Abstract
In 1997, an outbreak of virulent H5N1 avian influenza virus occurred in poultry in Hong Kong (HK) and was linked to a direct transmission to humans. The factors associated with transmission of avian influenza virus to mammals are not fully understood, and the potential risk of other highly virulent avian influenza A viruses infecting and causing disease in mammals is not known. In this study, two avian and one human HK-origin H5N1 virus along with four additional highly pathogenic H5 avian influenza viruses were analyzed for their pathogenicity in 6- to 8-week-old BALB/c mice. Both the avian and human HK H5 influenza virus isolates caused severe disease in mice, characterized by induced hypothermia, clinical signs, rapid weight loss, and 75 to 100% mortality by 6 to 8 days postinfection. Three of the non-HK-origin isolates caused no detectable clinical signs. One isolate, A/tk/England/91 (H5N1), induced measurable disease, and all but one of the animals recovered. Infections resulted in mild to severe lesions in both the upper and lower respiratory tracts. Most consistently, the viruses caused necrosis in respiratory epithelium of the nasal cavity, trachea, bronchi, and bronchioles with accompanying inflammation. The most severe and widespread lesions were observed in the lungs of HK avian influenza virus-infected mice, while no lesions or only mild lesions were evident with A/ck/Scotland/59 (H5N1) and A/ck/Queretaro/95 (H5N2). The A/ck/Italy/97 (H5N2) and the A/tk/England/91 (H5N1) viruses exhibited intermediate pathogenicity, producing mild to moderate respiratory tract lesions. In addition, infection by the different isolates could be further distinguished by the mouse immune response. The non-HK-origin isolates all induced production of increased levels of active transforming growth factor β following infection, while the HK-origin isolates did not.
Influenza A virus, a member of the Orthomyoxoviridae family, infects a wide range of species, including poultry, swine, horses, seals, and humans (10). Wild aquatic birds serve as reservoirs for all 15 different hemagglutinin (HA) and 9 neuraminidase subtypes of influenza A virus (32, 47). Of the 15 HA subtypes, only H1, H2, and H3 influenza virus subtypes have previously been associated with causing disease and death in humans (28, 46). It has been proposed that human strains arise from avian strains following evolution in a mammalian intermediate host (13). In the spring of 1997, however, nearly identical highly pathogenic (HP) H5N1 avian influenza A viruses were isolated from both diseased chickens and an ill child in Hong Kong (HK) (6, 40). HP H5 avian influenza viruses have been isolated from previous outbreaks of influenza in poultry (1, 3, 5, 17, 29, 37, 41, 44); however, this was the first documented case of a direct H5 avian influenza virus infection in humans (8, 40). Poultry workers exposed to chickens during an outbreak of HP H5N2 in Pennsylvania poultry farms in 1983 showed no susceptibility to infection with the H5N2 virus, A/ck/Pennsylvania/1/83, and did not seroconvert (3). In contrast, in HK some exposed poultry workers demonstrated a measurable seroconversion to the H5 subtype (6). Avian influenza virus isolates were able to infect a very small percentage of the human population and cause severe respiratory disease and mortality without any prior adaptation to the mammalian host. Throughout the course of the influenza outbreak from May to December 1997, six human deaths were recorded from 18 confirmed H5N1 influenza cases (6, 8, 39, 50). Sequences of all of the human isolates demonstrated greater than 99% sequence identity to isolates obtained from infected poultry, confirming that the outbreak resulted from direct transmission of H5N1 virus from infected poultry to humans (39). The human and chicken H5 viruses contained the multiple basic amino acids in the HA cleavage site characteristic of HP avian influenza virus H5 and H7 isolates (29). Furthermore, both the avian and human HK-origin H5N1 virus isolates contained an N1 gene with a shortened stalk due to a 57-nucleotide (19-amino-acid) deletion (4, 7, 51).
The fact that the avian HK H5N1 viruses are capable of replicating and causing disease in humans without prior adaptation in a mammalian host is a cause of concern. One factor thought to affect interspecies transmission is differences in host surface receptors on the target cells. Avian cells contain predominantly Sia2-3Galactose-containing surface receptors, whereas human cells contain Sia2-6Galactose-containing receptors (18). Binding of the HA to the surface receptors is crucial for virus entry and replication. However, in the case of these viruses initial avian-to-human transmission of highly virulent HK-origin avian influenza H5N1 viruses is not strictly prevented by host surface receptor specificity. The avian HK-origin H5N1 viruses along with the HK-origin H5N1 viruses isolated from humans retained the Sia2-3Galactose receptor specificity of the parent avian species (26). While the HK-origin H5N1 viruses were still able to replicate and cause disease and death in humans without the Sia2-6Galactose receptor specificity, they were clearly not easily transmitted from human to human.
The potential risk of other highly virulent avian influenza H5 viruses crossing the avian-to-human species barrier and infecting and causing disease and death in humans is not known. The human HK H5N1 viruses have previously been shown to be pathogenic to mice without prior adaptation, and it was suggested that mice serve as a good model to study mammalian pathogenesis and immune responses to these viruses (11, 15, 23, 25). In this present study, avian and human HK H5N1 viruses were analyzed and directly compared with four additional highly virulent H5 avian influenza viruses for their pathogenicity in BALB/c mice under strictly controlled experimental conditions. In addition, the immune response to infection with HK-origin H5 influenza A viruses, as measured by transforming growth factor β (TGF-β) cytokine levels, was evaluated and compared with the other highly virulent H5 avian influenza viruses. Previous studies showed that the neuraminidase of A/Turkey/Ontario/7732/66, a highly virulent H5 avian influenza virus, directly activated TGF-β both in vitro and in a mouse model (35). TGF-β is a potent proinflammatory cytokine that activates monocytes to induce the expression and release of various growth factors and inflammatory mediators (9). The decreased level of TGF-β noted during infection by the HK-origin viruses may contribute to the severe pathology observed in the infected mice.
MATERIALS AND METHODS
Viruses.
