Abstract
Hematopoietic cells are attractive targets for gene therapy. However, no satisfactory vectors are currently available. A major problem with the most commonly used adenovirus vectors, based on adenovirus type 2 (Ad2) or Ad5, is their low binding efficiency for hematopoietic cells. In this study we identify two adenovirus serotypes with high affinity for hematopoietic cells. The binding efficiency of prototype serotypes Ad4p, Ad11p, and Ad35p for different committed hematopoietic cell lines representing T cells (Jurkat), B cells (DG75), monocytes (U937-2), myeloblasts (K562), and granulocytes (HL-60) was evaluated and compared to that of Ad5v, the commonly used adenovirus vector, using flow cytometry. In contrast to Ad5v, which bound to less than 10% of the cells in all experiments, Ad11p and Ad35p showed high binding efficiency for all of the different hematopoietic cell lines. Ad4p bound to the lymphocytic cell lines to some extent but less well to the myelomonocytic cell lines. The abilities of the different serotypes to infect, replicate, and form complete infectious particles in the hematopoietic cell lines were also investigated by immunostaining, 35S labeling of viral proteins, and titrations of cell lysates. Ad11p and Ad35p infected the highest proportion of cells, and Ad11p infected all of the cell lines investigated. The Ad11p hexon was expressed equally well in K562 and A549 cells. Jurkat cells also showed high levels of expression of Ad11p hexons, but the production of infectious particles was low. The binding properties of virions were correlated to their ability to infect and be expressed.
The interest in adenoviruses is partly due to the perspective of using them as vectors for gene therapy. The hematopoietic system is a particularly suitable target for gene therapy, as techniques for bone marrow and blood cell transplantation are well established and the transductions can be performed ex vivo. However, there are currently no suitable adenovirus vectors available for this application.
Most adenovirus vector gene transfer systems are derived from adenovirus serotypes 2 and 5 (Ad2 and Ad5) since they are the best studied and nucleotide sequences are available for both (8, 43). However, vectors based on Ad2 or Ad5 are known to transduce resting hematopoietic cells with very low efficiency (5). Adenovirus entry into host cells involves interactions with two separate receptors. The fiber knob binds with high affinity to a specific cellular attachment receptor (32); subsequent interactions between the penton base RGD motif and αv integrins (second-step receptors) lead to endocytosis of the virus (41) in clathrin-coated vesicles (11). The coxsackievirus and adenovirus receptor (CAR) serves as the attachment receptor for at least the subgenus C adenoviruses Ad2 and Ad5 (4, 38). Furthermore, soluble CAR protein has been shown to bind to adenovirus serotypes from all subgenera except subgenus B (33).
The adenovirus family consists of 49 known serotypes, which have been divided into six subgenera (A to F) with distinctly different organ tropisms. Although other factors may influence infectivity and replication, the high-affinity attachment of virions to host cell fiber receptors represents a key determinant in cell and tissue tropism. The purpose of this study was to find adenovirus serotypes with tropism for hematopoietic cells. The binding properties of Ad4p (prototype), Ad5v (vector strain), and the subgenus B:2 viruses, Ad11p and Ad35p, to committed hematopoietic cells were evaluated by flow cytometry. Ad4p and Ad5v are epitheliotropic viruses causing airway infections, and Ad11p and Ad35p have been described as causing infections of the kidneys and urinary tract (20, 31). Many isolates from patients with acute hemorrhagic cystitis after bone marrow or renal transplants have been identified as Ad11p (25, 34). The ability of these viruses to replicate and form new infectious particles in hematopoietic cells was also investigated by immunostaining for viral structural proteins, 35S labeling of proteins after adenovirus infection, and titrations in A549 cells of lysates collected at different time points after infection.
MATERIALS AND METHODS
Cell lines and culture conditions.
The cell lines used, all of human origin, were as follows: Jurkat, an acute T-cell leukemia cell line; DG75, established from a Burkitt's B-cell lymphoma; U937-2, from a diffuse histiocytic lymphoma still expressing many monocyte-like characteristics; K562, established from a chronic myelogenous leukemia in terminal blast crisis; HL-60, from a promyelocytic leukemia with phagocytotic activity; and A549, from a human oat cell carcinoma of the lung. K562 was previously classified as an erythroleukemia line (2), but more recent studies indicate that the cells are multipotential blasts that spontaneously can differentiate into progenitors of the erythrocytic, granulocytic, and monocytic series (26). All hematopoietic cell lines were grown in RPMI 1640 containing 10% fetal calf serum (FCS), 20 mM HEPES, NaCO3 (0.75 g/liter), and 1× penicillin G (100 IU/ml)-streptomycin sulfate (100 μg/ml) (PEST) at 37°C. They were split 1:5 to 1:10 when they reached a concentration of about 1 million cells/ml, on average every second to third day. A549 cells were grown in Dulbecco's modified Eagle medium (DMEM) containing 5% FCS, 20 mM HEPES NaHCO3 (0.75 g/liter), and 1× PEST at 37°C; upon virus infection, the FCS concentration was lowered to 0.5 to 1.0%.
Viruses.
The adenovirus serotypes used in this study, 4p (RJ-67), 5v (pFG140), 11p (Slobitski), and 35p (S-761), were all typed with respect to their restriction patterns (1). All serotypes were raised in A549 cells and purified on CsCl gradients as previously described (27).
Virus labeling.
The virions were desalted on a NAP-10 column (Pharmacia Upjohn, Uppsala, Sweden) equilibrated in labeling buffer (50 mM NaHCO3, 2 mM MgCl2, 135 mM NaCl [pH 8.8]); 100 μl of N-hydroxysuccinimidobiotin (1 mg/ml; Sigma Chemical Co., St. Louis, Mo.) in dimethyl sulfoxide was added to 1 ml of the virions (1 to 4 mg/ml) (19). The solution was then mixed on a rocker platform overnight at 4°C under dark conditions. The buffer was then changed, and free biotin was removed by passing the solution thorough a NAP-10 column equilibrated in phosphate-buffered saline (PBS). The concentration of biotinylated virions was determined by spectrophotometry at 260 and 330 nm. One unit of optical density at A260 − A330 = 1012 particles/ml = 280 μg/ml of virions. Glycerol was added to a final concentration of 10%, and the virions were aliquoted in small volumes and kept at −70°C until used.
Binding experiments using a FACScan flow cytometer.
For each binding experiment, 5 × 105 cells were used. The cells were incubated with five different concentrations, 0.5, 1.0, 3.0, 6.0, and 10.0 pg/cell (corresponding to ca. 1,800, 3,600, 10,700, 21,400, and 35,700 particles/cell) of biotinylated Ad5v, Ad4p, Ad11p, and Ad35p virions in a total volume of 100 μl of PBS supplemented with 2% FCS and 0.01% NaN3 (PBS-FCS-NaN3) on a rocker platform at 4°C for 30 min. The cell samples were then washed in 150 μl of PBS-FCS-NaN3 buffer. Streptavidin-fluorescein isothiocyanate (FITC; DAKO) in a dilution of 1:100 in PBS-FCS-NaN3 was then added, and the samples were incubated for 30 min under the same conditions as before. The samples were washed again and finally resuspended in 300 μl of PBS-FCS-NaN3 buffer with an addition of propidium iodine (1 μg/ml) to exclude dead cells from the fluorescence-activated cell sorting (FACS) analysis. Throughout the experiment, the samples were kept on ice so that they never reached a temperature higher than 4°C, making virus internalization very unlikely. The samples were evaluated by a FACScan (Becton Dickinson) flow cytometer, and the measurements consisted of 10,000 events per sample. The data were analyzed with the LYSYS II software program (Becton Dickinson). Measurements of relative mean fluorescence were corrected for autofluorescence of control cells.
