Abstract
Background:
Abusive head trauma (AHT) is a mechanism of pediatric traumatic brain injury (TBI) with high morbidity and mortality. Multiorgan dysfunction syndrome (MODS), defined as organ dysfunction in two or more organ systems, is also associated with morbidity and mortality in critically ill children. Our objective was to compare the frequency of MODS and evaluate its association with outcome between AHT and accidental TBI (aTBI).
Methods:
This was a single center, retrospective cohort study including children under 3 years old admitted to the pediatric intensive care unit with nonpenetrating TBI between 2014 and 2021. Presence or absence of MODS on days 1, 3, and 7 using the Pediatric Logistic Organ Dysfunction-2 score and new impairment status (Functional Status Scale score change > 1 compared with preinjury) at hospital discharge (HD), short-term timepoint, and long-term timepoint were abstracted from the electronic health record. Multiple logistic regression was performed to examine the association between MODS and TBI mechanism with new impairment status.
Results:
Among 576 children, 215 (37%) had AHT and 361 (63%) had aTBI. More children with AHT had MODS on days 1 (34% vs. 23%, p = 0.003), 3 (28% vs. 6%, p < 0.001), and 7 (17% vs. 3%, p < 0.001) compared with those with aTBI. The most common organ failures were cardiovascular ([AHT] 66% vs. [aTBI] 66%, p = 0.997), neurologic (33% vs. 16%, p < 0.001), and respiratory (34% vs. 15%, p < 0.001). MODS was associated with new impairment in multivariable logistic regression at HD (odds ratio 19.1 [95% confidence interval 9.8–38.6, p < 0.001]), short-term discharge (7.4 [3.7–15.2, p < 0.001]), and long-term discharge (4.3 [2.0–9.4, p < 0.001])]. AHT was also associated with new impairment at HD (3.4 [1.6–7.3, p = 0.001]), short-term discharge (2.5 [1.3–4.7, p = 0.005]), and long-term discharge (2.1 [1.1–4.1, p = 0.036]).
Conclusions:
Abusive head trauma as a mechanism was associated with MODS following TBI. Both AHT mechanism and MODS were associated with new impairment at all time points.
Keywords: Child abuse, Traumatic brain injury, Multiorgan dysfunction syndrome, Pediatric intensive care units, Long-term effects
Introduction
Abusive head trauma (AHT) is a common mechanism of traumatic brain injury (TBI) in infants and young children [1, 2]. AHT is associated with acquisition of new cognitive and physical impairments and epilepsy [1, 3–9]. Impairment may be exacerbated by secondary hypoxia and hypotension and injury to nonneurologic organs that may lead to multiple organ dysfunction syndrome (MODS) [10, 11].
MODS is associated with increased morbidity and mortality in general pediatric critical care populations and accidental TBI (aTBI); however, MODS is less characterized in the AHT population [12–15]. In pediatric TBI, MODS occurred in 20% of patients; AHT mechanism was identified as a risk factor, with 1,382/3,530 (39%) patients with abuse having MODS [16].
The most commonly used trauma scoring system, the Injury Severity Score (ISS), underestimates the risk of mortality and impaired functional outcome in patients who have experienced child abuse [17]. The Pediatric Logistic Organ Dysfunction-2 (PELOD-2) score is validated in pediatric critical illness for prediction of mortality, but it has not been evaluated in the AHT population [18]. In a previous study comparing various MODS scoring systems, PELOD-2 performed better than the pediatric multiple organ dysfunction score [19].
The objectives for this study were to compare the frequency of MODS between patients with AHT and patients with aTBI and analyze the association of TBI mechanism and MODS occurrence in the first week of admission with mortality and new functional impairment.
Methods
Study Design and Setting
The University of Pittsburgh Institutional Review Board approved this retrospective, observational cohort study.
Inclusion and Exclusion Criteria
Patients less than 3 years of age admitted to the pediatric intensive care unit with TBI between 2014 and 2021 were included. Patients admitted with penetrating trauma were excluded.
Data Collection
The Benedum Trauma Center database supplied the eligible patients, and the electronic health record was used to collect patient demographics, TBI details, brain computed tomography or magnetic resonance imaging results, laboratory results, and vital signs. AHT was defined by the conclusion of the multidisciplinary child protection team; patients designated as having injuries that were “highly concerning” or “diagnostic” for AHT were included [2, 20, 21]. ISS was obtained on admission. Glasgow Coma Score (GCS) scores between 13 and 15 were classified as mild TBI, between 9 and 12 as moderate TBI, and equal to or below 8 as severe TBI [22]. PELOD-2 was calculated at day 1, day 3, and day 7 of admission. PELOD-2 uses mean blood pressure and lactate to calculate cardiovascular dysfunction; GCS and pupillary reaction for neurologic dysfunction; PaO2 to FiO2 ratio, PCO2, and mechanical ventilation for respiratory dysfunction; white blood cell and platelet count for hematologic dysfunction; and creatinine for renal dysfunction [18]. After discharge, patients were assumed to be negative for MODS, and after patients died, they were removed from the day analysis after death. Organ failure is defined as a PELOD-2 score or greater than 1 for each specific organ system. MODS is defined as two or more organ failures.
Outcomes
The primary outcome was new impairment in children who survived, defined as an increase in validated Functional Status Scale of more than one between preinjury, hospital discharge (HD), short-term timepoint (closest to 1 year after discharge), and long-term timepoint (closest to 5 years after discharge) from clinical documentation of neurologic examination and developmental history in the electronic health record [23].
