Abstract
Introduction
The development of new and changing melanocytic lesions has been increasingly reported as an adverse dermatologic toxicity of BRAF inhibitor therapy. Melanocytic lesions and melanomas induced by BRAF inhibitor therapy that lack BRAF V600E expression have been less commonly described. One mechanism that has been proposed for the development of BRAF inhibitor-induced melanocytic lesions, including those lacking BRAF V600E expression, is the paradoxical activation of the MAPK signaling pathway in BRAF wild-type (BRAFWT) cells.
Case Presentation
Herein, we report a rare case of a 39-year-old woman who developed numerous BRAF V600E-negative eruptive melanocytic nevi following encorafenib, cetuximab, and binimetinib combination therapy, the current standard of care for the treatment of BRAF-mutant metastatic colorectal cancer.
Conclusion
Patients treated with BRAF inhibitors, with or without related combination therapies, who develop BRAFWT melanocytic lesions are at risk for developing both dysplastic nevi and melanoma, thereby warranting baseline dermatoscopic evaluation prior to the initiation of therapy as well as regular follow-up during and after treatment.
Keywords: Eruptive melanocytic nevi, BRAF inhibitor, EGFR inhibitor, MEK inhibitor, BRAF V600E, Encorafenib, Cetuximab, Binimetinib, Case report
Introduction
The development of eruptive melanocytic nevi (EMN) and other melanocytic lesions including melanoma have been increasingly reported as a dermatologic toxicity of BRAF inhibitor therapy, representing up to 8% of biopsied cutaneous lesions [1–8]. One criteria used by Perry et al. to define EMN is the development of greater than 10 widespread melanocytic nevi during a 6-month period in association with biologic chemotherapeutics therapy, including BRAF inhibitors. EMN typically presents with the simultaneous appearance of hundreds of melanocytic lesions on previously uninvolved skin with intermittent sun exposure [3]. A mechanism that has been proposed for the development of melanocytic lesions following BRAF inhibitor therapy is the paradoxical activation of the MAPK signaling pathway in BRAFWT cells [7].
Few reports have described BRAFWT EMN induced by BRAF inhibitor therapy [1–4, 7]. Herein, we report a rare case of a patient who presented with numerous new BRAF V600E-negative EMN following a combined regimen of encorafenib, cetuximab, and binimetinib, the current standard of care for previously treated BRAF V600E-mutant mCRC.
Case Presentation
A 39-year-old woman with a history of ulcerative colitis and three-month diagnosis of BRAF V600E-mutant colorectal cancer with metastases to the liver presented to the dermatology clinic after developing innumerable 1–5 mm dark brown macules and papules. Prior to clinical presentation, the patient received 2 months of chemotherapeutic treatment with FOLFOXIRI plus bevacizumab. Due to limited treatment response, she was transitioned to combination therapy with encorafenib (300 mg daily), cetuximab (370 mg weekly), and binimetinib (45 mg twice daily).
