Abstract
Melasma is a prevalent hyperpigmentation condition known for its challenging treatment due to its resemblance to photoaged skin disorders. Numerous studies have shed light on the intricate nature of melasma, which often bears similarity to photoaging disorders. Various therapeutic approaches, encompassing topical and systemic treatments, chemical peeling, and laser therapy, have exhibited efficacy in managing melasma in previous research. However, melasma often reoccurs despite successful treatment, primarily due to its inherent photoaged properties. Given that melasma shares features with photoaging disorders, including disruptions in the basement membrane, solar elastosis, angiogenesis, and mast cell infiltration in the dermal layer, a comprehensive treatment strategy is imperative. Such an approach might involve addressing epidermal hyperpigmentation while concurrently restoring dermal components. In this article, we provide a comprehensive review of conventional treatment methods frequently employed in clinical practice, as well as innovative treatments currently under development for melasma management. Additionally, we offer an extensive overview of the pathogenesis of melasma.
Keywords: Melanosis, Melasma, Pathology, Skin aging, Therapy
INTRODUCTION
Melasma is a common, acquired hyperpigmentation disorder characterized by asymptomatic, irregular-bordered, symmetrically deposited light-to-dark brown macules and patches on the sun-exposed area. It is known to usually affect women in their third or fourth decades with Fitzpatrick skin types III-IV1,2. The most cited etiologic factors of melasma are genetic susceptibility, sexual hormone, and ultraviolet (UV) exposure1,2,3. Melasma is often resistant to conventional treatments and often recurs even after successful treatment. To understand the challenges of treating melasma, it is essential to understand its pathophysiology. Several recent studies have updated our understanding of melasma.
PATHOGENESIS OF MELASMA
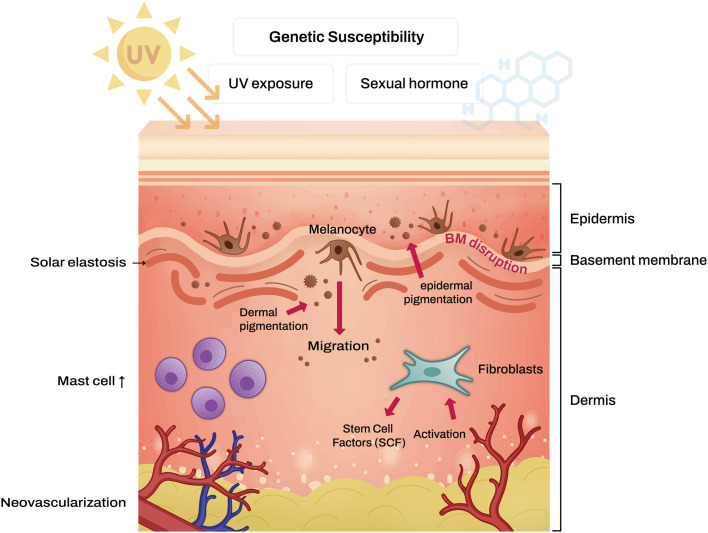
Previously, melasma was regarded as a melanocyte disorder. However, recent studies have integrated the roles of dermal components such as mast cells, solar elastosis, and neovascularization in melasma pathogenesis alongside melanocytes. When UV radiation accumulates, chronic dermal inflammation occurs, activating fibroblasts4. Subsequently, UV irradiated fibroblasts secrete stem cell factor (SCF), which induces melanogenesis by signaling with its receptor, c-kit, located in the epidermis5. Additionally, senescent fibroblasts are elevated in the lesional skin of melasma compared to perilesional normal skin. Senescent fibroblasts are believed to produce more skin-aging-related proteins, such as SCF6. Moreover, in the lesional skin of melasma, there is an upregulation of modulators associated with Wnt signaling7. Furthermore, there is a reduction in the expression of Wnt inhibitory factor-1 (WIF-1) in the hyperpigmented skin of melasma patients8. The downregulation of WIF-1, which can occur in epidermal keratinocytes and dermal fibroblasts, is implicated in the development of melasma due to its role in stimulating melanogenesis and the transfer of melanosomes through the upregulation of both canonical and noncanonical Wnt signaling pathways8. Meanwhile, the release of frizzled-related protein 2 (FRP2) serves as a stimulant for melanogenesis through the activation of the β-catenin signaling pathway9. Additionally, UV-induced cyclooxygenase-2 (COX-2) is recognized for its ability to stimulate melanocytes further10.
In the lesional skin of melasma, pendulous melanocytes dropped into the dermis, characteristic of the disease, and dermal melanophages were observed11. Dermal melanin content is thought to be promoted by disruption of the basement membrane4. Using periodic acid-Schiff-Diastase (D-PAS) staining and anti-collagen type IV immunohistochemistry, 95.5% and 83% of skin samples from melasma patients with Fitzpatrick skin types IV and V showed basement membrane disruption, respectively12. Chronic UV exposure activates metalloproteinases2 (MMP2) and MMP9 to degrade type IV and VI collagen in the basement membrane2. The decent of melanocytes and melanin in the dermis facilitated by the basement membrane makes melasma refractory to treatments that target epidermal pigmentation.
Mast cells are more frequently observed in melasma-affected skin than in normal skin13. UV radiation appears to promote histamine release from dermal mast cells14. The role of histamine in melanogenesis is still unclear, but it is thought to be related to growth-differentiation factor-15 (GDF-15), which belongs to the transforming growth factor (TGF-β) superfamily15. Also, histamine is known to stimulate human melanocytes through protein kinase A activation via H2 receptors16. Furthermore, chronic UV irradiation increases the tryptase released by mast cells13. Mast cell-released tryptase facilitates the degradation of type IV collagen by activating latent forms of MMPs or directly damaging extracellular matrix (ECM) components17. Consequently, the increased tryptase released by mast cells can induce basal cell disruptions in melasma patients. Moreover, mast cells release diverse angiogenic factors, including basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and TGF-β18. Furthermore, prolonged exposure to UV radiation leads to the development of solar elastosis, characterized by the abnormal accumulation of elastic tissue in the dermis, a phenomenon frequently observed in the affected skin of individuals with melasma2,19. It is proposed that mast cell-secreted tryptase plays a role in inducing solar elastosis by stimulating fibroblasts to produce elastin2. Additionally, granzyme B, which is secreted by mast cells, is known to promote ECM degradation after extended UV exposure20.
Previous studies have reported a significant increase in the number and size of blood vessels in the affected skin of individuals with melasma21,22. Within these altered blood vessels, there is an upregulation of VEGF expression22. VEGF is known to trigger the release of arachidonic acid and the phosphorylation and activation of cytosolic phospholipase A222. Although the exact mechanism remains unclear, there is a suggestion that VEGF may induce melanogenesis by elevating the expression of protease-activated receptor-2 (PAR-2)23. Furthermore, endothelin 1, secreted by the endothelial cells of microvasculature, is recognized for its ability to stimulate melanogenesis. It achieves this by activating the microphthalmia-associated transcription factor (MITF) through the activation of endothelin receptor B24.