Statistical Analyses
Nonparametric tests (Wilcoxon rank-sum test, Pearson’s χ2 test, and Fisher’s exact test) compared characteristics between AHT and aTBI groups. Multivariate logistic regressions were conducted to analyze the association between TBI mechanism, MODS status, and new impairment status at the three study time points. In a univariate regression, the following variables were assessed for association with impairment at each time point: TBI mechanism (AHT), age in months, ISS at admission, MODS on day 1, 3, or 7, race, seizure on electroencephalogram, and presence of subdural hemorrhage on initial head computed tomography. These variables were chosen because they have been shown to impact outcomes; then, via backward stepwise regression for p values < 0.05, the variables included in the multivariate logistic regression were TBI mechanism (AHT vs. aTBI), age in months, ISS at admission, seizure, and presence of MODS on day 1, 3, or 7. In the logistic regression for patients with AHT, age in months was not significant in univariate analysis, so this was not included (Supplemental Table 2). In the logistic regression for patients with aTBI, race, and subdural hemorrhage were not significant in univariate analysis, so these were not included (Supplemental Table 3). There were no concerns for collinearity. All statistical analyses were performed by using RStudio version 2022.12.0 + 353 (Rstudio, Boston, MA) and R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) with the following packages: broom, gtsummary, haven, knitr, lubridate, modelr, ggplot2, and tidyverse. Missing data were not imputed. Statistical significance was defined as p < 0.05.
Results
Patients
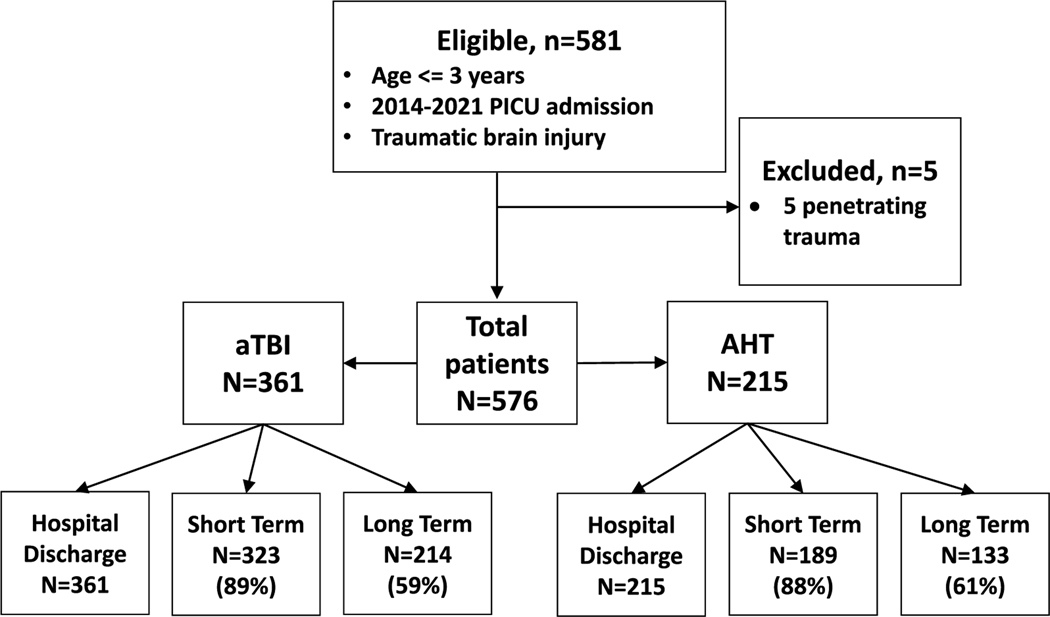
There were 576 patients eligible for inclusion. Two hundred fifteen (37%) children had AHT and 361 (63%) had aTBI (Fig. 1). There was no difference in age, sex, race, or history of medical comorbidities between mechanism groups (Table 1).
Fig. 1.
Patient flow diagram. AHT, abusive head trauma, aTBI, accidental traumatic brain injury, PICU, pediatric intensive care unit
Table 1.
Patient characteristics by TBI mechanism (N = 576)
| Characteristic | Overall (N = 576)a | aTBI (n = 361)a | AHT (n = 215)a | p valueb |
|---|---|---|---|---|
| Age (months) | 5 (2–15) | 5 (2–20) | 4 (2–10) | 0.110 |
| Male sex | 351 (61) | 220 (61) | 131 (61) | 0.998 |
| Race | 0.817 | |||
| White | 420 (73) | 265 (73) | 155 (72) | |
| Black | 79 (14) | 47 (13) | 32 (15) | |
| Other | 77 (13) | 49 (14) | 28 (13) | |
| Previously healthy TBI severity by GCS | 465 (81) | 299 (83) | 166 (77) | 0.085 |
| Mild | 468 (81) | 307 (85) | 161 (75) | 0.003 |
| Moderate | 24 (4.2) | 17 (4.7) | 7 (3.3) | < 0.001 |
| Severe | 84 (15) | 37 (10) | 47 (22) | 0.399 |
| Admission ISS | 13 (9–18) | 10 (8–17) | 17 (10–26) | < 0.001 |
| Extracranial fracture | 118 (20) | 39 (11) | 79 (37) | < 0.001 |
| Mechanical ventilation | 123 (21) | 54 (15) | 69 (32) | < 0.001 |
| Seizure | 93 (16) | 27 (7.5) | 66 (31) | < 0.001 |
| Intracranial hemorrhage | 490 (85) | 295 (82) | 195 (91) | 0.003 |
| Neurosurgical intervention | 66 (11) | 27 (7.5) | 39 (18) | < 0.001 |
| Mortality | 27 (5) | 5 (1) | 22 (10) | < 0.001 |
| Total length of stay | 2 (2–5) | 2 (1–3) | 4 (3–12) | < 0.001 |
| Hospital discharge impairment | 88 (16) | 35 (10) | 53 (27) | < 0.001 |
| Short-term impairment | 84 (16) | 28 (9) | 56 (30) | < 0.001 |
| Long-term impairment | 64 (18) | 22 (10) | 42 (32) | < 0.001 |
AHT, abusive head trauma, aTBI, accidental traumatic brain injury, GCS, Glasgow Coma Score, ISS, injury severity score, IQR, interquartile range, TBI, traumatic brain injury
Median (IQR); n (%)
Wilcoxon rank-sum test; Pearson’s χ2 test
Hospitalization Details
At admission, more patients with AHT had a severe TBI classified by admission GCS (47/215 [22%] vs. 37/361 [10%], p < 0.001) and higher ISS scores (median 17 [interquartile range, 10–26] vs. 10, p < 0.001) than patients with aTBI. Patients with AHT were more frequently mechanically ventilated (69/215 [32%] vs. 54/361 [15%], p < 0.001) and diagnosed with seizures (66/215 [31%] vs. 27/361 [8%], p < 0.001) during hospitalization. They also had more intracranial hemorrhage (195/215 [91%] vs. 295/361 [82%], p = 0.003) and neurosurgical intervention (39/215 [18%] vs. 27/361 [8%], p < 0.001) (Table 1).
