Abstract
A natural “medicine and food” plant, Rhodiola rosea (RR) is primarily made up of organic acids, phenolic compounds, sterols, glycosides, vitamins, lipids, proteins, amino acids, trace elements, and other physiologically active substances. In vitro, non-clinical and clinical studies confirmed that it exerts anti-inflammatory, antioxidant, and immune regulatory effects, balances the gut microbiota, and alleviates vascular circulatory disorders. RR can prolong life and has great application potential in preventing and treating suboptimal health, non-communicable diseases, and COVID-19. This narrative review discusses the effects of RR in preventing organ damage (such as the liver, lung, heart, brain, kidneys, intestines, and blood vessels) in non-communicable diseases from the perspective of predictive, preventive, and personalised medicine (PPPM/3PM). In conclusion, as an adaptogen, RR can provide personalised health strategies to improve the quality of life and overall health status.
Keywords: Rhodiola rosea, Adaptogens, Neocoronitis, Non-communicable illnesses, Suboptimal health, Predictive preventive personalised medicine (PPPM / 3PM)
Introduction
The four most prevalent Non-communicable Chronic Disease (NCDs)—diabetes, cancer, chronic respiratory diseases, and cardiovascular diseases—are the primary causes of morbidity and mortality worldwide, accounting for nearly 75% of NCD-related deaths (31.4 million) in developing nations. NCDs are increasingly recognised as an urgent threat to global health and development [1]. It is anticipated that by 2023, these diseases will result in a combined mortality rate of 510.54 deaths per 100,000 people, with NCDs accounting for 75.26% of all fatalities globally [2]. Beginning in 2019, New Crown pneumonia has affected approximately 1 billion people worldwide, claiming the lives of 500,000 people annually, and imposing significant adverse effects on the economy and social life [3].
Neocoronary pneumonia and NCDs are linked to immune dysregulation, oxidative damage, inflammation, blood circulation issues, and gut flora imbalances [4]. Redox reactions mostly occur within the mitochondria, and maintaining the functional integrity of the mitochondria is essential for maintaining regular redox reactions. Oxidative stress is characterised by an imbalance in redox reactions that can result in the generation of excess reactive oxygen species (ROS) and oxidative damage to cells. Blood circulation and inflammation disorders are closely linked to oxidative stress [5]. Additionally, faecal problems can potentially worsen oxidative stress, inflammatory responses, immunological dysregulation, and disorders associated with blood circulation [6]. The involvement of gut flora in diabetes, hypertension, and neurodegenerative illnesses has recently been proven [7].
As awareness of health advances, the goal of healthcare currently includes improving the overall quality of life while effectively preventing and treating illnesses. Personal efforts, such as adopting healthy eating habits, can significantly contribute to long-term health benefits. Many individuals opt to incorporate specific nutraceuticals into their everyday diet to maintain their health and well-being. Certain nutraceuticals are preferred by people to take in their everyday lives in order to keep their health in check [8]. Thomas Edison envisioned a shift in medicine and stated that, “The physician of the future will no longer treat the human body with drugs, but with nutritional therapy to prevent disease” [9]. This underscores the importance of nutrition in improving health and preventing diseases. Traditional Chinese medicine (TCM) is a range of medical practices and health interventions that have been used in China for over four millennia. While TCM continues to impact healthcare and population health in the Asia–Pacific region, it has received growing attention globally over the past 20 years, particularly with the advancement of genomics [10]. Natural tonic medicinal plants play an essential role in health intervention strategies in TCM, as they have the potential to maintain the body in a state of harmony and balance while preserving health. Rhodiola rosea (RR) and its extracts affect the transmission of neurotransmitters, the functioning of the central nervous system, the cardiovascular system, and other essential body functions. The Defense Research and Development Organization (DRDO) of India has established several regulatory procedures for RR-related health foods, supplements, and nutraceuticals [11].
Rhodiola rosea extract’s oligomeric proanthocyanidins (OPCRR) have potent antioxidant activity across a range of organs, including the ability to scavenge free radicals, boost the activity of glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD), and lower the levels of malondialdehyde (MDA), a dangerous byproduct of peroxidation [12]. Prolonged RR supplementation decreases lactate levels, reduces signs of damage to skeletal muscles during intense exercise, and improves fatty acid uptake by regulating cellular energy metabolism. Additionally, it enhances the body’s capacity to adjust to intense physical activity [13]. The extract has demonstrated efficacy in treating stress-induced injuries, enhancing exercise capacity, and significantly decreasing lactate dehydrogenase (LDH) and creatine kinase (CPK) levels during physical stress [14]. When administered in conjunction with chemotherapy, various preparations of RR have been shown to enhance patients’ immune systems, mitigate chemotherapy-induced adverse effects on the liver and kidneys, hasten the healing of drug-induced oral ulcers, and improve the prognosis of patients with breast cancer [15]. Through transcription factor skinhead-1(SKN-1) and insulin/insulin-like growth factor (IGF) signalling, this natural plant could potentially slow the ageing process and assist the body in repairing age-related damage [16]. Additionally, it can restore the balance of the intestinal flora [17]. Moreover, the byproducts of this plant, such as amino acids and terpenoid carbohydrates, can help preserve the variety of intestinal flora, safeguard beneficial genera, and regulate neurotransmitter and endocrine signalling through flora metabolites, reducing brain damage and enhancing memory and cognitive functions [18]. The glycosides from RR exhibit a colony-dependent effect on intestinal inflammation, reduce M1 macrophages, and increase mucin-expressing proteins (mucin-2) and tight junction proteins (occludin and zonula occludens-1 (ZO-1)) within the gut [19]. This plant serves as a natural prebiotic to produce anti-fatigue benefits and boost physical endurance by regulating the bacterial flora [20]. Sun et al. [21, 22], by blocking the Toll-like receptor-2(TLR4) / NF-κB signalling pathway, discovered that this plant extract could effectively reduce the secretion of inflammatory factors such as tumour necrosis factor-α (TNF-α) and interleukin 6 (IL-6), as well as mitigate the excessive inflammatory response during acute lung injury.
Rhodiola exerts its antioxidant, anti-inflammatory, and immune regulatory effects via multiple pathways. As a TCM nutrient, it plays a significant role in combating fatigue, improving exercise endurance, and preventing and treating cardiovascular and cerebrovascular diseases and COVID-19, as well as cancer. Given the current trend of various chronic diseases and the ongoing COVID-19 pandemic, there is a need to develop a health strategy that integrates multidisciplinary expertise. This strategy is timely and different from the previously used reactive medicine. From an ethical perspective, this strategy prioritises the health rights of individuals and assists them with self-care and nutrition under professional guidance before resorting to hospital visits [23].
Rhodiola rosea is a dietary supplement that “can be used as both food and medicine” and has a long history of use in strengthening the body and replenishing energy, and promoting vitality. By describing the mechanisms of action and research advancements related to suboptimal health and disease states, this narrative review aims to explore examples of the potential of RR in the context of PPPM and offer a possible strategy for adaptable medicine reform.
History of Rhodiola rosea
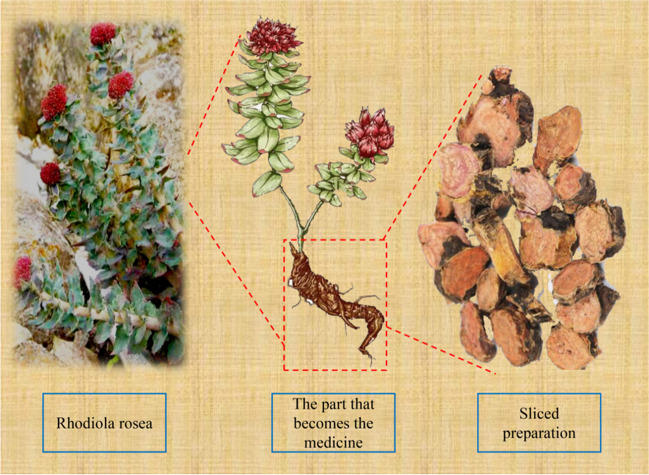
Rhodiola rosea was so named by the Swedish botanist Linnaeus because of the rose-like smell produced by its freshly cut rhizome. It is a perennial herb that grows to a height of 10–30 cm and belongs to the genus Rhodiola of the Sedum family. The rhizome is short, robust, and cylindrical, covering many imbricate, scale-like leaf arrangements. The root is sturdy, conical, fleshy, and tawny, with several fibrous roots at the root neck (Fig. 1). It is predominantly found in the alpine zone of the Northern Hemisphere, growing in the understory or on the grassy slopes of mountain slopes in the alpine unpolluted zone between 1800 and 2500 m above sea level [24]. Its strong vitality and unique adaptability result from its ability to endure harsh growing environments, which include low oxygen levels, dryness, wind, exposure to UV rays, and a wide range of temperatures between day and night. However, owing to unrestricted human harvesting, wild RR resources are becoming increasingly scarce. In certain countries, they are classified as protected plants, with illicit harvesting being prohibited [25]. Around 77 AD, the Greek physician Dioscorides noted in his book De Materia Medica that RR contains biological supplements that can provide anti-fatigue, anti-stress, and antidepressant effects. To our knowledge, this marks the first detailed account of the medical significance of RR. Referred to as the “immortal herb”, RR was also described by Li Shizhen in the Ming Dynasty as “a superior product of this scripture, dispelling evils and evil qi” in the book Compendium of Materia Medica. Its properties include “sweetness, bitterness, and flatness, and are associated with the Lung and Heart meridians”. It has been demonstrated to stimulate blood circulation, stop bleeding, and change blood composition. Its therapeutic functions include lowering oedema, resolving blood stasis, controlling bleeding, and promoting blood circulation. Plants are commonly used to treat ischaemic injury, neurodegenerative diseases, diabetes, hypertension, coronary heart disease, cancer, new coronary viruses, and infectious pneumonia, among other conditions. Modern pharmacological research shows that this plant has various functions, including anti-ageing [26], anti-inflammatory [27], immune enhancement, vascular protection, antioxidant properties, anti-fatigue, antidepressant effects, anti-allergy, antibacterial, cognitive function improvement, anticancer properties, and regulation of intestinal flora, among others [28–32]. Additionally, it is commonly used in the treatment of ischemic injury-related diseases, neurodegenerative diseases, diabetes, hypertension, coronary heart disease, cancer, and pneumonia caused by the novel coronavirus infection, as well as in the recuperative process of suboptimal health [33, 34]. RR has been used in traditional medicine in Russia, China, and Scandinavia for centuries. In 1969, the USSR Ministry of Health authorised and registered the plant for medicinal use, and in 1985, it was included on the list of Swedish botanical medicinal medicines. The European Medicine Agency (http://www.Ea.europa.eu/EMA/) now includes clinical applications of the plant. Moreover, it is frequently used as a strengthening and energising tonic herb in Denmark [35].
Fig. 1.
Morphology and pharmacological components of Rhodiala rosea. Created with WPS Office and Adobe Photoshop 2020
Active ingredients found in Rhodiola rosea
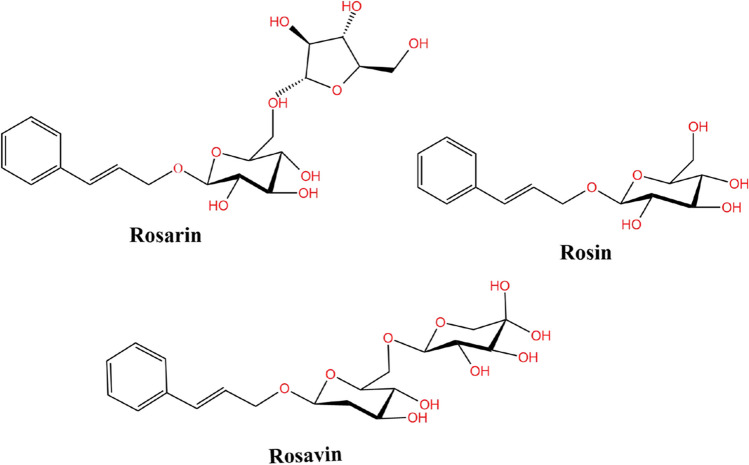
The natural medicine RR offers numerous medicinal advantages. Research initiated by the USSR on the standardisation of RR resulted in significant advancement, and in the 1970s, it was suggested that the glycoside from RR (content ≥ 0.8%) served as an effective marker for its quality. However, in 1986, owing to the widespread prevalence of adulterated RR, specific active ingredients, Rosavin, Rosarin, and Rosin (referred to as Rosavins, Fig. 2), were discovered in RR for the first time. Currently, conventional RR compounds utilised in clinical applications maintain a 3:1 ratio, with at least 3% Rosavins and 0.8–1% Rosin [36]. The plant has a complex composition; ≥ 140 different types of active ingredients have been isolated, including tannins, vitamins, fats, proteins, essential amino acids (17 types—including seven that the human body is unable to synthesise), sterols, glycosides, organic acids (oxalic, citric, malic, gallic, and succinic), and more than 40 different types of phenolics [37]. The majority of the active substances listed above are beneficial to human health [38, 39]. The extraction of its compounds is relatively straightforward, with the most common techniques being water and ethanol extraction. Both the water and ethanol extracts exhibit different levels of phenolic compounds and antioxidant and neurobiological activities. Gallic acid (2.33 mg/g DW) shows the highest concentration and bioactivity in the ethanol extract, while erucic acid (386.44 µg/g DW) has the highest [40]. Thus, RR and its extracts show significant promise for therapeutic applications. Pharmacological studies reveal rhodioloside and lorcetin as the primary active compounds. Numerous pharmacological studies on RR and its active ingredients have demonstrated their therapeutic effect on various diseases via various pathways. For example, RR glycosides exert anticancer activity by activating autophagy and apoptosis and inhibiting the PI3k/AKT, JAK/STAT, and MEK/ERK pathways. In diabetes management, they function via the AMPK pathway. The plant extract reduces inflammation and confers neuroprotection by modulating the NF-κB, Nrf2/HO-1, and PI3K/AKT pathways and balancing oxidative and antioxidative mechanisms [41]. Losevi inhibits hepatic inflammatory responses to mitigate hepatocyte injury from non-steatohepatitis, reduces lung injury from PM2.5 in rats by inhibiting the PI3K/Akt/Nrf2 signalling pathway, and inhibits NF-κB/mitogen-activated protein kinase (MAPK) to treat orthopaedic diseases linked to excessive osteoclastogenesis [42–44]. Several phenolic compounds in this plant have been found to possess potent antioxidant and anticancer activities [10]. RR enhances patient prognosis in addition to its ability to prevent and treat diseases in a targeted manner through specific signalling pathways. Predictive dosing can be deployed to manage pre-disease states or suboptimal health. A suboptimal health state lies between health and disease; its prognosis dictates whether the illness will manifest. In some instances, suboptimal health regulation can reduce the financial burden of healthcare on families and society as a whole and decrease medical resource waste [45]. Due to their broad spectrum of actions and fewer adverse effects on the human body, herbal medications play an active and unmistakable role in reversing suboptimal health status [46]. As an adaptogen, RR has anti-inflammatory and antioxidant properties [47, 48]. These studies above are consistent with the medical principles of primary prevention.
Fig. 2.
Molecular structure and formula of key components of Rhodiola rosea. Created with KingDraw
Possible Rhodiola rosea drug carriers
Rhodiola rosea exhibits an intricate chemical makeup, low drug stability, and possible interactions with its active ingredients. The delivery method often results in low bioavailability. Currently, oral administration is the primary route of administering RR and its related preparations. However, a significant portion of these drugs are rapidly cleared in the gastrointestinal tract when taken orally, leading to premature metabolism before reaching their targeted site of action [49, 50]. Additionally, the efficacy of active ingredients in treating different diseases is influenced by the complexity of their composition and the specific location where they exert their effect. Consequently, establishing a consistent quality management system for herbal medicine is challenging.
Rhodiola rosea is typically non-toxic. However, given the current advancements in toxicology and pharmacology, it is imperative to consider some low-probability adverse effects [51–53]. Most bioactive components from plant-derived medications encounter low absorption and bioavailability, which limits their effectiveness. Silk protein, a naturally occurring polymer with unique qualities, can transport drugs and serve as a carrier material by incorporating monomers from herbal medicine, facilitating the development of innovative drug release systems that are biocompatible in vivo, demonstrate good stability, and are devoid of any noticeable harmful side effects when employed to treat illnesses [54, 55]. Alternatively, the problems above can be effectively addressed through the Filipin protein delivery medication method. While this approach still requires clinical validation, many preclinical validations are sufficient to demonstrate its enormous potential for its application in ageing and ageing-related illness prevention and treatment techniques in the future [56]. Because of its tunable mechanical characteristics, regulated biodegradability, and biocompatibility, filipin protein has been shown to reduce inflammatory response during skin wound healing and/or soft tissue repair. Additionally, it also provides an opportunity for the widespread use of filipin proteins in RR’s health strategy owing to their low cost and ease of use [57]. Recent developments in nanomaterials have enabled us to address the shortcomings of RR in the drug delivery process. These materials can alter drug clearance in the body, increase drug efficacy, facilitate RR penetration to particular sites, and customise RR medication according to individual requirements [58]. Clinical data supports the ability of anticancer drug nano-delivery devices (NDD) to precisely treat cancer cells during targeted drug release while ensuring biosafety [59]. Several examples of the primary liposomal and polymer-based therapeutic nanoparticles that have been licensed for clinical use in the US market include Oncaspar® (poly(ethylene glycol) asparaginase), Abraxane® (albumin-conjugated paclitaxel), and Doxil® (liposome-encapsulated adriamycin) [60]. Lipid nanocapsules, polymer nanoparticles, solid lipid nanoparticles, nanoliposomes, and nanovesicles are examples of nanocarriers that can enhance the bioavailability of antiarrhythmic medications and decrease the frequency of adverse drug reactions [61]. Preparing formulations of RR nanoparticles is consistent with sustainable production practices and holds great promise for use in the biomedical field [62]. Silk proteins and nanocarriers have the potential to mitigate the shortcomings of current drug delivery techniques, maximise medication efficacy, enhance the effectiveness of drug therapy, and provide customised dosage schedules. In the future, using RR in precision medicine might be a worthwhile endeavour. However, there is still a considerable distance to cover, as neither the nanocarrier delivery system nor the filipin protein delivery system currently possesses sufficient clinical evidence to validate their efficacy.