Based on accumulated knowledge, it’s clear that melasma is not solely an epidermal hyperpigmentary disorder; instead, it is a complex condition with characteristics of photoaging disorders (Fig. 1). These factors make it challenging to treat melasma effectively. To address these issues, research efforts have been directed toward developing treatments that target the underlying pathophysiology of melasma.
Fig. 1. Schematic representation of melasma pathogenesis.
Sunscreen
Wavelength spectrum of solar irradiation is classified as infrared (780–5,000 nm), visible light (VL) (400–780 nm) and UV (290–400 nm) segments25. It is well known that both UV and VL exposure cause pigmentary changes, which are explained by physiological mechanism, where generation of reactive oxygen species (ROS) results in release of inflammatory cytokines and matrix-degrading enzymes in the skin. Recent study also revealed that VL induces long-lasting hyperpigmentation via activation of opsin 3-regulated calcium-dependent microphthalmia-associated transcription factor26. Together with this finding, photoprotection against VL is important in melasma patients. Indeed, Castanedo-Cazares et al. assigned sixty-eight patients with melasma into two groups to receive either UV-VL sunscreen or UV-only sunscreen randomly25. At 8 weeks, the former group showed 15%, 28%, and 4% greater improvements than the latter group in Melasma Area and Severity Index (MASI) scores, colorimetric values, and melanin assessment, respectively. Similarly, Boukari et al.27 designed another randomized controlled trial comparing two sunscreen groups differed by the presence or absence of iron oxide, an absorbing pigment blocking VL. The authors reported significantly lower MASI score at 6 months in the iron oxide group. Given this converging line of evidence, sunscreen containing either iron oxide or large size (>200 nm) of titanium dioxide and zinc oxide is highly recommended despite undesirable cosmetical issue represented by white turbidity28.
Topical treatments
1) Skin-lightening agents
Topical skin-lightening agents are mainstream in treating melasma. These compounds target tyrosinase, a rate-limiting enzyme of the melanogenesis pathway29. Hydroquinone, one of tyrosinase inhibitors, has solidified for decades as benchmark in the treatment of hyperpigmentation. It is hypothesized that hydroquinone inhibits tyrosinase by binding with the enzyme or interacting with copper molecule at the enzyme’s active site. This results in decreased formation of melanosomes, a marked alteration in the internal structure of melanosome, an increased degradation of melanosome, and ultimately, a destruction of the membranous organelles in the melanocytes30. Along with this underlying mechanism, indeed, hydroquinone led dose-dependent decrease in pigmentation in the clinical examination, where it was applied topically onto the dorsum of hands with solar lentigines at different concentrations (2%, 3%, and 5%)31. Further studies also suggested its skin lightening effect. Ennes et al. compared the proportions showing complete clinical response between melasma patient groups applied with 4% hydroquinone cream and placebo, respectively. The researchers found that there was significant gap between two groups (38% versus 8%)32. Despite these accumulated evidences guaranteeing its efficacy, safety issues have been raised continuously, and they often used to make hesitating its use. For instance, concerns regarding the systemic absorption of the drug and drug-induced carcinogenesis have been addressed33. Other concerns that several reports of exogenous ochronosis are presumably due to hydroquinone use were also raised34.
In another perspective, these safety issues have stimulated researchers to excavate novel compounds with a low risk of developing adverse events. 4-n-butylresorcinol, niacinamide, ascorbic acid, resveratrol, azelaic acid and kojic acid were proposed as alternative agents35.
2) Topical retinoids
Topical retinoids have shown their effectiveness in the treatment of melasma. Retinoids are suspected to stimulate turnover of keratinocytes, inhibit transfer of melanosome, and allow trans-epidermal penetration of other topical therapies36. Comparing 0.1% tretinoin cream to vehicle over a 40-week period, Griffiths et al. found that 68% of treatment group showed improvement in colorimetry and histological evaluation versus only 5% of vehicle group. Notably, the effects of this therapy were unclear until 24 weeks and many of treated patients (88%) experienced side effects, including erythema and desquamation37. Considering longer treatment required to lead clinical benefit and frequent occurrence of irritation, tretinoin may not be good mono-therapeutic option38.
Adapalene, a synthetic retinoid, has been applied for melasma patients. Dogra et al.39 conducted study comparing adapalene 0.1% gel with tretinoin 0.05% cream for Asian Indian melasma patients. The authors reported similar efficacy in both groups, indicated by 37% and 41% reduction of MASI scores in adapalene- and tretinoin-treated groups, respectively with superior tolerability. It was noteworthy that adapalene-treated patients reported quite fewer side effects and its superior tolerability.
3) Combined topical agent
Triple combination cream (TCC), consisted of hydroquinone, retinoid, and topical steroid, is widely used for melasma treatment. Taylor et al.40 showed its higher efficacy compared with any dual combination of the three active ingredients in a large, multicenter, randomized controlled trial. 77% of TCC-treated participants reached complete or near complete clearing, in contrast only 47% of dual-combination group achieved the very endpoints. Ferreira Cestari et al. also displayed its superiority to 4% hydroquinone in the view of efficacy41. Clearance of melasma, meaning lesions nearly equivalent to perilesional skin, was observed in 35% of subjects using TCC versus 5% using HQ alone.
One hypothesis explains the higher efficacy of this product based on the synergistic effects of its ingredients. Specifically, the topical steroid is thought to ameliorate irritation caused by the other two ingredients and inhibit melanin synthesis, while the retinoid is believed to interrupt the oxidation of hydroquinone and facilitate its trans-epidermal penetration42.
Given that hydroquinone in concentrations above 4% and in treatment courses longer than 3 months may be associated with new onset ochronosis43, the combination formula limiting the concentration of hydroquinone as 4% thanks to combined effects with other constituents may account for the lower risk of ochronosis44.
4) Investigational therapeutic approaches
Since academic attempts have unveiled the pathophysiologic mechanisms involved in onset and progression of melasma, various topical agents, targeting each step of cutaneous hyperpigmentation, have been proposed.
Microphthalmia-associated transcription factor-siRNA (MITF-siRNA) cream effectively lightened both brown facial hypermelanosis and normal skin in Asian individuals, by inhibiting the tyrosinase pathway45.
Topical proton pump inhibitors (PPIs), such as omeprazole, may also inhibit melanogenesis, and present a promising treatment for melasma. Omeprazole topically applied onto the skin of UV-irradiated human subjects elicited significant reduction of pigment levels after 3 weeks compared to untreated controls46. It is hypothesized that omeprazole decreases melanogenesis by inhibiting ATP7A and enhancing degradation of tyrosinase. Together with this finding, it is also noteworthy that PPIs may trigger or aggravate vitiligo, supported by the clinical case reports in which patients experienced relapse or development of their vitiligo after the use of oral PPIs47.