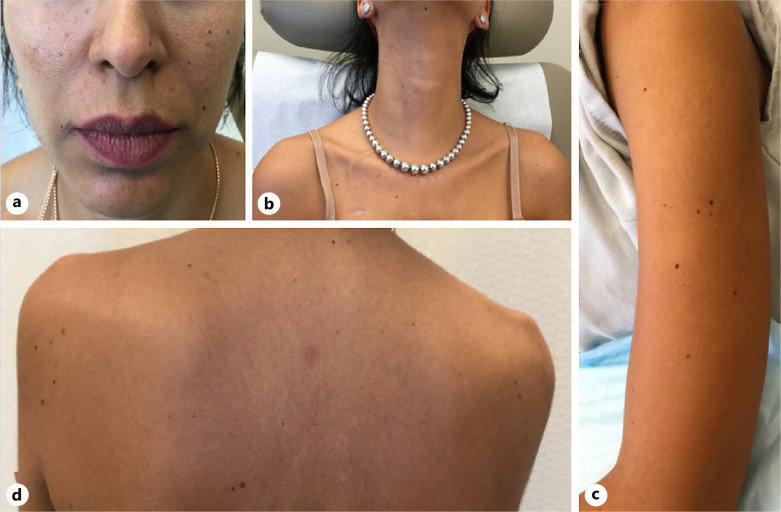
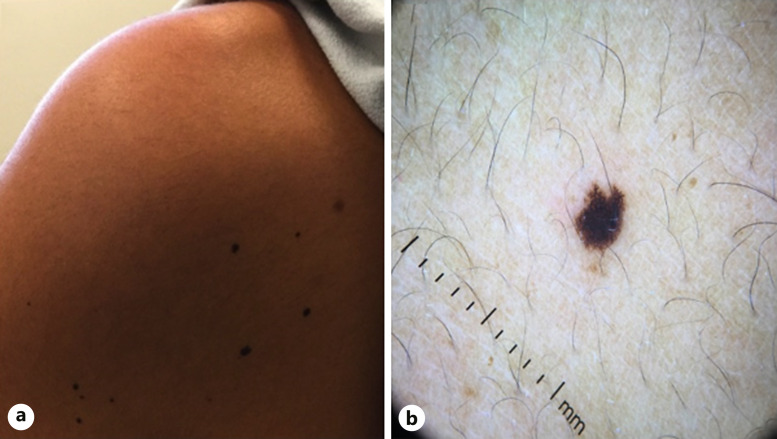
After completing 2 cycles of the triplet regimen over the course of 2 weeks, she noted multiple new melanocytic lesions on her face. Over the next 8 weeks while continuing on this regimen, she continued to develop over 200 additional lesions on her oral labia, shoulders, arms, palms, and buttocks (Fig. 1a–d). On dermatoscopy, the lesions demonstrated a fibrillar network pattern with focal areas of irregularity and non-perifollicular pigment dropout (Fig. 2a, b). No ocular lesions were noted. Note that the patient temporarily stopped binimetinib treatment 7 weeks after initiation due to significant menorrhagia. She resumed binimetinib after menorrhagia was controlled with 2 weeks of leuprolide acetate therapy.
Fig. 1.
New dark brown to black macules and papules of the face (a), neck (b), left arm (c), and back (d) that developed within 10 weeks after initiating encorafenib, cetuximab, and binimetinib combination therapy.
Fig. 2.
New dark brown to black papules on the posterior left shoulder (a), with fibrillar pigment network on dermoscopy (b).
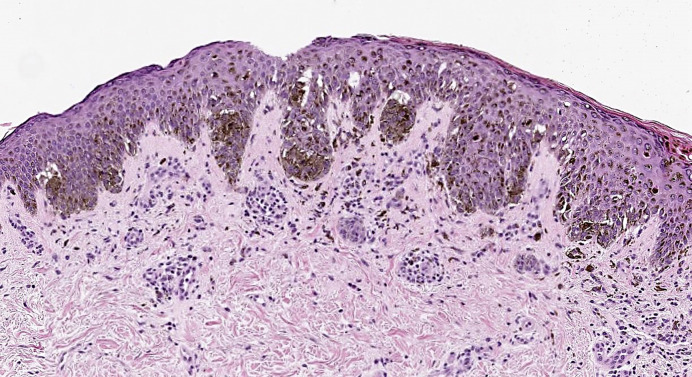
A punch biopsy of a lesion on the right upper arm was performed, which demonstrated a compound melanocytic proliferation with moderate atypia and negative staining for BRAF V600E (Fig. 3). Histopathologic findings did not meet diagnostic criteria for melanoma given that there was no significant pagetoid scatter, no severe cytologic atypia or dermal mitotic figures present, and maturation was present with depth and small size. Close follow-up with full-body skin exams every six to 8 weeks was recommended to the patient to monitor for malignant transformation.
Fig. 3.
Punch biopsy of a 4-mm dark brown macule on the patient’s right upper arm was obtained. Immunohistochemistry for BRAF V600E showed negative staining in rare dermal melanocytic nests. H&E ×10 demonstrated a moderately atypical compound melanocytic nevus.