MODS
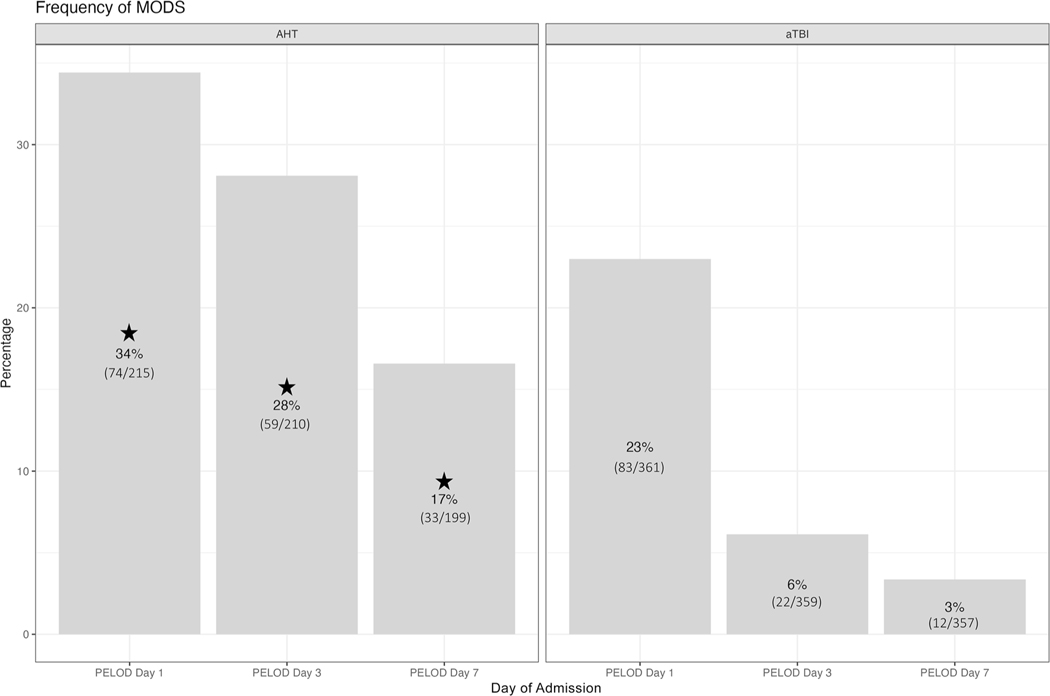
Of the 576 patients, 288 remained hospitalized on day 3 and 110 on day 7. Seven patients died before day 3 and 13 patients died before days 4–7, leaving 569 patients for analysis on day 3 and 556 on day 7. MODS was more frequent in patients with AHT than patients with aTBI on days 1 (74/215 [34%] vs. 83/361 [23%], p = 0.003), 3 (59/210 [28%] vs. 22/359 [6%], p < 0.001)], and 7 (33/199 [17%] vs. 12/357 [3%], p < 0.001)] (Fig. 2).
Fig. 2.
The frequency of multiorgan dysfunction syndrome (MODS) compared by TBI etiology is shown calculated by PELOD-2. More MODS was present in AHT on day 1 and day 3. The percentages per patient cohort at each time point are displayed in the appropriate areas, and stars indicate p values less than 0.05. AHT, abusive head trauma, aTBI, accidental traumatic brain injury, PELOD-2, Pediatric Logistic Organ Dysfunction-2, TBI, traumatic brain injury
On day 1, more patients with AHT had three organs (3/215 (15%) vs. 34/361 (9%), p = 0.032) and 4–5 organs involved (25/215 (12%) vs. 14/361 (4%), p = 0.003). All 27 patients who died qualified for MODS on at least one day, highest on day 1 (26/27 [96%]), and all 84 patients with severe TBI by admission GCS also qualified for MODS on day 1. Forty out of 576 patients (7%) had MODS on day 1, day 3, and day 7 with 45/576 patients (8%) having MODS on day 7. (Table 2).
Table 2.
