Abstract
Anaplasma phagocytophilum, Anaplasma platys and Ehrlichia canis, responsible of diseases in dogs, are tick-borne pathogens with a proven or potential zoonotic role that have shown increasing prevalence worldwide. The aims of this retrospective study were to assess the frequency of Anaplasma spp. and Ehrlichia spp. exposure in dogs tested in a veterinary teaching hospital in Italy over a 9-year period, to compare the performance of the diagnostic tests used, to evaluate correlations with clinical data, and to genetically analyse the identified bacteria. During the study period, 1322 dogs tested by at least one of the rapid immunoenzymatic test, indirect immunofluorescent antibody test or end-point PCR assay for Anaplasmataceae detection were included. Dogs were tested if they had clinical signs or clinicopathological alteration or risk factors related to infection, and if they were potential blood-donor animals. Ninety-four of 1322 (7.1%) dogs tested positive for at least one pathogen: 53 (4.3%) for A. phagocytophilum, one (0.1%) for A. platys and 63 (4.6%) for E. canis. The number of dogs tested increased and the positivity rate progressively declined over the years. Comparison of tests showed a near-perfect agreement between serological tests and a poor agreement between PCR and indirect assays. A breed predisposition has been highlighted for A. phagocytophilum infection in hunting breed dogs and for E. canis infection in mixed breed dogs. Phylogeny confirmed potential zoonotic implications for A. phagocytophilum and showed no correlation of the identified bacteria with the geographical origin. Our study provides new insights into possible risk factors in dogs and evidenced discordant results between different tests, suggesting that a combination of serological and molecular assays is preferable for a correct diagnosis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11259-024-10358-4.
Keywords: Diagnosis, PCR, Phylogeny, Serological tests, Tick-borne pathogen
Introduction
In a scenario of increased density and geographical expansion of ticks, due to climate change and global warming, and of increased animal traveling and more frequent contact between wild and domestic species, tick-borne pathogens (TBP) are gaining importance due to their huge impact on animal and human health (Sanchez-Vicente et al. 2019; Wilson et al. 2020).
Anaplasma phagocytophilum, Anaplasma platys and Ehrlichia canis, which are responsible for granulocytic anaplasmosis, canine infectious cyclic thrombocytopenia and canine monocytic ehrlichiosis (CME), respectively, are three examples of TBP with a proven (A. phagocytophilum) or potential (A. platys and E. canis) zoonotic role (Breitschwerdt et al. 2014; Sainz et al. 2015; Saito and Walker 2016) and have shown increasing prevalence worldwide in recent decades (Sainz et al. 2015). These microorganisms belong to the family Anaplasmataceae, order Rickettsiales, and are obligate intracellular bacteria, whose cycle in the environment involves complex interactions between invertebrate vectors and vertebrate hosts (Granick et al. 2023; Harrus et al. 2023). Indeed, numerous wild animal species are considered reservoir hosts (Ebani et al. 2008; Maia et al. 2014; Stuen et al. 2013; Torina et al. 2013; Pereira et al. 2016; Carvalho et al. 2017).
The geographical distribution of Anaplasmataceae species is related to tick survival and consequently to the temperature and the humidity of the area. They are widespread in states with temperate climates, usually with temperatures between 10 and 30 °C (Kidd 2019¸ El Hamiani Khatat et al. 2021). A. platys and, after the first recent detection in Australia, E. canis are now present in all continents (Sykes 2014; Carvalho et al. 2017; Neave et al. 2022), while for A. phagocytophilum Oceania is the only continent where no cases have yet been reported (Sykes and Foley 2014). In Italy, the presence of members of the Anaplasmataceae family is known from screening studies performed on dogs; however, results are extremely variable in relation to the diagnostic test used, geographical area investigated and inclusion criteria adopted (Petruccelli et al. 2021; Piantedosi et al. 2017). In Italy, A. phagocytophilum seroprevalence in dogs is estimated between 29% and 41.5% and molecular prevalence between 0% and 5.1%, but there are discordant studies on positivity in the different Italian regions (Solano-Gallego et al. 2006; Trotta et al. 2009; Mendoza-Roldan et al. 2021). There are few studies about the prevalence of A. platys in Italy, Trotta and colleagues estimated a molecular prevalence of 3.7% in dogs (2009), while a recent study estimated a molecular prevalence of 0.8% in dog-ticks (Zanet et al. 2020). Moreover, the true prevalence of these two pathogens is questioned due to a possible cross-reactivity between Anaplasma species, which cannot be discriminated by serological tests (Petruccelli et al. 2021). E. canis, on the other hand, shows a seroprevalence between 28.7% and 44%, while molecular positivity varies between 2.9% and 9.7% with the highest percentages detected in dogs in southern regions (Solano-Gallego et al. 2006; Mendoza-Roldan et al. 2021).
In dogs, the clinical signs related to these pathogens are often similar but highly variable depending on the virulence of the strain, the host’s immune response and the presence of coinfections or comorbidities (Sykes 2014). Nevertheless, granulocytic anaplasmosis is usually considered an acute disease with the development of non-specific clinical signs such as fever, lethargy, anorexia, and sometimes musculoskeletal pain and lameness (Greig et al. 1996; Granick et al. 2023). Ecchymosis, petechiae and gastrointestinal signs are rarely reported (Kohn et al. 2008). Differently, CME potentially recognise three clinical phases: an acute stage with non-specific signs and sometimes bleeding (petechiae, ecchymosis, epistaxis) (Sainz et al. 2015; Harrus et al. 2023), a subclinical stage without evident signs (Mylonakis et al. 2019), and a chronic stage characterised by the same, but more severe, clinical signs of the acute stage (Harrus et al. 2011). In the latter stage, neurological impairment, ocular lesions, ulcerative stomatitis and glomerulonephritis may be observed (Sainz et al. 2015; Mylonakis et al. 2019; Ziliani et al. 2019). Thrombocytopenia, anaemia and hyperglobulinaemia and proteinuria are the most common laboratory findings associated with Anaplasma and Ehrlichia infection (Poitout et al. 2005; Sainz et al. 2015).
A confirmed diagnosis of these diseases is particularly complex because of the frequent subclinical course of the disease itself or its manifestation with non-specific clinical signs (Clark et al. 1996). Currently, the diagnosis of Anaplasmataceae species infection in dogs is achieved by the combined use of different methods: (i) cytologic examination of Romanowsky-stained peripheral blood and buffy-coat smear, as well as lymph nodes and spleen aspirates, evidencing intracytoplasmic inclusion bodies (morulae) in granulocytes, mononuclear cells or platelets (Olano and Aguero-Rosenfeld 2008; Harrus and Waner 2011); (ii) rapid in-clinic immunoenzymatic (enzyme-linked immunosorbent assay, ELISA) tests validated to detect specific antibodies in serum samples (Little et al. 2014; Liu et al. 2018); (iii) serologic assays as indirect fluorescent antibody test (IFAT) and ELISA (Neer et al. 2002); and (iv) molecular assays able to detect pathogen DNA mainly in blood samples (Sainz et al. 2015). All the diagnostic tests have strengths and limitations in the different stage of infection (Allison and Little 2013; Diniz and Moura de Aguiar 2022). Direct tests show a better performance in the acute phase of infection because morulae and pathogen DNA are readily detected in whole blood samples (Harrus et al. 1998). Indirect testing is more effective in the later stages of infection, as antibodies against pathogens can take several weeks to become detectable after exposure, or when antibiotics have been administered before diagnosis, making direct testing ineffective (Egenvall et al. 2000).
The primary aim of this study was to assess the frequency of Anaplasma spp. and Ehrlichia spp. exposure in dogs tested by different diagnostic assays in a veterinary teaching hospital (VTH) in Italy. Secondary aims were to compare the performance of the different tests used, to evaluate correlations between infection and clinical data, and to genetically analyse the identified bacteria.
Materials and methods
Study design and inclusion criteria
For the purposes of the study, dogs referred to VTH of the University of Bologna (Italy) between 2012 and 2020 and tested with at least one among rapid immunoenzymatic test (RIT), IFAT or end-point PCR assay (PCR) for Anaplasma spp. or Ehrlichia spp. detection, were retrospectively included. Dogs were tested if they had clinical signs or clinicopathological alteration or risk factors related to infection, and if they were potential blood-donor animals. RIT tests were carried out in accordance with the manufacturer’s recommendation and IFAT and PCR tests were carried out on fresh material or samples stored at − 20 °C for a maximum of seven days until examination. All laboratory tests were performed no later than 2020. The study was carried out only on data retrieved from medical records and sequencing of PCR products obtained for diagnostic purposes following owner’s informed consent. No sampling or analyses were carried out for the purpose of this study and no experimental animals were involved.
