Abstract
Systemic inflammatory response (SIR) is a crucial determinant of disease progression and survival in patients with colorectal cancer. This study investigated the prognostic relevance of changes in the platelet count on survival and the predictive value of changes in the platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) on the pathological tumor response to preoperative chemoradiotherapy (CRT) in patients with microsatellite instability-high (MSI-H) rectal cancer. From 2011 to 2022, data of 46 consecutive patients with MSI-H rectal cancer who were treated with preoperative CRT followed by curative surgery at Kyungpook National University Chilgok Hospital (Daegu, South Korea) were retrospectively analyzed. A 235 cut-off value was used to define whether PLR was high or low. Any change in the PLR or NLR was calculated on the basis of subtracting the pre-CRT PLR or NLR from the post-CRT values. Both pre-CRT and post-CRT values of the NLR and PLR were not significantly associated with clinical outcomes. Simple logistic regression analysis showed that a change in the PLR following CRT was not significantly associated with survival outcomes; however, patients who maintained a high change in the PLR following CRT showed significantly better pathologic T-stage. No statistically significant association was noted between changes in the platelet count and clinical outcomes of patients. The results suggested that changes in the PLR following CRT are associated with pathologic T-stage of the group. However, the SIR markers showed no prognostic values on the survival outcomes of the patients with MSI-H/mismatch repair-deficient (dMMR) locally advanced rectal cancer (LARC).
Keywords: Rectal Neoplasms, Microsatellite Instability, Chemoradiotherapy, Prognosis
INTRODUCTION
Preoperative chemoradiotherapy (CRT) followed by total mesorectal excision (TME) is the standard treatment for locally advanced rectal cancer (LARC).1 However, a major limitation of this strategy is the poor impact on distant control, with metastasis rates remaining between 25% and 35%.2 In this situation, single-agent programmed death 1 blockade was remarkably effective in microsatellite instability-high (MSI-H)/mismatch repair–deficient (dMMR) in LARC, providing a clinical complete response in all 12 patients.3
MSI is a molecular marker of a defective function of the DNA MMR system that results in the accumulation of insertion or deletion mutations within microsatellite DNA regions.4 dMMR or MSI-H tumors are well known to exhibit a high tumor mutation burden, neoantigen load and immune infiltration, thereby making them well to immune checkpoint inhibitors (ICIs) in colorectal cancer (CRC).5,6 Among all patients diagnosed with rectal cancer, dMMR or MSI-H tumors account for approximately 3% of the population. Therefore, the identification of immune response related biomarkers is essential for facilitating patient selection and increasing the clinical benefit from a novel treatment including ICIs in rectal cancer.7,8
Laboratory parameters reflecting systemic inflammation are relatively economical to evaluate, easily measurable, repeatable, and ready to use in daily clinical practice.9 There is increasing evidence that the neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) can be effective prognostic indicators for various malignant tumors.10,11 Particularly, a high NLR has been reported as an indicator of poor clinical outcomes in patients with LARC.12 Additionally, the PLR has been correlated with the progression of CRC, and changes in the platelet count following chemotherapy were recently reported as a potential biomarker evaluating response.13 However, the prognostic impact of the NLR and PLR in patients with dMMR or MSI-H rectal cancer has not yet been investigated.
Accordingly, this study aimed at investigating the association between the NLR and PLR and changes in the platelet count following CRT with clinical outcomes in patients with dMMR or MSI-H LARC.
MATERIALS AND METHODS
1. Patient eligibility
This retrospective study included 46 patients with pathologically confirmed, locally advanced dMMR or MSI-H rectal adenocarcinoma. The participants completed preoperative CRT and underwent radical resection between January 2011 and January 2023 at Kyungpook National University Chilgok Hospital (KNUCH) (Daegu, Korea).