The following viruses were analyzed for their pathogenicity in BALB/c mice: the HK avian influenza viruses A/ck/HK/220/97 (H5N1) (HK220) and A/ck/HK/728/97 (H5N1) (HK728); the human HK isolate A/HK/156/97 (H5N1) (HK156); and the non-HK-origin avian influenza A viruses A/ck/Scotland/59 (H5N1) (Scot/59), A/tk/England/91 (H5N1) (Eng/91), A/ck/Italy/1485-330/97 (H5N2) (Italy/97), and A/ck/Queretaro/7653-20/95 (H5N2) (Q20/95). All seven viruses are HP for chickens (5, 11, 22, 29, 37, 39, 40, 42, 44, 45). Each virus was propagated in the allantoic cavity of 10-day specific pathogen-free embryonated chicken eggs. Infectious allantoic fluid was harvested and stored at −70°C for use as inocula. All working stocks consisted of either first or second egg-passaged virus from the original stock as received at our lab. Fifty percent embryo lethal dose (ELD50) titers were calculated according to Reed and Muench (31). Virus stocks were diluted in phosphate-buffered saline (PBS) and standardized to 106 ELD50/0.05 ml. All virus stocks were handled under biosafety level 3 agriculture conditions. Laboratory personnel wore protective HEPA-filtered respirator units during infection of mice with HK-origin influenza viruses.
Mice.
Seven- to 8-week-old male BALB/c mice were purchased from Simonsen Laboratories, Inc. (Gilroy, Calif.). Groups of eight mice each were housed in individual cages within separate negative-pressure stainless steel isolators in a high-containment biosafety level 3 agriculture facility at the Southeast Poultry Research Laboratory (SEPRL). Feed and water were provided ad libitum.
Experimental design.
Mice were anesthetized with 30 μl of ketamine-xylazine (66 mg of ketamine per ml and 6.6 mg of xylazine per ml), and implantable programmable temperature transponders (IPTT-100, Electronic Laboratory Animal Monitoring Systems; BioMedic Data Systems, Inc., Seaford, Del.) were inserted subcutaneously in the middorsal region. Anesthetized mice were inoculated intranasally with 50 μl of 106 ELD50 influenza virus isolates. One group of mice was inoculated with diluent PBS and served as age-matched sham-infected negative controls. Mice were monitored daily throughout the 14-day experiment for clinical signs and mortality. Body weights were recorded for individual mice within each group on days 0, 4, 6, 8, and 12 postinfection (p.i.), and body temperatures were measured on days 1 to 4, 6, 8, and 12 p.i. with a DAS-5007 Pocket Scanner System (BioMedic Data Systems, Inc.). Blood was collected by tail vein puncture at 8, 48, and 96 p.i. The serum was pooled for each group and analyzed for TGF-β levels. Surviving mice were bled by cardiac puncture on day 14 p.i. for analysis of anti-H5 antibodies.
Virus isolation and serology.
Tissue samples from the trachea, lungs, and kidneys of three mice per group were processed for virus isolation at day 4 p.i. Tissues were homogenized in brain heart infusion broth and inoculated into 10-day specific pathogen-free embryonated chicken eggs. Embryos were monitored daily for mortality, and dead embryos were assayed for HA activity. The titer for an antibody to avian influenza virus H5 in serum from mice was determined by using a competitive inhibition enzyme-linked immunosorbent assay (CI-ELISA) developed at SEPRL (unpublished data). Briefly, microtiter plates were coated with baculovirus-vectored influenza virus H5 HA recombinant protein derived from the HK156 isolate (Protein Sciences Corporation, Meriden, Conn.). Sixfold serial dilutions of serum samples were incubated on plates for 1 h. The plates were washed and incubated for 1 h with a horseradish peroxidase-conjugated H5 HA-specific monoclonal antibody produced at SEPRL. Plates were washed, and substrate solution was added to the plates. The reaction was stopped after 15 min by addition of 2 M H2SO4. Plates were measured for optical density at 495 nm with a Biotech Microplate Reader. The antibody present in the serum samples competed with the monoclonal antibody for binding to the antigen on the plate. The titer of the antibody in the serum sample was determined by a decreased optical density of the sample as a result of the inhibition, compared to the negative control sample, with values of ≤3 standard deviations from the negative control sample considered positive. The competitive inhibition titer was expressed as the highest dilution that tested positive.
Histopathology, ultrastructural pathology, and immunohistochemistry.
Mice were euthanized by intraperitoneal sodium pentobarbital injection on days 4 and 14 p.i. On day 4 p.i., tissues were excised from the respiratory tract and visceral organs, including the trachea, lung, thymus, heart, bronchi, kidney and attached adrenal gland, spleen, stomach, intestine, pancreas, liver, seminiferous tubules, testes, and femur, in addition to the brain and nasal cavity. The tissues were fixed in 10% neutral buffered formalin solution, sectioned, and stained with hematoxylin and eosin. Respiratory tract tissue samples were removed from any mice that died during the duration of the 14-day experiment and from all survivors on day 14 p.i. Duplicate histologic sections were stained immunohistochemically to determine influenza virus nucleoprotein distribution in individual tissues. A monoclonal antibody against influenza A virus nucleoprotein was used at a 1:2,000 dilution according to previously published procedures (38, 43).
NRK assay for TGF-β activity.
TGF-β activity was assessed by determining the colony-forming activity of normal rat kidney (NRK) cells, in the presence of epidermal growth factor (EGF), in soft agar as previously described (36). Briefly, 5% Noble agar (Difco, Detroit, Mich.) was diluted 10-fold in 10% calf serum–Dulbecco's modified Eagle's medium, and 0.5 ml of this 0.5% agar dilution was added per well to a 24-well tissue culture plate as a base layer and allowed to solidify. Serum samples (0.2 ml) containing EGF (1 ng) were combined with 0.6 ml of 0.5% agar and 0.2 ml (2 × 103) of a NRK cell suspension in 10% calf serum-Dulbecco's modified Eagle's medium, and 0.5 ml of this 0.3% agar sample solution was added to the cooled base layer. EGF is required for the assay and serves as the baseline for colony formation. The samples were incubated for 7 days at 37°C in 5% CO2 and stained with a 1% solution of neutral red (Sigma Chemicals, St. Louis, Mo.) in PBS, and colonies greater than 62 μm (>8 to 10 cells) in diameter were counted. Experiments were performed in triplicate.
