Abstract
The distinctive characteristics of nanoparticles and their potential applications have been given considerable attention by scientists across different fields, particularly agriculture. However, there has been limited effort to assess the impact of copper nanoparticles (CuNPs) in modulating physiological and biochemical processes in response to salt-induced stress. This study aimed to synthesize CuNPs biologically using Solenostemma argel extract and determine their effects on morphophysiological parameters and antioxidant defense system of barley (Hordeum vulgare) under salt stress. The biosynthesized CuNPs were characterized by (UV–vis spectroscopy with Surface Plasmon Resonance at 320 nm, the crystalline nature of the formed NPs was verified via XRD, the FTIR recorded the presence of the functional groups, while TEM was confirmed the shape (spherical) and the sizes (9 to 18 nm) of biosynthesized CuNPs. Seeds of barley plants were grown in plastic pots and exposed to different levels of salt (0, 100 and 200 mM NaCl). Our findings revealed that the supplementation of CuNPs (0, 25 and 50 mg/L) to salinized barley significantly mitigate the negative impacts of salt stress and enhanced the plant growth-related parameters. High salinity level enhanced the oxidative damage by raising the concentrations of osmolytes (soluble protein, soluble sugar, and proline), malondialdehyde (MDA) and hydrogen peroxide (H2O2). In addition, increasing the activities of enzymatic antioxidants, total phenol, and flavonoids. Interestingly, exposing CuNPs on salt-stressed plants enhanced the plant-growth characteristics, photosynthetic pigments, and gas exchange parameters. Furthermore, CuNPs counteracted oxidative damage by lowering the accumulation of osmolytes, H2O2, MDA, total phenol, and flavonoids, while simultaneously enhancing the activities of antioxidant enzymes. In conclusion, the application of biosynthesized CuNPs presents a promising approach and sustainable strategy to enhance plant resistance to salinity stress, surpassing conventional methods in terms of environmental balance.
Subject terms: Plant physiology, Nanoparticles
Introduction
Salinity stress is a significant environmental challenge that adversely affects agricultural productivity especially in arid and semiarid regions of the world1. Salt stress affects approximately 50% of irrigated land and about 20% of global cropland, leading to a severe reduction in crop growth and yield2,3. Salinity have led to alterations in various physiological responses in plants like harming the integrity of the plasma membrane, disrupting stomatal conductivity, hinders gaseous exchange and reducing photosynthetic efficiency4,5. It also possess a detrimental effects by producing reactive oxygen species (ROS) including hydroxyl free radical (OH*), singlet oxygen (1O2), and oxide ions (O2−)6. The accumulation of salt in the root zone creates a negative water potential, which hinders the absorption of water and the uptake of minerals by root cells7. Additionally, prolonged exposure to saline environments results in the accumulation of sodium ions (Na+) and chloride ions (Cl−) within cellular compartments of above-ground plant tissues, resulting in ionic toxicity8.
Throughout the years, several stress management strategies have been developed to enhance salinity stress tolerance in cereal crops for improving their productivity. These approaches encompass on-farm practices, screening of more resilient genotypes, introduction of genes that promote tolerance, and modification of traditional breeding methods9,10. However, these methods have encountered limitations due to their time-consuming, high costs, and limited adaptability. In this context, there is an increasing need for innovative and sustainable solutions to mitigate the impact of salinity stress on crops.
Nanotechnology has arisen as a versatile, effective, and widely embraced scientific method in human life, it played a crucial role in revolutionizing the field of agriculture11,12. Recent advancements in this field offer a promising avenue for significantly improving crop production and assuring sustainability. This potential improvement stems from the integration of nanoparticles (NPs) into strategies aimed at achieving sustainable and equitable utilization of agricultural resources13. Nanoparticles (NPs) are substances characterized by having a size smaller than 100 nm in at least one of their dimensions14. NPs exhibit significant attributes, such as a high surface area relative to their volume, diminutive size, shape adjustability, and the ability to effectively transport various substances to cells; thus, they are currently under examination for their potential applications in agriculture as nanofertilizers, nanopesticides, and carriers for a variety of plant growth regulators15,16. Furthermore, utilizing nanoparticles represents a novel approach to enhance plant growth and performance in saline conditions17. Many recent studies have highlighted the capability of NPs to act as agents that alleviate abiotic stress and promote the growth and development of plants18,19. This is mainly linked to their diminutive dimensions and their efficiency in transporting minerals and chemicals at the cellular level20. Singh et al.21 conducted a study involving the use of ZnO NPs, their findings revealed improved agronomic traits and increased antioxidant enzyme activities in rice (Oryza sativa) when exposed to salinity conditions. Additionally, the application of calcium nanoparticles to tomato plants was found to enhance plant defense mechanisms against salt stress by lessening the oxidative damage to membranes and maintaining balance in ionic state22.
Among the various nanoparticles under investigation, copper nanoparticles (CuNPs) have gained attention for their multifaceted properties and biocompatibility with plants23. Copper nanoparticles have been synthesized using various methods, including chemical, physical, and biological approaches24. The biological synthesis of nanoparticles, using plant materials offers biocompatibility and eco-friendly alternative to conventional chemical methods25,26. Where, many studies have documented the eco-friendly creation of copper nanoparticles through various plant extracts like Calotropis gigantea27,28. Such biosynthesized nanoparticles exhibit distinctive traits that render them well-suited for mitigating salinity stress in crops29. Copper plays a vital role as a fundamental element in numerous enzymes that are essential for redox and electron transfer processes within plant cells, thus it serves as a significant micronutrient capable of enhancing both growth and various developmental stages30. Reckoning this significant fact, CuNPs have been developed and examined for their ability to promote growth, improve development, manage diseases, and induce drought tolerance in plants31–33. To our knowledge, there have no comprehensive studies been conducted to assess the physiological and biochemical responses of barley plants to copper nanoparticles application in saline conditions.
Barley (Hordeum vulgare) is an important cereal crop that is often grown in regions prone to salinity stress for forage purposes and as a grain crop. The crop is known for its adaptability and resilience, yet its growth and yield can be significantly hampered by high soil salinity34. Where the susceptibility of the crop to salinity is most pronounced during the germination and early seedling stages35. The purpose of this study was to examine the potential impacts of biosynthesized copper nanoparticles in enhancing salinity stress tolerance in barley.
Material and methods
Synthesis of CuNPs
Solenostemma argel, a medicinal plant collected from botanical garden of Department of Botany, King Saud University, is commonly known as argel and thrives in desert environments. Previous studies, supported by GC–MS analysis, have revealed a plethora of phytochemicals within the plant36,37. These phytochemical constituents play a crucial role in the NPs formation as they act as reducing agents in the biological synthesis of various nanoparticles.
In this study, copper nanoparticles were synthesized in a green approach using Solenostemma argel. Copper (II) sulfate pentahydrate (CuSO4⋅5H2O) was purchased from Sigma-Aldrich Chemical Corp. All the solutions were made using deionized Milli-Q water. In the typical synthesis of copper nanoparticles, 5 g of dried leaves of S. argel were meticulously cleaned, then transferred in a round-bottom flask with 100 mL of deionized water. The mixture was boiled for 10 min, and the resulting aqueous extract was filtered and stored in a refrigerator at 4 °C for further use. 50 ml of the leaf extract was added into 100 ml of 1 mM of copper sulfate solution and kept in stirring for 24 h at room temperature. The color of the copper sulfate aqueous solution changed from blue to dark green confirming the initial formation of the copper nanoparticles synthesis.
Characterization techniques of synthesized CuNPs
The characterization of green-synthesized Cu nanoparticles was analyzed by different techniques. First, the surface plasmon resonance (SPR) band for the synthesized NPs was identified via ultraviolet–visible (UV–Vis) spectroscopy analysis. The presence of potential biomolecules and functional groups that acted as reduction agents for CuNPs synthesis were monitored using Fourier transform infrared spectroscopy (FT-IR). The crystalline behavior of the biosynthesized CuNPs was investigated using the X-ray diffraction method (XRD). The transmission electron microscope (SEM) was used to identify the size and shape of the biosynthesized nanoparticles.
Plant material and growth conditions
Seeds of barley (Hordeum vulgare) were surface sterilized with 0.1% sodium hypochlorite (NaOCl) solution for 15 min and then thoroughly washed with sterile distilled water under aseptic conditions before being used in the experiments.
The study was performed in controlled conditions at the Botany and Microbiology Department of King Saud University as a factorial experiment in a completely randomized design (CRD). The experiment consisted of nine treatments, three levels of salinity (0, 100, and 200 mM NaCl) as well as three levels of CuNPs (0, 25, and 50 mg/L). Each treatment was implemented in triplicates. The sterile barley seeds were planted in a plastic pot. Each pod contained 800 g of soil as well as ten seeds of H. vulgare. The plants were collected 25 days post-sowing for growth and physiological assessments.