Total colonoscopy, computed tomography (CT), pelvic magnetic resonance imaging, and whole-body positron emission tomography/CT according to the tumor, node, and metastasis (TNM) staging system, eighth edition, 2018 (AJCC, 2018) were performed to assess clinical staging.14 Measurements of neutrophil, lymphocyte, and platelet counts and serum carcinoembryonic antigen and carbohydrate antigen 19-9 concentrations were performed before and after CRT. The CRT consisted of 45 Gy delivered in 25 daily fractions over 5 weeks either with concurrent 5-fluorouracil (400 mg/m2) and leucovorin (20 mg/m2) on days 1-4 and 29-32, or with concurrent oral administration of capecitabine (825 mg/m2 twice daily) for 25 days. The TME was performed 6-8 weeks following CRT completion. This study was approved by the Institutional Review Board of KNUCH.
2. MSI analysis
Tumor tissue samples were obtained from each patient during surgery. Laboratory analysis was subsequently performed at KNUCH, wherein the DNA extracted from each sample was amplified by polymerase chain reaction, and MSI testing was performed on the basis of five mononucleotide locus markers proposed by the National Cancer Institute.15 Using capillary electrophoresis, the samples showing instability at the two most sensitive markers (BAT25 and BAT26) among the five-marker panel were classified as exhibiting MSI-H. Additionally, a tumor sample was classified as MSI-L if one locus showed instability and MSI-S if all the loci were stable. Moreover, mismatch repair status was determined locally by immunohistochemistry analysis of the mismatch repair proteins MLH1, MSH2, MSH6, and PMS2; mismatch repair deficiency was defined as an absent expression of one or more of these proteins.
3. Definition of laboratory parameters