Methimazole is an oral anti-thyroid medication commonly used to treat hyperthyroidism. It is noteworthy that topical application of methimazole causes depigmentation, therefore it can be used for therapeutic purposes in patients with melasma and post-inflammatory hyperpigmentation (PIH). It is believed that methimazole blocks melanin synthesis as a potent peroxidase inhibitor. Kasraee et al.48 reported moderate to marked improvement of the hyperpigmented lesions with topical methimazole 5% in a 27-year-old male with PIH. Its side effects on thyroid function were neglectable since there were no significant changes in serum thyroid-stimulating hormone, free thyroxine, and free triiodothyronine levels in 20 patients applied by methimazole 5% daily.49
Systemic treatments
1) Oral tranexamic acid (TXA)
TXA, originally designed for its hemostatic properties, acts as an antifibrinolytic agent. By interfering with the plasminogen/plasmin system, TXA affects the communication between keratinocytes and melanocytes. Furthermore, TXA hinders the plasmin activity induced by UV light exposure, leading to a reduction in mast cell activity and the inhibition of fibroblast growth factor50. This, in turn, results in a decrease in the number of mast cells in the dermis and a reduction in the formation of new blood vessels13,50. Moreover, a recent novel study suggested that TXA can activate the autophagy system by increasing the expression of autophagy-related proteins51. Earlier research indicates that the autophagy system plays a crucial role in determining skin color by regulating the degradation of melanosomes in keratinocytes, which is enhanced by activators of autophagy52. These autophagy-related proteins include Beclin-1, the autophagic modulator WIPI1, and microtubule-associated protein light chain 3 (LC3)51.
Numerous studies have investigated the appropriate dosage of TXA. Karn et al.53 reported improved MASI scores in a group that received 500 mg of TXA daily in addition to topical treatments (topical hydroquinone [HQ] with sunscreen) compared to a group receiving topical treatments alone 12 weeks. Eunice et al.54 demonstrated a 49% reduction in modified Melasma Area Severity Index (mMASI) in a group treated with 500 mg of TXA daily for three months, as opposed to an 18% reduction in a control group applying only sunscreen. Minni et al.55 showed that in a group receiving 500 mg of TXA daily alongside the application of a triple combination cream, 65.6% of patients experienced a mMASI improvement of 75% or more at 12 weeks, while only 27.1% in the topical treatment-only group achieved this level of improvement. In a study by Lajevardi et al.56, the combination therapy group, receiving oral TXA at a dosage of 250 mg three times daily in conjunction with 4% HQ, exhibited better outcomes after three months of treatment. The possibility of achieving improved efficacy using higher therapeutic doses has also been explored. In a study by Zhu et al., individuals with melasma were randomly assigned to receive daily doses of TXA at 500 mg, 750 mg, 1,000 mg, or 1,500 mg. There were no significant differences observed in the MASI score or melanin index among the four different dosage groups, although faster results were achieved with higher doses57. As the appliance of light- or laser-based therapy on melasma has been increasing recently, several studies about oral TXA use in conjunction with laser therapy have been conducted. The study conducted by Cho et al.58 reported a more significant reduction in the mMASI score in the group that received a combination therapy of oral TXA at a dose of 500 mg per day along with intense pulsed light (IPL) and low fluence 1,064 nm QS Nd:YAG laser, compared to the group that underwent only IPL and laser treatment. Subsequently, Shin et al.59 demonstrated that the combined treatment of 750 mg TXA with low fluence QS ND:YAG laser resulted in a higher mean reduction in the mMASI score at eight weeks after treatment compared to treatment with the laser alone.
There are concerns about the thrombogenic risk due to the use of TXA as a hemostatic agent. However, this risk is known to be very low in young adults without underlying medical conditions and who are not taking other medications at the same time53,56. However, it is crucial to conduct comprehensive screening for individuals with additional thromboembolic risk factors, including those with cardiovascular disease and current anticoagulant therapy. Such individuals should be contraindicated for systemic TXA therapy.
Despite the relatively good safety profile of oral TXA, there have been attempts to explore different delivery methods. Topical TXA in various formulations, such as gel or solution, has been investigated60,61. Moreover, to enhance the efficacy of topical TXA, several strategies have been employed to promote its delivery, including microneedling or CO2 fractional laser techniques62,63,64. Additionally, intradermal microinjection of TXA has also proven to be effective in previous studies65.
2) Other systemic agents
Others
Polypodium leucotomos (PL) is a tropical fern originating from Central and South Africa66. PL is known to have antioxidant effects, anti-inflammatory effects, as well as photoprotective effects by scavenging several ROS and inhibiting the formation of lipid peroxidation67,68. Several attempts have been made to utilize oral PLE to treat melasma, but the results have been inconclusive.
Other systemic agents studied for treatment for melasma include Vitamin C, Vitamin E69,70, Proanthocyanidin-rich extract from grape seeds71, Korean red ginseng72, carotenoids73, or French maritime pine bark extract74. Although they have been shown to have beneficial and promising effects on melasma due to their antioxidative effects, the evidence supporting their use is limited, and further research is needed.
Chemical peels
Chemical peels are a well-known treatment option for melasma and are typically considered a secondary approach to managing the condition. Their effectiveness in addressing the epidermal component of melasma is attributed to their ability to induce controlled epidermal separation and subsequent regeneration75. Additionally, they may aid in removing stagnant melanin through phagocytosis in the dermal layers75. However, it is essential to note that chemical peels carry a substantial risk of causing PIH, particularly in individuals with Fitzpatrick skin types III to VI.
1) Glycolic acid (GA) peels
GA peel is the most commonly used α-hydroxy peel, which has the smallest molecular weight, penetrating the epidermis easily76. Several studies have examined the effectiveness of GA peels. However, most of them have not demonstrated any superiority over topical therapy. In a split-face study conducted by Lim and Tham, they compared a combination approach (20%–70% GA peels every three weeks and a topical product containing 10% GA and 2% HQ) to a topical-only treatment. The results showed no significant difference between the two sides77. Similarly, in another split-face study conducted by Hurley et al.78, patients received 20%–30% GA peels every two weeks on one side of their face, along with a twice-daily full-face application of 4% HQ. This study also found no significant difference between the combination therapy and HQ-only treatment.
In contrast, certain studies have demonstrated a promising effect of combining GA peels with topical therapy. Sakar et al.79 reported a statistically significant improvement in the MASI score 21 weeks after treatment in the group that received 30%–40% GA peels combining TCC compared to the TCC-only group. Similarly, in a study conducted by Dayal et al., the combination of a GA peel with a topical 20% azelaic acid cream showed a statistically significant improvement in the MASI score compared to the topical-only group80. However, it is important to note that in the combination group, there were higher side effects such as erythema, a burning sensation, and PIH.