Blocking experiments using a FACScan flow cytometer.
The same cell lines as used in the binding experiments were incubated with unlabeled Ad11p (5, 10, 20, and 40 pg/cell) for 30 min in 4°C before addition of biotinylated Ad4p, Ad11p, and Ad35p. The amounts of biotinylated viruses used per cell in all reactions were 1 pg of Ad11p and Ad35p, 6 pg of Ad4p for DG75 cells, and 10 pg of Ad4p for Jurkat cells. To reach an initial minimum binding of 25% of the cell population, different concentrations of biotinylated Ad4p were used.
Immunostaining procedures.
Jurkat, DG75, U937-2, K562, and HL-60 cells (3 × 105 of each cell line) were cultured in a 24-well plate (2 cm2/well) in RPMI 1640 containing 2% FCS. After inoculation with Ad4p, Ad5v, Ad11p, and Ad35p (each at 2 pg/cell), the cell cultures were incubated for 1 or 48 h at 37°C. The medium was then aspirated, and the cells were washed once in PBS and allowed to dry. The cells were fixed in 100% ice-cold methanol at 4°C for 10 min followed by incubation with the primary antibody for 1 h at 37°C; as the primary antibody, unfractionated serum from rabbits immunized with whole virions (Ad5v, Ad4p, Ad11p, and Ad35p) was used at a dilution of 1:200 in PBS supplemented with 0.1% bovine serum albumin (BSA). The cells were then washed twice in PBS for 5 min each time and incubated with the secondary antibody, an FITC-conjugated swine anti-rabbit immunoglobulin G (DAKO), diluted 1:40 in PBS-BSA, for 30 min at 37°C. Again the cells were washed as previously described and examined under a fluorescence microscope. The stained cells were stored in PBS containing 50% glycerol. Micrographs were taken at 200× enlargement.
[35S]methionine-cysteine labeling of proteins after infection with Ad11p.
Jurkat, DG75, U937-2, K562, HL-60, and A549 cells (1.5 million of each cell line) were infected with 2 pg of Ad5v and Ad11p virions per cell. Virions were adsorbed in a minimal amount of medium, DMEM for A549 cells and RPMI 1640 for the hematopoietic cell lines, containing 5% FCS for 90 min on a rocker platform. Unbound viruses were then removed by washing with PBS. At 22 h postinfection (p.i.), the infected cell cultures were washed once with PBS and incubated for 2 h in 2.5 ml of methionine- and cysteine-free DMEM or RPMI 1640 (ICN Biomedicals, Inc.) containing 5% FCS, 20 mM HEPES, and 1× PEST to deplete endogenous methionine and cysteine. At 24 h p.i., the cells were labeled with 0.46 mCi of Tran35S-label (1,175 Ci/mmol, 10.5 mCi/ml; ICN Biomedicals, Inc.) per bottle. After labeling for 1 h, 26 μl of unlabeled cysteine (200 mM) was added, after another 4.5 h 26 μl of unlabeled methionine (100 mM) was added, and after labeling for 24 h both unlabeled methionine and unlabeled cysteine (26 μl of each) were added to final concentrations of 2 and 4 mM, respectively. The infected cultures were harvested at 72 h p.i. and washed twice in 0.1 M Tris-HCl (pH 8.0) containing 5 mM EDTA and 1 mM phenylmethylsulfonyl fluoride. The cells were dissolved in 90 μl of the same buffer, and the volumes were adjusted to 100 μl; 10 μl of each sample (∼250,000 cells) was taken for protein separation sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% gel. The gel was Coomassie blue stained, dried, autoradiographed for 16 to 24 h, and also analyzed in a Molecular Dynamics PhosphorImager system. Two separate labeling experiments were performed.
Production of infectious particles in Jurkat, K562, U937-2, and A549 cells.
Jurkat, K562, U937-2, and A549 cells (105 of each cell line) were incubated with Ad11p (0.2 pg/cell) for 1, 16, 48, 96, and 144 h. Duplicate samples for each time point were used; after 1 h of adsorption in 200 μl of DMEM or RPMI 1640 containing 2% FCS on a rocker platform at 37°C, all samples were washed twice in 1 ml of PBS and then given 1 ml of new medium. The duplicates were pooled at the end of the incubation and freeze-thawed three times. The lysates were diluted in 10-fold steps, inoculated in five parallel A549 cell tubes for each dilution, and read every second day for 12 days.
RESULTS
Ad11p and Ad35p bind with high efficiency to lymphocytic and myelomonocytic cell lines.
To compare the levels of binding of Ad5v, Ad4p, Ad11p, and Ad35p to different committed blood cell lineages, the continuous human blood cell lines Jurkat (T cells), DG75 (B cells), U937-2 (with monocyte-like characteristics), K562 (myeloblasts), and HL-60 (granulocyte-like) were used. Virus binding was evaluated by flow cytometry at five different virus concentrations (0.5, 1.0, 3.0, 6.0, and 10.0 pg/cell).
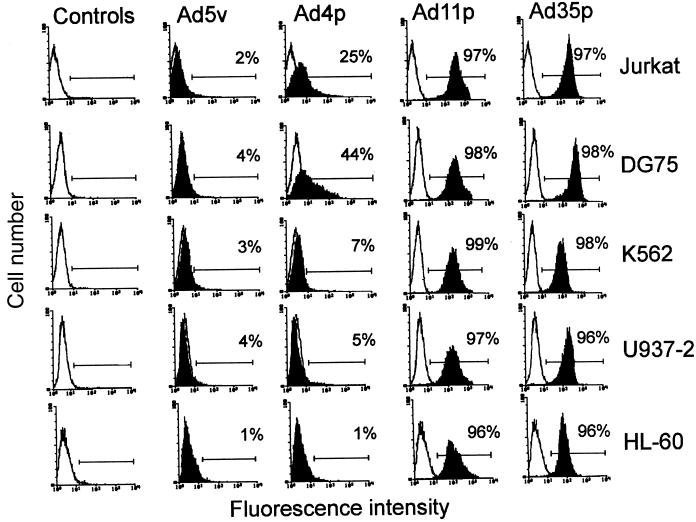
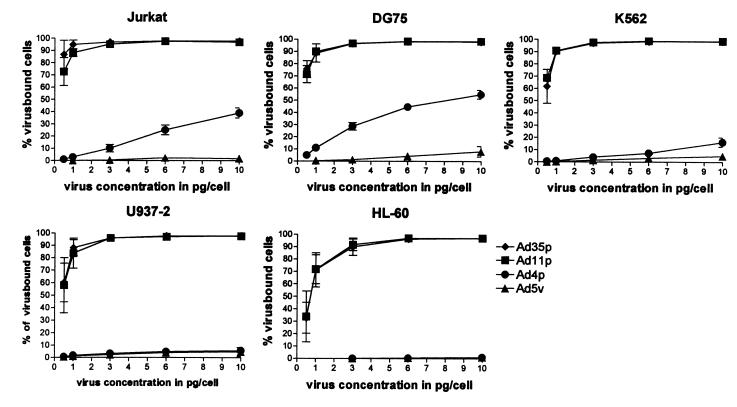
The two subgenus B:2 members, Ad11p and Ad35p, showed very efficient binding to all cell lines investigated (Fig. 1 and 2) and had nearly identical binding profiles (Fig. 2). They bound especially well to the two lymphocytic cell lines (DG75 and Jurkat) and to myeloblasts (K562), monocytes (U937), and granulocytes (HL-60) (listed in the order of descending affinity). At a virus concentration of only 0.5 pg/cell, Ad11p and Ad35p virions were bound to over 70% of DG75 and Jurkat cells, over 60% of K562 cells, and around 50% of U937-2 and 30% of HL-60 cells (Fig. 2). At a virus concentration of 3 pg/cell, there was almost 100% binding of Ad11p and Ad35p to all cell lines investigated. The most commonly used adenovirus vector, Ad5v, exhibited a very low affinity for all cell lines investigated. At the virus concentrations used, it never bound to more than 10% of the cells of any cell line (Fig. 2). Ad4p bound to the lymphocytic cell lines to some extent, but Ad4p and Ad5v showed comparatively low affinity for the myelomonocytic cell lines except perhaps for K562 cells (Fig. 1 and 2).