Peripheral blood samples were obtained from the patients during their first visit to the clinic (pre-CRT) and 2-4 weeks before surgery (post-CRT). For the platelet count, a cut-off value of 370×103/µL was selected according to a previous study;16 any changes in platelet counts were calculated by subtracting pre-CRT values from the post-CRT values. According to our previous study, the NLR was classified into the following two categories: low <5 or high ≥5. When calculating the PLR, PLR ≥235 was defined as high.17 Any changes in the NLR or PLR were determined as follows: pre-CRT NLR or PLR minus post-CRT NLR or PLR.
4. Statistical analysis
Statistical analyses were performed using R statistical software 4.3.1 (the R Foundation for Statistical Computing, Vienna, Austria, available at http://www.r-project.org). Disease-free survival (DFS) was calculated as the time from diagnosis to disease recurrence. Overall survival (OS) was defined as the date from diagnosis until death from any cause. Student’s t-test and a chi-square test were both applied to qualitative variables. The survival analysis used the Kaplan–Meier method with a log rank test. Two-sided p-values of <0.05 were considered statistically significant.
RESULTS
1. Patient characteristics
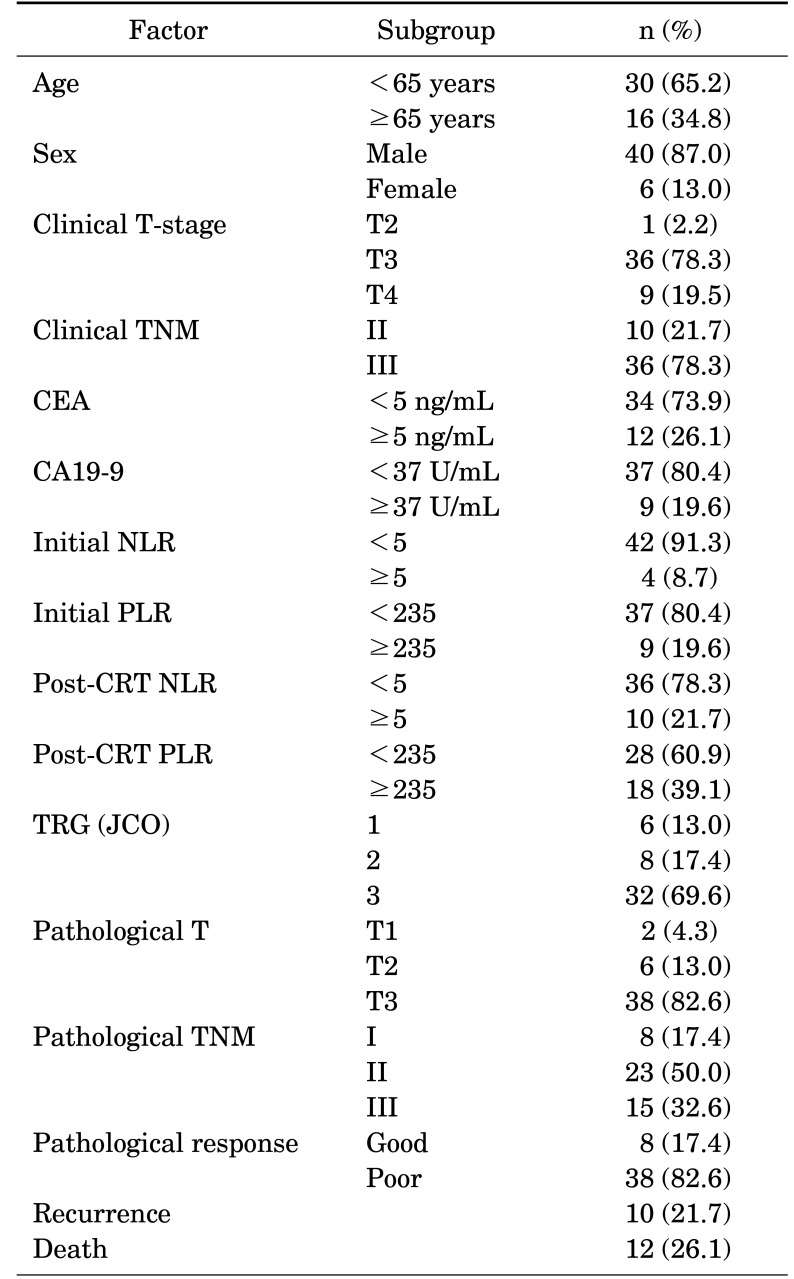
The baseline clinical characteristics and laboratory findings are summarized in Table 1. The median age of the patients was 60 (range, 24-84); the percentages of male and female patients were 87% and 13%, respectively. The following were the clinical stages before CRT: stage II (n=10, 21.7%) and stage III (n=36, 78.3%). Forty-two (91.3%) and thirty-seven (80.4%) patients had high initial NLR and PLR, respectively. Among the 46 patients, 10 (21.7%) experienced recurrence following CRT followed by surgical resection.
TABLE 1. Patient characteristics (n=46).
CEA: carcinoembryonic antigen, CA19-9: carbohydrate antigen 19-9, NLR: neutrophil-to-lymphocyte ratio, PLR: Platelet-to-lymphocyte ratio, CRT: chemoradiotherapy, TNM: the tumor, node, and metastasis, Good: pT1 and pT2, Poor: pT3 or node-positive, TRG: tumor regression grade, JCO: Journal of Clinical Oncology.
