Abstract
Background
DNA methylation is an important mechanism in epigenetics, which can change the transcription ability of genes and is closely related to the pathogenesis of ovarian cancer (OC). We hypothesize that DNA methylation is significantly different in OCs compared to controls. Specific DNA methylation status can be used as a biomarker of OC, and targeted drugs targeting these methylation patterns and DNA methyltransferase may have better therapeutic effects. Studying the key DNA methylation sites of immune-related genes (IRGs) in OC patients and studying the effects of these methylation sites on the immune microenvironment may provide a new method for further exploring the pathogenesis of OC, realizing early detection and effective monitoring of OC, identifying effective biomarkers of DNA methylation subtypes and drug targets, improving the efficacy of targeted drugs or overcoming drug resistance, and better applying it to predictive diagnosis, prevention, and personalized medicine (PPPM; 3PM) of OC.
Method
Hypermethylated subtypes (cluster 1) and hypomethylated subtypes (cluster 2) were established in OCs based on the abundance of different methylation sites in IRGs. The differences in immune score, immune checkpoints, immune cells, and overall survival were analyzed between different methylation subtypes in OC samples. The significant pathways, gene ontology (GO), and protein-protein interaction (PPI) network of the identified methylation sites in IRGs were enriched. In addition, the immune-related methylation signature was constructed with multiple regression analysis. A methylation site model based on IRGs was constructed and verified.
Results
A total of 120 IRGs with 142 differentially methylated sites (DMSs) were identified. The DMSs were clustered into a high-level methylation group (cluster 1) and a low-level methylation group (cluster 2). The significant pathways and GO analysis showed many immune-related and cancer-associated enrichments. A methylation site signature based on IRGs was constructed, including RORC|cg25112191, S100A13|cg14467840, TNF|cg04425624, RLN2|cg03679581, and IL1RL2|cg22797169. The methylation sites of all five genes showed hypomethylation in OC, and there were statistically significant differences among RORC|cg25112191, S100A13|cg14467840, and TNF|cg04425624 (p < 0.05). This prognostic model based on low-level methylation and high-level methylation groups was significantly linked to the immune microenvironment as well as overall survival in OC.
Conclusions
This study provided different methylation subtypes for OC patients according to the methylation sites of IRGs. In addition, it helps establish a relationship between methylation and the immune microenvironment, which showed specific differences in biological signaling pathways, genomic changes, and immune mechanisms within the two subgroups. These data provide ones to deeply understand the mechanism of immune-related methylation genes on the occurrence and development of OC. The methylation-site signature is also to establish new possibilities for OC therapy. These data are a precious resource for stratification and targeted treatment of OC patients toward an advanced 3PM approach.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13167-024-00359-3.
Keywords: Ovarian cancer, Immune-related genes, DNA methylation, Differentially methylated sites, Methylation subtypes, Biomarker, Tumor immune microenvironment, Risk score, Methylation-site signature, Patient stratification, Targeted treatment, Overall survival, Predictive preventive personalized medicine (PPPM; 3PM)
Introduction
The biomarkers of ovarian cancer (OC) are urgently needed in prediction, prevention, and personalized medicine (PPPM)
Every year, more than 310,000 women are diagnosed with OC worldwide, with nearly 210,000 deaths [1]. OC spreads through systemic circulation and intraperitoneal diffusion. There are various histological types of OC, which can be divided into epithelial, germ cells, sex cord stroma, and metastatic OC according to different sources, 90% of which are epithelial, and ovarian serous adenocarcinoma (OSC) is the most common histological subtype in epithelial tumors. Traditionally, OC subtypes were classified according to histopathology, molecular genetics, and immunophenotype, which could not predict the prognosis well. Since then, epithelial OC was classified into types I and II based on pathological observation and clinical and molecular biological characteristics, and its treatment methods were completely different from the prognosis, which made a great contribution to the study of OC. This classification method did not fully consider the heterogeneity of OC, and accurate classification based on molecular level was the basis for individualized diagnosis and treatment. Molecular targeted therapy was successful in many malignant tumors. Genetic, hormonal, ovulation, environmental, and other factors make OC prone to metastasis and recurrence, leading to a very poor prognosis [2–4]. OC has strong heterogeneity characteristics; indeed, one of the primary elements favoring OC’s early metastasis is the heterogeneicity of the tumor immune microenvironment [5]. The key factors for OC prognosis are early diagnosis, appropriate surgery, and systemic treatment (chemotherapy, radiotherapy, hormonal therapy, and immunotherapy); however, OC does not have apparent indicators in the early stage, and lesions are usually found in the late stage. The mortality rate of late-stage OC is extremely high. According to the data of the International Federation of Obstetrics and Gynecology, the 5-year survival rate for advanced OC is 34%, and that of stage IV is only 15% [6]. Conventional screening methods for OC include CA-125 and transvaginal ultrasound, but studies have shown that using these two screening methods at the same time cannot reduce the mortality rate of OC [7]. Patients with advanced OC still have the phenomenon of recurrence and increased drug resistance during treatment. To explain this abnormality, many studies were conducted, and it was found that epigenetic regulation, such as methylation which is the most studied epigenetic modification, affects tumor development and drug resistance. Methylation has long been used as a marker for early detection of OC due to its good tissue specificity changes in the early stage and various other modifications [8]. DNA methylation has great biomarker potential in screening OC, monitoring treatment response, and predicting prognosis. The relative stability of the methylation state, only a small amount of tissues are needed for methylation analysis, the relative accuracy of results can be ensured by quantitative determination, and it can be used for non-invasive diagnosis and prognosis follow-up, which makes methylation more advantageous as a biomarker. Detecting methylation levels by liquid biopsy technology can realize early detection and effective monitoring of OC.
Importance of methylation in cancer
Methylation is a chemical modification, which adds methyl groups to the bases of DNA molecules through DNA methyltransferases (DNMTs). In eukaryotes, methyl groups only transfer to the fifth carbon of cytosine to form 5-methylcytosine (5mC). Seventy percent to 80% of methylcytosine exists in CG dinucleotide, and the region rich in CG is called CpG island, where methylation is the most active [9]. DNMT is an important mechanism to control the cycle, transformation, apoptosis, differentiation, and proliferation of eukaryotic cells. Methylation regulates the expression of coding genes in two ways. One way is direct inhibition of the binding of DNA to transcription factors. For instance, in the case of the CmCGG site located in the binding sequence of AP-2, its methylation prevents the binding of AP-2, indicating that methylation of the CmCGG site inhibits the expression of human proenkephalin-CAT fusion gene by directly interfering with the binding of transcription factors [10]. In a similar example, mCpmCpG methylation at the Sp1 site inhibits the binding of Sp1 [11]. The other way is the aggregation of the histone deacetylase complex and methylcytosine-binding protein to indirectly inhibit transcription factor binding. As an example, one study found the mechanism of methylation inhibiting transcription and that methylation indirectly inhibited transcription through MeCP-1 [12]. Methylcytosine-binding protein MBD2 interacts with the histone deacetylase complex composed of NURD and Sin3A and mediates the transcription inhibition of genes [13]. TRD in MeCP2 can recruit large co-repressor complexes of histone deacetylase HDAC1 and 2, such as mSin3A, thus inhibiting transcription in vitro and in vivo [14]. Hypermethylation of promoters is common in the development of cancer. Indeed, the hypermethylation of the p16INK4A promoter leads to cell cycle dysfunction, which is very common in many tumors such as prostate, breast, and kidney cancers [15]. In addition, hypermethylation of the p16INK4A gene leads to poor prognosis in some cancer patients [16]. Hypermethylation can directly down-regulate gene expression by blocking the combination of transcription factors or indirectly silence gene expression by silencing transcription factors and DNA repair genes, which will promote the occurrence of cancer [17]. Another study showed that transcription factors (GATA-4, GATA-5, and RUNX3) were silenced by hypermethylation, which leads to the inactivation of their downstream targets, thus affecting various cell processes [18]. For instance, RUNX3 regulates gene expression and participates in the TGF-β signaling pathway. Therefore, its silencing caused by hypermethylation affects the progress of lung cancer [19]. Global hypomethylation also leads to genomic instability and cell transformation. For example, the deletion of methylation in satellite near the central point 2 (Sat2) and satellite near the central point α (Satα) in breast cancer is related to the early occurrence of tumor. However, the deletion of methylation in Sat2 and Satα promotes the progress of tumors and is considered an important indicator of poor prognosis of OC [20–22]. Global hypomethylation of DNA is found in many genomic sites, including CpG deletion promoters, repetitive sequences, retrotransposons, and many other sites, which are all important in tumor formation [23]. The hypomethylation of CPG island is related to transcription activity, growth factor, accessibility of transcription factor, open chromatin state, and overexpression of proto-oncogene. Increased methylation of tumor suppressor genes or decreased methylation of tumor oncogenes can promote tumor proliferation, migration, and invasion. For example, as tumor suppressor genes, the increased methylation of CDH1 and CDH13 leads to the down-regulation of their gene products, which influences tumor cell adhesion, invasion, and metastasis [24]. Deletion of promoter methylation of the CDH3 gene promotes migration, motility, and invasion of colorectal and breast cancer [25–27]. DNA hypermethylation can also induce the silencing of the miR-424/503 cluster, thereby inhibiting KIF23 expression, thus preventing the proliferation and migration of OC [28]. The miRNA expression can also be regulated by methylation of promoter sequences in various cancers, including liver, colorectal, oral, gynecological, and breast cancers [29–33]. Pharmacological intervention in the reversibility of methylation is undoubtedly an attractive drug target. For example, the overactivity of DNMTs leads to the hypermethylation of tumor suppressor gene promoters. Therefore, 5-aza-2′-deoxycytidine (5-Aza-CdR), a DNA hypomethylation agent, has been used in targeted therapy of cancer. Studies have shown that the interaction between protein kinase and DNMT1 can promote tumor drug resistance [34]. It has also been found that DNMT inhibitors induce gene demethylation and change the expression of tumor suppressor genes. Inhibition of gene methylation may lead to down-regulation of metabolism and tumor genes, indicating that gene methylation may be a potential therapeutic target [35]. The mechanism of action of DNMTs is diverse and complex, and its biological effects may vary according to the disease state, cell type, and drug dosage. Therefore, when using such drugs, it is necessary to make individualized treatment plans according to specific conditions.
Contribution of immune-related gene methylation to OC pathogenesis
Tumor immune microenvironment (TIME) includes cytokines, immune cells, cell surface molecules, and tumor cells. Methylation regulates the proliferation, differentiation, and function of immune cells and then affects the immune response process. Studies have shown that methylation mediates the expression of CD4 in T cells, and CD4 and CD8 promote the binding of T-cell antigen receptors (TCRs) with MHC molecules, which is important for the expansion of helper and cytotoxic T cells [36]. Methylation regulates the expression of immune-related genes and affects the intensity of immune response. Methylation blocks the activation of the IFN signaling pathway in melanoma, thus preventing immune cells from recognizing tumors [37]. Methylation can also affect T-cell differentiation. The increase of Methylation leads to gene silencing, which makes naive T cells differentiate into Treg, while the decrease of methylation makes naive T cells differentiate into effector T cells. Methylation plays a key role in maintaining the balance of the immune system and coping with the invasion of external pathogens. With the deepening of the research on the mechanism of methylation, we are expected to provide new ideas and methods for the prevention and treatment of immune-related diseases. In contrast, the state of the immune system may also regulate gene expression by affecting the pattern and characteristics of methylation. In OC, AlkB family genes are hypomethylated globally, and the infiltration of immune cells such as macrophages and CD8 + T cells affects their expression [38]. TLE3, a member of the TLE gene family and a predictor of OC, is expressed in neutrophils, and its mRNA expression level is significantly related to the methylation of OC and MMR gene, a biomarker of OC immunotherapy. Thus, TLE3 is used as a biomarker of OC immunotherapy and prognosis evaluation [39]. A follow-up analysis showed that TAP1 and PSMB9 were enriched in the immune response process, the hypomethylation of CpGs of 6p21.3 was related to the up-regulation of these genes, and the regulation of immune-related pathway genes and immune cell infiltration increase. The hypermethylation near the TAP1 promoter region had a shorter TTR, and the non-expression of TAP1 and PSMB9 leads to OC cells escaping from the immune response mediated by specific CD8 T cells. This article confirms that the differential CpG genes of OC play an important role in OC anti-tumor immunity [40]. Therefore, during the pathogenesis of OC, the methylation status of immune-related genes may change, thus affecting the activity and expression of related genes. The regulation of gene expression can further affect the functions of the immune system, including the activation of immune cells and the generation of immune responses. However, at present, there is relatively little research in this field, and further research is needed to reveal the specific relationship and mechanism between methylation of immune-related genes and OC.
Working hypothesis in the framework of 3P medicine
The methylation of immune-related genes is significantly associated with OC pathogenesis, which is a lack of effective biomarkers for OC early-stage diagnosis, targeted treatment, and prognostic prediction. We hypothesize that the methylation of immune-related genes in OCs is significantly different from ovarian controls. The methylation data of immune-related genes was obtained in OC and control tissues. The differential methylation sites of immune-related genes are significantly associated with different OC stages. The methylation signature biomarkers based on immune-related genes can be used for patient stratification, predictive diagnosis, prognostic assessment, and targeted medical services of OC.
Study design
In this work, 27,578 methylation sites were obtained from Illumina human methylation 27 K array, and 2448 immune biomarker–related genes were screened from the ImmPort database; 120 immune-related genes with 142 methylation sites were obtained. The consistent clustering of methylation sites was divided into two subtypes: hypermethylation and hypomethylation. According to the identified immune-related methylation sites, KEGG and GO enrichment analyses were carried out, and the immune checkpoints and immune infiltration of the two subtypes were compared. Based on the high-level methylation and low-level methylation groups, a prognosis model was established to show the influence of methylation on the immune microenvironment in OC. Further, the IRG methylation sites in the prognostic model were verified in human OC cells and tissues. The flow diagram of our investigation is represented (Fig. 1).
Fig. 1.
Experiment flow-chart to identify immune-related gene methylation signature in ovarian cancers. IRGs: immune-related genes. DMSs: differentially methylated sites. DMGs: differentially methylated genes
Materials and methods
Data processing
A total of 613 OC patients were investigated in this study. UCSC Xena (https://xenabrowser.net/datapages/) was used to obtain methylation data from Illumina human methylation 27 K array (Supplementary Table 1), and ImmPort Shared data (https://www.immport.org/shared/home) was used to get the immune cell–associated biomarkers (Supplementary Table 2). The corresponding clinical characteristics (anatomic subdivision, age, clinical stage, tumor residual disease, radiation therapy, intermediate dimension, longest dimension, histologic grade, cancer status, and therapy outcome) (Supplementary Table 16) were obtained from The Cancer Genome Atlas (TCGA) website (https://portal.gdc.cancer.gov/).
Unsupervised hierarchical cluster analysis
The methylation sites of immune-related genes were obtained through various analyses. The R and limma packages were employed to resolve differentially methylated sites (DMSs) of immune-related genes (IRGs) with fold-change > 1.5 and adjusted p-value < 0.05, in OC samples compared to normal samples (Supplementary Table 3). Unsupervised hierarchical clustering was used based on the IRG-DMS data to recognize subtypes of OC (Supplementary Table 4) with the “ConsensusClusterPlus” R software package (http://bioconductor.org/packages/release/bioc/html/ConsensusClusterPlus.html). The unsupervised clustering method based on the proportion of fuzzy clusters is an uncomplicated and effective approach to infer the optimal K (K-means) for classifying patients for further research, looping 1000 times to ensure reliable classification sex and stability. The heatmap of DMSs of immune-related genes was drawn with the “pheatmap” R package (https://cran.r-project.org/web/packages/pheatmap/index.html) between different methylation subtypes. A boxplot was established to demonstrate the total methylation level between different low- and high-methylation subtypes. Overall survival (OS) curves for the OC subset were acquired using the Kaplan–Meier approach as well as the R “survival” package.
The ssGSEA based on different methylation subtypes
The single-sample Gene Set Enrichment Analysis (ssGSEA) was utilized to calculate the scores of immune-related events with the GSEABase R package on the basis of RNA-seq gene expression data (https://www.biostars.org/p/452249/). Totally, 28 immune-related events were analyzed, including APC co-stimulation, type I IFN response, aDCs, macrophages, APC co-inhibition, B cells, HLA, DCs, NK cells, iDCs, CCR, inflammation-promoting, neutrophils, cytolytic activity, parainflammation, type II IFN response, MHC class I, CD8 + T cells, T-cell co-stimulation, pDCs, T-cell co-inhibition, Tfh, Th1 cells, Th2 cells, checkpoint, mast cells, and TIL (Supplementary Table 5). The distributions of immune-related events between different methylation subtypes were carried out by the “pheatmap” R package (https://cran.r-project.org/web/packages/pheatmap/index.html).