Measurement of growth parameters
The roots and shoots of plants uprooted at 25 days of age were washed with deionized water. Subsequently, the plant tissues soaked in water were dried on Whatman filter paper before measuring growth parameters. The lengths of plant roots and shoots were measured using a measuring scale, and the samples’ fresh weight was then determined using a sensitive electronic balance. To determine the dry weights of roots and shoots, the samples were subjected to oven-drying at 80 °C for a duration of 48 h. The leaf area index was assessed using a portable leaf area meter (ADC Bioscientific, UK). The diameter of the stem was measured in the middle internode of the barley stem using a Vernier caliper.
Gas exchange parameters
The gas exchange parameters such as net photosynthetic rate (Pn), stomatal conductance (gs), transpiration rate (Tr), and internal CO2 (Ci) content were calculated via infrared portable photosynthetic system (LI-COR 6400, LICOR, Lincoln, Nebraska, USA). All the parameters are determined in the intact plant leaves at room temperature and relative humidity (60%). Photosynthetic photo-flux density (PPFD) and CO2 concentration were kept constant at 800 μmol mol−2 s−1 and 600 ppm, respectively.
Biochemical parameters
Determination of chlorophyll and carotenoid contents
The amounts of photosynthetic pigments were estimated using the method reported by Arnon38. Fresh leaves of barley (100 mg) were crushed in 10 ml of chilled 80% acetone using pre-chilled mortar and pestle to obtain a fine pulp. The pulp was centrifuged in a High-Speed Refrigerated Microcentrifuge (M1324R, USA) at 1000g, at 4 °C for 10 min. The absorbance of the supernatant was measured against 80% acetone as blank at 470, 645 and 663 nm using UV-1800 spectrophotometer (Shimadzu, Japan) for the calculation of chlorophyll and carotenoid contents. The chlorophyll a and b, and carotenoid contents were calculated as follows.
where A663, A645, and A470 are the absorbance value read at 663 nm, 645 nm, and 470 nm, respectively.
Electrolyte leakage (EL) measurement
Electrolyte Leakage was calculated as per method described by Bajji et al.39. Removal of dust and contamination that occurred during sampling was performed by a slow wash of the leaves using deionized water. The leaf blade samples were placed in a test tube containing 20 mL of deionized water at room temperature, then after, an electrical conductivity meter (DDSJ-308A, Shanghai) was used to determine the electrical conductivity (E0) of the solution immediately. Following a day of incubation at 35 °C, the electrical conductivity (E1) was read again. The tubes were then flooded in a 120 °C water bath for 30 min and cooled to 25 °C before recalculating the electrical conductivity (E2). Finally, the electrolyte leakage percent (EL %) of the leaf cells was measured as follows:
Total protein and soluble sugars contents determination
Soluble sugars were extracted using the phenol sulfuric acid technique40. In this method, 300 mg of plant tissue was immersed in ethanol (10 mL). The extract at 10,000 rpm for 10 min was centrifuged and then treated with 5% phenol and 98% H2SO4. The mixture was subjected to a boiling water bath for 30 min and then cooled. The absorbance was measured at 490 nm using UV–Vis spectroscopy. A glucose standard curve was used to estimate the concentration of the total soluble sugars.
Lipid peroxidation and proline assay
The level of MDA concentration in fresh leaves was estimated according to Jiang and Zhang41 method. Fresh leaf (0.4 g) was homogenized in 5 mL of 0.1% (w/v) trichloroacetic acid, and the homogenate was centrifuged at 7000 rpm for 10 min. Then, 1 mL of the supernatant was mixed with 4 mL of 0.5% thiobarbituric acid (TBA, in 20% TCA), and the blend was heated to 95 °C for half an hour. Then, the mixture immediately was placed in an ice bath for cooling and centrifuged at 7000 rpm for 10 min; the supernatant absorbance (containing MDA) was read against a reagent blank (0.5% TBA in 20% (w/v) TCA) at 532 nm and corrected to non-specific turbidity by subtracting the value at 600 nm on a UV–1800 spectroscopy.
Bates et al.42 procedure was used to estimate the proline levels in barley leaf. Fresh barley leaves (0.5 g) were crushed in 10 mL of 3% sulfosalicylic acid and centrifuged at 10,000g for 15 min. Subsequently, the 2 mL supernatant was taken in a test tube and combined with 2 mL of ninhydrin reagent and glacial acetic. Afterward, the samples were placed in a water path at 100 °C for 30 min before incubating the mixture in an ice bath for 5 min to end the reaction. Then, 5 mL of toluene was added and vigorously mixed and stand for 15 min at room temperature. The absorbance of the developed color in the upper phase at 520 nm was measured using a UV–Vis spectroscopy and toluene was used as a blank.
Quantitation of hydrogen peroxide (H2O2)
Hydrogen peroxide (H2O2) level was determined as per Velikova et al.43 method. A known weight of barley fresh leaves was ground in 5 mL of 0.1% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuge at 7000g for 30 min; thereafter, 0.5 mL of the supernatant was blended with 0.5 mL of 10 mM potassium phosphate buffer (pH 7.2) and 1.0 mL of 1.0 M KI solution. The absorbance was read via spectrophotometer at 390 nm.
Estimation of antioxidant enzyme activities
Different parts of plant samples (Shoot and Roots) were initially blended in liquid nitrogen and dissolved in 100 mM sodium phosphate buffer (pH 7.2) for further sample preparation according to the specific protocols listed below. After centrifugation at 7000×g for 15 min at 4 °C, the upper phase was placed in a falcon tube and used to estimate the activity of enzymatic antioxidants. The protein content in the mixture was determined as per method described by Bradford44.
Superoxide dismutase (EC 1.15.1.1)
The activity of superoxide dismutase (SOD) was determined according to Marklund and Marklund45 procedure’s. The reaction mixture consisted of 1.9 mL 100 mM sodium phosphate buffer (pH 7.2), 0.25 mM pyrogallol, and 100 μL of plant extract. The absorbance was recorded at 420 nm. The SOD activity (U g−1 protein) was defined as the amount of enzyme that inhibits 50% of pyrogallol oxidation.
Catalase (EC 1.11.1.6)
The catalase (CAT) activity was measured spectrophotometrically by recording the absorbance at 240 nm using Claiborne46 method’s. The reaction mixture contained 1 mL of 0.059 M H2O2 in 0.1 M sodium phosphate buffer (pH 7.2), 1.8 mL of distilled water, and 200 μL of plant extract. The CAT activity was expressed as unit g−1 of protein.
Glutathione reductase (EC 1.6.4.2)
The activity of glutathione reductase (GR) enzyme was assessed as per protocol reported by Schaedle and Bassham47. The homogenate was followed oxidation of nicotinamide adenine dinucleotide phosphate (NADPH) and rea via spectrophotometer at 340 nm (e, 6.2 mM_1 cm_1) for 3 min in 2 mL of an assay mixture consists of 3 mM Na2EDTA, 50 mM potassium-phosphate buffer (pH 7.2), 0.15 mM NADPH, 0.5 mM GSSG, and 100 μL of plant extract. The glutathione reductase activity was expressed as EU mg−1 protein.
Ascorbate peroxidase (EC 1.11.1.11)
Ascorbate peroxidase (APX) activity was assessed by reducing the ascorbate content as described by Nakano and Asada48. The reaction mixture contained 0.5 mM ascorbic acid, 2 mL of 50 mM sodium phosphate buffer (pH 7.2), 0.1 mM EDTA, 0.1 mM H2O2, and 100 μL of plant extract. The absorbance of the mixture was read spectrophotometry at 290 nm for 180 s. The activity of APX was expressed as unit g−1 of protein.
Estimation of phenolic and flavonoid contents
The total phenolic content (TPC) in barley leaves was estimated using The Folin–Ciocalteu reagent in accordance with the method described by Velioglu et al.49. A hundred milligram of fresh leaf was digested in 99% Ethanol. The samples were subjected to an orbital shaker at 200 rpm for 2 h. The mixtures were centrifuged for 5 min at 10,000 rpm and the supernatant was poured into a 2 mL Eppendorf tube. Then, 100 μL of the extract was mixed with 0.75 mL of Folin–Ciocalteu reagent in a test tube. The mixture was allowed to stand for 5 min at room temperature, and then 0.75 mL of Na2CO3 solution was added to the mixture. After 30 min of incubation at room temperature, the absorbance was read at 765 nm via a UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan). The Gallic acid was subjected to plot the standard calibration curve and phenolic content was expressed in Gallic acid equivalent. For total flavonoids content (TFC), 20 μL plant extract, 0.16 mL di-ionized water, 10 μL CH3CO2K, and 10 μL AlCl3, were thoroughly jumbled and then stood for 15 min. Gallic acid was used to plot the standard calibration curve. TPC content was detected as mg/g FW at 420 nm using a (UV–Vis) spectroscopy.