Organ systems and number of organs involved in MODS by TBI mechanism (N = 576)
| Parameter | Overall (N = 576)a | aTBI (n = 361)a | AHT (n = 215)a | p valueb |
|---|---|---|---|---|
| Cardiovascular | 381 (66) | 239 (66) | 142 (66) | 0.969 |
| Day 1 | 350 (61) | 230 (64) | 120 (56) | 0.060 |
| Day 3 | 90 (16) | 31 (9) | 59 (28) | < 0.001 |
| Day 7 | 42 (8) | 16 (5) | 26 (13) | < 0.001 |
| Neurologic | 128 (22) | 58 (16) | 70 (33) | < 0.001 |
| Day 1 | 122 (21) | 57 (16) | 65 (30) | < 0.001 |
| Day 3 | 69 (12) | 20 (6) | 49 (23) | < 0.001 |
| Day 7 | 37 (7) | 11 (3) | 26 (13) | < 0.001 |
| Respiratory | 128 (22) | 54 (15) | 74 (34) | < 0.001 |
| Day 1 | 118 (20) | 53 (15) | 65 (30) | < 0.001 |
| Day 3 | 80 (14) | 23 (6) | 57 (27) | < 0.001 |
| Day 7 | 41 (7) | 10 (3) | 31 (16) | < 0.001 |
| Hematologic | 45 (8) | 20 (6) | 25 (12) | 0.008 |
| Day 1 | 33 (6) | 16 (4) | 17 (8) | 0.083 |
| Day 3 | 22 (4) | 7 (2) | 15 (7) | 0.002 |
| Day 7 | 9 (2) | 4 (1) | 5 (3) | 0.305 |
| Renal | 108 (19) | 53 (15) | 55 (26) | 0.001 |
| Day 1 | 101 (18) | 51 (14) | 50 (23) | 0.005 |
| Day 3 | 18 (3) | 4 (1) | 14 (7) | < 0.001 |
| Day 7 | 6 (1) | 1 (0) | 5 (2) | 0.024 |
| Day 1 | ||||
| 0 organs | 166 (29) | 102 (28) | 64 (30) | 0.698 |
| 1 organ | 253 (44) | 176 (49) | 77 (36) | 0.002 |
| 2 organs | 51 (9) | 35 (10) | 16 (7) | 0.357 |
| 3 organs | 67 (12) | 34 (9) | 33 (15) | 0.032 |
| 4–5 organs | 39 (7) | 14 (4) | 25 (12) | 0.003 |
| Day 3 | ||||
| 0 organs | 443 (77) | 314 (87) | 129 (60) | < 0.001 |
| 1 organ | 52 (9) | 25 (7) | 27 (13) | 0.019 |
| 2 organs | 29 (5) | 9 (3) | 20 (9) | < 0.001 |
| 3 organs | 41 (7) | 10 (3) | 31 (14) | < 0.001 |
| 4–5 organs | 11 (2) | 3 (1) | 8 (4) | 0.013 |
| Day 7 | ||||
| 0 organs | 511 (89) | 341 (94) | 170 (79) | < 0.001 |
| 1 organ | 20 (4) | 8 (2) | 12 (6) | 0.021 |
| 2 organs | 26 (5) | 5 (1) | 21 (10) | < 0.001 |
| 3 organs | 14 (2) | 5 (1) | 9 (4) | 0.024 |
| 4–5 organs | 5 (1) | 2 (0) | 3 (1) | 0.257 |
AHT, abusive head trauma, aTBI, accidental traumatic brain injury, IQR, interquartile range, MODS, multiorgan dysfunction syndrome, TBI, traumatic brain injury
n (%)
Wilcoxon rank-sum test; Pearson’s χ2 test
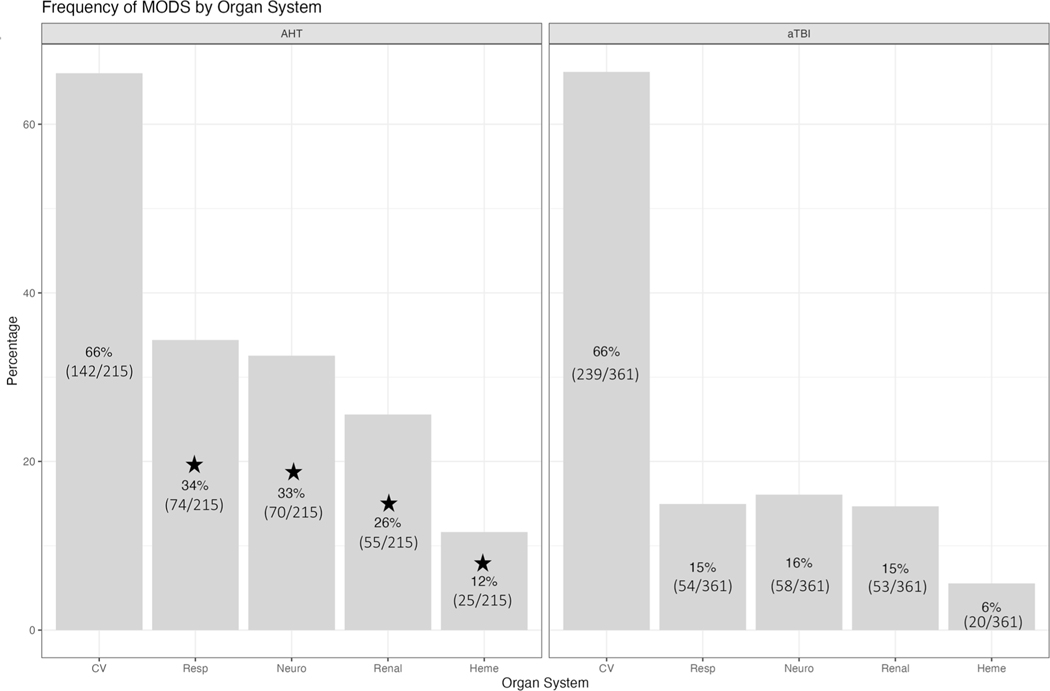
The most frequent organ system failures overall were cardiovascular (381/576 [66%]), neurologic (128/576 [22%]), and respiratory (128/576 [22%]). In the AHT group, more children had neurologic, respiratory, hematologic, and renal failure compared to the aTBI group [Neurologic (70/215 [33%] vs. 58/361 [16%], p < 0.001), Respiratory (74/215 [34%] vs. 54/361 [15%], p < 0.001), Hematologic (25/215 [12%] vs. 20/361 [6%], p = 0.008), Renal (55/215 [26%] vs. 53/361 [15%], p = 0.001)] (Fig. 3).