Adaptogens
The concept of “adaptogens” was proposed by the Soviet scientist Lazarev in 1940. In 1998, the U.S. Food and Drug Administration (FDA) defined “adaptogens” as a new class of metabolic regulators that demonstrated the ability to increase an organism’s ability to adapt to its environment and mitigate external damage [63]. Beneficial stress protection is achieved mainly through the involvement of the “hypothalamic–pituitary–adrenal axis” and the “stress counter pathway” [64]. Adaptogens are characterised by the following three features: (1) non-specific effects, capable of resisting a wide spectrum of harmful physical, chemical, or biological stressors; (2) homeostatic effects, capable of reversing disorders caused by external stressors; and (3) non-toxic to normal body functions [65]. RR was among the first “adaptogenic” herbs to be discovered, and since its discovery, it has captured the attention of many researchers. This stress response is formed by the body’s adaptation to sudden changes in the internal and external environments by enhancing nonspecific defences and generating nonspecific resistance to neutralise unfavourable physical, chemical, and biological factors [66]. In our modern society, unfavourable natural environmental factors (including abnormal climate and anthropogenic emission of radioactive substances into the sea) and social survival pressures (including heavy workloads, high-intensity work pressures, and the spread of various viruses) not only increase anxiety and tension but also reduce the body’s endurance and resilience. Although changing the natural environment and social pressures is difficult, people still prefer to live a more relaxed and healthier life. Adaptogens have been found to be helpful in managing stress and emotions. In addition, adaptogens can enhance endurance and physical resilience [47]. RR, as an “adaptogenic” herb, can enhance the body’s resistance to various external chemical, physical, and biostimulatory stresses [67]. Feeding RR glycosides to rats with cognitive and emotional impairments has been shown to improve their cognition, increase attention span, boost fatigue resistance, and mitigate the deleterious effects of negative emotions on the body [68, 69]. Similar benefits have also been observed clinically [70]. The biological mechanism by which RR, as an “adaptogen”, involves increasing the vitality of the central nervous system by increasing the expression level and activity of monoamine neurotransmitters as well as opioid peptides [71]. In the treatment of psychiatric and cognitive disorders, RR operates essentially the same way as conventional drugs. However, in contrast to conventional drugs, RR does not lead to drug dependence or addiction, nor does it surpass the body’s stress defence ability or hinder its ability to return to a normal state of functioning once the drug’s effects have subsided [30].
Rhodiola rosea’s role in protecting the mitochondria
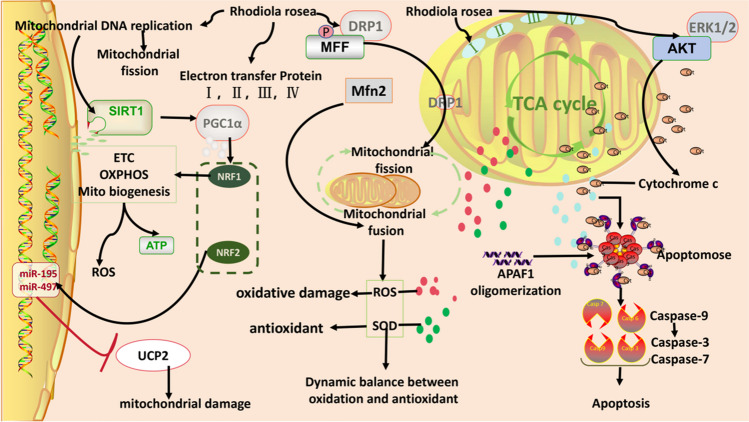
Mitochondria are important organelles in the body’s redox response, and their normal morphology and function are essential for coping with oxidative damage and energy metabolism. Mitochondrial damage is closely related to systemic multi-organ dysfunction, and protecting mitochondrial function is crucial for cellular health in the context of PPPM/3PM [72]. Diseases such as neuropathy, cancer, and cardiovascular disorders are usually caused by malfunctioning mitochondria [73]. Mitochondrial failure in cells could increase H4K8 acetylation and accelerate telomere depletion, ultimately leading to an age-dependent decline in cell function [74, 75]. Additionally, this is accompanied by an unbalanced mitochondrial oxidative and antioxidant capacity, a blocked oxidative phosphorylation mechanism (OXPHOS), a damaged mitochondrial network, excessive ROS generation, compromised communication between the nucleus and mitochondria, and an aggravated inflammatory reaction to infectious diseases [76]. Furthermore, mitochondrial dysfunction is an accelerator of degenerative diseases of the nervous system, such as Parkinson’s disease, metabolic disorders, and cancer [77]. RR glycosides can increase the expression of the mitochondrial movement-associated protein dynamin-related protein 1 (Drp1), upregulate the expression of mitotic fusion protein 2 (Mfn2), protect the mitochondrial respiratory chain, increase SOD levels, decrease ROS levels, and maintain the dynamic balance of mitochondrial oxidative and antioxidant systems [78]. These glycosides can enhance mitochondrial DNA replication and electron transport chain protein function and increase the silent information regulator sirtuin 1 (SIRT1) and peroxisome proliferator-activated receptor ϒ coactivator-1 alpha (PGC-1α) release to maintain normal mitochondrial activity [79]. RR also activates the extracellular regulated protein kinase 1/2(ERK1/2)-AKT signalling pathway and promotes cell survival and proliferation by inhibiting mitochondria-mediated caspase apoptosis [80]. RR and its key components can positively regulate the treatment of various diseases by modulating mitochondrial function; for example, RR glycosides can increase the activity of mitochondrial complex I, restore mitochondrial membrane potential, and increase the antioxidant capacity of mitochondria through the MEF2D-ND6 pathway, thus exerting a protective effect on dopaminergic (DA) neurons [81] (Fig. 3).
Fig. 3.
Key signalling pathway involved in Rhodiola rosea exerting mitochondrial protective effects. Created with WPS Office and scienceslides 2016
Yu et al. [82] demonstrated that RR ameliorates adverse moods, such as anxiety and depression, by inhibiting ROS and lipid peroxidation in the mitochondrial respiratory chain.
Anti-inflammatory effects of Rhodiola rosea
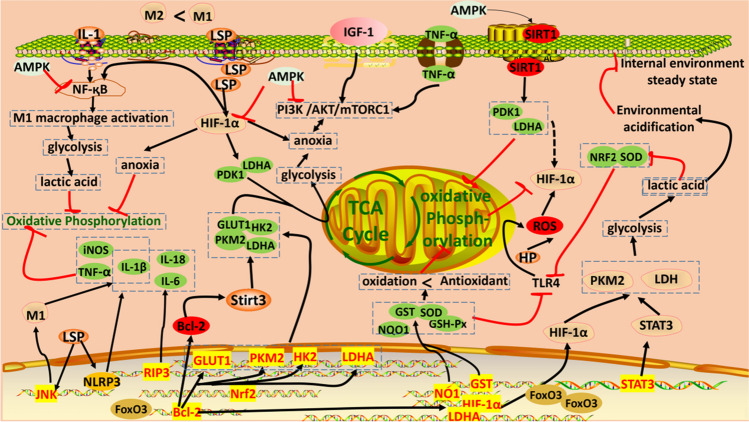
Acute and chronic inflammatory responses are essential triggers for developing suboptimal health and disease [83]. Furthermore, chronic inflammation is closely related to tumours, posing a lethal threat. Therefore, it is crucial to prevent inflammation, address it promptly, and mitigate its progression when resolution is challenging. These efforts are essential for the prevention and treatment of tumours, as well as various other inflammatory disorders [84]. AMPK regulates energetic homeostasis and is a key facilitator of the upstream inflammatory response [85]. It interacts with the mammalian target of rapamycin (mTOR), UNC-51-like kinase 1 (ULK1), Forkhead box O (FOXO), p53, SIRT1, and NF-κB, as well as other key regulatory proteins in inflammatory response-related pathways [86]. NF-κB is a chronic inflammatory transcription factor and demonstrates an age-related increase in activity and quantity [87]. AMPK and NF-κB promote the activation of NLRP3 inflammasomes and the release of IL-1β, IL-6, and IL-18, among other inflammatory cytokines, triggering an inflammatory cascade response that activates macrophages [88]. These macrophages can be activated into either pro-inflammatory M1 or anti-inflammatory M2 macrophages. Under normal conditions, both M1 and M2 are in a dynamic equilibrium, maintaining optimal body functioning. The continued accumulation of proinflammatory cytokines during ageing leads to an imbalance between M1 and M2 macrophages [89]. Exposure of the body to ageing, viral infections, and radiation decreases the expression of the transcription factor FoxO3. This decrease prompts the polarisation of macrophages into M1 macrophages, which facilitates the conversion of M2 macrophages to M1 cells. Subsequently, this leads to an increased release of pro-inflammatory cytokines and a sustained chronic inflammatory response within the body [90]. RR glycosides decrease the expression of IGF-1 and IGF-1R, downregulate the expression of p-PI3K and p-Akt, and significantly increase the expression of transcription factors SIRT1 and FOXO3a [91]. RR glycosides can inhibit the release of inflammatory cytokines and reduce inflammatory responses and oxidative stress damage through AMPK/NF-κB/NLRP3 and other signal transduction pathways [92–94]. Additionally, RR glycosides inhibit NF-κB via activation of the Notch-hes signalling pathway, which significantly reduces TNF-α, IL-1β, and IL-6 levels in macrophages [21, 95] and regulates early cytokine levels by blocking the activation of NF-κB and ERK/MAPKS responses [96]. These glycosides reduce the polarisation of M1 macrophages, the release of pro-inflammatory cytokines, and the various chronic inflammatory responses. In vivo studies confirmed that RR glycosides reduce inflammatory cell infiltration, mir-323-3p expression, IgE, IL-13, IL-4, IL-5, collagen type I (Col-I), and collagen type III (Col-III) levels; and decrease the number of inflammatory cells in a mouse model of asthma [97, 98] (Fig. 4).
Fig. 4.
Signal map associated with the anti-inflammatory response of Rhodiola rosea. Created with WPS Office and scienceslides 2016
Enhancement of the immune system by Rhodiola rosea
CD3 + and CD4 + are the primary immune cells of the T lymphocyte subsets involved in the body’s immune response, and the CD4 + /CD8 + ratio reflects the body’s cellular immune status. Myeloid-derived suppressor cells (MDSC) are a diverse population of immature heterogeneous cells in the bone marrow, with M-MDSCs being a pivotal MDSC subset that exerts an immunosuppressive effect. These cells secrete cytokines that recruit regulatory T cells with high expression levels and can significantly inhibit T lymphocyte responsiveness [99]. The decreased numbers and functions of T lymphocyte subsets and the increased numbers of MDSC are the leading causes of immune senescence. With advancing age, the proportion of CD3 + and CD4 + cells in splenic tissue decreases, and the proportion of CD4 + /CD8 + cells and the number of MDSC increases [100]. Immunosenescence is an important marker of the ageing process. Long-term, chronic low-grade inflammation is also an important factor affecting immune senescence in older adults. The secretion of the pro-inflammatory cytokine TNF-α increases with age, and studies have confirmed that TNF-β significantly inhibits the activity of Toll-like receptor-2 (TLR2) and TLR4. TLR is a vital molecule in the body, serving as a naturally occurring and specific immune protein. When this activity is inhibited, the immune response capacity decreases [101]. RR glycosides can significantly inhibit the TNF-α-activated MAPK and NF-κB signalling pathways. This leads to an improved inflammatory response and immune status in the body [102]. In this case, the intrinsic mechanism underlying RR’s action likely involves increasing the killing capacity of T cells by promoting the maturation of dendritic cells and antigen-presenting cells and enhancing the immune response of senescent cells to deal with various injurious factors [103].
Regulation of intestinal flora by Rhodiola rosea
The gut possesses a large microbiota of over 10 trillion bacteria, which are involved in physiological and pathological processes, such as immune response, tryptophan metabolism, vagus nerve function, and the enteric nervous system. These processes are mediated primarily through microbial metabolites, such as short-chain fatty acids, branched-chain amino acids, and peptidoglycan [104, 105]. The intestinal flora undergoes dynamical changes, and a healthy microbiota can mitigate the detrimental effects of various factors on the human body; therefore, the resilience of the intestinal flora plays a crucial role in maintaining health and preventing disease. When the intestinal flora becomes dysbiotic, the organism becomes vulnerable to diseases [106]. A decrease in the diversity and number of intestinal flora reduces the protective effect of the gut microbiota, accelerates metabolic disorders of insulin and leptin, and induces a decline in the functioning of the organism and the onset of diseases [107, 108]. Short-chain fatty acids (SCFAs) are key mediators of the gut flora during ageing and other stressful conditions. Under these conditions, faecal bacilli associated with SCFAs decrease, oxygen-resistant and pathogenic bacteria that cause damage to the body increase, the ratio of beneficial and pathogenic bacteria in the intestine becomes unbalanced, and the dynamic balance of the intestinal flora is disrupted, leading to a decrease in SCFA content [109, 110]. Butyric acid, an essential component of SCFAs, is produced by the digestion and fermentation of dietary fibre in the lower gastrointestinal tract and promotes the secretion of fibroblast growth factor-21 (FGF-21), a cytokine associated with longevity that is a benign regulator of energy and lipid metabolism in the body [111]. Endotoxins, such as lipopolysaccharides (LSP), are produced in the gut when the dynamic balance between beneficial bacteria, such as Prevotella and Bifidobacterium, and pathogenic bacteria, such as Enterobacteriaceae, Staphylococcus, Streptococcus, and Candida albicans, is disrupted [112]. Trimethylamine (TMA) is also an essential metabolite of intestinal bacteria, and gets oxidised in the liver and elsewhere to form trimethylamine oxide (TMAO). TMAO increases platelet aggregation and adhesion to form collagen, which promotes thrombosis; bacterial catabolism of choline leads to choline deficiency, exacerbating metabolic disorders by reducing DNA methylation and affecting epigenetics [113, 114]. RR protects the diversity of the intestinal flora in mice, increases the production of butyric acid and SCFAs, and reduces the production of LPS and TMA [115]. Rhodioloside inhibits the apoptosis of intestinal epithelial cells and maintains intestinal barrier function, diversity, and the dynamic balance of intestinal flora by upregulating the expression of tight junction proteins, ZO-1 and occludin [17]. RR glycosides downregulate circulating levels of serum LPS, decrease the release of pro-inflammatory cytokines IL-6 and TNF-α, increase the production of anti-inflammatory cytokine IL-10, significantly inhibit the production of bacterial akkermansia, and reduce the number of LPS-producing bacteria from Aspergillus spp. [31].
Rhodiola rosea alleviates blood circulation issues
With the advancement of modern societies and changes in dietary habits and lifestyles, an increasing number of individuals are suffering from cardiovascular and cerebrovascular diseases caused by circulatory disorders. An imaging study of cerebral haemodynamics using an arterial spin labelling magnetic resonance imaging (MRI) system revealed that the degree of cerebrovascular haemodynamic disturbances is directly proportional to the degree of ageing [116]. With age, the body’s average blood flow rate decreases, and arterial pulsation and resistance increase. This stresses the circulatory system and increases the risk of cardiovascular diseases such as atherosclerosis and dementia [117, 118]. In adipose tissue, with increasing age, insulin acts on endothelial cells to decrease the bioavailability of the vasodilator factor nitrous oxide (NO), thereby impairing the vasodilatory function of NO and damaging the vascular endothelium, which in turn disrupts vascular endothelial function. In addition, in an ageing body, impaired angiogenesis leads to reduced vascular density. Vascular density in aged adipose tissue is inversely proportional to age. Reduced vascular density deprives tissues of oxygen and nutrients, thereby triggering oxidative stress, inflammation, metabolic disorders, and other complications, accelerating the decline in bodily function [119]. Impaired angiogenesis is caused by a combination of factors. In a retrospective study, low vascular endothelial growth factor (AGF) levels were observed in senescent bodies [120]. RR can effectively ameliorate haemodynamic disturbances and angiogenic dysfunction by inhibiting excessive contraction of vascular smooth muscle by restoring Ca2+ and K+ channels in smooth muscle cells, thereby restoring vasodilatory function and ameliorating cerebral haemodynamic disturbances [121, 122]. Additionally, RR activates the PI3K/AKT signalling pathway to reverse cardiac haemodynamic disturbances and improve cardiac ejection fraction [123]. It can ameliorate oxidative stress, reduce damage to the vascular endothelial barrier by ROS, and ameliorate atherosclerosis via the CAMP/PKA/RHOA signalling pathway [124]. RR glycosides specifically inhibit oestrogen receptor α (ERα) and prolyl hydroxylase structural domain 3 (PHD3), enhance endothelial and smooth muscle cell migration through paracrine signalling in skeletal muscle cells, and promote angiogenesis in hindlimb ischaemic mice [125]. RR reduces the damage of TNF-α and other inflammatory cytokines on cardiac microvascular endothelial cells (CMEC) through the MAPK/NF-κB signalling pathway [42].
Anti-ageing properties of Rhodiola rosea
Ageing is unavoidable in all organisms. It is accompanied by telomere depletion, mitochondrial dysfunction, activation of nuclear signalling pathways, inflammatory responses, and circulatory and metabolic disorders, which disrupt the homeostasis of the organism and predispose individuals to various ageing-related diseases. These diseases include metabolic disorders related to senile obesity (and its associated cardiovascular and cerebrovascular adverse events) [126], chronic liver disease, decreased pulmonary ventilation, neurological dysfunction (accelerated onset and progression of Alzheimer’s disease and cerebral neurological damage after ischaemic stroke), progression of chronic kidney disease to renal fibrosis, and various cancers (such as lung, gastric, liver, and breast). The proportion of the population aged 60 years or older is projected to reach 22% of the total population by 2050 [127], suggesting that the age-related deterioration of physical functions may not only pose an existential challenge to the older adult population but also impose enormous pressure on social healthcare systems, which in turn affects economic and social development and progress. Ageing is a dynamic process of multi-system and multi-organ co-development. Accurate assessment of this dynamic process, followed by stratifying patients according to the assessment results, and implementing targeted preventive measures for high-risk groups according to the assessment and stratification results, is essential, and adopting personalised protective measures tailored to the individual conditions of each patient is essential. This approach seizes the window of opportunity before the onset of the disease states during ageing to prevent ageing-related diseases [128–130]. Ageing often is an independent risk factor for disease progression and death in individuals affected by illness. Conventional reactive medicine seems to be unfavourable towards ageing. Consequently, achieving primary prevention (providing targeted prevention to individuals at high risk of disease and subfertility), secondary prevention (offering improved treatment to those already afflicted by the disease, thereby reducing complications and adverse effects of conventional treatment), and tertiary prevention (alleviating the suffering of patients with incurable diseases and improving their quality of life at the final stage of the disease to ensure personalisation of healthcare) is highly desirable in the current social context. This approach enables individuals, families, and society as a whole to achieve the most significant personal health outcomes at the lowest cost [131, 132].
Continuous cell division and replication are necessary for the growth of most human cells. Telomeres at the ends of the chromosome continue to shorten during cell division, and the cells move towards senescence and functional decline. Although telomere length varies from cell to cell, it is innate to all cells. Shortened telomeres are important markers of human senescence [133]. Changes in mitochondrial dysfunction, inflammatory responses, oxidative stress [134], circulatory disorders, reduced immune responses, and gut flora disorders can induce telomere shortening [135]. Conversely, telomere shortening can cause the aforementioned reactions and accelerate ageing [136].