Statistical analysis
The data was analyzed statistically using a two-way analysis of variance (ANOVA) and mean separation between treatments was performed using Duncan’s new multiple range test with a significance level set at (p ≤ 0.05). The entire analysis was performed using SPSS v. 20 for Windows. Principal component analysis (PCA) and Pearson's correlation analysis were conducted using Origin Pro software, version 2023.
Ethical approval
The authors confirm that all materials/methods used in this study comply with relevant institutional, national, and international guidelines legislation.
Results
Green synthesis and characterization of CuO nanoparticle
Visual identification
During the synthesis process of CuNPs, a different color array appeared. Where the extract of S. argel plant consists of several phytochemicals that act as reducing agents and converts the copper sulfate into copper nanoparticles. The biosynthesized CuNPs were identified visually when the color of the reaction mixture turned to dark green within 24h of stirring confirming the initial formation of CuNPs (Fig. 1).
Figure 1.
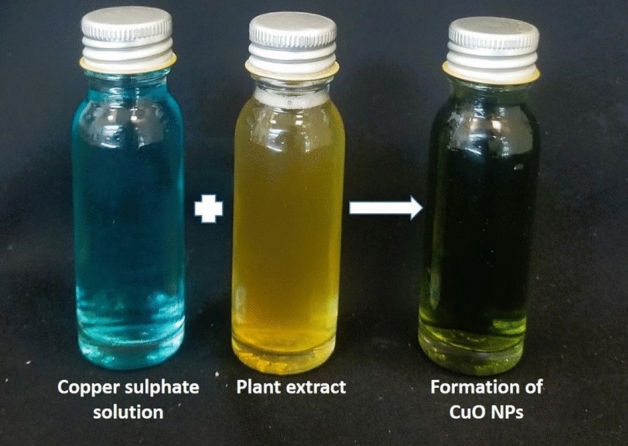
Photographic images of the synthesized CuNPs.
UV–Vis spectroscopic analysis of copper nanoparticles
The surface plasmon resonance characteristic of the bio-fabricated CuNPs were observed via UV–Vis spectroscopy at a different wavelength from 200 to 800 nm as shown in Fig. 2a. The UV–Vis spectrum analysis illustrated ideal absorption peak at 320 nm.
Figure 2.
Characterization of biosynthesized CuNPs from S. argel leaf extract, (a) Ultraviolet–visible absorption spectrum, (b) XRD patterns, (c) Fourier-transform infrared spectroscopy (FTIR), and (d) transmission electron microscope (TEM).
XRD analysis
The successful synthesis of copper nanoparticles was validated by examining the X-Ray diffraction. Bragg’s reflection analysis of copper nanoparticles revealed distinctive diffraction peaks at approximately 2θ = 38.34°, 43.4°, 51.3°, 73.39° and 78.36°. These peaks correspond to the crystallographic planes [110], [111], [200], [220] and [311], respectively, indicating the face-centered cubic (fcc) structure of the nanoparticles, in comparison to the standard powder diffraction card from JCPDS, specifically copper file No. 01–078-2076 (Fig. 2b).
FT-IR spectral analysis
The functional groups of the biosynthesized CuNPs due to chemical interaction between S. Argel leaf extract and Cu2+ were predicted using FTIR spectra. The FT-IR analysis revealed six peaks at 432 cm−1, 760 cm−1, 1110 cm−1, 1650 cm−1, 2098 cm−1, and 3440 cm−1 (Fig. 2c). The shift in the peaks was clearly attributed to the reduction of Cu2+ into CuNPs.
Transmission electron microscope (TEM) analysis
Transmission electron microscope (TEM) analysis of copper nanoparticles biosynthesized from the leaf extract of S. argel revealed spherical particles with sizes below 19 nm, as depicted in Fig. 2d.
Barley growth and development
The application of NaCl (100 and 200 mM) salt to barley plants caused a remarkable suppression in the fresh weight, dry weight, and plant length for both shoots and roots, in addition it reduced leaf area index and stem dimeter compared to control plants. On the other hand, it caused a significant increase in EL over the control (Table 1). However, the application of Cu NPs to salt-stressed barley plants successfully alleviates the mischievous affects symptoms caused by the NaCl stress through increasing all morphological attributes (Fig. 3). While significantly reduced the EL.
Table 1.
The impact of CuNPs and salt-induced stress, whether applied individually or in conjunction with each other on the gas exchange parameters in barley (H. vulgare).
| NaCl | CuNPs | Fresh weight (g) | Dry weight (g) | Plant height (cm) | LA(mm) | SD (mm) | EL (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | Root | Leaf | Root | Leaf | Root | |||||
| 0 NaCl | 0 mg/L NPs | 10.8 ± 0.2c | 4.4 ± 0.3d | 3.7 ± 0.15 | 1.5 ± 0.01 | 23.1 ± 0.3e | 22.7 ± 0.4d | 70.2 ± 1.3c | 10.2 ± 0.1d | 14.2 ± 1.3e |
| 25 mg/L NPs | 14.9 ± 0.2a | 7.4 ± 0.2a | 5.9 ± 0.15 | 2.5 ± 0.02 | 36 ± 0.5a | 41.8 ± 1.0a | 84.6 ± 0.6a | 24.7 ± 0.3a | 17.8 ± 0.6de | |
| 50 mg/L NPs | 14.0 ± 0.3ab | 7.0 ± 0.2a | 5.7 ± 0.1 | 2.6 ± 01 | 35.5 ± 0.4a | 40.7 ± 0.5a | 80.9 ± 0.8a | 24.5 ± 0.2a | 18.6 ± 0.8d | |
| 10 NaCl | 0 mg/L NPs | 7.5 ± 0.40d | 3.3 ± 0.3e | 3.0 ± 0.1 | 1.4 ± 0.01 | 17.1 ± 0.4d | 17.8 ± 0.3e | 52.5 ± 1.4d | 9.3 ± 0.2de | 27.0 ± 1.4a |
| 25 mg/L NPs | 13.7 ± 0.6ab | 6.7 ± 0.3a | 5.2 ± 0.2 | 2.3 ± 01 | 31.2 ± 0.3b | 32.1 ± 0.3b | 79.8 ± 0.8b | 22.3 ± 0.2b | 17.8 ± 0.8d | |
| 50 mg/L NPs | 13.6 ± 0.4ab | 5.4 ± 0.4c | 4.9 ± 0.1 | 2.2 ± 01 | 29.4 ± 0.3c | 29.1 ± 0.3c | 76.2 ± 1.1b | 18.6 ± 0.2c | 19.2 ± 1.1bc | |
| 200 NaCl | 0 mg/L NPs | 6.1 ± 0.45e | 2.8 ± 0.4f | 2.6 ± 0.02 | 1.2 ± 0.02 | 15.6 ± 0.40f | 15.1 ± 0.3f | 43.9 ± 0.8e | 8.4 ± 0.3e | 29.9 ± 0.8a |
| 25 mg/L NPs | 11.1 ± 0.35b | 6.3 ± 0.3b | 4.5 ± 0.1 | 1.9 ± 0.02 | 28.8 ± 0.4c | 29.6 ± 0.3b | 71 ± 1.0c | 17.5 ± 0.2c | 18.9 ± 1.0c | |
| 50 mg/L NPs | 10.4 ± 0.4c | 4.6 ± 0.3d | 4. 3 ± 0.15 | 1.8 ± 01 | 25.1 ± 0.4d | 26.3 ± 0.3e | 68.6 ± 1.6c | 18.2 ± 0.3c | 21.0 ± 1.6b | |
Figure 3.
Barley (H. vulgare) plants under NaCl stress and application of biosynthesized silver nanoparticles. (a) (shoots), (b) roots. Where, 0 (control), 1 (25 mg/L NPs), 2 (50 mg/L NPs), 3 (100 mM NaCl), 4 (25 mg/L NPs + 100 mM NaCl), 5 (50 mg/L NPs + 100 mM NaCl), 6 (100 mM NaCl), 7 (25 mg/L NPs + 200 mM NaCl), 8 (50 mg/L NPs + 200 mM NaCl).
All the data are means of three replicates ± standard deviation. Different letters indicate significant differences between treatments according to Duncan’s multiple range test (p ≤ 0.05).
