in the brain was previously demonstrated in MCP-3 and MCP-7 lines, without dissection of different brain sections (23). Here, cell suspensions were prepared from three brain structures (frontal cortex, hippocampus, and cerebellum) and analyzed by cytofluorometry for CD46 expression. Figure 1 shows that all transgenic lines express CD46 on all brain structures analyzed. These results are in agreement with previously demonstrated ubiquitous expression of CD46 on all tested cell types (23). Furthermore, lines MCP-8 and MCP-10 express more CD46 than lines MCP-3 and MCP-7, which correlates with the expression level observed in the periphery (lymphoid tissue) from these mice (data not shown). Finally, CD46-specific staining was not detected on the brain structures obtained from nontransgenic mice (Fig. 1).
FIG. 1.
CD46 expression in the brains of transgenic mice. Brain structures were dissected from PBS-perfused CD46 C-Cyt1 transgenic (MCP-8 and MCP-10), CD46 C-Cyt2 transgenic (MCP-3 and MCP-7), and nontransgenic (BALB/c) mice. Frontal cortex (A), hippocampus (B), and cerebellum (C) were gently dissociated and stained with anti-CD46 rabbit polyclonal serum, followed by FITC-conjugated goat anti-rabbit IgG. Dead cells were excluded by propidium iodide staining. Negative control histograms (nontransgenic mice) are filled in gray. Results are representative of three different experiments.
CD46 transgenic mice are highly sensitive to i.c. MV inoculation.
We assessed the susceptibility of CD46 transgenic lines to brain infection with MV Edmonston strain. Newborn mice (24 to 72 h after birth) were inoculated i.c. with the indicated doses of MV (Table 1). All CD46 transgenic mice were highly sensitive to inoculation of 3 × 105 TCID50 of MV, with 100% lethality, while only 1 of 54 nontransgenic mice succumbed to inoculation of the same dose of MV. In addition, the mean survival times after MV inoculation were similar (5 to 7 days) in all transgenic lines when the high dose of virus was used. Mice which died within 3 days of inoculation (less than 3% of all injected mice) were excluded from the data, since death was assumed to have resulted from inoculation trauma. We next tested the sensitivity of CD46 transgenic lines to the different doses of MV (Table 1). The MCP-10 line, which expresses the highest level of CD46 (Fig. 1), was highly sensitive to MV infection: only 60 TCID50 of MV induced 71% mortality, and as little as 6 TCID50 of MV was lethal in 17% of cases. Sensitivity of the MCP-8 line to MV infection seemed to be similar to that seen in the MCP-10 line. The MCP-7 line, expressing around 20 times less CD46 in the brain (Fig. 1), was less sensitive to the infection with the low dose of MV: 60 TCID50 of MV induced only 16% mortality, with a longer survival time (13 days) than for the MCP-10 line (8.4 days). The MCP-3 line was even less sensitive: 300 TCID50 of MV induced only 25% mortality.
TABLE 1.
Sensitivity of CD46 transgenic mice to MV infection of the brain
| Mice | CD46 isoform | TCID50 inoculateda | Dead/ inoculated | Mean survival timeb |
|---|---|---|---|---|
| Transgenic linesc | ||||
| MCP-8 | C-Cyt1 | 3 × 105 | 9/9 | 6.0 ± 2.0 |
| 6 × 101 | 3/4 | 9.0 ± 1.0 | ||
| MCP-10 | C-Cyt1 | 3 × 105 | 9/9 | 6.2 ± 1.2 |
| 6 × 102 | 4/4 | 8.0 ± 3.4 | ||
| 6 × 101 | 5/7 | 8.4 ± 2.2 | ||
| 6 × 100 | 1/6 | 8.0 | ||
| MCP-3 | C-Cyt2 | 3 × 105 | 7/7 | 7.0 ± 1.2 |
| 3 × 104 | 8/8 | 6.4 ± 1.1 | ||
| 3 × 103 | 6/7 | 7.5 ± 0.8 | ||
| 3 × 102 | 1/4 | 10.0 | ||
| MCP-7 | C-Cyt2 | 3 × 105 | 17/17 | 5.7 ± 0.9 |
| 3 × 104 | 16/16 | 6.0 ± 0.8 | ||
| 6 × 103 | 12/12 | 6.3 ± 1.4 | ||
| 6 × 102 | 9/9 | 8.7 ± 0.7 | ||
| 6 × 101 | 1/6 | 13 | ||
| Nontransgenic littermates | 3 × 105 | 0/42 | ||
| BALB/c | 3 × 105 | 1/12 | 22 | |
| 3 × 104 | 0/5 |
Suckling mice were inoculated intracerebrally with the indicated dose of MV (Edmonston strain) and observed daily for 2 months thereafter.
Average day of death ± SD after MV inoculation, calculated from mice not surviving MV infection.
Transgenic mice were used as homozygotes for the MCP-3 and MCP-7 lines and as heterozygotes for the MCP-8 and MCP-10 lines.