2. Pre-CRT NLR/PLR and clinical outcomes
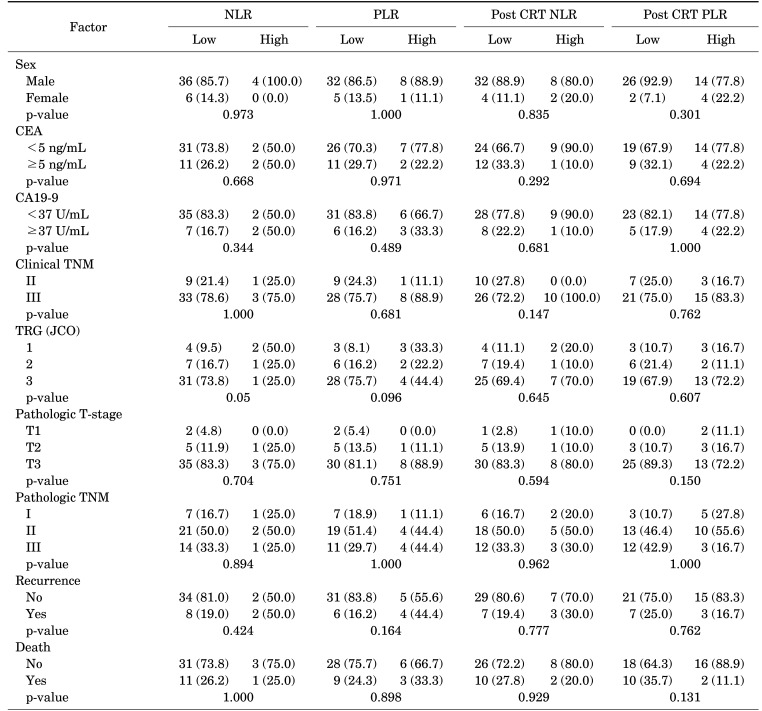
A comparison between the clinicopathological characteristics of each group is presented in Table 2. High initial NLR and PLR were not significantly associated with clinical outcomes. Patients in the high NLR and PLR groups did not show differences in pathologic stage, tumor regression grade (TRG), and the rates of recurrence or death. However, the patients with high initial NLR had a tendency to experience better TRG status following CRT followed by surgery (p=0.05). Moreover, the group with high initial PLR had comparably lower TRG scores (p=0.096). However, the post-CRT NLR and PLR values were not associated with any clinical outcomes.
TABLE 2. Association between clinicopathological features and the NLR and PLR.
CRT: chemoradiotherapy, CEA: carcinoembryonic antigen, CA19-9: carbohydrate antigen 19-9, NLR: neutrophil-to-lymphocyte ratio, PLR: Platelet-to-lymphocyte ratio, TNM: the tumor, node, and metastasis, TRG: tumor regression grade, JCO: Journal of Clinical Oncology.
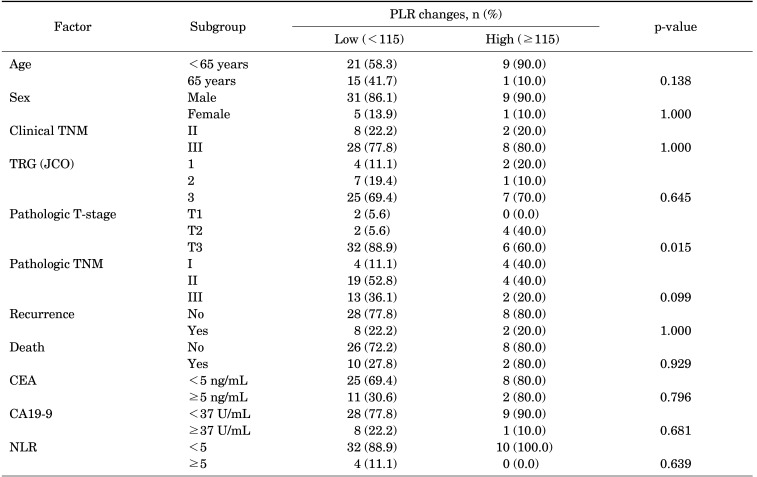
3. PLR changes and tumor response following CRT
The patients were categorized into two groups using a cut-off value of 115 (pre-CRT PLR subtracted by post-CRT PLR, low <115 and high ≥115) (Table 3). The patients with high PLR change showed a statistically significant association with pathologic T-stage (p=0.015) but not with TRG status (p=0.645) and recurrence rate (p=1.000). No significant correlation was observed between PLR change and pathologic TNM (p=0.099). Univariate analysis indicated that patients’ DFS and OS (DFS, p=0.8; OS, p=0.8) were not significantly different between the two groups (Fig. 1).