Year of sampling and signalment data (sex, age, breed and geographical origin) of the enrolled dogs were retrieved from medical records. Dogs included were grouped according to the four different age categories proposed by Harvey (2021): (i) puppy, juvenile and young adult dogs (≤ 24 months); (ii) mature adult dogs (25–84 months); (iii) senior adult dogs (85–156 months); and (iv) geriatric dogs (> 156 months). Purebred dogs were divided according to the size, attitude and possible risk factors in: (i) toy breeds group, (ii) hunting breeds group, (iii) shepherd and guardian breeds group, and (iv) “other” breeds group when the dogs did not fit into any of the previous classes. Furthermore, the study population was divided in three groups according to the geographical area of origin in: Northern Italy (including dogs from Emilia-Romagna, Friuli Venezia Giulia, Liguria, Lombardia, Piemonte, Trentino Alto Adige, Valle d’Aosta, and Veneto regions), Central Italy (including dogs from Abruzzo, Lazio, Marche, Toscana, and Umbria regions) and Southern Italy (including dogs from Puglia, Basilicata, Calabria, Campania, Molise, Sardegna, and Sicilia regions) groups. In dogs tested positive, clinical and clinicopathological data including complete blood count (CBC), serum biochemistry and urine protein to creatinine ratio (UPC) were retrieved from medical records (Online Resource 1).
Diagnosis of infection by rapid immunoenzymatic, serological and molecular tests
A RIT for the simultaneous detection of Dirofilaria immitis antigen and antibodies against A. phagocytophilum and A. platys, E. canis and E. ewingii, and Borrelia burgdorferi (SNAP 4DX, IDEXX Laboratories, Westbrook, ME, USA) was carried out on blood or serum specimens, following the manufacturer’s instructions.
Anaplasma phagocytophilum and E. canis IFAT for IgG detection (MegaFLUO ANAPLASMA phagocytophilum and MegaFLUO EHRLICHIA canis, MEGACOR Diagnostik, Horbranz, Austria) were performed on serum samples, following the manufacturer’s instructions. Briefly, slides coated with A. phagocytophilum and E. canis infected cells were probed with sera serially diluted in phosphate-buffered saline (PBS) starting with a concentration of 1:40 until reaching a concentration of 1:1280, incubated at 37 °C for 30 min and washed two times with PBS. Internal canine positive and negative sera controls were included on each slide. The slides were probed with 20 μL of fluorescein isothiocyanate (FITC) conjugated anti-dog IgG antibody diluted in PBS at a concentration of 1:64 (Anti-Dog IgG-FITC antibody produced in rabbit; Sigma-Aldrich, Saint Louis, MO, USA) at 37 °C for 30 min and were washed two times with PBS and examined under a fluorescent microscope. The highest dilution showing fluorescence was the final antibody titre. Samples that showed no fluorescence were considered negative.
DNA was extracted from blood samples using the NucleoSpin Tissue Kit (Macherey-Nagel, Düren, Germany), according to the manufacturer’s instructions. The extracted DNA were stored at − 20°C until use. The detection of all known Anaplasma spp. and Ehrlichia spp. DNA was carried out with a previously described end-point PCR assay (Balboni et al. 2021) using the Taq DNA Polymerase Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. An internal positive control and a no template control, consisting of ultrapure water, underwent analysis simultaneously. A fragment of about 600 bp of the groEL gene of Anaplasma spp. and Ehrlichia spp. was amplified using the primers groEL_For (5’-ACT GAT GGT ATG CAR TTT GAY CG-3’) and groEL_Rev (5’-TCT TTR CGT TCY TTM ACY TCA ACT TC-3’) (Barber et al. 2010). The thermal cycling consisted of an initial denaturation at 94 °C for 5 min followed by 45 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s and elongation at 72 °C for 45 s, followed by a final elongation step at 72 °C for 10 min. PCR products were visualized under UV after electrophoresis migration on a 1.5% agarose gel stained with ethidium bromide or Midori Green Advance DNA Stain (Nippon Genetics, Düren, Germany) in 1X standard tris-acetate-EDTA (TAE) buffer. Amplicons of the expected size were considered positive.
Dogs tested positive for more than one pathogen with the tests used were considered potentially coinfected.
Sequence analysis
Amplicons of the expected size were purified using the QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions and directly sequenced by Sanger method (BioFab Research, Italy) using both forward and reverse primers. The nucleotide sequences obtained were assembled and translated into amino acid sequences to evaluate their correct translation using BioEdit sequence alignment editor version 7.2.5. The assembled nucleotide sequences were analysed using the BLAST web interface to determine which species they belonged to (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and aligned with 55 A. phagocytophilum, 17 A. platys, eight A. platys-like and nine E. canis reference sequences available in the GenBank database (https://www.ncbi.nlm.nih.gov/nucleotide/), using the ClustalW method implemented in the BioEdit software. Phylogeny was carried out with the MEGA 11 software version 11.0.11 (Tamura et al. 2021) using Maximum Likelihood method and the Tamura 3-parameter model. The robustness of individual nodes on the phylogenetic tree was estimated using 1,000 bootstrap replicates and relevant bootstrap values were indicated at the corresponding node.
Statistical analysis
All the collected data were captured in Microsoft Excel 2019 and analysed using a commercially available statistical software (MedCalc Statistical Software version 19.5.1, Ostend, Belgium). The Shapiro-Wilk test was used to assess the distribution of continuous variables. Descriptive statistics was performed for all the evaluated variables and data are reported as mean ± standard deviation or median and range (minimum-maximum values), based on their distribution. Categorical data, such as year of sampling, sex, age and breed categories, and geographic origin, were analysed using the Fisher’s exact P-value test or Pearson’s chi-squared (χ2) test. Continuous data (e.g. clinicopathological findings) were analysed using the Mann-Whitney or Kruskal-Wallis tests. Inter-rater agreement test (Cohen’s kappa coefficient) was calculated to compare the results obtained by RIT, IFAT and PCR in dogs tested with two or more assays. Kappa values ≤ 0 were interpreted as indicating no agreement, 0.01–0.20 as no to mild agreement, 0.21–0.40 as fair agreement, 0.41–0.60 as moderate agreement, 0.61–0.80 as substantial agreement and 0.81–1.00 as near-perfect agreement between the compared tests. A P-value < 0.05 was considered significant.
Results
Study population
One thousand three hundred twenty-two dogs were tested with at least one assay for Anaplasma spp. or Ehrlichia spp. detection and were included in the study. Year of sampling, signalment data and clinicopathological findings of the enrolled dogs are reported in Table 1. The number of dogs tested increased during the years: 50/1322 (3.8%) dogs were tested in the first year of the study (2012), an initial peak was reached in 2014 (155/1322, 11.7% dogs) followed by a decrease in the following two years (2015 and 2016) and then a progressive increase up to 216 dogs tested was reported in each of the last two years of the study (2019 and 2020). As potential blood-donor animals, 102/1322 (7.7%) apparently healthy dogs were tested, all in the last four years (2017–2020). Among the dogs included, 657/1322 (49.7%) were male (130/657, 19.8% castrated) and 665/1322 (50.3%) were female (356/665, 53.5% spayed). The median age was 5 years and 7 months (range 1 month − 17 years). For four dogs the age was not available. In particular, most of the dogs were mature or senior adults: 542/1318 (41.1%) and 445/1318 (33.8%), respectively. Among the dogs included, 482/1322 (36.5%) were mixed breed and 840/1322 (63.5%) purebreds, with 93 different breeds reported. Based on the above reported categories 137/840 (16.3%) were toy dogs, 199/840 (23.7%) hunting dogs, 234/840 (27.9%) shepherd and guardian dogs, and 270/840 (32.1%) were dogs of other breeds. Most of the dogs (1232/1322, 93.2%) were from Northern Italy, whereas 57/1322 (4.5%) and 33/1322 (2.5%) were from Central and Southern Italy, respectively.
Table 1.