FIG. 1.
Flow cytometry analysis of the human hematopoietic cell lines Jurkat, DG75, U937, K562, and HL-60 exposed to 6.0 pg of biotin-streptavidin-FITC labeled adenoviruses per cell to evaluate the binding of various adenovirus serotypes to different hematopoietic lineages.
FIG. 2.
Binding of Ad4p, Ad5v, Ad11p, and Ad35p to hematopoietic cell lines Jurkat (T cells), DG75 (B cells), K562 (myeloblasts), U937-2 (monocytes), and HL-60 (granulocytes), evaluated by flow cytometry using biotin-streptavidin-FITC-labeled adenoviruses. Percentages of virus-bound cells were plotted against virus concentration (0.5, 1.0, 3.0, 6.0, and 10.0 pg/cell). The results are presented as the means ± 95% confidence interval of at least three independent experiments.
Ad11p and Ad35p uses the same, but Ad4p uses a different, attachment receptor.
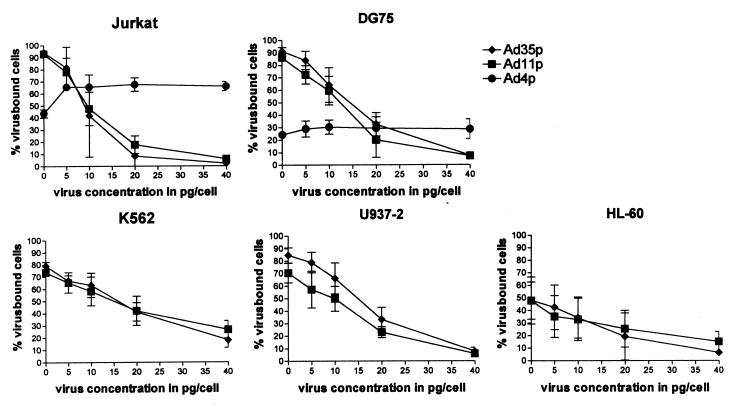
The binding profile of Ad4p was somewhat different from those of the other adenoviruses studied, and we wanted to test the hypothesis that Ad4p uses a different attachment receptor than Ad11p and Ad35p. The subgenus B:2 adenoviruses Ad11p and Ad35p displayed very similar binding profiles, and we also wanted to determine whether they were binding to the same receptor. The different cell lines were preincubated with unlabeled Ad11p virions up to 40 pg/cell, and then binding of biotinylated Ad11p and Ad35p (1 pg/cell) and Ad4p (6 to 10 pg/cell) was investigated by flow cytometry as before. Ad11p virions blocked the binding of Ad35p to all of the investigated cell lines similarly to the self-blocking of the binding (Fig. 3). Thus, they bind to the same receptor with similar affinities. However, unlabeled Ad11p virions displayed no blocking effect on the binding of Ad4p to the two lymphocytic cell lines. Surprisingly, there was an increase in the binding of Ad4p after addition of Ad11p virions (Fig. 3).