The different immune scores, immune-related checkpoints, and immune cells between different methylation subtypes in OC
Based on expression data, immune and stromal cells in tumor tissues were evaluated using the ESTIMATE algorithm. The algorithm is derived from public online resources (https://sourceforge.net/projects/estimateproject/), which is based on tumor samples relevant to matrix and immune cell infiltration in specific biomarkers to ESTIMATE the immune score. The immune score of every sample was computed (Supplementary Table 6) as well as assessed across distinct methylated subtypes of OC.
LM22 gene markers and the CIBERSORT algorithm were used to identify 22 human immune cell phenotypes with high specificity and sensitivity to evaluate immune cells in OC. Gene expression profiles were created using standard annotation files, and data were uploaded to the CIBERSORT web interface (http://cibersort.stanford.edu/), where the LM22 signature and 1000 permutations were used to run the algorithm (Supplementary Table 7). The distribution of immune cells among independent OC methylation subtypes was determined using an unpaired Student’s t-test. The immune cells included resting memory CD4 T cells, M0 macrophages, naive B cells, plasma cells, naive CD4 T cells, mast cells activated, memory-activated CD4 T cells, regulatory T cells (Tregs), neutrophils, resting NK cells, activated NK cells, monocytes, memory B cells, M1 macrophages, CD8 T cells, M2 macrophages, follicular helper T cells, resting dendritic cells, eosinophils, activated dendritic cells, resting mast cells, and gamma delta T cells.
The Level 3 RNA-seq data of immune checkpoints (Supplementary Table 8) were taken from TCGA (https://portal.gdc.cancer.gov/). The immune checkpoint levels in OC methylation subtypes, including CD86, PDCD1LG2, CD276, PDCD1, CTLA4, CD80, CD274, and VTCN1, were assessed by unpaired Student’s t-test.
The enriched significant pathways, GO, and PPI network of the identified DMSs of immune-related genes
All DMSs of immune-related genes were examined by pathways, gene ontology terms (GO), and protein–protein interaction (PPI) network analysis. The significantly enriched pathways were clustered with the Kyoto Encyclopedia of Genes and Genomes (KEGG) (https://david.ncifcrf.gov/). The significant signaling pathways of DMSs between normal samples and OC samples are listed in Supplementary Table 9 (p < 0.05 and FDR < 0.05). Cytoscape ClueGO was utilized to reveal the biological processes (BPs) enriched from DMSs (bilateral hypergeometry test, p < 0.05 after Benjamini-Hochberg’s correction). The significant BP analysis of DMSs is listed in Supplementary Table 10. STRING (https://cn.string-db.org/) was employed for the establishment of the PPI network (Supplementary Table 11) (combined score > 0.9).
Selection of methylation-driven genes and drug sensitivity analysis
All DMSs of immune-related genes and mRNA expression of immune-related genes (Supplementary Table 12) were selected. The correlation coefficient between mRNA expression and methylation of immune-related genes was calculated with Pearson’s correlation coefficient (Supplementary Table 13). A gene was selected when its methylation level was negatively related to the mRNA expression level (p < 0.05, the correlation |coefficient|> 0.4). CellMiner (https://discover.nci.nih. Gov/CellMiner/), a web-based genomic and pharmacologic tool usually employed to investigate transcripts and drug patterns in the NCI-60 cell line set, was used for screening. Based on CellMiner’s data in OC (Supplementary Table 14), corrplot R package + Spearman’s method were utilized to evaluate the association between methylation-driven gene expression and drug sensitivity (p < 0.05).
Construction and verification of Cox regression model for OC
The DMSs of methylation-driven genes were screened to construct the Cox regression model. OC samples were arbitrarily split into train and test groups. The risk scores on the basis of the DMSs of methylation-driven genes were used to divide train groups (or test groups) into low-risk and high-risk score groups (Supplementary Table 15). The Kaplan–Meier approach was employed to assess the presence of a prediction model between low-risk and high-risk score groups in train and test groups, respectively. TCGA database was used to extract clinical data, such as age, anatomic subdivision, clinical stage, tumor residual disease, radiation therapy, intermediate dimension, longest dimension, cancer status, therapy outcome, and histologic grade. PheATMAP R package was used to do clinical correlation analysis for high- and low-risk groups (Supplementary Table 16). A univariate Cox regression model was utilized to evaluate the clinical characteristics relevant to the OS of OC patients and a risk score assessment map was utilized to estimate the prognosis in OC patients (1-, 3-, and 5-year survival).
Cell culture
Four human OC cells (OVCAR-3, TOV-21G, SK-OV-3, and A2780) were used for the experiments along with one human normal ovarian epithelial cell (IOSE80) as control. IOSE80 and SK-OV-3 were cultured in DMEM basic (1x), A2780 was cultured in MEM basic (1x), and TOV-21G and OVCAR-3 were cultured in RPMI Medium 1640 basic (1x), all supplemented by 10% FBS and 1% penicillin streptomycin solution. All cells were incubated in 5% CO2 at 37 °C for their growth.
Patients and samples
Three ovarian serous cystadenocarcinoma tissues and three control ovarian tissues were obtained from Shandong Provincial Hospital (department of Gynecology) Affiliated with Shandong First Medical University. The sample collection was approved by the Ethical Committee of Shandong First Medical University.
Methylation‑specific polymerase chain reaction (MSP)
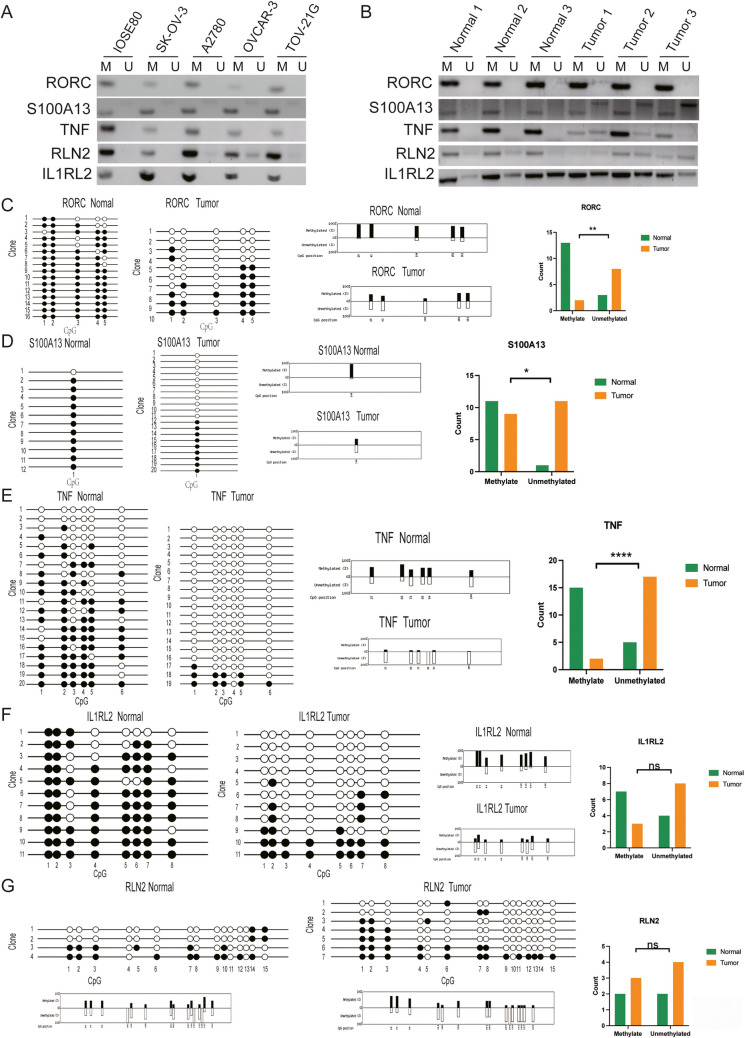
Genomic DNA was isolated from human OC tissues and cells using the TIANGEN TIANamp Genomic DNA Kit, and a microspectrophotometer was used to assert the sample’s concentration and purity. Vazyme EpiArt Methylation Bisulfite Kit was used to process the isolated DNA by converting the unmethylated C into U while maintaining the methylated C unchanged. Two pairs of primers were designed for the methylated and unmethylated DNA transformed by sulfite for five specific genes, namely, RORC|cg25112191, S100A13|cg14467840, TNF|cg04425624, RLN2cg03679581, and IL1RL2|cg22797169. An amount (60 ng) of transformed single-stranded DNAs was used for the PCR amplification using Vazyme 2 × EpiArt ® HS Taq Master Mix. The primers were designed and synthesized by Shandong Jipeng Biology Technology Company, and the primer sequences and circulation conditions are summarized in Supplementary Table 17. The PCR amplicons were visualized using 2% agarose gel electrophoresis.
Bisulfite sequencing polymerase chain reaction (BSP)
Three control ovarian tissues and three ovarian serous cystadenocarcinoma tissues were mixed separately and used for DNA extraction. The DNA extraction, sulfite transformation, and PCR amplification steps were done following the same procedures mentioned in the “Methylation‑specific polymerase chain reaction (MSP)” section. The primer sequences and circulating conditions to detect the methylation status of RORC, S100A13, TNF, RLN2, and IL1RL2 CpG islands at 25112191, 14467840, 04425624, 03679581, and 22797169 are shown in the Supplementary Table 17. A volume (8 µl) of PCR products was used for the second round of PCR amplification, and an amount (100 µl) of the resulting amplified PCR products was purified with the Tiantian Quick MIDI Purification Kit. TaKaRa pMD19-T Vector Cloning Kit was used to connect the purified PCR products. The ligation solution was transformed into DH5α competent cells, and a total of 24 colonies were selected for each sample. PCR identification of bacterial liquid by 2X Taq Plus Master Mix II (Dye Plus) was used for verification before sending the samples to Huada Gene Technology incorporated company for sequencing. The sequencing results were compared and analyzed with QUMA (http://quma.cdb.riken.jp).
Statistical analysis
For normally distributed variables, the p-value was determined using unpaired Student’s t-tests, p < 0.05 was considered statistically significant. p-values for DMS, GO, and pathway analyses were corrected with FDR and Benjamini-Hochberg’s multiple assays. The survival curve was generated by the Kaplan–Meier method, and the difference was statistically significant by the log-rank (Mantel-Cox) test, with p < 0.05 considered statistical significance. The hazard ratio of the univariate Cox proportional hazard regression model was also calculated (p < 0.05).
Results
High- and low-methylation subtypes in OC identified with ConsensusCluster
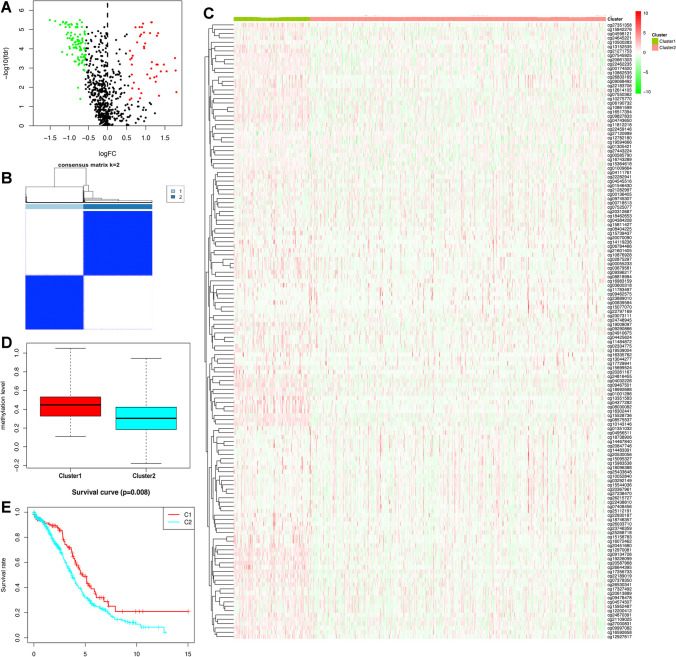
In total, 27,578 methylation sites were obtained from Illumina Human Methylation 27 K arrays (Supplementary Table 1). A total of 2448 immune-related genes were selected from the ImmPort database, including TCR signaling pathway, antigen processing and presentation, interferon receptor, chemokine receptors, chemokines, cytokines, cytokine receptors, interferons, interleukins, interleukin receptor, natural killer cell cytotoxicity, TGF-β family member receptor, BCR signaling pathway, antimicrobials, TGF-β family member, TNF receptors, and TNF family members (Supplementary Table 2). A total of 120 IRGs with 142 DMSs were identified (Fig. 2A; Supplementary Table 3). DMSs were defined as hypermethylated for probes revealing the fold-change > 1.5 and p-value < 0.05, and hypomethylated for fold-change < − 1.5 and p-value < 0.05. Thus, 97 DNA hypomethylation sites and 45 hypermethylation sites were acquired. The consensus clustering of DMSs was arranged into two methylation subtypes—hypermethylation (cluster 1) and hypomethylation (cluster 2) (Fig. 2B; Supplementary Table 4). Cluster 1 (n = 113) showed a high methylation level, and cluster 2 (n = 451) showed a low methylation level (Fig. 2C, D). Chi-square test with a p-value < 0.05 was used to compare the methylation level between the two subgroups. Figure 2C shows distinguishing immune-related methylation levels. Cluster 1 displayed a high methylation level, while cluster 2 displayed a low methylation level. The result in Fig. 2D is in agreement with Fig. 2C. Additionally, the OS curve of OC methylation subsets was acquired with the Kaplan–Meier approach (Fig. 2E), and cluster 1 showed a better prognosis than cluster 2.
Fig. 2.
The DNA methylation subtypes of ovarian cancer based on hierarchical cluster analysis of differentially methylated sites of immune-related genes. A The volcano plots of differentially methylated sites in ovarian cancer tissues compared to normal controls. B Clustering heat map of samples at consensus k = 2. Different colors reflect different cluster numbers; the color gradient is from white to blue, indicating the consensus of progression. C Clustering analysis of 142 differentially methylated sites divided into ovarian cancer samples into hypermethylation (cluster 1) and hypomethylation (cluster 2) groups. Red represents hypermethylation level, and green represents hypomethylation level. D Total methylation level between hypermethylation and hypomethylation subtypes. E The overall survival analysis between hypermethylation and hypomethylation subtypes in ovarian cancer
Immune status in different methylation subtypes
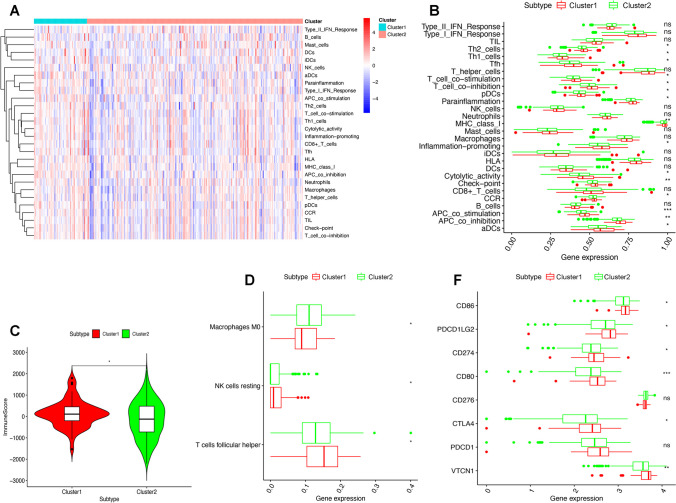
A total of 28 immune-related events were calculated using ssGSEA on the basis of RNA-seq gene expression data of the GSEABase R package (Supplementary Table 5). A high-level immune infiltration was found in cluster 1, not in cluster 2 (Fig. 3A). The biomarkers of checkpoint, aDCs, T-cell co-inhibition, APC co-inhibition, CCR, APC co-stimulation, cytolytic activity, T-cell co-stimulation, MHC class I, parainflammation, Th1 cells, pDCs, Tfh, Th2 cells, and inflammation-promoting were greatly divergent among methylation subtypes (Fig. 3B). There were obvious differences between the two methylation subtypes in the immune score based on ESTIMATE algorithm (Fig. 3C; Supplementary Table 6). LM22 gene signature and CIBERSORT algorithm were used to identify 22 human immune cell phenotypes with high specificity and sensitivity and evaluate immune cell ratio in OC (Supplementary Table 7). Resting NK cells, follicular helper T cells, and M0 macrophages were significantly distributed differentially between low-risk and high-risk score groups (Fig. 3D). Obviously, the immune checkpoints including CD86, CD274, PDCD1LG2, CTLA4, CD80, and VTCN1 showed clear differences between the two subtypes (Fig. 3E; Supplementary Table 8).
Fig. 3.