Gas exchange parameters
As shown in Table 2, all the gas exchange parameters including net photosynthetic rate (Pn), intracellular CO2 concentration (Ci), transpiration rate (Tr), and stomatal conductance (gs) were considerably decreased in salt-treated barley, the highest decreased was recorded in 200 mM NaCl compared to control. Application of copper nanoparticles significantly affected leaf gas exchange attributes under NaCl stress conditions. Treatment of 25 and 50 mg/L CuNPs to stressed plants resulted in a considerable increase in the values of gaseous exchange parameters under salt stress. where, treatment of 25 mg/L NPs alone, yielded the highest values for all leaf gas exchange parameters.
Table 2.
The impact of CuNPs and salt-induced stress, whether applied individually or in conjunction with each other on the gas exchange parameters in barley (H. vulgare).
| NaCl | CuNPs | Pn (μmol m−2 s−) | Ci (μmol mol−1) | Tr (μmol m−2 s−) | gs (μmol m−2 s−) |
|---|---|---|---|---|---|
| 0 NaCl | 0 mg/L NPs | 1.5 ± 0.05d | 722.3 ± 1.2e | 0.44 ± 0.02d | 0.09 ± 0.002e |
| 25 mg/L NPs | 3.1 ± 0.1a | 793.7 ± 2.4a | 0.64 ± 0.04a | 0.113 ± 0.002b | |
| 50 mg/L NPs | 3.0 ± 0.1a | 753.8 ± 2.3b | 0.58 ± 0.02b | 0.115 ± 0.003a | |
| 100 NaCl | 0 mg/L NPs | 1.3 ± 0.05e | 715.6 ± 2.1f | 0.32 ± 0.03e | 0.072 ± 0.002f |
| 25 mg/L NPs | 2.6 ± 0.1b | 743.2 ± 1.3c | 0.57 ± 0.01b | 0.106 ± 0.002c | |
| 25 mg/L NPs | 2.3 ± 0.1c | 726.6 ± 2.1d | 0.52 ± 0.03c | 0.104 ± 0.002c | |
| 200 NaCl | 0 mg/L NPs | 1.0 ± 0.1f | 704.2 ± 2.3g | 0.28 ± 0.02f | 0.058 ± 0.002g |
| 25 mg/L NPs | 1.6 ± 0.15d | 728.4 ± 2.0d | 0.46 ± 0.03d | 0.098 ± 0.001d | |
| 50 mg/L NPs | 1.5 ± 0.1d | 727.7 ± 1.3d | 0.44 ± 0.05d | 0.095 ± 0.002d |
All the data are means of three replicates ± standard deviation. Different letters indicate significant differences between treatments according to Duncan’s multiple range test (p ≤ 0.05).
Photosynthetic pigments contents
Salt stress provoked a significant reduction in photosynthetic pigments including chlorophyll a, chlorophyll b and carotenoids comparing to control group. The most significant reduction was observed in plants subjected to 200 mM NaCl treatment. Nevertheless, the application of CuNPs, either alone or in combination with NaCl, predominantly improved photosynthetic pigments in both unstressed and stressed barley plants (Fig. 4).
Figure 4.
The impact of CuNPs and salt-induced stress, whether applied individually or in conjunction with each other on the photosynthetic pigments contents, (a) Ch a, (b) Ch b, and (c) carotenoids in barley (H. vulgare). All the data are means of three replicates ± standard deviation. Different letters indicate significant differences between treatments according to Duncan’s multiple range test (p ≤ 0.05).
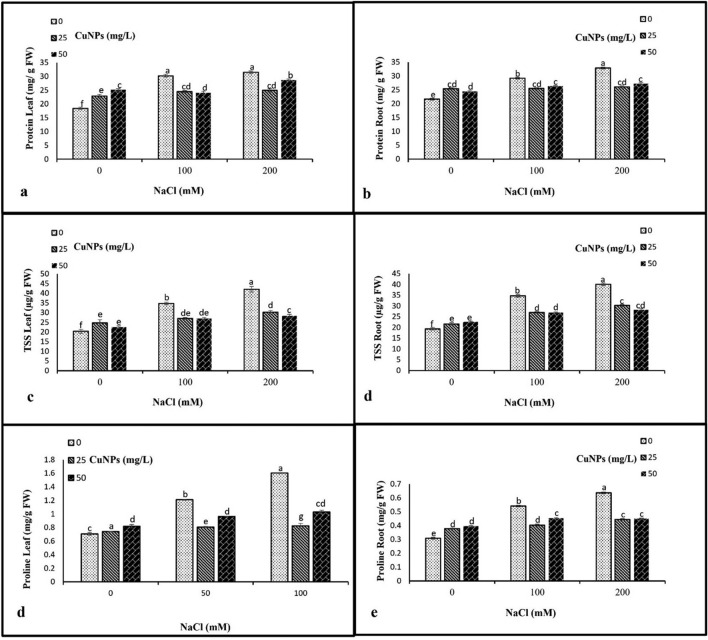
Soluble proteins, soluble sugars, and proline contents
Exposure of barley plants to salinity stress led to a significant accumulation of osmolytes including soluble proteins, soluble sugar, and proline contents. The plants exposed to 200 mM salt without CuNPs application recorded the highest levels in soluble proteins, soluble sugar, and proline for both leaves and roots. The application of CuNPs in its all concentrations (25 and 50 mg/L) successfully overcame the harmful effect of salt stress and caused a remarkable reduction in all osmoregulatory substances compared to only salt-stressed plants (Fig. 5).
Figure 5.
The impact of CuNPs and salt-induced stress, whether applied individually or in conjunction with each other on the osmolytes contents, (a) soluble proteins leaf, (b) soluble proteins root, (c) soluble sugars leaf, (d) soluble sugars root, (e) proline leaf, and (f) proline root in barley (H. vulgare). All the data are means of three replicates ± standard deviation. Different letters indicate significant differences between treatments according to Duncan’s multiple range test (p ≤ 0.05).
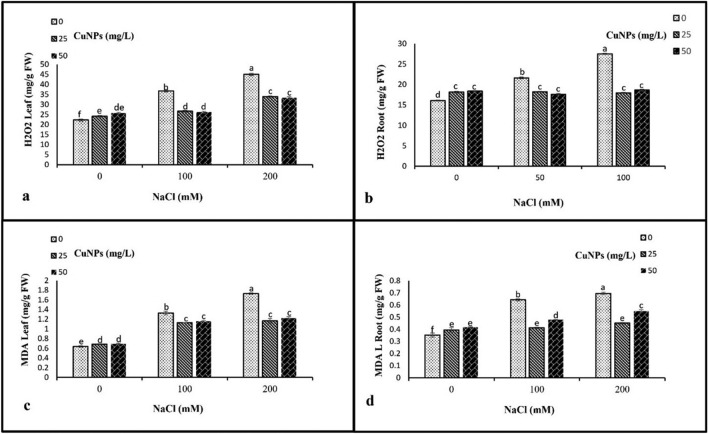
Hydrogen peroxide (H2O2) and lipid peroxidation analyses (MDA)
Our results indicate that the application of only salinity treatments (100 and 200 mM) significantly increased the levels of H2O2 in leaves, as well as in roots compared to the control barley. The introduction of CuNPs to salt-stressed barley plants led to a notable decrease in MDA and H2O2 levels in both leaves and roots. The most significant reduction was observed in plants treated with 25 mg/L of CuNPs supplementation under 100 and 200 mM NaCl stress, as compared to their respective control treatments (Fig. 6).
Figure 6.
The impact of CuNPs and salt-induced stress, whether applied individually or in conjunction with each other on the hydrogen peroxide (H2O2) and lipid peroxidation analyses (MDA), (a) H2O2 leaf, (b) H2O2 root, (c) MDA leaf, (d) MDA root in barley (H. vulgare). All the data are means of three replicates ± standard deviation. Different letters indicate significant differences between treatments according to Duncan’s multiple range test (p ≤ 0.05).