Data analysis.
Data were analyzed by one-way analysis of variance followed by Tukey analysis by using GraphPad InStat version 3.01 (GraphPad Software, Inc., San Diego, Calif.).
RESULTS
H5 virus replication in BALB/c mice.
The H5 HP avian influenza viruses replicated in mice without prior adaptation. Although all seven H5 viruses analyzed were highly pathogenic in their respective avian host (5, 11, 22, 29, 37, 39, 40, 42, 44, 45), they varied in their ability to cause disease in mice. The HK156-, HK220-, HK728-, and Eng/91-infected mice demonstrated clinical signs of disease characterized by ruffled fur, inappetance, hunched-back posture, and labored breathing within 4 days of infection. Death in the HK H5N1 infected mice was associated with severe interstitial pneumonia and alveolar edema, indicating that alterations in the O2-CO2 exchange surface impacted disease pathogenesis and death. This primary respiratory tract involvement is similar to that reported for mice inoculated with other mouse-adapted influenza viruses isolated from humans and animals (30, 48).
No fever was observed in avian influenza virus-infected mice; instead, the body temperatures of HK156- and HK220-infected mice decreased significantly from 36°C (±0.20) to 32°C (±0.25) (P < 0.001) by day 6 p.i. (Fig. 1A). Concurrently, HK156- and HK220-infected mice rapidly lost weight (Fig. 1B). By day 4 p.i., HK156- and HK 220-infected mice had lost approximately 15% (P < 0.01) of their initial weight at challenge and 25 to 26% (P < 0.001) body weight by day 6 p.i. Kodihalli et al. (23) also observed a 25% decrease in body weight of HK156- and HK258 (chicken isolate related to HK220)-infected mice by day 5 p.i. and progressive weight loss until death. The HK156- and HK220-infected mice were unable to recover from viral infection and died within 6 to 8 days p.i. with a mean death time (MDT) of 6.33 days (Table 1).
FIG. 1.
Physiological effect of H5 influenza virus infection of BALB/c mice. (A) Body temperatures of BALB/c mice after inoculation with HP avian influenza viruses. Body temperatures of individual mice were read on days 1 to 4, 6, 8, and 12 p.i. with a scanner. Values represent the means ± standard errors of the mean of eight mice per group. (B) Percent change in initial body weights of BALB/c mice after inoculation with HP avian influenza viruses. Body weights were taken on days 0, 4, 6, 8, and 12 p.i. The percent change in body weight was calculated for each mouse based on the initial starting weight at day 0 before virus inoculation. Each value represents the average percent change in weight ± standard error of the mean for eight mice per group. (a), All HK156 and HK220 mice died by day 8 p.i.; (b), two of eight HK728 mice died by day 8 p.i.; (c), four of six HK728 mice died by day 10 p.i.; ∗, significant at a P value of <0.05; ∗∗, significant at a P value of <0.001.
TABLE 1.
Virus isolation and serology
| Group | Virus isolation (4 days p.i.)a
|
Serologyb (14 days p.i.) | MDT (days) | ||
|---|---|---|---|---|---|
| Lung (n = 3) | Trachea (n = 3) | Kidney (n = 3) | |||
| Sham infected | 0 | 0 | 0 | Negative | NDc |
| Italy/97 | 3 | 2 | 0 | 36 | ND |
| Scot/59 | 1 | 1 | 1 | 6 | ND |
| Eng/91 | 2 | 3 | 0 | 216 | 9d |
| Q20/95 | 2 | 3 | 0 | 36 | ND |
| HK156 | 3 | 3 | 1 | —e | 6.33 |
| HK220 | 3 | 3 | 1 | —e | 6.33 |
| HK728 | 3 | 3 | 0 | —f | 9.00 |
Number of mice in which virus was isolated.
Values represent CI-ELISA titer.
ND, no deaths occurred.
One mouse died on day 9 p.i. in experiment 2.
All HK infected mice were dead by day 14 p.i. when serum samples were taken for CI-ELISA.
Mouse serum not analyzed at 14 days p.i. since only two mice were alive.
The other avian HK H5N1 virus, HK728, also caused morbidity and mortality in BALB/c mice. However, the influenza infection was not as acute as that observed with HK156 and HK220, with only 75% mortality and a MDT of 9 days p.i. (Table 1). The HK728-infected mice demonstrated a decrease in body temperature and body weight but at a slower rate than HK156 and HK220 (Fig. 1A and B). Infected mice exhibited a significant decrease in body temperature to 34°C (±1.25) (P < 0.001) and 22% (±0.6) (P < 0.001) reduction in body weight on day 8 p.i. (Fig. 1A and B). Two HK728-infected mice survived to day 14 p.i., when the experiment was terminated.
The non-HK HP avian influenza viruses varied in their ability to cause disease and mortality in BALB/c mice. Only Eng/91 caused clinical disease in BALB/c mice. The Eng/91-infected mice began losing weight by day 4 p.i. and had lost 20% (P > 0.001) of their starting weight by day 8 p.i. (Fig. 1B). The mice experienced a slight decrease in body temperature on day 6 p.i. (Fig. 1A); however, this decrease in body temperature was not significant (P > 0.001). The Eng/91-infected mice quickly recovered from avian influenza virus infection and regained most of their initial weight by day 12 p.i. (Fig. 1A and B). None of the non-HK origin influenza viruses killed any mice except Eng/91, where one mouse died on day 9 during experiment 2 (Table 1).
Virus isolation and serology.
Virus was reisolated from the lungs and trachea of mice infected with the seven influenza virus isolates on day 4 p.i. (Table 1). Virus was isolated inconsistently from the kidneys of infected mice with one positive mouse of three in each of the three groups infected with HK156, HK220, or Scot/59 (Table 1). Virus replication was further observed in mice infected with Italy/97, Eng/91, and Q20/95 as evidenced by circulating anti-H5 antibody titers detected in serum taken on day 14 p.i. (Table 1). These viruses caused mild to intermediate lesions in the respiratory tracts of infected mice. Lower anti-H5 titers were detected in serum from Scot/59-infected mice. No virus isolation and no circulating anti-H5 antibody was observed in the sham-infected controls. All HK156- and HK220-infected mice were dead by day 14 p.i., when blood was taken and serum analyzed by CI-ELISA.