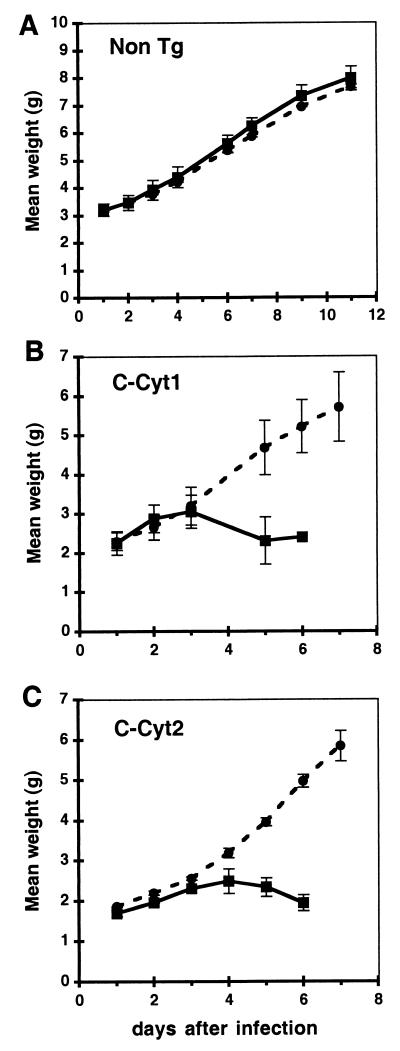
Signs of neurological disease corresponding to acute encephalitis appeared within 4 to 7 days after infection, with lack of mobility and tremors followed by seizures, paralysis, and death of infected animals. Signs of illness appeared in all MV-inoculated transgenic mice, even those which recovered after inoculation of the low dose of virus (MCP-3 line), suggesting that the mice were infected but later recovered. Transgenic mice inoculated with UV-inactivated MV or nontransgenic mice that received the infectious MV inoculum did not become ill or show any evidence of virus infection. Mice were weighed daily, and weight gain (mean ± standard deviation [SD]) is presented in Fig. 2. While there was no difference in weight gain between MV-infected and mock-infected nontransgenic animals (Fig. 2A), this difference was evident in CD46 transgenic mice, expressing either the C-Cyt1 or C-Cyt2 isoform (Fig. 2B and C); in this group, all MV-infected animals succumbed to MV infection on day 7 after injection. In contrast to i.c. inoculation, injection of 1- to 5-day-old mice with MV by the intraperitoneal or intranasal routes did not produce any detectable disease or infection (data not shown).
FIG. 2.
Retarded weight gain in infected mice. Litters from nontransgenic (A), CD46 C-Cyt1 transgenic MCP-8 (B), and CD46 C-Cyt2 transgenic MCP-7 (C) lines were inoculated 2 days after birth with either 3 × 105 TCID50 of MV Edmonston strain (squares) or mock preparation (circles) and weighed daily. Two to five mice per group were analyzed. Mean weight ± SD is presented.
Susceptibility to i.c. infection was age dependent: mice inoculated after day 10 of age showed symptoms of illness but survived the infection (data not shown). Furthermore, transgenic mice (MCP-7 line) were tested for susceptibility to i.c. infection by injecting 3 × 105 TCID50 of two other MV strains, vaccinal strain Hallé and CD46-dependent wild-type strain TT (2). While all infected transgenic mice showed clinical signs of acute encephalitis, mortality was lower than with the Edmonston strain, being 75% for Hallé and 30% for TT, with longer survival times (10.7 and 15 days, respectively). As with the Edmonston strain, nontransgenic mice were not affected, confirming the CD46 dependence of MV infection by these strains of MV.
MV replication in the brain.
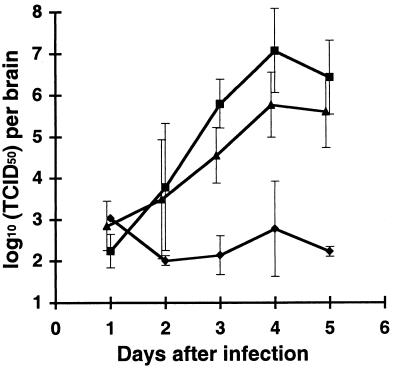
We next analyzed the capacity of MV to replicate in CD46 transgenic mice. Newborn mice were infected i.c. with MV (3 × 105 TCID50), brains were isolated at different times after infection and weighed, and brain homogenates were stored at −70°C. The virus titer in thawed brain homogenate was determined on a Vero cell monolayer. MV was reproducibly recovered from all infected CD46 transgenic mice starting from day 3 after infection; results for the MCP-7 and MCP-10 lines are presented in Fig. 3. We isolated from the brain of infected transgenic animals up to 100 times more virus (2 × 107 TCID50/brain) than the dose of the inoculated MV, indicating a productive virus replication in the brain. Similar results were obtained for two other CD46 transgenic lines, MCP-3 and MCP-8 (data not shown). In addition, MV was isolated from the spinal cord of infected transgenic mice but not from the peripheral organs (spleen and thymus) after i.c. infection (data not shown). In infected nontransgenic mice, only residual virus could be detected (Fig. 3).
FIG. 3.
Isolation of infectious virus from brains of infected animals. CD46 C-Cyt1 transgenic MCP-10 (triangles), CD46 C-Cyt2 transgenic MCP-7 (squares), and nontransgenic (diamonds) mice were inoculated 2 days after birth with 3 × 105 TCID50 of MV (Edmonston strain). At indicated times, animals were sacrificed, and brains were dissected, weighed, and homogenized. After freezing-thawing, viral titers in homogenates were evaluated on Vero monolayers. Mean log10 (TCID50) per brain ± SD is presented. Two to five mice per group were analyzed. The detection limit of the method used was 2 log10 per brain.