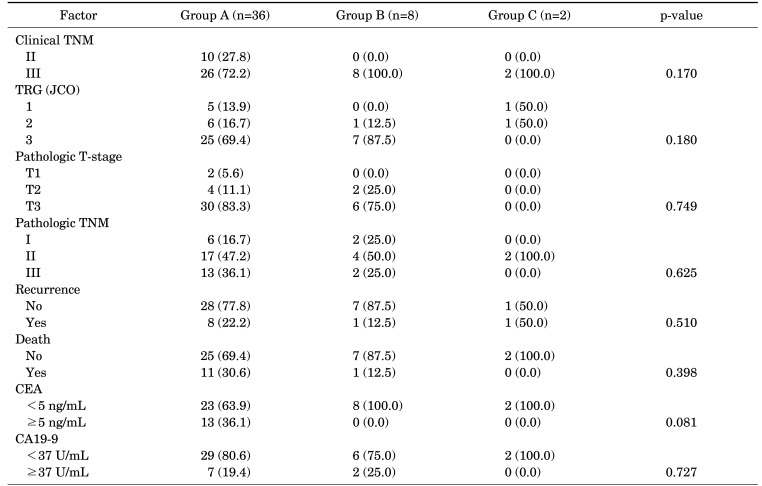
TABLE 3. Association of changes in the platelet count with clinical outcomes in patients categorized using a cut-off value of 370×103/µL: low pre-CRT count, regardless of the post-CRT count (group A); high pre-CRT count and low post-CRT count (group B) and high pre-CRT and post-CRT count (group C).
CRT: chemoradiotherapy, CEA: carcinoembryonic antigen, CA19-9: carbohydrate antigen 19-9, TNM: the tumor, node, and metastasis, TRG: tumor regression grade, JCO: Journal of Clinical Oncology.
FIG. 1. Kaplan–Meier cumulative disease-free (DFS) (A) and overall (OS) (B) survival curves according to changes in the platelet count. Patients are divided using a platelet count cut-off value of 370×103/µL: low pre-chemoradiotherapy (CRT) count, regardless of the post-CRT count (group A); high pre-CRT count and low post-CRT count (group B); and high pre-CRT and post-CRT count (group C). (A) The 5-year DFS for group A is 66.7% versus 87.5% in group B versus 50.0% in group C (p=0.423). (B) The 5-year OS group A is 83.3% versus 87.5% in group B versus 100.0% in group C (p=0.793).
4. Association of changes in the platelet count with clinical outcomes
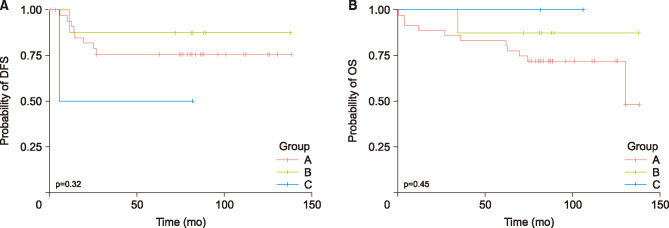
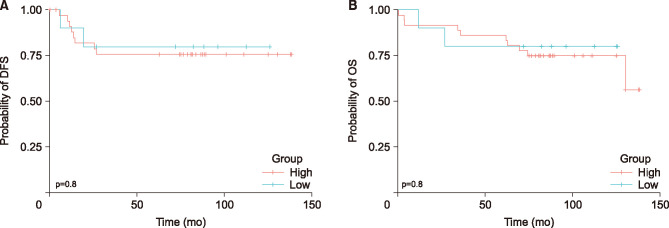
The patients were categorized into three groups on the basis of their pre- and post-CRT platelet counts with a cut-off of value 370×103/µL: low pre-CRT count, regardless of the post-CRT count (group A); high pre-CRT count and low post-CRT count (group B); and high pre-CRT and post-CRT count (group C) (Table 4). No statistically significant association was noted between changes in the platelet count and clinical outcomes of patients in the three groups. In the univariate analysis, the changes in the platelet count were not significantly related with patients’ DFS and OS (DFS, p=0.32; OS, p=0.45, Fig. 2).