Signalment data and year of sampling of dogs tested for Anaplasma spp. and Ehrlichia spp. exposure
| Variables | Total | Total positive | P value | Tested for A. ph | Positive to A. ph | P value | Tested for A. pl | Positive to A. pl | P value | Tested for E. ca. | Positive to E. ca. | P value | Tested for A. ph and E. ca. | Positive to A. ph and E. ca. | P value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of dogs | 1322 | 94 (7.1%) *2 | 1246 | 53 (4.3%) | 920 | 1 (0.1%) | 1312 | 63 (4.8%) | 1237 | 24 (1.9%) | ||||||
| Year of sampling | 0.0041 | 0.0001 | 0.2880 | 0.2619 | 0.2105 | |||||||||||
| 2012 | 50 | 6 (12%) | 41 | 4 (9.8%) | 1 | 0 (0%) | 50 | 2 (4.0%) | 41 | 0 (0%) | ||||||
| 2013 | 129 | 11 (8.5%) | 109 | 8 (7.3%) | 81 | 0 (0%) | 127 | 6 (4.7%) | 107 | 3 (2.8%) | ||||||
| 2014 | 155 | 20 (12.9%) | 142 | 13 (9.2%) | 76 | 0 (0%) | 151 | 12 (7.9%) | 138 | 5 (3.6%) | ||||||
| 2015 | 97 | 6 (6.2%) | 89 | 4 (4.5%) | 22 | 0 (0%) | 95 | 4 (4.2%) | 87 | 2 (2.3%) | ||||||
| 2016 | 93 | 10 (10.8%) | 82 | 5 (6.1%) | 26 | 0 (0%) | 93 | 8 (8.6%) | 82 | 3 (3.7%) | ||||||
| 2017 | 188 | 7 (3.7%) | 182 | 4 (2.2%) | 144 | 0 (0%) | 188 | 4 (2.1%) | 182 | 1 (0.5%) | ||||||
| 2018 | 178 | 8 (4.5%) | 173 | 4 (2.3%) | 154 | 0 (0%) | 177 | 8 (4.5%) | 172 | 4 (2.3%) | ||||||
| 2019 | 216 | 15 (6.9%) | 214 | 6 (2.6%) | 207 | 0 (0%) | 215 | 12 (5.6%) | 214 | 4 (1.9%) | ||||||
| 2020 | 216 | 11 (5.1%) | 214 | 5 (2.3%) | 209 | 1 (0.5%) | 216 | 7 (3.2%) | 214 | 2 (0.9%) | ||||||
| Sex | 0.0486 | 0.1990 | 0.9844 | 0.0787 | 0.5875 | |||||||||||
| Male | 657 | 37 (5.6%) | 613 | 21 (3.4%) | 451 | 1 (0.2%) | 652 | 24 (3.7%) | 609 | 10 (1.6%) | ||||||
| Female | 665 | 57 (8.6%) | 633 | 32 (5.1%) | 469 | 0 (0%) | 660 | 39 (5.9%) | 628 | 14 (2.2%) | ||||||
| Age (months) *1 | 67 (1-212) | 82 (2-194) | 0.4645 | 68 (1-212) | 85 (8-194) | 0.0794 | 76 (1-212) | 10 | 67 (1-212) | 71 (2-194) | 0.7671 | 68 (1-212) | 78 (8-194) | 0.5397 | ||
| Age groups | 0.1611 | 0.0299 | 0.1709 | 0.9502 | 0.5328 | |||||||||||
| Puppy, juvenile and young adult dogs | 247 | 14 (5.7%) | 231 | 6 (1.7%) | 153 | 1 (0.7%) | 246 | 11 (4.5%) | 230 | 2 (0.9%) | ||||||
| Mature adult dogs | 542 | 35 (6.5%) | 507 | 22 (4.3%) | 349 | 0 (0%) | 537 | 25 (4.7%) | 502 | 12 (2.4%) | ||||||
| Senior adult dogs | 445 | 40 (9.0%) | 426 | 26 (6.1%) | 346 | 0 (0%) | 441 | 22 (5.0%) | 423 | 9 (2.1%) | ||||||
| Geriatric dogs | 84 | 3 (3.6%) | 80 | 1 (1.3%) | 71 | 0 (0%) | 84 | 3 (3.6%) | 80 | 1 (1.3%) | ||||||
| Breed | < 0.0001 | 0.2925 | 0.7850 | < 0.0001 | 0.0034 | |||||||||||
| Mixed breed | 482 | 56 (11.6%) | 450 | 24 (5.3%) | 339 | 1 (0.3%) | 479 | 47 (9.8%) | 447 | 16 (3.6%) | ||||||
| Purebred | 840 | 38 (4.5%) | 796 | 29 (3.6%) | 581 | 0 (0%) | 833 | 16 (1.9%) | 790 | 8 (1.0%) | ||||||
| Purebred groups | 0.0847 | 0.0156 | 0.6695 | 0.0974 | ||||||||||||
| Toy breeds | 137 | 4 (2.9%) | 131 | 3 (2.3%) | 112 | 0 (0%) | 137 | 3 (2.2%) | 131 | 2 (1.5%) | ||||||
| Hunting breeds | 199 | 14 (7.0%) | 190 | 14 (7.4%) | 150 | 0 (0%) | 196 | 4 (2.0%) | 187 | 4 (2.1%) | ||||||
| Shepherd and guardian breeds | 234 | 13 (5.6%) | 220 | 7 (3.2%) | 152 | 0 (0%) | 231 | 6 (2.6%) | 218 | 1 (0.5%) | ||||||
| Other breeds | 270 | 7 (2.6%) | 255 | 5 (2.0%) | 167 | 0 (0%) | 269 | 3 (1.1%) | 254 | 1 (0.4%) | ||||||
| Geographical origin | 0.8617 | 0.9319 | < 0.0001 | 0.8756 | 0.7280 | |||||||||||
| Northern Italy group | 1232 | 87 (7.1%) | 1160 | 50 (4.3%) | 859 | 0 (0%) | 1233 | 59 (4.8%) | 1152 | 23 (2.0%) | ||||||
| Central Italy group | 57 | 5 (8.8%) | 55 | 2 (3.6%) | 42 | 1 (2.4%) | 56 | 3 (5.4%) | 54 | 1 (1.9%) | ||||||
| Southern Italy group | 33 | 2 (6.1%) | 31 | 1 (3.2%) | 19 | 0 (0%) | 33 | 1 (3.0%) | 31 | 0 (0%) |
A. ph: Anaplasma phagocytophilum; A. pl: Anaplasma platys; E. ca.: Ehrlichia canis
*1 Data are reported as median and (range minimum-maximum). *2 One dog tested positive was excluded from the statistical analyses referring to the different pathogens investigated because it had tested positive only by PCR for all known Anaplasmataceae but its nucleotide sequence had not obtained for characterisation
Diagnosis of infection by rapid immunoenzymatic, serological and molecular tests
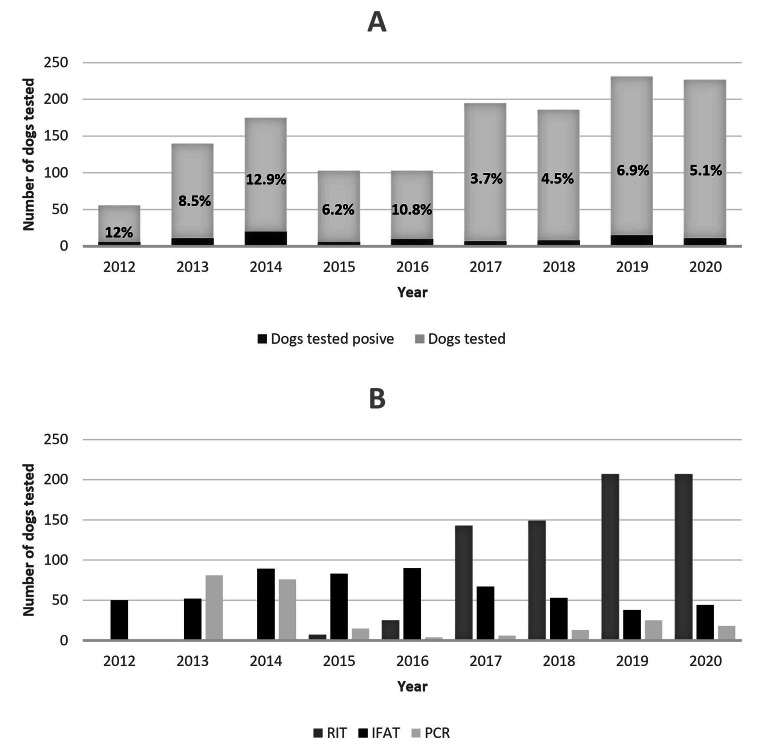
Ninety-four of the 1322 (7.1%) dogs tested positive for at least one pathogen investigated in the study. The frequency of dogs tested positive had fluctuations but it significantly decreased over the study period (P = 0.0041, Table 1; Fig. 1). All the 102 potential blood-donor dogs tested negative and even excluding them the positivity rate significantly decreased over the study period (P = 0.019).
Fig. 1.
Dogs tested positive (A) and type of test used (B) during the study period
A) highlighted in bold: percentages of positives on number of dogs tested per year
B) IFAT: indirect fluorescent antibody test; NT: not tested; PCR: polymerase chain reaction, RIT: rapid immunoenzymatic test
A significant association was found between positive result to at least one pathogen investigated and the sex of dogs, with females (57/665, 8.6%) more frequently positive than males (37/657, 5.6%) (P = 0.0486). The median age of dogs tested positive was 6 years and 10 months (range 2 months − 16 years) with no significance difference between age categories. The frequency of positive dogs was significantly higher in mixed breed (56/482, 11.6%) than in purebred (38/840, 4.5%) dogs (P < 0.0001). Differently, no significant association was evidenced between the four breed groups: toy breeds, hunting breeds, shepherd and guardian breeds, and other breeds. No other significant association was found between the frequency of infection and signalment data analysed (Table 1), including the geographical origin of the dogs tested positive, which appears equally distributed between Northern (87/1232, 7.1%), Central (5/57, 8.8%) and Southern (2/33, 6.1%) Italy. Clinical signs and clinicopathological variables of the dogs tested positive had no significant association with the presence of infections with the different pathogens considered in the study (Online Resources 2, 3 and 4). An exception was proteinuria, assessed by UPC values for 58 of 94 (61.7%) dogs tested positive. Indeed, of the 26/58 (44.8%) proteinuric dogs, those tested positive for A. phagocytophilum presented proteinuria more frequently compared to those tested positive for the other pathogens (P = 0.0487).
Fifty-three of the 1246 (4.3%) dogs tested for A. phagocytophilum were positive. The frequency of dogs tested positive for A. phagocytophilum significantly decreased over the study period (P < 0.0001, Table 1; Fig. 1), with 34/463 (7.3%) dogs tested positive in the first five years (2012–2016) and 19/783 (2.4%) dogs tested positive in the last four years (2017–2020). A slightly significant higher frequency of A. phagocytophilum detection was found in senior adult (26/426, 6.1%) dogs compared to other age groups (P = 0.0299), and in hunting breed dogs (14/190, 7.4%) compared to the other breed groups (P = 0.0156). No other significant association was found between A. phagocytophilum infection and signalment data analysed (Table 1).