Pathology and immunohistochemistry.
Mice inoculated with the seven H5 HP avian influenza virus isolates either lacked lesions or exhibited lesions in the respiratory tract similar to those reported in previous experimental studies with human, swine, equine, and avian influenza viruses (23, 41). At 4 days p.i., mice from the sham-infected control group lacked lesions and avian influenza virus nucleoprotein (NP) was not demonstrated. The Scot/59- and Q20/95-infected mice exhibited mild infrequent inflammation and rare avian influenza virus NP in trachea and secondary bronchi (Table 2). Similarly, infection of mice with non-HP H5N1 virus, A/Dk/Singapore/645/97, caused no lesions in BALB/c mice, and the only antigen was localized in a few respiratory epithelial cells in the trachea (data not shown).
TABLE 2.
Lesion severity and frequency of avian influenza virus NP in mice on day 4 p.i.
| Group | Lesionsa
|
Avian influenza virus NPb
|
||||||
|---|---|---|---|---|---|---|---|---|
| Nasal cavity | Trachea or primary bronchi | Lung | Nonrespiratory tissuesc | Nasal cavity | Trachea or primary bronchi | Lung | Nonrespiratory tissuesc | |
| Sham | − | − | − | − | − | − | − | − |
| Italy/97 | − | ++ | ++ | − | − | ++ | ++ | − |
| Scot/59 | − | − | ± | − | − | + | − | − |
| Eng/91 | ++ | +++ | +++ | − | ++ | +++ | +++ | − |
| Q20/95 | − | + | + | − | − | + | + | − |
| HK156 | ++ | +++ | ++++ | −d | +++ | ++ | +++ | +e |
| HK220 | − | ++ | ++++ | −d | + | + | ++ | − |
| HK728 | + | +++ | +++ | −d | ++ | + | +++ | − |
Histological lesion scores for severity: −, none; ±, very mild; +, mild; ++, mild to moderate; +++, moderate; and ++++, severe.
Localization of avian influenza virus NP: −, none; +, rare; ++, uncommon; +++, frequent.
Nonrespiratory tissues examined include the spleen, thymus, stomach, duodenum, pancreas, liver, large intestine, brain, heart, kidney/adrenal gland, skeletal muscle, femur, bone marrow, testis, and male sex glands (seminal vesicles and coagulating gland).
Increase in myeloid band cells in bone marrow.
Olfactory epithelium and neurons of autonomic ganglion adjacent to bronchial hilus.
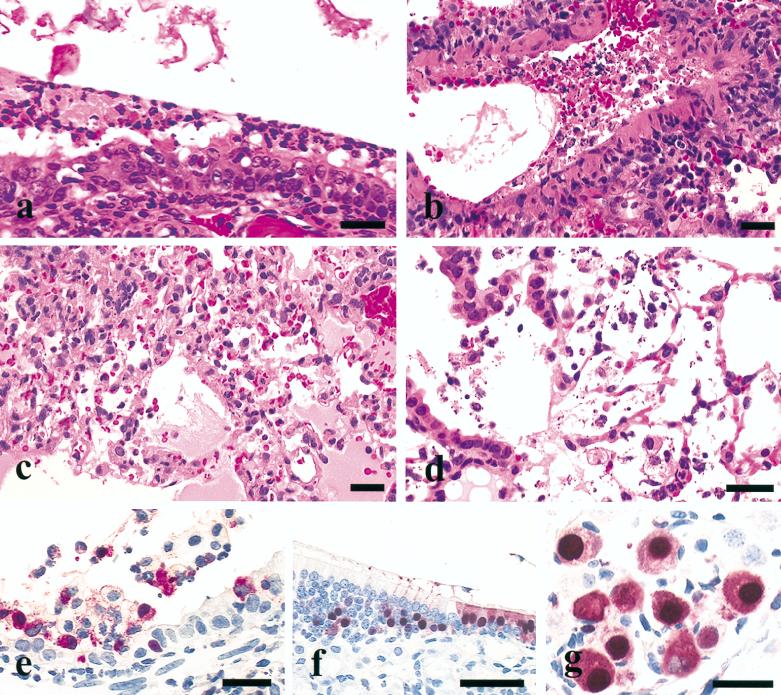
Mice from the remaining five groups, which included mice infected with Italy/97, Eng/91, HK156, HK220, and HK728, had variable degrees of degeneration and necrosis of respiratory epithelium in the nasal cavity, trachea, bronchi, and bronchioles, with accompanying fibrin and neutrophils (Fig. 2a and b). Commonly, the lungs had peribronchial alveolitis characterized by intra-alveolar serofibrinous exudate, erythrocytes and neutrophils, and increased numbers of alveolar macrophages (Fig. 2c). In some cases, necrotizing alveolitis was present adjacent to terminal bronchioles (Fig. 2d). This bronchointerstitial pattern of pneumonia was most frequent, but in the most severe cases, a diffuse interstitial pneumonia pattern affected entire lung lobes. Avian influenza virus NP was most prevalent in degenerating or necrotic respiratory epithelium of the nasal cavity and pulmonary bronchi and bronchioles (Fig. 2e and f) and associated lumenal debris. Occasionally, avian influenza virus NP was identified in alveolar macrophages. In addition, avian influenza virus NP was demonstrated in olfactory epithelium of the nasal cavity (Fig. 2f) and some ganglion cells within autonomic neurons of the bronchial hilus (Fig. 2g) of only HK156-infected mice. The severity of the lesions and distribution and quantity of avian influenza virus NP varied between these five avian influenza virus groups (Table 2). The lesions were mild in mice infected with Italy/97, moderate in mice infected with Eng/91, and moderate to severe in mice infected with the three HK viruses. The lung and nasal cavity lesions were most severe with HK156 virus. The greatest quantity of avian influenza virus NP was demonstrated in mice infected with the HK viruses followed by the Eng/91 virus. These HP avian influenza viruses did not cause lesions in visceral organs or the brain, and avian influenza virus NP was not demonstrated within these tissues.