Fig. 3.
The frequency of dysfunction by organ system compared by TBI etiology is shown calculated by PELOD-2. The neurologic, respiratory, hematologic, and renal organ systems were more frequently dysfunctional in AHT than in aTBI. The percentages per patient cohort at each time point are displayed in the appropriate areas and stars indicate p values less than 0.05. AHT, abusive head trauma, aTBI, accidental traumatic brain injury, CV, cardiovascular, Heme, hematologic, Neuro, neurologic, PELOD-2, Pediatric Logistic Organ Dysfunction-2, Resp, respiratory, TBI, traumatic brain injury
Table 2 also details the organ systems by day. On day 1, AHT had more failure than aTBI in the neurologic (65/215 [30%] vs. 57/361 [16%], p < 0.001), respiratory (65/215 [30%] vs. 53/361 [15%], p < 0.001), and renal (50/215 [23%] vs. 51/361 [14%], p = 0.005) organ systems. On day 3, AHT had more failure all organ systems than aTBI. On day 7, AHT had more failure than aTBI in cardiovascular (26/199 [13%] vs. 16/357 [5%], p < 0.001), neurologic (26/199 [13%] vs. 11/357 [3%], p < 0.001), respiratory (31/199 [16%] vs. 10/357 [3%], p < 0.001), and renal (5/199 [2%] vs. 1/357 [0%], p = 0.024) organ systems.
When divided into presenting GCS severity, patients with AHT had more MODS on days 3 (19/161 [12%] vs. 3/307 [1%], p < 0.001) and 7 (12/161 [8%] vs. 1/307 [0%], p < 0.001) in the mild GCS category than aTBI (Supplemental Table 1). In the severe GCS category, there was more MODS in patients with AHT on days 3 (36/47 [86%] vs. 14/37 [40%], p < 0.001) and 7 (18/47 [58%] vs. 10/37 [29%], p = 0.016). In both TBI mechanisms, there were similar rates of MODS in mild, moderate, and severe GCS categories on day 1.
Outcomes
There were more deaths in the AHT versus aTBI group (22/215 [10%] vs. 5/361 [1%], p < 0.001). Patients with AHT also had longer hospital length of stay (4 [3, 12] vs. 2 [1, 3], p < 0.001). The short-term time point was performed at a median of 11 months (5, 12) post hospitalization for patients with AHT and 10 months (2, 12) for patients with aTBI (p = 0.015). The long-term time point was performed at a median of 43 months (25, 62) post hospitalization for patients with AHT and 44 months (30, 61) for patients with aTBI (p = 0.3). Patients with AHT had a higher frequency of new impairment compared with patients with aTBI at all time points: HD (53/193 [27%] vs. 35/356 [10%], p < 0.001), short-term discharge (56/189 [30%] vs. 28/323 [9%], p < 0.001), and long-term discharge (42/133 [32%] vs. 22/214 [10%], p < 0.001) (Table 1).
Multivariable Logistic Regression for the Association with New Impairment at Each Study Time Points
Table 3 displays the results of univariate and multivariate logistic regressions for the association with new impairment by study time point. MODS was associated with new impairment after adjustment for age, subdural hemorrhage, and admission ISS in multivariable logistic regression at HD (odds ratio 19.1 [95% confidence interval 9.8, 38.6, p < 0.001]), short-term (7.4 [3.7, 15.2, p < 0.001]), and long-term (4.3 [2.0, 9.4, p < 0.001]). AHT was associated with new impairment in multivariable logistic regression at HD (3.4 [1.6, 7.3, p = 0.001]), short-term (2.5 [1.3, 4.7, p = 0.005]), and long-term (2.1 [1.1, 4.1, p = 0.036]). Seizures on electroencephalogram during admission were also associated with new impairment at HD (4.8 [2.4, 10.1, p < 0.001]), short-term (6.1 [3.2, 11.7, p < 0.001]), and long-term (4.1 [2.0, 9.2, p < 0.001]).
Table 3.