One of the 920 (0.1%) dogs tested for A. platys was positive. It was a mixed-breed male dog of ten months old from a kennel in Rome (Central Italy) in 2020 (Table 1; Fig. 1).
Sixty-three of the 1312 (4.8%) dogs tested for E. canis were positive. The frequency of dogs tested positive for E. canis showed a constant distribution over the study period (Table 1; Fig. 1), whereas this frequency was significantly higher in mixed breed (47/479, 9.8%) than in purebred (16/833, 1.9%) dogs (P < 0.0001). No other significant association was found between E. canis infection and the signalment data analysed (Table 1).
Twenty-four of the 1237 (2.1%) dogs tested for A. phagocytophilum and E. canis were positive to both pathogens, evidencing a state of potential coinfection. No significant association was found between potential coinfection and the signalment data analysed, with the exception of a higher frequency of potential coinfection in mixed breed (16/447, 3.6%) than in purebred (8/790, 1.0%) dogs (P = 0.0034, Table 1).
One dog that tested positive only in PCR was excluded from the statistical analysis regarding the specific pathogens investigated because its nucleotide sequence was not obtained for the bacterial species identification.
During the study period 1544 tests were carried out: 739 (47.9%) RIT, 566 (36.7%) IFAT and 239 (15.5%) PCR. IFAT was used since the start of the study period, with an increasing number of tests carried out in the first years and a progressive decrease starting from 2017. PCR was introduced into the diagnostic routine since 2013, with a large number of tests carried out in the first two years, followed by significantly lower and constant numbers since 2015. RIT was introduced into the diagnostic routine since 2015 and the number of tests carried out progressively increased until 2020 (Fig. 1). The distribution of positive results according to the tests used was: 38/739 (5.1%) for RIT, 79/566 (14.0%) for IFAT and 15/239 (6.3%) for PCR. Several dogs were tested by more than one type of assay: 112/1322 (8.5%) were tested with RIT and IFAT, 24/1322 (1.8%) with RIT and PCR, 20/1322 (1.5%) with IFAT and PCR, and 33/1322 (2.5%) with all three assays. Of the 94 dogs tested positive, 45 were tested by RIT and 38/45 (84.4%) were positive, 82 were tested by IFAT and 79/82 (96.3%) were positive, and 39 were tested by PCR and 15/39 (38.5%) were positive (Online Resource 5). In Table 2 (A, B and C), the results obtained with the different tests used were reported and compared. In particular, 145 dogs were tested with both IFAT and RIT, 57 with both RIT and PCR, and 53 with both IFAT and PCR. The values of Cohen’s kappa coefficient were consistent with a near-perfect agreement between RIT and IFAT (0.88) and a poor agreement between PCR and RIT (0.21) or PCR and IFAT (0.17).
Table 2.
Comparison between tests results in dogs positive for at least one pathogen
| Pos | Neg | Total | ||
|---|---|---|---|---|
| A | IFAT | |||
| RIT | Pos | 29 (20.0%) | 4 (2.8%) | 33 (22.8%) |
| Neg | 2 (1.4%) | 110 (75.9%) | 112 (77.2%) | |
| Total | 31 (21.4%) | 114 (78.6%) | 145 (100%) | |
| B | PCR | |||
| RIT | Pos | 6 (10.5%) | 16 (28.1%) | 22 (38.6%) |
| Neg | 3 (5.3%) | 32 (56.1%) | 35 (61.4%) | |
| Total | 9 (15.8%) | 48 (84.2%) | 57 (100%) | |
| C | IFAT | |||
| PCR | Pos | 7 (13.2%) | 1 (1.9%) | 8 (15.1%) |
| Neg | 23 (43.4%) | 22 (41.5%) | 45 (84.9%) | |
| Total | 30 (56.6%) | 23 (43.4%) | 53 (100%) | |
A: Comparison between RIT and IFAT test results
B: Comparison between RIT and PCR test results
C: Comparison between PCR and IFAT test results
IFAT: indirect fluorescent antibody test; Neg: negative result; PCR: polymerase chain reaction; Pos: positive result; RIT: rapid immunoenzymatic test
Sequence analysis
Partial groEL gene nucleotide sequences of Anaplasma spp. or Ehrlichia spp. were obtained for 13/15 PCR positive dogs: on the basis of BLAST analysis, four were A. phagocytophilum, one was A. platys and eight were E. canis. In two dogs, nucleotide sequences were not obtained due to the low amount of PCR product: the first was PCR-positive only and it was excluded from the specific statistical analyses; the second one was tested positive by both RIT and IFAT tests for A. phagocytophilum and was considered infected with the latter. The sequences of A. phagocytophilum obtained in this study (lab and GenBank IDs: 393/2012 KF778380, 862/2014 KT970678, 901/2014 KT970679, and 909/2014 KT970680, the first three sequences were previously reported in Dondi et al. 2014 and De Arcangeli et al. 2018) showed a nucleotide identity of 99.5–100% among them. The only A. platys detected was sequenced in the dog 682/2020 (GenBank ID: OR209783) and IFAT and SNAP tests identified it as E. canis. This A. platys sequence showed a nucleotide identity of 99.7–100%, 71.9% and ≤ 77.9% with A. platys, E. canis and A. phagocytophilum reference sequences, respectively. The sequences of E. canis obtained in this study (lab and GenBank IDs: 598/2014 OR209777, 810/2014 OR209778, 1382/2016 OR209779, 421/2019 OR209780, 438/2019 OR209781, 501/2019 OR209782, 636/2019 ON245149, 637/2019 ON245150, the last two sequences were previously reported in Urbani et al. 2022) were identical.
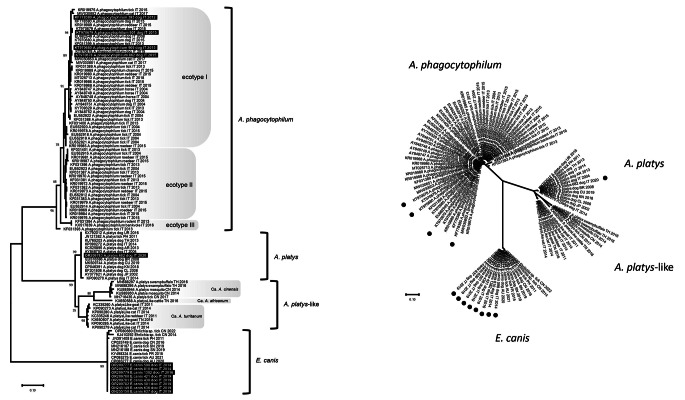
The phylogenetic tree showed four main clusters, corresponding to the species of pathogen investigated (Fig. 2).
Fig. 2.
Phylogenetic tree based on partial heat shock gene (groEL) nucleotide sequences of Anaplasma phagocytophilum, A. platys and Ehrlichia canis. Phylogeny was evaluated using the Maximum Likelihood method and the Tamura 3-parameter model implemented on MEGA 11 software version 11.0.11 on multiple alignment constructed with nucleotide sequences obtained in this study, 55 reference sequences of A. phagocytophilum, 17 of A. platys, 8 of A. platys-like and 9 of E. canis retrieved from GenBank. On the left a traditional rectangular branch style of the tree and on the right a radiation branch style of the tree. Identification of the sequences undergoes the following nomenclature: GenBank accession number, species, strain (only for sequences obtained in this study), host, country (AR: Argentina, AU: Australia, BR: Brazil, CL: Chile, CN: China, CU: Cuba, FR: France, IT: Italy, JP: Japan, KN: Saint Kitts and Navis, PH: Philippines, SN: Senegal, TH: Thailand, TN: Tunisia, UR: Uruguay), and collection date (or date of database submission). Statistical support was provided by bootstrapping with 1000 replicates and values indicated on the respective branches. Highlighted in black or black circles: sequences generated in this study. Main clusters of A. phagocytophilum are labelled following the ecotype classification proposed by Jahfari and colleagues (Jahfari et al. 2014)
In the A. phagocytophilum cluster, composed by sequences from Italy, three subgroups corresponding to three ecotypes proposed by Jahfari and colleagues (2014) were evidenced. The A. phagocytophilum sequenced in this study clustered in the ecotype I subgroup with other sequences identified in ticks and several mammalian hosts (dog, horse, red deer and chamois). The nucleotide sequence 682/2020 grouped in the A. platys cluster with reference strains from different geographical areas of the world. The so-called A. platys-like cluster had three subgroups composed by A. cinensis, A. africanum and A. turritanum candidates, respectively (Zobba et al. 2022a, b). Nucleotide sequences of E. canis obtained in this study grouped in a single cluster with the E. canis reference strains identified in ticks and dogs from various countries.