FIG. 3.
Binding of biotin-streptavidin-FITC-labeled virions of Ad11p, Ad35p, and Ad4p to various hematopoietic cell lines after preincubation with unlabeled Ad11p. Flow cytometry was used to determine the blocking effect of unlabeled Ad11p (5, 10, 20, and 40 pg/cell) on the binding of biotinylated Ad11p or Ad35p (1 pg/cell) or biotinylated Ad4p (6 pg/cell for DG75 cell and 10 pg/cell for Jurkat cells). Different concentrations of Ad4p were used to reach a minimum initial binding of 25% of the cell population for both cell lines. The results are presented as the means ± 95% confidence interval of three independent experiments.
Ad11p and Ad35p are more infectious to lymphocytic and myelomonocytic cell lines than Ad4p and Ad5v.
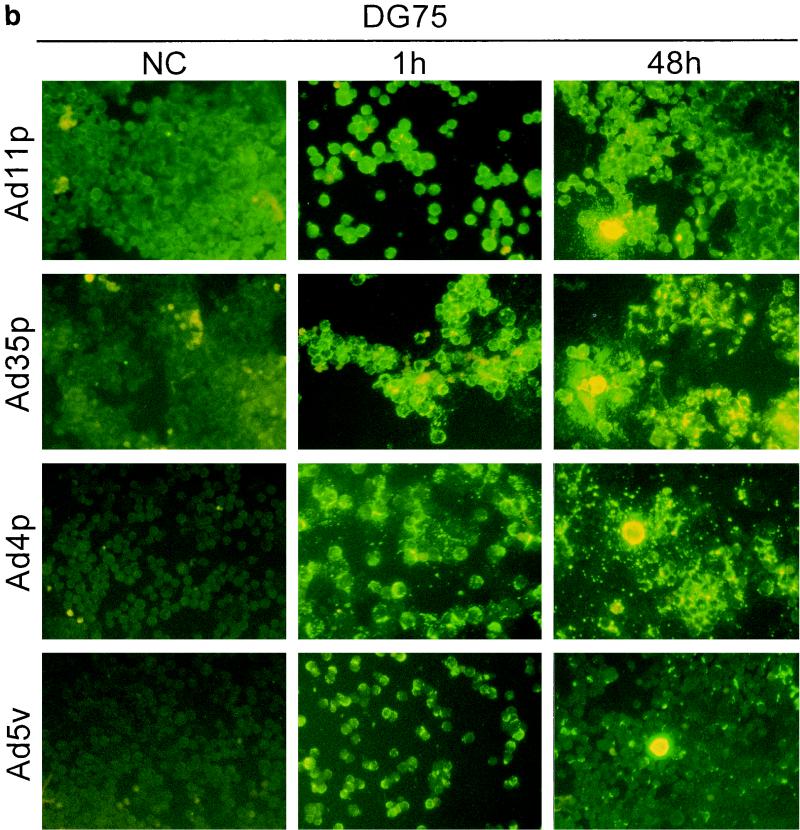
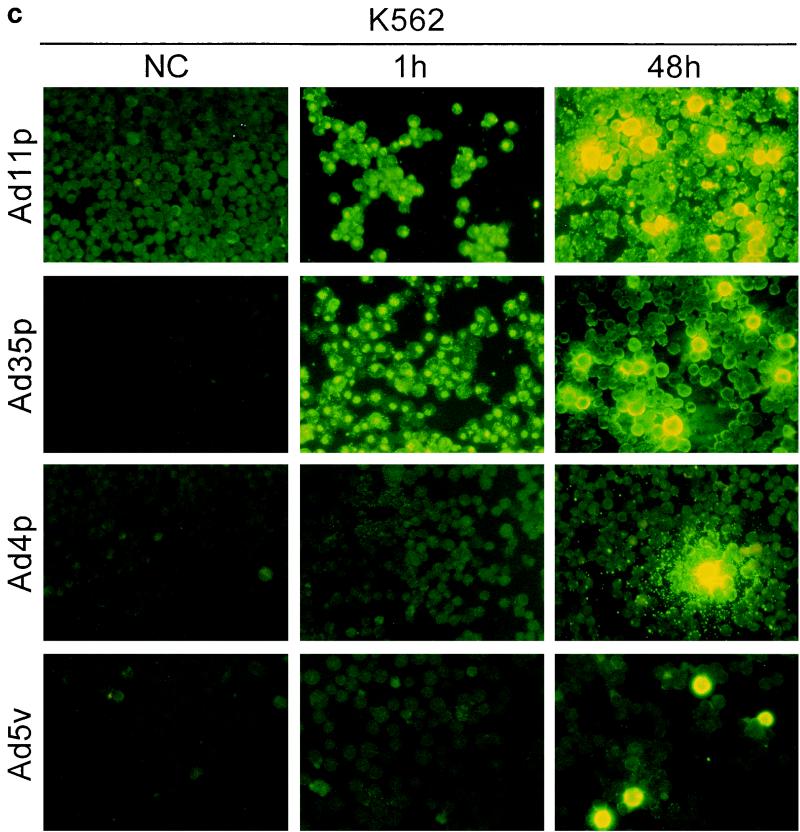
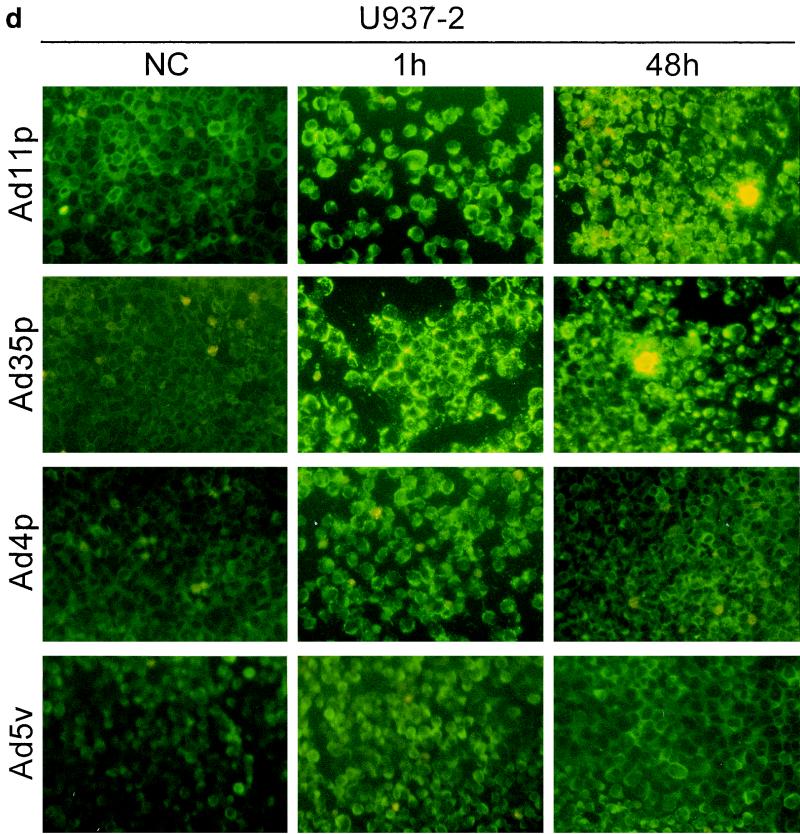
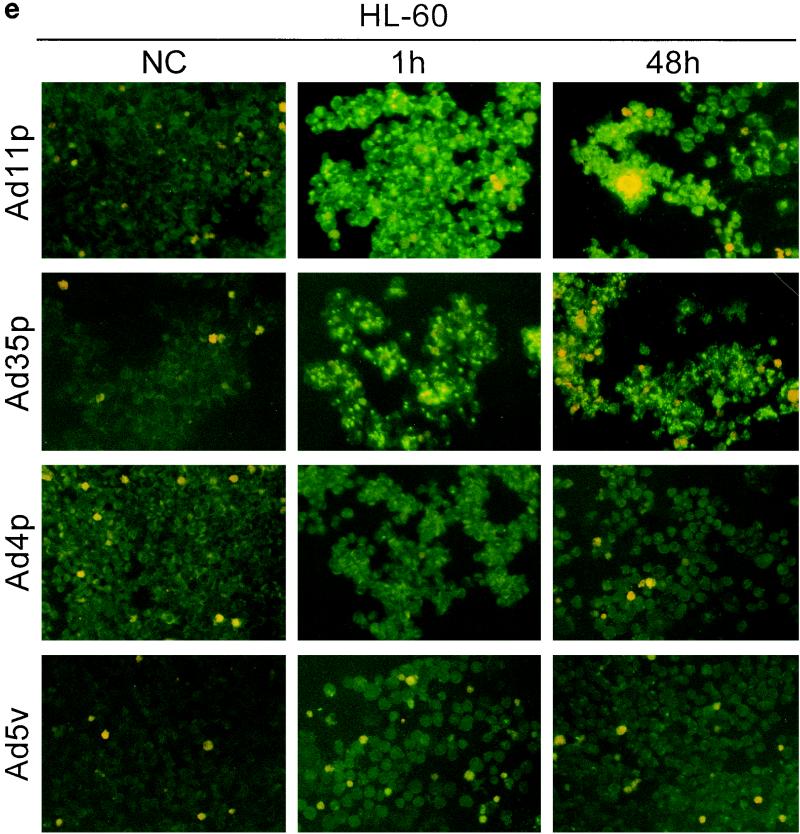
To determine whether the adenoviruses under study also were infectious to and could propagate in the hematopoietic cells, immunostainings against viral structural proteins were done on cultures of Jurkat, DG75, K562, U937-2, and HL-60 cells 1 and 48 h after infection with Ad4p, Ad5v, Ad11p, and Ad35p at a concentration of 2 pg/cell, which corresponds to a multiplicity of infection (MOI) of 100 for Ad11p and probably more for Ad5v (15). The original inoculum virus could be detected on the cell surfaces of the hematopoietic cell lines after 1 h of incubation, especially for adenovirus serotypes that display high binding efficiency, as previously determined by FACS (Fig. 4). However, the location and intensity of the fluorescence became clearly different after 48 h of incubation in cases of infection. The infected cells were also in many cases larger than surrounding uninfected cells, reflecting a cytopathogenic effect caused by the productive infection (Fig. 4).