Immune status between hyper- and hypomethylation subtypes of ovarian cancers. A Immune cell infiltration status between hyper- and hypomethylation subtypes of ovarian cancers. B Immune-related events based on ssGSEA between hyper- and hypomethylation subtypes of ovarian cancers. C Immune score between hyper- and hypomethylation subtypes of ovarian cancers. D Innate immune cells and adaptive immune cells between hyper- and hypomethylation subtypes of ovarian cancers. E Immune checkpoint genes between hyper- and hypomethylation subtypes of ovarian cancers. *p < 0.05, **p < 0.01, and ***p < 0.001
Significant pathways, functional characteristics, and PPI of IRG-DMSs
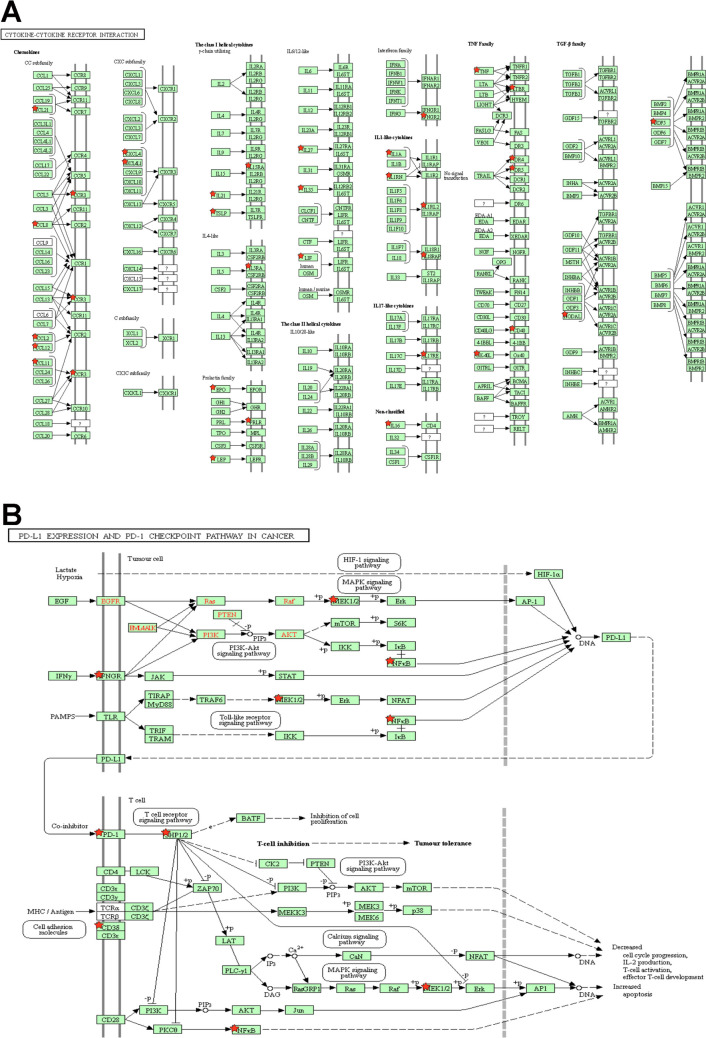
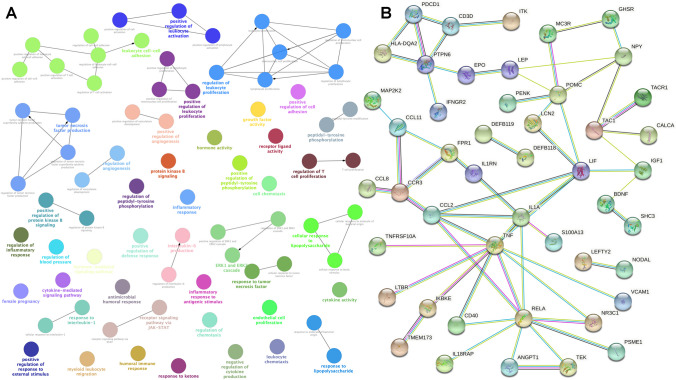
The limma analysis identified a total of 120 immune-related genes with 142 DMSs in OCs. KEGG pathway analysis of IRG-DMSs revealed 19 important signaling pathways (Fig. 4; Supplement Table 9), including viral protein-cytokine, cytokine receptor interactions and cytokine-cytokine receptor interactions, JAK-STAT, IL-17, MAPK, adipocytokine, NF-kappa B, T-cell receptor, Ras, chemokine, HIF-1, Toll-like receptor, PI3K-Akt, cAMP, Rap1 signaling pathway, PD-1 checkpoint pathway, Th17 cell differentiation, PD-L1 expression and natural killer cell–mediated cytotoxicity in cancer, and cell adhesion molecules. GO analysis of IRG-DMSs revealed 75 significant biological processes (Fig. 5A; Supplementary Table 10). The larger lymph node size corresponds to a smaller p-value (< 0.05) and a more distinct enrichment. Matching color indicates an identical function group. In these groups, representative of the most important term lag highlights were chosen, such as cytokine activity, cytokine-mediated signaling pathway, leukocyte cell–cell adhesion, myeloid leukocyte migration, negative regulation of cytokine production, positive regulation of lymphocyte activation, protein kinase B signaling, receptor signaling pathway via JAK-STAT, tumor necrosis factor production, and T-cell proliferation regulation as well as leukocyte. The IRG-DMSs were uploaded to STRING for PPI analysis. The comprehensive score of each node ranged from 0.900 to 0.999 (Fig. 5B; Supplementary Table 11). Some PPI network gave high-combined scores (> 0.99), such as RELA and TNF, TNF and RELA, CCL2 and TNF, NR3C1 and RELA, RELA and NR3C1, TNF and CCL2, IL1A and S100A13, S100A13 and IL1A, MC3R and POMC, POMC and MC3R, CD40 and RELA, RELA and CD40, ANGPT1 and TEK, CCL11 and CCR3, CCR3 and CCL11, TAC1 and TACR1, TACR1 and TAC1, and TEK and ANGPT1.
Fig. 4.
DNA methylation–mediated signaling pathway changes identified with DMSs of immune-related genes in ovarian cancers. A DNA methylation–mediated cytokine-cytokine receptor interaction pathway changes in ovarian cancer. B DNA methylation–mediated PD-L1 expression and PD-1 checkpoint pathway changes in ovarian cancers
Fig. 5.
DNA methylation–mediated biology processes and protein–protein interaction (PPI) network identified with DMSs of immune-related genes in ovarian cancers. A The significant biology processes of DMSs of immune-related genes in ovarian cancers. B The PPI network of DMSs of immune-related genes in ovarian cancers
Determination of methylation-driven genes
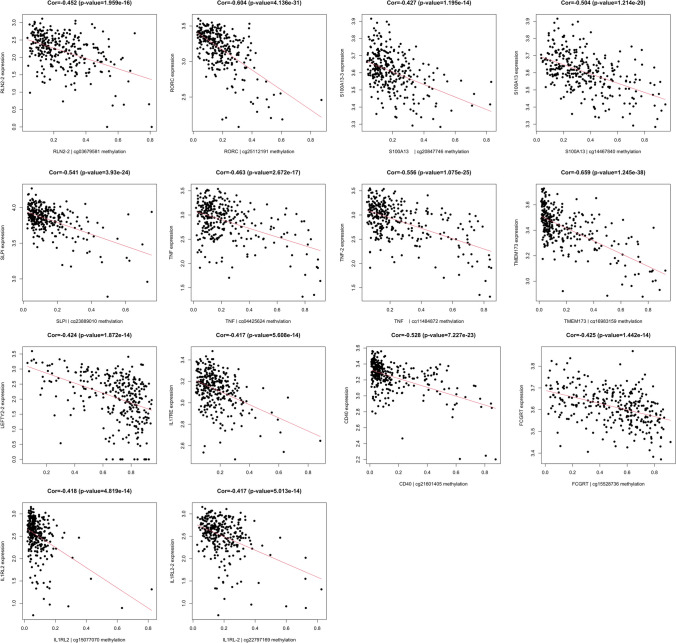
All DMSs and mRNA expressions of immune-related genes were selected (Supplementary Table 12). The correlation coefficient between mRNA expression and methylation of immune-related genes was calculated using the Pearson correlation coefficient (Supplementary Table 13). Some pairs of methylations and mRNA expressions of immune-related genes showed high negative correlation (p < 0.05; correlation coefficient < − 0.4), which were identified as methylation-driven genes (Fig. 6), including TMEM173 (Cor = − 0.659, p = 1.25E − 38), RORC (Cor = − 0.604, p = 4.14E − 31), TNF-2 (Cor = − 0.556, p = 1.08E − 25), SLPI (Cor = − 0.541, p = 3.93E − 24), CD40 (Cor = − 0.528, p = 7.23E − 23), S100A13 (Cor = − 0.504, p = 1.21E − 20), TNF (Cor = − 0.463, p = 2.67E − 17), RLN2-2 (Cor = − 0.452, p = 1.96E − 16), S100A13-3 (Cor = − 0.427, p = 1.20E − 14), FCGRT (Cor = − 0.425, p = 1.44E − 14), LEFTY2-2 (Cor = − 0.424, p = 1.87E − 14), IL1RL2 (Cor = − 0.418, p = 4.82E − 14), IL17RE (Cor = − 0.417, p = 5.61E − 14), and IL1RL-2 (Cor = − 0.417, p = 5.01E − 14).
Fig. 6.
The correlation coefficient between methylation and mRNA expression of immune-related genes in ovarian cancers
The drug sensitivity of methylation-driven genes
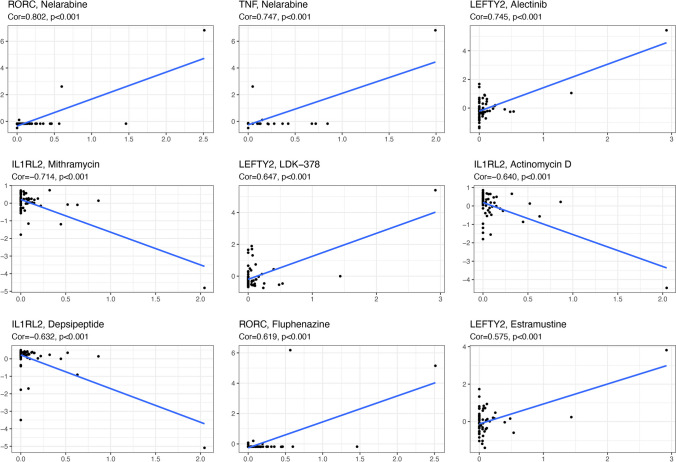
Some identified methylation-driven genes gave information about meaningful associations with drug sensibility with p < 0.05 (Supplementary Tables 14), such as RORC and nelarabine (Cor = 0.802, p = 1.33E − 14), TNF and nelarabine (Cor = 0.747, p = 6.94E − 12), LEFTY2 and alectinib (Cor = 0.744, p = 8.75E − 12), IL1RL2 and mithramycin (Cor = − 0.713, p = 1.51E − 10), LEFTY2 and LDK-378 (Cor = 0.647, p = 2.31E − 08), IL1RL2 and actinomycin D (Cor = − 0.640, p = 3.61E − 08), IL1RL2 and depsipeptide (Cor = − 0.631, p = 6.23E − 08), and RORC and fluphenazine (Cor = 0.618, p = 1.36E − 07). Some of them were plotted (Fig. 7).
Fig. 7.
The drug sensitivity of the identified methylation-driven sites in ovarian cancers
Build a risk-scoring model based on 5 methylation-driven sites
Fourteen overlapping genes in the Cox regression model were utilized to find the gene that most accurately predicts methylation, including TMEM173|cg16983159, RORC|cg25112191, TNF|cg11484872, SLPI|cg23889010, CD40|cg21601405, S100A13|cg20847746, TNF|cg04425624, RLN2|cg03679581, S100A13|cg14467840, FCGRT|cg15528736, LEFTY2|cg22462235, IL1RL2|cg22797169, IL17RE|cg15095327, and IL1RL2|cg15077070.
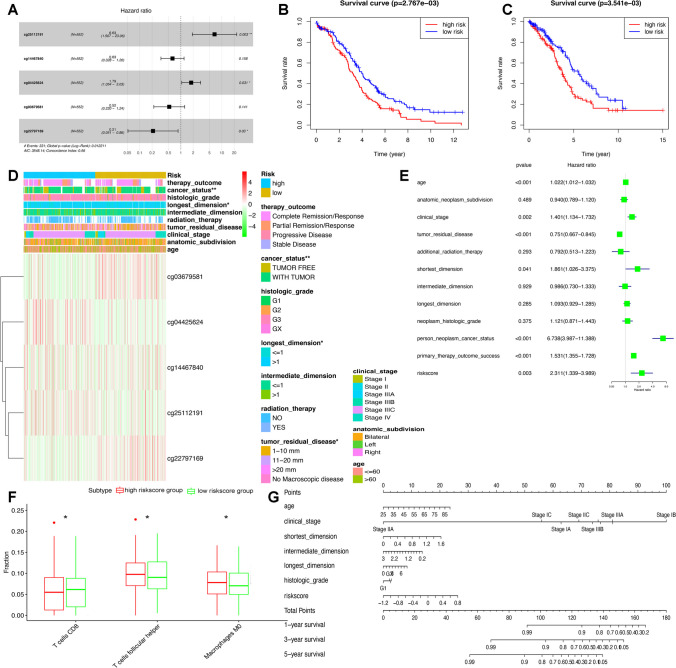
We randomly divided the OC samples into two groups: train (n = 276) and test (n = 276) groups. The risk scores based on the DMSs of methylation-driven genes further split the groups into high- and low-risk score groups (Supplementary Table 15). A set of five DMSs (RORC|cg25112191, S100A13|cg14467840, TNF|cg04425624, RLN2|cg03679581, and IL1RL2|cg22797169) was discovered to elevate the risk of a bad prognosis in OC through the Cox regression analysis (Fig. 8A). The risk-scoring formula was computed as follows: risk score = (1.8918* RORC|cg25112191) + (− 0.4667*S100A13|cg14467840) + (0.5812* TNF|cg04425624) + (− 0.6500* RLN2|cg03679581) + (− 1.5596* IL1RL2|cg22797169).
Fig. 8.
Construction of risk score model based on five methylation-driven sites in ovarian cancer. A Cox regression identified the prognostic model in ovarian cancer. B Overall survival analysis between high- and low-risk score groups in train cohort. C Overall survival analysis between high- and low-risk score groups in test cohort. D The heatmap of clinical features between high- and low-risk score groups. E The univariate Cox regression analysis of risk factors in ovarian cancer. F The different immune cells between high- and low-risk score groups. G The risk score assessment nomogram to evaluate prognosis in ovarian cancer (1-, 3-, and 5-year survival rates). *p < 0.05, **p < 0.01, and ***p < 0.001
In both the train and test cohorts in OC, the Kaplan–Meier approach was employed for OS determination comparing high- and low-risk score groups. KM curve revealed that risk scores of the training group and test group were significantly associated with OC overall survival (Fig. 8B, C). Heat maps showed a significant association between risk groups and clinical features (Supplementary Table 16), including cancer status, longest dimension, tumor residual disease, and risk score (Fig. 8D). Furthermore, the univariate Cox regression investigation discovered that age at diagnosis, clinical stage, cancer residual disease, shortest dimension, cancer status, therapy outcomes, and risk score were significantly connected with OS (Fig. 8E). Additionally, CD8 T cells, follicular helper T cells, and M0 macrophages were significantly distributed differentially between high- and low-risk score groups (Fig. 8F). The nomogram was created to provide a more straightforward and user-friendly way for determining patient survival rates based on basic clinical data along with risk score (Fig. 8G).
Evaluation of methylation status of 5 methylation-driven sites
MSP analysis revealed that all five immune-related genes were methylated in OC cells (Fig. 9A) and tissues (Fig. 9B). Specifically, BSP sequencing analysis revealed that 5 immune-related genes were hypomethylated in overall cancer tissues compared to control ovarian tissues. The methylation rates of the five CpG sites of RORC in the control ovarian tissue were 93.75%, 100%, 81.25%, 81.25%, and 75%. The methylation rates in OC tissues were 50%, 30%, 20%, 60%, and 65% (Fig. 9C). In the third CpG site RORC|cg25112191, the methylation rate in overall survival was the lowest, and a significant difference was discovered in OC in comparison to control tissues (p = 0.0040). The methylation rate of S100A13|cg14467840 was 91.7% in control ovarian tissue and 45% in OC tissue, with a significant divergence in methylation rate in control ovarian tissue compared to OC tissue (p = 0.0107) (Fig. 9D). The methylation rates of the six CpG sites of TNF were 60%, 75%, 45%, 55%, 55%, and 40% in control ovarian tissues and 15.8%, 10.5%, 10.5%, 0, 10.5%, and 5.3% in OC tissues, and the second CpG site TNF|cg04425624 had significant divergence in methylation rate between control ovarian tissue compared to OC tissues (p < 0.0001) (Fig. 9E). The methylation rates of 8 CpG sites of IL1RL2 were 100%, 100%, 54.5%, 72.3%, 72.3%, 81.8%, 90.9%, and 63.6% in control ovarian tissues and 27.3%, 54.5%, 18.2%, 18.2%, 27.3%, 18.2%, 45.5%, and 27.3% in OC tissues (Fig. 9F); the eighth CpG site IL1RL2|cg22797169 showed a low methylation rate in OC without significant variation (p > 0.05). The 15 CpG sites of RLN2 methylation rates were 50%, 50%, 50%, 0, 33.3%, 25%, 50%, 25%, 25%, 50%, 0, 25%, 0, 75%, and 50% in control ovarian tissues and 71.4%, 71.4%, 57.1%, 28.6%, 16.7%, 42.9%, 42.9%, 42.9%, 14.3%, 0, 14.3%, 14.3%, 14.3%, 14.3%, and 14.3% in OC tissues; the seventh CpG site RLN2|cg03679581 had a low methylation rate in OC tissues without significant difference (p > 0.05) (Fig. 9G).