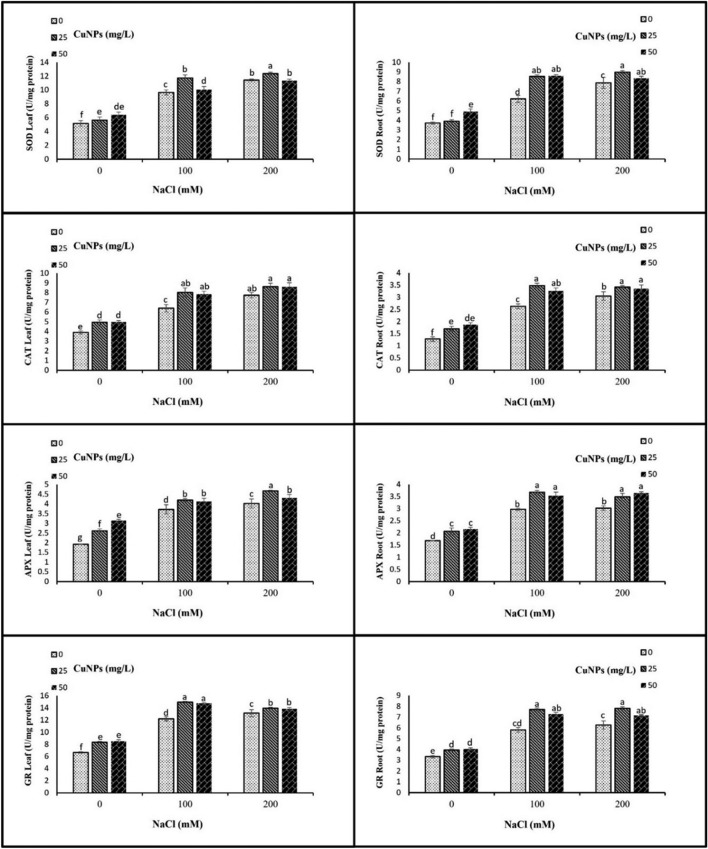
Antioxidant enzymes activities
The present results demonstrate that NaCl treatments at various concentrations led to a significant increase in the activities of enzymatic antioxidants. A treatment of 100 and 200 mM NaCl notably enhanced the activities of SOD, CAT, APX, and GR compared to the control group. The application of CuNPs salinized plants caused more increment in the antioxidant enzyme activities compared with their respective control (only salt-treated plants) in both shoots and roots. These changes were more pronounced with 25 mg/L CuNPs application than 50 mg/L CuNPs application (Fig. 7).
Figure 7.
The impact of CuNPs and salt-induced stress, whether applied individually or in conjunction with each other on the antioxidant enzymes activity, (a) SOD leaf, (b) SOD root, (c) CAT leaf, (d) CAT root, (e) APX leaf, (f) APX root, (g) GR leaf, and (h) GR root in barley (H. vulgare). All the data are means of three replicates ± standard deviation. Different letters indicate significant differences between treatments according to Duncan’s multiple range test (p ≤ 0.05).
Nonenzymatic antioxidants
The nonenzymatic antioxidants, including total phenol (TPC) and total flavonoids (TFC) contents, exhibited a significant increment in plants treated with 100 and 200 mM NaCl in comparison to control plants. The most substantial increase was observed in plants exposed to 200 mM NaCl. Interestingly, the application of 25 mg/L CuNPs in the two salinity levels (100 and 200 mM) significantly reduced the TPC and TFC barley-leaves and roots compared to their respective controls (Fig. 8).
Figure 8.
The impact of CuNPs and salt-induced stress, whether applied individually or in conjunction with each other on the non-enzymatic antioxidant compounds, (a) TPC leaf, (b) TPC root, (c) TFC leaf, and (d) TFC root in barley (H. vulgare). All the data are means of three replicates ± standard deviation. Different letters indicate significant differences between treatments according to Duncan’s multiple range test (p ≤ 0.05).
Correlation study
PCA and Pearson's correlation analyses were conducted to comprehend the connections between different CuNPs treatments and the diverse morpho-physiological parameters in barley under salinity. Figure 9 presents a PCA biplot which explained 86.59% (73.34% and 13.25%) of the total variance. Furthermore, Pearson’s correlation matrix reveals a significant correlation among the different morpho-physiological characteristics as shown in (Fig. 10). In summary, the growth parameters exhibited positive correlations with fresh weight, dry weight, plant height, chlorophyll, and gas exchange parameters content. Conversely, they showed negative correlations with electrolyte leakage, lipid peroxidation, H2O2 and proline contents.
Figure 9.

Principal component analysis (PCA) of morpho-physiological characteristics of barley plants exposed to different concentrations salt stress and application of CuNPs.
Figure 10.
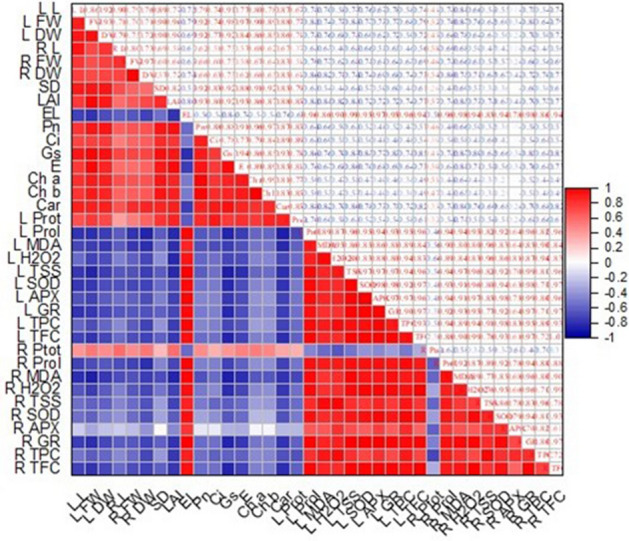
The correlation between different morpho-physiological characteristics of barley plants exposed to different salt stress and application of CuNPs.
Discussion
Characterization of biosynthesized nanoparticles involves common techniques such as UV, XRD, FTIR spectroscopy, and TEM microscopy. In the current study, the formation of the CuNPs was visually confirmed by the observable change in the color of the reaction mixture, which turned to dark green after the application of heat. The UV–vis spectra serve as an indirect method for verifying and analyzing the bioreduction of CuNPs within the CuSO4 solution. The spectrum observed at 320 nm confirmed the presence of surface plasmon resonance (SPR) of the CuNPs and suggests that the particles were dispersed without any signs of aggregation. The peak observed at 3430 cm−1 in the FTIR spectrum signifies the stretching of O–H bonds in alcohols and phenols, peak observed at 2098 cm−1 corresponding to C=H indicates a robust stretching associated with the alkyl methylene group. The peak at 1650 cm−1 signifies the carbonyl groups (C=O), and band at 760 cm−1 most likely due to the peroxide formation. The presence of these peaks indicated the existence of flavonoid and phenolic acids in the S. Argel leaves extract; thus, the reduction of metal ions and the subsequent formation of nanoparticles may be associated with the presence of flavonoids and phenolic functional groups in the extract. The TEM images demonstrated that the synthesized CuNPs were spherical in morphology, with the average size ranging from 9 to 18 nm.
Nanobiotechnology has significantly broadened the horizons of agricultural research, particularly in enhancing the growth and yield of food crops when faced with challenging environmental conditions. This field has opened innovative avenues for utilizing nanoparticles (NPs) to increase plant resilience and protect them against both biotic and abiotic stresses50. Among various abiotic stresses, salt-induced stress poses a severe threat to plants, where, several scientists stated that salt stress dramatically hamper germination, decreases plant growth, photosynthesis, stomatal conductance, root length, plant biomass, and generates osmotic stress by disturbing the ion balance and osmoregulation in plants51–53.
The application of the biologically synthesized CuNPs has played a significant role in alleviating salt stress on barley growth and positively improved its profile. Under salt stress conditions (100 and 200 mM NaCl), plant growth was adversely affected as indicated by a reduction in various developmental attributes, including stem diameter, leaf area index, fresh weight, dry weight, and the length of both shoots and roots compared to unstressed plants. Naturally, the hindered growth of plants in saline conditions can be ascribed to the excessive accumulation of sodium (Na+) and chloride (Cl−) ions within various cell compartments in both the roots and above-ground parts of the plant8,54. The accumulation of these ions to mischievous levels disrupts genetic expression, hinders protein synthesis, impairs enzymatic processes, affects energy metabolism, and inhibits cell division; this also results in damage to the structural integrity of the cells, ultimately leading to the possibility of cell death1,55,56. However, when copper nanoparticles (CuNPs) were applied to the salt-stressed plants, these detrimental effects were reversed, and a notable improvement in growth characteristics was observed. The enhanced growth of barley plants treated with CuNPs can be attributed to the elevation of cellular antioxidant levels, which in turn, helped alleviate oxidative stress by effectively scavenging ROS57. These findings align with previous report by Hernández-Hernández et al.58, they found that CuNPs positively affected the growth and physiology of tomato plants under saline stress by controlling oxidative stress.