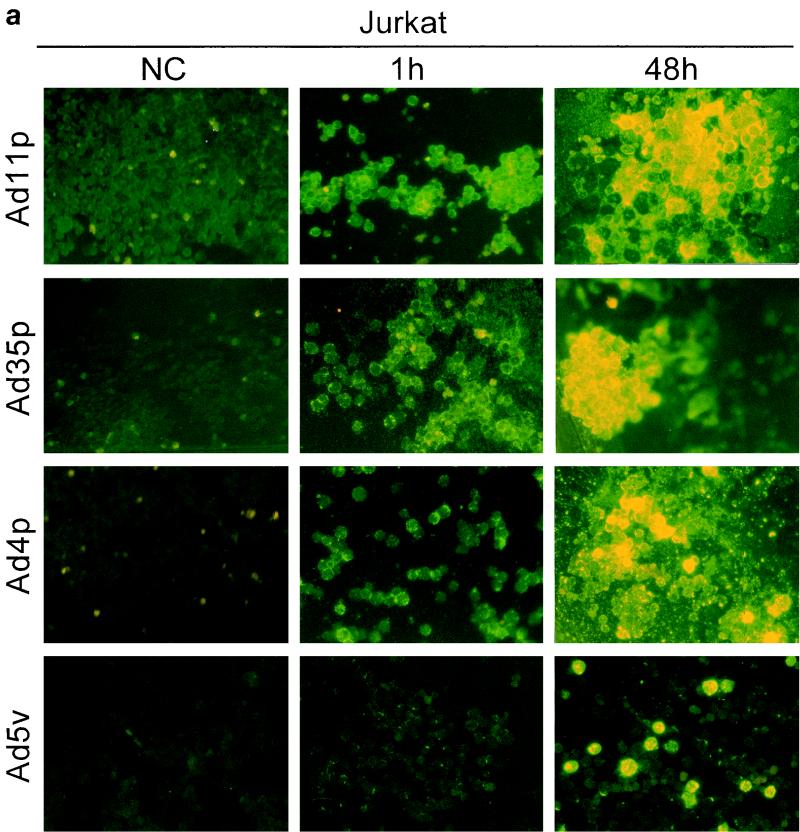
FIG. 4.
Infectivity of Ad4p, Ad5v, Ad11p, and Ad35p in various hematopoietic cell lines. Immunofluorescence with virion-specific antisera was performed on cultures of Jurkat (a), DG75 (b), K562(c), U937-2 (d), and HL-60 (e) cells 1 and 48 h after inoculation with Ad11p, Ad35p, Ad4p, and Ad5v. Micrographs show immunostainings of cells infected with 2 pg of adenoviruses per cell. NC, negative control (uninfected cells). Representative micrographs were taken at 200× enlargement.
Ad11p and Ad35p were the serotypes most infectious to the hematopoietic cell lines and thus infected the highest proportion of cells of all cell lines eventually except DG75. Ad11p and Ad35p infected almost all of the Jurkat cells and many K562 cells at the dose given (Fig. 4a and c). However, just a few DG75 or U937-2 cells produced viral structural proteins, though capped uninternalized virus particles could still be detected on many uninfected cells 48 h p.i. (Fig. 4b and d). Only one or two Ad11p antigen-positive HL-60 cells were occasionally seen per well (Fig. 4e). Ad11p was the only serotype found to cause productive infections in HL-60 cells. Ad4p and Ad5v structural proteins could be detected primarily in Jurkat and K562 cells and also in a few DG75 cells but in neither U937-2 nor HL-60 cells (Fig. 4). Jurkat and K562 were thus the cell lines most susceptible to infection by all adenoviruses investigated, whereas DG75, U937-2, and HL-60 (listed in order of increasing refractivity) were refractory.
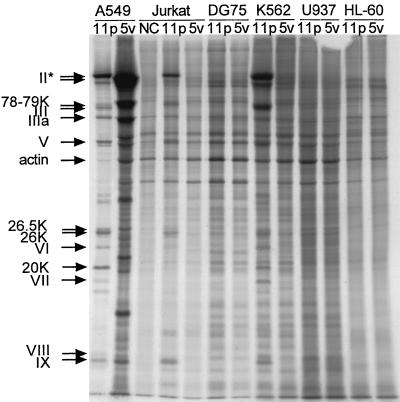
Ad11p structural proteins are highly expressed in Jurkat and K562 cells.
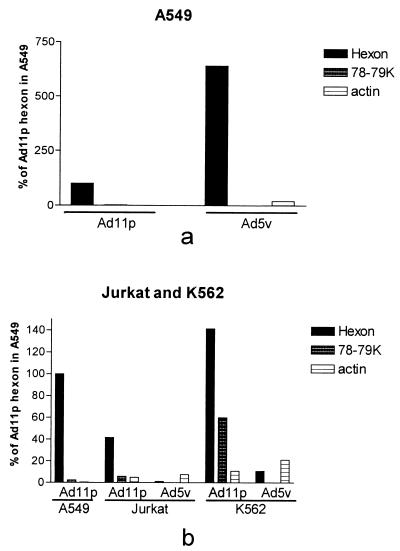
To confirm the immunofluorescence results and determine which viral proteins were produced in the hematopoietic cell lines, Jurkat, DG75, K562, U937-2, and HL-60 cells were infected with Ad11p or Ad5v (2 pg/cell) and pulsed with [35S]methionine and [35S]cysteine 24 h p.i. A549 cells were infected with the same amount of adenoviruses and pulsed for comparison with expression in permissive epithelial cells. To distinguish the viral proteins from cellular proteins, a mock-infected control of Jurkat cells was included. Viral proteins of both serotypes could be detected in Jurkat and K562 cells but not in the other cell lines (Fig. 5), which were found to be refractory to adenovirus infection also in the immunofluorescence experiment. The production of Ad5v hexons in A549 cells was more than sixfold higher than that of Ad11p, indicating that the amount of virions added corresponded to a higher MOI for Ad5v than for Ad11p (Fig. 6a). However, in the hematopoietic cell lines the production of Ad11p hexon was about 30-fold higher in Jurkat and 14-fold higher in K562 compared to the production of Ad5v hexon, which was the only detectable viral protein for Ad5v in both Jurkat and K562 cells, clearly showing that Ad11p is more effectively expressed in these cells (Fig. 6b). All of the main structural proteins of Ad11p seemed to be produced both in Jurkat and K562 cells, although there seemed to be a low production of protein III (penton base). In addition, there was a viral or induced cellular band with an apparent size of 78 to 79 kDa which was more than 2 times stronger in Jurkat cells and 24 times stronger in K562 cells than in A549 cells (Fig. 5 and 6).
FIG. 5.
Expression of viral structural proteins in Jurkat, DG75, K562, U937-2, HL-60, and permissive A549 cells. Cultures of 1.5 million cells of the different cell lines were infected with Ad11p or Ad5v (2 pg/cell) and pulsed with [35S]methionine-cysteine 24 h p.i. Equal amounts of cell lysates obtained from the different cultures were separated by SDS-PAGE on a 12% gel and autoradiographed for 16 to 24 h. Purified Ad11p and Ad5v separated on the same gel were used to identify the viral structural proteins. The arrows show the Ad11p structural proteins and the Ad5v hexon band, which was the only Ad5v band detected in the hematopoietic cell lines. Mock-infected Jurkat cells (negative control [NC]) were used to distinguish between viral and cellular bands. *, the hexon band has apparent sizes of 120 kDa for Ad11p and 110 kDa for Ad5v (39).