Fig. 9.
Experimental verification of methylation status of five immune-related gene CpG sites in human ovarian cancer tissue. A MSP qualitative analysis of methylation at CpG sites of five immune-related genes in ovarian cancer cells vs. control cells. B MSP qualitative analysis of methylation at CpG sites of five immune-related genes in ovarian cancer tissues vs. control tissues. C BSP quantitative analysis of methylation at RORC CpG sites in ovarian cancer tissues vs. control tissues. D BSP quantitative analysis of methylation at S100A13 CpG sites in ovarian cancer tissues vs. control tissues. E BSP quantitative analysis of methylation at TNF CpG sites in ovarian cancer tissues vs. control tissues. F BSP quantitative analysis of methylation at IL1RL2 CpG sites in ovarian cancer tissues vs. control tissues. G BSP quantitative analysis of methylation at RLN2 CpG site in ovarian cancer tissues vs. controls
Discussion
Importance of methylation in OC
Methylation is an epigenetic modification, which can change DNA stability and chromatin structure and regulate gene transcription and expression, thus having a great impact on the development of tumors [41]. Therefore, methylation has high specificity and sensitivity and is suitable for diagnosis as a biomarker. Due to its rapid development, heterogeneity, and lack of appropriate early detection methods, OC has a very high mortality rate. Gene methylation is important in the development of OC, and blood methylation can also be used for OC early detection [42, 43]. Methylation detection of multiple genes is generally more sensitive than the detection of a single gene, thus making the detection of multiple gene methylation particularly important. The methylation in most OC cells presents an abnormal state of the whole genome, which leads to the disorder and mutation of tumor suppressor genes including BRCA1, OPCML, and RASSF1A. BRCA1 gene is directly involved in maintaining the stability and repair mechanism of DNA, and its disorder is closely related to the genetic susceptibility of OC. RASSF1A is a gene that regulates cell morphology, and it shows abnormal methylation in many cancers. Compared with healthy women or patients with benign OC, the methylation frequency of BRCA1, OPCML, and RASSF1A in OC is significantly higher. The expression of the OPCML gene affects the aggregation and growth of OC cells, and it is a specific methylation biomarker with the best performance [44]. Methylation has important biomarker significance and affects the survival rate of patients with OC. For example, hypermethylation of FOXD3 prevents the migration and proliferation of OC cells and promotes apoptosis [45]. Studies have identified 35 different methylation sites and methylation-driven genes in OC. It has been found that LAPTM5 was an up-regulated hypomethylation gene, DLK1 was a down-regulated hypermethylation gene, and LAPTM5 was negatively correlated with 5′-UTR methylation. DLK1 encoded a reverse transcription membrane protein, which contains various EGF repeats participating in cell proliferation regulation. The overexpression of DLK1 participated in the development of OC through Notch activation and MT induction [46]. The 5-year OS rate of OC cases with highly methylated sFRP5 gene promoter was significantly lower than that of unmethylated sFRP5 [47]. Methylation of RASSF1A and ESR1 promoters was significantly correlated with OS rate [48, 49]. By detecting methylation in tumor tissues, we can judge the invasiveness, malignant degree, and metastatic ability of tumor cells and then predict the prognosis of patients. For patients with abnormal methylation, more active treatment measures are needed to improve the survival rate. When abnormal methylation occurs, OC patients may be resistant to platinum drugs, which may affect the chemotherapy and prognosis of OC patients. It was found that CPG islands of MINT25, OPCML1, HIC1, DCR1, RASSF1A, and sFRP1 had the same methylation in OC, and at least one of the three genes, BRCA1, MGMT, and GSTP1, was related to chemotherapy [50]. In another study, the hypermethylation of the sFRP5 promoter activated Wnt signal transduction in OC, promoted the invasion and growth of OC cells, and affected chemotherapy resistance of OC through AKT2 and TWIST-mediated EMT pathways [51]. In order to improve this situation, demethylation drugs have been studied to restore the sensitivity of chemotherapy-resistant OC patients to platinum drugs. By regulating methylation, these drugs may improve the prognosis of patients with OC and become potential therapeutic targets for OC.
Significance of methylation in OC TIME and immunotherapy
The TIME, which is influenced by methylation, is involved in the initiation and progress of anti-tumor immunity. For example, one study screened three differential methylation-driven genes (DMDGs) with poor prognosis in uterus corpus endometrial cancer (UCEC), namely, PARVG, CDO1, and SNE4, and built methylation driver signatures based on DMDGs to evaluate their prognosis. The results displayed divergences in immune-related pathways in low- and high-risk groups. The expression levels of M0 macrophages and M2 macrophages were increased significantly in the high-risk group, while the CD8 T cells, neutrophils, and eosinophils were increased in the low-risk group. Some molecules including PD-1, PD-L2, PD-L1, CD86, CTLA4, LAG3, CD70, CD270, and CD58 were highly expressed, and the independent prognostic index was negatively correlated with the gene expressions of these immune detection points, which showed that methylation is closely linked to UCEC TIME, thus providing a novel perspective for the potential mechanism of prognosis of UCEC [52]. One study collected methylation data and other information from 451 patients with gliomas and screened autophagy-related genes related to prognosis and their methylation to establish a risk prediction model. A model constructed with WIPI2, ARSB, RB1, CFLAR, RAB24, and ERN1 showed a good prognostic prediction effect on low-grade gliomas. In addition, the expressions of seven immune checkpoints (LAG3, PD-1, TIM3, PD-L1, B7H3, PD-L2, and CTLA4) and the level of immune cell infiltration were also examined. The expression of M2 macrophages, Tregs, CD4 T cells, resting NK cells, and resting mast cells in immune checkpoints was significantly increased, while activated mast cells, plasma cells, and naive CD4 T cells were significantly decreased. The methylation of six genes had strong predictive potential in low-grade gliomas, and methylation regulation had an important influence on the TIME of low-grade gliomas [53]. Another study used 350 pancreatic cancer (PACA) samples from the database and seventy-five methylation-driven genes (MDGs). Four genes (GPRC5A, ARNTL2, S100A14, and SOWAHC) were selected as prognostic genes to establish and verify the prediction model. In the low-group CD8 + T, M0 macrophages, and Treg had low infiltration; VEGFA, TGFB1, and PD-L1 were positively associated with the risk score; and MDGs were associated with the immunosuppressive microenvironment of PACA, which provided a basis for the prediction and treatment of PACA [54]. Also, the study used 242 DMGs and found that the function-enriched DMGs participated in some immune-related pathways, including regulation of antigen presentation and B-cell receptor signaling, Th17 cell differentiation, and cytokine-cytokine receptor interaction [46]. Altogether these studies provide support for more accurate immunotherapy strategies for OC patients. Methylation may affect the infiltration and prognosis of immune cells, thus affecting the effect of immunotherapy. Some components in TIME can promote tumor growth, while others can inhibit tumor growth, and their interaction determines the trend of anti-tumor immunity. Tumor immunotherapy has become one of the most promising treatment methods by overcoming tumor-induced immunosuppression and enhancing individual anti-tumor immune responses. A new binding method is to enhance the activity of an immune checkpoint inhibitor (ICI) by inhibiting methylation and identifying immune activation markers in vivo. Some studies have shown that in myeloid leukemia [55], with the increase of methyltransferase inhibitors, the gene expressions of immune checkpoints, such as PD-1, CTLA4, and PD-L1, were up-regulated. DNA methyltransferase inhibitors (DNMTis) significantly increased local infiltration of lymphocytes, thus improving anti-tumor efficacy [56]. One study found that EMT mediated by DNMT1 and H3K27me3 mediated by ezh2 inhibited the production of Th1 chemokines and that the epigenetic silencing of Th1 chemokines might be a new mechanism of tumor immune escape [57]. The comprehensive analysis of the data of 21 tumor types revealed that the global methylation level may directly affect the signal transduction and effector function of immune cells, such as immune infiltration, cytochrome-cytochrome receptor interaction, antigen processing and presentation, MHC, and interferon. The global hypomethylation was related to immune evasion, which reduced the effect of tumor immunity. Finally, a scheme for combining epigenetics and immunotherapy was proposed [58]. A study on methylation in different immune cells, lymphomas, and myeloid leukemia found that the methylation level of B cells and T lymphocytes was decreased but increased in macrophages. Methylation contributed to the development of lymphoma including leukemia, a fact that might affect the effect of demethylation agents in leukemia and lymphoma [59]. The immune system can naturally eliminate tumor cells; however, TIME gradually forms an immunosuppressive state. In a research work, DNMTi reversed the microenvironment of immunosuppressive tumors in OC by inducing IFN signal transduction, promoting the transcription of immunogenic dsRNA and the secretion of pro-inflammatory chemokines, and inducing lymphocyte migration. Also, adenosine deaminase 1’s (ADAR1) ability to edit dsRNA was demonstrated, and its knockout elevated the sensitivity of cancer cells to IFN. A combination therapy of knockout ADAR1 and DNMTi increased lymphocytes in TIME, including NK and CD8 + T cells, controlled the growth of cancers, and extended the survival rate [60]. Methylation may also affect the TIME by regulating the metabolic pathway of OC cells. Methylation may regulate the metabolic process of OC cells by affecting the expression of metabolism-related genes and then affect their interaction with immune cells. This interaction may include the recognition and response of immune cells to the metabolites of cancer cells and the subsequent immune effects. The effect of methylation on the TIME of OC is a complex process, involving the interaction of multiple genes, signal pathways, and cell types. Therefore, to fully understand this process, further research and exploration are needed. At the same time, the therapeutic strategy for methylation may also provide new ideas and methods for the treatment of OC. By regulating methylation, it is possible to restore the balance of TIME and enhance the killing ability of immune cells to OC cells, thus achieving the purpose of treating tumors. To summarize, the combination of epigenetic modifiers with vaccines, ICI, or other immunization strategies is being actively studied in clinics. Therefore, epigenome will become an important therapeutic target for cancer.
The significance of the identified DMSs in OC
In this study, 142 immune-related DMSs were obtained, which affected the proliferation and invasion of OC cells. For example, ANGPT1 expression was increased in OC, which promoted tumor angiogenesis and the aggregation of cancer-related fibroblasts in the OC microenvironment, and enhanced the invasion and proliferation of cancers in vivo, and BDNF can activate TrkB/PLCγ1 signaling pathway and inhibit apoptosis to stimulate the invasion and proliferation of tumors [61, 62]. Some DMSs can be used as prognostic markers of OC. A study detected LEP expression quantity in 208 samples, measured the methylation of LEP in 63 OC tissues, and evaluated the LEP methylation in OC along with its pertinence with clinical pathology and prognosis. These results showed that the expression pattern of No/low LEP protein was significantly related to the hypermethylation of the CpG island of LEP promoter, and the overexpression of LEP affected tumor subtype, tumor size, and staging. Thus, indicating that hypermethylation of LEP promoter regulated the expression of LEP [63]. TRAIL induced apoptosis in transformed tissues, and the increased TRAIL expression was related to good OS in OC [64]. The methylation of the TRAIL receptor occurred frequently in OC, and the decrease of TRAIL receptor expression leads to the accelerated growth of tumors. The key to inhibiting the progress and expansion of the cancer was the non-impairment of TRAIL receptor activity. Therefore, TRAIL is a tumor detection factor of OC and plays a major role [64]. Members of the TNF receptor superfamily, such as CD40, were among the DMS identified in this study. CD40 regulates humoral and cellular immunity. In OC, CD40 represses the development of cancer cells, promotes cell apoptosis, promotes the generation of IL-8 and IL-6, and activates NF-κB and JNK pathways [65]. Cytokines were also part of the DMSs identified in the current study. The level of IL-16 in malignant and late OC is higher than that in early and benign OC; furthermore, its high expression in late OC is connected to increased OC microvessels [66]. Some of the obtained DMSs were chemokines or chemokine receptors, including CCL11 and its receptors CCR3 and CCL2 [67]. CCL11 can bind to CCR3 and is secreted by chemotactic eosinophils, Th2 lymphocytes, basophils, dendritic cells, and mast cells through CCR3. CCL2 can also bind to CCR3, and CCL2 is secreted by T cells, monocytes, fibroblasts, and tumor cells. Chemokines regulate angiogenesis, proliferation, tumor immune response, tumor infiltration, and migration of tumor cells by binding to GPCRs. Previous studies have found that chemokines, such as overexpression of CCL11 and its receptor OC drug resistance [67], may stimulate the migration and proliferation of OC cells by activating MEK1, STAT3 phosphoproteins, KINSE 1/2, and other cytokines. Neutralizing antibodies can inhibit the tumor-promoting effect of CCL11 and raise the sensitivity of OC to cisplatin, indicating that targeting CCL11 can be a new way to treat OC and that CCL11 can serve as a biomarker and prognostic factor of OC [67]. CCL2 and its receptor CCR2 are highly expressed in paclitaxel-resistant OC. CCL2-CCR2 signal axis might induce paclitaxel resistance by activating NF-κB and PI3K-Akt pathways and might also induce OC resistance by inducing macrophage chemotaxis [68]. Targeting CCL11 and its receptors is a novel and resultful method to treat OC, given that CCL11 has pro-tumor effects and interacts with stromal cells to promote angiogenesis and remodeling. To sum up, the immune-related DMSs identified in this investigation play a role in OC occurrence, progression, and treatment.
Associations of methylation with differential immune cells/differential immune checkpoints in OC
The differential immune cells identified in this study were resting NK cells, follicular helper T cells, and M0 macrophages, while the differential immune checkpoints were CTL A4, CD80, CD274 (PD-L1), PDC D1LG 2 (PD-L2), CD86, and VTCN1. NK cells constituted heterogeneous groups, showing a state of rest, memory, activation, fatigue, and repression. Methylation regulated NK cell–mediated anti-tumor immunity, such as NK cells obtaining higher IFN-γ through hypomethylation of IFN-γ, which ultimately affected the immune regulation function of IFN-γ [69]. The gene expression of many NK cell receptors was related to methylation. For example, methylation maintained the expressions of KIR and 3DL1 in NK cells, and KIR and MHC class I molecules enabled NK cells to identify unusual cells [70]. Methylation mediated the expression of Siglec-7 in NK cells through CpG 8 and CpG 9, and Siglec-7 induced inhibitory signals to inhibit the function of NK cell effector, thus causing tumor immune escape [71]. In the mouse model, DNMTi targeted OC to recruit NK and CD8 + T cells to eliminate the cancer cells [72]. Some studies used WGCNA to discover the key genes in connection with the immune score in 376 cases of OCs. It was further found that these genes were actively correlated with M1 macrophages and CD8 T cells and inactively correlated with activated dendritic cells (DCs) and M0 macrophages. TFH cells were highly represented, which could better predict the prognosis of OC [73]. Methylation participates in the metabolic reprogramming of M1-like macrophages and promotes tumor metastasis [74]. The expression of TGR5 is relevant to M2 macrophage immune infiltration level and methylation in OC. Indeed, TGR5 could activate NF-κB, Akt, and other pathways and regulate the activation of immune cells and cytokines [75]. DNMTi 5-Aza-CdR stimulates macrophages to switch to the M1 phenotype, activates T cells, and inhibits tumor progression [76]. TFH cells hold a crucial part in long-lived antibody production, which contributes to the success of vaccination. Basically, TET2-mediated demethylation activates the downstream Runx1 and Foxo1 signal pathways which increases the production of THF cells [77]. BCL6 is the principal transcription factor in TFH cell differentiation and proliferation. Hypermethylation generated by TET2 mutation causes the expression of BCL6 to be out of control, which in turn affects the differentiation of TFH cells [78]. Immunological checkpoints such as CD80, CTLA4, and CD274 were up-regulated in the high hypoxia group of OC, which provides a new immunotherapy target for OC. In addition, CTLA4, CD274, and PDCD1LG2 were inactively correlated with OC risk score, and the accumulation of soluble PD-L1 in the peritoneal cavity and PD-L1/PD-L2 in tissues of OC in MDCs, PDCs, and MA might be a sign of immune modulation in OC [79–81]. CTLA4 and its ligands CD80 and CD86 were the main co-stimulatory molecules of the B7 family, which regulate T-cell differentiation. Methylation regulates the mRNA expressions of CTLA4, CD80, and CD86, CD8 + T-cell infiltration, and IFN-γ characteristics, all related to the prognosis [82]. Methylation also regulates the mRNA expression of immune checkpoint genes such as CTLA4, PD-L1, and PD-L2 [83]. miRNA methylation intensity in OC regulates the level of sPD-L1, and the methylation intensity of the MIR9-1 gene is correlated with the OC survival rate [84]. Finally, DNMT1 improves the therapeutic effect of PD-L1 blockade in tumors [57].