Multiple studies have confirmed that RR can prevent high-risk diseases during ageing by intervening in numerous physiological and pathological stages of the ageing process to exert a protective effect on the organism [137]. RR glycosides can inhibit mir-22 expression by activating SIRT1, improving mitochondrial mass; maintaining mitochondrial activity; increasing the production of PGC-1, nuclear respiratory factor 1 (NRF-1), and mitochondrial transcription factor A (TFAM); reducing the release of senescence-associated molecules such as p52, p21, and p16; and delaying division and replicative senescence in human fibroblasts [138]. Additionally, RR glycosides exert mitochondrial protective and antioxidant effects in different tissues and organs of the human body, such as the heart, lungs, and brain, and attenuate the ageing-induced degenerative organ lesions [139, 140]. Reportedly, oral administration of RR can reduce post-exercise muscle discomfort and enhance exercise endurance by reducing oxidative stress and increasing muscle antioxidant capacity [141]. This is beneficial for older adults because it helps them maintain their physical strength and improve their quality of life. Anti-ageing research has mainly focused on glycosides, the critical component of RR, with less research on other components and few high-quality clinical studies. Therefore, further research is required to identify the precise mechanisms.
Improvement of suboptimal health by Rhodiola rosea
The term “suboptimal health” was first proposed by Berkman, a Soviet scholar, in the 1980s as a critical state between health and disease, with the most common symptoms being fatigue, poor sleep quality, amnesia, fatigue that cannot be relieved by rest, dry throat, dizziness, dry eyes, flatulence, pain, and early awakening [142]. With the rapid development of science and technology in modern society, the pace of life is rapidly increasing, and the state of suboptimal health is becoming increasingly prevalent. Approximately 46.3% of the people in southern China are in suboptimal health [143]. Among students, approximately 55.9% were in a state of suboptimal health [144]. The prevalence of suboptimal health states is directly proportional to age, and the risk of their occurrence increases with age. Suboptimal health is a pre-disease condition, and currently, most diseases are treated only after the disease has manifested, leading to significant delays that result in a waste of medical resources. Preventive treatment of suboptimal health states before disease onset can reduce healthcare expenditures and contribute to the advancement of PPPM [46]. Oral administration of RR extract SHR-5 reduces tiredness in patients with fatigue syndrome, enables them to concentrate, and reduces cortisol response to arousal stress [72, 145]. Therefore, RR supplementation may be an effective treatment option for patients experiencing long-term or chronic fatigue. Moreover, the extract exhibits a good safety and tolerability profile [146]. Possible mechanisms by which it exerts its anti-fatigue effects involve pathways such as mitochondrial protection of cells, inhibition of inflammatory responses, and modulation of immunity [48].
Effect of Rhodiola rosea on obesity
Obesity leads to fat accumulation in various tissues and organs. Chronic low-grade inflammation and the insulin/insulin-like growth factor (IGF) axis are intrinsic mechanisms in developing obesity-related diseases [147]. Obesity-induced sarcopenic obesity syndrome involves multiple systems and serves as an accelerator of cardiovascular diseases, metabolic disorders, non-alcoholic fatty liver disease, cancer, and other acute and chronic diseases [148, 149]. With the advent of an ageing society, the number of obese older adults is gradually increasing. In the UK, 37% of people over 60 years of age are obese, 30% are obese in the USA, and over 50% of adults in some countries in North Africa and the Middle East are obese [150]. Age and obesity are significant triggers of oxidative stress. The development of cardiovascular-related diseases is another adverse event associated with high mortality in the obese population. Obesity has emerged as an important risk factor for cardiovascular disease due to its poor haemodynamics. Patients with obesity usually exhibit elevated blood lipid levels, leading to increased blood viscosity and higher kinetic demands on the circulatory process. If the existing haemodynamics fail to meet these demands, it can lead to haemodynamic disturbances [58]. Cells are more susceptible to hypoxia when the organism is overly obese, coinciding with heightened Na+/K+-ATPase activity. Na +/K+-ATPase is an important switch in the cellular oxidative response. Its overactivation promotes the accumulation of ROS in cells, causing peroxidative damage [151]. Increased body fat impairs health and reduces the quality of life. Medical research and intervention aim to prevent the onset of certain diseases in a timely manner to improve the quality of life [152]. Notably, the benefits of RR in controlling obesity and its associated complications align with PPPM/3PM principles. RR has been shown to inhibit AMPK-PKC by suppressing the ξ-signalling pathway to reduce Na + /K + -ATPase activation [153]. It protects telomeres at the ends of the chromosome strands against shortening caused by fragmentation through the activation of the SIRT1 signalling pathway and the inhibition of the atrophy of skeletal muscle gene fragments. It also improves mitochondrial quality control, activates the SIRT1/p53/drp1 signalling pathway to maintain dynamic mitochondrial homeostasis [25], and ultimately ameliorates chronic inflammation and oxidative stress-induced muscle dysfunction caused by obesity [154]. Thus, RR and its extracts may attenuate cardiomyocyte damage in patients with obesity by increasing the antioxidant capacity of cardiomyocytes. Additionally, this plant has been shown to exhibit lipid-lowering properties and may reduce ischaemia–reperfusion injury [155]. These studies suggest the potential of RR and its extracts for combating obesity and related diseases. Table 1 provides a summary of the studies on RR and its related components for combating obesity.
Table 1.
Studies on the effects of Rhodiola rosea on obesity
| Model | Interventional components | Disease model | Bridge | Results | References |
|---|---|---|---|---|---|
| 3T3-L1 cells | Phenolic tyrosol | The pentose phosphate pathway (PPP) disrupts proline-mediated energy generation and endogenous antioxidant enzyme response (AER) | SOD↑, PDH↓, G6PDH↓, ROS↓, lipogenesis↓, lipid accumulation↓ | [156] | |
| Foodborne obese mice | Salidroside | Intestinal dysfunction | Regulating gut microbiota and increasing the abundance of gut microbiota | mucin-2↑, Occludin↑, Zonula Occludens-1↑, mouse weight ↓, fat accumulation ↓, genes related to lipid synthesis ↓, liver inflammation ↓, intestinal damage ↓ | [157] |
| Obese mice on a high-fat diet | Salidroside | Muscle dysfunction | SIRT1 pathway | Mitochondrial quality control ↑, atrophy factor ↓ | [156] |
| Foodborne obese rats | Rhodiola rosea | Central monoamine pathway | White adipose tissue in the viscera ↓, norepinephrine in the hypothalamus ↑ | [158] | |
| High-fat diet mice | Rhodiola rosea | Non-alcoholic fatty liver disease | AMPK-dependent TXNIP/NLRP3 pathway | Insulin sensitivity ↑, obesity ↓, blood glucose variability ↓, liver lipid deposition ↓, oxidative stress ↓, TXNIP ↓, NLRP3 inflammasome activation ↓ | [159] |
| Obese mice | Salidroside | Diabetes |
Fat generation and inflammation in eWAT Stimulating leptin signaling in the hypothalamus |
Leptin signal transduction in the hypothalamus ↑, hyperglycemia ↓, gluconeogenesis ↓, G6Pase ↓, PGC-1 α ↓, Fat generation in eWAT ↓. Macrophage infiltration in eWAT ↓, pro-inflammatory cytokines ↓ | [160] |
SOD superoxide dismutase, PDH pyruvate dehydrogenase complex, G6PDH glucose-6-phosphate dehydrogenase, ROS reactive oxygen species, TXNIP thioredoxin-interacting protein, NLRP3 NOD-like receptor thermal protein domain associated protein 3, PGC-1 α peroxisome proliferators-activated receptor γ coactivator l alpha
Protective effect of Rhodiola rosea on the liver
The liver is the central hub of the body’s metabolism and plays crucial roles in regulating sugar metabolism and blood circulation, providing heat, and organising the body’s defence functions. Humans cannot survive without a functional liver. Liver-related diseases rank among the leading causes of disease incidence and death worldwide. Epidemiological surveys show that, globally, two million people die annually from liver disease, accounting for 4% of all deaths. Cirrhosis, hepatocellular carcinoma, and related complications are the most common causes of death [161]. Age plays an essential role in the development of chronic liver diseases. Therefore, the daily protection of liver function (especially in older adults with poor liver function) is a requirement for PPPM.
Reportedly, phosphorylated mlkl, ripk3, and ripk1 associated with programmed necrosis and apoptosis were significantly higher in the liver of aged rats than in young rats. Programmed necrosis and apoptosis were proportional to age. With age, programmed necrosis and apoptosis mechanisms were activated in hepatocytes and intrahepatic macrophages, and M1 macrophages were activated to produce TNFα, IL-6, and IL-1β, and increase the risk of liver fibrosis and other chronic liver diseases in the rats [162]. Additionally, oxidative stress and inflammatory responses increase with increasing age. The production of large amounts of cytokines during inflammatory response can increase mitochondrial membrane permeability and hepatic mitochondrial dysfunction, decrease antioxidant capacity, and increase the generation of ROS. The increase in mitochondrial membrane permeability exacerbates the cellular damage caused by ROS and other free radicals, increases oxidative damage to DNA fragments, accelerates telomere shortening, inhibits the repair and regeneration of damaged hepatocytes, and increases hepatic fibre scarring [163]. During ageing, the antioxidant capacity of the liver is diminished due to increased glutaminase levels, a decreased glutamine level in hepatocytes, and a decline in raw material for glutathione (GSH) synthesis [164]. Excess ROS acts on the nucleus, leading to increased mRNA expression of genes related to gluconeogenesis and cholesterol synthesis, glucose transporter type 2 (Glut2), glucokinase (GK), and sterol regulatory element-binding protein (SREBP)-2, 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), and an increase in mitochondrial glucose uptake and acetyl coenzyme A production. Consequently, glycolysis and lipid synthesis increase, and hepatocyte energy metabolism is abnormal [165]. During the ageing process, disturbances in the intestinal flora and decreased intestinal barrier function lead to the entry of intestinal endotoxins into the hepatic circulation via the mesenteric venous reflux system, which increases the levels of bacterial endotoxins and lipopolysaccharide-binding proteins (LBPs) in the hepatic bloodstream, increases the expression of TLR-1, TLR-2, TLR-4, TLR-6, and TLR-9 mRNAs in the hepatocytes, activates relevant inflammatory signalling pathways, and initiates a cascade of hepatic inflammatory signalling. This increases the levels of endotoxin-induced liver injury, mitochondrial dysfunction, insulin resistance, and hepatocyte apoptosis and further accelerates the inflammation and fibrosis associated with hepatic ageing [166, 167].
Pathological changes, such as age-related hepatic steatosis and ROS-induced cellular stress, can be reversed by modulating hepatocyte autophagy and promoting hepatocyte proliferation. Although autophagy can alleviate the inflammatory response, it exacerbates hepatic fibrosis when inflammation fails to resolve [168]. The induction of autophagy through mTOR-dependent pathways can severely impair liver regeneration. Conversely, recent observations suggest that the induction of autophagy via mTOR-independent pathways may help promote liver regeneration [169]. RR has been shown to inhibit inflammatory responses in the serum and liver tissues in vivo, which may inhibit hepatocyte apoptosis and autophagy, promote hepatocyte regeneration, and reduce hepatic fibrosis, mainly by inhibiting M1 macrophage activation through the Notch signalling pathway [33]. Additionally, RR reduces the release of inflammatory cytokines and activates the PI3K/AKT signalling pathway [170]. In contrast to the inhibitions above, RR extract increased the expression of SIRT1mRNA, PPARα, and PGC-1α proteins in fatty liver disease. Simultaneously, it increased the expression of autophagy markers and the autophagy lysosomal marker p62 and reduced cellular steatosis [171]. RR can reduce hepatic lipid accumulation in non-alcoholic fatty liver disease (NAFLD) by promoting the macrophage MIF pathway, downstream lipophagy, and lipid metabolism [172]. RR has a benign bidirectional modulation of autophagy in different chronic liver diseases, which can be multi-targeted to exert therapeutic effects and has important applications in preventive and personalised medicine frameworks for predicting the prevention and treatment of chronic liver diseases during the ageing process (Table 2).
Table 2.
Studies on the hepatoprotective effects of Rhodiola rosea
| Model | Interventional components | Study design/experimental model | Bridge | Results | References |
|---|---|---|---|---|---|
| Vitro research and CCl-treated mice | RRP1: It is composed of mannose, rhamnose, galacturonic acid, glucose, galactose, and arabinose | Toxic chemical induced liver injury | Oxidative stress | GSH↑, SOD↑, GAT↑, DPPH ↓, hydroxyl ↓, superoxide anion radicals↓, ALT↓, AST↓, MDA↓ | [173] |
| Vitro study and high-altitude hypoxia animal model | Salidroside (Sal) | High-altitude hypoxia induced liver injury | Nrf2 and JAK2/STAT3 signaling pathways | IL-1β ↓, TNF-α ↓, IL-6 ↓, MCP-1 ↓, ROS↓, MDA↓, SOD↑, CAT↑, GSH-Px↑, Nrf2 ↑, HO-1 ↑, NQO-1↑ | [174] |
GSH L-glutathione, GAT gamma glutamyl transferase, DPPH 1,1-diphenyl-2-picryl-hydrazyl radical, ALT alanine aminotransferase, AST aspartate transaminase, MDA malondialdehyde, IL-1β interleukin-1β, TNF-α tumour necrosis factor-α, IL-6 interleukin-6, MCP-1 monocyte chemoattractant protein-1, CAT catalase, GSH-Px glutathione peroxidase, Nrf2 nuclear respiratory factor 2, HO-1 Heme Oxygenase-1, NQO-1 NAD(P)H:quinone oxidoreductase 1, STAT3 signal transducer and activator of transcription 3, JAK2 Janus kinase-2
Antitumour effects of Rhodiola rosea
Malignant tumours are a common challenge for the medical community owing to their unclear mechanisms and high mortality rates. The American Cancer Society estimated the number of new cancer cases and deaths in the USA in 2022 to be 19,180,030 and 60,936,060, respectively, of which approximately 350 people are estimated to die of cancer daily [175]. Targeted cancer control interventions and early investment in treatment will help reduce cancer-related mortality. Phytochemicals are recommended for use in the tertiary treatment of cancer owing to their ability to exert therapeutic effects on tumours via multiple targets, improving frailty following conventional cancer treatment and improving patient tolerance to anticancer drugs [176, 177]. RR and its compounds have been widely demonstrated to exert antitumour effects. It inhibits tumour cell proliferation and metastasis by modulating the mir-103-3p/mzb1 signalling pathway [178]. Moreover, it is rich in flavonoids, which have been reported to inhibit the Warburg effect in the treatment of tumours by stimulating the reprogramming of energy metabolism in the tumour environment through the M2 isoform of pyruvate kinase (PKM2), human kallikrein 2(HK2), Glut1, and hypoxia-inducible factor-1 (HIF-1) regulatory sites, improving the energy metabolism of normal cells, blocking the energy supply of cancerous cells, and promoting tumour cell death [179, 180]. Its antitumour advantage may not only involve multiple pathways and targets within tumour cells, but may also exhibit the opposite effect in certain normal and tumour cells, ultimately exerting an anti-tumour effect [181]. In normal tissues, RR extract activates the mTOR pathway, inhibits the expression of phosphohydroxylase 3 (PHD3), and promotes HIF-1α. It reduces vascular damage, protects against angiogenesis, and provides adequate oxygen and energy to normal tissues [54]. It also has bi-directional regulatory effects: in cancer, its extract can inhibit the mTOR pathway by downregulating HIF-1α/HIF-2α to reduce angiogenesis; it also inhibits the JAK2/STAT3 signalling pathway, which inhibits the activity of matrix metalloproteinases (MMPs), and inhibits angiogenesis, migration, and growth of tumour cells [182]. In normal tissues, RR extract activates the mTOR pathway, inhibits the expression of PHD3, and promotes HIF-1α. It reduces vascular damage, protects normal angiogenesis, and provides adequate oxygen and energy to normal tissues [182]. However, the mechanism by which it exerts diametrically opposing effects in these two environments is unclear. Rhodioloside is water-soluble, and after oral administration, its final metabolite accumulates in the urine and is excreted by the kidneys; therefore, using Rhodioloside in the diagnosis and treatment of bladder cancer can inhibit the mTOR pathway and downregulate the expression of HIF-1α/HIF-2α to reduce angiogenesis, thereby alleviating the bladder pain experienced by patients with end-stage bladder cancer [182]. RR glycosides have great potential for clinical use as palliative therapies for patients with end-stage bladder cancer. It can also reduce the adverse effects of radiotherapy and chemotherapy in patients with cancer and enhance therapeutic effects. Skin damage is a major complication of cancer radiotherapy, and RR extract can selectively protect normal skin cells from ionising radiation and reduce radiotherapy-associated complications [183] (Table 3).
Table 3.