FIG. 6.
PhosphorImager analysis of Ad11p and Ad5v expression in Jurkat, K562, and permissive A549 cells. The SDS-PAGE-separated 35S-labeled lysates of the cell lines infected with Ad11p or Ad5v (Fig. 5) were also analyzed in a PhosphorImager system, and values obtained from a representative experiment were normalized against the value of the Ad11p hexon band in A549 cells. The values were determined for hexons, the unknown 78- to 79-kDa protein and actin.
Cellular protein synthesis was more effectively turned off in A549 cells infected with Ad11p than Ad5v, despite higher production of Ad5v proteins in this cell line. In Jurkat cells, the cellular proteins became equally labeled in the Ad11p-infected culture and the mock-infected control. The actin band was about 10 times stronger in Jurkat than in A549 cells after Ad11p infection (Fig. 5 and 6b). The immunofluorescence experiment indicated that almost all Jurkat cells became infected with Ad11p at an MOI of 100. Thus, the level of labeled cellular proteins, which was the same as in the mock-infected control, indicates that cellular protein synthesis is not turned off in Jurkat cells during Ad11p infection; alternatively, cellular protein synthesis is turned off later during infection (Fig. 5).
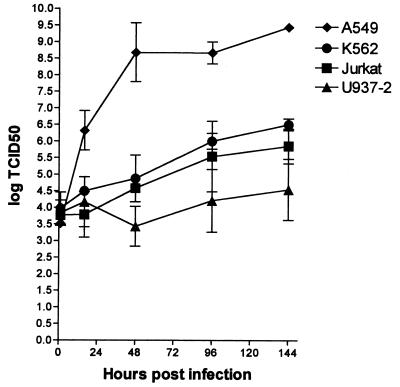
Infectious Ad11p particles are produced to only a small extent in Jurkat, K562, and U937-2 cells.
To investigate if any infectious virions were produced in the hematopoietic cell lines, the lymphocytic cell line Jurkat the myeloblastic cell line K562, and the monocytic cell line U937-2 were chosen. In earlier experiments, Jurkat and K562 appeared to be permissive to Ad11p infection, while U937-2 cells were refractory. We wanted to investigate if the production of particles was as high in Jurkat and K562 as the expression of structural proteins would indicate and if there was any production of infectious particles in the refractory U937-2 cells. Cultures of Jurkat, K562, and U937-2 cells were incubated for 1, 16, 48, 96, and 144 h with Ad11p at an MOI of 10. The samples were freeze-thawed, and the lysates were titered in A549 cells. To obtain a reference system, A549 cells were incubated in the same way. In Jurkat and K562 cells, the titer increased 2 and 2.5 logs, respectively, from ∼4.0 log 50% tissue culture infective doses (TCID50) at 1 h, which was the lowest point, to 5.8 and 6.5 log TCID50 at 144 h, respectively (Fig. 7). In U937-2, the lowest titer was obtained 48 h p.i.; from this time point to 144 h p.i., the titer increased 1 log (Fig. 7). However, the total increase in titer was only 0.5 log in the U937-2 cells. In A549 cells, the titer increased to 9.4 log TCID50 after 144 h of incubation, compared to 6.5, 5.8, and 4.5 log TCID50 in K562, Jurkat, and U937-2 cells, respectively, which corresponds to at least 1,000-fold higher production of infectious particles in A549 cells than in the hematopoietic cell lines (Fig. 7). The kinetics of the infection in Jurkat, K562, and U937-2 cells was also delayed in comparison with that in A549 cells. In A549 cells the production of complete virions started before 16 h p.i. and peaked before 48 h p.i. In Jurkat and K562 cells, the titer had increased by 48 h p.i., and in U937-2 cells an increase in titer was not seen until 96 h p.i. After 48 h p.i., the increase in titer was parallel in Jurkat, K562, and U937-2 cells (Fig. 7). The production of infectious particles was thus low and seemed delayed in the hematopoietic cell lines.
FIG. 7.
Production of Ad11p infectious particles in Jurkat, K562, U937-2, and permissive A549 cells. Cultures of 105 Jurkat, K562, and U937-2 cells were infected with Ad11p at an MOI of 10 (0.2 pg/cell) and incubated for 1, 16, 48, 96, and 144 h. The cultures were freeze-thawed three times at the end of the incubation, and endpoint titrations of the lysates were performed in A549 cells to determine the production of infectious particles at the different time points. To obtain a reference system, A549 cells were incubated in the same way. The results are presented as the means ± 95% confidence interval of at least two independent experiments.
DISCUSSION
The subgenus C serotypes Ad5 and Ad2, which most adenovirus vectors are based on, have been reported to have very limited ability to infect human leukocytes (3, 9, 13, 16, 35). This nonpermissiveness of hematopoietic cells to subgenus C adenoviruses could be explained in part by the limited capacity of subgenus C adenoviruses to bind to hematopoietic cells (9, 16, 35), which was also confirmed by our experiments. CAR, which has been shown to function as a fiber attachment receptor for Ad2 and Ad5, has also been found to be expressed at very low levels on resting peripheral blood leukocytes (16, 18, 30, 35, 38). Fiberless vectors have recently been created and found to have significantly reduced infectivity, demonstrating the importance of the high-affinity fiber binding to the target cell for the establishment of an infection (23, 44). Ectopic CAR expression has also been seen to be sufficient to render almost resistant lymphocytic cell lines susceptible to subgenus C infection (24). In this study we searched for and found adenovirus serotypes, namely, the subgenus B:2 serotypes Ad11p and Ad35p, that show remarkably high binding efficiency for all of the hematopoietic cell lines studied. The two lymphocytic cell lines Jurkat and DG75 cells seemed to express the highest amounts of the subgenus B:2 receptor since Ad11p and Ad35p showed particularly high binding to these cell lines. It has been known for a long time that subgenus B uses a different attachment receptor than the subgenus C adenoviruses (12, 14, 21, 36), and it has been shown that the subgenus B adenoviruses cannot bind CAR (33). Therefore, finding the receptor of these adenoviruses with apparent differences in tropism would be valuable. Chimeric vectors expressing the fiber genes or parts of these genes of Ad3 or Ad7 (subgenus B:1) have been created (14, 21, 36) and shown to be more infectious than the subgenus C adenoviruses to hematopoietic cells (36), in accordance with what we have observed for the subgenus B:2 serotypes, Ad11p and Ad35p. However, there could be two different subgenus B attachment receptors since members of this subgenus can be further divided into two different DNA clusters, B:1 and B:2, with tropism for the respiratory and urinary tracts, respectively. Ad4p showed some affinity for the two lymphocytic cell lines (DG75 and Jurkat) but not for the myelogenous ones and thus expressed a pattern of binding different from those of both Ad5v and the two subgenus B:2 serotypes used in this study. Blocking experiments confirmed that Ad11p and Ad35p share the same attachment receptor, which they bind to with similar affinities although they have very different fiber compositions (28, 29). However, Ad11p could not block the binding of Ad4p to the lymphocytic cell lines, indicating that Ad4p uses a cellular attachment receptor other than the receptor used by the subgenus B:2 adenoviruses, probably a receptor other than CAR since Ad5v showed no affinity to any of the hematopoietic cell lines used in this study. Ad4p has been shown to attach to the CAR protein (33) but has not been proven to use this protein as its receptor, or as its only receptor. On the contrary, preincubation with Ad11p seems to increase the binding efficiency of Ad4p, especially to Jurkat cells. Since the experiments were performed on ice, this phenomenon could hardly be explained by receptor induction.