FIG. 2.
Experimental studies in BALB/c mice inoculated intranasally with different H5 avian influenza viruses. Photomicrographs of hematoxylin-and-eosin-stained tissue sections (a to d) or sections stained immunohistochemically to demonstrate avian influenza virus NP (e to g). (a) Disorganization, degeneration, and necrosis of respiratory epithelium of the nasal turbinate with lumenal fibrinopurulent exudate from a mouse euthanatized 4 days after inoculation with HK156 (bar = 25 μm). (b) Necrotizing bronchitis with lumenal hemorrhage and necrotic debris from a mouse euthanatized 4 days after inoculation with HK220 (bar = 25 μm). (c) Peribronchial alveolitis characterized by intra-alveolar serofibrinous exudate, erythrocytes and neutrophils, increased alveolar macrophages, and inflammatory cells in alveolar walls from a mouse euthanatized 4 days after inoculation with HK156 (bar = 25 μm). (d) Necrotizing alveolitis with intra-alveolar macrophages, neutrophils, and fibrin from a mouse euthanatized 4 days after inoculation with HK728 (bar = 25 μm). (e) Avian influenza virus NP in respiratory epithelium, macrophages, and neutrophils in the bronchus from a mouse euthanatized 4 days after inoculation with Eng/91 (bar = 25 μm). (f) Avian influenza virus NP in respiratory (right) and olfactory (left) epithelia of the nasal turbinate from a mouse euthanatized 4 days after inoculation with HK156 (bar = 50 μm). (g) Intranuclear and intracytoplasmic avian influenza virus NP in ganglial neurons adjacent to the bronchial lymph node from a mouse euthanatized 4 days after inoculation with HK156 (bar = 25 μm).
The HK156- and HK220-infected mice that died had postmortem lesions similar to those of mice euthanized on day 4 p.i., except that the alveolitis was more severe and diffuse and severe pulmonary edema with hyaline membranes in alveoli was present in the mice that died. The two surviving HK 728 avian influenza virus-infected mice had chronic histiolymphocytic bronchointerstitial pneumonia with associated alveolar septal fibrosis and type 2 pneumocyte hyperplasia. The mice inoculated with the other four avian influenza viruses euthanized on day 14 p.i. lacked lesions or had mild chronic lymphohistioytic bronchointerstitial pneumonia. No avian influenza virus NP was identified in respiratory tissues of these mice on day 14 p.i.
TGF-β levels in HP avian influenza virus-infected mice.
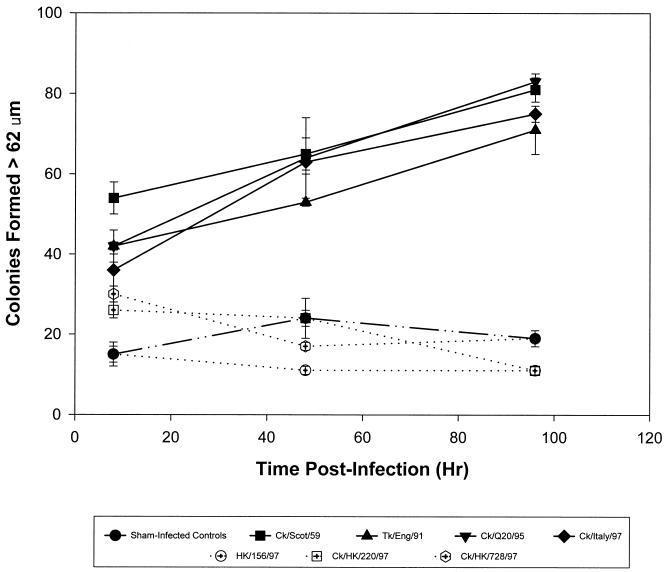
Previous studies showed that a HP avian influenza virus (A/Tk/Ontario/7732/66) (H5N9) caused a rapid and dramatic increase in serum TGF-β levels in vitro and in vivo (35). Similarly all of the non-HK-origin HP avian influenza viruses induced an increase in TGF-β levels in infected mice. TGF-β levels in mice infected with Italy/97, Scot/59, Eng/91, and Q20/95 began to increase as early as 8 h p.i. and continued to increase throughout the 96-h p.i. time course. In contrast, the HK156-, HK220-, and HK728-infected mice showed no increase in TGF-β activity (Fig. 3). Similar results were observed in vitro by using Madin-Darby canine kidney cells and an avian macrophage cell line (HD11), suggesting that the HK viruses fail to activate latent TGF-β (data not shown).
FIG. 3.
Active TGF-β level in infected mice. Mice were infected intranasally with Italy/97 (105.8 ELD50), Scot/59 (106.0 ELD50), Eng/91 (106.2 ELD50), Q20/95 (106.0 ELD50), HK728 (105.2 ELD50), HK156 (105.8 ELD50), HK220 (106.2 ELD50), or PBS (sham-inoculated controls). Blood was collected from mice at 8, 48, and 96 h p.i., and serum was analyzed for TGF-β activity by the NRK colony-forming soft agar assay. Three mice per group were analyzed, and the results represent the means ± standard deviations of triplicate determinations from the pooled sera.
DISCUSSION
The infection of a very small percentage of the human population with a lethal avian influenza virus in HK in 1997 may have signaled a new era in influenza epidemiology. For the first time, purely avian viruses were associated with severe disease and death in humans without prior adaptation in an intermediate host. It is uncertain whether other chicken lethal avian influenza viruses are capable of crossing the species barrier and infecting and causing disease and death in humans or if this is a unique phenotype of the HK avian influenza viruses. Previous studies showed that the viruses involved in the HK outbreak were also lethal in mice without prior adaptation and that the mouse was useful as a mammalian model of disease (11, 23, 25). However, these past studies were limited to analysis of only the HK H5N1 viruses. We wished to determine whether the mouse model could be useful toward predicting the pathogenicity of emerging HP avian influenza viruses for humans. In this study, a representative set of highly lethal avian influenza viruses were evaluated in direct comparison with the HK-origin isolates for their pathogenicity in mice.