Methylation-regulated signaling pathway changes in OC
Methylation regulates signaling pathway changes which play important roles in OCs. This study found that methylation-regulated signaling pathways were related to OC immunity, including JAK-STAT, IL-17, MAPK, adipocytokine, NF-kappa B, T-cell receptor, Ras, chemokine, HIF-1, Toll-like receptor, PI3K-Akt, cAMP, Rap1 signaling pathways, natural killer cell–mediated cytotoxicity, viral protein-cytokine and cytokine receptor interactions, Th17 cell differentiation, PD-L1 expression and PD-1 checkpoint pathway in cancer, and cell adhesion molecules. Previous studies reported the implication of these signaling pathways in tumor immunity, MET, and other events. For example, it has been shown that MET of OC was enriched in MAPK, JAK-STAT, Toll-like receptor, cytokine-cytokine receptor interaction pathways, and T-cell receptor signaling pathway [85, 86]. The activation of Toll-like receptors on OC cells by inflammatory proteins through MAPK and NF-κB leads to invasive phenotype and OC progress [87–89]. In gastric adenocarcinoma and cutaneous melanoma, DNMT3B and DNMT2 significantly affected the expression of TRL4 [90]. Methylation was involved in the process that some plasma cells up-regulate IL-10 through the TRL mechanism [91]. One study established a TLR-based gene signature that was significantly related to tumor prognosis and microenvironment in hepatocellular carcinoma, including MAP2K2, MAP3K7, TRAF3, SPP1, RAC1, and IRAK1, consistent with the results of MAP2K2 and SPP1 genes found on TLR pathway in the current study. In addition, knocking down MAP2K2 affected TLR function and promoted apoptosis of liver cancer cells [92]. JAK-STAT pathway mediated anti-proliferative response and IFN regulatory events, adjusted cytokine signals, and was related to drug resistance [93]. Methylation of DNA promoter determined the expression of the JAK-STAT signaling pathway in breast cancer stem cells [94]. The activation of the JAK-STAT signaling pathway promoted the development of OC leading to poor prognosis [95]. MAPK signaling pathway induced Fas ligand to induce apoptosis of OC cells and responded to chemotherapy drugs [96], while DNA hypermethylation regulated SOS2, KRAS, and ERBB4 in GRB7 and MAPK/ERK signaling pathways by silencing miR-193a-3p, thus enhancing the carcinogenicity of OC [97]. The signal intensity of the T-cell receptor and methylation of the Bim promoter predicted the differentiation and death of CD8 + T cells [98]. The activation of the TCR signal enriched DNMT1 and DNMT3b at the Foxp3 + site, increased CpG methylation, inhibited Foxp3 transcription, and induced regulatory T-cell differentiation [99].
A study used 298 OC patients to establish a weighted gene co-expression network analysis, which found that genes in the module were basically enriched in cytokine-cytokine receptor interaction and chemokine signaling pathways [100]. In OC, overexpressed IL1B, KIT, TNFRST11B, and BMPR2 were enriched in the cytokine-cytokine receptor interaction pathway. These differential gene data sets were related to OS, including chemokine receptor, CXCL family, and IL member–coding proteins, which were encapsulated in immune cell membranes, including NK cells and T cells [101]. The current study found that 29 genes were enriched in this pathway, including IL members and the TNF family and its receptors, which was consistent with the above conclusions. NF-κB is a transcription factor family, which regulates the expression of more than 400 genes. NF-κB signaling pathway involves complex cascade events triggered by immune signaling proteins and is involved in promoting apoptosis, angiogenesis, proliferation, and inflammation of many cancers, including OC [102]. Perfluorooctanoic acid (PFOA) treatment can induce the up-regulation of MMP-2/-9 expression, activate the ERK1/2/NF-κB signaling pathway, stimulate the migration and invasion of OC cells, and also eliminate this reaction by inhibiting NF-κB or ERK1/2 signaling pathway [103]. CD40, a member of the TNF receptor family, and TNE were some of the important molecules in the NF-κB signaling pathway identified in the current study. Some studies found that CD40 was expressed in OC. Briefly, CD40 binding instigated IL-6 and IL-8, which activated the NF-κB signaling pathway, indicating that CD40 affected OC cytokines, cell growth, and apoptosis, as well as OC phenotype and survival rate, and had certain functional roles in OC [104]. HOTAIR-related methylation was resistant to carboplatin [105]. It is noteworthy that the methylation regulation signaling pathway was relevant to OC immunity in this study, which showed that multiple immune cell-related genes participated in multiple significant signaling pathways corresponding to multiple methylation sites. For instance, TNF has a role in natural killer cell–mediated cytotoxicity, viral protein interactions with cytokines and cytokine receptors, cytokine-cytokine receptor interactions, and some signaling pathways (IL-17, MAPK, adipocytokine, toll-like receptor, NF-kappa B, T-cell receptor as well as other signaling pathways). MAP2K2 was expressed in natural killer cell–mediated cytotoxicity, PD-L1 expression and PD-1 checkpoint pathway, MAPK, HIF-1, Toll-like receptor, T-cell receptor, Ras, PI3K-Akt signaling pathways in cancer, cAMP signaling pathway, and Rap1 signaling pathway. Thereby, these genes might play important roles in OC.
Association of methylation with drug sensitivity in OC
Targeting tumor microenvironment (TME) is an important means to assist tumor cell chemotherapy and molecular therapy. Methylation affects tumor drug sensitivity and resistance. For example, a study showed that the methylation of SAMHD1 promoter in T-ALL was significantly higher than that in B-ALL, and the sensitivity of corresponding T-ALL cells to nelarabine was also higher than that in B-ALL, indicating that the methylation of SAMHD1 promoter affected the sensitivity of tumor cells to nelarabine [106]. Asparaginase therapy played an important part in the chemotherapy of T-ALL patients and was a biomarker of poor prognosis in children with T-ALL. The promoter of the asparaginase gene had a typical CpG island, and the hypermethylation of ASNS was significantly more sensitive to asparaginase. The hypomethylation of ASNS was related to the drug resistance of asparaginase [107]. Methylation plays an important role in drug biosynthesis and metabolism. Mithramycin contains 9 methyl groups, belonging to the aromatic polyketone family, which is methylated twice in its synthesis process. It was established that the aromatic C-7 of MtmMII methyltransferase is used to introduce the methyl groups so that the synthesized mithramycin can reach the nucleus and combine with the DNA [108]. In addition, CpG methylation enhances covalent DNA change through doxorubicin [109]. Some drugs also affect the methylation of tumor cells. For instance, bortezomib inhibits proteasome activity and is used in various myeloma treatments. It prevents DNMT1 gene promoter and Sp1/NF-κB complex combination and induces DNA hypomethylation of cancer cells [110]. Dimethylaminoporphanolide (DMAPT) is an epigenetic regulator, which strongly inhibits NF-κB, increases the levels of NSD1 of SETD2 and H3K36 trimethyl enzymes, and directly increases the trimethylation of KMT5C and H4K20 trimethyl enzymes [111].
In OC, different genes have different sensitivities to different drugs. For example, the RGS family is the drug target of malignant tumors, and some studies probed into RGS family genes on OC prognosis [112]. Some genes showed a positive association with immune cell scores, including RGS18 and RGS19. Patients with high expression of RGS18 were more sensitive to nelarabine and cyclophosphamide, while those with high expression of RGS19 were more sensitive to nelarabine and cladribine [112]. Some drugs affected the progress of OC. For example, a study found that L-asparaginase could damage tumor cells and cause autophagy by inhibiting the angiogenesis activity and invasiveness of OC, leading L-asparaginase to be considered a target for the treatment of OC [113]. Another study determined that global DNA hypermethylation was significantly related to platinum resistance, poor prognosis, and recurrence of OC. Combined therapy with demethylation agents such as decitabine prevented the progress of the G2/M cell cycle, delayed the development of OC tumor cells, and increased cisplatin-mediated cytotoxicity [114].
Carboplatin/paclitaxel combination therapy is the main chemotherapy scheme for OC [115]. The expression of S100A10 was positively associated with carboplatin resistance and prognosis. Knockout of MCM2 heightened the sensitivity of OC cells to carboplatin, and the combination therapy of MCM2 knockout and carboplatin up-regulated p53 and significantly improved the sensibility of OC cells [116, 117]. COL5A1 was overexpressed in paclitaxel-resistant OC cells. COL5A1 promoted tumor migration and proliferation, and knocking out COL5A1 could lower OC cells’ drug resistance to paclitaxel [118]. SNHG5 enhanced the sensitivity of OC cells to paclitaxel, which provided a new mechanism for OC chemotherapy [119].
The model significance of five-gene methylation signature in OC
In the current study, five IRG-DMSs (RORC|cg25112191, S100A13|cg14467840, TNF|cg04425624, RLN2|cg03679581, and IL1RL2|cg22797169) were found to be hypomethylated in OC and to elevate the risk of bad outcome in OC. Hypomethylation in the promoter region was found in many tumors, and the methylation status regulated the mRNA expression. Generally speaking, hypomethylation in the promoter region increases the expression of the related gene in cancer, which affects cancer cell proliferation, impacting patient survival. Five-gene expressions in cancers were retrieved through the GEPIA (http://Gepia.cancer-pku.cn) website, and it was discovered that all five genes were up-regulated in OC. RORC, TNF, and RLN2 were significantly up-regulated in OC (Supporting Fig. 9). We also found the methylation status of 5 genes whose methylation status was less studied in tumors. RORC, also known as RORγ, has the function of a DNA-binding transcription factor. A study ascertained that the hypomethylation state of the RORC promoter was associated with asthma [120]. A study evaluated abnormally methylated genes in OC and noted that TNF was the hub gene of OC hypomethylated up-regulated genes. They further verified that the methylation of TNF was negatively correlated with TNF mRNA levels, the higher the TNF level, the longer the OS and PPS time. TNF is considered the target of OC epigenetic diagnosis and treatment and also a potential marker for OC prognosis evaluation [121]. Significant hypomethylation of TNF in Crohn’s disease participates in the immune pathway of IL-17 [122]. S100A13 belongs to the S100 family. A study determined that DMS in diabetic retinopathy (DR) patients exists in the S100A13 gene, and DMS in S100A13 was considered to be a potential biomarker for DR [123]. In Crohn’s disease, S100A13 alters immune activation, and its methylation was significantly altered compared to healthy controls. No relevant literature on methylation of IL1RL2 and RLN2 was found.
RORC is also a key specific transcription factor of T helper cell subsets and participates in regulating cell metastasis and proliferation and chemotherapy resistance of various tumors [124]. A study showed that the expression of RORC in breast cancer is decreased, which was also negatively associated with the histological grade of the human cohort [125]. Another study on bladder cancer found that RORC expression was down-regulated and significantly low in patients with high-stage cancer and chemotherapy resistance, and cell proliferation and chemotherapy resistance were regulated by inhibiting the PD-L1/ITGB6/STAT3 signal axis [126]. The results are consistent with this study, and the methylation driving sites (Cor = 0.802, p = 1.33E − 14) of RORC and nelarabine were significantly related to drug sensitivity. TNF initiated different cellular reactions through different signal transduction pathways; activated the proliferation and survival pathways of cancer cells; migrated and invaded tumor cells; caused inflammatory cells to infiltrate tumors, promote angiogenesis, and induce cancer cell death; and paved the way for more selective targeting of TNF signals in tumor therapy. In NSCLC, TNF was up-regulated rapidly under the inhibition of EGFR, which resulted in the activation of NF-κB. TNF up-regulation and NF-κB activation were involved in mediating the resistance of NSCLC to EGFR inhibition. In the experimental model, the combined TNF and EGFR inhibition overcame the treatment resistance. Drugs that inhibit TNF might be helpful for combined targeted therapy of NSCLC [127]. S100A13 belongs to the S100 family, and the up-regulation of S100A13 was negatively correlated with progression-free survival and disease-specific survival of oral squamous cell carcinoma (OSCC) patients. Genome enrichment analysis gave information on the increased number of DNA repair genes in the high S100A13 expression group. Knockout of S100A13 raised the sensitivity of cisplatin, while overexpression reduced the sensitivity [128]. The OS rate of patients with S100A13 overexpression in NSCLC was significantly lower. Multivariate analysis revealed that the overexpression of S100A13 was an independent factor for the poor prognosis of early NSCLC [129]. One study explored the effect of relaxin 2 (RLN2) on the human endometrial carcinoma cell line, and the results indicated that RLN2 induced the invasion of Ishikawa and hec-1B cells. RXFP1 siRNA and XAV939 inhibited cell invasion induced by rln2. The immunoreactivity of RLN2 was examined in human endometrial carcinoma cells, and it was actively associated with histological grade [130]. Interleukin-1 receptor-like 2 (IL-1RL2) participated in inflammatory reaction and was further linked to breast cancer distant metastasis and TME [131]. In colorectal cancer, IL1RL2 was up-regulated in contrast to normal tissues [132]. To sum up, the five DMSs (RORC|CG25112191, S100A13|CG14467840, TNF|CG04425624, RLN2|CG03679581, and IL1RL2|CG22797169) identified in the current study were all related to immune microenvironment and OS rate.
Future perspectives
The completion of human genome sequencing has promoted the implementation of the human epigenome project, which aims to map the methylation variable sites of the human genome. Since then, epigenome research has provided new research ideas for cancer. More and more genomic information has been used for disease prediction, prevention, and personalized medical care, which has laid the foundation for the development of the 3P medical care era. In the development of PPPM, the methods of screening and early diagnosis of OC are insufficient, and the clinical treatment is not personalized enough. Methylation has become an important biomarker and prognostic evaluation index, such as methyl group analysis, and has become a powerful tool for brain tumor classification, the WHO CNS stratification can be identified according to the methylation profile, and recent technological progress, including sulfite sequencing, can realize individualized targeted drug therapy for individual genetic changes. In OC, the plasma homocysteine (Hcy) level is increased [133]. Hcy plays an important role in maintaining cell homeostasis and cell cycle progress, and its metabolism is the key way to regulate methylation. The innovative strategy under the framework of 3P medicine takes the Hcy metabolic pathway as the specific target of in vitro diagnosis, predictive medical methods, economical and effective preventive measures, multi-parameter patient stratification, and advanced screening scheme and optimizes the treatment for individualized patients with primary, secondary, and tertiary medical care. SAM, as a donor in methylation catalyzed by DNMTs, is unbalanced in the state of folic acid deficiency, which leads to methylation damage, which in turn leads to widespread hypomethylation of the genome. Hcy metabolism is helpful to the formation of folic acid and SAM. Hcy has clinical significance for the diagnosis and prognosis of chronic immune diseases and cancer, and the regulation of immune cell function and differentiation is directly affected by Hcy metabolism [134]. Although Hcy and its metabolites put forward new strategies for the prevention of diseases, methylation is still affected by gender and immune and gene mutation, and methylation affects gene transcription. In-depth study of immune-related methylation sites can deeply understand the regulatory mechanism of the genome, reveal the occurrence and development mechanism of OC, and provide new targets and new personalized targeted drugs for OC.
An article published in Cell Magazine shows that early hypomethylation-mediated gene silencing points to a specific tumorigenic pathway with biological and diagnostic significance, and the inhibition of methylation is helpful to the expression of immune monitoring genes in new tumors, to protect adjacent genes that promote cell proliferation, and to drive the early targeting of immune-rich loci [135]. A more extensive and in-depth study of immune-related methylation is of great significance for studying the molecular mechanism of OC, as well as prevention, prediction, and personalized treatment.
We suggest expanding and strengthening the study of immune-related methylation in OC from the following aspects:
(i) The model constructed in this study was applied to liquid biopsy. The analysis of cancer-related methylation and blood immune cell subsets is of great molecular diagnostic significance for non-invasive cancer detection. Many rapid, specific, and sensitive new methods for early detection of cancer methylation have been used to manage patients more effectively, thus improving their quality of life. Liquid biopsy uses urine, whole blood, peripheral white blood cells, serum, plasma, and saliva to detect the epigenetics of tumor cells in real time. To some extent, it can avoid the influence of tissue heterogeneity on tumor molecular typing and allow personalized treatment strategies. Circulating tumor cells (CTC), circulating extracellular nucleic acid (cell-free DNA; cfDNA), and circulating tumor DNA (ctDNA) can be separated from blood and other body fluids. It is used to predict the blocking reaction of the immune checkpoint, predict the response to immunization and targeted therapy, assess risk stratification, monitor the response to treatment, implement individualized adjuvant therapy and early detection of cancer stage, identify the driving factors and the chance of drug resistance mutation, and provide prognosis and prediction information (Table 1). Studies have proved that the combination of cfDNAme and CA-125 is more sensitive to the high risk of OC than either detection method alone, which improves the screening of OC in high-risk populations [143]. Liquid biopsy is a tool for prediction, and diagnosis, targeted prevention, and personalized medical service (PPPM/3P medicine) are new paradigms for overall OC management.
(ii) Global hypomethylation is mainly found in partial methylation domains (PMDs), and PMD has the potential as an epigenetic biomarker. The important mechanism of methylation loss in PMD is that CpG sites show low de novo methylation frequency and methylation maintenance rate, and some unsustainably maintained CpG sites gradually lose methylation during continuous mitosis. Some studies have shown that DNA replication related to proliferation directly drives DNA hypomethylation [144]. The silencing of genes in the core hypomethylation domain is aimed at the immune-related gene [135], and the study of PMD provides new insights for studying the influence of hypomethylation on OC.