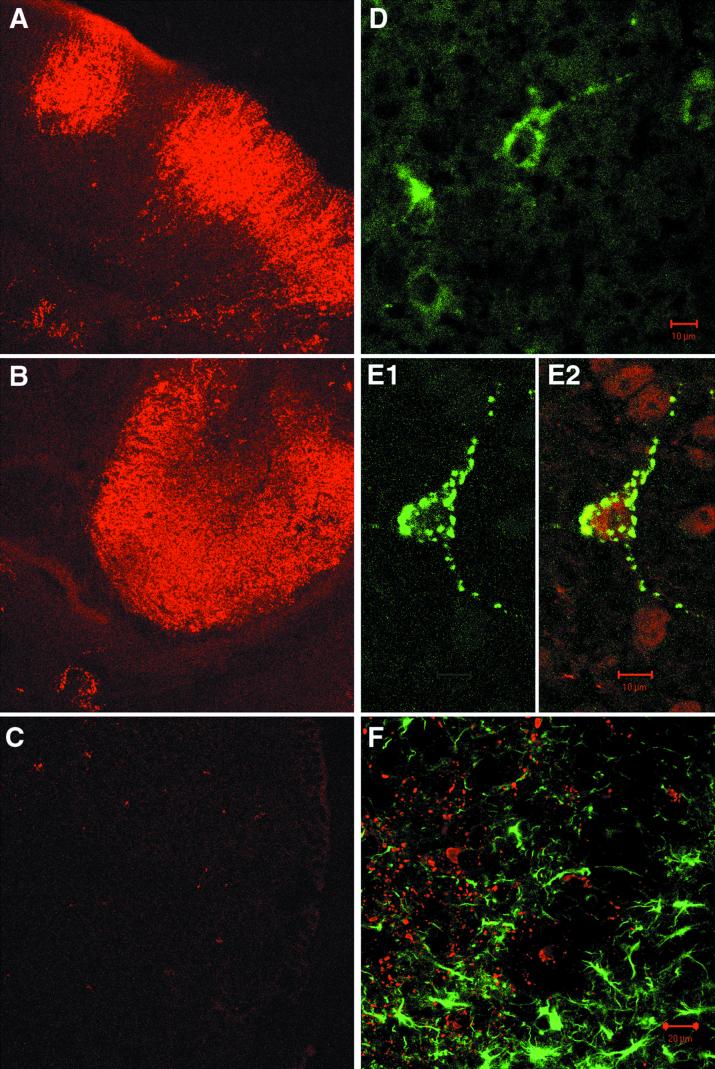
We analyzed brain tissue sections immunocytochemically for the presence of MV proteins in brain. Brain sections were prepared 5 days after MV infection from either CD46 transgenic or nontransgenic mice. Widespread, intensive NP staining was detected on numerous brain sections obtained from CD46 transgenic mice: cortex and hippocampus (Fig. 4A and B, respectively), as well as cerebellum and diencephalon (data not shown). On the brain sections prepared in the same way from infected nontransgenic littermates, only rare NP-positive cells were detected occasionally (Fig. 4C). The distribution of NP-positive cells throughout the cerebral cortex and hippocampus in CD46 transgenic mice suggests that cortical neurons and pyramidal neurons in the hippocampus could be one of the major targets of MV infection in the brain. Less intensive but reproducible staining was obtained with antibodies specific for MV protein H, where according to morphological criteria, the positive cells seemed to be neurons as well (Fig. 4D). Further microscopic examination of transgenic brain sections confirmed that neurons were replicating MV. Figure 4E1 shows a double-fluorescence-labeled thalamic neuron with granular staining characteristic for MV nucleocapsid in the cell body as well as in extensions. The same cell stained positively for neuron-specific marker NF, confirming its neuronal origin (Fig. 4E2). To further determine the cell specificity of MV replication in the brain, we performed double-fluorescence immunohistochemistry with the astrocyte marker GFAP and antibody specific for MV NP (Fig. 4F). GFAP staining revealed numerous reactive astrocytes in various brain areas. However, analysis by confocal microscopy did not show any colocalization of GFAP and NP staining, indicating that MV does not replicate in astrocytes. Finally, infiltration with T and B lymphocytes and microgliosis were not significant on analyzed brain sections (data not shown). Altogether, these results suggest that neurons are the principal MV target in the brain of newborn CD46 transgenic mice.
FIG. 4.
Immunohistochemical analysis of MV replication in brains of infected animals. CD46 transgenic and nontransgenic mice were inoculated 2 days after birth with 3 × 105 TCID50 of MV (Edmonston strain). At day 5, animals were sacrificed, serial frontal sections from frozen brains were prepared and stained for virus proteins NP and H, neuron-specific NF, and astrocyte-specific GFAP. Sections stained for NP (red) in cerebral cortex (A) and zone CA3 of hippocampus (B) of CD46 transgenic animals and cerebral cortex (C) from nontransgenic mouse are shown at a magnification of ×40. (D) Staining for MV H protein (green) in the thalamus. (E1 and E2) Double staining for NP and NF in the thalamus (E1, FITC staining for NP only; E2, the same field with NP [green] and NF [red]). (F) Double staining for NP (red) and GFAP (green) in the thalamus. Results are representative for two to five mice analyzed for each type of staining.
MV induces apoptosis in the brains of CD46 transgenic mice.
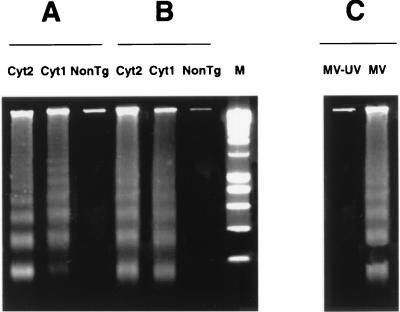
To further analyze pathogenic consequences of MV replication in the brain, we assessed DNA fragmentation in different brain regions. DNA was prepared from the cortex, hippocampus, and cerebellum dissected from infected transgenic and nontransgenic mice and analyzed by electrophoresis for the presence of a DNA ladder specific for apoptosis. Figure 5 shows a typical internucleosomal fragmentation of DNA, characteristic for apoptotic DNA degradation, in three brain structures obtained from MV-infected transgenic mice; however, DNA degradation was not detected in brain structures obtained from MV-inoculated nontransgenic mice or from transgenic animals inoculated by MV-UV. Mice containing the Cyt1 and Cyt2 cytoplasmic tail of CD46 showed the same pattern of brain apoptosis (Fig. 5A and B and data not shown).
FIG. 5.
Nucleosomal DNA fragmentation in brains of infected animals. Transgenic and nontransgenic (NonTg) mice were inoculated 2 days after birth with 3 × 105 TCID50 of MV (Edmonston strain). At day 5, animals were sacrificed, different brain structures were dissected, and DNA was isolated and analyzed on a 1.5% agarose gel. Nucleosomal DNA fragmentation in the cerebellum (10 μg of low-MW DNA) (A) and hippocampus (5 μg of low-MW DNA) (B) of CD46 Cyt1 transgenic (MCP-10), CD46 Cyt2 transgenic (MCP-7), and nontransgenic MV-infected mice is shown. (C) Nucleosomal fragmentation in the frontal cortex (50 μg of low-MW DNA) of MV- but not MV-UV-inoculated CD46 Cyt2 (MCP-7) mice. Lane M, 200-bp DNA marker. Results are representative of three different experiments.