16 weeks after initiating therapy, the patient developed new lung lesions with interval increases in liver metastases. Patient changed treatment to regorafenib and pembrolizumab combination therapy at an external institution and was noted to develop a drug rash and fever 2 weeks after initiation of treatment. Upon resolution of her drug rash, general physical exam described the patient’s skin as having no suspicious lesions. No specific comments regarding EMN after stopping encorafenib/cetuximab/binimetinib were made. The patient died due to complications secondary to mCRC, 11 months after diagnosis. The CARE Checklist has been completed by the authors for this case report, attached as supplementary material (online suppl. material; for all online suppl. material, see https://doi.org/10.1159/000539058) [18].
Discussion
Paradoxical upregulation of the MAPK signaling pathway in BRAFWT cells has been proposed as the underlying mechanism for the development of new EMN and the increase in size and pigmentation of preexisting nevi following BRAF inhibitor therapy [5, 7]. In BRAF-mutant cancer cells, BRAF inhibition results in decreased downstream ERK signaling in the MAPK pathway, arresting tumor growth [4, 9]. However, in BRAFWT cells, BRAF inhibitor treatment has been hypothesized to upregulate the MAPK cascade, either by transactivation of upstream RAF dimers [9]; or translocation of RAS mutant, wild-type BRAF to the cell membrane to dimerize with CRAF, stimulating CRAF signaling [10]. Subsequently, rapid downstream activation of MEK/ERK signaling stimulates proliferation and survival of BRAFWT cells, which may develop into dysplastic melanocytic lesions and melanoma [4, 7, 9]. Although it is known that patients with a history of melanoma are at risk for developing a second or additional primary melanomas, the incidence of developing a second primary melanoma on BRAF inhibitor therapy appears to be higher at ∼21% [1]. Almost all patients who were diagnosed with a second primary melanoma were diagnosed within 1 year of treatment with a BRAF inhibitor [1].
Paradoxical MAPK activation may play a role in our patient’s development of widespread eruptive EMN given the administration of a BRAF inhibitor (encorafenib), but further investigation is needed to elucidate how the addition of EGFR inhibitors (e.g., cetuximab) and MEK inhibitors (e.g., binimetinib) may affect paradoxical upregulation of the MAPK signaling cascade (online suppl. material 1).
Among biopsies of BRAF inhibitor-induced melanocytic nevi, more than 75% exhibit histologic atypia, often of moderate to severe degree [7]. This cytologic atypia, similarly observed in our patient’s biopsy, may be associated with increased cyclin D1 expression downstream of paradoxical MAPK activation in BRAFWT cells [7].
Our patient developed over 200 new EMN within 10 weeks of combination treatment with encorafenib, cetuximab, and binimetinib. There is one other case by Meneguzzo et al. [5] of a patient who presented with eruptive nevi after treatment with encorafenib and cetuximab co-therapy for BRAF V600E-mutant mCRC. Within the first 2 months of therapy, multiple eruptive nevi developed. Increased size and darkening of preexisting nevi were also observed. BRAF V600E expression was not described for the new or preexisting nevi.
According to the Naranjo algorithm for causality assessment of adverse drug reactions, our patient’s score of 8 indicates that it was “probable” that her development of EMN was caused by encorafenib, cetuximab, and binimetinib therapy, given that her adverse cutaneous reaction followed a reasonable temporal sequence after initiating triplet therapy and also followed a recognized response to the suspected drug, encorafenib [11]. Additionally, the WHO-UMC causality scale indicates that it was “possible” that the patient’s adverse cutaneous reaction was caused by her triplet regimen given that there was no information, or uncertainty, with regarding the nature of her EMN after stopping encorafenib, cetuximab, and binimetinib [12]. To elucidate how each drug in our patient’s triplet regimen may contribute to EMN and melanoma development, we review these specific cutaneous findings in encorafenib, cetuximab, and binimetinib monotherapy.