Associations with impairment at each time point
| Parameter | Univariate, OR (95% CI) | p value | Multivariate, OR (95% CI) | p valuel |
|---|---|---|---|---|
| Hospital discharge | ||||
| Race | 0.006 | |||
| Black | Ref | |||
| White | 0.4 (0.2–0.8) | |||
| Other | 0.8 (0.4–1.8) | |||
| Subdural hemorrhage | 3.2 (1.9–5.7) | < 0.001 | ||
| AHT | 3.5 (2.2–5.6) | < 0.001 | 3.4 (1.6–7.3) | 0.001 |
| Age (months) | 1.0 (1.0–1.1) | < 0.001 | 1.1 (1.0–1.1) | 0.001 |
| Admission ISS | 1.1 (1.1–1.2) | < 0.001 | 1.1 (1.0–1.1) | 0.001 |
| Seizure on EEG during admission | 12.2 (7.2–21.0) | < 0.001 | 4.4 (2.1–9.1) | < 0.001 |
| PELOD-2 day 1, 3, or 7 | 26.5 (14.9–49.7) | < 0.001 | 17.2 (8.9–35.3) | < 0.001 |
| Short term | ||||
| Race | 0.12 | |||
| Black | Ref | |||
| White | 0.6 (0.3–1.1) | |||
| Other | 0.9 (0.4–2.1) | |||
| Subdural hemorrhage | 2.3 (1.4–3.9) | 0.002 | ||
| AHT | 4.5 (2.7–7.4) | < 0.001 | 2.5 (1.3–4.7) | 0.004 |
| Age (months) | 1.0 (0.9–1.0) | 0.85 | 0.9 (0.9–1.0) | 0.6 |
| Admission ISS | 1.1 (1.1–1.1) | < 0.001 | 1.0 (1.0–1.1) | 0.06 |
| Seizure on EEG during admission | 15.4 (8.9–27.2) | < 0.001 | 6.0 (3.1–11.3) | < 0.001 |
| PELOD-2 day 1, 3, or 7 | 8.3 (5.0–13.9) | < 0.001 | 4.7 (2.5–8.6) | < 0.001 |
| Long term | ||||
| Race | 0.42 | |||
| Black | Ref | |||
| White | 0.7 (0.3–1.4) | |||
| Other | 1.0 (0.4–2.5) | |||
| Subdural hemorrhage | 2.0 (1.1–3.8) | 0.017 | ||
| AHT | 4.0 (23–73) | < 0.001 | 2.2 (1.1–4.3) | 0.027 |
| Age (months) | 0.9 (0.9–1.0) | 0.13 | 0.9 (0.9–1.0) | 0.100 |
| Admission ISS | 1.1 (1.0–1.1) | < 0.001 | 1 (0.9–1.1) | 0.4 |
| Seizure on EEG during admission | 10.8 (5.7–20.8) | < 0.001 | 4.4 (2.0–9.6) | < 0.001 |
| PELOD-2 day 1, 3, or 7 | 5.0 (2.8–8.9) | < 0.001 | 2.9 (1.5–6.0) | 0.003 |
Univariate regression analysis was performed for variables that impact outcomes. A backward stepwise multivariable regression analysis was performed by removing variables with p values < 0.05
AHT, abusive head trauma, aTBI, accidental traumatic brain injury, CI, confidence interval, EEG, electroencephalogram, GCS, Glasgow Coma Score, ISS, injury severity score, OR, odds ratio, PELOD-2, Pediatric Logistic Organ Dysfunction-2, Ref, reference
To examine the impact of MODS specifically on each TBI mechanism, multivariable logistic regressions were conducted on the patients with AHT and patients with aTBI separately. AHT survivors had higher odds ratios for MODS at each time point: HD (14.5 [5.7, 9.7, p < 0.001]), short-term (5.4 [2.3, 13.3, p < 0.001]), and long-term (4.7 [1.7, 13.7, p = 0.004]) (Supplemental Table 2). MODS was only significantly associated with impairment in aTBI survivors at the first two time points: HD (19.2 [7.5, 57.4, p < 0.001]), short-term (6.8 [2.9, 17.1, p < 0.001]), and long-term (1.4 [0.5, 4.1, p = 0.500]) (Supplemental Table 3).
Discussion
This single center, observational study evaluated MODS prevalence and patient outcomes by etiology (AHT vs. aTBI). When compared with children with aTBI, patients with AHT more frequently had MODS on days 1, 3, and 7 with failure most often due to neurologic, respiratory, hematologic, and renal organ dysfunction. Furthermore, in a multivariate logistic regression, AHT and MODS were both independently associated with new functional impairment at all study time points.
In a large, retrospective cohort study examining MODS in pediatric patients with trauma by Killien et al. [16], published in 2022, 23.1% (8592/37177) of patients qualified for MODS with two or more organ systems. Mortality was higher in patients who developed MODS (20.1% vs. 0.5%); in survivors, there was a decline in function at discharge (58.9% vs. 31.8%) [16]. The analysis of our data over the first week post TBI shows that patients with AHT experience more MODS compared with patients with aTBI. All the patients who died, regardless of mechanism, qualified for MODS on at least one analyzed day. In both mechanisms, MODS still contributed to impairment in short-term outcomes and it seemed to impact AHT in the long term, as well.
A recent pediatric TBI outcome study published in 2023 by Keenan et al. [9] demonstrated prospectively that infants and toddlers with severe TBI have diffuse multidomain deficits that persist, whereas those with mild and moderate TBI have similar outcomes to an orthopedic injury group. As seen in other studies, AHT was a large contributor to the severe TBI group and the poor outcome trajectory [9]. There are many hypotheses on why patients with AHT have worse outcomes. One of those is that there is a delay in presentation, leading to secondary insults such as hypoxia, hypotension, and seizures [10, 24]. These secondary insults lead to MODS due to inflammation and hypoxic ischemic injury [25, 26]. Repeated injury is another hypothesis and with each additional injury, increased inflammation could trigger MODS and thus lead to more critical illness [27]. Seizures were also common in AHT and were associated with poor outcomes in this data set. It is unknown why seizures occur more frequently than in aTBI. Seizures, especially status epilepticus, could cause MODS, or perhaps the multiorgan inflammation from AHT could lead to seizures [28, 29].
Children with TBI, particularly AHT, frequently experience apnea due to impact, seizures, or polytrauma, causing hypoxia, and hypercarbia, which can potentially worsen neurologic outcomes [10]. Acute respiratory distress due to pneumonia, direct or indirect lung injury, and/or neurogenic pulmonary edema may affect later outcomes as well [30, 31]. Cardiovascular dysfunction was also common in both aTBI and AHT in our cohort, which may be related to hemorrhagic shock, multiple injury, sepsis, secondary effect of analgesic and sedating medications, and critical illness associated decreased systolic function after trauma [32]. Hematologic dysfunction is prevalent after TBI with international normalized ratio being an indicator of trauma related coagulopathy [33]. Last, in the setting of severe TBI, acute kidney injury is associated with increased mortality and is related to abdominal trauma, shock, or toxic medications [34, 35]. In our study, there was more significant involvement of neurologic, respiratory, hematologic, and renal organ systems in patients with AHT, implying organ failure needs to be monitored and treated promptly to potentially improve outcomes.