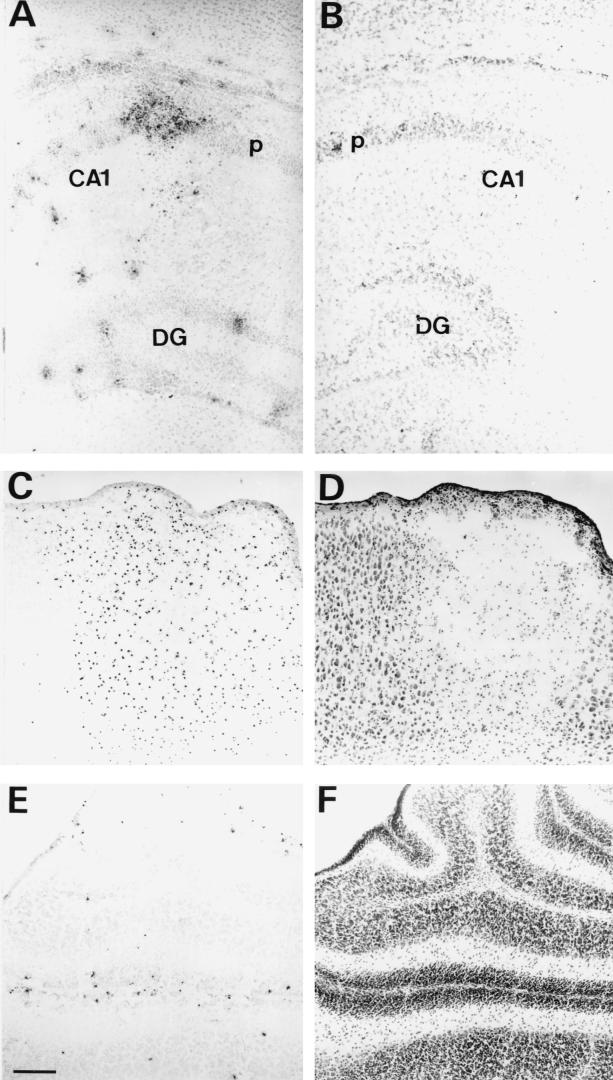
Occurrence of apoptotic cell death in vivo after MV infection was further confirmed by histological study of serial brain sections in parallel with the in situ TUNEL assay. Histological staining demonstrated changes typical of apoptosis, including shrinkage and formation of micronuclei, associated with the destruction of the normal tissue architecture in specific brain areas (motor cortex, hippocampus, and cerebellum [Fig. 6A to D]), as well as in the anterior olfactory nucleus, bed nucleus of stria terminalis, paraventricular nucleus of the thalamus, preoptic area of the hypothalamus, periaqueductal gray matter, and metencephalic reticular formation (data not shown). These alterations did not appear at the same time in all areas: the earliest were detected by 4 days postinfection and regularly included the hippocampus, motor cortex, and anterior olfactory nucleus, whereas the cerebellum became extensively damaged in its medial division shortly before death. TUNEL labeling demonstrated numerous apoptotic cells in areas with neuronal loss (Fig. 6A, C, and E). Brain regions abundant with apoptotic cells were more restricted than regions of intensive MV NP expression and appeared after virus replication was detected. Brain sections obtained from infected nontransgenic mice did not show similar pathology, presenting only rare apoptotic cells, similarly to normal noninfected mice of the same age (Fig. 6B).
FIG. 6.
Neuronal lesions associated with apoptosis in brains of MV-infected animals. CD46 transgenic and nontransgenic mice were inoculated 2 days after birth with 3 × 105 TCID50 of MV (Edmonston strain). At day 5 animals were sacrificed, and serial frontal sections from frozen brains were prepared and stained as described in Materials and Methods. Hippocampal areas of TUNEL-stained, phloxine-counterstained transverse sections from brains of MV-infected CD46 transgenic (A) and nontransgenic (B) mice are shown. Pairs of adjacent transverse sections of MV-infected CD46 transgenic mice were processed for TUNEL (C and E) or cresyl violet (D and F) staining at the levels of motor cortex (C and D) and cerebellum (E and F). TUNEL-positive nuclei appear as black dots restricted to neuronal perikarya-enriched layers of the hippocampus (A) and cerebellum (E) and within anatomical boundaries of the primary motor cortex (C). Results are representative for three animals analyzed per group. The scale bar (E) represents 150 μm (A and B) and 100 μm (C to F). CA1 field of the hippocampus Ammonis horn (CA1), dentate gyrus (DG), and pyramidal cell layer of the hippocampus (p) are labeled.
Maternally transferred immunity delays lethal outcome of MV infection in offspring.
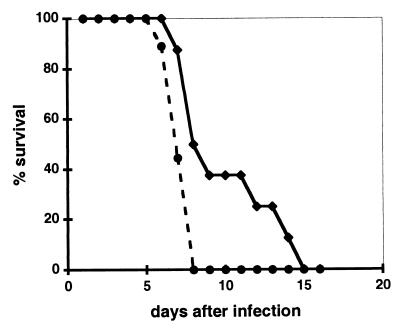
It has been well documented that in humans, transfer of maternal antibodies during pregnancy can protect infants from measles infection for several months, causing at the same time a problem for successful vaccination in early infancy (11). To test if similar protection could be reproduced in our murine model, we immunized intraperitoneally female CD46 transgenic mice with MV-UV (5 × 105 TCID50 per mouse), using a protocol known to induce production of MV-specific antibody in CD46 transgenic mice (48). Mice were challenged 5 weeks later with the same dose of MV-UV and subsequently mated. This immunization procedure gave reproducibly high titers of anti-H antibodies (reference 48 and data not shown). Offspring were infected with MV 1 to 3 days after birth. Survival of mice born to immunized mothers was compared to survival of those from nonimmunized CD46 transgenic mice (Fig. 7). Immunization of mothers significantly prolonged the survival time of the offspring after MV infection (P = 0.019), indicating that transfer of maternal immunity takes place in this CD46 transgenic murine model and can significantly delay death due to MV infection.