The HK H5N1 viruses are unique and are clearly distinguishable from the non-HK-origin H5 viruses by clinical and immunological parameters. Infection of mice with the HK H5N1 viruses HK156, HK220, and HK728 resulted in severe respiratory tract lesions with variable degrees of degeneration and necrosis of respiratory epithelium in the nasal cavity, trachea, bronchi, and bronchioles, with accompanying fibrin and neutrophils, whereas the non-HK-origin HP avian influenza viruses caused mild to severe lesions in the respiratory tract of intranasally infected mice. Where others observed systemic lesions with some of the HK-origin viruses (11, 25), we observed no lesions outside the respiratory tract. Thus, our studies do not indicate a role for systemic infection in pathogenesis. It is possible that low levels of virus, not inducing observable lesions, might still induce systemic organ failure. Expansion of myeloid cells in the bone marrow was noted in the HK-origin strains, probably in response to the severity of the lung lesions. We utilized avian origin viruses and/or viruses that were propagated once in chicken embryos as inoculum. It is possible that passage in mammalian cells or serial passage in mice could increase the likelihood of systemic lesions in the mice. Studies are underway in our lab to answer this question. What seems clear, however, is that these HK-origin avian and human viruses cause disease, tissue damage, and death in mice through their dramatic effect on the respiratory tract and primarily the lungs.
Differences in the severity of pathological lesions may be attributed in part to differences in the host response to avian influenza virus. Influenza A virus neuraminidase directly activates latent TGF-β (35). TGF-β is secreted by virtually all cells as a biological inactive molecule termed latent TGF-β. Latent TGF-β must be activated in order to bind to cellular receptors and elicit a biological response. Once activated, TGF-β is a potent immunomodulator and functions as both a pro- and anti-inflammatory agent. In addition, influenza-activated TGF-β is partially involved in inflammatory-mediated cell death (apoptosis). The HK156, HK220, and HK728 viruses failed to activate TGF-β either in vitro (data not shown) or in vivo, whereas infection of mice with HP avian influenza viruses Q20/95, Eng/91, Italy/97, and Scot/59 increased TGF-β activity within the first 96 h p.i. The reason for this is unclear. The HK viruses have less neuraminidase enzymatic activity, as measured by cleavage of fetuin, compared to the other N1 viruses, Eng/91 and Scot/59 (data not shown). This may partially explain the lack of TGF-β activation, although H5N1 viruses that are only mildly pathogenic in chickens and nonpathogenic in mice have little neuraminidase activity and still activate TGF-β in vitro and in vivo (unpublished data). Thus, the role of TGF-β in influenza pathogenesis is unclear. However, the severity of the HK infection suggests that decreased levels of TGF-β and the subsequent effect on other cytokines may be important in the pathogenesis.
Since there was no indication that any H5 isolates from outbreaks other than in HK are implicated in human infection, it is tempting to speculate that this mouse model may indeed reflect an accurate phenotypic correlate for human infection by an avian influenza virus. However, there are limitations with this direct extrapolation. First, there is a lack of significant data on seroconversion rates in humans exposed to other HP avian influenza viruses. Secondly, the HK viruses were nearly 100% infectious and lethal in the mice, whereas infection and lethality rates in humans were much lower. Influenza A virus infections in humans are normally widespread in the population and typically exhibited by a mild tracheobronchitis, rarely leading to viral pneumonia and death (20, 28). In addition, in order to obtain a consistent rate of infection and lethality in mice, it was necessary to anesthetize the mice. The rates of infection and lethality were much more variable without prior anesthesia and ranged from 0 to 50% (data not shown). Furthermore, the rapid decrease in temperature was also a significant difference distinguishing the mouse infection from a typical human infection. Influenza infection in humans results in fever; however, we were unable to detect any fever response whatsoever in mice, even as early as 8 h p.i. Previous infection of mice with a mouse-adapted strain of influenza A virus, A/Puerto Rico/8/34 (H1N1), resulted in conflicting reports. Two independent groups observed a decrease in mouse body temperature upon infection with the mouse-adapted strain (2, 21), whereas another observed a fever cascade (24). These discrepancies may be due to the method of determining body temperature and the times at which temperatures are recorded. Thus, relative to reflecting the human infection, the mouse infection data must then be viewed with caution and not overinterpreted.
The striking difference in pathology in mice between these chicken lethal groups cannot be discounted, however, and it is believed that analysis in the mouse system will yield valuable information. The only non-HK-origin HP avian influenza virus to cause pathological lesions in mice was the Eng/91 isolate. The Eng/91 strain may represent an intermediate between the chicken lethal North American strains and the HK-origin viruses with respect to mouse lethality. The existence of two distinct lineages of avian influenza virus strains has been known for some time (47), and data have indicated that the Eurasian lineage is more likely to make the jump into mammalian populations. Indeed, the proposal that currently circulating human strains arose from avian influenza viruses from southern China supports the notion that Eurasian-origin genes are more adaptable to humans than North American-origin genes (46). Recent avian isolates of the H9N2 subtype from HK have a nearly identical set of six internal protein genes as found in the 1997 H5N1 isolates (14). These six genes were proposed to have reassorted with the surface glycoproteins from waterfowl isolates to generate the HK isolates (E. Hoffman, J. Stech, S. Krauss, K. Shortridge, and R. Webster. Abstr. 1999 Meeting Am. Soc. Virol., abstr. P.63, 1999). Thus, infection of mice by these Eurasian- and avian-origin strains may indeed reflect a unique and perhaps stable mammalian affinity. Reassortment assays that involve mating the Q20/95 Mexican strain and the chicken HK220 strain are currently underway to help identify mammalian-specific versus chicken-specific lethal genes. Molecular epidemiologic studies are also ongoing in our lab to evaluate the occurrence of these avian genes and their distribution in nature.
The data in this report demonstrate distinct differences in mouse pathology between the HK-origin and the other HP avian influenza viruses studied. These differences may potentially be important predictors, identifying avian influenza virus isolates that can cause serious infections in mammals, including humans. It will be important to fully analyze and utilize these strains as tools in furthering our understanding of influenza virus pathogenesis.
ACKNOWLEDGMENTS
We are grateful to Patsy Decker, Joan Beck, Liz Turpin, and Roger Brock for their excellent technical assistance.