Role of Rhodiola rosea in different malignant tumours
| Disease | Study design/experimental model | Signaling pathways/mechanism of action | Results | Reference |
|---|---|---|---|---|
| Lung cancer | Lewis lung cancer-bearing mice were randomly divided into the normal saline group, 500 mg/kg RRL ethanol extract treatment group, and 10 mg/kg cyclophosphamide (CTX) treatment group. All the groups underwent the treatment for 10 days | Has a positive role in enhancing the anti-tumour immunity by regulating the number and function of immunocytes | CD4 + T↑, CD8 + T↑, CD4 + /CD25 + T↓, IFN-γ↑, IL-2↑, killing capacity of spleen CTL ↑ | [184] |
| Lung cancer | H1975 cells | Responsible for the synergy among anti-inflammatory, anti-angiogenic, and pro-apoptotic | VEGF↓, eNOS↓, COX-2↓, iNOS↓, TNF-α↓, Bcl-2↓, Bax↑, apoptosis ↑ | [185] |
| Glioblastoma multiforme (GBM) | Human U87 GBM cells were treated with 200 μg/mL of R crenulata or vehicle control | Wnt/β-catenin | Induced glial fibrillary acidic protein ↓, cadherin ↓, Wnt promoter activity↓, β-catenin↓, proliferation↓, differentiation↑ | [186] |
| Breast cancer | MCF7 breast cancer cell | Estrogen receptor | β-catenin↓, ER responses ↑, proliferation↓, tumour sphere formation↓ | [187] |
| Gastric cancer | The human gastric cancer cell line, BGC-823 | EMT/Src (its effects on EMT via Src-associated signaling pathways) | Src↓, Akt↓, STAT3↓, ERK↓, FAK↓, E-cadherin↑ | [188] |
| Gastric cancer | Human gastric cancer AGS cells | The PI3K/Akt/mTOR pathway | Apoptosis↑, autophagy↑, PI3K↓, Akt ↓, IGF-1↓ | [189] |
| Gastric cancer | SGC-7901 and MKN-45 human gastric cancer cells | ENO1/PKM2/GLUT1 | Apoptosis↑, proliferation↓, nuclear fragmentation↑, glycolysis↓, PKM2↓, ENO1↓, GLUT1↓ | [180] |
| Colorectal cancer (CRC) | CRC cells, HCT-116, were incubated with Sal alone or in combination with conventional chemotherapy agents including oxaliplatin (OXA), 5-fluorouracil (5-FU), and doxorubicin (ADM) | AMPK/mTOR, NF-κB, TGFβ1, JAK2/STAT3 | LC3↑, Becline-1↑, p-AMPK↑, p-mTOR↓, p-NF-κB(p65) ↓, TGF-β↓, p-JAK2↓, p-STAT3↓ | [190] |
| Human chondrosarcoma | SW1353 cells | ROS signaling pathways | Apoptosis↑, TFEB (Ser142) dephosphorylation↑, LC3-II↑, P62↓, TFEB nuclear translocation ↑, TFEB reporter activity↑, lysosomal biogenesis↑, the expression of autophagy-related genes↑, ROS↑ | [191] |
| Hepatocellular carcinoma | Highly metastatic hepatocellular carcinoma (HCC) cells (MHCC97H) | Inhibits HCC cell metastasis by modulating the activity of the Notch1 signaling pathway | Notch1↓, Snail↓, COX-2↓, MMP-2↓, MMP-9↓, cadherin↑, Hey1↓, Hes1↓, Hes5↓ | [192] |
CD4 + cluster of differentiation 4 receptors, IFN-γ interferon-γ, IL-2 interleukin-2, CTL cytotoxic t lymphocyte, VEGF vascular endothelial growth factor, eNOS endothelial nitric oxide synthase, COX-2 cyclooxygenase-2, iNOS inducible nitric oxide synthase, Bcl-2 B-cell lymphoma-2, Bax BCL2-associated X, ERK extracellular regulated protein kinases, FAK focal adhesion kinase, PI3K phosphoinositide 3-kinase, Akt also known as protein kinase B or PKB, IGF-1 insulin-like growth factor 1, ENO1 alpha enolase, LC3 Microtubule-Associated Protein 1 Light Chain 3, AMPK adenosine 5′-monophosphate (AMP)-activated protein kinase, mTOR mammalian target of rapamycin, NF-κB nuclear factor kappa-B, TGF-β transforming growth factor beta, TFEB transcription factor EB, COX-2 cyclooxygenase-2, MMP-2 matrix metalloproteinase 2, MMP-9 matrix metalloproteinase 9, Hey1 Hes Related Family BHLH Transcription Factor With YRPW Motif 1, Hes1 hairy and enhancer of split 1, Hes5 hairy and enhancer of split 5
Improvement of lung function in patients with lung disease by Rhodiola rosea
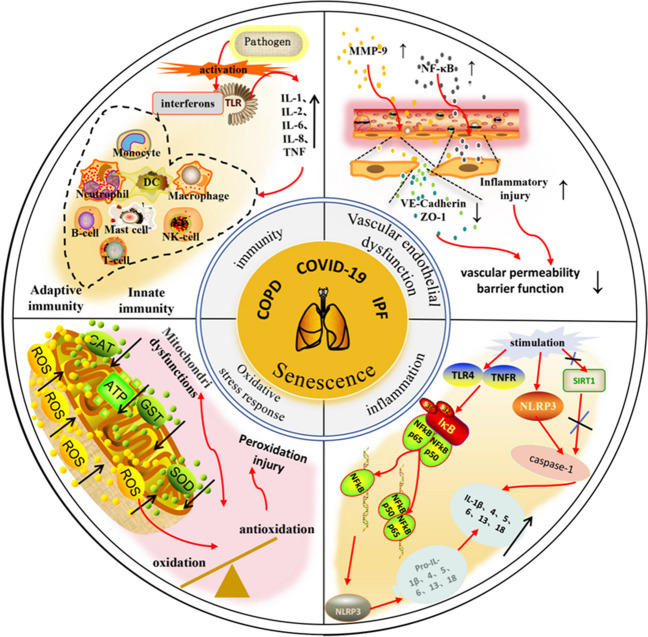
Age-related immune dysregulation and decreased anti-stress capacity accompanying the ageing process can lead to immune ageing and sustained low-grade inflammation. This accelerates the deterioration of lung function in patients with idiopathic pulmonary fibrosis (IPF), chronic obstructive pulmonary disease (COPD), COVID-19, and other conditions [193, 194]. Age increases the risk of mortality in most patients with lung diseases. As in the case of the COVID-19 global epidemic, older patients over 65 years of age had the highest probability of progressing to severe disease and mortality compared to younger patients, and although the molecular mechanisms underlying these epidemiological findings are not well understood, it has been demonstrated that the damage caused by neocoronary pneumonia in older patients can be reduced by improving the ageing state [195]. The prevalence of COPD is directly proportional to age, and COPD treatment approaches have not effectively improved in modern medical research, except for smoking cessation. Experts have suggested that cellular and molecular signalling pathways associated with ageing may become new targets for COPD treatment [196]. IPF also lacks effective treatment at present, except for relief during the symptomatic phase of the disease. Repeated episodes of lung diseases are prone to drug resistance during conventional treatment. RR, as an adaptogen, is used in the treatment of both acute and chronic lung diseases to mitigate the risk of drug resistance. Moreover, when used with conventional therapies, RR can enhance treatment efficacy. Therefore, using RR preparations for treating various acute and chronic lung diseases is consistent with the concept of PPPM/3P medicine [197]. This herb improves tidal breathing and ventilation efficiency during incremental exercises and reduces the incidence of acute exacerbations in patients with COPD [198]. RR glycosides inhibit matrix MMP-9 expression during pulmonary ventilation dysfunction, upregulate vascular endothelial calreticulin (VE calreticulin) and tight junction protein ZO-1 expression, and ameliorate pulmonary endothelial barrier dysfunction, as well as reduce inflammatory damage in lung tissues through inhibition of the NF-κB signalling pathway [199]. The active ingredients from RR significantly inhibited the NLRP3 inflammasome and its associated caspase-1 cleavage and IL-1 activation in mechanical ventilation-induced lung injury by upregulating the expression of SIRT1β. The plant extract also downregulated the expression of mir-323-3p in asthmatic mice and reduced the release of IgE, IL-13, IL-4, IL-5, COL-I, and COL-III. Reducing the resistance index (RI) of airway hyperresponsiveness ultimately reduces airway inflammation and remodelling [200]. Acute exacerbation of COVID-19 is a fatal acute lung injury that manifests as excessive lung inflammation. RR extracts inhibit the production of inflammatory cytokines such as TNFα and IL-6, which play a role in suppressing the inflammatory response, ultimately slowing it down and reducing acute lung injury. RR acts as a natural adaptogen and plays a positive role across all phases of COVID-19, including the modulation of immunity (both innate and adaptive), anti-inflammatory effects, antioxidant effects, inhibition of viral docking or replication, and improvement of the quality of life during recovery [201]. Clinical evidence suggests that the RR complex is effective in reducing the duration of fatigue and chronic pain in patients with COVID-19 and preventing renal failure by modulating creatinine metabolism [202]. It can protect lung function and enhance immunity, thus proving to be an attractive potential therapeutic option for symptomatic relief, prevention of severe infections, and reduction of mortality in neocoronary arthritis, ultimately improving health (Fig. 5).
Fig. 5.
Signalling pathways potentially involved in Rhodiola rosea treatment of various lung diseases. Created with WPS Office and scienceslides 2016
Neuroprotective effects of Rhodiola rosea
Neurodegenerative diseases often occur in the older adult population and are a major cause of disease and cognitive impairment in old age, with their incidence being directly proportional to age [203]. Their causative factors include changes in the natural environment, such as global warming and oceans contaminated with nuclear effluents, in addition to age and social factors, which contribute to the development of neurodegenerative disease [204]. Alzheimer’s disease is an age-related neurodegenerative disease characterised by excessive sedimentation of amyloid-β and its typical pathology is characterised by metabolic disturbances in nerve cells, followed by cell damage and apoptosis. It has been suggested that the decline in the expression level of the rbfox1 gene in the nucleus as individuals age contributes to elevated amyloid-β accumulation in the brain, thereby exacerbating amyloidosis and sustaining cognitive decline [205]. Currently, the clinical efficacy of treatment for Alzheimer’s disease is limited and easily hampered by adverse effects, making its widespread clinical application difficult [206]. Therefore, targeted prevention of Alzheimer’s disease and the search for therapeutic drugs with fewer adverse effects to enhance the well-being of older adults are requirements for 3PM medicine. RR has been demonstrated to increase the adverse effects of antioxidants such as GSH and reverse amyloid-β peptide content on neuronal cells by improving glutathione and arachidonate metabolisms in the brain neurons of older adult patients [207]. RR also upregulates the expression level of nicotinamide phosphate (Nampt) protein through the Nampt signalling pathway to increase the synthesis of NAD + and the increase of NAD + /nicotinamide adenine dinucleotide hydrogenation ratio. These mechanisms confer protective effects on neuronal cells [208]. This herb can reduce soluble and insoluble amyloid- β and increase the expression of PSD95, NMDAR1, and calmodulin-dependent protein kinase II by upregulating the phosphatidylinositide PI3K/AKT/mTOR signalling pathway in a rat model of Alzheimer’s disease, thereby exerting a protective effect on neurons and damaged synapses in mice [209]. Certain important components of RR can inhibit mitochondrial damage and apoptosis by inducing the early apoptotic pathway; modulating the PKC/ERK signalling pathway; increasing the protein expression levels of PKC, BCL-2, and phosphorylated ERK1/2; and decreasing the expression of Bax, Cyt-C, and cleaved caspase-3 proteins to protect the normal energy metabolism of neuronal cells and reduce nerve damage caused by mitochondrial dysfunction during ageing [33]. Additionally, RR ameliorates brain damage in patients with ischaemic stroke. Besides, it enhances the antioxidant capacity of neuronal cells, reduces ROS production, decreases mitochondrial permeability, and ameliorates mitochondrial and cellular damage. It activates the PINK1 parkin signalling pathway, increases mitophagy in the mitochondria, promotes apoptosis of damaged cells, maintains the integrity of the blood–brain barrier, and reduces ischaemia–reperfusion injury in the nervous system [210, 211]. A randomised, double-blind, controlled trial (ClinicalTrials.gov identifier: NCT03262376) revealed that RR extracts exhibit significant clinical advantages by positively modulating brain neural activity and improving body stress resistance [212].
Renal fibrosis reversal by Rhodiola rosea
Renal fibrosis develops in many chronic kidney diseases (CKD) and affects the prognosis of patients with renal disease and their disease-related healthcare expenditure; however, the current clinically available strategies focus on improving or slowing the progression of CKD rather than reversing renal fibrosis [213]. Older adult individuals are at a higher risk of developing renal fibrosis than younger ones. Renal fatty acid oxidation (FAO) pathway, mediated by the PPAR signalling pathway, plays a vital role in renal fibrosis [214]. As mentioned previously, ageing is often accompanied by mitochondrial dysfunction. During ageing, upregulation of Wnt/β-strand protein signalling and renin-angiotensin system (RAS) activity also decreases mitochondrial mass and function, exacerbating renal cell damage and favouring the progression of renal fibrosis [215]. RR and its related components have been shown to improve mitochondrial dysfunction, attenuate renal cell injury through multiple pathways and targets, and have significant clinical potential for reversing renal fibrosis. During the treatment of diabetic nephropathy, RR glycosides can enhance the expression levels of mitochondrial DNA copy and electron transfer chain proteins mediated by the SIRT1/PGC-1α signalling pathway, upregulate the expression of nephrin and podoplanin, and significantly reduce related renal injury indices such as urinary albumin, blood urea nitrogen, and serum creatinine. These actions can improve the structure of the kidneys and prevent and reverse the progression of diabetic nephropathy to renal fibrosis [12]. RR inhibits the TLR4/NF-κB/MAPK signalling pathway to downregulate α-SMA, waveform protein, and TGF-β, reverses renal tubular injury and deposition of extracellular matrix (ECM) components (including collagen III and collagen I), improves renal function, and reverses interstitial fibrosis [216]. Much more needs to be done to prevent and treat renal fibrosis using RR, from laboratory research to clinical application.
Current status and prospects of health management strategies using Rhodiola rosea
Rhodiola rosea has been used in traditional medicines for thousands of years. RR can exhibit a wide range of antioxidant, anti-inflammatory, anti-apoptotic, anti-fatigue, anti-stress, vasculoprotective, and immunomodulatory effects [27, 217]. Despite their extensive history of use and notable clinical efficacy, RR and other herbal medicines have gradually been overlooked with the advancement of modern or traditional Western medicine. This is partly due to the complexity of their compositions, the absence of rigorous and high-quality modern clinical trials to verify their effectiveness, and the lack of improved clinical safety monitoring and enhancement of quality control systems for herbal medicines [218, 219]. Recently, as the drawbacks of the traditional reactive medicine paradigm have continued to emerge, the potential application of RR in the new medical paradigm of 3PM for human health has been highlighted. The bioactive substances from this herb can play a role in improving mitochondrial function, reducing telomere depletion, improving haemodynamics and angiogenesis, modulating the inflammatory response, improving immune responsiveness and other pathways, enhancing the function of multiple systems (circulatory, neurological, immune, digestive, urinary, and respiratory), decreasing the damage of radiotherapeutic agents on normal tissues of the human body, and decreasing adverse reactions. Moreover, RR exhibits low toxicity and side effects and is easily and readily available for pre- and post-clinical use at a low cost. Nevertheless, the clinical application of RR and its related active ingredients remains challenging. Currently, animal and in vitro experiments are the mainstay, and there is limited evidence of the clinical application of single drugs, a lack of dosage standards for clinical application, and insufficient evidence for high-quality randomised clinical trials [220]. However, this does not stop the ongoing research into its potential benefits for overall human health. With the advancement of modern advanced pharmacological research techniques, such as computer-aided drug design, network pharmacology, phage display, affinity fishing, drug affinity response target stability, cellular thermal displacement assays [221], and the development of advanced nanodrug release systems [222], a large number of preclinical studies have demonstrated that RR, as a prophylactic or therapeutic agent, is important in driving health management strategies from the traditional medicine paradigm to the 3PM medicine paradigm. This transition is poised to exert greater influence than previously anticipated. Here, we propose relevant signalling pathways and the active ingredients (mainly RR glycosides) acting on the organism within the medical framework of PPPM/3PM to provide ideas for future health management strategies.
Conclusions and expert recommendations within the framework of 3PM
Primary prevention is the first step towards transitioning from reactive to predictive, preventive, and personalised disease management paradigms
The rapid progress of science and technology in our modern society has led to global advancement. However, the heterogeneous and uncontrollable states of an individual’s health are undeniable realities [46], and numerous negative factors in the natural and social environment affect human health, including increased pressure for survival, global warming, and the danger of nuclear radiation [223]. Genomics can help TCM build a stronger evidence-based practice. These advancements can be tailored to closely align with real-world personalised healthcare needs across global health landscapes, encompassing developing and developed countries [10]. A decade ago, China actively participated in a new initiative, the Human Glycome Project (HGP) (https://human-glycome.org), which is currently progressing at a rapid pace [224]. HGP promotes the development of personalised medicine and lays the foundation for personalised approaches within TCM. China boasts a rich civilization spanning 5000 years and TCM stands as one of its most prestigious medical heritages, with over 2 millennia of clinical practices. The latest research reveals the general TCM treatment principle as the topological relationship between disease symptoms and TCM herb targets on the human protein interactome [225]. This reaffirms the scientific foundation of TCM. Since ancient times, TCM has identified the physical status of health and disease, which we now term suboptimal health, and several attempts have been made to address this condition [46]. The first level of intervention is the prevention of the onset of the disease, which is aimed at targeting those individuals who have not been clinically diagnosed with the disease (including those in a state of suboptimal health or those in high-risk environments). Even before the disease is diagnosed, the body may already be undergoing pathological processes, such as oxidative stress, inflammatory response, immune dysregulation, and flora disorders. RR is a natural herbal nutritional plant with potent antioxidant, anti-inflammatory, intestinal flora-regulating, and immunomodulatory effects; low adverse and toxic side effects, and a high safety index for human application. Primary prevention requires cultivating a good behavioural lifestyle that includes reasonable nutritional intake. RR is a natural health food product that can replenish one’s energy, make the body lighter and more harmonious, and improve work efficiency [49]. Individuals at a high risk of disease RR have the potential to increase the body’s ability to fight diseases and reduce the risk of disease. RR, as an adaptogen, can enhance the radioprotective effects of Ex-RAD by increasing its antioxidant effects and ROS-scavenging potentials, accelerating haematopoietic recovery and DNA repair, and modulating apoptosis and signalling pathway repair [226]. The process of disruption from a healthy equilibrium state of the organism to disease onset is, often reversible, presenting a window of opportunity for early intervention between the pre-disease state, in which the equilibrium state is disrupted, and tissue and organ dysfunction occur. Personalised nutrition can effectively prevent the onset of disease. Primary prevention seizes the initial opportunity to prevent disease onset and marks the beginning of the paradigm shift from reactive to predictive, preventive, and personalised disease management [227, 228]. RR has been proven to have enormous potential for disease prevention and treatment at the laboratory level; however, cohort studies on its use are insufficient. We hope to address this issue in our future studies. Personalised parameters, such as genes, proteins, gut microbiota, routine blood tests, liver function, and individual symptoms, can be used to evaluate an individual’s health status and determine whether preventive strategies should be applied.
Secondary prevention is an effective strategy to control the development and deterioration of diseases
Secondary prevention focuses on achieving the “three earlies”: early detection, early diagnosis, and early treatment at the onset of the disease, which is crucial for optimising the initial diagnosis and treatment plan and preventing disease deterioration. TCM focuses on health maintenance, enhancing the body’s resistance to diseases, and has significant advantages in early intervention, personalised treatment, and combination therapies. Systems biology, an emerging science of the twenty-first century, bears striking resemblance to TCM in many aspects, such as study method and design. It represents a current key technological component that serves as the primary driving force for translating the personalised medicine revolution of TCM principles into practice and will advance personalised therapy principles into healthcare management tools for both individuals and populations [229]. The development of systems biology has also driven personalised research on RR. In the content of anti-ageing research, RR exerts an oestrogen-like role in the treatment of menopausal syndromes to prevent and treat cognitive, psychological, cardiovascular, and osteoporotic conditions related to menopause, which can prevent the development and deterioration of diseases caused by the decline in hormone levels during the ageing process in menopausal women. Moreover, as a purely botanical preparation, RR is a superior treatment option to hormonal drugs [230]. Rosavin, an RR extract, attenuates disease progression, inhibits hepatocyte death, and prevents the progression of NAFLD to liver fibrosis [43]. Medical ethics require that the optimal treatment plan be given to each patient, and the integration of RR in disease treatment facilitates the personalisation of disease control. Utilizing personalised parameters, such as genetic profiles, protein expression, gut microbiota composition, routine blood checks, liver function assessment, and individual symptoms, enables the evaluation of an individual’s health status and determines whether preventive strategies should be implemented. Developing genetic and glycomic parameters will provide new strategies for personalised RR treatment. The development of various neurotransmitters has enabled the precise use of RR [60–63].