TABLE 4. Relationship between clinicopathological characteristics and PLR changes following CRT.
CRT: chemoradiotherapy, CEA: carcinoembryonic antigen, CA19-9: carbohydrate antigen 19-9, PLR: Platelet-to-lymphocyte ratio, NLR: neutrophil-to-lymphocyte ratio, TNM: the tumor, node, and metastasis, TRG: tumor regression grade, JCO: Journal of Clinical Oncology.
FIG. 2. Kaplan–Meier cumulative disease-free (DFS) (A) and overall (OS) (B) survival curves according to changes in the platelet-to-lymphocyte ratio (PLR). Patients are categorized using a cut-off value of 115 into low (<115) and high (≥115) changes following chemoradiotherapy (CRT). (A) The 5-year DFS for those with low PLR change is 69.4% versus 70.0% for those with high PLR change (p=1.000). (B) The 5-year OS for those with low PLR change is 86.1% versus 80.0% for those with high PLR change (p=1.000).
DISCUSSION
This study investigated the prognostic values of blood-based systemic inflammatory response (SIR) markers including the NLR, PLR, and changes in the platelet count in patients with d-MMR/MSI-H LARC, and identified that the PLR changes following CRT were significantly associated with pathologic T-stage. However, the SIR markers were not associated with survival in these patient groups.
Recognized as inflammation and homeostasis regulators, high neutrophil and platelet counts play a key role in inducing angiogenesis, facilitating tumor cell extravasation, and motivating metastatic spreading.18,19 In contrast, lymphocytes act as suppressors of the proliferation and spread of cancer cells through their cytotoxic activity and anti-tumor cytokine production. Based on this background, previous studies have investigated the possibility of SIR markers as effective prognostic markers and predictive indicators.20,21 In various types of malignancies, a high NLR and PLR resulted in worse OS and DFS.22,23 Furthermore, our previous study has reported that a high PLR change was a significant independent predictive marker of good responses to CRT in LARC.18 However, results have been inconsistent across several studies, and a retrospective study conducted by An et al.24 observed that neither PLR nor NLR was associated with 5-year DFS in patients with LARC who received neoadjuvant CRT followed by curative resection. Furthermore, there remains insufficient evaluation of SIR in patients with MSI-H/dMMR. Recent findings provided by Corti et al.25 reported that an immune-inflammatory blood-based biomarker had an independent role in patients with MSI-H/dMMR metastatic CRC receiving ICIs. Considering the very small number of patients with MSI-H/dMMR, the current findings may provide meaningful information for this subgroup, that is, SIR exhibits a host cellular immune response against tumors.26
The current study showed that changes in the PLR following CRT were predictive or prognostic indicators of the pathological tumor response to preoperative CRT. Platelet is significant hematologic component maintaining homeostasis and the tissue repair system; previous studies have demonstrated that initial thrombocytosis is considered a factor predicting poor pathological tumor regression and shorter recurrence-free survival in CRC.17,27 In the present study, the pathologic T-stage was significantly related with a change in the PLR following preoperative CRT. For example, the patient group that maintained a low PLR change had advanced pathologic T-stage (T3: 88.9% vs. 60.0%) compared with the group with a high PLR change following CRT. This is consistent with a previous study, wherein the PLR was identified as an independent factor of the T-stage of the patients with CRC.28 It is well known that down-staging provided better oncologic outcomes in terms of disease-free survival and local recurrence in locally advanced rectal cancer patients.29 Thus, a more suitable treatment with TNT or ICIs is needed to improve oncological outcomes in these low PLR change group. Meanwhile, a retrospective study of individuals in a Chinese population by Liu et al.30 reported that MSI-H showed comparably low platelet volume and reduced platelet glycoprotein. These findings point to the possibility of platelet or the PLR as a biomarker in MSI-H/dMMR tumors, suggesting that platelet could be involved in immune response by incorporating into multiple significant cellular pathways.