(iii) Single-cell methylation and single-cell omics sequencing. Single-cell sequencing can study the gene expression in a single cell and solve the problem of tumor heterogeneity. By methylation sequencing of single-cell DNA, 40 PMDs were detected, and all of them were hypomethylated [133]. Bian et al. used single-cell triple recombination sequencing (scTrio-seq) technology to detect the methyl group, transcription group, and mutation in colorectal cancer and metastasis and detected that the whole genome DNA was hypomethylated in a single cancer cell, and the whole genome DNA demethylation pattern was consistent [145]. Single-cell omics sequencing provides insights into the molecular changes during CRC progression and metastasis. Single-cell methylation and single-cell omics sequencing technology will help to clarify the precise function of DNA in gene regulation in the next few years. Single-cell methylation and omics sequencing in tumor microenvironment can provide the possibility for finding important factors of OC progress and possible targets of treatment intervention.
(iv) Methylation is combined with other epigenetic modifications (such as histone modification). Methylation, histone modification, and nucleosome composition are the key determinants of epigenetics. Nucleosomes block DNA methyltransferase, but they can be remodeled by reducing the main regulatory factor of epigenetics, DNA methylation 1 (DDM1). DDM1 co-located with H3.1, H3.3, and DNA methyltransferase in the cell cycle but was blocked by H4K16 acetylation [146]. The combination of methyaltion and other modifications is of potential significance for understanding the etiology of cancer driven by chromatin abnormality and developing biomarkers and therapies. The combination of methylation and other modifications is of potential significance for understanding the etiology of cancer driven by chromatin abnormality and developing biomarkers and therapies.
(v) Epigenetic disorder is one of the key drivers of T-cell failure and one of the biggest obstacles in the field of immunotherapy, which reduces the efficiency of immune cell therapy. CRISPR system can target methylation and demethylation. The study of epigenome modifiers based on CRISPR has made a great contribution to the realization of epigenome editing and targeted gene activation, which may affect anti-tumor and immune response [147]. According to the differential methylation sites, a cell line or mouse model with stable mutation sites or overexpression was constructed, and its upstream influence mechanism and downstream functional changes were further detected to find possible therapeutic targets for OC.
Table 1.
Liquid biopsy and its application
| Type of biomarker | Method | Tumor | Result | Reference |
|---|---|---|---|---|
| cfDNA | Multimodal epigenetic sequencing analysis (MESA) | Colorectal cancer | MESA comprehensively analyzed the comprehensive and complementary epigenetic profile of cfDNA, which improved non-invasive cancer detection | [136] |
| cfDNA | Bisulfite sequencing; constructed a classifier (OvaPrint) | High-grade serous ovarian carcinoma (HGSOC) | OvaPrint can highly sensitively and specifically evaluate symptomatic patients with HGSOC, so it is necessary to further develop its non-HGSOC EOC type for women with symptomatic and asymptomatic masses | [137] |
| ccfDNA (circulating cell-free DNA) | Methylation-specific real-time PCR | Metastatic prostate cancer | Monitor the therapeutic effect | [138] |
| Bone marrow (BM); blood plasma (BP) | Quantitative methylation-specific PCR (qMSP); bisulfite amplicon sequencing (BAS) | Acute lymphoblastic leukemia | Cyclic biomarkers based on epigenetics may be used as a substitute for B- and T-ALL subtype classifiers and BM biopsy | [139] |
| ccfDNA | quantitative methylation-specific polymerase chain reaction | metastatic melanoma | SHOX2 ccfDNAm is an early predictor of the prognosis of patients with melanoma treated with anti-PD-1. The SHOX2 ccfDNAm test may be helpful for individualized treatment decision | [140] |
| Pleural fluid | Sentinel-MPE liquid biopsy assay | malignancy in pleural fluid | Sentinel-MPE test for detecting tumor-specific methylation based on ten genomic loci can be a supplementary tool for cytological diagnosis of MPE | [141] |
| ctDNA | ctDNA methylation, quantitative polymerase chain reaction assay | Colorectal Cancer | ctDNA methylation has potential in postoperative management, which can help to detect residual diseases, risk stratification, chemotherapy guidance, and recurrence monitoring in clinical environment | [142] |
Conclusion and expert recommendation in the framework of 3P medicine
This work established a five-gene methylation signature model, which can predict the survival and prognosis of patients with OC, help to better understand the development and occurrence mechanism of OC from the level of individual gene methylation, and promote the development of precision medicine. The hypomethylation of the 5 genes was proven by experiments, and significant divergences in methylation of RORC|CG 25112191, S100A13|CG 14467840, and TNF|CG 04425624 between normal and OC tissues were uncovered. We believe that the five genes and their methylation sites can be used as the target genes for the prognosis of patients with OC, and the expression of these five genes and the methylation modification of the sites with significant correlation can be changed by drugs or gene knockout or other means to upgrade the prognosis and survival rate of patients. The current study also found that molecules related to early differentiation of tumor cells may play the most important role in prognosis, which provides a new idea for immune intervention to inhibit OC. More molecular biology experiments are necessary to elucidate the role and mechanism of these differential immune cells and differential methylation sites in OC to bring forth new clues on the immune microenvironment and immune-related treatment of OC.
We strongly recommend paying attention to the research and practice of immune-related methylation genes in OC. The in-depth study of these methylations in OC has made great innovations in the following three aspects.
(i) Predictive approach. Biomarkers such as methylation can be used to accurately identify OC. Abnormal methylation can be found in the early development of OC and can be detected in DNA in blood circulation [148]. In this study, a risk prediction model based on differential methylation sites was established, and its various effects on OC and its immune microenvironment were comprehensively evaluated. Differential methylation sites can not only be used as prognostic indicators for OC patients, but also predict the response to immunotherapy, by detecting and analyzing the methylation level of immune-related genes, the susceptibility of individuals to diseases can be predicted, and the purpose of early intervention and prevention can be achieved. In-depth study of immune-related methylation and other predictive factors can provide non-invasive screening tools for cancer patients, provide new criteria for clinicians to diagnose OC, and provide OC prediction methods. OSC lacks specific diagnostic methods and typical clinical manifestations. Our experiment found that there are obviously differentially expressed immune-related methylation sites in OSC tissues and normal tissues, which can well predict the prognosis and survival of patients. Whether these methylation sites only affect the occurrence and development mechanism of OSC subtypes is worthy of in-depth study, providing more accurate diagnosis and treatment methods for OC patients’ typing. The detection of differential methylation sites of 5 genes has the potential as a prognostic and immune biomarker of OC, which provides a new basis for early diagnosis and typing of OC [149].
(ii) Targeted prevention. Immune checkpoint inhibitors (ICIs) such as PD-1 and CTLA4 inhibit the inhibition of cancer cells on the immune system and activate the immune system to fight cancer. Global hypomethylation increases the expression of inhibitory cytokines and induces drug resistance in ICB immunotherapy. DNA methyltransferase inhibitors (DNMTis) can enhance the anti-tumor effect of ICIs [150], and DNMTis is a potentially effective therapeutic drug for targeted prevention, which inhibits the occurrence and development of tumors. Methylation can also induce up-regulation of gene expression, and through demethylation treatment, the inhibited genes can be reactivated, thus inhibiting the malignant phenotype of tumor cells [151]. Therefore, drug targets/drugs based on methylation have the potential for targeted prevention.
(iii) Personalization of medical services. Human immunity is affected by many aspects, such as age, nutrition, psychology, and lifestyle. The prognosis model based on methylation is closely related to clinical characteristics such as drug sensitivity, immune microenvironment, and metabolic state. Clinicians can make more accurate treatment plans according to the individual immune-related methylation characteristics and improve the treatment effect [152].
In a word, our risk prediction model can help to generate a customized and targeted PPPM method, which is beneficial to both individuals and medical systems to establish an innovative state-of-the-art contributing to the paradigm shift from reactive medicine to PPPM in OC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge The Cancer Genome Atlas (TCGA) project organizers as well as all study participants to provide the publicly available TCGA RNA-seq data and clinical data.
Abbreviations
- ADAR1
Adenosine deaminase 1
- BPs
Biological processes
- CTC
Circulating tumor cells
- cfDNA
Cell-free DNA
- ctDNA
Circulating tumor DNA
- DCs
Dendritic cells
- DMAPT
Dimethylaminoporphanolide
- DMDGs
Differential methylation-driven genes
- DMSs
Differentially methylated sites
- DNMTs
DNA methyltransferases
- DNMTi
DNA methyltransferase inhibitors
- GO
Gene ontology terms
- Hcy
Homocysteine
- ICI
Immune checkpoint inhibitor
- IL-1RL2
Interleukin-1 receptor-like 2
- IRGs
Immune-related genes
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MDGs
Methylation-driven genes
- MESA
Multimodal epigenetic sequencing analysis
- MSP
Methylation‑specific polymerase chain reaction
- OC
Ovarian cancer
- OS
Overall survival
- PACA
Pancreatic cancer
- PPI
Protein-protein interaction
- Sat2
Satellite near the central point 2
- Satα
Satellite near the central point α
- ssGSEA
Single-sample Gene Set Enrichment Analysis
- TCGA
The Cancer Genome Atlas
- TCRs
T-cell antigen receptors
- TIME
Tumor immune microenvironment
- TME
Tumor microenvironment
- Tregs
T-cell regulatory
- UCEC
Uterus corpus endometrial cancer
- Aza-CdR
5-Aza-2′-deoxycytidine
- 5mC
5-Methylcytosine
Author contribution
W.J. analyzed partial data, performed methylation experiments, and wrote the manuscript draft. N.L. conceived the concept, analyzed data, and wrote the manuscript. J.W., X.G., Y.W., and J.Z. analyzed partial data. S.Y.O and G.G. reviewed and critically revised the manuscript. L.C. supervised the data and edited the manuscript. X.Z. conceived the concept, coordinated and critically revised the manuscript, and was responsible for the corresponding works. All authors approved the final manuscript.
Funding
This work was supported by the Shandong Provincial Taishan Scholar Engineering Project Special Funds (to X.Z.), the Shandong Provincial Natural Science Foundation (ZR2021MH156; ZR2022QH112; ZR2020LZL012), the Shandong First Medical University Talent Introduction Funds (to X.Z.), the Shandong First Medical University High-level Scientific Research Achievement Cultivation Funding Program (to X.Z.), the China National Nature Scientific Funds (82203592), the CSCO-CSPC Cancer Research Fund Project (Y-SY2021QN-0152), the Clinical Research Fund Project of Shandong Medical Association (YXH2022ZX02149), and the Beijing Science and Technology Innovation Medical Development Foundation (No. KC2021-JX-0186–138).
Data availability
All data and materials are provided in this article and supplemental materials, which can be available publicly.
Code availability
All protein and gene accession codes can be available in the Swiss-Prot and Genbank databases.
Declarations
Ethical approval
All investigations conformed to the principles outlined in the Declaration of Helsinki, and the research protocol was approved by the Medical Ethics Committee of Shandong First Medical University, China. All the patients were informed about the purposes of the study and consequently have signed their “consent of the patient.”. It includes the following four aspects:
- Approval of the research protocol by an Institutional Reviewer Board: Yes.
- Informed Consent: Yes.
- Registry and the Registration No. of the study: 202105200158.
- Animal Studies: N/A.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenshuang Jia and Na Li equally contributed to this work.
Contributor Information
Liang Chen, Email: 155338644@qq.com.
Xianquan Zhan, Email: yjzhan2011@gmail.com.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Devouassoux-Shisheboran M, Genestie C. Pathobiology of ovarian carcinomas. Chin J Cancer. 2015;34(1):50–55. doi: 10.5732/cjc.014.10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S, Nag S, Aggarwal S, Rauthan A, Warrier N. Maintenance therapy for recurrent epithelial ovarian cancer: current therapies and future perspectives - a review. J Ovarian Res. 2019;12(1):103. doi: 10.1186/s13048-019-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roett MA, Evans P. Ovarian cancer: an overview. Am Fam Physician. 2009;80(6):609–616. [PubMed] [Google Scholar]
- 5.Yu L, Ding Y, Wan T, Deng T, Huang H, Liu J. Significance of CD47 and its association with tumor immune microenvironment heterogeneity in ovarian cancer. Front Immunol. 2021;12:768115. doi: 10.3389/fimmu.2021.768115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Usach I, Blansit K, Chen LM, Ueda S, Brooks R, Kapp DS, Chan JK. Survival differences in women with serous tubal, ovarian, peritoneal, and uterine carcinomas. Am J Obstet Gynecol. 2015;212(2):188.e1–6. doi: 10.1016/j.ajog.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: the prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial. JAMA. 2011;305(22):2295–2303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 8.Cohen JD, Li L, Wang Y, Thoburn C, Afsari B, Danilova L, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359(6378):926–930. doi: 10.1126/science.aar3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinson C, Chatterjee R. CG methylation. Epigenomics. 2012;4(6):655–663. doi: 10.2217/epi.12.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comb M, Goodman HM. CpG methylation inhibits proenkephalin gene expression and binding of the transcription factor AP-2. Nucleic Acids Res. 1990;18(13):3975–3982. doi: 10.1093/nar/18.13.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark SJ, Harrison J, Molloy PL. Sp1 binding is inhibited by (m)Cp(m)CpG methylation. Gene. 1997;195(1):67–71. doi: 10.1016/s0378-1119(97)00164-9. [DOI] [PubMed] [Google Scholar]
- 12.Boyes J, Bird A. DNA methylation inhibits transcription indirectly via a methyl-CpG binding protein. Cell. 1991;64(6):1123–1134. doi: 10.1016/0092-8674(91)90267-3. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Ng HH, Erdjument-Bromage H, et al. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 1999;13(15):1924–1935. doi: 10.1101/gad.13.15.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nan X, Ng HH, Johnson CA, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393(6683):386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 15.Herman JG, Merlo A, Mao L, et al. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995;55(20):4525–4530. [PubMed] [Google Scholar]
- 16.Xing XB, Cai WB, Liang L, et al. The prognostic value of p16 hypermethylation in cancer: a meta-analysis. Plos One. 2013;8. 10.1371/journal.pone.0066587. [DOI] [PMC free article] [PubMed]
- 17.Lahtz C, Pfeifer GP. Epigenetic changes of DNA repair genes in cancer. J Mol Cell Biol. 2011;3(1). 10.1093/jmcb/mjq053. [DOI] [PMC free article] [PubMed]
- 18.Biswas S, Rao CM. Epigenetics in cancer: fundamentals and beyond. Pharmacol Therapeutics. 2017;173:118–134. doi: 10.1016/j.pharmthera.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Li QL, Kim HR, Kim WJ, et al. Transcriptional silencing of the RUNX3 gene by CpG hypermethylation is associated with lung cancer. Bioch Biophys Res Commun. 2004;314(1):223–228. doi: 10.1016/j.bbrc.2003.12.079. [DOI] [PubMed] [Google Scholar]
- 20.Costa FF, Paixao VA, Cavalher FP, et al. SATR-1 hypomethylation is a common and early event in breast cancer. Cancer Genetics Cytogenetics. 2006;165(2):135–143. doi: 10.1016/j.cancergencyto.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Widschwendter M, Jiang G, Woods C, et al. DNA hypomethylation and ovarian cancer biology. Cancer Res. 2004;64(13):4472–4480. doi: 10.1158/0008-5472.CAN-04-0238. [DOI] [PubMed] [Google Scholar]