Enhancing the quality of life and reducing the side effects and complications of conventional therapies of the affected population through tertiary care interventions
Tertiary care can help reduce the side effects and complications of medications and ultimately improve patients’ quality of life. Syndrome differentiation (Bian Zheng) in TCM is a comprehensive analysis of clinical information gained through four primary diagnostic TCM procedures: observation, listening, questioning, and pulse analysis, which can guide the choice of treatments. The same disease type may be classified into different syndrome types, leading to variations in the medications administered. Additionally, the same individual may exhibit different syndromes and medications at various disease stages. This diagnosis and treatment approach represents an early application of personalised medicine in humans. Dialectical treatment with TCM could achieve personalised clinical treatment with RR. As a personalised herbal supplement, RR can effectively address the inevitable side effects in patients requiring lifelong medication. Nutritional interventions encompass individual provisions. For example, in the management of traditional type 2 diabetes mellitus, drugs such as metformin and acarbose are mainly used to regulate the blood glucose levels of patients; however, these drugs not only manage the disease but also trigger drug-related discomfort by interfering with the intestinal microbiome and its related metabolic pathways [231]. Conversely, RR may improve fasting blood glucose levels by regulating the gut microbiota and maintaining the integrity of the intestinal barrier while reducing or even avoiding the side effects of conventional hypoglycaemic drugs [232]. In patients with cancer, RR combined with conventional anti-tumour treatments can effectively induce tumour cell apoptosis, regulate immune cell function, and reduce the possibility of adverse reactions to traditional antitumour therapies, such as radiotherapy and chemotherapy [182]. The synergistic effect with modern medicine maximises the therapeutic effect and improves the quality of patient survival. Moreover, advancements in various drug delivery transporters offer possible avenues for the personalised application of RR [233, 234]. RR intervenes in the progression of cardiovascular and neurodegenerative diseases, diabetes, sepsis, and cancer through various targets and pathways. The development of traditional medicine’s syndrome differentiation and treatment, as well as modern medicine’s gene technology, glycomic metabolism technology, network pharmacology, and new drug carriers, has increasingly revealed the potential of TCM in personalised medication while providing possibilities for the personalised application of RR. As a complementary and food-based therapy, RR can effectively compensate for the shortcomings and side effects of conventional reactive medicine [27]. While animal and cellular experiments with clinical experience suggest that Rhodiola has prospects for broad application in personalised medical frameworks, high-quality clinical cohort studies are currently lacking, and the study conditions we faced have many challenges including insufficient sample size, potential biases, inaccessible/limited data. Therefore, future efforts should focus on conducting high-quality clinical cohort studies to provide evidence-based findings for the application of RR in the field of 3PM. With these advancements, we believe that RR will achieve personalised applications in preventive medicine in the near future.
Author contributions
All authors contributed to the review conception and design. The frst draft of the manuscript was written by X.W.,Y. T.,Z. J..L.H. and G.M. corrected the versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Natural Science Foundation of China (grant no. 81904161), Henan Province Key Specialty for the Treatment of Major Diseases (grant no. CZ0271-04), and the National Key Specialty Construction Project of Traditional Chinese Medicine (TCM).
Data availability
No datasets were generated or analysed during the current study.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Wenqian Xu and Heguo Li are considered as the first authors of this article, and their contributions are the same.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhattacharya S, Heidler P, Varshney S. Incorporating neglected non-communicable diseases into the national health program-a review. Front Public Health. 2023;10(10):1093170. doi: 10.3389/fpubh.2022.1093170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Wang J. Modelling and prediction of global non-communicable diseases. BMC Public Health. 2020;20:1–13. doi: 10.1186/s12889-020-08890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kazakov SD, Kamenskikh EM, Sokolova TS, Fedorova OS. [The present-day epidemiology: challenges of public health and possibilities to settle them: the publications review]. Probl Sotsialnoi Gig Zdravookhranenniiai Istor Med. 202331(3):368–78. Russian. 10.32687/0869-866X-2023-31-3-368-378. [DOI] [PubMed]
- 4.Crisóstomo L, Oliveira PF, Alves MG. Antioxidants, oxidative stress, and non-communicable diseases. Antioxidants (Basel) 2022;11(6):1080. doi: 10.3390/antiox11061080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong G, Logue SE. Unfolding the interactions between endoplasmic reticulum stress and oxidative stress. Antioxidants (Basel) 2023;12(5):981. doi: 10.3390/antiox12050981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng J, Yang K, Nie H, Yuan L, Wang S, Zeng L, Ge A, Ge J. The mechanism of intestinal microbiota regulating immunity and inflammation in ischemic stroke and the role of natural botanical active ingredients in regulating intestinal microbiota: a review. Biomed Pharmacother. 2023;157:114026. doi: 10.1016/j.biopha.2022.114026. [DOI] [PubMed] [Google Scholar]
- 7.Sun D, Xiang H, Yan J, He L. Intestinal microbiota: a promising therapeutic target for hypertension. Front Cardiovasc Med. 2022;15(9):970036. doi: 10.3389/fcvm.2022.970036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao X, Tan X, Shi H, Xia D. Nutrition and traditional Chinese medicine (TCM): a system's theoretical perspective. Eur J Clin Nutr. 2021;75(2):267–73. doi: 10.1038/s41430-020-00737-w. [DOI] [PubMed] [Google Scholar]
- 9.Isaak CK, Siow YL. The evolution of nutrition research. Can J Physiol Pharmacol. 2013;91(4):257–67. doi: 10.1139/cjpp-2012-0367. [DOI] [PubMed] [Google Scholar]
- 10.Yun H, Hou L, Song M, Wang Y, Zakus D, Wu L, Wang W. Genomics and traditional Chinese medicine: a new driver for novel molecular-targeted personalized medicine? Curr Pharmacogenomics Pers Med. 2012;10(1):16–21. doi: 10.2174/1875692111201010016. [DOI] [Google Scholar]
- 11.Khanum F, Bawa AS, Singh B. Rhodiola rosea: a versatile adaptogen. Compr Rev Food Sci Food Saf. 2005;4(3):55–62. doi: 10.1111/j.1541-4337.2005.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Q, Yin ZP, Ma L, Zhao W, Hao HW, Li HL. Free radical-scavenging activities of oligomeric proanthocyanidin from Rhodiola rosea L. and its antioxidant effects in vivo. Nat Prod Res. 2014;28(24):2301–3. 10.1080/14786419.2014.921786. [DOI] [PubMed]
- 13.Parisi A, Tranchita E, Duranti G, Ciminelli E, Quaranta F, Ceci R, Cerulli C, Borrione P, Sabatini S. Effects of chronic Rhodiola rosea supplementation on sport performance and antioxidant capacity in trained male: preliminary results. J Sports Med Phys Fitness. 2010;50(1):57–63. [PubMed] [Google Scholar]
- 14.Bang VMJ, Aranão ALC, Nogueira BZ, Araújo AC, Bueno PCDS, Barbalho SM, de Souza MDSS, Guiguer EL. Effects of Rhodiola rosea and Panax ginseng on the metabolic parameters of rats submitted to swimming. J Med Food. 2019;22(10):1087–90. doi: 10.1089/jmf.2019.0062. [DOI] [PubMed] [Google Scholar]
- 15.Loo WT, Jin LJ, Chow LW, Cheung MN, Wang M. Rhodiola algida improves chemotherapy-induced oral mucositis in breast cancer patients. Expert Opin Investig Drugs. 2010;19(Suppl 1):S91–100. doi: 10.1517/13543781003727057. [DOI] [PubMed] [Google Scholar]
- 16.Jiang S, Deng N, Zheng B, Li T, Liu RH. Rhodiola extract promotes longevity and stress resistance of Caenorhabditis elegans via DAF-16 and SKN-1. Food Funct. 2021;12(10):4471–83. doi: 10.1039/d0fo02974b. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Tao H, Huang H, Xiao Y, Wu X, Li M, Shen J, Xiao Z, Zhao Y, Du F, Ji H, Chen Y, Cho CH, Wang Y, Wang S, Wu X. The dietary supplement Rhodiola crenulata extract alleviates dextran sulfate sodium-induced colitis in mice through anti-inflammation, mediating gut barrier integrity and reshaping the gut microbiome. Food Funct. 2021;12(7):3142–58. doi: 10.1039/d0fo03061a. [DOI] [PubMed] [Google Scholar]
- 18.Jiao Y, Zhao Z, Li X, Li L, Xiao D, Wan S, Wu T, Li T, Li P, Zhao R. Salidroside ameliorates memory impairment following long-term ethanol intake in rats by modulating the altered intestinal microbiota content and hippocampal gene expression. Front Microbiol. 2023;9(14):1172936. doi: 10.3389/fmicb.2023.1172936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Cai J, Fan P, Dong X, Zhang N, Tai J, Cao Y. Salidroside alleviates dextran sulfate sodium-induced colitis in mice by modulating the gut microbiota. Food Funct. 2023;14(16):7506–19. doi: 10.1039/d3fo01929b. [DOI] [PubMed] [Google Scholar]
- 20.Zhu H, Shen F, Wang X, Cheng Y, Guo Y, Qian H, Liu Y. Reshaped gut microbial composition and functions associated with the antifatigue effect of salidroside in exercise mice. Mol Nutr Food Res. 2023;67(12):e2300015. doi: 10.1002/mnfr.202300015. [DOI] [PubMed] [Google Scholar]
- 21.Song D, Zhao M, Feng L, Wang P, Li Y, Li W. Salidroside attenuates acute lung injury via inhibition of inflammatory cytokine production. Biomed Pharmacother. 2021;142:111949. doi: 10.1016/j.biopha.2021.111949. [DOI] [PubMed] [Google Scholar]
- 22.Jia X, Zhang K, Feng S, Li Y, Yao D, Liu Q, Liu D, Li X, Huang J, Wang H, Wang J. Total glycosides of Rhodiola rosea L. attenuate LPS-induced acute lung injury by inhibiting TLR4/NF-κB pathway. Biomed Pharmacother. 2023;158:114186. 10.1016/j.biopha.2022.114186. [DOI] [PubMed]
- 23.Golubnitschaja O, Watson ID, Topic E, Sandberg S, Ferrari M, Costigliola V. Position paper of the EPMA and EFLM: a global vision of the consolidated promotion of an integrative medical approach to advance health care. EPMA J. 2013;4(1):12. doi: 10.1186/1878-5085-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeChaine EG, Forester BR, Schaefer H, Davis CC. Deep genetic divergence between disjunct Refugia in the Arctic-Alpine King's Crown, Rhodiola integrifolia (Crassulaceae) PLoS One. 2013;8(11):e79451. doi: 10.1371/journal.pone.0079451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinckmann JA, Cunningham AB, Harter DEV. Running out of time to smell the roseroots: reviewing threats and trade in wild Rhodiola rosea L. J Ethnopharmacol. 2021;6(269):113710. doi: 10.1016/j.jep.2020.113710. [DOI] [PubMed] [Google Scholar]
- 26.Rutledge GA, Phang HJ, Le MN, Bui L, Rose MR, Mueller LD, Jafari M. Diet and botanical supplementation: combination therapy for healthspan improvement? Rejuvenation Res. 2021;24(5):331–344. doi: 10.1089/rej.2020.2361. [DOI] [PubMed] [Google Scholar]
- 27.Pu WL, Zhang MY, Bai RY, Sun LK, Li WH, Yu YL, Zhang Y, Song L, Wang ZX, Peng YF, Shi H, Zhou K, Li TX. Anti-inflammatory effects of Rhodiola rosea L.: a review. Biomed Pharmacother. 2020;121:109552. 10.1016/j.biopha.2019.109552. [DOI] [PubMed]
- 28.Wang Y, Su Y, Lai W, Huang X, Chu K, Brown J, Hong G. Salidroside restores an anti-inflammatory endothelial phenotype by selectively inhibiting endothelial complement after oxidative stress. Inflammation. 2020;43(1):310–25. doi: 10.1007/s10753-019-01121-y. [DOI] [PubMed] [Google Scholar]
- 29.Kosakowska O, Bączek K, Przybył JL, Pióro-Jabrucka E, Czupa W, Synowiec A, Gniewosz M, Costa R, Mondello L, Węglarz Z. Antioxidant and antibacterial activity of roseroot (Rhodiola rosea L.) dry extracts. Molecules. 2018;23(7):1767. 10.3390/molecules23071767. [DOI] [PMC free article] [PubMed]
- 30.Limanaqi F, Biagioni F, Busceti CL, Polzella M, Fabrizi C, Fornai F. Potential antidepressant effects of Scutellaria baicalensis, Hericium erinaceus and Rhodiola rosea. Antioxidants (Basel) 2020;9(3):234. doi: 10.3390/antiox9030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan Y, Wu X, Zhang X, Hong Y, Yan H. Ameliorative effect of salidroside from Rhodiola rosea L. on the gut microbiota subject to furan-induced liver injury in a mouse model. Food Chem Toxicol. 2019;125:333–40. 10.1016/j.fct.2019.01.007. [DOI] [PubMed]
- 32.Labachyan KE, Kiani D, Sevrioukov EA, Schriner SE, Jafari M. The impact of Rhodiola rosea on the gut microbial community of Drosophila melanogaster. Gut Pathog. 2018;10:12. 10.1186/s13099-018-0239-8. [DOI] [PMC free article] [PubMed]
- 33.Yang S, Wang L, Xie Z, Zeng Y, Xiong Q, Pei T, Wei D, Cheng W. The combination of salidroside and Hedysari radix polysaccharide inhibits mitochondrial damage and apoptosis via the PKC/ERK pathway. Evid Based Complement Alternat Med. 2022;25(2022):9475703. doi: 10.1155/2022/9475703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekhon-Loodu S, Rupasinghe HPV. Evaluation of antioxidant, antidiabetic and antiobesity potential of selected traditional medicinal plants. Front Nutr. 2019;25(6):53. doi: 10.3389/fnut.2019.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao H, Wu X, Cao J, et al. Rhodiola species: a comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med Res Rev. 2019;39(5):1779–1850. doi: 10.1002/med.21564. [DOI] [PubMed] [Google Scholar]
- 36.Perinskaya YuS, Sakanyan EI. Current state and prospects of developing drugs based on rhizomes and roots of Rhodiola rosea L. Med Plants. 2014;48(8):525–528. [Google Scholar]
- 37.Panossian A, Wikman G, Sarris J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 2010;17(7):481–93. doi: 10.1016/j.phymed.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Tayade AB, Dhar P, Kumar J, Sharma M, Chaurasia OP, Srivastava RB. Trans-Himalayan Rhodiola imbricate Edgew. root: a novel source of dietary amino acids, fatty acids and minerals. J Food Sci Technol. 2017;54(2):359–67. 10.1007/s13197-016-2469-4. [DOI] [PMC free article] [PubMed]
- 39.Ruan X, Hou P, Zhou J, Wang Q, Li G. [Analysis on the trace element and amino acid content in Xinjiang 6 series Rhodiola L. plant]. Guang Pu Xue Yu Guang Pu Fen Xi. 2001;21(4):542–4. [PubMed]
- 40.Polumackanycz M, Konieczynski P, Orhan IE, Abaci N, Viapiana A. Chemical composition, antioxidant and anti-enzymatic activity of golden root (Rhodiola rosea L.) commercial samples. Antioxidants (Basel). 2022;11(5):919. 10.3390/antiox11050919. [DOI] [PMC free article] [PubMed]
- 41.Magani SKJ, Mupparthi SD, Gollapalli BP, Shukla D, Tiwari AK, Gorantala J, Yarla NS, Tantravahi S. Salidroside - can it be a multifunctional drug? Curr Drug Metab. 2020;21(7):512–24. doi: 10.2174/1389200221666200610172105. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Zhao S, Jia N, Shen Z, Huang D, Wang X, Wu Y, Pei C, Shi S, He Y, Wang Z. Pretreatment with rosavin attenuates PM2.5-induced lung injury in rats through antiferroptosis via PI3K/Akt/Nrf2 signaling pathway. Phytother Res. 2023;37(1):195–210. 10.1002/ptr.7606. [DOI] [PubMed]
- 43.Albadawy R, Hasanin AH, Agwa SHA, Hamady S, Aboul-Ela YM, Raafat MH, Kamar SS, Othman M, Yahia YA, Matboli M. Rosavin ameliorates hepatic inflammation and fibrosis in the NASH rat model via targeting hepatic cell death. Int J Mol Sci. 2022;23(17):10148. doi: 10.3390/ijms231710148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Zhang W, Huo L, Chai Y, Liu Z, Ren Z, Yu C. Rosavin suppresses osteoclastogenesis in vivo and in vitro by blocking the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways. Ann Transl Med. 2021;9(5):383. 10.21037/atm-20-4255. [DOI] [PMC free article] [PubMed]
- 45.Wang W, Yan Y, Guo Z, Hou H, Garcia M, Tan X, Anto EO, Mahara G, Zheng Y, Li B, Kang T, Zhong Z, Wang Y, Guo X, Golubnitschaja O; Suboptimal Health Study Consortium and European Association for Predictive, Preventive and Personalised Medicine. All around suboptimal health - a joint position paper of the Suboptimal Health Study Consortium and European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2021;12(4):403–33. 10.1007/s13167-021-00253-2. [DOI] [PMC free article] [PubMed]
- 46.Wang W, Russell A, Yan Y; Global Health Epidemiology Reference Group (GHERG). Traditional Chinese medicine and new concepts of predictive, preventive and personalized medicine in diagnosis and treatment of suboptimal health. EPMA J. 2014;5(1):4. 10.1186/1878-5085-5-4. [DOI] [PMC free article] [PubMed]