2) Other chemical peels
Various agents, including SA, TCA, and lactic acid, have been explored for chemical peeling in patients with melasma. While the evidence supporting these methods is limited, they could be considered as an option for individuals who have melasma that does not respond well to topical treatments.
Laser and light treatment
Various light devices have been employed in melasma treatment, with IPL demonstrating effectiveness in both standalone and combined therapies, as indicated by several small-scale studies81,82,83. IPL emits a wide spectrum of light (500 to 1,200 nm), making it suitable for various dermatological conditions, including pigmentary disorders. In a study by Choi et al.82, 30 Asian patients treated with fractionated IPL over 14 weeks exhibited moderate efficacy against melasma. Wang et al.81 reported IPL’s efficacy when combined with TCC and sunscreen, noting the absence of serious side effects. Furthermore, when combined with Q-switched ruby laser (QSRL), MASI scores decreased and were sustained at the 3-month follow-up83.
Laser therapy, involving various devices like ablative lasers (including CO2 laser and Erbium:YAG [Er:YAG] laser) and nonablative lasers (such as Q-switched Nd:YAG laser [QSNYL], QSRL, and pulsed dye laser [PDL]), has undergone extensive study. However, ablative lasers like CO2 laser and Er:YAG laser are considered prone to postprocedural dyspigmentation84. In a study conducted by Hassan et al., melasma patients treated with PDL exhibited improvements in mMASI scores and a significant reduction in VEGF expression levels85.
Among the available laser devices, the QSNYL is the preferred choice. In the past, laser treatment for melasma was not recommended due to the risk of hyperpigmentation or hypopigmentation. However, since the introduction of the concept of “laser toning,” lasers have been increasingly utilized for melasma treatment. Laser toning involves the repetitively applying a 1064-nm Nd:YAG laser with a large diameter and lower fluence to the melasma-affected areas. This repetitive treatment approach has been popularized for its effectiveness in improving melasma. Furthermore, with the theoretical background of subcellular selective photothermolysis, more physicians have adopted this technique. Kim et al.86 suggested subcellular selective photothermolysis as the mechanism of “laser toning.” They observed dendrite shortening of melanocytes after treatment with 3-dimensional EM. By targeting melanocytes and Stage IV melanosomes, this laser therapy minimizes collateral damage and offers a promising approach to melasma treatment with fewer adverse effects. However, punctate leukoderma has become a concern, and with frequent and repeated treatments, it leads to the destruction of melanocytes87.
Also, there was a development of a pico-second laser. Feng et al.88 compared the picosecond and nanosecond Nd:YAG 1064-nm lasers in the treatment of melasma by split-face randomized clinical trial. They concluded that the efficacy is about the same in the treatment of melasma. However, the picosecond laser was less painful during the procedure, with a lower potential risk of exacerbation of melasma.
Moreover, melasma is currently regarded as a photoaging disease89, and there is a growing trend to combine treatments that target the dermis, given its impact on melasma. In this context, devices utilizing alternative energy sources, such as radiofrequency (RF) devices, exhibit promising results in melasma treatment. In one study, the use of monopolar RF alongside kojic acid demonstrated improved MASI scores. However, the lack of controls limits the interpretation of these results90. Microneedle RF, known for minimal epidermal ablation, proves effective in skin rejuvenation and holds potential for treating melasma by enhancing the impaired ECM and promoting melanin elimination91,92. Recent research suggests that pulsed-type microneedling RF could be effectively employed for refractory melasma due to its effects, including enhanced permeability for topical treatments and induction of various dermal changes such as alterations in vasculature, melanin washout, and neocollagenesis93,94.
Clinicians should carefully consider the available evidence and patient-specific factors when selecting the most appropriate treatment modality for melasma, aiming to improve both efficacy and patient satisfaction in addressing this challenging pigmentary disorder. While hydroquinone monotherapy and triple combination cream remain the gold standard for melasma treatment, considering the damaged dermal components of melasma and its challenging characteristics, light-based therapy can be used as an adjunct for treatment. Currently, QSNYL is the first choice in laser treatment, but for recalcitrant melasma patients, light-based therapies targeting the photoaged dermis, such as RF or PDL, should be considered. The treatment modalities affecting dermal components are summarized in Table 1.
Table 1. Treatment modalities affecting dermal components of melasma.
| Variables | Mechanism | References | |
|---|---|---|---|
| Topical | |||
| Hydroquinone | Inhibiting pendulous melanocytes which refer to melanocytes that protrude into the dermal layer and are related to the hyperactivity of melanocytes | 95 | |
| Retinoid | Decrease in solar elastosis and perivascular inflammation | 95,96 | |
| Formation of new dermal collagen | |||
| Azelaic acid | Reversing PUVA-induced senescence of dermal fibroblasts by the activation of PPARγ | 35 | |
| Oral | |||
| Tranexamic acid | Decrease in the number and activity of mast cells in the dermis | 50 | |
| Reduction in the formation of new blood vessels | |||
| Light-based therapy | |||
| Pulsed dye laser | Reduction in VEGF expression levels | 85 | |
| Microneedle radiofrequency | Enhancing the impaired extracellular matrix | 91,92 | |
| Microneedle pulsed type radiofrequency | Alterations in vasculature | 93,94 | |
| Neocollagenesis | |||
PUVA: psoralen plus ultraviolet-A radiation, PPARγ: Peroxisome proliferator-activated receptor gamma, VEGF: vascular endothelial growth factor.
CONCLUSION
Considering that melasma exhibits characteristics of a photoaging disorder, such as the disruption of the basement membrane, solar elastosis, angiogenesis, and mast cell infiltration in the dermis, it is important that we must take its pathogenesis into account when treating melasma. Various cell types, including melanocytes, keratinocytes, sebocytes, mast cells, and endothelial cells, are involved in melasma. Therefore, when it comes to effectively treating melasma, addressing these photoaging-related characteristics should be a priority.
Indeed, a combined treatment approach that includes both epidermal depigmentation and the enhancement of dermal photoaging is expected to be necessary in order to minimize the risk of recurrence in melasma. It is crucial to develop safer and more effective depigmenting agents. Additionally, therapeutic agents or light-based therapies that can restore dermal components, including the disrupted basement membrane or dysregulated dermal components, should be developed.
Footnotes
FUNDING SOURCE: None.
CONFLICTS OF INTEREST: The authors have nothing to disclose.