- 22.Widschwendter M, Siegmund KD, Müller HM, et al. Association of breast cancer DNA methylation profiles with hormone receptor status and response to tamoxifen. Cancer Res. 2004;64(11):3807–3813. doi: 10.1158/0008-5472.CAN-03-3852. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez J, Frigola J, Vendrell E, et al. Chromosomal instability correlates with genome-wide DNA demethylation in human primary colorectal cancers. Cancer Res. 2006;66(17):8462–9468. doi: 10.1158/0008-5472.CAN-06-0293. [DOI] [PubMed] [Google Scholar]
- 24.Qian ZR, Sano T, Yoshimoto K, Asa SL, Yamada S, Mizusawa N, Kudo E. Tumor-specific downregulation and methylation of the CDH13 (H-cadherin) and CDH1 (E-cadherin) genes correlate with aggressiveness of human pituitary adenomas. Mod Pathol. 2007;20(12):1269–1277. doi: 10.1038/modpathol.3800965. [DOI] [PubMed] [Google Scholar]
- 25.Milicic A, Harrison LA, Goodlad RA, et al. Ectopic expression of P-cadherin correlates with promoter hypomethylation early in colorectal carcinogenesis and enhanced intestinal crypt fission in vivo. Cancer Res. 2008;68(19):7760–7768. doi: 10.1158/0008-5472.CAN-08-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paredes J. P-cadherin overexpression is an indicator of clinical outcome in invasive breast carcinomas and is associated with CDH3 promoter hypomethylation. Clinical Cancer Res. 2005;11(16):5869–5877. doi: 10.1158/1078-0432.CCR-05-0059. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro AS, Albergaria A, Sousa B, et al. Extracellular cleavage and shedding of P-cadherin: a mechanism underlying the invasive behaviour of breast cancer cells. Oncogene. 2010;29(3):392–402. doi: 10.1038/onc.2009.338. [DOI] [PubMed] [Google Scholar]
- 28.Li T, Li Y, Gan Y, et al. Methylation-mediated repression of MiR-424/503 cluster promotes proliferation and migration of ovarian cancer cells through targeting the hub gene KIF23. Cell Cycle. 2019;18(14):1601–1618. doi: 10.1080/15384101.2019.1624112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bandres E, Agirre X, Bitarte N, et al. Epigenetic regulation of microRNA expression in colorectal cancer. Int J Cancer. 2009;125(11):2737–2743. doi: 10.1002/ijc.24638. [DOI] [PubMed] [Google Scholar]
- 30.He Y, Cui Y, Wang W, et al. Hypomethylation of the hsa-miR-191 locus causes high expression of hsa-miR-191 and promotes the epithelial-to-mesenchymal transition in hepatocellular carcinoma. Neoplasia. 2011;13(9):841–853. doi: 10.1593/neo.11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minor J, Wang X, Zhang F, et al. Methylation of microRNA-9 is a specific and sensitive biomarker for oral and oropharyngeal squamous cell carcinomas. Oral Oncol. 2011;48(1):73–78. doi: 10.1016/j.oraloncology.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simonini P, Breiling A, Gupta N, et al. Epigenetically deregulated microRNA-375 is involved in a positive feedback loop with estrogen receptor alpha in breast cancer cells. Cancer Res. 2010;70(22):9175–9184. doi: 10.1158/0008-5472.CAN-10-1318. [DOI] [PubMed] [Google Scholar]
- 33.Soto-Reyes E, Gonzalez-Barrios R, Cisneros-Soberanis F, et al. Disruption of CTCF at the miR-125b1 locus in gynecological cancers. BMC Cancer. 2012;12(1):40. doi: 10.1186/1471-2407-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan F, Shen N, Pang J, Molina JR, Yang P, Liu S. The DNA methyltransferase DNMT1 and tyrosine-protein kinase KIT cooperatively promote resistance to 5-Aza-2’-deoxycytidine (Decitabine) and Midostaurin (PKC412) in lung cancer cells. J Biol Chem. 2015;290(30):18480–18494. doi: 10.1074/jbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X, Han H, De Carvalho DD, et al. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell. 2014;26(4):577–590. doi: 10.1016/j.ccr.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sellars M, Huh JR, Day K, et al. Regulation of DNA methylation dictates Cd4 expression during the development of helper and cytotoxic T cell lineages. Nat Immunol. 2015;16(7):746–754. doi: 10.1038/ni.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emran AA, Chatterjee A, Rodger EJ, et al. Targeting DNA methylation and EZH2 activity to overcome melanoma resistance to immunotherapy. Trends Immunol. 2019;40(4):328–344. doi: 10.1016/j.it.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Cai Y, Wu G, Peng B, et al. Expression and molecular profiles of the AlkB family in ovarian serous carcinoma. Aging (Albany NY). 2021;13(7):9679–9692. 10.18632/aging.202716. [DOI] [PMC free article] [PubMed]
- 39.Li T, Liu R, Zhang G, et al. Pan-cancer analysis of TLE3 revealed its value in tumor microenvironment and prognosis. J Oncol. 2022;2022:4085770. doi: 10.1155/2022/4085770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Cicek MS, Charbonneau B, et al. Tumor hypomethylation at 6p21.3 associates with longer time to recurrence of high-grade serous epithelial ovarian cancer. Cancer Res. 2014;74(11):3084–91. doi: 10.1158/0008-5472.CAN-13-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barton CA, Hacker NF, Clark SJ, et al. DNA methylation changes in ovarian cancer: implications for early diagnosis, prognosis and treatment. Gynecologic Oncol. 2008;109(1):129–139. doi: 10.1016/j.ygyno.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 43.Zheng M, Hu Y, Gou R, et al. Integrated multi-omics analysis of genomics, epigenomics, and transcriptomics in ovarian carcinoma. Aging (Albany NY). 2019;11(12). 10.18632/aging.102047. [DOI] [PMC free article] [PubMed]
- 44.Terp SK, Stoico MP, Dybkær K, Pedersen IS. Early diagnosis of ovarian cancer based on methylation profiles in peripheral blood cell-free DNA: a systematic review. Clin Epigenetics. 2023;15(1):24. doi: 10.1186/s13148-023-01440-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo GF, Chen CY, Wang J, et al. FOXD3 may be a new cellular target biomarker as a hypermethylation gene in human ovarian cancer. Cancer Cell Int. 2019;19:44. doi: 10.1186/s12935-019-0755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Zhang T, Yang L, et al. Comprehensive genomic analysis of microenvironment phenotypes in ovarian cancer. Peer J. 2020 doi: 10.7717/peerj.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho CM, Lai HC, Huang SH, et al. Promoter methylation of sFRP5 in patients with ovarian clear cell adenocarcinoma. Eur J Clin Invest. 2010;40(4):310–318. doi: 10.1111/j.1365-2362.2010.02266.x. [DOI] [PubMed] [Google Scholar]
- 48.Giannopoulou L, Chebouti I, Pavlakis K, et al. RASSF1A promoter methylation in high-grade serous ovarian cancer: a direct comparison study in primary tumors, adjacent morphologically tumor cell-free tissues and paired circulating tumor DNA. Oncotarget. 2017;8(13): 21429–21443. 10.18632/oncotarget.15249. [DOI] [PMC free article] [PubMed]
- 49.Giannopoulou L, Mastoraki S, Buderath P, et al. ESR1 methylation in primary tumors and paired circulating tumor DNA of patients with high-grade serous ovarian cancer. Gynecol Oncol. 2018;150(2):355–360. doi: 10.1016/j.ygyno.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 50.Smyth C, Curto J, Siddiqui N, et al. CpG island methylation of DNA damage response genes in advanced ovarian cancer. Cancer Res. 2005;65(19):8961–8967. doi: 10.1158/0008-5472.CAN-05-1187. [DOI] [PubMed] [Google Scholar]
- 51.Su HY, Lai HC, Lin YW, et al. Epigenetic silencing of SFRP5 is related to malignant phenotype and chemoresistance of ovarian cancer through Wnt signaling pathway. Int J Cancer. 2010;127(3):555–567. doi: 10.1002/ijc.25083. [DOI] [PubMed] [Google Scholar]
- 52.Liu J, Ji C, Wang Y, Zhang C, Zhu H. Identification of methylation-driven genes prognosis signature and immune microenvironment in uterus corpus endometrial cancer. Cancer Cell Int. 2021;21(1):365. doi: 10.1186/s12935-021-02038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiao Q, Wang Y, Zhang R, Pang Q. Autophagy related DNA methylation signature predict clinical prognosis and immune microenvironment in low-grade glioma. Transl Cancer Res. 2022;11(7): 2157–2174. 10.21037/tcr-22-310. [DOI] [PMC free article] [PubMed]
- 54.Xiao M, Liang X, Yan Z, et al. A DNA-methylation-driven genes based prognostic signature reveals immune microenvironment in pancreatic cancer. Front Immunol. 2022;13:803962. doi: 10.3389/fimmu.2022.803962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang H, Bueso-Ramos C, Dinardo C, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28(6):1280–1288. doi: 10.1038/leu.2013.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dongjun P, Ilona K, Nisha N, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2018;527(7577):249–253. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, Sun Y, Zhao E, Vatan L, Szeliga W, Kotarski J, Tarkowski R, Dou Y, Cho K, Hensley-Alford S, Munkarah A, Liu R, Zou W. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527(7577):249–253. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jung H, Kim HS, Kim JY, Sun JM, Ahn JS, Ahn MJ, Park K, Esteller M, Lee SH, Choi JK. DNA methylation loss promotes immune evasion of tumours with high mutation and copy number load. Nat Commun. 2019;10(1):4278. doi: 10.1038/s41467-019-12159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schuyler RP, Merkel A, Raineri E, et al. Distinct trends of DNA methylation patterning in the innate and adaptive immune systems. Cell Rep. 2016;17(8):2101–2111. doi: 10.1016/j.celrep.2016.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gomez S, Cox OL, Walker RR, 3rd, et al. Inhibiting DNA methylation and RNA editing upregulates immunogenic RNA to transform the tumor microenvironment and prolong survival in ovarian cancer. J Immunother Cancer. 2022;10(11):e004974. doi: 10.1136/jitc-2022-004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brunckhorst MK, Xu Y, Lu R, et al. Angiopoietins promote ovarian cancer progression by establishing a procancer microenvironment. Am J Pathol. 2014;184(8):2285–2296. doi: 10.1016/j.ajpath.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang W-G, Wang H-C, et al. BDNF activates TrkB/PLCγ1 signaling pathway to promote proliferation and invasion of ovarian cancer cells through inhibition of apoptosis. Eur Rev Med Pharmacol Sci. 2019;23(12):5093–5100. doi: 10.26355/eurrev_201906_18173. [DOI] [PubMed] [Google Scholar]
- 63.Abu-Elmagd M. Leptin protein expression and promoter methylation in ovarian cancer: a strong prognostic value with theranostic promises. Int J Mol Sci. 2021;22. 10.3390/ijms222312872. [DOI] [PMC free article] [PubMed]
- 64.Braga Lda C, Silva LM, Piedade JB, Traiman P, da Silva Filho AL. Epigenetic and expression analysis of TRAIL-R2 and BCL2: on the TRAIL to knowledge of apoptosis in ovarian tumors. Arch Gynecol Obstet. 2014;289(5):1061–1069. doi: 10.1007/s00404-013-3060-0. [DOI] [PubMed] [Google Scholar]
- 65.Gallagher NJ, Eliopoulos AG, Agathangelo A, et al. CD40 activation in epithelial ovarian carcinoma cells modulates growth, apoptosis, and cytokine secretion. Mol Pathol. 2002;55(2):110–120. doi: 10.1136/mp.55.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yellapa A, Bitterman P, Sharma S, et al. Interleukin 16 expression changes in association with ovarian malignant transformation. Am J Obstet Gynecol. 2014;210(3):272.e1–10. doi: 10.1016/j.ajog.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 67.Levina V, Nolen BM, Marrangoni AM, et al. Role of eotaxin-1 signaling in ovarian cancer. Clin Cancer Res. 2009;15(8):2647–2656. doi: 10.1158/1078-0432.CCR-08-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang YI, Wang YY, Ahn JH, et al. CCL2 overexpression is associated with paclitaxel resistance in ovarian cancer cells via autocrine signaling and macrophage recruitment. Biomed Pharmacother. 2022;153:113474. doi: 10.1016/j.biopha.2022.113474. [DOI] [PubMed] [Google Scholar]
- 69.Luetke-Eversloh M, Cicek BB, Siracusa F, et al. NK cells gain higher IFN-γ competence during terminal differentiation. Eur J Immunol. 2014;44(7):2074–2084. doi: 10.1002/eji.201344072. [DOI] [PubMed] [Google Scholar]
- 70.Chan HW, Kurago ZB, Stewart CA, et al. DNA methylation maintains allele-specific KIR gene expression in human natural killer cells. J Exp Med. 2003;197(2):245–255. doi: 10.1084/jem.20021127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang HT, Su SC, Chiou TJ, et al. DNA methylation-mediated Siglec-7 regulation in natural killer cells via two 5’ promoter CpG sites. Immunology. 2020;160(1):38–51. doi: 10.1111/imm.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McDonald JI, Diab N, Arthofer E, et al. Epigenetic therapies in ovarian cancer alter repetitive element expression in a TP53-dependent manner. Cancer Res. 2021;81(20):5176–5189. doi: 10.1158/0008-5472.CAN-20-4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quan Q, Xiong X, Wu S, Yu M. Identification of immune-related key genes in ovarian cancer based on WGCNA. Front Genet. 2021;12:760225. doi: 10.3389/fgene.2021.760225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang M, Pan X, Fujiwara K, et al. Pancreatic cancer cells render tumor-associated macrophages metabolically reprogrammed by a GARP and DNA methylation-mediated mechanism. Signal Transduct Target Ther. 2021;6(1):366. doi: 10.1038/s41392-021-00769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guan Z, Luo L, Liu S, et al. The role of TGR5 as an onco-immunological biomarker in tumor staging and prognosis by encompassing the tumor microenvironment. Front Oncol. 2022;12:953091. doi: 10.3389/fonc.2022.953091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi R, Zhao K, Wang T, et al. 5-aza-2’-deoxycytidine potentiates anti-tumor immunity in colorectal peritoneal metastasis by modulating ABC A9-mediated cholesterol accumulation in macrophages. Theranostics. 2022;12(2):875–890. doi: 10.7150/thno.66420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baessler A, Novis CL, Shen Z, et al. Tet2 coordinates with Foxo1 and Runx1 to balance T follicular helper cell and T helper 1 cell differentiation. Sci Adv. 2022;8(24):eabm4982. doi: 10.1126/sciadv.abm4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishizawa S, Sakata-Yanagimoto M, Hattori K, et al. BCL6 locus is hypermethylated in angioimmunoblastic T-cell lymphoma. Int J Hematol. 2017;105(4):465–469. doi: 10.1007/s12185-016-2159-z. [DOI] [PubMed] [Google Scholar]
- 79.Wei C, Liu X, Wang Q, Li Q, Xie M. Identification of hypoxia signature to assess the tumor immune microenvironment and predict prognosis in patients with ovarian cancer. Int J Endocrinol. 2021;2021:4156187. doi: 10.1155/2021/4156187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun D, Zhao X, Yu Y, et al. Comprehensive characterization of the alternative splicing landscape in ovarian cancer reveals novel events associated with tumor-immune microenvironment. Biosci Rep. 2022;42(2):BSR20212090. doi: 10.1042/BSR20212090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pawłowska A, Kwiatkowska A, Suszczyk D, et al. Clinical and prognostic value of antigen-presenting cells with PD-L1/PD-L2 expression in ovarian cancer patients. Int J Mol Sci. 2021;22(21):11563. doi: 10.3390/ijms222111563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Vos L, Grünwald I, Bawden EG, et al. The landscape of CD28, CD80, CD86, CTLA4, and ICOS DNA methylation in head and neck squamous cell carcinomas. Epigenetics. 2020;15(11):1195–1212. doi: 10.1080/15592294.2020.1754675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Vos L, Carrillo Cano TM, et al. CTLA4, PD-1, PD-L1, PD-L2, TIM-3, TIGIT, and LAG3 DNA methylation is associated with BAP1-aberrancy, transcriptional activity, and overall survival in uveal melanoma. J Immunother. 2022;45(7):324–334. doi: 10.1097/CJI.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 84.Kushlinskii NE, Loginov VI, Utkin DO, et al. Novel miRNAs as potential regulators of PD-1/PD-L1 immune checkpoint, and prognostic value of MIR9–1 and MIR124–2 methylation in ovarian cancer. Mol Biol (Mosk). 2020;54(6):990–996. doi: 10.31857/S0026898420060075. [DOI] [PubMed] [Google Scholar]
- 85.Hu WL, Zhou XH. Identification of prognostic signature in cancer based on DNA methylation interaction network. BMC Med Genomics. 2017;10(Suppl 4):63. doi: 10.1186/s12920-017-0307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Daniela, Matei, Fang, et al. Epigenetic resensitization to platinum in ovarian cancer. Cancer Res. 2012. 10.1158/0008-5472.CAN-11-3909. [DOI] [PMC free article] [PubMed]
- 87.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nature Rev Immunol. 2009;9(8):535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nature Rev Cancer. 2009;9(1):57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 89.Gata V, Florin LI. The role of Toll-like receptors in ovarian cancer. J BUON. 2017;22(5):1092–1096. [PubMed] [Google Scholar]