- 47.Panossian A, Wagner H. Stimulating effect of adaptogens: an overview with particular reference to their efficacy following single dose administration. Phytother Res. 2005;19(10):819–38. doi: 10.1002/ptr.1751. [DOI] [PubMed] [Google Scholar]
- 48.Luo C, Xu X, Wei X, Feng W, Huang H, Liu H, Xu R, Lin J, Han L, Zhang D. Natural medicines for the treatment of fatigue: bioactive components, pharmacology, and mechanisms. Pharmacol Res. 2019;148:104409. doi: 10.1016/j.phrs.2019.104409. [DOI] [PubMed] [Google Scholar]
- 49.Zhang S, Deng N, Zheng B, Li T, Liu RH. The effect of in vitro gastrointestinal digestion on the phenolic profiles, bioactivities and bioaccessibility of Rhodiola. Food Funct. 2022;13(10):5752–65. 10.1039/d2fo00469k. [DOI] [PubMed]
- 50.Subramanian DA, Langer R, Traverso G. Mucus interaction to improve gastrointestinal retention and pharmacokinetics of orally administered nano-drug delivery systems. J Nanobiotechnol. 2022;20(1):362. doi: 10.1186/s12951-022-01539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Björnsson HK, Björnsson ES, Avula B, Khan IA, Jonasson JG, Ghabril M, Hayashi PH, Navarro V. Ashwagandha-induced liver injury: a case series from Iceland and the US Drug-Induced Liver Injury Network. Liver Int. 2020;40(4):825–9. doi: 10.1111/liv.14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen B, Truong J, Helliwell R, Govindaraghavan S, Sucher NJ. An in vitro study of neuroprotective properties of traditional Chinese herbal medicines thought to promote healthy ageing and longevity. BMC Complement Altern Med. 2013;27(13):373. doi: 10.1186/1472-6882-13-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tao H, Wu X, Cao J, Peng Y, Wang A, Pei J, Xiao J, Wang S, Wang Y. Rhodiola species: a comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med Res Rev. 2019;39(5):1779–850. doi: 10.1002/med.21564. [DOI] [PubMed] [Google Scholar]
- 54.Yu B, Sun Z, Li X, Qv A, Sohail M, Li Y, Xu H, Xiang P. Research progress of novel drug delivery systems of Chinese medicine monomers based on natural silk fibroin: a mini-review. Curr Drug Deliv. 2022 doi: 10.2174/1567201819666220413111439. [DOI] [PubMed] [Google Scholar]
- 55.Liu J, Sun H, Peng Y, Chen L, Xu W, Shao R. Preparation and characterization of natural silk fibroin hydrogel for protein drug delivery. Molecules. 2022;27(11):3418. doi: 10.3390/molecules27113418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tu P, Ma Y, Pan Y, Wang Z, Sun J, Chen K, Yang G, Wang L, Liu M, Guo Y. [Effect of silk fibroin microcarrier loaded with clematis total saponins and chondrocytes on promoting rabbit knee articular cartilage defects repair]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2022;36(3):343–51. Chinese. 10.7507/1002-1892.202107061. [DOI] [PMC free article] [PubMed]
- 57.Gholipourmalekabadi M, Sapru S, Samadikuchaksaraei A, Reis RL, Kaplan DL, Kundu SC. Silk fibroin for skin injury repair: where do things stand? Adv Drug Deliv Rev. 2020;1(153):28–53. doi: 10.1016/j.addr.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 58.He B, Sui X, Yu B, Wang S, Shen Y, Cong H. Recent advances in drug delivery systems for enhancing drug penetration into tumors. Drug Deliv. 2020;27(1):1474–90. doi: 10.1080/10717544.2020.1831106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maharjan S, Gautam M, Poudel K, Yong CS, Ku SK, Kim JO, Byeon JH. Streamlined plug-in aerosol prototype for reconfigurable manufacture of nano-drug delivery systems. Biomaterials. 2022;284:121511. doi: 10.1016/j.biomaterials.2022.121511. [DOI] [PubMed] [Google Scholar]
- 60.Kim B, Park J-E, Im E, Cho Y, Lee J, Lee H-J, Sim D-Y, Park W-Y, Shim B-S, Kim S-H. Recent advances in nanotechnology with nano-phytochemicals: molecular mechanisms and clinical implications in cancer progression. Int J Mol Sci. 2021;22:3571. doi: 10.3390/ijms22073571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nadimi AE, Ebrahimipour SY, Afshar EG, Falahati-Pour SK, Ahmadi Z, Mohammadinejad R, Mohamadi M. Nano-scale drug delivery systems for antiarrhythmic agents. Eur J Med Chem. 2018;5(157):1153–63. doi: 10.1016/j.ejmech.2018.08.080. [DOI] [PubMed] [Google Scholar]
- 62.Kapoor S, Sood H, Saxena S, Chaurasia OP. Green synthesis of silver nanoparticles using Rhodiola imbricata and Withania somnifera root extract and their potential catalytic, antioxidant, cytotoxic and growth-promoting activities. Bioprocess Biosyst Eng. 2022;45(2):365–80. doi: 10.1007/s00449-021-02666-9. [DOI] [PubMed] [Google Scholar]
- 63.Todorova V, Ivanov K, Delattre C, Nalbantova V, Karcheva-Bahchevanska D, Ivanova S. Plant adaptogens-history and future perspectives. Nutrients. 2021;13(8):2861. doi: 10.3390/nu13082861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Panossian A, Wikman G. Evidence-based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress-protective activity. Curr Clin Pharmacol. 2009;4(3):198–219. doi: 10.2174/157488409789375311. [DOI] [PubMed] [Google Scholar]
- 65.Esmaealzadeh N, Iranpanah A, Sarris J, Rahimi R. A literature review of the studies concerning selected plant-derived adaptogens and their general function in body with a focus on animal studies. Phytomedicine. 2022;105:154354. doi: 10.1016/j.phymed.2022.154354. [DOI] [PubMed] [Google Scholar]
- 66.Zhang JQ, Meng SY, Rao GY. Phylogeography of Rhodiola kirilowii (Crassulaceae): a story of Miocene divergence and quaternary expansion. PLoS One. 2014;9(11):e112923. doi: 10.1371/journal.pone.0112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiang HM, Chen HC, Wu CS, Wu PY, Wen KC. Rhodiola plants: chemistry and biological activity. J Food Drug Anal. 2015;23(3):359–69. doi: 10.1016/j.jfda.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ross SM. Rhodiola rosea (SHR-5), Part I: a proprietary root extract of Rhodiola rosea is found to be effective in the treatment of stress-related fatigue. Holist Nurs Pract. 2014;28(2):149–54. 10.1097/HNP.0000000000000014. [DOI] [PubMed]
- 69.Palmeri A, Mammana L, Tropea MR, Gulisano W, Puzzo D. Salidroside, a bioactive compound of Rhodiola rosea, ameliorates memory and emotional behavior in adult mice. J Alzheimers Dis. 2016;52(1):65–75. doi: 10.3233/JAD-151159. [DOI] [PubMed] [Google Scholar]
- 70.Ivanova Stojcheva E, Quintela JC. The effectiveness of Rhodiola rosea L. preparations in alleviating various aspects of life-stress symptoms and stress-induced conditions-encouraging clinical evidence. Molecules. 2022;27(12):3902. 10.3390/molecules27123902. [DOI] [PMC free article] [PubMed]
- 71.Petkov VD, Yonkov D, Mosharoff A, Kambourova T, Alova L, Petkov VV, Todorov I. Effects of alcohol aqueous extract from Rhodiola rosea L. roots on learning and memory. Acta Physiol Pharmacol Bulg. 1986;12(1):3–16. [PubMed]
- 72.Koklesova L, Mazurakova A, Samec M, Kudela E, Biringer K, Kubatka P, Golubnitschaja O. Mitochondrial health quality control: measurements and interpretation in the framework of predictive, preventive, and personalized medicine. EPMA J. 2022;13(2):177–93. doi: 10.1007/s13167-022-00281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan DC. Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol. 2020;24(15):235–59. doi: 10.1146/annurev-pathmechdis-012419-032711. [DOI] [PubMed] [Google Scholar]
- 74.Guha M, Srinivasan S, Johnson FB, Ruthel G, Guja K, Garcia-Diaz M, Kaufman BA, Glineburg MR, Fang J, Nakagawa H, Basha J, Kundu T, Avadhani NG. hnRNPA2 mediated acetylation reduces telomere length in response to mitochondrial dysfunction. PLoS One. 2018;13(11):e0206897. doi: 10.1371/journal.pone.0206897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y, Weng W, Gao R, Liu Y. new insights for cellular and molecular mechanisms of aging and aging-related diseases: herbal medicine as potential therapeutic approach. Oxid Med Cell Longev. 2019;12(2019):4598167. doi: 10.1155/2019/4598167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andrieux P, Chevillard C, Cunha-Neto E, Nunes JPS. Mitochondria as a cellular hub in infection and inflammation. Int J Mol Sci. 2021;22(21):11338. doi: 10.3390/ijms222111338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148(6):1145–59. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhuang X, Maimaitijiang A, Li Y, Shi H, Jiang X. Salidroside inhibits high-glucose induced proliferation of vascular smooth muscle cells via inhibiting mitochondrial fission and oxidative stress. Exp Ther Med. 2017;14(1):515–24. doi: 10.3892/etm.2017.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xue H, Li P, Luo Y, Wu C, Liu Y, Qin X, Huang X, Sun C. Salidroside stimulates the Sirt1/PGC-1α axis and ameliorates diabetic nephropathy in mice. Phytomedicine. 2019;15(54):240–247. doi: 10.1016/j.phymed.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 80.Liao ZL, Su H, Tan YF, Qiu YJ, Zhu JP, Chen Y, Lin SS, Wu MH, Mao YP, Hu JJ, Yu EY. Salidroside protects PC-12 cells against amyloid β-induced apoptosis by activation of the ERK1/2 and AKT signaling pathways. Int J Mol Med. 2019;43(4):1769–77. 10.3892/ijmm.2019.4088. [DOI] [PMC free article] [PubMed]
- 81.Li T, Zhang W, Kang X, Yang R, Li R, Huang L, Chen J, Yang Q, Sun X. Salidroside protects dopaminergic neurons by regulating the mitochondrial MEF2D-ND6 pathway in the MPTP/MPP+ -induced model of Parkinson’s disease. J Neurochem. 2020;153(2):276–89. 10.1111/jnc.14868. [DOI] [PubMed]
- 82.Yu HL, Zhang PP, Zhang C, Zhang X, Li ZZ, Li WQ, Fu AS. [Effects of Rhodiola rosea on oxidative stress and negative emotional states in patients with obstructive sleep apnea]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019;33(10):954–7. 10.13201/j.issn.1001-1781.2019.10.013. [DOI] [PubMed]
- 83.Fioranelli M, Roccia MG, Flavin D, Cota L. Regulation of inflammatory reaction in health and disease. Int J Mol Sci. 2021;22(10):5277. doi: 10.3390/ijms22105277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qian S, Golubnitschaja O, Zhan X. Chronic inflammation: key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019;10(4):365–81. doi: 10.1007/s13167-019-00194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Michalik A, Jarzyna R. Kluczowa rola kinazy białkowej aktywowanej przez AMP (AMPK) w procesach starzenia [The key role of AMP-activated protein kinase (AMPK) in aging process] Postepy Biochem. 2016;62(4):459–471. [PubMed] [Google Scholar]
- 86.Ge Y, Zhou M, Chen C, Wu X, Wang X. Role of AMPK mediated pathways in autophagy and aging. Biochimie. 2022;195:100–13. doi: 10.1016/j.biochi.2021.11.008. [DOI] [PubMed] [Google Scholar]
- 87.Haga Masatoshi, Okada Mariko. Systems approaches to investigate the role of NF-κB signaling in aging. Biochem J. 2022;479(2):161–83. doi: 10.1042/BCJ20210547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cordero, Mario D et al. AMP-activated protein kinase regulation of the NLRP3 inflammasome during aging. Trends Endocrinol Metab: TEM. (2018);29(1):8–17. 10.1016/j.tem.2017.10.009. [DOI] [PubMed]
- 89.van Beek AA, Van den Bossche J, Mastroberardino PG, de Winther MPJ, Leenen PJM. Metabolic alterations in aging macrophages: ingredients for inflammaging? Trends Immunol. 2019;40(2):113–27. doi: 10.1016/j.it.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 90.Matacchione G, Perugini J, Di Mercurio E, Sabbatinelli J, Prattichizzo F, Senzacqua M, Storci G, Dani C, Lezoche G, Guerrieri M, Giordano A, Bonafè M, Olivieri F. Senescent macrophages in the human adipose tissue as a source of inflammation. Geroscience. 2022. 10.1007/s11357-022-00536-0. [DOI] [PMC free article] [PubMed]
- 91.Wang X, Ren Y, Du X, Song L, Chen F, Su F. Effects of late-onset dietary intake of salidroside on insulin/insulin-like growth factor-1 (IGF-1) signaling pathway of the annual fish Nothobranchius guentheri. Arch Gerontol Geriatr. 2020;17(91):104233. doi: 10.1016/j.archger.2020.104233. [DOI] [PubMed] [Google Scholar]
- 92.Hu R, Wang MQ, Ni SH, Wang M, Liu LY, You HY, Wu XH, Wang YJ, Lu L, Wei LB. Salidroside ameliorates endothelial inflammation and oxidative stress by regulating the AMPK/NF-κB/NLRP3 signaling pathway in AGEs-induced HUVECs. Eur J Pharmacol. 2020;867:172797. 10.1016/j.ejphar.2019.172797. [DOI] [PubMed]
- 93.Chen P, Liu J, Ruan H, Zhang M, Wu P, Yimei D, Han B. Protective effects of salidroside on cardiac function in mice with myocardial infarction. Sci Rep. 2019;9(1):18127. doi: 10.1038/s41598-019-54713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xie N, Fan F, Jiang S, Hou Y, Zhang Y, Cairang N, Wang X, Meng X. Rhodiola crenulate alleviates hypobaric hypoxia-induced brain injury via adjusting NF-κB/NLRP3-mediated inflammation. Phytomedicine. 2022;103:154240. doi: 10.1016/j.phymed.2022.154240. [DOI] [PubMed] [Google Scholar]
- 95.Li JS, Fan LY, Yuan MD, Xing MY. Salidroside inhibits lipopolysaccharide-ethanol-induced activation of proinflammatory macrophages via Notch signaling pathway. Curr Med Sci. 2019;39(4):526–33. doi: 10.1007/s11596-019-2069-4. [DOI] [PubMed] [Google Scholar]
- 96.Guan S, Feng H, Song B, Guo W, Xiong Y, Huang G, Zhong W, Huo M, Chen N, Lu J, Deng X. Salidroside attenuates LPS-induced pro-inflammatory cytokine responses and improves survival in murine endotoxemia. Int Immunopharmacol. 2011;11(12):2194–9. doi: 10.1016/j.intimp.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 97.Ming X, Yu X, Li J, Wang J, Zheng J, Xiong L. Salidroside attenuates airway inflammation and remodeling via the miR-323–3p/SOCS5 axis in asthmatic mice. Int Arch Allergy Immunol. 2022;183(4):424–34. doi: 10.1159/000520444. [DOI] [PubMed] [Google Scholar]
- 98.Wang X, Tang Y, Xie N, Bai J, Jiang S, Zhang Y, Hou Y, Meng X. Salidroside, a phenyl ethanol glycoside from Rhodiola crenulata, orchestrates hypoxic mitochondrial dynamics homeostasis by stimulating Sirt1/p53/Drp1 signaling. J Ethnopharmacol. 2022;15(293):115278. doi: 10.1016/j.jep.2022.115278. [DOI] [PubMed] [Google Scholar]
- 99.Tan TT, Lang YZ, Liu C, Mi J, Yan YM, Cao YL, Ran LW, Yang W. Effect of natural aging on immune function in mice. Jiangsu Med J. 2021;47(12):1189–1192. [Google Scholar]
- 100.Borgoni S, Kudryashova KS, Burka K, de Magalhães JP. Targeting immune dysfunction in aging. Ageing Res Rev. 2021;70:101410. doi: 10.1016/j.arr.2021.101410. [DOI] [PubMed] [Google Scholar]
- 101.Bailey KL, Smith LM, Heires AJ, Katafiasz DM, Romberger DJ, LeVan TD. Aging leads to dysfunctional innate immune responses to TLR2 and TLR4 agonists. Aging Clin Exp Res. 2019;31(9):1185–93. doi: 10.1007/s40520-018-1064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li R, Dong Z, Zhuang X, Liu R, Yan F, Chen Y, Gao X, Shi H. Salidroside prevents tumor necrosis factor-α-induced vascular inflammation by blocking mitogen-activated protein kinase and NF-κB signaling activation. Exp Ther Med. 2019;18(5):4137–43. doi: 10.3892/etm.2019.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang XW, Zhang YL, Wen ZX, Li PF, Cui L, Zhang M. Salidroside regulates DC through TLR4 to increase the lethality of T cells to lung cancer 3LL cells. Chin J Cancer Biother. 2020;27:01, 37–41. 10.3872/j.issn.1007-385x.2020.01.006.
- 104.Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O'Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 105.[Morais LH, Schreiber HL 4th, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19(4):241–55. 10.1038/s41579-020-00460-0. [DOI] [PubMed]
- 106.Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 2017;15(10):630–8. doi: 10.1038/nrmicro.2017.58. [DOI] [PubMed] [Google Scholar]
- 107.Binyamin D, Werbner N, Nuriel-Ohayon M, Uzan A, Mor H, Abbas A, Ziv O, Teperino R, Gutman R, Koren O. The aging mouse microbiome has obesogenic characteristics. Genome Med. 2020;12(1):87. doi: 10.1186/s13073-020-00784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leite G, Pimentel M, Barlow GM, Chang C, Hosseini A, Wang J, Parodi G, Sedighi R, Rezaie A, Mathur R. Age and the aging process significantly alter the small bowel microbiome. Cell Rep. 2021;36(13):109765. doi: 10.1016/j.celrep.2021.109765. [DOI] [PubMed] [Google Scholar]
- 109.Fusco W, Lorenzo MB, Cintoni M, Porcari S, Rinninella E, Kaitsas F, Lener E, Mele MC, Gasbarrini A, Collado MC, Cammarota G, Ianiro G. Short-chain fatty-acid-producing bacteria: key components of the human gut microbiota. Nutrients. 2023;15(9):2211. doi: 10.3390/nu15092211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao YT, Yang L, Pang HT, Luo DS. The correlation between the content of intestinal short chain fatty acid and microflora characteristics in aged mice. Chin J Biochem Mol Biol. 2021:37;12, 1682–90. 10.13865/j.cnki.cjbmb.2021.10.1307.