This research was supported in its entirety by the USDA Agricultural Research Service, CRIS research unit no. 6612-3200-22.
REFERENCES
- 1.Alexander D J, Lister S A, Johnson M J, Randall C J, Thomas P J. An outbreak of highly pathogenic avian influenza in turkeys in Great Britain in 1991. Vet Rec. 1993;132:535–536. doi: 10.1136/vr.132.21.535. [DOI] [PubMed] [Google Scholar]
- 2.Baus F, Droge W, Mannel D N. Tumor necrosis factor mediates endotoxic effects in mice. Infect Immun. 1987;55:1622–1625. doi: 10.1128/iai.55.7.1622-1625.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bean W J, Kawaoka Y, Wood J M, Pearson J E, Webster R G. Characterization of virulent and avirulent A/chicken/Pennsylvania/83 influenza viruses: potential role of defective interfering RNAs in nature. J Virol. 1985;54:151–160. doi: 10.1128/jvi.54.1.151-160.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender C, Hall H, Huang J, Klimov A, Cox N, Hay A, Gregory V, Cameron K, Lim W, Subbarao K. Characterization of the surface proteins of influenza A (H5N1) viruses isolated from humans in 1997–1998. Virology. 1999;254:115–123. doi: 10.1006/viro.1998.9529. [DOI] [PubMed] [Google Scholar]
- 5.Capula I, Marangon S, Selli L, Alezander D J, Swayne D E, Pozza M D, Parenti E, Cancellotti F M. Outbreaks of highly pathogenic avian influenza (H5N2) in Italy during October 1997 to January 1998. Avian Pathol. 1999;28:455–460. doi: 10.1080/03079459994470. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Update: isolation of avian influenza A (H5N1) viruses from humans—Hong Kong, 1997–1998. Morbid Mortal Weekly Rep. 1998;46:1245–1247. [PubMed] [Google Scholar]
- 7.Claas E C J, Osterhaus A D M E, van Beek R, De Jong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 8.De Jong J C, Claas E C J, Osterhaus A D M E. A pandemic warning? Nature. 1997;389:554. doi: 10.1038/39218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derynck R. TGF-β. In: Thomson A, editor. The cytokine handbook. 2nd ed. San Diego, Calif: Academic Press, Inc.; 1994. pp. 318–342. [Google Scholar]
- 10.Easterday B C, Hinshaw V S. Influenza. In: Calnek B W, Barnes H J, Beard C W, Reid W M, Yoder H W Jr, editors. Diseases of poultry. 9th ed. Ames: Iowa State University Press; 1991. pp. 532–551. [Google Scholar]
- 11.Gao P, Watanabe S, Ito T, Goto H, Wells K, McGregor M, Cooley A J, Kawaoka Y. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J Virol. 1999;73:3184–3189. doi: 10.1128/jvi.73.4.3184-3189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia M, Suarez D L, Crawford J M, Latimer J W, Slemons R D, Swayne D E, Perdue M L. Evolution of H5 subtype avian influenza A viruses in North America. Virus Res. 1997;51:115–124. doi: 10.1016/s0168-1702(97)00087-7. [DOI] [PubMed] [Google Scholar]
- 13.Gorman O T, Bean W J, Webster R G. Evolutionary processes in influenza viruses: divergence, rapid evolution, and stasis. Curr Top Microbiol. 1992;172:75–97. doi: 10.1007/978-3-642-77011-1_6. [DOI] [PubMed] [Google Scholar]
- 14.Guan Y, Shortridge K F, Krauss S, Webster R G. Molecular characterization of H9N2 influenza viruses: were they the donors of internal genes of H5N1 viruses in Hong Kong? Proc Natl Acad Sci USA. 1999;96:9363–9367. doi: 10.1073/pnas.96.16.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gubareva L V, McCullers J A, Bethell R C, Webster R G. Characterization of influenza A/HongKong/156/97 (H5N1) virus in a mouse model and protective effect of zanamivir on H5N1 infection in mice. J Infect Dis. 1998;178:1592–1596. doi: 10.1086/314515. [DOI] [PubMed] [Google Scholar]
- 16.Hinshaw V S, Webster R G, Easterday B C, Bean W J., Jr Replication of avian influenza A viruses in mammals. Infect Immun. 1981;34:354–361. doi: 10.1128/iai.34.2.354-361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horimoto T, Rivera E, Pearson J, Senne D, Krauss S, Kawaoka Y, Webster R G. Origin and molecular changes associated with emergence of a highly pathogenic H5N2 influenza virus in Mexico. Virology. 1995;213:223–230. doi: 10.1006/viro.1995.1562. [DOI] [PubMed] [Google Scholar]
- 18.Kawaoka Y. Difference in receptor specificity among influenza A viruses from different species of animals. J Vet Med Sci. 1991;53:357–358. doi: 10.1292/jvms.53.357. [DOI] [PubMed] [Google Scholar]
- 19.Kawaoka Y. Equine H7N7 influenza A viruses are highly pathogenic in mice without adaptation: potential uses as an animal model. J Virol. 1991;65:3891–3894. doi: 10.1128/jvi.65.7.3891-3894.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilbourne E D. Influenza. New York, N.Y: Plenum Publishing Corporation; 1987. Influenza in man; pp. 157–218. [Google Scholar]
- 21.Klein M S, Conn C A, Kluger M J. Behavioral thermoregulation in mice inoculated with influenza virus. Physiol Behav. 1992;52:1133–1139. doi: 10.1016/0031-9384(92)90472-e. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi Y, Horimoto T, Kawaoka Y, Alexander D J, Itakura C. Pathological studies of chickens experimentally infected with two highly pathogenic avian influenza viruses. Avian Pathol. 1996;25:285–304. doi: 10.1080/03079459608419142. [DOI] [PubMed] [Google Scholar]