Encorafenib is a second-generation RAF kinase inhibitor that suppresses the MAPK signaling pathway in BRAF V600E-mutant tumors (online suppl. material 1) [5]. Currently, there is one report of noncancerous EMN development following encorafenib monotherapy [2]. Another report described the appearance of multiple second primary melanomas within a preexisting nevus in a patient undergoing encorafenib monotherapy for metastatic melanoma treatment [6]. Unlike encorafenib, first-generation BRAF inhibitors, such as vemurafenib, have been commonly associated with EMN, and there are novel reports of EMN induced by third-generation BRAF inhibitors, such as ponatinib [3, 13].
Cetuximab is an EGFR inhibitor that targets a tyrosine kinase upstream of the MAPK cascade (online suppl. material 1) [14]. There are currently no reports of EMN in association with cetuximab monotherapy. However, there are multiple reported cases of erlotinib, another intracellular EGFR inhibitor, inducing the development of EMN and melanomas as well as increased pigmentation of preexisting nevi [14, 15].
Binimetinib is a highly selective inhibitor of MEK, a central kinase downstream of RAF (online suppl. material 1) [16]. Although the addition of binimetinib to the doublet therapy of encorafenib and cetuximab has not been shown to make a difference in the overall survival of mCRC patients, our patient maintained binimetinib treatment due to possible improvement of EMN [4, 17]. Chen et al. [4] describe a metastatic melanoma patient who developed numerous nonmalignant EMN within 3 months of vemurafenib (BRAF inhibitor) therapy. Four months after adding cobimetinib (MEK inhibitor) to her drug regimen, many EMN faded in color, and most of her vemurafenib-induced EMN involuted. However, since this patient’s nevi were not biopsied, it is unknown whether a BRAF V600E mutation was present.
During the interval in which our patient paused binimetinib treatment until her menorrhagia was controlled with leuprolide acetate, she continued to develop more EMN. From the time of binimetinib reinitiation until her death, resolution of EMN was not observed in our patient.
The strengths of this study include recognizing and describing BRAF V600E-negative EMN as an uncommon but possible adverse cutaneous reaction following encorafenib, cetuximab, and binimetinib combination therapy, used in the treatment of a growing number of mCRC patients, which may progress into dysplastic nevi and melanoma. In addition to increasing physician awareness regarding the importance of pretreatment evaluation and regular follow-up full-body skin exams during and after treatment, this case report offers insights into possible mechanisms behind this adverse skin reaction. Limitations of this case report include not performing multiple biopsies as the lesions were new, eruptive (over 100+), small-sized (1–5 mm), and developed simultaneously. Additionally, as this patient was undergoing multiple anticancer therapies for metastatic carcinoma, it was favored to not go overboard with procedures from a clinician and patient point of view. Furthermore, no information was available regarding the nature of the patient’s EMN after stopping encorafenib, cetuximab, and binimetinib.
Conclusion
BRAF WT EMN and melanomas are a recently reported dermatologic adverse effect of BRAF inhibitor therapy and rarely have been described secondary to a combined regimen of encorafenib (BRAF inhibitor), cetuximab (EGFR inhibitor), and binimetinib (MEK inhibitor) therapy [1–4, 7, 17]. There is currently limited knowledge regarding the clinical significance of EMN, but multiple studies demonstrate that patients on BRAF inhibitor therapy who develop melanocytic lesions lacking BRAF V600E expression are at risk for developing both dysplastic nevi and melanoma [1–4, 7].
For this reason, full-body skin examinations with baseline dermatoscopic evaluation of existing pigmented lesions are recommended prior to the initiation of BRAF inhibitor therapy with or without combination treatments. Regular follow-up is recommended for the duration of treatment, as well as following treatment, especially if new nevi continue to develop [3]. Referral to a Pigmented Lesion Clinic or expert should also be considered. Furthermore, the threshold should be low for biopsy for histopathologic analysis and immunohistochemical staining of newly developed lesions or existing nevi with alterations in color or size, given the possible risk of progression to melanoma [3].