- 111.Martin A, Ecklu-Mensah G, Ha CWY, Hendrick G, Layman DK, Gilbert J, Devkota S. Gut microbiota mediate the FGF21 adaptive stress response to chronic dietary protein-restriction in mice. Nat Commun. 2021;12(1):3838. doi: 10.1038/s41467-021-24074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qin XY, Li XP, Wu X, Wang ZH, Cui ZT, Dong B, Zhao HY. Research progress on the relationship between the gutmicrobiota and geriatric diseases. Chin J Geriatr. 2021;40(07):937–41. doi: 10.3760/cma.j.issn.0254-9026.2021.07.026. [DOI] [Google Scholar]
- 113.Lv S, Wang Y, Zhang W, Shang H. Trimethylamine oxide: a potential target for heart failure therapy. Heart. 2022;108(12):917–22. doi: 10.1136/heartjnl-2021-320054. [DOI] [PubMed] [Google Scholar]
- 114.Li J, Li Y, Ivey KL, Wang DD, Wilkinson JE, Franke A, Lee KH, Chan A, Huttenhower C, Hu FB, Rimm EB, Sun Q. Interplay between diet and gut microbiome, and circulating concentrations of trimethylamine N-oxide: findings from a longitudinal cohort of US men. Gut. 2022;71(4):724–33. doi: 10.1136/gutjnl-2020-322473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang ZR, Zeng GM, Peng LH, Zhang MM, Cheng JL, Zhan RT. Preliminary study on effect of Rhodiolae crenulatae radix et rhizoma cell wall-broken decoction pieces on intestinal flora of mice. Chin Mater Med. 2015;40(15):3053–8. doi: 10.4268/cjcmm20151526. [DOI] [PubMed] [Google Scholar]
- 116.Juttukonda MR, Li B, Almaktoum R, Stephens KA, Yochim KM, Yacoub E, Buckner RL, Salat DH. Characterizing cerebral hemodynamics across the adult lifespan with arterial spin labeling MRI data from the Human Connectome Project-Aging. Neuroimage. 2021;15(230):117807. doi: 10.1016/j.neuroimage.2021.117807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang D, Cabral D, Gaspard EN, Lipton RB, Rundek T, Derby CA. Cerebral hemodynamics in the elderly: a transcranial Doppler study in the Einstein Aging Study Cohort. J Ultrasound Med. 2016;35(9):1907–14. doi: 10.7863/ultra.15.10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.43Kim LB, Melnikov VN, Putyatina AN. [Relationships between aging indicators, central hemodynamics and arterial stiffness in men in the European North of Russia.]. Adv Gerontol. 2021;34(1):39–47. [PubMed]
- 119.Islam MT, Henson GD, Machin DR, Bramwell RC, Donato AJ, Lesniewski LA. Aging differentially impacts vasodilation and angiogenesis in arteries from the white and brown adipose tissues. Exp Gerontol. 2020;142:111126. doi: 10.1016/j.exger.2020.111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ambrose CT. Pro-angiogenesis therapy and aging: a mini-review. Gerontology. 2017;63(5):393–400. doi: 10.1159/000477402. [DOI] [PubMed] [Google Scholar]
- 121.Ma YG, Wang JW, Bai YG, Liu M, Xie MJ, Dai ZJ. Salidroside contributes to reducing blood pressure and alleviating cerebrovascular contractile activity in diabetic Goto-Kakizaki rats by inhibition of L-type calcium channel in smooth muscle cells. BMC Pharmacol Toxicol. 2017;18(1):30. doi: 10.1186/s40360-017-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ma YG, Wang JW, Zhang YB, Wang BF, Dai ZJ, Xie MJ, Kang HF. Salidroside improved cerebrovascular vasodilation in streptozotocin-induced diabetic rats through restoring the function of BKCa channel in smooth muscle cells. Cell Tissue Res. 2017;370(3):365–77. 10.1007/s00441-017-2671-3. [DOI] [PubMed]
- 123.Liu SH, Hsiao YW, Chong E, Singhal R, Fong MC, Tsai YN, Hsu CP, Chen YC, Chen YJ, Chiou CW, Chiang SJ, Chang SL, Chen SA. Rhodiola inhibits atrial arrhythmogenesis in a heart failure model. J Cardiovasc Electrophysiol. 2016;27(9):1093–101. doi: 10.1111/jce.13026. [DOI] [PubMed] [Google Scholar]
- 124.Li L, Yang Y, Zhang H, Du Y, Jiao X, Yu H, Wang Y, Lv Q, Li F, Sun Q, Qin Y. Salidroside ameliorated intermittent hypoxia-aggravated endothelial barrier disruption and atherosclerosis via the cAMP/PKA/RhoA signaling pathway. Front Pharmacol. 2021;12:723922. 10.3389/fphar.2021.723922. [DOI] [PMC free article] [PubMed]
- 125.Zhang J, Kasim V, Xie YD, Huang C, Sisjayawan J, Dwi Ariyanti A, Yan XS, Wu XY, Liu CP, Yang L, Miyagishi M, Wu SR. Inhibition of PHD3 by salidroside promotes neovascularization through cell-cell communications mediated by muscle-secreted angiogenic factors. Sci Rep. 2017;7:43935. doi: 10.1038/srep43935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fuster JJ, Walsh K. Somatic mutations and clonal hematopoiesis: unexpected potential new drivers of age-related cardiovascular disease. Circ Res. 2018;122(3):523–32. doi: 10.1161/CIRCRESAHA.117.312115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hair R, Sakaki JR, Chun OK. Anthocyanins, microbiome and health benefits in aging. Molecules. 2021;26(3):537. doi: 10.3390/molecules26030537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bizzarri M, Fedeli V, Monti N, Cucina A, Jalouli M, Alwasel SH, Harrath AH. Personalization of medical treatments in oncology: time for rethinking the disease concept to improve individual outcomes. EPMA J. 2021;12(4):545–58. doi: 10.1007/s13167-021-00254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Koklesova L, Mazurakova A, Samec M, Biringer K, Samuel SM, Büsselberg D, Kubatka P, Golubnitschaja O. Homocysteine metabolism as the target for predictive medical approach, disease prevention, prognosis, and treatments tailored to the person. EPMA J. 2021;12(4):1–29. doi: 10.1007/s13167-021-00263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Koklesova L, Samec M, Liskova A, Zhai K, Büsselberg D, Giordano FA, Kubatka P, Golunitschaja O. Mitochondrial impairments in aetiopathology of multifactorial diseases: common origin but individual outcomes in context of 3P medicine. EPMA J. 2021;12(1):1–14. doi: 10.1007/s13167-021-00237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Golubnitschaja O, Costigliola V; EPMA. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3(1):14. 10.1186/1878-5085-3-14. [DOI] [PMC free article] [PubMed]
- 132.Wang W, Yan Y, Guo Z, Hou H, Garcia M, Tan X, Anto EO, Mahara G, Zheng Y, Li B, et al. All around suboptimal health-a Joint Position Paper of the Suboptimal Health Study Consortium and European Association for Predictive Preventive and Personalised Medicine. EPMA J. 2021;12:403–33. 10.1007/s13167-021-00253-2. [DOI] [PMC free article] [PubMed]
- 133.Rossiello F, Jurk D, Passos JF, d'Adda di Fagagna F. Telomere dysfunction in ageing and age-related diseases. Nat Cell Biol. 2022;24(2):135–47. 10.1038/s41556-022-00842-x. [DOI] [PMC free article] [PubMed]
- 134.Yadav S, Maurya PK. Correlation between telomere length and biomarkers of oxidative stress in human aging. Rejuvenation Res. 2022;25(1):25–9. doi: 10.1089/rej.2021.0045. [DOI] [PubMed] [Google Scholar]
- 135.Vatner SF, Zhang J, Oydanich M, Berkman T, Naftalovich R, Vatner DE. Healthful aging mediated by inhibition of oxidative stress. Ageing Res Rev. 2020;64:101194. doi: 10.1016/j.arr.2020.101194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 137.Panossian A, Seo EJ, Efferth T. Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine. 2018;15(50):257–84. doi: 10.1016/j.phymed.2018.09.204. [DOI] [PubMed] [Google Scholar]
- 138.Mao GX, Xu XG, Wang SY, Li HF, Zhang J, Zhang ZS, Su HL, Chen SS, Xing WM, Wang YZ, Dai JH, Wang GF, Leng SX, Yan J. Salidroside delays cellular senescence by stimulating mitochondrial biogenesis partly through a miR-22/SIRT-1 pathway. Oxid Med Cell Longev. 2019 doi: 10.1155/2019/5276096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huangfu ZM, Xu Q, Wang X, Wang EP, Feng Y, Zeng J, Zhu R, Zhao CL. Salidroside can improve lung injury in mouse models of chronic intermittent hypoxia. Zhongguo Zuzhi Gongcheng Yanjiu. 2019;23(31):5036–40. doi: 10.3969/j.issn.2095-4344.1490. [DOI] [Google Scholar]
- 140.Ma Y, Liu J, Pan T. Protective effect and mechanism of salidroside on H9C2 cardiomyocytes under oxidative stress and hyperglycemia. J Nanjing Univ Tradit Med. 2019;35:04 442–7. 10.14148/j.issn.1672-0482.2019.0442.
- 141.Lu Y, Deng B, Xu L, Liu H, Song Y, Lin F. Effects of Rhodiola rosea supplementation on exercise and sport: a systematic review. Front Nutr. 2022;7(9):856287. doi: 10.3389/fnut.2022.856287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xie YM, Liu BY, Piao HY. Exploration on the common characters of suboptimal Healthy people based on clinical epidemiology. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006;26(7):612–616. [PubMed] [Google Scholar]
- 143.Wu S, Xuan Z, Li F, Xiao W, Fu X, Jiang P, Chen J, Xiang L, Liu Y, Nie X, Luo R, Sun X, Kwan H, Zhao X. Work-recreation balance, health-promoting lifestyles and suboptimal health status in Southern China: a cross-sectional study. Int J Environ Res Public Health. 2016;13(3):339. doi: 10.3390/ijerph13030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bi J, Huang Y, Xiao Y, Cheng J, Li F, Wang T, Chen J, Wu L, Liu Y, Luo R, Zhao X. Association of lifestyle factors and suboptimal health status: a cross-sectional study of Chinese students. BMJ Open. 2014;4(6):e005156. doi: 10.1136/bmjopen-2014-005156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Olsson EM, von Schéele B, Panossian AG. A randomised, double-blind, placebo-controlled, parallel-group study of the standardised extract shr-5 of the roots of Rhodiola rosea in the treatment of subjects with stress-related fatigue. Planta Med. 2009;75(2):105–12. doi: 10.1055/s-0028-1088346. [DOI] [PubMed] [Google Scholar]
- 146.Lekomtseva Y, Zhukova I, Wacker A. Rhodiola rosea in subjects with prolonged or chronic fatigue symptoms: results of an open-label clinical trial. Complement Med Res. 2017;24(1):46–52. doi: 10.1159/000457918. [DOI] [PubMed] [Google Scholar]
- 147.Gil-Iturbe E, Félix-Soriano E, Sáinz N, Idoate-Bayón A, Castilla-Madrigal R, Moreno-Aliaga MJ, Lostao MP. Effect of aging and obesity on GLUT12 expression in small intestine, adipose tissue, muscle, and kidney and its regulation by docosahexaenoic acid and exercise in mice. Appl Physiol Nutr Metab. 2020;45(9):957–67. doi: 10.1139/apnm-2019-0721. [DOI] [PubMed] [Google Scholar]
- 148.Nunan E, Wright CL, Semola OA, Subramanian M, Balasubramanian P, Lovern PC, Fancher IS, Butcher JT. Obesity as a premature aging phenotype - implications for sarcopenic obesity. Geroscience. 2022;44(3):1393–405. doi: 10.1007/s11357-022-00567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020;126(11):1477–500. doi: 10.1161/CIRCRESAHA.120.316101. [DOI] [PubMed] [Google Scholar]
- 150.Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016;118(11):1752–70. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- 151.Bartlett DE, Miller RB, Thiesfeldt S, Lakhani HV, Shapiro JI, Sodhi K. The role of Na/K-ATPase signaling in oxidative stress related to aging: implications in obesity and cardiovascular disease. Int J Mol Sci. 2018;19(7):2139. doi: 10.3390/ijms19072139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Trovato FM, Catalano D, Musumeci G, Trovato GM. 4Ps medicine of the fatty liver: the research model of predictive, preventive, personalized and participatory medicine-recommendations for facing obesity, fatty liver and fibrosis epidemics. EPMA J. 2014;5(1):21. doi: 10.1186/1878-5085-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee SY, Shi LS, Chu H, Li MH, Ho CW, Lai FY, Huang CY, Chang TC. Rhodiola crenulata and its bioactive components, salidroside and tyrosol, reverse the hypoxia-induced reduction of plasma-membrane-associated Na, K-ATPase expression via inhibition of ROS-AMPK-PKC ξ pathway. Evid Based Complement Alternat Med. 2013;2013:284150. doi: 10.1155/2013/284150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.You B, Dun Y, Fu S, Qi D, Zhang W, Liu Y, Qiu L, Xie M, Liu S. The treatment of Rhodiola Mimics exercise to resist high-fat diet-induced muscle dysfunction via Sirtuin1-dependent mechanisms. Front Pharmacol. 2021;15(12):646489. doi: 10.3389/fphar.2021.646489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen Y, Tang M, Yuan S, Fu S, Li Y, Li Y, Wang Q, Cao Y, Liu L, Zhang Q. Rhodiola rosea: a therapeutic candidate on cardiovascular diseases. Oxid Med Cell Longev. 2022;27(2022):1348795. doi: 10.1155/2022/1348795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee OH, Kwon YI, Apostolidis E, Shetty K, Kim YC. Rhodiola-induced inhibition of adipogenesis involves antioxidant enzyme response associated with pentose phosphate pathway. Phytother Res. 2011;25(1):106–15. doi: 10.1002/ptr.3236. [DOI] [PubMed] [Google Scholar]
- 157.Liu J, Cai J, Fan P, Dong X, Zhang N, Tai J, Cao Y. Salidroside protects mice from high-fat diet-induced obesity by modulating the gut microbiota. Int Immunopharmacol. 2023;120:110278. doi: 10.1016/j.intimp.2023.110278. [DOI] [PubMed] [Google Scholar]
- 158.Verpeut JL, Walters AL, Bello NT. Citrus aurantium and Rhodiola rosea in combination reduce visceral white adipose tissue and increase hypothalamic norepinephrine in a rat model of diet-induced obesity. Nutr Res. 2013;33(6):503–12. doi: 10.1016/j.nutres.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zheng T, Yang X, Li W, Wang Q, Chen L, Wu D, Bian F, Xing S, Jin S. Salidroside attenuates high-fat diet-induced nonalcoholic fatty liver disease via AMPK-dependent TXNIP/NLRP3 pathway. Oxid Med Cell Longev. 2018 doi: 10.1155/2018/8597897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang M, Luo L, Yao L, Wang C, Jiang K, Liu X, Xu M, Shen N, Guo S, Sun C, Yang Y. Salidroside improves glucose homeostasis in obese mice by repressing inflammation in white adipose tissues and improving leptin sensitivity in hypothalamus. Sci Rep. 2016;5(6):25399. doi: 10.1038/srep25399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. 2023;79(2):516–37. doi: 10.1016/j.jhep.2023.03.017. [DOI] [PubMed] [Google Scholar]
- 162.Mohammed S, Thadathil N, Selvarani R, Nicklas EH, Wang D, Miller BF, Richardson A, Deepa SS. Necroptosis contributes to chronic inflammation and fibrosis in aging liver. Aging Cell. 2021;20(12):e13512. doi: 10.1111/acel.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Maeso-Díaz R, Dalton GD, Oh S, Du K, Tang L, Chen T, Dutta RK, Hartman JH, Meyer JN, Diehl AM. Aging reduces liver resiliency by dysregulating Hedgehog signaling. Aging Cell. 2022;21(2):e13530. doi: 10.1111/acel.13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Simón J, Martínez-Chantar ML, Delgado TC. Glutamine, fatty liver disease and aging. Aging (Albany NY). 2021;13(3):3165–6. 10.18632/aging.202666. [DOI] [PMC free article] [PubMed]
- 165.Seo E, Kang H, Choi H, Choi W, Jun HS. Reactive oxygen species-induced changes in glucose and lipid metabolism contribute to the accumulation of cholesterol in the liver during aging. Aging Cell. 2019;18(2):e12895. doi: 10.1111/acel.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jin CJ, Baumann A, Brandt A, Engstler AJ, Nier A, Hege M, Schmeer C, Kehm R, Höhn A, Grune T, Witte OW, Bergheim I. Aging-related liver degeneration is associated with increased bacterial endotoxin and lipopolysaccharide binding protein levels. Am J Physiol Gastrointest Liver Physiol. 2020;318(4):G736–47. doi: 10.1152/ajpgi.00345.2018. [DOI] [PubMed] [Google Scholar]
- 167.Baumann A, Hernández-Arriaga A, Brandt A, Sánchez V, Nier A, Jung F, Kehm R, Höhn A, Grune T, Frahm C, Witte OW, Camarinha-Silva A, Bergheim I. Microbiota profiling in aging-associated inflammation and liver degeneration. Int J Med Microbiol. 2021;311(4):151500. doi: 10.1016/j.ijmm.2021.151500. [DOI] [PubMed] [Google Scholar]
- 168.Xu F, Tautenhahn HM, Dirsch O, Dahmen U. Modulation of autophagy: a novel "rejuvenation" strategy for the aging liver. Oxid Med Cell Longev. 2021;10(2021):6611126. doi: 10.1155/2021/6611126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xu F, Hua C, Tautenhahn HM, Dirsch O, Dahmen U. The role of autophagy for the regeneration of the aging liver. Int J Mol Sci. 2020;21(10):3606. doi: 10.3390/ijms21103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Feng J, Niu P, Chen K, Wu L, Liu T, Xu S, Li J, Li S, Wang W, Lu X, Yu Q, Liu N, Xu L, Wang F, Dai W, Xia Y, Fan X, Guo C. Salidroside mediates apoptosis and autophagy inhibition in concanavalin A-induced liver injury. Exp Ther Med. 2018;15(6):4599–614. doi: 10.3892/etm.2018.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yuan C, Jin Y, Yao L, Liu L, Li J, Li H, Lai Y, Chen Z, Pan Z, Han T, Ke D, Li C, Wang S, Wang M, Yamahara J, Wang J. Rhodiola crenulata root extract ameliorates fructose-induced hepatic steatosis in rats: association with activating autophagy. Biomed Pharmacother. 2020;125:109836. doi: 10.1016/j.biopha.2020.109836. [DOI] [PubMed] [Google Scholar]
- 172.Liu J, Li D, Dun Y, Li H, Ripley-Gonzalez JW, Zhang J, Qiu L, You B, Liu S. Rhodiola activates macrophage migration inhibitory factor to alleviate non-alcoholic fatty liver disease. Life Sci. 2022;308:120949. doi: 10.1016/j.lfs.2022.120949. [DOI] [PubMed] [Google Scholar]
- 173.Xu Y, Jiang H, Sun C, Adu-Frimpong M, Deng W, Yu J, Xu X. Antioxidant and hepatoprotective effects of purified Rhodiola rosea polysaccharides. Int J Biol Macromol. 2018;1(117):167–78. doi: 10.1016/j.ijbiomac.2018.05.168. [DOI] [PubMed] [Google Scholar]