DATA SHARING STATEMENT: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Sarkar R, Bansal A, Ailawadi P. Future therapies in melasma: what lies ahead? Indian J Dermatol Venereol Leprol. 2020;86:8–17. doi: 10.4103/ijdvl.IJDVL_633_18. [DOI] [PubMed] [Google Scholar]
- 2.Kwon SH, Hwang YJ, Lee SK, Park KC. Heterogeneous pathology of melasma and its clinical implications. Int J Mol Sci. 2016;17:824. doi: 10.3390/ijms17060824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Handel AC, Lima PB, Tonolli VM, Miot LD, Miot HA. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171:588–594. doi: 10.1111/bjd.13059. [DOI] [PubMed] [Google Scholar]
- 4.Rajanala S, Maymone MB, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:25. [PubMed] [Google Scholar]
- 5.Kang HY, Hwang JS, Lee JY, Ahn JH, Kim JY, Lee ES, et al. The dermal stem cell factor and c-kit are overexpressed in melasma. Br J Dermatol. 2006;154:1094–1099. doi: 10.1111/j.1365-2133.2006.07179.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim M, Kim SM, Kwon S, Park TJ, Kang HY. Senescent fibroblasts in melasma pathophysiology. Exp Dermatol. 2019;28:719–722. doi: 10.1111/exd.13814. [DOI] [PubMed] [Google Scholar]
- 7.Kang HY, Suzuki I, Lee DJ, Ha J, Reiniche P, Aubert J, et al. Transcriptional profiling shows altered expression of Wnt pathway- and lipid metabolism-related genes as well as melanogenesis-related genes in melasma. J Invest Dermatol. 2011;131:1692–1700. doi: 10.1038/jid.2011.109. [DOI] [PubMed] [Google Scholar]
- 8.Kim JY, Lee TR, Lee AY. Reduced WIF-1 expression stimulates skin hyperpigmentation in patients with melasma. J Invest Dermatol. 2013;133:191–200. doi: 10.1038/jid.2012.270. [DOI] [PubMed] [Google Scholar]
- 9.Kim M, Han JH, Kim JH, Park TJ, Kang HY. Secreted frizzled-related protein 2 (sFRP2) functions as a melanogenic stimulator; the role of sFRP2 in UV-induced hyperpigmentary disorders. J Invest Dermatol. 2016;136:236–244. doi: 10.1038/JID.2015.365. [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, Shin JY, Kim MR, Hann SK, Oh SH. siRNA-mediated knock-down of COX-2 in melanocytes suppresses melanogenesis. Exp Dermatol. 2012;21:420–425. doi: 10.1111/j.1600-0625.2012.01483.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee DJ, Park KC, Ortonne JP, Kang HY. Pendulous melanocytes: a characteristic feature of melasma and how it may occur. Br J Dermatol. 2012;166:684–686. doi: 10.1111/j.1365-2133.2011.10648.x. [DOI] [PubMed] [Google Scholar]
- 12.Torres-Álvarez B, Mesa-Garza IG, Castanedo-Cázares JP, Fuentes-Ahumada C, Oros-Ovalle C, Navarrete-Solis J, et al. Histochemical and immunohistochemical study in melasma: evidence of damage in the basal membrane. Am J Dermatopathol. 2011;33:291–295. doi: 10.1097/DAD.0b013e3181ef2d45. [DOI] [PubMed] [Google Scholar]
- 13.Na JI, Choi SY, Yang SH, Choi HR, Kang HY, Park KC. Effect of tranexamic acid on melasma: a clinical trial with histological evaluation. J Eur Acad Dermatol Venereol. 2013;27:1035–1039. doi: 10.1111/j.1468-3083.2012.04464.x. [DOI] [PubMed] [Google Scholar]
- 14.Siiskonen H, Smorodchenko A, Krause K, Maurer M. Ultraviolet radiation and skin mast cells: Effects, mechanisms and relevance for skin diseases. Exp Dermatol. 2018;27:3–8. doi: 10.1111/exd.13402. [DOI] [PubMed] [Google Scholar]
- 15.Lee HJ, Park MK, Lee EJ, Kim YL, Kim HJ, Kang JH, et al. Histamine receptor 2-mediated growth-differentiation factor-15 expression is involved in histamine-induced melanogenesis. Int J Biochem Cell Biol. 2012;44:2124–2128. doi: 10.1016/j.biocel.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida M, Takahashi Y, Inoue S. Histamine induces melanogenesis and morphologic changes by protein kinase A activation via H2 receptors in human normal melanocytes. J Invest Dermatol. 2000;114:334–342. doi: 10.1046/j.1523-1747.2000.00874.x. [DOI] [PubMed] [Google Scholar]
- 17.Iddamalgoda A, Le QT, Ito K, Tanaka K, Kojima H, Kido H. Mast cell tryptase and photoaging: possible involvement in the degradation of extra cellular matrix and basement membrane proteins. Arch Dermatol Res. 2008;300(Suppl 1):S69–S76. doi: 10.1007/s00403-007-0806-1. [DOI] [PubMed] [Google Scholar]
- 18.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang WH, Yoon KH, Lee ES, Kim J, Lee KB, Yim H, et al. Melasma: histopathological characteristics in 56 Korean patients. Br J Dermatol. 2002;146:228–237. doi: 10.1046/j.0007-0963.2001.04556.x. [DOI] [PubMed] [Google Scholar]
- 20.Parkinson LG, Toro A, Zhao H, Brown K, Tebbutt SJ, Granville DJ. Granzyme B mediates both direct and indirect cleavage of extracellular matrix in skin after chronic low-dose ultraviolet light irradiation. Aging Cell. 2015;14:67–77. doi: 10.1111/acel.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang HY, Bahadoran P, Suzuki I, Zugaj D, Khemis A, Passeron T, et al. In vivo reflectance confocal microscopy detects pigmentary changes in melasma at a cellular level resolution. Exp Dermatol. 2010;19:e228–e233. doi: 10.1111/j.1600-0625.2009.01057.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim EH, Kim YC, Lee ES, Kang HY. The vascular characteristics of melasma. J Dermatol Sci. 2007;46:111–116. doi: 10.1016/j.jdermsci.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Kim JM, Lee SE, Jeong SK, Lee SH. Upregulation of protease-activated receptor-2 in keratinocytes by epidermal vascular endothelial growth factor in vitro and in vivo. Exp Dermatol. 2017;26:286–288. doi: 10.1111/exd.13183. [DOI] [PubMed] [Google Scholar]
- 24.Regazzetti C, De Donatis GM, Ghorbel HH, Cardot-Leccia N, Ambrosetti D, Bahadoran P, et al. Endothelial cells promote pigmentation through endothelin receptor B activation. J Invest Dermatol. 2015;135:3096–3104. doi: 10.1038/jid.2015.332. [DOI] [PubMed] [Google Scholar]