FIG. 7.
Maternally transferred immunity increases survival of infected animals. Offspring from untreated (circles) or MV-UV-immunized (diamonds) CD46 transgenic (MCP-7) females were inoculated 2 days after birth with 600 TCID50 of MV (Edmonston strain) and observed daily for clinical symptoms and death. The percentage of animals alive at each indicated time is presented. Eight and nine newborn animals from MV-UV-immunized and control females, respectively, were infected. Survival in two tested groups was significantly different (P = 0.019, Student t test).
DISCUSSION
In this report, we show for the first time reproducible productive MV replication in brains of CD46 transgenic mice. Neurons seemed to be the principal cell population replicating MV. Infection was widespread in specific brain areas, and granular staining characteristic for MV nucleocapsid, resembling measles inclusion bodies seen in human infectious encephalitis, could be easily identified. In addition, positive staining of brain sections for MV envelope protein H suggested that MV is capable of completing its replication cycle in CD46 transgenic neurons. In progressive measles encephalitis and SSPE, MV-infected cells were shown to be essentially neurons and oligodendrocytes (14). In mice, oligodendrocytes develop after birth and are very rare in the brain during the first week (7, 8), which makes them unlikely targets for MV infection in the newborn murine brain in our transgenic model. One of the hallmarks of SSPE disease in humans is a persistent MV infection, where virus is only cell associated. In contrast, in progressive infectious measles encephalitis, absence of an efficient immune response seems to allow productive MV replication in the brain, and infectious virus could be recovered from brain tissue in some cases (43). Pathological analysis of this progressive measles encephalitis showed intracellular viral inclusions in neurons and glial cells, with widespread distribution within the CNS, associated with the lack of brain inflammation (14). Similar histopathology was observed in our transgenic model after MV infection. The less competent immune system in neonates (1), associated with the characteristic histopathological picture of infected brain as well as isolation of infectious virus, argues in favor of a murine model of progressive infectious measles encephalitis.
Several CD46 transgenic rodent models have been generated and extensively analyzed for susceptibility to MV replication. In two models, MV replication was detected in the brain; however, infectious virus could not be reproducibly isolated, mimicking what has been seen in human MV-induced SSPE (9, 39, 45). In one of these models, adult CD46 transgenic mice were shown to be resistant to MV brain infection unless they were crossed in genetically modified background with a nonfunctional alpha/beta interferon receptor system (9, 39). In these double-transgenic mice, virus RNA and antigen were detected in neurons, ependymal cells, and oligodendrocytes and were associated with marked neuronal necrosis, without reproducible production of infectious virus. The difference in neuropathology between this model and our transgenic mice could be related to MV infection of animals at an adult age and with a nonfunctional interferon system. In the second model, expression of CD46 was under the control of a neuron-specific promoter (NSE-CD46 mice), targeting MV entry to neurons (45). Clinical signs and neuropathology developing after MV infection in this model were similar to those observed in our mice. The major difference between NSE-CD46 mice and our model involved the lack of productive brain infection in NSE-CD46 animals, whereas infectious virus could be easily isolated from our transgenic mice. In addition, we could not detect any significant lymphocyte infiltration in infected transgenic brains, as has been recently shown in NSE-CD46 mice (29, 33). The difference in promoter used to govern the expression of CD46 may be responsible for that disparity: it is possible that the HMGCR promoter allows CD46 expression in some additional types of NSE-negative neurons, capable of productive MV replication. In addition, although astrocytes were not infected in our model, the interaction between MV and CD46 expressed on astrocytes could induce cytokine secretion as has been recently demonstrated with a human astrocytoma cell line (19) and human embryonic astrocytes (53). These cytokines could further modulate the outcome of neuronal MV infection. Understanding the differences between these transgenic models will be important for a better understanding of the cellular factors determining lytic and persistent MV brain infection.
Expression of CD46 was required to confer MV brain infection in mice and induce neurological disease by three strains of MV. CD46 is not a unique molecular entity, and due to alternative RNA splicing, several CD46 isoforms are expressed in all human tissue except erythrocytes (32). The various isoforms differ in the extracellular region close to the membrane, designated STP-A, -B, and -C, and cytoplasmic tails, Cyt1 and Cyt2. Although different tissues usually express several CD46 isoforms, the C-Cyt2 isoform is preferentially expressed in the human brain (4, 25). The abilities of various CD46 isoforms to function as MV receptors in vivo have been compared in this study for the first time. Here, we show that C-Cyt1 and C-Cyt2 isoforms, although having different amino acid sequences in their cytoplasmic tails, are functionally similar in mediating MV entry into the brain. Although higher sensitivity to low doses of MV was detected in C-Cyt1-expressing mice, this could be associated to the higher level of CD46 expression in these lines. Analogous results obtained with C-Cyt1 and C-Cyt2 isoforms in vivo are in accord with previous in vitro data (18, 34) and further demonstrate that the C-Cyt1 isoform of CD46, not normally expressed in the human brain, is functionally similar to the C-Cyt2 isoform as an MV receptor. Previous in vitro studies suggested that a large STP domain could hinder CD46 receptor function (24) and that increasing the distance between the MV binding site and the transmembrane domain enhanced virus binding but reduced fusion efficiency (5). Whether the difference in the size of the extracellular STP domain between NSE-CD46 mice (BC-Cyt1) and our model (C-Cyt1) could be responsible for the distinct pathology seen in these two models remains to be determined.