- 174.Xiong Y, Wang Y, Xiong Y, Teng L. Protective effect of salidroside on hypoxia-related liver oxidative stress and inflammation via Nrf2 and JAK2/STAT3 signaling pathways. Food Sci Nutr. 2021;9(9):5060–9. doi: 10.1002/fsn3.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 176.Mazurakova A, Samec M, Koklesova L, Biringer K, Kudela E, Al-Ishaq RK, Pec M, Giordano FA, Büsselberg D, Kubatka P, Golubnitschaja O. Anti-prostate cancer protection and therapy in the framework of predictive, preventive and personalised medicine - comprehensive effects of phytochemicals in primary, secondary and tertiary care. EPMA J. 2022;13(3):461–86. doi: 10.1007/s13167-022-00288-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mazurakova A, Koklesova L, Samec M, Kudela E, Kajo K, Skuciova V, Csizmár SH, Mestanova V, Pec M, Adamkov M, Al-Ishaq RK, Smejkal K, Giordano FA, Büsselberg D, Biringer K, Golubnitschaja O, Kubatka P. Anti-breast cancer effects of phytochemicals: primary, secondary, and tertiary care. EPMA J. 2022;13(2):315–34. doi: 10.1007/s13167-022-00277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhu X, Liu D, Wang Y, Dong M. Salidroside suppresses nonsmall cell lung cancer cells proliferation and migration via microRNA-103–3p/Mzb1. Anticancer Drugs. 2020;31(7):663–71. doi: 10.1097/CAD.0000000000000926. [DOI] [PubMed] [Google Scholar]
- 179.Samec M, Liskova A, Koklesova L, Samuel SM, Zhai K, Buhrmann C, Varghese E, Abotaleb M, Qaradakhi T, Zulli A, Kello M, Mojzis J, Zubor P, Kwon TK, Shakibaei M, Büsselberg D, Sarria GR, Golubnitschaja O, Kubatka P. Flavonoids against the Warburg phenotype-concepts of predictive, preventive and personalised medicine to cut the Gordian knot of cancer cell metabolism. EPMA J. 2020;11(3):377–98. doi: 10.1007/s13167-020-00217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dai Z, Zhang X, Li W, Tang J, Pan T, Ma C, Guan Q. Salidroside induces apoptosis in human gastric cancer cells via the downregulation of ENO1/PKM2/GLUT1 expression. Biol Pharm Bull. 2021;44(11):1724–31. doi: 10.1248/bpb.b21-00443. [DOI] [PubMed] [Google Scholar]
- 181.Kang DY, Sp N, Kim DH, Joung YH, Lee HG, Park YM, Yang YM. Salidroside inhibits migration, invasion and angiogenesis of MDA-MB 231 TNBC cells by regulating EGFR/Jak2/STAT3 signaling via MMP2. Int J Oncol. 2018;53(2):877–85. 10.3892/ijo.2018.4430. [DOI] [PubMed]
- 182.Li Y, Pham V, Bui M, Song L, Wu C, Walia A, Uchio E, Smith-Liu F, Zi X. Rhodiola rosea L.: an herb with anti-stress, anti-aging, and immunostimulating properties for cancer chemoprevention. Curr Pharmacol Rep. 2017;3(6):384–95. 10.1007/s40495-017-0106-1. [DOI] [PMC free article] [PubMed]
- 183.Lin KT, Chang TC, Lai FY, Lin CS, Chao HL, Lee SY. Rhodiola crenulata attenuates γ-ray induced cellular injury via modulation of oxidative stress in human skin cells. Am J Chin Med. 2018;46(1):175–90. doi: 10.1142/S0192415X18500106. [DOI] [PubMed] [Google Scholar]
- 184.Zhang Y, Zhang X, Yue Q, Wen Z, Zhang M. [Ethanol extract of Rhodiola rosea L. regulates the number of tumor infiltrating T cells to enhance antitumor effect in Lewis lung cancer-bearing mice]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2019;35(2):103–8. [PubMed]
- 185.Zhang X, Zhu J, Yan J, Xiao Y, Yang R, Huang R, Zhou J, Wang Z, Xiao W, Zheng C, Wang Y. Systems pharmacology unravels the synergic target space and therapeutic potential of Rhodiola rosea L. for non-small cell lung cancer. Phytomedicine. 2020;79:153326. 10.1016/j.phymed.2020.153326. [DOI] [PubMed]
- 186.Mora MC, Bassa LM, Wong KE, Tirabassi MV, Arenas RB, Schneider SS. Rhodiola crenulata inhibits Wnt/β-catenin signaling in glioblastoma. J Surg Res. 2015;197(2):247–55. doi: 10.1016/j.jss.2015.02.074. [DOI] [PubMed] [Google Scholar]
- 187.Bassa LM, Jacobs C, Gregory K, Henchey E, Ser-Dolansky J, Schneider SS. Rhodiola crenulata induces an early estrogenic response and reduces proliferation and tumorsphere formation over time in MCF7 breast cancer cells. Phytomedicine. 2016;23(1):87–94. doi: 10.1016/j.phymed.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 188.Qi Z, Tang T, Sheng L, Ma Y, Liu Y, Yan L, Qi S, Ling L, Zhang Y. Salidroside inhibits the proliferation and migration of gastric cancer cells via suppression of Src-associated signaling pathway activation and heat shock protein 70 expression. Mol Med Rep. 2018;18(1):147–56. doi: 10.3892/mmr.2018.8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rong L, Li Z, Leng X, Li H, Ma Y, Chen Y, Song F. Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway. Biomed Pharmacother. 2020;122:109726. doi: 10.1016/j.biopha.2019.109726. [DOI] [PubMed] [Google Scholar]
- 190.Li H, Chen C. Inhibition of autophagy enhances synergistic effects of salidroside and anti-tumor agents against colorectal cancer. BMC Complement Altern Med. 2017;17(1):538. doi: 10.1186/s12906-017-2046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zeng W, Xiao T, Cai A, Cai W, Liu H, Liu J, Li J, Tan M, Xie L, Liu Y, Yang X, Long Y. Inhibiting ROS-TFEB-dependent autophagy enhances salidroside-induced apoptosis in human chondrosarcoma cells. Cell Physiol Biochem. 2017;43(4):1487–502. doi: 10.1159/000481971. [DOI] [PubMed] [Google Scholar]
- 192.Lu L, Liu S, Dong Q, Xin Y. Salidroside suppresses the metastasis of hepatocellular carcinoma cells by inhibiting the activation of the Notch1 signaling pathway. Mol Med Rep. 2019;19(6):4964–72. doi: 10.3892/mmr.2019.10115. [DOI] [PubMed] [Google Scholar]
- 193.Chen Y, Klein SL, Garibaldi BT, Li H, Wu C, Osevala NM, Li T, Margolick JB, Pawelec G, Leng SX. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65:101205. doi: 10.1016/j.arr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lynch SM, Guo G, Gibson DS, Bjourson AJ, Rai TS. Role of senescence and aging in SARS-CoV-2 infection and COVID-19 disease. Cells. 2021;10(12):3367. doi: 10.3390/cells10123367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schneider JL, Rowe JH, Garcia-de-Alba C, Kim CF, Sharpe AH, Haigis MC. The aging lung: physiology, disease, and immunity. Cell. 2021;184(8):1990–2019. doi: 10.1016/j.cell.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yoon YS, Jin M, Sin DD. Accelerated lung aging and chronic obstructive pulmonary disease. Expert Rev Respir Med. 2019;13(4):369–80. doi: 10.1080/17476348.2019.1580576. [DOI] [PubMed] [Google Scholar]
- 197.Yang F, Wendusubilige, Kong J, Zong Y, Wang M, Jing C, Ma Z, Li W, Cao R, Jing S, Gao J, Li W, Wang J. Identifying oxidative stress-related biomarkers in idiopathic pulmonary fibrosis in the context of predictive, preventive, and personalized medicine using integrative omics approaches and machine-learning strategies. EPMA J. 2023;14(3):417–42. 10.1007/s13167-023-00334-4. [DOI] [PMC free article] [PubMed]
- 198.Chuang ML, Wu TC, Wang YT, Wang YC, Tsao TC, Wei JC, Chen CY, Lin IF. Adjunctive treatment with Rhodiola crenulata in patients with chronic obstructive pulmonary disease–a randomized placebo controlled double blind clinical trial. PLoS One. 2015;10(6):e0128142. doi: 10.1371/journal.pone.0128142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang H, Dong W, Li S, Zhang Y, Lv Z, Yang L, Jiang L, Wu T, Wang Y. Salidroside protects against ventilation-induced lung injury by inhibiting the expression of matrix metalloproteinase-9. Pharm Biol. 2021;59(1):760–8. doi: 10.1080/13880209.2021.1967409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang Y, Xu CF, Liu YJ, Mao YF, Lv Z, Li SY, Zhu XY, Jiang L. Salidroside attenuates ventilation induced lung injury via SIRT1-dependent inhibition of NLRP3 inflammasome. Cell Physiol Biochem. 2017;42(1):34–43. doi: 10.1159/000477112. [DOI] [PubMed] [Google Scholar]
- 201.Panossian A, Brendler T. The role of adaptogens in prophylaxis and treatment of viral respiratory infections. Pharmaceuticals (Basel) 2020;13(9):236. doi: 10.3390/ph13090236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Karosanidze I, Kiladze U, Kirtadze N, Giorgadze M, Amashukeli N, Parulava N, Iluridze N, Kikabidze N, Gudavadze N, Gelashvili L, Koberidze V, Gigashvili E, Jajanidze N, Latsabidze N, Mamageishvili N, Shengelia R, Hovhannisyan A, Panossian A. Efficacy of adaptogens in patients with long COVID-19: a randomized, quadruple-blind, placebo-controlled trial. Pharmaceuticals (Basel) 2022;15(3):345. doi: 10.3390/ph15030345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Erkkinen MG, Kim MO, Geschwind MD. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2018;10(4):a033118. doi: 10.1101/cshperspect.a033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bongioanni P, Del Carratore R, Corbianco S, Diana A, Cavallini G, Masciandaro SM, Dini M, Buizza R. Climate change and neurodegenerative diseases. Environ Res. 2021;201:111511. doi: 10.1016/j.envres.2021.111511. [DOI] [PubMed] [Google Scholar]
- 205.Raghavan NS, Dumitrescu L, Mormino E, Mahoney ER, Lee AJ, Gao Y, Bilgel M, Goldstein D, Harrison T, Engelman CD, Saykin AJ, Whelan CD, Liu JZ, Jagust W, Albert M, Johnson SC, Yang HS, Johnson K, Aisen P, Resnick SM, Sperling R, De Jager PL, Schneider J, Bennett DA, Schrag M, Vardarajan B, Hohman TJ, Mayeux R; Alzheimer’s Disease Neuroimaging Initiative. Association between common variants in RBFOX1, an RNA-binding protein, and brain amyloidosis in early and preclinical Alzheimer disease. JAMA Neurol. 2020;77(10):1288–98. 10.1001/jamaneurol.2020.1760. [DOI] [PMC free article] [PubMed]
- 206.Brown AJH, Bradley SJ, Marshall FH, Brown GA, Bennett KA, Brown J, Cansfield JE, Cross DM, de Graaf C, Hudson BD, Dwomoh L, Dias JM, Errey JC, Hurrell E, Liptrot J, Mattedi G, Molloy C, Nathan PJ, Okrasa K, Osborne G, Patel JC, Pickworth M, Robertson N, Shahabi S, Bundgaard C, Phillips K, Broad LM, Goonawardena AV, Morairty SR, Browning M, Perini F, Dawson GR, Deakin JFW, Smith RT, Sexton PM, Warneck J, Vinson M, Tasker T, Tehan BG, Teobald B, Christopoulos A, Langmead CJ, Jazayeri A, Cooke RM, Rucktooa P, Congreve MS, Weir M, Tobin AB. From structure to clinic: design of a muscarinic M1 receptor agonist with potential to treatment of Alzheimer's disease. Cell. 2021;184(24):5886–901.e22. doi: 10.1016/j.cell.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li X, Wang Y, Su M, Chu X, Li S, Yue Y, Zhang X, Wang J, Han F. Brain metabolomics study for the protective effects of Rhodiola crenulata extract on Alzheimer's disease by HPLC coupled with Fourier transform-ion cyclotron resonance mass spectrometry. J Sep Sci. 2020;43(16):3216–23. doi: 10.1002/jssc.201901314. [DOI] [PubMed] [Google Scholar]
- 208.Huang X, Xing S, Chen C, Yu Z, Chen J. Salidroside protects PC12 cells from Aβ1–40-induced cytotoxicity by regulating the nicotinamide phosphoribosyltransferase signaling pathway. Mol Med Rep. 2017;16(3):2700–6. doi: 10.3892/mmr.2017.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang H, Li Q, Sun S, Chen S. Neuroprotective effects of salidroside in a mouse model of Alzheimer's disease. Cell Mol Neurobiol. 2020;40(7):1133–42. doi: 10.1007/s10571-020-00801-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gu C, Li L, Huang Y, Qian D, Liu W, Zhang C, Luo Y, Zhou Z, Kong F, Zhao X, Liu H, Gao P, Chen J, Yin G. Salidroside ameliorates mitochondria-dependent neuronal apoptosis after spinal cord ischemia-reperfusion injury partially through inhibiting oxidative stress and promoting mitophagy. Oxid Med Cell Longev. 2020;23(2020):3549704. doi: 10.1155/2020/3549704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.98Li Y, Cai M, Mao GX, Shu QF, Liu XB, Liu XL. Preclinical evidence and possible mechanisms of Rhodiola rosea L. and its components for ischemic stroke: a systematic review and meta-analysis. Front Pharmacol. 2021;12:736198. 10.3389/fphar.2021.736198. [DOI] [PMC free article] [PubMed]
- 212.Boyle NB, Billington J, Lawton C, Quadt F, Dye L. A combination of green tea, Rhodiola, magnesium and B vitamins modulates brain activity and protects against the effects of induced social stress in healthy volunteers. Nutr Neurosci. 2022;25(9):1845–59. doi: 10.1080/1028415X.2021.1909204. [DOI] [PubMed] [Google Scholar]
- 213.Nastase MV, Zeng-Brouwers J, Wygrecka M, Schaefer L. Targeting renal fibrosis: mechanisms and drug delivery systems. Adv Drug Deliv Rev. 2018;129:295–307. doi: 10.1016/j.addr.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 214.Chung KW, Lee EK, Lee MK, Oh GT, Yu BP, Chung HY. Impairment of PPARα and the fatty acid oxidation pathway aggravates renal fibrosis during aging. J Am Soc Nephrol. 2018;29(4):1223–37. 10.1681/ASN.2017070802. [DOI] [PMC free article] [PubMed]
- 215.Miao J, Liu J, Niu J, Zhang Y, Shen W, Luo C, Liu Y, Li C, Li H, Yang P, Liu Y, Hou FF, Zhou L. Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell. 2019;18(5):e13004. doi: 10.1111/acel.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Li R, Guo Y, Zhang Y, Zhang X, Zhu L, Yan T. Salidroside ameliorates renal interstitial fibrosis by inhibiting the TLR4/NF-κB and MAPK signaling pathways. Int J Mol Sci. 2019;20(5):1103. doi: 10.3390/ijms20051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tao H, Wu X, Cao J, Peng Y, Wang A, Pei J, Xiao J, Wang S, Wang Y. Rhodiola species: a comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med Res Rev. 2019;39(5):1779–850. doi: 10.1002/med.21564. [DOI] [PubMed] [Google Scholar]
- 218.Pan S-Y, Litscher G, Gao S-H, Zhou S-F, Yu Z-L, Chen H-Q, Zhang S-F, Tang M-K, Sun J-N, Ko K-M. Historical perspective of traditional indigenous medical practices: the current renaissance and conservation of herbal resources. Evid Based Complement Altern Med ECAM. 2014;2014:525340. doi: 10.1155/2014/525340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wei RL, Wang LX, Xie YM. Cognition of traditional Chinese medicine properties and thinking of clinical rational use of drug. Zhongguo Zhong Yao Za Zhi. 2021;46(21):5462–5467. doi: 10.19540/j.cnki.cjcmm.20210330.501. [DOI] [PubMed] [Google Scholar]
- 220.Tang JY. Suggestions on technical requirements for traditional Chinese medicine new drug research and development. Zhongguo Zhong Yao Za Zhi. 2020;45(16):4009–4016. [PubMed] [Google Scholar]
- 221.Lu F, Wang D, Li RL, He LY, Ai L, Wu CJ. Current strategies and technologies for finding drug targets of active components from traditional Chinese medicine. Front Biosci (Landmark Ed). 2021;26(9):572–89. 10.52586/4968. [DOI] [PubMed]
- 222.Sun R, Dai J, Ling M, Yu L, Yu Z, Tang L. Delivery of triptolide: a combination of traditional Chinese medicine and nanomedicine. J Nanobiotechnology. 2022;20(1):194. doi: 10.1186/s12951-022-01389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Feng T, Wang L, Zhou N, Liu C, Cui J, Wu R, Jing J, Zhang S, Chen H, Wang S. Salidroside, a scavenger of ROS, enhances the radioprotective effect of Ex-RAD® via a p53-dependent apoptotic pathway. Oncol Rep. 2017;38(5):3094–102. doi: 10.3892/or.2017.5940. [DOI] [PubMed] [Google Scholar]
- 224.Wang W. Glycomics research in China: the current state of the art. OMICS. 2019;23(12):601–2. doi: 10.1089/omi.2019.0163. [DOI] [PubMed] [Google Scholar]
- 225.Gan X, Shu Z, Wang X, Yan D, Li J, Ofaim S, Albert R, Li X, Liu B, Zhou X, Barabási AL. Network medicine framework reveals generic herb-symptom effectiveness of traditional Chinese medicine. Sci Adv. 2023;9(43):eadh0215. 10.1126/sciadv.adh0215. [DOI] [PMC free article] [PubMed]
- 226.Golubnitschaja O, Liskova A, Koklesova L, Samec M, Biringer K, Büsselberg D, Podbielska H, Kunin AA, Evsevyeva ME, Shapira N, et al. Caution, “normal” BMI: health risks associated with potentially masked individual underweight-EPMA Position Paper. EPMA J. 2021;2021:1–22. doi: 10.1007/s13167-021-00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wang W, Yan Y, Guo Z, Hou H, Garcia M, Tan X, Anto EO, Mahara G, Zheng Y, Li B, et al. All around suboptimal health — a Joint Position Paper of the Suboptimal Health Study Consortium and European Association for Predictive Preventive and Personalised Medicine. EPMA J. 2021;12:403–33. 10.1007/s13167-021-00253-2. [DOI] [PMC free article] [PubMed]
- 228.Gerbarg PL, Brown RP. Pause menopause with Rhodiola rosea, a natural selective estrogen receptor modulator. Phytomedicine. 2016;23(7):763–9. doi: 10.1016/j.phymed.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 229.Zhang A, Sun H, Wang P, Han Y, Wang X. Future perspectives of personalized medicine in traditional Chinese medicine: a systems biology approach. Complement Ther Med. 2012;20(1–2):93–9. 10.1016/j.ctim.2011.10.007. [DOI] [PubMed]
- 230.Dong Y, Sui L, Yang F, Ren X, Xing Y, Xiu Z. Reducing the intestinal side effects of acarbose by baicalein through the regulation of gut microbiota: an in vitro study. Food Chem. 2022;15(394):133561. doi: 10.1016/j.foodchem.2022.133561. [DOI] [PubMed] [Google Scholar]
- 231.Jafari M, Juanson Arabit JG, Courville R, Kiani D, Chaston JM, Nguyen CD, Jena N, Liu ZY, Tata P, Van Etten RA. The impact of Rhodiola rosea on biomarkers of diabetes, inflammation, and microbiota in a leptin receptor-knockout mouse model. Sci Rep. 2022;12(1):10581. doi: 10.1038/s41598-022-14241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Xiong YX, Li N, Han MM, Ye F, Liu T, Ye HY, Zheng TT, Wu JJ, Li Y, Lv S, Zhang YH, Zhang Y, Dong ZQ. Rhodiola rosea polysaccharides-based nanoparticles loaded with DOX boosts chemo-immunotherapy for triple-negative breast cancer by re-educating Tumor-associated macrophages. Int J Biol Macromol. 2023;1(239):124110. doi: 10.1016/j.ijbiomac.2023.124110. [DOI] [PubMed] [Google Scholar]
- 233.Udintsev SN, Krylova SG, Fomina TI. Povshenie éffektivnosti adriamitsina s pomoshch'iu gepatoprotektorov rastitel'nogo proiskhozhdeniia pri metastazakh adenokartsinomy Erlikha v pechen' u mysheĭ [The enhancement of the efficacy of adriamycin by using hepatoprotectors of plant origin in metastases of Ehrlich's adenocarcinoma to the liver in mice] Vopr Onkol. 1992;38(10):1217–1222. [PubMed] [Google Scholar]
- 234.Borovskaia TG, Fomina TI, Iaremenko KV. Snizhenie toksicheskogo deĭstviia rubomitsina na tonkuiu kishku myshcheĭ s perevivaemoĭ opukhol'iu s pomoshch'iu ékstrakta rodioly [A decrease in the toxic action of rubomycin on the small intestine of mice with a transplantable tumor through the use of a Rhodiola extract] Antibiot Khimioter. 1988;33(8):615–617. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.
Not applicable.