Discussion
This study was primarily aimed to describe the frequency of Anaplasma spp. and Ehrlichia spp. exposure in a veterinary hospital population of dogs in Italy, retrospectively enrolled in a 9-year period from 2012 to 2020. Performance of the tests adopted, correlations between infection and clinical data, and phylogeny of the identified bacteria were also investigated. A total of 94/1322 dogs tested positive to Anaplasma spp. or Ehrlichia spp. antibodies or DNA, with an overall frequency of infection of 7.1%. In particular, the values of frequency of infection of A. phagocytophilum (53/1246, 4.3%), A. platys (1/920, 0.1%), and E. canis (63/1312, 4.8%) obtained in this study were lower compared with previous surveys carried out in Italy which, however, were highly variable in relation to the diagnostic tests used, geographical area investigated and inclusion criteria adopted (Piantedosi et al. 2017; Petruccelli et al. 2021). In fact, some authors reported seroprevalence in dogs between 29% and 41.5% for A. phagocytophilum and between 28.7% and 44% for E. canis (Trotta et al. 2009; Mendoza-Roldan et al. 2021), while other surveys reported molecular prevalence in dogs between 0% and 5.1% for A. phagocytophilum, 0.8% and 3.7% for A. platys, and between 2.9% and 9.7% for E. canis (Solano-Gallego et al. 2006; Trotta et al. 2009; Zanet et al. 2020).
During the study period, the frequency of exposure detected decreased significantly, with highest frequency in 2012 (6/50, 12%) and 2014 (20/155, 12.9%) and a progressive decline from 2016 to 2020 (11/216, 5.1%). This trend was predominantly associated with the number of dogs tested positive to A. phagocytophilum and could be related to the climatic conditions, that are known to strongly influence the presence of ticks in the environment and consequently the spreading of vector-borne pathogens (Ebani 2019). Indeed, the year 2014, which showed the highest frequency of positivity detected, was a very warm and rainy year in Italy, with the average annual temperature 1.63 °C higher than the normal value and a total annual rainfall overall higher of about 13% than the climatic average (Desiato et al. 2014). Furthermore, the progressive decrease in frequency of positivity detected could be linked to the increasing use of ectoparasiticides by dog-owners or to several factors which led to carrying out a greater number of tests for screening purposes, testing animals at risk of infection and not only those with associated clinical signs, therefore with a lower probability of testing positive: (i) the introduction into the diagnostic routine of a rapid and easy-to-use test such as the RIT (since 2015), which has also led to a reduction in the use of IFAT and PCR; (ii) the increasing sensitivity of veterinarians to these pathogens, given the increasing prevalence of the associated diseases; and (iii) the launch in 2017 of our veterinary blood bank (Veterinary Blood Solutions – VeBS) in the VTH with routine tests of blood donor dogs with low suspicion of infection. Consistently, Morganti and colleagues (2022) reported a lower prevalence of infection in potential blood donor dogs compared to a non-selected dog population.
Several studies reported that sex is not a risk factor for Anaplasma spp. and Ehrlichia spp. infection or reported a slight predisposition in male dogs, probably related to their behaviour which exposes them more to ticks (Sainz et al. 2015; Ebani 2019; Hazelrig et al. 2023). Differently, in our study a slight predisposition to infection was found in female dogs, a result probably linked to random sampling. In our study, moreover, a slight association between dog age and infection was found for A. phagocytophilum only, with senior adult dogs (6–10 years of age) being more predisposed to contract the infection, a finding consistent with other studies (Watanabe et al. 2004; Costa et al. 2007; Ebani et al. 2013). This predisposition is probably due to an increased risk of exposure of the senior adult dogs to TBP over time, rather than an increased susceptibility to them (Egenvall et al. 2000; Kohn et al. 2011). Some authors reported a higher prevalence of Ehrlichia spp. infection in adult dogs (Ebani 2019; Hazelrig et al. 2023), while in several studies age was not a risk factor for any of the TBP considered (Ebani et al. 2014; Sainz et al. 2015). A significantly higher prevalence of A. phagocytophilum infection was found in hunting breed dogs enrolled in this study, an association never reported in literature (Sainz et al. 2015; Piantedosi et al. 2017). On the other hand, some studies reported a link between TBP infection and the lifestyle or environments frequented by dogs of hunting breeds, due to closer contact with wooded and rural areas, cohabitation in outdoor kennels and potentially less consistent use of acaricide products. For these reasons, it is possible to speculate that hunting dogs have a predisposition to contracting the infection due to exposure to risk factors rather than to the breed (Kordick et al. 1999; Solano-Gallego et al. 2006; Piantedosi et al. 2017). Furthermore, Ixodes ricinus tick, vector of A. phagocytophilum, is widespread in environments and animals to which hunting dogs are more exposed than other dog breeds (Santoro et al. 2016; Westmoreland et al. 2016). Indeed, I. ricinus is found in mixed and deciduous forests, open pastures and other areas with high humidity and its wide distribution is also related to a broad host range, including many mammalian species and birds (Medlock et al. 2013). Differently, a predisposition to contract infection sustained by E. canis was found in this study for mixed-breed dogs compared to purebred ones. This finding is not in agreement with the literature which reports mixed-breed dogs as generally more resistant to infection (Harrus et al. 1997). As for hunting breed dogs, also the predisposition of mixed-breed dogs to contract TBP infection may be related to the lifestyle of the dogs investigated. Moreover, mixed-breed dogs are often adopted without knowing their infectious status or the epidemiological situation of the geographical area of origin, thus increasing the possibility that they were infected prior to adoption. Analysing the geographical origin of the dogs included in this study, the majority of enrolled dogs come from Northern Italy (1237) compared to other areas (57 and 33 dogs for Central and Southern Italy, respectively), as a result of the location of our veterinary hospital. Nevertheless, no significant differences in the frequency of exposure were found in our study in the different Italian geographical areas. Differently, several studies found significantly higher prevalence in Central and Southern Italian regions (Solano-Gallego et al. 2006; Vascellari et al. 2016; Colombo et al. 2021). This discrepancy in the results may be due to an underestimation of the prevalence of Anaplasma spp. and Ehrlichia spp. in Central and Southern Italy for a limited sampling of dogs from these regions. Proteinuria was the only clinicopathological variable that showed a significantly higher frequency in A. phagocytophilum infections compared to dogs tested positive for the other pathogens investigated in this study. This finding was already reported to be associated with this pathogen and this clinicopathological variable could be considered to differentiate the diagnosis of these TBP (Dondi et al. 2014; Ravnik et al. 2014).
The tests used in this study showed a different performance. The serological methods (RIT and IFAT) had high concordance among them and allowed the identification of a higher number of positive dogs compared to PCR. This result is in agreement with the characteristics of serological tests which, detecting antibodies against pathogens even after the infection has resolved, identify more positive dogs than the molecular methods. Conversely, PCR can only diagnose active infections and therefore identifies fewer positive dogs. In fact, molecular assays applied to blood samples are highly sensitive and specific but false-negative results may occur as consequence of pathogen load (Sainz et al. 2015), presence of inhibitors, variations in levels of circulating pathogens due to intermittent bacteraemia and antibiotics administration (Allison and Little 2013). Furthermore, E. canis often play a role in chronic infections which may not be easily identified by direct methods. For these reasons, negative results in molecular tests only indicate that the respective nucleic acid sequence is not detected in a particular sample at a particular point of time and should not be interpreted as conclusive evidence of absence of infection (Allison and Little 2013; Sainz et al. 2015; Diniz and Moura de Aguiar 2022). Furthermore, a major therapeutic dilemma is posed by asymptomatic dogs with positive tests results, because the treatment of choice against infections with bacteria of the Anaplasmataceae family is long-term antibiotic therapy. At a time when antimicrobial resistance and the reduction of the use of antimicrobial drugs are becoming increasingly important, it is difficult to assess the real need to treat positive dogs even in the absence of clinical or clinicopathological abnormalities (Harrus et al. 2023).
Phylogeny of the pathogens sequenced in this study showed a clear distinction between the three species examined. All the A. phagocytophilum obtained in this study clustered in the ecotype I subgroup (Jahfari et al. 2014; Jaarsma et al. 2019), which exhibits the widest host range and includes all strains identified in humans in Europe, suggesting potential zoonotic implications and reservoir role of wild animals (Balboni et al. 2021; Grassi et al. 2021). The A. platys obtained in this study clustered with other A. platys detected in dogs worldwide, revealing a high genetic similarity between strains from different geographical areas (de la Fuente et al. 2006; Zobba et al. 2015). All the E. canis obtained in this study clustered and showed an identity of 100% with reference strains identified in dogs and ticks from Europe, Asia and Africa, excluding correlation with host and geographical origin (Alhassan et al. 2021).
Of interest, the A. platys sequenced in this study was identified in a dog (682/2020) tested positive by RIT and IFAT, which were apparently indicative for E. canis exposure. This result can have two explanations. The dog could have antibodies against E. canis from a previous exposure and at the same time an active infection with A. platys, without detectable levels of specific antibodies. This type of coinfection has already been reported and may be related to sharing the same tick vector (Rhipicephalus sanguineus, Piratae et al. 2019; Garcia Ribeiro et al. 2023). Otherwise, an antibody cross-reactivity between the two pathogens may have occurred, thus distorting the RIT and IFAT tests results. Few cases of cross-reactivity have been reported, but never between E. canis and A. platys. They often occur between different Ehrlichia species, or between A. phagocytophilum and A. platys, or even between A. phagocytophilum and E. canis (Al-Adhami et al. 2011; Qurollo et al. 2021). Further studies are needed to investigate the occurrence of potential cross-reactions between these two species.