As expected, the binding properties of the serotypes studied correlated with their abilities to infect and replicate in the hematopoietic cell lines, as seen both in the immunofluorescence experiment, where Ad11p and Ad35p infected the highest proportion of cells, and in the [35S]methionine-cysteine labeling experiment, showing 10- and 30-fold higher expression of Ad11p hexons than Ad5v hexons in K562 and Jurkat cells, respectively. However, Ad11p and Ad35p are relatively uncharacterized serotypes, and there could be several additional differences between them and Ad5v that also account for their remarkably high expression in Jurkat and K562 cells. For example, it has been shown that there are differences between subgenus B and subgenus C in endosome lysis and route to the nucleus (6, 7, 12). The cell lines studied also differed significantly in permissiveness to adenovirus infection. Jurkat cells and K562 cells were much more susceptible to adenovirus infection than DG75, U937-2, and HL-60 cells, as shown in the immunofluorescence and 35S protein labeling experiments. Varying levels of permissiveness of established hematopoietic cell lines to adenovirus infection have also been observed by others (22, 40). Primary leukocytes are known to express low levels of the adenovirus internalization receptors, αvβ3 and αvβ5 integrins. The nonpermissiveness of these cells can be partly overcome by stimulation of the cells with several different stimulation protocols (5, 17, 18, 30), and the activated cells have also been seen to upregulate the αvβ3 and αvβ5 integrins (5, 18). Adenovirus aggregates were found to be trapped on surfaces of cells with low permissiveness and still detectable at 48 h p.i. This would indicate that lack of adenovirus internalization receptors is the major obstacle to adenovirus infection in the refractory cell lines. Jurkat cells express αvβ3 and αvβ5 integrins according to leukocyte typing VI (Becton Dickinson). Different expression levels of the αvβ3 and αvβ5 integrins may thus explain the highly variable permissiveness observed by us and others between different hematopoietic cell lines.
Although Jurkat and K562 cells seem susceptible to Ad11p infection, as seen in both the immunofluorescence and 35S labeling experiments, the production of Ad11p infectious particles is 1,000-fold less than in A549 cells, indicating that virus replication is somehow disturbed in these cells. The 35S labeling of viral proteins shows that all of the major structural proteins seem to be produced in both Jurkat and K562 cells, although protein III (penton base) seems to be produced to a lesser extent in Jurkat and K562 cells than in A549 cells. There is also a viral or induced cellular protein with an apparent size 78 to 79 kDa that seems to be produced in excess in both Jurkat and K562 cells. However, the penton base is not known to have any precursor. The only viral protein migrating in this region known to have a precursor is IIIa, which for Ad5 has an apparent size of 67 kDa, and this precursor could be of a different size for Ad11p. Faucon and coworkers detected an increase of the 64- to 66-kDa band in lymphoblastoid cell lines after infection with Ad5 (13). However, the 78- to 79-kDa protein appearing in Jurkat and K562 cells after infection with Ad11p remains to be identified.
To summarize, hematopoietic cells seem to be semipermissive to Ad11p and probably also other subgenus B:2 infections. From a vector perspective this must be advantageous since the risk of getting productive infections from leaky vectors will be reduced. These findings regarding adenovirus serotypes with high binding efficiency for hematopoietic cells we hope will contribute to the development of more efficient gene delivery vectors in relation to hematopoietic cells. The problems with cytotoxicity (10, 37, 42) observed with the currently used adenovirus vectors, based on Ad2 or Ad5, will probably be overcome by using a vector with high affinity for its target since such a vector can be used in lower concentrations.
ACKNOWLEDGMENTS
This work was financed by grants from the Swedish Cancer Foundation.
We thank Eva Maria Fenyö, Karolinska Institutet, Stockholm, Sweden, who gave us some of the hematopoietic cell lines.
REFERENCES
- 1.Adrian T H, Wadell G, Hierholzer J C, Wigand R. DNA restriction analysis of adenovirus prototypes 1 to 41. Arch Virol. 1986;91:277–290. doi: 10.1007/BF01314287. [DOI] [PubMed] [Google Scholar]
- 2.Andersson L C, Nilsson K, Gahmberg C G. K562—a human erythroleukemic cell line. Int J Cancer. 1979;23:143–147. doi: 10.1002/ijc.2910230202. [DOI] [PubMed] [Google Scholar]
- 3.Andiman W A, Miller G. Persistent infection with adenovirus types 5 and 6 in lymphoid cells from humans and woolly monkeys. J Infect Dis. 1982;145:83–88. doi: 10.1093/infdis/145.1.83. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson J M, Cunningham J A, Droguett G, Kurt Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 5.Cantwell M J, Sharma S, Friedmann T, Kipps T J. Adenovirus vector infection of chronic lymphocytic leukemia B cells. Blood. 1996;88:4676–4683. [PubMed] [Google Scholar]
- 6.Chardonnet Y, Dales S. Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome. Virology. 1970;40:462–477. doi: 10.1016/0042-6822(70)90189-3. [DOI] [PubMed] [Google Scholar]
- 7.Chardonnet Y, Dales S. Early events in the interaction of adenoviruses with HeLa cells. II. Comparative observations on the penetration of types 1, 5, 7, and 12. Virology. 1970;40:478–485. doi: 10.1016/0042-6822(70)90190-x. [DOI] [PubMed] [Google Scholar]
- 8.Chroboczek J, Bieber F, Jacrot B. The sequence of the genome of adenovirus type 5 and its comparison with the genome of adenovirus type 2. Virology. 1992;186:280–285. doi: 10.1016/0042-6822(92)90082-z. [DOI] [PubMed] [Google Scholar]
- 9.Chu Y, Sperber K, Mayer L, Hsu M T. Persistent infection of human adenovirus type 5 in human monocyte cell lines. Virology. 1992;188:793–800. doi: 10.1016/0042-6822(92)90534-v. [DOI] [PubMed] [Google Scholar]
- 10.Connelly S, Gardner J M, Lyons R M, McClelland A, Kaleko M. Sustained expression of therapeutic levels of human factor VIII in mice. Blood. 1996;87:4671–4677. [PubMed] [Google Scholar]
- 11.Dales S, Chardonnet Y. Early events in the interaction of adenoviruses with HeLa cells. IV. Association with microtubules and the nuclear pore complex during vectorial movement of the inoculum. Virology. 1973;56:465–483. doi: 10.1016/0042-6822(73)90050-0. [DOI] [PubMed] [Google Scholar]
- 12.Defer C, Belin M T, Caillet-Boudin M, Boulanger P. Human adenovirus-host interactions: comparative study with members of subgroups B and C. J Virol. 1990;64:3661–3673. doi: 10.1128/jvi.64.8.3661-3673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faucon N, Ogier G, Chardonnet Y. Changes in human adenovirus 5 propagated in Burkitt's lymphoma cells. J Natl Cancer Inst. 1982;69:1215–1220. [PubMed] [Google Scholar]