Statement of Ethics
Ethical approval is not required for this study in accordance with local or national guidelines. This manuscript contains original, unpublished work that is not being considered for publication elsewhere at the same time. Written informed consent was obtained from the patient’s next of kin for publication of this case report and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
All the listed authors fulfill ICMJE’s four criteria for authorship. Karen Lam, BS: conceptualization, data curation, project administration, writing – original draft, and writing – review and editing; Gregory A. Gates, DO, Daniel Q. Bach, MD, MPH, and Kyle Cheng, MD, MS: conceptualization, data curation, project administration, supervision, and writing – review and editing.
Funding Statement
This study was not supported by any sponsor or funder.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary Material
References
- 1. Zimmer L, Hillen U, Livingstone E, Lacouture ME, Busam K, Carvajal RD, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30(19):2375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anforth RM, Carlos GR, Scolyer RA, Chou S, Fernandez-Peñas P. Eruptive naevi in a patient treated with LGX818 for BRAF mutant metastatic melanoma. Melanoma Res. 2015;25(1):91–4. [DOI] [PubMed] [Google Scholar]
- 3. Cohen PR, Bedikian AY, Kim KB. Appearance of new vemurafenib-associated melanocytic nevi on normal-appearing skin: case series and a review of changing or new pigmented lesions in patients with metastatic malignant melanoma after initiating treatment with vemurafenib. J Clin Aesthet Dermatol. 2013;6(5):27–37. [PMC free article] [PubMed] [Google Scholar]
- 4. Chen FW, Tseng D, Reddy S, Daud AI, Swetter SM. Involution of eruptive melanocytic nevi on combination BRAF and MEK inhibitor therapy. JAMA Dermatol. 2014;150(11):1209–12. [DOI] [PubMed] [Google Scholar]
- 5. Meneguzzo A, Lazzarotto A, Alaibac M. Eruptive melanocytic nevi secondary to encorafenib for BRAF mutant metastatic colorectal cancer. In Vivo. 2020;34(1):441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Donati M, Zelano G, Coppola R, et al. Personalized and targeted mutational analysis of multiple second primary melanomas under kinase inhibitors. G Ital Dermatol Venereol. 2019. [DOI] [PubMed] [Google Scholar]
- 7. Mudaliar K, Tetzlaff MT, Duvic M, Ciurea A, Hymes S, Milton DR, et al. BRAF inhibitor therapy-associated melanocytic lesions lack the BRAF V600E mutation and show increased levels of cyclin D1 expression. Hum Pathol. 2016;50:79–89. [DOI] [PubMed] [Google Scholar]
- 8. Perry BM, Nguyen A, Desmond BL, Blattner CM, Thomas RS, Young RJ. Eruptive nevi associated with medications (ENAMs). J Am Acad Dermatol. 2016;75(5):1045–52. [DOI] [PubMed] [Google Scholar]
- 9. Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464(7287):427–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140(2):209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naranjo CA, Busto U, Sellers EM, Ruiz, I, Roberts, EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45. [DOI] [PubMed] [Google Scholar]
- 12. Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Case Rep. 2013;2(5):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim YH, Kim Y, Park TJ, Kang HY. Ponatinib-induced eruptive nevi and melanocytic proliferation. Melanoma Res. 2022;32(1):59–62. [DOI] [PubMed] [Google Scholar]
- 14. Santiago F, Gonçalo M, Reis JP, Figueiredo A. Adverse cutaneous reactions to epidermal growth factor receptor inhibitors: a study of 14 patients. An Bras Dermatol. 2011;86(3):483–90. [DOI] [PubMed] [Google Scholar]
- 15. Seidman JS, Eichenfield DZ, Orme CM. Nevoid melanoma and eruptive nevi from erlotinib. Dermatol Online J. 2020;26(6). [PubMed] [Google Scholar]
- 16. Subbiah V, Baik C, Kirkwood JM. Clinical development of BRAF plus MEK inhibitor combinations. Trends Cancer. 2020;6(9):797–810. [DOI] [PubMed] [Google Scholar]
- 17. Tabernero J, Grothey A, Van Cutsem E, Yaeger R, Wasan H, Yoshino T, et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600e–mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol. 2021;39(4):273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D, et al. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Health Med. Sep 2013;2(5):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.