Several viruses have been shown to cause cell death in the CNS by apoptosis, including alphavirus (21) and HIV (17). Recently, apoptosis was detected in numerous CNS areas analyzed in three SSPE patients (36). Apoptosis was seen in the cortex, hippocampus, and thalamus of MV-infected NSE-CD46 transgenic mice (33). In our model, MV-induced apoptosis was largely disseminated in specific brain areas after MV replication reached its maximal level and was easily detectable by DNA laddering in brain structures isolated later during the infection as well as by TUNEL. Brain cell death by apoptosis preceded the death of animals and was probably responsible for it. Similarly to the absence of productive MV replication, apoptosis was not detected in MV-inoculated nontransgenic mice. Furthermore, similar levels of apoptosis were detected in transgenic lines expressing Cyt1 and Cyt2 CD46 cytoplasmic tails but not in MV-UV-treated mice, indicating that apoptosis is a consequence of MV replication in brain cells. This model of MV-induced encephalitis provides a new means of analyzing the nature of MV brain infection and evaluating of the importance of cerebral apoptosis in MV infection in vivo.
This study demonstrated delayed clinical disease and lethality in newborn transgenic mice following a transfer of maternal immunity, which suggests its resemblance to the situation seen in humans (11). It is likely that i.c. inoculation of MV causes the rupture of blood-brain barrier and allows entry of maternal antibodies to the site of infection. In addition, it is possible that in these protected mice, the longer course of disease could change the acute encephalitis to the subacute form, similar to the human disease SSPE, where a high titer of MV antibodies is regularly found. Indeed, antibodies to MV H were shown to change the course of acute encephalitis caused by neuroadapted MV in adult mice (46) and rats (31) by inducing a switch from acute cytopathic effect to a persistent MV infection.
In summary, MV brain infection in this CD46 transgenic model mimics in many aspects the human acute progressive measles encephalitis seen in immunosuppressed patients. This fatal neurological disease caused by MV still occurs among immunocompromised patients (16, 37, 40), particularly in children infected with HIV (6, 35, 44). In some rare cases, vaccinal virus could also be involved in the pathogenesis of MV encephalitis (51). Our transgenic model provides a means to obtain greater insight into the pathogenesis of this fatal neurological disease.
ACKNOWLEDGMENTS
We are grateful to D. Aubert for microinjection of the transgenic construct and to B. Loveland for the generous gift of polyclonal anti-CD46 antibody. The useful comments of D. Bass, E. Derington, F. Wild, H. Hosseini, M. Pechansky, A. Astier, C. Servet-Delprat, and P. O. Vidalain are greatly appreciated.
This work was supported in part by institutional grants from INSERM and Ministere de l'Education Nationale et de la Recherche et de la Technologie (PRFMM IP) and by grants from Ligue National Contre le Cancer (B.H.) and ARC (CRC 6108). A.E. was supported by a fellowship from the MENESR-MAF (1997 to 1998) and Fondation pour la Recherche Medicale (1998 to 1999).
REFERENCES
- 1.Adkins B. T-cell function in newborn mice and humans. Immunol Today. 1999;20:330–335. doi: 10.1016/s0167-5699(99)01473-5. [DOI] [PubMed] [Google Scholar]
- 2.Bartz R, Firsching R, Rima B, ter Meulen V, Schneider-Schaulies J. Differential receptor usage by measles virus strains. J Gen Virol. 1998;79:1015–1025. doi: 10.1099/0022-1317-79-5-1015. [DOI] [PubMed] [Google Scholar]
- 3.Blixenkrone-Moller M, Bernard A, Bencsik A, Sixt N, Diamond L E, Logan J S, Wild T F. Role of CD46 in measles virus infection in CD46 transgenic mice. Virology. 1998;249:238–248. doi: 10.1006/viro.1998.9301. [DOI] [PubMed] [Google Scholar]
- 4.Buchholz C J, Gerlier D, Hu A, Cathomen T, Liszewski M K, Atkinson J P, Cattaneo R. Selective expression of a subset of measles virus receptor-competent CD46 isoforms in human brain. Virology. 1996;217:349–355. doi: 10.1006/viro.1996.0122. [DOI] [PubMed] [Google Scholar]
- 5.Buchholz C J, Schneider U, Devaux P, Gerlier D, Cattaneo R. Cell entry by measles virus: long hybrid receptors uncouple binding from membrane fusion. J Virol. 1996;70:3716–3723. doi: 10.1128/jvi.70.6.3716-3723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budka H, Urbanits S, Liberski P P, Eichinger S, Popow-Kraupp T. Subacute measles virus encephalitis: a new and fatal opportunistic infection in a patient with AIDS. Neurology. 1996;46:586–587. doi: 10.1212/wnl.46.2.586. [DOI] [PubMed] [Google Scholar]
- 7.Cameron R S, Rakic P. Glial cell lineage in the cerebral cortex: a review and synthesis. Glia. 1991;4:124–137. doi: 10.1002/glia.440040204. [DOI] [PubMed] [Google Scholar]
- 8.Campagnoni A T, Pribyl T M, Campagnoni C W, Kampf K, Amur-Umarjee S, Landry C F, Handley V W, Newman S L, Garbay B, Kitamura K. Structure and developmental regulation of Golli-mbp, a 105-kilobase gene that encompasses the myelin basic protein gene and is expressed in cells in the oligodendrocyte lineage in the brain. J Biol Chem. 1993;268:4930–4938. [PubMed] [Google Scholar]