This study had some limitations. First, although the current study had a homogeneous ethnicity and equivalent treatment application, the sample size of 46 patients was too small to retrospectively analyze. Second, a standard cut-off value for the SIR markers had not yet been clearly established. Lastly, as the level of laboratory markers was measured in the blood at a single timepoint, reflecting dynamic changes was highly difficult.
In conclusion, the current study suggests that the changes in the PLR following CRT are associated with the pathologic T-stage of the group. However, the SIR markers particularly the NLR, PLR, and platelet count showed no prognostic value for the survival outcomes of patients with MSI-H/dMMR LARC.
ACKNOWLEDGEMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR22C1832).
Footnotes
CONFLICT OF INTEREST STATEMENT: None declared.
References
- 1.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Aschele C, Glynne-Jones R. Selecting a TNT schedule in locally advanced rectal cancer: can we predict who actually benefits? Cancers (Basel) 2023;15:2567. doi: 10.3390/cancers15092567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. 2022;386:2363–2376. doi: 10.1056/NEJMoa2201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richman S. Deficient mismatch repair: read all about it (Review) Int J Oncol. 2015;47:1189–1202. doi: 10.3892/ijo.2015.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–2087.e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383:2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 7.Xiao W, Luo H, Yao Y, Wang Y, Liu S, Sun R, et al. Total neoadjuvant treatment and PD-1/PD-L1 checkpoint inhibitor in locally advanced rectal cancer. Front Immunol. 2023;14:1149122. doi: 10.3389/fimmu.2023.1149122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koukourakis IM, Kouloulias V, Tiniakos D, Georgakopoulos I, Zygogianni A. Current status of locally advanced rectal cancer therapy and future prospects. Crit Rev Oncol Hematol. 2023;186:103992. doi: 10.1016/j.critrevonc.2023.103992. [DOI] [PubMed] [Google Scholar]
- 9.Kang BW, Chau I. Current status and future potential of predictive biomarkers for immune checkpoint inhibitors in gastric cancer. ESMO Open. 2020;5:e000791. doi: 10.1136/esmoopen-2020-000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi: 10.1016/j.lungcan.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Graziano V, Grassadonia A, Iezzi L, Vici P, Pizzuti L, Barba M, et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast. 2019;44:33–38. doi: 10.1016/j.breast.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Huang CM, Huang MY, Tsai HL, Huang CW, Su WC, Chang TK, et al. Pretreatment neutrophil-to-lymphocyte ratio associated with tumor recurrence and survival in patients achieving a pathological complete response following neoadjuvant chemoradiotherapy for rectal cancer. Cancers (Basel) 2021;13:4589. doi: 10.3390/cancers13184589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ergen ŞA, Barlas C, Yıldırım C, Öksüz DÇ. Prognostic role of peripheral neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) in patients with rectal cancer undergoing neoadjuvant chemoradiotherapy. J Gastrointest Cancer. 2022;53:151–160. doi: 10.1007/s12029-020-00578-7. [DOI] [PubMed] [Google Scholar]
- 14.Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. 2018;25:1454–1455. doi: 10.1245/s10434-018-6462-1. [DOI] [PubMed] [Google Scholar]
- 15.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 16.Kim HJ, Choi GS, Park JS, Park S, Kawai K, Watanabe T. Clinical significance of thrombocytosis before preoperative chemoradiotherapy in rectal cancer: predicting pathologic tumor response and oncologic outcome. Ann Surg Oncol. 2015;22:513–519. doi: 10.1245/s10434-014-3988-8. [DOI] [PubMed] [Google Scholar]