Regulating the parameters of leaf gas exchange is a crucial factor in enhancing crops resilience to different biotic and abiotic stressors. Our findings showed that the exposure of barley plants to salt stress (100 and 200 mM) caused a drastic reduction in barley leaf gas exchange. The salt-induced decreases in leaf gas exchange parameters, including Ci, Tr, gs, and Pn, have been observed in barley plants59 and oil palm60. Interestingly, CuNPs have been shown to increase the values of barley leaf gas parameters under salt stress in comparison to salinized plants. A similar result was reported by Javeed et al.61 who found that the gas exchange parameters were increased after application of zinc oxide nanoparticle in Lagenaria siceraria L.
The decrease in photosynthetic pigments in the leaves of barley plants under saline conditions aligns perfectly with the discovery made by Narimani et al.62. The reduction in pigment levels was most likely attributed to increased damage of chloroplast structure, instability in pigment-protein complexes, and heightened chlorophyllase activity63. Our findings designated a similar decrease in photosynthetic pigments content in barley plants treated with salt stress alone. Interestingly, the application of CuNPs at different concentrations resulted in a considerable increase in photosynthetic pigments in both salt-treated and non-treated barley plants. The highest point of this increase was observed in plants that had been exposed to 25 mg/L CuNPs. Copper nanoparticles enhance nutrient uptake in plants, which can lead to better pigment synthesis and maintenance64. This can result in an augmentation of chlorophylls or other supplementary pigments in the plant leaves.
Plants have an inherent ability to boost the synthesis of osmolytes such as soluble protein, soluble sugars and proline within their cytosol and various organelles, which helps them counteract the adverse impact of salt toxicity65. In this study, the levels of soluble sugars, soluble proteins, and proline were significantly elevated in barley plants subjected to NaCl stress. Several studies have revealed that salt-induced stress resulted in increased levels of osmoregulatory substances including soluble sugars, soluble proteins, and proline in rice, faba bean and artichoke plants66–68. The application of CuNPs resulted in a reduction in the accumulation of total soluble proteins, total soluble sugars and proline in the stressful barley plants compared to their respective control. Our findings align with the results presented in the study by Mohamed et al.69, in which they observed that nanoparticles decreased soluble sugar, and proline levels in plants under salinity stress compared to their respective salt-treated plants alone.
The excessive salt accumulation in the cytoplasm leads to hyperosmotic stress and ionic imbalance, hence, triggers the production of ROS like hydrogen H2O2 as well as overproduction of MDA. In this study, a notable rise in the levels of MDA and H2O2 was observed in barley plants subjected to salt stress alone, aligning with previous findings in soybean70 and strawberry71, where MDA and H2O2 concentrations were found to increase in response to salinity. This accumulation most likely attributed to the shock and photo-oxidative stress induced to salinity stress as suggested by70,72. Furthermore, salinity causes a drastic reduction in the water status of the stressed plants due to higher accumulation of the sodium ions; this is probably another reason responsible for the increase in H2O2 and MDA concentrations.73. The application of CuNPs to salinized plants resulted in a significant reduction in oxidative stress and lipid peroxidation as indicated by the observed decrease in the levels of H2O2 and MDA .The positive impacts of CuNPs on barley plants in mitigating oxidative damage caused by salinity appear to be linked to the upregulated activity of enzymatic antioxidants by swiftly disposal of H2O2, thereby promoting and sustaining plant growth74.
Numerous studies have indicated that exposure to salt stress can trigger the production of reactive oxygen species, leading to heightened activity of antioxidant enzymes as a protective mechanism74–76. This is consistent with our results, where the levels of antioxidant enzymes including SOD, CAT, APX, and GR activity significantly increased in barley plants as response to salt stress. Interestingly, our findings demonstrated that the use of CuNPs led to more enhancement in the activity of antioxidant enzymes in comparison to only salt-stressed plants. The increment in the activities of antioxidant enzymes in the salt-stressed plants after application of NPs suggests that this nanoparticle effectively mitigated the effects of salinity stress on barley plants. In line with our results, the application of CuNPs promoted the activities of enzymatic antioxidants in maize and tomato under salinity conditions57,77.
Phenolic and flavonoid compounds are vital for safeguarding plants against various biotic and abiotic stressors. They also function as co-factors for enzymes, influencing the growth and development of plants, starting from their early stages and continuing through senescence78,79. Our results in response to salt stress indicate significant enhancements in TPC and TFC compared to control group. The elevations in phenolic contents might be due to higher expression of genes in phenolic biosynthetic pathway80, while, increment in flavonoids considered as a part of the adaptive reaction to salt stress81. The CuNPs successfully reduced phenols and flavonoid content on barley plants treated with NaCl. This findings in alignment with Hanif et al.82 report, who found that NPs caused reduction in TPC and TFC on Coriandrum sativum plants under salinity. The reduction in total phenolic and total flavonoid contents indicates that they are needed in lower quantities because of reduced ROS production following the application of copper nanoparticles9.
The findings of this research suggest that the negative impacts of salt stress can be reduced by using CuNPs. Thus, these CuNPs, produced through biological synthesis, can be utilized in salt-affected agricultural settings as a cost-effective and environmentally friendly solution to boost crop yield. However, Further research in this area is necessary, with an emphasis on exploring the potential of next-generation technologies, including nanomodified stimulants and nano-based smart sensors, to address agricultural challenges such as salinity stress.
Conclusion
This study underscores the significant connection between sustainable agricultural practices and nanoscience. It particularly highlights the positive impact of biologically synthesized copper nanoparticles (CuNPs) from S. argel leaf extract in mitigating the detrimental effects of salinity stress on various plant responses. We can infer that the application of biologically synthesized CuNPs induced a significant change into barley plants under salinity conditions, where mitigated the adverse effects of NaCl stress by promoting plant growth, improving gas exchange parameters, boosting photosynthetic pigments, regulating osmoregulation, and reducing the accumulation of MDA and H2O2. Additionally, protection against NaCl-induced stress was achieved by minimizing total phenols, flavonoids, and enhancing the activities of antioxidant enzymes. According to the results, this study concluded that the application of biosynthesized CuNPs to barley plants could serve as a tactic to enhance their salt tolerance, thereby improving plant growth, physiological parameters, and overall yield. However, further research efforts are needed to comprehensively understand how copper nanoparticles alleviate the detrimental impacts of salt in plants at both molecular and genetic levels.
Acknowledgements
The authors extend their appreciation to the Researcher Supporting Project No (RSP-2024/73) at King Saud University, Riyadh, Saudi Arabia).
Author contributions
H.O.S. proposed and designed the experiments. H.O.S, A.M.S., and M.T. performed nanoparticles synthesis and all characterization experiments. H.O.S, AA and S.K. analyzed the morphological and biochemical parameters. H.O.S. performed the statistical analysis via SPSS and prepared all the figures. H.O.S. and F.A. wrote the manuscript and performed the manuscript revision. All authors read and approved the final manuscript.
Data availability
The datasets generated and analyzed in the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh A. Soil salinity: A global threat to sustainable development. Soil Use Manag. 2022;38:39–67. doi: 10.1111/sum.12772. [DOI] [Google Scholar]
- 2.Etesami, H. & Noori, F. Soil salinity as a challenge for sustainable agriculture and bacterial-mediated alleviation of salinity stress in crop plants. In Saline Soil-based Agriculture by Halotolerant Microorganisms Vol. 13, 1–22 (2019).
- 3.Song P, et al. Effects of Bacillus subtilis HS5B5 on maize seed germination and seedling growth under NaCl stress conditions. Agronomy. 2023;13:1874. doi: 10.3390/agronomy13071874. [DOI] [Google Scholar]
- 4.Bano, A. et al. Mechanistic role of reactive oxygen species and its regulation via the antioxidant system under environmental stress. Plant Stress Physiol.—Perspect. Agric.11, 1–18 (2021).