- 25.Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, Fuentes-Ahumada C, Torres-Álvarez B. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35–42. doi: 10.1111/phpp.12086. [DOI] [PubMed] [Google Scholar]
- 26.Regazzetti C, Sormani L, Debayle D, Bernerd F, Tulic MK, De Donatis GM, et al. Melanocytes sense blue light and regulate pigmentation through opsin-3. J Invest Dermatol. 2018;138:171–178. doi: 10.1016/j.jid.2017.07.833. [DOI] [PubMed] [Google Scholar]
- 27.Boukari F, Jourdan E, Fontas E, Montaudié H, Castela E, Lacour JP, et al. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: a prospective randomized comparative trial. J Am Acad Dermatol. 2015;72:189–190.e1. doi: 10.1016/j.jaad.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Verallo-Rowell VM, Pua JM, Bautista D. Visible light photopatch testing of common photocontactants in female filipino adults with and without melasma: a cross-sectional study. J Drugs Dermatol. 2008;7:149–156. [PubMed] [Google Scholar]
- 29.Arrowitz C, Schoelermann AM, Mann T, Jiang LI, Weber T, Kolbe L. Effective tyrosinase inhibition by thiamidol results in significant improvement of mild to moderate melasma. J Invest Dermatol. 2019;139:1691–1698.e6. doi: 10.1016/j.jid.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Jimbow K, Obata H, Pathak MA, Fitzpatrick TB. Mechanism of depigmentation by hydroquinone. J Invest Dermatol. 1974;62:436–449. doi: 10.1111/1523-1747.ep12701679. [DOI] [PubMed] [Google Scholar]
- 31.Spencer MC. Topical use of hydroquinone for depigmentation. JAMA. 1965;194:962–964. [PubMed] [Google Scholar]
- 32.Ennes SB, Paschoalick RC, Alchorne MM. A double-blind, comparative, placebo-controlled study of the efficacy and tolerability of 4% hydroquinone as a depigmenting agent in melasma. J Dermatolog Treat. 2000;11:173–179. [Google Scholar]
- 33.Westerhof W, Kooyers TJ. Hydroquinone and its analogues in dermatology - a potential health risk. J Cosmet Dermatol. 2005;4:55–59. doi: 10.1111/j.1473-2165.2005.40202.x. [DOI] [PubMed] [Google Scholar]
- 34.Mishra SN, Dhurat RS, Deshpande DJ, Nayak CS. Diagnostic utility of dermatoscopy in hydroquinone-induced exogenous ochronosis. Int J Dermatol. 2013;52:413–417. doi: 10.1111/j.1365-4632.2011.05305.x. [DOI] [PubMed] [Google Scholar]
- 35.Kwon SH, Na JI, Choi JY, Park KC. Melasma: updates and perspectives. Exp Dermatol. 2019;28:704–708. doi: 10.1111/exd.13844. [DOI] [PubMed] [Google Scholar]
- 36.Ortonne JP. Retinoid therapy of pigmentary disorders. Dermatol Ther. 2006;19:280–288. doi: 10.1111/j.1529-8019.2006.00085.x. [DOI] [PubMed] [Google Scholar]
- 37.Griffiths CE, Finkel LJ, Ditre CM, Hamilton TA, Ellis CN, Voorhees JJ. Topical tretinoin (retinoic acid) improves melasma. A vehicle-controlled, clinical trial. Br J Dermatol. 1993;129:415–421. doi: 10.1111/j.1365-2133.1993.tb03169.x. [DOI] [PubMed] [Google Scholar]
- 38.Sheth VM, Pandya AG. Melasma: a comprehensive update: part II. J Am Acad Dermatol. 2011;65:699–714. doi: 10.1016/j.jaad.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Dogra S, Kanwar AJ, Parsad D. Adapalene in the treatment of melasma: a preliminary report. J Dermatol. 2002;29:539–540. doi: 10.1111/j.1346-8138.2002.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 40.Taylor SC, Torok H, Jones T, Lowe N, Rich P, Tschen E, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72:67–72. [PubMed] [Google Scholar]
- 41.Ferreira Cestari T, Hassun K, Sittart A, de Lourdes Viegas M. A comparison of triple combination cream and hydroquinone 4% cream for the treatment of moderate to severe facial melasma. J Cosmet Dermatol. 2007;6:36–39. doi: 10.1111/j.1473-2165.2007.00288.x. [DOI] [PubMed] [Google Scholar]
- 42.Lynde CB, Kraft JN, Lynde CW. Topical treatments for melasma and postinflammatory hyperpigmentation. Skin Therapy Lett. 2006;11:1–6. [PubMed] [Google Scholar]
- 43.Ishack S, Lipner SR. Exogenous ochronosis associated with hydroquinone: a systematic review. Int J Dermatol. 2022;61:675–684. doi: 10.1111/ijd.15878. [DOI] [PubMed] [Google Scholar]
- 44.Ahmad Nasrollahi S, Sabet Nematzadeh M, Samadi A, Ayatollahi A, Yadangi S, Abels C, et al. Evaluation of the safety and efficacy of a triple combination cream (hydroquinone, tretinoin, and fluocinolone) for treatment of melasma in Middle Eastern skin. Clin Cosmet Investig Dermatol. 2019;12:437–444. doi: 10.2147/CCID.S202285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yi X, Zhao G, Zhang H, Guan D, Meng R, Zhang Y, et al. MITF-siRNA formulation is a safe and effective therapy for human melasma. Mol Ther. 2011;19:362–371. doi: 10.1038/mt.2010.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsui MS, Petris MJ, Niki Y, Karaman-Jurukovska N, Muizzuddin N, Ichihashi M, et al. Omeprazole, a gastric proton pump inhibitor, inhibits melanogenesis by blocking ATP7A trafficking. J Invest Dermatol. 2015;135:834–841. doi: 10.1038/jid.2014.461. [DOI] [PubMed] [Google Scholar]
- 47.Shin JM, Lee JY, Lee DY, Yoon TY, Lee JC, Lim EH, et al. Proton pump inhibitors as a possible cause of vitiligo: an in vivo and in vitro study. J Eur Acad Dermatol Venereol. 2014;28:1475–1479. doi: 10.1111/jdv.12317. [DOI] [PubMed] [Google Scholar]
- 48.Kasraee B, Handjani F, Parhizgar A, Omrani GR, Fallahi MR, Amini M, et al. Topical methimazole as a new treatment for postinflammatory hyperpigmentation: report of the first case. Dermatology. 2005;211:360–362. doi: 10.1159/000088509. [DOI] [PubMed] [Google Scholar]
- 49.Kasraee B, Safaee Ardekani GH, Parhizgar A, Handjani F, Omrani GR, Samani M, et al. Safety of topical methimazole for the treatment of melasma. Transdermal absorption, the effect on thyroid function and cutaneous adverse effects. Skin Pharmacol Physiol. 2008;21:300–305. doi: 10.1159/000148222. [DOI] [PubMed] [Google Scholar]