The present study has some limitations due to its retrospective design. Firstly, the animals enrolled were not all tested with the three tests used, limiting the comparison between the results obtained and the evaluation of the sensitivity and specificity of the assays. Furthermore, only for 13/94 positive dogs the identified pathogen was typed by sequencing. In the other 81/94 positive dogs, the species identification was carried out on a serological basis, by RIT or IFAT, and could be subjected to errors, as demonstrated by the A. platys genetically identified in a dog with serological test results apparently indicative of E. canis exposure. Twenty-four of 94 (25.5%) dogs were considered potentially coinfected because tested positive for more than one pathogen. In dogs where multiple positivity was detected only by a serological test (RIT or IFAT) it was not possible to exclude with certainty (gene sequencing of the bacteria identified by PCR) that antibody cross-reactivity between different pathogens or serological positivities related to previous resolved infections have occurred, resulting in incorrect identification of the status of coinfection. Although sequencing of end-point PCR products is a very useful tool in single-pathogen infections, it alone is not conclusive in the correct identification of multiple pathogens during coinfections. Another limit was the geographical distribution of the enrolled dogs, mostly from Northern Italy, which was linked to our VTH location. Consequently, the dog population analysed does not adequately represent all of Italy, but allows to draw more reliable conclusions for dogs from Northern Italy and to compare them with the dogs moving from Central and Southern Italy to be visited in a veterinary hospital of reference in the North.
Conclusion
Our study reports the trend over time of the dogs tested positive for Anaplasma spp. and Ehrlichia spp. according to the tests used and the population analysed and provides new insights into possible risk factors for infection in dogs in Italy. Some risks factors as adult age for A. phagocytophilum and breed (hunting breed for A. phagocytophilum and mixed breed for E. canis) were evidenced. This study also reports discordant results between different tests, showing that single diagnostic tests often fail to detect positive subjects in different stage of infection and confirming the importance of a diagnostic approach that combines molecular and serological tests. Considering a wider spread of the vectors due to climate change and an increasingly sharing of environment between companion animals and owners, constant surveillance of animal populations is necessary from a One Health perspective. The discovery of zoonotic strains in several animal cases suggests that companion animals can act as sentinels for human infections, and dogs with undiagnosed Anaplasmataceae infection may play an important epidemiological role by acting as source of infection for invertebrate hosts, with an increased risk of transmission to humans and posing a threat to public health.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by EU funding within the NextGenerationEU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT).
Author contributions
A. B., F. D. and M. B. conceptualization. M. C. S., L.U., A. T., M. M. and F. L. methodology. V. F., A. B. and F. D. formal analysis. V. F., M. C. S., and L.U. writing - original draft. A. B., F. D. and M. B. writing - review & editing. M. B. supervision. All authors have read, reviewed, and approved the final manuscript.
Funding
This research was supported by EU funding within the NextGenerationEU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT).
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. The nucleotide sequences generated and analyzed during the current study are available in the International Nucleotide Sequence Database Collaboration repository (INSDC, http://www.insdc.org/) with the IDs: OR209777-OR209783.
Declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained from all owners of the animals involved in the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Adhami B, Scandrett WB, Lobanov VA, Gajadhar AA. Serological cross-reactivity between Anaplasma marginale and an Ehrlichia species in naturally and experimentally infected cattle. J Vet Diagn Invest. 2011;23:1181–1188. doi: 10.1177/1040638711425593. [DOI] [PubMed] [Google Scholar]
- Alhassan A, Hove P, Sharma B, Matthew-Belmar V, Karasek I, Lanza-Perea M, Werners AH, Wilkerson MJ, Ganta RR. Molecular detection and characterization of Anaplasma platys and Ehrlichia canis in dogs from the Caribbean. Ticks Tick Borne Dis. 2021;12:101727. doi: 10.1016/j.ttbdis.2021.101727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison RW, Little SE. Diagnosis of rickettsial diseases in dogs and cats. Vet Clin Pathol. 2013;42:127–144. doi: 10.1111/vcp.12040. [DOI] [PubMed] [Google Scholar]
- Balboni A, Urbani L, Morini M, Dondi F, Battilani M. Molecular detection of Anaplasma phagocytophilum in hair and spleen of cats revealed a possible underestimation of feline vector-borne pathogens. Res Vet Sci. 2021;137:144–149. doi: 10.1016/j.rvsc.2021.05.003. [DOI] [PubMed] [Google Scholar]
- Barber RM, Li Q, Diniz PP, Porter BF, Breitschwerdt EB, Claiborne MK, Birkenheuer AJ, Levine JM, Levine GJ, Chandler K, Kenny P, Nghiem P, Wei S, Greene CE, Kent M, Platt SR, Greer K, Schatzberg SJ. Evaluation of brain tissue or cerebrospinal fluid with broadly reactive polymerase chain reaction for Ehrlichia, Anaplasma, spotted fever group Rickettsia, Bartonella, and Borrelia species in canine neurological diseases (109 cases) J Vet Intern Med. 2010;24:372–378. doi: 10.1111/j.1939-1676.2009.0466.x. [DOI] [PubMed] [Google Scholar]
- Breitschwerdt EB, Hegarty BC, Qurollo BA, Saito TB, Maggi RG, Blanton LS, Bouyer DH (2014) Intravascular persistence of Anaplasma platys, Ehrlichia chaffeensis, and Ehrlichia ewingii DNA in the blood of a dog and two family members. Parasit Vectors 7: 1–7. 10.1186/1756-3305-7-298 [DOI] [PMC free article] [PubMed]
- Carvalho L, Armua-Fernandez MT, Sosa N, Félix ML, Venzal JM. Anaplasma platys in dogs from Uruguay. Ticks Tick Borne Dis. 2017;8:241–245. doi: 10.1016/j.ttbdis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Clark AM, Hopkins GF, MacLean IA. Tick-borne fever in dogs. Vet Rec. 1996;139:268. [PubMed] [Google Scholar]
- Colombo M, Morelli SM, Simonato G, Di Cesare A, Veronesi F, Frangipane di Regalbono A, Grassi L, Russi I, Tiscar PG, Morganti G, Hattab J, Rizzo V, Traversa D. Exposure to major Vector-Borne diseases in dogs subjected to different preventative regimens in endemic areas of Italy. Pathogens. 2021;10:507. doi: 10.3390/pathogens10050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LM, Jr, Rembeck K, Ribeiro MF, Beelitz P, Pfister K, Passos LM. Seroprevalence and risk indicators for canine ehrlichiosis in three rural areas of Brazil. Vet J. 2007;174:673–676. doi: 10.1016/j.tvjl.2006.11.002. [DOI] [PubMed] [Google Scholar]
- De Arcangeli S, Balboni A, Serafini F, Battilani M, Dondi F. Anaplasma phagocytophilum infection in thrombocytopenic dogs. Vet Ital. 2018;54:73–78. doi: 10.12834/VetIt.1070.5796.2. [DOI] [PubMed] [Google Scholar]
- de la Fuente J, Torina A, Naranjo V, Nicosia S, Alongi A, La Mantia F, Kocan KM. Molecular characterization of Anaplasma platys strains from dogs in Sicily, Italy. BMC Vet Res. 2006;2:24. doi: 10.1186/1746-6148-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiato F, Fioravanti G, Fraschetti P, Perconti W, Piervitali E, Pavan V (2014) Gli Indicatori del Clima in Italia nel 2014. Anno XI ISPRA. Stato dell’Ambiente 57/2015. ISBN 978-88-448-0722-1. Available online: https://www.isprambiente.gov.it/files/pubblicazioni/statoambiente/SA_57_15_Indicatori_clima_2014.pdf (accessed on 18 Aug 2023)
- Diniz PPVP, Moura de Aguiar D. Ehrlichiosis and anaplasmosis: an update. Vet Clin North Am Small Anim Pract. 2022;52:1225–1266. doi: 10.1016/j.cvsm.2022.07.002. [DOI] [PubMed] [Google Scholar]
- Dondi F, Russo S, Agnoli C, Mengoli N, Balboni A, Alberti A, Battilani M (2014) Clinicopathological and molecular findings in a case of canine Anaplasma phagocytophilum infection in Northern Italy. ScientificWorldJournal 810587. 10.1155/2014/810587 [DOI] [PMC free article] [PubMed]