- 5.Abideen Z, et al. Phragmites karka plants adopt different strategies to regulate photosynthesis and ion flux in saline and water deficit conditions. Plant Biosyst. 2021;155:524–534. doi: 10.1080/11263504.2020.1762783. [DOI] [Google Scholar]
- 6.Negacz K, Vellinga P, Barrett-Lennard E, Choukr-Allah R, Elzenga T. Future of Sustainable Agriculture in Saline Environments. Taylor & Francis; 2021. [Google Scholar]
- 7.Talat N. Climate Change and Soil Interactions. Elsevier; 2020. pp. 305–329. [Google Scholar]
- 8.Singh A. Soil salinization management for sustainable development: A review. J. Environ. Manag. 2021;277:111383. doi: 10.1016/j.jenvman.2020.111383. [DOI] [PubMed] [Google Scholar]
- 9.Farooq T, Nisa ZU, Hameed A, Ahmed T, Hameed A. Priming with copper-chitosan nanoparticles elicit tolerance against PEG-induced hyperosmotic stress and salinity in wheat. BMC Chem. 2022;16:23. doi: 10.1186/s13065-022-00813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajihashemi S, Skalicky M, Brestic M, Pavla V. Cross-talk between nitric oxide, hydrogen peroxide and calcium in salt-stressed Chenopodium quinoa Willd. At seed germination stage. Plant Physiol. Biochem. 2020;154:657–664. doi: 10.1016/j.plaphy.2020.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Salam A, et al. Nano-priming against abiotic stress: A way forward towards sustainable agriculture. Sustainability. 2022;14:14880. doi: 10.3390/su142214880. [DOI] [Google Scholar]
- 12.Duhan JS, et al. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017;15:11–23. doi: 10.1016/j.btre.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wahid I, et al. Silver nanoparticle regulates salt tolerance in wheat through changes in ABA concentration, ion homeostasis, and defense systems. Biomolecules. 2020;10:1506. doi: 10.3390/biom10111506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinha A, et al. The translational paradigm of nanobiomaterials: Biological chemistry to modern applications. Mater. Today Bio. 2022;17:100463. doi: 10.1016/j.mtbio.2022.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahid M, Naeem-Ullah U, Khan W, Saeed D, Razzaq K. Application of nanotechnology for insect pests management: A review. J. Innov. Sci. 2021;7:28–39. [Google Scholar]
- 16.Sharma, S. et al. Nanotechnology: An efficient tool in plant nutrition management. Ecosyst. Serv. Types Manag. Benefits (2022).
- 17.Shaikhaldein HO, et al. Assessment of the impacts of green synthesized silver nanoparticles on Maerua oblongifolia shoots under in vitro salt stress. Materials. 2022;15:4784. doi: 10.3390/ma15144784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Saadony MT, et al. Role of nanoparticles in enhancing crop tolerance to abiotic stress: A comprehensive review. Front. Plant Sci. 2022;13:946717. doi: 10.3389/fpls.2022.946717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mali SC, Raj S, Trivedi R. Nanotechnology a novel approach to enhance crop productivity. Biochem. Biophys. Rep. 2020;24:100821. doi: 10.1016/j.bbrep.2020.100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seleiman MF, Ahmad A, Alhammad BA, Tola E. Exogenous application of zinc oxide nanoparticles improved antioxidants, photosynthetic, and yield traits in salt-stressed maize. Agronomy. 2023;13:2645. doi: 10.3390/agronomy13102645. [DOI] [Google Scholar]
- 21.Singh A, Sengar RS, Rajput VD, Minkina T, Singh RK. Zinc oxide nanoparticles improve salt tolerance in rice seedlings by improving physiological and biochemical indices. Agriculture. 2022;12:1014. doi: 10.3390/agriculture12071014. [DOI] [Google Scholar]
- 22.Abeed AH, et al. Calcium nanoparticles mitigate severe salt stress in Solanum lycopersicon by instigating the antioxidant defense system and renovating the protein profile. S. Afr. J. Bot. 2023;161:36–52. doi: 10.1016/j.sajb.2023.08.005. [DOI] [Google Scholar]
- 23.Mali SC, Dhaka A, Sharma S, Trivedi R. Review on biogenic synthesis of copper nanoparticles and its potential applications. Inorganic Chem. Commun. 2023;149:110448. doi: 10.1016/j.inoche.2023.110448. [DOI] [Google Scholar]
- 24.Kumar B, Smita K, Debut A, Cumbal L. Andean Sacha Inchi (Plukenetia volubilis L.) leaf-mediated synthesis of Cu2O nanoparticles: a low-cost approach. Bioengineering. 2020;7:54. doi: 10.3390/bioengineering7020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gholami M, Azarbani F, Hadi F, Murthy HA. Eco-friendly synthesis of copper nanoparticles using Mentha pulegium leaf extract: Characterisation, antibacterial and cytotoxic activities. Materi. Technol. 2022;37:1523–1531. doi: 10.1080/10667857.2021.1959214. [DOI] [Google Scholar]
- 26.Verma, S. K., Jha, E., Panda, P. K., Thirumurugan, A. & Suar, M. Biological effects of green-synthesized metal nanoparticles: A mechanistic view of antibacterial activity and cytotoxicity. Adv. Nanostruct. Mater. Environ. Remediat.25, 145–171 (2019).
- 27.Kumari P, et al. Molecular insight to in vitro biocompatibility of phytofabricated copper oxide nanoparticles with human embryonic kidney cells. Nanomedicine. 2018;13:2415–2433. doi: 10.2217/nnm-2018-0175. [DOI] [PubMed] [Google Scholar]
- 28.Kumari P, et al. Mechanistic insight to ROS and apoptosis regulated cytotoxicity inferred by green synthesized CuO nanoparticles from Calotropis gigantea to embryonic zebrafish. Sci. Rep. 2017;7:16284. doi: 10.1038/s41598-017-16581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh A, Rajput VD, Sharma R, Ghazaryan K, Minkina T. Salinity stress and nanoparticles: Insights into antioxidative enzymatic resistance, signaling, and defense mechanisms. Environ. Res. 2023;235:116585. doi: 10.1016/j.envres.2023.116585. [DOI] [PubMed] [Google Scholar]
- 30.Yruela I. Copper in plants. Brazil. J. Plant Physiol. 2005;17:145–156. doi: 10.1590/S1677-04202005000100012. [DOI] [Google Scholar]
- 31.Ahmed F, Javed B, Razzaq A, Mashwani ZUR. Applications of copper and silver nanoparticles on wheat plants to induce drought tolerance and increase yield. IET Nanobiotechnol. 2021;15:68–78. doi: 10.1049/nbt2.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shende S, Rathod D, Gade A, Rai M. Biogenic copper nanoparticles promote the growth of pigeon pea (Cajanus cajan L.) IET Nanobiotechnol. 2017;11:773–781. doi: 10.1049/iet-nbt.2016.0179. [DOI] [Google Scholar]
- 33.Iliger KS, et al. Copper nanoparticles: Green synthesis and managing fruit rot disease of chilli caused by Colletotrichum capsici. Saudi J. Biol. Sci. 2021;28:1477–1486. doi: 10.1016/j.sjbs.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammami Z, et al. Modeling the effects of irrigation water salinity on growth, yield and water productivity of barley in three contrasted environments. Agronomy. 2020;10:1459. doi: 10.3390/agronomy10101459. [DOI] [Google Scholar]
- 35.Walia H, et al. Expression analysis of barley (Hordeum vulgare L.) during salinity stress. Funct. Integr. Genomics. 2006;6:143–156. doi: 10.1007/s10142-005-0013-0. [DOI] [PubMed] [Google Scholar]
- 36.El-Sonbaty SM, Mansour SZ, Mahdy E, El-Mezayen HA, Salem F. Gas chromatography/mass spectrography (GC/MS) analysis and biological properties of probiotic fermented Solenostemma argel. Eur. J. Med. Plants. 2016;16:27662. doi: 10.9734/EJMP/2016/27662. [DOI] [Google Scholar]
- 37.Elsanhoty RM, et al. Pharmacological activities and characterization of phenolic and flavonoid compounds in Solenostemma argel extract. Molecules. 2022;27:8118. doi: 10.3390/molecules27238118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bajji M, Kinet J-M, Lutts S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant growth Regul. 2002;36:61–70. doi: 10.1023/A:1014732714549. [DOI] [Google Scholar]
- 40.Jogeswar G, et al. Antioxidative response in different sorghum species under short-term salinity stress. Acta Physiol. Plantarum. 2006;28:465–475. doi: 10.1007/BF02706630. [DOI] [Google Scholar]
- 41.Jiang M, Zhang J. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001;42:1265–1273. doi: 10.1093/pcp/pce162. [DOI] [PubMed] [Google Scholar]
- 42.Bates L, Waldren RA, Teare I. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 43.Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. doi: 10.1016/S0168-9452(99)00197-1. [DOI] [Google Scholar]
- 44.Bradford N. A rapid and sensitive method for the quantitation microgram quantities of a protein isolated from red cell membranes. Anal. Biochem. 1976;72:e254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 45.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 46.Claiborne A. Handbook of Methods for Oxygen Radical Research. CRC Press; 1985. pp. 283–284. [Google Scholar]