- 17.Lee IH, Hwang S, Lee SJ, Kang BW, Baek D, Kim HJ, et al. Systemic inflammatory response after preoperative chemoradiotherapy can affect oncologic outcomes in locally advanced rectal cancer. Anticancer Res. 2017;37:1459–1465. doi: 10.21873/anticanres.11470. [DOI] [PubMed] [Google Scholar]
- 18.Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, et al. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6:630–649. doi: 10.1158/2159-8290.CD-15-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toiyama Y, Inoue Y, Kawamura M, Kawamoto A, Okugawa Y, Hiro J, et al. Elevated platelet count as predictor of recurrence in rectal cancer patients undergoing preoperative chemoradiotherapy followed by surgery. Int Surg. 2015;100:199–207. doi: 10.9738/INTSURG-D-13-00178.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18:360. doi: 10.1186/s12916-020-01817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang J, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One. 2014;9:e101119. doi: 10.1371/journal.pone.0101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho U, Park HS, Im SY, Yoo CY, Jung JH, Suh YJ, et al. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PLoS One. 2018;13:e0200936. doi: 10.1371/journal.pone.0200936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henriksen JR, Nederby L, Donskov F, Waldstrøm M, Adimi P, Jakobsen A, et al. Prognostic significance of baseline T cells, B cells and neutrophil-lymphocyte ratio (NLR) in recurrent ovarian cancer treated with chemotherapy. J Ovarian Res. 2020;13:59. doi: 10.1186/s13048-020-00661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An SH, Kim IY. Can pretreatment platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios predict long-term oncologic outcomes after preoperative chemoradiation followed by surgery for locally advanced rectal cancer? Ann Coloproctol. 2022;38:253–261. doi: 10.3393/ac.2021.00633.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corti F, Lonardi S, Intini R, Salati M, Fenocchio E, Belli C, et al. The Pan-Immune-Inflammation Value in microsatellite instability-high metastatic colorectal cancer patients treated with immune checkpoint inhibitors. Eur J Cancer. 2021;150:155–167. doi: 10.1016/j.ejca.2021.03.043. [DOI] [PubMed] [Google Scholar]
- 26.Sahin IH, Akce M, Alese O, Shaib W, Lesinski GB, El-Rayes B, et al. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br J Cancer. 2019;121:809–818. doi: 10.1038/s41416-019-0599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai K, Kitayama J, Tsuno NH, Sunami E, Watanabe T. Thrombocytosis before pre-operative chemoradiotherapy predicts poor response and shorter local recurrence-free survival in rectal cancer. Int J Colorectal Dis. 2013;28:527–535. doi: 10.1007/s00384-012-1594-4. [DOI] [PubMed] [Google Scholar]
- 28.Jia J, Zheng X, Chen Y, Wang L, Lin L, Ye X, et al. Stage-dependent changes of preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in colorectal cancer. Tumour Biol. 2015;36:9319–9325. doi: 10.1007/s13277-015-3667-9. [DOI] [PubMed] [Google Scholar]
- 29.Cui Y, Liu X, Li S, Wang H, Xiang Y, Zhang Y, et al. The ypT may better predict the efficacy of neoadjuvant chemoradiotherapy than tumor regression grade in locally advanced rectal cancer patients diagnosed ypT1-4N0. Clin Transl Oncol. 2024;26:1012–1021. doi: 10.1007/s12094-023-03343-x. [DOI] [PubMed] [Google Scholar]
- 30.Liu ZY, Jia QC, Wang W, Liu YX, Wang RT, Li JY. The association between platelet glycocalicin and high microsatellite instability in colorectal cancer. Gastroenterol Res Pract. 2022;2022:9012063. doi: 10.1155/2022/9012063. [DOI] [PMC free article] [PubMed] [Google Scholar]