- Ebani VV. Serological survey of Ehrlichia canis and Anaplasma phagocytophilum in dogs from Central Italy: an update (2013–2017) Pathogens. 2019;8:3. doi: 10.3390/pathogens8010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebani V, Cerri D, Fratini F, Ampola M, Andreani E. Seroprevalence of Anaplasma phagocytophilum in domestic and wild animals from central Italy. New Microbiol. 2008;31:371–375. [PubMed] [Google Scholar]
- Ebani VV, Bertelloni F, Turchi B, Cerri D. Serological and molecular survey of Anaplasma phagocytophilum in Italian hunting dogs. Ann Agric Environ Med. 2013;20:289–292. [PubMed] [Google Scholar]
- Ebani VV, Bertelloni F, Torracca B, Cerri D. Serological survey of Borrelia burgdorferi Sensu Lato, Anaplasma phagocytophilum, and Ehrlichia canis infections in rural and urban dogs in Central Italy. Ann Agric Environ Med. 2014;21:671–675. doi: 10.5604/12321966.1129912. [DOI] [PubMed] [Google Scholar]
- Egenvall AE, Bonnett BN, Gunnarsson A, Hedhammar A, Shoukri M, Bornstein S, Artursson K. Sero-prevalence of granulocytic Ehrlichia spp. and Borrelia burgdorferi sensu lato in Swedish dogs 1991-94. Scand J Infect Dis. 2000;32:19–25. doi: 10.1080/00365540050164164. [DOI] [PubMed] [Google Scholar]
- El Hamiani Khatat S, Daminet S, Duchateau L, Elhachimi L, Kachani M, Sahibi H. Epidemiological and clinicopathological features of Anaplasma phagocytophilum infection in dogs: a systematic review. Front Vet Sci. 2021;8:686644. doi: 10.3389/fvets.2021.686644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Ribeiro M, da Silva CPC, Pchevuzinske LM, Portilho FVR, Siqueira AK, Takahira RK, Paschoal NR, de Souza AAL, Rodrigues CA, de Almeida BO, Bello TS, Filho MFÁ, de Lima Paz PJ, Dutra V, Nakazato L, Pereira NA, de Aguiar DM. Pleural effusion-related Nocardia otitidiscaviarum, Anaplasma platys and Ehrlichia canis coinfection in a dog. Braz J Microbiol. 2023 doi: 10.1007/s42770-023-01029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick J, Lappin MR, Waner T, Harrus S, Mylonakis ME. Anaplasmosis. In: Sykes JE, editor. Greene’s infectious diseases of the dog and cat. 5. St. Louis: Saunders Elsevier; 2023. pp. 542–554. [Google Scholar]
- Grassi L, Franzo G, Martini M, Mondin A, Cassini R, Drigo M, Pasotto D, Vidorin E, Menandro ML. Ecotyping of Anaplasma phagocytophilum from wild ungulates and ticks shows circulation of zoonotic strains in Northeastern Italy. Animals. 2021;11:310. doi: 10.3390/ani11020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig B, Asanovich KM, Armstrong PJ, Dumler JS. Geographic, clinical serologic, and molecular evidence of granulocytic ehrlichiosis, a likely zoonotic disease, in Minnesota and Wisconsin dogs. J Clin Microbiol. 1996;34:44–48. doi: 10.1128/jcm.34.1.44-48.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrus S, Waner T. Diagnosis of canine monocytotropic ehrlichiosis (Ehrlichia canis): an overview. Vet J. 2011;187:292–296. doi: 10.1016/j.tvjl.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Harrus S, Kass PH, Klement E, Waner T. Canine monocytic ehrlichiosis: a retrospective study of 100 cases, and an epidemiological investigation of prognostic indicators for the disease. Vet Rec. 1997;141:360–363. doi: 10.1136/vr.141.14.360. [DOI] [PubMed] [Google Scholar]
- Harrus S, Waner T, Aizenberg I, Foley JE, Poland AM, Bark H. Amplification of ehrlichial DNA from dogs 34 months after infection with Ehrlichia canis. J Clin Microbiol. 1998;36:73–76. doi: 10.1128/jcm.36.1.73-76.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrus S, Perlman-Avrahami A, Mumcluoglu KY, Morick D, Eyal O, Baneth G. Molecular detection of E. canis A. Bovis. A. platys, Candidatus Midichloria mitochondrii and Babesia canis vogeli in ticks from Israel. Clin Microbiol Infect. 2011;17:459–463. doi: 10.1111/j.1469-0691.2010.03316.x. [DOI] [PubMed] [Google Scholar]
- Harrus S, Waner T, Mylonakis ME, Sykes JE, Qurollo B. Ehrlichiosis. In: Sykes JE, editor. Greene’s infectious diseases of the dog and cat. 5. St. Louis: Saunders Elsevier; 2023. pp. 522–541. [Google Scholar]
- Harvey ND. How old is my dog? Identification of rational Age groupings in Pet Dogs based upon normative age-linked processes. Front Vet Sci. 2021;8:643085. doi: 10.3389/fvets.2021.643085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelrig CM, Gettings JR, Cleveland CA, Varela-Stokes A, Majewska AA, Hubbard K, Burton KW, Yabsley MJ. Spatial and risk factor analyses of vector-borne pathogens among shelter dogs in the Eastern United States. Parasit Vectors. 2023;16:197. doi: 10.1186/s13071-023-05813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaarsma RI, Sprong H, Takumi K, Kazimirova M, Silaghi C, Mysterud A, Rudolf I, Beck R, Földvári G, Tomassone L, Groenevelt M, Everts RR, Rijks JM, Ecke F, Hörnfeldt B, Modrý D, Majerová K, Votýpka J, Estrada-Peña A. Anaplasma phagocytophilum evolves in geographical and biotic niches of vertebrates and ticks. Parasit Vectors. 2019;12:1–17. doi: 10.1186/s13071-019-3583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Coipan EC, Fonville M, van Leeuwen AD, Hengeveld P, Heylen D, Heyman P, van Maanen C, Butler CM, Földvári G, Szekeres S, van Duijvendijk G, Tack W, Rijks JM, van der Giessen J, Takken W, van Wieren SE, Takumi K, Sprong H. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasit Vectors. 2014;7:365. doi: 10.1186/1756-3305-7-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd L. Optimal vector-borne disease screening in dogs using both serology-based and polymerase chain reaction–based diagnostic panels. Vet Clin North Am Small Anim Pract. 2019;49:703–718. doi: 10.1016/j.cvsm.2019.02.011. [DOI] [PubMed] [Google Scholar]
- Kohn B, Galke D, Beelitz P, Pfister K. Clinical features of canine granulocytic anaplasmosis in 18 naturally infected dogs. J Vet Intern Med. 2008;22:1289–1295. doi: 10.1111/j.1939-1676.2008.0180.x. [DOI] [PubMed] [Google Scholar]
- Kohn B, Silaghi C, Galke D, Arndt G, Pfister K. Infections with Anaplasma phagocytophilum in dogs in Germany. Res Vet Sci. 2011;91:71–76. doi: 10.1016/j.rvsc.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Kordick SK, Breitschwerdt EB, Hegarty BC, Southwick KL, Colitz CM, Hancock SI, Bradley JM, Rumbough R, Mcpherson JT, MacCormack JN. Coinfection with multiple tick-borne pathogens in a walker hound kennel in North Carolina. J Clin Microbiol. 1999;37:2631–2638. doi: 10.1128/jcm.37.8.2631-2638.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SE, Beall MJ, Bowman DD, Chandrashekar R, Stamaris J (2014) Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2010–2012. Parasit Vectors 7: 1–9. 10.1186/1756-3305-7-257 [DOI] [PMC free article] [PubMed]
- Liu J, Eberts M, Bewsey H, O’Connor TP, Chandrashekar R, Breitschwerdt EB. Sensitivity and specificity levels of two rapid assays for antibodies to Anaplasma spp. in dogs. J Vet Diagn Invest. 2018;30:290–293. doi: 10.1177/1040638717745932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia C, Ramos C, Coimbra M, Bastos F, Martins A, Pinto P, Nunes M, Vieira ML, Cardoso L, Campino L. Bacterial and protozoal agents of feline vector-borne diseases in domestic and stray cats from southern Portugal. Parasit Vectors. 2014;7:115. doi: 10.1186/1756-3305-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock JM, Hansford KM, Bormane A, Derdakova M, Estrada-Peña A, George JC, Golovljova I, Jaenson TG, Jensen JK, Jensen PM, Kazimirova M, Oteo JA, Papa A, Pfister K, Plantard O, Randolph SE, Rizzoli A, Santos-Silva MM, Sprong H, Vial L, Hendrickx G, Zeller H, Van Bortel W. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1. doi: 10.1186/1756-3305-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Roldan JA, Benelli G, Bezerra-Santos MA, Nguyen VL, Conte G, Iatta R, Furlanello T, Otranto D. Seropositivity to canine tick-borne pathogens in a population of sick dogs in Italy. Parasit Vectors. 2021;14:292. doi: 10.1186/s13071-021-04772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti G, Miglio A, Moretta I, Misia AL, Rigamonti G, Cremonini V, Antognoni MT, Veronesi F. Retrospective longitudinal survey on Canine Vector-Borne pathogens: trends and challenges of 10 years of activities of a Veterinary Blood Bank. Vet Sci. 2022;9:274. doi: 10.3390/vetsci9060274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonakis ME, Harrus S, Breitschwerdt EB. An update on the treatment of canine monocytic ehrlichiosis (Ehrlichia canis) Vet J. 2019;246:45–53. doi: 10.1016/j.tvjl.2019.01.015. [DOI] [PubMed] [Google Scholar]