- 50.Maeda K, Tomita Y. Mechanism of the inhibitory effect of tranexamic acid on melanogenesis in cultured human melanocytes in the presence of keratinocyte-conditioned medium. J Health Sci. 2007;53:389–396. [Google Scholar]
- 51.Cho YH, Park JE, Lim DS, Lee JS. Tranexamic acid inhibits melanogenesis by activating the autophagy system in cultured melanoma cells. J Dermatol Sci. 2017;88:96–102. doi: 10.1016/j.jdermsci.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 52.Murase D, Hachiya A, Takano K, Hicks R, Visscher MO, Kitahara T, et al. Autophagy has a significant role in determining skin color by regulating melanosome degradation in keratinocytes. J Invest Dermatol. 2013;133:2416–2424. doi: 10.1038/jid.2013.165. [DOI] [PubMed] [Google Scholar]
- 53.Karn D, Kc S, Amatya A, Razouria EA, Timalsina M. Oral tranexamic acid for the treatment of melasma. Kathmandu Univ Med J. 2012;10:40–43. doi: 10.3126/kumj.v10i4.10993. [DOI] [PubMed] [Google Scholar]
- 54.Del Rosario E, Florez-Pollack S, Zapata L, Jr, Hernandez K, Tovar-Garza A, Rodrigues M, et al. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate-to-severe melasma. J Am Acad Dermatol. 2018;78:363–369. doi: 10.1016/j.jaad.2017.09.053. [DOI] [PubMed] [Google Scholar]
- 55.Minni K, Poojary S. Efficacy and safety of oral tranexamic acid as an adjuvant in Indian patients with melasma: a prospective, interventional, single-centre, triple-blind, randomized, placebo-control, parallel group study. J Eur Acad Dermatol Venereol. 2020;34:2636–2644. doi: 10.1111/jdv.16598. [DOI] [PubMed] [Google Scholar]
- 56.Lajevardi V, Ghayoumi A, Abedini R, Hosseini H, Goodarzi A, Akbari Z, et al. Comparison of the therapeutic efficacy and safety of combined oral tranexamic acid and topical hydroquinone 4% treatment vs. topical hydroquinone 4% alone in melasma: a parallel-group, assessor- and analyst-blinded, randomized controlled trial with a short-term follow-up. J Cosmet Dermatol. 2017;16:235–242. doi: 10.1111/jocd.12291. [DOI] [PubMed] [Google Scholar]
- 57.Zhu CY, Li Y, Sun QN, Takada A, Kawada A. Analysis of the effect of different doses of oral tranexamic acid on melasma: a multicentre prospective study. Eur J Dermatol. 2019;29:55–58. doi: 10.1684/ejd.2018.3494. [DOI] [PubMed] [Google Scholar]
- 58.Cho HH, Choi M, Cho S, Lee JH. Role of oral tranexamic acid in melasma patients treated with IPL and low fluence QS Nd:YAG laser. J Dermatolog Treat. 2013;24:292–296. doi: 10.3109/09546634.2011.643220. [DOI] [PubMed] [Google Scholar]
- 59.Shin JU, Park J, Oh SH, Lee JH. Oral tranexamic acid enhances the efficacy of low-fluence 1064-nm quality-switched neodymium-doped yttrium aluminum garnet laser treatment for melasma in Koreans: a randomized, prospective trial. Dermatol Surg. 2013;39:435–442. doi: 10.1111/dsu.12060. [DOI] [PubMed] [Google Scholar]
- 60.Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, Nakakes A. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150–154. doi: 10.3109/14764172.2012.685478. [DOI] [PubMed] [Google Scholar]
- 61.Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753–757. [PMC free article] [PubMed] [Google Scholar]
- 62.Xu Y, Ma R, Juliandri J, Wang X, Xu B, Wang D, et al. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: a randomized, self-controlled, split-face study. Medicine (Baltimore) 2017;96:e6897. doi: 10.1097/MD.0000000000006897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mekawy KM, Sadek A, Seddeik Abdel-Hameed AK. Micro-needling versus fractional carbon dioxide laser for delivery of tranexamic acid in the treatment of melasma: a split-face study. J Cosmet Dermatol. 2021;20:460–465. doi: 10.1111/jocd.13537. [DOI] [PubMed] [Google Scholar]
- 64.Tawfic SO, Abdel Halim DM, Albarbary A, Abdelhady M. Assessment of combined fractional CO2 and tranexamic acid in melasma treatment. Lasers Surg Med. 2019;51:27–33. doi: 10.1002/lsm.23032. [DOI] [PubMed] [Google Scholar]
- 65.Lee JH, Park JG, Lim SH, Kim JY, Ahn KY, Kim MY, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626–631. doi: 10.1111/j.1524-4725.2006.32133.x. [DOI] [PubMed] [Google Scholar]
- 66.Nestor M, Bucay V, Callender V, Cohen JL, Sadick N, Waldorf H. Polypodium leucotomos as an adjunct treatment of pigmentary disorders. J Clin Aesthet Dermatol. 2014;7:13–17. [PMC free article] [PubMed] [Google Scholar]
- 67.González S, Pathak MA. Inhibition of ultraviolet-induced formation of reactive oxygen species, lipid peroxidation, erythema and skin photosensitization by polypodium leucotomos. Photodermatol Photoimmunol Photomed. 1996;12:45–56. doi: 10.1111/j.1600-0781.1996.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 68.Gomes AJ, Lunardi CN, Gonzalez S, Tedesco AC. The antioxidant action of Polypodium leucotomos extract and kojic acid: reactions with reactive oxygen species. Braz J Med Biol Res. 2001;34:1487–1494. doi: 10.1590/s0100-879x2001001100018. [DOI] [PubMed] [Google Scholar]
- 69.Hayakawa R, Ueda H, Nozaki T, Izawa Y, Yokotake J, Yazaki K, et al. Effects of combination treatment with vitamins E and C on chloasma and pigmented contact dermatitis. A double blind controlled clinical trial. Acta Vitaminol Enzymol. 1981;3:31–38. [PubMed] [Google Scholar]
- 70.Handog EB, Galang DA, de Leon-Godinez MA, Chan GP. A randomized, double-blind, placebo-controlled trial of oral procyanidin with vitamins A, C, E for melasma among Filipino women. Int J Dermatol. 2009;48:896–901. doi: 10.1111/j.1365-4632.2009.04130.x. [DOI] [PubMed] [Google Scholar]
- 71.Yamakoshi J, Sano A, Tokutake S, Saito M, Kikuchi M, Kubota Y, et al. Oral intake of proanthocyanidin-rich extract from grape seeds improves chloasma. Phytother Res. 2004;18:895–899. doi: 10.1002/ptr.1537. [DOI] [PubMed] [Google Scholar]