- 14.Gall J, Kass Eisler A, Leinwand L, Falck Pedersen E. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J Virol. 1996;70:2116–2123. doi: 10.1128/jvi.70.4.2116-2123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green M, Pina M, Kimes R C. Biochemical studies on adenovirus multiplication. XII. Plaquing efficiencies of purified human adenoviruses. Virology. 1967;31:562–565. doi: 10.1016/0042-6822(67)90241-3. [DOI] [PubMed] [Google Scholar]
- 16.Horvath J, Weber J M. Nonpermissivity of human peripheral blood lymphocytes to adenovirus type 2 infection. J Virol. 1988;62:341–345. doi: 10.1128/jvi.62.1.341-345.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang M R, Olsson M, Kallin A, Pettersson U, Totterman T H. Efficient adenovirus-mediated gene transduction of normal and leukemic hematopoietic cells. Gene Ther. 1997;4:1093–1099. doi: 10.1038/sj.gt.3300499. [DOI] [PubMed] [Google Scholar]
- 18.Huang S, Endo R I, Nemerow G R. Upregulation of integrins αvβ3 and αvβ5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inghirami G, Nakamura M, Balow J E, Notkins A L, Casali P. Model for studying virus attachment: identification and quantitation of Epstein-Barr virus-binding cells by using biotinylated virus in flow cytometry. J Virol. 1988;62:2453–2463. doi: 10.1128/jvi.62.7.2453-2463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito M, Hirabayashi N, Uno Y, Nakayama A, Asai J. Necrotizing tubulointerstitial nephritis associated with adenovirus infection. Hum Pathol. 1991;22:1225–1231. doi: 10.1016/0046-8177(91)90104-w. [DOI] [PubMed] [Google Scholar]
- 21.Krasnykh V N, Mikheeva G V, Douglas J T, Curiel D T. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70:6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavery D, Fu S M, Lufkin T, Chen Kiang S. Productive infection of cultured human lymphoid cells by adenovirus. J Virol. 1987;61:1466–1472. doi: 10.1128/jvi.61.5.1466-1472.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legrand V, Spehner D, Schlesinger Y, Settelen N, Pavirani A, Mehtali M. Fiberless recombinant adenoviruses: virus maturation and infectivity in the absence of fiber. J Virol. 1999;73:907–919. doi: 10.1128/jvi.73.2.907-919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leon R P, Hedlund T, Meech S J, Li S, Schaack J, Hunger S P, Duke R C, DeGregori J. Adenoviral-mediated gene transfer in lymphocytes. Proc Natl Acad Sci USA. 1998;95:13159–13164. doi: 10.1073/pnas.95.22.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Londergan T A, Walzak M P. Hemorrhagic cystitis due to adenovirus infection following bone marrow transplantation. J Urol. 1994;151:1013–1014. doi: 10.1016/s0022-5347(17)35153-4. [DOI] [PubMed] [Google Scholar]
- 26.Lozzio B B, Lozzio C B, Bamberger E G, Feliu A S. A multipotential leukemia cell line (K-562) of human origin. Proc Soc Exp Biol Med. 1981;166:546–550. doi: 10.3181/00379727-166-41106. [DOI] [PubMed] [Google Scholar]
- 27.Mei Y F, Lindman K, Wadell G. Two closely related adenovirus genome types with kidney or respiratory tract tropism differ in their binding to epithelial cells of various origins. Virology. 1998;240:254–266. doi: 10.1006/viro.1997.8904. [DOI] [PubMed] [Google Scholar]
- 28.Mei Y F, Wadell G. Hemagglutination properties and nucleotide sequence analysis of the fiber gene of adenovirus genome types 11p and 11a. Virology. 1993;194:453–462. doi: 10.1006/viro.1993.1284. [DOI] [PubMed] [Google Scholar]
- 29.Mei Y F, Wadell G. Highly heterogeneous fiber genes in the two closely related adenovirus genome types Ad35p and Ad34a. Virology. 1995;206:686–689. doi: 10.1016/s0042-6822(95)80089-1. [DOI] [PubMed] [Google Scholar]
- 30.Mentel R, Dopping G, Wegner U, Seidel W, Liebermann H, Dohner L. Adenovirus-receptor interaction with human lymphocytes. J Med Virol. 1997;51:252–257. [PubMed] [Google Scholar]
- 31.Mufson M A, Belshe R B, Horrigan T J, Zollar L M. Cause of acute hemorrhagic cystitis in children. Am J Dis Child. 1973;126:605–609. doi: 10.1001/archpedi.1973.02110190487004. [DOI] [PubMed] [Google Scholar]
- 32.Philipson L, Lonberg Holm K, Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968;2:1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roelvink P W, Lizonova A, Lee J G, Li Y, Bergelson J M, Finberg R W, Brough D E, Kovesdi I, Wickham T J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shindo K, Kitayama T, Ura T, Matsuya F, Kusaba Y, Kanetake H, Saito Y. Acute hemorrhagic cystitis caused by adenovirus type 11 after renal transplantation. Urol Int. 1986;41:152–155. doi: 10.1159/000281186. [DOI] [PubMed] [Google Scholar]
- 35.Silver L, Anderson C W. Interaction of human adenovirus serotype 2 with human lymphoid cells. Virology. 1988;165:377–387. doi: 10.1016/0042-6822(88)90582-x. [DOI] [PubMed] [Google Scholar]
- 36.Stevenson S C, Rollence M, Marshall Neff J, McClelland A. Selective targeting of human cells by a chimeric adenovirus vector containing a modified fiber protein. J Virol. 1997;71:4782–4790. doi: 10.1128/jvi.71.6.4782-4790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teramoto S, Johnson L G, Huang W, Leigh M W, Boucher R C. Effect of adenoviral vector infection on cell proliferation in cultured primary human airway epithelial cells. Hum Gene Ther. 1995;6:1045–1053. doi: 10.1089/hum.1995.6.8-1045. [DOI] [PubMed] [Google Scholar]
- 38.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wadell G, Hammarskjold M L, Winberg G, Varsanyi T M, Sundell G. Genetic variability of adenoviruses. Ann N Y Acad Sci. 1980;354:16–42. doi: 10.1111/j.1749-6632.1980.tb27955.x. [DOI] [PubMed] [Google Scholar]
- 40.Wattel E, Vanrumbeke M, Abina M A, Cambier N, Preudhomme C, Haddada H, Fenaux P. Differential efficacy of adenoviral mediated gene transfer into cells from hematological cell lines and fresh hematological malignancies. Leukemia. 1996;10:171–174. [PubMed] [Google Scholar]
- 41.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 42.Wilmott R W, Amin R S, Perez C R, Wert S E, Keller G, Boivin G P, Hirsch R, De Inocencio J, Lu P, Reising S F, Yei S, Whitsett J A, Trapnell B C. Safety of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator cDNA to the lungs of nonhuman primates. Hum Gene Ther. 1996;7:301–318. doi: 10.1089/hum.1996.7.3-301. [DOI] [PubMed] [Google Scholar]
- 43.van Ormondt H, Galibert F. Nucleotide sequences of adenovirus DNAs. Curr Top Microbiol Immunol. 1984;110:73–142. doi: 10.1007/978-3-642-46494-2_4. [DOI] [PubMed] [Google Scholar]
- 44.Von Seggern D J, Chiu C Y, Fleck S K, Stewart P L, Nemerow G R. A helper-independent adenovirus vector with E1, E3, and fiber deleted: structure and infectivity of fiberless particles. J Virol. 1999;73:1601–1608. doi: 10.1128/jvi.73.2.1601-1608.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]