- 47.Schaedle M, Bassham JA. Chloroplast glutathione reductase. Plant Physiol. 1977;59:1011–1012. doi: 10.1104/pp.59.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- 49.Velioglu Y, Mazza G, Gao L, Oomah B. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998;46:4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- 50.Kandhol N, Jain M, Tripathi DK. Nanoparticles as potential hallmarks of drought stress tolerance in plants. Physiol. Plantarum. 2022;174:e13665. doi: 10.1111/ppl.13665. [DOI] [PubMed] [Google Scholar]
- 51.Sharmin S, Lipka U, Polle A, Eckert C. The influence of transpiration on foliar accumulation of salt and nutrients under salinity in poplar (Populus× canescens) PLoS ONE. 2021;16:e0253228. doi: 10.1371/journal.pone.0253228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh P, et al. Nanoparticles enhances the salinity toxicity tolerance in Linum usitatissimum L. by modulating the antioxidative enzymes, photosynthetic efficiency, redox status and cellular damage. Ecotoxicol. Environ. Saf. 2021;213:112020. doi: 10.1016/j.ecoenv.2021.112020. [DOI] [PubMed] [Google Scholar]
- 53.Zhu G, et al. Different types of fertilizers enhanced salt resistance of oat and associated physiological mechanisms in saline soils. Agronomy. 2022;12:317. doi: 10.3390/agronomy12020317. [DOI] [Google Scholar]
- 54.Chaudhry S, Sidhu GPS. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2022;41:1–31. doi: 10.1007/s00299-021-02759-5. [DOI] [PubMed] [Google Scholar]
- 55.Kesawat MS, et al. Regulation of reactive oxygen species during salt stress in plants and their crosstalk with other signaling molecules—Current perspectives and future directions. Plants. 2023;12:864. doi: 10.3390/plants12040864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Z, Li J-L, Liu L-N, Xie Q, Sui N. Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front. Plant Sci. 2020;10:1722. doi: 10.3389/fpls.2019.01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noman M, et al. Biogenic copper nanoparticles produced by using the Klebsiella pneumoniae strain NST2 curtailed salt stress effects in maize by modulating the cellular oxidative repair mechanisms. Ecotoxicol. Environ. Saf. 2021;217:112264. doi: 10.1016/j.ecoenv.2021.112264. [DOI] [PubMed] [Google Scholar]
- 58.Hernández-Hernández H, et al. Effects of chitosan–PVA and Cu nanoparticles on the growth and antioxidant capacity of tomato under saline stress. Molecules. 2018;23:178. doi: 10.3390/molecules23010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ali B, et al. Mitigation of salinity stress in barley genotypes with variable salt tolerance by application of zinc oxide nanoparticles. Front. Plant Sci. 2022;13:973782. doi: 10.3389/fpls.2022.973782. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Vieira LR, et al. Morphophysiological responses of young oil palm plants to salinity stress. Pesqui. Agropecu. Bras. 2023;55:1835. [Google Scholar]
- 61.Javeed A, Ahmed S, Sardar R. Alleviation of salinity stress in zinc oxide nanoparticle-treated Lagenaria siceraria L. by modulation of physiochemical attributes, enzymatic and non-enzymatic antioxidative system. Funct. Plant Biol. 2023;50:941–954. doi: 10.1071/FP23069. [DOI] [PubMed] [Google Scholar]
- 62.Narimani T, Toorchi M, Tarinejad A, Mohammadi S, Mohammadi H. Physiological and biochemical evaluation of barley (Hordeum vulgare L.) under salinity stress. J. Agric. Sci. Technol. 2020;22:1009–1021. [Google Scholar]
- 63.Kapoor RT, Hasanuzzaman M. Exogenous kinetin and putrescine synergistically mitigate salt stress in Luffa acutangula by modulating physiology and antioxidant defense. Physiol. Mol. Biol. Plants. 2020;26:2125–2137. doi: 10.1007/s12298-020-00894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao Y, et al. Copper accumulation and physiological markers of soybean (Glycine max) grown in agricultural soil amended with copper nanoparticles. Ecotoxicol. Environ. Saf. 2022;229:113088. doi: 10.1016/j.ecoenv.2021.113088. [DOI] [PubMed] [Google Scholar]
- 65.Singh A, et al. How to cope with the challenges of environmental stresses in the era of global climate change: An update on ROS stave off in plants. Int. J. Mol. Sci. 2022;23:1995. doi: 10.3390/ijms23041995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang H, Takano T, Liu S. Screening and evaluation of saline–alkaline tolerant germplasm of rice (Oryza sativa L.) in soda saline–alkali soil. Agronomy. 2018;8:205. doi: 10.3390/agronomy8100205. [DOI] [Google Scholar]
- 67.Abdel Latef AAH, Tahjib-Ul-Arif M, Rhaman MS. Exogenous auxin-mediated salt stress alleviation in faba bean (Vicia faba L.) Agronomy. 2021;11:547. doi: 10.3390/agronomy11030547. [DOI] [Google Scholar]
- 68.Dawood MF, Sohag AAM, Tahjib-Ul-Arif M, Latef AAHA. Hydrogen sulfide priming can enhance the tolerance of artichoke seedlings to individual and combined saline-alkaline and aniline stresses. Plant Physiol. Biochem. 2021;159:347–362. doi: 10.1016/j.plaphy.2020.12.034. [DOI] [PubMed] [Google Scholar]
- 69.Mohamed AKS, et al. Interactive effect of salinity and silver nanoparticles on photosynthetic and biochemical parameters of wheat. Arch. Agron. Soil Sci. 2017;63:1736–1747. doi: 10.1080/03650340.2017.1300256. [DOI] [Google Scholar]
- 70.Abdalla H, Adarosy MH, Hegazy HS, Abdelhameed RE. Potential of green synthesized titanium dioxide nanoparticles for enhancing seedling emergence, vigor and tolerance indices and DPPH free radical scavenging in two varieties of soybean under salinity stress. BMC Plant Biol. 2022;22:1–18. doi: 10.1186/s12870-022-03945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lamnai K, et al. Impact of exogenous application of salicylic acid on growth, water status and antioxidant enzyme activity of strawberry plants (Fragaria vesca L.) under salt stress conditions. Gesunde Pflanzen. 2021;73:465–478. doi: 10.1007/s10343-021-00567-1. [DOI] [Google Scholar]
- 72.Jbir-Koubaa R, et al. Investigation of the response to salinity and to oxidative stress of interspecific potato somatic hybrids grown in a greenhouse. Plant Cell Tissue Organ Cult. 2015;120:933–947. doi: 10.1007/s11240-014-0648-4. [DOI] [Google Scholar]
- 73.Rehman S, et al. Effect of salinity on cadmium tolerance, ionic homeostasis and oxidative stress responses in conocarpus exposed to cadmium stress: Implications for phytoremediation. Ecotoxicol. Environ. Saf. 2019;171:146–153. doi: 10.1016/j.ecoenv.2018.12.077. [DOI] [PubMed] [Google Scholar]
- 74.Singh S, Husen A. Role of nanomaterials in the mitigation of abiotic stress in plants. Nanomaterials Plant Potential. 2019;3:441–471. doi: 10.1007/978-3-030-05569-1_18. [DOI] [Google Scholar]
- 75.Singh D. Juggling with reactive oxygen species and antioxidant defense system—A coping mechanism under salt stress. Plant Stress. 2022;5:100093. doi: 10.1016/j.stress.2022.100093. [DOI] [Google Scholar]
- 76.Azizi S, Seyed Hajizadeh H, Aghaee A, Kaya O. In vitro assessment of physiological traits and ROS detoxification pathways involved in tolerance of Damask rose genotypes under salt stress. Sci. Rep. 2023;13:17795. doi: 10.1038/s41598-023-45041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pérez-Labrada F, et al. Responses of tomato plants under saline stress to foliar application of copper nanoparticles. Plants. 2019;8:151. doi: 10.3390/plants8060151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bistgani ZE, et al. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 2019;135:311–320. doi: 10.1016/j.indcrop.2019.04.055. [DOI] [Google Scholar]
- 79.Cheynier V, Comte G, Davies KM, Lattanzio V, Martens S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013;72:1–20. doi: 10.1016/j.plaphy.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 80.Akhavan Hezaveh T, Pourakbar L, Rahmani F, Alipour H. Effects of ZnO NPs on phenolic compounds of rapeseed seeds under salinity stress. J. Plant Process Funct. 2020;8:11–18. [Google Scholar]
- 81.Sarker U, Islam MT, Oba S. Salinity stress accelerates nutrients, dietary fiber, minerals, phytochemicals and antioxidant activity in Amaranthus tricolor leaves. PLoS ONE. 2018;13:e0206388. doi: 10.1371/journal.pone.0206388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hanif S, Sajjad A, Javed R, Mannan A, Zia M. Proline doped ZnO nanocomposite alleviates NaCl induced adverse effects on morpho-biochemical response in Coriandrum sativum. Plant Stress. 2023;9:100173. doi: 10.1016/j.stress.2023.100173. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed in the current study are available from the corresponding author upon reasonable request.