- 9.Cathomen T, Mrkic B, Spehner D, Drillien R, Naef R, Pavlovic J, Aguzzi A, Billeter M A, Cattaneo R. A matrix-less measles virus is infectious and elicits extensive cell fusion: consequences for propagation in the brain. EMBO J. 1998;17:3899–3908. doi: 10.1093/emboj/17.14.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattaneo R, Billeter M A. Mutations and A/I hypermutations in measles virus persistent infections. Curr Top Microbiol Immunol. 1992;176:63–74. doi: 10.1007/978-3-642-77011-1_5. [DOI] [PubMed] [Google Scholar]
- 11.Clements C J, Cutts F T. The epidemiology of measles: thirty years of vaccination. Curr Top Microbiol Immunol. 1995;191:13–33. doi: 10.1007/978-3-642-78621-1_2. [DOI] [PubMed] [Google Scholar]
- 12.Dorig R E, Marcil A, Chopra A, Richardson C D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 13.Duprex W P, Duffy I, McQuaid S, Hamill L, Cosby S L, Billeter M A, Schneider-Schaulies J, ter Meulen V, Rima B K. The H gene of rodent brain-adapted measles virus confers neurovirulence to the Edmonston vaccine strain. J Virol. 1999;73:6916–6922. doi: 10.1128/jvi.73.8.6916-6922.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esiri M M, Oppenheimer D R, Brownell B, Haire M. Distribution of measles antigen and immunoglobulin-containing cells in the CNS in subacute sclerosing panencephalitis (SSPE) and atypical measles encephalitis. J Neurol Sci. 1982;53:29–43. doi: 10.1016/0022-510x(82)90078-8. [DOI] [PubMed] [Google Scholar]
- 15.Gautier C, Mehtali M, Lathe R. A ubiquitous mammalian expression vector, pHMG, based on a housekeeping promoter. Nucleic Acids Res. 1989;17:8389. doi: 10.1093/nar/17.20.8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gazzola P, Cocito L, Capello E, Roccatagliata L, Canepa M, Mancardi G L. Subacute measles encephalitis in a young man immunosuppressed for ankylosing spondylitis. Neurology. 1999;52:1074–1077. doi: 10.1212/wnl.52.5.1074. [DOI] [PubMed] [Google Scholar]
- 17.Gelbard H A, James H J, Sharer L R, Perry S W, Saito Y, Kazee A M, Blumberg B M, Epstein L G. Apoptotic neurons in brains from paediatric patients with HIV-1 encephalitis and progressive encephalopathy. Neuropathol Appl Neurobiol. 1995;21:208–217. doi: 10.1111/j.1365-2990.1995.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 18.Gerlier D, Loveland B, Varior-Krishnan G, Thorley B, McKenzie I, Rabourdin-Combe C. Measles virus receptor properties are shared by several CD46 isoforms differing in extracellular regions and cytoplasmic tails. J Gen Virol. 1994;75:2163–2171. doi: 10.1099/0022-1317-75-9-2163. [DOI] [PubMed] [Google Scholar]
- 19.Ghali M, Schneider-Schaulies J. Receptor (CD46)- and replication-mediated interleukin-6 induction by measles virus in human astrocytoma cells. J Neurovirol. 1998;4:521–530. doi: 10.3109/13550289809113496. [DOI] [PubMed] [Google Scholar]
- 20.Giraudon P, Wild F T. Correlation between epitopes on hemagglutinin of measles virus and biological activities: passive protection by monoclonal antibodies is related to their hemagglutination inhibiting activity. Virology. 1985;144:46–58. doi: 10.1016/0042-6822(85)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Griffin D E, Levine B, Tyor W R, Tucker P C, Hardwick J M. Age-dependent susceptibility to fatal encephalitis: alphavirus infection of neurons. Arch Virol Suppl. 1994;9:31–39. doi: 10.1007/978-3-7091-9326-6_4. [DOI] [PubMed] [Google Scholar]
- 22.Hogan B, Costantini F, Lacy E. Manipulation of the mouse embryo: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 23.Horvat B, Rivailler P, Varior-Krishnan G, Cardoso A, Wild F, Gerlier D, Rabourdin-Combe C. Transgenic mice expressing human measles virus (MV) receptor CD46 provide cells exhibiting different permissivities to MV infection. J Virol. 1996;70:6673–6681. doi: 10.1128/jvi.70.10.6673-6681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwata K, Seya T, Ueda S, Ariga H, Nagasawa S. Modulation of complement regulatory function and measles virus receptor function by the serine-threonine-rich domains of membrane cofactor protein (CD46) Biochem J. 1994;304:169–175. doi: 10.1042/bj3040169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnstone R W, Russell S M, Loveland B E, McKenzie I F C. Polymorphic expression of CD46 protein isoforms due to tissue-specific RNA splicing. Mol Immunol. 1993;30:1231–1241. doi: 10.1016/0161-5890(93)90038-d. [DOI] [PubMed] [Google Scholar]
- 26.Jourdan F, Moyse E, De Bilbao F, Dubois-Dauphin M. Olfactory neurons are protected from apoptosis in adult transgenic mice over-expressing the bcl-2 gene. Neuroreport. 1998;9:921–926. doi: 10.1097/00001756-199803300-00029. [DOI] [PubMed] [Google Scholar]
- 27.Katz M. Clinical spectrum of measles. Curr Top Microbiol Immunol. 1995;191:1–12. doi: 10.1007/978-3-642-78621-1_1. [DOI] [PubMed] [Google Scholar]
- 28.Laird P W, Zijderveld A, Linders K, Rudnicki M A, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawrence D M, Vaughn M M, Belman A R, Cole J S, Rall G F. Immune response-mediated protection of adult but not neonatal mice from neuron-restricted measles virus infection and central nervous system disease. J Virol. 1999;73:1795–1801. doi: 10.1128/jvi.73.3.1795-1801.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liebert U G, Finke D. Measles virus infections in rodents. Curr Top Microbiol Immunol. 1995;191:149–166. doi: 10.1007/978-3-642-78621-1_10. [DOI] [PubMed] [Google Scholar]