There are currently no specific treatments for MODS, although there are studies evaluating the use of immune modulating medications to decrease TBI-associated inflammation [36–38]. To improve outcomes, patients with AHT need close monitoring in a pediatric intensive care unit setting immediately after presentation so that MODS can be prevented and organ failure can be addressed specifically. More research is needed to explore the organ dysfunction in this population and other aspects to their care because public health measures at prevention have not proven to be effective [39].
Limitations for this study include the retrospective nature and the dependence on electronic health record documentation, which led to a relatively low proportion traumatic brain injury, CI, confidence interval, EEG, electroencephalogram, GCS, Glasgow Coma Score, ISS, injury severity score, OR, odds ratio, PELOD-2, Pediatric Logistic Organ Dysfunction-2, Ref, reference of patients with long-term outcome data available. Limitations of the PELOD-2 score and other MODS score performance in patients with TBI also may have affected our results, especially in relation to more traditional assessments of neurologic function using GCS [40]. The PELOD-2 cutoff for neurologic failure is a GCS of 11, whereas more traditional GCS cutoffs are defined as 3–8 (severe), 9–13 (moderate), and 14–15 (mild), and thus overlap exists. In addition, patients who meet the PELOD-2 neurologic failure threshold are frequently intubated, thus having coexisting respiratory failure. Further, there was a high frequency of patients with cardiovascular failure (66% on day 1) using PELOD-2 scoring that are based on a wide range of blood pressures, leading to a high frequency of MODS in this cohort. The recent Pediatric Organ Dysfunction Information Update Mandate, which is a summary of multiple MODS scores, details narrower age range limits for heart rate and systolic blood pressure; this measure was not available during the conduct of this study [41]. When using these scores, MODS was developed to assess risk for mortality and morbidity, which needs to be kept in mind when interpreting our impairment results.
Conclusions
Children with TBI due to AHT had more frequent MODS than children with aTBI; both AHT as a mechanism and MODS occurrence during the first week post TBI were associated with new impairment at HD, short-term discharge (11 months), and long-term discharge (4 years). MODS scores such as PELOD-2 may serve as a useful tool to identify children at high risk for poor outcomes needing personalized organ support. Our study suggests that children with TBI, especially AHT, need close monitoring and treatment of organ dysfunction.
Supplementary Material
Source of Support
National Institutes of Health number T32 5T32HD040686-22.
Footnotes
Conflicts of interest
No authors have any personal or financial affiliations that could present a conflict of interest.
Ethical Approval/Informed Consent
This article complied with ethical approval and received approval from the University of Pittsburgh Institutional Review Board.
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.1007/s12028-023-01887-y.
References
- 1.Rebbe R, Mienko JA, Martinson ML. Incidence and risk factors for abusive head trauma: a population-based study. Child Abuse Rev. 2020;29(3):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shanahan ME, Zolotor AJ, Parrish JW, Barr RG, Runyan DK. National, regional, and state abusive head trauma: Application of the CDC algorithm. Pediatrics. 2013;132(6):e1546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araki T, Yokota H, Morita A. Pediatric traumatic brain injury: characteristic features, diagnosis, and management. Neurol Med Chir (Tokyo). 2017;57(2):82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson C, Xu L, Florence C, Parks SE. Annual cost of US hospital visits for pediatric abusive head trauma. Child Maltreat. 2015;20(3):162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keenan HT, Runyan DK, Nocera M. Child outcomes and family characteristics 1 year after severe inflicted or noninflicted traumatic brain injury. Pediatrics. 2006;117(2):317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haviland J, Ross Russell RI. Outcome after severe non-accidental head injury. Arch Dis Child. 1997;77(6):504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson JE, Beres AL, Theodorou CM, Ugiliweneza B, Boakye M, Nuño M. Long-term impact of abusive head trauma in young children: Outcomes at 5 and 11 years old. J Pediatr Surg. 2021;56(12):2318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eismann EA, Theuerling J, Cassedy A, Curry PA, Colliers T, Makoroff KL. Early developmental, behavioral, and quality of life outcomes following abusive head trauma in infants. Child Abuse Negl. 2020;108:104643. [DOI] [PubMed] [Google Scholar]
- 9.Keenan HT, Clark A, Holubkov R, Ewing-Cobbs L. Longitudinal Developmental Outcomes of Infants and Toddlers With Traumatic Brain Injury. JAMA Netw Open [Internet]. 2023;6(1):e2251195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller Ferguson N, Sarnaik A, Miles D, Shafi N, Peters MJ, Truemper E, et al. Abusive head trauma and mortality-an analysis from an international comparative effectiveness study of children with severe traumatic brain injury. Crit Care Med. 2017;45(8):1398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paek D, Kwon DI. A review on four different paths to respiratory arrest from brain injury in children; implications for child abuse. J Forensic Leg Med. 2020;1:71. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson JD, Pollack MM, Ruttimann UE, Glass NL, Yeh TS. Outcome of pediatric patients with multiple organ system failure. Crit Care Med [Internet]. 1986;14(4):271–4. [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson JD, Pollack MM, Glass NL, Kanter RK, Katz RW, Steinhart CM. Mortality associated with multiple organ system failure and sepsis in pediatric intensive care unit. J Pediatr. 1987;111(3):324–8. [DOI] [PubMed] [Google Scholar]