- 23.Kodihalli S, Goto H, Kobasa D L, Krauss S, Kawaoka Y, Webster R G. DNA vaccine encoding hemagglutinin provides protective immunity against H5N1 influenza virus infection in mice. J Virol. 1999;73:2094–2098. doi: 10.1128/jvi.73.3.2094-2098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurokawa M, Imakita M, Kumeda C A, Shiraki K. Cascade of fever production in mice infected with influenza virus. J Med Virol. 1996;50:152–158. doi: 10.1002/(SICI)1096-9071(199610)50:2<152::AID-JMV8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Lu X, Tumpey T M, Morken T, Zaki S R, Cox N J, Katz J M. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J Virol. 1999;73:5903–5911. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matrosovich M, Zhou N, Kawaoka Y, Webster R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J Virol. 1999;73:1146–1155. doi: 10.1128/jvi.73.2.1146-1155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mo I P, Brugh M, Fletcher O J, Rowland G N, Swayne D E. Comparative pathology of chickens experimentally inoculated with avian influenza viruses of low and high pathogenicity. Avian Dis. 1997;41:125–136. [PubMed] [Google Scholar]
- 28.Murphy B R, Webster R G. Orthomyxoviruses. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1397–1445. [Google Scholar]
- 29.Perdue M L, Garcia M, Senne D, Fraire M. Virulence-associated sequence duplication at the hemagglutinin cleavage site of avian influenza viruses. Virus Res. 1997;49:173–186. doi: 10.1016/s0168-1702(97)01468-8. [DOI] [PubMed] [Google Scholar]
- 30.Raut S, Hurd J, Blandford G, Heath R B, Cureton R J. The pathogenesis of infections of the mouse caused by virulent and avirulent variants of an influenza virus. J Med Microbiol. 1975;8:127–136. doi: 10.1099/00222615-8-1-127. [DOI] [PubMed] [Google Scholar]
- 31.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 32.Rohm C, Nannan Z, Suss J, Mackenzie J, Webster R G. Characterization of a novel influenza hemagglutinin, H15: criteria for determination of influenza A subtypes. Virology. 1996;217:508–516. doi: 10.1006/viro.1996.0145. [DOI] [PubMed] [Google Scholar]
- 33.Saito T, Horimoto T, Kawaoka Y, Senne D A, Webster R G. Emergence of a potentially pathogenic H5N2 influenza virus in chickens. Virology. 1994;201:277–284. doi: 10.1006/viro.1994.1292. [DOI] [PubMed] [Google Scholar]
- 34.Scheiblauer H, Kendal A P, Rott R. Pathogenicity of influenza A/Seal/Mass/1/80 virus mutants for mammalian species. Arch Virol. 1995;140:341–348. doi: 10.1007/BF01309867. [DOI] [PubMed] [Google Scholar]
- 35.Schultz-Cherry S, Hinshaw V S. Influenza virus neuraminidase activates latent transforming growth factor β. J Virol. 1996;70:8624–8629. doi: 10.1128/jvi.70.12.8624-8629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schultz-Cherry S, Murphy-Ullrich J E. Thrombospondin causes activation of latent transforming growth factor-β secreted by endothelial cells by a novel mechanism. J Cell Biol. 1993;122:923–932. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shortridge K F, Zhou N N, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markhill D, Murti G, Norwood M, Senne D, Sims L, Takada A, Webster R G. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998;252:331–342. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- 38.Slemons R D, Swayne D E. Replication of a waterfowl-origin influenza virus in the kidney and intestine of chickens. Avian Dis. 1990;34:277–284. [PubMed] [Google Scholar]
- 39.Suarez D L, Perdue M L, Cox N, Rowe T, Bender C, Huang J, Swayne D E. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J Virol. 1998;72:6678–6688. doi: 10.1128/jvi.72.8.6678-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 41.Swayne D E. Pathobiology of H5N2 Mexican avian influenza viruses for chickens. Vet Pathol. 1997;34:557–567. doi: 10.1177/030098589703400603. [DOI] [PubMed] [Google Scholar]
- 42.Swayne D E, Beck J R, Garcia M, Perdue M L, Brugh M. Pathogenicity shifts in experimental avian influenza virus infections in chickens. In: Swayne D E, Slemons R D, editors. Proceedings of the Fourth International Symposium on Avian Influenza. U.S. Richmond, Va: Animal Health Association; 1998. pp. 171–181. [Google Scholar]
- 43.Swayne D E, Beck J R, Perdue M L, Brugh M, Slemons R D. Assessment of the ability of ratite-origin influenza viruses to infect and produce disease in rheas and chickens. Avian Dis. 1996;40:438–447. [PubMed] [Google Scholar]
- 44.Swayne D E, Perdue M L, Garcia M, Rivera-Cruz E, Brugh M. Pathogenicity and diagnosis of H5N2 Mexican avian influenza viruses in chickens. Avian Dis. 1997;41:335–346. [PubMed] [Google Scholar]
- 45.Uys C J, Becker W B. Experimental infection of chickens with influenza A/Tern/South Africa/1961 and chicken/Scotland/1959 viruses. J Comp Pathol. 1967;77:167–173. doi: 10.1016/0021-9975(67)90007-2. [DOI] [PubMed] [Google Scholar]
- 46.Webster R G. Influenza: an emerging disease. Emerg Infect Dis. 1998;4:436–441. doi: 10.3201/eid0403.980325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Webster R G, Bean W J, Gorman W T, Chambers T M, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wells M A, Albrecht P, Ennis F A. Recovery from a viral respiratory infection. I. Influenza pneumonia in normal and T-deficient mice. J Immunol. 1981;126:1036–1041. [PubMed] [Google Scholar]
- 49.Yilma T, Zee Y C, Osebold J W. Immunofluorescence determination of the pathogenesis of infection with influenza virus in mice following exposure to aerosolized virus. J Infect Dis. 1979;139:458–464. doi: 10.1093/infdis/139.4.458. [DOI] [PubMed] [Google Scholar]
- 50.Yuen K Y, Chan P K S, Peiris M, Tsang D N C, Que T L, Shortridge K F, Cheung P T, To W K, Ho E T F, Sung R, Cheng A F B Members of the H5N1 Study Group. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 51.Zhou N N, Shortridge K F, Claas E C J, Krauss S L, Webster R G. Rapid evolution of H5N1 influenza viruses in chickens in Hong Kong. J Virol. 1999;73:3366–3374. doi: 10.1128/jvi.73.4.3366-3374.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]