- 31.Liebert U G, Schneider-Schaulies S, Baczko K, ter Meulen V. Antibody-induced restriction of viral gene expression in measles encephalitis in rats. J Virol. 1990;64:706–713. doi: 10.1128/jvi.64.2.706-713.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liszewski M K, Post T W, Atkinson J P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 33.Manchester M, Eto D S, Oldstone M B A. Characterization of the inflammatory response during acute measles encephalitis in NSE-CD46 transgenic mice. J Neuroimmunol. 1999;96:207–217. doi: 10.1016/s0165-5728(99)00036-3. [DOI] [PubMed] [Google Scholar]
- 34.Manchester M, Liszewski M K, Atkinson J P, Oldstone M B. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc Natl Acad Sci USA. 1994;91:2161–2165. doi: 10.1073/pnas.91.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McQuaid S, Cosby S L, Koffi K, Honde M, Kirk J, Lucas S B. Distribution of measles virus in the central nervous system of HIV-seropositive children. Acta Neuropathol (Berlin) 1998;96:637–642. doi: 10.1007/s004010050945. [DOI] [PubMed] [Google Scholar]
- 36.McQuaid S, McMahon J, Herron B, Cosby S L. Apoptosis in measles virus-infected human central nervous system tissues. Neuropathol Appl Neurobiol. 1997;23:218–224. [PubMed] [Google Scholar]
- 37.Monfort-Gouraud M, Robain O, Boccara F, Badoual J. Delayed measles encephalitis in a leukemic child. Arch Fr Pediatr. 1990;47:275–277. [PubMed] [Google Scholar]
- 38.Morgan B P, Gasque P. Expression of complement in the brain: role in health and disease. Immunol Today. 1996;17:461–466. doi: 10.1016/0167-5699(96)20028-f. [DOI] [PubMed] [Google Scholar]
- 39.Mrkic B, Pavlovic J, Rulicke T, Volpe P, Buchholz C J, Hourdcade D, Atkinson J P, Aguzzi A, Cattaneo R. Measles virus spread and pathogenesis in genetically modified mice. J Virol. 1998;72:7420–7427. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mustafa M M, Weitman S D, Winick N J, Bellini W J, Timmons C F, Siegel J D. Subacute measles encephalitis in the young immunocompromised host: report of two cases diagnosed by polymerase chain reaction and treated with ribavirin and review of the literature. Clin Infect Dis. 1993;16:654–660. doi: 10.1093/clind/16.5.654. [DOI] [PubMed] [Google Scholar]
- 41.Naniche D, Varior-Krishnan G, Cervoni F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niewiesk S, Schneider-Schaulies J, Ohnimus H, Jassoy C, Schneider-Schaulies S, Diamond L, Logan J S, ter Meulen V. CD46 expression does not overcome the intracellular block of measles virus replication in transgenic rats. J Virol. 1997;71:7969–7973. doi: 10.1128/jvi.71.10.7969-7973.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norrby E, Kristensson K. Measles virus in the brain. Brain Res Bull. 1997;44:213–220. doi: 10.1016/s0361-9230(97)00139-1. [DOI] [PubMed] [Google Scholar]
- 44.Poon T P, Tchertkoff V, Win H. Subacute measles encephalitis with AIDS diagnosed by fine needle aspiration biopsy. A case report. Acta Cytol. 1998;42:729–733. doi: 10.1159/000331835. [DOI] [PubMed] [Google Scholar]
- 45.Rall G F, Manchester M, Daniels L R, Calahan E M, Belman A R, Oldstone M B A. A transgenic mouse model for measles virus infection in brain. Proc Natl Acad Sci USA. 1997;94:4569–4663. doi: 10.1073/pnas.94.9.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rammohan K W, McFarland H F, McFarlin D E. Induction of subacute murine measles encephalitis by monoclonal antibody to virus haemagglutinin. Nature. 1981;290:588–589. doi: 10.1038/290588a0. [DOI] [PubMed] [Google Scholar]
- 47.Reed J L, Muench H. A simple method of estimating fifty percent end points. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 48.Rivailler P, Trescol-Biemont M C, Gimenez C, Rabourdin-Combe C, Horvat B. Enhanced MHC class II-restricted presentation of measles virus (MV) hemagglutinin in transgenic mice expressing human MV receptor CD46. Eur J Immunol. 1998;28:1301–1314. doi: 10.1002/(SICI)1521-4141(199804)28:04<1301::AID-IMMU1301>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 49.Schulz T F, Hoad J G, Whitby D, Tizard E J, Dillon M J, Weiss R A. A measles virus isolate from child with Kawasaki disease: sequence comparison with contemporaneous isolates from ‘classical’ cases. J Gen Virol. 1992;73:1581–1586. doi: 10.1099/0022-1317-73-6-1581. [DOI] [PubMed] [Google Scholar]
- 50.Thorley B R, Milland J, Christiansen D, Lanteri M B, McInnes B, Moeller I, Rivailler P, Horvat B, Rabourdin-Combe C, Gerlier D, McKenzie I F C, Loveland B E. Transgenic expression of a CD46 (membrane cofactor protein) minigene: studies of xenotransplantation and measles virus infection. Eur J Immunol. 1997;27:726–734. doi: 10.1002/eji.1830270322. [DOI] [PubMed] [Google Scholar]
- 51.Valmari P, Lanning M, Tuokko H, Kouvalainen K. Measles virus in the cerebrospinal fluid in postvaccination immunosuppressive measles encephalopathy. Pediatr Infect Dis J. 1987;6:59–63. doi: 10.1097/00006454-198701000-00015. [DOI] [PubMed] [Google Scholar]
- 52.Weiss R. Measles battle loses potent weapon. Science. 1992;258:546–547. doi: 10.1126/science.1329205. [DOI] [PubMed] [Google Scholar]
- 53.Xiao B G, Mousa A, Kivisakk P, Seiger A, Bakhiet M, Link H. Induction of beta-family chemokines mRNA in human embryonic astrocytes by inflammatory cytokines and measles virus protein. J Neurocytol. 1998;27:575–580. doi: 10.1023/a:1006918110952. [DOI] [PubMed] [Google Scholar]