- Neave MJ, Mileto P, Joseph A, Reid TJ, Scott A, Williams DT, Keyburn AL. Comparative genomic analysis of the first Ehrlichia canis detections in Australia. Ticks Tick Borne Dis. 2022;13:101909. doi: 10.1016/j.ttbdis.2022.101909. [DOI] [PubMed] [Google Scholar]
- Neer TM, Breitschwerdt EB, Greene RT, Lappin MR (2002) Consensus statement on ehrlichial disease of small animals from the infectious disease study group of the ACVIM. American College of Veterinary Internal Medicine. J Vet Intern Med 16:309–315. 10.1892/0891-6640(2002)016<0309:csoedo>2.3.co;2 [DOI] [PubMed]
- Olano JP, Aguero-Rosenfeld ME (2008) Ehrlichia, Anaplasma, and related intracellular Bacteria. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA (eds) Manual of Clinical Microbiology, vol 1. American Society for Microbiology, Washington DC, USA
- Pereira A, Parreira R, Nunes M, Casadinho A, Vieira ML, Campino L, Maia C. Molecular detection of tick-borne bacteria and protozoa in cervids and wild boars from Portugal. Parasit Vectors. 2016;9:1–9. doi: 10.1186/s13071-016-1535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruccelli A, Ferrara G, Iovane G, Schettini R, Ciarcia R, Caputo V, Pompameo M, Pagnini U, Montagnaro S (2021) Seroprevalence of Ehrlichia spp., Anaplasma spp., Borrelia burgdorferi Sensu Lato, and Dirofilaria Immitis in stray dogs, from 2016 to 2019, in Southern Italy. Animals 11(9). 10.3390/ani11010009 [DOI] [PMC free article] [PubMed]
- Piantedosi D, Neola B, D’Alessio N, Di Prisco F, Santoro M, Pacifico L, Sgroi G, Auletta L, Buch J, Chandrashekar R, Breitschwerdt EB, Veneziano V. Seroprevalence and risk factors associated with Ehrlichia canis, Anaplasma spp., Borrelia burgdorferi Sensu Lato, and D. Immitis in hunting dogs from southern Italy. Parasitol Res. 2017;116:2651–2660. doi: 10.1007/s00436-017-5574-z. [DOI] [PubMed] [Google Scholar]
- Piratae S, Senawong P, Chalermchat P, Harnarsa W, Sae-Chue B. Molecular evidence of Ehrlichia canis and Anaplasma platys and the association of infections with hematological responses in naturally infected dogs in Kalasin, Thailand. Vet World. 2019;12:131–135. doi: 10.14202/vetworld.2019.131-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitout FM, Shinozaki JK, Stockwell PJ, Holland CJ, Shukla SK. Genetic variants of Anaplasma phagocytophilum infecting dogs in western Washington State. J Clin Microbiol. 2005;43:796–801. doi: 10.1128/jcm.43.2.796-801.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qurollo BA, Stillman BA, Beall MJ, Foster P, Hegarty BC, Breitschwerdt EB, Chandrashekar R. Comparison of Anaplasma and Ehrlichia species-specific peptide ELISAs with whole organism-based immunofluorescent assays for serologic diagnosis of anaplasmosis and ehrlichiosis in dogs. Am J Vet Res. 2021;82:71–80. doi: 10.2460/ajvr.82.1.71. [DOI] [PubMed] [Google Scholar]
- Ravnik U, Bajuk BP, Lusa L, Tozon N. Serum protein profiles, circulating immune complexes and proteinuria in dogs naturally infected with Anaplasma phagocytophilum. Vet Microbiol. 2014;173:160–165. doi: 10.1016/j.vetmic.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Sainz Á, Roura X, Miró G, Estrada-Peña A, Kohn B, Harrus S, Solano-Gallego L. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit Vectors. 2015;8:75. doi: 10.1186/s13071-015-0649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito TB, Walker DH. Ehrlichioses: an important one health opportunity. Vet Sci. 2016;3:20. doi: 10.3390/vetsci3030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vicente S, Tagliafierro T, Coleman JL, Benach JL, Tokarz R. Polymicrobial nature of tick-borne diseases. mBio. 2019;10:e02055–e02019. doi: 10.1128/mbio.02055-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M, Veneziano V, D’Alessio N, Di Prisco F, Lucibelli MG, Borriello G, Cerrone A, Dantas-Torres F, Latrofa MS, Otranto D, Galiero G. Molecular survey of Ehrlichia canis and Coxiella burnetii infections in wild mammals of southern Italy. Parasitol Res. 2016;115:4427–4431. doi: 10.1007/s00436-016-5213-0. [DOI] [PubMed] [Google Scholar]
- Solano-Gallego L, Trotta M, Razia L, Furlanello T, Caldin M. Molecular survey of Ehrlichia canis and Anaplasma phagocytophilum from blood of dogs in Italy. Ann N Y Acad Sci. 2006;1078:515–518. doi: 10.1196/annals.1374.101. [DOI] [PubMed] [Google Scholar]
- Stuen S, Granquist EG, Silaghi C. Anaplasma phagocytophilum—A wide spread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol. 2013;3:1–33. doi: 10.3389/fcimb.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes JE. Ehrlichiosis. In: Sykes JE, editor. Canine and Feline Infectious diseases. 1. St. Louis: Elsevier; 2014. pp. 278–289. [Google Scholar]
- Sykes JE, Foley JE. Anaplasmosis. In: Sykes JE, editor. Canine and Feline Infectious diseases. 1. St. Louis: Elsevier; 2014. pp. 290–300. [Google Scholar]
- Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torina A, Blanda V, Antoci F, Scimeca S, D’Agostino R, Scariano E, Piazza A, Galluzzo P, Giudice E, Caracappa S (2013) A molecular survey of Anaplasma spp., Rickettsia spp., Ehrlichia canis and Babesia microti in foxes and fleas from Sicily. Transbound Emerg Dis 60: 125–130. 10.1111/tbed.12137 [DOI] [PubMed]
- Trotta M, Fogliazza A, Furlanello T, Solano-Gallego L. A molecular and serological study of exposure to tick-borne pathogens in sick dogs from Italy. Clin Microbiol Infect. 2009;2:62–63. doi: 10.1111/j.1469-0691.2008.02279.x. [DOI] [PubMed] [Google Scholar]
- Urbani L, Tirolo A, Balboni A, Troia R, Dondi F, Battilani M. Concomitant infections with Canine Parvovirus type 2 and intracellular tick-borne pathogens in two puppy dogs. Front Vet Sci. 2022;9:964177. doi: 10.3389/fvets.2022.964177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vascellari M, Ravagnan S, Carminato A, Cazzin S, Carli E, Da Rold G, Lucchese L, Natale A, Otranto D, Capelli G. Exposure to vector-borne pathogens in candidate blood donor and free-roaming dogs of northeast Italy. Parasit Vectors. 2016;9:369. doi: 10.1186/s13071-016-1639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Okuda M, Tsuji M, Inokuma H. Seroepidemiological study of canine ehrlichial infections in Yamaguchi prefecture and surrounding areas of Japan. Vet Parasitol. 2004;124:101–107. doi: 10.1016/j.vetpar.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Westmoreland LS, Stoskopf MK, Maggi RG. Prevalence of Anaplasma phagocytophilum in North Carolina eastern black bears (Ursus americanus) J Wildl Dis. 2016;52:968–970. doi: 10.7589/2016-02-036. [DOI] [PubMed] [Google Scholar]
- Wilson AL, Courtenay O, Kelly-Hope LA, Scott TW, Takken W, Torr SJ, Lindsay SW. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl Trop Dis. 2020;14:e0007831. doi: 10.1371/journal.pntd.0007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanet S, Battisti E, Pepe P, Ciuca L, Colombo L, Trisciuoglio A, Ferroglio E, Cringoli G, Rinaldi L, Maurelli MP. Tick-borne pathogens in Ixodidae ticks collected from privately-owned dogs in Italy: a country-wide molecular survey. BMC Vet Res. 2020;16:46. doi: 10.1186/s12917-020-2263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziliani TF, Castilho AR, Poletto D, Mendonça AJ, Sousa VRF, Dutra V. Kidney disease in natural infection by Ehrlichia canis in dogs. Semin Cienc Agrar. 2019;40:981–986. doi: 10.5433/1679-0359.2019v40n2p981. [DOI] [Google Scholar]
- Zobba R, Anfossi AG, Visco S, Sotgiu F, Dedola C, Pinna Parpaglia ML, Battilani M, Pittau M, Alberti A. Cell tropism and molecular epidemiology of Anaplasma platyslike strains in cats. Ticks Tick Borne Dis. 2015;6:272–280. doi: 10.1016/j.ttbdis.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Zobba R, Murgia C, Dahmani M, Mediannikov O, Davoust B, Piredda R, Schianchi E, Scagliarini A, Pittau A, Alberti A. Emergence of anaplasma species related to A. phagocytophilum and A. platys in Senegal. Int J Mol Sci. 2022;24:35. doi: 10.3390/ijms24010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobba R, Schianchi E, Ben Said M, Belkahia H, Messadi L, Piredda R, Pittau M, Alberti A. gltA typing of Anaplasma strains related to A. platys: Taxonomical and one health implications. Ticks Tick Borne Dis. 2022;13:101850. doi: 10.1016/j.ttbdis.2021.101850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files. The nucleotide sequences generated and analyzed during the current study are available in the International Nucleotide Sequence Database Collaboration repository (INSDC, http://www.insdc.org/) with the IDs: OR209777-OR209783.