- 14.Hanna K, Hamidi M, Vartanyan P, Henry M, Castanon L, Tang A, et al. Non-neurologic organ dysfunction plays a major role in predicting outcomes in pediatric traumatic brain injury ☆. J Pediatr Surg [Internet]. 2020;55(8):1590–5. 10.1016/j.jpedsurg.2020.01.051. [DOI] [PubMed] [Google Scholar]
- 15.Typpo KV, Petersen NJ, Markovitz BPMMM. Day one MODS is associated with poor functional outcome and mortality in the pediatric intensive care unit. Pediatr Crit Care Med. 2009;10(5):562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Killien EY, Zahlan JM, Lad H, Watson RS, Vavilala MS, Huijsmans RLN, et al. Epidemiology and outcomes of multiple organ dysfunction syndrome following pediatric trauma. J Trauma Acute Care Surg [Internet]. 2022;93(6):829–37. 10.1097/TA.0000000000003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown JB, Gestring ML, Leeper CM, Sperry JL, Peitzman AB, Billiar TR, et al. The value of the injury severity score in pediatric trauma: Time for a new definition of severe injury? J Trauma Acute Care Surg. 2017;82(6):995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F. PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med. 2013;41(7):1761–73. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Wu Y, Huang H, Liu C, Cheng Y, Xu L, et al. Performance of PRISM III, PELOD-2, and P-MODS scores in two pediatric intensive care units in China. Front Pediatr. 2021;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamalin D, Hamlin I, Shults J, Katherine Henry M, Campbell KA, Anderst JD, et al. (2023) Predictors of making a referral to child protective services prior to expert consultation. Acad Pediatr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood JN, Henry MK, Berger RP, Lindberg DM, Anderst JD, Song L, et al. Use and utility of skeletal surveys to evaluate for occult fractures in young injured children. Acad Pediatr [Internet]. 2018. 10.1016/j.acap.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petridou ET, Antonopoulos CN. Injury epidemiology. Int Encycloped Public Health. 2017;1:258–74. [Google Scholar]
- 23.Pollack MM, Holubkov R. The functional status score (FSS): a new pediatric outcome measure. Pediatrics. 2009;124(1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy JM, Ma J, Lyden ER, Haney SB. Abusive head trauma and a delay in presentation for care. Pediatr Emerg Care. 2020;38(1):e170–2. [DOI] [PubMed] [Google Scholar]
- 25.Sulhan S, Lyon KA, Shapiro LA, Huang JH. Neuroinflammation and blood–brain barrier disruption following traumatic brain injury: pathophysiology and potential therapeutic targets. J Neurosci Res. 2020;98:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carcillo JA, Podd B, Aneja R, Weiss SL, Hall MW, Cornell TT, et al. Pathophysiology of pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2017;18(3):S32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas A, Krishnan P, Albalkhi I, Mankad K, Shroff M. Imaging of Abusive Head Trauma in Children. W.B. Saunders: Neuroimaging Clinics of North America; 2023. [DOI] [PubMed] [Google Scholar]
- 28.Dingman AL, Stence NV, O’Neill BR, Sillau SH, Chapman KE. Seizure severity is correlated with severity of hypoxic-ischemic injury in abusive head trauma. Pediatr Neurol [Internet]. 2018;82:29–35. 10.1016/j.pediatrneurol.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Oh A, Olson LD, Chern JJ, Kim H. Clinical characteristics and nonconvulsive seizures in young children with abusive head trauma. J Child Neurol. 2019;34(12):713–9. [DOI] [PubMed] [Google Scholar]
- 30.Komisarow JM, Chen F, Vavilala MS, Laskowitz D, James ML, Krishnamoorthy V. Epidemiology and outcomes of acute respiratory distress syndrome following isolated severe traumatic brain injury. J Intensive Care Med. 2022;37(1):68–74. [DOI] [PubMed] [Google Scholar]
- 31.Humayun M, Premraj L, Shah V, Cho SM. Mechanical ventilation in acute brain injury patients with acute respiratory distress syndrome. Front Med (Lausanne) [Internet]. 2022;6:9. 10.3389/fmed.2022.999885/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollenberg SM, Singer M. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol. 2021;18:424–34. [DOI] [PubMed] [Google Scholar]
- 33.Christiaans SC, Duhachek-Stapelman AL, Russell RT, Lisco SJ, Kerby JD, Pittet JF. Coagulopathy after severe pediatric trauma. Shock. 2014;41:476–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almuqamam M, Novi B, Rossini CJ, Mammen A, DeSanti RL. Association of hyperchloremia and acute kidney injury in pediatric patients with moderate and severe traumatic brain injury. Child’s Nervous Syst. 2023;39:1267–75. [DOI] [PubMed] [Google Scholar]
- 35.De Vlieger G, Meyfroidt G. Kidney dysfunction after traumatic brain injury: pathophysiology and general management, Neurocritical Care. Berlin: Springer; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss SL, Carcillo JA, Leclerc F, Leteurtre S, Schlapbach LJ, Tissieres P, et al. Refining the Pediatric Multiple Organ Dysfunction Syndrome. Pediatrics. 2022;1:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radermacher P, Billiar TR, Ghezzi P, Martin L, Thiemermann C. Editorial: translational insights into mechanisms and therapy of organ dysfunction in sepsis and trauma. Front Immunol. 2020;11:1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.. Asim M, Amin F, El-Menyar A. Multiple organ dysfunction syndrome: Contemporary insights on the clinicopathological spectrum. Qatar Med J. 2020;2020(2):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson LW, Bass KD, Agyei JO, Naseem HUR, Borngraber E, Wang J, et al. Incidence of nonaccidental head trauma in infants: a call to revisit prevention strategies. J Neurosurg Pediatr [Internet]. 2019;24(6):689–96. [DOI] [PubMed] [Google Scholar]
- 40.Schlapbach LJ, Weiss SL, Bembea MM, Carcillo JA, Leclerc F, Leteurtre S, et al. Scoring systems for organ dysfunction and multiple organ dysfunction: the PODIUM consensus conference. Pediatrics [Internet]. 2022;149:S23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bembea MM, Agus M, Akcan-Arikan A, Alexander P, Basu R, Bennett TD, et al. Pediatric organ dysfunction information update mandate (PODIUM) contemporary organ dysfunction criteria: executive summary. Pediatrics [Internet]. 2022;149:S1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.