- 72.Song M, Mun JH, Ko HC, Kim BS, Kim MB. Korean red ginseng powder in the treatment of melasma: an uncontrolled observational study. J Ginseng Res. 2011;35:170–175. doi: 10.5142/jgr.2011.35.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teo W, Gan EY, Jinghan A, Chuah S, Alain K, Goh C, Thng S. Double blind placebo controlled trial to evaluate of the effectiveness of a dietary supplement rich in carotenoids as adjunct to topical lightening cream for the treatment of melasma: a pilot study. J Pigment Disord. 2015;2:1000164 [Google Scholar]
- 74.Ni Z, Mu Y, Gulati O. Treatment of melasma with Pycnogenol. Phytother Res. 2002;16:567–571. doi: 10.1002/ptr.1085. [DOI] [PubMed] [Google Scholar]
- 75.Sarkar R, Arsiwala S, Dubey N, Sonthalia S, Das A, Arya L, et al. Chemical peels in melasma: a review with consensus recommendations by Indian Pigmentary Expert Group. Indian J Dermatol. 2017;62:578–584. doi: 10.4103/ijd.IJD_490_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sarkar R, Garg V, Bansal S, Sethi S, Gupta C. Comparative evaluation of efficacy and tolerability of glycolic acid, salicylic mandelic acid, and phytic acid combination peels in melasma. Dermatol Surg. 2016;42:384–391. doi: 10.1097/DSS.0000000000000642. [DOI] [PubMed] [Google Scholar]
- 77.Lim JT, Tham SN. Glycolic acid peels in the treatment of melasma among Asian women. Dermatol Surg. 1997;23:177–179. doi: 10.1111/j.1524-4725.1997.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 78.Hurley ME, Guevara IL, Gonzales RM, Pandya AG. Efficacy of glycolic acid peels in the treatment of melasma. Arch Dermatol. 2002;138:1578–1582. doi: 10.1001/archderm.138.12.1578. [DOI] [PubMed] [Google Scholar]
- 79.Sarkar R, Kaur C, Bhalla M, Kanwar AJ. The combination of glycolic acid peels with a topical regimen in the treatment of melasma in dark-skinned patients: a comparative study. Dermatol Surg. 2002;28:828–832. doi: 10.1046/j.1524-4725.2002.02034.x. [DOI] [PubMed] [Google Scholar]
- 80.Dayal S, Sahu P, Dua R. Combination of glycolic acid peel and topical 20% azelaic acid cream in melasma patients: efficacy and improvement in quality of life. J Cosmet Dermatol. 2017;16:35–42. doi: 10.1111/jocd.12260. [DOI] [PubMed] [Google Scholar]
- 81.Wang CC, Hui CY, Sue YM, Wong WR, Hong HS. Intense pulsed light for the treatment of refractory melasma in Asian persons. Dermatol Surg. 2004;30:1196–1200. doi: 10.1111/j.1524-4725.2004.30371.x. [DOI] [PubMed] [Google Scholar]
- 82.Yun WJ, Lee SM, Han JS, Lee SH, Chang SY, Haw S, et al. A prospective, split-face, randomized study of the efficacy and safety of a novel fractionated intense pulsed light treatment for melasma in Asians. J Cosmet Laser Ther. 2015;17:259–266. doi: 10.3109/14764172.2015.1027227. [DOI] [PubMed] [Google Scholar]
- 83.Tong LG, Wu Y, Wang B, Xu XG, Tu HD, Chen HD, et al. Combination of fractional QSRL and IPL for melasma treatment in Chinese population. J Cosmet Laser Ther. 2017;19:13–17. doi: 10.1080/14764172.2016.1228980. [DOI] [PubMed] [Google Scholar]
- 84.Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther (Heidelb) 2017;7:305–318. doi: 10.1007/s13555-017-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hassan AM, Elfar NN, Rizk OM, Eissa NY. Pulsed dye laser versus intense pulsed light in melasma: a split-face comparative study. J Dermatolog Treat. 2018;29:725–732. doi: 10.1080/09546634.2018.1441487. [DOI] [PubMed] [Google Scholar]
- 86.Mun JY, Jeong SY, Kim JH, Han SS, Kim IH. A low fluence Q-switched Nd:YAG laser modifies the 3D structure of melanocyte and ultrastructure of melanosome by subcellular-selective photothermolysis. J Electron Microsc (Tokyo) 2011;60:11–18. doi: 10.1093/jmicro/dfq068. [DOI] [PubMed] [Google Scholar]
- 87.Kim T, Cho SB, Oh SH. Punctate leucoderma after 1,064-nm Q-switched neodymium-doped yttrium aluminum garnet laser with low-fluence therapy: is it melanocytopenic or melanopenic? Dermatol Surg. 2010;36:1790–1791. doi: 10.1111/j.1524-4725.2010.01751.x. [DOI] [PubMed] [Google Scholar]
- 88.Feng J, Huang L. Comparison of picosecond and nanosecond Nd:YAG 1064-nm lasers in the treatment of melasma: a split-face randomized clinical trial. Plast Reconstr Surg. 2023;151:772–777. doi: 10.1097/PRS.0000000000009994. [DOI] [PubMed] [Google Scholar]
- 89.Kim JC, Park TJ, Kang HY. Skin-aging pigmentation: who is the real enemy? Cells. 2022;11:2541. doi: 10.3390/cells11162541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cameli N, Abril E, Mariano M, Berardesca E. Combined use of monopolar radiofrequency and transdermal drug delivery in the treatment of melasma. Dermatol Surg. 2014;40:748–755. doi: 10.1111/dsu.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 91.Lolis MS, Goldberg DJ. Radiofrequency in cosmetic dermatology: a review. Dermatol Surg. 2012;38:1765–1776. doi: 10.1111/j.1524-4725.2012.02547.x. [DOI] [PubMed] [Google Scholar]
- 92.Jung JW, Kim WO, Jung HR, Kim SA, Ryoo YW. A face-split study to evaluate the effects of microneedle radiofrequency with Q-switched Nd:YAG laser for the treatment of melasma. Ann Dermatol. 2019;31:133–138. doi: 10.5021/ad.2019.31.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Choi M, Choi S, Kang JS, Cho SB. Successful treatment of refractory melasma using invasive micro-pulsed electric signal device. Med Lasers. 2015;4:39–44. [Google Scholar]
- 94.Kim HM, Lee MJ. Therapeutic efficacy and safety of invasive pulsed-type bipolar alternating current radiofrequency on melasma and rebound hyperpigmentation. Med Lasers. 2017;6:17–23. [Google Scholar]
- 95.Phansuk K, Vachiramon V, Jurairattanaporn N, Chanprapaph K, Rattananukrom T. Dermal pathology in melasma: an update review. Clin Cosmet Investig Dermatol. 2022;15:11–19. doi: 10.2147/CCID.S343332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gilchrest BA. Treatment of photodamage with topical tretinoin: an overview. J Am Acad Dermatol. 1997;36:S27–S36. doi: 10.1016/s0190-9622(97)70058-6. [DOI] [PubMed] [Google Scholar]