- 90.Hu J, Xu J, Feng X, Li Y, Hua F, Xu G. Differential expression of the TLR4 gene in pan-cancer and its related mechanism. Front Cell Dev Biol. 2021;9:700661. doi: 10.3389/fcell.2021.700661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lino AC, Dang VD, Lampropoulou V, et al. LAG-3 inhibitory receptor expression identifies immunosuppressive natural regulatory plasma cells. Immunity. 2018;49(1):120–133.e9. doi: 10.1016/j.immuni.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu L, Liu B, Yu J, Zhang D, Shi J, Liang P. Development of a Toll-like receptor-based gene signature that can predict prognosis, tumor microenvironment, and chemotherapy response for hepatocellular carcinoma. Front Mol Biosci. 2021;8:729789. doi: 10.3389/fmolb.2021.729789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carmo CR, Lyons-Lewis J, Seckl MJ, et al. A novel requirement for Janus kinases as mediators of drug resistance induced by fibroblast growth factor-2 in human cancer cells. PLoS ONE. 2011;6(5):e19861. doi: 10.1371/journal.pone.0019861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hernandez-Vargas H, Ouzounova M, Le Calvez-Kelm F, et al. Methylome analysis reveals Jak-STAT pathway deregulation in putative breast cancer stem cells. Epigenetics. 2011;6(4):428–439. doi: 10.4161/epi.6.4.14515. [DOI] [PubMed] [Google Scholar]
- 95.Shang AQ, Wu J, Bi F, et al. Relationship between HER2 and JAK/STAT-SOCS3 signaling pathway and clinicopathological features and prognosis of ovarian cancer. Cancer Biol Ther. 2017;18(5):314–322. doi: 10.1080/15384047.2017.1310343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mansouri A, Ridgway LD, Korapati AL, et al. Sustained activation of JNK/p38 MAPK pathways in response to Cisplatin leads to Fas ligand induction and cell death in ovarian carcinoma cells. J Biol Chem. 2003;278(21):19245–19256. doi: 10.1074/jbc.M208134200. [DOI] [PubMed] [Google Scholar]
- 97.Chen K, Liu MX, Mak CS, Yung MM, Leung TH, Xu D, Ngu SF, Chan KK, Yang H, Ngan HY, Chan DW. Methylation-associated silencing of miR-193a-3p promotes ovarian cancer aggressiveness by targeting GRB7 and MAPK/ERK pathways. Theranostics. 2018;8(2):423–436. doi: 10.7150/thno.22377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li KP, Ladle BH, Kurtulus S, et al. T-cell receptor signal strength and epigenetic control of Bim predict memory CD8+ T-cell fate. Cell Death Differ. 2020;27(4):1214–1224. doi: 10.1038/s41418-019-0410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li C, Ebert PJ, Li QJ. T cell receptor (TCR) and transforming growth factor β (TGF-β) signaling converge on DNA (cytosine-5)-methyltransferase to control forkhead box protein 3 (foxp3) locus methylation and inducible regulatory T cell differentiation. J Biol Chem. 2013;288(26):19127–19139. doi: 10.1074/jbc.M113.453357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pan X, Chen Y, Gao S. Four genes relevant to pathological grade and prognosis in ovarian cancer. Cancer Biomark. 2020;29(2):169–178. doi: 10.3233/CBM-191162. [DOI] [PubMed] [Google Scholar]
- 101.Graudenzi A. Pathway-based classification of breast cancer subtypes. Front Bioscience. 2017;22(10):1697. doi: 10.2741/4566. [DOI] [PubMed] [Google Scholar]
- 102.White KL, Rider DN, Kalli KR, et al. Genomics of the NF-κB signaling pathway: hypothesized role in ovarian cancer. Cancer Causes Control. 2011;22(5):785–801. doi: 10.1007/s10552-011-9745-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li X, Bao C, Ma Z, Xu B, Ying X, Liu X, Zhang X. Perfluorooctanoic acid stimulates ovarian cancer cell migration, invasion via ERK/NF-κB/MMP-2/-9 pathway. Toxicol Lett. 2018;15(294):44–50. doi: 10.1016/j.toxlet.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 104.Gallagher NJ, Eliopoulos AG, Agathangelo A, Oates J, Crocker J, Young LS. CD40 activation in epithelial ovarian carcinoma cells modulates growth, apoptosis, and cytokine secretion. Mol Pathol. 2002;55(2):110–120. doi: 10.1136/mp.55.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rothenburger T, McLaughlin KM, Herold T, et al. SAMHD1 is a key regulator of the lineage-specific response of acute lymphoblastic leukaemias to nelarabine. Commun Biol. 2020;3(1):324. doi: 10.1038/s42003-020-1052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Teschendorff AE, Lee SH, Jones A, et al. HOTAIR and its surrogate DNA methylation signature indicate carboplatin resistance in ovarian cancer. Genome Med. 2015;7:108. doi: 10.1186/s13073-015-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Akahane K, Kimura S, Miyake K, et al. Association of allele-specific methylation of the ASNS gene with asparaginase sensitivity and prognosis in T-ALL. Blood Adv. 2022;6(1):212–224. doi: 10.1182/bloodadvances. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rodríguez D, Quirós LM, Salas JA. MtmMII-mediated C-methylation during biosynthesis of the antitumor drug mithramycin is essential for biological activity and DNA-drug interaction. J Biol Chem. 2004;279(9):8149–8158. doi: 10.1074/jbc.M312351200. [DOI] [PubMed] [Google Scholar]
- 109.Evison BJ, Bilardi RA, Chiu FC, Pezzoni G, Phillips DR, Cutts SM. CpG methylation potentiates pixantrone and doxorubicin-induced DNA damage and is a marker of drug sensitivity. Nucleic Acids Res. 2009;37(19):6355–6370. doi: 10.1093/nar/gkp700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu S, Liu Z, Xie Z, et al. Bortezomib induces DNA hypomethylation and silenced gene transcription by interfering with Sp1/NF-kappaB-dependent DNA methyltransferase activity in acute myeloid leukemia. Blood. 2008;111(4):2364–2373. doi: 10.1182/blood-2007-08-110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nakshatri H, Appaiah HN, Anjanappa M, et al. NF-κB-dependent and -independent epigenetic modulation using the novel anti-cancer agent DMAPT. Cell Death Dis. 2015;6(1):e1608. doi: 10.1038/cddis.2014.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu Y, Zheng M, Wang S, et al. Identification of a five-gene signature of the RGS gene family with prognostic value in ovarian cancer. Genomics. 2021;113(4):2134–2144. doi: 10.1016/j.ygeno.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 113.Yu M, Henning R, Walker A, et al. L-asparaginase inhibits invasive and angiogenic activity and induces autophagy in ovarian cancer. J Cell Mol Med. 2012;16(10):2369–2378. doi: 10.1111/j.1582-4934.2012.01547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chan DW, Lam WY, Chen F, et al. Genome-wide DNA methylome analysis identifies methylation signatures associated with survival and drug resistance of ovarian cancers. Clin Epigenetics. 2021;13(1):142. doi: 10.1186/s13148-021-01130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boyd LR, Muggia FM. Carboplatin/paclitaxel induction in ovarian cancer: the finer points. Oncology (Williston Park). 2018;32(8):418–20, 422–4. [PubMed]
- 116.Wang L, Yan W, Li X, et al. S100A10 silencing suppresses proliferation, migration and invasion of ovarian cancer cells and enhances sensitivity to carboplatin. J Ovarian Res. 2019;12(1):113. doi: 10.1186/s13048-019-0592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Deng M, Sun J, Xie S, Zhen H, et al. Inhibition of MCM2 enhances the sensitivity of ovarian cancer cell to carboplatin. Mol Med Rep. 2019;20(3):2258–2266. doi: 10.3892/mmr.2019.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang J, Zhang J, Wang F, et al. Overexpressed COL5A1 is correlated with tumor progression, paclitaxel resistance, and tumor-infiltrating immune cells in ovarian cancer. J Cell Physiol. 2021;236(10):6907–6919. doi: 10.1002/jcp.30350. [DOI] [PubMed] [Google Scholar]
- 119.Lin H, Shen L, Lin Q, et al. SNHG5 enhances paclitaxel sensitivity of ovarian cancer cells through sponging miR-23a. Biomed Pharmacother. 2020;123:109711. doi: 10.1016/j.biopha.2019.109711. [DOI] [PubMed] [Google Scholar]
- 120.Leija-Martínez JJ, Giacoman-Martínez A, Del-Río-Navarro BE, Sanchéz-Muñoz F, Hernández-Diazcouder A, Muñoz-Hernández O, Romero-Nava R, Villafaña S, Marchat LA, Hong E, Huang F. Promoter methylation status of RORC, IL17A, and TNFA in peripheral blood leukocytes in adolescents with obesity-related asthma. Heliyon. 2022;8(12):e12316. doi: 10.1016/j.heliyon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gong G, Lin T, Yuan Y. Integrated analysis of gene expression and DNA methylation profiles in ovarian cancer. J Ovarian Res. 2020;13(1):30. doi: 10.1186/s13048-020-00632-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nimmo ER, Prendergast JG, Aldhous MC, Kennedy NA, Henderson P, Drummond HE, Ramsahoye BH, Wilson DC, Semple CA, Satsangi J. Genome-wide methylation profiling in Crohn’s disease identifies altered epigenetic regulation of key host defense mechanisms including the Th17 pathway. Inflamm Bowel Dis. 2012;18(5):889–899. doi: 10.1002/ibd.21912. [DOI] [PubMed] [Google Scholar]
- 123.Li T, Xu Y, Shi Y, Chen J, Lin S, Zhu J, Xu X, Lu L, Zou H. Genome-wide analysis of DNA methylation identifies S100A13 as an epigenetic biomarker in individuals with chronic (≥ 30 years) type 2 diabetes without diabetic retinopathy. Clin Epigenetics. 2020;12(1):77. doi: 10.1186/s13148-020-00871-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gérard Benoit, Cooney A, Giguere V, et al. International union of pharmacology. LXVI. Orphan nuclear receptors. Pharmacol Rev. 2007;58(4):798–836. 10.1124/pr.58.4.10. [DOI] [PubMed]
- 125.Muscat GEO, Eriksson NA, Karen B, et al. Research resource: nuclear receptors as transcriptome: discriminant and prognostic value in breast cancer. Mol Endocrinol. 2013;27(2). 10.1210/me.2012-1265. [DOI] [PMC free article] [PubMed]
- 126.Dalong, Zihao, Pang, et al. Retinoic acid-related orphan receptor C regulates proliferation, glycolysis, and chemoresistance via the PD-L1/ITGB6/STAT3 signaling axis in bladder cancer. Cancer Res. 2019;79(10): 2604–2618. 10.1158/0008-5472.CAN-18-3842. [DOI] [PubMed]
- 127.Li R, Li W, He F, et al. Systematic screening identifies a TEAD4-S100A13 axis modulating cisplatin sensitivity of oral squamous cell carcinoma cells. J Oral Pathol Med. 2021;50(9):882–890. doi: 10.1111/jop.13224. [DOI] [PubMed] [Google Scholar]
- 128.Miao S, Qiu T, Zhao Y, et al. Overexpression of S100A13 protein is associated with tumor angiogenesis and poor survival in patients with early-stage non-small cell lung cancer. Thoracic Cancer. 2018;9(9):1136–1144. doi: 10.1111/1759-7714.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gong K, Guo G, Beckley N, et al. Tumor necrosis factor in lung cancer: complex roles in biology and resistance to treatment. Neoplasia. 2021;23(2):189–196. doi: 10.1016/j.neo.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Misaki F, Yasuhiro M, Kiyoshi T, et al. Relaxin 2/RXFP1 signaling induces cell invasion via the β-catenin pathway in endometrial cancer. Int J Mol Sci. 2018;19(8):2438. doi: 10.3390/ijms19082438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen YC, Gonzalez ME, Burman B, et al. Mesenchymal stem/stromal cell engulfment reveals metastatic advantage in breast cancer. Cell Rep. 2019;27(13):3916–3926. doi: 10.1016/j.celrep.2019.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wen S, He L, Zhong Z, et al. Prognostic model of colorectal cancer constructed by eight immune-related genes. Front Mol Biosci. 2020;27(7):604252. doi: 10.3389/fmolb.2020.604252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bukhari SA, Zafar K, Rajoka M, İbrahim Z, Javed S, Sadiq R. Oxidative stress-induced DNA damage and homocysteine accumulation may beinvolved in ovarian cancer progression in both young and old patients. Turk J Med Sci. 2016;46(3):583–589. doi: 10.3906/sag-1406-17. [DOI] [PubMed] [Google Scholar]
- 134.Mazurakova A, Samec M, Biringer K, et al. Homocysteine metabolism as the target for predictive medical approach, disease prevention, prognosis, and treatments tailored to the person. EPMA J. 2021;12(4):477–505. doi: 10.1007/s13167-021-00263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Endicott JL, Nolte PA, Shen H, Laird PW. Cell division drives DNA methylation loss in late-replicating domains in primary human cells. Nat Commun. 2022;13(1):6659. 150. 10.1038/s41467-022-34268-8. [DOI] [PMC free article] [PubMed]
- 136.Guo H, Vuille JA, Wittner BS, et al. DNA hypomethylation silences anti-tumor immune genes in early prostate cancer and CTCs. Cell. 2023;186(13):2765–2782.e28. doi: 10.1016/j.cell.2023.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li Y, Xu J, Chen C, et al. Multimodal epigenetic sequencing analysis (MESA) of cell-free DNA for non-invasive colorectal cancer detection. Genome Med. 2024;16(1):9. doi: 10.1186/s13073-023-01280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Buckley DN, Lewinger JP, Gooden G, et al. OvaPrint-a cell-free DNA methylation liquid biopsy for the risk assessment of high-grade serous ovarian cancer. Clin Cancer Res. 2023;29(24):5196–5206. doi: 10.1158/1078-0432.ccr-23-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Büttner T, Dietrich D, Zarbl R, et al. Feasibility of monitoring response to metastatic prostate cancer treatment with a methylation-based circulating tumor DNA approach. Cancers (Basel) 2024;16(3):482. doi: 10.3390/cancers16030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hussan SS, Ali MS, Fatima M, Altaf M, Sadaf S. Epigenetically dysregulated NOTCH-Delta-HES signaling cascade can serve as a subtype classifier for acute lymphoblastic leukemia. Ann Hematol. 2024;103(2):511–523. doi: 10.1007/s00277-023-05515-9. [DOI] [PubMed] [Google Scholar]
- 141.Fietz S, Diekmann E, de Vos L, et al. Circulating cell-free SHOX2 DNA methylation is a predictive, prognostic, and monitoring biomarker in adjuvant and palliative anti-PD-1-treated melanoma. Clin Chem. 2024:hvad230. 144. 10.1093/clinchem/hvad230. [DOI] [PubMed]
- 142.Bixby B, Vrba L, Lenka J, et al. Cell-free DNA methylation analysis as a marker of malignancy in pleural fluid. Sci Rep. 2024;14(1):2939.145. 10.1038/s41598-024-53132-x. [DOI] [PMC free article] [PubMed]
- 143.Mo S, Ye L, Wang D, et al. Early detection of molecular residual disease and risk stratification for stage I to III colorectal cancer via circulating tumor DNA methylation. JAMA Oncol. 2023;9(6):770–778.147. 10.1001/jamaoncol.2023.0425. [DOI] [PMC free article] [PubMed]
- 144.Herzog C, Jones A, Evans I, et al. Plasma cell-free DNA methylation analysis for ovarian cancer detection: analysis of samples from a case-control study and an ovarian cancer screening trial. Int J Cancer. 2024;154(4):679–691. doi: 10.1002/ijc.34757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bian S, Hou Y, Zhou X, et al. Single-cell multiomics sequencing and analyses of human colorectal cancer. Science. 2018;362(6418):1060–1063. doi: 10.1126/science.aao3791. [DOI] [PubMed] [Google Scholar]
- 146.Lee SC, Adams DW, Ipsaro JJ, et al. Chromatin remodeling of histone H3 variants by DDM1 underlies epigenetic inheritance of DNA methylation. Cell. 2023;186(19):4100–4116.e15. doi: 10.1016/j.cell.2023.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yahsi B, Palaz F, Dincer P. Applications of CRISPR epigenome editors in tumor immunology and autoimmunity. ACS Synth Biol. 2024;13(2):413–427. doi: 10.1021/acssynbio.3c00524. [DOI] [PubMed] [Google Scholar]
- 148.Barton CA, Hacker NF, Clark SJ, O’Brien PM. DNA methylation changes in ovarian cancer: implications for early diagnosis, prognosis and treatment. Gynecol Oncol. 2008;109(1):129–39. doi: 10.1016/j.ygyno.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 149.Grech G, Zhan X, Yoo BC, Bubnov R, Hagan S, Danesi R, Vittadini G, Desiderio DM. EPMA position paper in cancer: current overview and future perspectives. EPMA J. 2015;6(1):9. doi: 10.1186/s13167-015-0030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chiappinelli KB, Strissel PL, Desrichard A, Li H, Henke C, Akman B, Hein A, et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell. 2015;162(5):974–86. doi: 10.1016/j.cell.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hou X, Liao Q, Wu Y, Wang L, Zhao J, Liao X. Hypomethylation-mediated upregulation of NFE2L3 promotes malignant phenotypes of clear cell renal cell carcinoma cells. Mol Biotechnol. 2024;66(2):198–207. doi: 10.1007/s12033-023-00727-w. [DOI] [PubMed] [Google Scholar]
- 152.Mazurakova A, Samec M, Koklesova L, Biringer K, Kudela E, Al-Ishaq RK, Pec M, Giordano FA, Büsselberg D, Kubatka P, Golubnitschaja O. Anti-prostate cancer protection and therapy in the framework of predictive, preventive and personalised medicine - comprehensive effects of phytochemicals in primary, secondary and tertiary care. EPMA J. 2022;13(3):461–486. doi: 10.1007/s13167-022-00288-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials are provided in this article and supplemental materials, which can be available publicly.
All protein and gene accession codes can be available in the Swiss-Prot and Genbank databases.